Abstract
A 12-year-old girl with acute lymphoblastic leukemia was referred to King Faisal Specialist Hospital and Research Center. The diagnosis without central nervous system (CNS) involvement was confirmed on admission, and chemotherapy was initiated according to the Children Cancer Group (CCG) 1882 protocol for high-risk-group leukemia. During neutropenia amphotericin B (AMB) (1 mg/kg of body weight/day) was initiated for presumed fungal infection when a computed tomography (CT) scan of the chest revealed multiple nodular densities. After 3 weeks of AMB therapy, a follow-up chest CT revealed progression of the pulmonary nodules. The patient subsequently suffered a seizure, and a CT scan of the brain was consistent with infarction or hemorrhage. Because of progression of pulmonary lesions while receiving AMB, antifungal therapy was changed to liposomal AMB (LAMB) (6 mg/kg/day). Despite 26 days of LAMB, the patient continued to have intermittent fever, and CT and magnetic resonance imaging of the brain demonstrated findings consistent with a brain abscess. Aspiration of brain abscess was performed and the Gomori methenamine silver stain was positive for hyphal elements. Culture of this material grew Acrophialophora fusispora. Lung biopsy showed necrotizing fungal pneumonia with negative culture. The dosage of LAMB was increased, and itraconazole (ITRA) was added; subsequently LAMB was discontinued and therapy was continued with ITRA alone. The patient demonstrated clinical and radiological improvement. In vitro, the isolate was susceptible to low concentrations of AMB and ITRA. A. fusispora is a thermotolerant, fast-growing fungus with neurotropic potential. We report the first case of human infection involving the CNS. Acrophialophora resembles Paecilomyces but differs in having colonies that become dark and in the development of phialides along the sides or at the tips of echinulate brown conidiophores. Conidia are borne in long chains and are smooth or ornamented with fine-to-coarse echinulations, sometimes in spiral bands. The taxonomy of the genus Acrophialophora is reviewed, and Acrophialophora nainiana and Acrophialophora levis are considered as synonyms of A. fusispora.
During the past 2 decades invasive fungal infections have emerged as a major cause of morbidity and mortality in an expanding spectrum of high-risk immunocompromised patients (27). The numbers and types of saprobic and opportunistic filamentous fungi that have been documented as etiologic agents of invasive diseases continue to escalate (15, 18, 26). Candida, Aspergillus, Trichosporon, and Fusarium species are the leading fungal pathogens in patients with hematologic malignancies (1), but less common molds are increasingly being documented as occasional pathogens (2, 12). Acrophialophora fusispora is a thermotolerant soil fungus that grows well at 45°C or higher temperatures. It has been reported once from a human corneal infection (22) and implicated in disseminated infection involving the brain in two dogs. In one of the latter cases, the etiologic agent was originally identified as Scopulariopsis chartarum (29), suggesting that A. fusispora may go unrecognized by diagnostic laboratories. We describe the first case of human cerebral brain abscess due to A. fusispora and use this case to compare microbiologic features and antifungal susceptibilities of pathogenic and nonpathogenic isolates and to review the taxonomy of the genus Acrophialophora.
CASE REPORT
Clinical course.
The patient was a 12-year-old Sudanese girl diagnosed with acute lymphoblastic leukemia on 12 July 1998. She received four courses of induction chemotherapy with vincristine and prednisone for 4 weeks in Sudan prior to admission to the King Faisal Specialist Hospital and Research Center (KFSH&RC) on 14 August 1998. The diagnosis of acute lymphoblastic leukemia without central nervous system (CNS) involvement was confirmed, and chemotherapy was initiated according to the CCG 1882 protocol for high-risk leukemia. This protocol included weekly vincristine and daunomycin and daily dexamethazone. After 2 weeks of chemotherapy, hyperglycemia and hypertension developed, following which dexamethasone was changed to prednisone to complete the 4 weeks of induction therapy. The patient received nine doses of asparaginase over 4 weeks. A bone marrow aspirate at 28 days postinduction showed remission of leukemia. The consolidation phase of chemotherapy consisted of cytosine arabinoside, 6-mercaptopurine, and cyclophosphamide, as well as intrathecal methotrexate. Subsequently the patient continued on maintenance chemotherapy.
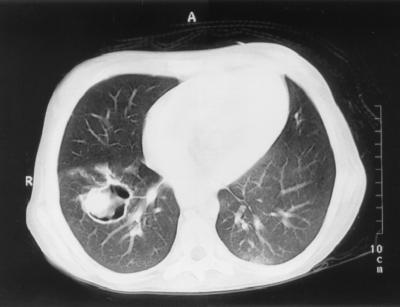
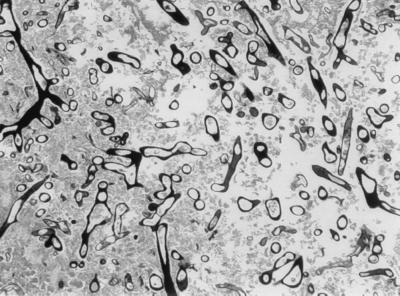
At her initial presentation to the KFSH&RC, the patient was febrile with no focus of infection. Empirical broad-spectrum antibiotics (ceftazidime and gentamicin) were initiated. Subsequently vancomycin was added. As her fever persisted during neutropenia, while she was on broad-spectrum antibiotics, amphotericin B (AMB), 1 mg/kg of body weight/day, was initiated on the 5th day for presumed fungal infection when the chest computed tomography (CT) scan revealed multiple nodular densities. A lung biopsy was deferred because of coagulopathy. After 3 weeks of AMB therapy, a follow-up chest CT scan revealed an increase in size and numbers of pulmonary nodules (Fig. 1). The patient subsequently suffered a seizure, and a CT scan of the brain was consistent with infarction or hemorrhage. Antifungal therapy was changed to liposomal AMB (LAMB) (AmBisome), 6 mg/kg/day, due to the progression of pulmonary lesions while receiving AMB. Despite 26 days of LAMB, the patient continued to have intermittent fever, and a follow-up CT and magnetic resonance imaging of the brain demonstrated a ring-enhancing lesion at the site of previously reported infarct. These findings were consistent with a brain abscess in the parieto-occipital area (Fig. 2). Aspiration of the brain abscess was performed. Thick yellowish pus was drained from the abscess, and the Gomori methenamine silver (GMS) stain was positive for a large number of septate hyphae (Fig. 3). Culture of the brain abscess aspirate grew a fungus closely resembling a Paecilomyces species.
