Abstract
Background
The coronavirus disease 2019 (COVID-19) pandemic caused disruptions to healthcare systems, consequently endangering tuberculosis (TB) control. We investigated delays in TB treatment among notified patients during the first wave of the COVID-19 pandemic in Korea.
Methods
We systemically collected and analyzed data from the Korea TB cohort database from January to May 2020. Groups were categorized as ‘before-pandemic’ and ‘during-pandemic’ based on TB notification period. Presentation delay was defined as the period between initial onset of symptoms and the first hospital visit, and healthcare delay as the period between the first hospital visit and anti-TB treatment initiation. A multivariate logistic regression analysis was performed to evaluate factors associated with delays in TB treatment.
Results
Proportion of presentation delay > 14 days was not significantly different between two groups (48.3% vs. 43.7%, P = 0.067); however, proportion of healthcare delay > 5 days was significantly higher in the during-pandemic group (48.6% vs. 42.3%, P = 0.012). In multivariate analysis, the during-pandemic group was significantly associated with healthcare delay > 5 days (adjusted odds ratio = 0.884, 95% confidence interval = 0.715–1.094).
Conclusion
The COVID-19 pandemic was associated with healthcare delay of > 5 days in Korea. Public health interventions are necessary to minimize the pandemic’s impact on the national TB control project.
Keywords: COVID-19, SARS-CoV-2, Tuberculosis, Healthcare, Diagnosis, Anti-Tuberculosis Treatment
Graphical Abstract
INTRODUCTION
Coronavirus disease 2019 (COVID-19) was declared as a pandemic on March 11, 2020 by the World Health Organization (WHO).1 This caused a global public health crisis that triggered disruptions to healthcare systems and endangered the control and prevention of common endemic diseases. Korea's healthcare capabilities and efforts were focused mainly on the management of COVID-19, which consequently resulted in severe reductions in the availability of health services and access to care for patients with other chronic diseases.2 Furthermore, the management of tuberculosis (TB), one of the oldest endemic infectious diseases, was unavoidably affected by the COVID-19 pandemic.3
Early diagnosis and prompt initiation of treatment are among the most essential interventions for an effective TB control program. In this context, the Republic of Korea implemented the national Public-Private Mix (PPM) TB control project that provides such comprehensive patient management.4,5 The long-standing pandemic could disrupt the PPM project, which may delay TB diagnosis and treatment.6 In addition, we hypothesized that the COVID-19 pandemic could alter the patient’s health seeking behavior; fearful of contacting the disease or being stigmatized, and may delay visiting the clinics as much as possible. In this study, we investigated delays of TB treatment among notified patients under the PPM project during the first wave of the COVID-19 pandemic in Korea.
METHODS
Study design and the Korea TB cohort database
We constructed a prospective observational cohort database, called the ‘Korea TB Cohort database’. Data was systematically collected from TB patients who visited hospitals under the national PPM TB control project and were notified to the national TB surveillance system. All the notified TB patients were followed at regular intervals during anti-TB treatment, as recommended by the Korean TB guideline. For the purpose of this database, every TB patient notified from the first to the tenth days each month were consecutively enrolled across the country. Data regarding the participants during this period were collected by TB specialist nurses using the prespecified questionnaire and case report form and were encoded into Microsoft Access (Redmond, WA, USA). Subsequently, monthly data gathered by the regional data managers from local hospitals were organized and sent to central data managers every quarter. In order to improve and maintain data quality, regional and central data managers performed audits to identify missing and erroneous data. For the purpose of this study, we retrieved data from the Korea TB cohort database from January to May 2020, and retrospectively analyzed them.
