Abstract
Background
Topical antibiotics are widely prescribed as prophylaxis for surgical site infection (SSI). Despite giving high drug concentrations at local wound sites, their efficacy remains controversial. This study is a systematic review and meta-analysis designed to compare the efficacy and safety of topical antibiotics with non-antibiotic agents in preventing SSI.
Methods
Randomized controlled trials (RCTs) comparing topical antibiotics in patients with clean and clean-contaminated postsurgical wounds were included. Relevant trials published before 30 September 2020, were searched in the PubMed, Embase, and Cochrane databases, without language restrictions. The primary outcome was the incidence of SSIs, presented as the event rate. The secondary outcome was the incidence of contact dermatitis (safety outcome). Data were synthesized using the random-effects model, with the results expressed as risk ratio (RR) with 95 per cent confidence intervals (c.i.).
Results
Thirteen RCTs were included. The incidence of SSIs and contact dermatitis showed no significant difference between topical antibiotics and non-antibiotic agents (RR 0.89, 95 per cent c.i. 0.59 to 1.32 (P = 0.56, I2 = 48 per cent); and RR 2.79, 95 per cent c.i. 0.51 to 15.19 (P = 0.24, I2 = 0 per cent), respectively). In the subgroup analyses, a reduction in SSIs was also not observed in dermatological (RR 0.77, 95 per cent c.i. 0.39 to 1.55; P = 0.46, I2 = 65 per cent), ocular (RR 0.08, 95 per cent c.i. 0.00 to 1.52; P = 0.09), spinal (RR 1.34, 95 per cent c.i. 0.65 to 2.77; P = 0.43, I2 = 0 per cent), orthopaedic (RR 0.69, 95 per cent c.i. 0.37 to 1.29; P = 0.25, I2 = 0 per cent), or cardiothoracic surgeries (RR 1.60, 95 per cent c.i. 0.79 to 3.25; P = 0.19).
Conclusion
Given the current evidence, the routine application of topical antibiotics to surgical wounds did not reduce the incidence of SSI. Further trials are needed to assess their effectiveness in high-risk surgeries or in selected patient groups.
Our results indicate that topical antibiotics did not have a benefit in reducing surgical site infections (SSIs). In addition, topical antibiotics did not result in any reduction in SSIs in different types or different phases of surgery.
Introduction
Surgical site infection (SSI) is a common postoperative complication and a substantial cause of morbidity, prolonged hospitalization, and death1. Of note, SSI was the most common healthcare-associated infection from 2015 to 2017, followed by catheter-associated urinary tract infection and central line-associated bloodstream infection2. As such, SSI remains one of the most common preventable infections today3. Based on the concept that infection impairs the process of wound healing, prophylactic antibiotics play an essential role in wound management4.
Preoperatively, prophylactic antibiotics are primarily administered intravenously (i.v.). Extensive studies of the preoperative i.v. administration of antibiotic prophylaxis have shown it to be effective in reducing SSIs5. However, with the rise of Staphylococcus aureus-related healthcare infections2, the preoperative administration of intranasal mupirocin has also been suggested, owing to its role in the decolonization of methicillin-resistant S. aureus (MRSA), thereby decreasing SSIs6.
The evidence for using topical antibiotics intraoperatively has been a matter of debate. A meta-analysis demonstrated that the use of topical antibiotic agents before wound closure could not be recommended7. According to recent guidelines, the irrigation of incisional wounds with antibiotic agents before closure should not be performed owing to the risk of multiple drug resistance3,8,9. However, the question over the intraoperative administration of vancomycin powder remains unsolved owing to the growing number of cases of MRSA infection in recent years10.
Postoperatively, topical antibiotics are an option with several advantages, including a high drug concentration at the application site, a low incidence of systemic side effects, and good patient compliance11. Nevertheless, there is still controversy over their use owing to possible detrimental effects, such as local allergic reactions, poor skin penetration, and the emergence of resistant organisms with antibiotic exposure11. The Centers for Disease Control and Prevention 2017 guideline for the prevention of SSI states that additional prophylactic antibiotics should not be administered after the closure of the surgical incision in clean and clean-contaminated procedures3. Furthermore, despite the low-quality evidence, it also recommended against the administration of antimicrobial agents into surgical incisions for the prevention of SSIs.
Although there is no robust evidence of whether topical antibiotic prophylaxis is beneficial in patients undergoing clean and clean-contaminated surgery, it remains common practice during postsurgical wound care. The aim of this systematic review and meta-analysis was to compare the efficacy and safety of topical antibiotics with non-antibiotic agents for the prevention of SSI.
Methods
Inclusion and exclusion criteria
Surgical wounds were grouped into four classes, according to the National Academy of Sciences and the National Research Council: clean (I); clean-contaminated (II); contaminated (III); and infected/dirty (IV) (Table S1)12. The prophylaxis strategy was defined as the administration of topical antibiotics to wounds before the development of infection. Randomized controlled trials (RCTs) evaluating the outcome of using prophylactic topical antibiotics in patients undergoing surgery specifically classified as clean (I) or clean-contaminated (II) were included. Trials that contained other classes of wounds were included if the data from individual classes could be extracted. Additionally, trials were required to document their inclusion and exclusion criteria.
Different forms of topical antibiotics were included, such as ointment, cream, lotion, and powder. Trials that used antiseptic agents were also included. Studies of the use of irrigation solutions during surgery, the use of antibiotic dressings for wounds, and other delivery forms (e.g. collagen implants and antibiotic-impregnated sponges) were excluded. Observational and duplicate studies were excluded from this study. In addition, trials regarding catheter infection, therapeutic and decolonization effects, and the use of polypropylene mesh were also excluded.
