Abstract
The oncological necessity of submandibular gland removal during neck dissection for oral cavity squamous cell carcinoma surgery has remained controversial. This study was aimed to determine the rate of SMG involvement and assess the feasibility of submandibular gland (SMG) preservation. We present a prospective study conducted at a tertiary cancer center from June 2017 to May 2019. All patients of oral squamous cell carcinoma who underwent primary surgery with neck dissection were included and analyzed for incidence and predictive factors for incidence of level IB nodal and SMG involvement as per CAP guidelines. A total of 60 patients were inducted in the study, wherein 63 neck dissections were performed including bilateral dissection in three cases. There was involvement of SMG in 6 patients with two cases each in floor of mouth cancer, gingivo-buccal, and alveolar lesions. The SMG was involved by direct contiguous spread from the primary lesion in two cases, extra-capsular extension from level IB lymph nodes in one and by both mode of spread in three glands. Perineural invasion was seen in 83.33% (n = 5) patients with SMG involvement (p- < 0.001), while 66.67% (4/6) patients had lympho vascular invasion (p-0.006) and all the cases with SMG involvement had extra-capsular extension (p < 0.001), suggesting PNI, LVI, and ECE as the strongest predictors of SMG involvement. This study demonstrates that oral cavity squamous cell carcinoma has low potential to metastasize to the SMG; however, high-risk factors include primary tumor site in floor of mouth or tongue, heavy level IB nodal burden, presence of LVI, PNI, and ECE. In the absence of these high-risk factors, SMG preservation with complete nodal clearance in level IB is a promising technique for reducing future complications.
Keywords: Neck dissection, Submandibular gland involvement, Submandibular gland preservation
Introduction
Squamous cell carcinoma (SCC) of oral cavity most commonly metastasizes to cervical lymph nodes. Formal neck dissection (ND) either therapeutic or prophylactic along with composite resection of primary lesion is the mainstay surgical procedure for management of oral cancers. With neck dissection we can achieve loco-regional oncologic control as well as it allows for surgical staging of neck in order to determine need for adjuvant therapy. The submandibular gland (SMG) is located in level IB region of neck in close proximity to level IB nodes. Level IB group includes lymph nodes in pre- and post-glandular region, pre- and post-vascular regions, and intra-glandular lymph nodes. In addition, there may be other perifacial lymph nodes in close proximity to the SMG [1]. In view of close proximity of SMG to adjacent level 1B lymph nodes, traditionally ND has always included excision of SMG along with level IB nodal clearance in order to have complete resection of disease and more so for technical ease of nodal clearance. The oncological necessity of SMG removal during neck dissection however remains controversial.
The SMGs, along with the minor and parotid salivary glands, secrete saliva which facilitates deglutition and gustation and helps to maintain oral health. Both submandibular glands are responsible for 65% of total unstimulated saliva production [2, 3]. Resection of either one or both SMG may result in reduced salivary outflow leading to development of xerostomia. Jacob et al. reported post-operative xerostomia in one-third patients undergoing ND with concomitant SMG excision [4]. Dünne et al. reported incidence of xerostomia in 21% patients undergoing ND with unilateral excision of SMG compared to only 7% in patients with preservation of SMG [5]. Post-operative xerostomia may result in sleep disturbances, gingival irritation, dental decay, impairment of taste, mastication, and swallowing [6–9]. There is also risk of injury to nearby structures in some cases including the marginal mandibular nerve (7.7%), hypoglossal nerve (2.9%), and lingual nerve (1.4%) [10]. Excision of SMG also leads to external contour deformity in upper neck.
In several studies, anatomic dissection did not find any lymph nodes within the parenchyma of SMG [11]. In addition, there is very low risk of unpredicted SMG invasion by adjacent pathologic lymph nodes in patients with primary oral cavity SCC [12, 13]. The reason for such low rate of involvement has been attributed to the presence of a fibrous capsule around the gland and paucity of lymphatics and vasculature within the gland [13–15]. Dhiwakar et al. demonstrated that level IB nodal clearance was possible without excision of SMG as direct invasion was unlikely and there were no parenchymal LN within the gland [16]. In light of a new trend towards more conservative and individually tailored neck dissection, where non-lymphatic structures are being conserved in an oncologically safe way, preservation of SMG in ND may be a viable option and hence it is imperative to determine the actual incidence of SMG involvement in oral cavity SCC.
