Abstract
Saponins are potent and safe vaccine adjuvants, but their mechanisms of action remain incompletely understood. Here, we explored the properties of several saponin formulations, including immunostimulatory complexes (ISCOMs) formed by the self-assembly of saponin and phospholipids in the absence or presence of the Toll like receptor (TLR) 4 agonist monophosphoryl lipid A (MPLA). We found that MPLA self-assembles with saponins to form particles physically resembling ISCOMs, which we termed saponin/MPLA nanoparticles (SMNP). Saponin-containing adjuvants exhibited distinctive mechanisms of action, altering lymph flow in a mast cell-dependent manner and promoting antigen entry into draining lymph nodes. SMNP was particularly effective, exhibiting even greater potency than the compositionally-related adjuvant AS01B in mice, and primed robust germinal center B cell, TFH, and HIV tier 2 neutralizing antibodies in non-human primates. Altogether, these findings shed new light on mechanisms by which saponin adjuvants act to promote the immune response and suggest SMNP may be a promising adjuvant in the setting of HIV, SARS-CoV-2, and other pathogens.
One Sentence Summary:
An adjuvant composed of saponin and a TLR4 agonist acts by enhancing lymph flow and antigen entry into lymph nodes.
INTRODUCTION
Adjuvants are important components of vaccines, promoting protective immune responses to poorly immunogenic antigens. Very few adjuvants have been approved as part of licensed human vaccines to date, and the development of new adjuvants that can safely augment adaptive immune responses is of great interest for vaccine efforts against infectious diseases for which billions of people are at risk, such as malaria, tuberculosis, human immunodeficiency virus (HIV) (1, 2), and now COVID-19 (3). Saponins are triterpene glycosides isolated from natural sources such as the Quillaja Saponaria tree, which have been under extensive study as vaccine adjuvants (4, 5). Although free saponins are toxic, formulation of saponins with lipids and cholesterol maintains their adjuvant activity in a non-toxic state, and even enables saponins to be safely combined with additional innate immune stimulators such as Toll-like receptor (TLR) agonists. Such formulation advances have led to the first licensed vaccines with saponin adjuvants, which employ liposomal saponin and MPLA (Glaxo Smith Kline’s AS01 adjuvant used in the Shingrix® and Mosquirix® vaccines) (6).
Another intensively investigated form of saponins are immune stimulating complexes (ISCOMs) – cage-like nanoparticles with diameters of approximately 40 nm formed by the self-assembly of saponins, cholesterol, and phospholipids. ISCOM-based vaccine formulations have been shown to induce adaptive immune responses in small animals (7, 8), non-human primates (NHPs) (9-13) and humans (14-17). Promising phase 3 clinical trial results for Novavax’s SARS-CoV-2 vaccine employing the saponin adjuvant Matrix M® may lead to the first FDA-approved vaccine incorporating an ISCOM-based adjuvant (18). ISCOMs were originally described as a multivalent antigen-delivery system with built-in adjuvant (i.e. saponins) (7). However, physical association with antigen was later found to be not important (16, 17, 19). Rather, “antigen-free” ISCOMs can be simply added to improve the immunogenicity of soluble antigens.
ISCOMs and other saponin-containing adjuvants are known to induce local proinflammatory cytokine and chemokine responses and activate dendritic cells (DCs) (20-23), and several mechanisms have been defined relating to enhanced CD8+ T cell priming through effects on DCs and promotion of antigen cross presentation (24-27). However, saponin adjuvants are also effective for inducing antibody responses, and how saponins promote antibody responses remains obscure. Saponins exhibit strong synergy with Toll-like receptor (TLR) 4 agonists (23, 28), but mechanisms underlying this synergy are also poorly defined. To gain further insight into these issues, we compared the immunological effects of ISCOMs versus a diverse panel of clinical and experimental adjuvants in mouse models enabling detailed dissection of antigen-specific B and T cell responses. Further, we designed ISCOMs incorporating the TLR4 agonist monophosphoryl lipid A (MPLA), forming saponin-MPLA nanoparticles (hereafter, SMNP), to evaluate potential synergistic effects of saponins with this TLR agonist.
As the majority of licensed vaccines are thought to function by inducing a protective antibody response (29, 30), we focused on analyzing adjuvant impacts on humoral immunity. Here, we demonstrate that saponin adjuvants exhibited potent activity in mice, eliciting robust germinal center (GC), T cell, and class-switched antibody responses superior to a range of alternative adjuvants, with SMNP exhibiting particularly strong potency. Mechanistically, we observed ISCOMs and SMNP altered lymph flow and lymph node permeability, leading to enhanced antigen acquisition by B cells in draining lymph nodes (dLNs), with maximal effects obtained for the combined saponin/TLR4 agonist adjuvant. In non-human primates, vaccination with HIV Env trimer and SMNP induced robust and durable GCs and autologous tier 2 neutralizing antibody responses. Altogether, these data provide insights into the mechanisms of action underlying saponin adjuvants and suggest that SMNP represents a promising new adjuvant.
RESULTS
Development and characterization of saponin/MPLA-based nanoparticle adjuvants
We first evaluated the ability of a panel of innate immune stimulators containing lipid moieties to co-assemble with Quil-A saponin, cholesterol, and phospholipids to form ISCOMs. Unlike lipid-linked NOD1, TLR2/7, TLR7, or TLR9 ligands (fig. S1A-B), the synthetic TLR4 agonist MPLA (PHAD®) could be incorporated into ISCOM particles at a 10:10:2.5:1 Quil-A:cholesterol:DPPC:MPLA (molar ratio) to yield homogenous ~40 nm nanocage structures (Fig. 1A-C). We focused on this particular formulation for further vaccine studies, and termed this adjuvant saponin/MPLA nanoparticles (hereafter, SMNP). Stimulation of TLR4 reporter cells confirmed that MPLA incorporated in SMNP had bioactivity comparable to free MPLA (Fig. 1D). In contrast to traditional ISCOMs, SMNP induced murine bone marrow-derived dendritic cells to secrete cytokines TNF-α and IL-6 in vitro (Fig. 1E), which are known to promote adaptive immune responses. We also assessed stimulation of human peripheral blood mononuclear cells (PBMCs), and observed robust production of IFN-γ, IL-12, TNF-α and other cytokines in response to SMNP in a dose-dependent manner (Fig. 1F). Importantly, SMNP was non-toxic to human PBMCs (fig. S1C).
Figure 1: Characterization of saponin-based nanoparticle adjuvants.
(A) Dynamic Light Scattering (DLS) size measurements of ISCOMs and SMNP.
(B) Size exclusion chromatogram of SMNP particles.
(C) Representative transmission electron microscope (TEM) images of ISCOM and SMNP particles. Scale bars, 50 nm.
(D) Activation analysis of Raw-Blue TLR reporter cell line after 18hr incubation with ISCOMS, SMNP, or free MPLA. ISCOMs were added at molar equivalent concentrations to SMNP as a control. Symbols represent mean values from duplicate wells.
(E) Analysis of cytokine secretion by bone marrow-derived DCs 6hrs after incubation with 1 μg/mL SMNP or ISCOMs. Each symbol represents a replicate well (n = 3) and the bar represents the mean.
(F) SMNP was added at indicated concentrations to human PBMCs in vitro for 24 hr and then cytokines and chemokines in the supernatant were analyzed by multiplexed ELISA. Shown are means ± SEM.
(G-H) Analysis of proinflammatory cytokine secretion in draining inguinal LNs of mice at 3, 6, and 18 hrs after adjuvant injection. Adjuvant was injected subcutaneously at the base of the tail in Balb/C mice. Representative data from 2 independent experiments (n=3 mice/group). Shown are the experiment timeline (G) and mean cytokine concentrations at each timepoint (H). Symbols represent mean values. Error bars are standard error of the mean (SEM).
Statistical analysis for (E) was performed by one-way ANOVA, followed by Tukey’s post-test. Statistical analysis for (H) is comparing responses to SMNP using two-way ANOVA, followed by Dunnett’s post test. *, p < 0.05; **, p < 0.01; ***, p < 0.001; ****, p < 0.0001.
We next evaluated cytokine responses induced by SMNP in mice. A panel of proinflammatory cytokines and chemokines in dLNs were measured at 3h, 6h, or 18h after injection of ISCOMs, SMNP, MPLA, or ISCOMs mixed with free MPLA (Fig. 1G). Compared to traditional ISCOMs, SMNP rapidly induced 10-100-fold higher levels of the proinflammatory cytokines IFN-α, IFN-γ, IL-6, and TNF-α (Fig. 1H and fig. S1D). Free MPLA, administered in a dose equivalent to SMNP (0.5μg/mouse), failed to induce any response above background (Fig. 1G and fig. S1D). Notably, ISCOMs mixed with free MPLA at the same ratio used in SMNP did not show appreciable differences from ISCOMs alone (Fig. 1H and fig. S1D), indicating that physical incorporation of MPLA into the nanoparticle structure was essential for the activity of SMNP. Despite the high levels of inflammatory cytokines induced in LNs, cytokine and chemokine levels in the serum remained low following vaccination with the exception of IL-6, which showed a transient elevation at 6 hr that returned to baseline by 18 hr (fig. S1E). Together, these data show that incorporating MPLA into ISCOM nanoparticles synergistically enhances innate immune activation in dLNs.
SMNP supports potent antibody responses in mice
Saponin particulate adjuvants have been reported to elicit potent immune responses in mice, but we first sought to benchmark them against a panel of alternative experimental and clinical adjuvants. For these studies we assessed the capacity of ISCOMs or SMNP to stimulate humoral responses in Balb/c mice against a poorly immunogenic HIV antigen, a stabilized HIV Env BG505 SOSIP trimer (termed MD39) (31), representative of SOSIP Env trimers currently in clinical trials (Fig. 2A). Mice were immunized subcutaneously at the base of the tail, and draining inguinal lymph nodes (iLN) were harvested for analysis. SMNP elicited GC B cell (BGC) responses greater than traditional ISCOMs, and outperformed other adjuvants, including AddaVax (an MF59-like adjuvant), Ribi (an oil-in-water emulsion adjuvant containing MPLA), AS01B (a clinical liposomal saponin/MPLA adjuvant (6)), and alum (Fig. 2B, fig. S2A). Follicular helper CD4+ T cells (TFH) that drive GC responses were also increased by SMNP 2-3 fold over all other adjuvants tested (Fig. 2C and fig. S2B), suggesting a significant impact of combining saponin with MPLA in an ISCOM particle. We also analyzed BGC and TFH responses in the axillary lymph nodes (axLN), which drain efferent lymph from the iLNs. Interestingly, we saw that ISCOMs and SMNP elicited robust GC responses at these distal dLNs, in contrast to all other adjuvants tested, including AS01B (Fig. 2D). Hence, SMNP and ISCOMs elicit robust GC and TFH responses that extend beyond the proximal dLNs in mice.
Figure 2. SMNP is a potent adjuvant for humoral immunity in mice.
(A) Schematic of the experiment for (A-F). Balb/C mice were immunized s.c. with specified adjuvants and 2 μg of MD39 HIV Env trimer, and were boosted with identical formulations 4 weeks later. Germinal center B cell (BGC) and T follicular helper (TFH) responses were analyzed 12 days post prime immunization and antibody titers were measured 2, 4, 5, and 7 weeks post prime. Pooled data from 2 independent experiments. Each symbol represents a mouse and the bar represents the mean. n = 9 or 10 mice per group.
(B) Representative flow plots and quantification of BGC cells in draining iLN on day 12. Live, single cells were gated on B220+, CD4−, CD38lo, GL7hi
(C) Representative flow plots and quantification of TFH cells in draining iLN on day 12. Live, single cells were gated on B220−, CD4+, CXCR5hi, PD1hi.
(D) Enumeration of BGC and TFH cells in axillary LNs on day 12.
(E) Total HIV MD39 Env-specific IgG Ab titers.
(F) HIV MD39 Env-specific IgG1 Ab titers.
(G) HIV MD39 Env-specific IgG2a Ab titers.
Statistical analysis (B-D) was performed by One-way Anova, followed by Dunnett’s post-test. Statistical analysis (E-G) was performed by Two-way Anova, followed by Benjamin, Krieger and Yekitieli’s post-test. Log-distributed datasets (E-G) were log-transformed before statistical analysis. *, p < 0.05; **, p < 0.01; ***, p < 0.001; ****, p < 0.0001.
Serum Ig responses were measured longitudinally after prime and booster immunization with MD39 HIV Env trimer and the same panel of diverse adjuvants. Analysis of Env-specific IgG responses revealed that the saponin adjuvants elicited the strongest IgG responses following the priming immunization, with a hierarchy in potency of SMNP > AS01B ~ ISCOMs (Fig. 2E). Following boosting, the saponin adjuvants, Ribi, and Addavax all elicited high total IgG titers, but IgG titers with SMNP were 7-fold higher than the next best comparator. SMNP was also superior over the other adjuvants for priming an IgG2a response (Fig. 2F-G). Comparing two most potent adjuvants for generating high IgG titers, SMNP and ISCOMs, the strongest plasmablast responses were elicited by SMNP (fig. S2C-D). SMNP additionally induced superior humoral responses to other vaccine antigens in separate experiments, including diphtheria toxin (DTx) and influenza hemagglutinin (fig. S2E-G).
We also compared SMNP formulations prepared using Quil-A versus QS-21, a purified sub-fraction of Quil-A saponin used in the licensed AS01B adjuvant. Both SMNP formulations elicited comparable BGC, TFH and MD39 Env-specific antibody responses (fig. S2H-J). We compared serum cytokine profiles and animal weight loss following immunization with QS-21 SMNP, ISCOMs, AS01B, or alum to gain insights into potential differences in toxicity. QS-21 SMNP induced transient elevations in proinflammatory cytokine and chemokine levels in circulation that were comparable to or less than AS01B (fig. S2K-L). AS01B also induced transient weight loss 24hrs after injection, which was not observed with SMNP (p<0.001, fig. S2M). Overall, these experiments suggest that saponin adjuvants are highly effective, with saponin/MPLA combination adjuvants promoting a broader range of antibody effector functions, and SMNP in particular capable of enhancing multiple facets of the humoral response elicited by subunit vaccination while maintaining a safety profile that appears comparable to an approved saponin adjuvant.
