Abstract
Background
The effectiveness of patient decision aids (PtDAs) and other shared decision making (SDM) interventions for socially disadvantaged populations has not been well studied.
Purpose
To assess if PtDAs and other SDM interventions improve outcomes or decrease health inequalities among socially disadvantaged populations and determine the critical features of successful interventions.
Data Sources
MEDLINE, CINAHL, Cochrane, PsycINFO and Web of Science from inception to October 2019. Cochrane systematic reviews on PtDAs.
Study Selection
Randomized controlled trials of PtDAs and SDM interventions that included socially disadvantaged populations.
Data Extraction
Independent double data extraction using a standardized form and the Template for Intervention Description and Replication checklist.
Data Synthesis
Twenty-five PtDA and 13 other SDM intervention trials met our inclusion criteria. Compared to usual care, PtDAs improved knowledge (mean difference=13.91, 95% CI 9.01, 18.82 [I2=96%]) and patient-clinician communication (relative risk=1.62, 95% CI 1.42, 1.84 [I2=0%]). PtDAs reduced decisional conflict (mean difference=−9.59; 95% CI −18.94, −0.24 [I2=84%]) and the proportion undecided (standardized mean difference=0.39; 95% CI 0.28, 0.53 [I2=75%]). PtDAs did not affect anxiety (standardized mean difference=0.02, 95% CI −0.22, 0.26 [I2=70%]). Only one trial looked at clinical outcomes (hemoglobin A1C). Five out of the twelve PtDA studies that compared outcomes by disadvantaged standing found that outcomes improved more for socially disadvantaged participants. No evidence indicated which intervention characteristics were most effective. Results were similar for SDM intervention trials.
Limitations
Sixteen PtDA studies had overall unclear risk of bias. Heterogeneity was high for most outcomes. Most studies only had short-term follow-up.
Conclusions
PtDAs led to better outcomes among socially disadvantaged populations but did not reduce health inequalities. We could not determine which intervention features were most effective.
Introduction
Clinical equipoise warrants patient involvement in decision making (shared decision making [SDM]).(1) Patient decision aids (PtDAs) and other SDM interventions are often used to facilitate SDM, which has been shown to improve knowledge, risk perception and congruence between informed values and health choices.(2) PtDAs and other SDM interventions come in many forms, including paper-based interventions, computer-based interventions, or health professional training.(2,3) Each type is likely to provide different advantages and disadvantages to patients who are socially disadvantaged with respect to race, ethnicity, literacy, health literacy education, or income when compared to more socially advantaged people.
People from socially disadvantaged groups, and particularly those with lower literacy and/or health literacy, represent a substantial proportion of the population. It is estimated that about 36% of Americans have limited health literacy skills.(4) Australia and European countries report that up to 60% of their citizens have inadequate health literacy.(5,6) This global public health problem affects both high, low, and middle income countries.(7,8) Social disadvantage, whether due to lower health literacy or other characteristics such as lower education, lower income, or race, has a strong link to health inequalities.(9–12)
We published a systematic review and meta-analysis in 2014 that assessed whether SDM interventions reduced health inequalities.(13) We used a broad definition of social disadvantage in order to determine if people who are socially disadvantaged might benefit differently from PtDAs and other SDM interventions compared to non-disadvantaged people. We found that these interventions improved outcomes for socially disadvantaged groups. A narrative synthesis suggested that socially disadvantaged groups might stand to benefit the most, provided content was tailored to their needs. Most of the studies had been conducted within the prior two years, signaling a growing focus. Therefore we have updated this 2014 review to incorporate more recent evidence and to inform recommendations for the 2.0 update to the International Patient Decision Aid Standards (IPDAS). Our three objectives were:
to assess if PtDAs improved outcomes and decreased health inequalities for lower health literacy and socially disadvantaged groups,
to assess if other SDM interventions improved outcomes and decreased health inequalities for lower health literacy and socially disadvantaged groups, and
to determine the critical features of PtDAs and other SDM interventions that best support SDM for lower health literacy and disadvantaged populations.
Methods
Protocol and registration
We revised and re-registered the protocol for the 2014 review through PROSPERO (CRD42012002200).(13) We planned and reported this review using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses and the Cochrane Handbook for Systematic Reviews of Interventions.(14,15) See supplemental file 1.
Inclusion criteria
We included published randomized controlled trials that assessed the effect of PtDAs and other SDM interventions on socially disadvantaged groups and/or health inequalities. We included articles reporting at least 50% of participants from a socially disadvantaged group or if a separate analysis was conducted for this group. We considered multiple criteria for being socially disadvantaged as health literacy is well-correlated with other socially-defined characteristics.(16,17) Using this definition allowed us to broadly examine outcomes among people who might be socially disadvantaged based on one or multiple criteria. We defined a socially disadvantaged group as meeting at least one of the following criteria based on published definitions:(18,19)
People who are socially disadvantaged with respect to poverty or lower socioeconomic status
People who are socially disadvantaged as a result of their ethnicity or race
People who have lower educational attainment (no college degree)
People who have lower literacy and/or lower health literacy
People who are socially disadvantaged with respect to geographical location (areas described as disadvantaged and/or medically underserved)
People who are uninsured or on public health insurance
People who have lower numeracy
People who are socially disadvantaged as a result of speaking a primary language that differs from the official language(s) of their country of residence
We had no language restrictions. We included all conditions and clinical settings. Interventions were considered PtDAs if they appeared in the 2014, 2017, or upcoming 2021 Cochrane systematic review of PtDAs.(2,20) We defined other SDM interventions as interventions or strategies designed to engage patients in medical decision-making and/or facilitate SDM, patient involvement in medical decision-making, or patient activation.(13) This included professional coaching/training, patient coaching, skills workshops, and patient prompts, provided the aim was to increase patient engagement in decision-making. We included educational or self-management interventions that targeted activation and involvement in medical decision making. We did not prespecify any required outcomes. We allowed multiple definitions of a control group, as long as there was a group not exposed to the tested PtDA or SDM intervention.
Search strategy and study selection
To find PtDAs, we searched the 2014, 2017, and upcoming 2021 Cochrane systematic review of PtDAs.(2) To find other SDM interventions, we adapted our search strategy from the 2014 review, consulted a research librarian, and piloted it in MEDLINE via Ovid (see supplemental file 2, figure 1). We also searched CINAHL, Cochrane Central Register of Controlled Trials, Cochrane Database of Systematic Reviews, PsycINFO, and Web of Science from inception to October 2019. We hand-searched the reference list and performed a “cited by” and “related articles” search through PubMed of all included primary articles. In Google Scholar, one reviewer looked at the first 100 results to check for relevant records not already captured. We included all randomized trials reported in Durand and colleagues’ 2014 review. We independently screened the title and abstract and subsequent full text of retrieved records (two per record: RWY, M-A D, SS, LPP, GE, JE, JS, OM).(21) We resolved disagreements as a team.
Data extraction and risk of bias assessment
We conducted independent double data extraction using a pre-designed, piloted form adapted from previous reviews (two per record: RWY, M-A D, JE, SS, LPP, JM, DM, JS, OM, KM, TC, AG, AL, AH).(13,22) We extracted all intervention characteristics using an adapted version of the Template for Intervention Description and Replication (TIDieR) checklist.(23) We used the Cochrane Risk of Bias tool, version 2, to assess risk of bias (two per record: RWY, M-A D, JE, SS, LPP, JM).(24)
Data synthesis and analysis
We used a random-effects model since the included studies did not come from the same population.(25) Using R, we pooled studies in a meta-analysis to calculate a weighted effect and 95% confidence interval (CI) for outcome measures that were reported at least three times across the included studies.(26,27) For studies with more than two arms, we analyzed the arms that were closest to a control and PtDA or SDM intervention. If measures were repeated, we selected the time point that was the most conservative estimate to understand the intervention’s effect on the outcome.
For continuous outcome measures, we calculated standardized mean difference (SMD) when the tools or surveys used to measure the outcome varied using Hedges’ g method and mean difference (MD) when the outcome measurement tool was the same across studies.[21] For dichotomous outcome measures, we calculated a relative risk (RR). We conducted sensitivity analyses when findings were significant using the Hartung-Knapp method given the varying study sizes and heterogeneity across the included studies.(28–30) We excluded studies from the meta-analysis that only conducted separate analyses by socially disadvantaged status but did not have >50% participants considered socially disadvantaged as these separate analyses were not reported in sufficient detail to be included.
We conducted a narrative synthesis guided by the UK’s Economic and Social Research Council (ESRC) Methods Program to assess the PtDA or other SDM intervention’s effect on health inequalities by looking at studies that reported the impact of the interventions by socially disadvantaged versus non-socially disadvantaged status. We also used the narrative synthesis to determine the critical features of included interventions, and for all outcomes that could not be included in the meta-analysis.(31) We combined the evidence by looking at the outcomes and heterogeneity of the included studies to compare and contrast the combined results. To look at the critical features of included interventions, we stratified by whether the outcomes tested for that intervention did or did not favor its use.
Dealing with missing data
If outcomes data were not available in the primary paper, we extracted it from Stacey et al.’s review, if applicable.(2) If standard deviation data were not available, we used methods in the Cochrane Handbook to calculate standard deviation by its relationship to p-values, standard errors, or 95% confidence intervals.(32)
Assessment of heterogeneity
We looked for statistical heterogeneity using the I2 statistic to determine the percentage of variation across included studies for each outcome in the meta-analysis.(15) We assumed an I2>50% to indicate significant heterogeneity. For studies or outcomes included in the narrative synthesis, we looked at study quality, outcome measurement, and intervention differences to assess heterogeneity.(31)
Assessment of reporting bias
We used funnel plots to visually assess for potential publication bias for each outcome included in the meta-analysis.(15) Publication bias was assessed quantitatively using Egger’s regression test for asymmetry for outcomes reported by at least 10 studies.(33) A p-value of less than 0.05 indicated significant publication bias.
Subgroup and sensitivity analysis
Our primary goal was to look at the effect on outcomes for PtDAs. However, given the sufficient number of SDM interventions not considered a PtDA, we also conducted a secondary meta-analysis of outcomes including all intervention types. All studies reporting decisional conflict used either the original Decisional Conflict Scale or the low literacy Decisional Conflict Scale.(34,35) We therefore conducted a subgroup analysis for decisional conflict based on the type of scale used.
