Abstract
Background
Plasmodium falciparum malaria dominates throughout sub-Saharan Africa, but the prevalence of Plasmodium malariae, Plasmodium ovale spp., and Plasmodium vivax increasingly contribute to infection in countries that control malaria using P. falciparum-specific diagnostic and treatment strategies.
Methods
We performed quantitative polymerase chain reaction (qPCR) on 2987 dried blood spots from the 2015–2016 Malawi Demographic and Health Survey to identify presence and distribution of nonfalciparum infection. Bivariate models were used to determine species-specific associations with demographic and environmental risk factors.
Results
Nonfalciparum infections had broad spatial distributions. Weighted prevalence was 0.025 (SE, 0.004) for P. malariae, 0.097 (SE, 0.008) for P. ovale spp., and 0.001 (SE, 0.0005) for P. vivax. Most infections (85.6%) had low-density parasitemias ≤ 10 parasites/µL, and 66.7% of P. malariae, 34.6% of P. ovale spp., and 40.0% of P. vivax infections were coinfected with P. falciparum. Risk factors for P. malariae were like those known for P. falciparum; however, there were few risk factors recognized for P. ovale spp. and P. vivax, perhaps due to the potential for relapsing episodes.
Conclusions
The prevalence of any nonfalciparum infection was 11.7%, with infections distributed across Malawi. Continued monitoring of Plasmodium spp. becomes critical as nonfalciparum infections become important sources of ongoing transmission.
Keywords: Malaria, Malawi, P. malariae, P. ovale spp, P. vivax
Using dried blood spots from the 2015–2016 Malawi Demographic and Health Survey, we assess spatial distribution of P. malariae, P. ovale spp., and P. vivax infection nationally, and compare differences in prevalence due to demographic and environmental risk factors.
Malaria is a major public health problem in Malawi with an estimated 393 malaria cases per 1000 people annually [1]. In Africa, 4 species of Plasmodium parasites lead to human disease. Plasmodium falciparum is the most prevalent, causing 99.7% of infections in sub-Saharan Africa [2], but Plasmodium malariae, Plasmodium ovale spp., and Plasmodium vivax also contribute to the burden of infection. While P. falciparum causes the most severe forms of clinical disease [3], nonfalciparum infections are increasingly recognized as sources of clinical morbidity and mortality [4, 5]. Reservoirs of nonfalciparum infection hinder efforts by malaria control and elimination programs to reduce the malaria burden using conventional toolkits.
Little has been published on the prevalence of nonfalciparum malaria in Malawi. The latest national prevalence estimates were taken from the Malawi Malaria Indicator Survey (MIS) in 2017 among children aged 6–59 months. Of all malaria parasite infections diagnosed by microscopy, 95.3% were due to P. falciparum, 8.0% to P. malariae, 0.5% to P. ovale spp., 0.0% to P. vivax, and 3.8% to mixed species infections [6]. However, the MIS 2017 did not determine the geospatial distribution of parasite species or include school-age children, adolescents, and adults, all of whom are key reservoirs for transmission [7]. Existing research studies documenting the presence of nonfalciparum parasite species in Malawi have been limited by narrow geographic range or outdated projections [8, 9], and national up-to-date estimates among adults are necessary to determine the landscape of nonfalciparum infection.
Malawi primarily uses rapid diagnostic test (RDT) kits that detect P. falciparum antigens only, providing no data on the burden of nonfalciparum malaria. Individuals with nonfalciparum infections have the potential to remain undiagnosed by the health system and may continue to transmit parasites within their communities if left untreated. Diagnosis of nonfalciparum infections are difficult, especially as current RDTs do not reliably or specifically detect P. malariae, P. ovale spp., and P. vivax. In addition, P. vivax and P. ovale spp. life cycles include dormant liver stages, called hypnozoites, which allow for relapse weeks to months after primary infection. The dormant stages of infection are not detectable by current diagnostics. Malawi’s first-line treatment for P. falciparum, artemisinin combination therapy (ACT), is effective against the blood stages of all Plasmodium spp. but does not treat hypnozoite liver stages in P. ovale spp. and P. vivax infections [10]. Thus, latent infection is resilient to diagnosis, prevention, and treatment mechanisms focused on the blood stages of the life cycle [11, 12]. The only effective therapies for preventing relapse are primaquine and tafenoquine, both of which are not routinely used through most of Africa [10]. Achieving malaria elimination will require a comprehensive approach to treatment and prevention, one that addresses all Plasmodium spp. and the populations contributing most to nonfalciparum transmission [13].
Malawi’s National Malaria Control Program is currently implementing the National Malaria Strategic Plan 2017–2022 alongside in-country partners, with a vision to achieve universal coverage of malaria interventions and reduce the malaria burden to a level of negligible public health significance [14]. Malaria parasite prevalence detected through microscopy decreased from 33% in the MIS 2014 [15] to 24% in the MIS 2017 [6], primarily all due to P. falciparum infections. As the burden of P. falciparum malaria declines worldwide, nonfalciparum malaria is becoming increasingly important. Surges in P. malariae and P. ovale spp. have been reported in Uganda and Zanzibar in response to P. falciparum-targeted interventions such as repeat ACT treatments and P. falciparum-specific RDTs [16, 17]. Increases in P. ovale spp. have also occurred in the Democratic Republic of the Congo (DRC) [18, 19] and Kenya [20] during the last 2 decades. In Tanzania, the prevalence of P. malariae and P. ovale spp. increased from 1.1% and 0.6% in 2010 to 2.4% and 3.6% in 2016, despite declines in P. falciparum during the same time period [21]. Little is known about nonfalciparum malaria in Malawi, as speciation is not a routine part of clinical diagnosis.
The main objective of this study was to assess the occurrence and geospatial distribution of nonfalciparum malaria parasite species among adolescents and adults in Malawi. We determine the national prevalence of infection using dried blood spots (DBS) collected as part of the 2015–2016 Malawi Demographic and Health Survey (MDHS), assess the spatial distribution of infection, and compare differences in prevalence due to demographic and environmental risk factors. Characterizing the presence of nonfalciparum infection provides insight into potential sources of ongoing transmission as policy makers determine the most appropriate antimalarial and diagnostic tools to counter changing malaria epidemiology in Malawi.
METHODS
Study Design and Population
The 2015–2016 MDHS was a cross-sectional, nationally representative survey enrolling 26 361 households from 850 clusters between October 2015 and February 2016 [22]. Women aged 15–49 years and men aged 15–54 years were interviewed and asked to contribute DBS to measure HIV prevalence. There were 7393 of 15 125 (48.9%) total DBS from eligible individuals remaining for use and previously screened for P. falciparum [23]. For this study, a random subset of 2987 DBS (19.7% of 15 125) was tested for P. malariae, P. ovale spp., and P. vivax. Informed consent was obtained from all individuals and/or their parent or legal guardian for additional DBS testing by the 2015–2016 MDHS. Ethical approval for this analysis was obtained from Institutional Review Boards through the National Health Sciences Research Committee at the Malawi Ministry of Health (No. 19/08/2381) and the University of North Carolina at Chapel Hill (No. 19-2882).