SMNP promotes antigen capture by cognate B cells
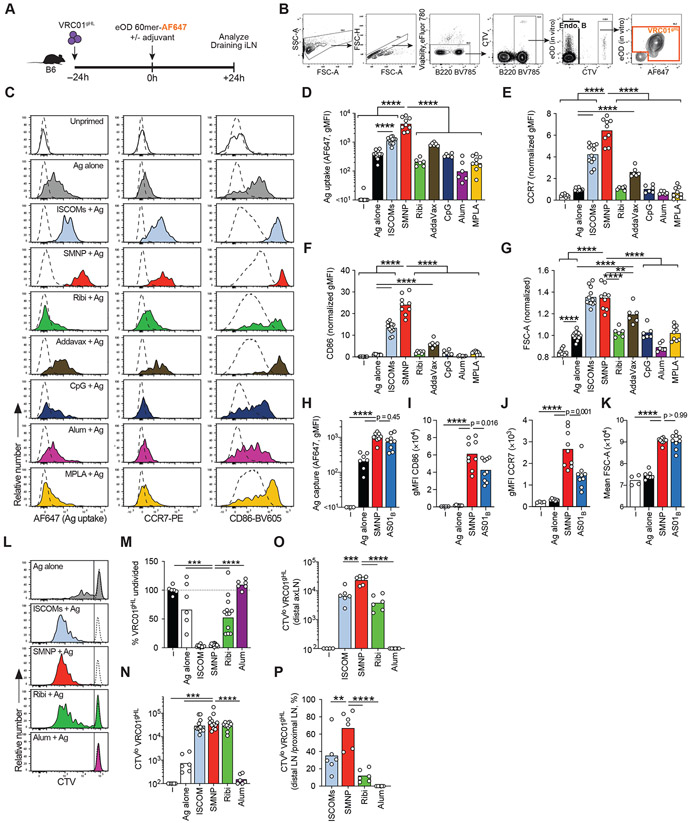
We next sought to test potential mechanisms of action underlying SMNP adjuvanticity. Based on the rapid induction of cytokines in dLNs, we suspected that SMNP might influence early B cell responses. To investigate this possibility, we made use of an adoptive transfer model in which B cells expressing the inferred germline-reverted VRC01 B cell receptor (VRC01gHL B cells) are seeded in C57BL/6 recipient mice at defined precursor frequencies, enabling assessment of antigen-specific B cell responses in vivo under challenging conditions designed to be relevant for vaccine regimens intended to induce HIV broadly neutralizing antibodies in humans (32-34). Mice adoptively transferred with VRC01gHL B cells were immunized with a 60mer form of the HIV gp120 immunogen eOD-GT5 (engineered outer domain germline-targeting, version 5, eOD-GT5 60mer) labeled with Alexa Fluor 647 (eOD-GT5 60mer-AF647) to assess antigen uptake and early activation of antigen-specific B cells in dLNs (Fig. 3A, B). This experiment models germline-targeting immunizations for HIV that are currently undergoing initial clinical trials (35). We compared the panel of adjuvants evaluated earlier in wild type mice, adding CpG as an additional comparator targeting a distinct Toll-like receptor (TLR9).
Figure 3. SMNP enhances antigen uptake, activation, and proliferation of B cells in draining LNs.
(A-G) 106 CTV+ VRC01gHL B cells were adoptively transferred into B6 mice prior to vaccination with eOD-60mer-AF647 with various adjuvants. Antigen uptake and activation of VRC01gHL B cells in draining iLNs was assessed at 24h post-immunization.
(A) Schematic of the experiment.
(B) Gating strategies to identify endogenous B cells (Endo B) and VRC01gHL B cells. Cells were stained in vitro using AF488-labeled eOD-60mer to identify VRC01gHL B cells. AF647 fluorescence indicates in vivo antigen uptake. Representative plots from B6 mice immunized with ISCOMs + eOD-60mer-AF647 are shown.
(C) Representative histograms showing VRC01gHL B cell phenotype in LN. The uptake of eOD-60mer was inferred from AF647 fluorescence (left column). Surface expression of early activation markers, CCR7 (middle) and CD86 (right), was assessed. Dotted line represents endogenous B cells.
(D) Quantification of AF647 uptake (geometric MFI) in LN.
(E) Quantification of CCR7 expression (geometric MFI normalized to Ag alone group) in iLNs.
(F) Quantification of CD86 expression (geometric MFI normalized to Ag alone group) in iLNs.
(G) Quantification of cell size (mean FSC-A normalized to Ag alone group) in iLNs.
(D-G) Each symbol represents a mouse and the bar represents the mean (e-g) or geometric mean (d). Pooled data from 2-4 independent experiments. n = 8 mice (unprimed; 4 experiments), n = 12 mice (Ag alone, ISCOM; 4 experiments), n = 9 mice (SMNP, MPLA; 3 experiments), n = 6 mice (AddaVax, Ribi, amph-CpG, Alum; 2 experiments).
(H-K) Antigen uptake and activation of VRC01gHL B cells in draining iLNs was assessed 24h after immunization with eOD-60mer-AF647 and SMNP, AS01B or no adjuvant.
(H) Quantification of AF647 uptake (geometric MFI).
(I) Quantification of CCR7 expression (geometric MFI).
(J) Quantification of CD86 expression (geometric MFI).
(K) Quantification of cell size (mean FSC-A).
(H-K) Each symbol represents a mouse and the bar represents the mean (K) or geometric mean (H-J). Pooled data from 2 independent experiments. N = 4 mice (unprimed), n = 7 mice (Ag alone), n = 9 mice (SMNP, AS01B).
(L-N) 106 CTV+ VRC01gHL B cells were adoptively transferred into B6 mice prior to vaccination with eOD-GT5 60mer with various adjuvants. VRC01gHL B cell proliferative responses in draining iLNs were assessed at d3.
(L) Representative histograms (tinted) showing VRC01gHL B cell proliferation following different immunizations. Unprimed VRC01gHL cells (dotted histograms) are overlaid. The vertical line divides CTVhi (undivided) and CTVlo (divided) populations.
(M) Normalized frequency of undivided VRC01gHL B cells in proximal draining (inguinal) LNs. Frequency of undivided VRC01gHL B cells of the total B cells were calculated for each mouse. Normalization was performed for each experiment by dividing the frequencies with the average (mean) in unimmunized mice, and the values were expressed as percentages.
(N) Quantification of divided VRC01gHL B cell numbers in draining inguinal LNs.
(O) Quantification of divided VRC01gHL B cell numbers in distal draining axillary LNs.
(P) Ratio of divided cells in the distal axillary LN vs the proximal inguinal LN.
(L-P) Each symbol represents a mouse and the bar represents the mean. (L-N) Pooled data from 2 independent experiments. n = 6 mice (unprimed; 4 experiments), n = 6 mice (Ag alone, alum; 2 experiments), n = 12 mice (ISCOM, SMNP; 4 experiments). (O, P) Pooled data from 2 independent experiments, n = 6 mice per group.
Statistical analysis was performed by One-way Anova, followed by Tukey’s post-test (D, H) or Dunnett’s post-test (E-G, I-P). **, p < 0.01; ***, p < 0.001; ****, p < 0.0001. Log-distributed datasets (D, H) were log-transformed before statistical analysis.
Injection of eOD-GT5 60mer-AF647 alone resulted in a moderate shift of AF647 fluorescence on VRC01gHL B cells, indicating that in vivo uptake of antigen within dLNs is inefficient in the absence of adjuvant (Fig. 3C left column, D). Most adjuvants did not alter the in vivo uptake of antigen by specific B cells (Fig. 3C, D). Notably, saponin-containing ISCOMs and SMNP promoted substantial antigen acquisition by VRC01gHL B cells in LNs (Fig. 3C, D), which was largely internalized (fig. S3A-D). SMNP resulted in a 10-fold increase in antigen uptake compared to the non-adjuvanted control. Free MPLA, or Ribi (containing 50 μg MPLA), did not impact antigen uptake by the B cells, in contrast to SMNP (Fig. 3C, D). VRC01gHL B cells robustly upregulated activation markers including CCR7, CD86, CD69, and CD44 in response to ISCOMs or SMNP vaccination, with SMNP triggering maximal rapid B cell activation compared to other adjuvants (Fig. 3C, E-F, fig. S3E-G). In addition, ISCOMs and SMNP exhibited a rapid induction of metabolic activity by the antigen-specific B cells, based on increased cell size, which was significantly greater than any of the other adjuvants tested (Fig. 3G, fig. S3E). SMNP had much weaker effects on polyclonal B cells (e.g., no cell size increase, Fig. 3C, fig. S3E, H-M), indicating the adjuvant predominantly enhanced cognate BCR-dependent activation. To rule out the possibility that other adjuvants might be as effective as SMNP at triggering B cell activation but with slower kinetics, we carried out 48 hr time course experiments of antigen acquisition and B cell activation. SMNP elicited higher overall antigen uptake and B cell activation signatures over time compared to non-saponin-containing adjuvants (fig. S3N-Q). Given the striking impact of SMNP on B cell antigen acquisition, we repeated these experiments including AS01B from the commercial Shingrix® vaccine, to assess if a liposomal form of saponin and MPLA would have similar effects. AS01B also increased early antigen-specific B cell uptake of antigen, activation, and increased metabolic activity (Fig. 3H-K). These data suggest that the capacity to promote antigen uptake by cognate B cells is a distinctive attribute of saponin-containing particulate adjuvants, and integration of MPLA amplifies this process in ways that are not accomplished by saponin or MPLA individually.
To explore adjuvant effects on B cell proliferation in vivo, CellTrace Violet (CTV)-labelled VRC01gHL B cells were tracked for three days post-immunization. Approximately 50% of CTV+ VRC01gHL B cells in dLNs remained undivided at d3 after immunization with eOD-60mer without adjuvant (Fig. 3L-M), and there was minimal accumulation of CTVlo (divided) VRC01gHL B cells (Fig. 3N). Alum did not promote early B cell proliferation (Fig. 3L-N), and 50% of the B cells did not proliferate in response to Ribi (Fig. 3L, M). In contrast, nearly all antigen-specific B cells proliferated in the presence of SMNP or ISCOMs, in an antigen-dependent manner (Fig. 3L-N, fig. S3R-T). SMNP also led to substantial proliferation of VRC01gHL B cells in distal axillary LNs (axLNs) (Fig. 3O). Interestingly, with SMNP, VRC01gHL B cell proliferation was similar in distal and proximal LNs (Fig. 3P). In sum, enhanced cognate B cell activation induced by saponin formulations subsequently leads to robust B cell proliferation in vivo, explaining an additional important mechanism of action of these adjuvants.
Saponin/MPLA combination adjuvants induce an antiviral TFH functionality profile
GCs and the development of most neutralizing antibodies depend on TFH cells. To explore effects of SMNP on CD4+ T cell responses, we used a similar adoptive transfer model approach, now employing T cell receptor (TCR) transgenic CD4+ T cells to track antigen-specific T cell responses. SMARTA CD4+ T cells specific for the lymphocytic choriomeningitis virus (LCMV) gp66–77 antigen were transferred into C57BL/6 recipient mice prior to immunization with eOD-GT5 60mer containing the LCMV gp66–77 epitope (eOD-GT5gp61 60mer) in the presence of SMNP or other adjuvants (Fig. 4A). Immunization with SMNP led to the greatest early (24h) upregulation of activation markers on SMARTA CD4+ T cells, including ICOS, IL-2Rα (CD25) and CD69, without affecting non-cognate T cells (Fig. 4B-C, fig. S4A-D). To assess T cell differentiation, we analyzed dLNs 4 days after immunization (Fig. 4D). Accumulation of SMARTA T cells at d4 was equivalent when immunizing with SMNP, ISCOMs, AS01B, or Ribi (Fig. 4E-F, fig. S4E). However, production of the cytokines IL-21 and IFN-γ by SMARTA non-TFH and TFH cells was notably increased by SMNP compared to the other adjuvants, including relative to the other saponin adjuvants ISCOMs and AS01B (Fig. 4G-J). IL-21 was particularly notable, as IL-21 is the signature cytokine of TFH cells, and detection of IL-21 production by antigen-specific TFH cells is typically very challenging (36, 37). In sum, SMNP potently enhances the quality of CD4+ T cell help provided to B cells.
Figure 4. SMNP enhances TFH differentiation and IL-21 production by CD4+ T cells in draining LN.
(A–C) 106 CTV+ SMARTA CD4+ T cells were adoptively transferred into B6 mice prior to vaccination with eODgp61-60mer with various adjuvants. Activation of SMARTA CD4+ T cells in draining iLN was assessed at 24h post-immunization.
(A) Schematic of the experiment.
(B) Representative histograms showing surface expression of ICOS (left) and CD25 (right) by SMARTA (solid line) and endogenous CD4+ T cells (dotted line).
(C) Quantification (geometric MFI) of ICOS (left) and CD25 (right). Each symbol represents a mouse, bars indicate the mean.
(B, C) Pooled data from two independent experiments. n = 3 mice (unimmunized) or n = 6 mice (no adjuvant, ISCOM, SMNP, Ribi).
(D–J) 2×104 SMARTA CD4+ T cells were adoptively transferred into B6 mice prior to vaccination with eODgp61-60mer with various adjuvants. SMARTA responses in iLNs were analyzed at d4 post-immunization.
(D) Schematic of the experiment.
(E) Quantification of total SMARTA CD4+ T cells.
(F) Quantification of CXCR5hi PD1hi SMARTA TFH.
(G–J) Cells were re-stimulated ex vivo with LCMV gp66–77 or irrelevant OVA323–339 to assess cytokine responses.
(G) SMARTA CD4+ T cells stimulated with LCMV gp66–77. Numbers in quadrants indicate the percentages.
(H) Quantification of IL-21+ cells as a percentage of SMARTA CD4+ T cells.
(I) Quantification of IFNγ + cells as a percentage of SMARTA CD4+ T cells.
(J) Quantification of IL-21+ cells as a percentage of SMARTA TFH.
(K) Quantification of IFNγ + cells as a percentage of SMARTA TFH.
(E-K) Pooled data from 3-5 independent experiments. Ag alone, n = 6 (Ag alone; 2 experiments), n = 15 (ISCOMs, SMNP; 5 experiments), n = 11 (Ribi; 4 experiments), n = 9 (AS01B; 3 experiments). Each symbol represents a mouse, bars represent the geometric mean (C, E, F) or the mean (H-K). **, p < 0.01; ***, p < 0.001; ****, p < 0.0001 by One-way Anova with Tukey’s test. Log-distributed data sets (E-F) were log-transformed before statistical analysis.
