Abstract
Background
African American men have a higher burden of prostate cancer compared with other populations. We sought to determine if they experience disparities in access to prostate cancer clinical trials.
Methods
We created a database of all US counties by linking prostate cancer clinical trial data with county-level socioeconomic, demographic, and health-care facility data derived from several external data sources. Using this data linkage, we examined 2 potential access barriers. We investigated the relationship between the proportion of African Americans and access to cancer facilities, adjusting for county population size and other characteristics. Additionally, among counties with cancer facilities, we investigated the relationship between the proportion of African Americans and number of available prostate cancer trials per capita per year. We addressed these questions using logistic and negative binomial regression, respectively.
Results
Between 2008 and 2015, 613 prostate cancer trial sites were found among 3145 US counties. Counties with a higher proportion of African Americans were less likely to have cancer facilities (adjusted odds ratio = 0.85, 95% confidence interval = 0.78 to 0.92). Among counties with cancer facilities, those with a higher proportion of African Americans had statistically significantly fewer prostate cancer trials per capita per year (rate ratio per 10% increase in African Americans = 0.90, 95% confidence interval = 0.83 to 0.96).
Conclusions
Counties with higher proportions of African Americans seem less likely to have access to cancer facilities. Among counties with cancer facilities, those with higher proportions of African Americans appear to have fewer prostate cancer trials available per capita per year. Clinical trials in prostate cancer therapy should ensure adequate availability of enrollment sites in regions with high concentrations of African Americans.
Participation of minority populations in cancer clinical trials is vital to ensure generalizability of the results generated, facilitate discovery of novel therapies and therapeutic responses that are particularly relevant to traditionally underrepresented populations, and ensure equitable access to new and promising treatments. Yet more than 20 years after Congress mandated that the National Institutes of Health ensure “sufficient and appropriate” participation of minorities in clinical research, the participation rate of many minority populations remains substantially lower than the composition of the overall US population (1,2). A 2017 study showed that the enrollment rate of minority populations in 2003-2016 was even lower than in the past (1996-2002) (3,4).
We focus in particular on the underrepresentation of African Americans in prostate cancer trials. The incidence rate of prostate cancer is 70% higher and the mortality rate is approximately 2.5 times higher among African American men compared with White men (5). It is critical to understand if, despite the disproportionately higher burden of prostate cancer, African American men experience disparities in access to prostate cancer clinical trials. A better understanding of disparities in access can help with designing interventions to improve participation of African American men in such trials.
Previous studies have found that factors such as lack of knowledge about clinical trials and fear and mistrust of clinical research represent barriers to participation of African Americans in clinical trials to which they have access (6-8). However, to our knowledge, no previous studies have examined whether access barriers exist. Lack of facilities that can conduct prostate cancer trials or fewer trials conducted at the available facilities both represent access barriers, which would systematically limit the ability of African Americans to participate in such trials. Accordingly, in this study, we created an extensive county-level data linkage of all US counties and used it to investigate these 2 potential access barriers. Specifically, we investigated the relationship between the proportion of African Americans in the county and access to cancer facilities. Additionally, among counties with cancer facilities, we investigated the relationship between the percentage of African Americans in the county and number of available prostate cancer treatment trials per capita per year from 2008 to 2015.
Methods
Data Linkage
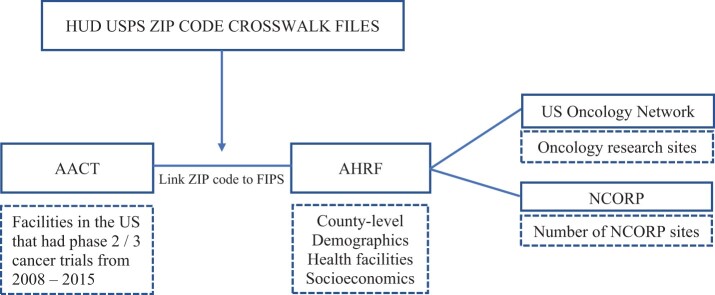
Figure 1 shows an overview of our data linkage. We used the 2016 Aggregate Analysis of ClincalTrials.gov database to identify phase II and III prostate cancer clinical trials open for recruitment in the United States from 2008 to 2015. The Aggregate Analysis of ClincalTrials.gov database reflects the clinical trials registered on ClinicalTrials.gov, including the location of each trial’s recruitment sites (9). We used Medical Subject Headings terms “prostate” and “prostatic neoplasms” to screen and identify prostate cancer trials in this study (10).
