Abstract
Background:
The prognosis of relapsed and unresectable Ewing sarcoma and osteosarcoma is dismal and unchanged over the last decades. Management of patients is based on the used of various cytotoxic regimens. However, pharmacologic inhibition of Met signaling and of aberrant angiogenesis has shown promising results in several preclinical models of Ewing sarcoma and osteosarcoma. This study aims to investigate the activity of the MET/VEGFR2 inhibitor, cabozantinib in patients with advanced Ewing and osteosarcoma.
Methods:
These are two multi-centre single-arm two-stage phase 2 trials assessing the efficacy and safety of cabozantinib in patients with advanced Ewing sarcoma or osteosarcoma. Main eligibility criteria included: age ≥ 12 years, ECOG Performance status ≤ 1, metastatic or unresectable locally advanced disease and documented disease progression (as per RECIST v1.1) before study entry. The number of previous lines of treatment was not limited. Patients received cabozantinib (oral route; adults: 60 mg, children: 40 mg/m2), daily until progressive disease or unacceptable toxicity. The primary endpoint was objective response for Ewing sarcoma and a dual one based on a 6-month objective response and non-progression of osteosarcoma.
Findings:
From April 16 2015 to July 12 2018, 90 patients were recruited (Ewing sarcoma: 45; Osteosarcoma: 45). Median follow-up was 31.3 months (95%CI: [12.4–35.4]) and 31.1 months (95%CI: [24.4–31.7]), for Ewing sarcomas and osteosarcomas, respectively. Thirty-nine (86.7%) Ewing sarcoma and 42 (93.3%) osteosarcoma were assessable for efficacy after histological and radiological review. Seven patients with osteosarcoma (16.7%) had partial response and 14 (33.3%) had stable disease. Ten patients with Ewing sarcoma (25.6%) had partial response and 15 (38.4%) had stable disease. Fourteen osteosarcoma patients (33.3%) and 10 Ewing sarcoma patients (25.6%) were progression-free at six months. Therapy was well tolerated, although grade 1 or grade 2 fatigue, diarrhea, mucositis and liver transaminitis were common. The most common grade 3 or 4 adverse events were hypophosphatemia (n=8, 8.9%), aspartate aminotransferase increase (n=5,5.6%), palmo-plantar syndrome (n=5, 5.6%), pneumothorax (=5, 5.6%), neutropenia (n=5, 5.6%). At least one serious adverse event was reported in 61 patients (67.8%).
Clinical Trial Registration: NCT02243605
Interpretation:
In this study, cabozantinib showed marked antitumor activity in patients with advanced Ewing sarcoma and osteosarcoma and may represent a new therapeutic option in this setting.
INTRODUCTION
Treatment of patients with recurrent Ewing sarcoma or osteosarcoma remains an important clinical challenge. The outcome is particularly poor, with a median overall survival of less than 12 months and a standard management remaining to be established [1, 2].
MET was originally identified as the protein product of the translocated promoter region (TPR)-MET transforming oncogene, which was derived from an osteosarcoma cell line [3]. Several studies have demonstrated how the HGF/SF and MET receptors might function together in activating biological properties that may contribute to osteosarcoma progression [4]. Indeed, wild-type or constitutively activated Met has been shown to drive osteoblast transformation [4]. Moreover, introduction of dominant-negative Met inhibits the in-vivo tumorigenicity of osteosarcoma cells [4]. A role for MET has also been demonstrated in Ewing sarcoma tumorigenesis [5]. Aberrant angiogenesis is also crucial for sustained osteosarcoma and Ewing sarcoma growth and metastasis. VEGFA is abundantly expressed in 74.1% of osteosarcoma cases, and patients with VEGFA-positive osteosarcomas had significantly worse tumor-free survival rates than patients with VEGFA-negative osteosarcomas [6]. A similar prognostic impact has been observed in Ewing sarcoma patients [7].
Cabozantinib (XL184) is the only VEGFR2 tyrosine kinase inhibtor which has also a specific MET receptor inhibitory activity [8] and has shown in vitro and in vivo anti-tumor activity in several osteosarcomas and Ewing sarcoma tumor models [9]. In collaboration with the National Cancer Institute (Cancer Therapy Evaluation Program), the French Sarcoma Group has conducted a clinical study (CABONE) comprising two phase II trials investigating cabozantinib in patients with advanced Ewing sarcomas and advanced osteosarcomas respectively. This study was registered with ClinicalTrials.gov number NCT02243605.
PATIENTS AND METHODS
Study design and participants
The CABONE study consisted of two phase 2 trials enrolling Ewing sarcomas and osteosarcomas patients respectively in 10 centers from the French Sarcoma Group. Patients were eligible if they were at least 12 years of age, had histologically confirmed Ewing sarcoma or osteosarcoma after central review, Eastern Cooperative Oncology Group Performance status 0–1, adequate renal, hepatic and cardiac functions (see Study protocol) and any type and number of previous treatment. For patients with Ewing sarcoma, the histological diagnosis had to be confirmed by fluorescence in situ hybridization or RT-PCR for assessment of EWS gene rearrangement. Blood tests included an assessment of blood cell count, alanine transaminase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), albumin, bilirubin, creatinine and urea nitrogen. A washout period of 21 days for previous chemotherapy was mandatory (see Study protocol). Key exclusion criteria included previous treatment with cabozantinib (see Study protocol). All patients with osteosarcoma had centrally documented progressive disease according to RECIST 1.1 [10] based on two imaging assessments obtained within less than a 6-month interval. As required by the French regulation, the protocol was centrally approved by a central IRB (the Comité de Protection des Personnes Sud-Ouest et Outre Mer III,Bordeaux, France) which reviewed the appropriateness of the clinical trial protocol as well as the risks and benefits to study participants. All patients provided written informed consent.
Study procedures
After an assessment of eligibility and a signature of informed consent, patients received cabozantinib 60 mg/day (or 40 mg/m2 in patients less than 16 years old) orally once daily in cycles of 28 days. Treatment was continued until disease progression, unacceptable toxicity, investigator’s decision or patient consent withdrawal. Subjects were monitored continuously for adverse events throughout the study. Adverse events were graded according to NCI-CTCAE v.4.0. Laboratory assessments were performed at baseline week two, week four and every four weeks afterwards. To manage adverse events, dose modifications were allowed that included dose interruptions and reductions. The dose of cabozantinib could be reduced to 40-mg and then to 20-mg from the starting dose of 60-mg. Tumor lesions were assessed according to RECIST v1.1 at baseline within 14 days before the first dose of cabozantinib, and every eight weeks until disease progression or the start of another treatment. 18F-FDG PET image was also performed at baseline and at week 4 after treatment onset [11].
Outcomes
For Ewing sarcomas, the primary endpoint was the best objective response within six months of treatment onset (six-month ORR). For osteosarcomas, we relied on a dual endpoint encompassing six-month ORR and the proportion of patients who had not progressed at 6 months (6-months NP) with six-month NP defined as the percentage of patients with complete response (CR), partial response (PR) or stable disease (SD) at six months of treatment onset as per RECIST 1.1 [12]. These endpoints were assessed based on a blinded and central review of the radiological data.