Methods
We prospectively analyzed data of 112 patients of head and neck cancers treated at a tertiary cancer center from June 2017 to May 2019. Out of these, 88 patients had oral cavity SCC, where in 60 patients underwent primary surgery for cancer while 28 patients had surgery for recurrent cancer. We present results of clinic-radiological and histopathological correlation in 60 patients undergoing neck dissection for primary oral cancer.
Patient demographics studied included age and gender. Tumor characteristics evaluated included tumor site, tumor size (T), neck nodes (N), and preoperative tumor grade and histology. Along with clinical examination, radiological evaluation with either contrast-enhanced computerized tomography (CECT) or magnetic resonance imaging (MRI) depending on the site of tumor was undertaken. All patients underwent primary tumor excision for oral cavity lesions (composite oral resection) with simultaneous neck dissections; levels I–III or more depending on presence and location of suspicious lymph nodes in neck. Patients undergoing surgery for recurrent oral cavity SCC with history of prior neck dissection or irradiation and patients with metastatic disease were excluded in our study.
The extent of neck dissection performed (ipsilateral/bilateral), number of neck nodes resected, number of positive neck nodes on histopathological examination (HPE), levels of neck nodes involved, SMG involvement, pattern of SMG involvement, presence of lympho vascular invasion (LVI), perineural invasion (PNI), or extra-capsular extension (ECE) were recorded in final histopathology. The final histopathology was reported as per College of American Pathologists (CAP) guidelines. The data of preoperative parameters (clinic-radiological staging) and final histopathology reports were interpreted to find the incidence of level IB and SMG involvement in oral cavity SCC. The clinical and pathological staging of oral cavity cancer was followed as per 7th AJCC manual.
The data’s were summarized using frequencies and/or mean ± sd.
Quantitative variables are expressed as mean ± sd and compared between groups using Mann–Whitney test. Qualitative variables are expressed as frequencies/percentages and analyzed using Fisher’s exact test. A p-value < 0.05 is considered statistically significant. The data analysis performed using “R” programming language.
Results
We present data of 60 patients who underwent neck dissection as part of surgical management of oral cancer over 2 years. A total of 63 neck dissections (ND) were performed including bilateral ND in three cases. The mean age of our study population was 53 years (range 30–78 years) with 51 males (85%) and 9 females (15%). Among these 60 patients, 24 patients (40%) had carcinoma tongue, followed by carcinoma alveolus in 10 (16.67%), carcinoma retromolar trigone (RMT) in 9 (15%), carcinoma gingivobuccal sulcus (GBS) in 7 (11.67%), carcinoma floor of mouth (FOM) in 6 (10%), and carcinoma lip and hard palate in 2 (3.33%) cases each. Most of the patients had grade I and II SCC with 28 (46.67%) cases in each group while 4 cases (6.67%) had grade III tumors. Nearly half of the patients had T2 tumors (31/60, 51.67%), followed by T1 and T4 stage in 14 patients each (23.33%) and T3 (1/60, 1.67%). Fifty-three percent patients had clinically N0 disease, 25% had N1, and 22% had N2 disease. There were no patients with N3 disease in our series (Table 1).
Table 1.
Patient demographics
In the 63 neck dissections performed including bilateral dissection in three cases, level IB node was involved in 31.67% cases (n = 19), followed by level II in 23.33% (n = 14), level III and IV in 11.67% (n = 7) each, and level V in 5.0% (n = 3). The frequency of level IB lymph node metastasis by primary site was 60% (6/10) for carcinoma alveolus, 42.86% (3/7) for GBS lesions, 57.14% (4/7) for FOM lesions, 22.2% (2/9) for RMT tumors, 12.5% (3/21) for carcinoma tongue, and half of the patients with carcinoma lip. There was no nodal involvement in hard palate lesions. There was higher incidence of level IB LN involvement in T4 stage lesions 64.3% (9/14) followed by T2 stage lesions 29% (9/31), T1 lesions 5.26% (1/14), and no involvement in T3 stage lesions. Similarly, the involvement of level IB LNs in N0, N1, and N2 was 9.3% (3/32), 26.6% (4/15), and 92.3% (12/13) respectively. The average level IB nodal burden was calculated as follows: Number of positive level IB nodes / Total positive nodes. The nodal burden in NI and N2 disease was determined to be 78.5% (11/14) and 40% (28/70) respectively (Table 2).