Study setting and participants
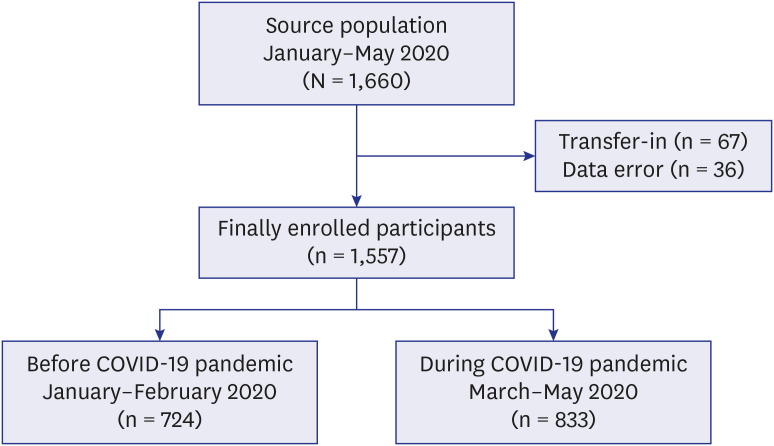
Since its nationwide expansion in 2011, the national PPM TB control project plays a key role in patient management in Korea, an intermediate TB-burden country. In 2018, approximately 70.7% of newly notified TB patients in Korea were treated at hospitals participating in the PPM project. During the study period, 1,660 TB patients were enrolled (Fig. 1). After excluding those who were transferred-in and those with inaccurate data regarding date of symptom onset, hospital visit, and treatment initiation, 1,557 TB patients remained and were finally enrolled. Patients notified between January and February were categorized as ‘before-pandemic group’ and those notified between March and May as ‘during-pandemic group’.7
Fig. 1. Flow chart of enrollment of tuberculosis patients before and during the COVID-19 pandemic.
COVID-19 = coronavirus disease 2019.
Outcome variables
Presentation and healthcare delays were defined by applying the median values of time components in diagnosis and treatment while considering local clinical settings. In order to define presentation delay, we first identified the period between initial onset of symptoms and the first hospital visit, and then calculated its median time among the enrolled participants, which was 13 days (interquartile range [IQR]; 4–32 days). As the Korean TB guideline recommends investigation if cough lasts for more than 2 weeks, we defined presentation delay as the time lag greater than 14 days. In order to define healthcare delay, we identified the period between the first hospital visit and anti-TB treatment initiation, then calculated its median time among the enrolled participants, which was 5 days (interquartile range; 2–13 days). Since the results of acid-fast bacilli (AFB) smear and nucleic acid amplification tests (NAATs) are mostly reported within 1 or 2 days in Korea, we defined healthcare delay as the time lag greater than 5 days. Healthcare delay included time taken for COVID-19 testing.
Independent variables
Demographic and clinical data were collected based on in-depth interviews by TB specialist nurses at PPM participating hospitals. Baseline characteristics such as age, sex, co-existing comorbidities, initial presenting symptoms, record of TB history, sites of TB involvement, and laboratory findings were collected. All the data were coded as bivariate variables, except for microbiological test results, which were coded as categorical variables.
Statistical analyses
Both presentation and healthcare delays were expressed as median and IQR. We performed a Mann-Whitney U test to compare both delays between two groups. The five regions with the most reported cases of COVID-19 were selected during the early pandemic period and re-categorized into two regions based on their proximity8; Daegu-Gyeongbuk, and Seoul Metropolitan Area (Seoul, Incheon, and Gyeonggi), and both presentation and healthcare delays were also calculated in the two regions.
Discrete variables were expressed as frequency or percentage. Between-group comparisons were conducted using univariate analysis via the χ2 test and binary logistic regression. We hypothesized that delays would be associated with comorbidities and clinical presentation. Accordingly, clinical variables with a P value of < 0.20 in the univariate analysis were selected in addition to age, sex, and prior TB history and included in the multivariate binary logistic regression to evaluate the possible association between variables and delays. A P value of < 0.05 was considered statistically significant. Statistical analyses were performed using SPSS version 17.0 (Statistical Product and Service Solutions, Chicago, IL, USA).
Sample size
We selected 12 variables a priori for inclusion into our model. To minimize bias in logistic regression analysis, 120 events are required to ensure a minimum of 10 events per variable. Because we coded both presentation and healthcare delays as bivariate data using median values, we assumed that proportions of both delays are approximately 50%. Thus, at least 480 patients were required for the sample size.
Ethics statement
The Institutional Review Board of Incheon St. Mary’s Hospital, the Catholic University of Korea approved the study protocol (IRB No. OC21ZNSI0063) and waived the need for informed consent as none of the patients were at risk.
