Abstract
Objective:
Lung volume reduction surgery continues to have a high morbidity despite National Emphysema Treatment Trial selection criteria. This study evaluated the association between analytic morphomics on chest computed tomography scans and outcomes after lung volume reduction surgery.
Methods:
In a retrospective review of 85 lung volume reduction surgery patients from 1998–2013, dorsal muscle group area, subcutaneous and visceral fat area, and bone mineral density were assessed using analytic morphomics. Lung density was divided into five levels of increasing density (Lung density 1, emphysema; 2, normal lung; 4–5, scarring). Outcomes including survival, hospital length of stay, readmission at 30 days, and pulmonary complications were analyzed using univariate and multivariable techniques.
Results:
Pulmonary complications developed in 27.1% (23/85). Mortality at 90 days was 9.4% (8/85). On multivariable analysis, lower bone mineral density (Odds ratio 0.61; 95% confidence interval 0.39–0.95) was associated with decreased survival, longer length of stay (0.83; 0.77–0.89), and readmissions (0.39; 0.15–1.00). Higher lung density 5:lung density 2 volume (1.84; 1.05–3.23), possibly due to scarring, was associated with pulmonary complications and longer length of stay (1.32; 1.23–1.41) while lower subcutaneous fat area:height was associated with readmissions which may reflect decreased metabolic reserve (0.35; 0.13–0.93).
Conclusions:
Patients with signs of frailty including lower bone mineral density may be at increased risk of adverse outcomes including decreased survival after lung volume reduction surgery. The results of this hypothesis-generating study will need to be confirmed in larger, multicenter trials to determine whether analytic morphomics can improve risk stratification and patient selection.
Keywords: Morphomics, lung volume reduction surgery, emphysema, bone mineral density, frailty
INTRODUCTION
Lung volume reduction surgery (LVRS) was proposed as a treatment for severe emphysema, allowing the remainder of the lung to expand and improving diaphragmatic function by removing the most diseased portions of the lung. The National Emphysema Treatment Trial (NETT) randomized patients to LVRS or medical management.1 Patients with upper lobe predominant emphysema and low exercise capacity had improved survival while those with upper lobe disease and high exercise capacity only had a quality of life benefit. A high-risk group was identified with an FEV1<20% and either DLCO<20% or homogenous emphysema. Despite increasing experience and use of patient selection criteria from the NETT trial, LVRS continues to be associated with significant morbidity, and concerns over outcomes may have limited more widespread adoption.2
Analytic morphomics is a novel method that assesses individual patient characteristics on computed tomography (CT) and may allow for better preoperative risk assessment. Body composition, such as core muscle area, has been correlated with surgical outcomes in other patient populations.3, 4 Applying morphomic analysis in patients undergoing LVRS may add important morphomic factors to existing clinical criteria with the potential to improve patient selection and risk stratification for LVRS. The goal of the current study was to evaluate the association of analytic morphomics with outcomes following LVRS using standard preoperative chest CT scans. We hypothesize that lower bone mineral density and dorsal muscle group area, morphomic features associated with frailty in previous publications from our analytic morphomics group, would be associated with worse outcomes after LVRS. The primary outcome evaluated was overall survival with secondary outcomes including pulmonary complications, hospital length of stay, and readmission at 30 days.
MATERIAL AND METHODS
Approval from the University of Michigan Institutional Review Board was obtained. Consent was waived for this retrospective review. We identified 114 consecutive patients undergoing bilateral LVRS, either thoracoscopically (Video 1) or by sternotomy, from 1998–2013 at the University of Michigan Health System, one of 17 institutions in the NETT Trial. Adequate preoperative chest CT imaging was available for 85 patients.
Data Collection
Clinical data were obtained from our institutional LVRS database and the medical record. The primary outcome evaluated was overall survival. Secondary outcomes included pulmonary complications, hospital length of stay, and readmission at 30-days. Pulmonary complications included pneumonia and reintubation were combined as a composite outcome, and length of stay was a continuous outcome measure. Morphomic factors included lung density; bone mineral density (BMD), dorsal muscle group (DMG) normal density area, subcutaneous fat area, visceral fat area, fascia area, and total body area (Supplemental Table 1). Aside from lung density, all values were taken at the T11 level. Area variables were all indexed to height. High and low BMD were defined as above or below the median bone mineral density at T11.