Figure 1.
Flowchart of data linkage. We linked clinical trial sites from the 2016 Aggregate Analysis of ClincalTrials.gov (AACT) database to county-level characteristics from the Area Health Resource File (AHRF). We also collected additional geographic information from the US Oncology Network and NCI Community Oncology Research Program (NCORP). FIPS = Federal Information Processing Standards; HUD = US Department of Housing and Urban Development; NCI = National Cancer Institute.
To address the question of racial disparities while controlling for geographic and socioeconomic factors, we aggregated the number of open cancer clinical trial sites by county and linked to county-level information derived from several external data sources (Figure 1). We derived county-level socioeconomic, demographic, and health-care facility data from the 2014-2015 Area Health Resource File (11). The Area Health Resource File database is a widely used collection of data from more than 50 sources, including the American Medical Association, the US Census Bureau, and the American Community Survey, that contains information on health-care facilities (eg, American College of Surgeons [ACS], Commission on Cancer Accredited Cancer Centers) (12), health professions, measures of resource scarcity, health status, economic activity, health training programs, and socioeconomic and population characteristics. The database also contains data on the number of physicians for multiple subspecialties, including the number of urologists who completed residency training.
Finally, to characterize cancer care facilities by county, we obtained the location of all National Cancer Institute (NCI) Comprehensive Cancer Centers and the NCI Community Oncology Research Program (NCORP; or before 2014, the program’s predecessor) sites directly from the NCI website (13). We also included sites from the US Oncology Network (USON), which is a clinical trial management system that includes community-based physician practices, that is, independent practices not affiliated with hospitals (14).
Outcomes
Outcomes were at the county level and included 1) availability of cancer care facilities within the county, and 2) among counties with cancer care facilities, the rate of prostate cancer clinical trials in the county per year from 2008 to 2015. We defined availability of cancer facilities as a binary indicator of whether the county had at least 1 NCORP site, NCI comprehensive cancer center, ACS cancer center (12), or USON site.
To derive the number of prostate cancer trials being offered in each county per year, we first matched the sites offering trials with their respective counties. The locations of trial sites were primarily determined by ZIP codes, whereas counties were identified by Federal Information Processing Standards codes. We linked the ZIP codes of the sites to county Federal Information Processing Standards codes using the US Department of Housing and Urban Development United States Postal Service crosswalk files (15). Because trial information from clinicaltrials.gov was self-reported by investigators, we encountered some invalid ZIP codes of trial sites. We looked up these sites and manually fixed the miscoded ZIP codes. For cases where ZIP code was unavailable but the name of the site was available, we developed a matching algorithm to assign a county to each site using its name, city, and state. Finally, for the sites that did not have a valid ZIP code or site name and had information only on city and state, we created a city-county-state list and assigned counties to those sites. Sites (n = 87) without any information of city and state were excluded because they could not be matched to a county.
Once all sites had been matched to their respective counties, we calculated the number of prostate cancer trials per county. Because the ZIP code of a trial site may be split across multiple counties, we used the ratio of total residential addresses within a county compared with total addresses within a ZIP code to weight the number of trials belonging to that county. The weights for all counties belonging to 1 ZIP code added up to 1.
Statistical Analyses
We compared characteristics of counties with lower vs higher proportions of African Americans using means and SDs for continuous variations and frequencies and proportions for categorical variables. To define low and high proportions of African Americans in a county, we chose a cut-off of 13%, because this is the prevalence of African Americans in the overall US population (16).