FIG. 1.
Chest CT showing nodular lung lesions with cavitation.
FIG. 2.
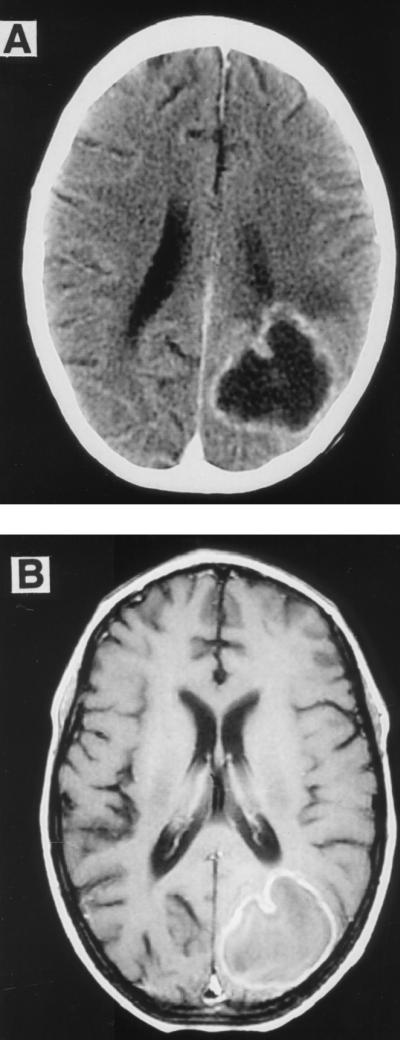
(A) CT of the brain with contrast demonstrating the brain lesion in the left parieto-occipital region. (B) Brain magnetic resonance imaging with contrast showing the ring-enhancing lesion in the left parieto-occipital region with minimal surrounding edema.
FIG. 3.
GMS stain of the brain abscess aspirate showing multiple fungal elements.
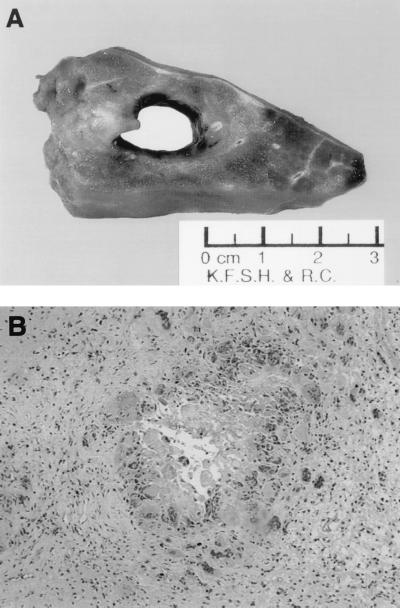
The patient also underwent right thoracotomy, and there was a cavitating fungal lesion in the lower lobe penetrating through the diaphragm. Histopathology of this lesion was consistent with necrotizing fungal pneumonia with septate hyphae (Fig. 4); however, the culture of this tissue yielded no growth. Because of the radiological and histopathologic findings the dosage of LAMB was increased to 10 mg/kg/day and itraconazole (ITRA), 7.5 mg/kg/day, was added and increased to 7.5 mg/kg every 12 h according to levels in serum. The patient also received granulocyte colony-stimulating factor, 5 μg/kg/day, as adjunctive therapy. Clinical and radiological improvement occurred after the LAMB dose was increased and ITRA was added. This combination was continued for 8 months, following which LAMB was discontinued and ITRA was continued as suppressive therapy. Repeated CT of the chest and brain 3 months after LAMB was discontinued showed no progression of the lesions. The child currently remains stable on ITRA, 15 mg/kg/day, with concomitant reduction in size of the brain abscess and resolution of the lung nodules.
FIG. 4.
Lung biopsy. (A) Cavitary lesion in the right lobe; (B) hematoxylin and eosin stain showing necrotizing cavitating pneumonia.
Histopathology.
The specimen from the brain aspirate consisted of multiple tiny pieces of grayish soft and necrotic white tissue. The frozen section showed necrotic brain tissue with granulomatous inflammation suggestive of fungal infection. GMS stain revealed numerous branching septate hyphae (Fig. 3). The lung biopsy consisted of a central rounded irregular cavity measuring 3 cm in diameter filled with creamy gray solid mass separated from the surrounding tissue (Fig. 4A). The microscopic findings were consistent with necrotizing cavitating invasive fungal pneumonia (Fig. 4B). The GMS stain demonstrated multiple hyphal elements similar to those in the brain.
MATERIALS AND METHODS
Mycology.