We conducted two sensitivity analyses to determine whether risk of bias in the randomization of participants or deviations from the intended interventions affected the results of all outcomes with at least six studies in the meta-analysis. Finally, we used the Cochrane standard deviation calculator for a proportion of the studies in the knowledge meta-analysis and conducted a sensitivity analysis to see if the results changed when the calculator was used.
Results
Identified studies
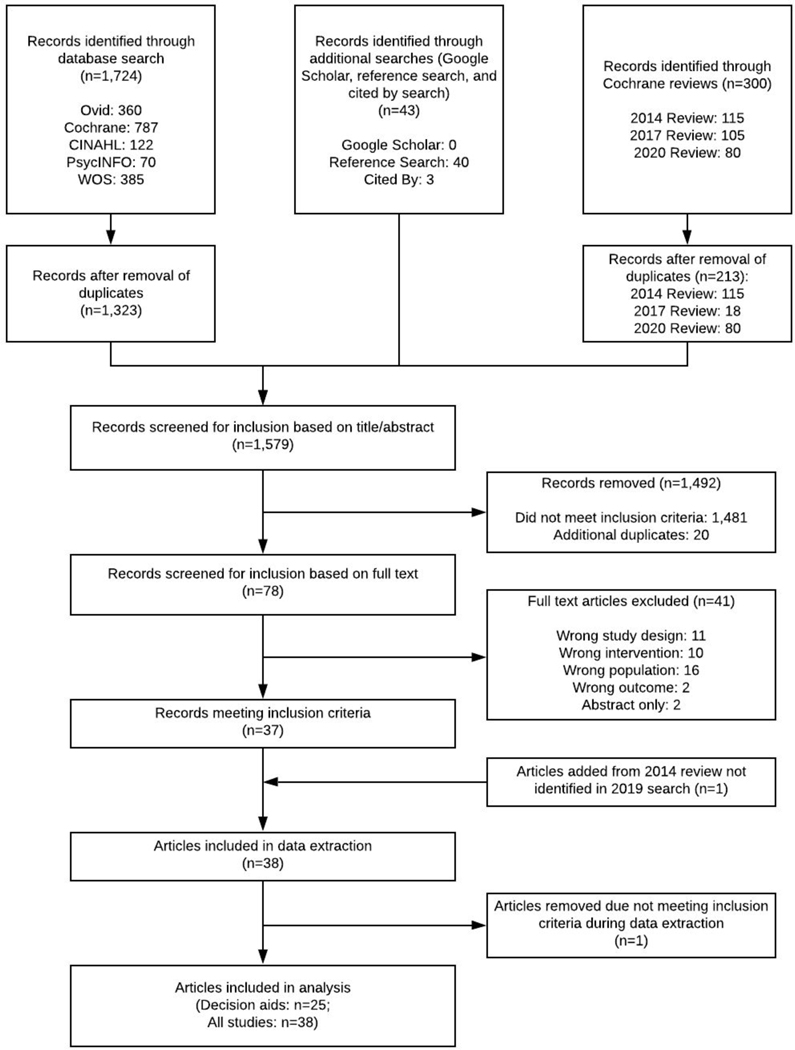
We retrieved 300 articles from the Cochrane reviews, 1,724 records from the database searches, and 43 from additional search methods. After removing duplicates, we screened the title and abstract of 1,366 records then reviewed the full text of 78 articles. Twenty-five PtDAs were included in the primary analysis.(36–60) An additional 13 studies of SDM interventions were included in the secondary analysis (figure 1).(61–73)
Figure 1. PRISMA flow diagram.
Study and participant characteristics
The number of participants ranged from 60 to 1,270, representing 9,591 in total, with an average of 384 participants/study (Table 1). Eighteen PtDA studies focused on screening behavior, six examined treatment options for various health conditions, and one focused on referral for early childhood developmental delays. Fifteen had greater than 50% of participants from racial or ethnic minorities. Eleven included participants of lower educational attainment. Six included participants reporting low annual household income. Thirteen of the studies included participants that met more than one criteria of social disadvantage. Characteristics of the other SDM intervention studies are available in supplementary file, table 1.
Table 1.
Study characteristics of included patient decision aid trials
| Author and Year | Country | Baseline N‘ (intervention | control) | Poverty, income, or SES | Race/ethnicity | Education | Low (health) literacy | Geographical location | Underinsured | Lower numeracy | Different language | Separate analysis | Medical Area/Decision |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Boulware et al. 2018§(31) | United States | 61 | 31 | 59% with income of <$20,000/year | 100% Black | 73% high school degree or less | 45% on low-income insurance (Medicaid) | Live kidney transplants for patients on hemodialysis | |||||
| Brenner et al. 2016(32) | United States | 131 | 131 | 61% Latinx, 17% Black | 29% on low-income insurance (Medicaid) | 71% of Latinx participants (61% of total) preferred Spanish | Colorectal cancer screening | ||||||
| Diefenbach et al. 2018(33) | United States | 181 | 168 | Race, education | Prostate cancer | ||||||||
| Hoffman et al. 2017(34) | United States | 59 | 29 | 100% Black | Colorectal cancer screening | ||||||||
| Ibrahim et al. 2017(35) | United States | 168 | 168^ | 50.2% household income <$15,000/year | 100% Black | Total knee replacement | |||||||
| Jibaja-Weiss et al. 2011(36) | United States | 40 | 36 | 53% Hispanic/Latina, 32% Black | Majority lower health literacy (exact % unknown) | 100% no insurance | Breast cancer surgery | ||||||
| Jimenez et al. 2017(37) | United States | 31 | 33 | 88% Black (parents) | Health literacy | Early intervention for developmental concerns | |||||||
| Kuppermann et al. 2009(38) | United States | 244 | 252 | 15.7% Black, 18.0% Latina, 13.5% Asian, 5.7% Other | Education | Prenatal genetic testing | |||||||
| Kuppermann et al. 2014(39) | United States | 357 | 353 | 48% earning ≤$25,000/year | 45% Hispanic/Latina, 16% Black, 9% Asian/Pacific Islander | 46% high school degree or less | 45% Lower numeracy | Prenatal genetic testing | |||||
| Lepore et al. 2012(40) | United States | 244 | 246 | 100% Black | 63% high school degree or less | Prostate cancer screening | |||||||
| Marteau et al. 2010(41) | United Kingdom | 633 | 639 | Socioeconomic status | Diabetes screening | ||||||||
| Miller et al. 2011(42) | United States | 132 | 132 | 70% earning <$20,000/year | 73% Black | 77% high school degree or less | 56% limited health literacy | Health literacy | Colorectal cancer screening | ||||
| Miller et al. 2018(43) | United States | 223 | 227 | 53% earning <$20,000/year | 53% high school degree or lesss | Income, health literacy, race | Colorectal cancer screening | ||||||
| Myers et al. 2005(44) | United States | 121 | 121 | 100% Black | 62% high school education or less | Prostate cancer screening | |||||||
| Rising et al. 2017(45) | United States | 451 | 457 | 62% lower health literacy | Race, income, insurance, education, health literacy and numeracy | Chest pain testing | |||||||
| Ruffin et al. 2007(46) | United States | 87 | 87 | 47% Black | Insurance, education, and race | Colorectal cancer screening | |||||||
| Schroy et al. 2011(47) | United States | 212 | 231 | 63% Black, 6% Hispanic/Latinx | 66% Medicaid, Medicare, free care, or none | Colorectal cancer screening | |||||||
| Smith et al. 2010§(48) | Australia | 384 | 188 | Recruited in low-income communities | 58% Lower educational attainment (0–10 years) | Colorectal cancer screening | |||||||
| Street et al. 1995(49) | United States | 30 | 30 | 73% Less than college education | Education | Breast cancer surgery | |||||||
| Taylor et al. 2006(50) | United States | 164 | 74 | 100% Black | 71% Less than a college degree | Prostate cancer screening | |||||||
| Trevena et al. 2008(51) | Australia | 157 | 157 | 78% Completed secondary (High school) education or less | Education | Colorectal cancer screening | |||||||
| Vina et al. 2016(52) | United States | 240 | 253 | 52% household income < $15,000 | 100% Black | Knee replacement | |||||||
| Volk et al. 2008(53) | United States | 224 | 226 | Low-literacy site: 72% Black, 9% Hispanic/Latinx | Low-literacy site: 77% High school education or less | Literacy | Prostate cancer screening | ||||||
| Williams et al. 2013§(54) | United States | 272 | 271 | 46% earning less than $50,000/year | 61% Black | 51% Less than a college degree | Race | Prostate cancer screening | |||||
| Wolf et al. 1996(55) | United States | 103 | 102 | 65% household income <$15,000 | 69% Less than high school education | 59.3% Public insurance | Prostate cancer screening |
Almost met the threshold or met the threshold at one site
Patient participants only
Intention-to-treat analyses
Included multiple intervention groups
included a clinician intervention however in order to isolate the effect of the patient-facing intervention, we only reported data from the arms that focused on patient interventions.
Description of included decision aids
Across all PtDAs, one was a verbal script with no visual component (Table 2). Five were paper-based. Thirteen were virtual, computer- or web-based interventions. Of these, nine were interactive (e.g., being able to select specific options or alter the path through the intervention) and four were static (e.g., videos only or click-through design). Six combined mediums.(36,37,49,53,55,57) Fifteen studies mentioned including consumers in the development of the PtDA and fourteen reported user-testing among socially disadvantaged groups. Seven reported readability scores which ranged from second to tenth grade. An in-depth assessment of the readability of included PtDAs is available in our companion manuscript.(74)
Table 2.