To investigate the association of the proportion of the African American population with the availability of cancer facilities, we used a multivariable logistic regression model. We defined the outcome as the availability of at least 1 cancer facility in the county and the predictor of interest as the percentage of African Americans in the county. We included covariate adjustment for county population, percentage of residents who were male vs female, percentage of residents who were 65 years and older vs younger than 65 years, racial and ethnic composition (percentages of Hispanic and Asian American residents), and whether the county was in a metropolitan, suburban, or rural area, which was categorized based on Rural-Urban Continuum Codes (metropolitan: 01-03, suburban: 04-06, rural: 07-09).
Among counties with at least 1 cancer facility, we investigated the association of the proportion of the African American population with the rate of prostate cancer clinical trials per year using a negative binomial regression model. The model included county population as an offset term and adjusted for percentage of residents who were male vs female, percentage of residents who were 65 years and older vs younger than 65 years, racial and ethnic composition (percentages of Hispanic and Asian American residents), and whether the county was in a metropolitan, suburban, or rural area. Additionally, because NCORP sites, NCI-designated cancer centers, ACS cancer centers, USON sites, and facilities with medical school affiliations may be more likely to conduct cancer clinical trials, we also adjusted for the density of cancer facilities by type (NCORP, NCI, ACS, USON) and whether there were any facilities with medical school affiliations in the county. Finally, because many prostate cancer studies are run in urology practices, we also adjusted for density of urologists. The model accounted for correlation among multiple records from the same county (1 record per year from 2008 to 2015) by clustering on county.
All analyses were conducted using R, version 3.4.2 (R Foundation for Statistical Computing) and STATA, version 15 (STATACorp LLC). All tests were 2-sided, and a P less than .05 was considered statistically significant.
Results
County Characteristics
Characteristics of counties with lower vs higher proportions of African Americans are reported in Table 1. A total of 3145 US counties were identified: among 688 (21.9%) counties with at least 13% African Americans, 91.3% of them were in the South (vs 32.4% of counties with <13% African Americans) and approximately 49.6% were in urban areas (vs 33.6% of counties with <13% African Americans). The higher population density (per square mile) was also observed among these counties compared with counties with less than 13% African Americans.
Table 1.
Characteristics among counties by density of African Americansa
| County-level characteristics | County density of African American individuals |
|
|---|---|---|
| Lower density, <13% (n = 2457) | Higher density, ≥13% (n = 688) | |
| Region, No. (%) | ||
| Midwest | 1022 (41.6) | 33 (4.8) |
| Northeast | 191 (7.8) | 26 (3.8) |
| South | 795 (32.4) | 628 (91.3) |
| West | 449 (18.3) | 1 (0.1) |
| Metropolitan, No. (%) | ||
| Rural | 933 (38.0) | 144 (20.9) |