RESULTS
Comparison of baseline characteristics
Among 1,557 enrolled patients, 833 patients were categorized in the during-pandemic group. Demographic profiles were similar between two groups. The proportion of patients who initially had cough was lower in the during-pandemic group (56.9% vs. 50.1%, P = 0.007). Compared to the before-pandemic group, initial coverages of NAAT (87.4% vs. 84.6%, P = 0.113) and AFB smear test (87.2% vs. 83.9%, P = 0.071) were lower in the during-pandemic group, but their differences were not significant (Table 1).
Table 1. Comparison of baseline characteristics of all enrolled participants with tuberculosis before and during the COVID-19 pandemic.
| Variables | Before COVID-19 (n = 724) | During COVID-19 (n = 833) | Total (n = 1,557) | P value | |
|---|---|---|---|---|---|
| Male | 425 (58.7) | 500 (60.0) | 925 (59.4) | 0.596 | |
| Age ≥ 65 yr | 370 (51.1) | 399 (47.9) | 769 (49.4) | 0.207 | |
| Prior TB treatment | 113 (15.6) | 140 (16.8) | 253 (16.2) | 0.522 | |
| Extrapulmonary involvement | 229 (31.6) | 312 (37.5) | 541 (34.7) | 0.016 | |
| Comorbidities | |||||
| Diabetes | 143 (19.8) | 177 (21.2) | 320 (20.6) | 0.466 | |
| Solid malignancy | 42 (5.8) | 65 (7.8) | 107 (6.9) | 0.119 | |
| Initial symptoms | |||||
| Cough ± sputum | 412 (56.9) | 417 (50.1) | 829 (53.2) | 0.007 | |
| Fever | 138 (19.1) | 189 (22.7) | 327 (21.0) | 0.080 | |
| Alarming symptomsa | 282 (39.0) | 327 (39.3) | 609 (39.1) | 0.902 | |
| NAAT performed | 633 (87.4) | 705 (84.6) | 1,338 (85.9) | 0.113 | |
| AFB smear test performed | 631 (87.2) | 699 (83.9) | 1,330 (85.4) | 0.071 | |
| Presentation delay,b days | 14 [4–32] | 11 [3–33] | 13 [4–32] | 0.077 | |
| Healthcare delay,c days | 4 [2–11] | 5 [2–13] | 5 [2–13] | 0.027 | |
Values are expressed as numbers and percentage.
Both presentation and healthcare delays were expresses as median and interquartile.
COVID-19 = coronavirus disease 2019, TB = tuberculosis, NAAT = nucleic acid amplification test, AFB = acid-fast bacilli.
aAlarming symptoms were defined as sum of chest discomfort, hemoptysis, or dyspnea; bPresentation delay was defined as period between initial onset of symptoms and the first hospital visit; cHealthcare delay was defined as period between the first hospital visit and anti-TB treatment initiation.
Symptoms and Laboratory findings among pulmonary TB patients
Among patients with pulmonary TB, the proportions of patients with bilateral infiltration (37.2% vs. 32.7%, P = 0.109) and cavitation (14.7% vs. 13.8%, P = 0.682) on chest radiography were lower in the during-pandemic group, but not statistically significant (Table 2). Proportions of sputum smear test results ≥ 3+ between the two groups were not statistically different (10.8% vs. 8.6%, P = 0.204).
Table 2. Clinical profiles and laboratory findings of 1,138 participants with pulmonary tuberculosis before and during the COVID-19 pandemic.
| Variables | Before COVID-19 (n = 545) | During COVID-19 (n = 593) | Total (n = 1,138) | P value | |
|---|---|---|---|---|---|
| Initial symptoms | 217 (39.8) | 222 (37.4) | 439 (38.6) | 0.410 | |
| Cough ± sputum | 176 (32.3) | 230 (38.8) | 406 (35.7) | 0.022 | |
| Fever | 104 (19.1) | 135 (22.8) | 239 (21.0) | 0.128 | |
| Alarming symptomsa | 217 (39.8) | 222 (37.4) | 439 (38.6) | 0.410 | |
| Chest X-ray | |||||
| Bilateral disease | 203 (37.2) | 194 (32.7) | 397 (34.9) | 0.109 | |
| Cavitary disease | 80 (14.7) | 82 (13.8) | 162 (14.2) | 0.682 | |
| NAAT result | 0.389 | ||||
| Not performed | 12 (2.2) | 19 (3.2) | 31 (2.7) | ||
| Negative result | 183 (33.6) | 212 (35.8) | 395 (34.7) | ||
| Positive result | 350 (64.2) | 362 (61.0) | 712 (62.6) | ||
| AFB smear test result | 0.278 | ||||
| Not performed | 25 (4.6) | 39 (6.6) | 64 (5.6) | ||
| Negative result | 289 (53.0) | 319 (53.8) | 608 (53.4) | ||
| Positive result | 231 (42.4) | 235 (28.2) | 466 (40.9) | ||
| Sputum smear test result ≥ 3+ | 59 (10.8) | 51 (8.6) | 110 (9.7) | 0.204 | |
Values are expressed as numbers and percentage.