Characteristics of the included decision aid interventions*
| Study | Intervention summary | Length of Intervention/Time to Use Intervention | How Delivered | When Delivered | Where Delivered | Who Delivered | How Many Times | Tailored to the individual | Readability | Acceptable reading age | Strategies to reduce cognitive demand | Use of pictures or graphs (including icon arrays) | Use of audiovisual | Consumer involvement in development | User testing with disadvantaged groups | Language adaptation | Communication/literacy experts |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Boulware et al. 2018§(31) | The PREPARED DVD decision aid describes living donor kidney transplant and a 162-page book, written at 4th grade reading level, summarizes the evidence about treatment effects on aspects of patients’ lives. | DVD: 45 minutes Book: 162 pages |
Visual digital Paper | Before appointment | Clinic | NA - online/computer program | Once | No | Booklet: 4th grade | ✓ | ✓ | ✓ | ✓ | ✓ | |||
| Brenner et al. 2016*(32) | The CHOICE/OPCIONES video decision aid has three parts: 1) review of fecal testing and colonoscopy, 2) comparison of the two testing options, and 3) selection of screening readiness where viewers are prompted to select one of three color-coded, printed brochures based on their screening readiness. | Video: 14 minutes Brochure: Not reported |
Visual digital - interactive Paper | Before appointment | Clinic | Video: Online/computer program Brochure: Research staff |
Once | Yes | Not reported | ✓ | ✓ | ✓ | ✓ | ||||
| Diefenbach et al. 2018(33) | The Healing Choices CD-ROM decision aid includes 4 sections (1) information about prostate cancer treatment, (2) videos of ethnically diverse patient stories, (3) physician’s opinion on treatment and recovery, and (4) an opportunity to clarify personal values/preferences. | Average of 3 hours per week | Visual digital - interactive | Independent of a clinic visit | Home | NA - online/computer program | Daily to 1–2x/week | Yes | 7th grade | ✓ | ✓ | ✓ | ✓ | ||||
| Hoffman et al. 2017(34) | The video decision aid describes the anatomy of the digestive system and colon, how colorectal cancer forms, who is at risk, morbidity/mortality rates, and how it can be prevented. It then reviews three screening options and information to encourage participants to discuss options and concerns with their provider. | 30 minutes | Visual digital - static | Before appointment | Clinic | NA - online/computer program | Once | Yes | Not reported | ✓ | ✓ | ✓ | |||||
| Ibrahim et al. 2017(35) | The video decision aid includes knee osteoarthritis treatment options including lifestyle changes, medications, injections, complementary therapy and surgery, risks, benefits and known efficacy of each option, clinical indications, rehabilitative care, recovery time, effort and cost. Same decision aid as Vina 2016. | 40 minutes | Visual digital - static | Before appointment | Clinic | NA - online/computer program | Once | No | Not reported | ✓ | ✓ | ||||||
| Jibaja-Weiss* et al. 2011(36) | Patchwork of Life is a interactive, computer-based decision aid that includes 1) a soap opera of a woman’s journey through breast cancer diagnosis and treatment and 2) interactive learning modules and values clarification exercises. | Not reported | Visual digital - interactive | After appointment | Clinic | NA - online/computer program | Once | Yes | Not reported | ✓ | ✓ | ✓ | ✓ | ✓ | |||
| Jimenez et al. 2017(37) | A 3-minute decision aid video plus text message explaining child development and Early Intervention (EI). | 3 minutes | Visual digital - static | After appointment | Clinic | NA - online/computer program | Once | No | Not applicable | ✓ | ✓ | ✓ | |||||
| Kuppermann et al. 2009(38) | The “Prenatal Testing: Exploring Your Options” decision support guide is a interactive video computer program narrated by a bilingual actress and includes 1) prenatal testing education including accuracy, 2) role of preference in decision-making, 3) description of conditions associated with common mutations (e.g., Down syndrome). Same decision aid as Kuppermann 2014. | 45–60 minutes | Visual digital - interactive | Before appointment | Research facility | NA - online/computer program | Not reported | Yes | Not reported | ✓ | ✓ | ✓ | ✓ | ||||
| Kuppermann et al. 2014(39) | The “Prenatal Testing: Exploring Your Options” decision support guide is a interactive video computer program narrated by a bilingual actress and includes 1) prenatal testing education including accuracy, 2) role of preference in decision-making, 3) description of conditions associated with common mutations (e.g., Down syndrome). Same decision aid as Kuppermann 2009. | 45–60 minutes | Visual digital - interactive | Independent of a clinic visit | Not reported | NA - online/computer program | Once | Yes | Not reported | ✓ | ✓ | ✓ | ✓ | ||||
| Lepore et al. 2012(40) | The information booklet (“Prostate Cancer: Your Life - You Decide”) includes Black and White physicians and laypeople discussing the advantages and disadvantages of prostate cancer testing. It was designed for men with low literacy. The telephone counselling session provided tailored information about testing options and their risk, help clarify prefrerences, and preparation to discuss testing with their physician. | Booklet: Not reported Phone call: first, mean of 20 minutes, second, mean of 5 minutes |
Paper Over the phone | Independent of a clinic visit | Home | Trained interventionists | Three times | Yes | Flesch-Kincaid: 2.7 | ✓ | ✓ | ✓ | ✓ | ||||
| Marteau et al. 2010(41) | The informed choice intervention includes information that the participant might have an elevated risk of having diabetes, includes information about the risk of diabetes, its complications and screening/treatment outcomes, including possible harms. It included two pie charts in-line with the text. | 3 pages | Paper | Independent of a clinic visit | Home | NA - mailed to participants | Once | No | Age 11, Flesch-Kincaid 5.76 | ✓ | ✓ | ✓ | |||||
| Miller et al. 2011(42) | The CHOICE (Communicating Health Options through Interactive Computer Education) interactive video decision aid includes 1) colorectal cancer prevalence, 2) the reason for screening, and 3) a description of common screening tests. Participants can then choose to learn more about a specifıc test, view comparisons of the tests, or end the program. At the end, participants select their screening decision and the program prints a corresponding one-page color handout. | 10 minutes | Visual digital - interactive | Before appointment | Clinic | NA - online/computer program | Once | Yes | Not reported | ✓ | ✓ | ✓ | |||||
| Miller et al. 2018(43) | The mPATH-CRC program is a 8.6 minute decision aid video about colorectal cancer screening that goes over the two most common tests and lets patients “order” their own screening test. If participants “order” they provide their contact information to help get the screening procedure started. | 8.6 minutes | Visual digital - static | Before appointment | Clinic | NA - online/computer program | Once | Yes | Not reported | ✓ | ✓ | ✓ | |||||
| Myers et al. 2005(44) | Booklet provided information on prostate anatomy, prostate-related problems, risk factors and symptoms of prostate cancer, pros/cons of screening, options with an abnormal screening result, and treatment options. A decision education session for values clarification and preference scoring based on top three decision factors selected by participants. | Not reported | Paper Over the phone |
Independent of a clinic visit | Home | Trained health educator | Once | Yes | Not reported | ✓ | ✓ | ✓ | ✓ | ✓ | |||
| Rising et al. 2017(45) | The Chest Pain Choice decision aid includes the reason for and results of their emergency department evaluation, an icon array showing personal risk for acute coronary syndrome within 45 days, and potential utility of additional testing. Color coded according to the level of risk. | 1 page | Paper | During meeting with clinician | Hospital | Clinician | Once | Yes | Not reported | ✓ | ✓ | ✓ | ✓ | ||||
| Ruffin et al. 2007(46) | Colorectal Web is an online, interactive tool that presents the available screening options. It is designed to help adults determine which colorectal screening option they prefer by having them select three variables that they are considering while making a choice. At the end they select the suggested test to commit to that test. It has limited text and emphasizes graphics. | Not reported | Visual digital - interactive | Independent of a clinic visit | Research facility | NA - online/computer program | Once | Yes | Not reported | ✓ | ✓ | ✓ | |||||
| Schroy et al. 2011§(47) | Two intervention groups: (1) an interactive decision aid with touch screen features with modules on CRC screening, test features, audiovisual comparison of the options (e.g., traffic lights) and a decision-making module to help users clarify test preference. (2) same decision aid with additional “Your Disease Risk” to include personalized risk information. | Not reported | Visual digital - interactive | Before appointment | Clinic | NA - online/computer program | Once | Yes | Not reported | ✓ | ✓ | ||||||
| Smith et al. 2010§(48) | The paper based, interactive decision aid booklet (and accompanying DVD) combines words and number to provide information about colorectal cancer and colorectal cancer screening, uses simplified text and bullet points, has a glossary of medical terms, and a medical diagram of the bowl. | 33 pages + DVD | Paper Visual digital - interactive |
Independent of a clinic visit | Home | NA - mailed to participants | Not reported | Yes | 7th grade | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |
| Street et al. 1995(49) | An interactive multimedia program (“Options for Treating Breast Cancer”) utilizing touchscreen, graphics, audio, music, and video clips with four sections: (1) introduction, (2) understanding the problem, (3) treatment options, and (4) experiences of other women. | 30–45 minutes | Visual digital - interactive | Before appointment | Clinic | NA - online/computer program | Once | Yes | Not reported | ✓ | ✓ | ||||||
| Taylor et al. 2006*§(50) | Two intervention groups: (1) a 16-page booklet with information about prostate cancer screening, questions to as a doctor, glossary, and celebrity spokesperson, (2) a 25-minute video narrated by a celebrity spokesperson of a middle-aged Black man discussing screening with friends and family then his doctor to facilitate the decision process. | Booklet: 16 pages Video: 25 minutes |
Paper Visual digital - static |
Independent of a clinic visit | Home | NA - mailed to participants NA - online/computer program |
Once | No | Not reported | ✓ | ✓ | ✓ | ✓ | ✓ | |||
| Trevena et al. 2008(51) | The paper-based decision aid booklet addresses 1) definition of FOBT (fecal occult blood test) screening, 2) baseline probability based on personal risk factors for colorectal cancer over 10 years, 3) risk reduction with screening, 4) probability of FOBT outcomes over 10 years, and 5) how to use FOBT kit. | 20 pages | Paper | Independent of a clinic visit | Home | NA - mailed to participants | Once | Yes | 10th grade | ✓ | ✓ | ||||||
| Vina et al. 2016(52) | Two components: (1) the video decision aid tested in Ibrahim 2017, (2) face-to-face motivational interviewing session with trained, certified interventionists to discuss their thoughts regarding total knee arthoplasty and their goals/values. | Video: 40 minutes Interview: 30 minutes |
Video: Visual digital - static Interview: Person to person |
Independent of a clinic visit | Not reported | Video: NA - online/computer program Interview: Trained interventionist |
Once | Yes | Not reported | ✓ | |||||||
| Volk et al. 2008(53) | The computer-based decision aid included 1) soap opera segments on prostate cancer facts, risk factors, screening, treatment, and complications and 2) interactive learning modules. There are celebrity testimonials. The last part was a values-clarification exercise using a social-matching scenario (“Pick who is most like you”) | 53–68 minutes | Visual digital - interactive | Before appointment | Clinic | NA - online/computer program | Once | Yes | Not reported | ✓ | ✓ | ✓ | ✓ | ||||
| Williams et al. 2013§(54) | The Prostate Cancer Screening: Making an Informed Decision decision aid booklet includes the information on 1) the leading causes of death among men, 2) the accuracy of the prostate-specific antigen test, 3) guidelines for prostate cancer screeing, and 4) how prostate cancer is diagnosed and treated. There is a 10-item values clarification section. | 20 minutes to read (24 pages) | Paper | Before appointment | Clinic Home | Researcher or mailed to participants | Once | No | 8th grade | ✓ | ✓ | ✓ | |||||
| Wolf et al. 1996(55) | A script read by a trained research assistant that included an overview of PSA screening, lifetime probability of developing and dying from prostate cancer, known risk factors, management options, and major complications. | Not reported | Person to person | Before appointment | Clinic | Trained research assistant | Once | No | Not applicable | ✓ |
Intervention characteristics pulled from the published protocol or other publications on the tool.(38–45)
Included multiple intervention groups
included a clinician intervention however in order to isolate the effect of the patient-facing intervention, we only reported data from the arms that focused on patient interventions.