| Suburban | 696 (28.4) | 203 (29.5) |
| Urban | 826 (33.6) | 341 (49.6) |
| Income, No. (%) | ||
| ≥$50 000 | 604 (24.6) | 110 (16.0) |
| <$50 000 | 1852 (75.4) | 578 (84.0) |
| High poverty, No. (%) | ||
| ≥20% | 418 (17.0) | 349 (50.7) |
| <20% | 2037 (82.9) | 339 (49.3) |
| Any facilities with medical school affiliations, No. (%) | ||
| Medical school affiliations (yes) | 428 (17.5) | 151 (21.9) |
| Medical school affiliations (no) | 2024 (82.5) | 537 (78.1) |
| Have at least 1 cancer facility, No. (%) | 688 (28.0) | 243 (35.3) |
| Density of prostate cancer trials (per 100 000) | 2.01 (6.67) | 1.80 (4.17) |
| Demographics, mean % (SD) | ||
| Sex | ||
| Percentage of male | 50.0 (1.86) | 49.4 (2.96) |
| Percentage of female | 50.0 (1.89) | 50.5 (2.98) |
| Age | ||
| Percentage of age ≥65 y | 16.7 (4.32) | 14.4 (3.25) |
| Percentage of age <65 y | 80.9 (5.21) | 81.5 (5.84) |
| Race, mean % (SD) | ||
| Percentage of African American | 2.7 (3.09) | 32.1 (15.49) |
| Percentage of Asian | 1.2 (2.64) | 1.4 (2.09) |
| Percentage of Hispanic/Latino | 8.9 (14.30) | 6.2 (7.26) |
| Percentage of White | 91.3 (10.48) | 63.8 (14.83) |
| Health-care infrastructure, mean (SD) | ||
| No. of NCORP sites, per 100 000 | 0.55 (4.20) | 0.27 (0.95) |
| No. of NCI sites, per 100 000 | 0.00 (0.03) | 0.01 (0.06) |
| No. of total hospitals, per 100 000 | 6.24 (9.69) | 3.59 (3.71) |
| No. of ACS cancer centers, per 100 000 | 0.24 (0.64) | 0.29 (0.68) |
| No. of community-based physician practices (USON sites), per 100 000 | 0.05 (0.74) | 0.03 (0.19) |
| No. of urologists, per 100 000 | 1.23 (2.56) | 1.76 (2.62) |
| Population density (per square mile), mean (SD) | 145.91 (554.52) | 676.44 (3577.19) |
| Percentage of less educated, mean (SD) | 14.4 (6.76) | 19.3 (6.05) |
| Percentage of uninsured, mean (SD) | 17.6 (5.77) | 19.5 (4.04) |
| Percentage of unemployment, mean (SD) | 7.3 (2.60) | 9.1 (2.32) |
ACS = American College of Surgeons; NCI = National Cancer Institute; NCORP = NCI Community Oncology Research Program; USON = US Oncology Network.
Counties with higher proportions of African Americans had similar demographics in terms of age and sex compared with those with lower proportions of African Americans, but the former included larger proportions of low-income, higher poverty, less educated, and uninsured individuals.
Availability of Cancer Facilities
Among a total of 3145 counties, there were 931 (29.6%) counties with at least 1 cancer care facility (data not shown). Specifically, there were 637 NCORP sites among 501 counties and 41 NCI comprehensive cancer centers among 40 counties. A total of 1421 ACS cancer centers were found among 673 counties, and 172 USON sites were found in 108 counties.
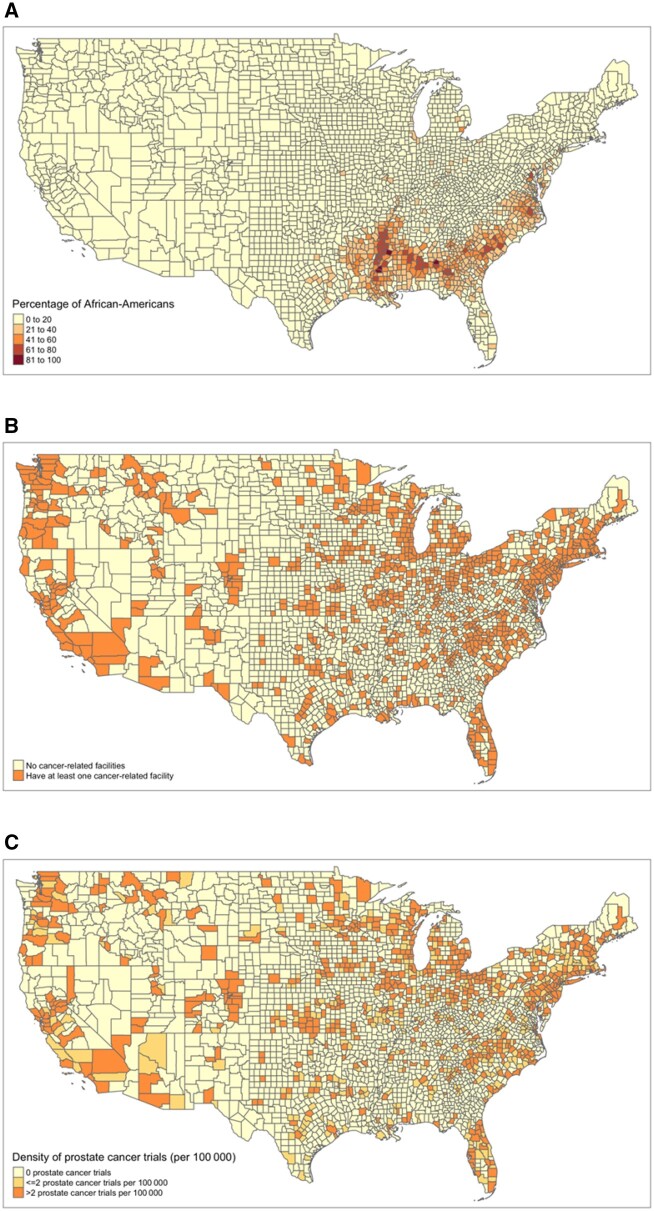
Table 1 shows that although counties with a higher proportion of African Americans were more likely, on average, to have at least 1 cancer facility (35.3% of counties vs 28.0% of counties), they also tended to have lower total numbers of hospitals (mean [SD] = 3.59 [3.71] per 100 000 vs 6.24 [9.69] per 100 000) and NCORP sites (mean [SD] = 0.27 [0.95] per 100 000 vs 0.55 [4.20] per 100 000). Figure 2, A and B shows that counties with the highest proportions of African Americans are in the South, where most counties are without any cancer facility.