Secondary endpoints included safety as per NCI CTC-AE v4.0, best overall response (BOR), one- and two-year progression-free survival (PFS), overall survival (OS), and metabolic response as assessed by 18F-FDG PET. BOR was defined as the best response obtained from the start of treatment to the time of progression and was categorized as complete response, partial response, stable disease or progressive disease (PD) as per RECIST 1.1. PFS was defined as the time from treatment onset to the time of progression or death from any cause, whichever occurred first. Data for patients alive and progression-free were censored at the date of the last follow-up. OS was defined as the time from treatment onset to the time of death from any cause or last patient contact. Metabolic response based on standard [18F] fluoro-2-deoxy-D-glucose positron emission tomography was defined as per PERCIST criteria and classified as complete (CMR) or partial metabolic response (PMR), stable metabolic disease (SMD), or progressive metabolic disease (PMD) [11].
Exploratory analyses of potential plasma biomarkers of cabozantinib were performed by carrying plasma analysis for the following markers: vascular endothelial growth factor A (VEGF-A), hepatocyte growth factor (HGF), soluble vascular endothelial growth factor receptor 2 (sVEGFR2) and soluble (s)MET receptor.
Statistical Analysis
For Ewing sarcomas, we relied on a single-arm phase 2 trial, based on a two-stage Simon’s optimal design [14], with ORR as the primary endpoint (binary variable following a binomial distribution). Assuming 5% (H0: null hypothesis) and 20% (H1: alternative hypothesis) six-month ORR, 5% type I error rate and 90% power, 41 eligible and assessable patients were necessary (21 in the first stage and 20 in the second stage). At the final stage, cabozantinib would be considered promising if at least five patients had objective response.
For osteosarcomas, we relied on a single-arm phase 2 trial, based on a dual-endpoint design with ORR and NP as the primary endpoints (binary variables following a binomial distribution). Assuming 5% (H0) and 20% (H1) ORR, 25% (H0) and 50% (H1) NP, 5% type I error rate and 90% power, 41 assessable patients were necessary (21 in the first stage and 20 in second stage). At the final stage, cabozantinib would be considered promising if at least five patients had objective response or if at least 16 patients were progression-free at six months.
All enrolled patients who received at least one dose of cabozantinib were included in the safety analysis. The efficacy population included all subjects who met the eligibility criteria and who received at least one complete or two incomplete treatment cycles. The median follow-up was calculated using the reverse Kaplan-Meier method. Survival endpoints (PFS and OS) were described using the Kaplan-Meier method. Quantitative variables were described using the median and range, and qualitative variables were described using frequency and percentage. All eligible and assessable patients for efficacy were included in the denominator for the calculation of the proportions.
The growth modulation index (GMI) was estimated in patients who received prior chemotherapy for advanced disease as defined by Von Hoff [13]. For each patient, we calculated as a post-hoc analysis the ratio of time to progression with cabozantinib (TTPn) to the most recent prior line of therapy (TTPn-1). We considered GMI ≥ 1.33 as a marker of meaningful clinical activity [13].
Estimated parameters were reported with their two-sided 95% confidence interval (95%CI). p-value less than 0.05 (typically ≤ 0.05) were considered as statistically significant. Statistical analyses were performed using SAS version 9.4 software (SAS Institute, North Carolina, USA).
Role of the funding source
The study was sponsored by Institut Bergonié, Comprehensive Cancer Center (Bordeaux, France). NCI-CTEP provided cabozantinib under a Cooperative and Development Research Agreement established with Exelixis. The funding source (French National Cancer Institute) played no role in the design of this study and did not play any role during its execution, analysis, or in interpretation of the data or the decision to submit results. The corresponding author had full access to all of the data and the final responsibility to submit for publication
RESULTS
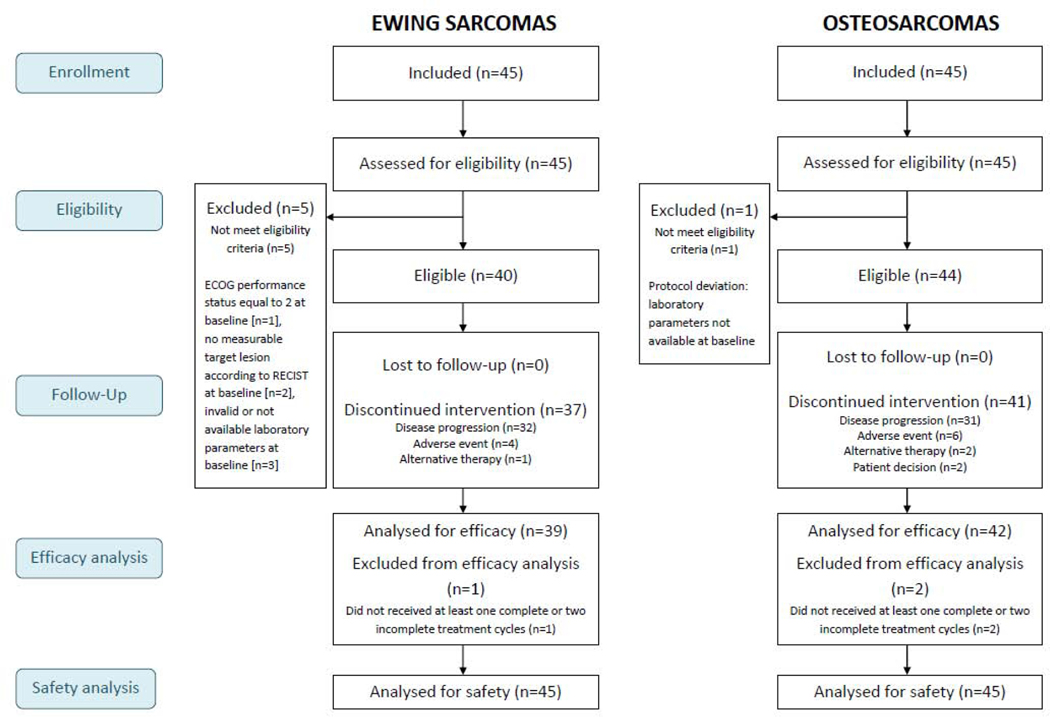
Between April 16 2015 and July 12 2018, 90 patients (45 Ewing sarcoma, 45 osteosarcomas) were recruited in ten study centers. Six and three patients were not eligible for the efficacy assessment for Ewing sarcomas and osteosarcomas respectively due to protocol deviations (Figure 1). Hence, 39 Ewing sarcoma and 42 osteosarcoma patients were included in the efficacy population. Characteristics of the patients are summarized in Table 1. Forty-two of 45 patients (93.3%) with Ewing sarcoma and 28 of 45 patients (62.2%) with osteosarcoma received previous lines of systemic therapy for advanced disease with a median of 2 lines (Q1-Q3 : 1–3) in both histologies. Most patients with osteosarcoma were rechallenged with methotrexate and /or platinum-anthracycline based regimen whereas patients with Ewing sarcoma received various regimens including: topotecan combined with cyclophophamide, irinotecan plus temozolomide, docetaxel combined with gemcitabine and high dose ifosfamide.