Twelve of the PtDAs were delivered before a specific clinic visit, two were delivered after a specific clinic visit, ten were delivered independent of a specific clinic visit, and one was delivered during the clinic visit. The majority (n=21) were used by or delivered to participants only once. One was given to patients to use repeatedly at home.(38) Eighteen had components tailored to the participant. Our companion paper presents more details regarding how PtDAs were tailored for people who are socially disadvantaged.(74) The other SDM interventions had a similar mix of attributes (supplementary file 2, table 2).
Risk of bias in included studies
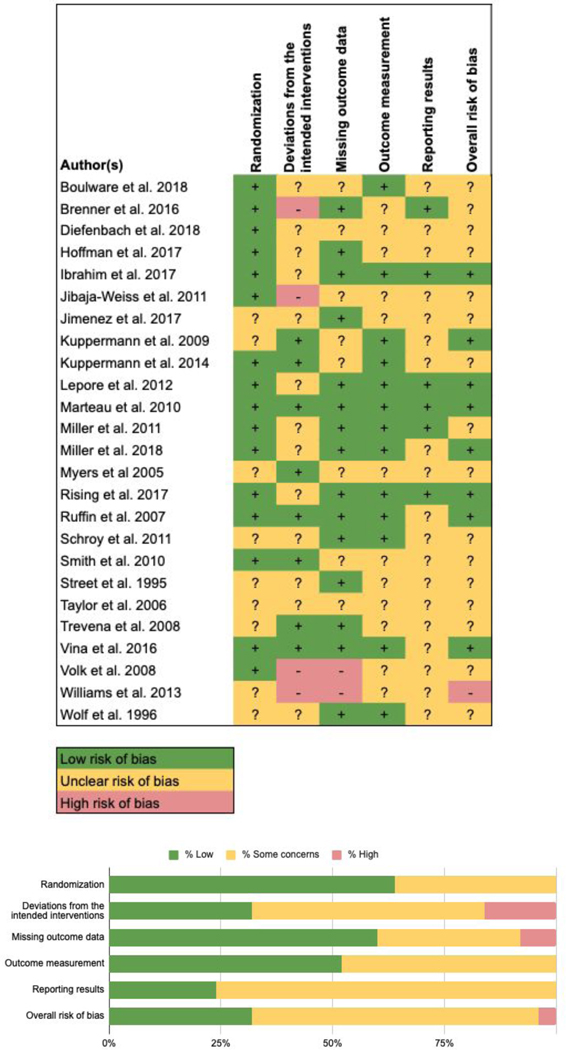
A large majority of the PtDAs studies had overall unclear risk of bias (16/25) (figure 2). Reasons for unclear risk of bias varied but the largest domain was reporting results (19/25). This typically occurred because information on a protocol or trial registry was limited or missing, or there was disagreement between the published study and published protocol or trial registry. Most studies had low risk of bias due to randomization procedures (16/25). The highest risk of bias domain was due to deviations from the intended interventions (4/25) which was usually related to a lack of blinding. Risk of bias analysis was similar for the other SDM interventions (supplementary file 2, figure 2).
Figure 2. Risk of bias for included patient decision aid studies.
Meta-analysis
Knowledge
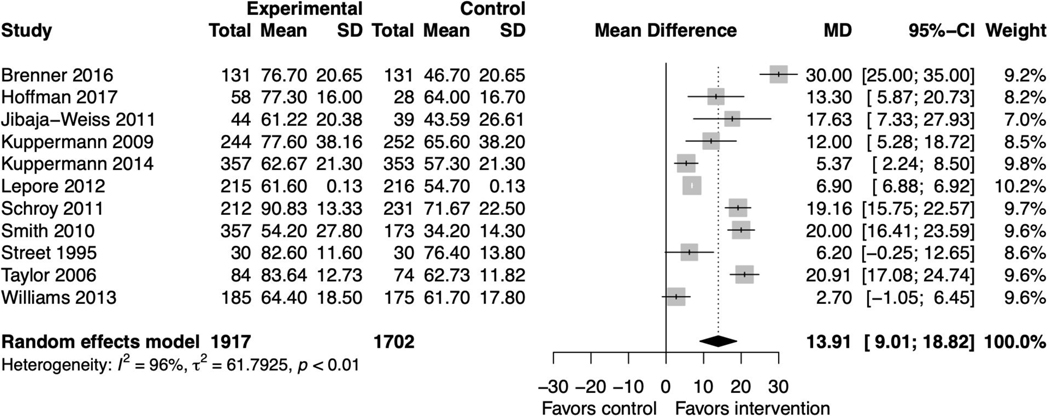
Fourteen PtDA studies reported knowledge as an outcome measure.(37,39,41–45,52–56,58,59) The pooled mean difference was 13.91 (95% CI 9.01, 18.82) favoring the intervention with substantial heterogeneity (I2=96%, p<0.01) (figure 3). Sensitivity analyses did not affect this finding (supplemental file 2, figures 3–6). Among the three studies not included in the meta-analysis due to insufficient information, one reported no significant difference,(58) and two reported differences favoring the intervention.(42,56) When including other SDM intervention studies, results remained significant, favoring the interventions (supplemental file 2, figure 7).(63,65,67,69,71,73)
Figure 3. Forest plot for decision aid studies reporting knowledge.
Decisional conflict
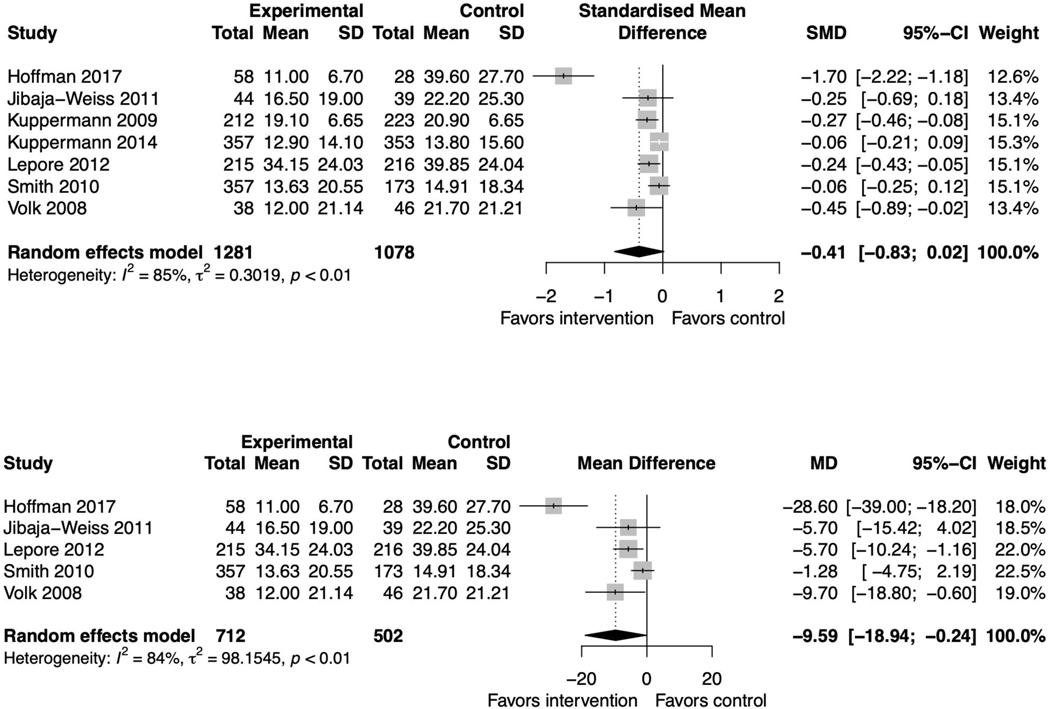
Fourteen PtDA studies reported decisional conflict as an outcome measure.(39,41,43–45,53,55,56,58,59) The pooled standardized mean difference was −0.41 points (95% CI −0.83, 0.02) favoring the intervention but not statistically significant with significant heterogeneity (I2=85%, p<0.01) (figure 4A). There were no changes to statistical significance in the sensitivity analyses (supplemental file 2, figures 8–9). Among the three studies with insufficient information for inclusion in the meta-analysis, two reported no significant differences between the intervention and control groups.(56,59) One reported that a print-based intervention reduced decisional conflict more than their video-based intervention or usual care.(55)
Figure 4. Primary forest plots for decisional conflict.