Figure 2.
Geographic distribution of the African American population, cancer facilities, and prostate cancer trials. A) The distribution of the percentage of the African American individuals in the population is shown on the US map with lines indicating US counties. B) The distribution of cancer facilities, as either no cancer-related facilities or having at least 1 cancer-related facility, is shown. C) The distribution of prostate cancer clinical trials (0, ≤2, or >2 per 100 000) between 2008 and 2015 is shown.
After adjusting for potential confounders, a higher proportion of African Americans was statistically significantly associated with a lower likelihood of having at least 1 cancer facility (Table 2). Specifically, a 10-percentage point increase in the proportion of African Americans (eg, comparing counties with 10% African Americans vs 20% African Americans) was associated with 15% lower odds of a cancer center in the county (adjusted odds ratio = 0.85, 95% confidence interval [CI] = 0.78 to 0.92, P < .001).
Table 2.
Adjusteda association between the availability of cancer facilities and county-level characteristics among all counties (n = 3145)
| County-level characteristics | Odds ratio (95% CI) | P b |
|---|---|---|
| Race (per 10%) | ||
| Percentage of African American | 0.85 (0.78 to 0.92) | <.001 |
| Percentage of Asian | 2.11 (0.57 to 7.80) | .26 |
| Percentage of Hispanic/Latino | 0.69 (0.59 to 0.79) | <.001 |
| Sex | ||
| Percentage of male | 0.81 (0.74 to 0.88) | <.001 |
| Age | ||
| Percentage of ≥65 y | 0.98 (0.95 to 1.01) | .25 |
| Metropolitan | ||
| Rural | Ref | |
| Suburban | 1.72 (1.27 to 2.34) | <.001 |
| Urban | 1.55 (1.07 to 2.24) | .02 |
Also adjusted for total population. CI = confidence interval.
Logistic regression was used; all tests were 2-sided.
Availability of Prostate Cancer Clinical Trials
The number of prostate cancer clinical trials in a county was calculated over all trial facilities in that county. Our sample included 613 prostate cancer clinical trials conducted at 16 866 sites in the United States between 2008 and 2015. On average, there were 1.2 open prostate cancer trials per 100 000 residents across all counties. We found that 19 counties conducted the largest number of prostate cancer trials in our study period (>100 trials over 8 years). The average proportion of African Americans in these counties was 20.5%. Figure 2, A and C show that counties with the highest proportions of African Americans are in the South, where most counties have few to no prostate cancer trials. Overall, approximately 85.3% of African Americans resided in a county with a prostate cancer trial.
