Abstract
The Alaska blackfish (Dallia pectoralis) is a facultative air-breather endemic to northern latitudes where it remains active in winter under ice cover in cold hypoxic waters. To understand the changes in cellular Ca2+ cycling that allow the heart to function in cold hypoxic water, we acclimated Alaska blackfish to cold (5 °C) normoxia or cold hypoxia (2.1–4.2 kPa; no air access) for 5–8 weeks. We then assessed the impact of the acclimation conditions on intracellular Ca2+ transients (Δ[Ca2+]i) of isolated ventricular myocytes and contractile performance of isometrically-contracting ventricular strips. Measurements were obtained at various contractile frequencies (0.2–0.6 Hz) in normoxia, during acute exposure to hypoxia, and reoxygenation at 5 °C. The results show that hypoxia-acclimated Alaska blackfish compensate against the depressive effects of hypoxia on excitation-contraction coupling by remodelling cellular Δ[Ca2+]i to maintain ventricular contractility. When measured at 0.2 Hz in normoxia, hypoxia-acclimated ventricular myocytes had a 3.8-fold larger Δ[Ca2+]i peak amplitude with a 4.1-fold faster rate of rise, compared to normoxia-acclimated ventricular myocytes. At the tissue level, maximal developed force was 2.1-fold greater in preparations from hypoxia-acclimated animals. However, maximal attainable contraction frequencies in hypoxia were lower in hypoxia-acclimated myocytes and strips than preparations from normoxic animals. Moreover, the inability of hypoxia-acclimated ventricular myocytes and strips to contract at high frequency persisted upon reoxygenation. Overall, the findings indicate that hypoxia alters aspects of Alaska blackfish cardiac myocyte Ca2+ cycling, and that there may be consequences for heart rate elevation during hypoxia, which may impact cardiac output in vivo.
Keywords: Force-frequency relationship, Fura-2, Calcium, Cardiomyocyte, Oxygen, Temperature
Highlights
-
•
The air-breathing Alaska blackfish remains active under ice cover in hypoxic waters.
-
•
Maintained activity is supported by compensation of intracellular Ca2+ transients.
-
•
The compensation permits greater ventricular maximal developed force.
-
•
However, maximal attainable contraction frequencies are limited by hypoxia exposure.
1. Introduction
The regulation of the intracellular Ca2+ transient (Δ[Ca2+]i) is essential for cardiomyocyte and whole heart function but can be disrupted by low oxygen (hypoxia) and lack of oxygen (anoxia) (Piper et al., 1993; Yellon and Hausenloy, 2007). Insufficient intracellular Ca2+ levels can occur because of cellular energy deficit, intracellular acidosis and accumulation of inorganic phosphate (amongst other things) which impacts excitation-contraction coupling and reduces cardiac contractile function when oxygen is too low (Gómez et al., 1997; Nielsen and Gesser, 1984). Conversely, intracellular Ca2+ overload can also occur with low oxygen levels. Here, diminished extrusion of Ca2+ stemming from failure of Ca2+ pumps and/or the Na+-K + -ATPase and resultant downstream effects on the Na+/Ca2+ exchanger (NCX), also leads to decreased contractility and cardiac arrhythmias (Dhalla et al., 2001; Vassalle and Lin, 2004; Wang et al., 2020). By comparison, anoxia-tolerant vertebrate species that survive prolonged periods of oxygen deprivation, such as the red-eared slider turtle (Trachemys scripta), Western painted turtle (Chrysemys picta belli), snapping turtle (Chelydra serpentina) and crucian carp (Carassius carassius), employ strategies to successfully regulate intracellular Ca2+ in critical tissues, including the heart (Buck and Pamenter, 2018; Bundgaard et al., 2020; Lutz and Milton, 2004; Ruhr et al., 2019; Stecyk et al., 2007, 2021). Consequently, the heart of anoxia-tolerant vertebrates can continue to beat for hours, days, weeks and even months in the absence of oxygen (Herbert and Jackson, 1985; Stecyk et al., 2004, 2008; Stecyk, 2017; Tikkanen et al., 2017).
The Alaska blackfish (Dallia pectoralis) is a facultative air-breather endemic to the tundra wetlands of western and northern mainland Alaska, the Bering Sea Islands and Eastern Siberia (Campbell and Lopéz, 2014; Campbell et al., 2015). Unlike most extant air-breathing fishes, which reside in tropical freshwaters and are rarely, if ever, restricted from accessing atmospheric oxygen for prolonged time periods, the Alaska blackfish is forcibly submerged by ice and snow cover for many months in winter, which not only precludes air-breathing, but also results in aquatic hypoxia. In winter, the PO2 of the lakes and ponds in which the fish resides ranges from 9 to 75% air saturation; ∼1.9–15.8 kPa (Haynes et al., 2014; Lefevre et al., 2014; Leppi et al., 2016). Yet, remarkably, cold-acclimated Alaska blackfish deprived of atmospheric and aquatic oxygen remain active. Wild fish can be caught by baited hook and line from waters covered with more than a metre of ice, with temperatures of between 2 and 4 °C and with PO2 ranging from 0.8 kPa to 3.6 kPa (Lefevre et al., 2014). Moreover, in winter when local conditions become untenable, Alaska blackfish migrate through the shallow drainage ditches that connect the numerous lakes and ponds of the Arctic tundra to seek out less hypoxic and/or deeper unfrozen refugia (Haynes et al., 2014; Leppi et al., 2016). In captivity, 5 °C-acclimated Alaska blackfish chronically exposed to aquatic hypoxia (6–8 weeks at 6.3–8.4 kPa) and restricted from air-breathing actively feed and exhibit behavioural aggression towards conspecifics (Stecyk et al., 2020).
Modification of ventricular Ca2+ cycling with cold acclimation in normoxia, as well as with chronic hypoxic submergence at cold temperature, is thought to be central to the continued heart beat in the cold and under oxygen limited conditions in the Alaska blackfish (Kubly and Stecyk, 2015, 2019; Stecyk et al., 2020). With acclimation to 5 °C from 15 °C in normoxia, peak ventricular L-type Ca2+ current (ICa) density is reduced by 8-fold, but alterations of the ventricular ICa Ca2+-dependent and voltage-dependent inactivation properties serve to limit the reduction of total Ca2+ transferred through the L-type Ca2+ channel (QCa) (Kubly and Stecyk, 2015). Additionally, ventricular relaxation is prolonged (Kubly and Stecyk, 2015), the plateau phase of the action potential (AP) lengthened (Stecyk et al., 2020), the inotropic responsiveness of the ventricular myocardium to adrenergic stimulation enhanced (Kubly and Stecyk, 2019) and ventricular gene expression of the NCX (slc8a1) upregulated (Stecyk et al., 2020). With chronic hypoxic submergence at 5 °C (6–8 weeks at 6.3–8.4 kPa; no air access), ventricular QT interval and AP duration are shortened compared to 5 °C-acclimated, normoxic fish, but the duration of the AP plateau is unchanged (Stecyk et al., 2020). Moreover, the ventricular gene expression of proteins involved in sarcolemmal (SL) and sarcoplasmic reticulum (SR) Ca2+ cycling, including the L-type Ca2+ channel Cav1.2 (cacna1c), the ryanodine receptor-Ca2+ release channel (ryr2), the SR Ca2+-ATPase SERCA2 (atp2a2) and phospholamban (pln), a regulator of SERCA2, as well as ventricular gene expression of the Na+-K+-ATPase (atp1a1), is substantially upregulated in Alaska blackfish exposed to chronic hypoxic submergence compared to normoxic fish acclimated to warm and/or cold temperature (Stecyk et al., 2020). Nevertheless, whether these adjustments to excitation-contraction coupling (E-C) coupling pathways translate to changes in Δ[Ca2+]i and cardiac contractility remains unknown.
Here, to address this information gap and understand the changes in cellular Ca2+ cycling that allow the Alaska blackfish heart to function in cold hypoxia, we investigated the effects of chronic hypoxic submergence at cold acclimation temperature (5 °C) on Δ[Ca2+]i of isolated ventricular myocytes and contractile performance of isometrically-contracting ventricular muscle strips. We hypothesized that Δ[Ca2+]i will be sustained or upregulated when Alaskan blackfish fish are acclimated to hypoxia without air access, thereby offsetting the depressive effects of oxygen limitation on E-C coupling (Nielsen and Gesser, 1984) and cross-bridge cycling (Allen and Orchard, 1987; Matthews et al., 1986; Orchard and Kentish, 1990) and allowing ventricular contractility to be maintained. We also hypothesized that Alaska blackfish exposed to chronic hypoxic submergence avoid ventricular intracellular Ca2+ overload, especially at increased pacing frequencies under hypoxic conditions.
2. Materials and methods
2.1. Experimental animals and exposure conditions
Animals were collected under appropriate Alaska Department of Fish and Game permitting (SF-2016-30d) and the University of Alaska Anchorage (UAA) Institutional Animal Care and Use Committee approved all procedures (852440, 852441 and 852442). A total of twenty Alaskan blackfish (Dallia pectoralis) of both sexes and with a body mass of 25.5 ± 12.2 g (mean ± S.D.; ranging from 9.6 to 48.6 g) were utilized. Fish were captured with minnow traps from Duck Hunter's Training pond (Palmer, AK, USA) in summer and transported to the UAA vivarium. Fish were maintained indoors under a 12 h:12 h light:dark photoperiod in two 300 L (190 × 40 × 40 cm WxHxD) fiberglass aquaria containing recirculating, dechlorinated and aerated water. Holding temperature initially matched natural habitat temperature (10–12 °C). Then, after one week, water temperature was lowered by 1 °C per day to 5 °C. Water temperature was regulated using Teco-TR20 heater/cooler systems (Senkor Group, Inc., Terrell, TX, USA).