Search strategy and study selection
Relevant trials published up to 30 September 2020 were identified from the PubMed, Cochrane, and Embase databases. Unpublished trials were collected from the ClinicalTrials.gov registry (http://clinicaltrials.gov/). The following medical subject headings terms were used: surgical wound; surgical wound infection; wound healing; antibacterial agents; antibiotic prophylaxis; administration; topical; staphylococcal infections; topical anti-infective agent; local topical anti-infective agent; bacterial infection; postoperative complications; surgical wound dehiscence; dermatitis; and allergic contact (Table S2). All retrieved abstracts, trials, and citations were reviewed. In addition, other trials were identified using the reference sections of relevant papers and through correspondence with subject experts. No language restrictions were imposed.
Methodological quality appraisal
Two reviewers (Y.M.H. and M.C.L.) independently assessed the methodological quality of each trial by using the risk of bias method, as recommended by the Cochrane Collaboration13. Several domains were evaluated, including the adequacy of randomization, concealment of allocation, blinding of the patients and the outcome assessors, follow-up duration, the information provided to the patients regarding study withdrawals, whether an intention-to-treat (ITT) analysis was performed, and freedom from other biases.
Data and outcome extraction
Baseline and outcome data were independently extracted by two reviewers (Y.M.H. and M.C.L.). The trial design, population characteristics, inclusion and exclusion criteria, surgery type, patient source, regimen of drug administration, and postsurgical wound infection rates were extracted. Disagreements were resolved by a third reviewer (P.J.C.).
The primary outcome was the incidence of SSI, presented as the event rate. The secondary outcome was the incidence of contact dermatitis, which represents the safety outcome.
Statistical analyses
Data were entered and analysed using Review Manager (version 5.4; The Cochrane Collaboration, Oxford, UK). The meta-analysis was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines14. Standard deviations were estimated from the provided confidence interval (c.i.) limits or standard error. Furthermore, dichotomous outcomes were analysed using risk ratios (RRs) as the summary statistics. The precision levels of the effect sizes are reported as 95 per cent confidence intervals. A pooled estimate of the RR and weighted mean difference was computed using the DerSimonian and Laird random-effects model15.
To evaluate the statistical heterogeneity and inconsistency of prophylaxis effects across the trials, the Cochrane Q tests and I2 statistics were used. Statistical significance was set at P < 0.10 for the Cochrane Q tests. Statistical heterogeneity across the trials was assessed using I2 statistics, which quantified the outcome variability across the trials. Heterogeneity was categorized as low (I2 ≤ 25 per cent), moderate (25 per cent < I2 < 75 per cent) or high (I2 ≥ 75 per cent). Additionally, a sensitivity analysis was performed to strengthen the robustness of the results when I2 > 50 per cent. A one-by-one exclusion method was applied for analysis, and subgroup analyses were performed to investigate the effect of the different types and phases of surgery (preoperative, intraoperative, and postoperative).
Results
Characteristics of the included trials and patients
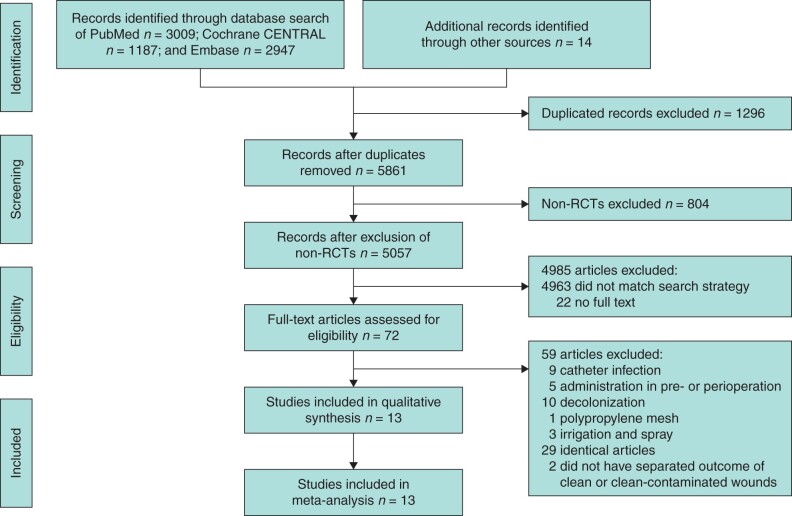
Figure 1 shows the PRISMA flow diagram. The initial search strategy yielded 7157 studies, and after removing the duplicates and non-RCTs, 5057 studies were eligible for title and abstract screening. Seventy-two full-text articles were retrieved and, after further exclusions, 13 trials with complete data were included in the meta-analysis16–28.
Fig. 1.
Flowchart of study selection for systematic review and meta-analysis
Twelve trials compared topical antibiotics to placebo, paraffin, petrolatum, and other non-antibiotic ingredients16–26,28, and one trial compared topical antibiotics to placebo and antiseptic agents27. Regarding type of surgery, there were five trials in dermatological surgery16–20, one in abdominal surgery21, two in orthopaedic surgery22,23, two in spinal surgery24,25, one in ocular surgery26, and two in cardiothoracic surgery27,28. Most trials enrolled clean (I) wounds (12 of 13)16–20,22–28, and only one trial enrolled clean-contaminated (II) wounds21. The administration of topical antibiotics included a nasal administration with mupirocin preoperatively22,28, vancomycin powder intraoperatively24,25 and other topical applications postoperatively16–21,23,26,27. The use of prophylactic antibiotics before surgery in the included trials was inconsistent, and only seven used i.v. prophylactic antibiotics21–25,27,28 (Table 1). Other perioperative management related to SSI and definition of outcomes varied among these trials (Tables S4 and S5).