DNA Genotyping and Risk Factors
DBS were punched into 96 well plates and DNA was extracted from filter paper using Chelex; the full extraction protocol has been previously published [23]. We tested each sample with a real-time polymerase chain reaction (PCR) assay targeting the 18s ribosomal subunit (18S rSU) to identify individuals with P. malariae, P. ovale spp., and/or P. vivax infection [24–26]. PCR was run on the Applied Biosystems QuantStudio 6 Flex Real-Time PCR System using Roche Universal Probe Mastermix (Roche Life Science). Samples were run against nontemplate controls and dilutions of 18S plasmids (MR4: MRA-178, MRA-180, MRA-179) to allow quantification of 18S rSU copies, using a trendline based on the cycle threshold values of known levels of positive control standards. Parasitemia, or genomic equivalents of parasites in the extracted DNA, was estimated using an average of 6 copies of 18S rSU per parasite. The PCR assay used to identify P. ovale spp. infections contains a variable region to nondiscriminately amplify both P. ovale spp., P. ovale curtisi and P. ovale wallikeri [24]. Additional real-time PCR assays were conducted on a selection of high-parasitemia samples to differentiate between the 2 subspecies [27, 28]. Primers, reaction conditions, and control reaction results are listed in Supplementary Tables 1–3.
Deidentified 2015–2016 MDHS survey and geospatial data [29] were linked to PCR results through random sample barcode. Clusters with individuals who were PCR positive for any nonfalciparum infection were mapped using geospatial coordinates, offset according to Demographic and Health Survey methodology. Individual-level factors included sex, age group, wealth quintile, education level, owning livestock, source of drinking water, living in a household with a bed net, sleeping under a long-lasting insecticide-treated net (LLIN), LLIN insecticide type, living in a household with at least 1 net per 1.8 household members, and anemia (women only). Cluster level covariates included region, urban/rural place of residence, elevation, month of data collection, the proportion of a cluster living in a household with a bed net, the proportion of a cluster that slept under an LLIN, landcover, and current month’s average daily maximum temperature and the prior month’s precipitation. Landcover estimates were obtained from the Regional Center for Mapping of Resources for Development and SERVIR-Eastern and Southern Africa [30], and temperature and precipitation values were acquired from the Climate Hazards Center at the University of California, Santa Barbara [31, 32]; clusters were assigned values within buffers as previously described [23, 33].
Statistical Analysis
We used prevalence differences (PDs) to determine bivariate associations between risk factors and the probability of being infected with nonfalciparum malaria parasites. The 2015–2016 MDHS HIV sample weights were incorporated into linear risk regression models fit using generalized estimating equations to account for the 2015–2016 MDHS complex sample design. The subset of DBS was not a simple random sample (Supplementary Table 4), so we also incorporated standardized inverse probability of selection weights [34] into regression models. Bivariate models were run between all risk factors and prevalence outcomes, to determine areas for targeted interventions and characterize high-risk populations. All weighted counts, maps, and models were run using R 3.6.2 (R Foundation for Statistical Computing), using the sf (version 0.9-2; Pebesma, 2020) and survey (version 3.35-1; Lumley, 2019) packages.
RESULTS
Participant Characteristics
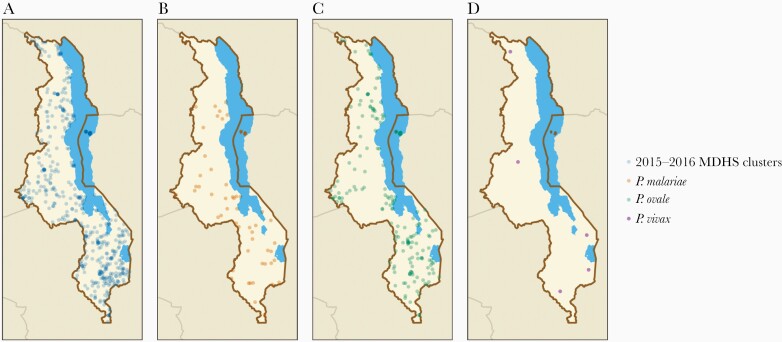
A total of 2987 samples were analyzed for nonfalciparum infection, representing 470 out of 850 (55.3%) 2015–2016 MDHS clusters (Figure 1). The median number of individuals per cluster was 6 (interquartile range, 4–8; range, 1–19). After incorporating survey and selection weights, 52.4% of the study population was female and 44.7% were 15–24 years of age (Table 1). The majority (69.8%) had a primary school education or no education, and 83.9% lived in rural areas. Most households (65.7%) owned a bed net, but only 35.4% slept under an LLIN the previous night. Nearly all (99.7%) individuals lived in a household that did not meet the World Health Organization’s criteria of having at least 1 net per 1.8 household members.
Figure 1.
A, Location of 2015–2016 Malawi Demographic and Health Survey (MDHS) clusters with samples in the current analysis (n = 470). B, Location of clusters with ≥ 1 positive Plasmodium malariae infection (n = 55). C, Location of clusters with ≥ 1 positive Plasmodium ovale spp. infection (n = 179). D, location of clusters with ≥ 1 positive Plasmodium vivax infection (n = 5).
Table 1.
