Abstract
Mn-doped lead halide perovskites exhibit long-lived dopant luminescence and enhanced host excitonic quantum yield. The contention between energy and charge transfer in sensitizing dopant luminescence in Mn-doped perovskites is investigated by state-of-the-art DFT calculations on APbX3 perovskites (X = Cl, Br, and I). We quantitatively simulate the electronic structure of Mn-doped perovskites in various charge and spin states, providing a structural/mechanistic analysis of Mn sensitization as a function of the perovskite composition. Our analysis supports both energy- and charge-transfer mechanisms, with the latter probably preferred in Mn:CsPbCl3 due to small energy barriers and avoidance of spin and orbital restrictions. An essential factor determining the dopant luminescence quantum yield in the case of charge transfer is the energetics of intermediate oxidized species, while bandgap resonance can well explain energy transfer. Both aspects are mediated by perovskite host band edge energetics, which is tuned in turn by the nature of the halide X.
Doping semiconductors by transition metal ions has been widely proved to impart specific optical and magnetic properties to the semiconducting host. In particular, Mn2+ doping has been successfully implemented in binary semiconductor nanocrystals (NCs), such as CdSe, ZnS, ZnSe, and ZnO, with a direct impact on their stability and optoelectronic properties.1−3 As an example, doping CdSe NCs by Mn2+ substantially increases the excitonic photoluminescence (PL) lifetime. Another behavior which is also typically observed is the appearance of two PL features, i.e., the excitonic CdSe band at ∼2.4 eV and the typical 4T1→6A1 ligand field transition of the Mn2+ ion at ∼2 eV.4 Of technological relevance for optoelectronics and biomedical applications, the dopant emission is usually confined to a narrow energy range; thus, a variable Stokes shift can be realized by playing with both the size and the composition of the NC host. Furthermore, incorporation of manganese ions into the crystal lattice imparts magnetic properties to the host, clearly manifested at cryogenic temperatures.5,6
Metal halide perovskites (MHPs) are a game-changer class of materials in photovoltaics and optoelectronics.7−16 Besides their success in solar cells, perovskite NCs have shown outstanding optical properties in light-emitting diodes, with near unity emission quantum yield and wide color tuning.17−24 To further diversify the emission color gamut of MHP NCs, Mn2+ doping has been successfully implemented.5,25,26 Mn doping can be introduced in variable concentrations, covering a range up to ∼10%.5,25−31 Previous studies indicate incorporation of the transition metal in the bulk of the material rather than on the NC surface, with the transition metal located in a lead substitutional position within an octahedral coordination environment.25,29,31,32
Similar to what is observed in binary semiconductors, in MHPs a dual-color emission from the perovskite host exciton is observed, along with a broad Mn2+ (4T1→6A1) ligand field transition. The PL decay of the Mn emission is single exponential with a ∼ms lifetime,27,30,32−34 and it is moderately tunable by playing with the dopant content, varying from the yellow–orange region (2.14 eV) to the orange–red one (1.98 eV).5,33,35,36 Mn doping was also reported to enhance the MHP band gap PL quantum yield.13,25,28,30,25,31,37−43 The possible mechanism behind the dopant-induced PL quantum yield increase has been ascribed in some cases to dopant-to-material back transfer, although a plausible explanation is also defect passivation, e.g., Mn→Pb vacancy filling,44 which coherently suppresses material degradation and progressively stabilizes the host’s cohesive energy.45
Most notably, the Mn2+ luminescence quantum yield in perovskite NCs is strongly halide-dependent, with lead bromide perovskites showing a significantly less intense emission intensity compared to their lead chloride counterpart, while, to our knowledge, Mn2+ luminescence is not detected in lead iodide perovskites.37−40,5,25,41−43 This peculiar behavior raises questions about the interplay between dopant and host electronic energy levels and how this would affect the population of dopant states from initially populated host states. It is generally accepted that population of the 4T1 excited Mn2+ state occurs through energy transfer from the host exciton to the Mn dopant. In this framework, Liu et al. proposed that the more intense Mn2+ luminescence of lead chloride perovskites, compared to lead bromide and lead iodide compounds, can be ascribed to the progressively less resonant band gap of the perovskite host with the ligand field dopant transition.5 Pinchetti et al. pointed out the presence of a dopant sensitization process in CsPbCl3 occurring via an intermediate step which involves a long-lived shallow trap state mediating excitation of the Mn2+ center by the host band-edge excitons.30 Consistent with the proposed mechanism, an activation energy of ∼0.3 eV was measured for the sensitization process. Sun et al. confirmed the presence of an activation barrier for Mn2+ luminescence, though these authors found a smaller barrier of ∼0.1 eV.29 A similar effect was also detected by Zeng et al., with Mn2+ luminescence quantum yield decreasing with temperature.46
Despite available information, the nature of the mechanism responsible for the possible sensitization of the excited manganese 4T1 state remains elusive. Temperature-dependent Raman spectroscopy performed by Pradeep et al. on Mn:CsPb(Cl/Br)3 shows a progressive sharpening of the 132 cm–1 Pb–Br stretching phonon mode with increasing temperature, which is strongly coupled to Mn dopant modes.41 The presence of a transient Mn3+ species in Mn2+-doped II–IV semiconducting NCs was proposed by Gahlot et al.47 The presence of Mn3+ in perovskite samples was also invoked to rationalize the ferromagnetic coupling in MAPb1–xMnxI3, proposed to arise from Mn2+–I––Mn3+ motifs.48 Very recently, Babu et al. revealed the formation of a transient charge-transfer state in Mn:CsPbBr3, suggesting that a spin-allowed charge-transfer process (concurrent to energy transfer) related to Mn3+ formation is likely to occur.49
Motivated by the huge interest in Mn-doped MHPs and by the puzzling mechanism of Mn2+ sensitization, we here explore the structural and electronic properties of Mn-doped APbX3 perovskites (X = Cl, Br, and I) by state-of-the-art first-principles modeling studies based on hybrid density functional theory (DFT) and spin–orbit coupling (SOC) on fairly large perovskite models, Figure 1a. We accurately simulate the alignment of host/dopant energy levels in both Mn2+ and Mn3+ states considering different electronic and spin states and the associated structural rearrangement. Based on this data we provide an in-depth analysis on both the possible energy- and charge-transfer processes in Mn:APbX3 perovskites, highlighting points in favor of the former or the latter. In the framework of a hole (h) charge transfer to Mn2+ in CsPbCl3, we found a thermally activated mechanism with a barrier in fair agreement with that reported by Pinchetti et al.30 This suggests the same mechanism to be a major pathway in lead chloride perovskites, proposing Mn itself as a shallow trap mediating the sensitization of 4T1, later decaying to 6A1. We finally investigate the charge transfer/energy transfer dependency on different halides, extending our study to lead bromide and lead iodide perovskites.
Figure 1.