Figure 1. Flow-chart of patients included in the CABONE study.
Note: 5 Ewing sarcoma patients not eligible (protocol deviations: ECOG performance status equal to 2 at baseline [n=1], no measurable target lesion according to RECIST at baseline [n=2], invalid or not available laboratory parameters at baseline [n=3]. One Osteosarcoma not eligible (Protocol deviation: laboratory parameters not available at baseline).
Table 1.
Patient characteristics (N=90)
| Ewing Sarcoma |
Osteosarcoma |
|||
|---|---|---|---|---|
| Included / Safety population (N=45) | Efficacy population (N=39) | Included / Safety population (N=45) | Efficacy population (N=42) | |
| Sex n (%) | ||||
| Male | 31 (68.9) | 27 (69.2) | 27 (60.0) | 26 (61.9) |
| Female | 14 (31.1) | 12 (30.8) | 18 (40.0) | 16 (38.1) |
|
| ||||
| Age | ||||
| Median, years (Q1-Q3) | 33 (24–45) | 36 (23–45) | 34 (20–53) | 35 (21–53) |
| Age < 18 years old | 2 (4.4%) | 2 (5.1%) | 6 (13.3%) | 6 (14.3%) |
| Age ≥ 18 years old | 43 (95.6%) | 37 (94.9%) | 39 (86.7%) | 36 (85.7%) |
| ECOG PS n (%) | ||||
| 0 | 15 (33.3) | 15 (38.5) | 17 (37.8) | 16 (38.1) |
| 1 | 29 (64.4) | 24 (61.5) | 26 (57.8) | 25 (59.5) |
| 2 | 1 (2.2) | 0 (0.0) | 1 (2.2) | 1 (2.4) |
| 3 | 0 (0.0) | 0 (0.0) | 1 (2.2) | 0 (0.0) |
| Metastatic sites n (%) | ||||
| Lung | 32 (71.1) | 28 (71.8) | 39 (86.7) | 37 (88.1) |
| Pleura | 5 (11.1) | 4 (10.3) | 4 (8.9) | 4 (9.5) |
| Bone | 17 (37.8) | 13 (33.3) | 10 (22.2) | 10 (23.8) |
| Liver | 2 (4.4) | 2 (5.1) | 1 (2.2) | 1 (2.4) |
| Other | 8 (17.8) | 7 (17.9) | 5 (11.1) | 5 (11.9) |
| Prior lines of treatment for advanced disease n (%) a | ||||
| 0b | 3 (6.7) | 3 (7.7) | 17 (37.8) | 15 (35.7) |
| 1 | 12 (26.7) | 11 (28.2) | 10 (22.2) | 10 (23.8) |
| 2 | 13 (38.9) | 9 (23.1) | 10 (22.2) | 10 (23.8) |
| > 2 | 17 (37.8) | 16 (41.0) | 8 (17.8) | 7 (16.7) |
Patients received standard chemotherapy for locoregional disease, Previous lines of treatment included
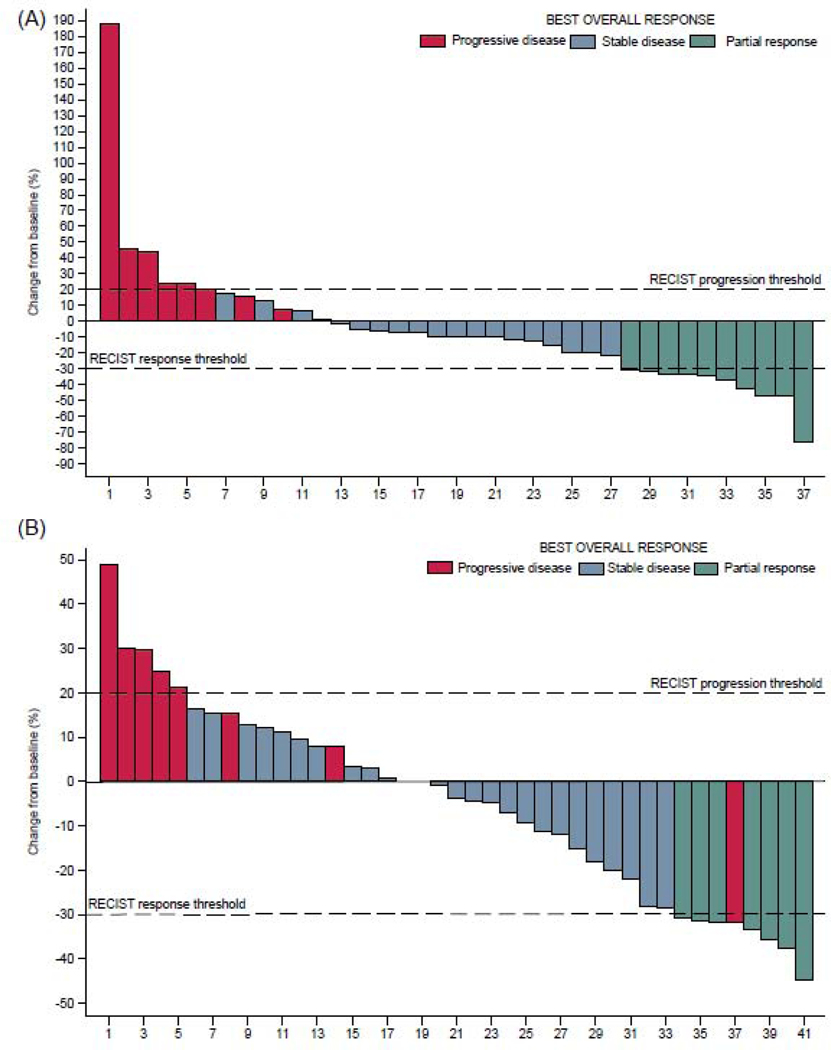
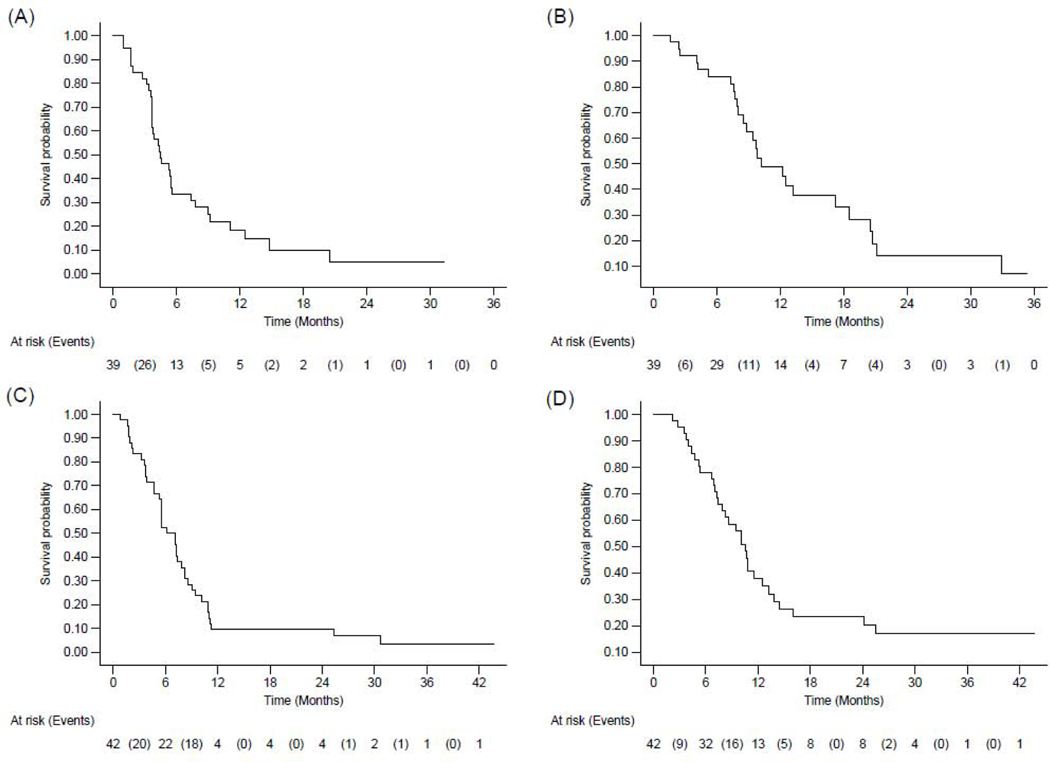
In the efficacy population and after a median follow-up of 31.3 months (95%CI: [12.4–35.4]), 13 out of 39 Ewing sarcoma patients (33.3%) were still alive, with three (7.7%) still under treatment. With regard to the primary endpoint, ten of 39 patients achieved a partial response leading to a 25.6% (95%CI: 13.0%−42.1%) objective response rate within six months, and as such, primary efficacy criterion was reached. With regard to the best overall response, among 39 patients with Ewing sarcoma in the efficacy population, 19 (48.7%) showed stable disease, including 15 (38.4%) with tumor shrinkage (range from −21.6% to −1.5%) (Figure 2). Eight (20.5%) had progressive disease as best overall response. Twenty-six eligible and assessable patients with Ewing sarcoma died during the course of the trial and 34 eligible patients had progressive disease (n=32) or died (n=2).Median PFS and OS were 4.4 months (95%CI: [3.7–5.6]) and 10.2 months (95%CI: [8.5–18.5]) respectively (Figure 3). The 6-month non-progression rate was 25.6% (13.0–42.1). The 6-, 12- and 24-month PFS were 33.3% (95%CI: [19.3–48.0]), 18.3% (95%CI: [7.6; 32.6]), and 4.9% (95%CI: [0.4– 19.0]) respectively. The 6-, 12- and 24-month OS rate were 84.0% (95%CI: [67.9; 92.5]), 48.8% (95%CI: [30.7; 64.7]), and 14.2% (95%CI: [3.8; 31.1]) respectively.
Figure 2: Waterfall plot of best overall response in Ewing sarcoma (A) and osteosarcoma patients (B) treated with cabozantinib (Response based on central review assessment according to RECIST 1.1).