Figure 4A. Forest plot for all decision aids studies reporting decisional conflict
Figure 4B: Forest plot for studies using the low literacy Decisional Conflict Scale
When we sub-grouped by scale used, the pooled mean difference for PtDA studies using the low literacy scale (n=5) was −9.59 points (−18.94, −0.24) favoring the intervention with significant heterogeneity (I2=84%, p<0.01) (figure 4B). This lost significance in sensitivity analysis (supplemental file 2, figure 10). Of the two studies that measured decisional conflict using the original scale, one found lower decisional conflict in the intervention arm; one found no differences.(43,44) These findings were similar when including all SDM interventions (supplemental file 2, figures 11–12).(65,71)
Patient participation in care
Three PtDA studies reported patient participation in care but all three did not report with sufficient detail to perform a meta-analysis. Two found higher patient participation in the intervention arm, (39,58) and one found no differences.(54) When including other SDM interventions, there were six studies to include in the meta-analysis. The pooled standardized mean difference was 0.23 (95% CI 0.05, 0.42) favoring the intervention with low heterogeneity (I2=35%, p=0.17) (supplemental file 2, figure 13).(39,58,61,66,70,71)
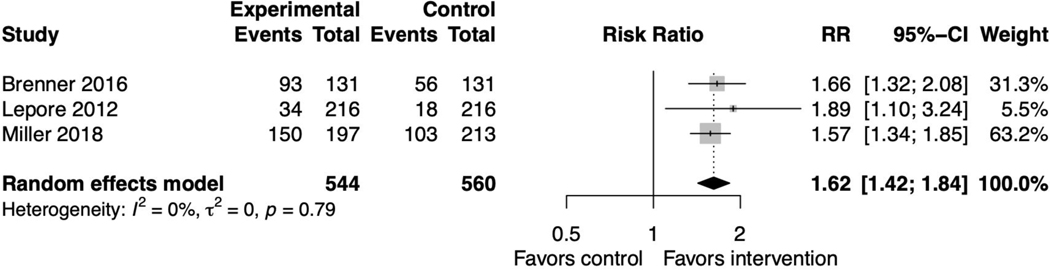
Patient-clinician communication
Four PtDA studies reported patient-clinician communication about the decision being made.(36,37,45,47) The pooled relative risk was 1.62 (95% CI 1.42, 1.84) favoring the intervention with no heterogeneity (I2=0%, p=0.79) (figure 5). Sensitivity analyses did not affect this outcome (supplemental file 2, figure 14). One study did not distinguish between communication with a clinician versus a family member and was not included in the meta-analysis.(36) These findings were similar when including all SDM interventions (supplemental file 2, figure 15).
Figure 5. Forest plot for decision aid studies reporting patient-clinician communication.
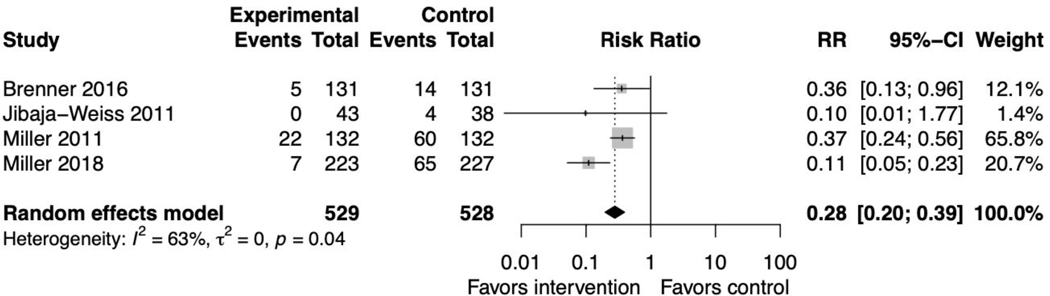
Proportion undecided
Four PtDA studies reported whether participants were undecided about their treatment/screening approach after study participation.(37,41,47,48) The pooled relative risk was 0.28 (95% CI 0.20, 0.39) favoring the intervention with reasonably high heterogeneity (I2=63%, p=0.04) (figure 6). This remained significant in the sensitivity analysis (supplemental file 2, figure 16). When including all SDM intervention studies, results remained significant, favoring the interventions (supplemental file 2, figure 17).(71,73)
Figure 6. Forest plot for decision aid studies reporting proportion undecided.
Informed choice
Three PtDA studies measured whether participants made an informed choice.(37,53,56) but some did not report with enough detail to conduct a meta-analysis. All three found that more participants made an informed choice in the intervention arm. When including SDM interventions, three studies could be pooled into the meta-analysis where the pooled relative risk was 2.23 (95% CI 1.24, 4.01) favoring the intervention with substantial heterogeneity (I2=83%, p<0.01) (supplemental file 2, figure 18).(53,56,71) One additional study did not report findings with sufficient detail to be included in the meta-analysis and found no differences.(65)
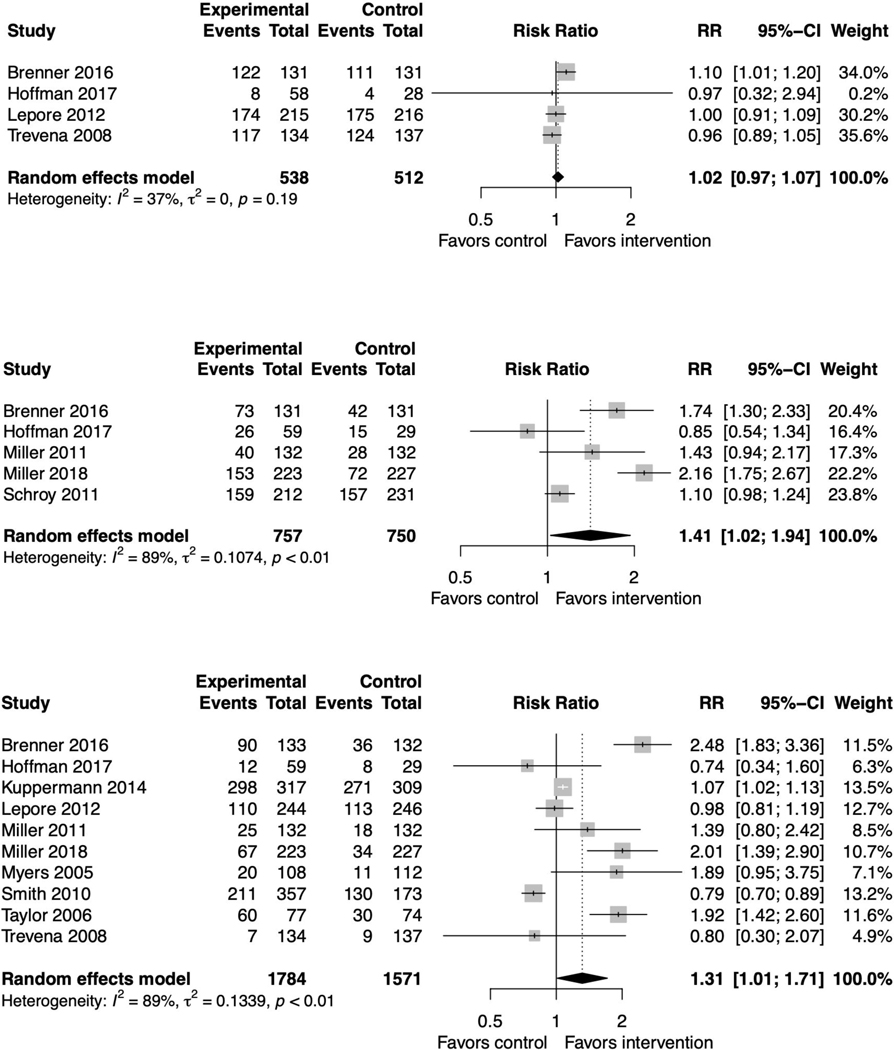
Screening behavior
Six studies measured screening intent.(37,39,45,52,55,56) The pooled relative risk was 1.02 (95% CI 0.97, 1.07) favoring neither the intervention nor the control with non-significant heterogeneity (I2=47%, p=0.19) (figure 7a). Sensitivity analysis did not affect this outcome (supplemental file 2, figure 19). Two studies did not report their findings with sufficient detail to be included in the meta-analysis; one found significantly higher intent in the intervention arm,(52) and one found high intent at baseline regardless of arm.(55) These findings remained consistent when including all SDM interventions (supplemental file 2, figure 20).(63) Two additional studies reported outcomes on screening readiness and screening interest, and reported that the interventions increased screening readiness and interest.(47,60)
Figure 7. Forest plots for decision aid studies reporting screening behavior.
Figure 7A. Intent to be screened
Figure 7B. Screening test ordered
Figure 7C. Screening test done
Five reported whether screening tests were ordered.(37,39,47,48,52) The pooled relative risk was 1.41 (95% CI 1.02, 1.94) slightly favoring the intervention with high heterogeneity (I2=89%, p<0.01) (figure 7b). Sensitivity analyses did not affect this result (supplemental file 2, figure 21). These findings held when including all SDM interventions (supplemental file 2, figure 22).(68)
Ten studies measured screening test uptake.(37,39,44,45,47–49,53,55,56,59) The pooled relative risk was 1.31 (95% CI 1.01, 1.71) slightly favoring the intervention with high heterogeneity (I2=89%, p<0.01) (figure 7c). One study did not report this outcome with sufficient detail; it found no differences between arms.(59) Sensitivity analyses resulted in loss of statistical significance (supplemental file 2, figures 23–25). These findings held when including all SDM interventions (supplemental file 2, figure 26).(65,68,72)
Anxiety
Three PtDA studies measured anxiety, but not all with sufficient detail to perform a meta-analysis. All found no differences.(45,53,56) There were enough studies when including other SDM intervention studies to perform a meta-analysis. The pooled standardized mean difference was 0.02 (−0.22, 0.26) favoring neither the intervention nor the control with significant heterogeneity (I2=70%, p=0.02) (supplemental file, figure 27).(45,53,65,72) One study that did not report with sufficient detail for the meta-analysis found no difference.(63) One reported their outcome as distress and thus was not pooled in the meta-analysis, it found the intervention reduced distress.(67)
Publication bias
For the two outcomes with at least 10 studies, the Egger’s regression indicated there was significant publication bias for knowledge (p=0.03) but not for screening completed (p=0.30). We did not observe publication bias when examining the funnel plots for the remaining outcomes in the meta-analysis (supplemental file, figures 28–34).
Narrative synthesis
Effect on health inequalities
Twelve PtDA studies reported findings that compared the effect of the intervention among who were and were not socially disadvantaged.(38,42,43,46–48,50,51,54,56,58,59) Five found that their interventions were more effective among socially disadvantaged participants based on literacy (n=3), education (n=2), and race (n=1).(38,42,50,56,58) Seven found no differences based on race (n=4), socioeconomic status, income or deprivation (n=4), insurance (n=1), numeracy (n=1), education (n=1), or literacy (n=1).(46–48,50,51,54,59) Two found more improvements in non-disadvantaged populations when stratifying by education, race, or numeracy.(43,50) See supplemental file 2, table 3 for a summary by outcome.