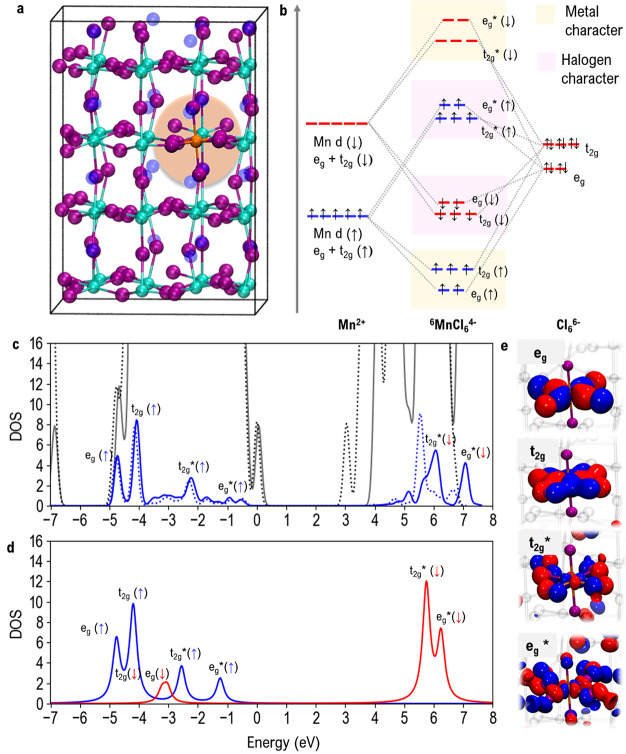
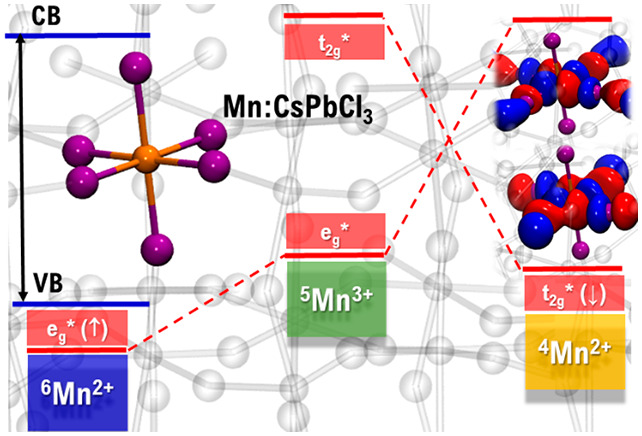
(a) Tetragonal 2×2×2 supercell (32 formula units) employed to model Mn:CsPbCl3. Orange, Mn; purple, Cl; cyan, Pb; and blue, Cs atoms. (b) Qualitative molecular orbital scheme for the complex with spin (↑) (blue) and spin (↓) (red) manifolds. (c) Total (gray) and manganese (blue) density of states (DOS) of 6Mn2+:CsPbCl3 computed at the PBE0 and PBE0-SOC levels (solid and dashed lines, respectively), with Mn molecular orbitals labeled. (d) Aligned manganese DOS for the 6MnCl64– model complex. (e) Isodensity plots of representative 6Mn2+:CsPbCl3 spin α single-particle states with eg, t2g, t2g*, and eg* character.
We model three key states of Mn-doped APbX3 perovskites. While the perovskite host has an even number of electrons, paired to form a singlet ground state, Mn doping introduces additional d-states of the dopant which can give rise to different electronic patterns, namely the sextet ground state of the Mn2+ ion with five Mn d-unpaired electrons (6A1, t2g3eg2, hereafter 6Mn2+), the quartet excited state (4T1, t2g4eg1, hereafter 4Mn2+) obtained by pairing two electrons from the high-spin sextet, and an oxidized state obtained by removing an electron from the sextet ground state, formally corresponding to a Mn3+ ion with a quintet ground state (t2g3eg1, hereafter 5Mn3+), which may account for hole trapping at the Mn2+ site.
We immediately face an issue with the coherent description of the electronic structure of the perovskite host—which requires the inclusion of SOC—and the interest in various local Mn spin multiplet states, which are not purely defined in the presence of SOC. In addition, we need to combine SOC with a hybrid functional, such as PBE0,50,51 for a correct description of the perovskite band edge energies and band gap. At the same time, a hybrid functional is required to correctly describe the energetics of the dopant d-shell orbitals and the structural relaxation associated with the various local Mn spin states.52 As a practical solution, by taking Mn:CsPbCl3 as a benchmark, we first calculate the electronic structure at the PBE0-SOC level, which provides results in quantitative agreement with the experiment (e.g., a host band gap of 3.04 eV, Figure 1c).53 Based on this analysis, we notice that unoccupied Mn states lie sufficiently above the host conduction band (CB) edge (>2 eV) that they are less likely to intrude in the gap when neglecting SOC (SOC basically downshifts the host CB states by ∼1 eV, while its effect on the valence band (VB) is negligible in this case, Figure 1c). We thus confidently employ PBE0 with no SOC for structural optimizations and for electronic structure analysis, checking the impact of SOC in selected cases.
The local electronic structure of 6Mn2+:CsPbCl3 shares many analogies with that typical of octahedral transition metal complexes with π-donor ligands,54 as schematized in Figure 1b, where a qualitative molecular orbital diagram for a 6MnCl64– model complex is reported. In the octahedral symmetry (Oh), the Mn d orbitals labeled as eg (dx2–y2, dz2) and t2g (dxy, dxz, dyz), Figure 1b, interact with the halogen orbitals of the same symmetry, generating bonding molecular orbitals, i.e., t2g and eg, and antibonding ones, i.e., t2g* and eg*, Figure 1e. The molecular orbitals with spins α and β are shown separately, referred to as (↑) and (↓) respectively, Figure 1b. The Mn contributions to the density of states (DOS) of Figure 1c for 6Mn2+:CsPbCl3 can be classified by comparison with the ones of 6MnCl64– in Figure 1d as following: eg (↑) orbitals at ∼ –4.77 eV below the VB maximum, which are originated from the σ bonding interaction between the Mn dx2–y2, dz2 and Cl p orbitals, followed at ∼ –4.13 eV by t2g (↑) orbitals, which originate from the π bonding interaction between the Mn dxy, dxz, dyz and Cl p orbitals. Corresponding antibonding interactions can be found at ∼ −2.29 eV (t2g* (↑) orbitals) and at ∼ –0.70 eV (eg* (↑) states). Antibonding eg* and t2g* unoccupied (↓) states are found 1.63 eV above the SOC-calculated CB edge, Figure 1c. Notice that, in agreement with the model complex (Figure 1b–d), while the α bonding combinations show a predominant Mn character, α antibonding states have more halide character. The computed energy of occupied Mn (↑) 3d levels with respect to the material band edges appears in good accordance with that found for typical Mn-doped binary semiconductors like Cd1–xMnxTe. The 3d5 levels of Mn(II)-doped Cd1–xMnxTe are empirically located at 9.7 eV vs vacuum; thus, for a given work function (Wf, eV), subtract the Wf from 9.7 eV to find the binding energy (BE) of the Mn(II) levels below the Fermi level.55,56 In our case, with an experimental VB for CsPbCl3 located at −5.82 eV.57 we can expect the Mn levels to be 3–4 eV below the VB, in line with the calculated position of the t2g (↑) and eg (↑) states.
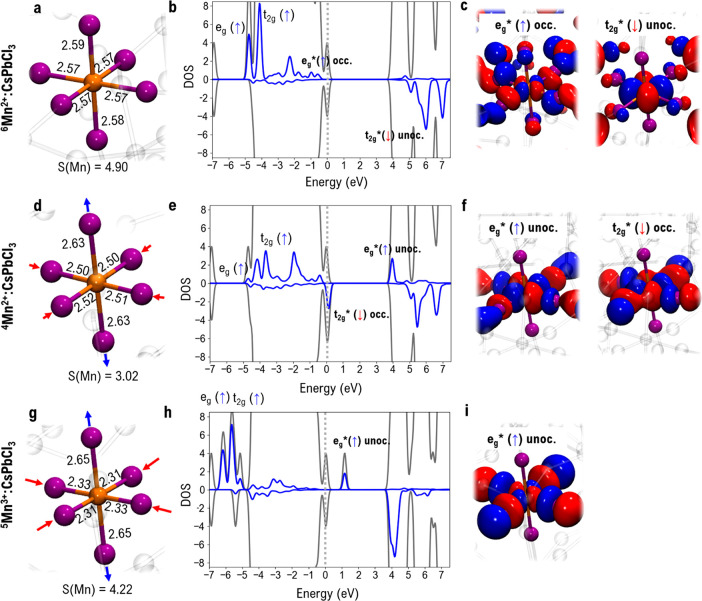
The 6Mn2+:CsPbCl3 ground state has an almost symmetric Mn–ligand coordination environment, Figure 2a, with average Mn–Cl distances of 2.57 Å, in good agreement with previous results reported by Ma et al.58 Significant structural differences in the MnCl6 coordination sphere are calculated for 4Mn2+:CsPbCl3 and 5Mn3+:CsPbCl3, Figure 2d–g, with compression (elongation) along the equatorial (axial) bond distances in 4Mn2+, Figure 2d, and average distances of 2.51 Å (2.63 Å). These structural differences can be understood in terms of the variation in the electronic structure associated with the different spin/charge states. 4Mn2+:CsPbCl3 is originated from 6Mn2+:CsPbCl3 by promotion of an electron from an eg* (↑) to an unoccupied t2g* (↓) state, initially lying within the perovskite host CB, Figure 2e,f. Although the octahedral symmetry is not retained, we continue to use octahedral labels for simplicity rather than D4h labels; see Figure S1 in the Supporting Information.
Figure 2.