Characteristics of the Study Population, Stratified by Plasmodium spp. and PCR Status, and Weighted According to 2015–2016 Malawi Demographic and Health Survey Weights
| Characteristic | P. malariae (n=2987) | P. ovale spp. (n=2987) | P. vivax (n=2987) | ||||
|---|---|---|---|---|---|---|---|
| PCR Positive | PCR Negative | PCR Positive | PCR Negative | PCR Positive | PCR Negative | Total | |
| Unweighted total numbera | 63 | 2924 | 292 | 2695 | 5 | 2982 | 2987 |
| Weighted count proportionb | 72 | 2741 | 274 | 2539 | 3 | 2809 | 2812 |
| Individual-level covariatesb | |||||||
| Sex | |||||||
| Male | 36 (50.1) | 1302 (47.5) | 138 (50.6) | 1200 (47.3) | 2 (76.7) | 1336 (47.6) | 1338 (47.6) |
| Female | 36 (49.9) | 1438 (52.5) | 135 (49.4) | 1339 (52.7) | 1 (23.3) | 1473 (52.4) | 1474 (52.4) |
| Age group, y | |||||||
| 15–24 | 44 (61.5) | 1213 (44.3) | 120 (43.7) | 1137 (44.8) | 2 (56.5) | 1255 (44.7) | 1257 (44.7) |
| 25–34 | 16 (22.3) | 804 (29.3) | 71 (25.9) | 749 (29.5) | 1 (20.2) | 819 (29.2) | 819 (29.1) |
| 35–44 | 11 (15.5) | 496 (18.1) | 63 (22.9) | 445 (17.5) | 1 (23.3) | 507 (18.0) | 507 (18.0) |
| 45–54 | 1 (0.8) | 228 (8.3) | 21 (7.5) | 208 (8.2) | 0 (0.0) | 228 (8.1) | 228 (8.1) |
| Wealth quintiles | |||||||
| Poorest | 20 (28.4) | 484 (17.7) | 52 (19.1) | 452 (17.8) | 0 (0.0) | 505 (18.0) | 505 (17.9) |
| Poorer | 19 (26.2) | 544 (19.8) | 51 (18.6) | 512 (20.2) | 2 (79.8) | 560 (19.9) | 563 (20.0) |
| Middle | 12 (17.1) | 555 (20.2) | 53 (19.4) | 514 (20.2) | 0 (0.0) | 567 (20.2) | 567 (20.2) |
| Richer | 15 (21.4) | 530 (19.3) | 49 (18.0) | 496 (19.5) | 0 (0.0) | 545 (19.4) | 545 (19.4) |
| Richest | 5 (6.9) | 628 (22.9) | 68 (24.9) | 564 (22.2) | 1 (20.2) | 632 (22.5) | 633 (22.5) |
| Education | |||||||
| None/preschool | 12 (17.2) | 213 (7.8) | 26 (9.7) | 198 (7.8) | 0 (0.0) | 225 (8.0) | 225 (8.0) |
| Primary | 45 (63.2) | 1694 (61.8) | 167 (61.0) | 1572 (61.9) | 2 (70.3) | 1737 (61.8) | 1739 (61.8) |
| Secondary | 14 (19.6) | 733 (26.7) | 71 (26.0) | 676 (26.6) | 1 (29.7) | 746 (26.6) | 747 (26.6) |
| Higher | 0 (0.0) | 92 (3.3) | 8 (2.9) | 84 (3.3) | 0 (0.0) | 92 (3.3) | 92 (3.3) |
| Owns livestock, herds, or farm animals | |||||||
| No | 35 (49.0) | 1247 (45.5) | 134 (49.1) | 1147 (45.2) | 2 (49.9) | 1280 (45.6) | 1282 (45.6) |
| Yes | 36 (51.0) | 1494 (54.5) | 139 (50.9) | 1391 (54.8) | 2 (50.1) | 1529 (54.4) | 1530 (54.4) |
| Source of drinking water | |||||||
| Piped | 6 (9.0) | 584 (21.3) | 71 (26.1) | 519 (20.4) | 1 (20.2) | 590 (21.0) | 590 (21.0) |
| Unpiped | 65 (91.0) | 2157 (78.7) | 202 (73.9) | 2020 (79.6) | 2 (79.8) | 2219 (79.0) | 2222 (79.0) |
| Household has a bednet | |||||||
| No | 31 (43.3) | 932 (34.0) | 101 (37.0) | 862 (34.0) | 2 (70.3) | 961 (34.2) | 963 (34.3) |
| Yes | 41 (56.7) | 1808 (66.0) | 172 (63.0) | 1677 (66.0) | 1 (29.7) | 1848 (65.8) | 1849 (65.7) |
| Slept under an LLIN last night | |||||||
| No | 56 (78.5) | 1760 (64.2) | 178 (64.9) | 1639 (64.5) | 3 (100.0) | 1813 (64.5) | 1816 (64.6) |
| Yes | 15 (21.5) | 981 (35.8) | 96 (35.1) | 900 (35.5) | 0 (0.0) | 996 (35.5) | 996 (35.4) |
| Permethrin | 10 (13.6) | 629 (23.0) | 62 (64.6) | 577 (64.1) | 0 (0.0) | 639 (64.1) | 639 (64.1) |
| Nonpermethrin | 6 (7.8) | 351 (12.8) | 34 (35.4) | 323 (35.9) | 0 (0.0) | 357 (35.9) | 357 (35.9) |
| At least 1 net per 1.8 household members | |||||||
| No | 72 (100.0) | 2733 (99.7) | 273 (99.7) | 2532 (99.8) | 3 (100.0) | 2802 (99.7) | 2805 (99.7) |
| Yes | 0 (0.0) | 5 (0.2) | 1 (0.3) | 4 (0.2) | 0 (0.0) | 5 (0.2) | 5 (0.2) |
| Anemia, women only | |||||||
| Not anemic | 26 (72.0) | 964 (67.0) | 91 (67.5) | 898 (67.1) | 1 (100.0) | 989 (67.1) | 990 (67.1) |
| Mild | 10 (28.0) | 368 (25.6) | 31 (22.6) | 347 (25.9) | 0 (0.0) | 378 (25.6) | 378 (25.6) |
| Moderate | 0 (0.0) | 91 (6.3) | 13 (9.6) | 78 (5.8) | 0 (0.0) | 91 (6.2) | 91 (6.2) |
| Severe | 0 (0.0) | 14 (1.0) | 0 (0.2) | 14 (1.0) | 0 (0.0) | 14 (0.9) | 14 (0.9) |
| Cluster-level covariatesb | |||||||
| Region | |||||||
| Northern | 9 (12.6) | 444 (16.2) | 43 (15.5) | 411 (16.2) | 0 (9.5) | 453 (16.1) | 453 (16.1) |
| Central | 24 (33.7) | 1001 (36.5) | 88 (32.1) | 938 (36.9) | 1 (23.3) | 1025 (36.5) | 1025 (36.5) |
| Southern | 38 (53.7) | 1295 (47.2) | 143 (52.3) | 1190 (46.9) | 2 (67.2) | 1331 (47.4) | 1333 (47.4) |
| Place of residence | |||||||
| Urban | 2 (3.0) | 450 (16.4) | 52 (18.9) | 401 (15.8) | 0 (0.0) | 452 (16.1) | 452 (16.1) |
| Rural | 69 (97.0) | 2290 (83.6) | 222 (81.1) | 2138 (84.2) | 3 (100.0) | 2357 (83.9) | 2360 (83.9) |
| Elevation, m | |||||||
| <500 | 15 (21.6) | 371 (13.5) | 53 (19.2) | 334 (13.1) | 1 (20.2) | 386 (13.7) | 386 (13.7) |
| ≥500 and <1000 | 36 (50.8) | 1017 (37.1) | 128 (46.9) | 925 (36.4) | 1 (47.0) | 1052 (37.5) | 1054 (37.5) |
| ≥1000 and <1500 | 20 (27.6) | 1262 (46.0) | 88 (32.2) | 1194 (47.0) | 1 (23.3) | 1281 (45.6) | 1282 (45.6) |
| ≥1500 | 0 (0.0) | 91 (3.3) | 5 (1.7) | 86 (3.4) | 0 (9.5) | 90 (3.2) | 91 (3.2) |
| Month of data collection | |||||||
| October 2015 | 20 (28.2) | 572 (20.9) | 66 (24.0) | 526 (20.7) | 1 (20.2) | 591 (21.1) | 592 (21.1) |
| November 2015 | 23 (32.1) | 886 (32.3) | 84 (30.8) | 824 (32.5) | 0 (9.5) | 908 (32.3) | 909 (32.3) |
| December 2015 | 12 (16.9) | 352 (12.8) | 28 (10.2) | 336 (13.2) | 0 (0.0) | 364 (13.0) | 364 (12.9) |
| January 2016 | 11 (15.6) | 824 (30.1) | 80 (29.4) | 754 (29.7) | 2 (70.3) | 833 (29.6) | 835 (29.7) |
| February 2016 | 5 (7.2) | 107 (3.9) | 15 (5.6) | 97 (3.8) | 0 (0.0) | 113 (4.0) | 113 (4.0) |