Other outcomes
For all outcomes that could not be pooled into a meta-analysis, the results of the narrative synthesis are available in supplemental file, table 4. Briefly, PtDAs influenced treatment choice but there were no observed differences in satisfaction, attitudes, or self-efficacy.
Other clinical outcomes
Only one PtDA study measured a clinical outcome not already included in the meta-analysis. It found no differences in hemoglobin A1C improvement by arm.(67) Three additional SDM intervention studies measured clinical outcomes.(62,64,72) Two saw no differences, one found that blood pressure improved more in the intervention arm.(62)
Characteristics of successful interventions
There was limited evidence on which characteristics of the interventions or attributes of the intervention development were more effective at promoting SDM. There were no patterns with respect to intervention length, mode of delivery, time of delivery, involvement of consumers, or user-testing with the socially disadvantaged participants of interest to indicate what might be more likely to improve any of the included outcomes. Tailoring to the individual participants did not differentially affect outcomes.
Across the studies that analyzed their results by socially disadvantaged status, from the five that saw greater benefit for those who are socially disadvantaged, three were computer-based and two were paper-based. Two were independent of a clinic visit, one was delivered before the visit, one was delivered during the visit, and one was delivered after the visit. Three were delivered at a clinic or hospital via a computer, care manager, or physician and two were delivered at home. Four were tailored to the participant and one was not.
Discussion
Summary of main findings
In this meta-analysis, we found that PtDAs tested among socially disadvantaged populations improved knowledge, patient-clinician communication, and ordering or receipt of a screening test. They reduced decisional conflict and the proportion of people undecided. They did not have an effect on anxiety. These findings held when including all SDM interventions. When including the additional SDM studies, interventions also improved informed choice and had a weak effect on patient participation in care. There was limited information on the PtDAs’ or other SDM interventions’ effects on clinical outcomes. In the narrative synthesis, we found that PtDAs influenced treatment choice. There were no differences in satisfaction, attitudes, or self-efficacy.
Among the twelve PtDA studies that included an analysis of outcomes based on being a member of a socially disadvantaged group relative to those not defined as disadvantaged, less than half found that their interventions were more successful among those who were socially disadvantaged. There was significant heterogeneity regarding key features of the PtDAs and no specific features led to improved outcomes for socially disadvantaged populations. Tailoring the intervention to the user did not disproportionately improve outcomes.
We therefore concluded that while most outcomes of interest were better in PtDA or other SDM intervention arms, there was no evidence of a reduction in health inequalities through the use of these interventions (aims 1 and 2). Additionally, there were no critical features that stood out as exceptionally improving outcomes among socially disadvantaged groups (aim 3).
Strengths and limitations
Strengths and limitations of the included studies
We limited our analysis to randomized controlled trials that represented multiple complex definitions of social disadvantage. A large number of studies had an overall unclear risk of bias. This was often because there was not enough information in the article about study personnel blinding or prespecified outcomes. The range of included interventions and controls could be seen as a limitation because of the heterogeneity this might have introduced. The overwhelming majority of the studies were from the US and all included studies were from wealthy countries.
Strengths and limitations of the review method
We built on and strengthened the meta-analysis conducted in 2014.(13) The 2014 review included seven randomized controlled trials compared to the 38 in this analysis (including PtDAs and SDM interventions). We used the newest version of the Cochrane Risk of Bias tool, strengthening our risk of bias assessment. We included a critical appraisal of the included interventions using the validated TIDieR checklist.(23) We included a primary analysis of PtDAs as well as a secondary analysis of all included SDM interventions, which allowed us to confirm our results within the larger body of SDM literature.
There was substantial heterogeneity for most outcomes so we must take this into account. Using the Hartung-Knapp method was a methodological strength but showed that some results were sensitive to this emerging analysis approach. We captured three studies in our database search that are reported as PtDAs but are not in Stacey and colleagues’ updated 2021 Cochrane review so were not included as PtDAs in this analysis.(65,69,71)
Comparison with other studies
Our findings align with the conclusions from the 2014 review regarding knowledge, informed choice, and patient participation.(13) Different from this previous review however, our results showed that PtDAs and other SDM interventions did not reduce health inequalities since there was no differential benefit to socially disadvantaged populations. This could be because of the larger number of studies in the updated review. Our findings align with Stacey and colleagues’ 2017 Cochrane, indicating that PtDAs may improve knowledge and informed choice, lower decisional conflict, and but have no association with anxiety.(2) They found significant reductions in people having prostate-specific antigen testing but found no significance for other screening decisions. Stacey and colleagues’ 2017 review includes 105 PtDAs, over four times the number of PtDAs included in our more narrowly-focused review. While our analysis is robust with important findings that can inform future work, additional rigorous randomized controlled designs are needed to examine interventions among socially disadvantaged groups. Specifically, there is a lack of evidence demonstrating the effectiveness of decision-making interventions among people who are socially disadvantaged compared to those who are not disadvantaged.
Implications for research and clinical practice
The differences in how people who are socially disadvantaged receive care have been well documented.(75–79) These differences are compounded by the likelihood that people who are socially disadvantaged are less likely to seek out health information.(80,81) We need additional research on how PtDAs and other decision-making interventions improve longer term outcomes such as clinical indicators and decision satisfaction. Additionally, we need further research on the differential impact these interventions might have on the health care experiences among people from socially disadvantaged groups. For example, PtDAs for common health conditions that have been specifically tested among socially disadvantaged groups.
National policy in recent years has highlighted the need for improvements in patient-centered care with limited discussion on how this shift might affect populations differently depending on their background, literacy, or socioeconomic status.(82–84) The current IPDAS criteria and the SUNDAE (Standards for UNiversal reporting of patient Decision Aid Evaluation) checklist encourage developers to write interventions in plain language. However, in addition to the barrier of lower health literacy other factors can result in poorer care and worse outcomes, including lower education, minority race/ethnicity, lower socioeconomic status, or lower income.(75–79) Only including recommendations for plain language might not fully address the various complex needs of those who are socially disadvantaged.(85,86) Both the IPDAS criteria and the SUNDAE checklist include user-testing or stakeholder involvement, however it could be emphasized that patient involvement and user-testing should include a range of participants the PtDAs are designed for, particularly those who are socially disadvantaged. Future research could delve more into the complexities of PtDAs and other decision-making interventions across the range of patient backgrounds. Redefining how policymakers and researchers think about what it means to be socially disadvantaged in a complex healthcare system will help us create and implement interventions that are appropriately able to change the care these populations receive.
Conclusions
This updated review shows strong evidence that PtDAs and other SDM interventions for socially disadvantaged populations can improve patient-reported outcomes. However, this review did not reveal what PtDA characteristics best support populations who are socially disadvantaged. Despite the evidence presented here, the development of tailored, effective interventions for socially disadvantaged populations is not keeping up with the broader global trajectory focused on the development of SDM interventions. It is critical to keep using interventions proven to be effective, and develop, adapt, and evaluate, interventions that ensure socially disadvantaged groups can benefit the most from their implementation.
Supplementary Material
Highlights.
Systematic review and meta-analysis of patient decision aids and other shared decision making (SDM) interventions for disadvantaged populations
Patient decision aids and other SDM interventions improve patient-reported outcomes for disadvantaged populations
There was no evidence on what intervention characteristics best supported disadvantaged populations
Acknowledgments
Thank you to Paige Scudder, Dartmouth research librarian, for her assistance with developing the search strategy. Thank you to Myrtle Mitchell, our patient partner, who helped us design this study and reviewed the protocol.
There was no financial support for this study.
M-A D has contributed to the development of the Option Grid patient decision aids, which are licensed to EBSCO Health. She receives consulting income from EBSCO Health, and may receive royalties in the future.
Glyn Elwyn has edited and published books that provide royalties on sales by the publishers: the books include Shared Decision Making (Oxford University Press) and Groups (Radcliffe Press). Glyn Elwyn’s academic interests are focused on shared decision making and coproduction. He owns copyright in measures of shared decision making and care integration, namely collaboRATE, integRATE (measure of care integration, consideRATE (patient experience of care in serious illness), coopeRATE (measure of goal setting), incorpoRATE (clinician attitude to shared decision making, Observer OPTION-5 and Observer OPTION-12 (observer measures of shared decision making). He has in the past provided consultancy for organizations, including: 1) Emmi Solutions LLC who developed patient decision support tools; 2) National Quality Forum on the certification of decision support tools; 3) Washington State Health Department on the certification of decision support tools. He is the Founder and Director of &think LLC which owns the registered trademark for Option GridsTM patient decision aids; Founder and Director of SHARPNETWORK LLC, a provider of online training for shared decision making. He has provided advice in the domain of shared decision making and patient decision aids to: 1) Access Community Health Network, Chicago (Adviser to Federally Qualified Medical Centers); 2) EBSCO Health (Consultant); 3) Bind On-Demand Health Insurance (Consultant), 4) abridge AI Inc (Chief Clinical Research Scientist).
Footnotes
Conflicts of Interest
None for RWY, JS, JE, DMM, SS, JM, LPP, AJO, JKL, OM, TC, AG, AJH, AL, KM.
