Abstract
A significant correlation has been shown between the binding antibody responses against original severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spike protein and vaccine efficacy of 4 approved coronavirus disease 2019 vaccines. We therefore assessed the immune response against original SARS-CoV-2 elicited by the adjuvanted S-Trimer vaccine, SCB-2019 + CpG/alum, in the same assay and laboratory. Responses to SCB-2019 were comparable or superior for antibody to original and Alpha variant when compared with 4 approved vaccines. The comparison accurately predicted success of the recently reported efficacy trial of SCB-2019 vaccine. Immunogenicity comparisons to original strain and variants of concern should be considered as a basis for authorization of vaccines.
Keywords: COVID-19, vaccine, immunogenicity, variants of concern
The antibody-binding responses to the adjuvanted S-Trimer vaccine, SCB-2019 + CpG/alum, were compared with approved vaccines and accurately predicted success of the recently reported efficacy trial of SCB-2019 vaccine. Immunogenicity comparisons should be considered as a basis for authorization of vaccines.
Intensive research efforts to develop novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccines to protect against the global coronavirus disease 2019 (COVID-19) pandemic have resulted in many different vaccines but with only a few currently approved for use. Most are based on the spike (S) protein sequence of the original wild-type SARS-CoV-2 virus isolated in Wuhan, China, or inactivated whole virus [1, 2]. Immunological results from the various vaccine candidates assessed in multiple studies by different laboratories use their own unique antibody binding or neutralization assays. Differences in these assays makes it difficult to compare and interpret the relative potencies of the various S-protein vaccines. The use of different vaccine platforms theoretically further complicates comparison among vaccines. However, despite these differences, a strong correlation of antibody responses and efficacy has been reported even when using the imperfect calibration with antibody responses of convalescent patients measured in the same assay [3, 4]. Recently Goldblatt and colleagues have overcome these limitations by comparing binding antibody responses in subjects vaccinated with 1 or 2 doses of approved vaccines measured in a single laboratory using an immunoglobulin G (IgG) enzyme-linked immunosorbent assay (ELISA) against S-protein calibrated with the World Health Organization (WHO) international standard and reported as binding antibody units (BAU)/mL. A highly statistically significant correlation was found between their calibrated geometric mean antibody concentrations and the clinical efficacies of those vaccines obtained from large-scale clinical trials [5].
Clover Biopharmaceuticals has applied its innovative S-Trimer technology to develop a subunit vaccine candidate, SCB-2019 + CpG/alum, a stabilized prefusion form of the SARS-CoV-2 S-protein adjuvanted with the Toll-like receptor agonist, CpG-1018, and alum. In a phase 1 study, SCB-2019 + CpG/alum was shown to be well tolerated and immunogenic in adults from 18 to 75 years of age [6]. Subsequent follow-up has shown persistence of the immune responses to 6 months and provided an initial demonstration of neutralizing activity against several SARS-CoV-2 variants of concern (VOCs) [7].
A major phase 2/3 trial (SPECTRA) is ongoing to assess the efficacy of SCB-2 019 + CpG/alum in preventing COVID-19 illness (EudraCT number, 2020-004272-17) and preliminary results have just recently been reported by Clover Biopharmaceuticals (see https://www.cloverbiopharma.com/news/83.html). Due to the current epidemiology in the countries where the vaccine trial is being conducted, all of the documented cases with sequence data available were VOCs and predominantly the Delta variant. Thus, it was not possible to estimate efficacy to the original strain nor compare to the efficacy against the original strain of approved vaccines. However, in this report (before the results of the SPECTRA trial were known), we compare vaccine-induced antibody responses of a subset of subjects in the SPECTRA trial to responses of subjects who were immunized with the 4 COVID-19 vaccines currently in clinical use. All of the assays were conducted in the same laboratory using the same assay calibrated to the WHO international standard.