Restricting to counties with at least 1 cancer facility and after adjusting for the number of cancer facilities and other potential confounders, a higher proportion of African Americans was statistically significantly associated with a lower rate of prostate cancer trials per year. Specifically, a 10-percentage point increase in the proportion of African Americans (eg, comparing counties with 10% African Americans vs 20% African Americans) was associated with a 10% lower rate of prostate cancer trials per year (adjusted incidence rate ratio = 0.90, 95% CI = 0.83 to 0.96, P = .004) (Table 3). As expected, the rate of prostate cancer trials had a statistically significant positive association with the number of urologists (per 100 000) (adjusted incidence rate ratio = 1.11, 95% CI = 1.07 to 1.16, P < .001).
Table 3.
Adjusteda association between number of prostate cancer trials and county-level characteristics among counties with at least 1 cancer facility (n = 931)
| County-level characteristics | Incidence rate ratio (95% CI) | P b |
|---|---|---|
| Race (per 10%) | ||
| Percentage of African American | 0.90 (0.83 to 0.96) | .004 |
| Percentage of Asian | 0.95 (0.82 to 1.10) | .48 |
| Percentage of Hispanic/Latino | 0.81 (0.75 to 0.88) | <.001 |
| Percentage of male | 1.11 (1.00 to 1.23) | .04 |
| Percentage of age ≥65 y | 0.99 (0.96 to 1.03) | .91 |
| Health care infrastructure | ||
| No. of urologists, per 100 000 | 1.11 (1.07 to 1.16) | <.001 |
| No. of NCORP sites, per 100 000 | 1.04 (0.99 to 1.09) | .13 |
| No. of NCI-designated cancer centers, per 100 000 | 2.28 (0.62 to 8.34) | .21 |
| No. of ACS cancer centers, per 100 000 | 0.97 (0.86 to 1.09) | .60 |
| No. of community-based physician practices (USON sites), per 100 000 | 0.83 (0.71 to 0.97) | .02 |
| Any facilities with medical school affiliations | ||
| Medical school affiliations (yes) | 1.65 (1.35 to 2.01) | <.001 |
| Medical school affiliations (no) | Ref | |
| Metropolitan | ||
| Rural | Ref | – |
| Suburban | 1.36 (0.79 to 2.32) | <.001 |
| Urban | 1.04 (0.59 to 1.83) | <.001 |
Also adjusted for year, and using total population as offset. ACS = American College of Surgeons; CI = confidence interval; NCI = National Cancer Institute; NCORP = NCI Community Oncology Research Program; USON = US Oncology Network.
Negative binomial regression was used; all tests were 2-sided.
Discussion
We investigated whether African Americans are less likely to have access to prostate cancer treatment trials despite experiencing a disproportionately higher burden of prostate cancer than other populations. We created a novel, extensive data linkage and studied 2 specific access barriers—availability of cancer facilities that can run clinical trials and availability of prostate cancer clinical trials at those facilities. Adjusting for county population and other county-level characteristics, we found that counties with higher proportions of African Americans have access to both fewer cancer facilities and to fewer prostate cancer trials at the available facilities.
The existing literature has identified potential barriers to trial enrollment of racial and ethnic minorities at the system, individual, and interpersonal levels (17). For example, minorities may be less likely to enroll in clinical trials due to lack of awareness of the trials, mistrust in their health providers, or fear of participating in clinical trials (7,8). Minorities are also more affected by barriers related to lower socioeconomic status, such as transportation, inadequate insurance, childcare, and poor access to health care (18). At the system level, a recent meta-analysis of barriers to cancer clinical trial participation showed that more than one-half of cancer patients report that a trial was unavailable for their cancer type at their institution (19). Consistent with these reports, our study adds to current knowledge of the factors that may contribute to the underrepresentation of African Americans in prostate cancer clinical trials by showing that patients are unable to enroll in trials simply because fewer trials are available to them. Our findings are critical for ensuring generalizability of clinical trial results and the subsequent adoption of clinical trial evidence to inform decision-making and policy.
In a secondary analysis, we included socioeconomic variables (ie, percent low income in county, percent low education in county, percent uninsured in county, and percent unemployed in county) in the models and found the effect of race was eliminated after adjusting for these socioeconomic variables, potentially suggesting no racial disparities. However, we believe that these results are misleading; the very high association between race and socioeconomic status means that the effect of each cannot be disentangled cleanly using population-level data. It is also possible that socioeconomic variables should be considered mediators between race and outcome rather than confounders. Future study is needed to explore the role of socioeconomic variables in racial disparities.