COVID-19 = coronavirus disease 2019, NAAT = nucleic acid amplification test, AFB = acid-fast bacilli.
aAlarming symptoms were defined as sum of chest discomfort, hemoptysis, or dyspnea.
TB care delays across the country before and during the COVID-19 pandemic
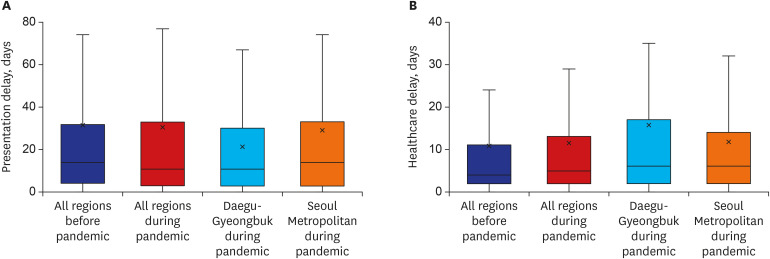
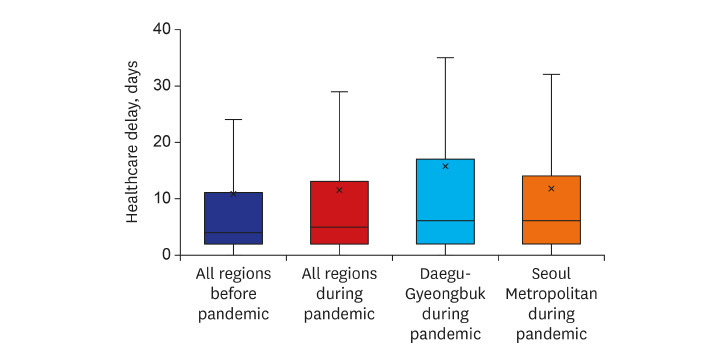
Median intervals between initial onset of symptoms and the first hospital visit were not different before and during COVID-19 (14 [IQR = 4–32] days vs. 11 [IQR = 3–33], P = 0.077). Presentation delays in the Daegu-Gyeongbuk and Seoul metropolitan areas during the pandemic were 11 (IQR = 3–30) days and 10 (IQR = 3–33) days, respectively (Fig. 2). Median intervals between the first hospital visit and treatment initiation were longer during the pandemic compared to before (4 [IQR = 2–11] days vs. 5 [IQR = 2–13], P = 0.027). Healthcare delays in the Daegu-Gyeongbuk and Seoul metropolitan areas during the pandemic were 6 (IQR = 2–17) days and 6 (IQR = 2–14) days, respectively.
Fig. 2. Box plots of tuberculosis care delays across the country before and during the COVID-19 pandemic. (A) Presentation delay. (B) Healthcare delays.
The five regions with the most reported cases of COVID-19 were selected during early pandemic and re-categorized into two regions based on their proximity; Daegu-Gyeongbuk, and Seoul Metropolitan Area (Seoul, Incheon, and Gyeonggi), and both presentation and healthcare delays were also calculated in two regions.
COVID-19 = coronavirus disease 2019.
Presentation delay
Proportion of presentation delay > 14 days in the during-pandemic group was lower than in the before-pandemic group (48.3% vs. 43.7%, P = 0.067), but not significant (Table 3). A multivariate analysis (Table 3) showed similar findings regarding the during-pandemic group (aOR, 0.883; 95% CI, 0.714–1.092). Patients with cough were associated with presentation delay > 14 days (aOR, 2.454; 95% CI, 1.945–3.095). Initial alarming symptoms (aOR, 0.603; 95% CI, 0.483–0.752), fever (aOR, 0.377; 95% CI, 0.286–0.496) and age ≥ 65 years (aOR, 0.658; 95% CI, 0.529–0.819) were associated with presentation delay ≤ 14 days.