Structural properties, DOSs with Mn contributions, and key orbitals involved in spin/charge transitions for Mn:CsPbCl3. (a) 6Mn2+:CsPbCl3 first coordination sphere with distances in Å and spin moments from Mulliken population analysis. Orange spheres represent Mn atoms, purple ones chloride atoms; species outside the Mn first coordination shell are transparent. (b) Total (gray) and manganese (blue) DOS (with negative/positive spin differentiation) for 6Mn2+:CsPbCl3 computed at the PBE0 level of theory, with Mn active molecular orbitals labeled. The dashed vertical bar represents the Fermi level. (c) Isodensity plots of orbitals involved in spin/charge transitions for occupied eg* (↑) and unoccupied t2g* (↓) single-particle states of 6Mn2+:CsPbCl3. (d) 4Mn2+:CsPbCl3 first coordination sphere. (e) Total (gray) and manganese (blue) DOS for 4Mn2+:CsPbCl3. (f) Isodensity plot of orbitals involved in spin/charge transitions for unoccupied eg* (↑)and occupied t2g* (↓) single-particle states of 4Mn2+:CsPbCl3. (g) 5Mn3+:CsPbCl3 first coordination sphere. (h) Total (gray) and manganese (blue) DOS for 5Mn3+:CsPbCl3. (i) Isodensity plot of orbitals involved in spin/charge transitions for unoccupied eg* (↑) single-particle state of 5Mn3+:CsPbCl3.
The PBE0-calculated energy difference between the optimized 4Mn2+ and 6Mn2+ structures is 2.05 eV, in excellent agreement with the 4T1→6A1 PL recorded experimentally.5,25,30,34,59,60 A value of 1.48 eV was calculated by using the non-hybrid PBE functional, confirming the importance of the use of a hybrid functional for a quantitative assessment of Mn2+ electronic state energetics (see the related section in the Supporting Information to look for further GGA-hybrid comparative data).52,61−63 The charge hole left on the eg*(↑) state in 4Mn2+:CsPbCl3 is largely destabilized, now lying 0.01 eV above the CB, while the occupied t2g*(↓) state is strongly stabilized, now lying 0.13 eV above the VB; compare the DOS for the two states in Figure 2b–e and Figure S2 in the Supporting Information for additional analyses. A significant orbital reorganization occurs in parallel for eg (↑) and t2g (↑) states, whose density significantly decreases in 4Mn2+:CsPbCl3 compared to that in 6Mn2+:CsPbCl3, Figure 2b–e.
Upon removing one electron from the 6Mn2+:CsPbCl3 ground state, we find two structural minima with different energies and electronic structures; see Figure 2g and Figure S3a in the Supporting Information. The most stable oxidized species has a geometry similar to that of 6Mn2+:CsPbCl3, and inspection of the DOS (Figure S3b, Supporting Information) shows that it indeed corresponds to removal of one electron from the perovskite host VB, leading to a [6Mn2+/h+:CsPbCl3] electronic structure; i.e., the host rather than the dopant is oxidized. A secondary minimum corresponding to manganese oxidation, 5Mn3+:CsPbCl3, is calculated 0.18 eV above [6Mn2+/h+:CsPbCl3], which shows a significant equatorial compression (average Mn–Cl distance of 2.31 Å), Figure 2g, due to the typical Jahn–Teller effect of 5Mn3+ ions in weak ligand fields. The electronic structure of the 5Mn3+:CsPbCl3 DOS is reported in Figure 2h,i, which clearly shows hole localization in the eg* state, along with energy relaxation of bonding manganese states by ∼1.5 eV due to the increased manganese effective charge. It is interesting to notice that the [6Mn2+/h+:CsPbCl3] and 5Mn3+:CsPbCl3 minima are connected by an energy barrier of 0.33 eV, Figure 3, as determined by a linear transit calculation. On overall, while in 4Mn2+:CsPbCl3 a significant orbital reorganization occurs compared to 6Mn2+:CsPbCl3, in 5Mn3+:CsPbCl3 the distribution of t2g (↑)(↓) and eg (↑)(↓) states is almost unaltered, except for the aforementioned energy shift, Figure 2h. This behavior parallels what was found for the model complexes 4MnCl64– and 5MnCl63– (Figure S1, Supporting Information).
Figure 3.
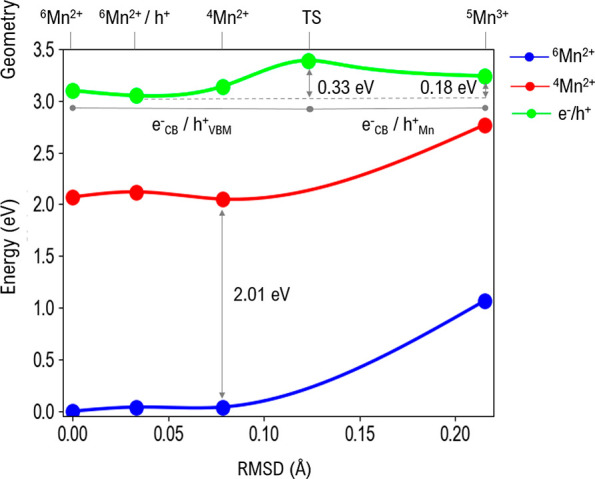
Configurational energy diagram of Mn:CsPbCl3. The energies of the sextet state (blue dots/lines), quartet state (red dots/lines), and electron/hole pair (green dots) are plotted against the root-mean-square deviation (RMSD) of the MnCl6 moiety in the perovskite. The reference is set as the sextet ground state. Larger values of RMSD correspond to MnCl6 equatorial bond compression and axial bond elongation.
In Figure 3 we propose a global spin/charge configurational diagram for Mn-doped CsPbCl3, with the three involved potential energy surfaces, i.e., 6Mn2+, 4Mn2+, and that obtained upon band gap excitation, lying 3.04 eV above the ground state. In this latter case we aim at describing the formation of an electron/hole pair, where the electron is delocalized into a host CB state, while the hole can be either VB-delocalized, as in [6Mn2+/h+:CsPbCl3], or trapped by manganese in 5Mn3+:CsPbCl3.
The potential energy surface of the three considered states is almost flat for low root-mean-square deviation (RMSD) values of MnCl6 (i.e., small structural distortions from the sextet ground-state geometry), while the total energy significantly increases when the Mn–Cl equatorial distances are compressed, moving to the 5Mn3+:CsPbCl3 minimum region, Figure 3. While the energies of the 6Mn2+:CsPbCl3 and 4Mn2+:CsPbCl3 states increase, that of the 5Mn3+:CsPbCl3 state goes through a transition state (TS) (0.33 eV above the [6Mn2+/h+:CsPbCl3] minimum) and then reaches a local minimum corresponding to hole trapping at the Mn center, which we calculate to be 0.18 eV higher than [6Mn2+/h+:CsPbCl3], Figure 3.
Upon band gap excitation of the CsPbCl3 perovskite host, there are two possible decay pathways involving the dopant:
-
(i)
energy transfer from the host to the excited 4Mn2+:CsPbCl3 state;
-
(ii)
hole trapping to form 5Mn3+:CsPbCl3, followed by non-radiative decay to 4Mn2+:CsPbCl3.