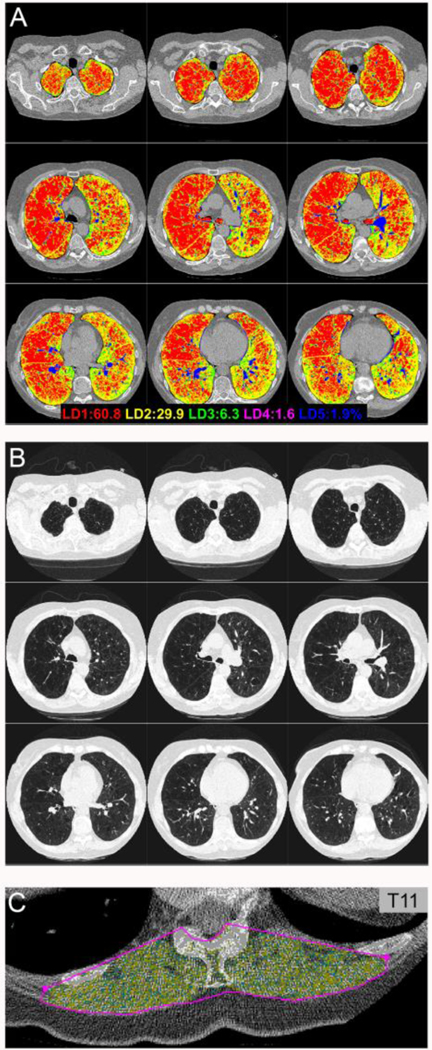
The volume of both lungs was divided into five levels (LD 1–5) of increasing density (Figure 1): LD1 included emphysema (less than or equal to −875 Hounsfield Units (HU)); LD2, normal lung (−874 HU to −725 HU); LD3 (−724 HU to −425 HU); LD 4 (−424 HU to −175 HU); and LD5 (> −175 HU). The pixel spacing and density were available for each pixel from the CT scan and its corresponding DICOM (Digital Imaging and COmmunications in Medicine) headers. A 3D volume was created by integrating these parameters for each slice that included the segmented lung parenchyma. The individual density group volumes are subsets within the lung parenchyma volume where each LD group captures a different range of HU values. The sum of all cross-sectional areas within this range of Hounsfield Units (less than or equal to −875 HU) from both the right and left lungs make up the total LD1 volume and indicates the presence of emphysema in the lung. For the purposes of this study, only the most emphysematous (LD1:LD2 volume) and dense (LD5:LD2 volume) portions of the lungs, consistent with parenchymal changes such as scarring, consolidation, and mucous impaction, were included in the analyzed morphomic factors.
Figure 1.
In patients undergoing lung volume reduction surgery, morphomic variables were evaluated including (A) lung density (LD), where LD1 (red) includes emphysema and LD2 (yellow) includes normal lung. (B) Matching images from a standard chest CT scan show severe upper-lobe predominant emphysema. (C) Normal density dorsal muscle group area (yellow) was also evaluated using morphomic techniques.
Image Processing
Chest CT scans were quality-controlled to confirm they included the entire lung. Studies were non-contrast, and scanners at the University of Michigan were calibrated daily using a phantom. Morphomic variables were acquired by our Morphomics Analysis Group using automated algorithms in MATLAB version 13.0 (MathWorks, Natick, MA). Morphomic parameters were determined based on fixed landmarks such as vertebral levels or the carina point. Definitions of the morphomic measures can be found at www.med.umich.edu/surgery/morphomics/data_dictionary and in Supplemental Table 1. While automated algorithms provided standardization between scans, decreasing observer bias, the automated results were evaluated by the morphomics team to confirm the accuracy of the results.
Statistics
Univariate analysis was conducted to determine the association of clinical and morphomic variables with pulmonary complications. Fischer’s exact test was used for binary variables while the Wilcoxon signed-rank test was used for continuous variables. Generalized linear models were used in the multivariable analysis to investigate the association between morphomic variables and short-term outcomes after LVRS. Logistic regression was used for binary outcomes while Poisson regression was used for count data such as length of stay (LOS). The Cox proportional hazards model was performed to analyze the differential hazard of death during follow-up. The Kaplan-Meier estimator was used to analyze the survival probabilities between patients with high versus low bone mineral density at the vertebral level T11. The primary procedure evaluated was lung volume reduction, and overall survival was evaluated whether or not the patient subsequently underwent lung transplant. The median bone mineral density was used to dichotomize patients into high and low BMD groups. Patients were censored at the time of last follow-up. The log rank test was used to compare the survival curves between the two groups.
The initial variables were the same for all multivariable models including FEV1, exercise capacity, LD1:LD2 volume, LD5:LD2 volume, bone mineral density (BMD) HU T11, subcutaneous fat area:height T11, visceral fat area:height T11, fascia area:height T11, total body area:height T11, and DMG normal density area:height T11 representing different aspects of body composition including muscle, bone, fat, and lung.4–7 A forward and backward selection procedure was employed to determine which variables were included in the final model, and Akaike’s Information Criterion (AIC) was optimized during variable selection. All analyses were performed using R version 3.2 (http://www.r-project.org).
