Abstract
Despite the global efforts, schistosomiasis remains a public health problem in several tropical and subtropical countries. One of the major challenges in the fight against schistosomiasis is the interruption of the parasite life cycle. Here, we evaluated the anticercarial, cytotoxicity, and phytochemical profiles of Sida acuta (HESa) and Sida rhombifolia (HESr) hydroethanolic extracts (Malvaceae). Schistosoma mansoni cercaria was collected from fifteen Biomphalaria pfeifferi-infected snails. Twenty-five cercariae were incubated in duplicate with different concentrations (31.25–1,000 μg/mL) of HESa or HESr. The cercaria viability was monitored at 30 min time intervals for 150 min, and the concentration-response curve of each plant extract was used to determine their respective lethal concentration 50 (LC50). Additionally, the cytotoxicity profile of each plant extract was evaluated on the Hepa 1–6 cell line at a concentration range of 15.625–1,000 µg/mL using the WST-8 assay method and its inhibitory concentration 50 (IC50) was calculated. Moreover, phytochemical characterization of each plant extract was carried out by HPLC-MS. Both extracts exhibited cercaricidal activity in a time- and concentration-dependent manner. At 30 min time point, HESa (LC50 = 28.41 ± 3.5 µg/mL) was more effective than HESr (LC50 = 172.42 ± 26.16 µg/mL) in killing S. mansoni cercariae. Regarding the cytotoxicity effect of both extracts, the IC50 of HESa (IC50 = 109.67 µg/mL) was lower than that of HESr (IC50 = 888.79 µg/mL). The selectivity index was 3.86 and 5.15 for HESa and HESr, respectively. Fifteen compounds were identified from HESa and HESr after HPLC-MS analysis. N-Feruloyltyramine, a polyphenol, and thamnosmonin, a coumarin, were identified in both extracts. HESa and HESr displayed cercaricidal activity and were not toxic on Hepa 1–6 cell line. Based on the selectivity index of these extracts, S. rhombifolia extract could be more effective on S. mansoni cercariae than S. acuta extract. This study could provide baseline information for further investigations aiming to develop plant-based alternative drugs against S. mansoni.
1. Introduction
Schistosomiasis, also known as bilharzia, is an infectious disease caused by trematodes flatworms of the genus Schistosoma. It is known as one of the most prevalent tropical diseases worldwide. It is estimated that 229 million people required preventive treatment in 2018 and close to 800 million are at risk of infection [1]. After an infected person releases Schistosoma eggs into the water by defecation or urination, the ripe miracidia hatch out and invade the intermediate host freshwater snail where they form sporocysts. They then matured to give cercariae that emerges from snails and swims to penetrate the skin of humans and/or animals that are definitive hosts [2]. Therefore, to control schistosomiasis, the life cycle of the parasite can be interrupted by killing cercariae and miracidia [3–5]. The use of chemical compounds to control the aquatic snails, cercariae, or miracidia is not recommended because of their adverse effects on the environment [6]. A large number of plant families with potential schistosomicidal activity have been identified through plant screening, which represents a continuous effort to find new bioactive molecules. The anti-cercarial activities of several plants such as Rauwolfia vomitoria [7], Nigella sativa [8] Balanites aegyptica, Azadirachta indica, Nauclea latifolia, Morinda lucida, Phyllanthus amarus, Vernonia amygdalina [9], and Ozoroa pulcherrima [10] have been reported. In several countries, Sida L. (Malvaceae) has been used for centuries in traditional medicines for the prevention and treatment of different diseases such as diarrhea, dysentery, gastrointestinal and urinary infections, cancer, malaria, and helminth infections [11–18]. Moreover, during the last decade, Jatsa et al. [19–21] have demonstrated the in vitro and in vivo anti-schistosomal potency of Sida pilosa. In addition, previous investigations on two of the plants of the genus Sida, namely Sida acuta and Sida rhombifolia, reported their anthelmintic and larvicidal activities [22–24]. Considering the above, this study was therefore carried out to evaluate the in vitro cercaricidal activity of S. acuta and S. rhombifolia hydroethanolic extracts against Schistosoma mansoni cercaria, their cytotoxicity profile, and determine their phytoconstituents.
2. Materials and Methods
2.1. Collection, Preparation, and Extraction Process of Plant Specimens
The whole plant of S. acuta (Figure 1(a)) was collected in February 2017 at Nkoemvone near Ebolowa in the South Region while the aerial parts of S. rhombifolia (Figure 1(b)) were harvested in March 2017 at Bazou, a village of Ndé division in the West region of Cameroon. The plant specimens were identified and authenticated at Cameroon's National Herbarium under a voucher specimen HNC n°46188 (S. acuta) and HNC n°20113 (S. rhombifolia). Each plant species was air-dried for 2 weeks, and the dried plant materials were ground into powder. Exactly 400 g of each pulverized plant material was macerated with 800 mL of ethanol/water mixture (70/30) at room temperature for 48 h. The macerates were filtered, and the filtrate was concentrated using a rotary evaporator (Buchi Rotavapor, R 200) under reduced pressure at 40°C. The crude extract was finally air-dried, and the yield of obtained hydroethanolic extracts of S. acuta (HESa) and S. rhombifolia (HESr) was calculated.
Figure 1.

Aerial parts of (a) Sida acuta and (b) Sida rhombifolia.
2.2. In Vitro Cercaricidal Activity of the Plant Extracts
2.2.1. Snails Infection and Preparation of the Cercarial Suspension
Schistosome cercariae were obtained from experimentally infected juvenile Biomphalaria pfeifferi snails that were maintained in the snail laboratory at the Centre Schistosomiasis and Parasitology of Yaoundé, Cameroon, under standard laboratory conditions of 18–26°C. Briefly, each snail was exposed for 12 hours to 4–5 miracidia obtained after hatching of eggs isolated from S.-mansoni-infected mice liver. Four weeks later, 14 infected snails, known to be shedding cercariae, were pooled into a glass Beaker with 20 mL of distilled water and allowed to shed cercariae by exposing them to artificial light for 60 min. After the exposition time, snails were removed from the beaker and 100 μL of the cercariae suspension was transferred on three microscope slides. A volume of 100 µL of Lugol was added on each slide to stain and fix cercariae, and the slides were transferred onto a light microscope to estimate the number of cercariae per 100 µL, and the volume of the cercariae suspension containing approximately 25 viable cercariae was determined [7, 9].