After 4 weeks at 5 °C, fish were assigned to the control (normoxia-acclimated) or treatment (hypoxia-acclimated) group. Normoxia-acclimated fish were maintained at 5 °C in aerated water and had access to atmospheric air. Hypoxia-acclimated fish were exposed to chronic hypoxic submergence following procedures previously described (Stecyk et al., 2020). Briefly, hypoxia-acclimated fish were denied access to atmospheric O2 by the placement of an impenetrable grate below the water surface, and water PO2 was progressively lowered (by ∼3–4 kPa every 3 days) and then maintained between 2.1 kPa and 4.2 kPa. The final level of hypoxia was selected to represent the lower limits of the range of dissolved oxygen levels (∼1.9–15.8 kPa) that Alaska blackfish experience in winter in their natural environment (Haynes et al., 2014; Leppi et al., 2016). Water PO2 was measured and maintained at appropriate levels using a one-channel oxygen regulator system (Loligo Systems, Tjele, Denmark) that regulated the bubbling of the water with 100% N2. The system was calibrated daily following the manufacturer's protocol. Water PO2 was also confirmed once or twice a day using a fibre optic FDO 925 oxygen probe and Multi 3410 m (WTW, Weilheim, Germany). Normoxia-acclimated and hypoxia-acclimated fish were held under the above conditions for a minimum of 5 and maximum of 8 weeks until use.
2.2. Animal husbandry
Animal husbandry followed protocols previously detailed (Kubly and Stecyk, 2015, 2019; Stecyk et al., 2020). Briefly, fish were fed bloodworms ad libitum bi-daily throughout the temperature acclimation and experimental exposure periods, but food was withheld 24 h prior to experimental measurements. Water changes occurred weekly to maintain levels of nitrite, nitrate, and ammonium below recommended levels (Tetra EasyStrips, Tetra, Blacksburg, VA, USA). The fresh water added to the tanks was pre-chilled to 5 °C to ensure minimal temperature fluctuation. Also, to prevent hypoxia-acclimated fish from gaining air access and/or experiencing increased water oxygen levels, water changes were accomplished with as little disturbance to the water column as possible, without lowering the water level below the level of the submerged grating and with the water pre-bubbled with 100% N2 to match the oxygen level within the tank.
2.3. Ventricular myocytes
Fish were euthanized by cranial concussion and pithed, the heart dissected and ventricular cardiomyocytes isolated by enzymatic dissociation, as previously described (Kubly and Stecyk, 2015; Stecyk et al., 2020). Briefly, the outflow tract was cannulated and the heart retrograde perfused for 30–35 min with low-Na+ isolation solution containing (in mmol l−1): NaCl 100, KCl 10, KH2PO4∙2H2O 1.2, MgSO4∙7H2O 4, taurine 50, glucose 10 and HEPES 10 at pH of 6.9, supplemented with proteolytic enzymes (collagenase type IA, 1.5 mg ml−1; trypsin type IX, 1 mg ml−1) and fatty acid free bovine serum albumin (1.5 mg ml−1). The ventricle was then minced and triturated with a Pasteur pipette to obtain individual myocytes. Cells were maintained in low-Na+ solution at 4 °C for up to 8 h prior to use.
To measure Δ[Ca2+]i, cardiomyocytes were loaded with the AM-ester of the cell-permeant fluorescent indicator Fura-2 (0.075 μmol l−1 for 10 min; Invitrogen, Carlsbad, CA, USA). Following loading, cells were resuspended in fresh isolation solution for 15–20 min to allow de-esterification. An aliquot of cardiomyocytes was placed into a recording chamber (RC-22C; Warner Instruments, Hamden, CT, USA, volume by depth: 138 μl/mm) mounted on the stage of an inverted microscope (Nikon Ti–S, Tokyo, Japan) and allowed to settle. Myocytes were then superfused at a rate of 1–2 ml min−1 with physiological saline containing (in mmol l−1): 150 NaCl, 5.4 KCl, 1.5 MgSO4, 0.4 NaH2PO4, 2 CaCl2, 10 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) and 10 glucose (Stecyk et al., 2020). Saline pH was titrated to 7.6 with NaOH at 21 °C using an Orion Star A211 pH meter with Orion ROSS Ultra Glass Triode pH/ATC combination electrode (Thermo Fisher Scientific, Waltham, MA, USA) and was allowed to change with temperature (temperature coefficient of HEPES: -0.014/°C) such that pH was 7.82 at 5 °C. Saline temperature was continuously monitored with a thermistor positioned close to the myocytes and was set to 5 °C using a SC-20 dual in-line solution heater/cooler and a CL-100 bipolar temperature controller (Warner Instruments).
Cardiomyocytes were stimulated to contract using a SD9 Stimulator (Grass Instruments, West Warwick, RI, USA) connected to platinum electrodes. Stimulation frequency was 0.2 Hz and then 0.4 Hz under oxygenated conditions, after 5 and 15 min of hypoxic perfusion (2.1 kPa) and finally, after 5 min of reoxygenation. The routine stimulation frequency of 0.2 Hz (=12 beats min−1) was selected to approximate the in vivo resting heart rate of 5 °C normoxia- and hypoxia-acclimated Alaska blackfish (Stecyk et al., 2020), as well as to algin with previous in vitro investigations of Alaska blackfish cardiac physiology at cold temperature (Kubly and Stecyk, 2015, 2019). Oxygen level of the perfusate was measured continuously throughout the protocol with a TROXROB3 robust trace oxygen miniprobe and FireSting fiber-optic oxygen meter (PyroScience GmbH, Aachen, Germany). Preliminary studies showed no effect of field stimulation on the oxygen levels in the bath while perfusion was running.
2.4. Fluorescent imaging
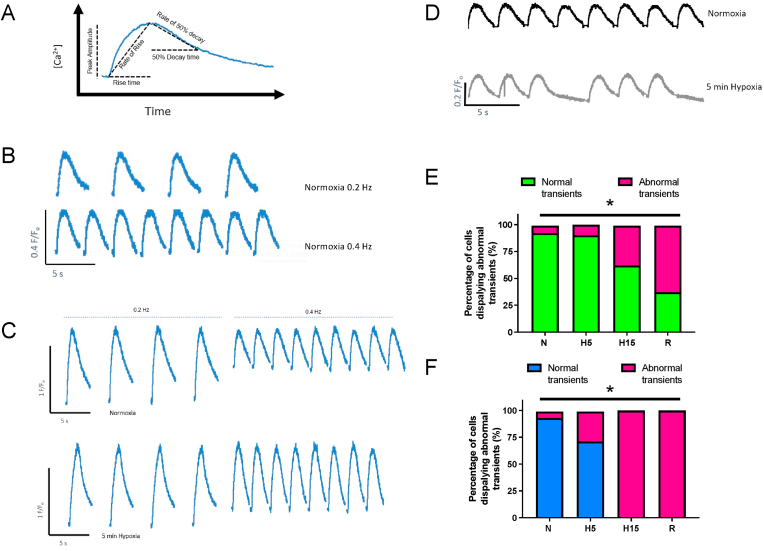
The inverted microscope was coupled to an Optoscan photomultiplier tube, monochromator, and high-intensity xenon arc lamp (Cairn Research Instruments, Faversham, UK). Signals were digitized with a Digidata 1440A and analyzed with pClamp 10 software (Axon Instruments, Sunnyvale, CA). Fura-2 was excited by alternating wavelengths of 340/380 nm and light was collected at 515 nm, with input and exit slit widths each set to 10 nm. The ratio of emitted fluorescence at 340 nm/380 nm was calculated to give an index of [Ca2+]i. Fura-2 fluorescence data is presented as arbitrary units after background correction. Δ[Ca2+]i peak amplitude (F/Fo; calculated by subtracting the value of the diastolic [Ca2+]i from the peak systolic [Ca2+]i), diastolic Ca2+ (F/Fo), rise time (time in ms from 0 to 90% peak of the Δ[Ca2+]i), 50% decay time (time in ms from 100 to 50% decay of the peak of the transient), and rates of Ca2+ rise ((F/Fo)−ms; linear regression between 0 and 90% of peak transient) and 50% decay ((F/Fo)−ms; linear regression between 100 and 50% decay of peak transient) were measured from the transient recordings in Clampfit10 software (Axon Instruments, Sunnyvale, CA). Please see Fig. 1A for schema of various parameters measured.
Fig. 1.
Impact of acclimation, contraction frequency and acute hypoxia/normoxia on Ca2+ transients. (A) Representative trace of intracellular Ca2+ transient from a normoxia-acclimated cell under hypoxic perfusion and stimulated at 0.2 Hz used to illustrate the various parameters measured. (B) Impact of increasing contraction frequency from 0.2 Hz (top panel) to 0.4 Hz (bottom panel) in a ventricular myocyte from a normoxia-acclimated fish during normoxic perfusion. There was no increase in diastolic Ca2+ levels between top and bottom panel. (C) Impact of increasing contraction frequency from 0.2 Hz to 0.4 Hz (top panel) in a ventricular myocyte from a hypoxia-acclimated fish during normoxic perfusion, and again in the same cell following 5 min hypoxia perfusion (lower panel). Note the increase in diastolic Ca2+ levels with increased contraction frequency in the hypoxia-acclimated myocytes under both normoxic and hypoxic recording conditions. (D) An example of an abnormal Ca2+ transient (missed beat) at 0.4 Hz during hypoxic perfusion (grey, bottom panel) compared with the same cell at 0.4 Hz during normoxic perfusion (black, upper panel). Ca2+ transient data were recorded in sweeps of 3 s duration with a 2 s inter-sweep interval where data was not recorded for the 0.2 Hz recordings. This configuration meant that in some instances the cell had not fully relaxed during the recording. However, diastolic Ca2+ had returned to baseline before the next transient began. This did not affect decay measurements as 50% decay and rate of 50% decay were always measured within the recording time frame under each condition and at each frequency (see panel A). In panels B and C, data are presented with the inter-sweep duration shown, thus the traces are not continuous. In panel D, the sweeps have been concatenated to enable a longer time series to be shown. Bar charts show percentage of abnormal transients (including skipped beats, rapid beats, and/or biphasic beats) under each recording condition in ventricular myocytes contracting at 0.4 Hz from (E) normoxia-acclimated fish (*indicates significant effect of treatment on incidence of abnormal transients, Chi-Square, P = 0.025) and (F) Hypoxia-acclimated fish (* indicates significant effect of treatment on incidence of abnormal transients, Chi-Square, P = 0.007). There is also a difference in the incidence of abnormal transients between normoxia- and hypoxia-acclimated fish (i.e., between panels E and F; not demarcated; Chi-Square, P < 0.001). Importantly, abnormal transients were rarely (in 2 out of all cells) observed at 0.2 Hz for either acclimation group (not shown).