METHODS
In Clover’s SPECTRA phase 2/3 efficacy trial approximately 30000 volunteers aged 18–75 years have been recruited in the Philippines, Colombia, Belgium, Brazil, and South Africa and randomized to receive 2 immunizations 3 weeks apart with either SCB-2019 + CpG/alum or placebo (0.9% saline). The trial is being performed in concordance with International Council for Harmonisationof Technical Requirements for Pharmaceuticals for Human Use and Good Clinical Practice guidelines and with the signed informed consent of all volunteer participants. The study protocol and amendments were approved by institutional review board and registered (EudraCT number 2020-004272-17). A subset of phase 2 participants was evaluated for B- and T-cell immune responses at 2 weeks after the second vaccination (day 36), and sera were collected from all participants for use in estimating correlates of protection. Sera at baseline were also assessed for prior exposure to SARS-CoV-2 with the Elecsys SARS-CoV-2 S ELISA kit (Roche, Basel, Switzerland). For the additional analysis reported here, an unblinded statistician randomly selected day 36 sera from participants who were seronegative at baseline, 100 from vaccinees and 20 from placebo recipients, stratified across 2 age ranges of 18–60 years and ≥60 years. The selection of 100 sera from vaccine recipients included participants from the Philippines (n=40), Colombia (n=42), Belgium (n=13), and South Africa (n=5). The samples for vaccine recipients included 90 from participants aged 18–59 years (32 aged <30 years, 35 aged 30–39 years, 23 aged 40–59 years) and 10 aged ≥60 years. Before the recently available efficacy results were known, the selected sera were tested in the Goldblatt laboratory in a blinded manner using a multiplex assay for IgG binding the S-protein and spike receptor-binding domain (RBD) of the original Wuhan strain of SARS-CoV-2 and of the Alpha variant (B.1.1.7) as previously described [5]. After the efficacy results were reported, the sera were also tested for IgG binding to S-protein of the Delta variant (B.1.617.2). Results were calibrated with the WHO international standard serum and expressed in BAU/mL. The final data from the vaccinees are presented as BAU concentrations for individual sera and as geometric mean concentrations (GMCs) for each age group; data for placebo recipients, which were all negative, are not included in this report to be consistent with the reported data for the 4 approved vaccines. The demographic characteristics of the naive vaccine cohorts for the 4 approved vaccines are detailed previously [5]. The antibody GMCs were compared by rank-sum paired tests corrected by Dunn multiple test.
RESULTS
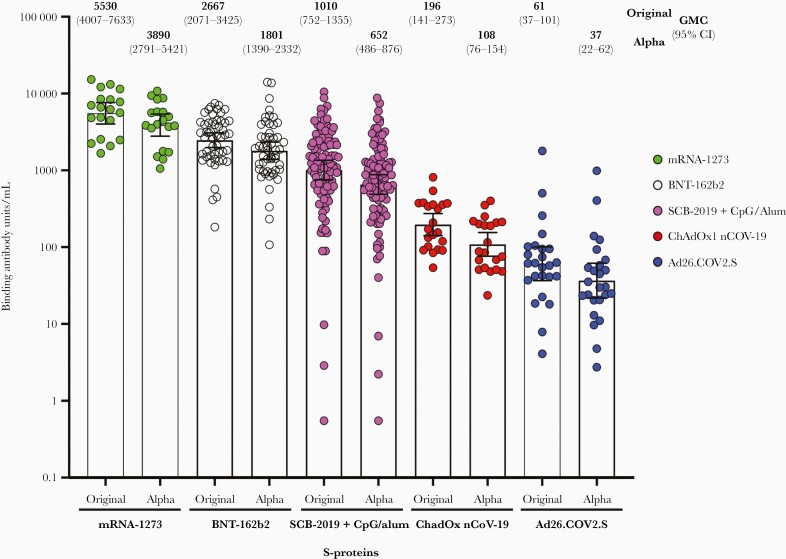
Individual results for IgG binding to S-protein from the original strain and Alpha variant expressed as BAU/mL for all 5 cohorts are shown in Figure 1. The GMC of Clover vaccine recipients following 2 doses for original spike was 1010 BAU/mL (95% confidence interval [CI], 752–1355) compared with GMCs following 2 doses for mRNA-1273 (5530 BAU/mL [95% CI, 4007–7633]), BNT162b2 (2667 BAU/mL [95% CI, 2071–3425]), and ChAdOx1 nCoV-19 (196 BAU/mL [95% CI, 141–273]) and after 1 dose of Ad.26COV2.s (61 BAU/mL [95% CI, 37–101]). The Clover vaccine recipients’ GMCs were significantly higher for both original prototype and Alpha variant viruses than ChadOx1nCoV-19 vaccine cohort (P<.0001) and Ad.26COV2.s vaccine cohort (P<.0001) and significantly lower when compared with BNT 162b2 (P=.0004) and mRNA-1273 (P<.0001). Responses to the RBD for original prototype and Alpha variant viruses showed similar results with Clover GMC significantly higher for ChadOxInCoV-19 (P=.0009 for original and P=.0013 for Alpha) and Ad.26COVV2.s cohorts (P<.0001 for both). After the recent efficacy study results were available, we also assessed antibody to the Delta variant for the Clover recipients as shown in Table 1. Of note, the younger Clover adults generally displayed higher responses against S-protein and RBD for original prototype and Alpha variant, and against S-protein for Delta variant compared with the older adults (Table 1). However, the older adults had a GMC that was still higher than the GMC for both the ChAdOx1nCoV-19 and Ad.26COV2.S for the original and Alpha variant for the entire vaccine cohorts which each contained older adults [5]. Antibody data in this assay for Delta variant for the approved vaccines are not yet available for comparison (submitted for publication and under review).