While the decay channel (i) requires negligible structural deformation, similar to band gap recombination, pathway (ii) requires overcoming an energy barrier associated with hole trapping with significant structural deformation. Previous literature typically ascribed population of the 4Mn2+ state to pathway (i), i.e., band gap exciton to manganese dopant energy transfer. A factor in favor of this mechanism is the small required structural rearrangement. An experimental finding which is also a point in favor of energy transfer is the mono-exponential Mn luminescence decay, suggesting the absence of intermediates.27 The different local Mn spin states could, however, hinder such a process; as a matter of fact, the direct 6T1→4T1 excitation has a negligible cross section due to the spin-forbidden nature of this process. Also, the significant orbital reorganization associated with the inner manganese shell discussed above could further inhibit such a process. Pathway (ii) requires a significant structural reorganization but no spin restriction, as 5Mn3+:CsPbCl3 can accept one electron in the t2g* state to form 4Mn2+:CsPbCl3, keeping the eg* orbital unoccupied. This mechanism of manganese 4T1 sensitization is associated with a calculated activation barrier of 0.33 eV, corresponding to the evolution of the transient 5Mn3+ species, which is in good agreement with the activation energy measured by Pinchetti et al.30 and consistent with the behavior of Mn:ZnSe NCs reported by Gahlot et al.47 The shallow trap in our case is represented by hole trapping at 5Mn3+:CsPbCl3. Coherently, Pradeep et al. reported temperature-dependent Raman spectra with sharpening of the 132 cm–1 peak (Mn/Pb–Br stretching mode) on Mn:CsPb(Cl/Br)3 NCs at temperatures >173 K, consistent with the occurrence of a significant Jahn–Teller distortion related to Mn oxidation.41
Since both energy- and charge-transfer processes are strongly dependent on the dopant/host relative energy levels, energetic overlap variations in the perovskite composition, in particular in terms of the halide nature, could afford significantly different electronic structures with different associated decay channels. To evaluate the electronic and structural changes occurring in different halide perovskites, we carried out a comparative study on CsPbCl3 and MAPbBr3/MAPbI3. The impact of A-site cations on the electronic structure is minimal; as a matter of fact, the 4T1→6A1 Mn luminescence is still observed,64 so we used pre-existing models for the bromide and iodide perovskites. Structural analyses indicate a progressive Mn–X bond increase with the series Mn–Cl ∼ 2.57 Å > Mn–Br ∼ 2.74 Å > Mn–I ∼ 2.98 Å, Table 1, in line with the increasing ionic radii trend.
Table 1. Averaged Mn–X Axial (dMn-X(ax)) and Equatorial (dMn-X(eq)) Distances (Å), Mn Spin from Mulliken Population Analysis (e), Band Gaps (ΔEgap, V), and 4T1→6A1 Spin Gaps (ΔE4 T1/6A1, eV).
| CsPbCl3 | |||
|---|---|---|---|
| ΔEgap = 3.03 |
ΔE4T1/6A1 = 2.05 | ||
| dMn-X(ax) | dMn-X(eq) | Mn spin | |
| 6Mn2+ | 2.58 | 2.57 | 4.90 |
| 4Mn2+ | 2.63 | 2.51 | 3.02 |
| 6Mn2+/h+ | 2.59 | 2.56 | 4.90 |
| TS | 2.64 | 2.42 | 4.74 |
| 5Mn3+ | 2.65 | 2.32 | 4.22 |
| MAPbBr3 | |||
|---|---|---|---|
| ΔEgap = 2.34 |
ΔE4T1/6A1 = 2.04 | ||
| dMn-X(ax) | dMn-X(eq) | Mn spin | |
| 6Mn2+ | 2.77 | 2.72 | 4.92 |
| 4Mn2+ | 2.83 | 2.65 | 3.07 |
| 6Mn2+/h+ | 2.77 | 2.73 | 4.92 |
| TS | 2.83 | 2.56 | 4.62 |
| 5Mn3+ | 2.85 | 2.52 | 4.48 |
| MAPbI3 | |||
|---|---|---|---|
| ΔEgap = 1.80 |
ΔE4T1/6A1 = 1.99 | ||
| dMn-X(ax) | dMn-X(eq) | Mn spin | |
| 6Mn2+ | 3.04 | 2.95 | 4.92 |
| 4Mn2+ | 3.09 | 2.89 | 3.15 |
| 6Mn2+/h+ | 3.04 | 2.95 | 4.92 |
| 5Mn3+ | 3.09 | 2.72 | 4.59 |
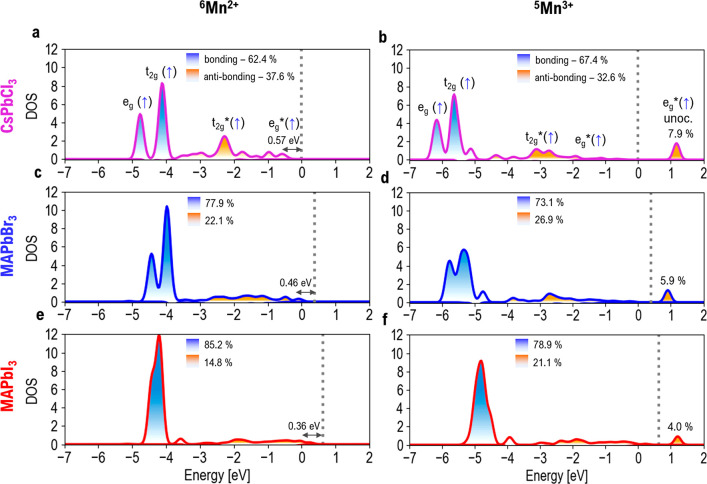
We notice that our calculations retrieve the experimental trend of VB rising and band gap closing observed for the Cl → Br → I series, while the electronic structure of the Mn-doped systems is qualitatively similar along the halide series, although quantitative differences are found in the distribution of the bonding/antibonding states. Figure 4, panels a, c, and e, clearly show that the Mn–Cl bonding states, i.e., eg and t2g, contain progressively smaller halide contributions when moving from Cl to I, in line with 6MnX64– models (Figure S4, Supporting Information). This could be ascribed to the reduced covalency in the Mn–X bond and to the reduced ligand field strength. The inner manganese states become harder because of the combined less covalent character of the chemical bond and weaker ligand field provided by the halides according to the I– < Br– < Cl– trend.
Figure 4.
Manganese DOS contribution in 6Mn2+ and 5Mn3+ electronic states for Mn-doped CsPbCl3, MAPbBr3, and MAPbI3 perovskites. The manganese–halide bonding (antibonding) states are highlighted in blue (orange), and the percentage weight of each region compared to the total is also reported. The VB of CsPbCl3 is set to zero, while the energies of Mn-doped MAPbBr3 and MAPbI3 perovskites are aligned to that of Mn-doped CsPbCl3. The dashed lines represent the aligned VB edges.
In line with results on CsPbCl3, in the bromide and iodide perovskites, the 4Mn2+ structures present a slight equatorial compression and axial expansion; see Table 1. The energy of the quartet 4T1 vs 6A1 is ∼2 eV for the three perovskites, irrespective of the nature of the halide. These values well agree with the emission detected experimentally,5,41,65 confirming the insensitivity of the Mn-dopant emission energy to the host chemical structure. A significantly higher energy state is found in the case of Mn:MAPbI3, characterized by a Mn spin moment of 1.12 (see results in the Supporting Information), related to the reduced covalency and ligand strength of the Mn–X bond.
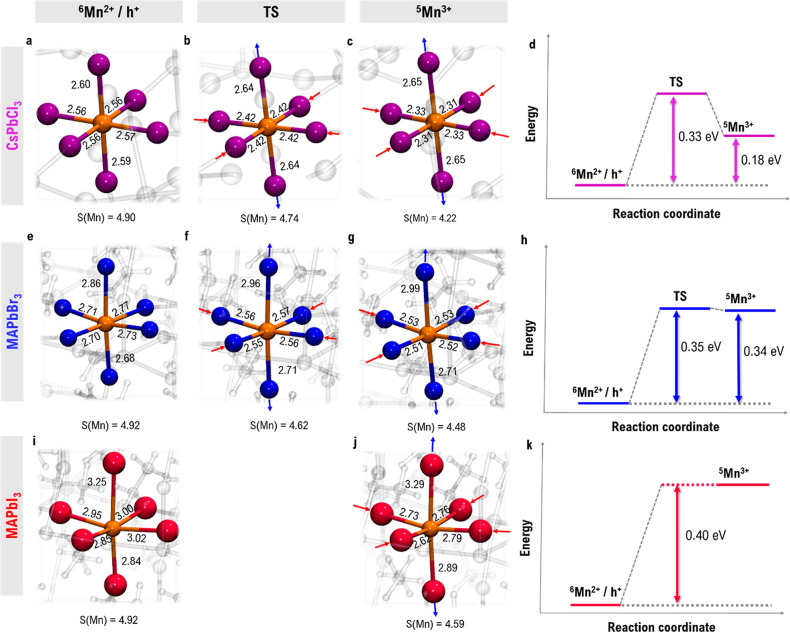
Similar to what has already been discussed for CsPbCl3, when a positive charge is allocated to the lead bromide or lead iodide perovskites, a global minimum is found where the perovskite host VB is oxidized, followed by a higher energy local minimum where Mn is oxidized, with subsequent equatorial (axial) compression (elongation), Figure 5g–j. In the case of bromide, the 5Mn3+ minimum is 0.34 eV above the 6Mn2+/h+ state, and it can be reached by overcoming a barrier of 0.35 eV, while in the case of the iodide, 5Mn3+ minimum is 0.40 eV above 6Mn2+/h+, Figure 5h–k.
