Abstract
Age-related immunosenescence, defined as an increase in inflammaging and the decline of the immune system, leads to tissue dysfunction and increased risk for metabolic disease. The elderly population is expanding, leading to a heightened need for therapeutics to improve health span. With age, many alterations of the immune system are observed, including shifts in the tissue-resident immune cells, increased expression of inflammatory factors, and the accumulation of senescent cells, all of which are responsible for a chronic inflammatory loop. Adipose tissue and the immune cell activation within are of particular interest for their well-known roles in metabolic disease. Recent literature reveals that adipose tissue is an organ in which signs of initial aging occur, including immune cell activation. Aged adipose tissue reveals changes in many innate and adaptive immune cell subsets, revealing a complex interaction that contributes to inflammation, increased senescence, impaired catecholamine-induced lipolysis, and impaired insulin sensitivity. Here, we will describe current knowledge surrounding age-related changes in immune cells while relating those findings to recent discoveries regarding immune cells in aged adipose tissue.
Introduction
The aging population is expected to expand by fourfold to represent 20% of the global population by 2050 (1). The elderly suffer from multiple chronic diseases, including metabolic diseases, cancer, and Alzheimer disease. These diseases are now recognized to be driven by low-grade, chronic inflammation, termed “inflammaging,” that accumulates with age (2). Inflammaging is one component of immunosenescence, the dysregulated and dysfunctional immune system. The connections between inflammation, immunosenescence, and metabolic disease are well established. A central feature of aging and the associated risk for metabolic disease is the increased adipose tissue (AT) mass that occurs. White AT is an endocrine organ with active roles in regulating inflammation during metabolic disease. Recent publications used transcriptomics and proteomics across multiple murine tissues, ages, and sexes. They revealed that the visceral AT uniquely exhibits immune cell activation, inflammation, and a reduction in oxidative phosphorylation compared with the other tissues examined, including brown AT, liver, kidney, and lymphoid tissues, among others (3,4). This work, which formed the Tabula Muris Senis, or “Mouse Aging Cell Atlas,” describes the activation of the immune cells in the white AT that occurs during middle age, prior to the immune activation in other tissues (3). This helps to establish the AT as an initiator of aging. Immune cells and their tissue-maintaining versus inflammatory properties are critical in modulating the adipocyte response to hormones, including catecholamines and insulin, and the adipocyte ability to differentiate from pre-adipocytes. Aged immune cells are altered through both cell-intrinsic mechanisms and cell-extrinsic mechanisms that are driven by changes in the aged environment. Work over the last few years has greatly progressed our understanding of immune cells from older individuals. This article focuses on the systemic changes in immune cells that were recently described and then discusses identified changes in AT immune cells. This Perspective presents a model where the inflammatory microenvironment driven by resident immune cells leads to impaired adipocyte function during aging.
Mouse Models in Aging Research
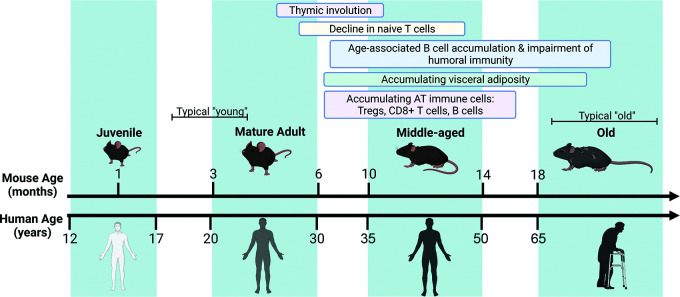
Mouse models are widely used in aging and immunological research due to their short life span, immune system similarities, and ease of genetic manipulations. In aging studies, it is important to consider the biological age of the mouse. Phases of the life span have been highlighted in Fig. 1. A well-known phenomenon, the involution of the thymus, is seen at middle age in both mice and humans (9 months of age in mice and at 30–40 years old in humans). Other changes in the immune system and the time frame in which those changes develop in mice are also listed. Given the initial immune cell activation that is seen in the AT that is seen in middle-aged mice, it is important to distinguish those from old age. A clear delineation of those changes would permit a greater understanding of if, when, and how to intervene with therapeutics to target the aged immune system.
Figure 1.
Aging of the mouse immune system. Shown is a timeline to compare mouse and human aging, along with broad phases of life: juvenile, mature adult, middle-aged, and old. The identified changes in the immune system of mice are listed at the top, and the typical comparison for mouse experiments are highlighted in brackets. Created in BioRender (BioRender.com).
Sex as a Variable in Aging
Women have a longer life span, a tendency toward reduced basal inflammation, and protection from metabolic disease compared with males (5,6). Men and women also tend to have differential distribution of AT, where men are more likely to have greater amounts of AT located around internal organs. Rodents also show sex-specific differences, where even in young, lean conditions, white visceral AT from male and female mice shows distinct cellular and molecular differences. These include the composition of the immune cells, the lipolytic response, ability to store lipid, and adipocyte differentiation, all of which are further altered with age (7). The production of sex hormones declines with advancing age and is associated with a greater risk for obesity and type 2 diabetes in females (5). Although aged female mice show increased AT mass and impaired glucose uptake when placed on a high-fat diet, the visceral AT maintains its lipolysis-induced metabolic flexibility and still shows an attenuated inflammatory response compared with aged male mice (8). Some recent research focuses on sex-specific differences in aged AT immune cells (highlighted in specific sections of this Perspective). A greatly understudied area is the cell-specific response to sex hormones, which can be mediated through signaling receptors that are widely expressed. Immune cells also express these receptors, but whether those receptors change with age and the impact of the decline in hormones on aged immune cells is unclear (6). This helps to highlight the need for studies that evaluate both sexes.
