Abstract
The phoenixin (PNX) peptide is linked to the control of reproduction, food intake, stress, and inflammation. However, little is known about what regulates its gene and protein expression, information that is critical to understand the physiological role of PNX. In this review, we summarize what is known about the transcriptional control of Pnx and its receptor Gpr173. A main function of PNX is as a positive regulator of the hypothalamic-pituitary-gonadal axis, but there is a lack of research on its control by reproductive hormones and peptides. PNX is also associated with food intake, and its expression is linked to feeding status, fatty acids, and glucose. It is influenced by environmental and hormonal-induced stress. The regulation of Pnx in most contexts remains an enigma, in part due to conflicting and negative results. An extensive analysis of the response of the Pnx gene to factors related to reproduction, metabolism, stress, and inflammation is required. Analysis of the Pnx promoter and epigenetic regulation must be considered to understand how this level of control contributes to its pleiotropic effects. PNX is now linked to a broad range of functions, but more research on its gene regulation is required to understand its place in overall physiology and therapeutic potential.
Keywords: phoenixin, hypothalamus, reproduction, obesity, gene expression, signal transduction, GPR173
In the 8 years since its identification, the peptide phoenixin (PNX) has been linked to numerous physiological processes and possible therapeutic applications [1-5]. PNX was discovered by Yosten et al using a bioinformatics algorithm based on data from the Human Genome Project that searched for conserved, secreted peptides [6]. PNX was detected most highly in the rat hypothalamus but was also present in the heart, spleen, thymus, and pancreas among several other tissues. Within the hypothalamus, PNX immunostaining identified its presence across several nuclei including the arcuate nucleus (ARC), paraventricular nucleus (PVN), supraoptic nucleus, and median eminence. PNX is cleaved from the C-terminal of small integral membrane protein 20 (SMIM20), a protein involved in cellular respiration through its role in assembly of mitochondrial cytochrome c oxidase [7]. PNX is predominantly produced in 2 amidated isoforms: a 14 amino acid peptide (PNX-14) and an N-terminal extended 20 amino acid peptide (PNX-20). PNX-14 is identical in humans, rodents, and pigs, whereas PNX-20 differs by 1 amino acid in pigs. Although the distribution patterns of PNX isoform expression have not been entirely delineated, studies to date suggest that the isoforms show a slightly different tissue expression pattern; for example, PNX-20 is more highly expressed in the hypothalamus, yet PNX-14 and PNX-20 appear thus far to be functionally similar [6,8,9].
This first paper on PNX demonstrated its clear link with reproduction, as intracerebroventricular (ICV) small interfering RNA–mediated knockdown of the peptide in female rats extended the estrous cycle by 2.3 days. A study from our laboratory demonstrated that PNX stimulated Gnrh and Kisspeptin (Kiss1) gene expression in hypothalamic neurons [10]. Thus, evidence indicates that PNX is a positive regulator of the hypothalamic-pituitary-gonadal (HPG) axis. At the level of the pituitary, PNX potentiated gonadotropin-releasing hormone (GnRH) activity and stimulated luteinizing hormone secretion. Subsequent studies indicated that PNX injection could impact a wide array of physiologic processes, including feeding patterns, inflammation, memory, anxiety, and water balance [11].
Three years after the first paper was published on PNX, a putative receptor for PNX was identified. Three possible receptor candidates were identified for PNX using a deductive ligand-receptor matching strategy and the orphan receptor G protein-coupled receptor 173 (GPR173) was found to mediate the effects of PNX on Gnrh and Kiss1 gene expression in hypothalamic neurons [10,12]. Small interfering RNA knockdown of Gpr173 approximately doubled the estrous cycle length to over 8 days [12]. Of note, GPR173 levels following knockdown were significantly lower in the hypothalamic ARC but not in the pituitary, mediobasal hypothalamus, or anteroventral periventricular nucleus, signifying that a broader knockdown in these other key reproductive centers of the brain may exacerbate the delay to the estrous cycle. GPR173 was also expressed in several other hypothalamic nuclei, including the PVN, ventromedial nucleus, and medial preoptic area. Additionally, it was broadly expressed in peripheral tissues, including the heart, pancreas, and ovary.
Significant progress has been made since the initial PNX identification whereby this neuropeptide is now implicated with multiple physiological processes. These functions are extensively described in recent reviews [1-5]. Still, there remains many questions; especially important is what controls PNX expression, and when is it induced to carry out its many pleiotropic functions? It is without a doubt critical to understand the physiological purpose of PNX, but without understanding how its transcription, translation, and secretion are regulated, we cannot fully appreciate the physiological role of the PNX peptide. From this point forward, for clarity, we will refer to the PNX host gene as Pnx, instead of Smim20, although it is clear that the Pnx transcript originates from the Smim20 gene. To date, there are only a handful of studies on the regulation of Pnx, some with negative or contradictory results. Herein, we summarize the information available on Pnx gene regulation by nutrients, reproductive status, stress, and inflammation, and we propose future avenues of investigation (Fig. 1).