Saponin adjuvants promote antigen entry in lymph nodes and enhance lymph flow
Motivated by the findings of enhanced antigen acquisition, rapid B cell activation, and robust responses in distal LNs, we hypothesized that saponin adjuvants might impact antigen delivery and/or access into lymph nodes. We first examined antigen distribution within dLNs. Four hours post-injection, fluorescent 70 kDa dextran injected without adjuvant or with AddaVax was observed primarily in the subcapsular sinus and collagen conduits (Fig. 5A, fig. S5A). By contrast, dextran administered with SMNP exhibited widespread distribution deep into the LN parenchyma (Fig. 5A-C). Flow cytometric analysis revealed that SMNP was captured by numerous LN cell types, including CD169+ subcapsular sinus macrophages (SSM, Fig. 5D-E, fig. S5B). SMNP rapidly induced a >50% loss of CD169+ SSM in the dLN within 4h of injection (Fig. 5F). ISCOMs also induced SSM depletion, although to a lower extent, whereas other adjuvants had no effect on CD169+ SSM at this timepoint (Fig. 5F). Immunofluorescence imaging of dLN sections confirmed that injection of antigen (eOD-GT5 60mer) with SMNP was accompanied by loss of CD169+ cells in the subcapsular sinus (Fig. 5G-H). Injection of AS01B also caused a loss of CD169+ SSMs (fig. S5C), similar to previous reports showing that liposomal saponins can deplete this population (21). Prior studies have suggested that saponin adjuvant effects may involve inflammasome activation. However, neither the NLRP3 inflammasome nor non-canonical inflammasomes regulated by caspase-1 or caspase-11 were required for SMNP-mediated macrophage depletion (Fig. 5I).
Figure 5. SMNP adjuvant enhances lymph flow and promotes antigen delivery to draining lymph nodes.
(A) Histological images of fluorescent 70kDa dextran drainage to iLN 4h after subcutaneous base-of-tail injection in balb/C mice. Scale bar = 200 um.
(B) Quantification of fluorescent 70kDa dextran diffusion into the LN parenchyma 4h after injection in balb/C mice. Dextran intensity values were measured every 10um from LN surface. n = 6 mice per group. Each symbol represents an analyzed LN section. 9 LN sections were evaluated for Naïve, 7 for SMNP, and 13 for AddaVax. Data representative of 2 independent experiments.
(C) Area under curve (AUC) analysis for (B).
(D-E) C57Bl/6 mice were subcutaneously injected with Cy-5 labeled SMNP (SMNP-Cy5). Cellular uptake of Cy5 was assessed at 2h post-injection.
(D) Representative flow cytometry histograms of uptake of Cy5-labeled SMNP by the indicated cell type (tinted red histogram) in the draining iLN 2h after subcutaneous injection. The vertical line divides Cy5+ and Cy5− cells. The number indicates the frequency (percentage) of Cy5+ cells from a representative sample. The gate was set based on background signals from mice immunized with non-fluorescent SMNP (dotted histogram). Subcapsular sinus macrophage (SSM), medullary macrophage (MSM).
(E) Quantification of percent Cy5+ cells for each indicated cell type. Each symbol represents a mouse and bars indicate the mean. n = 6 mice for SMNP-Cy5, n = 3 mice for SMNP. Pooled data from two independent experiments. Gating strategy is depicted in fig. S5B.
(F) Flow cytometry analysis of CD169+ SSM remaining in the draining iLN 4hrs after subcutaneous adjuvant injection. n = 6 mice per group. Live single cells were gated on B220−, CD3−, Ly6G−, Ly6C−, CD11b+, CD169hi. Pooled data from 2 independent experiment. Each symbol represents a mouse and the bar represents the mean.
(G) Representative confocal images of CD169+ SSM in the draining LN at 24h after injection of adjuvants with eOD-GT5 60mer. CD169 staining is shown in red, IgD staining is shown in blue. Scale bar = 100μm.
(H) Quantification of loss of SSM from (G). Each symbol represents a mouse and bars represent the mean. n = 6 mice per group. Pooled data from two independent experiments.
(I) Casp-1/Casp-11 KO, NLRP3 KO, or WT C57Bl/6 mice were injected with 5ug of SMNP. 24hrs later, the draining LNs were harvested for flow cytometry analysis of SSMs. Each symbol represents a mouse. Bars represent the mean. n = 3 or 4 mice per group.
(J-K) B6 mice were pre-treated with clodronate liposome (CLL) to deplete CD169+ macrophages or with control liposome. 106 CTV+ VRC01gHL B cells were then adoptively transferred one day before immunization with eOD-60mer:AF647 alone or with SMNP.
(J) Quantification of antigen uptake by VRC01gHL B cells in draining iLNs at 24h post-immunization. Each symbol represents a mouse. Bars represent the geometric mean. Results from two independent experiments, n = 6 mice per group.
(K) Activation of VRC01gHL B cells as assessed by CCR7 expression (geometric MFI). Each symbol represents a mouse. Bars represent the mean. Results from two independent experiments, n = 6 per group.
(L-P) B6 mice were pre-treated with CLL or control liposome. 2×104 SMARTA CD4+ T cells were then adoptively transferred prior to vaccination with eOD-GT5gp61 60mer with SMNP.
(L) SMARTA CD4+ T cell numbers in iLNs at d4 post-vaccination.
(M) CXCR5hi PD1hi SMARTA TFH cell numbers in iLNs at d4 post-vaccination.
(N) Percentage of IL-21+ SMARTA CD4+ T cells upon ex vivo gp66-77 re-stimulation.
(O) Percentage of IFNγ + SMARTA CD4+ T cells upon ex vivo gp66-77 re-stimulation.
(P) Percentage of IL-21+ SMARTA TFH cells upon ex vivo gp66-77 re-stimulation.
For (C, E, F, H, I, J and K) statistical analysis was performed by One-way Anova, followed by Tukey’s post-test. Datasets in (K) were log-transformed before statistical analysis. For (L-P), statistical analysis was performed using Mann Whitney U test. *, p < 0.05; **, p < 0.01; ***, p < 0.001; ****, p < 0.0001.
To assess the impact of SSM depletion on the function of SMNP, we tested the effects of pre-depleting SSMs using clodronate liposomes (CLL) in the VRC01gHL adoptive transfer model. Pre-depletion of macrophages prior to injection of eOD-GT5 60mer alone enhanced antigen uptake and showed a trend toward enhanced early activation of VRC01gHL B cells in dLNs (Fig. 5J, K), consistent with the idea that CD169+ SSMs limit antigen access to naïve B cells in dLNs. By contrast, injection of eOD-GT5 60mer together with SMNP led to the same level of antigen uptake whether or not CLL was used to pre-deplete macrophages (Fig. 5J). Early VRC01gHL B cell activation was blunted when antigen and SMNP were administered after CLL (Fig. 5K), but subsequent serum antibody titers were not impacted (fig. S5D). We similarly assessed the impact of macrophage pre-depletion on responses to eOD-GT5 60mer and SMNP immunization in the SMARTA T cell adoptive transfer model. Overall SMARTA T cell proliferation and TFH expansion in response to SMNP immunization were not impacted by macrophage pre-depletion with CLL (Fig. 5L, M), but cytokine polyfunctionality of antigen-specific T cells was significantly reduced (Fig. 5N-P, fig. S5E). Thus, SMNP acting on lymph node macrophages does not have an obvious impact on early antibody responses, but does impact T cell polyfunctionality.
CLL treatment followed by eOD-GT5 60mer administered alone led to 2.6-fold lower antigen uptake by VRC01gHL B cells compared to eOD-GT5 60mer administered with SMNP (Fig. 5J, +CLL No Adj vs. −CLL SMNP), demonstrating that depletion of SSMs alone cannot explain the enhanced antigen acquisition observed with SMNP adjuvant. We thus next examined lymph flow and antigen accumulation in dLNs following injection of SMNP or other adjuvants. SMNP increased the rate of fluorescent antigen (DTx) clearance from the injection site 24h after immunization (Fig. 6A), implicating enhanced antigen drainage. Strikingly, transport of the lymphatic tracer indocyanine green (ICG) or fluorescent DTx to dLNs was increased by SMNP and ISCOMs, but not other adjuvants (Fig. 6B-D). Intravital imaging revealed that SMNP increased the diameter of lymphatic vessels draining the injection site within 1h after injection (Fig. 6E-F). Hence, uniquely among a panel of common vaccine adjuvants, saponin-containing adjuvants appear to alter lymphatic drainage. These data show that enhanced lymphatic drainage and disruption of LN CD169+ SSMs are two mechanisms of action of saponin adjuvants that contribute to enhanced antigen capture and B cell activation, with saponin/MPLA particulate adjuvants having the greatest impact on these events.
Figure 6. SMNP adjuvant enhances lymph flow in a mast cell-dependent manner.
(A) Fluorescent antigen (DTx) remaining at injection site 24hrs after injection. Wistar rats were injected with 20 μg of fluorescent antigen intradermally in 20 or 40 μl of PBS. Representative data from 2 independent experiments. (n=4 animals, 4 separate injection sites were evaluated in each animal per group).
(B-C). Balb/C mice were injected with 5 μg Indocyanine Green (ICG) dye in the footpad and the draining popliteal LN (pLN) was monitored. Shown are intravital images showing ICG drainage to popliteal lymph-node in mice 1hr following injection (B) and quantification of ICG in pLN (C). Representative data from 2 separate experiments. n=4 mice (no adj) or n=6 mice (adjuvant groups). Each symbol represents a mouse and the bar represents the mean.
(D) Quantification of DTx trafficking to iLN in balb/C mice 4hr after subcutaneous base-of-tail injection (one on each side) with 20ug of fluorescent DTx. Representative data from 2 separate experiments. n = 4 mice per group. Each symbol represents an individual iLN measurement (2 per mouse) and the bar represents the mean.
(E) Representative images of lymphatic vessels 1hr after subcutaneous injection. Scale bar = 50 μm.
(F) Quantification of lymphatic vessel diameter in (E). n = 6 mice per group. Each symbol represents one mouse and the bar represents the mean. Shown are results from 2 independent experiments.
(G) Quantification of ICG drainage into inguinal LNs of Balb/C mice 4 hrs after base of tail injection. A subset of mice were treated with antihistamine (diphenhydramine) just prior to ICG injection. Representative data from 2 separate experiments. n=4 mice (naive) or n=10 mice (ICG groups). Each symbol represents a mouse and the bar represents the mean.
(H) Quantification of ICG drainage into inguinal LNs of C57/b6 or cKitw-sh (mast cell-deficient) mice 4 hrs after base of tail injection. Representative data from 2 separate experiments. n=10 mice (C57b/6) or n=8 mice (cKitw-sh). Each symbol represents a mouse and the bar represents the mean.
(J-L) 106 CTV+ VRC01gHL B cells were adoptively transferred into B6 or cKitw-sh mice prior to vaccination with eOD-60mer-AF488 with SMNP. Antigen uptake and activation of VRC01gHL B cells in draining iLNs was assessed at 24h post-immunization.
(I) Quantification of eOD-60mer AF488 uptake (geometric MFI).
(J) Quantification of CCR7 expression (geometric MFI).
(K) Quantification of CD86 expression (geometric MFI normalized).
(I-K) Pooled data from 2 independent experiments. n = 7 (B6) or n = 8 (cKitw-sh). Each symbol represents a mouse, bars represent the geometric mean (I) or the mean (J and K).
(L-Q) 2×104 SMARTA CD4+ T cells were adoptively transferred into B6 or cKitw-sh mice prior to vaccination with eODgp61-60mer with SMNP. SMARTA responses in iLNs were analyzed at d4 post-immunization.
(L) Quantification of total SMARTA CD4+ T cells.
(M) Quantification of CXCR5hi PD1hi SMARTA TFH
(N–Q) Cells were re-stimulated ex vivo with LCMV gp66–77 or irrelevant OVA323–339 to assess cytokine responses.
(N) Quantification of IL-21+ cells as a percentage of SMARTA CD4+ T cells.
(O) Quantification of IFNγ + cells as a percentage of SMARTA CD4+ T cells.
(P) Quantification of IL-21+ cells as a percentage of SMARTA TFH cells.
(Q) Quantification of IFNγ + cells as a percentage of SMARTA TFH cells.
(N-Q) Pooled data from 2 independent experiments. n = 8 per group. Each symbol represents a mouse, bars represent the geometric mean (L, M) or the mean (N-Q). For (A, H-Q) statistical analysis performed by Mann-Whitney test. For (C -D, F-G) statistical analysis was One-way Anova, followed by Dunnett’s post-test. *, p < 0.05; **, p < 0.01; ***, p < 0.001; ****, p < 0.0001.
Given the unexpected finding of lymph flow alterations, we assessed candidate pathways that might be triggered by SMNP to impact lymphatic vessels. Nitric oxide (NO), VEGF-C, and histamine have been reported to impact lymph flow in certain conditions (38-41). We tested the effect of administering inhibitors of NO or histamine or blocking antibodies against VEGF-R together with SMNP and the lymphatic tracer dye ICG. While NO or VEGF inhibition had no effect on dye accumulation in dLNs, histamine inhibitors modestly reduced ICG uptake in the dLNs (Fig. 6G). As mast cells are key cellular producers of histamine, we next tested whether the lymph flow effects of SMNP were preserved in cKitW-sh mice, which have a mutation in the 5’ regulatory region of the Kit locus that leads to mast cell abolishment shortly after birth. The enhanced ICG accumulation in dLNs triggered by SMNP was ablated in mast cell-deficient animals (Fig. 6H). To determine if mast cells contributed to the enhanced antigen capture by cognate B cells in dLNs seen with SMNP, we evaluated cognate B cell responses in the VRC01gHL B cell adoptive transfer model. Absence of mast cells reduced uptake of eOD-GT5 60mer by ~2-fold (Fig. 6I), correlating with weaker VRC01gHL B cell activation (Fig. 6J, K). While expansion and TFH differentiation of CD4+ T cells was not impacted by mast cell deficiency (Fig. 6L, M), the cytokine polyfunctionality of antigen-specific TFH cells was reduced (Fig. N-Q). Together, these data suggest that mast cells are one cell population targeted by SMNP for initiating enhanced vaccine immune responses.