2.2.2. In Vitro Cercaricidal Assay
The concentrations ranging from 1,000 to 31.25 µg/mL are widely used to evaluate the cercaricidal activity of medicinal plant crude extracts [7, 9]. According to this protocol, serial dilutions (1,000, 500, 250, 125, 62.5, and 31.25 μg/mL) of each of the test plant extract were prepared using distilled water as diluent. A volume of 1 mL of each dilution was added into a well of a 24-well culture plate. Niclosamide-olamide 5% (1 µg/mL; Jiangsu Aijin Agrochemical Co. Ltd., China) was used as a reference control. Approximately 25 cercariae were pipetted into each of the wells. Cercaria mortality was observed for 150 min at 30 min intervals since the infectivity of cercariae is known to be rapidly lost after 12 h [25]. The bottom of each well was observed at 4X magnification using an inverted microscope and cercariae survival and mortality were recorded at specific 30 min time intervals (30, 60, 90, 120, and 150 min). Cercariae were presumed dead when they stopped movement and sank or their tail was detached [26]. All experiments were carried out in duplicate and repeated twice. The viability percentage and the lethal concentration 50 (LC50) values of plant extracts on schistosome cercariae were calculated at each time point.
2.3. Cytotoxicity Assay of the Extracts against Hepatocyte Cell Lines
Cytotoxicity of S. acuta and S. rhombifolia extracts was investigated on C57/L mouse melanoma liver cells line (Hepa 1-6, ATCC CRL-1830) using WST-8-based assay as previously described by Murata et al. [27]. The cells were cultured in high-glucose Dulbecco's Modified Eagle Medium (DMEM) with pyruvate and L-glutamine (Gibco, Life Technologies, USA), supplemented with 10% (v/v) fetal bovine serum (FBS) heat-inactivated (Serana, Australia) and 1% (v/v) penicillin/streptomycin (Gibco, Life Technologies, USA). The cells were grown in a growth medium at 37°C in a 95% air, 5% CO2-humidified incubator. Monolayer cultures reaching a confluence between 80 and 90% were detached using trypsin solution (Sigma Aldrich, Germany) and calibrated using a cell counter chamber (Fast Read 102). The calibrated cell suspension was seeded into 96-well tissue culture microtiter plates at a density of 1 × 104 cells per well and incubated overnight at 37°C in a 5% CO2 incubator for cell adhesion. Following incubation, the medium was removed from the cells and replaced with a fresh one followed by the addition of extracts at different concentrations (1,000, 500, 250, 125, 62.5, 31.25, and 15.625 µg/mL). Control wells with cells only were added, and the plates were incubated for 24 h in the same culture conditions. After incubation, cell viability was measured by mitochondrial activity in reducing 2-(2-methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,4disulfophenyl)-2H-tetrazolium monosodium salt to formazan using the cell counting kit-8 (WST-8, Abcam, ab228554, UK) according to the manufacturer instructions. After 4 hours of incubation, optical density was measured at 450 nm using a Dynex MRX TC II microplate reader (Dynex Technologies, USA). The results were expressed as a percentage growth of the control cells and the inhibitory concentration 50 (IC50) values were calculated as the concentration of the resulting in 50% reduction of absorbance compared to untreated cells. Tests were carried out in duplicate, and each experiment was repeated three times.
2.4. Selectivity Index
In the present study, the degree of selectivity of each hydroethanolic plant extract is expressed as the ratio of the IC50 obtained for the cell line to the LC50 for S. mansoni cercariae.
| (1) |
2.5. Phytochemical Analysis
2.5.1. Phytochemical Screening
The hydroethanolic extracts of S. acuta and S. rhombifolia were subjected to qualitative chemical tests to identify phytochemical constituents. The screening of alkaloids, anthraquinones, and cardiac glycosides was performed by Mayer's test, Bontrager's test, and the Keller–Killiani's test, respectively. Ferric chloride (FeCl3) test was used for the identification of phenols and tannins, Fehling's test for reducing sugars, foam test for saponins, and Liebermann–Burchard's test for steroids. The presence of flavonoids, lipids, and terpenoids was performed using the ammonia test, the grease spot test, and Salkowski's test, respectively [28].
2.5.2. Qualitative Determination of Compounds Using LC-DAD-(HR) ESI-MS
Sample Preparation. HESa and HESr were dissolved in HPLC grade methanol at a concentration of 5 mg/mL and then filtrated through a syringe-filter-membrane. Aliquots of 5 µL were injected into the UPLC-DAD/MS Dionex Ultimate 3000 HPLC (Germany).
HPLC-MS Conditions. High-resolution mass spectra were obtained with a quadrupole-time-of-flight (QTOF) spectrometer (Bruker, Germany) equipped with a HESI source. The spectrometer was operated in positive mode (mass range: 100–1,500, with a scan rate of 1.00 Hz) with automatic gain control to provide high-accuracy mass measurements within 0.40 ppm deviation using Na formate as calibrant. The following parameters were used for experiments: spray voltage of 4.5 kV and the capillary temperature of 200°C. Nitrogen was used as sheath gas (10 L/min). The spectrometer was attached to an Ultimate 3000 UHPLC system (Thermo Fisher, USA) consisting of an LC-pump, Diode Array Detector (DAD; λ: 190–600 nm), autosampler (injection volume: 10 μL), and a column oven (40°C). The separations were performed using a Synergi MAX-RP 100A (50 × 2 mm, 2.5 μ particle size) with a H2O (+0.1% HCOOH) (A)/acetonitrile (+0.1% HCOOH) (B) gradient (flow rate: 500 μL/min and injection volume: 5 μL). Samples were analyzed using a gradient program as follows: A linear gradient starting with 95% eluent A isocratic over 1.5 min to linear gradient with 100% eluent B over 6 min; 2 min after, the system returned to its initial condition (90% of eluent A) within 1 min and was equilibrated for 1 min.
Identification of Peaks. Identification of all constituents was performed by UPLC-DAD-MS analysis and by comparing the UV and MS spectra and MS/MS fragmentation of the peaks in the samples with those of data reported in the literature of the SciFinder, NIST/EPA/NIH Mass Spectral Library (NIST 14), and Mass Bank of North America (MoNA) databases.
2.6. Statistical Analysis
Graph drawing and statistical analysis were performed using GraphPad Software version 8.01 (GraphPad Software, San Diego, USA). The results were expressed as means ± SEM, and Student's t-test and two-way ANOVA followed by Tukey multiple comparison post-test were used to determine the significance of differences between mean values. A p-value less than 0.05 was considered statistically significant.
3. Results
3.1. Cercaricidal Activity of Plant Extracts
The mortality of S. mansoni cercariae following in vitro exposure to different concentrations of S. acuta and S. rhombifolia extracts was both time- and concentration-dependent increased. In the absence of the plant extract, cercariae showed normal viability without any morphological changes (tail loss) for up to 2 h (Figures 2(a) and 3(a)).