Table 2.
Level IB nodal involvement
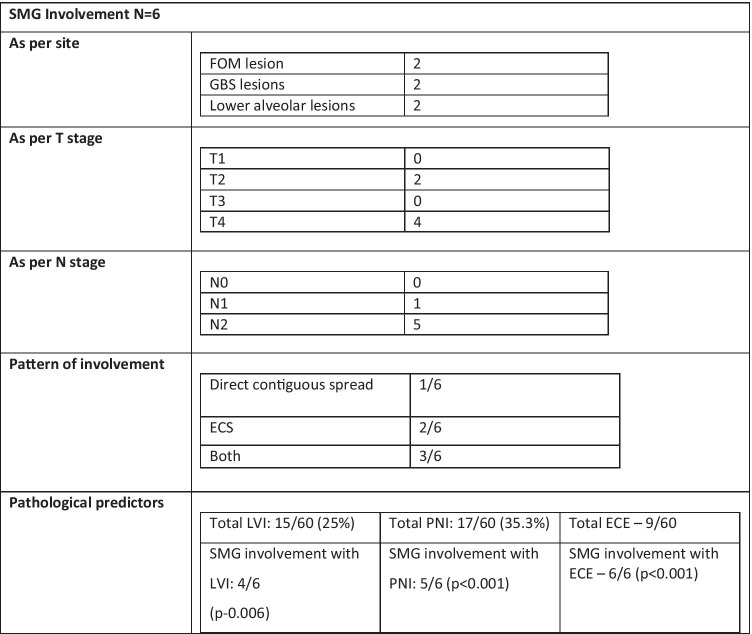
There was involvement of SMG in 6 patients only with two cases each in FOM lesion, GBS lesions, and lower alveolus lesions. Out of the six SMG cases with involvement three cases had grade II lesion, one had grade III lesion while two cases had grade I lesion (p = NS). There was no SMG involvement in T1 and T3 lesions while 2/31 in T2 and 4/14 in T4 lesions had involvement of SMG. One patient out of six in N1 staged and five out of six in N2 staged patients showed SMG involvement (P < 0.001) (Table 3).
Table 3.
Submandibular gland involvement
In our study, out of 6 cases of SMG involvement, two had direct contiguous spread from the primary lesion, while one showed extracapsular extension from level IB lymph nodes and glandular involvement by both modes in three glands. There was no evidence of intraglandular metastasis in any of the positive SMG cases. Five cases (83.33%) had PNI (p < 0.001), four patients (66.67%) had LVI (p = 0.006), and all the six cases had ECE (p < 0.001), suggesting that PNI, LVI, and ECE were strong predictors of SMG involvement.
Discussion
The involvement of SMG can be either by direct spread from primary oral cavity tumors or from regional lymph node involvement. Hematogenous spread to SMG from distant primary cancers, e.g., genitourinary malignancy or lung cancer is however rare [1, 17–19]. Although level I and level II lymph nodes are commonly involved in oral cavity cancers, metastasis to SMG parenchyma is uncommon [1, 13, 20]. Risk factors for SMG involvement include location of primary tumor in tongue or floor of mouth, extensive nodal burden in level IB, and presence of LVI, PNI, or ECE in level IB lymph nodes [5]. In a study by Subramaniam et al., high risk of peri-glandular lymphadenopathy was seen in patients with tumor depth of invasion (DOI) more than 10 mm. In addition, presence of LVI or PNI, moderate or poorly differentiated tumor was associated with a higher incidence of SMG involvement [21].
In our study, median patient age was 53 years. Eighty-five percent patients were males and rest were females. There is no correlation between age and gender of patients with SMG involvement. In a review of 157 patients by N.K. Panda et al., median age was 49 years. Thirty-four patients were females and 141 were males. They did not find any correlation between age or patient gender with level IB LN metastasis and SMG involvement [22].
In our study, carcinoma tongue was the most common site of primary tumor (40%). Level IB lymph node metastasis was seen in 19 cases and distribution by primary site was 60% (6/10) for alveolus lesions, 42.86% (3/7) for GBS, 57.14% (4/7) for FOM, 22.22% (2/9) for RMT, 12.5% (3/24) for tongue, 50% (1/2) for lip, and no involvement in hard palate (0/2). There was involvement of SMG in total six patients in our study with two cases each with FOM, GBS, and alveolar lesions.