2.5. Contractile performance of ventricular tissue
Fish were euthanized as described above. The ventricle was dissected and splayed lengthwise to expose the lumen. Two longitudinal ventricular strips cut by razor blade were suspended vertically between a fixed post and isometric transducer (FT03C; Grass Instruments) and submerged in 30 ml water-jacketed organ baths containing physiological saline at 5 °C and continuously bubbled with 100% O2. This increases the PO2 of the perfusate ensuring adequate oxygen in the contracting tissue and was thus designated the normoxic condition. Temperature was maintained with a refrigerated re-circulating water bath (VWR, Radnor, PA, USA). Strips were allowed a 20 min equilibration period, after which slack was removed. After an additional 20 min, the strips were stimulated at ∼30 V (1.5x the voltage required to initiate contraction), 5 ms pulse duration and frequency of 0.2 Hz using a SD9 Stimulator (Grass Instruments) connected to platinum electrodes positioned on either side of each strip. The length at which active tension was maximal (Lmax) was determined and the tissue was allowed to stabilize at this length for 25 min in the presence of tonic (10 nmol l−1) adrenergic stimulation (Graham and Farrell, 1989) prior to being subjected to the experimental protocol. Strips that failed to contract regularly with stimulation or exhibited spontaneous contractions were excluded from experimentation and data analysis. Length, width, and wet mass of the n = 12 viable strips were 3.68 ± 1.3 mm, 1.05 ± 0.23 mm and 2.03 ± 1.3 mg (means ± SD), respectively.
One strip was designated the time control, which was exposed to oxygenated saline throughout the protocol, and the other strip was designated the experimental. At the conclusion of the 25 min stabilization period, the physiological saline solution bathing the strips was refreshed, 10 nmol−1 adrenaline administered and 5 min later a force-frequency trial was conducted. Stimulation frequency was increased by 0.1 Hz increments from the routine stimulation frequency of 0.2 Hz until a maximum of 0.6 Hz or until the muscle failed to show regular contractions, at which point stimulation frequency was reduced to the routine level (Kubly and Stecyk, 2019). 5 min after completion of the force-frequency trial, the tissue bath of the experimental strip was bubbled with 100% N2 to decrease the PO2 of the physiological saline to 2.1 kPa over a 10 min period. After 10 min at 2.1 kPa, 10 nmol−1 adrenaline was administered to both strips and 5 min later a second force-frequency trial was conducted. The bath saline of the hypoxia-exposed strip was then reoxygenated over a period of 10 min by changing bubbling from N2 to O2, and following 20 min exposure to oxygenated saline, 10 nmol−1 adrenaline administered to both strips and 5 min later a final force-frequency trial was conducted. The duration of the protocol was approximately 75 min, throughout which TROXROB3 robust trace oxygen miniprobes and FireSting fiber-optic oxygen meter (PyroScience GmbH, Aachen, Germany) were used to monitor oxygenation levels in both tissue baths.
Force transducer outputs were amplified with CP122 AC/DC strain gage amplifiers (Grass Instruments) and the signals digitized at 100 Hz with a PowerLab 8/35 data acquisition system (AD Instruments, Colorado Springs, CO, USA). Recordings were analyzed offline using the Peak Analysis module of LabChart 8 (AD Instruments). 8–25 peaks were analyzed for each stimulation frequency of the force-frequency trials. The following parameters were measured: resting tension, maximal developed force (Fmax; the difference between resting tension and maximal force after electrical stimulation), time-to-peak force (TPF) and time-to-half relaxation (T0.5R). Average rate of contraction (Raterise) and average rate of 50% relaxation (Rate50% relax) were calculated by dividing Fmax by TPF and 0.5 x Fmax by T0.5R, respectively (Galli et al., 2009; Joyce et al., 2019; Kubly and Stecyk, 2019). Fmax is expressed as mN mm−2, where mean cross-sectional area was calculated using the strip wet mass, length and an assumed muscle density of 1.06 g cm−3 (Layland et al., 1995).
2.6. Statistical analysis
Results are reported as means ± SEM. N represents number of fish. n represents number cells or strips. Statistical analysis was performed using SigmaPlot 14.5 (Systat Sofware, San Jose, CA, USA) and GraphPad Prism 9.2 (GraphPad Sofware, San Diego, CA, USA) and results are detailed in the figure and table legends. In all instances, differences were considered statistically significant when P < 0.05.
3. Results
3.1. Relative heart mass
Heart mass (inclusive of the outflow tract, ventricle, atrium and sinus venosus) relative to body mass was measured for the fish from which ventricular myocytes were obtained and did not differ (P = 0.251) between normoxia-acclimated (0.19 ± 0.02%; N = 8) and hypoxia-acclimated (0.17 ± 0.01%; N = 6) fish.
3.2. Intracellular Ca2+ transient of ventricular myocytes
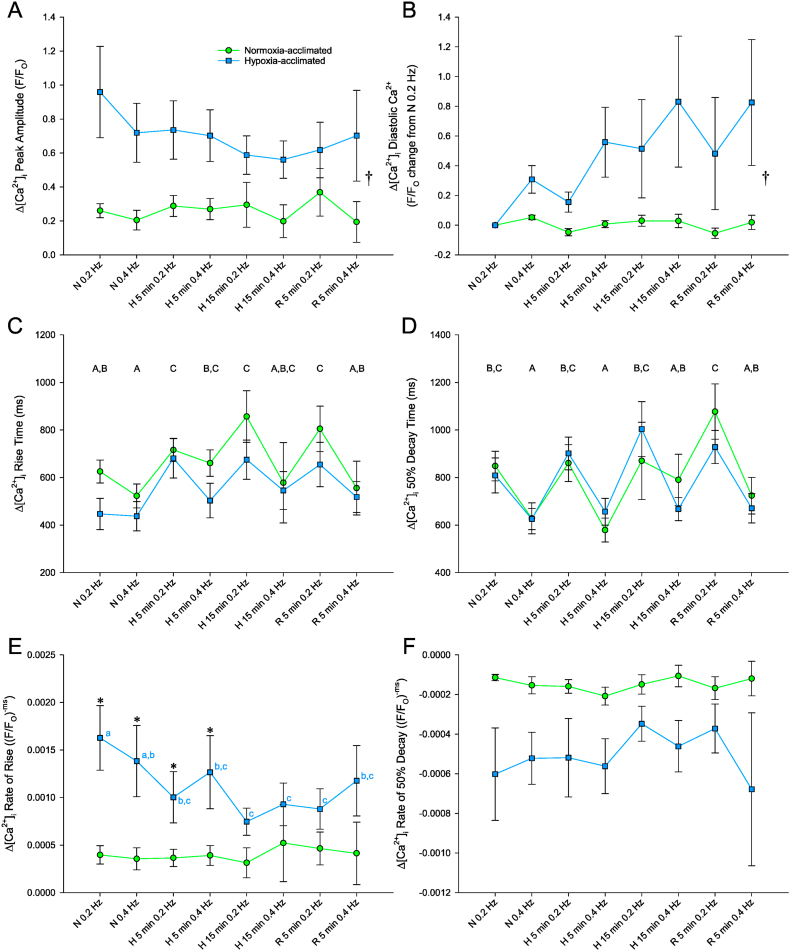
Hypoxia acclimation resulted in ventricular myocytes with larger Δ[Ca2+]i peak amplitudes and faster rising time courses compared with those from normoxia-acclimated myocytes (Table 1; Fig. 1B and C; Fig. 2A, C and E). When cells were stimulated at 0.2 Hz in normoxic perfusate, the Δ[Ca2+]i peak amplitude was 3.8-fold greater (P = 0.008) and Δ[Ca2+]i rise time was 178 ms shorter (P = 0.034) in hypoxia-acclimated than normoxia-acclimated ventricular myocytes (Table 1; Fig. 1B and C; Fig. 2A and C). Consequently, Δ[Ca2+]i rate of rise was 4.1-fold faster (P < 0.001) in ventricular myocytes from hypoxia-acclimated fish (Table 1; Fig. 2E), whereas Δ[Ca2+]i 50% decay time (P = 0.682) and Δ[Ca2+]i rate of 50% decay (P = 0.059) were similar between acclimation groups (Table 1; Fig. 2D and F).
Table 1.
Comparison of intracellular Ca2+ transients (Δ[Ca2+]i) and isometric contractile properties of the ventricular myocardium of 5 °C normoxia-acclimated and hypoxia-acclimated Alaska blackfish.
| 1234 | Normoxia-acclimated | Hypoxia-acclimated |
|---|---|---|
| Ventricular Myocytes | ||
| Δ[Ca2+]i Peak Amplitude (F/Fo) | 0.26 ± 0.04 | 0.96 ± 0.27∗ |
| Δ[Ca2+]i Rise Time (ms) | 625.1 ± 48.3 | 447.0 ± 65.6∗ |
| Δ[Ca2+]i 50% Decay Time (ms) | 848.4 ± 62.0 | 808.9 ± 73.5 |
| Δ[Ca2+]i Rate of Rise ((F/Fo)−ms) | 0.00040 ± 0.00010 | 0.00163 ± 0.00034∗ |
| Δ[Ca2+]i Rate of 50% Decay ((F/Fo)−ms) |
−0.00012 ± 0.00002 |
−0.00060 ± 0.00023∗ |
| Ventricular Strips | ||
| Fmax (mN mm−2) | 6.7 ± 1.3 | 14.4 ± 2.2∗ |
| Resting Tension (mN mm−2) | 7.8 ± 2.7 | 4.3 ± 1.0 |
| TPF (ms) | 1010.1 ± 40.8 | 1328.1 ± 6.4∗ |
| T0.5R (ms) | 615.1 ± 41.7 | 1151.0 ± 46.3∗ |
| Raterise (mN mm−2 s−1) | 6.6 ± 1.3 | 10.9 ± 1.7 |
| Rate50% relax (mN mm−2 s−1) | −5.5 ± 1.2 | −6.1 ± 0.8 |
Measurements were obtained under oxygenated conditions at the routine stimulation frequency of 0.2 Hz.