Figure 2.

Effect of Sida acuta hydroethanolic extract on Schistosoma mansoni cercariae viability (a) and mortality rate (b). All bars are expressed as mean ± SEM. ∗p < 0.05 and ∗∗∗p < 0.001, significantly different from controls (distilled water). #p < 0.05 and ###p < 0.001, significantly different from reference control (niclosamide).
Figure 3.

Effect of Sida rhombifolia hydroethanolic extract on Schistosoma mansoni cercariae viability (a) and mortality rate (b). All bars are expressed as mean ± SEM. ∗p < 0.05 and ∗∗∗p < 0.001, significantly different from controls (distilled water). ###p < 0.001, significantly different from reference control (niclosamide).
3.1.1. Cercaricidal Activity of Sida acuta Hydroethanolic Extract
As shown in Figure 2(b), following 30 min incubation of cercariae with S. acuta hydroethanolic extract with 1,000, 500, or 250 µg/mL, 100% of the cercariae died. At the same time, we recorded mortality rates of 83%, 88%, and 91% of cercariae incubated with 125, 62.5, and 31.25 µg/mL of the hydroethanolic extract of S. acuta, respectively. However, from 60 min to 150 min, we obtained a mortality rate of 100% with all concentrations of the extract. At 30 minutes time point, the cercaricidal activity of HESa at all concentrations was significantly higher (p < 0.01) than the niclosamide-olamide one (Figure 2(b)).
3.1.2. Cercaricidal Activity of Sida rhombifolia Hydroethanolic Extract
The incubation of cercariae with S. rhombifolia hydroethanolic extract at 1,000, 500, and 250 µg/mL induced after 30 min, mortality rates of 99%, 97%, and 66%, respectively. At the 60 min time point, we observed a mortality rate of 100% from the cercariae incubated with niclosamide-olamide or HESr at 1,000 and 500 µg/mL, while the same rate (100%) was obtained after 90 min and 120 min of incubation respectively with 250 and 125 µg/mL of HESr. The concentrations of 62.5 and 31.25 µg/mL showed a 100% cercariae mortality rate at the 150 min time point. In addition, at 30 minutes time-point, HESr at 500 and 1,000 µg/mL showed a significantly high cercaricidal activity (p < 0.001) in comparison to niclosamide one (Figure 3(b)).
3.1.3. Median Lethal Concentration of Sida acuta and Sida rhombifolia Hydroethanolic Extracts
As shown in Table 1, the cecaricidal median lethal concentration of S. acuta extract was almost constant from 60 to 150 mins of incubation. Conversely, the LC50 profile was gradually decreased after cercariae incubation with S. rhombifolia extract. The LC50 of HESa was then 28.41 ± 3.45 µg/mL and 18.63 ± 0.28 µg/mL after 30 min and 60 min of incubation respectively, while that of HESr were 172.42 ± 26.16 µg/mL and 56.60 ± 4.07 µg/mL at the same time points. After 150 min of incubation, both extracts disclosed the same LC50 (18.15 ± 0.00 µg/mL).
Table 1.
LC50 values of Sida acuta and Sida rhombifolia hydroethanolic extracts on Schistosoma mansoni cercariae at different time points.
| LC50 (µg/mL) | ||
|---|---|---|
| Time (min) | HESa | HESr |
| 30 | 28.41 ± 3.45 | 172.42 ± 26.16 |
| 60 | 18.63 ± 0.28 | 56.60 ± 4.07 |
| 90 | 18.74 ± 0.59 | 32.29 ± 4.05 |
| 120 | 18.15 ± 0.00 | 21.95 ± 1.54 |
| 150 | 18.15 ± 0.00 | 18.15 ± 0.00 |
3.2. Effect of Sida acuta and Sida rhombifolia Hydroethanolic Extracts on Mouse Hepatic (Hepa 1–6) Cell Growth
As indicated in Figure 4, the results of the effect of HESa and HESr extracts on the growth of the Hepa 1–6 cells at different concentrations (15.625–1,000 µg/mL) showed a decrease in the viability of cells in a concentration-dependent manner after 24 h of incubation.
Figure 4.
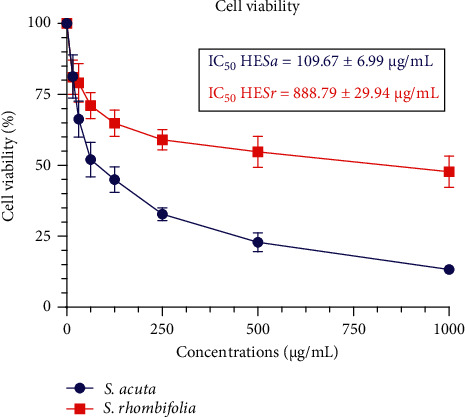
Concentration-response curve of the effect of Sida acuta and Sida rhombifolia hydroethanolic extracts on Hepa 1–6 cells.
The inhibitory rates of S. acuta and S. rhombifolia extracts are shown in Table 2. At 31.25 µg/mL, the inhibitory rate was 28.69% and 14.78% for HESa and HESr, respectively. The same tendency was observed at 125 µg/mL where, after 24 h of incubation, inhibitory rates of 51.66% and 29.91% were recorded for HESa and HESr, respectively. Furthermore, the incubation of cells with the highest concentration (1,000 µg/mL) shows inhibition rates of 85.69% for HESa and 51.49% for HESr (Table 2). Based on their inhibitory activity on the Hepa 1–6 cells, the IC50 of HESa was 109.67 ± 6.99 µg/mL, and that of HESr was 888.79 ± 29.94 µg/mL.
Table 2.
Inhibition rates of the Sida acuta and Sida rhombifolia hydroethanolic extracts on the growth of the Hepa 1–6 cells.
| Percentage of inhibition | ||
|---|---|---|
| Concentrations | HESa | HESr |
| 15.625 µg/mL | 12.42 ± 1.54 | 12.42 ± 0.54 |
| 31.25 µg/mL | 28.69 ± 1.33 | 14.78 ± 0.73 |
| 62.5 µg/mL | 44.20 ± 2.33 | 23.11 ± 1.33 |
| 125 µg/mL | 51.66 ± 1.12 | 29.91 ± 0.91 |
| 250 µg/mL | 64.53 ± 1.21 | 36.12 ± 1.41 |
| 500 µg/mL | 75.62 ± 1.80 | 41.11 ± 1.44 |
| 1000 µg/mL | 85.69 ± 0.41 | 51.49 ± 0.45 |
Based on their cercaricidal activity and their cytotoxicity, the selectivity index of S. acuta and S. rhombifolia hydroethanolic extracts was calculated. At 30 min post-incubation, this index was 3.86 for HESa and 5.15 for HESr.