SMNP is a highly effective adjuvant in non-human primates
Given the effectiveness and distinctive mechanisms of action observed for SMNP in mice, we next sought to evaluate the potency of this new adjuvant in rhesus monkeys. We previously found that NHPs immunized with Quil-A ISCOMs plus a stabilized SOSIP-type HIV Env trimer based on MD39 (BG505 Olio6 (42)) made robust GC responses and anti-Env IgG binding titers, but little or no autologous tier 2 neutralizing antibodies (10), consistent with HIV Env being a difficult target for eliciting neutralizing antibodies (43). We therefore immunized NHPs with SMNP combined with the MD39 HIV Env trimer. Autologous tier 2 neutralizing antibody titers after SMNP + BG505 MD39 were elicited in all animals and were much higher compared to the previous study (p = 0.0014, Fig. 7A). These responses are comparable to the best BG505 autologous tier 2 neutralizing antibody titers reported to date following 3 immunizations in NHPs by a conventional (not slow delivery (9, 10)) immunization regimen (44-46). High quality neutralizing antibodies are generally the result of successful GC responses. Impressively robust Env-specific BGC responses were observed in lateral and central axillary LNs proximal to the immunization site (Fig. 7B-D, fig. S6A-D). Notably, Env-specific BGC were also induced in more distal pectoral and apical LNs, indicating broad recruitment of LNs by SMNP (Fig. 7B-D, fig. S6A-D), and consistent with the enhanced lymphatic drainage observed in mice. As a measure of the safety of SMNP, we immunized animals and collected serum for analysis of systemic cytokines/chemokines over 48 hr post injection. This data revealed transient elevations in IL-6, G-CSF, IL-10, and the chemokines MCP-1 and MIP-1β, and low levels of IFN-γ; however all of these responses returned to baseline by 48 hr (fig. S6E). TNF-α, IL-12, and MIP-1α did not rise above baseline levels (data not shown). Thus, SMNP is a potent and likely safe adjuvant in NHPs.
Figure 7. SMNP is a potent adjuvant in non-human primates.
(A–D) RMs were subcutaneously immunized in the deltoid area with soluble native-like Env trimer BG505 MD39 with SMNP at week 0, 10 and 24. LN necropsies were performed at week 30.
(A) Autologous BG505 pseudovirus ID50 neutralization titers assessed at week 26. Each symbol represents an animal, bars indicate the geometric mean. Previously published data (ref. (10)) of RMs subcutaneously immunized in the mid-thighs with soluble native-like Env trimer BG505 Olio6CD4ko with ISCOMs in the same schedule are shown as a reference. n = 6 animals per group. Data from one experiment.
(B) Schematic showing the cluster of axillary LNs (lateral, central, pectoral and apical LNs) draining the deltoid region (triangle) in NHPs with approximate distances from the injection site.
(C) Top panel, Representative FACS plots pre-gated on B cells, showing CD38− CD71+ total BGC responses. Bottom panel, MD39 Env-binding cells within the BGC gate are identified. Numbers indicate percentages.
(D) Quantification of MD39 Env+ BGC cells in draining LNs identified as in (C), expressed as number per 106 lymphocytes. n = 6 animals. Left and right LNs were separately examined. Each symbol represents one side of an animal. Data from one experiment.
(E) Schematic of experimental design for (F-M) to compare the adjuvanticity of CDN, amph-CpG, and SMNP in NHPs. RMs were immunized subcutaneously in each inner thigh with MD39-ferritin NPs along with the specified adjuvant at week 0, 10 and 24. n = 5 (no antigen), n = 6 (adjuvant groups).
(F) HIV MD39 Env binding titers at various time points post prime and boost immunization. Symbols represent mean values. Error bars are SEM.
(G) Antibody dependent cellular phagocytosis, neutrophil mediated phagocytosis, and complement deposition from purified Ab 25 wks post immunization. Data is displayed in arbitrary units (AU).
(H) ID50 autologous BG505 pseudovirus neutralizing titers analyzed 25wks post immunization.
(I, J) BGC responses of biopsied dLNs at (I) 6 and (J) 14 wks post immunization. Data is shown as percent of total B cells.
(K) HIV MD39 Env binding BGC cells 6 wks post immunization. Data is shown as percent of total BGC cells.
(L-M) TFH responses of biopsied dLNs at 6 and 14 wks post immunization. Data is shown as percent of total CD4+ T cells.
For (A, G-M), each symbol represents one animal and the bar represents the mean. Statistical analysis was performed by Mann-Whitney test (A), or One-way Anova, followed by Dunnett’s post-test (G-M). *, p < 0.05; **, p < 0.01; ***, p < 0.001; ****, p < 0.0001.
To gain further insight into the nature of immune responses elicited by SMNP in NHPs, we compared SMNP with two other adjuvants capable of promoting type I IFN responses, cyclic dinucleotides (CDNs) (47) and CpG (48). CDNs and CpG are potent adjuvants in mice and act on distinct innate pathways (STING or TLR9, respectively). An appropriate CDN capable of effectively stimulating NHP STING alleles was selected based on rhesus PBMC activation by CDN variants in vitro. 2’3’-c-di-AM(PS)2(Rp,Rp) was selected as the most potent inducer of cytokine release (fig. S6F). NHPs were vaccinated with a ferritin-based nanoparticle presenting 8 copies of MD39 Env trimer (MD39-NP) (49) combined with SMNP, CDN or CpG at weeks 0, 10, and 24 (Fig. 7E). Adjuvant alone controls were also included. Immunization with MD39-NP and CDNs or MD39-NP and CpG elicited no Env trimer-specific IgG until after the week 10 booster immunization (Fig. 7F). By contrast, immunization with MD39-NP and SMNP elicited substantial IgG titers following a single immunization, with all (6/6) animals seroconverted by week 8 (Fig. 7F). Env IgG titers remained ~100 to ~1000-times higher in animals that received SMNP following the second and third immunizations (Fig. 7F). SMNP also induced more polyfunctional humoral responses, evident by higher antibody-dependent monocyte and neutrophil phagocytosis, and complement deposition 25 weeks post-immunization (Fig. 7G). Additionally, SMNP induced significantly higher autologous neutralization titers compared to the other adjuvants (p < 0.01, Fig. 7H). Flow cytometry analysis of dLNs revealed robust BGC responses at week 6 following immunization with MD39-NP and SMNP, while BGC responses were near baseline in other conditions (Fig. 7I); SMNP-induced GC responses were also stronger than the other groups at week 14 (Fig. 7J). Similarly, vaccination with SMNP induced the most robust Env trimer-specific BGC cells (Fig. 7K). TFH responses were also significantly greater in animals that received SMNP compared to other adjuvants (Fig 7L-M). Taken together, these data demonstrate that SMNP is a potent adjuvant for humoral immunity in NHPs, consistent with outcomes and mechanisms of action in mice.
DISCUSSION
Saponins incorporated into particulate lipid carriers are safe for use in humans, and are now part of licensed vaccines and ongoing clinical development campaigns. Despite their clinical importance, their means of promoting humoral immunity remain poorly understood. Here, we analyzed immunological mechanisms of action underlying the potency of saponin-based adjuvants, and developed a new ISCOM-type adjuvant physically incorporating the TLR4 agonist MPLA. We found that compared to a broad panel of experimental and clinical alternatives, saponin-containing adjuvants exhibit superior potency, with the saponin/MPLA combination adjuvants SMNP and AS01B exhibiting the most robust humoral immune responses in mice. HIV Env trimers are poorly immunogenic, and single immunizations with various adjuvants were previously found to induce little or no Env-specific IgG in NHPs (9, 46, 50). Here, we found that vaccination with Env trimers combined with the saponin/MPLA adjuvant SMNP led to seroconversion of all animals following a single immunization and had the capacity to elicit excellent HIV neutralizing antibody titers. Mechanistically, we found that saponins are distinctive in their capability of inducing increases in lymphatic flow, antigen access to cognate B cells, secondary LN engagement, and improved TFH cell quality.
We found that saponin exhibited substantial synergy when combined with the TLR4 agonist MPLA. TLR agonists have long been viewed as important candidate vaccine adjuvants. Both MPLA and TLR9 agonists (CpG DNA) have reached approval as adjuvant components for licensed vaccines, and there remains high interest in TLR7/8 ligands as candidate adjuvants (51). Although some studies have suggested an advantage of TLR7/8 ligands over other TLR stimuli in NHPs (52, 53), this now seems less clear. For example, a recent study (54) found that a nanoemulsion formulation of TLR4 agonist outperformed alum/TLR7 agonist or TLR7 agonist in the same nanoemulsion, and alum/TLR4 agonist formulations were found to promote greater antibody titer durability than alum/TLR7 agonist (55). Further, a recent NHP study with a candidate COVID-19 subunit vaccine (3) showed that an alum/TLR7 agonist combination was worse than traditional alum in promoting neutralizing responses to SARS-CoV-2. TLR agonists have also been combined with ISCOMs in preclinical studies, and shown the potential for synergy with saponins (11, 12, 27, 56). However, unformulated TLR agonists, especially low molar mass compounds such as imidazoquinoline TLR7 ligands or CpG DNA, exhibit problematic pharmacokinetics with rapid dissemination into the bloodstream from injection sites that can trigger unacceptable systemic inflammation (48, 57). This has motivated a number of approaches to formulate TLR agonists in carriers that can help retain the adjuvants at the injection site and/or dLNs, including alum, oil-in-water emulsions, or formulation in nanoparticles (48, 52, 54, 55, 58-60). Such formulation strategies are capable of retaining the potent immunostimulatory capacity of the TLR agonist while achieving an acceptable safety profile in NHPs. This likely contributes to the success of liposomal AS01 adjuvants (6). SMNP’s nanoparticle form both promotes efficient lymphatic trafficking and enables synergy between saponin and MPLA at a very low dose of the TLR4 agonist; together these factors likely lead to the limited and transient systemic inflammation following parenteral immunization in mice or NHPs.
Vaccine adjuvants have often been reported to recruit phagocytic cells to injection sites, which subsequently acquire and transport antigen to draining lymph nodes (61-63). However, an effect of adjuvants on direct lymphatic transport of antigens has not been previously described. Using B cell adoptive transfers, we unambiguously show that SMNP strongly improves access of cognate B cells to intact antigen in dLNs. We found that AddaVax only had marginal effects on cognate B cell antigen uptake and activation, despite earlier studies implicating this adjuvant class in enhancing antigen accumulation in dLNs (62, 63). Despite the technical challenges involved, this finding underscores the value of analyzing antigen-specific B cells.
Disruption of CD169+ SSM may also contribute to enhanced antigen accumulation in dLNs by SMNP. CD169+ SSM capture lymph-born particulate antigens (64-66), which can be relayed to follicular B cells (64, 67-70). However, SSM can also limit lymph-born particulate antigens access to follicular B cells (71). Previous studies have shown adjuvants such as LPS and CpG can disrupt CD169+ SSM over the course of 4-7 days (68), but the only adjuvants tested herein capable of inducing substantial loss of CD169+ SSM within 24h—when antigen trafficking is most concentrated—were saponin-based adjuvants including SMNP and ISCOMs. This finding is consistent with prior work reported similarly rapid SSM loss in response to liposomal saponin (21).
SMNP had profound effects on cognate CD4+ T cell responses. CD4+ T cells primed in the presence of SMNP produced high levels of IL-21, a key TFH cytokine. IL-21 derived from TFH cells is important for B cell proliferation, isotype-switching and differentiation (72, 73). Robust early expression of IFN-α and IL-6 in dLN induced by SMNP likely drive this phenotype (74-77). Although compositionally-related AS01B also led to early expression of IFN-α and IL-6, SMNP induced substantially more IL-21+ TFH cells. The exact cell types induced to express IFN-α and/or IL-6 by SMNP and AS01B may underlie the difference. Notably, we found that SMNP also primed a substantial IFN-γ+IL-21+ TH1 population, which is important for controlling viral infections. High levels of ICOS and CD25 induced rapidly on cognate CD4+ T cells by SMNP likely facilitated efficient differentiation of highly polarized TFH and TH1 (73).
We have reported that immunization regimens enabling extended vaccine kinetics can also be used to substantially enhance humoral response to Env trimers (9, 10). Notably, these “extended delivery” methods are based on concepts related to the kinetics of adaptive immune responses and, as such, are complementary to mechanisms of action of adjuvants such as SMNP. Extended delivery immunizations still rely on the use of adjuvants, and we expect that more potent adjuvants such as SMNP will further enhance the efficacy of such approaches.
A limitation of these studies is the inherent inability to predict the safety profile of SMNP in humans without clinical testing. The licensed AS01B adjuvant provides a useful comparator, however. AS01B is comprised of saponin and MPLA inserted in 100 nm diameter liposomes at a ~1:1:20 saponin:MPLA:lipid mass ratio. The ~40 nm diameter ISCOMs “honeycomb cages” of SMNP are formed by a 1:0.1:0.1 mass ratio of the same components; both AS01B and SMNP are dosed in mice at 5 μg saponin. SMNP elicited transient serum cytokine elevations similar to or lesser than AS01B, and elicited no weight loss in mice. No injection site reactions or gross effects on animal behavior have been reported across more than 74 individual immunizations of NHPs with SMNP to date (including unpublished ongoing studies), suggesting no obvious safety issues. Hence, while safety must ultimately be determined by human testing, these preclinical studies are encouraging. A second limitation is that TLR4 expression exhibits some differences between mice and humans, with TLR4 expressed by naive mouse B cells but not human naive B cells (78). However, as we observed little or no detectable SMNP uptake by B cells in mouse lymph nodes (Fig. 5E), that species difference is not expected to be relevant for SMNP mechanisms of action. Further, the potent cytokine induction in human PBMCs in vitro and humoral responses elicited by SMNP in NHPs—where TLR expression is similar to humans—suggests this adjuvant is promising for human translation.
Overall, we have shown that SMNP is a potent and safe vaccine adjuvant that strongly promotes humoral immunity in mice and NHPs. SMNP augments the immune response via multiple mechanisms of action that synergize to enhance high quality humoral immune responses. These findings suggest SMNP is a promising candidate vaccine adjuvant for clinical translation.
Methods
Study design
The major objective of this study was to evaluate the adjuvant activity of ISCOMs-like saponin nanoparticles in the presence of absence of co-incorporated TLR4 agonist, comparing against well-known experimental and clinical adjuvants in small and large animal models, and to defined mechanisms of action underlying the action of these saponin-based adjuvants. Mice and non-human primates were immunized with clinically-relevant subunit protein immunogens combined with the saponin or alternate adjuvants, and both early (antigen uptake, TFH and germinal center induction) and later (serum antibody, plasmablast) responses were assessed over time. Mechanistic studies employed adoptive transfer models to allow a focused analysis of antigen acquisition and activation of antigen-specific B cells and T cells, as well as analysis of the immune response in mice genetically modified to lack certain key genes/cell populations or chemically treated to remove target cell subsets such as macrophages.