Figure 5.
Mn coordination spheres and energy features of key species along the route of Mn oxidation in the quintet spin state: 6Mn2+/h+ with a free hole delocalized in the material VB, the transition state (TS) connecting 6Mn2+/h+ to 5Mn3+, and 5Mn3+ corresponding to the oxidized manganese. Mn spin moments are reported along with octahedron distances in Å. Orange spheres represent manganese atoms, purple ones chloride atoms, blue ones bromide atoms, and red ones iodide atoms; species outside the Mn first coordination shell are transparent.
The energy difference between 5Mn3+ and 6Mn2+/h+ increases when moving from Cl (0.18 eV) to Br (0.34 eV) and I (0.40 eV), highlighting a progressive thermodynamical destabilization of the oxidized form, although their kinetic barriers are similar. Considering the energy-transfer process of pathway (i), we should expect, as proposed by Liu et al.,5 to observe a band-gap-dependent Mn-dopant luminescence. Basically, to observe Mn PL, the host band gap should be larger than ∼2.0 eV, i.e., the energy of the (4T1→6A1) ligand field transition. The charge-transfer pathway (ii) is instead modulated by the different thermodynamic stability of the oxidized forms and the corresponding kinetic barriers, Figure 5. We predict that formation of 5Mn3+ requires similar activation energies in the three materials, which should induce a similar PL quantum yield temperature trend for the three halides. The reduced stability of 5Mn3+ in the order Cl > Br > I, however, indicates that the concentration of dopant luminescent centers is significantly reduced in the latter two cases, progressively hindering the dopant PL quantum yield.
To sum up, our accurate theoretical analysis has allowed us to quantify the dopant/host alignment of energy levels for a series of manganese-doped lead halide perovskites, identifying the key factors responsible for energy- vs charge-transfer population of the 4Mn2+ excited state. Our analysis supports both energy-transfer and charge-transfer mechanisms, with the latter probably preferred in Mn:CsPbCl3 due to avoidance of spin and orbital restrictions, though requiring a significant structural modification at the dopant site. Also in favor of a charge-transfer mechanism in this case is the calculated activation energy for manganese sensitization, agreeing with experimental data for the 4T1→6A1 luminescence. The reported quantitative comparison of the electronic structure of different halide perovskites may well rationalize the progressive disappearance of dopant luminescence on the basis of both energy-transfer and charge-transfer mechanisms. For the latter we found that an essential factor in determining the Mn quantum yield, in addition to the host band gap/Mn 4T1→6A1 resonance, is provided by the thermodynamics/kinetics of 5Mn3+ formation. The kinetics is similar, and this predicts PL temperature trends analogous to those of to Mn:CsPbCl3, but the Mn oxidized species is thermodynamically more stable in chloride than in bromide or iodide perovskites, implying a reduced concentration of Mn chromocenters in the latter two systems.
Computational Details
Mn-doped perovskites CsPbCl3, MAPbBr3, and MAPbI3 were modeled in the 2×2×2 supercell of tetragonal APbX3. The dopant Mn was disposed in a substitutional position, in place of the metal lead, assuming a doping concentration of 3.12%. Equilibrium structures were found by relaxing ion positions in the supercell defects by using the PBE0 functional and fixing cell parameters to the experimental values: for CsPbCl3, a = b = 15.811 Å, c = 22.520 Å; for MAPbBr3, a = b = 16.690 Å, c = 23.604 Å; and for MAPbI3, a = b = 17.711 Å, c = 25.320 Å.66,67 Hybrid functional PBE0 calculations were performed by using the CP2K code,68,69 keeping the fraction of Fock exchange α at its original value (0.25). Calculations were carried out with Goedecker–Teter–Hutter pseudopotentials, six double-ζ polarized basis sets for the wave functions, and a cutoff of 500 Ry for the expansion of the electron density in plane waves.70 We used the auxiliary density matrix method with the cFIT auxiliary basis set to speed up the hybrid functional calculations. To simulate the different spin properties of our systems, the calculations were all performed with the local spin density (LSD) approximation by fixing the spin multiplicity to the respective value, i.e., 6 for 6Mn2+:APbX3, 4 for 4Mn2+:APbX3, and 5 for 5Mn3+:APbX3 and 6Mn2++h+:APbX3. The atomic Mn spin moments of the minima were calculated by employing the Mulliken population analysis.71
Additional PBE0-SOC calculations for 6Mn2+:CsPbCl3/MAPbBr3/MAPbI3 were performed by means of single points on the PBE0 structures using a PBE0 hybrid functional50,51 together with SOC corrections.72 Norm-conserving pseudopotentials were used with a cutoff on the wave function of 40 Ry and a cutoff on the Fock grid of 80 Ry, sampling at the Γ point of the Brillouin zone. The fraction of exact exchange was kept to its original value of α = 0.25. To this aim we used the Quantum Espresso package.73
To compare energy values of positively charged systems, such as 5Mn3+ and 6Mn2++h+, to those of the neutral ground state, we computed the respective redox level (0/+),44 making use of the Freysoldt approach for the finite size correction.74
The alignment of valence bands and Mn levels for different halides perovskites in Figure 4 was achieved using the tridimensional averaged Hartree potential.
Comparative molecular calculations on the model complexes MnX64–/MnX63– were carried out by employing the Amsterdam Density Functional program (ADF) using the PBE0 functional,50 a Slater-type triple-zeta basis set augmented with two polarization functions (TZ2P), and a scalar Zero Order Regular Approximation to the Dirac Equation (ZORA) Hamiltonian.75,76 Molecular orbital diagrams were constructed through the symmetrized fragment orbital approach by dividing the complex in two fragments, i.e., Mn2+/Mn3+ and 6Cl–. We applied octahedral (Oh) molecular symmetry for 6MnX64– and tetragonally distorted (D4h) symmetry for 4MnX64– and 5MnX63–.75
Acknowledgments
The authors acknowledge support from the Ministero Istruzione dell’Università e della Ricerca (MIUR) and the University of Perugia through the program “Dipartimenti di Eccellenza 2018–2022” (grant AMIS) and from the European 531 Union’s Horizon 2020 research and innovation program under Grant Agreement No. 764047 of the Espresso project. E.M. and A.A.A wish to thank the Distinguished Scientist Fellowship Program (DSFP) of King Saud University, Riyadh, Saudi Arabia.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsenergylett.1c00553.