Table 3. Multivariate analysis for factors associated with presentation delay > 14 days.
| Variables | ≤ 14 days (n = 843) | > 14 days (n = 714) | P value | Multivariate analysis | |
|---|---|---|---|---|---|
| aOR (95% CI) | P value | ||||
| During-COVID-19 group | 469 (55.6) | 364 (51.0) | 0.067 | 0.884 (0.715–1.094) | 0.257 |
| Age ≥ 65 years | 463 (54.9) | 306 (42.9) | < 0.001 | 0.658 (0.529–0.819) | < 0.001 |
| Male | 511 (60.6) | 414 (58.0) | 0.292 | 0.910 (0.731–1.133) | 0.400 |
| Prior TB treatment | 140 (16.6) | 113 (15.8) | 0.677 | 0.919 (0.689–1.227) | 0.590 |
| Extrapulmonary involvement | 316 (37.5) | 225 (31.5) | 0.014 | 1.070 (0.837–1.366) | 0.567 |
| Cough ± sputum | 363 (43.1) | 466 (65.3) | < 0.001 | 2.454 (1.945–3.095) | < 0.001 |
| Fever | 232 (27.5) | 95 (13.3) | < 0.001 | 0.375 (0.285–0.494) | < 0.001 |
| Alarming symptomsa | 380 (45.1) | 229 (32.1) | < 0.001 | 0.604 (0.484–0.753) | < 0.001 |
| Diabetes | 192 (22.8) | 128 (17.9) | 0.018 | 0.828 (0.632–1.084) | 0.170 |
| Solid malignancy | 67 (7.9) | 40 (5.6) | 0.068 | 0.848 (0.551–1.304) | 0.452 |
Values are expressed as numbers and percentage.
COVID-19 = coronavirus disease 2019, aOR = adjusted odds ratio, CI = confidence interval, TB = tuberculosis.
aAlarming symptoms were defined as sum of chest discomfort, hemoptysis, or dyspnea.
Healthcare delay
Proportion of healthcare delay > 5 days was significantly higher in the during-pandemic group (48.6% vs. 42.3%, P = 0.012) (Table 4). In a multivariate analysis, the during-pandemic group was significantly associated with healthcare delay > 5 days (aOR, 1.267; 95% CI, 1.032–1.555). Extrapulmonary involvement were also associated with healthcare delay > 5 days (aOR, 1.588; 95% CI, 1.259–2.004). Initial alarming symptoms (aOR, 0.644; 95% CI, 0.519–0.798) and coughing (aOR, 0.766; 95% CI, 0.614–0.955) were associated with healthcare delay ≤ 5 days.
Table 4. Multivariate analysis for factors associated with healthcare delay > 5 days.
| Variables | ≤ 5 days (n = 846) | > 5 days (n = 711) | P value | Multivariate analysis | |
|---|---|---|---|---|---|
| aOR (95% CI) | P value | ||||
| During COVID-19 pandemic | 428 (50.6) | 405 (57.0) | 0.012 | 1.267 (1.032–1.555) | 0.024 |
| Age ≥ 65 years | 412 (48.7) | 357 (50.2) | 0.552 | 1.154 (0.937–1.421) | 0.177 |
| Male | 529 (62.5) | 396 (55.7) | 0.006 | 0.795 (0.644–0.981) | 0.032 |
| Prior TB treatment | 136 (16.1) | 117 (16.5) | 0.840 | 1.117 (0.845–1.478) | 0.438 |
| Extrapulmonary involvement | 248 (29.3) | 293 (41.2) | < 0.001 | 1.588 (1.259–2.004) | < 0.001 |
| Cough ± sputum | 489 (57.8) | 340 (47.8) | < 0.001 | 0.766 (0.614–1.018) | 0.068 |
| Fever | 188 (22.2) | 139 (19.5) | 0.197 | 0.791 (0.614–1.018) | 0.068 |
| Alarming symptomsa | 364 (43.0) | 245 (34.5) | 0.001 | 0.644 (0.519–0.798) | < 0.001 |
| Diabetes | 183 (21.6) | 137 (19.3) | 0.250 | ||
| Solid malignancy | 52 (6.1) | 55 (7.7) | 0.217 | ||
Values are expressed as numbers and percentage.
aOR = adjusted odds ratio, CI = confidence interval, COVID-19 = coronavirus disease 2019, TB = tuberculosis.
aAlarming symptoms were defined as sum of chest discomfort, hemoptysis, or dyspnea.