Fmax: maximal developed force; TPF: time-to-peak force; T0.5R: time-to-half relaxation; Raterise: average rate of contraction; Rate50% relax: average rate of 50% relaxation.
n myocytes: 16 (normoxia-acclimated) and 13 (hypoxia-acclimated) cells from N = 8 (normoxia-acclimated) and N = 6 (hypoxia-acclimated) fish.
n strips: 6 strips from N = 3 fish per acclimation group.
Data are means ± SEM.
Statistically significant differences between acclimation conditions (P < 0.05; t-test) are indicated with an asterisk.
Fig. 2.
Comparison of Δ[Ca2+]i peak amplitude (A), Δ[Ca2+]i diastolic Ca2+ (B), Δ[Ca2+]i rise time (C), Δ[Ca2+]i 50% decay time (D), Δ[Ca2+]i rate of rise (E) and Δ[Ca2+]i rate of 50% decay (F) of 5 °C normoxia-acclimated and hypoxia-acclimated Alaska blackfish ventricular myocytes stimulated to contract at 0.2 and 0.4 Hz in normoxia (N), after 5 and 15 min of acute hypoxia (H; 2.1 kPa) and 5 min of subsequent reoxygenation (R). Statistical analysis: 2-way repeated-measures ANOVA and Student-Newman-Keuls multiple comparison post hoc test. A dagger (†) indicates a main effect of acclimation condition and a difference (P < 0.05) between normoxia- and hypoxia-acclimated preparations. Dissimilar uppercase letters indicate a main effect of and differences (P < 0.05) among recording conditions. In panel E, where an interaction was found between acclimation and recording conditions, an asterisk (*) indicates a difference (P < 0.05) between acclimation groups within a recording condition, whereas dissimilar lowercase letters indicate differences (P < 0.05) among recording conditions within an acclimation group. Data are means ± SEM. Cells were obtained from N = 8 normoxia-acclimated fish and N = 6 hypoxia-acclimated fish. Number of cells (n) for each recording condition (reading from left to right) was n = 16, 16, 16, 14, 7, 3, 8, 3 for normoxia-acclimated fish and n = 13, 13, 13, 10, 11, 8, 10, 8 for hypoxia-acclimated fish. The decrease in myocyte numbers occurred due to cells blowing away or failing to contract during the long imaging protocol.
The greater Δ[Ca2+]i peak amplitude in hypoxia-acclimated ventricular myocytes recorded at 0.2 Hz in normoxia persisted under all subsequent recording conditions, including acute hypoxia exposure, reoxygenation, and increased stimulation frequency to 0.4 Hz. A significant main effect of acclimation condition was found for Δ[Ca2+]i peak amplitude (F1,170 = 7.579; P = 0.009; Fig. 2A). A significant main effect of acclimation condition (F1,162 = 4.323; P = 0.039) was also found for the response of Δ[Ca2+]i diastolic Ca2+ to acute hypoxia exposure, reoxygenation and increased stimulation frequency to 0.4 Hz (Fig. 2B). The change in Δ[Ca2+]i diastolic Ca2+ from 0.2 Hz in normoxia was greater for hypoxia-acclimated than normoxia-acclimated myocytes (Fig. 1B and C; Fig. 2B).
A significant interaction between acclimation condition and recording conditions was found for Δ[Ca2+]i rate of rise (F7,169 = 2.848; P = 0.009; Fig. 2E). The faster Δ[Ca2+]i rate of rise of hypoxia-acclimated ventricular myocytes compared to normoxia-acclimated myocytes in normoxia persisted at both stimulation frequencies after 5 min of acute hypoxia exposure (Fig. 2E). However, Δ[Ca2+]i rate of rise of hypoxia-acclimated myocytes slowed after 15 min of acute hypoxia exposure such that no difference in Δ[Ca2+]i rate of rise existed between hypoxia- and normoxia-acclimated cells (Fig. 2E). The slowed Δ[Ca2+]i rate of rise in hypoxia-acclimated cells by acute hypoxia exposure persisted upon reoxygenation (Fig. 2E).
Regardless of acclimation condition, Δ[Ca2+]i rise time was slowed by acute hypoxia exposure (significant main effect of recording conditions: F7,170 = 9.211; P < 0.001; Fig. 2C). The slower Δ[Ca2+]i rise time induced by acute hypoxia exposure persisted upon reoxygenation at 0.2 Hz stimulation frequency. A significant main effect of recording conditions was also found for Δ[Ca2+]i 50% decay time (F7,170 = 9.211; P < 0.001; Fig. 2D). However, in contrast to Δ[Ca2+]i rise time, Δ[Ca2+]i 50% decay time was unaffected by acute hypoxia exposure, but was shortened at 0.4 Hz stimulation frequency in normoxia, at 5 min of exposure to hypoxic perfusate and upon reoxygenation (Fig. 2D). A similar trend towards a shortened Δ[Ca2+]i 50% decay time was apparent at 15 min of acute hypoxia exposure (Fig. 2D). Δ[Ca2+]i rate of 50% decay was not affected by acute hypoxia exposure or reoxygenation, regardless of stimulation frequency (F7,168 = 0.544; P = 0.883; Fig. 2F).
3.3. Effect of increased pacing frequency during acute hypoxia exposure
Ca2+ transients from both acclimation groups sometimes failed to keep pace (i.e., skipped beats, rapid beats and/or biphasic beats) as pacing frequency was increased from 0.2 Hz to 0.4 Hz (Fig. 1D). The incidence of cells displaying abnormal transients increased during the exposure protocol in both normoxia-acclimated myocytes [X2 (1, n = 16) = 7.16, P = 0.007; Fig. 1E] and hypoxia-acclimated myocytes [X2 (1, n = 13) = 4.96, P = 0.025; Fig. 1F]. Moreover, the incidence of abnormal transients was greater in hypoxia-acclimated than normoxia-acclimated myocytes [X2 (1, n = 29) = 22.31, P < 0.001; Fig. 1E and F).
3.4. Stability of the ventricular strip preparations over time
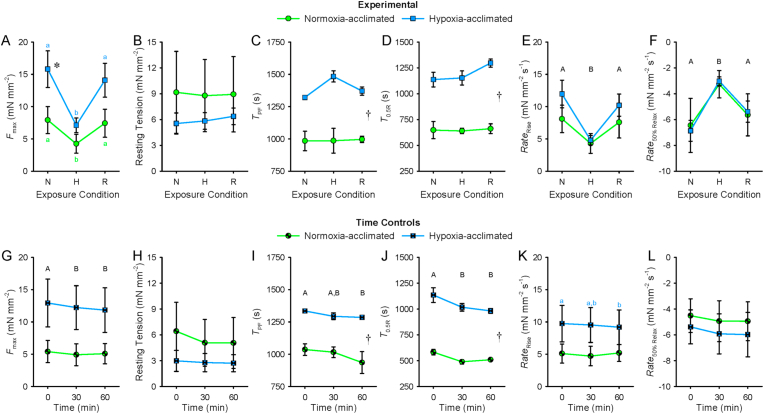
Control experiments conducted to assess the stability of the isometrically-contracting ventricular strip preparations over time revealed that the preparations were viable throughout the duration of the experiment (Fig. 3). Fmax, TPF, T0.5R and Raterise of time control strips from normoxia-acclimated and hypoxia-acclimated fish decreased by only 6–9%, 4–10%, 13% and 9%, respectively, over time.
Fig. 3.
Comparison of maximal developed force (Fmax; A and G), resting tension (B and H), time-to-peak force (TPF; C and I), time-to-half relaxation (T0.5R; D and J), average rate of contraction (RateRise; E and K) and average rate of 50% relaxation (RateRelax 50%; F and L) of isometrically-contracting ventricular strips from 5 °C Alaska blackfish acclimated to normoxia or hypoxia. Experimental preparations exposed to normoxia (N), acute hypoxia (H) and subsequent reoxygenation (R) are presented in panels A–F. Time control preparations maintained in oxygenated saline are presented in panels G–L. Statistical analysis: 2-way repeated-measures ANOVA and Student-Newman-Keuls multiple comparison post hoc test. A dagger (†) indicates a main effect of acclimation condition and a difference (P < 0.05) between normoxia- and hypoxia-acclimated preparations. Dissimilar uppercase letters indicate a main effect of and differences (P < 0.05) among recording conditions (or times). In panels A and K, where an interaction was found between acclimation and recording conditions (or times), an asterisk (*) indicates a difference (P < 0.05) between acclimation groups within a recording condition, whereas dissimilar lowercase letters indicate differences (P < 0.05) among recording conditions (or times) within an acclimation group. Data are means ± SEM. In all instances, n = 3 strips from N = 3 fish per acclimation group.
3.5. Effect of hypoxia-acclimation on ventricular contractile properties
Consistent with the increased Δ[Ca2+]i peak amplitude found for hypoxia-acclimated ventricular myocytes, hypoxia-acclimated ventricular strips exhibited a 2.1-fold greater Fmax compared to normoxia-acclimated strips when measured in normoxia and stimulated at 0.2 Hz (P = 0.012; Table 1; Fig. 3A). Unlike the shorter Ca2+ rise time found for hypoxia-acclimated ventricle myocytes, hypoxia-acclimated ventricular strips exhibited a 30% longer TPF (P < 0.001; Table 1; Fig. 3C and I). Hypoxia-acclimated ventricular strips also exhibited a nearly doubled T0.5R compared to normoxia-acclimated ventricular strips (P < 0.001), which contrasts with the unaltered Ca2+ 50% decay time found for hypoxia-acclimated ventricular myocytes (Table 1; Fig. 3D and J). The similar Raterise between normoxia- and hypoxia-acclimated strips (P = 0.074; Table 1; Fig. 3E and K) was inconsistent with the faster Ca2+ rate of rise found for hypoxia-acclimated ventricular myocytes, whereas the similar Rate50% relax between normoxia- and hypoxia-acclimated strips (P = 0.660; Table 1; Fig. 3F and L) was consistent with the unchanged Ca2+ rate of 50% decay of normoxia- and hypoxia-acclimated ventricular myocytes.