This study has some limitations. First, we used a county-level analysis. Although hospital service areas might be more appropriate for reflecting the use of health care, demographics at the hospital service area level were not available. Furthermore, a county-level analysis allowed us to take into account the status of the clinical trials (open or closed) that could provide more accurate information regarding the availability of clinical trials. Second, we used oncology practices to capture cancer facilities that can conduct prostate cancer trials. Although many prostate cancer studies are conducted in urology practices, not oncology practices, county-level data on the number of urology practices were not available. To address this issue, we adjusted for the number of urologists in our analysis as a proxy for number of urology practices. Third, we did not consider spatial dependency among counties. It is possible that residents may travel to other counties and states for cancer trials. If this is the case, our analysis may have overestimated African American men’s lack of access, but we do not believe it would not change the direction of our results. Finally, we focused on the relationship between the total number of prostate cancer trials and county-level characteristics in this study. Trial-level characteristics, such as phase and type of sponsor, and facility-level characteristics, such as size and capability, may have an impact on the availability of clinical trials as well. This requires even more extensive data linkages, and we leave this to future work.
This study adds to a growing body of evidence that African Americans possibly face barriers in access to cancer clinical trials by showing that counties with higher proportions of African Americans appear to be less likely to have access to cancer facilities and to fewer prostate cancer trials at the available facilities. Health disparities are likely to widen without appropriate representation of diverse populations in cancer clinical trials. Future clinical trials in prostate cancer therapy should account for these barriers and include targeted strategies to ensure adequate availability of enrollment sites in regions with high concentrations of African Americans. Furthermore, because African Americans experience the highest death rate of any racial or ethnic group for most cancers, future studies should investigate whether similar disparities hold in other cancers (20).
Funding
This research was supported by the National Cancer Institute of the National Institutes of Health under grant R03CA219621.
Notes
Role of the funder: The funder had no role in the design of the study; the collection, analysis, and interpretation of the data; the writing of the manuscript; or the decision to submit the manuscript for publication.
Disclosures: All the authors declared there is no conflict of interest.
Author contributions: Wei-Jhih Wang, PhD: Methodology, Formal analysis, Writing—original draft. Scott D. Ramsey, PhD: Conceptualization, Writing—review and editing. Caroline S. Bennette, PhD: Conceptualization, Funding acquisition, Writing—review and editing. Aasthaa Bansal, PhD: Conceptualization, Funding acquisition, Methodology, Supervision, Writing—review and editing.
Disclaimer: The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Data Availability
The datasets were derived from publicly available sources, including the Aggregate Analysis of ClincalTrials.gov (AACT) database at https://aact.ctti-clinicaltrials.org/ and Area Health Resource File (AHRF) at https://datawarehouse.hrsa.gov/topics/ahrf.aspx.
References
- 1. Chen MS Jr, Lara PN, Dang JHT, Paterniti DA, Kelly K.. Twenty years post-NIH Revitalization Act: Enhancing Minority Participation in Clinical Trials (EMPaCT): laying the groundwork for improving minority clinical trial accrual: renewing the case for enhancing minority participation in cancer clinical trials. Cancer. 2014;120 Suppl 7(0 7):1091–1096. doi: 10.1002/cncr.28575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. US Congress. National Institutes of Health Revitalization Act of 1993: Act to Amend the Public Health Service Act to Revise and Extend the Programs of the National Institutes of Health, and for Other Purposes. Public Law 103-43. 1994.
- 3. Murthy VH, Krumholz HM, Gross CP.. Participation in cancer clinical trials: race-, sex-, and age-based disparities. JAMA. 2004;291(22):2720–2726. doi: 10.1001/jama.291.22.2720. [DOI] [PubMed] [Google Scholar]
- 4. Duma N, Vera Aguilera J, Paludo J, et al. Representation of minorities and women in oncology clinical trials: review of the past 14 years. J Oncol Pract. 2018;14(1):e1–e10. doi: 10.1200/JOP.2017.025288. [DOI] [PubMed] [Google Scholar]
- 5. American Cancer Society. Cancer facts and figures for African Americans 2016-2018. https://www.cancer.org/research/cancer-facts-statistics/cancer-facts-figures-for-african-americans.html. Accessed June 20, 2020.