DISCUSSION
In this cross-sectional study, we evaluated the factors that were associated to delays of TB care, especially focusing on the impact of the COVID-19 pandemic in Korea. Since the pandemic is lasting longer than we previously expected, we are witnessing a severe impact on essential TB services across the globe. The WHO advised that such disruption of TB services could lead to fewer TB diagnoses, an increase in diagnostic delay, and consequently, an increase in TB mortality.9 More data and research are necessary to identify the impact of the COVID-19 pandemic on TB services and to promote efforts that ensure the continuity of essential TB services during the pandemic.
Our key finding is that the healthcare delay of TB during the first wave of the pandemic significantly increased compared to the before-pandemic period in Korea. Due to the similar respiratory symptoms present in TB and COVID-19, almost all patients with cough and fever were redirected to COVID-19 screening clinics, where mycobacterial testing was not usually performed with the low suspicion of TB disease. Inevitable further delays occurred in referring patients to pulmonologists, as many of whom were deployed as frontline workers to fight the pandemic. In addition, reporting of mycobacterial test results also could be delayed.10 For instance, Italy also experienced significant diagnostic delays during the first wave of the COVID-19 pandemic.11 Several strategies are possible to mitigate diagnosis and treatment delays, such as the use of digital health technologies, decentralizing TB treatment to community health workers and by integrating TB and COVID-19 services.3 For example, simultaneous testing of the same patient for both TB and COVID-19 is warranted particularly in high TB-burden countries.
Despite our initial hypothesis of longer delays seeking healthcare services during the pandemic, our study showed that presentation delays between two periods were not different. Presentation delays in our result were similar to the findings of a previous Korean single-center study, which was conducted before the COVID-19 pandemic.12 It is assumed that since TB and COVID-19 share similar symptoms, patients presenting these symptoms have become a high alert during the pandemic and thus had priority and relatively easy access to hospitals during the early pandemic in Korea. This could also be ascribed to Korea’s ability to have rapidly achieved disease control in early 2020 without a lockdown.13 In addition, drive-through screening centers enabled efficient screening of large number of patients with respiratory symptoms during the first wave of COVID-19 pandemic,14 which effectively reduced the influx of patients visiting hospitals. However, we must continue to assume the possibility that a substantial proportion of vulnerable patients with TB were unable to access healthcare services during the first wave of COVID-19 pandemic. Recent significant decrements of TB notification after the COVID-19 surge in Korea15 signals the underdiagnosis and under-notification of TB cases. Further, the ongoing pandemic disproportionately affects vulnerable populations, such as the elderly, children, women, and those with comorbid illnesses or disabilities.16,17 Further investigations on the impact caused by COVID-19 pandemic should focus on vulnerable groups and their health seeking behaviors in order to restore the continuity of TB care services.
In the current study, we only evaluated the impact of COVID-19 pandemic on TB services before initiating treatment on individual levels. Thus, further investigation and careful surveillance are necessary to assess how patient outcomes are jeopardized, and to avoid endangering the national TB control program. In a recent article, we demonstrated that the treatment success rate among smear-positive pulmonary TB at the national level significantly decreased during the early phase of the pandemic in Korea.18 Globally, it is estimated that the impact of the COVID-19 pandemic could result in half a million excess TB deaths, setting the world back to the TB mortality rate in 2010.19 Therefore, the national TB control program must prioritize on improving the health system’s resilience to cope with TB, the most life-threatening infectious disease, despite the ongoing pandemic.
This study has additional limitations. First, this study was conducted in Korea, a high-income country with an intermediate TB-burden. Impacts of COVID-19 pandemic on TB services could vary depending on different healthcare systems and burdens of the two diseases. For example, patient’s delay in seeking healthcare providers was significantly longer during the pandemic in China.20 Local authorities need to collect their own data in order to implement public health interventions to minimize disruptions in TB care. Second, our study population was recruited from PPM-participating hospitals in Korea. The exclusion of patients from non-PPM hospitals could limit generalizability of our results. Due to the unavailability of comprehensive patient management at non-PPM hospitals, we could anticipate a greater negative impact on TB care services. Third, we only draw conclusions from the TB patients who had survived to visit the clinics and enrolled in this study, which could lead to survival bias. A certain number of TB patients could not visit the clinic during the early phase of pandemic, which might lead to early TB death before starting anti-TB treatment. Fourth, because we retrospectively analyzed data, other variables affecting TB patient’s health-seeking behavior were not available. However, we prospectively collected data by well-trained nurses based on the standardized questionnaire, which could minimize information bias.