3.6. Contractile responses to acute hypoxia exposure and reoxygenation
Acute hypoxia exposure depressed Fmax by 48% (P = 0.021) in normoxia-acclimated strips and by 55% (P < 0.001) in hypoxia-acclimated strips (Fig. 3A). Notably, the Fmax of hypoxia-acclimated strips in hypoxia (7.1 ± 1.1 mN mm−2; Fig. 3A) was equivalent to the Fmax of normoxia-acclimated strips in normoxia (Table 1). While TPF (P = 0.188) and T0.5R (P = 0.757) were unaffected by acute hypoxia exposure (Fig. 3C and D), Raterise and Rate50% relax were slowed by 47–48% (P < 0.001) in normoxia-acclimated preparations and by 55–60% (P < 0.001) in hypoxia-acclimated preparations with acute hypoxia exposure (Fig. 3E and F). Reoxygenation recovered the contractile force and rates of contraction and relaxation lost during acute hypoxia exposure in preparations from both acclimation groups (Fig. 3A, E and F).
3.7. Fmax response to increased pacing frequencies
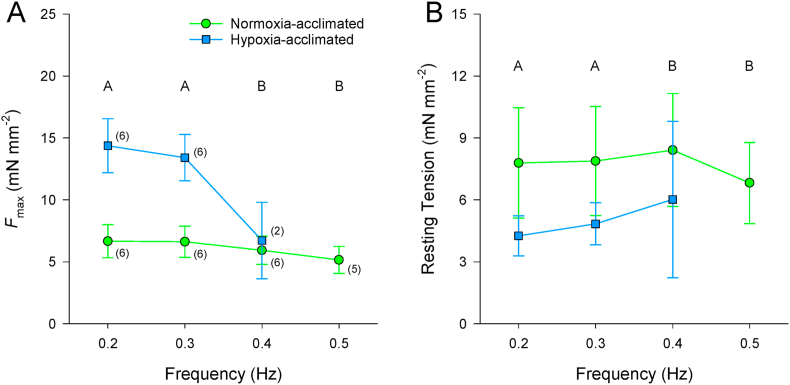
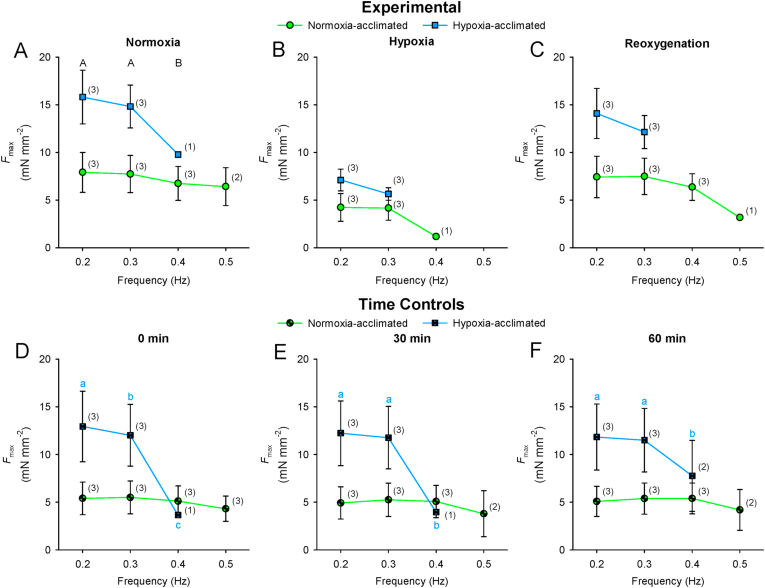
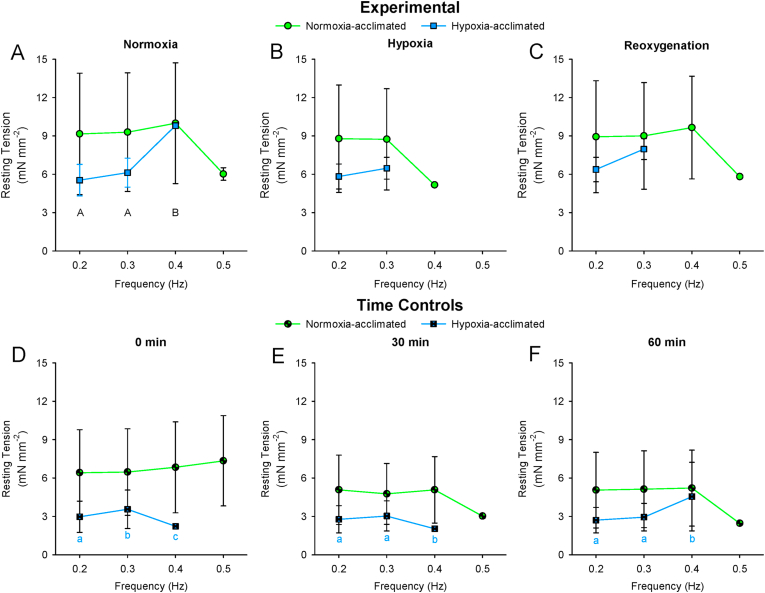
A main effect of stimulation frequency was found for Fmax (F3,36 = 11.115; P < 0.001; Fig. 4A) and resting tension (F3,36 = 14.461; P < 0.001; Fig. 4B). Fmax decreased, whereas resting tension increased in response to increased stimulation frequency in normoxia. Regular contractions persisted to higher frequencies in strips from normoxia-acclimated fish compared to strips from hypoxia-acclimated fish (Time Controls: F1,17 = 13.091; P = 0.022; Table 2; Fig. 4). However, the relative magnitude of decrease in Fmax from 0.2 Hz at the maximum attainable frequency did not differ between acclimation groups (Experimental: F1,17 = 0.305; P = 0.610; Time Controls: F1,17 = 0.358; P = 0.582; Table 3).
Fig. 4.
Comparison of the effects of increased stimulation frequency in normoxia on maximal developed force (Fmax; A) and resting tension (B) of isometrically-contracting ventricular strips from 5 °C Alaska blackfish acclimated to normoxia or hypoxia. Statistical analysis: 2-way repeated-measures ANOVA and Student-Newman-Keuls multiple comparison post hoc test. Dissimilar uppercase letters indicate a main effect of and differences (P < 0.05) among stimulation frequencies. Data are means ± SEM. n of strips at each stimulation frequency from N = 3 fish per acclimation group is indicated in parenthesis in panel A.
Table 2.
Maximum contraction frequency (Hz) of Alaska blackfish isometrically-contracting ventricle strips under each recording condition.
|
Experimental |
|||
|---|---|---|---|
| Normoxia | Hypoxia | Reoxygenation | |
| Normoxia-acclimated | 0.47 ± 0.03 a | 0.33 ± 0.03 b | 0.43 ± 0.03 a |
| Hypoxia-acclimated |
0.33 ± 0.03 ∗ |
0.30 ± 0.00 |
0.30 ± 0.00 ∗ |
| Time Controls | |||
| 0 min |
30 min |
60 min |
|
| Normoxia-acclimated | 0.50 ± 0.00 | 0.47 ± 0.03 | 0.47 ± 0.03 |
| Hypoxia-acclimated † | 0.33 ± 0.03 | 0.33 ± 0.03 | 0.37 ± 0.03 |
Statistical analysis: 2-way RM ANOVA and Student-Newman-Keuls post hoc test. A dagger.
n = 3 strips from N = 3 fish per acclimation group.
Data are means ± SEM.
Indicates a main effect of acclimation condition and a difference (P < 0.05) between normoxia-acclimated and hypoxia-acclimated preparations. An asterisk.
Indicates a difference (P < 0.05) between normoxia- and hypoxia-acclimated preparations within a recording condition. Dissimilar lowercase letters indicate differences (P < 0.05) among recording conditions within an acclimation group.
Table 3.
Maximal developed force of Alaska blackfish isometrically-contracting ventricle strips at their maximum attainable contraction frequency expressed as a percentage (%) of routine contraction frequency (02 Hz) under each recording condition.
|
Experimental |
|||
|---|---|---|---|
| Normoxia | Hypoxia | Reoxygenation | |
| Normoxia-acclimated | 74.2 ± 2.4 | 89.1 ± 16.0 | 81.1 ± 10.8 |
| Hypoxia-acclimated |
87.5 ± 5.9 |
80.5 ± 3.1 |
87.5 ± 3.8 |
| Time Controls | |||
| 0 min |
30 min |
60 min |
|
| Normoxia-acclimated | 80.2 ± 6.1 | 91.8 ± 9.3 | 100.7 ± 8.0 |
| Hypoxia-acclimated | 82.7 ± 9.2 | 88.2 ± 8.3 | 83.3 ± 7.1 |
Statistical analysis: 2-way RM ANOVA on log10 transformed data. No differences (P > 0.05) between normoxia- and hypoxia-acclimated preparations or among recording conditions within an acclimation group.
n = 3 strips from N = 3 fish per acclimation group.
Data are means ± SEM.
The force-frequency response of the time control strips was not compromised by the experiment duration (Table 2, Table 3; Fig. 5, Fig. 6). Acute hypoxia exposure limited the stimulation frequency at which regular contractions occurred in normoxia-acclimated, but not hypoxia-acclimated strips (significant interaction between acclimation condition and recording conditions: F2,17 = 4.500; P = 0.049; Table 2; Fig. 5, Fig. 6). Upon reoxygenation, the strips from normoxia-acclimated fish recovered the ability to contract at high stimulation frequencies (Table 2; Fig. 5, Fig. 6). By comparison, the response of resting tension to increased stimulation frequency was not altered by acute hypoxia exposure or reoxygenation in normoxia- or hypoxia-acclimated strips (Fig. 6).
Fig. 5.
Comparison of the effects of increased stimulation frequency on maximal developed force (Fmax) of isometrically-contracting ventricular strips from 5 °C Alaska blackfish acclimated to normoxia or hypoxia. Experimental preparations exposed to normoxia, acute hypoxia and subsequent reoxygenation are presented in panels A–C. Time control reparations maintained in oxygenated saline are presented in panels D–F. Statistical analysis: 2-way repeated-measures ANOVA and Student-Newman-Keuls multiple comparison post hoc test on data spanning 0.2–0.4 Hz. Dissimilar uppercase letters indicate a main effect of and differences (P < 0.05) among recording conditions. In panels D–F, where an interaction was found between acclimation group and recording times, dissimilar lowercase letters indicate differences (P < 0.05) among recording times within an acclimation group. Data are means ± SEM. n of strips at each frequency from N = 3 fish per acclimation group is indicated in parenthesis.