RESULTS
Sternotomy was performed in 52.9% (45/85) of patients and bilateral thoracoscopic LVRS in 47.1% (40/85). Pulmonary complications developed in 27.1% (23/85). Six patients (7.0%) were ultimately listed for lung transplant. The median follow-up was 69 months, and there were 36 deaths during follow-up. Patient characteristics and distribution of the morphomic variables are shown in Table 1 and are stratified by pulmonary complications.
Table 1.
Patient Characteristics Stratified by Pulmonary Complications
| All Patients | No Pulmonary Complications | Pulmonary Complications | p-value | |
|---|---|---|---|---|
| Total | 85 | 62 | 23 | |
| Age | 63 (56–67) | 62.5 (56–67) | 64 (57–67) | 1.00 |
| FEV1 % predicted | 27 (24–31) | 27 (24–31) | 26 (22.5–29) | 0.46 |
| DLCO % predicted | 34 (28–41) | 34 (28–41) | 33 (28.5–37.5) | 0.74 |
| Low Exercise Capacity | 44 (53.0%) | 32 (51.6%) | 12 (57.1%) | 0.80 |
| Lung Density 1: Lung Density 2 Volume |
2.4 (1.9–3.3) | 2.3 (1.8–3.3) | 3.2 (2.1–4.2) | 0.37 |
| Lung Density 5:Lung Density 2 Volume |
0.07 (0.03–0.09) | 0.06 (0.03–0.08) | 0.07 (0.03–0.11) | 0.06 |
| Bone Mineral Density HU T11 |
128.3 (106.9–152.1) | 134.7 (107.9–151.7) | 122.9 (98.4–158.8) | 0.37 |
| DMG Normal Density Area:Height T11 |
809.0 (680.9–993.4) | 776.7 (679.5–969.7) | 894.5 (788–6-1064.4) | 0.11 |
| Fascia Area:Height T11 | 33789.6 (30000.9–39125.0) | 32646.5 (29602.2–37355.9) | 38146.6 (34463.9–40917.6) | 0.002 |
| Subcutaneous Fat Area:Height T11 |
5336.0 (3130.6–7323.7) | 5593.8 (3189.8–7462.1) | 4399.2 (3016.4–5823.9) | 0.20 |
| Visceral Fat Area:Height T11 |
5008.5 (3319.4–7348.0) | 4897.9 (3066.9–7026.1) | 6593.5 (3721.3–8979.3) | 0.19 |
| Total Body Area:Height T11 |
41969.9 (36508.4–45903.9) | 40702.5 (35330.2–44573.3) | 44605.1 (42146.9–48324.0) | 0.007 |
| ICU LOS (days) | 1 (0–3) | 1 (0–2) | 3 (2–5.5) | 0.00002 |
| Hospital LOS (days) | 8 (6–14) | 7 (6–10) | 14 (8–21) | 0.004 |
| Readmission 30-Day | 11 (13.4%) | 5 (8.1%) | 6 (30.0%) | 0.02 |
| Hospital Mortality | 4 (4.7%) | 0 (0%) | 4 (17.4%) | 0.004 |
| 90-Day Mortality | 5 (6.0%) | 0 (0%) | 5 (21.7%) | 0.001 |
Continuous variables shown as median (interquartile range)
FEV1, Forced expiratory volume in one second; DLCO, Diffusion capacity of lung for carbon monoxide; HU, Hounsfield unit; DMG, Dorsal muscle group; ICU, intensive care unit; LOS, Length of stay.
Lung Morphomics
While both emphysematous (LD1:LD2) and dense (LD5:LD2) lung, consistent with parenchymal changes such as scarring, consolidation, and mucous impaction, were higher in patients with pulmonary complications, neither lung measure reached significance on univariate analysis (Table 1). However, on multivariable analysis, lung scarring (LD5:LD2) was significantly associated with pulmonary complications (Table 2) as well as increased hospital length of stay (Table 3).
Table 2.
Multivariable Analysis – Pulmonary Complications
| Variable | OR (95% CI) | p-value |
|---|---|---|
| Fascia Area:Ht T11 | 2.32 (1.35–4.00) | 0.002 |
| LD5:LD2 Volume | 1.84 (1.05–3.23) | 0.03 |
Ht, Height; LD, Lung density
Table 3.