| Landcover | |||||||
| Settlement | 2 (3.0) | 383 (14.0) | 48 (17.7) | 337 (13.3) | 0 (0.0) | 385 (13.7) | 385 (13.7) |
| Forest | 19 (26.9) | 333 (12.1) | 36 (13.1) | 316 (12.5) | 0 (9.5) | 352 (12.5) | 352 (12.5) |
| Grassland | 5 (7.5) | 215 (7.9) | 22 (8.0) | 199 (7.8) | 0 (0.0) | 221 (7.9) | 221 (7.9) |
| Cropland | 38 (53.7) | 1647 (60.1) | 148 (54.0) | 1538 (60.6) | 3 (90.5) | 1683 (59.9) | 1686 (59.9) |
| Wetland | 6 (8.9) | 121 (4.4) | 17 (6.3) | 110 (4.3) | 0 (0.0) | 127 (4.5) | 127 (4.5) |
| Other | 0 (0.0) | 41 (1.5) | 3 (1.0) | 39 (1.5) | 0 (0.0) | 41 (1.5) | 41 (1.5) |
| Proportion of cluster with bednets, mean (SE) | 0.6 (0.0) | 0.6 (0.0) | 0.6 (0.0) | 0.6 (0.0) | 0.6 (0.1) | 0.6 (0.0) | 0.6 (0.0) |
| Proportion of cluster that slept under an LLIN last night, mean (SE) | 0.3 (0.0) | 0.3 (0.0) | 0.3 (0.0) | 0.3 (0.0) | 0.2 (0.1) | 0.3 (0.0) | 0.3 (0.0) |
| Current month's average daily maximum temperature, °C, mean (SE) | 32.3 (0.5) | 31.1 (0.1) | 31.8 (0.2) | 31.1 (0.1) | 31.8 (1.5) | 31.2 (0.1) | 31.2 (0.1) |
| Prior month's precipitation, mm, mean (SE) | 56.2 (9.8) | 67.1 (4.2) | 66.4 (7.0) | 66.9 (4.3) | 136.1 (34.8) | 66.8 (4.2) | 66.8 (4.2) |
Data are No. (%) except where indicated.
Abbreviations: LLIN, long-lasting insecticide-treated net; PCR, polymerase chain reaction.
aCounts do not incorporate sample weights and are not representative of the weighted populations used in the table.
bSampling weights applied.
Weighted Prevalence
Infections of all species were geospatially distributed across the country; there were 63 cases of P. malariae, 292 cases of P. ovale spp., and 5 cases of P. vivax. The intraclass coefficient at the cluster level was 47.5% for P. malariae and 27.3% for P. ovale, indicating the lack of independence between sampling units. The weighted prevalence of P. malariae was 0.025 (SE, 0.004), P. ovale spp. was 0.097 (SE, 0.008), P. vivax was 0.001 (SE, 0.0005), and the weighted prevalence of any nonfalciparum infection was 0.117 (SE, 0.009) (Supplementary Table 5). The weighted prevalence of nonfalciparum infections excluding falciparum mixed infections was 0.070 (SE, 0.007). Most infections of all species types had low-density parasitemias ≤ 10 parasites/µL; 68.3% (n = 43/63) of P. malariae PCR-positive infections, 87.0% (n = 260/292) of P. ovale spp., and 100% (n = 5/5) of P. vivax (Figure 2).
Figure 2.
Distribution of polymerase chain reaction (PCR)-positive parasitemia values for Plasmodium malariae (n = 63), Plasmodium ovale spp. (n = 292), and Plasmodium vivax (n = 5) infections. Values < 10 parasites/µL are rounded up to 10 parasites/µL.
Mixed Species Infections
Several infections were mixed species infections with P. falciparum, including 42 (66.7%) P. malariae, 104 (35.6%) P. ovale spp., and 2 (40.0%) P. vivax infections. Individuals living in rural areas had a higher risk of coinfection with P. falciparum (risk difference (RD), 0.28; 95% confidence interval [CI], .13–.43) as compared to having a monoinfection. Individuals aged 25–34 years had a lower risk of coinfection with P. falciparum (RD, −0.17; 95% CI, −.32 to −.02) compared to individuals aged 15–24 years and individuals in the central region had the highest risk (0.54) of coinfection with P. falciparum. Sex was not associated with P. falciparum coinfection (P = .6). Four samples were positive for P. malariae and P. ovale spp., and 10 samples were positive for P. malariae, P. ovale spp., and P. falciparum. We successfully amplified 59/60 samples to determine P. ovale subspecies. We identified P. ovale curtisi in 44.1% (26/59) of samples, P. ovale wallikeri in 16.9% (10/59),, and 11.9% (7/59) remained undifferentiated (positive for both subspecies). There was no obvious difference in the geographical distribution of P. ovale subspecies.
Bivariate Associations
Bivariate regression models using weighted survey data found few associations between demographic and environmental risk factors, and prevalence of nonfalciparum infection (Figure 3 and Table 2). Increased age was associated with a lower prevalence of infection with P. malariae, with a PD of −0.03 (95% CI, −.05 to −.02) between those aged 45–54 years and those 12–24 years. Greater wealth (PD, −0.03; 95% CI, −.06 to −.01) and higher education (PD, −0.05; 95% CI, −.10 to −.01) were also associated with a lower prevalence of infection. Sleeping under an LLIN the night prior (PD, −0.02; 95% CI, −.03 to .00) and higher elevation (PD, −0.04; 95% CI, −.07 to −.01) were also protective against P. malariae. A lower prevalence of P. ovale spp. infection was associated with higher elevation, with a PD of −0.09 (95% CI, −.14 to −.03) comparing elevations ≥ 1500 m to < 500 m. Severe anemia was associated with infection with P. malariae (PD, −0.03; 95% CI, −.04 to −.01) and P. ovale spp. (PD, −0.07; 95% CI, −.12 to −.02) among women, relative to no anemia; however, these values are unadjusted and do not account for potential confounding factors. No associations were found between P. vivax and demographic and environmental risk factors, in part due to the small number of PCR-positive cases (n = 5), which limited precision. Infection with P. malariae was associated with P. falciparum infection (PD, 0.03; 95% CI, .02 to .05) and P. ovale infection (PD, 0.05; 95% CI, .01 to .09), likely due to similar risk factors. P. ovale and P. vivax were not associated with P. falciparum coinfection.