In conclusion, our analysis identified that healthcare delays increased during the COVID-19 pandemic. Further investigations are necessary to identity how such delays affect anti-TB treatment outcome during the pandemic. Access to healthcare services and diagnostic testing were likely reduced due to limited human and material resources. In addition, the COVID-19 pandemic exacerbated the social stigma of coughing and appearing unwell, which potentially drove TB patients to conceal their illness from others until the severity of the disease and infectiousness worsens.21,22 This disruption of TB care continuity jeopardized previous progress towards achieving the targets of the WHO’s End TB Strategy. Therefore, as one of the priority recommendations outlined by the WHO, we must ensure that TB care and management are safeguarded in the midst of the COVID-19 pandemic.
ACKNOWLEDGMENTS
The Korea Disease Control and Prevention Agency has the authority to hold and analyze surveillance data for public health and research purposes. The Korea Disease Control and Prevention Agency approved the use of data and provided data without personal identification information.
Footnotes
Funding: The national PPM TB control project is supported by the National Health Promotion Fund, funded by the Ministry of Health and Welfare, Republic of Korea in 2021.
Disclosure: All authors have no potential conflicts of interest.
- Conceptualization: Min J, Ko Y, Kim JS.
- Data curation: Ko Y, Kim HW, Koo HK, Oh JY, Kim JS.
- Format analysis: Min J, Ko Y, Kim HW, Koo HK, Oh JY, Jeong YJ, Kang HH, Kang JY, Kim JS.
- Funding acquisition: Park JS.
- Methodology: Min J, Ko Y, Kim JS.
- Supervision: Park KJ, Hwang YI, Kim JW, Ahn JH, Jegal Y, Kang JY, Lee SS, Park JS, Kim JS.
- Writing - original draft: Min J, Kang JY, Lee SS, Park JS, Kim JS.
- Writing - review & editing: Min J, Park KJ, Hwang YI, Kim JW, Ahn JH, Jegal Y, Kim JS.
References
- 1.World Health Organization. Coronavirus disease 2019 (COVID-19) Situation Report - 5. [Updated 2020]. [Accessed September 17, 2020]. https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200311-sitrep-51-covid-19.pdf?sfvrsn=1ba62e57_10 .
- 2.Oh J, Lee JK, Schwarz D, Ratcliffe HL, Markuns JF, Hirschhorn LR. National response to COVID-19 in the Republic of Korea and lessons learned for other countries. Health Syst Reform. 2020;6(1):e1753464. doi: 10.1080/23288604.2020.1753464. [DOI] [PubMed] [Google Scholar]
- 3.Alene KA, Wangdi K, Clements AC. Impact of the COVID-19 pandemic on tuberculosis control: an overview. Trop Med Infect Dis. 2020;5(3):123. doi: 10.3390/tropicalmed5030123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Min J, Kim HW, Ko Y, Oh JY, Kang JY, Lee J, et al. Tuberculosis surveillance and monitoring under the national public-private mix tuberculosis control project in South Korea 2016–2017. Tuberc Respir Dis (Seoul) 2020;83(3):218–227. doi: 10.4046/trd.2020.0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Son E, Jeon D. Current situation of tuberculosis and national strategic plan for tuberculosis control in Korea. J Korean Med Assoc. 2021;64(4):316–323. [Google Scholar]
- 6.Yang J, Kwon Y, Kim J, Jang Y, Han J, Kim D, et al. Delays in the diagnosis and treatment of tuberculosis during the COVID-19 outbreak in the Republic of Korea in 2020. Osong Public Health Res Perspect. 2021;12(5):293–303. doi: 10.24171/j.phrp.2021.0063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kang YJ. Characteristics of the COVID-19 outbreak in Korea from the mass infection perspective. J Prev Med Public Health. 2020;53(3):168–170. doi: 10.3961/jpmph.20.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Korean Society of Infectious Diseases; Korean Society of Pediatric Infectious Diseases; Korean Society of Epidemiology; Korean Society for Antimicrobial Therapy; Korean Society for Healthcare-associated Infection Control and Prevention; Korea Centers for Disease Control and Prevention. Report on the epidemiological features of coronavirus disease 2019 (COVID-19) outbreak in the Republic of Korea from January 19 to March 2, 2020. J Korean Med Sci. 2020;35(10):e112. doi: 10.3346/jkms.2020.35.e112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.World Health Organization. Global Tuberculosis Report 2020. Geneva, Switzerland: World Health Organization; 2020. [Google Scholar]