Multivariable Analysis – Hospital Length of Stay
| Variable | IRR (95% CI) | p-value |
|---|---|---|
| LD5:LD2 Volume | 1.32 (1.23–1.41) | <0.0001 |
| BMD HU T11 | 0.83 (0.77–0.89) | <0.0001 |
| Visceral Fat Area:Ht T11 | 1.13 (1.06–1.20) | 0.0002 |
| Preop FEV1 | 0.84 (0.77–0.92) | 0.0001 |
| Preop Exercise Capacity | 0.90 (0.84–0.97) | 0.006 |
| DMG Normal Density Area:Ht T11 | 1.08 (1.00–1.17) | 0.04 |
| Subcutaneous Fat Area:Ht T11 | 1.06 (0.99–1.15) | 0.06 |
IRR, incidence rate ratio; LD, Lung density; BMD, Bone mineral density; HU, Hounsfield Units; Ht, Height; FEV1, Forced expiratory volume in one second; DMG, Dorsal muscle group
Bone Mineral Density
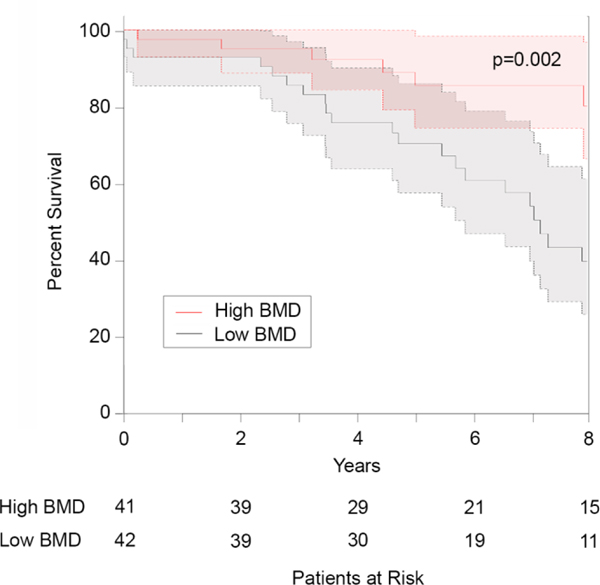
On multivariable analysis, lower BMD was significantly associated with increased hospital length of stay (Table 3). Lower bone mineral density was also associated with increased readmissions at 30 days (Table 4), and the risk of death was significantly increased in patients with lower BMD (Table 5). In addition, when high and low BMD were defined as above or below the median bone mineral density at T11, low BMD was associated with significantly decreased survival (Figure 2).
Table 4.
Multivariable Analysis – Readmission at 30 Days
| Variable | OR (95% CI) | p-value |
|---|---|---|
| BMD HU T11 | 0.39 (0.15–1.00) | 0.05 |
| Subcutaneous Fat Area:Ht T11 | 0.35 (0.13–0.93) | 0.03 |
| DMG Normal Density Area:Ht T11 | 1.71 (0.85–3.44) | 0.13 |
BMD, Bone mineral density; DMG, Dorsal muscle group; Ht, Height
Table 5.
Multivariable Analysis – Death
| Variable | HR (95% CI) | p-value |
|---|---|---|
| BMD HU T11 | 0.61 (0.39–0.95) | 0.03 |
| Visceral Fat Area:Ht T11 | 1.40 (0.98–1.99) | 0.06 |
BMD, Bone mineral density; Ht, Height
Figure 2.
Using the Kaplan-Meier estimator, low bone mineral density (BMD) was associated with significantly decreased survival after lung volume reduction surgery. High and low BMD were defined as above or below the median bone mineral density at T11. Confidence intervals are shown as shaded areas.
Muscle and Fat Morphomics
On multivariable analysis, higher DMG normal density area:height was associated with increased hospital LOS (Table 3). While higher visceral fat area:height was significantly association with increased hospital length of stay on multivariable analysis (Table 3), lower subcutaneous fat area:height was significantly associated with increased readmissions at 30 days which may reflect decreased metabolic reserve (Table 4). Higher fascia area:height was significantly associated with pulmonary complications on multivariable analyses (Table 2) and could be consistent with an emphysema patient with a barrel chest.
DISCUSSION
The NETT trial defined a subset of patients with increased survival and quality of life after LVRS.1 Others have shown that these results are durable over the long-term, and follow-up studies have confirmed increased survival and functional improvements after LVRS.8, 9 However, despite these results, only 538 patients underwent LVRS in the Society of Thoracic Surgeons (STS) Database between 2003–2011.10 Even with the use of NETT selection criteria, a subset of patients continues to do poorly. The goal of the current study was to evaluate the association of analytic morphomics with outcomes following LVRS using standard preoperative chest CT scans. We hypothesized that lower bone mineral density and dorsal muscle group area, morphomic features associated with frailty in previous publications from our analytic morphomics group, would be associated with worse outcomes after LVRS. The key findings include an association between lower BMD and the primary outcome, decreased survival, as well as other adverse outcomes including increased LOS and readmission at 30 days. Dense lung (LD5:LD2) likely represents consolidated or scarred lung from previous exacerbations and was associated with pulmonary complications and increased length of stay. Fat also appears to play a role in patients with emphysema with higher visceral fat area:height, which has been correlated with cardiac and metabolic comorbidities, associated with an increased LOS while lower subcutaneous fat area:height, which has been associated with frailty, was significantly associated with increased readmission at 30 days.