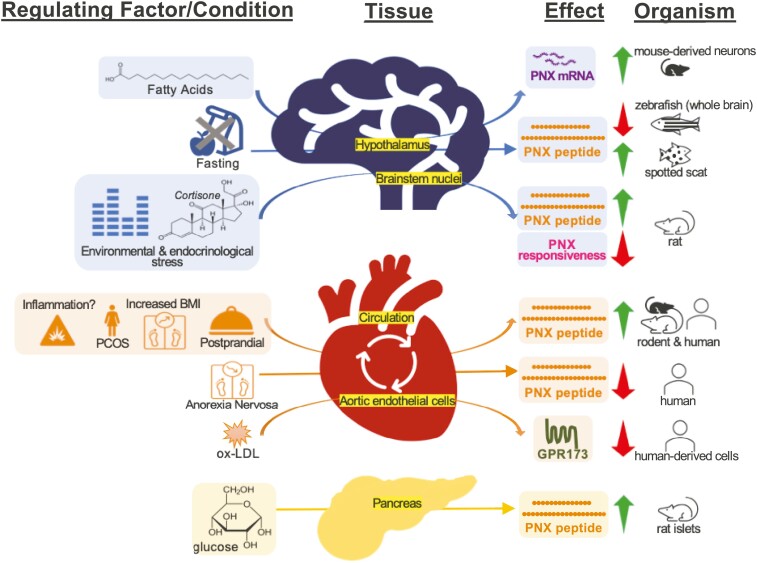
Figure 1.
Summary of the multidimensional effects of phoenixin with regards to metabolic, environmental, and physiological functions. Note that for certain results the horizontal arrows represent correlation and not necessarily causation.
Energy Homeostasis
In addition to reproductive control, PNX functions in the control of food intake and energy homeostasis. Energy homeostasis is, in part, centrally controlled by hypothalamic neurons that govern appetite and energy expenditure through secretion of neuropeptides [13]. For example, neuropeptide Y (NPY) is an orexigenic peptide, and alpha-melanocyte stimulating hormone is an anorexigenic peptide. These neuropeptides are secreted from the ARC and signal to second order neurons in the PVN, resulting in changes in food intake and energy expenditure. Given that PNX is expressed in hypothalamic nuclei associated with energy homeostasis, including the ARC and PVN, it is not unexpected that it appears to control food intake. PNX ICV injection in rats, but not intraperitoneal (IP) injection, caused an increase in food intake in the light phase at multiple doses and a reduction in food intake in the dark phase only at the highest dose used [14]. The rats had reduced satiety and satiation as measured by shorter intermeal intervals and larger meal sizes and duration. It is not clear whether the reduction in food intake in the dark phase represents a compensatory mechanism, as it only occurred at 1 dose, or an overall phase shift in the timing of food intake, which could be established with a further study assessing 24-hour food intake at more frequent time points. Another study corroborated the finding that PNX ICV injection during the light phase into rats increased food intake and additionally showed that it increased locomotor activity and drinking 2 hours following injection [15]. PNX also activated numerous brain regions associated with food intake including the supraoptic nucleus, PVN, and nucleus of the solitary tract (NTS), among several other brain regions, demonstrating that its receptors and activity are widespread through the brain. Recently it was shown that not only does PNX impact energy homeostasis from the brain, it can also act on gut cells. In MGN3-1 stomach endocrine cells, PNX increased ghrelin, while in STC-1 enteroendocrine cells, PNX suppressed cholecystokinin but had no effect on glucagon-like peptide-1, gastric inhibitory polypeptide, and peptide YY [16].
Emerging data demonstrate that PNX expression and secretion are controlled by metabolic cues. Preliminary evidence shows that PNX was correlated with body mass index in humans with mild cognitive impairment and in women with polycystic ovarian syndrome [17]. Moreover, postprandial circulating levels of PNX were elevated in chow-fed rats, indicating food consumption triggers a relatively rapid response for PNX secretion into the bloodstream [18]. This might suggest either an anorexigenic role for PNX, in contrast with the observed increase in food intake following ICV injection, or it could represent a separate metabolic peripheral function for PNX. This second option is more likely given that IP injection of PNX did not alter food intake. The postprandial increase in PNX is disrupted in metabolic disease, as it was blunted in high-fat diet (HFD)-fed rats, suggesting that elevated levels of fats interfere with normal PNX function, perhaps contributing to the pathogenesis of obesity [18].
Comparative studies in nonmammalian species are normally quite useful to shed light on evolutionary conserved functions, although in the case of PNX this leads to further questions. In zebrafish, Pnx gene expression was reduced in multiple tissues following 7 days of fasting, including in the brain, and PNX IP injection reduced food intake, leading the authors to conclude that PNX is anorexigenic [19]. Further support for an anorexigenic role of PNX was that it is a positive regulator of glycolysis [19]. In the spotted scat, Pnx gene expression was increased in the hypothalamus after 2 and 7 days of fasting and decreased after refeeding, suggesting it could be orexigenic [9]. This contradictory evidence suggests that PNX may have either anorexigenic or orexigenic effects in different species or that its function may be context specific and depend on the concentration or timing of PNX injection.