References
- 1.Elwyn G, Durand MA, Song J, Aarts J, Barr PJ, Berger Z, et al. A three-talk model for shared decision making: multistage consultation process. BMJ. 2017. Nov 6;359:j4891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stacey D, Légaré F, Lewis K, Barry MJ, Bennett CL, Eden KB, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev [Internet]. 2017;(4). Available from: http://doi.wiley.com/10.1002/14651858.CD001431.pub5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Légaré F, Adekpedjou R, Stacey D, Turcotte S, Kryworuchko J, Graham ID, et al. Interventions for increasing the use of shared decision making by healthcare professionals. Cochrane Database Syst Rev. 2018. Jul 19;7:CD006732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cutilli CC, Bennett IM. Understanding the health literacy of America: results of the National Assessment of Adult Literacy. Orthop Nurs. 2009. Jan;28(1):27–32; quiz 33–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Australian Institute of Health and Welfare 2018. Australia’s health 2018. Australia’s health series no. 16. AUS 221. Canberra: AIHW.; [Google Scholar]
- 6.Sørensen K, Pelikan JM, Röthlin F, Ganahl K, Slonska Z, Doyle G, et al. Health literacy in Europe: comparative results of the European health literacy survey (HLS-EU). Eur J Public Health. 2015. Dec;25(6):1053–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rajah R, Hassali MAA, Murugiah MK. A systematic review of the prevalence of limited health literacy in Southeast Asian countries. Public Health. 2019. Feb;167:8–15. [DOI] [PubMed] [Google Scholar]
- 8.McClintock HF, Alber JM, Schrauben SJ, Mazzola CM, Wiebe DJ. Constructing a measure of health literacy in Sub-Saharan African countries. Health Promot Int [Internet]. 2019. Aug 22; Available from: 10.1093/heapro/daz078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Paasche-Orlow MK, Wolf MS. The causal pathways linking health literacy to health outcomes. Am J Health Behav. 2007. Sep;31 Suppl 1:S19–26. [DOI] [PubMed] [Google Scholar]
- 10.Nutbeam D, Lloyd JE. Understanding and Responding to Health Literacy as a Social Determinant of Health. Annu Rev Public Health. 2021. Apr 1;42:159–73. [DOI] [PubMed] [Google Scholar]
- 11.Egede LE. Race, ethnicity, culture, and disparities in health care. J Gen Intern Med. 2006. Jun;21(6):667–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Braveman P, Gottlieb L. The social determinants of health: it’s time to consider the causes of the causes. Public Health Rep. 2014. Jan;129 Suppl 2:19–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Durand M-A, Carpenter L, Dolan H, Bravo P, Mann M, Bunn F, et al. Do Interventions Designed to Support Shared Decision-Making Reduce Health Inequalities? A Systematic Review and Meta-Analysis. Malaga G, editor. PLoS One. 2014. Apr 15;9(4):e94670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009. Jul 21;6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. , editors. Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019) [Internet]. Cochrane; 2019. [cited 2020 Jun 2]. Available from: https://training.cochrane.org/handbook [Google Scholar]
- 16.Adult Literacy and Life Skills Survey, Summary Results. Commonwealth of Australia; 2006. Report No.: 4228.0. [Google Scholar]
- 17.Martin LT, Ruder T, Escarce JJ, Ghosh-Dastidar B, Sherman D, Elliott M, et al. Developing predictive models of health literacy. J Gen Intern Med. 2009. Nov;24(11):1211–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kilbourne AM, Switzer G, Hyman K, Crowley-Matoka M, Fine MJ. Advancing health disparities research within the health care system: a conceptual framework. Am J Public Health. 2006. Dec;96(12):2113–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Enard KR, Dolan Mullen P, Kamath GR, Dixon NM, Volk RJ. Are cancer-related decision aids appropriate for socially disadvantaged patients? A systematic review of US randomized controlled trials. BMC Med Inform Decis Mak. 2016. Jun 6;16:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stacey D, Légaré F, Col NF, Bennett CL, Barry MJ, Eden KB, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev [Internet]. 2014. Jan 28;(1). Available from: http://doi.wiley.com/10.1002/14651858.CD001431.pub4 [DOI] [PubMed] [Google Scholar]
- 21.Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan-a web and mobile app for systematic reviews. Syst Rev. 2016. Dec 5;5(1):210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scholl I, Koelewijn-van Loon M, Sepucha K, Elwyn G, Légaré F, Härter M, et al. Measurement of shared decision making - a review of instruments. Z Evid Fortbild Qual Gesundhwes. 2011. May 4;105(4):313–24. [DOI] [PubMed] [Google Scholar]
- 23.Hoffmann TC, Glasziou PP, Boutron I, Milne R, Perera R, Moher D, et al. Better reporting of interventions: Template for intervention description and replication (TIDieR) checklist and guide. BMJ. 2014. Mar 7;348:g1687. [DOI] [PubMed] [Google Scholar]
- 24.Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019. Aug 28;366:l4898. [DOI] [PubMed] [Google Scholar]
- 25.Riley RD, Higgins JPT, Deeks JJ. Interpretation of random effects meta-analyses. BMJ. 2011. Feb 10;342:d549. [DOI] [PubMed] [Google Scholar]
- 26.Harrer M, Cuijpers P, Furukawa TA, Ebert DD. Doing Meta-Analysis in R: A Hands-on Guide [Internet]. 2019. [cited 2020 Jun 2]. Available from: https://bookdown.org/MathiasHarrer/Doing_Meta_Analysis_in_R/
- 27.R Core Team (2020). R: A language and environment for statistical computing [Internet]. Vienna, Austria; Available from: https://www.R-project.org/ [Google Scholar]
- 28.Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. , editors. Chapter 10: Analysing data and undertaking meta-analyses. In: Cochrane Handbook for Systematic Reviews of Interventions version 60 (updated July 2019). 2019. [Google Scholar]
- 29.Sidik K, Jonkman JN. A simple confidence interval for meta-analysis. Stat Med. 2002. Nov 15;21(21):3153–9. [DOI] [PubMed] [Google Scholar]
- 30.Hartung J, Knapp G. A refined method for the meta-analysis of controlled clinical trials with binary outcome. Stat Med. 2001. Dec 30;20(24):3875–89. [DOI] [PubMed] [Google Scholar]
- 31.Popay J, Roberts H, Sowden A, Petticrew M, Duffy S. Guidance on the conduct of narrative synthesis in systematic reviews: A product from the ESRC Methods Programme. 2006. Jan 1 [cited 2020 Jun 2]; Available from: https://www.researchgate.net/publication/233866356_Guidance_on_the_conduct_of_narrative_synthesis_in_systematic_reviews_A_product_from_the_ESRC_Methods_Programme
- 32.Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. , editors. Chapter 6: Choosing effect measures and computing estimates of effect. In: Cochrane Handbook for Systematic Reviews of Interventions version 60 (updated July 2019). 2019. [Google Scholar]
- 33.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997. Sep 13;315(7109):629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.O’Connor AM. User Manual - Decisional Conflict Scale (10 item question format) [document on the Internet] [Internet]. Ottawa: Ottawa Hospital Research Institute; © 1993. [updated 2010]. Available from: http://decisionaid.ohri.ca/docs/develop/User_Manuals/UM_Decisional_Conflict.pdf [Google Scholar]
- 35.O’Connor AM. Validation of a decisional conflict scale. Med Decis Making. 1995. Jan;15(1):25–30. [DOI] [PubMed] [Google Scholar]
- 36.Boulware LE, Ephraim PL, Ameling J, Lewis-Boyer L, Rabb H, Greer RC, et al. Effectiveness of informational decision aids and a live donor financial assistance program on pursuit of live kidney transplants in African American hemodialysis patients. BMC Nephrol. 2018. May 3;19(1):107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brenner AT, Hoffman R, McWilliams A, Pignone MP, Rhyne RL, Tapp H, et al. Colorectal Cancer Screening in Vulnerable Patients: Promoting Informed and Shared Decisions. Am J Prev Med. 2016. Oct;51(4):454–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Diefenbach MA, Benedict C, Miller SM, Stanton AL, Ropka ME, Wen K-Y, et al. Examining the impact of a multimedia intervention on treatment decision-making among newly diagnosed prostate cancer patients: results from a nationwide RCT. Transl Behav Med. 2018. Nov 21;8(6):876–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hoffman AS, Lowenstein LM, Kamath GR, Housten AJ, Leal VB, Linder SK, et al. An entertainment-education colorectal cancer screening decision aid for African American patients: A randomized controlled trial. Cancer. 2017. Apr 15;123(8):1401–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ibrahim SA, Blum M, Lee G-C, Mooar P, Medvedeva E, Collier A, et al. Effect of a Decision Aid on Access to Total Knee Replacement for Black Patients With Osteoarthritis of the Knee: A Randomized Clinical Trial. JAMA Surg. 2017. Jan 18;152(1):e164225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jibaja-Weiss ML, Volk RJ, Granchi TS, Neff NE, Robinson EK, Spann SJ, et al. Entertainment education for breast cancer surgery decisions: a randomized trial among patients with low health literacy. Patient Educ Couns. 2011. Jul;84(1):41–8. [DOI] [PubMed] [Google Scholar]
- 42.Jimenez ME, DuRivage NE, Bezpalko O, Suh A, Wade R, Blum NJ, et al. A Pilot Randomized Trial of a Video Patient Decision Aid to Facilitate Early Intervention Referrals From Primary Care. Clin Pediatr. 2017. Mar;56(3):268–77. [DOI] [PubMed] [Google Scholar]
- 43.Kuppermann M, Norton ME, Gates E, Gregorich SE, Learman LA, Nakagawa S, et al. Computerized Prenatal Genetic Testing Decision-Assisting Tool [Internet]. Vol. 113, Obstetrics & Gynecology. 2009. p. 53–63. Available from: 10.1097/aog.0b013e31818e7ec4 [DOI] [PubMed] [Google Scholar]
- 44.Kuppermann M, Pena S, Bishop JT, Nakagawa S, Gregorich SE, Sit A, et al. Effect of enhanced information, values clarification, and removal of financial barriers on use of prenatal genetic testing: a randomized clinical trial. JAMA. 2014. Sep 24;312(12):1210–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lepore SJ, Wolf RL, Basch CE, Godfrey M, McGinty E, Shmukler C, et al. Informed decision making about prostate cancer testing in predominantly immigrant black men: a randomized controlled trial. Ann Behav Med. 2012. Dec;44(3):320–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Marteau TM, Mann E, Prevost AT, Vasconcelos JC, Kellar I, Sanderson S, et al. Impact of an informed choice invitation on uptake of screening for diabetes in primary care (DICISION): randomised trial. BMJ. 2010. May 13;340:c2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Miller DP Jr, Spangler JG, Case LD, Goff DC Jr, Singh S, Pignone MP. Effectiveness of a web-based colorectal cancer screening patient decision aid: a randomized controlled trial in a mixed-literacy population. Am J Prev Med. 2011. Jun;40(6):608–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Miller DP Jr, Denizard-Thompson N, Weaver KE, Case LD, Troyer JL, Spangler JG, et al. Effect of a Digital Health Intervention on Receipt of Colorectal Cancer Screening in Vulnerable Patients: A Randomized Controlled Trial. Ann Intern Med. 2018. Apr 17;168(8):550–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Myers RE, Daskalakis C, Cocroft J, Kunkel EJS, Delmoor E, Liberatore M, et al. Preparing African-American men in community primary care practices to decide whether or not to have prostate cancer screening. J Natl Med Assoc. 2005. Aug;97(8):1143–54. [PMC free article] [PubMed] [Google Scholar]