Mice and Rats
Mouse and rat experiments were performed at MIT or the La Jolla Institute for Immunology (LJI). All experimental procedures were approved by institutional IACUC committees. Experiments were done using sex- and age-matched female mice between 6-12 weeks of age. Balb/C mice (strain: 000671) and C57B/6J mice (strain: 000664) were purchased form the Jackson Laboratory. VRC01gHL mice, BCR transgenic knock-in mice carrying inferred germline reverted VRC01 IgH and IgL (34), were maintained on the B6.GFP (strain 004353; Jackson Laboratory), C57BL/6J or B6.SJL-Ptprcapepcb/BoyJ (B6.CD45.1) (strain 002014; Jackson Laboratory) backgrounds as homozygous lines at LJI. SMARTA mice were maintained on the B6.CD45.1 background at LJI. Mast cell deficient c-Kitw-sh mice (strain 030764; Jackson Laboratory) were used for lymphatic drainage mechanism studies. Female 12-16 wk old Wistar rats (the Jackson Laboratory) were used for imaging antigen drainage from intradermal injections.
Antigens
eOD-GT5 60mer, a self-assembling protein nanoparticle formed by the fusion of the eOD-GT5 antigen with lumazine synthase, was generated as previously described(34, 79). eOD-GT5 has a monovalent binding affinity for VRC01gHL Fab of KD ~250 nM, which is near the high end of the spectrum of affinities of eOD-GT8 for naive VRC01-class precursors in HIV-uninfected humans(34). eOD-GT5 60mer was fluorescently labeled with Alexa Fluor 647 (ThermoFisher; A20173) according to the manufacturer’s protocol. In some experiments, we used eOD-GT5 60mer fused with the LCMV gp61-80 helper epitope (GLKGPDIYKGVYQFKSVEFD) to track CD4+ T cell responses. For this immunogen, a GGS linker (GGSGGSGGSGGSGGG) between the N-terminal lumazine synthase and C-terminal eOD was replaced with the LCMV gp61-80 sequence. All eOD constructs were analyzed by SEC-MALS and SDS-PAGE gels. 60mers were injected at a dose of 5-10 μg per mouse. MD39 HIV Env trimer was generated as previously described(31) and was administered at a dose of 2 μg per animal. Diphtheria toxin CRM197 was purchased from Scarab Genomics LLC (CRM197-10) and was administered at 2 ug per animal. Trimeric hemagglutinin (HA) ectodomain from A/New Caledonia/20/1999 (NC99) was expressed in 293F cells and purified as described previously(80, 81). Mice were immunized with 3.5 μg of HA trimer.
Adjuvants
ISCOMs adjuvant was prepared as previously described(9). Briefly, 20 mg/ml solutions of cholesterol (Avanti Polar Lipids Cat# 700000) and DPPC (Avanti Polar Lipids Cat# 850355) were prepared in Milli-Q water containing 20% w/vol MEGA-10 (Sigma D6277) detergent. Quil-A saponin (InvivoGen; vac-quil) was dissolved in Milli-Q water at a final concentration of 100 mg/ml. All components were mixed at a molar ratio of 10:10:5 (Quil-A:chol:DPPC) followed by dilution with PBS to a final concentration of 1 mg/ml cholesterol. The solution was allowed to equilibrate overnight at room temperature, followed by dialysis against PBS using a 10k MWCO membrane. The adjuvant solution was then sterile filtered, concentrated using 50k MWCO centricon spin filters, and further purified by FPLC using a Sephacryl S-500 HR size exclusion column. SMNP was formulated in a similar manner but included MPLA (Avanti Polar Lipids 699800P) at a molar ratio of 10:10:2.5:1 (Quil-A:chol:DPPC:MPLA). SMNP QS-21 was prepared in a similar manner, substituting QS-21 for Quil-A. QS-21 was purchased from DesertKing.com. SMNP Cy5 was produced in a similar manner to SMNP, substituting the lipid DPPC for DSPC-Cy5. Doses of ISCOMs and SMNP are reported in terms of the amount of saponin administered, calculated by measuring the concentration of cholesterol (Cholesterol Quantitation kit; Millipore Sigma; Cat# MAK043) in the preparation and assuming quantitative incorporation of the saponin during synthesis.
All adjuvant and antigens were prepared in sterile PBS, mixed, and injected bilaterally at the base of tail in 50-100 μL volume per injection site (100-200 μL per mouse). For ISCOMs and SMNP, each mouse received a dose corresponding to 5 μg Quil-A (corresponding to 0.5 μg MPLA for SMNP). AS01B was taken from licensed Shingrix vaccine doses; the adjuvant is packaged as pure AS01b in a separate vial from the antigen. Each mouse received 1/10th the human dose of AS01B, containing 5 μg QS-21 and 5 μg MPLA. This dose has been previously reported and is in line with FDA animals-to-human dosing conversion (82, 83). Ribi (Sigma Adjuvant System, Sigma-Aldrich; Cat# S6322), AddaVax (InvivoGen; Cat# vac-adx-10), or Alum (Alhydrogel adjuvant 2%, InvivoGen; Cat# vac-alu-250) were mixed 1:1 vol:vol with antigen prepared in sterile PBS. At this dose, Ribi contains 25-50 μg MPLA per mouse. In some experiments, 0.5-5 μg free MPLA (PHAD, Avanti Polar Lipids, Cat # 699800) or 1 μg LPS-B5 from E. coli 055:B5 (InvivoGen; Cat# tlrl-pb5lps) was injected in control mice. Amph-CpG 1826 was produced as previously described (48) and used at 1.24 nmol per mouse. CpG 1826 (Invivogen; Cat# vac-1826-1) was used at 50 ug per mouse.
Biophysical characterization of SMNP
Dynamic light Scattering (DLS) images were acquired using a DynaPro Dynamic Light Scatterer. Nanoparticle images were acquired using Transmission Electron Microscopy (TEM) on a JEOL 2100F microscope. The microscope was operated at 200 kV with a magnification range of 10,000-60,000X. All images were recorded on a Gatan 2kx2k UltraScan CCD camera. Size exclusion chromatography (SEC) was performed with an AKTA Pure FPLC system (GE Healthcare) using a Sephacryl S-500 HR column.
Adoptive transfer of T and B cells
B cells were purified from spleens using CD43 MACS MicroBeads (Miltenyi Biotec, Cat# 130-049-801) following the manufacturer’s protocol. Purity of VRC01gHL B cells (eOD-binding, B220+) purified from homozygous VRC01gHL mice was ~90%. Cell numbers for adoptive transfer were adjusted according to the purity of VRC01gHL B cells. SMARTA CD4+ T cells were purified from the spleens of SMARTA mice using the EasyStep Mouse CD4+ T Cell Isolation Kit (STEMCELL, Cat# 19852) according to the manufacturer’s protocol. Purity of SMARTA CD4+ T cells (CD4+ TCR Vα2+) typically exceeded 95%. Purified B or T cells were adoptively transferred via retro-orbital injection in 200 μL RPMI supplemented with 1% FCS 1-2d before vaccination. To examine VRC01gHL B cell responses in vivo, 106 VRC01gHL B cells from homozygous VRC01gHL mice were adoptively transferred. To examine SMARTA CD4+ T cells, 2×104 or 106 SMARTA CD4+ T cells were adoptively transferred.
In some experiments, purified B or T cells were labeled with CellTrace™ Violet (CTV) (ThermoFisher). For CTV labeling, B cells resuspended at 107 cells/mL in DPBS supplemented with 0.1% bovine serum albumin (Sigma-Aldrich) were incubated with 5 μM CTV for 9.5 minutes at 37°C. The reaction was quenched using 3x volume of ice-cold fetal calf serum (FCS), and washed once in RPMI supplemented with 5% FCS (RP5).
Mouse flow cytometry analyses
Single cell suspensions were generated by mechanical dissociation of spleens and lymph nodes. Erythrocytes in spleens were removed by washing cells in Gibco™ Ammonium-Chloride-Potassium (ACK) lysing buffer (ThermoFisher) at 4°C. To analyze CD169+ macrophages or dendritic cells, splenocytes were digested with liberase TM (Roche, Cat# 5401127001) and DNase I (Roche, Cat# 10104159001) at 20 μg/mL and 70 μg/mL, respectively, for 20 min at room temperature. Antibody staining was performed in FACS buffer (PBS, 1% BSA, 0.02% NaN3, 2mM EDTA). Fc-mediated binding was blocked using 1% normal rat serum (STEMCELL) and 2.5 μg/mL purified anti-CD16/32 (93; Biolegend) at 4°C for 15 min, prior to performing staining with primary antibodies. Staining panels that included CXCR5 or CCR7 was performed at room temperature for 45 min. All other surface staining was performed at 4°C for 30 min. To assess BGC cells, cells were stained with GL7 PerCPCy5.5 (GL7; Biolegend), CD38 AF488 (90; Biolegend), B220 PECy7 (RA3-6B2; Biolegend) and CD4 BV711(GK1.5; Biolegend). To assess TFH cells, cells were stained with CD4-BV711 (GK1.5; Biolegend), B220-PECy7 (RA3-6B2; Biolegend), CXCR5-PE (L138D7; Biolegend), PD1-BV421 (29F.1A12; Biolegend) To assess early B cell activation, cells were stained with B220-BV785 (RA3-6B2; Biolegend), CCR7-PE (4B12; Biolegend), CD86 BV605 (GL-1; Biolegend), CD69-PECy7 (H1.2F3; Biolegend), CD44-PerCPCy5.5 (IM7; Biolegend), and CD4 AF700 (RM4.4; Biolegend). eOD-GT8 60mer was generated in-house and conjugated to Alexa Fluor 488 (ThermoFisher; A10235) to detect VRC01gHL B cells. To assess early T cell responses, cells were stained with CD4 AF700, B220 BV785, CD69 PECy7, CD62L BV711 (Mel-14; Biolegend), CD25 PerCPCy5.5 (3C7; Biolegend), ICOS BB515 (7E.17G9; BD), CD44 BV605 (IM7; Biolegend) and CD45.1 BV510 (A20; Biolegend). Dead cells were stained using Fixable Viability Dye eFluor™ 780 (ThermoFisher). To analyze SMARTA CD4+ T cell cytokine responses, single cell suspension was prepared in sterile D10 (DMEM, 10% FCS, Gibco GlutaMAX, Gibco Penicillin-Streptomycin). 2-5×106 cells were restimulated with either LCMV gp66-77 or non-specific OVA323-339 at 2 μg/mL in the presence of GolgiPlug and BME in 300 μL D10 using F-bottom 96-well plates for 5h at 37C. Cells were then surface stained with CXCR5-biotin, CD4-AF700, CD45.1 BV510, B220 BV785, SA-PECy7, PD1 BV605 (29F.1A12; Biolegend), and Viability Dye e780. Cells were then fixed using BDCytofix/Cytoperm for 15min at 4C, then intracellularly stained with recombinant mouse IL-21R-Fc Chimera Protein (R&D System) for 60min at room temperature. After washing, cells were intracellularly stained with F(ab’)2 goat anti-human IgG PE (Jackson) and IFN-γ FITC. Background cytokine responses (OVA323-339 stimulation) were subtracted from LCMV gp66-77 stimulated samples Data acquisition was performed using FACSCelesta™ (BD Bioscience) and analyzed using FlowJo 10 (Tree Star).
Measurement of lymphatic trafficking and antigen distribution in LNs
Noninvasive fluorescence imaging of lymphatic drainage with Indocyanine Green (ICG): 10 μL aqueous ICG solution (0.5 mg/mL) was injected subcutaneously in the hind footpad of mice with or without the specified adjuvant. 1hr after the injection, the lymphatic clearance of ICG from the injection site into the popliteal lymph node was visualized noninvasively through intact mouse skin. An 808-nm continuous wave laser (CNI lasers, MDL-N-808) was used to illuminate the imaging area with a 50 mW/m2 power density. Fluorescence images were captured by an InGaAs SWIR camera (Princeton Instrument OMA V) with a 1,100-nm long-pass filter at 200 ms exposure time. ImageJ was used to calculate intensity values in the popliteal LN region of interest.
Imaging of antigen drainage to LNs using ICG or fluorescent proteins: DTx was labeled with Alexa-fluor 750 (ThermoFisher: A20111) according to the manufacturer’s instructions. 20 μg of fluorescent protein was injected subcutaneously with or without the specified adjuvant at the tail base of Balb/C mice in 50 ul volume. 5 hrs post-injection the draining inguinal LN was harvested and imaged using an IVIS imaging system (PerkinElmer: 124262) with imaging parameters optimized for Alexa-fluor 750 (Ex: 745, Em: 820). Living Image (PerkinElmer) was used to calculate total radiant efficiency values at each LN. IVIS imaging of draining lymph nodes was also performed on mice receiving adjuvants combined with 10 μL aqueous ICG solution (0.5 mg/mL). For some experiments, mice received pharmacological blockade of NO (1400W, 10mg/kg intraperitoneal injection; Tocris), VEGF (anti-VEGF receptor Ab, 20mg/kg intravenous injection; BioXcel DC101) or histamine (diphenhydramine hydrochloride, 10mg/kg intraperitoneal injection; Tocris) prior to ICG/SMNP injections.
Imaging of antigen drainage from injection site: Female 12-16 wk old Wistar rats (the Jackson Laboratory) were shaved then injected intradermally (ID) in the dorsal paraspinal region with 20 μg of DT-750 in 20 μL PBS with or without 5 μg of SMNP. Rats were anesthetized with isoflurane and imaged (Ex: 745, Em: 820) immediately and 24h post injection using an IVIS Spectrum bioluminescent and fluorescent imaging system (Perkin Elmer). Total radiant efficiency per injected area was analyzed with Living Image software (Perkin Elmer).