Note: Two Ewing sarcoma patients had no tumor assessment due to early discontinuation of treatment because of end of treatment after cycle 1 of Cabozantinib, for disease progression and toxicity respectively. One osteosarcoma patient had not tumor assessment due to early treatment discontinuation because of toxicity. These three patients are classified “Not evaluable” as per RECIST 1.1.
Despite tumor shrinkage one osteosarcoma patient (ID = 37) had progressive disease as best overall response. This patient had a single tumor assessment during treatment; although shrinkage of target lesion was observed, a new lesion was identified. He was as such classified as progressive disease as per RECIST 1.1.
Figure 3. Kaplan-Meier curves of progression-free survival (A, C) and overall survival (B,D) in Ewing sarcoma (A, B) and osteosarcoma patients (C, D).
Note: For overall survival, 26 eligible and assessable patients with Ewing sarcoma (B) and 32 eligible and assessable patients with osteosarcoma (D) died during the course of the trial. For progression-free survival, 34 eligible and assessable patients with Ewing sarcoma (A) and 40 eligible and assessable patients with osteosarcoma (C) had progressive disease or died during the course of the trial.
Among the 39 patients eligible and assessable for efficacy, 31 (7.9%) were evaluable for early metabolic response at the end of one cycle of cabozantinib. Of those, 13 (41.9%) had PMR, 9 (29.0%) had SMD, and 9 (29.0%) had PMD (Appendix Figure 1 page 4). The metabolic tumor response rate was therefore 41.9% (95%CI: [24 .5; 60.9]). Median PFS in months was 5.4 (95%CI: [3.7–8.9]), 4.2 (95%CI: [1.7–9.2]), 2.7 (95%CI: [0.9–4.4]) for patients with PMR, SMD and PMD, respectively (log-rank test: p = 0.002) (Appendix Figure 2 page 5).
As three patients did not receive prior chemotherapy for advanced disease, GMI was assessable as a post-hoc analysis for 36 patients (Appendix Table 1 page 3 and Figure 3 page 6). Twelve (33.3%) had GMI greater or equal to 1.33.
In the efficacy population, after a median follow-up of 31.1 months (95%CI: [24.4–31.7]), 10 (23.8%) out of 42 osteosarcoma patients were still alive with three patients still under treatment. With regard to the dual endpoint design, among 42 patients with osteosarcoma in the efficacy population, five patients (11.9%) had objective response (PR) and 14 (33.3%) were progression-free at six months (12 SD and 2 PR), and as such, primary efficacy criterion was reached. The six-month NP was 33.3% (95%CI: [(19.6–49.6)]) and ORR within six months was 11.9% (95%CI: [4.0; 25.6]). With regards to the BOR, out of 42 patients eligible and assessable for efficacy with osteosarcoma, seven (16.7%) patients achieved PR and 26 (61.9%) showed SD. Fourteen SD patients (33.3%) had tumor shrinkage (with a range of −28.4% to −0.9%) (Figure 2). Eight (19.0%) had progressive disease as best overall response. Thirty-two eligible and assessable patients with osteosarcoma died during the course of the trial and 40 eligible patients had progressive disease (n=31) or died (n=9). Median PFS and OS were 6.7 months (95%CI: [5.4–7.9]) and 10.6 months (95%CI: [7.4–12.5]) respectively (Figure 3). The 4-, 6-, 12- and 24-month PFS were 71.4% (95%CI: [55.2; 82.6]), 52.4% (95%CI: [36.4; 66.1]), 9.5% (95%CI: [3.0; 20.6]), and 9.5% (95%CI: [3.0; 20.6]) respectively. The 6-, 12- and 24-month OS were 78.0% (95%CI: [62.1; 87.9]), 37.9% (95%CI: [23.2; 52.6]) and 23.3% (95%CI: [11.4; 37.8]) respectively.
One patient with PR to cabozantinib had surgery of lung metastases. Histological assessment showed no residual tumor cells.