Figure 1.
Immunoglobulin G binding antibody to spike (S) protein from the original type severe acute respiratory syndrome coronavirus 2 and the Alpha variant of 100 adults vaccinated with SCB-2019 compared to the antibody responses of cohorts immunized with each of 4 approved vaccines. The geometric mean concentrations (GMCs) with 95% confidence interval (CIs) are shown above each group. Responses to SCB-2019 were significantly higher than both ChadOx1nCoV-19 and Ad26.COV2.s vaccine groups (P<.0001) and significantly lower than mRNA-1273 (P<.0001) and BNT-162b2 (P=.0009) vaccine groups for both original and Alpha variant, utilizing rank-sum unpaired tests adjusted by Dunn multiple test.
Table 1.
Age-Specific Geometric Mean Concentrations of Immunoglobulin G Binding Antibody Units Assessed by Enzyme-Linked Immunosorbent Assay Against S-Protein and Receptor-Binding Domain of the Original Prototype Severe Acute Respiratory Syndrome Coronavirus 2 and of the Alpha Variant (B.1.1.7), and Against the S-Protein of the Delta Variant (B.1.617.2) in Participants Vaccinated With SCB-2019
| Age Group | Virus and Antigen | ||||
|---|---|---|---|---|---|
| Original Prototype | Alpha Variant | Delta Variant | |||
| S-Protein | RBD | S-Protein | RBD | S-Protein | |
| 18–60 y | |||||
| No. | 90 | 90 | 90 | 90 | 90 |
| GMC, BAU/mL (95% CI) | 1097 (802–1502) | 1217 (889–1666) | 700 (511–961) | 1259 (933–1699) | 248 (187–329) |
| ≥60 y | |||||
| No. | 10 | 10 | 10 | 10 | 10 |
| GMC, BAU/mL (95% CI) | 477 (215–1057) | 430 (135–1365) | 345 (153–779) | 481 (150–1542) | 129 (55–307) |
Abbreviations: BAU, binding antibody unit; CI, confidence interval; GMC, geometric mean concentration; RBD, receptor-binding domain.
DISCUSSION
Major issues for licensure of new COVID-19 vaccines are 2-fold: first, the conduct of controlled field efficacy trials is hampered by the fact that placebo control is at this stage deemed unethical by most ethical review boards while the comparison of a new candidate to an approved vaccine is operationally problematic because of the limited access of approved COVID-19 vaccines for clinical trials. Second, the problem of assessing clinical efficacy against an evolving background of disease incidence due to new variants replacing the original prototype Wuhan-Hu-1 virus as has been documented by the recent results for Clover vaccine efficacy trial. In that trial, all cases were due to VOCs with Delta predominating. At the time of this report, COVID-19 in most of the world is caused predominantly by the Delta variant (B.1.617.2) with less caused by the Alpha (B.1.1.7), Beta (B.1.351), Gamma (P.1), Mu (B.1.621), and other variants. Many of the variants have reduced sensitivity to antibody compared with original and Alpha, and currently approved vaccines are showing lower efficacy/effectiveness against VOCs than original virus [8–10]. As it is no longer feasible to evaluate or compare clinical efficacy of new vaccines to approved vaccines against the original strain, it has become critically important to compare new vaccines to the approved vaccines based on immunogenicity to the original strain and VOCs rather than clinical efficacy. As noted, our group previously reported that binding antibody was correlated with efficacy and also highly correlated with neutralization antibody. We and others also suggest that binding antibody comparisons may be a superior choice due to technical simplicity, lower variability, easier standardization, and ability to assess neutralizing as well nonneutralizing antibody activities [4, 11].