Fig. 6.
Comparison of the effects of increased stimulation frequency on resting tension of isometrically-contracting ventricular strips from 5 °C Alaska blackfish acclimated to normoxia or hypoxia. Experimental preparations exposed to normoxia, acute hypoxia and subsequent reoxygenation are presented in panels A–C. Time control reparations maintained in oxygenated saline are presented in panels D–F. Statistical analysis: 2-way repeated-measures ANOVA and Student-Newman-Keuls multiple comparison post hoc test on data spanning 0.2–0.4 Hz. Dissimilar uppercase letters indicate a main effect of and differences (P < 0.05) among recording conditions. In panels D–F, where an interaction was found between acclimation condition and recording times, dissimilar lowercase letters indicate differences (P < 0.05) among recording times within an acclimation group. Data are means ± SEM. n of strips at each frequency from N = 3 fish per acclimation group is demarcated in Fig. 5.
4. Discussion
4.1. Compensation of ventricular Δ[Ca2+]i with chronic hypoxic submergence at cold temperature maintains contractility at routine heart rates
Our most striking discovery was that ventricular myocytes from Alaska blackfish chronically exposed to aquatic hypoxia and restricted from air breathing for 5–8 weeks remodelled cellular Δ[Ca2+]i to maintain ventricular contractility in the face of the depressive effects of oxygen limitation on E-C coupling (Nielsen and Gesser, 1984) and myofilament cross-bridge cycling (Allen and Orchard, 1987; Matthews et al., 1986; Orchard and Kentish, 1990). Hypoxia-acclimated fish displayed a 3.8-fold greater ventricular Δ[Ca2+]i peak amplitude and 4.1-times faster Δ[Ca2+]i rate of rise compared to normoxia-acclimated fish. The cellular response was mirrored at the tissue level by changes in Fmax that were prototypical of physiological compensation in response to a prolonged exposure to environmental change. Fmax of hypoxia-acclimated strips measured at 0.2 Hz in hypoxia was equivalent to the Fmax of normoxia-acclimated strips measured in normoxia, whereas it was 2.1-fold greater than that of normoxia-acclimated strips when measured under comparable (i.e., normoxic) conditions. The conclusion is consistent with the previous finding that cold-acclimated Alaska blackfish exposed to prolonged hypoxic submergence prioritize the continuation of cardiac performance to support an active lifestyle over reducing cardiac ATP demand and do not exhibit hypoxic bradycardia (Stecyk et al., 2020).
The altered Δ[Ca2+]i in hypoxia-acclimated ventricular myocytes aligns with the upregulated ventricular gene expression of proteins involved in SL and SR Ca2+ cycling in Alaska blackfish exposed to chronic hypoxic submergence (Stecyk et al., 2020). An increased density of SL and SR Ca2+ channels, receptors and/or transporters with prolonged hypoxic submergence could underly the greater Δ[Ca2+]i peak amplitude, as well as the faster Δ[Ca2+]i rise time and Δ[Ca2+]i rate of rise observed in the myocytes from hypoxia-acclimated fish. Indeed, Δ[Ca2+]i 50% decay time was not prolonged and Rate50% relax was not slowed in hypoxia-acclimated fish. Nevertheless, further studies are required to confirm the specific Ca2+ influx and efflux pathways that are upregulated to fully elucidate the contribution of the SR to Ca2+ cycling and determine if other mechanisms, such as modification of channel kinetics and intracellular Ca2+ buffering capacity (e.g., calmodulin), also contribute to the altered Δ[Ca2+] in ventricle of hypoxia-acclimated Alaska blackfish. An additional possible adaption to increase contractility would be an increased end diastolic volume to shift the heart up the Frank-Starling curve. Future measurements of resting end diastolic volume in vivo would be interesting in this regard.
Notably, the effect of prolonged hypoxic submergence on the kinetics of Δ[Ca2+]i in ventricle myocytes and the kinetics of contraction and relaxation in ventricle strips were not always consistent. In ventricle myocytes, Δ[Ca2+]i rise time was shortened, and Δ[Ca2+]i 50% decay time was unaltered by hypoxia acclimation. By comparison, TPF and T0.5R of isometrically-contracting ventricle strips were prolonged by hypoxia acclimation. Given that the kinetics of cardiac contraction are determined by the rate of Δ[Ca2+]i in cardiomyocytes (i.e., the rate of activation induced by Ca2+ ions and their removal) and by myofibrillar ATPase activity (i.e., the attachment and detachment rate of cross-bridges) (Hoh et al., 1988), the findings may indicate changes at the level of the myofibrils with prolonged hypoxia exposure. The fluorescence signals recorded here as F/Fo for amplitude and time course are influenced by the Ca2+-availability in the cytosol and by Ca2+-affinity of the myofilaments (Allen and Orchard, 1987), but the current set of cellular experiments cannot differentiate between these. However, in the anoxia-tolerant crucian carp, slowed isometric contraction kinetics following cold acclimation is associated with lower myofibrillar Ca2+-Mg2+-ATPase activity (Tiitu and Vornanen, 2001), as well as with the expression of a slow myosin heavy chain isoform that has a lower myosin-ATPase activity (Vornanen, 1994). The modifications served to improve the energetic economy of contraction. Whether similar modifications occur in Alaska blackfish with cold acclimation (see Kubly and Stecyk, 2019) and/or prolonged hypoxic submergence as an energy conserving mechanism remains to be determined.
4.2. Maximum contractile frequency is limited by chronic hypoxic submergence
While changes to Δ[Ca2+]i following hypoxia-acclimation facilitated maintained cellular performance in Alaska blackfish at routine contraction frequencies, they did not enhance cardiac performance at high contraction frequencies. Rather, maximal attainable contraction frequencies were lower in hypoxia-acclimated myocytes and strips than preparations from normoxic animals in hypoxia. Moreover, the inability of hypoxia-acclimated ventricular myocytes and strips to contract at high frequency persisted upon and was often exacerbated by reoxygenation. The findings indicate that there may be consequences for heart rate elevation during hypoxia, which may impact cardiac output in vivo.
The diminished ability of the hypoxic Alaska blackfish heart to beat at higher frequencies in hypoxia, and subsequently upon reoxygenation, may have arisen from cellular Ca2+ overload. Indeed, the change in Δ[Ca2+]i diastolic Ca2+ with acute hypoxia exposure and increased pacing frequency (from 0.2 Hz in normoxia) was greater in hypoxia-acclimated than normoxia-acclimated myocytes, and hypoxia-acclimated myocytes displayed a clear increase in diastolic Ca2+ levels with increased contraction frequency under both normoxic and hypoxic recording conditions. However, resting tension of ventricular strips from hypoxia-acclimated fish did not increase beyond that of normoxia-acclimated preparations as stimulation frequency increased. Nevertheless, the increased incidence of alternans and arrhythmias following acute hypoxia exposure at frequencies greater than 0.2 Hz signifies disruption to Ca2+ cycling. Notably, the reduced response to increased demand reflects changes that occur in the diseased mammalian heart, where shifts to anaerobic respiration pathways and substrate utilization leads to a reduced capacity to respond to sustained increased demand (Chen et al., 2019). Alternatively, the cellular mechanisms underlying the inability of the hypoxia-acclimated heart to beat at higher frequencies could reflect those associated with reperfusion of the ischemic mammalian heart (Hausenloy et al., 2016). Indeed, the cardiac tissue of hypoxia-acclimated fish was reoxygenated prior to the acute hypoxia exposure. Finally, the inability of the hypoxia hypoxia-acclimated heart to beat at higher frequencies could reflect differential responses of ventricular K+ channels to hypoxia. Chronically hypoxic Alaska blackfish exhibit a pronounced shortening of ventricular AP duration that is associated with an upregulation of IKr, a repolarizing K+ current via rapid delayed rectifier K+ channels (Stecyk et al., 2020). However, the effect of hypoxia-acclimation on the activity of cardioprotective sarcolemmal ATP-sensitive K+ (KATP) channels (Zhuo et al., 2005), remains unknown. A blunted response of KATP channels to oxygen limitation in hypoxia-acclimated Alaska blackfish, as occurs in cold-acclimated C. carrasius (Paajanen and Vornanen, 2002), could lead to a disruption of Ca2+ cycling at increased stimulation frequencies. Clearly, elucidating the effects of hypoxia-acclimation on the ventricular metabolic enzyme activities and KATP channel activity, as well as probing the ability of the Alaska blackfish heart to deal with reactive oxygen species, would be informative avenues of future research.
4.3. Insights into the hypoxia tolerance of the Alaska blackfish
The present study reveals that the Alaska blackfish is more hypoxia-tolerant than previously appreciated. In the present study, Alaska blackfish acclimated to 5 °C successfully tolerated up to 8 weeks exposure to a level of aquatic hypoxia (2.1 kPa–4.2 kPa) that was considerably more severe than previously employed (∼6.3 kPa–8.4 kPa) (Stecyk et al., 2020) and below the critical oxygen partial pressure (∼5 kPa) determined for 5 °C-acclimated, normoxic Alaska blackfish (Lefevre et al., 2014). The prolonged period at which Alaska blackfish were able to tolerate severe hypoxia in the present study also contrasts with the prior finding that cold-acclimated, normoxic Alaska blackfish acutely exposed to severe hypoxia (3.3 kPa) and restricted from air-breathing rapidly (within 13 h of exposure) lost equilibrium (Lefevre et al., 2014).
The striking difference in hypoxia tolerance of Alaska blackfish described in the former (Lefevre et al., 2014) and current study could reflect that the stepwise exposure aquatic hypoxia employed in the present study may have preconditioned tissues for more severe hypoxia exposure and/or provided adequate time for acclimatory physiological remodelling to occur. In this regard, future experiments investigating if gradual and long-term hypoxia acclimation alters the respiratory physiology of Alaska blackfish would be enlightening. Alternatively, seasonality may have influenced the hypoxia tolerance of Alaska blackfish. Previous experiments were conducted in spring on fish that had either been maintained in captivity at 12–15 °C for more than 12 months prior to acclimation to 5 °C, or captured from the wild in spring (Lefevre et al., 2014). In the present study, fish were captured in summer and acclimated to low temperature and exposed to chronic hypoxic submergence in autumn. If metabolism and organ function is altered seasonally in Alaska blackfish like it is in the anoxia-tolerant crucian carp, which accumulates energy reserves (i.e., tissue glycogen levels) in summer to support anoxic overwintering (reviewed by Vornanen et al., 2009), then a greater hypoxia tolerance in fall would be expected.