Measurement of lymphatic diameter
Lymphatic vessel diameter measurements were performed as previously described(84, 85). Mice were anesthetized with 250 μl of 100 mg/ml Ketamine/xylazine. A 5 μg injection of SMNP was administered in the right footpad followed by a 2 μl injection of 2% w/v FITC-dextran (2 million molecular weight; Sigma Aldrich cat # FD2000S) 1hr later in the same site. The afferent collecting lymphatic vessels draining to the popliteal lymph node (PLV) were surgically exposed and imaged. Intravital images of the exposed lymphatic vessels were captured with an inverted fluorescence microscope (Olympus IX70 Inverted Epi-Fluorescence Microscope).
ELISA
Serum anti-MD39 Env antibody ELISAs were performed as previously described (86). For anti-DTx and NC99 Influenza ELISAs, antigen was coated on MaxiSorp plates (ThermoFisher) at 2 ug/ml. Mouse sera was diluted in 2% BSA block starting at 1:100 with 3x serial dilutions and incubated for 1 hr, followed by detection with 1:5000 goat anti-mouse IgG-HRP, IgG1-HRP, or IgG2a-HRP conjugate in block. Titers are reported as inverse dilutions giving an 0.2 HRP absorbance.
Cytokine analysis
Draining LN cytokine measurements were performed using a LEGENDplex anti-virus response panel (Biolegend: 740622) following the manufacturer’s instructions. Serum cytokine samples were submitted for analysis by Eve Technologies using a 31-plex cytokine/chemokine array (MD31).
Subcapsular sinus macrophage depletion
For SSM depletion, clodronate or control liposomes (Encapsula NanoSciences) were injected subcutaneously 6 days prior to immunization according to the manufacturer’s instructions.
NHP studies
For studies in Fig. 7A-D, male and female Indian rhesus monkeys (RMs, Macaca mulatta) were housed at the Yerkes National Primate Research Center and were maintained in accordance with NIH guidelines. This study was approved by the Emory University Institutional Animal Care and Use Committee (IACUC). Animals were between 4 – 6.5 years of age at time of 1st immunization. Animals were immunized at 3 time points: week 0, week 10, and week 24. All immunizations were given subcutaneously in the left and right deltoid with 50μg of MD39 and 375ug of SMNP adjuvant per side.
At the end of the 30-week study, animals were sacrificed. Clusters of draining axillary lymph nodes were harvested and defined based on their location: lateral, central, pectoral or apical. For each animal, 1 lateral, 1 central, 1 pectoral, and 2 apical/subclavicular lymph nodes were collected from each side. To generate fluorescent antigen probes, biotinylated MD39-avi was tetramerized with fluorescently labeled streptavidin (Alexa Fluor 647 and BV421) at RT for 20 minutes. For the BGC panel, cells were incubated with the probes for 30 minutes at 4°C and then with surface antibodies [CD4 (APCe780; SK3; ThermoFisher), CD8α (APCe780; RPA-T8; ThermoFisher), CD16 (APCe780; eBioCB16; ThermoFisher), CD20 (Alexa Fluor 488; 2H7, Biolegend), IgG (PE-Cy7; G18-145; BD Biosciences), IgM (PerCPCy5.5; G20-127; BD Biosciences), CD38 (PE; OKT10; NHP Reagents), CD71 (PE-CF594; L01.1; BD Biosciences)] and Fixable Viability Dye e780 (ThermoFisher) for an additional 30 minutes. Validation of CD38 and CD71 as surface markers for BGC cells were previously described(10). For the GC-TFH panel, cells were incubated with surface antibodies [CD4 (BV650; OKT4; Biolegend), CD8 (Qdot705; 3B5; ThermoFisher), CD20 (PE-TexasRed; B9E9; Beckman), CXCR5 (PE; MU5UBEE ThermoFisher), PD1 (BV605; EH12.2H7; Biolegend), CD3 (BV786; SP34-2; BD Biosciences)] and Fixable Viability Dye e506 (ThermoFisher) for 30 minutes at 4°C, washed twice, and fixed with 1% paraformaldehyde. The samples stained for BGC were acquired and bulk sorted on a FACSAria II (BD Biosciences). The samples stained for GC TFH were acquired on LSR Fortessa (BD Biosciences). BGC and GC TFH data reported are normalized to the number of lymphocytes gated to account for the varying sizes and numbers of harvested lymph nodes.
For studies in Fig. 7E-M, male Indian rhesus monkeys (RMs, Macaca mulatta) were housed at the Southwest National Primate Research Center and were maintained in accordance with NIH guidelines. This study was approved by the Texas Biomedical Research Institutional Animal Care and Use Committee (IACUC). Animals were between 6.8-10.1 years of age at time of 1st immunization. Animals were immunized at 3 time points: week 0, week 10, and week 24. All immunizations were given subcutaneously in the left and right inner thigh (1mL per injection site).
MD39 Ferritin nanoparticles were produced as previously described(49) and were administered at a dose of 50 μg s.c. in the left and right inner thigh (100 ug per animal). Adjuvants used were amph-CpG (250 ug per site, 500 μg total per animal), cyclic dinucleotide (2’3’-c-di-AM(PS)2, 50 μg per site, 100 ug total per animal) or SMNP adjuvant (187.5 μg per site, 375 ug total per animal). Inguinal lymph node biopsies were collected at weeks 6 and 14 for flow cytometry analysis.
Fluorescent MD39 or Ferritin probes were generated by combining biotinylated antigen with fluorescent streptavidin (APC and BV605) at RT for 20 min. For the BGC panel, cells were incubated with fixable viability dye Live/Dead Acqua (ThermoFisher) for 20 minutes, followed by the probes for 30 minutes at 4°C and then with surface antibodies [CD3 (AF700; SP34-2; BD Bioscience), CD20 (BUV737; 2H7, Biolegend), IgG (PE-Cy5; G18-145; BD Bioscience), IgM (FITC; G20-127; BD Bioscience)]. Subsequently cells were fixed with True-Nuclear transcription factor buffer set (Biolegend) following manufacturer instructions, and stained with intracellular antibodies [Bcl-6 (PE; K112-91; BD Bioscience), Ki67 (BV421; B56, BD Bioscience)]. For the GC-TFH panel, cells were incubated with surface antibodies [CD3 (AF700; SP34-2; BD Bioscience), CD4 (APC-Cy7; OKT4; Biolegend), CD8a (BV711; RPA-T8; Biolegend), CXCR5 (PE-Cy7; MU5UBEE, Biolegend), PD1 (BV785; EH12.2H7; Biolegend)] and fixable viability dye Live/Dead Aqua (ThermoFisher) for 30 minutes at 4°C, washed twice, and fixed with 1% paraformaldehyde. All samples were acquired on LSR Fortessa (BD Biosciences).
Serum samples were collected at weeks 0, 6, 8, 10, 11, 12, 14, 24, 25 and 26. Antibody titers were measured by ELISA as follows: MAXIsorp 96 well plates (ThermoFisher) were coated with 1 ug/mL of rabbit anti-his tag antibody (GenScript) in PBS, followed by his-tagged MD39 trimer at 1 ug/mL. Sera was diluted in 2% BSA block starting at 1:50 with 4x serial dilutions and incubated for 2 hr, followed by detection with 1:5000 goat anti-human IgG-HRP (Jackson ImmunoReserach). Alternatively, samples were detected using anti anti-Rhesus IgG1, IgG2 or IgG3 (NHP Reagent Resource), followed by anti-mouse IgG-HRP in block. Titers are reported as inverse dilutions giving an 0.2 HRP absorbance.
Statistical Analysis
Statistical analyses were performed using GraphPad Prism v8. Bars plotted in linear scale represent the mean. Bars plotted in log scale represent the geometric mean. Symbols in bar graphs represent individual mice unless otherwise stated in figure legends. Data sets were tested for normality log-distributed datasets were transformed to linear scale before performing statistical analysis. Multiple group comparisons were performed by one-way ANOVA followed by a post hoc test specified in figure legends. Data containing two independent variables were analyzed using two-way Anova, followed by Benjamin, Krieger and Yekitieli’s post-test. Data were considered to be statistically significant at * p < 0.05, ** p < 0.01, *** p < 0.001, and **** p < 0.0001.
Supplementary Material
Acknowledgments
We thank the Koch Institute Swanson Biotechnology Center for technical support, specifically the Flow Cytometry, Microscopy, and Dong Soo Yun for collecting TEM images at the Nanotechnology Materials Core Facilities. We thank Elizabeth Curran and Dr. Sanjeev Gumber at the Yerkes National Primate Research Center for their expert veterinary services and knowledge. We thank Christina Corbaci for graphical design. We thank the staff of LJI Microscopy and Histology Core Facilities for their support with obtaining imaging data presented in this paper. Zeiss LSM880 was supported by NIH S10OD021831.
Funding:
This work was supported in part by the National Institute of Allergy and Infectious Diseases of the NIH under award numbers UM1AI100663 (D.R.B., W.R.S., G.S., S.C., and D.J.I.), UM1AI144462 (D.R.B., W.R.S., G.S., S.C., and D.J.I.), R01CA214913 (T.P.P.), R01AI125068 (S.C. and D.J.I.), R01AI137057 (D.L.), R01AI153098 (D.L.), P01AI104715 (D.J.I.), and P01AI048240 (R.M.R. and D.J.I.), the Marble Center for Cancer Nanomedicine (A.M.B. and D.J.I.), the U. S. Army Research Office through the Institute for Soldier Nanotechnologies at MIT under Cooperative Agreement Number W911NF-18-2-0048 (D.J.I.), the Koch Institute Support (core) Grant P30-CA14051 from the National Cancer Institute (A.M.B. and D.J.I.), the IAVI Neutralizing Antibody Center (NAC) (W.R.S. and D.R.B.), the Bill and Melinda Gates Foundation Collaboration for AIDS Vaccine Discovery funding for the IAVI NAC (W.R.S. and D.R.B.), and the Ragon Institute of MGH, MIT, and Harvard (G.A., D.L., D.R.B., W.R.S., and D.J.I.). Yerkes National Primate Research Center is supported by the base grant P51 OD011132. M.S. is an NRSA Fellow (T32 AI07386). D.J.I. is an investigator of the Howard Hughes Medical Institute.
Footnotes
Competing interests: The authors do not have competing financial interests related to this work. Intellectual property on SMNP has been filed (International Patent Application No. PCT/US2019/041580).
Data and materials availability: All data needed to evaluation the conclusions in the paper are present in the paper or the Supplementary Materials.