White AT Microenvironment During Aging
AT is a systemic regulator of hormone production, inflammation, and nutrient sensing. The major function of white adipocytes is to store lipids as triglycerides (9). The visceral depot, located internally and next to organs, is associated with the highest metabolic risk (10). The tissue is highly heterogeneous, containing immune cells, endothelial cells, pre-adipocytes, and stem cells within the stromal vascular fraction that function together to ensure homeostasis. Hormones, including insulin and catecholamines, regulate the balance of lipid storage as triglyceride versus hydrolysis to free fatty acid. Stem cells and pre-adipocytes make up the precursor cells that proliferate and differentiate into adipocytes. Precursor cells may become senescent or differentiate into fibroblasts and contribute to the inflammatory and profibrotic microenvironment of aged AT (11). The early accumulation of inflammation from immune cells results in reduced adipocyte response to insulin signaling through insulin receptor substrate (IRS) and the PI3K/AKT. There is also lower overall capacity to store excess energy as lipids due to impaired hyperplasia and differentiation of precursor cells. Additionally, aged AT has reduced catecholamine signaling through β-adrenergic receptors, activation of cAMP-dependent protein kinase (PKA), and the adipocyte lipases (hormone-sensitive lipase). This results in lower levels of lipolysis and fat mobilization under stimulation. The microenvironment is shifted toward fibrotic, reduced lipid storage/hydrolysis flexibility, and impaired differentiation to adipocytes. We will focus on the immune cell populations that contribute to the inflammatory microenvironment and impaired function of adipocytes.
Aged Macrophages Drive Inflammaging
Macrophages become activated through their expression of the NOD-, LRR-, and pryin domain-containing protein 3 (NLRP3) inflammasome, a pattern-like recognition receptor that recognizes damage-associated molecular patterns (DAMPs) and results in the release of cytokines interleukin-1β (IL-1β) and IL-18 (12). DAMPs include a diverse array of products, including ATP, pore-forming toxins, crystalline substances, nucleic acids, and endogenous products like ceramide or fatty acids (12), which increase during aging. Research implicates the NLRP3 inflammasome activation in driving chronic diseases. These include experiments using small-molecule inhibition of the NLRP3 inflammasome, where mouse models showed broadly beneficial responses in Alzheimer disease, atherosclerosis, and myocardial infarction, with reduced inflammation, preserved tissue function, and reduced disease severity after treatment (13–15). This research points toward the macrophage as an instigator of inflammaging after chronic exposure to DAMPS.
Aged macrophages also show reduced phagocytic ability and reduced antigen presentation but increased mitochondrial dysfunction and impaired cellular metabolism (16–21). Recent findings have highlighted the role of the sirtuin pathway and the de novo production of nicotinamide adenine dinucleotide (NAD+) via the kynurenine pathway in aged AT macrophages. Cell-autonomous production of NAD+ is reduced in aged macrophages, resulting in increased inflammation and impaired oxidative metabolism (20). Impaired NAD+ metabolism is linked to inflammasome activation through the activity of sirtuin 2 (SIRT2) (22). NAD+ supplementation, via nicotinamide mononucleotide, has a diverse array of positive effects in aged mouse models. These include reducing reactive oxygen levels, NLRP3 inflammasome activation, and bone loss, suggesting that metabolic defects in aged macrophages can be overcome (23). Strikingly, reprogramming myeloid glucose metabolism via blockade of the G-protein–coupled receptor, EP2, also reverses cognitive decline (24). These results highlight the importance of understanding which age-related changes are reversible and which are permanent.
Accumulation of Inflammatory AT Macrophages
Secreted factors regulate both the accumulation and the polarization of AT immune cells. The chemokines CCL2 and CCL11 are increased in aged murine and human AT (25). Paradoxically, research points toward a decrease in the chemokine-responding cells (subsets of macrophages, eosinophils, and innate lymphoid cells) (16,25,26), suggesting that old cells are not responsive. In this situation, eosinophils are recruited by CCL11 to young tissue and promote macrophage polarization to the M2 tissue-maintaining phenotype via IL-4 production (27). In the absence of IL-4 and other M2-polarizing factors, M1 proinflammatory macrophages are polarized by Toll-like receptor (TLR) ligands and interferon-γ (IFN-γ), commonly produced by T-cell subsets. These polarization states represent opposite ends of an activation spectrum, and it is now recognized that macrophages in vivo are exposed to multiple factors within the local microenvironment and, thus, have a more diverse phenotype (28,29). M2 macrophages are predominant in the young, lean environment and can be further divided into the subsets M2a, M2b, and M2c, which are polarized by IL-4/IL-13 (M2a), immune complexes (M2b), and IL-10 or glucocorticoids (M2c), respectively (30). Aged AT macrophages are of the proinflammatory phenotype, but reports differ on whether they express CD11c and other common markers to differentiate between the subsets (8,16,17). Transcriptomics reveal a spectrum of macrophage phenotypes present in the young and old male visceral AT, with IL-4–responsive macrophages predominant in the young and high-density lipoprotein-responsive macrophages in the old tissue (16). Overall, this research points toward a decrease in macrophages of the M2 phenotype and an increase in the inflammatory phenotype that occurs in both sexes, although to a lesser extent in female mice (8,16).