Table 1.
Study baseline (n = 13)
| Study | Type of surgery | Wound classification | Regimen | Administrated route |
|---|---|---|---|---|
| Dermatologic surgery | ||||
| Dixon 2006 | Skin lesion excision | I | Mupirocin ointment | Topical |
| Smack 1996 | Ambulatory surgery | I | Bacitracin | Topical |
| Taylor 2011 | Remove dermatosis papulosa nigra | I | Polymyxin B sulfate/bacitracin zinc | Topical |
| Heal 2009 | Minor skin excision | I | Chloromycetin ointment | Topical |
| Draelos 2011 | Remove seborrheic keratoses | I | Polymyxin B sulfate/bacitracin zinc bid | Topical |
| Abdominal surgery | ||||
| Neri 2008 | Laparoscopic cholecystectomy | II | Rifamycin | Topical |
| Orthopaedic surgery | ||||
| Kalmeijer 2002 | Prosthetic implant material | I | Mupirocin ointment bid | Nasal |
| Kamath 2005 | Femur fracture | I | Chloramphenicol | Topical |
| Spinal surgery | ||||
| Mirzashahi 2018 | Open spine surgery | I | 1-2 g vancomycin powder | Topical |
| Tubaki 2013 | Open spine surgery | I | 1 g vancomycin powder | Topical |
| Ocular surgery | ||||
| Ashraf 2020 | Periocular surgery | I | Erythromycin, bacitracin zinc, or bacitracin zinc plus polymyxin B sulfate ophthalmic ointment | |
| Cardiothoracic surgery | ||||
| Khalighi 2014 | Cardiac electronic implantable device procedure | I | Povidone iodine or neomycin ointment | Topical |
| Konvalinka 2006 | Elective open-heart surgery | I | 2% mupirocin ointment bid | Nasal |
| Study | Comparison | Number | Prophylactic systematic antibiotics | Prophylactic timing |
|---|---|---|---|---|
| Dermatologic surgery | ||||
| Dixon 2006 | Placebo or sterile paraffin |
Abx:262; placebo:247; paraffin:269 | None | After surgery |
| Smack 1996 | Petrolatum | Abx:444; placebo:440; | None | After surgery |
| Taylor 2011 | Aquaphor Healing Ointment | Abx:20; placebo:20 | None | 21 days after surgery |
| Heal 2009 | Paraffin ointment | Abx:488; placebo:484; | None | After suturing |
| Draelos 2011 | Petrolatum-based ointment | Abx:30; placebo:30 | None | 7 days after surgery |
| Abdominal surgery | ||||
| Neri 2008 | Placebo | Abx:24; placebo:24 | Ceftriaxone | 3 days after surgery |
| Orthopaedic surgery | ||||
| Kalmeijer 2002 | Placebo | Abx:315; placebo:299; | Cefamandole or clindamycin | At least 2 doses before surgery |
| Kamath 2005 | Placebo | Abx:47; placebo:45 | Cefuroxime | 3 days after surgery |
| Spinal surgery | ||||
| Mirzashahi 2018 | Placebo | Abx:193; placebo:187; | Cefazolin or clindamycin | Before surgery |
| Tubaki 2013 | Placebo | Abx:433; placebo:474 | Cefuroxime | Before surgery |
| Ocular surgery | ||||
| Ashraf 2020 | Ophthalmic lubricant ointments, mineral oil and petrolatum | Abx:201; placebo:187 | None | On the surgical site(s) 4 times daily for 7 days after surgery |
| Cardiothoracic surgery | ||||
| Khalighi 2014 | Povidone iodine sterile non-adherent pad or placebo |
Povidone iodine:257; neomycin:263; sterile non-adherent pad:240; placebo:248 | Gentamicin, cefazolin or vancomycin | 3 days after surgery |
| Konvalinka 2006 | Placebo | Abx:130; placebo:127 | Cefazolin or clindamycin | 7 days before surgery |
Abbreviation: ABx, antibiotics.
Quality of the trials
Table S3 summarizes the results of the trial quality assessment. Most trials (10 of 13) had adequate randomization and sequence descriptions, but only six utilized allocation concealment. In the blinding domains, five of 13 had a high risk of bias in participant blinding. In some trials, blinding could not be done completely owing to limitations in drug application; however, there were no deviations from the intended intervention. The risk of bias of assessor blinding was unclear in most trials (nine of 13). Nine of 13 used the ITT analytical method, and eight had a low risk of bias in the selective reporting domain.