In vivo studies are critical to understand PNX regulation in a whole-body context, but an in vitro approach allows for delineating the effects of individual compounds on Pnx expression and secretion. We previously treated characterized hypothalamic neuronal cell lines with metabolism-related nutrients and hormones to determine the direct regulation of the Pnx gene. The saturated free fatty acid palmitate, monounsaturated oleate, and polyunsaturated docosahexaenoic acid increased Pnx gene expression in immortalized hypothalamic neuronal cell lines, albeit by a significant, but relatively modest degree [20]. Palmitate also reduced the expression of Gpr173 messenger RNA (mRNA) in the same neuronal cell line [21]. A response to elevated fat levels in the hypothalamus may be related to changes in circulating levels of PNX observed with obesity. We also questioned whether the adipocyte-derived leptin would alter Pnx expression, as leptin signaling in the hypothalamus is essential for energy homeostasis puberty and fertility. However, leptin had no effect on Pnx mRNA levels [20]. Unpublished results from our laboratory indicate that insulin also had no effect on Pnx mRNA levels in immortalized hypothalamic neurons. The absence of regulation by these key hormones was puzzling. Either Pnx gene expression truly operates independently of these hormonal systems, or this response is specific to individual hypothalamic neurons. Protein levels and/or secretion should also be assessed in these conditions, since protein levels occasionally change without alterations in gene expression. Nutrients appear to regulate PNX secretion in the periphery as well. In pancreatic islets, exposure to high glucose increased PNX secretion, consistent with the theory that PNX could be anorexigenic [22]. PNX was detected in both alpha and beta cells and could stimulate insulin secretion from rat islets [22]. As an interesting aside, PNX was also able to induce INS-1E cell proliferation, a remarkable feat that needs to be assessed in vivo, as there exist few known inducers of beta cell proliferation. Collectively, these studies demonstrate that PNX is associated with weight and can be regulated by fats and glucose.
Given that PNX has several links to feeding, it would be interesting to study whether other metabolism-related hormones and nutrients could regulate its expression. For example, PNX expression could be controlled by hypothalamic feeding neuropeptides, such as NPY, orexin, or alpha-melanocyte stimulating hormone. It is possible that these peptides co-localize with PNX in the hypothalamus and could regulate PNX in an autocrine or paracrine manner, but that has not yet been established. Another factor that might affect Pnx expression in the hypothalamus is glucose, given that PNX is regulated by glucose in the pancreas. Glucose sensing is a defined function of the hypothalamus that coordinates whole-body energy homeostasis. A subset of hypothalamic neurons is glucose responsive, possessing glucose transporters and processing enzymes, including glucokinase [23]. If glucose were able to regulate PNX expression or secretion, it would point to an additional layer of complexity for PNX in energy homeostasis. Studies with HFD-fed mice, in which PNX expression is measured in multiple tissues, are necessary to firmly establish its apparent link to obesity. Further studies into PNX regulation in the pancreas, intestine, and stomach are also important to understand its role in the periphery.
Reproductive System
Given the key role for PNX in the HPG axis, it would be expected that the Pnx gene or protein are regulated by reproductive peptides and hormones. Yet, there are comparatively few studies on this aspect of Pnx regulation. A study from our laboratory examined immortalized hypothalamic neurons expressing Pnx that were treated with 17β-estradiol (E2), and found no change in Pnx mRNA levels over a 48-hour time course [20]. This result was unexpected given that this hypothalamic cell line expresses the nuclear estrogen receptors α and β and the membrane-bound estrogen receptor G protein-coupled estrogen receptor 1, and E2 alters Npy expression in this same cell model. We speculate that Pnx gene expression may be regulated indirectly by E2 through intermediate neurons. Although the hypothalamus expresses PNX and GPR173 at high levels, they are also localized to the ovary where their expression changes with follicle development, and this system appears to govern oocyte maturation. Pnx and Gpr173 expression increased throughout follicle development with the highest levels in the antral follicle and corpus luteum [24]. Treatment with PNX caused an increase in E2 secretion and mature oocytes from ovarian tissue slices. PNX also induced markers of oocyte maturation, including Fshr, Lhr, and Kitl in HGrC1 granulosa cells in a GPR173-dependent manner [24]. Reproductive disorders appear to impact levels of PNX and its receptor. Circulating levels of PNX-14 were elevated in a letrozole-induced rat model of polycystic ovary syndrome [25]. Pnx gene expression was elevated in the ovary and periovarian adipose tissue of rats with polycystic ovary syndrome, and the protein was significantly elevated in the ovary. In contrast, Gpr173 mRNA was reduced in adipose tissue, and the protein was altered in the ovary and adipose. More research is necessary to assess reproductive system control of PNX. For example, the hypothalamic PNX response should be tested against reproductive hormones, testosterone and progesterone, or other key reproductive peptides GnRH and Kiss. Circulating levels of PNX could also be measured in individuals with subfertility or infertility to determine whether PNX contributes to these conditions.