The tumor necrosis factor-α (TNF-α) and the NLRP3 inflammasome pathways are a critical part of macrophage activation and their ability to impair adipocyte insulin or catecholamine signaling (16,31,32). Macrophages from aged Nlrp3-deficient mice have reduced production of inflammatory cytokines, in part mediated through increased reactive oxygen levels and expression of growth differentiation factor 3 (GDF-3) (16,18). The NLRP3 activation increases expression of monoamine oxidase, leading to the degradation of catecholamines and reduced lipolytic signaling following activation of the sympathetic nervous system (16). Although a specific DAMP to activate the NLRP3 inflammasome in age-related inflammation is unknown, aged macrophages in the AT are constantly exposed to the lipid-rich environment. Lymphatic fluid from dysfunctional vessels (33) or necrotic adipocytes (34) are a source of DAMPs that drive the NLRP3 activation. Aged Nlrp3−/− mice have restored macrophage frequency, reduced AT inflammation, improved insulin sensitivity, and increased fasting-induced lipolysis, in line with a broad role for macrophage NLRP3 inflammasome activation in potentiating inflammaging (16,31,35).
Metabolic pathways are also critical in AT homeostasis and macrophage-specific inflammation. Aged AT shows reduced NAD metabolism and NAD+ levels, at least in part driven by increased expression of CD38, a NADase, on macrophages (36). Treatment to increase NAD+ levels improves glucose tolerance in obesity (36), providing a link between cellular NAD+ levels, inflammation, and adipocyte function. Future research should also consider antigen presentation, phagocytosis, lysosomal activity, lipid uptake capabilities of macrophages, and their requirement for insulin or catecholamine signaling in aged adipocytes.
The roles of other innate immune cells, including γδ T cells, natural killer (NK) cells, and innate lymphoid cells, are important in steady state and obesity (37), suggesting that they may also regulate age-related tissue inflammation and adipocyte function.
Age-Related Alterations in B-Cell Subsets
B-cell lymphopoiesis declines with advancing age, but significant changes also occur within subsets of B cells (38). Defects in hematopoietic stem cell components, common lymphoid progenitors, and the action of myeloid-derived suppressor cells or increased adipocytes in bone marrow led to altered lymphopoiesis and elevated numbers of plasma cells secreting inflammatory cytokines (39,40). Increases in a subset of mature, antigen-experienced B cells arise during T-cell–dependent immune responses and then persist as a distinct memory B-cell population, now termed age-associated B cells (ABCs) (41,42). ABCs are induced and regulate age-related diseases, infection, or autoimmune disease; therefore, they are an attractive target to improve outcomes under those conditions.
ABCs have low expression of CD21 and CD23 but express CD11c and Tbet and initially arise in females (42). They are refractory to B-cell receptor cross-linking but can be activated through TLR7 and TLR9, pattern recognition receptors that are localized in the endosomes and recognize DNA or single-stranded RNA. They do not require B-cell–activating factor for survival and are thought to outcompete follicular B cells, which depend on B-cell–activating factor for survival (42). ABCs increase inflammaging via their production of IL-6 and IFN-γ following TL7 or TLR9 activation (42). ABCs can skew CD4+ T cells toward the Th17 fate, producing IL-17. Other subsets of B cells are altered with age, such as 4-1BBL+ peritoneal B cells (B1 cells), that may also contribute to inflammaging via their production of CCL2 and regulation of macrophages and CD8+ T cells (43,44). Specific changes in B-cell metabolic signaling pathways are not well defined, although aged B cells have impaired glycolysis, mitochondrial energy production, and reduced expression of sirtuin 1 (SIRT1) (45).
AAB Expansion
Tissue-resident B cells are expanded between 2- and 10-fold in the white visceral AT (aged adipose B cells, also called AABs) in an inflammation-dependent manner and predominantly in female mice (3,46,47). B-cell accumulation is localized to inducible lymphoid clusters, termed fat-associated lymphoid clusters (FALCs). FALCs are a complex microenvironment of dense vasculature to support the accumulating immune cells, where formation is a result of local TNF-α–dependent inflammation in a macrophage-specific manner (48). AABs accumulate in FALCs through recruitment and proliferation. A portion of the AABs are Ki67+, and they proliferate in an IL-1β–dependent manner. AABs fail to accumulate in Cxcl13 knockout mice, a chemokine important for the recruitment of CXCR5+ B cells (48). Multiple cell populations, including stromal cells and macrophages, express CXCL13 (48). It is not clear whether these make up separate populations or whether a subset of B cells are first recruited and then undergo proliferation.