3.3. Phytochemical Constituents of Sida acuta and Sida rhombifolia Hydroethanolic Extracts
Qualitative phytochemical analysis of hydroethanolic extracts of S. acuta (HESa) and S. rhombifolia (HESr) revealed the presence of some secondary metabolites such as flavonoids, polyphenols, saponins, alkaloids, tannins, anthocyanins, triterpenes, sterols, anthraquinones, coumarins, and cardiac glycosides (Table 3).
Table 3.
Secondary metabolites of Sida acuta and Sida rhombifolia hydroethanolic extracts.
| Phytochemicals | HESa | HESr |
|---|---|---|
| Anthocyanins | + | − |
| Triterpenes | + | + |
| Sterols | + | − |
| Flavonoids | + | + |
| Polyphenols | + | + |
| Saponins | + | + |
| Essential oils | − | − |
| Anthraquinones | + | − |
| Alkaloids | + | + |
| Tannins | − | + |
| Coumarins | + | + |
| Reducing sugar | − | + |
| Cardiac glycosides | + | + |
(+): presence; (−): absence.
The HPLC-MS analysis of S. rhombifolia and S. acuta hydroethanolic extracts confirmed the presence of alkaloids, polyphenols, and flavonoids. The HPLC-MS chromatogram recorded for HESr was composed of peaks of chemical compounds, with retention times below 10 min (Figure 5) while those of HESa were below 15 min (Figure 6). The combination of the MS spectral data and the information from the literature allows tentative identification of 23 compounds (numbered 1–23 on the chromatograms).
Figure 5.

UPLC-DAD-MS UV profile of Sida rhombifolia hydroethanolic extract, identified peaks (1–16), and each identified compound spectrum (a–p).
Figure 6.

UPLC-DAD-MS UV profile of Sida acuta hydroethanolic extract, identified peaks, and each identified compound spectrum (a–i).
The peak eluted at 0.34 min (compound 1), corresponding to the molecular ion [M + Na]+ detected at m/z 203 was credited to glucose with a molecular mass of 203.05 (Figure 5(a)). Compound 2 appears at RT 3.06 min and produced the molecular ion [M + H]+ detected at m/z 219. According to the obtained data, it was possible to infer the molecular mass of 219.09 and identify it as quindoline, an alkaloid (Figure 5(b)). Compound 3 was observed at RT 3.13 min with a molecular ion [M + Na]+ detected at m/z 503 and was identified as 20-hydroxyecdysone, an ecdysteroid with the molecular mass of 503.29 (Figure 5(c)). Concerning compound 4 identification, it appeared at RT 3.50 min with a molecular ion [M + H]+ detected at m/z 314 and was identified as the polyphenol, N-feruloyltyramine with 314.13 as molecular mass (Figure 5(d)). The peak eluted at 4.39 min was allotted to compound 8 with a molecular ion [M + Na]+ at m/z 617 and was identified as tiliroside, a flavonoid with 617.12 as molecular mass (Figure 5(h)). The analysis of the HeSr spectrum also allowed to identify two coumarins, thamnosmonin (compound 9, RT 4.77 min with [M + H]+ detected at m/z 277) and scoparone (compound 11, RT 5.01 with [M + H]+ detected at m/z 207) with molecular masses of 277.14 and 207.09, respectively (Figures 5(i) and 5(k)). Moreover, at RT 6.30 min, compound 16 corresponding to the molecular ion [M + Na]+ detected at m/z 413 was identified as di-(2-ethylhexyl) phthalate with the molecular mass of 413.26 (Figure 5(p)). In addition to these identified compounds, compounds 10, 12, 13, 14, and 15 showed at 4.94, 5.07, 5.25, 5.38, and 5.57 min, respectively, were unidentified alkaloids. Also, compounds 5, 6, and 7, with RT 3.91 min, 3.98 min, and 4.20 min, respectively, showed a molecular ion [M + Na]+ at m/z 353, 239, and 259 (Figures 5(e)–5(g)) and were not identified (Table 4).
Table 4.
Main signals exhibited in the LC-DAD-MS spectra of compounds detected in the hydroethanolic extract from Sida rhombifolia aerial parts and proposed attribution.
| RT (min) | Exp. mass | Cald. mass | Molecular formula | Identified compounds | Structure |
|---|---|---|---|---|---|
| 0.34 | 203.0527; [M + Na]+ | 203.0526 | C6H12O6Na | Glucose (sugar) | 1 |
| 3.06 | 219.0986; [M + H]+ | 219.0922 | C15H11N2 | Quindoline (alkaloid) | 2 |
| 3.13 | 503.2959; [M + Na]+ | 503.2979 | C27H44O7Na | 20-Hydroxyecdysone (steroid) | 3 |
| 3.50 | 314.1371; [M + H]+ | 314.1387 | C18H20NO4 | N-Feruloyltyramine (polyphenol) | 4 |
| 3.91 | 353.2281; [M + Na]+ | 353.2298 | C20H34O5Na | NI | 5 |
| 3.98 | 239.1246; [M + Na]+ | 239.1254 | C11H20O4Na | NI | 6 |
| 4.20 | 259.1693; [M + Na]+ | 259.1669 | C15H24O2Na | NI | 7 |
| 4.39 | 617.1259; [M + Na]+ | 617.1271 | C30H26O13Na | Tiliroside (flavonoid) | 8 |
| 4.77 | 277.1409; [M + H]+ | 277.0895 | C15H17O5 | Thamnosmonin (coumarin) | 9 |
| 4.94 | 319.2227; [M + H]+ | 319.2227 | C15H31N2O5 | NI (alkaloids) | 10 |
| 5.01 | 207.0982; [M + H]+ | 207.0657 | C11H11O4 | Scoparone (coumarin) | 11 |
| 5.07 | 463.2637; [M + Na]+ | 463.2626 | C19H40N2O9 | NI (alkaloid) | 12 |
| 5.25 | 363.2484; [M + H]+ | 363.2490 | C17H35N2O6 | NI (alkaloid) | 13 |
| 5.38 | 635.4454; [M + Na]+ | 635.4453 | C30H64N2O10Na | NI (alkaloid) | 14 |
| 5.57 | 377.2641; [M + H]+ | 377.2646 | C18H37N2O6 | NI (alkaloid) | 15 |
| 6.30 | 413.2643; [M + Na]+ | 413.2663 | C24H38O4Na | Di-(2-ethylhexyl) phthalate | 16 |
NI: not identified.