Stress and Anxiety
Multiple studies have suggested that PNX is involved in the physiological response to diverse types of stress. PNX is expressed in NTS neurons [6], which are tightly linked to stress circuits due to their connectivity to forebrain regions implicated in the hypothalamic-pituitary-adrenal (HPA) axis [26]. Corticosterone (CORT) is the primary adrenal steroid produced in the HPA axis in laboratory rodents; hence, its administration can serve as a chronic stress model [27]. In coronal slices of the NTS from male Sprague-Dawley rats, bath-applied PNX depolarized and increased the firing frequency in populations of NTS neurons [28]. Intriguingly, these effects were diminished in the presence of environmental and endocrine stress. As measured by patch-clamp recordings, disturbances arising from animal facility construction markedly decreased neuronal responsiveness to PNX, which was restored after animal relocation to a construction-free building. In parallel, 2 weeks of CORT-infused drinking water, mimicking elevated circulating CORT upon exposure to environmental stress, resulted in a comparable loss of PNX responsiveness [28]. Both observations present evidence that stress can modulate neuronal sensitivity to PNX, potentially through GPR173 desensitization or internalization. Future studies should aim to determine the localization of GPR173, including whether it is localized in NTS neurons.
Central PNX protein levels also increase in response to restraint stress, which involves both physical and psychological stress in immobilized animals [29]. Specifically, 30 minutes of restraint stress significantly increased PNX protein expression in the rat dorsal motor nucleus of vagal nerve, raphe pallidus, and medial NTS. In these nuclei, PNX levels were positively correlated with c-Fos, a marker of neuronal activity [30]. Aside from changes in central PNX levels, the same group later reported peripheral changes as well. Restraint stress increased peripheral cortisol at 30, 60, 120, and 240 minutes while decreasing peripheral PNX at 15 minutes, although the PNX change was relatively small (0.8-fold, P < 0.05) [31]. While no correlation was identified between alterations in circulating PNX and cortisol levels in this study, these findings align with a previous study that found plasma PNX levels to be lower in patients with anorexia nervosa [32], a type of metabolic stress frequently associated with hypercortisolemia [33]. Therefore, along with evidence from the CORT experiments, the potential interplay between the HPA axis and PNX should be considered.
Stress signaling through glucocorticoids has modulating effects throughout the HPG axis [34] and central nervous system [35]. Given established roles of PNX in reproduction [10], metabolism [4], sensory [36], and more, it is probable that stress regulates both central and peripheral origins of PNX. However, it remains to be determined whether PNX levels are correlated with components of the HPA axis. It is also worthwhile to experimentally investigate whether PNX-expressing cells express glucocorticoid receptors or whether glucocorticoids act indirectly through other pathways. Furthermore, studies to date have only shown correlations and not causative links, so it is difficult to distinguish whether PNX is a downstream mediator of the cellular and systemic impacts of stress or rather activated to counterbalance those effects given its anxiolytic properties.
Stress can induce anxiety alongside neurogenic factors, and PNX dose-dependently produces anxiolytic effects, as demonstrated through anxiety-assessing behavioral tests. Following PNX-14 ICV administration into the lateral ventricles and anterior hypothalamic area, respectively, male mice spent more time in the open arms during the elevated plus maze test and in the center during the open field maze test as early as 15 minutes post injection. Both behaviors indicate reduced anxiety [37]. Notably, cetrorelix, a GnRH antagonist, uniquely blocked these effects, implicating the GnRH system as a necessary modulator of PNX-14 [38]. In obese men who are at risk for comorbid anxiety, PNX circulation levels showed a weak negative association with anxiety scores as measured by Generalized Anxiety Disorder-7 (r = −0.259) [39]. No association between PNX and perceived stress or depressiveness was found however, so more evidence is needed to elucidate the influence of PNX on stress, and vice versa. For instance, it would be interesting to examine whether central PNX is activated by corticotropin-releasing factor and whether anxiety symptoms are in turn alleviated by endogenous PNX production. In conclusion, stress may both regulate and be regulated by PNX, and further investigation of this relationship may prove advantageous for understanding the pathogenesis and treatment of stress-related disorders.
Inflammation
Aside from psychological and physiological stress, converging evidence corroborates the protective effects of PNX in oxidative stress and inflammatory pathways. In mouse astrocytes, PNX-14 mitigated bacterial endotoxin lipopolysaccharide-activated endoplasmic reticulum stress and the NLR family pyrin domain containing-3 inflammasome response and inhibited proinflammatory cytokines interleukin 1β and interleukin 18 through GPR173 [40]. The same suppressive effects of PNX (both PNX-14 and PNX-20) on the high mobility group box-1, toll-like receptor 4, myeloid differentiation factor 88, nuclear factor kappa B (NF-κB) signaling pathway, and reactive oxygen species-induced cytotoxicity extend to human dental pulp cells [41] and more. For instance, PNX ameliorates oxygen-glucose deprivation with reoxygenation injury in human bEnd0.3 brain endothelial cells through Kruppel-like factor-2–mediated occludin transcription [42] and in murine BV2 microglia through increased activation of sirtuin-1 [43] and the antioxidant glutathione [44].