The aspirate of the brain abscess and the lung biopsy were plated on Sabouraud dextrose agar (SDA) and brain heart infusion agar. No organisms grew from lung biopsy. The brain abscess aspirate yielded several colonies of a mold that was identified initially as a Paecilomyces species based on its formation of conidia in short chains. The isolate was referred to the Fungus Testing Laboratory, Department of Pathology, University of Texas Health Science Center at San Antonio for further identification and susceptibility testing. There, the isolate was examined on potato flakes agar (PFA) prepared in-house and incubated at room temperature in ambient air with alternating daylight and darkness. Based on its colonial and microscopic features, the fungus was identified as A. fusispora and given accession number UTHSC 98-2508. The isolate was subsequently referred to the University of Alberta Microfungus Collection and Herbarium, Edmonton, Alberta, Canada, accession number UAMH 9508, for comparison with six other isolates of this species (Table 1) (23). Cultural and microscopic features were examined on SDA, PFA, and potato dextrose agar (Difco Laboratories, Detroit, Mich.) plates incubated at 30 and 37°C. Terms for colony colors are according to Kornerup and Wanscher (14). PFA slant cultures were evaluated for growth at 25, 35, and 42°C. The case isolate (UTMB 3307 = UTHSC R-3122 = UAMH 9684) from a dog with systemic mycosis (Welsh, personal communication) was acquired later and included in some comparative analyses (Table 1).
TABLE 1.
Source and features of A. fusispora isolates grouped by conidial ornamentation
| UTHSCa (UAMHb) accession no. | Source | Conidium ornamentation seen by SEMc | Conidium appearance observed on slide culture after 7 days at 37°C
|
Colonial features on PDA after 7 days at 37°C
|
||
|---|---|---|---|---|---|---|
| Size (μm) | Color | Colony diam (cm) | Colony colord (Top/Reverse) | |||
| 98-2508 (9508)f | Human brain; Saudi Arabia; I. Z. Al-Mohsen (=MRN-435262) | Smooth to very slightly roughened | 4 to 6.5 by 2.5 to 3.5 | Hyaline to yellow | 6 | Pale orange (6A3/4) with pale grayish patches centrally/light brownish orange |
| 96-2378 (8781) | Human lung nodules and bronchoalveolar lavage fluid; California; N. McClenny (=SUH H2976) | Smooth to very slightly roughened | 4 to 6 by 2 to 3 | Hyaline to yellow | 6.5 | Gray (6D1) to brownish gray (6E2), zonate/brownish black |
| R-2942 (6731) | Soil; Zaire; T. Matsushima (=OS-178) | Smooth to slightly roughened | 5 to 6 by 2.5 to 3 | Hyaline to yellow | 6.1 | Pale orange (6A3) to grayish orange [6B3]/light yellow |
| R-3122 (9684) | Dog, systemic infection involving brain; Stillwater, Okla.; R. D. Welsh (=UTMBe 3307 [reference 29]) | Smooth to slightly roughened | 5 to 7 by 2.5 to 4 | Hyaline to yellow | 8 | Brownish gray (6E2) to pale gray/brownish black |
| R-2944 (7792) | Human corneal ulcer; South India (=CDC B-5574) | Fine spirals | 4 to 8 by 3.5 to 5 | Hyaline to yellow | 5 | Brownish gray (6E2) with lighter pale orange (6A3) sector/brownish black |
| R-2940 (2889) | Soil; Ontario, Canada; G. C. Bhatt (=Barron OAC 10156) | Coarse spirals | 4 to 8 by 2.5 to 3.5 | Pale brown | 7.8 | Pale orange (6A3) to pale gray, zonate/grayish brown |
| R-2941 (4425) | Dog, heart and brain tissue; Oklahoma; J. A. Jackson (=CDC B-3538 =CDC 81-057308) | Coarse spirals | 4 to 6 by 2 to 4 | Pale brown | 6.5 | Gray (6D1) to brownish gray (6E2), zonate/brown |
| R-2943 (6967) | Human bronchial wash fluid; Edmonton, Alberta, Canada; P. Kibsey (=MY 1246) | Coarse spirals | 5.5 to 9 by 3.5 to 6 | Pale brown | 6.5 | Grayish orange (6B3) darkening to gray near edge/patchy brownish orange |
UTHSC, Department of Pathology, University of Texas Health Science Center at San Antonio.
UAMH, Edmonton, University of Alberta Microfungus Collection and Herbarium.
SEM, scanning electron microscopy.
Color names are from Kornerup and Wanscher (14).
UTMB, University of Texas Medical Branch, Galveston.
Case isolate.
Scanning electron microscopy.
Stubs were mounted with Spot-O-Glue labels (Avery, Diamond Bar, Calif.) and touched to the fungal culture. Preps were coated with gold-palladium using a Balzers MED 010 vacuum evaporator (Technotrade International, Inc., Manchester, N.H.) and examined with a JEOL (Tokyo, Japan) 840A microscope.
Antifungal susceptibility testing.
The case isolate and six additional strains (Table 2) were tested in a broth macrodilution method using NCCLS reference standard M27-T or M27-A (16, 17) modified for filamentous fungi to determine their susceptibility to antifungal agents. Isolates were grown on PFA slants at 25°C for approximately 1 week. Mycelium was flooded with sterile distilled water and scraped to obtain a conidial suspension. The case isolate was tested in 1999 utilizing M27-A in which conidial suspensions were standardized by hemacytometer conidial counts, with a final inoculum concentration of 1.0 × 104 CFU/ml. Isolates 96-2378 and R-2940 through R-2944 were tested in 1997 utilizing M27-T in which the suspension was standardized spectrophotometrically at 95%T at 530 nm and then diluted 1:10 to provide a final inoculum concentration of 1.0 × 104 CFU/ml.