Survival
Naunheim et al. reported long-term follow-up after the NETT trial and found an overall survival advantage after LVRS at 5 years for patients with upper lobe predominant disease and low exercise capacity while those with high exercise capacity had no survival benefit.11 Kaplan et al. looked specifically at the long-term survival in the high-risk surgical subgroup from the NETT trial, with an FEV1<20% and either a DLCO<20% or homogeneous disease, and found that while survival was decreased in the first 3 years, after 4 years the quality-adjusted survival model favored surgery with comparable outcomes to the lower-risk subgroup.12 Ginsburg et al. reported 5-year survival rates of 79% after LVRS which is similar to the results in the current study.13
In analyzing survival, the six patients who underwent lung transplant were included with the entire cohort in our study since we were evaluating LVRS as the primary procedure. Inci et al. found that previous LVRS did not negatively affect survival after lung transplantation.14 In addition, with the limited numbers of donors available and the downward shift in lung allocation scores for patients with emphysema after revision of the allocation score in 2015, LVRS should be considered in carefully selected patients being evaluated for lung transplant.15
Lower BMD likely reflects frailty in patients with severe emphysema and may be decreased due to a combination of factors including oral steroids, malnutrition, and age. Decreased BMD has been associated with baseline chronic obstructive pulmonary disease (COPD) and related mortality in the National Health and Nutrition Examination Survey (NHANES III).16 Campos-Obando et al. also reported decreased BMD in chronic lung disease which was associated with increased mortality.17 By quantifying bone mineral density, it is possible to assess morphomic age. Englesbe et al. found that morphomic age, determined using abdominal aortic calcification, BMD, and psoas area and density, was more highly associated with mortality and LOS than the patient’s chronological age.5
Pulmonary Complications
Pulmonary complications are among the most common complications after LVRS and occurred in 18% of patients in the NETT trial.1 Naunheim et al. evaluated the NETT trial data and found higher age, declining FEV1, and decreasing DLCO were independently associated with pulmonary complications.18 Early extubation, chest physiotherapy, early ambulation, and pain management are all important in preventing pulmonary complications. Higher LD5:LD2 volume was also associated with increased pulmonary complications and likely reflects scarring, consolidation, or mucous plugging from previous COPD exacerbations.
Hospital Length of Stay
Several morphomic factors were associated with a longer LOS. Lower BMD likely reflects frailty with reduced functional performance. Yormaz et al. reported that 60.47% of patients with COPD had osteoporosis which correlated with the severity of the emphysema and was inversely related to the body mass index (BMI) suggesting a relationship with malnutrition.19 In a study by Balci et al., osteoporosis was found in 46% of lung transplant candidates with end-stage lung disease and was associated with a lower BMI and decreased 6-minute walk distance.20 Pienta et al. reported that higher BMD was associated with decreased LOS in lung transplant patients.21 While BMD is decreased in patients with severe emphysema, Mineo et al. found an improvement in BMD at 12 months postoperatively in patients who underwent LVRS compared to those on medical therapy who refused surgery.22
Higher LD5:LD2 volume also correlated with increased LOS. This likely reflects scarring, consolidation, or mucous plugging from previous COPD exacerbations and could increase LOS due to pulmonary complications. Increased pleural adhesions could also result in increased air leaks which are the most common cause of prolonged hospital LOS after LVRS.23
Higher visceral fat area:height was associated with increased LOS. Visceral fat has been associated with cardiac and metabolic comorbidities such as diabetes and is considered “bad” fat. Visceral fat is distinct from subcutaneous fat and has been correlated with decreased survival in patients undergoing resection for adrenocortical carcinoma7 and liver transplantation.24 Thijs et al. reported that pulmonary function was negatively correlated with visceral fat area in patients with metabolic syndrome.25 Furutate et al. found that patients with COPD have a larger visceral fat area which correlates with the severity of the COPD even in the absence of obesity.26
Interestingly, higher DMG ND area:height was associated with a longer LOS. Previous data has shown a relationship between sarcopenia of core muscles and postoperative morbidity and mortality following esophagectomy and liver transplant.27, 28 Patients with emphysema commonly have muscle wasting. Sarcopenia has been reported in 14.5% of patients with COPD and impairs functional performance although this can be reversed with pulmonary rehabilitation.29 However, Orozco-Levi et al, found differences in adaptive changes in response to COPD depending on the muscle compartment.30 Although he does not directly address the dorsal muscle group, the latissimus dorsi was found to increase in strength and fiber size in contrast to peripheral skeletal muscle. While the latissimus is not traditionally thought of as a muscle of respiration, electrophysiological studies have reported that the latissimus is involved as an accessory inspiratory muscle in patients with emphysema.31 The dorsal muscle group may have a similar function as accessory muscles of respiration in patients with emphysema.