Regarding the HESa chromatogram (Figure 6), the peak eluted at 4.80 min (compound 17), corresponding to the molecular ion [M + Na]+ detected at m/z 277 was credited to chrysin with a molecular mass of 277.09 (Figure 6(a)). Compound 18 appears at RT 5.19 min and produced the molecular ion [M + H]+ detected at m/z 233 and according to the obtained data, it was possible to infer the molecular mass of 219.10 and identify it as cryptolepine, an alkaloid (Figure 6(b)). Compound 19 was observed at RT 5.61 min with a molecular ion [M + H]+ detected at m/z 287 and was identified as kaempferol, a flavonoid with the molecular mass of 287.24 (Figure 5(c)). Concerning compound 20 identification, it appeared at RT 6.05 min with a molecular ion [M + H]+ detected at m/z 229 and was identified as the coumarin, xanthyletin with 229.24 as molecular mass (Figure 6(d)). The peaks eluted at 6.48 min and 7.48 min were N-feruloyltyramine and thamnosmonin corresponding to compounds 4 and 9, respectively. These compounds were previously identified in HESr (Figures 6(e) and 6(f)). The analysis of the HeSr spectrum also allowed to identify two flavonoids, luteolin (compound 22, RT 8.26 min with [M + Na]+ detected at m/z 309) and acacetin (compound 23, RT 12.30 min with [M + Na]+ detected at m/z 284) with molecular masses of 309.24 and 284.26, respectively (Figures 6(h) and 6(i)). Moreover, at RT 7.75 min, compound 21, an alkaloid identified as cryptolepinone was obtained with the molecular ion [M + Na]+ detected at m/z 249 and the molecular mass of 249.28 (Figure 6(g); Table 5). The chemical structures of all identified compounds from S. acuta and S. rhombifolia hydroethanolic extracts are shown in Figure 7.
Table 5.
Main signals exhibited in the LC-DAD-MS spectra of compounds detected in the hydroethanolic extract from Sida acuta the whole plant and proposed attribution.
| RT (min) | Exp. mass | Cald. mass | Molecular formula | Identified compounds | Structure |
|---|---|---|---|---|---|
| 4.80 | 277.0977; [M + Na]+ | 277.24 | C15H10O4Na | Chrysin (flavonoid) | 17 |
| 5.19 | 233.1090; [M + H]+ | 233.28 | C16H12N2 | Cryptolepine (alkaloid) | 18 |
| 5.61 | 287.0545; [M + H]+ | 287.24 | C15H10O6 | Kaempferol (flavonoid) | 19 |
| 6.05 | 229.0828; [M + H]+ | 229.24 | C14H13O3 | Xanthyletin (coumarin) | 20 |
| 6.48 | 314.1371; [M + H]+ | 314.13 | C18H21NO4 | N-Feruloyltyramine (polyphenol) | 4 |
| 7.48 | 277.2147; [M + H]+ | 277.14 | C11H20O4 | Thamnosmonin (coumarin) | 9 |
| 7.75 | 249.1035; [M + H]+ | 249.28 | C16H13N2O | Cryptolepinone (alkaloid) | 21 |
| 8.26 | 309.2067; [M + Na]+ | 309.24 | C15H10O6Na | Luteolin (flavonoid) | 22 |
| 12.30 | 284.2629; [M + Na]+ | 284.26 | C16H12O5Na | Acacetin (flavonoid) | 23 |
Figure 7.
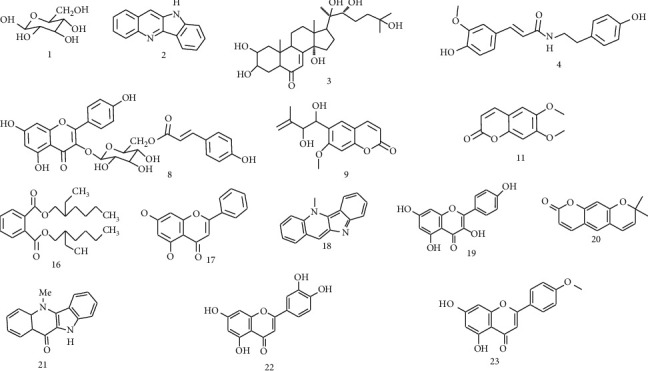
Chemical structures of identified compounds from Sida acuta and Sida rhombifolia hydroethanolic extracts.
4. Discussion
The present investigation showed that hydroethanolic (30:70) extracts of S. acuta whole plant and S. rhombifolia aerial parts disclosed cercaricidal activity against S. mansoni cercariae in both time- and concentration-dependent manner. This confirms the larvicidal activity of isolated compounds from S. rhombifolia previously highlighted by Islam et al. [23]. Previous studies conducted by some authors have also reported the cercaricidal activity of Glinus lotoides fruits aqueous extract [29], R. vomitoria stem bark, roots ethanolic extract [7], and O. pulcherrima roots methanolic extract and fractions [10]. Other studies have also reported a time- and concentration-dependent molluscicidal, cercaricidal, and/or schistosomicidal activity of various plant extracts such as A. indica and V. amygdalina [9], Millettia thonningii [30, 31], and Jatropha elliptica [32]. Based on the median lethal concentration LC50 and at any defined period, the cercaricidal activity of S. acuta hydroethanolic extract was more important than that of S. rhombifolia. As compared with results from previous studies, S. acuta and S. rhombifolia hydroethanolic 30:70 extracts were more potent, regarding the LC50 and the effective time, in killing S. mansoni cercariae than G. lotoides fruits aqueous extract [29], R. vomitoria stem bark and roots ethanolic extracts [7], A. indica and V. amygdalina methanolic extracts [9] but less effective than O. pulcherrima roots methanolic extract [10]. The cercaricidal activity of S. acuta and S. rhombifolia extracts might be due to their secondary metabolites such as flavonoids, alkaloids, and saponins. Abo-Zeid and Shohayeb [8] have shown the anti-miracidial and anti-cercarial activities of total alkaloids, saponins, and volatile oil extracted from N. sativa seeds hydroethanolic extract. It has also been demonstrated that alpinumisoflavone and robustic acid, two isoflavonoids isolated from M. thonningii seeds exhibit in vitro cercaricidal activity [30, 31]. Moreover, Dos Santos et al. [32] reported a strong cercaricidal activity of diethyl 4-phenyl-2,6-dimethyl-3,5 pyridine dicarboxylate, a penta-substituted pyridine alkaloid from the rhizome of J. elliptica with an LC100 of 2 µg/mL. The cercaricidal potential of these secondary metabolites could strongly support the efficacy of S. acuta and S. rhombifolia extracts in killing S. mansoni cercariae. Indeed, phytochemicals belonging to flavonoids, polyphenols, coumarins, alkaloids, and steroids were isolated from S. rhombifolia hydroethanolic 30:70 extract in this study. The cercaricidal activity of S. acuta and S. rhombifolia may be due to their ability to damage the cercariae tegument and disturb its motor activity. Xiao et al. [33] have previously demonstrated that exposure of S. japonicum cercariae to praziquantel results in intensive disturbance in motor activity and lysis of cercarial tissues, followed by an extensive release of gland contents and separation of the tail from the body. These observations were followed by the cercariae surface damages that are characterized by the decrease of the membranous glycocalyx, the swelling, and the degeneration of mitochondria distributed in the muscle and parenchymal cells as well as the lysis of the tegumental muscular layer. In fact, in our study, the incubation of S. mansoni cercaria with S. acuta and S. rhombifolia hydroethanolic extracts resulted in the separation of their tail from their body.