- 6. Owens OL, Jackson DD, Thomas TL, Friedman DB, Hébert JR.. African American men's and women's perceptions of clinical trials research: focusing on prostate cancer among a high-risk population in the South. J Health Care Poor Underserved. 2013;24(4):1784–1800. doi: 10.1353/hpu.2013.0187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ford JG, Howerton MW, Lai GY, et al. Barriers to recruiting underrepresented populations to cancer clinical trials: a systematic review. Cancer. 2008;112(2):228–242. doi: 10.1002/cncr.23157. [DOI] [PubMed] [Google Scholar]
- 8. Lara PN, Paterniti DA, Chiechi C, et al. Evaluation of factors affecting awareness of and willingness to participate in cancer clinical trials. J Clin Oncol. 2005;23(36):9282–9289. doi: 10.1200/JClinOncol.2005.02.6245. [DOI] [PubMed] [Google Scholar]
- 9. Clinical Trials Transformation Initiative (CCTI). The Aggregate Analysis of ClincalTrials.gov (AACT). 2016. https://aact.ctti-clinicaltrials.org/. Accessed July 1, 2016.
- 10. Bennette CS, Ramsey SD, McDermott CL, et al. Predicting low accrual in the National Cancer Institute's Cooperative Group Clinical Trials. JNCI J. 2016;108(2):djv324. doi: 10.1093/jnci/djv324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. U.S. Department of Health and Human Services HRSA. Area Health Resource Files (AHRF). https://datawarehouse.hrsa.gov/topics/ahrf.aspx. Accessed July 1, 2016.
- 12. The American College of Surgeons (ACoS). Commission on Cancer (CoC) Program. https://www.facs.org/quality-programs/cancer/coc. Accessed July 1, 2016.
- 13. National Cancer Institute (NCI). Community Oncology Research Program (NCORP). https://ncorp.cancer.gov. Accessed July 13, 2016.
- 14. The US Oncology Network (USON). https://www.usoncology.com. Accessed June 15, 2019.
- 15. Office of Policy Development and Research (PD&R). The U.S. Department of Housing and Urban Development (HUD) United States Postal Service (USPS) Crosswalk Files. https://www.huduser.gov/portal/datasets/usps_crosswalk.html. Accessed July 30, 2016.
- 16. U.S. Census Bureau. QuickFacts, United States. https://www.census.gov/quickfacts/fact/table/US/PST045221. Accessed February 27, 2020. [Google Scholar]
- 17. Giuliano AR, Mokuau N, Hughes C, et al. Participation of minorities in cancer research: the influence of structural, cultural, and linguistic factors. Ann Epidemiol 2000;10(8, Supplement 1):S22–S34. doi: 10.1016/S1047-2797(00)00195-2. [DOI] [PubMed] [Google Scholar]
- 18. Hamel LM, Penner L, Albrecht TL, et al. Barriers to clinical trial enrollment in racial and ethnic minority patients with cancer. Cancer Control. 2016;23(4):327–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Unger JM, Vaidya R, Hershman DL, Minasian LM, Fleury ME.. Systematic review and meta-analysis of the magnitude of structural, clinical, and physician and patient barriers to cancer clinical trial participation. J Natl Cancer Inst. 2019;111(3) :245–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. DeSantis CE, Miller KD, Goding Sauer A, Jemal A, Siegel RL.. Cancer statistics for African Americans, 2019. CA Cancer J Clin. 2019;69(3):211–233. doi: 10.3322/caac.21555 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets were derived from publicly available sources, including the Aggregate Analysis of ClincalTrials.gov (AACT) database at https://aact.ctti-clinicaltrials.org/ and Area Health Resource File (AHRF) at https://datawarehouse.hrsa.gov/topics/ahrf.aspx.