Structures, DOS, and orbitals of Mn:APbX3 and MnX4– (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Bussian D. A.; Crooker S. A.; Yin M.; Brynda M.; Efros A. L.; Klimov V. I. Tunable Magnetic Exchange Interactions in Manganese-Doped Inverted Core–Shell ZnSe–CdSe Nanocrystals. Nat. Mater. 2009, 8, 35–40. 10.1038/nmat2342. [DOI] [PubMed] [Google Scholar]
- Irvine S. E.; Staudt T.; Rittweger E.; Engelhardt J.; Hell S. W. Direct Light-Driven Modulation of Luminescence from Mn-Doped ZnSe Quantum Dots. Angew. Chem., Int. Ed. 2008, 47, 2685–2688. 10.1002/anie.200705111. [DOI] [PubMed] [Google Scholar]
- Chin P. T. K.; Stouwdam J. W.; Janssen R. A. J. Highly Luminescent Ultranarrow Mn Doped ZnSe Nanowires. Nano Lett. 2009, 9, 745–750. 10.1021/nl8033015. [DOI] [PubMed] [Google Scholar]
- Beaulac R.; Archer P. I.; van Rijssel J.; Meijerink A.; Gamelin D. R. Exciton Storage by Mn2+ in Colloidal Mn2+-Doped CdSe Quantum Dots. Nano Lett. 2008, 8, 2949–2953. 10.1021/nl801847e. [DOI] [PubMed] [Google Scholar]
- Liu W.; Lin Q.; Li H.; Wu K.; Robel I.; Pietryga J. M.; Klimov V. I. Mn2+-Doped Lead Halide Perovskite Nanocrystals with Dual-Color Emission Controlled by Halide Content. J. Am. Chem. Soc. 2016, 138, 14954–14961. 10.1021/jacs.6b08085. [DOI] [PubMed] [Google Scholar]
- Byan J. D.; Gamelin D. R. In Progress in Inorganic Chemistry, Vol. 53; Karlin K. D., Ed.; John Wiley & Sons, Inc.:New York, 2005; p 47. [Google Scholar]
- Kojima A.; Teshima K.; Shirai Y.; Miyasaka T. Organometal Halide Perovskites as Visible-Light Sensitizers for Photovoltaic Cells. J. Am. Chem. Soc. 2009, 131, 6050–6051. 10.1021/ja809598r. [DOI] [PubMed] [Google Scholar]
- Im J.-H.; Lee C.-R.; Lee J.-W.; Park S.-W.; Park N.-G. 6.5% efficient perovskite quantum-dot-sensitized solar cell. Nanoscale 2011, 3, 4088–4093. 10.1039/c1nr10867k. [DOI] [PubMed] [Google Scholar]
- Etgar L.; Gao P.; Xue Z.; Peng Q.; Chandiran A. K.; Liu B.; Nazeeruddin M. K.; Grätzel M. Mesoscopic CH3NH3PbI3/TiO2 Heterojunction Solar Cells. J. Am. Chem. Soc. 2012, 134, 17396–17399. 10.1021/ja307789s. [DOI] [PubMed] [Google Scholar]
- Burschka J.; Pellet N.; Moon S.-J.; Humphry-Baker R.; Gao P.; Nazeeruddin M. K.; Grätzel M. Sequential deposition as a route to high-performance perovskite-sensitized solar cells. Nature 2013, 499, 316–319. 10.1038/nature12340. [DOI] [PubMed] [Google Scholar]
- Heo J. H.; Im S. H.; Noh J. H.; Mandal T. N.; Lim C.-S.; Chang J. A.; Lee Y. H.; Kim H.-j.; Sarkar A.; Nazeeruddin Md. K.; et al. Efficient inorganic-organic hybrid heterojunction solar cells containing perovskite compound and polymeric hole conductors. Nat. Photonics 2013, 7, 486–491. 10.1038/nphoton.2013.80. [DOI] [Google Scholar]
- Liu M.; Johnston M. B.; Snaith H. J. Efficient planar heterojunction perovskite solar cells by vapour deposition. Nature 2013, 501, 395–398. 10.1038/nature12509. [DOI] [PubMed] [Google Scholar]
- Kim H.-S.; Lee C.-R.; Im J.-H.; Lee K.-B.; Moehl T.; Marchioro A.; Moon S.-J.; Humphry-Baker R.; Yum J.-H.; Moser J. E.; et al. Lead Iodide Perovskite Sensitized All-Solid-State Submicron Thin Film Mesoscopic Solar Cell with Efficiency Exceeding 9%. Sci. Rep. 2012, 2, 591. 10.1038/srep00591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H.; Chen Q.; Li G.; Luo S.; Song T.-b.; Duan H.-S.; Hong Z.; You J.; Liu Y.; Yang Y. Interface engineering of highly efficient perovskite solar cells. Science 2014, 345, 542–546. 10.1126/science.1254050. [DOI] [PubMed] [Google Scholar]
- Xing G.; Mathews N.; Sun S.; Lim S. S.; Lam Y. M.; Grätzel M.; Mhaisalkar S.; Sum T. C. Long-Range Balanced Electron- and Hole-Transport Lengths in Organic-Inorganic CH3NH3PbI3. Science 2013, 342, 344–347. 10.1126/science.1243167. [DOI] [PubMed] [Google Scholar]
- Stranks S. D.; Eperon G. E.; Grancini G.; Menelaou C.; Alcocer M. J. P.; Leijtens T.; Herz L. M.; Petrozza A.; Snaith H. J. Electron-Hole Diffusion Lengths Exceeding 1 Micrometer in an Organometal Trihalide Perovskite Absorber. Science 2013, 342, 341–344. 10.1126/science.1243982. [DOI] [PubMed] [Google Scholar]
- Cho H.; Jeong S.-H.; Park M.-H.; Kim Y.-H.; Wolf C.; Lee C.-L.; Heo J. H.; Sadhanala A.; Myoung N.; Yoo S.; et al. Overcoming the electroluminescence efficiency limitations of perovskite light-emitting diodes. Science 2015, 350, 1222–1225. 10.1126/science.aad1818. [DOI] [PubMed] [Google Scholar]
- Yuan M.; Quan L. N.; Comin R.; Walters G.; Sabatini R.; Voznyy O.; Hoogland S.; Zhao Y.; Beauregard E. M.; Kanjanaboos P.; et al. Perovskite energy funnels for efficient light-emitting diodes. Nat. Nanotechnol. 2016, 11, 872–877. 10.1038/nnano.2016.110. [DOI] [PubMed] [Google Scholar]
- Wang N.; Cheng L.; Ge R.; Zhang S.; Miao Y.; Zou W.; Yi C.; Sun Y.; Cao Y.; Yang R.; et al. Perovskite light-emitting diodes based on solution-processed self-organized multiple quantum wells. Nat. Photonics 2016, 10, 699–704. 10.1038/nphoton.2016.185. [DOI] [Google Scholar]
- Kim Y.-H.; Cho H.; Heo J. H.; Kim T.-S.; Myoung N.; Lee C.-L.; Im S. H.; Lee T.-W. Multicolored Organic/Inorganic Hybrid Perovskite Light-Emitting Diodes. Adv. Mater. 2015, 27, 1248–1254. 10.1002/adma.201403751. [DOI] [PubMed] [Google Scholar]
- Chiba T.; Hayashi Y.; Ebe H.; Hoshi K.; Sato J.; Sato S.; Pu Y.-J.; Ohisa S.; Kido J. Anion-exchange red perovskite quantum dots with ammonium iodine salts for highly efficient light-emitting devices. Nat. Photonics 2018, 12, 681–687. 10.1038/s41566-018-0260-y. [DOI] [Google Scholar]
- Protesescu L.; Yakunin S.; Bodnarchuk M. I.; Krieg F.; Caputo R.; Hendon C. H.; Yang R. X.; Walsh A.; Kovalenko M. V. Nanocrystals of Cesium Lead Halide Perovskites (CsPbX3, X = Cl, Br, and I): Novel Optoelectronic Materials Showing Bright Emission with Wide Color Gamut. Nano Lett. 2015, 15, 3692–3696. 10.1021/nl5048779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H.; Raith J.; Kershaw S. V.; Kalytchuk S.; Tomanec O.; Jing L.; Susha A. S.; Zboril R.; Rogach A. L. Growth mechanism of strongly emitting CH3NH3PbBr3 perovskite nanocrystals with a tunable bandgap. Nat. Commun. 2017, 8, 996. 10.1038/s41467-017-00929-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling Y.; Yuan Z.; Tian Y.; Wang X.; Wang J. C.; Xin Y.; Hanson K.; Ma B.; Gao H. Bright Light-Emitting Diodes Based on Organometal Halide Perovskite Nanoplatelets. Adv. Mater. 2016, 28, 305–311. 10.1002/adma.201503954. [DOI] [PubMed] [Google Scholar]