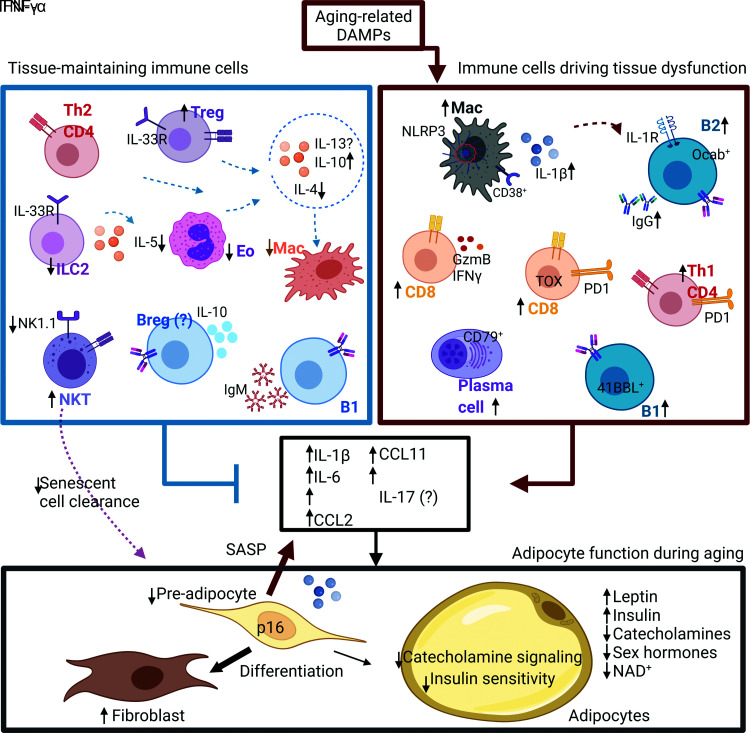
Phenotypically, AABs are unique from B cells in other tissues, although they share some similarities with ABCs that accumulate in the spleen. The AT environment and obesity drive the inflammatory ABC-like phenotype (46,49,50). AABs do not express CD11c and Tbet, which are expressed by splenic ABCs, but they express inflammatory cytokines (46). Whether AABs affect T-cell production of IL-17 has not been examined. The B-cell–specific transcription factor Oct coactivator (OcaB), important for transcription of the variable part of the κ light chain, is required for B cell numbers in aged AT, as hematopoietic deletion of OcaB reduced IgG2c antibody levels and improved insulin sensitivity (47). There is also an expansion of B1 cells and plasma cells that express the J chain and CD79 (3,46). It is currently unclear how these subsets interact and whether they independently support or inhibit inflammation or adipocyte function (Fig. 2).
Figure 2.
Aged immune cells drive AT dysfunction. Shown is a schematic to describe the tissue maintaining immune cells within the AT in young, lean conditions (left side) and the accumulation of inflammatory immune cells in aged AT. The net sum of the changes in the tissue-maintaining and inflammatory cells leads to an accumulation of inflammation and loss of adipocyte function. Pre-adipocytes accumulate features of senescence, secrete a SASP, and tend to differentiate into fibroblasts as opposed to lipid-storing adipocytes. Adipocytes show reduced catecholamine and insulin signaling. A question mark indicates that age-related changes are unknown. Arrows indicate the direction of change compared with young mice. Changes in both male and female mice are included in this summary. Breg, regulatory B cell; Eo, eosinophils; GzmB, granzyme B; ILC2, innate lymphoid cell; Mac, macrophage. Created in BioRender (BioRender.com).
The specific role of AABs in the aged AT is unclear. RNA sequencing reveals their proinflammatory nature (46), suggesting they contribute to the inflammatory milieu of aged AT. The adipokine leptin activates and increases cytokine production in B cells from aged humans (51) and could be a contributing factor to AT-related inflammation. Systemic depletion of B cells improves fasting-induced lipolysis, insulin sensitivity, and maintenance of core body temperature following cold challenge (46), indicating they also have roles in impairing adipocyte function during aging. Within the FALC, AABs also play a role in evaluating antigens within the microenvironment. FALCs constantly sample protein antigens within the local environment, including the peritoneal cavity (52). During acute inflammation, peritoneal B1 cells, expressing 4-1BBL and TNF, accumulate in FALCs to promote pathogen clearance (43). This paints a picture where accumulating AABs are intimately connected with AT inflammation and the ability of adipocytes to respond to insulin, leptin, and catecholamine, but more work is needed to understand molecular factors involved.
Age-Related Cytotoxicity and T-Cell Exhaustion
Thymic involution is a prominent and early visible change that negatively affects central tolerance, where inflammatory, self-reactive T cells escape the thymus and feed into inflammaging (53). Memory T cells show a restricted T-cell receptor with less antigen diversity. While all subsets of T cells are altered with age, regulatory CD4+ T cells (Tregs) and cytotoxic CD8+ T cells are expanded with age. Multiple recent publications have described the importance of CD8+ T cells in age-related inflammation.
CD8+ T cells expand with age, but there is a reduction in their cytotoxic capabilities and increased expression of markers of exhaustion, PD1 and TOX (54). These cells also express granzyme K, which increases senescence-associated secretory products (SASPs; discussed later) from in vitro–exposed fibroblasts (54). Virtual memory CD8+ T cells, CD49dlow, CD44high, and CD122high, which proliferate due to cytokines and not antigen, are also increased. They retain some effector functions, but exhibit features of senescence, including reduced proliferation. (55). Overall, these reports indicate that CD8+ T cells increase systemic inflammaging and senescence.