TABLE 2.
Antifungal susceptibility data
| UTHSCa (UAMHb) accession no. and origin | MLCf (μg/ml) of AMBc at 24 h (48 h) | MICe (μg/ml) of drug at 24 h (48 h)g
|
||||
|---|---|---|---|---|---|---|
| AMBc | 5-FCd | FLUd | ITRAd | MONd | ||
| 98-2508 (9508) human (case isolate) | 1.0 (1.0) | 1.0 (1.0) | >64 (>64) | NT | 0.125 (0.25) | NT |
| R-2943 (6967) human | 1.0 (1.0) | 0.5 (1.0) | >64 (>64) | 16 (32) | 0.06 (0.125) | ≤0.03 (≤0.03) |
| 96-2378 (8781) human | 1.0 (2.0) | 0.5 (1.0) | >64 (>64) | 8 (32) | 0.25 (0.25) | ≤0.03 (≤0.03) |
| R-2944 (7792) human | 1.0 (1.0) | 1.0 (1.0) | >64 (>64) | 32 (32) | 0.125 (0.125) | ≤0.03 (≤0.03) |
| R-2941 (4425) animal | 1.0 (16) | 0.25 (0.5) | >64 (>64) | 32 (32) | 0.125 (0.125) | ≤0.03 (≤0.03) |
| R-2942 (6731) soil | 2.0 (16) | 0.5 (2.0) | >64 (>64) | 16 (64) | 0.06 (0.125) | ≤0.03 (0.06) |
| R-2940 (2889) soil | 2.0 (8) | 0.25 (0.25) | >64 (>64) | 16 (32) | 0.06 (0.125) | ≤0.03 (≤0.03) |
UTHSC, Department of Pathology, University of Texas Health Science Center at San Antonio.
UAMH, University of Alberta Microfungus Collection and Herbarium.
Tested in antibiotic medium 3, pH 7.0, 35°C.
Tested in RPMI-1640, pH 7.0, 35°C.
MICs are defined on the basis of the first tube showing no growth (AMB) or the first tube with an 80% reduction in growth (the azoles and 5F-C) compared to the drug-free control.
MLC is defined on the basis of the tube, for which the MIC was lowest, that was plated onto drug-free SPA and yielded five colonies or fewer.
Tubes were read after 24 and 48 h of incubation.
Antifungal agents and ranges tested included AMB (E. R. Squibb & Sons, Princeton, N.J.), 0.03 to 16 μg/ml; 5-fluorocytosine (5-FC) (Roche Laboratories, Nutley, N.J.), 0.125 to 64 μg/ml; fluconazole (FLU) (Pfizer, Inc., New York, N.Y.), 0.125 to 64 μg/ml; ITRA (Janssen Pharmaceutica, Titusville, N.J.), 0.03 to 16 μg/ml; and miconazole (MON), 0.03 to 16 μg/ml. Tubes were incubated at 35°C and read at 24 and 48 h. MICs were defined based on the first tube with a score of 0 (optically clear) for AMB and a score of 2 (a reduction of 80% or more in turbidity as contrasted with the drug-free control tube) for the other agents tested. Minimum lethal concentrations (MLCs) were determined for AMB by plating 100 μl of the 24-h growth from the drug-free control tube, the MIC tube, and each tube with a concentration above the MIC onto an SDA plate. The MLCs, read at both 24 and 48 h, were defined as the lowest concentration of antifungal compound resulting in five or fewer colonies on the SDA plate. The Paecilomyces quality control strain UTHSC 90-459, which has known values, was run in conjunction with the test isolates in all antifungal susceptibility testing.
RESULTS
Features of case isolate UTHSC 98-2508.
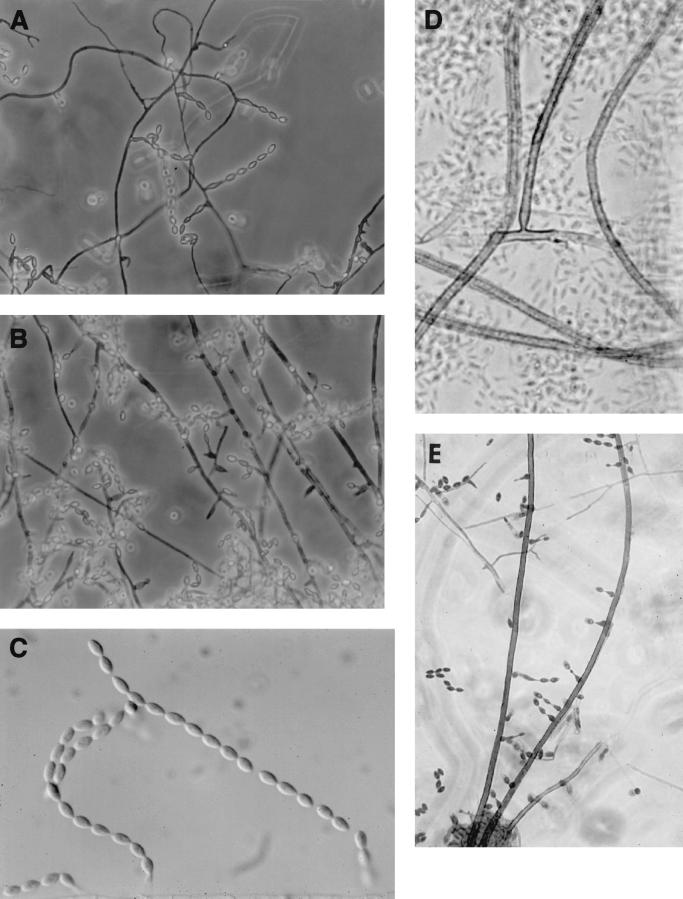
After 10 days of incubation at 30°C, colonies appeared white to buff with darker concentric circles on SDA and were a darker gray-brown on PFA. The reverse of colonies was brownish with centrally darker areas on SDA and a solid gray-brown on PFA. Basally inflated phialides (6 to 10 μm long by 3.5 to 6 μm wide) occurred along the sides of thin-walled, hyaline-to-pale brown septate hyphae (1.5 to 3.5 μm in diameter) (Fig. 5A) and on long, unbranched brown echinulate conidiophores (3 to 4 μm wide) that tapered at the tip. The phialides, sometimes proliferating, occurred singly or in pairs and occasionally in whorls (Fig. 5B). They bore long chains of limoniform-to-fusiform, one-celled, smooth, hyaline conidia (4.5 to 6.5 μm by 2.5 to 3.5 μm) (Fig. 5C). The echinulate brown conidiophores were prostrate on the subhyaline (very slightly pigmented) vegetative mycelium and were sometimes anchored by a definite basal hyphal cell (Fig. 5D), and they bore phialides sparingly along the sides and near the apex (Fig. 5E). Temperature studies on PFA revealed rapid growth at both 35 and 42°C.