- 50.Rising KL, Hollander JE, Schaffer JT, Kline JA, Torres CA, Diercks DB, et al. Effectiveness of a Decision Aid in Potentially Vulnerable Patients: A Secondary Analysis of the Chest Pain Choice Multicenter Randomized Trial. Med Decis Making. 2018. Jan;38(1):69–78. [DOI] [PubMed] [Google Scholar]
- 51.Ruffin MT 4th, Fetters MD, Jimbo M. Preference-based electronic decision aid to promote colorectal cancer screening: results of a randomized controlled trial. Prev Med. 2007. Oct;45(4):267–73. [DOI] [PubMed] [Google Scholar]
- 52.Schroy PC 3rd, Emmons K, Peters E, Glick JT, Robinson PA, Lydotes MA, et al. The impact of a novel computer-based decision aid on shared decision making for colorectal cancer screening: a randomized trial. Med Decis Making. 2011. Jan;31(1):93–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Smith SK, Trevena L, Simpson JM, Barratt A, Nutbeam D, McCaffery KJ. A decision aid to support informed choices about bowel cancer screening among adults with low education: randomised controlled trial. BMJ. 2010. Oct 26;341:c5370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Street RL Jr, Voigt B, Geyer C Jr, Manning T, Swanson GP. Increasing patient involvement in choosing treatment for early breast cancer. Cancer. 1995. Dec 1;76(11):2275–85. [DOI] [PubMed] [Google Scholar]
- 55.Taylor KL, Davis JL 3rd, Turner RO, Johnson L, Schwartz MD, Kerner JF, et al. Educating African American men about the prostate cancer screening dilemma: a randomized intervention. Cancer Epidemiol Biomarkers Prev. 2006. Nov;15(11):2179–88. [DOI] [PubMed] [Google Scholar]
- 56.Trevena LJ, Irwig L, Barratt A. Randomized trial of a self-administered decision aid for colorectal cancer screening. J Med Screen. 2008;15(2):76–82. [DOI] [PubMed] [Google Scholar]
- 57.Vina ER, Richardson D, Medvedeva E, Kent Kwoh C, Collier A, Ibrahim SA. Does a Patient-centered Educational Intervention Affect African-American Access to Knee Replacement? A Randomized Trial. Clin Orthop Relat Res. 2016. Aug;474(8):1755–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Volk RJ, Jibaja-Weiss ML, Hawley ST, Kneuper S, Spann SJ, Miles BJ, et al. Entertainment education for prostate cancer screening: a randomized trial among primary care patients with low health literacy. Patient Educ Couns. 2008. Dec;73(3):482–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Williams RM, Davis KM, Luta G, Edmond SN, Dorfman CS, Schwartz MD, et al. Fostering informed decisions: a randomized controlled trial assessing the impact of a decision aid among men registered to undergo mass screening for prostate cancer. Patient Educ Couns. 2013. Jun;91(3):329–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wolf AM, Nasser JF, Wolf AM, Schorling JB. The impact of informed consent on patient interest in prostate-specific antigen screening. Arch Intern Med. 1996. Jun 24;156(12):1333–6. [PubMed] [Google Scholar]
- 61.Alegria M, Nakash O, Johnson K, Ault-Brutus A, Carson N, Fillbrunn M, et al. Effectiveness of the DECIDE Interventions on Shared Decision Making and Perceived Quality of Care in Behavioral Health With Multicultural Patients: A Randomized Clinical Trial. JAMA Psychiatry. 2018. Apr 1;75(4):325–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Boulware LE, Ephraim PL, Hill-Briggs F, Roter DL, Bone LR, Wolff JL, et al. Hypertension Self-management in Socially Disadvantaged African Americans: the Achieving Blood Pressure Control Together (ACT) Randomized Comparative Effectiveness Trial. J Gen Intern Med. 2020. Jan;35(1):142–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chan ECY, McFall SL, Byrd TL, Mullen PD, Volk RJ, Ureda J, et al. A community-based intervention to promote informed decision making for prostate cancer screening among Hispanic American men changed knowledge and role preferences: a cluster RCT. Patient Educ Couns. 2011. Aug;84(2):e44–51. [DOI] [PubMed] [Google Scholar]
- 64.Cooper LA, Roter DL, Carson KA, Bone LR, Larson SM, Miller ER 3rd, et al. A randomized trial to improve patient-centered care and hypertension control in underserved primary care patients. J Gen Intern Med. 2011. Nov;26(11):1297–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gabel P, Edwards A, Kirkegaard P, Larsen MB, Andersen B. The LEAD trial-The effectiveness of a decision aid on decision making among citizens with lower educational attainment who have not participated in FIT-based colorectal cancer screening in Denmark: A randomised controlled trial. Patient Educ Couns. 2020. Feb;103(2):359–68. [DOI] [PubMed] [Google Scholar]
- 66.Gustafson DH, Hawkins R, Pingree S, McTavish F, Arora NK, Mendenhall J, et al. Effect of computer support on younger women with breast cancer. J Gen Intern Med. 2001. Jul;16(7):435–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Heisler M, Choi H, Palmisano G, Mase R, Richardson C, Fagerlin A, et al. Comparison of community health worker-led diabetes medication decision-making support for low-income Latino and African American adults with diabetes using e-health tools versus print materials: a randomized, controlled trial. Ann Intern Med. 2014. Nov 18;161(10 Suppl):S13–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kripalani S, Sharma J, Justice E, Justice J, Spiker C, Laufman LE, et al. Low-literacy interventions to promote discussion of prostate cancer: a randomized controlled trial. Am J Prev Med. 2007. Aug;33(2):83–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Krishnamurti L, Ross D, Sinha C, Leong T, Bakshi N, Mittal N, et al. Comparative Effectiveness of a Web-Based Patient Decision Aid for Therapeutic Options for Sickle Cell Disease: Randomized Controlled Trial. J Med Internet Res. 2019. Dec 4;21(12):e14462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Roter DL, Erby LH, Rimal RN, Smith KC, Larson S, Bennett IM, et al. Empowering Women’s Prenatal Communication: Does Literacy Matter? J Health Commun. 2015;20 Suppl 2:60–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Singh JA, Fraenkel L, Green C, Alarcón GS, Barton JL, Saag KG, et al. Individualized decision aid for diverse women with lupus nephritis (IDEA-WON): A randomized controlled trial. PLoS Med. 2019. May;16(5):e1002800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sudore RL, Schillinger D, Katen MT, Shi Y, Boscardin WJ, Osua S, et al. Engaging Diverse English- and Spanish-Speaking Older Adults in Advance Care Planning: The PREPARE Randomized Clinical Trial. JAMA Intern Med. 2018. Dec 1;178(12):1616–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Volandes AE, Paasche-Orlow MK, Barry MJ, Gillick MR, Minaker KL, Chang Y, et al. Video decision support tool for advance care planning in dementia: randomised controlled trial. BMJ. 2009. May 28;338:b2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Muscat DM, Smith J, Mac O, Cadet T, Giguere A, Housten A, et al. Addressing health literacy in patient decision aids: an update from the International Patient Decision Aid Standards. Under Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nancy Berkman PhD; Sheridan Stacey L. MD MPH; Donahue Katrina E. MD MPH; Halpern David J MD MAKC. Low Health Literacy and Health Outcomes: An Updated Systematic Review. Annals of Internal Medicine. 2014;155(2). [DOI] [PubMed] [Google Scholar]
- 76.Dewalt DA, Berkman ND, Sheridan S, Lohr KN, Pignone MP. Literacy and health outcomes: a systematic review of the literature. J Gen Intern Med. 2004. Dec;19(12):1228–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hawley ST, Lantz PM, Janz NK, Salem B, Morrow M, Schwartz K, et al. Factors associated with patient involvement in surgical treatment decision making for breast cancer. Patient Educ Couns. 2007. Mar;65(3):387–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Willems S, De Maesschalck S, Deveugele M, Derese A, De Maeseneer J. Socio-economic status of the patient and doctor-patient communication: does it make a difference? Patient Educ Couns. 2005. Feb;56(2):139–46. [DOI] [PubMed] [Google Scholar]
- 79.Siminoff LA, Graham GC, Gordon NH. Cancer communication patterns and the influence of patient characteristics: disparities in information-giving and affective behaviors. Patient Educ Couns. 2006. Sep;62(3):355–60. [DOI] [PubMed] [Google Scholar]
- 80.Richardson A, Allen JA, Xiao H, Vallone D. Effects of race/ethnicity and socioeconomic status on health information-seeking, confidence, and trust. J Health Care Poor Underserved. 2012. Nov;23(4):1477–93. [DOI] [PubMed] [Google Scholar]
- 81.Jacobs W, Amuta AO, Jeon KC. Health information seeking in the digital age: An analysis of health information seeking behavior among US adults. Alvares C, editor. Cogent Social Sciences. 2017. Mar 13;3(1):327. [Google Scholar]
- 82.Senate and House of Representatives. Patient Protection and Affordable Care Act. Washington, DC; 2010. [Google Scholar]
- 83.Trevena L, Shepherd HL, Bonner C, Jansen J, Cust AE, Leask J, et al. Shared decision making in Australia in 2017. Z Evid Fortbild Qual Gesundhwes. 2017. Jun;123–124:17–20. [DOI] [PubMed] [Google Scholar]
- 84.Great Britain: Department of Health. Equity and excellence: liberating the NHS. The Stationery Office; 2010. 59 p. [Google Scholar]
- 85.Elwyn G, O’Connor A, Stacey D, Volk R, Edwards A, Coulter A, et al. Developing a quality criteria framework for patient decision aids: online international Delphi consensus process. BMJ. 2006. Aug 26;333(7565):417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sepucha KR, Abhyankar P, Hoffman AS, Bekker HL, LeBlanc A, Levin CA, et al. Standards for UNiversal reporting of patient Decision Aid Evaluation studies: the development of SUNDAE Checklist. BMJ Qual Saf. 2018. May;27(5):380–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.