Readmissions at 30 days
Lower BMD was associated with readmissions and is likely related to patient frailty as described above, and frailty related to COPD has been associated with increased readmissions after acute exacerbations at 30 days.32 Lower subcutaneous fat area:height was also associated with readmissions. Decreased subcutaneous fat is another important indicator of frailty. While obesity and increased BMI have been correlated with poor outcomes after abdominal surgery, obesity has not correlated with worse outcomes after major lung resection.33 However, a low BMI<18.5 was associated with an increased risk of pulmonary complications and mortality. Subcutaneous fat may be an indicator of metabolic reserve that is important in patients with end-stage lung disease with their increased work of breathing combined with malnutrition. Grace et al. found that increased subcutaneous fat was associated with less progression of emphysema over time, and overweight patients with COPD appear to have a better prognosis which has become known as the “obesity paradox.”34
This study has several limitations. Our study only includes patients from a single institution which may limit the generalizability of our findings. In addition, with a relatively small study population of 85 patients, validation techniques such as leave-one-out cross validation were not performed. However, LVRS is a relatively uncommon operation with only 538 LVRS patients in the STS Database between 2003–2011.2 During this time period, our group performed 72 LVRS procedures, or 13.4% of the cases in the STS database, confirming our large LVRS experience. While the inclusion of LVRS patients undergoing lung transplant could affect survival of these patients, the changes would likely be consistent with our results showing that morphomic features of frailty including lower BMD were associated with decreased survival. These frail patients would also be less likely to meet the functional requirements to be considered for lung transplant since transplant candidates must meet minimal functional thresholds.
Another limitation is that there are possible interactions between variables in the morphomic analysis. We have chosen a limited number of morphomic variables representing different aspects of body composition including muscle, bone, fat, and lung to reduce interactions between variables. The long study period and changes in CT technology over this time are also limitations. To minimize this effect, we limited the patients included to those with CT studies of sufficient quality for morphomic analysis (for example, < 5 mm slice thickness). In addition, to evaluate for differences in technique (reconstruction kernel and tube current kVP), we have data on 1600 studies scanned with the same phantom, whose physical and material properties are known, with three channels of different densities. All scanners were from one manufacturer except one, and scanners at the University of Michigan were calibrated daily using a phantom. Patient management may have also changed during the study period with the proportion of thoracoscopic LVRS increasing over time. However, a review of LVRS cases in the STS Database since the NETT study showed similar results when compared to the NETT trial.2, 35
Conclusions
Despite the use of patient selection criteria from the NETT trial, LVRS continues to be associated with significant morbidity and concerns over outcomes may have limited more widespread adoption. In this study, we report significant associations between analytic morphomic factors and patient outcomes after LVRS. In patients with signs of frailty, lower bone mineral density was associated with decreased survival as well as other adverse outcomes including readmission at 30 days and increased length of stay while lower subcutaneous fat area was associated with increased readmission at 30 days. While this study is hypothesis-generating, if these results are validated in a larger, multicenter study, they could potentially have a valuable impact on selection and risk stratification of patients being considered for LVRS.
Supplementary Material
Video 1. This brief video demonstrates the port placement and standard techniques used during a bilateral thoracoscopic lung volume reduction surgery (LVRS) including the use of reinforced stapler loads to potentially decrease the risk of air leaks.
Central Picture.
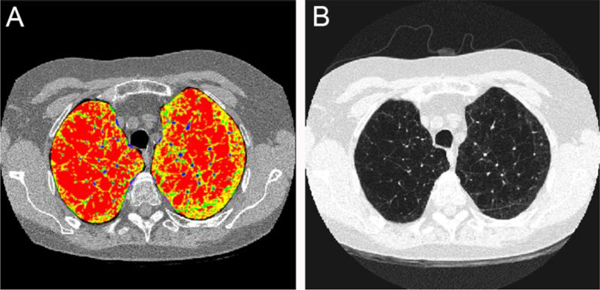
Lung density 1 (LD1) (red) includes emphysema while LD2 (yellow) includes normal lung.
Central Message
Using analytic morphomics, signs of frailty such as lower bone mineral density (BMD) were associated with decreased survival after lung volume reduction surgery.
Perspective Statement
Lung volume reduction surgery continues to result in high morbidity despite National Emphysema Treatment Trial selection criteria. In this study, signs of frailty such as lower bone mineral density were associated with adverse outcomes and decreased survival. The results need to be confirmed in larger trials to determine whether analytic morphomics can improve risk stratification and patient selection.
Acknowledgments
We would like to thank John Donkersloot and Steven Sun for their help with this study.
Funding: PZ was partially supported by NIDDK 1K01DK106296-01A1.