Efficacy outcomes
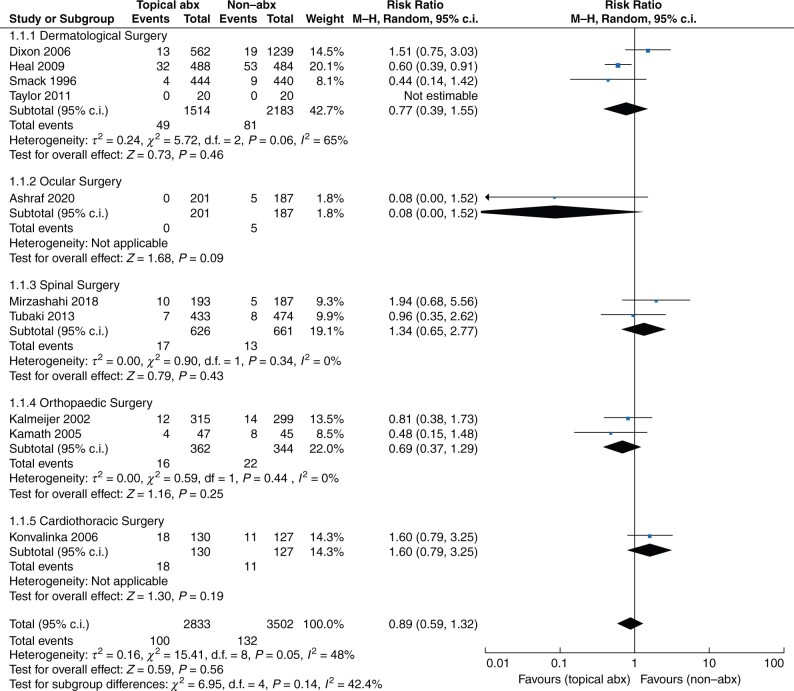
Ten of the included trials16–19,22–26,28 were pooled to compare the prophylactic effect of topical antibiotics to a placebo in clean post-surgical wounds. Figure 2 demonstrates the RR and incidence of SSI in both groups for each type of surgery. The total incidence of SSI was 100 of 2833 in the topical antibiotics group and 132 of 3502 in the non-antibiotic group. Compared with non-antibiotic agents, the use of topical antibiotics did not result in a statistically significant difference in SSI reduction in all populations (RR 0.89, 95 per cent c.i. 0.59 to 1.32; P = 0.56, I2 = 48 per cent). The use of topical antibiotics in all types of surgery was not associated with a reduction in SSI, including dermatological surgery (four trials; RR 0.77, 95 per cent c.i. 0.39 to 1.55 (P = 0.46, I2 = 65 per cent)), ocular surgery (one trial; RR 0.08, 95 per cent c.i. 0.00 to 1.52 (P = 0.09)), spinal surgery (two trials; RR 1.34, 95 per cent c.i. 0.65 to 2.77 (P = 0.43, I2 = 0 per cent)), orthopaedic surgery (two trials; RR 0.69, 95 per cent c.i. 0.37 to 1.29 (P = 0.25, I2 = 0 per cent)), and cardiothoracic surgery (one trial; RR 1.60, 95 per cent c.i. 0.79 to 3.25 (P = 0.19)).
Fig. 2.
Forest plot of surgical site infection
Abx, antibiotics.
Safety outcomes
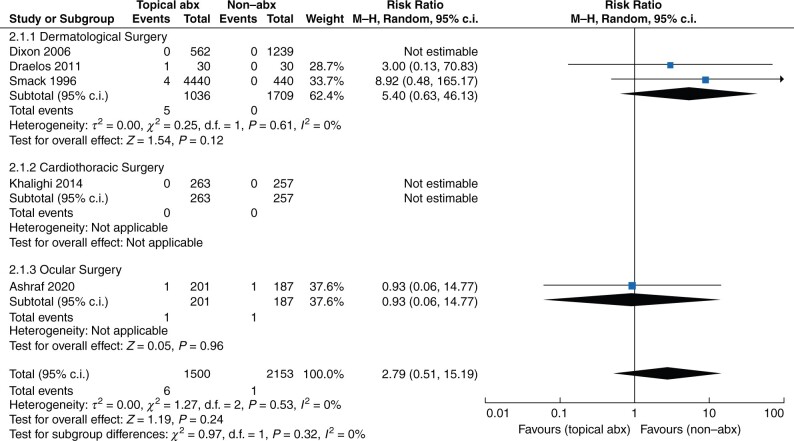
Five trials collected data on contact dermatitis (Fig. 3)16,17,20,26,27. The overall RR of contact dermatitis was not statistically significantly different between topical antibiotics and non-antibiotic agents (RR 2.79, 95 per cent c.i. 0.51 to 15.19 (P = 0.24, I2 = 0 per cent)). In dermatological (RR 5.40, 95 per cent c.i. 0.63 to 46.13 (P = 0.12, I2 = 0 per cent)) and ocular surgeries (RR 0.93, 95 per cent c.i. 0.06 to 14.77; P = 0.96), the risk of contact dermatitis with topical antibiotics was not statistically significant different compared with non-antibiotic agents.
Fig. 3.
Forest plot of contact dermatitis
Abx, antibiotics.
Sensitivity analysis
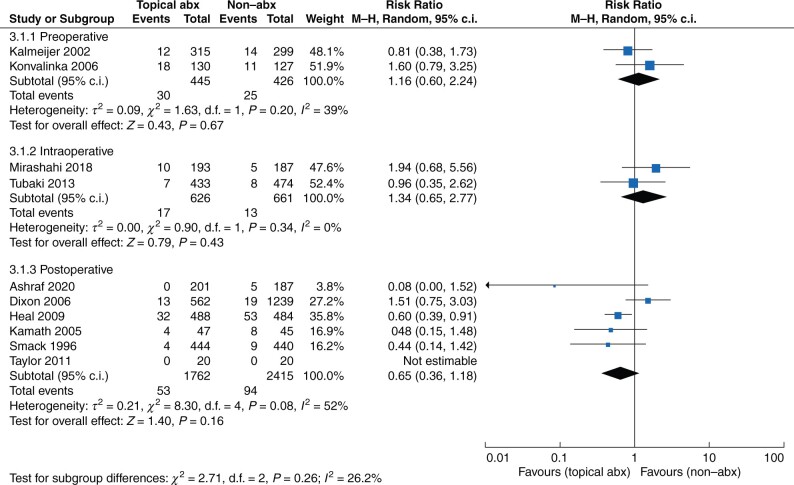
The subgroup analyses of administration according to the different operative phases are presented in Fig. 4. In the preoperative phase, nasal mupirocin did not reduce SSI versus non-antibiotic agents (RR 1.16, 95 per cent c.i. 0.60 to 2.24 (P = 0.67, I2 = 39 per cent)). Similarly, topical vancomycin did not reduce SSI versus non-antibiotic agents (RR 1.34, 95 per cent c.i. 0.65 to 2.77 (P = 0.43, I2 = 0 per cent)) in the intraoperative phase. Moreover, topical antibiotics did not reduce SSI versus non-antibiotic agents in the postoperative phase (RR 0.65, 95 per cent c.i. 0.36 to 1.18 (P = 0.16, I2 = 52 per cent)).