Neuroinflammation and its accompanying cytokines, neurotransmitters, and other signaling molecules communicate through microglia, neurons, and astrocytes to intermodulate cellular expression of necessary proteins [45]. Neuropeptides whose mRNA and protein expression are known to be changed by inflammatory stimuli include agouti-related peptide [46], NPY [47], and nesfatin-1 [48], all of which relate to PNX both in anatomical distribution and function [49]. Therefore, by induction of the above cerebroprotective effects of PNX, its expression could be regulated by the context of inflammation to help restore physiological function. Our group previously tested this possibility. Immortalized hypothalamic neurons were exposed to lipopolysaccharide, which induces inflammation, for up to 24 hours and noticed no change in Pnx gene expression [20]. It is possible that other inflammatory markers, such as tumor necrosis factor alpha, would influence Pnx expression in the brain or other tissues.
Beyond the central nervous system, the anti-inflammatory effects of PNX confer protection to organ systems subjected to ischemia followed by reperfusion. It is cardioprotective by activating reperfusion injury salvage kinase and survivor-activating factor enhancement cascades, thus inhibiting apoptosis [18], and hepatoprotective in mice with HFD-induced nonalcoholic fatty liver disease [50]. In atherosclerosis, GPR173 expression is reduced in human aortic endothelial cells upon exposure to oxidized low-density lipoprotein, thereby implying a role for PNX in disease onset. Moreover, PNX-20 agonism can improve disease progression by preventing the attachment of THP-1 monocytes to human aortic endothelial cells and attenuating the NF-κB pathway [51].
Taken together, the beneficial and widespread actions of PNX in injury and inflammation imply that inflammatory markers likely contribute to the control of peptide synthesis, release, degradation, and tissue availability. Transcriptomics, proteomic, and functional studies could thus effectively establish the spatial and temporal changes in PNX and shed light on its relationship with inflammatory processes. Considering the brief history of PNX and consequent lack of published data, ample ground exists for more research to unravel the intricacies of its regulatory processes and corresponding clinical implications.
Promoter and Epigenetic Regulation
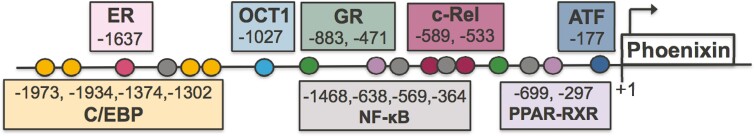
Two unexplored aspects of Pnx gene expression are analysis of its promoter region and consideration of epigenetic regulators, such as microRNAs (miRNAs). A preliminary analysis of the mouse Pnx gene 5’ upstream regulatory region using Genomatix revealed that there are numerous predicted response elements for transcription factors related to reproduction and inflammation (Fig. 2). These predicted sites will certainly need to be experimentally validated using chromatin immunoprecipitation, but they allow for speculation into what else might regulate Pnx mRNA levels. For example, there are NF-κB and cRel binding sites, and although lipopolysaccharide did not change Pnx gene expression in a hypothalamic cell model, it is possible other inflammatory factors might. There are also glucocorticoid response elements that suggest stress hormones could regulate Pnx transcription, as well as predicted sites for C/EBP, OCT1, and the CREB family. Oddly, when we treated a hypothalamic neuronal cell line to activate pathways upstream of these transcription factors, including cyclic AMP, protein kinase C and nitric oxide pathways, Pnx gene expression was unchanged; thus, the regulators of Pnx expression are still undefined [20]. When considering the regulation of Pnx, we must also consider that it is cleaved from SMIM20, a membrane protein linked to mitochondrial function, but there is even less published about Smim20 regulation than Pnx. Nonetheless, a detailed study of the Pnx gene promoter could yield promising information on its regulation.
Figure 2.
Putative transcription factor binding sites in the 5’ upstream regulatory region of the mouse Pnx gene. Predictions were generated using Genomatix and positions of each site are relative to a transcription start site (TSS) at position 53424425 on chromosome 5 (based on GRCm39 C57BL/6J NC_000071.7). Each circle represents a predicted binding site with the position number labeled in the corresponding coloured box. Putative sites were found for the following transcription factors: estrogen receptor (ER), CCAAT/enhancer binding protein (C/EBP), octamer-binding transcription factor 1 (OCT1), glucocorticoid receptor (GR), nuclear factor kappa B (NF-κB), c-Rel, peroxisome proliferator activated receptor (PPAR) and retinoid X receptor (RXR) heterodimer, and activating transcription factor (ATF) from the CREB family.
miRNAs posttranscriptionally regulate genes, and whether some regulate PNX remains to be determined. A simple in silico analysis on TargetScan 8.0 shows that only 2 miRNAs are predicted to have conserved binding sites in the 3’ untranslated region of the mouse Pnx transcript, and over 1000 miRNAs have poorly conserved sites. On another prediction program, miRDB, 34 miRNAs are predicted to bind Pnx. All of these putative miRNAs should be experimentally validated for their direct binding to the Pnx 3’ untranslated region. miRNAs are critical for fertility as loss of global miRNA expression in granulosa cells and the uterus cause infertility [52]. Specific miRNAs have crucial roles in fertility; for example, miR-30 controls puberty onset through targeting Mkrn3 [53]. Similar types of miRNA-Pnx pairings will likely be identified.