FIG. 5.
Microscopic morphology. (A) Basally inflated phialides of case isolate, UTHSC 99-2508, occurring along the sides of thin-walled, hyaline-to-pale brown septate hyphae (magnification, ×306). (B) Proliferating phialides of the case isolate, UTHSC 99-2508 (magnification, ×306). (C) Long chains of limoniform-to-fusiform, one-celled, smooth, hyaline conidia of the case isolate, UTHSC 99-2508 (magnification, ×613). (D) Echinulate, brown, prostrate conidiophore of isolate UTHSC 96-2378, anchored by a definite basal hyphal cell (magnification, ×580). (E) Basally inflated phialides of dog isolate R-3122, borne along the sides of brown, echinulate conidiophores (magnification, ×306).
Comparison with other isolates.
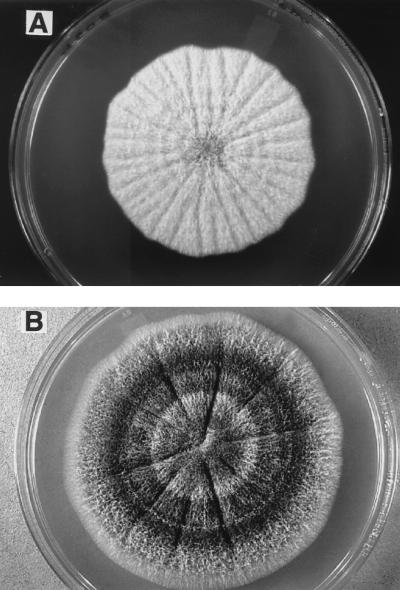
The isolates identified as A. fusispora varied in growth rates and colonial features (Table 1). All grew faster at 37°C (colony diameters, 5 to 8 cm after 7 days) (Table 1) than at 30°C (diameter 3.1 to 5.2 cm). Colonies of the case isolate (UTHSC 98-2508) (Fig. 6A) and isolates R2942, R2940, and R2943 were similar in being predominantly light orange with patches of pale gray on the top side and yellow to brownish orange, grayish brown, or uniformly brownish black on the reverse (Table 1). The other isolates were darker gray to brownish gray on the obverse and reverse (Fig. 6B). All produced dark, echinulate conidiophores, but none displayed the complex whorls of phialides borne at the tips as is usually depicted for the species (6, 13, 20). More commonly, phialides occurred singly and in pairs along the length of the conidiophore or on the vegetative hyphae. The brown conidiophores were prominant in isolates with darker colonies but developed in all isolates in older cultures and on media such as PFA. Sometimes they arose from sclerotium-like structures (round aggregations of hyphae). Conidial size and ornamentation varied among the isolates (Table 1). Walls were smooth or very slightly roughened (case isolate, 96-2378 and R-2942 [Fig. 7A]) to finely echinulate (R-2944 [Fig. 7B]) or coarsely echinulate in spiral bands (R-2940, R-2941, and R-2943 [Fig. 7C]) (Table 1). Conidium dimensions fell within a range of 4 to 9 μm long by 2 to 6 μm wide, with R-2943 having the largest dimensions. The conidia of the case isolate and R-2941 (dog) were similar in size, but they differed in color and ornamentation. Our observations were similar to those of Brown and Smith (4) for both microscopic features and growth habit. They had noted that isolates grew rapidly within 7 to 10 days but that by 21 days, colonies rarely reached the edge of the petri dish. Although some of our isolates did attain diameters of 100 mm within 15 days at 37°C, the average hourly growth rate after 3 days at 37°C was 0.46 mm/h, compared with 0.38 mm/h after 7 days (0.33 and 0.25 mm/h, respectively, at 30°C).
FIG. 6.
Colonial morphology after 7 days of incubation on PDA at 37°C. (A) Colony of the case isolate, UTHSC 99-2508; (B) colony of dog isolate R-3122.
FIG. 7.