References
- 1.Rao M, Alving CR, Adjuvants for HIV vaccines. Current Opinion in HIV and AIDS 11, 585–592 (2016). [DOI] [PubMed] [Google Scholar]
- 2.Noe AR, Kotraiah V, Gutierrez GM, Enabling Vaccine Delivery Platforms and Adjuvants for Malaria. Current Topics in Malaria, 387 (2016). [Google Scholar]
- 3.Arunachalam PS, Walls AC, Golden N, Atyeo C, Fischinger S, Li C, Aye P, Navarro MJ, Lai L, Edara VV, Roltgen K, Rogers K, Shirreff L, Ferrell DE, Wrenn S, Pettie D, Kraft JC, Miranda MC, Kepl E, Sydeman C, Brunette N, Murphy M, Fiala B, Carter L, White AG, Trisal M, Hsieh CL, Russell-Lodrigue K, Monjure C, Dufour J, Spencer S, Doyle-Meyers L, Bohm RP, Maness NJ, Roy C, Plante JA, Plante KS, Zhu A, Gorman MJ, Shin S, Shen X, Fontenot J, Gupta S, O'Hagan DT, Van Der Most R, Rappuoli R, Coffman RL, Novack D, McLellan JS, Subramaniam S, Montefiori D, Boyd SD, Flynn JL, Alter G, Villinger F, Kleanthous H, Rappaport J, Suthar MS, King NP, Veesler D, Pulendran B, Adjuvanting a subunit COVID-19 vaccine to induce protective immunity. Nature 594, 253–258 (2021). [DOI] [PubMed] [Google Scholar]
- 4.Lvgren Bengtsson K, Morein B, Osterhaus ADME, ISCOM technology-based Matrix M\texttrademark adjuvant: success in future vaccines relies on formulation. Expert review of vaccines 10, 401–403 (2011). [DOI] [PubMed] [Google Scholar]
- 5.Ragupathi G, Gardner JR, Livingston PO, Gin DY, Natural and synthetic saponin adjuvant QS-21 for vaccines against cancer. Expert Rev Vaccines 10, 463–470 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Didierlaurent A, Berger A, Heineman T, Henderickx V, Da Silva FT, Vekemans J, Voss G, Garçon N, in Immunopotentiators in Modern Vaccines. (Elsevier, 2017), pp. 265–285. [Google Scholar]
- 7.Morein B, Sundquist B, Hglund S, Dalsgaard K, Osterhaus A, Iscom, a novel structure for antigenic presentation of membrane proteins from enveloped viruses. Nature 308, 457–460 (1984). [DOI] [PubMed] [Google Scholar]
- 8.Takahashi H, Takeshita T, Morein B, Putney S, Germain RN, Berzofsky JA, Induction of CD8+ cytotoxic T cells by immunization with purified HIV-1 envelope protein in ISCOMs. Nature 344, 873–875 (1990). [DOI] [PubMed] [Google Scholar]
- 9.Pauthner M, Havenar-Daughton C, Sok D, Nkolola JP, Bastidas R, Boopathy AV, Carnathan DG, Chandrashekar A, Cirelli KM, Cottrell CA, Elicitation of robust tier 2 neutralizing antibody responses in nonhuman primates by HIV envelope trimer immunization using optimized approaches. Immunity 46, 1073–1088. e1076 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cirelli KM, Carnathan DG, Nogal B, Martin JT, Rodriguez OL, Upadhyay AA, Enemuo CA, Gebru EH, Choe Y, Viviano F, Nakao C, Pauthner MG, Reiss S, Cottrell CA, Smith ML, Bastidas R, Gibson W, Wolabaugh AN, Melo MB, Cossette B, Kumar V, Patel NB, Tokatlian T, Menis S, Kulp DW, Burton DR, Murrell B, Schief WR, Bosinger SE, Ward AB, Watson CT, Silvestri G, Irvine DJ, Crotty S, Slow delivery immunization enhances HIV neutralizing antibody and germinal center responses via modulation of immunodominance. Cell 177, 1153–1171.e1128 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sundling C, Forsell MN, O'Dell S, Feng Y, Chakrabarti B, Rao SS, Lore K, Mascola JR, Wyatt RT, Douagi I, Karlsson Hedestam GB, Soluble HIV-1 Env trimers in adjuvant elicit potent and diverse functional B cell responses in primates. J Exp Med 207, 2003–2017 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martinez P, Sundling C, O'Dell S, Mascola JR, Wyatt RT, Karlsson Hedestam GB, Primate immune responses to HIV-1 Env formulated in the saponin-based adjuvant AbISCO-100 in the presence or absence of TLR9 co-stimulation. Sci Rep 5, 8925 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ols S, Yang L, Thompson EA, Pushparaj P, Tran K, Liang F, Lin A, Eriksson B, Karlsson Hedestam GB, Wyatt RT, Lore K, Route of Vaccine Administration Alters Antigen Trafficking but Not Innate or Adaptive Immunity. Cell Rep 30, 3964–3971 e3967 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cebon JS, Gore M, Thompson JF, Davis ID, McArthur GA, Walpole E, Smithers M, Cerundolo V, Dunbar PR, MacGregor D, Fisher C, Millward M, Nathan P, Findlay MPN, Hersey P, Evans TRJ, Ottensmeier CH, Marsden J, Dalgleish AG, Corrie PG, Maria M, Brimble M, Williams G, Winkler S, Jackson HM, Endo-Munoz L, Tutuka CSA, Venhaus R, Old LJ, Haack D, Maraskovsky E, Behren A, Chen W, Results of a randomized, double-blind phase II clinical trial of NY-ESO-1 vaccine with ISCOMATRIX adjuvant versus ISCOMATRIX alone in participants with high-risk resected melanoma. Journal for immunotherapy of cancer 8, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chung KY, Coyle EM, Jani D, King LR, Bhardwaj R, Fries L, Smith G, Glenn G, Golding H, Khurana S, ISCOMATRIX \texttrademark adjuvant promotes epitope spreading and antibody affinity maturation of influenza A H7N9 virus like particle vaccine that correlate with virus neutralization in humans. Vaccine 33, 3953–3962 (2015). [DOI] [PubMed] [Google Scholar]
- 16.Fries LF, Smith GE, Glenn GM, A recombinant viruslike particle influenza A (H7N9) vaccine. New England Journal of Medicine 369, 2564–2566 (2013). [DOI] [PubMed] [Google Scholar]
- 17.Pedersen GK, Madhun AS, Breakwell L, Hoschler K, Sjursen H, Pathirana RD, Goudsmit J, Cox RJ, T-helper 1 cells elicited by H5N1 vaccination predict seroprotection. The Journal of infectious diseases 206, 158–166 (2012). [DOI] [PubMed] [Google Scholar]
- 18.Heath PT, Galiza EP, Baxter DN, Boffito M, Browne D, Burns F, Chadwick DR, Clark R, Cosgrove C, Galloway J, Goodman AL, Heer A, Higham A, Iyengar S, Jamal A, Jeanes C, Kalra PA, Kyriakidou C, McAuley DF, Meyrick A, Minassian AM, Minton J, Moore P, Munsoor I, Nicholls H, Osanlou O, Packham J, Pretswell CH, San Francisco Ramos A, Saralaya D, Sheridan RP, Smith R, Soiza RL, Swift PA, Thomson EC, Turner J, Viljoen ME, Albert G, Cho I, Dubovsky F, Glenn G, Rivers J, Robertson A, Smith K, Toback S, nCo VSG, Safety and Efficacy of NVX-CoV2373 Covid-19 Vaccine. N Engl J Med, (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cox RJ, Pedersen G, Madhun AS, Svindland S, Svik M, Breakwell L, Hoschler K, Willemsen M, Campitelli L, Nstbakken JK, Weverling GJ, Klap J, McCullough KC, Zambon M, Kompier R, Sjursen H, Evaluation of a virosomal H5N1 vaccine formulated with Matrix M \texttrademark adjuvant in a phase I clinical trial. Vaccine 29, 8049–8059 (2011). [DOI] [PubMed] [Google Scholar]
- 20.Windon RG, Chaplin PJ, Beezum L, Coulter A, Cahill R, Kimpton W, Drane D, Pearse M, Sjlander A, Tennent JM, Scheerlinck JY, Induction of lymphocyte recruitment in the absence of a detectable immune response. Vaccine 19, 572–578 (2000). [DOI] [PubMed] [Google Scholar]
- 21.Detienne S, Welsby I, Collignon C, Wouters S, Coccia M, Delhaye S, Van Maele L, Thomas S, Swertvaegher M, Detavernier A, Elouahabi A, Goriely S, Didierlaurent AM, Central role of CD169+ lymph node resident macrophages in the adjuvanticity of the QS-21 component of AS01. Scientific reports 6, 39475 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wilson NS, Yang B, Morelli AB, Koernig S, Yang A, Loeser S, Airey D, Provan L, Hass P, Braley H, Couto S, Drane D, Boyle J, Belz GT, Ashkenazi A, Maraskovsky E, ISCOMATRIX vaccines mediate CD8+ T-cell cross-priming by a MyD88-dependent signaling pathway. Immunology and Cell Biology 90, 540–552 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Coccia M, Collignon C, Hervé C, Chalon A, Welsby I, Detienne S, van Helden MJ, Dutta S, Genito CJ, Waters NC, Cellular and molecular synergy in AS01-adjuvanted vaccines results in an early IFNγ response promoting vaccine immunogenicity. NPJ vaccines 2, 1–14 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Den Brok MH, Büll C, Wassink M, De Graaf AM, Wagenaars JA, Minderman M, Thakur M, Amigorena S, Rijke EO, Schrier CC, Saponin-based adjuvants induce cross-presentation in dendritic cells by intracellular lipid body formation. Nature communications 7, 1–14 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wilson NS, Yang B, Morelli AB, Koernig S, Yang A, Loeser S, Airey D, Provan L, Hass P, Braley H, ISCOMATRIX vaccines mediate CD8+ T-cell cross-priming by a MyD88-dependent signaling pathway. Immunology and cell biology 90, 540–552 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schnurr M, Orban M, Robson NC, Shin A, Braley H, Airey D, Cebon J, Maraskovsky E, Endres S, ISCOMATRIX adjuvant induces efficient cross-presentation of tumor antigen by dendritic cells via rapid cytosolic antigen delivery and processing via tripeptidyl peptidase II. The Journal of Immunology 182, 1253–1259 (2009). [DOI] [PubMed] [Google Scholar]
- 27.Raaijmakers TK, van den Bijgaart RJE, den Brok MH, Wassink M, de Graaf A, Wagenaars JA, Nierkens S, Ansems M, Scheffer GJ, Adema GJ, Tumor ablation plus co-administration of CpG and saponin adjuvants affects IL-1 production and multifunctional T cell numbers in tumor draining lymph nodes. J Immunother Cancer 8, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Beck Z, Matyas GR, Jalah R, Rao M, Polonis VR, Alving CR, Differential immune responses to HIV-1 envelope protein induced by liposomal adjuvant formulations containing monophosphoryl lipid A with or without QS21. Vaccine 33, 5578–5587 (2015). [DOI] [PubMed] [Google Scholar]
- 29.Rappuoli R, Aderem A, A 2020 vision for vaccines against HIV, tuberculosis and malaria. Nature 473, 463–469 (2011). [DOI] [PubMed] [Google Scholar]
- 30.Plotkin SA, Correlates of protection induced by vaccination. Clinical and vaccine immunology 17, 1055–1065 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Steichen JM, Kulp DW, Tokatlian T, Escolano A, Dosenovic P, Stanfield RL, McCoy LE, Ozorowski G, Hu X, Kalyuzhniy O, HIV vaccine design to target germline precursors of glycan-dependent broadly neutralizing antibodies. Immunity 45, 483–496 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jardine JG, Kulp DW, Havenar-Daughton C, Sarkar A, Briney B, Sok D, Sesterhenn F, Ereño-Orbea J, Kalyuzhniy O, Deresa I, HIV-1 broadly neutralizing antibody precursor B cells revealed by germline-targeting immunogen. Science 351, 1458–1463 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kato Y, Abbott RK, Freeman BL, Haupt S, Groschel B, Silva M, Menis S, Irvine DJ, Schief WR, Crotty S, Multifaceted Effects of Antigen Valency on B Cell Response Composition and Differentiation In Vivo. Immunity, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Abbott RK, Lee JH, Menis S, Skog P, Rossi M, Ota T, Kulp DW, Bhullar D, Kalyuzhniy O, Havenar-Daughton C, Schief WR, Nemazee D, Crotty S, Precursor frequency and affinity determine B cell competitive fitness in germinal centers, tested with germline-targeting HIV vaccine immunogens. Immunity 48, 133–146.e136 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.ClinicalTrials.gov, A Phase I Trial to Evaluate the Safety and Immunogenicity of eOD-GT8 60mer Vaccine, Adjuvanted. (NCT03547245).
- 36.Dan JM, Lindestam Arlehamn CS, Weiskopf D, da Silva Antunes R, Havenar-Daughton C, Reiss SM, Brigger M, Bothwell M, Sette A, Crotty S, A Cytokine-Independent Approach To Identify Antigen-Specific Human Germinal Center T Follicular Helper Cells and Rare Antigen-Specific CD4+ T Cells in Blood. J Immunol, (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Havenar-Daughton C, Reiss SM, Carnathan DG, Wu JE, Kendric K, Torrents de la Pena A, Kasturi SP, Dan JM, Bothwell M, Sanders RW, Pulendran B, Silvestri G, Crotty S, Cytokine-Independent Detection of Antigen-Specific Germinal Center T Follicular Helper Cells in Immunized Nonhuman Primates Using a Live Cell Activation-Induced Marker Technique. J Immunol, (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sestito LF, Thomas SN, Lymph-directed nitric oxide increases immune cell access to lymph-borne nanoscale solutes. Biomaterials 265, 120411 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Breslin JW, Gaudreault N, Watson KD, Reynoso R, Yuan SY, Wu MH, Vascular endothelial growth factor-C stimulates the lymphatic pump by a VEGF receptor-3-dependent mechanism. Am J Physiol Heart Circ Physiol 293, H709–718 (2007). [DOI] [PubMed] [Google Scholar]
- 40.Haddy FJ, Scott JB, Grega GJ, Effects of histamine on lymph protein concentration and flow in the dog forelimb. Am J Physiol 223, 1172–1177 (1972). [DOI] [PubMed] [Google Scholar]
- 41.Brigham KL, Owen PJ, Increased sheep lung vascular permeability caused by histamine. Circ Res 37, 647–657 (1975). [DOI] [PubMed] [Google Scholar]
- 42.Kulp DW, Steichen JM, Pauthner M, Hu X, Schiffner T, Liguori A, Cottrell CA, Havenar-Daughton C, Ozorowski G, Georgeson E, Kalyuzhniy O, Willis JR, Kubitz M, Adachi Y, Reiss SM, Shin M, de Val N, Ward AB, Crotty S, Burton DR, Schief WR, Structure-based design of native-like HIV-1 envelope trimers to silence non-neutralizing epitopes and eliminate CD4 binding. Nature Communications 8, 1655 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Havenar-Daughton C, Lee JH, Crotty S, Tfh cells and HIV bnAbs, an immunodominance model of the HIV neutralizing antibody generation problem. Immunological reviews 275, 49–61 (2017). [DOI] [PubMed] [Google Scholar]