Figure 3.
Bivariate associations between demographic and environmental risk factors and Plasmodium spp. prevalence using weighted survey data. Models incorporate 2015–2016 Malawi Demographic and Health Survey weights and inverse probability of selection weights. Abbreviations: CI, confidence interval; LLIN, long-lasting insecticide-treated net; PD, prevalence difference.
Table 2.
Bivariate Associations Between Demographic and Environmental Risk Factors and Plasmodium spp. Prevalence Using Weighted Survey Data
| P. malariae (n=2987) | P. ovale spp. (n=2987) | P. vivax (n=2987) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Prevalence | PD (95% CI) | P Value | Prevalence | PD (95% CI) | P Value | Prevalence | PD (95% CI) | P Value | |
| Individual-level covariatesa | |||||||||
| Sex | |||||||||
| Male | 0.027 | … | … | 0.103 | … | … | 0.002 | … | … |
| Female | 0.024 | 0.00 (−.02 to .01) | .7 | 0.092 | −0.01 (−.04 to .01) | .4 | 0.000 | 0.00 (.00 to .00) | .2 |
| Age group, y | |||||||||
| 15–24 | 0.035 | … | … | 0.095 | … | … | |||
| 25–34 | 0.019 | −0.02 (−.03 to .00) | .1 | 0.086 | −0.01 (−.04 to .02) | .6 | |||
| 35–44 | 0.022 | −0.01 (−.03 to .01) | .2 | 0.124 | 0.03 (−.01 to .07) | .14 | |||
| 45–54 | 0.002 | −0.03 (−.05 to −.02) | <.001 | 0.090 | −0.01 (−.05 to .04) | .8 | |||
| Wealth quintiles | |||||||||
| Poorest | 0.040 | … | … | 0.103 | … | … | |||
| Poorer | 0.033 | −0.01 (−.04 to .02) | .6 | 0.090 | −0.01 (−.06 to .04) | .6 | |||
| Middle | 0.022 | −0.02 (−.04 to .01) | .1 | 0.094 | −0.01 (−.06 to .04) | .7 | |||
| Richer | 0.028 | −0.01 (−.04 to .02) | .4 | 0.090 | −0.01 (−.06 to .04) | .6 | |||
| Richest | 0.008 | −0.03 (−.06 to −.01) | .01 | 0.108 | 0.00 (−.05 to .06) | .9 | |||
| Education | |||||||||
| None/preschool | 0.055 | … | … | 0.118 | … | … | |||
| Primary | 0.026 | −0.03 (−.07 to .01) | .2 | 0.096 | −0.02 (−.09 to .04) | .5 | |||
| Secondary | 0.019 | −0.04 (−.08 to .01) | .1 | 0.095 | −0.02 (−.09 to .05) | .5 | |||
| Higher | 0.000 | −0.05 (−.10 to −.01) | .01 | 0.087 | −0.03 (−.14 to .07) | .6 | |||
| Owns livestock, herds, or farm animals | |||||||||
| No | 0.027 | … | … | 0.105 | … | … | 0.001 | … | … |
| Yes | 0.024 | 0.00 (−0.02 to .01) | .7 | 0.091 | −0.01 (−0.04 to .02) | .4 | 0.001 | 0.00 (.00 to .00) | .9 |
| Source of drinking water | |||||||||
| Piped | 0.011 | … | … | 0.121 | … | … | 0.001 | … | … |
| Unpiped | 0.029 | 0.02 (.01 to .03) | .01 | 0.091 | −0.03 (−.08 to .02) | .2 | 0.001 | 0.00 (.00 to .00) | 1.0 |
| Household has a bednet | |||||||||
| No | 0.032 | … | … | 0.105 | … | … | 0.002 | … | … |
| Yes | 0.022 | −0.01 (−.03 to .01) | .2 | 0.093 | −0.01 (−0.05 to .02) | .5 | 0.001 | 0.00 (.00 to .00) | .2 |
| Slept under an LLIN last night | |||||||||
| No | 0.031 | … | … | 0.098 | … | … | |||
| Yes | 0.015 | −0.02 (−.03 to .00) | .03 | 0.096 | 0.00 (−.03 to .03) | .9 | |||
| Permethrin | 0.015 | … | … | 0.097 | … | … | |||
| Nonpermethrin | 0.016 | 0.00 (−.02 to .02) | 1.0 | 0.095 | 0.00 (−.05 to .04) | .9 | |||
| At least 1 net per 1.8 household members | |||||||||
| No | 0.026 | … | … | 0.097 | … | … | |||
| Yes | 0.000 | −0.03 (−.03 to −.02) | <.001 | 0.173 | 0.08 (−.25 to .40) | .6 | |||
| Anemia, women only | |||||||||
| Not anemic | 0.026 | … | … | 0.092 | … | … | |||
| Mild | 0.026 | 0.00 (−.02 to .02) | 1.0 | 0.081 | −0.01 (−.05 to .03) | .6 | |||
| Moderate | 0.000 | −0.03 (−.04 to −.01) | <.001 | 0.144 | 0.05 (−.07 to .17) | .4 | |||
| Severe | 0.000 | −0.03 (−.04 to −.01) | <.001 | 0.024 | −0.07 (−.12 to −.02) | .01 | |||
| Cluster-level covariatesa | |||||||||
| Region | |||||||||
| Northern | 0.020 | … | … | 0.094 | … | … | 0.001 | … | … |
| Central | 0.024 | 0.00 (−.01 to .02) | .7 | 0.086 | −0.01 (−.04 to .03) | .7 | 0.001 | 0.00 (.00 to .00) | 1.0 |
| Southern | 0.029 | 0.01 (−.01 to .03) | .3 | 0.107 | 0.01 (−.02 to .05) | .5 | 0.002 | 0.00 (.00 to .00) | .4 |
| Place of residence | |||||||||
| Urban | 0.005 | … | … | 0.114 | … | … | |||
| Rural | 0.029 | 0.02 (.01 to .04) | <.001 | 0.094 | −0.02 (−.07 to .03) | .4 | |||
| Elevation, m | |||||||||
| <500 | 0.040 | … | … | 0.136 | … | … | 0.002 | … | … |
| ≥500 and <1000 | 0.034 | −0.01 (−.04 to .03) | .7 | 0.122 | −0.01 (−.06 to .04) | .6 | 0.001 | 0.00 (.00 to .00) | .9 |
| ≥1000 and <1500 | 0.015 | −0.02 (−.06 to .01) | .1 | 0.069 | −0.07 (−.11 to −.02) | .00 | 0.001 | 0.00 (.00 to .00) | .5 |
| ≥1500 | 0.000 | −0.04 (−.07 to −.01) | .009 | 0.050 | −0.09 (−.14 to −.03) | .002 | 0.003 | 0.00 (−.01 to .01) | .7 |
| Month of data collection | |||||||||
| October 2015 | 0.034 | … | … | 0.111 | … | … | |||
| November 2015 | 0.025 | −0.01 (−.03 to .02) | .5 | 0.093 | −0.02 (−.07 to .04) | .5 | |||
| December 2015 | 0.033 | 0.00 (−.03 to .03) | 1.0 | 0.077 | −0.03 (−.09 to .02) | .2 | |||
| January 2016 | 0.013 | −0.02 (−.04 to .00) | .1 | 0.096 | −0.01 (−.07 to .04) | .6 | |||
| February 2016 | 0.046 | 0.01 (−.02 to .05) | .5 | 0.135 | 0.02 (−.05 to .10) | .5 | |||
| Landcover | |||||||||
| Settlement | 0.125 | … | … | ||||||
| Forest | 0.102 | −0.02 (−.09 to .04) | .5 | ||||||
| Grassland | 0.099 | −0.03 (−.11 to .06) | .5 | ||||||
| Cropland | 0.088 | −0.04 (−.10 to .02) | .2 | ||||||
| Wetland | 0.136 | 0.01 (−.08 to .10) | .8 | ||||||
| Other | 0.064 | −0.06 (−.12 to −.01) | .03 | ||||||
| Proportion of cluster with bednets, scaled | |||||||||
| Mean | 0.042 | … | … | 0.097 | … | … | |||
| 10% increase | 0.016 | −0.03 (−.07 to .02) | .3 | 0.092 | −0.01 (−.02 to .00) | .26 | |||