- Parobek D.; Roman B. J.; Dong Y.; Jin H.; Lee E.; Sheldon M.; Son D. H. Exciton-to-Dopant Energy Transfer in Mn-Doped Cesium Lead Halide Perovskite Nanocrystals. Nano Lett. 2016, 16, 7376–7380. 10.1021/acs.nanolett.6b02772. [DOI] [PubMed] [Google Scholar]
- Guria A. K.; Dutta S. K.; Adhikari S. D.; Pradhan N. Doping Mn2+ in Lead Halide Perovskite Nanocrystals: Successes and Challenges. ACS Energy Lett. 2017, 2, 1014–1021. 10.1021/acsenergylett.7b00177. [DOI] [Google Scholar]
- Rossi D.; Parobek D.; Dong Y.; Son D. H. Dynamics of Exciton–Mn Energy Transfer in Mn-Doped CsPbCl3 Perovskite Nanocrystals. J. Phys. Chem. C 2017, 121, 17143–17149. 10.1021/acs.jpcc.7b06182. [DOI] [Google Scholar]
- He T.; Li J.; Ren C.; Xiao S.; Li Y.; Chen R.; Lin X. Strong two-photon absorption of Mn-doped CsPbCl3 perovskite nanocrystals. Appl. Phys. Lett. 2017, 111, 211105. 10.1063/1.5008437. [DOI] [Google Scholar]
- Sun Q.; Wang S.; Zhao C.; Leng J.; Tian W.; Jin S. Excitation-Dependent Emission Color Tuning from an Individual Mn-Doped Perovskite Microcrystal. J. Am. Chem. Soc. 2019, 141, 20089–20096. 10.1021/jacs.9b09143. [DOI] [PubMed] [Google Scholar]
- Pinchetti V.; Anand A.; Akkerman Q. A.; Sciacca D.; Lorenzon M.; Meinardi F.; Fanciulli M.; Manna L.; Brovelli S. Trap-Mediated Two-Step Sensitization of Manganese Dopants in Perovskite Nanocrystals. ACS Energy Lett. 2019, 4, 85–93. 10.1021/acsenergylett.8b02052. [DOI] [Google Scholar]
- Zou S.; Liu Y.; Li J.; Liu C.; Feng R.; Jiang F.; Li Y.; Song J.; Zeng H.; Hong M.; Chen X. Stabilizing Cesium Lead Halide Perovskite Lattice through Mn(II) Substitution for Air-Stable Light-Emitting Diodes. J. Am. Chem. Soc. 2017, 139, 11443–11450. 10.1021/jacs.7b04000. [DOI] [PubMed] [Google Scholar]
- Mir W. J.; Jagadeeswararao M.; Das S.; Nag A. Colloidal Mn-Doped Cesium Lead Halide Perovskite Nanoplatelets. ACS Energy Lett. 2017, 2, 537–543. 10.1021/acsenergylett.6b00741. [DOI] [Google Scholar]
- Li Q.; Ji S.; Yuan X.; Li J.; Fan Y.; Zhang J.; Zhao J.; Li H. Ultraviolet Light-Induced Degradation of Luminescence in Mn-Doped CsPbCl3 Nanocrystals. J. Phys. Chem. C 2019, 123, 14849–14857. 10.1021/acs.jpcc.9b03294. [DOI] [Google Scholar]
- Sun C.; Gao Z.; Deng Y.; Liu H.; Wang L.; Su S.; Li P.; Li H.; Zhang Z.; Bi W. Orange to Red, Emission-Tunable Mn-Doped Two-Dimensional Perovskites with High Luminescence and Stability. ACS Appl. Mater. Interfaces 2019, 11, 34109–34116. 10.1021/acsami.9b11665. [DOI] [PubMed] [Google Scholar]
- Das Adhikari S.; Guria A. K.; Pradhan N. Insights of Doping and the Photoluminescence Properties of Mn-Doped Perovskite Nanocrystals. J. Phys. Chem. Lett. 2019, 10, 2250–2257. 10.1021/acs.jpclett.9b00182. [DOI] [PubMed] [Google Scholar]
- Dutta S. K.; Dutta A.; Das Adhikari S.; Pradhan N. Doping Mn2+ in Single-Crystalline Layered Perovskite Microcrystals. ACS Energy Lett. 2019, 4, 343–351. 10.1021/acsenergylett.8b02349. [DOI] [Google Scholar]
- Meinardi F.; Akkerman Q. A.; Bruni F.; Park S.; Mauri M.; Dang Z.; Manna L.; Brovelli S. Doped Halide Perovskite Nanocrystals for Reabsorption-Free Luminescent Solar Concentrators. ACS Energy Lett. 2017, 2, 2368–2377. 10.1021/acsenergylett.7b00701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang G.; Wang C.; Xu S.; Zong S.; Lu J.; Wang Z.; Lu C.; Cui Y. Postsynthetic Doping of MnCl2 Molecules into Preformed CsPbBr3 Perovskite Nanocrystals via a Halide Exchange-Driven Cation Exchange. Adv. Mater. 2017, 29, 1700095. 10.1002/adma.201700095. [DOI] [PubMed] [Google Scholar]
- Zhu J.; Yang X.; Zhu Y.; Wang Y.; Cai J.; Shen J.; Sun L.; Li C. Room-Temperature Synthesis of Mn-Doped Cesium Lead Halide Quantum Dots with High Mn Substitution Ratio. J. Phys. Chem. Lett. 2017, 8, 4167–4171. 10.1021/acs.jpclett.7b01820. [DOI] [PubMed] [Google Scholar]
- Yao Z.; Zhao W.; Chen S.; Jin Z.; Liu S. F. Mn Doping of CsPbI3 Film Towards High-Efficiency Solar Cell. ACS Appl. Energy Mater. 2020, 3, 5190–5197. 10.1021/acsaem.9b02468. [DOI] [Google Scholar]
- K. R P.; Acharya D.; Jain P.; Gahlot K.; Yadav A.; Camellini A.; Zavelani-Rossi M.; Cerullo G.; Narayana C.; Narasimhan S.; Viswanatha R. Harvesting Delayed Fluorescence in Perovskite Nanocrystals Using Spin-Forbidden Mn d States. ACS Energy Lett. 2020, 5, 353–359. 10.1021/acsenergylett.9b02399. [DOI] [Google Scholar]
- Xu K.; Vickers E. T.; Luo B.; Allen A. L. C.; Chen E.; Roseman G.; Wang Q.; Kliger D. S.; Millhauser G. L.; Yang W.; et al. First Synthesis of Mn-Doped Cesium Lead Bromide Perovskite Magic Sized Clusters at Room Temperature. J. Phys. Chem. Lett. 2020, 11, 1162–1169. 10.1021/acs.jpclett.9b03700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mir W. J.; Mahor Y.; Lohar A.; Jagadeeswararao M.; Das S.; Mahamuni S.; Nag A. Postsynthesis Doping of Mn and Yb into CsPbX3 (X = Cl, Br, or I) Perovskite Nanocrystals for Downconversion Emission. Chem. Mater. 2018, 30, 8170–8178. 10.1021/acs.chemmater.8b03066. [DOI] [Google Scholar]
- Meggiolaro D.; De Angelis F. First-Principles Modeling of Defects in Lead Halide Perovskites: Best Practices and Open Issues. ACS Energy Lett. 2018, 3, 2206–2222. 10.1021/acsenergylett.8b01212. [DOI] [Google Scholar]
- Akkerman Q. A.; Meggiolaro D.; Dang Z.; De Angelis F.; Manna L. Fluorescent Alloy CsPbxMn1–xI3 Perovskite Nanocrystals with High Structural and Optical Stability. ACS Energy Lett. 2017, 2, 2183–2186. 10.1021/acsenergylett.7b00707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng M.; Locardi F.; Mara D.; Hens Z.; Van Deun R.; Artizzu F. Switching on Near-Infrared Light in Lanthanide-doped CsPbCl3 Perovskite Nanocrystals. ChemRXiv Preprints 2021, 13352549. 10.26434/chemrxiv.13352549. [DOI] [PubMed] [Google Scholar]
- Gahlot K.; K. R. P.; Camellini A.; Sirigu G.; Cerullo G.; Zavelani-Rossi M.; Singh A.; Waghmare U. V.; Viswanatha R. Transient Species Mediating Energy Transfer to Spin-Forbidden Mn d States in II–VI Semiconductor Quantum Dots. ACS Energy Lett. 2019, 4, 729–735. 10.1021/acsenergylett.9b00064. [DOI] [Google Scholar]
- Ren L.; Wang Y.; Wang M.; Wang S.; Zhao Y.; Cazorla C.; Chen C.; Wu T.; Jin K. Tuning Magnetism and Photocurrent in Mn-Doped Organic–Inorganic Perovskites. J. Phys. Chem. Lett. 2020, 11, 2577–2584. 10.1021/acs.jpclett.0c00034. [DOI] [PubMed] [Google Scholar]