Multiple T-Cell Subsets Accumulate in the Aged AT
CD3+ T cells make up approximately 10% of CD45+ in the visceral AT of adolescence to mature mice and expand to 30% in old male and female mice (17,46). CD3+ T-cell subsets that are present include CD4+ T cells (Tregs, Th1, Th2, and Th17) and cytotoxic CD8+ T cells. Tregs are a critical cell type maintaining homeostasis in AT via their production of IL-10 and expression of peroxisome proliferator–activated receptor γ (56). They are abundant in male mice but significantly expanded with age in both male and female visceral AT (17,46,57). Sex-specific differences in Tregs, in middle-aged male and female mice, result from differences in chromatin accessibility at over 3,000 loci and transcriptome expression specifically in the expression of genes related to inflammation (Tnfα, Ccl2, and Il1β), tissue fibrosis (Col6a5), and prostaglandin metabolism (Hpgds) (58). Female mice have a larger expansion of activated CD8+ CD69+ T cells than their male counterparts (59). They also express more IFN-γ and granzyme B. In contrast, approximately 50% of the CD8+ T cells in the male epididymal AT have an exhausted phenotype (TOX+ PD1+ granzyme K+) (54), suggesting a sex-specific accumulation of CD8+ T-cell subsets in the AT. Systemic depletion of CD3+ T cells resulted in efficient depletion in spleen, epididymal white AT, and liver, but not other tissues, in old male mice at 20–24 months of age (60). This depletion also improved glucose tolerance, gluconeogenesis, and reduced adiposity (60), but which subsets of T cells are responsible for improved adipocyte action needs further clarity.
Aged Senescent Immune Cells
Further complicating our understanding of immunosenescence is the cellular state of senescence, which was originally identified by irreversible cell cycle arrest (61). Senescent cells also show global omics changes, an accumulation of DNA damage, and secretion of SASPs. Aged immune cells may not strictly fall under this definition for cellular senescence but instead show characteristics overlapping those of senescent cells. Senescence-associated genes, including Chek2 and Gdf15, were increased in aged AT macrophages (16), and virtual memory T cells that accumulate with age show increased expression of Cdkn genes (e.g., p21), a marker of senescence (55). Telomere shortening, one mechanism to induce senescence, results in impaired STAT5a phosphorylation and proliferation in aged macrophages (62). Accumulating immune cell–specific DNA damage increases markers of senescence in immune cells and drives tissue-related accelerated aging in both male and female mice by 6 months (63). Furthermore, T cells with dysfunctional mitochondria, driven by a defect in mitochondrial DNA–stabilizing protein (TFAM), induce multimorbidity and premature senescence (64). These results indicate immune cells become senescent-like and are required for aging of AT and other tissues.
SASP and Accumulating Senescent Cell Burden in AT
The SASPs are responsible for spreading senescence and inflammation (65). SASP normally functions to alert the immune system of cell damage, but the inability of the aged immune cells to clear senescent cells feeds forward into accumulating senescence. Depletion of senescent cells in transgenic aged mouse models or using natural senolytics in aged wild-type mice extends life span, reduces SASP factors, and improves health span (66). Senescent cell depletion also improves whole-body insulin sensitivity and glucose metabolism in aged mouse models. Using whole-AT gene expression, male and female mice have similar markers of senescence (8), but analysis of individual cells is still needed. Mouse models which cannot clear senescent cells, for example, perforin knockout mice, show impaired cytotoxicity and exhibit a higher senescent-cell tissue burden and chronic inflammation (67). They also have increased adiposity and metabolic dysfunction associated with alterations in T-cell turnover (68). Invariant NK cells were revealed to have roles in clearing senescent cells in AT from diet-induced obese mice (69), but NK cells, NKT cells, and CD8+ T cells are additional candidates with cytotoxic activity. Current research suggests senescent cells are located through the parenchyma of AT, but whether they can be found in higher density in specific microenvironments, such as the FALCs, has not been addressed. Senescent cells, the SASP, and the immune cells needed to clear senescent cells are relevant targets for reducing inflammation and improving adipocyte function.
Age-Related Inflammation in “Healthy” Human AT
Less is known about AT from aged human, but some recent research reveals similarities to the research described in mice. Two critical research articles investigated subcutaneous AT changes in healthy, active individuals who are aged but not obese (70,71). CD4+ T cells and CD8+ T cells are increased, with no changes seen in overall macrophage numbers. B cells and other innate immune cells were not analyzed. Pathways that were significantly increased in the AT included biological processes associated with innate and acquired immune processes and the inflammatory processes. Adipocytes were significantly larger in old individuals, and, consistent with a decline of adipocyte function, they showed lower levels of proteins involved in glucose uptake (InsRB, AS160, and GLUT4). Overall, these changes in human subcutaneous tissue are comparable to those seen in the mouse visceral AT. They also support the findings that immune cell activation and the decline in insulin signaling in AT precedes the activation in muscle. It remains to be seen whether human visceral AT has similar features.