Among the 42 patients eligible and assessable for efficacy, 31 were evaluable for early metabolic response at the end of one cycle of cabozantinib. Of those, 20 (64.5%) had PMR, eight (25.8%) had SMD, and three (9.7%) had PMD (Appendix Figure 1 page 4). The metabolic tumor response rate was therefore 64.5% (95%CI: [45.4; 80.8]). Median PFS in months was 7.2 (95%CI: [4.7; 10.9]), 4.5 (95%CI: [1.8; 9.5]), 1.8 (95%CI: [0.8; 1.9]) for patients with PMR, SMD and PMD, respectively (log-rank test: p <.0001) (Appendix Figure 2 page 5).
As 15 patients did not receive prior chemotherapy for advanced disease, GMI was assessable as a post-hoc analysis for 27 patients (Appendix Table 1 page 3 and Figure 3 page 6). As observed in the Ewing sarcoma strata, more than one third of patients had a GMI greater or equal to 1.33 (n=10, 37.0%).
All included patients received at least one dose of cabozantinib, hence 90 patients were evaluated for safety. Treatment with cabozantinib was generally well tolerated, although virtually every patient had grade 1 or 2 adverse events (AE) related to therapy. Treatment-related AE and laboratory abnormalities that were reported in more than 5% of patients for grade 1–2 in one of the two cohorts and any for grade 3 and 4 are shown in Table 2. The most common treatment-related AE were fatigue, diarrhea, mucositis, hypothyroidism, nausea and anorexia. Among 90 patients assessable for safety, at least one serious adverse event was reported in 61 patients (67.8%). 19 patients out of 90 (21.1%) experienced dose reduction because of a drug-related adverse event. No patient died from drug-related toxicity.
Table 2.
Treatment-related Adverse Events during the treatment period in ≥ 5% of patients (N=90)
| Adverse event | Histology | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ewing sacoma (n=45) | Osteosarcoma (n=45) | |||||||||||
| G1/2 | G3 | G4 | G1/2 | G3 | G4 | |||||||
| n | (%) | n | (%) | n | (%) | n | (%) | n | (%) | n | (%) | |
| Fatigue | 26 | (57.8) | 3 | (6.7) | . | . | 29 | (64.4) | 1 | (2.2) | . | . |
| Diarrhea | 23 | (51.1) | 2 | (4.4) | . | . | 29 | (64.4) | 1 | (2.2) | . | . |
| Mucositis oral | 24 | (53.3) | 1 | (2.2) | . | . | 21 | (46.7) | 3 | (6.7) | . | . |
| Hypothyroidism | 22 | (48.9) | . | . | . | . | 20 | (44.4) | . | . | . | . |
| ASAT increase | 20 | (44.4) | 2 | (4.4) | . | . | 16 | (35.6) | 3 | (6.7) | . | . |
| ALAT increase | 17 | (37.8) | 2 | (4.4) | . | . | 18 | (40.0) | 2 | (4.4) | . | . |
| Nausea | 19 | (42.2) | . | . | . | . | 12 | (26.7) | . | . | . | . |
| Anorexia | 21 | (46.7) | . | . | . | . | 9 | (20.0) | . | . | . | . |
| Hair color changes | 15 | (33.3) | . | . | . | . | 15 | (33.3) | . | . | . | . |
| Palmar-plantar syndrom | 11 | (24.4) | 3 | (6.7) | . | . | 16 | (35.6) | 2 | (4.4) | . | . |
| Thrombocytopenia | 13 | (28.9) | . | . | . | . | 14 | (31.1) | 2 | (4.4) | . | . |
| Dry skin | 11 | (24.4) | . | . | . | . | 16 | (35.6) | . | . | . | . |
| Dysgeusia | 9 | (20.0) | . | . | . | . | 13 | (28.9) | . | . | . | . |
| Weight loss | 7 | (15.6) | 3 | (6.7) | . | . | 9 | (20.0) | . | . | . | . |
| Hypophosphatemia | 5 | (11.1) | 5 | (11.1) | . | . | 10 | (22.2) | 3 | (6.7) | . | . |
| Neutropenia | 7 | (15.6) | 2 | (4.4) | . | . | 5 | (11.1) | 3 | (6.7) | 1 | (2.2) |
| Dysphonia | 8 | (17.8) | . | . | . | . | 4 | (8.9) | . | . | . | . |
| Alopecia | 6 | (13.3) | . | . | . | . | 6 | (13.3) | . | . | . | . |
| Abdominal pain | 6 | (13.3) | . | . | . | . | 5 | (11.1) | . | . | . | . |
| Hypomagnesemia | 8 | (17.8) | 1 | (2.2) | 1 | (2.2) | 3 | (6.7) | 2 | (4.4) | . | . |
| Anemia | 4 | (8.9) | . | . | . | . | 6 | (13.3) | 1 | (2.2) | . | . |
| Vomiting | 4 | (8.9) | . | . | . | . | 6 | (13.3) | . | . | . | . |
| TSH increase | 6 | (13.3) | . | . | . | . | 4 | (8.9) | . | . | . | . |
| Hypokalemia | 6 | (13.3) | . | . | . | . | 4 | (8.9) | . | . | . | . |
| Headache | 4 | (8.9) | . | . | . | . | 6 | (13.3) | . | . | . | . |
| Proteinuria | 6 | (13.3) | . | . | . | . | 4 | (8.9) | . | . | . | . |
| Skin hypopigmentation | 5 | (11.1) | . | . | . | . | 5 | (11.1) | . | . | . | . |
| Constipation | 5 | (11.1) | . | . | . | . | 4 | (8.9) | . | . | . | . |
| Gastroesophageal reflux disease | 3 | (6.7) | . | . | . | . | 6 | (13.3) | . | . | . | . |
| Myalgia | 6 | (13.3) | . | . | . | . | 3 | (6.7) | . | . | . | . |
| PAL increased | 2 | (4.4) | 1 | (2.2) | . | . | 6 | (13.3) | . | . | . | . |
| Erythema multiforme | 4 | (8.9) | . | . | . | . | 4 | (8.9) | . | . | . | . |
| Lipase increased | 5 | (11.1) | 3 | (6.7) | 1 | (2.2) | 2 | (4.4) | 1 | (2.2) | 1 | (2.2) |
| Pneumothorax | 3 | (6.7) | 1 | (2.2) | . | . | 4 | (8.9) | 4 | (8.9) | . | . |
| Dry mouth | 1 | (2.2) | . | . | . | . | 6 | (13.3) | . | . | . | . |
| Hypocalcemia | 5 | (11.1) | . | . | . | . | 2 | (4.4) | . | . | . | . |
| Epistaxis | 3 | (6.7) | . | . | . | . | 4 | (8.9) | . | . | . | . |
| Leucopenia | 3 | (6.7) | . | . | . | . | 3 | (6.7) | 2 | (4.4) | . | . |
| Hypertension | 3 | (6.7) | 2 | (4.4) | . | . | 2 | (4.4) | 1 | (2.2) | . | . |
| Dysphagia | 2 | (4.4) | . | . | . | . | 3 | (6.7) | . | . | . | . |
| Hyperbilirubinemia | 3 | (6.7) | . | . | . | . | 2 | (4.4) | . | . | . | . |
| CPK increased | 1 | (2.2) | . | . | . | . | 4 | (8.9) | . | . | . | . |
| Cough | 2 | (4.4) | . | . | . | . | 3 | (6.7) | . | . | . | . |
| Rash maculo-papular | 4 | (8.9) | . | . | . | . | 1 | (2.2) | . | . | . | . |
ALAT: alanine aminotransferase; ALP: alkaline phosphatase; ASAT: asparte aminotransferase; CPK:creatine phosphokinase; TSH: thyroid-stimulating hormone
Twelve patients (13.3%, four osteosarcomas and eight Ewing sarcomas) out of 90 had a pneumothorax related to cabozantinib. A central review of imaging performed at baseline and at the occurrence of pneumothorax showed the presence of pleural or sub-pleural metastases at baseline in all cases and cavitation of lung metastases secondary to cabozantinib-induced tumor necrosis in seven cases. Ten patients underwent pleural drainage with a chest tube leading to full recovery in all cases but one. Treatment discontinuation due to pneumothorax occurred in three cases.