Conclusion
In summary, PNX is a dynamic peptide whose regulation is an ongoing mystery. The most evidence on Pnx gene regulation is with regards to energy homeostasis, where PNX appears to be regulated by nutritional status and, in particular, free fatty acids. PNX also appears to be anxiolytic and anti-inflammatory, although whether these processes directly regulate the PNX and GPR173 system requires further examination. PNX is crucial for reproductive function through stimulation of the hypothalamus, pituitary, and ovaries, but there is little known about its regulation by reproductive hormones and peptides. So, the question remains, what can control PNX expression? The answer is key to delineating the physiological roles of PNX, define its dysregulation in pathophysiological states, and establish potential therapeutic uses for PNX.
Acknowledgments
We acknowledge funding from the Canadian Institutes for Health Research (CIHR), Canada Foundation for Innovation and Canada Research Chairs Program (DDB). E.K.M. and N.Z. were supported by Natural Sciences and Engineering Research Council (NSERC) Studentships and the Banting and Best Diabetes Centre. E.K.M. was also supported by an Ontario Graduate Scholarship (OGS).
Disclosure Statement
The authors have no conflict of interest.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
References
- 1. Billert M, Rak A, Nowak KW, Skrzypski M. Phoenixin: more than reproductive peptide. Int J Mol Sci . 2020;21(21):8378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Clarke SA, Dhillo WS. Phoenixin and its role in reproductive hormone release. Semin Reprod Med. 2019;37(4):191-196. [DOI] [PubMed] [Google Scholar]
- 3. McIlwraith EK, Belsham DD. Phoenixin: uncovering its receptor, signaling and functions. Acta Pharmacol Sin. 2018;39(5):774-778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Schalla MA, Stengel A. The role of phoenixin in behavior and food intake. Peptides. 2019;114:38-43. [DOI] [PubMed] [Google Scholar]
- 5. Stein LM, Haddock CJ, Samson WK, Kolar GR, Yosten GLC. The phoenixins: From discovery of the hormone to identification of the receptor and potential physiologic actions. Peptides. 2018;106:45-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yosten GL, Lyu RM, Hsueh AJ, et al. A novel reproductive peptide, phoenixin. J Neuroendocrinol. 2013;25(2):206-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dennerlein S, Oeljeklaus S, Jans D, et al. MITRAC7 acts as a COX1-specific chaperone and reveals a checkpoint during cytochrome c oxidase assembly. Cell Reports. 2015;12(10):1644-1655. [DOI] [PubMed] [Google Scholar]
- 8. Prinz P, Scharner S, Friedrich T, et al. Central and peripheral expression sites of phoenixin-14 immunoreactivity in rats. Biochem Biophys Res Commun. 2017;493(1):195-201. [DOI] [PubMed] [Google Scholar]
- 9. Wang M, Deng SP, Chen HP, et al. Phoenixin participated in regulation of food intake and growth in spotted scat, Scatophagus argus. Comp Biochem Physiol B Biochem Mol Biol. 2018;226:36-44. [DOI] [PubMed] [Google Scholar]
- 10. Treen AK, Luo V, Belsham DD. Phoenixin activates immortalized GnRH and kisspeptin neurons through the novel receptor GPR173. Mol Endocrinol. 2016;30(8):872-888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Haddock CJ, Almeida-Pereira G, Stein LM, Yosten GLC, Samson WK. A novel regulator of thirst behavior: phoenixin. Am J Physiol Regul Integr Comp Physiol. 2020;318(6):R1027-R1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Stein LM, Tullock CW, Mathews SK, et al. Hypothalamic action of phoenixin to control reproductive hormone secretion in females: importance of the orphan G protein-coupled receptor Gpr173. Am J Physiol Regul Integr Comp Physiol. 2016;311(3):R489-R496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hill JW, Faulkner LD. The Role of the melanocortin system in metabolic disease: new developments and advances. Neuroendocrinology. 2017;104(4):330-346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Schalla M, Prinz P, Friedrich T, et al. Phoenixin-14 injected intracerebroventricularly but not intraperitoneally stimulates food intake in rats. Peptides. 2017;96:53-60. [DOI] [PubMed] [Google Scholar]
- 15. Friedrich T, Schalla MA, Scharner S, et al. Intracerebroventricular injection of phoenixin alters feeding behavior and activates nesfatin-1 immunoreactive neurons in rats. Brain Res. 2019;1715:188-195. [DOI] [PubMed] [Google Scholar]