- 10.Min J, Kang JY, Kim J, Yang J, Kwon Y, Shim E, et al. Impact of COVID-19 on TB services in Korea. Int J Tuberc Lung Dis. 2021;25(5):400–402. doi: 10.5588/ijtld.20.0942. [DOI] [PubMed] [Google Scholar]
- 11.Di Gennaro F, Gualano G, Timelli L, Vittozzi P, Di Bari V, Libertone R, et al. Increase in tuberculosis diagnostic delay during first wave of the COVID-19 pandemic: data from an Italian Infectious Disease Referral Hospital. Antibiotics (Basel) 2021;10(3):272. doi: 10.3390/antibiotics10030272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim SH, Min J, Cho JY, Kang H, Yang B, Shin YM, et al. Clinical profiles and outcomes of pulmonary tuberculosis patients with delayed treatment at a tertiary hospital in South Korea. Ann Palliat Med. 2021;10(3):2948–2957. doi: 10.21037/apm-20-1521. [DOI] [PubMed] [Google Scholar]
- 13.Yoo KJ, Kwon S, Choi Y, Bishai DM. Systematic assessment of South Korea’s capabilities to control COVID-19. Health Policy. 2021;125(5):568–576. doi: 10.1016/j.healthpol.2021.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kwon KT, Ko JH, Shin H, Sung M, Kim JY. Drive-through screening center for COVID-19: a safe and efficient screening system against massive community outbreak. J Korean Med Sci. 2020;35(11):e123. doi: 10.3346/jkms.2020.35.e123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kwak N, Hwang SS, Yim JJ. Effect of COVID-19 on Tuberculosis Notification, South Korea. Emerg Infect Dis. 2020;26(10):2506–2508. doi: 10.3201/eid2610.202782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jeong HE, Lee J, Shin HJ, Shin JY. Socioeconomic disparities in Korea by health insurance type during the COVID-19 pandemic: a nationwide study. Epidemiol Health. 2021;43(0):e2021007. doi: 10.4178/epih.e2021007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yoon WH. Why fast COVID-19 vaccination needed for people with disabilities and autistics in Korea? J Korean Med Sci. 2021;36(37):e267. doi: 10.3346/jkms.2021.36.e267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Min J, Kim HW, Koo HK, Ko Y, Oh JY, Kim J, et al. Impact of COVID-19 pandemic on the national PPM tuberculosis control project in Korea: the Korean PPM monitoring database between July 2019 and June 2020. J Korean Med Sci. 2020;35(43):e388. doi: 10.3346/jkms.2020.35.e388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Glaziou P. Predicted impact of the COVID-19 pandemic on global tuberculosis deaths in 2020. medRxiv. 2020 May 04; doi: 10.1101/2020.04.28.20079582. [DOI] [Google Scholar]
- 20.Wang X, He W, Lei J, Liu G, Huang F, Zhao Y. Impact of COVID-19 pandemic on pre-treatment delays, detection, and clinical characteristics of tuberculosis patients in Ningxia Hui Autonomous Region, China. Front Public Health. 2021;9:644536. doi: 10.3389/fpubh.2021.644536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bonadonna LV, Saunders MJ, Zegarra R, Evans C, Alegria-Flores K, Guio H. Why wait? The social determinants underlying tuberculosis diagnostic delay. PLoS One. 2017;12(9):e0185018. doi: 10.1371/journal.pone.0185018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saunders MJ, Evans CA. COVID-19, tuberculosis and poverty: preventing a perfect storm. Eur Respir J. 2020;56(1):2001348. doi: 10.1183/13993003.01348-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]