Fig. 4.
Forest plot of different operative phases of administration
Abx, antibiotics.
In addition, a sensitivity analysis was performed using the one-by-one exclusion method (Table S6). After combining the RR values of the remaining trials, no significant impact was found by excluding any individual trial from the final results. Furthermore, no individual trial was found to have a significant impact on the heterogeneity, based on I2.
Discussion
In the general population, topical antibiotics did not contribute to a reduction in SSIs, compared with non-antibiotics, during the perioperative period. There was also no benefit found in dermatological, spinal, orthopaedic, and cardiothoracic surgery. Regarding safety, the risk of contact dermatitis did not increase with the use of topical antibiotics in dermatological, cardiothoracic, or ocular surgery.
A previous guideline29 suggested that the use of topical antibiotics should be limited because of the unclear evidence and the potential adverse effects. Several meta-analyses have been performed to evaluate the effect of topical antibiotics. According to the meta-analysis conducted by Heal et al.30, topical antibiotics reduced the risk of SSI versus placebo (RR 0.61, 95 per cent c.i. 0.42 to 0.87). This effect remained when compared with antiseptics (RR 0.49, 95 per cent c.i. 0.30 to 0.80). Similar findings were reported in the study by Tong et al.11, where topical antibiotics were found to reduce the risk of SSI versus antiseptics or placebo (RR 0.56 (95 per cent c.i. 0.34 to 0.91) and RR 0.57 (95 per cent c.i. 0.37 to 0.86), respectively)11.
In contrast, the pooled results of this study showed that topical antibiotics tended to decrease the risk of an SSI, although these were not statistically significantly different. Compared to the previously mentioned meta-analyses, more recent RCTs were included in the current study, while quasi-randomized study designs were excluded to reduce the risk of selection bias.
In addition, to clarify the prophylactic effect of the topical antibiotics, the focus was only on clean and clean-contaminated wounds. In contrast, Heal et al.30 included clean, clean-contaminated, and contaminated wounds. In addition, the current study assessed the three phases of surgery (preoperative, intraoperative, and postoperative), while previous studies mainly focused on wounds after primary wound closure. Furthermore, subgroup and sensitivity analyses were carried out to assess the heterogeneity and efficacy in specific conditions.
The rates of SSIs in the modern-day outpatient dermatological setting are low, typically ranging from 0.7–4.0 per cent31. However, the prescription of topical antibiotics by dermatologists remains ubiquitous. Statistics show that dermatologists in the USA alone wrote three to four million prescriptions of topical antibiotics in 200331. Nevertheless, the current evidence supporting their use in patients undergoing clean dermatological surgery is conflicting. The current study did not support the use of topical antibiotics compared with placebo in the dermatological field (RR 0.77, 95 per cent c.i. 0.39 to 1.55). Another meta-analysis31 had a similar finding, and also highlighted the advantages of petrolatum-based management, suggesting that the moist environment provided by the ointment may benefit wound healing, rather than the bactericidal actions of the antibiotic32.
In addition to wounds in patients with diabetes, wounds located in the groin or below the knees, basal cell carcinoma and squamous cell carcinoma excisions, skin grafts, flaps on the nose or ears, and wedge resections of the ears or lip are associated with higher rates of SSI31. Even in wounds with a higher risk of developing an infection, petrolatum is equally efficacious in preventing postoperative wound infections as topical antibiotics, based on the study’s results31. Furthermore, oral prophylactic antibiotics might be another option for patients at high risk of infection31.
In intraocular surgery, topical antibiotics are regularly administered owing to the restricted effect of systemic antibiotics caused by the blood–ocular barriers, such as the blood–aqueous barrier and the blood–retinal barrier33. Hence, intracameral or subconjunctival administration of topical antibiotics for surgical prophylaxis has been advocated to achieve adequate tissue drug concentration. However, a recent review on infection prophylaxis for periorbital Mohs surgery and reconstruction contradicted the recommendation of antibiotic ointment use34. Although the rate of SSI in oculoplastic surgery is low, typically between 0.04 and 1.7 per cent26,35, the related complications of SSI can be devastating, possibly even vision-threatening. The included RCT of periocular surgery demonstrated that postoperative SSI was more common in the non-antibiotic group (5 versus 0 in the antibiotics group), although this did not reach statistical significance26. There is an overall trend toward increased prescription of topical antibiotics during intraorbital or oculofacial surgery by ophthalmologists.