AE led to dose modification or definitive treatment discontinuation in 35 (38.9%) patients out of 90. Among 84 patients assessable for safety who stopped treatment, overall reasons for treatment discontinuation included disease progression (78.6%), AE (14.3%), death on study due to disease progression (1.2%), patient decision (2.4%), initiation of a subsequent treatment (3.6%) (Appendix Table 2 page 3).
Among 39 eligible and assessable patients in Ewing sarcoma strata, biomarkers were available for 35 patients. Although levels of VEGFA, HGF, and VEGFR2 were not associated with outcome, we observed a non-significant trend for improved PFS in patients with high level of soluble c-MET receptor at baseline (median PFS: 5.5 [4.3–12.5] versus 3.7 [2.8–7.4], p=0.07). In osteosarcoma patients, this exploratory analysis suggests a correlation between low VEGF-A levels and improved OS (13.2 months [10.1-NA] versus 8.2 [4.0–10.8], p=0.014, log-rank test, α=5%) and high sMET levels and improved PFS (7.8 [5.6–10.9] versus5.4 [3.7–6.2] p=0.016, log-rank test, α=5%), among 36 patients eligible and assessable with available biomarkers (Appendix Figure 4 page 7).
DISCUSSION
The CABONE study reached its primary efficacy endpoint in both Ewing sarcomas and osteosarcomas cohorts. Indeed, ten Ewing sarcoma patients out of 39 achieved a partial response leading to a 25.6% (95%CI: 13.0%−42.1%) objective response rate and 14 osteosarcoma patients out of 42 (33.3%) were progression-free at six months.
Topotecan combined with cyclophophamide, irinotecan plus temozolomide, docetaxel combined with gemcitabine and high dose ifosfamide represent the most widespread regimens on the setting of refractory or recurrent ES. Published evidence of the activity of these regimens is weak, mainly based on a mixture of single institution retrospective reviews and early phase trials, each including small numbers of evaluable patients with Ewing sarcoma [15]. Importantly, most of the Ewing sarcoma patients included in the CABONE study had already received these salvage regimens before inclusion and therefore represented a heavily pre-treated population with highly refractory disease.
Among targeted therapies, IGF-1R inhibitors have been the most thoroughly studied in Ewing sarcoma. Despite preliminary promising results from early phase studies, phase 2 studies of four different single-agent IGF-1R antibodies have produced disappointing efficacy results with objective response rates of <15% and median progression-free survival of <2 months in adults and children with recurrent tumors. [16].
The majority of Ewing sarcoma patients treated with cabozantinib experienced tumor shrinkage. The objective response rate is among the highest ever observed with a tyrosine kinase inhibitor targeting the VEGFR2 pathway in solid tumors, with the exception of renal cell carcinoma, which has an exquisite sensitivity to drugs targeting the VEGR pathway [17]. These data are in line with pre-clinical studies that showed inhibition of Ewing sarcoma growth with VEGFR receptors inhibitors [18]. Interestingly, such compounds resulted in little in vitro but significant in vivo activity supporting an anti-angiogenic effect, rather than a direct tumor cell inhibitory effect. MET is ubiquitously expressed in Ewing sarcoma and high expression has been associated with adverse outcomes [7]. Whether VEGFR inhibition has no or minimal impact in vitro, cabozantinib induced growth inhibition in several Ewing sarcoma cell lines. This activity is correlated with the level of MET expression [7]. Therefore, MET inhibition may contribute to the clinical activity of cabozantinib in this setting, as suggested by the non-significant trend for better outcome in patients with high levels of soluble c-MET receptor at baseline.
Objective response may not be an appropriate surrogate marker for therapeutic activity in osteosarcoma. Indeed, due to the abundant bone matrix, substantial anti-tumor activity may not result in a marked decrease in overall tumor volume. Moreover, non-progression rate is a worldwide recognized endpoint to assess new investigational agents in advanced sarcoma patients [19]. For this reason, we chose for the osteosarcoma cohort a dual endpoint combining progression-free survival and objective response. A systematic review of summary of seven negative phase II trials, published after the activation of the CABONE study, recommended in fact to consider an agent worth further investigation in advanced ostesarcomas if it is associated with a 16-week progression-free survival greater than 30% [20]. The CABONE study establishes a 71.4% (95%CI: [55.2; 82.6]) 16-week progression-free survival with cabozantinib. This level of activity appears higher than those which were reported with other angiogenesis inhibitors even when used with in combinations with other anti-cancer agents [21–24]. One can argue that progression-free survival is not a direct reflection of drug activity and may be influenced by the natural history of the disease. However, and contrary to previous advanced osteosarcomas studies using progression-free survival as their first endpoint, all patients included in the CABONE study had to have their progressive disease confirmed by a central review of two imaging studies performed in less than six-month intervals. Moreover, 50% of patients experienced tumor shrinkage and 16.7% had an objective response (two being delayed occurring more than 6 months after treatment onset). This represents a more direct measure of anti-tumor activity attributable to the drug, these proportions being the highest ever reported with targeted therapy in osteosarcomas. It is also important to recognize that calcification or necrosis of osteosarcoma lesions can arise even in the absence of tumor shrinkageas illustrated by the patient who underwent surgery of lung metastases which showed complete histological response despite only partial response according to RECIST. The activity of cabozantinib observed in this study may be related at least in part to MET pathway inhibition. Indeed, pharmacologic c-MET inhibition with drugs lacking anti-angiogenic activity significantly inhibited tumor growth and associated osteolysis and osteoid production in several in vivo osteosarcoma models [25]. The significantly higher PFS observed in our study in patients with high levels of soluble c-MET at baseline suggest that this biomarker may be predictive of cabozantinib benefit in osteosarcoma patients. However, besides its action on the MET pathway, a recent study suggested that cabozantinib can impact osteosarcoma growth by decreasing the production of RANK ligand by osteoblasts and therefore the proliferation of RANK-positive overall survival cells [26].
Two recent meta-analyses have suggested the value of [18F] FDG-PET/CT for the diagnosis, staging and follow-up of patients with Ewing sarcomas and osteosarcomas [27, 28]. In this study, the prognostic value of early [18F] FDG-PET/CT response in patients with advanced Ewing sarcomas and osteosarcomas treated with a tyrosine kinase inhibitor was reported for the first time. Our results suggested that early metabolic response assessed by [18F] FDG-PET/CT is a potential biomarker for benefit of cabozantinib in this setting. Such data may represent a rationale to investigate modifications of therapy based on [18F] FDG-PE-guided strategies in further studies assessing cabozantinib and other regimens in Ewing sarcoma and osteosarcoma.