- 44.Havenar-Daughton C, Carnathan DG, Torrents de la Pea A, Pauthner M, Briney B, Reiss SM, Wood JS, Kaushik K, van Gils MJ, Rosales SL, van der Woude P, Locci M, Le KM, de Taeye SW, Sok D, Mohammed AUR, Huang J, Gumber S, Garcia A, Kasturi SP, Pulendran B, Moore JP, Ahmed R, Seumois G, Burton DR, Sanders RW, Silvestri G, Crotty S, Direct probing of germinal center responses reveals immunological features and bottlenecks for neutralizing antibody responses to HIV Env trimer. Cell reports 17, 2195–2209 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhou T, Doria-Rose NA, Cheng C, Stewart-Jones GB, Chuang G-Y, Chambers M, Druz A, Geng H, McKee K, Do Kwon Y, Quantification of the impact of the HIV-1-glycan shield on antibody elicitation. Cell reports 19, 719–732 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sanders RW, van Gils MJ, Derking R, Sok D, Ketas TJ, Burger JA, Ozorowski G, Cupo A, Simonich C, Goo L, HIV-1 neutralizing antibodies induced by native-like envelope trimers. Science 349, aac4223 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hanson MC, Crespo MP, Abraham W, Moynihan KD, Szeto GL, Chen SH, Melo MB, Mueller S, Irvine DJ, Nanoparticulate STING agonists are potent lymph node-targeted vaccine adjuvants. Journal of Clinical Investigation 125, 2532–2546 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu H, Moynihan KD, Zheng Y, Szeto GL, Li AV, Huang B, Van Egeren DS, Park C, Irvine DJ, Structure-based programming of lymph-node targeting in molecular vaccines. Nature 507, 519–522 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tokatlian T, Read BJ, Jones CA, Kulp DW, Menis S, Chang JY, Steichen JM, Kumari S, Allen JD, Dane EL, Innate immune recognition of glycans targets HIV nanoparticle immunogens to germinal centers. Science 363, 649–654 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Petitdemange C, Kasturi SP, Kozlowski PA, Nabi R, Quarnstrom CF, Reddy PBJ, Derdeyn CA, Spicer LM, Patel P, Legere T, Vaccine induction of antibodies and tissue-resident CD8+ T cells enhances protection against mucosal SHIV-infection in young macaques. JCI insight 4, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pulendran B, A PS, O'Hagan DT, Emerging concepts in the science of vaccine adjuvants. Nat Rev Drug Discov 20, 454–475 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kasturi SP, Rasheed MAU, Havenar-Daughton C, Pham M, Legere T, Sher ZJ, Kovalenkov Y, Gumber S, Huang JY, Gottardo R, Fulp W, Sato A, Sawant S, Stanfield-Oakley S, Yates N, LaBranche C, Alam SM, Tomaras G, Ferrari G, Montefiori D, Wrammert J, Villinger F, Tomai M, Vasilakos J, Fox CB, Reed SG, Haynes BF, Crotty S, Ahmed R, Pulendran B, 3M-052, a synthetic TLR-7/8 agonist, induces durable HIV-1 envelope-specific plasma cells and humoral immunity in nonhuman primates. Sci Immunol 5, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Thompson EA, Ols S, Miura K, Rausch K, Narum DL, Spangberg M, Juraska M, Wille-Reece U, Weiner A, Howard RF, Long CA, Duffy PE, Johnston L, O’Neil CP, Lore K, TLR-adjuvanted nanoparticle vaccines differentially influence the quality and longevity of responses to malaria antigen Pfs25. JCI Insight 3, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Francica JR, Sheng Z, Zhang Z, Nishimura Y, Shingai M, Ramesh A, Keele BF, Schmidt SD, Flynn BJ, Darko S, Lynch RM, Yamamoto T, Matus-Nicodemos R, Wolinsky D, Program NCS, Nason M, Valiante NM, Malyala P, De Gregorio E, Barnett SW, Singh M, O'Hagan DT, Koup RA, Mascola JR, Martin MA, Kepler TB, Douek DC, Shapiro L, Seder RA, Analysis of immunoglobulin transcripts and hypermutation following SHIV(AD8) infection and protein-plus-adjuvant immunization. Nature communications 6, 6565 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Francica JR, Zak DE, Linde C, Siena E, Johnson C, Juraska M, Yates NL, Gunn B, De Gregorio E, Flynn BJ, Valiante NM, Malyala P, Barnett SW, Sarkar P, Singh M, Jain S, Ackerman M, Alam M, Ferrari G, Salazar A, Tomaras GD, O'Hagan DT, Aderem A, Alter G, Seder RA, Innate transcriptional effects by adjuvants on the magnitude, quality, and durability of HIV envelope responses in NHPs. Blood Adv 1, 2329–2342 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Silva A, Mount A, Krstevska K, Pejoski D, Hardy MP, Owczarek C, Scotney P, Maraskovsky E, Baz Morelli A, The combination of ISCOMATRIX adjuvant and TLR agonists induces regression of established solid tumors in vivo. J Immunol 194, 2199–2207 (2015). [DOI] [PubMed] [Google Scholar]
- 57.Smirnov D, Schmidt JJ, Capecchi JT, Wightman PD, Vaccine adjuvant activity of 3M-052: an imidazoquinoline designed for local activity without systemic cytokine induction. Vaccine 29, 5434–5442 (2011). [DOI] [PubMed] [Google Scholar]
- 58.Wu TY-H, Singh M, Miller AT, De Gregorio E, Doro F, D’Oro U, Skibinski DA, Mbow ML, Bufali S, Herman AE, Rational design of small molecules as vaccine adjuvants. Science translational medicine 6, 263ra160–263ra160 (2014). [DOI] [PubMed] [Google Scholar]
- 59.Kasturi SP, Kozlowski PA, Nakaya HI, Burger MC, Russo P, Pham M, Kovalenkov Y, Silveira ELV, Havenar-Daughton C, Burton SL, Kilgore KM, Johnson MJ, Nabi R, Legere T, Sher ZJ, Chen X, Amara RR, Hunter E, Bosinger SE, Spearman P, Crotty S, Villinger F, Derdeyn CA, Wrammert J, Pulendran B, Adjuvanting a Simian Immunodeficiency Virus Vaccine with Toll-Like Receptor Ligands Encapsulated in Nanoparticles Induces Persistent Antibody Responses and Enhanced Protection in TRIM5alpha Restrictive Macaques. J Virol 91, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lynn GM, Laga R, Darrah PA, Ishizuka AS, Balaci AJ, Dulcey AE, Pechar M, Pola R, Gerner MY, Yamamoto A, Buechler CR, Quinn KM, Smelkinson MG, Vanek O, Cawood R, Hills T, Vasalatiy O, Kastenmuller K, Francica JR, Stutts L, Tom JK, Ryu KA, Esser-Kahn AP, Etrych T, Fisher KD, Seymour LW, Seder RA, In vivo characterization of the physicochemical properties of polymer-linked TLR agonists that enhance vaccine immunogenicity. Nat Biotechnol 33, 1201–1210 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Neeland MR, Shi W, Collignon C, Taubenheim N, Meeusen ENT, Didierlaurent AM, de Veer MJ, The lymphatic immune response induced by the adjuvant AS01: a comparison of intramuscular and subcutaneous immunization routes. Journal of immunology (Baltimore, Md. : 1950) 197, 2704–2714 (2016). [DOI] [PubMed] [Google Scholar]
- 62.Calabro S, Tortoli M, Baudner BC, Pacitto A, Cortese M, O'Hagan DT, De Gregorio E, Seubert A, Wack A, Vaccine adjuvants alum and MF59 induce rapid recruitment of neutrophils and monocytes that participate in antigen transport to draining lymph nodes. Vaccine 29, 1812–1823 (2011). [DOI] [PubMed] [Google Scholar]
- 63.Liang F, Lindgren G, Sandgren KJ, Thompson EA, Francica JR, Seubert A, De Gregorio E, Barnett S, O’Hagan DT, Sullivan NJ, Vaccine priming is restricted to draining lymph nodes and controlled by adjuvant-mediated antigen uptake. Science Translational Medicine 9, eaal2094 (2017). [DOI] [PubMed] [Google Scholar]
- 64.Junt T, Moseman EA, Iannacone M, Massberg S, Lang P. a., Boes M, Fink K, Henrickson SE, Shayakhmetov DM, Di Paolo NC, van Rooijen N, Mempel TR, Whelan SP, von Andrian UH, Subcapsular sinus macrophages in lymph nodes clear lymph-borne viruses and present them to antiviral B cells. Nature 450, 110–114 (2007). [DOI] [PubMed] [Google Scholar]
- 65.Kastenmuller W, Torabi-Parizi P, Subramanian N, Lmmermann T, Germain RN, A spatially-organized multicellular innate immune response in lymph nodes limits systemic pathogen spread. Cell 150, 1235–1248 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chtanova T, Schaeffer M, Han S-J, van Dooren GG, Nollmann M, Herzmark P, Chan SW, Satija H, Camfield K, Aaron H, Striepen B, Robey EA, Dynamics of neutrophil migration in lymph nodes during infection. Immunity 29, 487–496 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Carrasco YR, Batista FD, B cells acquire particulate antigen in a macrophage-rich area at the boundary between the follicle and the subcapsular sinus of the lymph node. Immunity 27, 160–171 (2007). [DOI] [PubMed] [Google Scholar]
- 68.Gaya M, Castello A, Montaner B, Rogers N, Reis e Sousa C, Bruckbauer A, Batista FD, Host response. Inflammation-induced disruption of SCS macrophages impairs B cell responses to secondary infection. Science (New York, N.Y.) 347, 667–672 (2015). [DOI] [PubMed] [Google Scholar]
- 69.Phan TG, Green J. a., Gray EE, Xu Y, Cyster JG, Immune complex relay by subcapsular sinus macrophages and noncognate B cells drives antibody affinity maturation. Nature Immunology 10, 786–793 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Phan TG, Grigorova I, Okada T, Cyster JG, Subcapsular encounter and complement-dependent transport of immune complexes by lymph node B cells. Nature Immunology 8, 992–1000 (2007). [DOI] [PubMed] [Google Scholar]
- 71.Pucci F, Garris C, Lai CP, Newton A, Pfirschke C, Engblom C, Alvarez D, Sprachman M, Evavold C, Magnuson A, von Andrian UH, Glatz K, Breakefield XO, Mempel TR, Weissleder R, Pittet MJ, SCS macrophages suppress melanoma by restricting tumor-derived vesicle-B cell interactions. Science (New York, N.Y.) 352, 242–246 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kurosaki T, Kometani K, Ise W, Memory B cells. Nature reviews. Immunology 15, 149–159 (2015). [DOI] [PubMed] [Google Scholar]
- 73.Crotty S, T follicular helper cell biology: A decade of discovery and diseases. Immunity 50, 1132–1148 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.De Giovanni M, Cutillo V, Giladi A, Sala E, Maganuco CG, Medaglia C, Di Lucia P, Bono E, Cristofani C, Consolo E, Giustini L, Fiore A, Eickhoff S, Kastenmuller W, Amit I, Kuka M, Iannacone M, Spatiotemporal regulation of type I interferon expression determines the antiviral polarization of CD4+ T cells. Nature Immunology 21, 321–330 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Choi YS, Eto D, Yang JA, Lao C, Crotty S, Cutting edge: STAT1 is required for IL-6-mediated Bcl6 induction for early follicular helper cell differentiation. Journal of immunology (Baltimore, Md. : 1950) 190, 3049–3053 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dienz O, Eaton SM, Bond JP, Neveu W, Moquin D, Noubade R, Briso EM, Charland C, Leonard WJ, Ciliberto G, Teuscher C, Haynes L, Rincon M, The induction of antibody production by IL-6 is indirectly mediated by IL-21 produced by CD4+ T cells. The Journal of experimental medicine 206, 69–78 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Eddahri F, Denanglaire S, Bureau F, Spolski R, Leonard WJ, Leo O, Andris F, Interleukin-6/STAT3 signaling regulates the ability of naive T cells to acquire B-cell help capacities. Blood 113, 2426–2433 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hornung V, Rothenfusser S, Britsch S, Krug A, Jahrsdorfer B, Giese T, Endres S, Hartmann G, Quantitative expression of toll-like receptor 1-10 mRNA in cellular subsets of human peripheral blood mononuclear cells and sensitivity to CpG oligodeoxynucleotides. J Immunol 168, 4531–4537 (2002). [DOI] [PubMed] [Google Scholar]
- 79.Jardine J, Julien J-P, Menis S, Ota T, Kalyuzhniy O, McGuire A, Sok D, Huang P-S, MacPherson S, Jones M, Nieusma T, Mathison J, Baker D, Ward AB, Burton DR, Stamatatos L, Nemazee D, Wilson IA, Schief WR, Rational HIV immunogen design to target specific germline B cell receptors. Science (New York, N.Y.) 340, 711–716 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sangesland M, Ronsard L, Kazer SW, Bals J, Boyoglu-Barnum S, Yousif AS, Barnes R, Feldman J, Quirindongo-Crespo M, McTamney PM, Rohrer D, Lonberg N, Chackerian B, Graham BS, Kanekiyo M, Shalek AK, Lingwood D, Germline-Encoded Affinity for Cognate Antigen Enables Vaccine Amplification of a Human Broadly Neutralizing Response against Influenza Virus. Immunity 51, 735–749.e738 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Weaver GC, Villar RF, Kanekiyo M, Nabel GJ, Mascola JR, Lingwood D, In vitro reconstitution of B cell receptor–antigen interactions to evaluate potential vaccine candidates. Nature protocols 11, 193 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Reagan Shaw S, Nihal M, Ahmad N, Dose translation from animal to human studies revisited. FASEB journal : official publication of the Federation of American Societies for Experimental Biology 22, 659–661 (2008). [DOI] [PubMed] [Google Scholar]
- 83.Didierlaurent AM, Collignon C, Bourguignon P, Wouters S, Fierens K, Fochesato M, Dendouga N, Langlet C, Malissen B, Lambrecht BN, Garçon N, Van Mechelen M, Morel S, Enhancement of Adaptive Immunity by the Human Vaccine Adjuvant AS01 Depends on Activated Dendritic Cells. The Journal of Immunology 193, 1920–1930 (2014). [DOI] [PubMed] [Google Scholar]
- 84.Jones D, Meijer EFJ, Blatter C, Liao S, Pereira ER, Bouta EM, Jung K, Chin SM, Huang P, Munn LL, Vakoc BJ, Otto M, Padera TP, Methicillin-resistant <em>Staphylococcus aureus</em> causes sustained collecting lymphatic vessel dysfunction. Science Translational Medicine 10, eaam7964 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Liao S, Jones D, Cheng G, Padera TP, Method for the quantitative measurement of collecting lymphatic vessel contraction in mice. Journal of biological methods 1, (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tokatlian T, Kulp DW, Mutafyan AA, Jones CA, Menis S, Georgeson E, Kubitz M, Zhang MH, Melo MB, Silva M, Yun DS, Schief WR, Irvine DJ, Enhancing Humoral Responses Against HIV Envelope Trimers via Nanoparticle Delivery with Stabilized Synthetic Liposomes. Scientific Reports 8, 16527 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mayordomo JI, Zorina T, Storkus WJ, Zitvogel L, Celluzzi C, Falo LD, Melief CJ, Ildstad ST, Kast WM, Deleo AB, Bone marrow-derived dendritic cells pulsed with synthetic tumour peptides elicit protective and therapeutic antitumour immunity. Nature Medicine 1, 1297–1302 (1995). [DOI] [PubMed] [Google Scholar]
- 88.Wei X, Decker JM, Liu H, Zhang Z, Arani RB, Kilby JM, Saag MS, Wu X, Shaw GM, Kappes JC, Emergence of resistant human immunodeficiency virus type 1 in patients receiving fusion inhibitor (T-20) monotherapy. Antimicrobial agents and chemotherapy 46, 1896–1905 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wei X, Decker JM, Wang S, Hui H, Kappes JC, Wu X, Salazar-Gonzalez JF, Salazar MG, Kilby JM, Saag MS, Antibody neutralization and escape by HIV-1. Nature 422, 307–312 (2003). [DOI] [PubMed] [Google Scholar]
- 90.Sarzotti-Kelsoe M, Bailer RT, Turk E, C.-l. Lin, M. Bilska, K. M. Greene, H. Gao, C. A. Todd, D. A. Ozaki, M. S. Seaman, Optimization and validation of the TZM-bl assay for standardized assessments of neutralizing antibodies against HIV-1. Journal of immunological methods 409, 131–146 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Fischinger S, Fallon JK, Michell AR, Broge T, Suscovich TJ, Streeck H, Alter G, A high-throughput, bead-based, antigen-specific assay to assess the ability of antibodies to induce complement activation. J Immunol Methods 473, 112630 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lu LL, Chung AW, Rosebrock TR, Ghebremichael M, Yu WH, Grace PS, Schoen MK, Tafesse F, Martin C, Leung V, Mahan AE, Sips M, Kumar MP, Tedesco J, Robinson H, Tkachenko E, Draghi M, Freedberg KJ, Streeck H, Suscovich TJ, Lauffenburger DA, Restrepo BI, Day C, Fortune SM, Alter G, A Functional Role for Antibodies in Tuberculosis. Cell 167, 433–443.e414 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ackerman ME, Moldt B, Wyatt RT, Dugast AS, McAndrew E, Tsoukas S, Jost S, Berger CT, Sciaranghella G, Liu Q, Irvine DJ, Burton DR, Alter G, A robust, high-throughput assay to determine the phagocytic activity of clinical antibody samples. J Immunol Methods 366, 8–19 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.