For spinal surgery, the rate of SSI is approximately 0.7–10 per cent, despite appropriate antibiotic prophylaxis; this can cause severe complications, such as spinal instability and neurological deficit36. SSIs are often caused by common skin flora, mainly staphylococci. In addition, there have been growing cases of MRSA infection in recent years10. Therefore, vancomycin has been postulated to decrease the rates of SSI. Intravenous vancomycin was initially espoused by investigators but was later proven to be of no benefit in postoperative wound infection, compared to intravenous cephalosporins37. However, the intraoperative administration of vancomycin powder has gradually gained attention from researchers because of its high concentration levels at the site of operative wounds without causing any systemic side effects24.
According to a recent guideline, for patients undergoing complicated spinal surgery, especially those with comorbidities, alternative prophylactic regimens such as intrawound vancomycin could be considered36. A meta-analysis, which pooled two RCTs and 19 retrospective cohort studies, found that vancomycin powder reduced SSI caused by Gram-positive bacilli and polymicrobial infections38. The current study only included two RCTs on spinal surgery24,25, and pooled analysis failed to show any difference in the reduction of SSI rates versus placebo. The difference in results, compared to the previous meta-analysis38, might be due to the potential confounding factors inherent to cohort studies.
Staphylococcus aureus is a leading cause of postoperative wound infections, and studies regarding orthopaedic and cardiothoracic surgery have explicitly shown that nasal colonization by S. aureus is a notable risk factor in the development of an SSI6,12,39,40. Of note, nasal decolonization has been shown to decrease the risk of S. aureus-related healthcare-associated infections in patients with known nasal carriage of S. aureus22,28. The evidence is particularly robust for patients undergoing cardiothoracic and orthopaedic surgery. Considering the high risk of SSI in cardiac surgery, reportedly up to 33 per cent41, and the possible need for implant removal if SSI occurs in orthopaedic procedures22, the preoperative intranasal application of mupirocin 2 per cent ointment for known S. aureus carriers is beneficial in decolonization and is highly supported by current evidence6,9,42,43.
A meta-analysis conducted by the WHO Guidelines Development Group concluded that nasal decolonization using mupirocin ointment, with or without the combination of chlorhexidine gluconate soap body wash preoperatively, had a significant benefit in reducing the incidence of S. aureus SSI in patients with known S. aureus carriage compared with placebo or no treatment (odds ratio 0.46, 95 per cent c.i. 031 to 069)6.
In the current meta-analysis, trials evaluating the efficacy of preoperative nasal mupirocin administration were also included. The two included RCTs demonstrated that prophylactic intranasal mupirocin did not decrease the overall SSI or S. aureus-related infection rate22,28. Therefore, there is no clear evidence that routine nasal decolonization with mupirocin in all patients resulted in a reduction of SSIs versus placebo. The different results obtained by this study and the published evidence may lie in the enrolled patient groups (whether there was nasal carriage of S. aureus or not). The current study included patients who underwent surgery and were noticeably distinct from the WHO study groups, which consisted mainly of S. aureus carriers.
The strengths of the current study are that only RCTs were included in the meta-analysis to minimize selection bias and confounding factors. Further, more trials were included and provided additional results from the different types and different phases of surgery were provided. Furthermore, the sensitivity analysis strengthened the robustness of the primary outcome. Nevertheless, there are several limitations. Firstly, heterogeneity could not be avoided because the study design, perioperative management related to SSI, definition of outcomes, and enrolled patients varied among the trials. Although a subgroup and sensitivity analysis was performed, moderate heterogeneity still existed. Secondly, not all surgical wounds were included and therefore the results are not applicable to other patient groups. Finally, the number of trials in each subgroup was still insufficient, and patients at a high risk of infection were not discussed separately. Further RCTs are required to resolve these clinical problems.
Overall, this study does not support the routine use of topical antibiotics to prevent SSIs in patients undergoing clean surgeries, especially dermatological procedures. However, there is a potential benefit during ocular surgery because of the devastating outcomes of SSIs in these scenarios. In other types of surgery, the number of enrolled trials was limited and thus no conclusions concerning clean-contaminated surgical wounds can be made.
Supplementary material
Supplementary material is available at BJS Open online.
Funding
None.
Supplementary Material
Acknowledgements
PCJ and YMH contributed equally to this study, including the conception or design of the work and drafting of the manuscript. MCL and HST contributed to the acquisition, analysis, and interpretation of the data for this study. All authors critically revised the manuscript, gave final approval, agreed to be accountable for all aspects of work, and ensured its integrity and accuracy. Po-Jung Chen and Yi-Ming Hua had an equal contribution to the present study.
Disclosure. The authors declare no conflicts of interest.
Contributor Information
Po-Jung Chen, Department of Emergency Medicine, Chi Mei Medical Center, Tainan, Taiwan.
Yi-Ming Hua, Department of Pharmacy, Chi Mei Medical Center, Tainan, Taiwan.
Han Siong Toh, Department of Intensive Care Medicine, Chi Mei Medical Center, Tainan, Taiwan; Institute of Clinical Medicine, College of Medicine, National Cheng Kung University, Tainan, Taiwan; Department of Health and Nutrition, Chia Nan University of Pharmacy & Science, Tainan, Taiwan.
Mei-Chuan Lee, Department of Pharmacy, Chi Mei Medical Center, Tainan, Taiwan; Department of Public Health, College of Medicine, National Cheng Kung University, Tainan, Taiwan.