The genus Sida L. is one of the most diverse in the Malvaceae family, with about 200 species scattered in every part of tropical and subtropical regions in the world [18, 34, 35]. In addition, 142 chemical constituents belonging to various classes have been reported for Sida sp. Alkaloids, flavonoids, and ecdysteroids were predominant and reported mostly from S. acuta, S. cordifolia, S. rhombifolia, S. glutinosa, and S. spinosa. [36]. In our study, alkaloids were the major bioactive principles of S. acuta and S. rhombifolia hydroethanolic 30:70 extracts. Moreover, among the 15 compounds identified in those extracts, only thamnosmonin and xanthyletin (coumarins), and tiliroside (flavonoid) were recently identified in S. rhombifolia hydroethanolic extract [37]. Some of these compounds have been previously identified and/or isolated from the Sida L. genus. In fact, quindoline has been isolated from S. cordifolia and S. rhombifolia [38, 39], while 20-hydroxyecdysone has been isolated from S. spinosa [40] S. cordifolia [41], S. tuberculata [42–44], and S. acuta [37]. Furthermore, N-feruloyltyramine and di-(2-ethylhexyl) phthalate have been isolated from S. acuta [45] and scoparone from S. rhombifolia [46]. Moreover, Das et al. [47] identified chrysin in S. glutinosa and Silva et al. [48] isolated luteolin from S. galheirensis. Acacetin and kaempferol were isolated from S rhombifolia [38, 39], while cryptolepine and cryptolepinone were characterized from S. acuta [38, 39]. These phytoconstituents exhibited various pharmacological activities such as anti-microbial, anti-plasmodial, vasorelaxant, anti-oxidant, and anti-inflammatory [38, 42, 44, 49, 50].
Regarding the cytotoxic activity of both extracts on the growth of the Hepa 1–6 cells, S. acuta extract disclosed the highest anti-proliferative activity than S. rhombifolia. Anti-proliferative activity of a plant extract may be linked to its phytochemical constituents. It has been shown that quindoline and cryptolepinone, as well as N-trans-feruloyltyramine identified in S. acuta and S. rhombifolia hydro ethanolic extracts have a significant anti-proliferative activity on mouse hepatoma cells (Hepa 1c1c7) by inhibiting the quinone reductase activity [46].
5. Conclusions
S. acuta and S. rhombifolia hydroethanolic extracts disclosed cercaricidal activity and were not toxic on Hepa 1–6 cell line. However, Based on the LC50, the cercaricidal activity was more pronounced with S. acuta extract. The cercaricidal activity of these plant extracts may be linked to the presence of several secondary metabolite such as alkaloids, flavonoids, and coumarins. Based on their activity and their selectivity index, S. rhombifolia extract could be more effective on S. mansoni cercariae than S. acuta extract. This study could provide baseline information for further investigations aiming to develop plant-based alternative drugs against S. mansoni.
Acknowledgments
The authors are grateful to the “Deutscher Akademischer Austauschdienst” (DAAD) for financial support through the Yaounde-Bielefeld Graduate School for Natural Products with Antiparasitic and Antibacterial Activities (YaBINaPA, grant no. 57316173).
Data Availability
The data used to support the findings of this study are available from the corresponding author upon request.
Conflicts of Interest
The authors declare that there are no conflicts of interest regarding the publication of this study.
References
- 1.WHO. Schistosomiasis . Geneva, Switzerland: WHO; 2020. https://www.who.int/news-room/fact-sheets/detail/schistosomiasis . [Google Scholar]
- 2.Castro A. P., Alves de Mattos A. C., Alves de Mattos A. C., Martins Souza R. L., José Marques M., Henrique Dos Santos M. Medicinal plants and their bioactive constituents: a review of bioactivity against Schistosoma mansoni. Journal of Medicinal Plants Research . 2013;7(21):1515–1522. doi: 10.5897/jmpr12.0750. [DOI] [Google Scholar]
- 3.Collins C., Xu J., Tang S. Schistosomiasis control and the health system. Infectious Diseases of Poverty . 2012;1 doi: 10.1186/2049-9957-1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abo Zaid K. H., El-Wakil H., El-Hussein A., Jomaa S., Shohayeb M. Evaluation of the molluscicidal activity of Punica granatum, Calotropis procera, Solanum incanum and Citrullus colocynthis against Biomphalaria arabica. World Applied Sciences Journal . 2013;26(7):873–879. [Google Scholar]
- 5.WHO. Report of an Informal Consultation on Schistosomiasis Control . Geneva, Switzerland: WHO; 2013. [Google Scholar]
- 6.Takougang I., Meli J., Wabo Poné J., Angwafo F. Community acceptability of the use of low-dose niclosamide (Bayluscide), as a molluscicide in the control of human schistosomiasis in Sahelian Cameroon. Annals of Tropical Medicine and Parasitology . 2007;101(6):479–486. doi: 10.1179/136485907x193833. [DOI] [PubMed] [Google Scholar]
- 7.Tekwu E. M., Bosompem K. M., Anyan W. K., et al. In vitro assessment of anthelmintic activities of Rauwolfia vomitoria (Apocynaceae) stem bark and roots against parasitic stages of Schistosoma mansoni and cytotoxic study. Journal of parasitology research . 2017;2017:11. doi: 10.1155/2017/2583969.2583969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abo-zeid K., Shohayeb M. Evaluation of the biocidal activity of alkaloids, saponins and volatile oil extracted from Nigella sativa seeds against miracidia and cercariae of Schistosoma mansoni. International Journal of Pharmaceutical Science Invention . 2015;4(1):47–54. [Google Scholar]