Electron microscopy. (A) Scanning electron micrograph of smooth-to-slightly roughened conidia, soil isolate R-2942 (magnification, ×10,000). (B) Scanning electron micrograph of conidia with fine spirals, from human corneal isolate R-2944 (magnification, ×10,000). (C) Scanning electron micrograph of conidia with coarse spirals, from human bronchial washing isolate R-2943 (magnification, ×10,000).
Antifungal susceptibility.
The MICs of AMB against isolates of A. fusispora ranged between 0.25 and 2.0 μg/ml, and the MLCs ranged from 1.0 to 16 μg/ml (Table 2). These concentrations are within or above the safely achievable levels in plasma. Human isolates had lower 48-h MLCs of AMB than did nonhuman isolates, suggesting cidal activity at generally achievable drug levels. The isolates were inhibited by relatively low concentrations of ITRA and MON, but only by relatively high concentrations of FLU, demonstrating dose-dependent susceptibility (a term employed to indicate susceptibility of an organism at escalated doses of the drug). All isolates were resistant to high concentrations of 5-FC.
DISCUSSION
Other cases.
A. fusispora is a thermotolerant fungus with a wide distribution in tropical and temperate regions. Although this fungus has been regarded as suspicious for potential pathogenicity due to its isolation from eye and lung specimens (Table 1) (23), data have been insufficient to establish etiology. There is only one confirmed report concerning an eye infection, in a patient from India, and in that case, the authors were able to induce keratitis experimentally in rabbits by using the organism recovered from the patient (22). A. fusispora, originally placed in the genus Paecilomyces, is seldom described in medical mycology texts (5, 24), raising the question as to whether lack of information on this fungus has led to its being misidentified or overlooked as a potential pathogen. We have determined that one case published under the name S. chartarum involves this fungus. Welsh (personal communication) described systemic infection with brain involvement in a stray dog captured in Edmond, Okla. Their isolate is reidentified here as A. fusispora (UTMB 3307 = UTHSC R-3122 = UAMH 9684 [Table 1]). Coincidentally, UAMH 4425, acquired in 1981, was isolated from heart and brain tissue of a dog from Stillwater, Okla. Although no further details are available concerning the second dog, R. D. Welsh (personal communication) has confirmed that these are two separate cases. Ours is the first case of human cerebral infection and demonstrates that A. fusispora is another dark fungus potentially able to cause brain infection (2, 25). However it should be noted that isolates of A. fusispora vary in pigmentation and are not uniformly dark, as demonstrated by our patient's isolate (Table 1).
Present case.
Our patient demonstrated clinical and radiological response only to combination therapy with high-dose LAMB and ITRA. The pneumonia in this case was most likely caused by the same pathogen, which failed to grow due to the prolonged antifungal therapy prior to the time of the lung biopsy. Brain abscesses caused by molds can be very difficult to treat and often have a fatal outcome. The clinical manifestations (seizures, pneumonia) and risk factors (history of neutropenia and corticosteroids) of CNS Acrophialophora infection, in this case, are very similar to those of CNS aspergillosis (28). Hagensee et al. (11) found a 97% mortality rate in a review of the records of 58 patients with brain abscess following bone marrow transplant for treatment of hematologic conditions such as leukemia and aplastic anemia. Fungi, mainly Aspergillus and Candida species, were isolated in 92% of cases. A Scopulariopsis species was identified as the etiologic agent in one of these cases. As noted above, the disseminated infection in the dog was also attributed to a species of Scopulariopsis (29). Baddley et al. (2), when reporting the dematiaceous ascomycete Microascus cinereus (anamorph Scopulariopsis) as the cause of brain abscess in a bone marrow transplant recipient, pointed out that careful examination is required to discriminate the conidial stage from the superficially similar genus Paecilomyces.
Susceptibility and therapy.
Although standardization of antifungal susceptibility testing for filamentous fungi is only commencing (9) and breakpoints for Candida do not apply (19), some assessment as to an isolate's in vitro susceptibility can be inferred by comparing normally achievable concentrations of the antifungal agents in serum in patients receiving the recommended dosages (10, 21) with the MICs or MLCs for the isolate. Utilizing this approach showed that all isolates appeared susceptible to ITRA and MON. Isolates also appeared susceptible to AMB based on MICs (Table 2), except R-2942, for which the 48-h MIC was 2 μg/ml. Curiously, most human isolates appeared susceptible to AMB based on MLCs, excluding 96-2378, while nonhuman (one animal and two soil) isolates appeared resistant. Although the isolates displayed dose-dependent susceptibility to FLU, this agent is generally less efficacious for opportunistic filamentous fungi.
The MIC of AMB for the index isolate was 1.0 μg/ml, which is near the higher range of the maximum safely achievable peak concentrations of this polyene in plasma. The patient's infection progressed despite a high dosage of AMB (1 mg/kg/day). Thus, the MIC of 1 μg/ml in this patient correlates with a response observed only at the highest dose range of amphotericin. ITRA also may have contributed substantially to treatment of this patient's infections and was instituted in order to provide a compound that achieved high concentrations in brain tissue (10). That the MICs and MLCs of AMB against other isolates of A. fusispora are relatively high suggests that therapy in immunocompromised patients may fail despite administration of conventional AMB or lipid formulation of AMB. The addition of high-dose ITRA (21) with documentation of therapeutic plasma concentrations above its MIC may have an important role in management of disseminated infections due to this new human pathogen.
Taxonomy.