- 16. Mukherjee K, Unniappan S. Mouse gastric mucosal endocrine cells are sources and sites of action of Phoenixin-20. Peptides. 2021;141:170551. [DOI] [PubMed] [Google Scholar]
- 17. Ullah K, ur Rahman T, Wu D-D, Lin X-H, Liu Y, Guo X-Y, Leung PCK, Zhang R-J, Huang H-F, Sheng J-Z. Phoenixin-14 concentrations are increased in association with luteinizing hormone and nesfatin-1 concentrations in women with polycystic ovary syndrome. Clinica Chimica Acta. 2017;471:243-247. [DOI] [PubMed] [Google Scholar]
- 18. Rocca C, Scavello F, Granieri MC, et al. Phoenixin-14: detection and novel physiological implications in cardiac modulation and cardioprotection. Cell Mol Life Sci. 2018;75(4):743-756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rajeswari JJ, Blanco AM, Unniappan S. Phoenixin-20 suppresses food intake, modulates glucoregulatory enzymes, and enhances glycolysis in zebrafish. Am J Physiol Regul Integr Comp Physiol. 2020;318(5):R917-R928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. McIlwraith EK, Loganathan N, Belsham DD. Phoenixin expression is regulated by the fatty acids palmitate, docosahexaenoic acid and oleate, and the endocrine disrupting chemical bisphenol a in immortalized hypothalamic neurons. Front Neurosci. 2018;12:838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. McIlwraith EK, Loganathan N, Belsham DD. Regulation of Gpr173 expression, a putative phoenixin receptor, by saturated fatty acid palmitate and endocrine-disrupting chemical bisphenol A through a p38-mediated mechanism in immortalized hypothalamic neurons. Mol Cell Endocrinol. 2019;485:54-60. [DOI] [PubMed] [Google Scholar]
- 22. Billert M, Kołodziejski PA, Strowski MZ, Nowak KW, Skrzypski M. Phoenixin-14 stimulates proliferation and insulin secretion in insulin producing INS-1E cells. Biochim Biophys Acta Mol Cell Res. 2019;1866(12):118533. [DOI] [PubMed] [Google Scholar]
- 23. Roh E, Song DK, Kim MS. Emerging role of the brain in the homeostatic regulation of energy and glucose metabolism. Exp Mol Med. 2016;48:e216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nguyen XP, Nakamura T, Osuka S, et al. Effect of the neuropeptide phoenixin and its receptor GPR173 during folliculogenesis. Reproduction. 2019;158(1):25-34. [DOI] [PubMed] [Google Scholar]
- 25. Kalamon N, Błaszczyk K, Szlaga A, et al. Levels of the neuropeptide phoenixin-14 and its receptor GRP173 in the hypothalamus, ovary and periovarian adipose tissue in rat model of polycystic ovary syndrome. Biochem Biophys Res Commun. 2020;528(4):628-635. [DOI] [PubMed] [Google Scholar]
- 26. Ter Horst GJ, de Boer P, Luiten PG, van Willigen JD. Ascending projections from the solitary tract nucleus to the hypothalamus. A Phaseolus vulgaris lectin tracing study in the rat. Neuroscience. 1989;31(3):785-797. [DOI] [PubMed] [Google Scholar]
- 27. Kaplowitz ET, Savenkova M, Karatsoreos IN, Romeo RD. Somatic and neuroendocrine changes in response to chronic corticosterone exposure during adolescence in male and female rats. J Neuroendocrinol. 2016;28(2):12336. [DOI] [PubMed] [Google Scholar]
- 28. Grover HM, Smith PM, Ferguson AV. Phoenixin influences the excitability of nucleus of the solitary tract neurones, effects which are modified by environmental and glucocorticoid stress. J Neuroendocrinol. 2020;32(6):e12855. [DOI] [PubMed] [Google Scholar]
- 29. Pare WP, Glavin GB. Restraint stress in biomedical research: a review. Neurosci Biobehav Rev. 1986;10(3):339-370. [DOI] [PubMed] [Google Scholar]
- 30. Friedrich T, Schalla MA, Lommel R, et al. Restraint stress increases the expression of phoenixin immunoreactivity in rat brain nuclei. Brain Res. 2020;1743:146904. [DOI] [PubMed] [Google Scholar]
- 31. Schalla MA, Goebel-Stengel M, Friedrich T, et al. Restraint stress affects circulating NUCB2/nesfatin-1 and phoenixin levels in male rats. Psychoneuroendocrinology. 2020;122:104906. [DOI] [PubMed] [Google Scholar]
- 32. Pałasz A, Tyszkiewicz-Nwafor M, Suszka-Świtek A, et al. Longitudinal study on novel neuropeptides phoenixin, spexin and kisspeptin in adolescent inpatients with anorexia nervosa - association with psychiatric symptoms. Nutr Neurosci. 2021;24(11):896-906. [DOI] [PubMed] [Google Scholar]