- 9.Acheampong D. O., Owusu-Adzorah N., Armah F. A., et al. Ethnopharmacological evaluation of schistosomicidal and cercaricidal activities of some selected medicinal plants from Ghana. Tropical Medicine and Health . 2020;48(1):p. 19. doi: 10.1186/s41182-020-00205-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feussom N. G., Jatsa H. B., Kenfack M. C., et al. In vitro activity of Ozoroa pulcherrima Schweinf. extracts and fractions on Schistosoma mansoni cercariae and adult worms. European Journal of Medicinal Plants . 2020;31:17–30. doi: 10.9734/ejmp/2020/v31i830256. [DOI] [Google Scholar]
- 11.Pawar R. S., Jain A., Sharma P., Chaurasiya P. K., Singour P. K. In vitro studies on Sida cordifolia Linn for anthelmintic and antioxidant properties. Chinese Medicine . 2011;2(2):47–52. doi: 10.4236/cm.2011.22009. [DOI] [Google Scholar]
- 12.Wake R., Patil N., Halde U. K. Genus Sida–the plants with ethno medicinal and therapeutic potential. Golden Research Thoughts . 2011;1(2231) [Google Scholar]
- 13.Coe F. G., Anderson G. J. Screening of medicinal plants used by the Garífuna of Eastern Nicaragua for bioactive compounds. Journal of Ethnopharmacology . 1996;53(1):29–50. doi: 10.1016/0378-8741(96)01424-9. [DOI] [PubMed] [Google Scholar]
- 14.Karou D., Dicko M. H., Sanon S., Simpore J., Traore A. S. Antimalarial activity of Sida acuta Burm. F. (Malvaceae) and Pterocarpus erinaceus Poir. (Fabaceae) Journal of Ethnopharmacology . 2003;89(2–3):291–294. doi: 10.1016/j.jep.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 15.Tene Tcheghebe O., Ngouafong Tatong F., Seukep A., Kamga J., Nenwa J. Ethnobotanic survey of medicinal plants used for malaria therapy in western Cameroon. Journal of medicinal plants studies . 2016;4(3):248–258. [Google Scholar]
- 16.Mallikarjuna G., Prabhakaran, Saratkumar Reddy B. Anticancer activity of Sida acuta Burm.F against nitrosodiethylamine and CCl-4 induced hepatocellular carcinoma. Indo American Journal of Pharmaceutical Research . 2013;3(54) [Google Scholar]
- 17.Adjanohoun J., Aboubakar N., Dramane K. Traditional Medicine and Pharmacopeia- Contribution to Ethnobotanical and Floristic Studies in Cameroon . Lagos, Nigeria: Scientific, Technical, and Research Commission of the Organization of African Unity; 1996. [Google Scholar]
- 18.Rodrigues F. C., de Oliveira A. F. M. The genus Sida L. (Malvaceae): an update of its ethnomedicinal use, pharmacology and phytochemistry. South African Journal of Botany . 2020;132:432–462. doi: 10.1016/j.sajb.2020.04.030. [DOI] [Google Scholar]
- 19.Boukeng Jatsa H., de Jesus Pereira C. A., Dias Pereira A. B., et al. In vitro evaluation of Sida pilosa Retz (Malvaceae) aqueous extract and derived fractions on Schistosoma mansoni. Pharmacology & Pharmacy . 2015;6(8):380–390. doi: 10.4236/pp.2015.68039. [DOI] [Google Scholar]
- 20.Jatsa H. B., Russo R. C., Pereira C. A. D. J., et al. Improvement of the liver pathology by the aqueous extract and the n-butanol fraction of Sida pilosa Retz in Schistosoma mansoni-infected mice. Journal of Ethnopharmacology . 2016;180:114–123. doi: 10.1016/j.jep.2016.01.017. [DOI] [PubMed] [Google Scholar]
- 21.Jatsa H. B., Femoe U. M., Njiaza J., et al. Efficacy of Sida pilosa Retz aqueous extract against Schistosoma mansoni- induced granulomatous inflammation in the liver and the intestine of mice: histomorphometry and gastrointestinal motility evaluation. BMC Complementary and Alternative Medicine . 2018;18(1):p. 247. doi: 10.1186/s12906-018-2318-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Akilandeswari S., Senthamarai R., Prema S., Valarmathi R. Antimicrobial activity of leaf extracts of Sida acuta Burm. International Journal of Pharma Sciences and Research . 2010;1(5):248–250. [Google Scholar]
- 23.Islam M. E., Khatune N. A. Larvicidal activity of a new glycoside, phenyl ethyl β-D glucopyranoside from the stem of the plant Sida rhombifolia. Pakistan Journal of Biological Sciences . 2002;6(1):73–75. doi: 10.3923/pjbs.2003.73.75. [DOI] [Google Scholar]
- 24.Govindarajan M. Larvicidal and repellent activities of Sida acuta Burm. F. (Family: Malvaceae) against three important vector mosquitoes. Asian Pacific Journal of Tropical Medicine . 2010;3(9):691–695. doi: 10.1016/s1995-7645(10)60167-8. [DOI] [Google Scholar]
- 25.Holtfreter M. C., Loebermann M., Klammt S., et al. Schistosoma mansoni: schistosomicidal effect of mefloquine and primaquine in vitro. Experimental Parasitology . 2011;127(1):270–276. doi: 10.1016/j.exppara.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 26.Eissa M. M., El Bardicy S., Tadros M. Bioactivity of miltefosine against aquatic stages of Schistosoma mansoni, Schistosoma haematobium and their snail hosts, supported by scanning electron microscopy. Parasites & Vectors . 2011;4(1):73–11. doi: 10.1186/1756-3305-4-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Murata S., Shiragami R., Kosugi C., et al. Antitumor effect of 1, 8-cineole against colon cancer. Oncology Reports . 2013;30(6):2647–2652. doi: 10.3892/or.2013.2763. [DOI] [PubMed] [Google Scholar]
- 28.Trease G. E., Evans W. C. Pharmacognosy . London, UK: 1989. [Google Scholar]
- 29.Kiros G., Erko B., Giday M., Mekonnen Y. Laboratory assessment of molluscicidal and cercariacidal effects of Glinus lotoides fruits. BMC Research Notes . 2014;7(1):220–227. doi: 10.1186/1756-0500-7-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Perrett S., Whitfield P. J., Bartlett A., Sanderson L. Attenuation of Schistosoma mansoni cercariae with a molluscicide derived from Millettia thonningii. Parasitology . 1994;109(5):559–563. doi: 10.1017/s0031182000076435. [DOI] [PubMed] [Google Scholar]