Conclusion
Aging research is an incredibly complex field, a fact supported by the intricacy seen when trying to interrogate and understand the changes in the aged immune system. The aged dysfunctional immune system clearly has a role in regulating metabolic disease through increased secretion of inflammatory factors, failure to maintain tissue homeostasis, and failure to clear senescent cells, although there is still a great deal to investigate regarding specific factors involved. The current research supports a model where AT is an organ of early immune activation, involving macrophage response to DAMPs and their shift to inflammatory phenotype, along with a loss of support from tissue-maintaining immune cells (eosinophils and Tregs) and the accumulation of inflammatory and exhausted lymphocytes. This inflammatory loop, including the expanding senescent cell burden, and the SASP are regulators of impaired lipid storage or mobilization in the aged adipocyte (Fig. 2). The AT poses as an organ to interconnect inflammation with metabolic dysregulation, leading to inflammaging and chronic disease. Ultimately, this research strives to understand how the immune system drives metabolic disease in efforts of reducing disease and improving health span.
Article Information
Acknowledgments. We thank Stephanie Cholensky (University of Minnesota) for comments and edits on the final draft.
Funding. This work was supported by National Institute on Aging grant R00 AG058800 (C.D.C.) and the Medical Discovery Team on the Biology of Aging (C.D.C.). C.D.C. also is supported by the Fesler-Lampert Chair in Aging Studies and the Glenn Foundation for Medical Research/AFAR Grants for Junior Faculty.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
References
- 1. United Nations . World Population Ageing 2017. Accessed 10 June 2021. Available from https://www.un.org/en/development/desa/population/publications/pdf/ageing/WPA2017_Highlights.pdf
- 2. Franceschi C, Garagnani P, Parini P, Giuliani C, Santoro A. Inflammaging: a new immune-metabolic viewpoint for age-related diseases. Nat Rev Endocrinol 2018;14:576–590 [DOI] [PubMed] [Google Scholar]
- 3. Schaum N, Lehallier B, Hahn O, et al.; Tabula Muris Consortium . Ageing hallmarks exhibit organ-specific temporal signatures. Nature 2020;583:596–602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yu Q, Xiao H, Jedrychowski MP, et al. Sample multiplexing for targeted pathway proteomics in aging mice. Proc Natl Acad Sci U S A 2020;117:9723–9732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Horstman AM, Dillon EL, Urban RJ, Sheffield-Moore M. The role of androgens and estrogens on healthy aging and longevity. J Gerontol A Biol Sci Med Sci 2012;67:1140–1152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Klein SL, Flanagan KL. Sex differences in immune responses. Nat Rev Immunol 2016;16:626–638 [DOI] [PubMed] [Google Scholar]
- 7. Varghese M, Griffin C, McKernan K, et al. Sex differences in inflammatory responses to adipose tissue lipolysis in diet-induced obesity. Endocrinology 2019;160:293–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Varghese M, Griffin C, McKernan K, Eter L, Abrishami S, Singer K. Female adipose tissue has improved adaptability and metabolic health compared to males in aged obesity. Aging (Albany NY) 2020;12:1725–1746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kahn CR, Wang G, Lee KY. Altered adipose tissue and adipocyte function in the pathogenesis of metabolic syndrome. J Clin Invest 2019;129:3990–4000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tchernof A, Després JP. Pathophysiology of human visceral obesity: an update. Physiol Rev 2013;93:359–404 [DOI] [PubMed] [Google Scholar]
- 11. Berry DC, Jiang Y, Arpke RW, et al. Cellular aging contributes to failure of cold-induced beige adipocyte formation in old mice and humans. Cell Metab 2017;25:166–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lamkanfi M, Dixit VM. Mechanisms and functions of inflammasomes. Cell 2014;157:1013–1022 [DOI] [PubMed] [Google Scholar]
- 13. van der Heijden T, Kritikou E, Venema W, et al. NLRP3 inflammasome inhibition by MCC950 reduces atherosclerotic lesion development in apolipoprotein E-deficient mice-brief report. Arterioscler Thromb Vasc Biol 2017;37:1457–1461 [DOI] [PubMed] [Google Scholar]
- 14. van Hout GP, Bosch L, Ellenbroek GH, et al. The selective NLRP3-inflammasome inhibitor MCC950 reduces infarct size and preserves cardiac function in a pig model of myocardial infarction. Eur Heart J 2017;38:828–836 [DOI] [PubMed] [Google Scholar]
- 15. Dempsey C, Rubio Araiz A, Bryson KJ, et al. Inhibiting the NLRP3 inflammasome with MCC950 promotes non-phlogistic clearance of amyloid-β and cognitive function in APP/PS1 mice. Brain Behav Immun 2017;61:306–316 [DOI] [PubMed] [Google Scholar]
- 16. Camell CD, Sander J, Spadaro O, et al. Inflammasome-driven catecholamine catabolism in macrophages blunts lipolysis during ageing. Nature 2017;550:119–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lumeng CN, Liu J, Geletka L, et al. Aging is associated with an increase in T cells and inflammatory macrophages in visceral adipose tissue. J Immunol 2011;187:6208–6216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Stout-Delgado HW, Cho SJ, Chu SG, et al. Age-dependent susceptibility to pulmonary fibrosis is associated with NLRP3 inflammasome activation. Am J Respir Cell Mol Biol 2016;55:252–263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pence BD, Yarbro JR. Aging impairs mitochondrial respiratory capacity in classical monocytes. Exp Gerontol 2018;108:112–117 [DOI] [PubMed] [Google Scholar]