For the 4 approved vaccines compared in this report, efficacies were 94.1% (95% CI, 89.3%–96.8%) [12], 94.6% (95% CI, 89.9%–97.3%) [13], and 80.7% (95% CI, 66.5%–88.9%) [14] against symptomatic disease of original virus after 2 doses for mRNA-1273, BNT162b2, and ChAdOx1 nCoV-19, respectively, and 72.0% (95% CI, 58.2%–81.7%) after 1 dose of Ad26.COV2.s [15]. The binding antibody response for SCB-2019 + CpG/alum to the original virus predicted an efficacy rate in a range of 81%–94% for original prototype virus. Furthermore, GMCs of binding antibody to Alpha variant compared favorably with the approved vaccine responses, and thus acceptable efficacy against Alpha variant was predicted within the ongoing trial by Clover.
However, after our data were submitted for this publication, it was reported that other VOCs with lower levels of cross-reactivity were predominant in this efficacy trial. In fact, no cases of original virus and only 1 case of Alpha variant were noted, with the majority of cases due to Delta variant. In this report, we demonstrate that the concentration of antibody directed to the spike protein of 1 VOC (Alpha variant) is consistent among vaccines based on the response to original spike (Figure 1) and propose this will be true for all variants including Delta. Thus, the favorable antibody response to SCB-2019 vaccine to original and Alpha variant, compared with approved vaccines, predicted comparable efficacy to those vaccines for Delta variant. In fact, the success of the trial was accurately predicted by the immunogenicity data in this report, with efficacy of 78.9% for the Delta variant documented, which is comparable to the effectiveness against Delta variant now being reported for the approved vaccines [9, 10].
We therefore suggest that immunogenicity comparisons need to be considered for authorization of new vaccines because the predominance of VOCs in ongoing trials will make current efficacy guidelines based on the original strain challenging or impossible to achieve. At least 1 regulatory authority has recently reported that authorization based solely on immunogenicity comparisons with an active comparator vaccine group will be acceptable (https://valneva.com/press-release/valneva-reports-positive-phase-3-results-for-inactivated-adjuvanted-covid-19-vaccine-candidate-vla2001/). We also propose an alternative approach to conduct such comparisons that would require cooperation among multiple stakeholders: namely, to assemble adequately sized banks of postimmunization sera from licensed vaccines to support direct comparisons with responses to new vaccines for noninferiority. The comparisons could be done in an independent blinded manner against the original strain and VOCs. We suggest that binding antibody may be best suited for these comparisons provided that strong correlations between binding and neutralization for original strain and VOCs have been established. Utilizing an established serum bank would avoid the requirement of securing comparator vaccines by all developers in many different locations and avoid lengthy head-to-head noninferiority trials, which would delay timely availability of much-needed safe and effective additional vaccines.
CONCLUSIONS
The adjuvanted SCB-2019 COVID-19 vaccine candidate demonstrates comparable or superior immunogenicity for both original and Alpha variant to 4 approved vaccines. The comparable immunogenicity responses accurately predicted success in the recently reported efficacy study by Clover against VOCs. Thus, comparable immunogenicity data to original virus and variants between new vaccines and approved vaccines should be considered for approval when conducted in a robust independent manner utilizing standardized appropriate assays, preferably within the same laboratory.
Notes
Author contributions. All authors contributed to the design of the study, writing of the study, and revision of the manuscript. Additionally, D. G., was responsible for sample collection for the 4 approved vaccine cohorts; H. H. H., B. H., and J. L. were responsible for design and collection of samples for SCB-2019 sera; and M. J. was responsible for laboratory evaluation of samples.
Acknowledgments. We acknowledge statistical advice from Andrew Fiore-Gartland and writing assistance from Keith Veitch.
Financial support. This study was partly funded by Clover Biopharmaceuticals and the Coalition for Epidemic Preparedness Innovations (grant numbers RRCL2001 and RCL2202). D. G. receives partial salary support from the National Institute for Health Research Great Ormond Street Biomedical Research Centre.