Limitations
The main limitation of the CABONE study is its non-randomized design. Indeed, by minimizing many sources of potential bias, randomized, controlled clinical trials provide the most robust information about the effects of investigational drugs. However, recurrent ES and osteosarcomas are rare diseases; approximately 250 ES and 300 osteosarcomas patients are estimated per year across the EU. Randomized controlled trials are challenging for these conditions. Therefore, innovative endpoints that incorporate patients as their own control can be particularly useful in this setting. Growth Modulation Index (GMI), is the ratio of time to progression with nth line of therapy (TTPn) to the most recent prior line of therapy (TTPn-1) and a GMI ≥ 1.33 has been proposed as a marker of meaningful clinical activity [13]. We found here that more than one patient out of three with Ewing sarcoma or osteosarcoma and treated with cabozantinib had a GMI > 1.33. Interestingly, this proportion is like that reported in the MOSCATO 01 trial (NCT01566019), which was one of the largest precision medicine studies using high-throughput molecular analysis to guide targeted therapy for patients with advanced solid tumors. In that study, GMI was >1.3 in 33% of the patients treated with an innovative drug matched to the tumor molecular profile [29]. This proportion was also similar with that observed in sarcoma patients treated with trabectedin, an alkylating agent recently approved by the FDA for the management of patients with advanced soft-tissue sarcomas [30]. Nevertheless, even if the usefulness of this endpoint has been recognized by regulatory authorities such as the European Medicine Agency, the analysis we performed here remains exploratory and further studies are needed to confirm the reliability of GMI to assess the efficacy of experimental drugs in Ewing sarcomas and osteosarcomas. The safety profile of cabozantinib was manageable. Twelve patients (13.3%) experienced pneumothorax. Sarcomas predominantly metastasizes to the lungs. A recent review investigating the incidence of pneumothorax in sarcoma patients demonstrated that this complication can occur in every kind of sarcoma histological subtype before or during treatment [31]. Pneumothorax have also been described, with an incidence of up 14%, in patients receiving pazopanib, a tyrosine kinase inhibitor targeting VEGFR2 and approved for the treatment of advanced soft tissue sarcoma in patients who have received previous chemotherapy [32–33]. The presence of sub-pleural or pleural metastases as well as of cavitary lung lesions cavitation have been identified as the main risk factors. In the CABONE study, all the patients with pneumothorax had subpleural or pleural metastases at baseline. Pneumothorax were observed in both progressive and responding patients, were manageable and lead to treatment discontinuation in only 2 cases.
In conclusion, cabozantinib is active in advanced Ewing and osteosarcoma and may represent a new therapeutic option in patients suffering from these devastating diseases.
Supplementary Material
RESEARCH IN CONTEXT.
Evidence before this study
We searched PubMed with the terms “recurrent osteosarcoma” OR “relapsed osteosarcoma” OR “metastatic osteosarcoma” OR “recurrent Ewing sarcoma” OR “relapsed Ewing sarcoma” OR “metastatic Ewing sarcoma” AND “clinical trial” NOT “review” for clinical trials done in humans published in English up to July 31, 2019.
Among targeted therapies, IGF-1R inhibitors and anti-angiogenic drugs have been the most thoroughly studied in Ewing sarcoma and osteosarcoma, respectively. Efficacy results of IGF-1R were disappointing with objective response rates of <15% and median progression-free survival of <2 months in adults and children with recurrent Ewing sarcomas. Two drugs targeting angiogenic receptors (sorafenib and regorafenib) have shown promising signs of activity in four phase 2 studies enrolling advanced osteosarcomas patients. To the best of our knowledge, no previous trial assessing the activity of cabozantinib or other drugs targeting c-MET in Ewing sarcoma and osteosarcoma has been published.
Added value of this study
Pre-clinical and translational studies have suggested the role of aberrant angiogenesis as well as of c-MET pathway activation in Ewing sarcomas and osteosarcomas. Our results show that cabozantinib, an inhibitor of c-MET and VEGFR2 kinase activity, already approved for the management of medullary thyroid cancer, renal cancer and hepatocarcinoma, induces RECIST partial response in 25% and 16% of heavily pre-treated patients with Ewing sarcoma and osteosarcoma respectively. Thirty-three percent and 25% of osteosarcoma and Ewing sarcoma patients were progression-free at six months, respectively.
Implications of all the available evidence
To our knowledge, the CABONE study is the first study investigating a therapy targeting both angiogenic and c-MET receptors in patients with advanced Ewing sarcomas and osteosarcomas. Our results indicate that cabozantinib may represent a new therapeutic option for patients suffering from these diseases.
ACKNOWLEDGMENTS
This study was funded by the Institut National du Cancer (INCA) and I’Association pour la Recherche contre le Cancer (ARC)
This study was sponsored by Institut Bergonié and funding support was provided by the French National Cancer Institute and Association pour la Recherche contre le Cancer.
DECLARATION OF INTERESTS
AC, CB, CL, CC, EB, ES, FD, HP, IRC, JW, MK, MT, NG, NP, NEW, PMB, SMP, SPN: nothing to disclose
JYB reports grants from Ipsen, grants from Exelixis, grants and personal fees from Bayer, grants and personal fees from Novartis, grants and personal fees from GSK, during the conduct of the study.