- 20. Minhas PS, Liu L, Moon PK, et al. Macrophage de novo NAD+ synthesis specifies immune function in aging and inflammation. Nat Immunol 2019;20:50–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Linehan E, Dombrowski Y, Snoddy R, Fallon PG, Kissenpfennig A, Fitzgerald DC. Aging impairs peritoneal but not bone marrow-derived macrophage phagocytosis. Aging Cell 2014;13:699–708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. He M, Chiang HH, Luo H, et al. An acetylation switch of the NLRP3 inflammasome regulates aging-associated chronic inflammation and insulin resistance. Cell Metab 2020;31:580–591.e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Liang H, Gao J, Zhang C, et al. Nicotinamide mononucleotide alleviates aluminum induced bone loss by inhibiting the TXNIP-NLRP3 inflammasome. Toxicol Appl Pharmacol 2019;362:20–27 [DOI] [PubMed] [Google Scholar]
- 24. Minhas PS, Latif-Hernandez A, McReynolds MR, et al. Restoring metabolism of myeloid cells reverses cognitive decline in ageing. Nature 2021;590:122–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Brigger D, Riether C, van Brummelen R, et al. Eosinophils regulate adipose tissue inflammation and sustain physical and immunological fitness in old age. Nat Metab 2020;2:688–702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Goldberg EL, Shchukina I, Camell CD, et al. Dysregulation of adipose ILC2 underlies thermogenic failure in aging. bioRxiv. 8 September 2020 [preprint]. DOI:10.1101/2020.09.08.288431 [Google Scholar]
- 27. Wu D, Molofsky AB, Liang HE, et al. Eosinophils sustain adipose alternatively activated macrophages associated with glucose homeostasis. Science 2011;332:243–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hill DA, Lim HW, Kim YH, et al. Distinct macrophage populations direct inflammatory versus physiological changes in adipose tissue. Proc Natl Acad Sci U S A 2018;115:E5096–E5105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Silva HM, Báfica A, Rodrigues-Luiz GF, et al. Vasculature-associated fat macrophages readily adapt to inflammatory and metabolic challenges. J Exp Med 2019;216:786–806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Koo SJ, Garg NJ. Metabolic programming of macrophage functions and pathogens control. Redox Biol 2019;24:101198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Youm YH, Grant RW, McCabe LR, et al. Canonical Nlrp3 inflammasome links systemic low-grade inflammation to functional decline in aging. Cell Metab 2013;18:519–532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Choe SS, Huh JY, Hwang IJ, Kim JI, Kim JB. Adipose tissue remodeling: its role in energy metabolism and metabolic disorders. Front Endocrinol (Lausanne) 2016;7:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zolla V, Nizamutdinova IT, Scharf B, et al. Aging-related anatomical and biochemical changes in lymphatic collectors impair lymph transport, fluid homeostasis, and pathogen clearance. Aging Cell 2015;14:582–594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cinti S, Mitchell G, Barbatelli G, et al. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J Lipid Res 2005;46:2347–2355 [DOI] [PubMed] [Google Scholar]
- 35. Bauernfeind F, Niepmann S, Knolle PA, Hornung V. Aging-associated TNF production primes inflammasome activation and NLRP3-related metabolic disturbances. J Immunol 2016;197:2900–2908 [DOI] [PubMed] [Google Scholar]
- 36. Camacho-Pereira J, Tarragó MG, Chini CCS, et al. CD38 dictates age-related NAD decline and mitochondrial dysfunction through an SIRT3-dependent mechanism. Cell Metab 2016;23:1127–1139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Khan S, Chan YT, Revelo XS, Winer DA. The immune landscape of visceral adipose tissue during obesity and aging. Front Endocrinol (Lausanne) 2020;11:267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Scholz JL, Diaz A, Riley RL, Cancro MP, Frasca D. A comparative review of aging and B cell function in mice and humans. Curr Opin Immunol 2013;25:504–510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Pioli PD, Casero D, Montecino-Rodriguez E, Morrison SL, Dorshkind K. Plasma cells are obligate effectors of enhanced myelopoiesis in aging bone marrow. Immunity 2019;51:351–366.e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kennedy DE, Knight KL. Inhibition of B lymphopoiesis by adipocytes and IL-1-producing myeloid-derived suppressor cells. J Immunol 2015;195:2666–2674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Cancro MP. Age-associated B cells. Annu Rev Immunol 2020;38:315–340 [DOI] [PubMed] [Google Scholar]
- 42. Hao Y, O’Neill P, Naradikian MS, Scholz JL, Cancro MP. A B-cell subset uniquely responsive to innate stimuli accumulates in aged mice. Blood 2011;118:1294–1304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bodogai M, O’Connell J, Kim K, et al. Commensal bacteria contribute to insulin resistance in aging by activating innate B1a cells. Sci Transl Med 2018;10:eaat4271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lee-Chang C, Bodogai M, Moritoh K, et al. Accumulation of 4-1BBL+ B cells in the elderly induces the generation of granzyme-B+ CD8+ T cells with potential antitumor activity. Blood 2014;124:1450–1459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kurupati RK, Haut LH, Schmader KE, Ertl HC. Age-related changes in B cell metabolism. Aging (Albany NY) 2019;11:4367–4381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Camell CD, Günther P, Lee A, et al. Aging induces an Nlrp3 inflammasome-dependent expansion of adipose B cells that impairs metabolic homeostasis. Cell Metab 2019;30:1024–1039.e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Carter S, Miard S, Caron A, et al. Loss of OcaB prevents age-induced fat accretion and insulin resistance by altering B-lymphocyte transition and promoting energy expenditure. Diabetes 2018;67:1285–1296 [DOI] [PubMed] [Google Scholar]