- 33. Lawson EA, Donoho D, Miller KK, et al. Hypercortisolemia is associated with severity of bone loss and depression in hypothalamic amenorrhea and anorexia nervosa. J Clin Endocrinol Metab. 2009;94(12):4710-4716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Whirledge S, Cidlowski JA. Glucocorticoids and reproduction: traffic control on the road to reproduction. Trends Endocrinol Metab. 2017;28(6):399-415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Godoy LD, Rossignoli MT, Delfino-Pereira P, Garcia-Cairasco N, de Lima Umeoka EH. A comprehensive overview on stress neurobiology: basic concepts and clinical implications. Front Behav Neurosci. 2018;12:127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lyu RM, Huang XF, Zhang Y, et al. Phoenixin: a novel peptide in rodent sensory ganglia. Neuroscience. 2013;250:622-631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Belovicova K, Bogi E, Csatlosova K, Dubovicky M. Animal tests for anxiety-like and depression-like behavior in rats. Interdiscip Toxicol. 2017;10(1):40-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Jiang JH, He Z, Peng YL, et al. Effects of Phoenixin-14 on anxiolytic-like behavior in mice. Behav Brain Res. 2015;286:39-48. [DOI] [PubMed] [Google Scholar]
- 39. Hofmann T, Weibert E, Ahnis A, et al. Phoenixin is negatively associated with anxiety in obese men. Peptides. 2017;88:32-36. [DOI] [PubMed] [Google Scholar]
- 40. Wang J, Zheng B, Yang S, Tang X, Wei D. The protective effects of phoenixin-14 against lipopolysaccharide-induced inflammation and inflammasome activation in astrocytes. Inflamm Res. 2020;69(8):779-787. [DOI] [PubMed] [Google Scholar]
- 41. Sun G, Ren Q, Bai L, Zhang L. Phoenixin-20 suppresses lipopolysaccharide-induced inflammation in dental pulp cells. Chem Biol Interact. 2020;318:108971. [DOI] [PubMed] [Google Scholar]
- 42. Zhang B, Li J. Phoenixin-14 protects human brain vascular endothelial cells against oxygen-glucose deprivation/reoxygenation (OGD/R)-induced inflammation and permeability. Arch Biochem Biophys. 2020;682:108275. [DOI] [PubMed] [Google Scholar]
- 43. Zeng X, Li Y, Ma S, Tang Y, Li H. Phoenixin-20 ameliorates lipopolysaccharide-induced activation of microglial NLRP3 inflammasome. Neurotox Res. 2020;38(3):785-792. [DOI] [PubMed] [Google Scholar]
- 44. Ma H, Su D, Wang Q, et al. Phoenixin 14 inhibits ischemia/reperfusion-induced cytotoxicity in microglia. Arch Biochem Biophys. 2020;689:108411. [DOI] [PubMed] [Google Scholar]
- 45. Bernaus A, Blanco S, Sevilla A. Glia crosstalk in neuroinflammatory diseases. Front Cell Neurosci. 2020;14:209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Clemenzi MN, Wellhauser L, Aljghami ME, Belsham DD. Tumour necrosis factor alpha induces neuroinflammation and insulin resistance in immortalised hypothalamic neurones through independent pathways. J Neuroendocrinol. 2019;31(1):e12678. [DOI] [PubMed] [Google Scholar]
- 47. Chandrasekharan B, Jeppsson S, Pienkowski S, et al. Tumor necrosis factor-neuropeptide Y cross talk regulates inflammation, epithelial barrier functions, and colonic motility. Inflamm Bowel Dis. 2013;19(12):2535-2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ramanjaneya M, Chen J, Brown JE, et al. Identification of nesfatin-1 in human and murine adipose tissue: a novel depot-specific adipokine with increased levels in obesity. Endocrinology. 2010;151(7):3169-3180. [DOI] [PubMed] [Google Scholar]
- 49. Palasz A, Rojczyk E, Bogus K, Worthington JJ, Wiaderkiewicz R. The novel neuropeptide phoenixin is highly co-expressed with nesfatin-1 in the rat hypothalamus, an immunohistochemical study. Neurosci Lett. 2015;592:17-21. [DOI] [PubMed] [Google Scholar]
- 50. Yang F, Huang P, Shi L, Liu F, Tang A, Xu S. Phoenixin 14 inhibits high-fat diet-induced non-alcoholic fatty liver disease in experimental mice. Drug Des Devel Ther. 2020;14:3865-3874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Wei X, Lin H, Zhang B, et al. Phoenixin-20 prevents ox-LDL-Induced attachment of monocytes to human aortic endothelial cells (HAECs): a protective implication in atherosclerosis. ACS Chem Neurosci. 2021;12(6):990-997. [DOI] [PubMed] [Google Scholar]
- 52. Nagaraja AK, Andreu-Vieyra C, Franco HL, et al. Deletion of dicer in somatic cells of the female reproductive tract causes sterility. Mol Endocrinol. 2008;22(10):2336-2352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Heras V, Sangiao-Alvarellos S, Manfredi-Lozano M, et al. Hypothalamic miR-30 regulates puberty onset via repression of the puberty-suppressing factor, Mkrn3. PLoS Biol. 2019;17(11):e3000532. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.