The genus Acrophialophora was described by Edward in 1959 with the type species A. nainiana for a fungus that was repeatedly recovered from Indian soil during the warmer months (7). The fungus was considered similar to Paecilomyces in forming chains of ellipsoidal to fusiform conidia from basally swollen phialides that were borne either on conidiophores or directly from the vegetative hyphae. However, the organism manifested several striking differences that included (i) unbranched, erect, brown, echinulate conidiophores that were fertile only near the apex; (ii) a basal, hyphal cell anchoring the conidiophore to the vegetative hyphae, somewhat like a foot cell in the genus Aspergillus; (iii) a distinct swelling at the base of the phialides; and (iv) phialides that did not curve or bend away from the main axis. Barron (3) did not accept the genus as being different from Paecilomyces, but Ellis (8) accepted it as distinct and listed A. fusispora as the type species based on an earlier published name (Paecilomyces fusisporus Saksena 1953). Ellis regarded A. nainiana Edward as a synonym of A. fusispora; however, he was apparently unaware of the redescription of Acrophialophora by Samson and Mahmood (20) that appeared about the same time. These authors compared 13 strains mostly isolated from the soil and recognized three species. They were differentiated mainly by conidial ornamentation and the degree of development of brown conidia but showed overlapping conidial dimensions: A. nainiana, with mature conidia that were hyaline and finely echinulate (measuring 4 to 10.5 μm long and 2 to 5 μm wide); A. fusispora (Saksena) Samson, with mature conidia brown, thick walled, and with echinulations in spiral bands (measuring 5 to 12 μm long and 3 to 6 μm wide); and A. levis Samson et T. Mahmood, with mature conidia smooth to slightly roughened and hyaline (measuring 4.5 to 8 μm long and 2 to 3.5 μm wide).
Although the case isolate could fit the description of A. levis following the species concepts proposed by Samson and Mahmood, we take the broader view that A. fusispora includes a continuum of conidial roughness from smooth to distinctly spiraled. This broad concept is supported by other common and overlapping characteristics, including thermotolerance, growth rates, colonial pigmentation, conidial dimensions, and in vitro susceptibility data. Although the four human isolates demonstrated lower MLCs of AMB (Table 2), these same isolates demonstrated variability in growth rates, colony color, and conidial wall ornamentation and size (Table 1).
Based on this broad species concept, the case isolate and other isolates are identified as A. fusispora. Features identifying the species include the following. (i) Isolates are thermotolerant, showing good growth at 42°C or higher. (ii) Colonies are initially buff or tan, usually becoming grayish brown with an uncolored or dark grayish brown reverse. Note that colonies of the case isolate remained lighter colored. (iii) Basally swollen phialides are borne mostly singly on the vegetative hyphae or along the length and near the tip of brown, echinulate conidiophores. (iv) Limoniform-to-fusiform or ellipsoidal conidia are borne in long chains and are smooth or finely to coarsely echinulate, sometimes in spiral bands. (v) Phialides do not curve away from the main axis. (vi) Phialides sometimes proliferate to form a second opening (Fig. 5B). Phialides producing more than one opening not delimited by a septum are also referred to as polyphialides. This recently recognized fungal pathogen differs from Paecilomyces in having colonies that turn dark and in forming brown echinulate conidiophores and differs from Scopulariopsis species in forming conidia from phialides rather than annellides.
As demonstrated by the present case, A. fusispora is capable of causing a devastating cerebral infection in a human, thus requiring aggressive antifungal therapy. This fungus has the potential to be neurotropic, as evidenced by our case and two cases involving dogs.
REFERENCES
- 1.Anaissie E, Bodey G P, Kantarjian H, Ro J, Vartivarian S E, Hopfer R, Hoy J, Rolston K. New spectrum of fungal infections with cancer. Rev Infect Dis. 1989;11:369–378. doi: 10.1093/clinids/11.3.369. [DOI] [PubMed] [Google Scholar]
- 2.Baddley J W, Moser S A, Sutton D A, Pappas P G. Microascus cinereus (anamorph Scopulariopsis) brain abscess in a bone marrow transplant patient. J Clin Microbiol. 2000;38:395–397. doi: 10.1128/jcm.38.1.395-397.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barron G L. The genera of Hyphomycetes from soil. Baltimore, Md: Williams & Wilkins Co.; 1968. [Google Scholar]
- 4.Brown A H S, Smith G. The genus Paecilomyces Bainer and its perfect stage Byssochlamys Wrestling. B Mycol Soc Trans. 1957;40:17–89. [Google Scholar]
- 5.De Hoog G S, Guarro J. Atlas of clinical fungi. Baarn, The Netherlands: Centraalbureau voor Schimmelcutlures; 1995. [Google Scholar]
- 6.Domsch K H, Gams W, Anderson T-H. Compendium of soil fungi. Eching, Germany: IHW-Verlag; 1980. . [Reprint, IHW-Verlag, Eching, Germany, 1993.] [Google Scholar]
- 7.Edward J C. A new genus of the Moniliaceae. Mycologia. 1959;51:781–786. [Google Scholar]
- 8.Ellis M G. Dematiaceous Hyphomycetes. Kew, United Kingdom: Commonwealth Mycological Institute; 1971. pp. 533–534. [Google Scholar]