Poster presentation at the 2016 AATS Annual Meeting (Winner of the General Thoracic Moderated Poster Competition)
Abbreviations:
- (BMD)
Bone mineral density
- (BMI)
body mass index
- (COPD)
chronic obstructive pulmonary disease
- (CT)
computed tomography
- (DLCO)
diffusion capacity of lung for carbon monoxide
- (DICOM)
Digital Imaging and Communications in Medicine
- (DMG)
dorsal muscle group
- (FEV1)
forced expiratory volume in one second
- (Ht)
height
- (HU)
Hounsfield Unit
- (ICU)
intensive care unit
- (LOS)
IRR, incidence rate ratio, length of stay
- (LD)
lung density
- (LVRS)
lung volume reduction surgery
- (NETT)
National Emphysema Treatment Trial
- (NHANES III)
National Health and Nutrition Examination Survey
- (ND)
normal density
- (STS)
Society of Thoracic Surgeons
Footnotes
Disclosures: Intuitive Surgical Site Mentor and Proctor (JL, RMR), Equity owner of Prenovo and Morphomic Analysis Group, LLC (SCW), Auris Health Consultant (RMR), Medtronic Advisory Board (RMR), None (WBW, TG, PZ, BAD, BE, JU, ACC)
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Fishman A, Martinez F, Naunheim K, et al. A randomized trial comparing lung-volume-reduction surgery with medical therapy for severe emphysema. N Engl J Med. 2003;348:2059–2073. [DOI] [PubMed] [Google Scholar]
- 2.Decker MR, Leverson GE, Jaoude WA, Maloney JD. Lung volume reduction surgery since the National Emphysema Treatment Trial: study of Society of Thoracic Surgeons Database. J Thorac Cardiovasc Surg. 2014;148:2651–2658 e2651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stidham RW, Waljee AK, Day NM, et al. Body fat composition assessment using analytic morphomics predicts infectious complications after bowel resection in Crohn’s disease. Inflamm Bowel Dis. 2015;21:1306–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Englesbe MJ, Lee JS, He K, et al. Analytic morphomics, core muscle size, and surgical outcomes. Ann Surg. 2012;256:255–261. [DOI] [PubMed] [Google Scholar]
- 5.Englesbe MJ, Terjimanian MN, Lee JS, et al. Morphometric age and surgical risk. J Am Coll Surg. 2013;216:976–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee CS, Cron DC, Terjimanian MN, et al. Dorsal muscle group area and surgical outcomes in liver transplantation. Clin Transplant. 2014;28:1092–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miller BS, Ignatoski KM, Daignault S, et al. Worsening central sarcopenia and increasing intra-abdominal fat correlate with decreased survival in patients with adrenocortical carcinoma. World J Surg. 2012;36:1509–1516. [DOI] [PubMed] [Google Scholar]
- 8.Ginsburg ME, Thomashow BM, Bulman WA, et al. The safety, efficacy, and durability of lung-volume reduction surgery: A 10-year experience. J Thorac Cardiovasc Surg. 2016;151:717–724 e711. [DOI] [PubMed] [Google Scholar]
- 9.Yusen RD, Lefrak SS, Gierada DS, et al. A prospective evaluation of lung volume reduction surgery in 200 consecutive patients. Chest. 2003;123:1026–1037. [DOI] [PubMed] [Google Scholar]
- 10.Decker MR, Leverson GE, Jaoude WA, Maloney JD. Lung volume reduction surgery since the National Emphysema Treatment Trial: study of Society of Thoracic Surgeons Database. J Thorac Cardiovasc Surg. 2014;148:2651–2658.e2651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Naunheim KS, Wood DE, Mohsenifar Z, et al. Long-term follow-up of patients receiving lung-volume-reduction surgery versus medical therapy for severe emphysema by the National Emphysema Treatment Trial Research Group. Ann Thorac Surg. 2006;82:431–443. [DOI] [PubMed] [Google Scholar]
- 12.Kaplan RM, Sun Q, Naunheim KS, Ries AL. Long-term follow-up of high-risk patients in the National Emphysema Treatment Trial. Ann Thorac Surg. 2014;98:1782–1789. [DOI] [PubMed] [Google Scholar]
- 13.Ginsburg M, Thomashow B, Bulman W, Jellen P, Whippo B, Sonett J. Surgical risk, functional outcomes, and late survival after lung volume reduction surgery (LVRS): Report of one hundred consecutive surgical cases. European Respiratory Journal. 2015;46:PA1832. [Google Scholar]
- 14.Inci I, Iskender I, Ehrsam J, et al. Previous lung volume reduction surgery does not negatively affect survival after lung transplantation. Eur J Cardiothorac Surg. 2018;53:596–602. [DOI] [PubMed] [Google Scholar]
- 15.Maloney J, Strieter N, Meyer K, Mack T, Cornwell R. Lung Volume Reduction Surgery (LVRS) in the Lung Allocation Score Era (LAS). CHEST. 2012;142:741A. [Google Scholar]