| Proportion of cluster that slept under an LLIN last night, scaled | |||||||||
| Mean | 0.097 | … | … | ||||||
| 10% increase | 0.089 | −0.01 (−.02 to .00) | .10 | ||||||
| Current month's average daily maximum temperature, °C, scaled | |||||||||
| Mean | 0.097 | … | … | −0.002 | … | … | |||
| 1°C increase | 0.109 | 0.01 (.01 to .02) | .001 | −0.002 | 0.00 (.00 to .00) | .7 | |||
| Prior month's precipitation, mm, scaled | |||||||||
| Mean | 0.028 | … | … | 0.097 | … | … | 0.000 | … | … |
| 100 mm increase | 0.028 | 0.00 (.00 to .00) | .4 | 0.097 | 0.00 (.00 to .00) | .9 | 0.000 | 0.00 (.00 to .00) | .2 |
Models incorporate 2015–2016 Malawi Demographic and Health Survey weights and inverse probability of selection weights. Continuous variables are scaled to their means.
Abbreviations: CI, confidence interval; LLIN, long-lasting insecticide treated net; PD, prevalence difference.
aSampling weights applied.
Discussion
Our findings show that nonfalciparum Plasmodium infections are more prevalent among adolescents and adults in Malawi than previously reported. The weighted prevalence of infection among our study population was 2.5% for P. malariae, 9.7% for P. ovale spp., and 0.1% for P. vivax. Most (85.6%) infections of all species types had low-density parasitemias ≤ 10 parasites/µL, the majority of these were likely asymptomatic infections. Although asymptomatic P. falciparum malaria has been shown to significantly increase the risk of symptomatic disease at 1 month, the impact of asymptomatic low-density nonfalciparum infection on future symptomatic disease remains unclear [35]. Several infections were mixed species; 39.9% of all P. malariae, P. ovale spp., and P. vivax infections were coinfected with P. falciparum. Tracking the presence of nonfalciparum infections highlights sources of ongoing transmission in Malawi and surveillance of all Plasmodium spp. is necessary to develop a comprehensive malaria elimination strategy.
The only previously published national estimates of nonfalciparum infection in Malawi stem from the 2017 MIS among children aged 6–59 months, which reported that of all microscopy-positive cases, 95.3% had P. falciparum, 8.0% had P. malariae, 0.5% had P. ovale spp., 00.0% had P. vivax, and 3.8% had mixed species infections [6]. Despite being collected in a similar time period, of all PCR-positive samples among our adult study population, 84.7% had P. falciparum, 9.5% had P. malariae, 30.0% had P. ovale spp., 0.3% had P. vivax, and 23.8% had a mixed species infection with P. falciparum after incorporating weighting. Our results may be detecting a larger proportion of nonfalciparum cases than the 2017 MIS as PCR is more sensitive than microscopy, particularly for low-density infections [36]. However, prior research among individuals aged > 6 months in 2 districts in Malawi in 2002 found that the PCR prevalence of P. malariae was between 7.7% and 13.2% and P. ovale spp. 3.3% and 7.8% [8]. No infections of P. vivax were detected. Our results suggest that the prevalence of P. ovale spp. and P. vivax have increased over the last 2 decades since the 2002 survey, although we are likely underestimating the true prevalence of P. malariae as our assay is not as sensitive as that of P. ovale spp. at detecting low parasitemias (Supplementary Table 3).
The overall 9.7% prevalence of P. ovale spp. found in this study is higher than what has been previously reported in studies of neighboring countries, although much lower than recent findings from Kenya showing increasing nonfalciparum species over time [20]. This may reflect the high level of malaria transmission intensity in Malawi, as over 35% of all P. ovale spp. infections occurred alongside another Plasmodium spp., as well as other social or ecological factors permitting ongoing and undetected transmission of P. ovale spp. P. vivax has been shown to occur in all regions of Africa [37, 38], but to our knowledge, this represents the first known report of P. vivax in Malawi. Risk factors for P. ovale spp. in the DRC included male sex, coprevalent P. falciparum, and rural residence [24], but there were few risk factors for P. vivax [25] and many P. falciparum-associated risk factors were not associated with either P. ovale spp. or P. vivax. Similarly, few risk factors were identified for P. ovale spp. and P. vivax in our study, perhaps due to our inability to differentiate between incident and relapsing infections, or low-parasite–density infections failing to drive the selective pressure that would result in identifiable risk factors.
Coinfection with P. falciparum was common among our study population, similar to previously published data from the DRC and Malawi [8, 24]. Rurality and younger age were associated with higher proportions of P. falciparum coinfection compared to monoinfections, consistent with risk factors previously identified for P. falciparum infection among our study population [23]. Mixed infections often go unrecognized and can lead to severe disease complications; patients with mixed infections have higher proportions of pulmonary complications and multiple organ failure than patients with P. falciparum infection alone [39]. In Malawi, mixed infections have been shown to have protective associations such as higher hemoglobin levels as compared to monoinfections, but associations vary by transmission intensity and seasonality [8]. Further research is necessary to determine the effects of mixed infections among our study population.