- 9.Espinel-Ingroff A, Dawson K, Pfaller M, Anaissie E, Breslin B, Dixon D, Fothergill A, Paetznick V, Peter J, Rinaldi M, Walsh T. Comparative and collaborative evaluation of standardization of antifungal susceptibility testing for filamentous fungi. Antimicrob Agents Chemother. 1995;39:314–319. doi: 10.1128/aac.39.2.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Groll A H, Piscitelli S C, Walsh T J. Clinical pharmacology of systemic antifungal agents: a comprehensive review of agents in clinical use, current investigational compounds, and putative targets for antifungal drug development. Adv Pharmacol. 1998;44:343–500. doi: 10.1016/s1054-3589(08)60129-5. [DOI] [PubMed] [Google Scholar]
- 11.Hagensee M E, Bauwens J E, Kjos B, Bowden R A. Brain abscess following marrow transplantation: experience at the Fred Hutchinson Cancer Research Center, 1984-1992. Clin Infect Dis. 1994;19:402–408. doi: 10.1093/clinids/19.3.402. [DOI] [PubMed] [Google Scholar]
- 12.Iwen P C, Sigler L, Tarantolo S, Sutton D A, Rinaldi M G, Lackner R P, McCarthy D I, Hinrichs S H. Pulmonary infection caused by Gymnascella hyalinospora in a patient with acute myelogenous leukemia. J Clin Microbiol. 2000;38:375–381. doi: 10.1128/jcm.38.1.375-381.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kirk P M. IMI Descriptions of fungi and bacteria 1051. Acrophialophora fusispora. Mycopathologia. 1991;115:131–132. [Google Scholar]
- 14.Kornerup A, Wanscher J H. Methuen handbook of color. 3rd ed. London, United Kingdom: Methuen; 1978. [Google Scholar]
- 15.Matsumoto T, Ajello L, Matsud T, Szaniszlo P J, Walsh T J. Developments in hyalohyphomycosis and phaeohyphomycosis. J Med Vet Mycol. 1994;32(Suppl. 1):329–349. doi: 10.1080/02681219480000951. [DOI] [PubMed] [Google Scholar]
- 16.National Committee for Clinical Laboratory Standards. Reference method for broth dilution antifungal susceptibility testing of yeasts. Tentative standard M27-T. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1995. [Google Scholar]
- 17.National Committee for Clinical Laboratory Standards. Reference method for broth dilution antifungal susceptibility testing of yeasts. Approved standard M27-A. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1997. [Google Scholar]
- 18.Perfect J R, Schell W A. The new fungal opportunists are coming. Clin Infect Dis. 1996;22(Suppl. 2):S112–S118. doi: 10.1093/clinids/22.supplement_2.s112. [DOI] [PubMed] [Google Scholar]
- 19.Rex J H, Pfaller M A, Galgiani J N, Bartlett M S, Espinel-Ingroff A, Ghannoum M A, Lancaster M, Odds F C, Rinaldi M G, Walsh T J, Barry A L Subcommittee on Antifungal Susceptibility Testing. Conceptual framework and analysis of in vitro-in vivo correlation data for fluconazole, itraconazole, and Candida infections. Clin Infect Dis. 1997;24:235–247. doi: 10.1093/clinids/24.2.235. [DOI] [PubMed] [Google Scholar]
- 20.Samson R A, Mahmood T. The genus Acrophialophora (Fungi Moniliales) Acta Bot Neerl. 1970;19:804–808. [Google Scholar]
- 21.Sharkey P K, Rinaldi M G, Dunn J F, Hardin T C, Fetchick R J, Graybill J R. High-dose itraconazole in the treatment of severe mycoses. Antimicrob Agents Chemother. 1991;35:707–713. doi: 10.1128/aac.35.4.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shukla P K, Khan Z A, Lal B, Agrawal P K, Srivastava O P. Clinical and experimental keratitis caused by the Collectotrichum state of Glomerella cingulata and Acrophialophora fusispora. Sabouraudia. 1983;21:137–147. [PubMed] [Google Scholar]
- 23.Sigler L, Flis A. Catalogue of the University of Alberta Microfungus Collection and Herbarium. 3rd ed. Edmonton, Alberta, Canada: University of Alberta; 1998. [Google Scholar]
- 24.Sigler L, Kennedy M J. Aspergillus, Fusarium, and other opportunistic moniliaceous fungi. In P. R. Murray, E. J. Baron, M. F. Pfaller, F. C. Tenover, and R. H. Yolken (ed.), Manual of clinical microbiology. 7th ed. Washington, D.C.: American Society for Microbiology; 1999. pp. 1212–1241. [Google Scholar]
- 25.Sutton D A, Slifkin M, Yakulis R, Rinaldi M G. U.S. case report of cerebral phaeohyphomycosis caused by Ramichloridium obovoideum (R. mackenziei): criteria for identification, therapy, and review of other known dematiaceous neurotropic taxa. J Clin Microbiol. 1998;36:708–715. doi: 10.1128/jcm.36.3.708-715.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vartivarian S, Anaissie E J, Bodey G P. Emerging fungal pathogens in immunocompromised patients: classification, diagnosis, and management. Clin Infect Dis. 1993;17:S487–S491. doi: 10.1093/clinids/17.supplement_2.s487. [DOI] [PubMed] [Google Scholar]
- 27.Walsh T J, Hiemenz J, Pizzo P A. Evolving risk factors for invasive fungal infections: all neutropenic patients are not the same. Clin Infect Dis. 1994;18:793–798. doi: 10.1093/clinids/18.5.793. [DOI] [PubMed] [Google Scholar]
- 28.Walsh T J, Hier D B, Caplan L R. Fungal infections of the central nervous system: analysis of risk factors and clinical manifestations. Neurology. 1985;35:1654–1657. doi: 10.1212/wnl.35.11.1654. [DOI] [PubMed] [Google Scholar]
- 29.Welsh R D, Ely R W. Scopulariopsis chartarum systemic mycosis in a dog. J Clin Microbiol. 1999;37:2102–2103. doi: 10.1128/jcm.37.6.2102-2103.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]