Potential conflicts of interest. G. S. reports personal fees and other from Clover Biopharmaceuticals; other from Vaxxinity; personal fees from AdVaccine, CanSino, CureVac, Valneva, and Vaxart; personal fees and other from Affinivax; fees and other from Everest Medicines; and fees from Senda, outside the submitted work. D. A. reports personal fees from Vaxxinity, personal fees and other from Clover Biopharmaceuticals, fees and other from Everest Medicines, and fees from Senda, outside the submitted work. R. C. reports personal fees from Clover Biopharmaceuticals and personal fees from CureVac, Valneva, and Icosavax, outside the submitted work. H. H. H., B. H., and J. L. are all employees of Clover Biopharmaceuticals. D.G. and M.J. report no potential conflicts of interest.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Huang Y, Yang C, Xu XF, Xu W, Liu SW.. Structural and functional properties of SARS-CoV-2 spike protein: potential antivirus drug development for COVID-19. Acta Pharmacol Sin 2020; 41:1141–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wu F, Zhao S, Yu B, et al. A new coronavirus associated with human respiratory disease in China. Nature 2020; 579:265–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Khoury DS, Cromer D, Reynaldi A, et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med 2021; 27:1205–11. [DOI] [PubMed] [Google Scholar]
- 4. Earle KA, Ambrosino DM, Fiore-Gartland A, et al. Evidence for antibody as a protective correlate for COVID-19 vaccines. Vaccine 2021; 39:4423–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Goldblatt D, Fiore-Gartland A, Johnson M, et al. A population-based threshold of protection for COVID-19 vaccines. Research Square [Preprint]. Posted online 31 August 2021. doi: 10.21203/rs.3.rs-832531/v1. [DOI] [Google Scholar]
- 6. Richmond P, Hatchuel L, Dong M, et al. Safety and immunogenicity of S-Trimer (SCB-2019), a protein subunit vaccine candidate for COVID-19 in healthy adults: a phase 1, randomised, double-blind, placebo-controlled trial. Lancet 2021; 397:682–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Richmond P, Hatchuel L, Pacciarini F, et al. Persistence of the immune responses and cross-neutralizing activity with variants of concern following two doses of adjuvanted SCB-2019 COVID-19 vaccine. J Infect Dis 2021; 224:1699–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Uriu K, Kimura I, Shirakawa K, et al. Ineffective neutralization of the SARS-CoV-2 Mu variant by convalescent and vaccine sera. bioRxiv [Preprint]. Posted online ahead of print 7 September 2021. doi: 10.1101/2021.09.06.459005. [DOI] [Google Scholar]
- 9. Lopez Bernal J, Andrews N, Gower C, et al. Effectiveness of Covid-19 vaccines against the B.1.617.2 (Delta) variant. N Engl J Med 2021; 385:585–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sheikh A, McMenamin J, Taylor B, Robertson C; Public Health Scotland and the EAVE II Collaborators. SARS-CoV-2 Delta VOC in Scotland: demographics, risk of hospital admission, and vaccine effectiveness. Lancet 2021; 397:2461–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Carreño J, Alshammary H, Singh G, et al. Reduced neutralizing activity of post-SARS-CoV-2 vaccination serum against variants B.1.617.2, B.1.351, B.1.1.7+E484K and a sub-variant of C.37. medRxiv [Preprint]. Posted online 23 July 2021. doi: 10.1101/2021.07.21.21260961. [DOI] [Google Scholar]
- 12. Baden LR, El Sahly HM, Essink B, et al. ; COVE Study Group. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med 2021; 384:403–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Polack FP, Thomas SJ, Kitchin N, et al. ; C4591001 Clinical Trial Group. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med 2020; 383:2603–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Voysey M, Costa Clemens SA, Madhi SA, et al. ; Oxford COVID Vaccine Trial Group. Single-dose administration and the influence of the timing of the booster dose on immunogenicity and efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine: a pooled analysis of four randomised trials. Lancet 2021; 397:881–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sadoff J, Gray G, Vandebosch A, et al. ; ENSEMBLE Study Group. Safety and efficacy of single-dose Ad26.COV2.S vaccine against Covid-19. N Engl J Med 2021; 384:2187–201. [DOI] [PMC free article] [PubMed] [Google Scholar]