References
- 1. Badia JM, Casey AL, Petrosillo N, Hudson PM, Mitchell SA, Crosby C. Impact of surgical site infection on healthcare costs and patient outcomes: a systematic review in six European countries. J Hosp Infect 2017;96:1–15. [DOI] [PubMed] [Google Scholar]
- 2. Weiner-Lastinger LM, Abner S, Edwards JR, Kallen AJ, Karlsson M, Magill SS et al. Antimicrobial-resistant pathogens associated with adult healthcare-associated infections: summary of data reported to the National Healthcare Safety Network, 2015–2017. Infect Control Hosp Epidemiol 2020;41:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Berríos-Torres SI, Umscheid CA, Bratzler DW, Leas B, Stone EC, Kelz RR et al. ; Healthcare Infection Control Practices Advisory Committee. Centers for Disease Control and Prevention guideline for the prevention of surgical site infection, 2017. JAMA Surg 2017;152:784–791. [DOI] [PubMed] [Google Scholar]
- 4. Lipsky BA, Hoey C. Topical antimicrobial therapy for treating chronic wounds. Clin Infect Dis 2009;49:1541–1549. [DOI] [PubMed] [Google Scholar]
- 5. McHugh SM, Collins CJ, Corrigan MA, Hill AD, Humphreys H. The role of topical antibiotics used as prophylaxis in surgical site infection prevention. J Antimicrob Chemother 2011;66:693–701. [DOI] [PubMed] [Google Scholar]
- 6. Allegranzi B, Bischoff P, de Jonge S, Kubilay NZ, Zayed B, Gomes SM et al. ; WHO Guidelines Development Group. New WHO recommendations on preoperative measures for surgical site infection prevention: an evidence-based global perspective. Lancet Infect Dis 2016;16:e276–e287. [DOI] [PubMed] [Google Scholar]
- 7. López-Cano M, Kraft M, Curell A, Puig-Asensio M, Balibrea J, Armengol-Carrasco M et al. Use of topical antibiotics before primary incision closure to prevent surgical site infection: a meta-analysis. Surg Infect (Larchmt) 2019;20:261–270. [DOI] [PubMed] [Google Scholar]
- 8. Allegranzi B, Zayed B, Bischoff P, Kubilay NZ, de Jonge S, de Vries F et al. ; WHO Guidelines Development Group. New WHO recommendations on intraoperative and postoperative measures for surgical site infection prevention: an evidence-based global perspective. Lancet Infect Dis 2016;16:e288–e303. [DOI] [PubMed] [Google Scholar]
- 9. National Institute for Health and Care Excellence: Clinical Guidelines. Surgical Site Infections: Prevention and Treatment. London: National Institute for Health and Care Excellence, 2020. [PubMed] [Google Scholar]
- 10. Thakkar V, Ghobrial GM, Maulucci CM, Singhal S, Prasad SK, Harrop JS et al. Nasal MRSA colonization: impact on surgical site infection following spine surgery. Clin Neurol Neurosurg 2014;125:94–97. [DOI] [PubMed] [Google Scholar]
- 11. Tong QJ, Hammer KD, Johnson EM, Zegarra M, Goto M, Lo TS. A systematic review and meta-analysis on the use of prophylactic topical antibiotics for the prevention of uncomplicated wound infections. Infect Drug Resist 2018;11:417–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mangram AJ, Horan TC, Pearson ML, Silver LC, Jarvis WR. Guideline for prevention of surgical site infection, 1999. Centers for Disease Control and Prevention (CDC) Hospital Infection Control Practices Advisory Committee. Am J Infect Control 1999;27:97–132. [PubMed] [Google Scholar]
- 13. Shuster JJ. Assessing risk of bias in included studies. In: Cochrane Handbook for Systematic Reviews of Interventions, version 5.1.0, Chap.8. https://handbook-5-1.cochrane.org (accessed 17 November 2021).
- 14. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 2009;339:b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177–188. [DOI] [PubMed] [Google Scholar]
- 16. Dixon AJ, Dixon MP, Dixon JB. Randomized clinical trial of the effect of applying ointment to surgical wounds before occlusive dressing. Br J Surg 2006;93:937–943. [DOI] [PubMed] [Google Scholar]
- 17. Smack DP, Harrington AC, Dunn C, Howard RS, Szkutnik AJ, Krivda SJ et al. Infection and allergy incidence in ambulatory surgery patients using white petrolatum vs bacitracin ointment. A randomized controlled trial. JAMA 1996;276:972–977. [PubMed] [Google Scholar]
- 18. Taylor SC, Averyhart AN, Heath CR. Postprocedural wound-healing efficacy following removal of dermatosis papulosa nigra lesions in an African American population: a comparison of a skin protectant ointment and a topical antibiotic. J Am Acad Dermatol 2011;64:S30–S35. [DOI] [PubMed] [Google Scholar]
- 19. Heal CF, Buettner PG, Cruickshank R, Graham D, Browning S, Pendergast J et al. Does single application of topical chloramphenicol to high risk sutured wounds reduce incidence of wound infection after minor surgery? Prospective randomised placebo controlled double blind trial. BMJ 2009;338:a2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Draelos ZD, Rizer RL, Trookman NS. A comparison of postprocedural wound care treatments: do antibiotic-based ointments improve outcomes? J Am Acad Dermatol 2011;64:S23–S29. [DOI] [PubMed] [Google Scholar]
- 21. Neri V, Fersini A, Ambrosi A, Tartaglia N, Valentino TP. Umbilical port-site complications in laparoscopic cholecystectomy: role of topical antibiotic therapy. JSLS 2008;12:126–132. [PMC free article] [PubMed] [Google Scholar]