- 48. Bénézech C, Luu NT, Walker JA, et al. Inflammation-induced formation of fat-associated lymphoid clusters. Nat Immunol 2015;16:819–828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Frasca D, Diaz A, Romero M, Vazquez T, Blomberg BB. Obesity induces pro-inflammatory B cells and impairs B cell function in old mice. Mech Ageing Dev 2017;162:91–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Frasca D, Ferracci F, Diaz A, Romero M, Lechner S, Blomberg BB. Obesity decreases B cell responses in young and elderly individuals. Obesity (Silver Spring) 2016;24:615–625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Gupta S, Agrawal S, Gollapudi S. Increased activation and cytokine secretion in B cells stimulated with leptin in aged humans. Immun Ageing 2013;10:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Rangel-Moreno J, Moyron-Quiroz JE, Carragher DM, et al. Omental milky spots develop in the absence of lymphoid tissue-inducer cells and support B and T cell responses to peritoneal antigens. Immunity 2009;30:731–743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Thomas R, Wang W, Su DM. Contributions of age-related thymic involution to immunosenescence and inflammaging. Immun Ageing 2020;17:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Mogilenko DA, Shpynov O, Andhey PS, et al. Comprehensive profiling of an aging immune system reveals clonal GZMK+ CD8+ T cells as conserved hallmark of inflammaging. Immunity 2020;54:99–115 [DOI] [PubMed] [Google Scholar]
- 55. Quinn KM, Fox A, Harland KL, et al. Age-related decline in primary CD8+ T cell responses is associated with the development of senescence in virtual memory CD8+ T cells. Cell Rep 2018;23:3512–3524 [DOI] [PubMed] [Google Scholar]
- 56. Cipolletta D. Adipose tissue-resident regulatory T cells: phenotypic specialization, functions and therapeutic potential. Immunology 2014;142:517–525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Bapat SP, Myoung Suh J, Fang S, et al. Depletion of fat-resident Treg cells prevents age-associated insulin resistance. Nature 2015;528:137–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Vasanthakumar A, Chisanga D, Blume J, et al. Sex-specific adipose tissue imprinting of regulatory T cells. Nature 2020;579:581–585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Ahnstedt H, Roy-O’Reilly M, Spychala MS, et al. Sex differences in adipose tissue CD8+ T cells and regulatory T cells in middle-aged mice. Front Immunol 2018;9:659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Trott DW, Islam MT, Buckley DJ, et al. T lymphocyte depletion ameliorates age-related metabolic impairments in mice. Geroscience 2021;43:1331–1347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. van Deursen JM. The role of senescent cells in ageing. Nature 2014;509:439–446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Sebastián C, Herrero C, Serra M, Lloberas J, Blasco MA, Celada A. Telomere shortening and oxidative stress in aged macrophages results in impaired STAT5a phosphorylation. J Immunol 2009;183:2356–2364 [DOI] [PubMed] [Google Scholar]
- 63. Yousefzadeh MJ, Flores RR, Zhu Y, et al. An aged immune system drives senescence and ageing of solid organs. Nature 2021;594:100–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Desdín-Micó G, Soto-Heredero G, Aranda JF, et al. T cells with dysfunctional mitochondria induce multimorbidity and premature senescence. Science 2020;368:1371–1376 [DOI] [PubMed] [Google Scholar]
- 65. Kale A, Sharma A, Stolzing A, Desprez PY, Campisi J. Role of immune cells in the removal of deleterious senescent cells. Immun Ageing 2020;17:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Baker DJ, Wijshake T, Tchkonia T, et al. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature 2011;479:232–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Ovadya Y, Landsberger T, Leins H, et al. Impaired immune surveillance accelerates accumulation of senescent cells and aging. Nat Commun 2018;9:5435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Revelo XS, Tsai S, Lei H, et al. Perforin is a novel immune regulator of obesity-related insulin resistance. Diabetes 2015;64:90–103 [DOI] [PubMed] [Google Scholar]
- 69. Arora S, Thompson PJ, Wang Y, et al. Invariant natural killer T cells coordinate removal of senescent cells. Med 2021;2:938–950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Trim WV, Walhin JP, Koumanov F, et al. Divergent immunometabolic changes in adipose tissue and skeletal muscle with ageing in healthy humans. J Physiol 2021 [DOI] [PubMed] [Google Scholar]
- 71. Ortega Martinez de Victoria E, Xu X, Koska J, et al. Macrophage content in subcutaneous adipose tissue: associations with adiposity, age, inflammatory markers, and whole-body insulin action in healthy Pima Indians. Diabetes 2009;58:385–393 [DOI] [PMC free article] [PubMed] [Google Scholar]