Trends of increasing prevalence of nonfalciparum species have been seen elsewhere in Africa, even in regions where malaria measures have reduced the presence of P. falciparum [16, 17, 21]. If Malawi follows suit, new diagnostic and treatment methods will be critical to continue malaria control and elimination. Currently health facilities in Malawi use RDT kits that diagnose P. falciparum antigens only (First Response, SD Bioline, CareStart, and Paracheck) in all outpatient settings [40], with microscopy only used in select inpatient settings, resulting in zero cases of P. malariae, P. ovale spp., P. vivax, or mixed infections reported to the global community in 2019 [41]. Infections that are undiagnosed could remain untreated; under Malawi Ministry of Health policy, negative RDT results are not treated with ACTs and remain as reservoirs for community transmission, although in practice some individuals may still receive treatment for symptoms consistent with malaria. In Zambia, where P. falciparum-specific RDTs also cause nonfalciparum malaria to remain undiagnosed, assessment of the prevalence of nonfalciparum malaria in the country was also higher than previously reported (10.3% of infections were mixed species) and may explain why malaria continues to dominate despite continued control measures [42]. Evolving parasite epidemiology prompts further consideration of organized sentinel surveillance of nonfalciparum species until field-deployable testing with species-specific RDTs can be achieved.
This analysis represents the first national assessment of nonfalciparum malaria infection among adolescents and adults in Malawi. Use of existing samples and individual-level data collected through a national household survey makes efficient use of both time and resources. Our methods can be replicated through future DHS and MIS iterations to track changes in nonfalciparum infection longitudinally in response to changes in malaria policy.
One limitation of our study design is the cross-sectional nature of 2015–2016 MDHS sample collection, which restricts inference into changes in nonfalciparum prevalence rates over time and focuses our analysis on marginal associations rather than causal relationships. Our study population was limited to 15–54 year olds, a population often neglected in malaria research; however we are missing data on children < 5 years and school-age children, who have the highest prevalence of P. falciparum infection in Malawi [7]. We could not capture information on gametocytes and could not determine if P. ovale spp. and P. vivax infections were newly acquired or the result of relapse from hypnozoites. Future research on nonfalciparum Plasmodium spp. in Malawi can better take advantage of novel data streams, such as molecular surveillance, to determine if there is a changing ecology of nonfalciparum malaria in the country and to determine locations for intervention. Reusing RDTs to conduct molecular surveillance [43] and use of mobile phone networks to examine the direction and intensity of parasite mixing between regions [44] could contribute to tools for ongoing surveillance.
This study represents the first depiction of nonfalciparum infection nationally among adolescents and adults in Malawi. The prevalence of nonfalciparum infection was 0.117, with all species present across the country; however, there are few risk factors that exist to identify target groups for intervention. Most infections had low-density parasitemias and many were coinfected with P. falciparum. Molecular surveillance techniques enable efficient use of national survey samples and allow for periodic evaluation of the burden of nonfalciparum species in response to ongoing interventions. Insight into the prevalence of nonfalciparum infection can aid the National Malaria Control Program in setting up surveillance programs and fine-tuning diagnostic and treatment guidelines as the malaria landscape changes in response to P. falciparum-targeted interventions.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. We thank Kyaw Thwai and Meredith Muller from the University of North Carolina (UNC) Infectious Disease Epidemiology and Ecology Laboratory and staff at the UNC Project-Malawi laboratory for assistance with processing samples. We are grateful to the individuals included in the 2015–2016 Malawi Demographic and Health Survey (MDHS) for their participation and continued contributions to research, as well as to the 2015–2016 MDHS funders and study team who coordinated original dried blood spots collection. We also thank the National Malaria Control Programme and the National HIV Reference Laboratory at the Malawi Ministry of Health for coordination and use of 2015–2016 MDHS samples. P. vivax and P. ovale spp. plasmid stocks used as positive controls were obtained through BEI Resources, National Institute of Allergy and Infectious Diseases, National Institutes of Health: diagnostic plasmid containing the small subunit ribosomal RNA gene (18S) from Plasmodium vivax, MRA-178, and from Plasmodium ovale, MRA-180, contributed by Peter A. Zimmerman.
Financial support. This work was supported by the Institute for Global Health and Infectious Diseases at the University of North Carolina; and the National Institutes of Health (grant number K24AI134990).
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Battle KE, Gumbo A, Hamuza G, et al. Consultative meeting that examined alignment and discrepancies between health facility and household survey data in Malawi. Malar J 2019; 18:411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. World Health Organization (WHO). World malaria report 2019. Geneva, Switzerland: WHO, 2019. [Google Scholar]
- 3. Fairhurst RM, Wellems TE. Malaria (Plasmodium species). In: Bennett JE, Dolin R, Blaser MJ, eds. Mandell, Douglas, and Bennett’s principles and practice of infectious diseases. 8th ed. Vol 2. Philadelphia: Elsevier/Saunders; 2015:3070–90.e9. [Google Scholar]
- 4. Kotepui M, Kotepui KU, Milanez GD, Masangkay FR. Severity and mortality of severe Plasmodium ovale infection: a systematic review and meta-analysis. PLoS One 2020; 15:e0235014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rahimi BA, Thakkinstian A, White NJ, Sirivichayakul C, Dondorp AM, Chokejindachai W. Severe vivax malaria: a systematic review and meta-analysis of clinical studies since 1900. Malar J 2014; 13:481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. National Malaria Control Programme (NMCP) Malawi and ICF International. Malawi malaria indicator survey 2017. Lilongwe, Malawi, and Rockville, Maryland: NMCP and ICF International, 2018. https://dhsprogram.com/pubs/pdf/MIS28/MIS28.pdf. [Google Scholar]
- 7. Walldorf JA, Cohee LM, Coalson JE, et al. School-age children are a reservoir of malaria infection in Malawi. PLoS One 2015; 10:e0134061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bruce MC, Macheso A, Kelly-Hope LA, Nkhoma S, McConnachie A, Molyneux ME. Effect of transmission setting and mixed species infections on clinical measures of malaria in Malawi. PLoS One 2008; 3:e2775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rantala AM, Taylor SM, Trottman PA, et al. Comparison of real-time PCR and microscopy for malaria parasite detection in Malawian pregnant women. Malar J 2010; 9:269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Phillips MA, Burrows JN, Manyando C, van Huijsduijnen RH, Van Voorhis WC, Wells TNC. Malaria. Nat Rev Dis Primers 2017; 3:17050. [DOI] [PubMed] [Google Scholar]