- 22. Kalmeijer MD, Coertjens H, van Nieuwland-Bollen PM, Bogaers-Hofman D, de Baere GAJ, Stuurman A et al. Surgical site infections in orthopedic surgery: the effect of mupirocin nasal ointment in a double-blind, randomized, placebo-controlled study. Clin Infect Dis 2002;35:353–358. [DOI] [PubMed] [Google Scholar]
- 23. Kamath S, Sinha S, Shaari E, Young D, Campbell AC. Role of topical antibiotics in hip surgery. A prospective randomised study. Injury 2005;36:783–787. [DOI] [PubMed] [Google Scholar]
- 24. Mirzashahi B, Chehrassan M, Mortazavi SMJ. Intrawound application of vancomycin changes the responsible germ in elective spine surgery without significant effect on the rate of infection: a randomized prospective study. Musculoskelet Surg 2018;102:35–39. [DOI] [PubMed] [Google Scholar]
- 25. Tubaki VR, Rajasekaran S, Shetty AP. Effects of using intravenous antibiotic only versus local intrawound vancomycin antibiotic powder application in addition to intravenous antibiotics on postoperative infection in spine surgery in 907 patients. Spine (Phila Pa 1976) 2013;38:2149–2155. [DOI] [PubMed] [Google Scholar]
- 26. Ashraf DC, Idowu OO, Wang Q, YeEun T, Copperman TS, Tanaboonyawat S et al. The role of topical antibiotic prophylaxis in oculofacial plastic surgery: a randomized controlled study. Ophthalmology 2020;127:1747–1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Khalighi K, Aung TT, Elmi F. The role of prophylaxis topical antibiotics in cardiac device implantation. Pacing Clin Electrophysiol 2014;37:304–311. [DOI] [PubMed] [Google Scholar]
- 28. Konvalinka A, Errett L, Fong IW. Impact of treating Staphylococcus aureus nasal carriers on wound infections in cardiac surgery. J Hosp Infect 2006;64:162–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Leggat PA. Therapeutic guidelines: antibiotic. J Mil Veterans Health 2010;18:35–36. [Google Scholar]
- 30. Heal CF, Banks JL, Lepper PD, Kontopantelis E, van Driel ML. Topical antibiotics for preventing surgical site infection in wounds healing by primary intention. Cochrane Database Syst Rev 2016;11:CD011426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Saco M, Howe N, Nathoo R, Cherpelis B. Topical antibiotic prophylaxis for prevention of surgical wound infections from dermatologic procedures: a systematic review and meta-analysis. J Dermatolog Treat 2015;26:151–158. [DOI] [PubMed] [Google Scholar]
- 32. Smith R, Russo J, Fiegel J, Brogden N. Antibiotic delivery strategies to treat skin infections when innate antimicrobial defense fails. Antibiotics 2020;9:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Urtti A. Challenges and obstacles of ocular pharmacokinetics and drug delivery. Adv Drug Deliv Rev 2006;58:1131–1135. [DOI] [PubMed] [Google Scholar]
- 34. DelMauro MA, Kalberer DC, Rodgers IR. Infection prophylaxis in periorbital Mohs surgery and reconstruction: a review and update to recommendations. Surv Ophthalmol 2020;65:323–347. [DOI] [PubMed] [Google Scholar]
- 35. Olds C, Spataro E, Li K, Kandathil C, Most SP. Postoperative antibiotic use among patients undergoing functional facial plastic and reconstructive surgery. JAMA Facial Plast Surg 2019;21:491–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Shaffer WO, Baisden JL, Fernand R, Matz PG; North American Spine Society. An evidence-based clinical guideline for antibiotic prophylaxis in spine surgery. Spine J 2013;13:1387–1392. [DOI] [PubMed] [Google Scholar]
- 37. Nguyen AV, Coggins WS, Jain RR, Branch DW, Allison RZ, Maynard K et al. Cefazolin versus vancomycin for neurosurgical operative prophylaxis - a single institution retrospective cohort study. Clin Neurol Neurosurg 2019;182:152–157. [DOI] [PubMed] [Google Scholar]
- 38. Li S, Rong H, Zhang X, Zhang Z, Wang C, Tan R et al. Meta-analysis of topical vancomycin powder for microbial profile in spinal surgical site infections. Eur Spine J 2019;28:2972–2980. [DOI] [PubMed] [Google Scholar]
- 39. Perl TM, Golub JE. New approaches to reduce Staphylococcus aureus nosocomial infection rates: treating S. aureus nasal carriage. Ann Pharmacother 1998;32:S7–16. [DOI] [PubMed] [Google Scholar]
- 40. Wenzel RP, Perl TM. The significance of nasal carriage of Staphylococcus aureus and the incidence of postoperative wound infection. J Hosp Infect 1995;31:13–24. [DOI] [PubMed] [Google Scholar]
- 41. Culver DH, Horan TC, Gaynes RP, Martone WJ, Jarvis WR, Emori TG et al. Surgical wound infection rates by wound class, operative procedure, and patient risk index. National Nosocomial Infections Surveillance System. Am J Med 1991;91:152s–157s. [DOI] [PubMed] [Google Scholar]
- 42. Bode LGM, Kluytmans JAJW, Wertheim HFL, Bogaers D, Vandenbroucke-Grauls CMJE, Roosendaal R et al. Preventing surgical-site infections in nasal carriers of Staphylococcus aureus. N Engl J Med 2010;362:9–17. [DOI] [PubMed] [Google Scholar]
- 43. Tai YJ, Borchard KL, Gunson TH, Smith HR, Vinciullo C. Nasal carriage of Staphylococcus aureus in patients undergoing Mohs micrographic surgery is an important risk factor for postoperative surgical site infection: a prospective randomised study. Australas J Dermatol 2013;54:109–114. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.