At the level of the heart, although experimental differences (e.g., stimulation frequency, temperature, composition of physiological saline solution, etc.) confound comparisons across species and studies, the ∼50% depression of ventricular Fmax of normoxia- and hypoxia-acclimated Alaska blackfish with acute hypoxia exposure, plus the recovery of Fmax to normoxic levels upon reoxygenation, signifies that the Alaska blackfish ventricle is relatively hypoxia-tolerant compared to other water and air-breathing teleosts. By comparison, oxygen limitation reduces myocardial force generation by 90% in rainbow trout (Gesser, 1977), 66% in common carp (Cyprinus carpio) (Gesser, 1977) and 50% in the red-bellied piranha (Joyce et al., 2019). In air-breathing fishes, oxygen limitation decreases ventricle contractility by 40–75% in the swamp eel (Monopterus albus) (Iversen et al., 2013), Amazonian armored catfish (Liposarcus pardalis) (MacCormack et al., 2003) and striped catfish (Pangasianodon hypophthalmus) (Joyce et al., 2015). Nevertheless, the reduction of Alaska blackfish ventricular Fmax by hypoxia surpasses that displayed by cold-acclimated anoxia-tolerant vertebrates. Twitch force of ventricular strips from 5 °C-acclimated, anoxia-exposed Western painted turtles only falls by 14% after 30–40 min of anoxia exposure (Overgaard et al., 2005) and maximal force generation of spontaneously contracting heart preparations from 6 to 8 °C-acclimated crucian carp only decreases by 38% after 60 min of anoxia at 6.5 °C (Stecyk et al., 2011).
4.4. Conclusions, perspectives and future directions
Overall, the present study provides further mechanistic insight into the cardiophysiological responses employed by Alaska blackfish to support continued activity during overwintering. Our results signify that chronic exposure to hypoxic submergence at cold temperature modifies ventricular Δ[Ca2+]i in a manner that serves to maintains cardiac contractility at the normoxic level, but only at routine contraction frequency. Comparatively, the response is in stark contrast with that of anoxia-tolerant freshwater turtles, which exhibit a marked bradycardia during anoxia exposure and have an anoxia-survival strategy of down-regulating ATP demand and ATP production in the heart (reviewed by Farrell and Stecyk, 2007; Jackson, 2000; Overgaard et al., 2007; Stecyk et al., 2008; Stecyk, 2017). Indeed, juvenile snapping turtles that embryonically developed in hypoxia display a smaller Δ[Ca2+]i than normoxic controls (Ruhr et al., 2019), cold, anoxic Western painted turtles present evidence of reduced ventricular E-C coupling and/or calcium cycling (Overgaard et al., 2005), and cold, anoxic red-eared slider turtles display unaltered ventricular L-type Ca2+ current (Stecyk et al., 2007) and trans-SL Ca2+ flux (Stecyk et al., 2021). Finally, the results point to an enhanced role of the SR in Ca2+ cycling, and modifications to the cardiac myofilaments and respiratory physiology of Alaska blackfish with chronic hypoxic submergence. The Alaska blackfish thus presents an interesting model for future investigations to probe the connections between oxygen, metabolism, and cardiac physiology.
Funding
Research reported in this publication was generously supported by funding from the National Science Foundation, Division of Integrative Organismal Systems (1557818) and a University of Alaska Anchorage (UAA) INNOVATE Award to J.A.W.S. D.H. was kindly supported by a University of Alaska Anchorage Undergraduate Discovery Grant Research Award and an Undergraduate Research Assistantship supported by an Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of the National Institutes of Health Award under grant number P20GM103395. S.R. was an undergraduate research participant in an NIH Diversity Program Consortium study that was supported by the National Institutes of Health Common Fund and Office of Scientific Workforce Diversity under three linked awards RL5GM118963, TL4GM118965, and UL1GM118964, administered by the National Institute of General Medical Sciences. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
CRediT authorship contribution statement
Holly A. Shiels: Conceptualization, Investigation, Formal analysis, Writing – review & editing. Ed White: Conceptualization, Investigation, Formal analysis, Writing – review & editing. Christine S. Couturier: Conceptualization, Investigation, Formal analysis, Writing – review & editing. Diarmid Hall: Investigation, Formal analysis. Shannon Royal: Investigation, Formal analysis. Gina L.J. Galli: Conceptualization, Investigation, Formal analysis, Writing – review & editing. Jonathan A.W. Stecyk: Conceptualization, Investigation, Formal analysis, Writing – original draft, Project administration, Funding acquisition.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Jonathan Stecyk reports financial support was provided by National Science Foundation. Diarmid Hall, Shannon Royal reports financial support was provided by National Institutes of Health.
References
- Allen D.G., Orchard C.H. Myocardial contractile function during ischemia and hypoxia. Circ. Res. 1987;60:153–168. doi: 10.1161/01.res.60.2.153. [DOI] [PubMed] [Google Scholar]
- Buck L.T., Pamenter M.E. The hypoxia-tolerant vertebrate brain: arresting synaptic activity. Comp. Biochem. Physiol. B. 2018;224:61–70. doi: 10.1016/j.cbpb.2017.11.015. [DOI] [PubMed] [Google Scholar]
- Bundgaard A., Ruhr I.M., Fago A., Galli G.L.J. Metabolic adaptations to anoxia and reoxygenation: new lessons from freshwater turtles and crucian carp. Cur. Opinion Endocrine Metabol. Res. 2020;11:55–64. [Google Scholar]
- Campbell M.A., Lopéz J.A. Mitochondrial phylogeography of a Beringian relict: the endemic freshwater genus of blackfish Dallia (Esociformes) J. Fish. Biol. 2014;84:523–538. doi: 10.1111/jfb.12314. [DOI] [PubMed] [Google Scholar]
- Campbell M.A., Takebayashi N., López J.A. Beringian sub-refugia revealed in blackfish (Dallia): implications for understanding the effects of Pleistocene glaciations on Beringian taxa and other Arctic aquatic fauna. BMC Evol. Biol. 2015;15:144. doi: 10.1186/s12862-015-0413-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L., Song J., Hu S. Metabolic remodeling of substrate utilization during heart failure progression. Heart Fail. Rev. 2019;24:143–154. doi: 10.1007/s10741-018-9713-0. [DOI] [PubMed] [Google Scholar]
- Dhalla N.S., Temsah R.M., Netticadan T., Sandhu M.S. In: Heart Physiology and Pathophysiology. fourth ed. Sperelakis N., Kurachi Y., Terzic A., Cohen M.V., editors. Academic Press; San Diego: 2001. Calcium overload in ischemia/reperfusion injury; pp. 949–965. [Google Scholar]
- Farrell A.P., Stecyk J.A. The heart as a working model to explore themes and strategies for anoxic survival in ectothermic vertebrates. Comp. Biochem. Physiol. A. 2007;147:300–312. doi: 10.1016/j.cbpa.2007.01.021. [DOI] [PubMed] [Google Scholar]
- Galli G.L.J., Shiels H.A., Brill R.W. Temperature sensitivity of cardiac function in pelagic fishes with different vertical mobilities: yellowfin tuna (Thunnus albacares), bigeye tuna (Thunnus obesus), mahimahi (Coryphaena hippurus), and swordfish (Xiphias gladius) Physiol. Biochem. Zool. 2009;82:280–290. doi: 10.1086/597484. [DOI] [PubMed] [Google Scholar]
- Gesser H. The effects of hypoxia and reoxygenation on force development in myocardia of carp and rainbow trout: protective effects of CO2/HCO3- J. Exp. Biol. 1977;69:199–206. doi: 10.1242/jeb.69.1.199. [DOI] [PubMed] [Google Scholar]
- Gómez A.M., Valdivia H.H., Cheng H., Lederer Miriam R., Santana L.F., Cannell M.B., McCune S.A., Altschuld R.A., Lederer W.J. Defective excitation-contraction coupling in experimental cardiac hypertrophy and heart failure. Science. 1997;276:800–806. doi: 10.1126/science.276.5313.800. [DOI] [PubMed] [Google Scholar]
- Graham M.S., Farrell A.P. The effect of temperature acclimation and adrenaline on the performance of a perfused trout heart. Physiol. Zool. 1989;62:38–61. [Google Scholar]
- Hausenloy D.J., Barrabes J.A., Bøtker H.E., Davidson S.M., Di Lisa F., Downey J., Engstrom T., Ferdinandy P., Carbrera-Fuentes H.A., Heusch G., Ibanez B., Iliodromitis E.K., Inserte J., Jennings R., Kalia N., Kharbanda R., Lecour S., Marber M., Miura T., Ovize M., Perez-Pinzon M.A., Piper H.M., Przyklenk K., Schmidt M.R., Redington A., Ruiz-Meana M., Vilahur G., Vinten-Johansen J., Yellon D.M., Garcia-Dorado D. Ischaemic conditioning and targeting reperfusion injury: a 30 year voyage of discovery. Basic Res. Cardiol. 2016;111:70. doi: 10.1007/s00395-016-0588-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes T.B., Rosenberger A.E., Lindberg M.S., Whitman M., Schmutz J.A. Patterns of lake occupancy by fish indicate different adaptations to life in a harsh Arctic environment. Freshw. Biol. 2014;59:1884–1896. [Google Scholar]
- Herbert C.V., Jackson D.C. Temperature effects on the responses to prolonged submergence in the turtle Chrysemys picta bellii. II. Metabolic rate, blood acid-base and ionic changes, and cardiovascular function in aerated and anoxic water. Physiol. Zool. 1985;58:670–681. [Google Scholar]