- 11. Hetzel MW, Reimer LJ, Gideon G, et al. Changes in malaria burden and transmission in sentinel sites after the roll-out of long-lasting insecticidal nets in Papua New Guinea. Parasit Vectors 2016; 9:340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chaumeau V, Kajeechiwa L, Fustec B, et al. Contribution of asymptomatic Plasmodium infections to the transmission of malaria in Kayin State, Myanmar. J Infect Dis 2019; 219:1499–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cotter C, Sturrock HJ, Hsiang MS, et al. The changing epidemiology of malaria elimination: new strategies for new challenges. Lancet 2013; 382:900–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. National Malaria Control Programme. Revised malaria strategic plan 2017–2022. Lilongwe: Malawi Ministry of Health, 2020. [Google Scholar]
- 15. National Malaria Control Programme (NMCP) Malawi and ICF International. Malawi malaria indicator survey 2014. Lilongwe, Malawi, and Rockville, Maryland: NMCP and ICF International, 2015. http://dhsprogram.com/pubs/pdf/MIS18/MIS18.pdf. [Google Scholar]
- 16. Betson M, Clifford S, Stanton M, Kabatereine NB, Stothard JR. Emergence of nonfalciparum Plasmodium infection despite regular artemisinin combination therapy in an 18-month longitudinal study of Ugandan children and their mothers. J Infect Dis 2018; 217:1099–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cook J, Xu W, Msellem M, et al. Mass screening and treatment on the basis of results of a Plasmodium falciparum-specific rapid diagnostic test did not reduce malaria incidence in Zanzibar. J Infect Dis 2015; 211:1476–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Taylor SM, Messina JP, Hand CC, et al. Molecular malaria epidemiology: mapping and burden estimates for the Democratic Republic of the Congo, 2007. PLoS One 2011; 6:e16420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mitchell CL, Topazian HM, Brazeau NF, et al. Household prevalence of P. falciparum, P. vivax, and P. ovale in the Democratic Republic of the Congo, 2013–2014 [published online ahead of print 26 November 2020]. Clin Infect Dis doi: 10.1093/cid/ciaa1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Akala HM, Watson OJ, Mitei KK, et al. Plasmodium interspecies interactions during a period of increasing prevalence of Plasmodium ovale in symptomatic individuals seeking treatment: an observational study. Lancet Microbe 2021; 2:e141–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yman V, Wandell G, Mutemi DD, et al. Persistent transmission of Plasmodium malariae and Plasmodium ovale species in an area of declining Plasmodium falciparum transmission in eastern Tanzania. PLoS Negl Trop Dis 2019; 13:e0007414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. National Statistical Office (NSO) Malawi and ICF. Malawi demographic and health survey 2015–16. Zomba, Malawi: and Rockville, Maryland: NSO; and ICF, 2017. http://dhsprogram.com/pubs/pdf/FR319/FR319.pdf. [Google Scholar]
- 23. Topazian HM, Gumbo A, Puerto-Meredith S, et al. Asymptomatic Plasmodium falciparum malaria prevalence among adolescents and adults in Malawi, 2015–2016. Sci Rep 2020; 10:18740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mitchell CL, Brazeau NF, Keeler C, et al. Under the radar: epidemiology of Plasmodium ovale in the Democratic Republic of the Congo. J Infect Dis 2020; 223: 1005–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Brazeau NF, Mitchell CL, Morgan AP, et al. The epidemiology of Plasmodium vivax among adults in the Democratic Republic of the Congo. Nat Commun 2021; 12: 4169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Veron V, Simon S, Carme B. Multiplex real-time PCR detection of P. falciparum, P. vivax and P. malariae in human blood samples. Exp Parasitol 2009; 121:346–51. [DOI] [PubMed] [Google Scholar]
- 27. Perandin F, Manca N, Calderaro A, et al. Development of a real-time PCR assay for detection of Plasmodium falciparum, Plasmodium vivax, and Plasmodium ovale for routine clinical diagnosis. J Clin Microbiol 2004; 42:1214–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Calderaro A, Piccolo G, Gorrini C, et al. A new real-time PCR for the detection of Plasmodium ovale wallikeri. PLoS One 2012; 7:e48033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Demographic and Health Surveys Program. GPS data collection. https://dhsprogram.com/What-We-Do/GPS-Data-Collection.cfm. Accessed 9 August 2019.
- 30. Regional Centre for Mapping of Resources for Development (RCMRD) SE and SA. Land cover maps for Malawi. http://geoportal.rcmrd.org/. Accessed 27 April 2020.
- 31. Funk C, Peterson P, Peterson S, et al. A high-resolution 1983-2016 T max climate data record based on infrared temperatures and stations by the Climate Hazard Center. J Climate 2019; 32: 5639–58. [Google Scholar]
- 32. Funk C, Peterson P, Landsfeld M, et al. The climate hazards infrared precipitation with stations—a new environmental record for monitoring extremes. Sci Data 2015; 2:1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mayala B, Fish TD, Eitelberg D, Dontamsetti T.. The DHS program geospatial covariate datasets manual. 2nd ed. Rockville, Maryland: ICF, 2018. [Google Scholar]
- 34. Cole SR, Hernán MA. Constructing inverse probability weights for marginal structural models. Am J Epidemiol 2008; 168:656–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sumner KM, Mangeni JN, Obala AA, et al. Impact of asymptomatic Plasmodium falciparum infection on the risk of subsequent symptomatic malaria in a longitudinal cohort in Kenya. Elife 2021; 10: e68812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Moody A. Rapid diagnostic tests for malaria parasites. Clin Microbiol Rev 2002; 15:66–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Twohig KA, Pfeffer DA, Baird JK, et al. Growing evidence of Plasmodium vivax across malaria-endemic Africa. PLoS Negl Trop Dis 2019; 13:e0007140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Battle KE, Lucas TCD, Nguyen M, et al. Mapping the global endemicity and clinical burden of Plasmodium vivax, 2000-17: a spatial and temporal modelling study. Lancet 2019; 394:332–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kotepui M, Kotepui KU, De Jesus Milanez G, Masangkay FR. Plasmodium spp. mixed infection leading to severe malaria: a systematic review and meta-analysis. Sci Rep 2020; 10:11068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. World Health Organization. Malawi country profile, 2018. https://www.who.int/malaria/publications/country-profiles/profile_mwi_en.pdf.
- 41. World Health Organization (WHO). World malaria report 2020: 20 years of global progress and challenges. Geneva, Switzerland: WHO, 2020. [Google Scholar]
- 42. Sitali L, Chipeta J, Miller JM, et al. Patterns of mixed Plasmodium species infections among children six years and under in selected malaria hyper-endemic communities of Zambia: population-based survey observations. BMC Infect Dis 2015; 15:204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Boyce RM, Hathaway N, Fulton T, et al. Reuse of malaria rapid diagnostic tests for amplicon deep sequencing to estimate Plasmodium falciparum transmission intensity in western Uganda. Sci Rep 2018; 8:10159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Chang HH, Wesolowski A, Sinha I, et al. Mapping imported malaria in Bangladesh using parasite genetic and human mobility data. Elife 2019; 8:e43481. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.