- 31.Lyddiard J. R. A., Whitfield P. J., Bartlett A. Antischistosomal bioactivity of isoflavonoids from Millettia thonningii (leguminosae) The Journal of Parasitology . 2002;88(1):163–170. doi: 10.1645/0022-3395(2002)088[0163:aboifm]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 32.Dos Santos A. F., Fonseca S. A., César F. A., de Azevedo Albuquerque M. C. P., Santana J. V., Santana A. E. G. A penta-substituted pyridine alkaloid from the rhizome of Jatropha elliptica (Pohl) Muell. Arg. is active against Schistosoma mansoni and Biomphalaria glabrata. Parasitology Research . 2014;113(3):1077–1084. doi: 10.1007/s00436-013-3743-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xiao S.-H., Keiser J., Chen M.-G., Tanner M., Utzinger J. Research and development of antischistosomal drugs in the people’s Republic of China. Important Helminth Infections in Southeast Asia: Diversity and Potential for Control and Elimination, Part B . 2010;73:231–295. doi: 10.1016/s0065-308x(10)73009-8. [DOI] [PubMed] [Google Scholar]
- 34.Brandão J. L., Baracho G. S., de Sales M. F., Filho M. P. V. Synopsis of Sida (Malvaceae, malvoideae, malveae) in the state of Pernambuco, Brazil. Phytotaxa . 2017;307(3):205–227. [Google Scholar]
- 35.Aminah N. S., Laili E. R., Rafi M., Rochman A., Insanu M., Tun K. N. W. Secondary metabolite compounds from Sida genus and their bioactivity. Heliyon . 2021;7(4):p. e06682. doi: 10.1016/j.heliyon.2021.e06682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dinda B., Das N., Dinda S., Dinda M., Silsarma I. The genus Sida L.-a traditional medicine: its ethnopharmacological, phytochemical and pharmacological data for commercial exploitation in herbal drugs industry. Journal of Ethnopharmacology . 2015;176:135–176. doi: 10.1016/j.jep.2015.10.027. [DOI] [PubMed] [Google Scholar]
- 37.Kamdoum B. C., Simo I., Wouamba S. C. N. Chemical constituents of two Cameroonian medicinal plants: Sida rhombifolia L. and Sida acuta Burm. f. (Malvaceae) and their antiplasmodial activity. Natural Product Research . 2021;14 doi: 10.1080/14786419.2021.1937156. [DOI] [PubMed] [Google Scholar]
- 38.Chaves O. S., Teles Y. C., Monteiro M. M., et al. Alkaloids and phenolic compounds from Sida rhombifolia L. (Malvaceae) and vasorelaxant activity of two indoquinoline alkaloids. Molecules . 2017;22(1) doi: 10.3390/molecules22010094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chaves O., Gomes R., Tomaz A., et al. Secondary metabolites from Sida rhombifolia L. (Malvaceae) and the vasorelaxant activity of cryptolepinone. Molecules . 2013;18(3):2769–2777. doi: 10.3390/molecules18032769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Darwish F. M. M., Reinecke M. G. Ecdysteroids and other constituents from Sida spinosa L. Phytochemistry . 2003;62(8):1179–1184. doi: 10.1016/s0031-9422(03)00021-9. [DOI] [PubMed] [Google Scholar]
- 41.Jadhav A. N., Rumalla C. S., Avula B., Khan I. A. HPTLC method for determination of 20-hydroxyecdysone in Sida rhombifolia L. and dietary supplements. Chromatographia . 2007;66(9–10):797–800. doi: 10.1365/s10337-007-0407-3. [DOI] [Google Scholar]
- 42.Da Rosa H. S., Koetz M., Santos M. C., et al. Extraction optimization and UHPLC method development for determination of the 20-hydroxyecdysone in Sida tuberculata leaves. Steroids . 2018;132:33–39. doi: 10.1016/j.steroids.2018.01.014. [DOI] [PubMed] [Google Scholar]
- 43.Rosa H. S., Coelho I. S., Silva M. D., et al. Sida tuberculata extract reduces the nociceptive response by chemical noxious stimuli in mice: implications for mechanism of action, relation to chemical composition and molecular docking. Phytotherapy Research . 2019;33(1):224–233. doi: 10.1002/ptr.6220. [DOI] [PubMed] [Google Scholar]
- 44.Kumar S., Lakshmi P. K., Sahi C., Pawar R. S. Sida cordifolia accelerates wound healing process delayed by dexamethasone in rats: effect on ROS and probable mechanism of action. Journal of Ethnopharmacology . 2019;235:279–292. doi: 10.1016/j.jep.2018.07.003. [DOI] [PubMed] [Google Scholar]
- 45.Da Rosa H. S., De Camargo V. B., Camargo G., Garcia C. V., Fuentefria A. M., Mendez A. S. L. Ecdysteroids in Sida tuberculata R.E. Fries (Malvaceae): chemical composition by LC-ESI-MS and selective anti-Candida krusei activity. Food Chemistry . 2015;182:193–199. doi: 10.1016/j.foodchem.2015.02.144. [DOI] [PubMed] [Google Scholar]
- 46.Jang D. S., Park E. J., Kang Y.-H., et al. Compounds obtained from Sida acuta with the potential to induce quinone reductase and to inhibit 7,12-dimethylbenz-[a]anthracene-induced preneoplastic lesions in a mouse mammary organ culture model. Archives of Pharmacal Research . 2003;26(8):585–590. doi: 10.1007/bf02976704. [DOI] [PubMed] [Google Scholar]
- 47.Das N., Nath J., Dinda B. Antioxidant phytochemicals from Sida glutinosa. Journal of Pharmacy Research . 2012;5(9):4845–4848. [Google Scholar]
- 48.Silva D. A. E., Silva T. M. S. D., Lins A. C. D. S., et al. Constituintes químicos e atividade antioxidante de Sida galheirensis Ulbr. (Malvaceae) Química Nova . 2006;29(6):1250–1254. doi: 10.1590/s0100-40422006000600020. [DOI] [Google Scholar]
- 49.Preethidan D. S., Arun G., Surendran M. P. Lipoxygenase inhibitory activity of some Sida species due to di (2-ethylhexyl) phthalate. Current Science . 2013;105:232–234. [Google Scholar]
- 50.Karou D., Savadogo A., Canini A. Antibacterial activity of alkaloids from Sida acuta. African Journal of Biotechnology . 2005;4(12):1452–1457. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.