- Hoh J.F., Rossmanith G.H., Kwan L.J., Hamilton A.M. Adrenaline increases the rate of cycling of crossbridges in rat cardiac muscle as measured by pseudo-random binary noise-modulated perturbation analysis. Circ. Res. 1988;62:452–461. doi: 10.1161/01.res.62.3.452. [DOI] [PubMed] [Google Scholar]
- Iversen N.K., Lauridsen H., Huong D.T.T., Van Cong N., Gesser H., Buchanan R., Bayley M., Pedersen M., Wang T. Cardiovascular anatomy and cardiac function in the air-breathing swamp eel (Monopterus albus) Comp. Biochem. Physiol. A. 2013;164:171–180. doi: 10.1016/j.cbpa.2012.08.007. [DOI] [PubMed] [Google Scholar]
- Jackson D.C. Living without oxygen: lessons from the freshwater turtle. Comp. Biochem. Physiol. A. 2000;125:299–315. doi: 10.1016/s1095-6433(00)00160-4. [DOI] [PubMed] [Google Scholar]
- Joyce W., Gesser H., Bayley M., Wang T. Anoxia and acidosis tolerance of the heart in an air-breathing fish (Pangasianodon hypophthalmus) Physiol. Biochem. Zool. 2015;88:648–659. doi: 10.1086/682701. [DOI] [PubMed] [Google Scholar]
- Joyce W., Williams C.J.A., Iversen S., Henriksen P.G., Bayley M., Wang T. The effects of endogenous and exogenous catecholamines on hypoxic cardiac performance in red-bellied piranhas. J. Exp. Zool. 2019;331:27–37. doi: 10.1002/jez.2233. [DOI] [PubMed] [Google Scholar]
- Kubly K.L., Stecyk J.A.W. Temperature-dependence of L-type Ca2+ current in ventricular cardiomyocytes of the Alaska blackfish (Dallia pectoralis) J. Comp. Physiol. B. 2015;185:845–858. doi: 10.1007/s00360-015-0931-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubly K.L., Stecyk J.A.W. Contractile performance of the Alaska blackfish (Dallia pectoralis) ventricle: assessment of the effects of temperature, pacing frequency, the role of the sarcoplasmic reticulum in contraction and adrenergic stimulation. Comp. Biochem. Physiol. A. 2019;238:110564. doi: 10.1016/j.cbpa.2019.110564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Layland J., Young I.S., Altringham J.D. The effect of cycle frequency on the power output of rat papillary muscles in vitro. J. Exp. Biol. 1995;198:1035. doi: 10.1242/jeb.198.4.1035. [DOI] [PubMed] [Google Scholar]
- Lefevre S., Damsgaard C., Pascale D.R., Nilsson G.E., Stecyk J.A.W. Air breathing in the Arctic: influence of temperature, hypoxia, activity and restricted air access on respiratory physiology of the Alaska blackfish (Dallia pectoralis) J. Exp. Biol. 2014;217:4387–4398. doi: 10.1242/jeb.105023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leppi J.C., Arp C.D., Whitman M.S. Predicting late winter dissolved oxygen levels in Arctic lakes using morphology and landscape metrics. Environ. Manag. 2016;57:463–473. doi: 10.1007/s00267-015-0622-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz P.L., Milton S.L. Negotiating brain anoxia survival in the turtle. J. Exp. Biol. 2004;207:3141–3147. doi: 10.1242/jeb.01056. [DOI] [PubMed] [Google Scholar]
- MacCormack T.J., Treberg J.R., Almeida-Val V.M.F., Val A.L., Driedzic W.R. Mitochondrial KATP channels and sarcoplasmic reticulum influence cardiac force development under anoxia in the Amazonian armored catfish Liposarcus pardalis. Comp. Biochem. Physiol. A. 2003;134:441–448. doi: 10.1016/s1095-6433(02)00315-x. [DOI] [PubMed] [Google Scholar]
- Matthews P.M., Taylor D.J., Radda G.K. Biochemical mechanisms of acute contractile failure in the hypoxic rat heart. Cardiovasc. Res. 1986;20:13–19. doi: 10.1093/cvr/20.1.13. [DOI] [PubMed] [Google Scholar]
- Nielsen K., Gesser H. Energy metabolism and intracellular pH in trout heart muscle under anoxia and different [Ca2+]o. J. Comp. Physiol. B. 1984;154:523–527. [Google Scholar]
- Orchard C.H., Kentish J.C. Effects of changes of pH on the contractile function of cardiac muscle. Am. J. Physiol. Cell Physiol. 1990;258:C967–C981. doi: 10.1152/ajpcell.1990.258.6.C967. [DOI] [PubMed] [Google Scholar]
- Overgaard J., Gesser H., Wang T. Tribute to P. L. Lutz: cardiac performance and cardiovascular regulation during anoxia/hypoxia in freshwater turtles. J. Exp. Biol. 2007;210:1687–1699. doi: 10.1242/jeb.001925. [DOI] [PubMed] [Google Scholar]
- Overgaard J., Wang T., Nielsen O.B., Gesser H. Extracellular determinants of cardiac contractility in the cold anoxic turtle. Physiol. Biochem. Zool. 2005;78:976–995. doi: 10.1086/432853. [DOI] [PubMed] [Google Scholar]
- Paajanen V., Vornanen M. The induction of an ATP-sensitive K+ current in cardiac myocytes of air- and water-breathing vertebrates. Pflug. Arch. Eur. J. Phy. 2002;444:760–770. doi: 10.1007/s00424-002-0870-5. [DOI] [PubMed] [Google Scholar]
- Piper H.M., Siegmund B., Ladilov Y.V., Schlüter K.D. Calcium and sodium control in hypoxic-reoxygenated cardiomyocytes. Basic Res. Cardiol. 1993;88:471–482. doi: 10.1007/BF00795413. [DOI] [PubMed] [Google Scholar]
- Ruhr I.M., McCourty H., Bajjig A., Crossley D.A., Shiels H.A., Galli G.L.J. Vol. 286. Proc. R. Soc.B Biol. Sci.; 2019. (Developmental plasticity of cardiac anoxia-tolerance in juvenile common snapping turtles (Chelydra serpentina)). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stecyk J.A., Galli G.L., Shiels H.A., Farrell A.P. Cardiac survival in anoxia-tolerant vertebrates: an electrophysiological perspective. Comp. Biochem. Physiol., C. 2008;148:339–354. doi: 10.1016/j.cbpc.2008.05.016. [DOI] [PubMed] [Google Scholar]
- Stecyk J.A., Paajanen V., Farrell A.P., Vornanen M. Effect of temperature and prolonged anoxia exposure on electrophysiological properties of the turtle (Trachemys scripta) heart. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007;293:R421–R437. doi: 10.1152/ajpregu.00096.2007. [DOI] [PubMed] [Google Scholar]
- Stecyk J.A., Stensløkken K.-O., Farrell A.P., Nilsson G.E. Maintained cardiac pumping in anoxic crucian carp. Science. 2004;306:77. doi: 10.1126/science.1100763. [DOI] [PubMed] [Google Scholar]
- Stecyk J.A.W. In: Gamperl A.K., Gillis T.E., Farrell A.P., Brauner C.J., editors. 36B. Academic Press; San Diego: 2017. Cardiovascular responses to limiting oxygen levels; pp. 299–371. (Fish Physiology). [Google Scholar]
- Stecyk J.A.W., Barber R.G., Cussins J., Hall D. Indirect evidence that anoxia exposure and cold acclimation alter transarcolemmal Ca2+ flux in the cardiac pacemaker, right atrium and ventricle of the red-eared slider turtle (Trachemys scripta) Comp. Biochem. Physiol. A. 2021;261:111043. doi: 10.1016/j.cbpa.2021.111043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stecyk J.A.W., Couturier C.S., Abramochkin D.V., Hall D., Arrant-Howell A., Kubly K.L., Lockmann S., Logue K., Trueblood L., Swalling C., Pinard J., Vogt A. Cardiophysiological responses of the air-breathing Alaska blackfish to cold acclimation and chronic hypoxic submergence at 5°C. J. Exp. Biol., jeb. 2020:225730. doi: 10.1242/jeb.225730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stecyk J.A.W., Larsen B.C., Nilsson G.E. Intrinsic contractile properties of the crucian carp (Carassius carassius) heart during anoxic and acidotic stress. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011;301:R1132–R1142. doi: 10.1152/ajpregu.00372.2010. [DOI] [PubMed] [Google Scholar]
- Tiitu V., Vornanen M. Cold adaptation suppresses the contractility of both atrial and ventricular muscle of the crucian carp heart. J. Fish. Biol. 2001;59:141–156. [Google Scholar]
- Tikkanen E., Haverinen J., Egginton S., Hassinen M., Vornanen M. Effects of prolonged anoxia on electrical activity of the heart in Crucian carp (Carassius carassius) J. Exp. Biol. 2017;220:445–454. doi: 10.1242/jeb.145177. [DOI] [PubMed] [Google Scholar]
- Vassalle M., Lin C.-I. Calcium overload and cardiac function. J. Biomed. Sci. 2004;11:542–565. doi: 10.1007/BF02256119. [DOI] [PubMed] [Google Scholar]
- Vornanen M. Seasonal and temperature-induced changes in myosin heavy chain composition of crucian carp hearts. Am. J. Physiol. 1994;267:R1567–R1573. doi: 10.1152/ajpregu.1994.267.6.R1567. [DOI] [PubMed] [Google Scholar]
- Vornanen M., Stecyk J.A.W., Nilsson G.E. In: Fish Physiology. Richards J.G., Farrell A.P., Brauner C.J., editors. Academic Press; 2009. The anoxia-tolerant crucian carp (Carassius Carassius L.) pp. 397–441. [Google Scholar]
- Wang R., Wang M., He S., Sun G., Sun X. Targeting calcium homeostasis in myocardial ischemia/reperfusion injury: an overview of regulatory mechanisms and therapeutic reagents. Front. Pharmacol. 2020;11:872. doi: 10.3389/fphar.2020.00872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yellon D.M., Hausenloy D.J. Myocardial reperfusion injury. N. Engl. J. Med. 2007;357:1121–1135. doi: 10.1056/NEJMra071667. [DOI] [PubMed] [Google Scholar]
- Zhuo M.-L., Huang Y., Liu D.-P., Liang C.-C. KATP channel: relation with cell metabolism and role in the cardiovascular system. Int. J. Biochem. Cell Biol. 2005;37:751–764. doi: 10.1016/j.biocel.2004.10.008. [DOI] [PubMed] [Google Scholar]