- Justice Babu K.; Kaur G.; Shukla A.; Kaur A.; Goswami T.; Ghorai N.; Ghosh H. N. Concurrent Energy- and Electron-Transfer Dynamics in Photoexcited Mn-Doped CsPbBr3 Perovskite Nanoplatelet Architecture. J. Phys. Chem. Lett. 2021, 12, 302–309. 10.1021/acs.jpclett.0c03267. [DOI] [PubMed] [Google Scholar]
- Adamo C.; Barone V. Toward reliable density functional methods without adjustable parameters: The PBE0 model. J. Chem. Phys. 1999, 110, 6158–6170. 10.1063/1.478522. [DOI] [Google Scholar]
- Perdew J. P.; Ernzerhof M.; Burke K. Rationale for mixing exact exchange with density functional approximations. J. Chem. Phys. 1996, 105, 9982–9985. 10.1063/1.472933. [DOI] [Google Scholar]
- Tran F.; Blaha P.; Schwarz K.; Novák P. Hybrid exchange-correlation energy functionals for strongly correlated electrons: Applications to transition-metal monoxides. Phys. Rev. B: Condens. Matter Mater. Phys. 2006, 74, 155108. 10.1103/PhysRevB.74.155108. [DOI] [Google Scholar]
- Tao S.; Schmidt I.; Brocks G.; Jiang J.; Tranca I.; Meerholz K.; Olthof S. Absolute energy level positions in tin- and lead-based halide perovskites. Nat. Commun. 2019, 10, 2560. 10.1038/s41467-019-10468-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Housecroft C. E.; Sharpe A. G.. Inorganic Chemistry; Pearson, University of Basel, Switzerland, 2012. [Google Scholar]
- Webb C.; Kaminska M.; Lichtensteiger M.; Lagowski J. Valence band states of semi-magnetic semiconductors: Cd1-xMnxTe. Solid State Commun. 1981, 40, 609–611. 10.1016/0038-1098(81)90586-X. [DOI] [Google Scholar]
- Kahn A. Fermi level, work function and vacuum level. Mater. Horiz. 2016, 3, 7–10. 10.1039/C5MH00160A. [DOI] [Google Scholar]
- Maculan G.; Sheikh A. D.; Abdelhady A. L.; Saidaminov M. I.; Haque M. A.; Murali B.; Alarousu E.; Mohammed O. F.; Wu T.; Bakr O. M. CH3NH3PbCl3 Single Crystals: Inverse Temperature Crystallization and Visible-Blind UV-Photodetector. J. Phys. Chem. Lett. 2015, 6, 3781–3786. 10.1021/acs.jpclett.5b01666. [DOI] [PubMed] [Google Scholar]
- Ma J.; Yao Q.; McLeod J. A.; Chang L.-Y.; Pao C.-W.; Chen J.; Sham T.-K.; Liu L. Investigating the luminescence mechanism of Mn-doped CsPb(Br/Cl)3 nanocrystals. Nanoscale 2019, 11, 6182–6191. 10.1039/C9NR00143C. [DOI] [PubMed] [Google Scholar]
- Chen D.; Fang G.; Chen X.; Lei L.; Zhong J.; Mao Q.; Zhou S.; Li J. Mn-Doped CsPbCl3 perovskite nanocrystals: solvothermal synthesis, dual-color luminescence and improved stability. J. Mater. Chem. C 2018, 6, 8990–8998. 10.1039/C8TC03139H. [DOI] [Google Scholar]
- Fei L.; Yuan X.; Hua J.; Ikezawa M.; Zeng R.; Li H.; Masumoto Y.; Zhao J. Enhanced luminescence and energy transfer in Mn2+ doped CsPbCl3–xBrx perovskite nanocrystals. Nanoscale 2018, 10, 19435–19442. 10.1039/C8NR05492D. [DOI] [PubMed] [Google Scholar]
- Stroppa A.; Kresse G. Unraveling the Jahn-Teller effect in Mn-doped GaN using the Heyd-Scuseria-Ernzerhof hybrid functional. Phys. Rev. B: Condens. Matter Mater. Phys. 2009, 79, 201201. 10.1103/PhysRevB.79.201201. [DOI] [Google Scholar]
- Stroppa A.; Kresse G.; Continenza A. Revisiting Mn-doped Ge using the Heyd-Scuseria-Ernzerhof hybrid functional. Phys. Rev. B: Condens. Matter Mater. Phys. 2011, 83, 085201. 10.1103/PhysRevB.83.085201. [DOI] [Google Scholar]
- Krcha M. D.; Janik M. J. Examination of Oxygen Vacancy Formation in Mn-Doped CeO2 (111) Using DFT+U and the Hybrid Functional HSE06. Langmuir 2013, 29, 10120–10131. 10.1021/la401747n. [DOI] [PubMed] [Google Scholar]
- Li X.; Guo Y.; Luo B. Improved Stability and Photoluminescence Yield of Mn2+-Doped CH3NH3PbCl3 Perovskite Nanocrystals. Crystals 2018, 8 (1), 4. 10.3390/cryst8010004. [DOI] [Google Scholar]
- Parobek D.; Dong Y.; Qiao T.; Son D. H. Direct Hot-Injection Synthesis of Mn-Doped CsPbBr3 Nanocrystals. Chem. Mater. 2018, 30, 2939–2944. 10.1021/acs.chemmater.8b00310. [DOI] [Google Scholar]
- Poglitsch A.; Weber D. Dynamic Disorder in Methylamomonium Trihaloplumbates(II) Observed by Millimeter-Wave Spectroscopy. J. Chem. Phys. 1987, 87, 6373–6378. 10.1063/1.453467. [DOI] [Google Scholar]
- Lim A. R.; Jeong S. Y. Twin structure by 133Cs NMR in ferroelastic CsPbCl3 crystal. Solid State Commun. 1999, 110, 131–136. 10.1016/S0038-1098(99)00052-6. [DOI] [Google Scholar]
- Hutter J.; Iannuzzi M.; Schiffmann F.; VandeVondele J. cp2k: atomistic simulations of condensed matter systems. Comp. Mol. Sci. 2014, 4, 15–25. 10.1002/wcms.1159. [DOI] [Google Scholar]
- VandeVondele J.; Krack M.; Mohamed F.; Parrinello M.; Chassaing T.; Hutter J. Quickstep: Fast and accurate density functional calculations using a mixed Gaussian and plane waves approach. Comput. Phys. Commun. 2005, 167, 103–128. 10.1016/j.cpc.2004.12.014. [DOI] [Google Scholar]
- Goedecker S.; Teter M.; Hutter J. Separable dual-space Gaussian pseudopotentials. Phys. Rev. B: Condens. Matter Mater. Phys. 1996, 54, 1703–1710. 10.1103/PhysRevB.54.1703. [DOI] [PubMed] [Google Scholar]
- Mulliken R. S. Electronic Population Analysis on LCAO–MO Molecular Wave Functions. I. J. Chem. Phys. 1955, 23, 1833–1840. 10.1063/1.1740588. [DOI] [Google Scholar]
- Dal Corso A.; Mosca Conte A. Spin-orbit coupling with ultrasoft pseudopotentials: Application to Au and Pt. Phys. Rev. B: Condens. Matter Mater. Phys. 2005, 71, 115106. 10.1103/PhysRevB.71.115106. [DOI] [Google Scholar]
- Giannozzi P.; Baroni S.; Bonini N.; Calandra M.; Car R.; Cavazzoni C.; Ceresoli D.; Chiarotti G. L.; Cococcioni M.; Dabo I.; et al. QUANTUM ESPRESSO: a modular and open-source software project for quantum simulations of materials. J. Phys.: Condens. Matter 2009, 21, 395502. 10.1088/0953-8984/21/39/395502. [DOI] [PubMed] [Google Scholar]
- Freysoldt C.; Grabowski B.; Hickel T.; Neugebauer J.; Kresse G.; Janotti A.; Van de Walle C. G. First-principles calculations for point defects in solids. Rev. Mod. Phys. 2014, 86, 253–305. 10.1103/RevModPhys.86.253. [DOI] [Google Scholar]
- te Velde G.; Bickelhaupt F. M.; Baerends E. J.; Fonseca Guerra C.; van Gisbergen S. J. A.; Snijders J. G.; Ziegler T. Chemistry with ADF. J. Comput. Chem. 2001, 22, 931–967. 10.1002/jcc.1056. [DOI] [Google Scholar]
- Wolff S. K.; Ziegler T.; van Lenthe E.; Baerends E. J. Density functional calculations of nuclear magnetic shieldings using the zeroth-order regular approximation (ZORA) for relativistic effects: ZORA nuclear magnetic resonance. J. Chem. Phys. 1999, 110, 7689–7698. 10.1063/1.478680. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.