- 16.Looker AC. Relationship between femur neck bone mineral density and prevalent chronic obstructive pulmonary disease (COPD) or COPD mortality in older non-Hispanic white adults from NHANES III. Osteoporos Int. 2014;25:1043–1052. [DOI] [PubMed] [Google Scholar]
- 17.Campos-Obando N, Castano-Betancourt MC, Oei L, et al. Bone mineral density and chronic lung disease mortality: the rotterdam study. J Clin Endocrinol Metab. 2014;99:1834–1842. [DOI] [PubMed] [Google Scholar]
- 18.Naunheim KS, Wood DE, Krasna MJ, et al. Predictors of operative mortality and cardiopulmonary morbidity in the National Emphysema Treatment Trial. J Thorac Cardiovasc Surg. 2006;131:43–53. [DOI] [PubMed] [Google Scholar]
- 19.Yormaz B, Cebeci H, Yilmaz F, Suerdem M. Bone mineral density in emphysema and chronic bronchitis phenotypes in hospitalized male chronic obstructive pulmonary disease patients. Clin Respir J. 2020;14:47–53. [DOI] [PubMed] [Google Scholar]
- 20.Balci MK, Ari E, Vayvada M, et al. Osteoporosis in Lung Transplantation Candidates: Association With 6-minute Walking Test and Body Mass Index. Transplant Proc. 2016;48:2147–2151. [DOI] [PubMed] [Google Scholar]
- 21.Pienta MJ, Zhang P, Derstine BA, et al. Analytic Morphomics Predict Outcomes After Lung Transplantation. Ann Thorac Surg. 2018;105:399–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mineo TC, Ambrogi V, Mineo D, Fabbri A, Fabbrini E, Massoud R. Bone mineral density improvement after lung volume reduction surgery for severe emphysema. Chest. 2005;127:1960–1966. [DOI] [PubMed] [Google Scholar]
- 23.DeCamp MM, Blackstone EH, Naunheim KS, et al. Patient and surgical factors influencing air leak after lung volume reduction surgery: lessons learned from the National Emphysema Treatment Trial. Ann Thorac Surg. 2006;82:197–206; discussion 206–197. [DOI] [PubMed] [Google Scholar]
- 24.Terjimanian MN, Harbaugh CM, Hussain A, et al. Abdominal adiposity, body composition and survival after liver transplantation. Clin Transplant. 2016;30:289–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thijs W, Alizadeh Dehnavi R, Hiemstra PS, et al. Association of lung function measurements and visceral fat in men with metabolic syndrome. Respir Med. 2014;108:351–357. [DOI] [PubMed] [Google Scholar]
- 26.Furutate R, Ishii T, Wakabayashi R, et al. Excessive visceral fat accumulation in advanced chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2011;6:423–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Englesbe MJ, Patel SP, He K, et al. Sarcopenia and mortality after liver transplantation. J Am Coll Surg. 2010;211:271–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sheetz KH, Zhao L, Holcombe SA, et al. Decreased core muscle size is associated with worse patient survival following esophagectomy for cancer. Dis Esophagus. 2013;26:716–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jones SE, Maddocks M, Kon SS, et al. Sarcopenia in COPD: prevalence, clinical correlates and response to pulmonary rehabilitation. Thorax. 2015;70:213–218. [DOI] [PubMed] [Google Scholar]
- 30.Orozco-Levi M. Structure and function of the respiratory muscles in patients with COPD: impairment or adaptation? Eur Respir J Suppl. 2003;46:41s–51s. [DOI] [PubMed] [Google Scholar]
- 31.Orozco-Levi M, Gea J, Monells J, Aran X, Aguar MC, Broquetas JM. Activity of latissimus dorsi muscle during inspiratory threshold loads. Eur Respir J. 1995;8:441–445. [DOI] [PubMed] [Google Scholar]
- 32.Nguyen HQ, Chu L, Amy Liu IL, et al. Associations between physical activity and 30-day readmission risk in chronic obstructive pulmonary disease. Ann Am Thorac Soc. 2014;11:695–705. [DOI] [PubMed] [Google Scholar]
- 33.Ferguson MK, Im HK, Watson S, Johnson E, Wigfield CH, Vigneswaran WT. Association of body mass index and outcomes after major lung resection. Eur J Cardiothorac Surg. 2014;45:e94–99; discussion e99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu TD, Ejike CO, Wise RA, McCormack MC, Brigham EP. Investigation of the Obesity Paradox in Chronic Obstructive Pulmonary Disease, According to Smoking Status, in the United States. Am J Epidemiol. 2019;188:1977–1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ahmad S, Taneja A, Kurman J, Dagar G, Kumar G. National trends in lung volume reduction surgery in the United States: 2000 to 2010. Chest. 2014;146:e228–e229. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video 1. This brief video demonstrates the port placement and standard techniques used during a bilateral thoracoscopic lung volume reduction surgery (LVRS) including the use of reinforced stapler loads to potentially decrease the risk of air leaks.