AB: personal fees from Immusmol/Explicyte
OM: Personal fees from Amgen, Bayer Healthcare, Blueprint Medicine, Novartis, Pfizer, Ipsen, Roche, Lilly, Lundbeck, Janssen
AI: research grants MSD , BMS, ROCHE, personal fees: Epizyme, Bayer, Lilly, Roche, Springworks; non-financial support: Merck
Footnotes
DATA SHARING STATEMENT
Individual participant data that underlie the results reported in this article will be available after deidentification beginning 24 months and ending 48 months following article publication to researchers who will provide a methodologically sound proposal. Request should be sent to the corresponding author.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.McTiernan A, Driver D, Michelagnoli MP, Kilby AM, Whelan JS. High dose chemotherapy with bone marrow or peripheral stem cell rescue is an effective treatment option for patients with relapsed or progressive Ewing’s sarcoma family of tumours. Ann Oncol. 2006;17:1301–5. [DOI] [PubMed] [Google Scholar]
- 2.Leary SE, Wozniak AW, Billups CA, et al. Survival of pediatric patients after relapsed osteosarcoma: the St. Jude Children’s Research Hospital experience.Cancer. 2013;119:2645–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cooper CS, Park M, Blair DG, Tainsky MA, Huebner K, Croce CM, et al. (1984) Molecular cloning of a new transforming gene from a chemically transformed human cell line. Nature 311: 29–33 [DOI] [PubMed] [Google Scholar]
- 4.Patanè S, Avnet S, Coltella N, et al. MET overexpression turns human primary osteoblasts into osteosarcomas. Cancer Res. 2006;66:4750–7. [DOI] [PubMed] [Google Scholar]
- 5.Fleuren ED, Roeffen MH, Leenders WP, et al. Expression and clinical relevance of MET and ALK in Ewing sarcomas. Int J Cancer. 2013;133:427–36. [DOI] [PubMed] [Google Scholar]
- 6.Yang J, Yang D, Sun Y, et al. Genetic amplification of the vascular endothelial growth factor (VEGF) pathway genes, including VEGFA, in human osteosarcoma. Cancer. 2011;117:4925–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DuBois SG, Marina N, Glade-Bender J. Angiogenesis and vascular targeting in Ewing sarcoma: a review of preclinical and clinical data. Cancer. 2010;116:749–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Uitdehaag JC, de Roos JA, van Doornmalen AM, et al. Comparison of the cancer gene targeting and biochemical selectivities of all targeted kinase inhibitors approved for clinical use. PLoS One. 2014;9(3):e92146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith M, Kang M, Reynolds P, et al. Pediatric Preclinical Testing Program (PPTP) stage 1 evaluation of cabozantinib. [abstract]. In: Proceedings of the 104th Annual Meeting of the American Association for Cancer Research; 2013 Apr 6–10; Washington, DC. Philadelphia (PA): AACR; Cancer Res; 2013;73(8 Suppl):Abstract nr LB-353. [Google Scholar]
- 10.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228–47. [DOI] [PubMed] [Google Scholar]
- 11.O JH, Lodge MA, Wahl RL. Practical PERCIST: A Simplified Guide to PET Response Criteria in Solid Tumors 1.0. Radiology. 2016;280:576–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goffin JR, Tu D. Phase II stopping rules that employ response rates and early progression. J Clin Oncol. 2008;26:3715–20. [DOI] [PubMed] [Google Scholar]
- 13.Von Hoff DD. There are no bad anticancer agents, only bad clinical trial designs--twenty-first Richard and Hinda Rosenthal Foundation Award Lecture. Clin Cancer Res. 1998;4:1079–86. [PubMed] [Google Scholar]
- 14.Simon R. Optimal two-stage designs for phase II clinical trials. Control Clin Trials. 1989;10:1–10. [DOI] [PubMed] [Google Scholar]
- 15.Van Mater D, Wagner L. Management of recurrent Ewing sarcoma: challenges and approaches. Onco Targets Ther. 2019;12:2279–2288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vornicova O, Bar-Sela G. Investigational therapies for Ewing sarcoma: a search without a clear finding. Expert Opin Investig Drugs. 2016;25:679–86. [DOI] [PubMed] [Google Scholar]
- 17.Iacovelli R, Sternberg CN, Porta C, et al. Inhibition of the VEGF/VEGFR pathway improves survival in advanced kidney cancer: a systematic review and meta-analysis. Curr Drug Targets. 2015;16:164–70 [DOI] [PubMed] [Google Scholar]
- 18.DuBois SG, Marina N, Glade-Bender J. Angiogenesis and vascular targeting in Ewing sarcoma: a review of preclinical and clinical data. Cancer. 2010;116:749–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Van Glabbeke M, Verweij J, Judson I, Nielsen OS; EORTC Soft Tissue and Bone Sarcoma Group. Progression-free rate as the principal end-point for phase II trials in soft-tissue sarcomas. Eur J Cancer. 2002;38:543–9. [DOI] [PubMed] [Google Scholar]
- 20.Lagmay JP, Krailo MD, Dang H, et al. Outcome of Patients With Recurrent Osteosarcoma Enrolled in Seven Phase II Trials Through Children’s Cancer Group, Pediatric Oncology Group, and Children’s Oncology Group: Learning From the Past to Move Forward. J Clin Oncol. 2016;34:3031–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Davis LE, Bolejack V, Ryan CW, et al. Randomized Double-Blind Phase II Study of Regorafenib in Patients With Metastatic Osteosarcoma. J Clin Oncol. 2019;37:1424–1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Duffaud F, Mir O, Boudou-Rouquette P, et al. Efficacy and safety of regorafenib in adult patients with metastatic osteosarcoma: a non-comparative, randomised, double-blind, placebo-controlled,phase 2 study. Lancet Oncol. 2019;20:120–133. [DOI] [PubMed] [Google Scholar]
- 23.Grignani G, Palmerini E, Ferraresi V, et al. Sorafenib and everolimus for patients with unresectable high-grade osteosarcoma progressing after standard treatment: a non-randomised phase 2 clinical trial. Lancet Oncol. 2015;16:98–107. [DOI] [PubMed] [Google Scholar]
- 24.Martin-Broto J, Redondo A, Valverde C, et al. Gemcitabine plus sirolimus for relapsed and progressing osteosarcoma patients after standard chemotherapy: a multicenter, single-arm phase II trial of Spanish Group for Research on Sarcoma (GEIS). Ann Oncol. 2017;28:2994–2999. [DOI] [PubMed] [Google Scholar]
- 25.Sampson ER, Martin BA, Morris AE, et al. The orally bioavailable met inhibitor PF-2341066 inhibits osteosarcoma growth and osteolysis/matrix production in a xenograft model. J Bone Miner Res. 2011. Jun;26(6):1283–94. [DOI] [PubMed] [Google Scholar]
- 26.Fioramonti M, Fausti V, Pantano F, et al. Cabozantinib Affects Osteosarcoma Growth Through A Direct Effect On Tumor Cells and Modifications In Bone Microenvironment. Sci Rep. 2018;8:4177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang T, Li F, Yan Z, et al. Effectiveness of 18F-FDG PET/CT in the diagnosis, staging and recurrence monitoring of Ewing sarcoma family of tumors: A meta-analysis of 23 studies. Medicine (Baltimore). 2018;97(48):e13457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu F, Zhang Q, Zhou D, Dong J. Effectiveness of 18F-FDG PET/CT in the diagnosis and staging of osteosarcoma: a meta-analysis of 26 studies. BMC Cancer. 2019;19:323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Massard C, Michiels S, Ferté C, et al. High-Throughput Genomics and Clinical Outcome in Hard-to-Treat Advanced Cancers: Results of the MOSCATO 01 Trial. Cancer Discov. 2017; 7:586–595. [DOI] [PubMed] [Google Scholar]
- 30.Penel N, Demetri GD, Blay JY, et al. Growth modulation index as metric of clinical benefit assessment among advanced soft tissue sarcoma patients receiving trabectedin as a salvage therapy. Ann Oncol. 2013;24:537–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hoag JB, Sherman M, Fasihuddin Q, Lund ME. A comprehensive review of spontaneous pneumothorax complicating sarcoma. Chest. 2010;138:510–8. [DOI] [PubMed] [Google Scholar]
- 32.Sabath B, Muhammad HA, Balagani A, et al. Secondary spontaneous pneumothorax in patients with sarcoma treated with Pazopanib, a case control study. BMC Cancer. 2018;18:937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Verschoor AJ, Gelderblom H. Pneumothorax as adverse event in patients with lung metastases of soft tissue sarcoma treated with pazopanib: a single reference centre case series. Clin Sarcoma Res. 2014. Oct 1;4:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.