Ali Razfar et al. in their study of 261 patients had carcinoma tongue as the most common site of involvement at 43.9% followed by FOM at 27.3%. They reported the incidence of level IB metastasis by primary site as 20% (2/10) for the hard palate, 18.2% (10/52) for FOM, 11.87% (2/17) for the alveolus, 8.6% (7/81) for the RMT, and 7.1% (1/14) for buccal mucosa lesions (BM). They had only one SMG involvement, which was directly involved by FOM lesion [23].
In the study by Panda et al., carcinoma tongue was the most common tumor site in 56 patients (36%) cases, followed by 36 (23%) and 33 (22%) cases of carcinoma buccal mucosa and alveolus respectively. Other less common sites were of FOM, RMT, lip, and hard palate lesions. They showed SMG involved in 6 out of 163 cases (3.68%); wherein, four were directly involved by FOM lesions tumor, one by carcinoma tongue, and one by the buccal mucosa cancer [22].
Okoturo et al. in their study of 194 patients demonstrated carcinoma buccal mucosa as the most common tumor in 36.6% (71/194) followed by carcinoma tongue in 26.3%. There was highest incidence of level IB nodal involvement with FOM lesions in 50% cases followed by GBS lesions in 42.9% cases. Only three cases had direct involvement of SMG from primary site tumor (two from carcinoma tongue and one from lower alveolus lesion), one being involved in T2 lesion and rest of two were involved in T4 lesions. The rate of SMG involvement in T1–2 lesion was only 8%, while 70% cases in T3–4 lesion had such involvement [24].
Although histological grade has been a prognostic factor in oral cavity SCC, there is no data for correlation between tumor grade and SMG involvement. In our study population, grade I and II tumors were equally distributed with 46.67% cases each while grade III was seen in 6.67% cases only. Out of six cases with SMG involvement, three cases had grade II tumors, two had grade I, and one had grade III tumor. Tumor grade was not a predictor for SMG involvement. In our study, T2 was the most common stage with 51.67% cases, followed by T4 (23.33%), T1 (23.33%), and T3 (1.67%). There was higher incidence of level IB involvement in T4 stage lesions 64.3% (9/14) followed by T2 stage lesions 29% (9/31), T1 lesions 7.14% (1/14), and no involvement in T3 stage lesions (0/1). Out of 60 patients, no patients with T1 and T3 stage lesions had submandibular gland involvement, while 2/31 cases with T2 lesions and 4/14 T4 staged lesions showed submandibular gland involvement.
Our study had 53% cases staged as clinical N0, 25% as N1, and 22% as N2 disease. It is noteworthy to mention that only in 17% cases nodal involvement was detected clinically while radiological imaging helped to detect in 30% cases. But on final HPE, the rate of nodal involvement was higher with distribution as follows: level IB—31.67%, level II—23.33%, level III—11.67%, level IV—11.67%, and level V—5.0%. The involvement of level IB in clinical N0, N1, and N2 was 9.3% (3/32), 26.6% (4/15), and 92.3% (12/13) respectively. Out of six cases with SMG involvement, one was in N1 stage and five in N2 staged patient which was statistically significant (p < 0.001). The average number of level IB nodes involved in NI and N2 is 78.5% (11/14) and 40% (28/70) respectively, based on final HPE reports (positive level IB nodes/total positive nodes). This explains high level IB nodal burden in N1 and N2 disease as a contributing factor for SMG gland involvement.
Ali Razfar and coworkers in their study reported the involvement of level IB in T1–2 lesions in 9.3% cases (13/140) and in T3–T4 lesions as 13/186 (15.1%). Only one SMG was involved by direct infiltration from FOM T2 lesion and had N2 nodal disease on final pathology. The incidence of level IB metastasis as per N stage was N0 12/167 (6.7%), N1 6/27 (22.2%), and N2 or greater 8/18(44.4%) [23].
Yao Yu et al. showed distribution of clinical N stage as N0–N2a 25 (35%), N2b–N3 46 (65%).The involvement of level IB in N0–N2a was only 5.6%, and N2b–N3 with 34%. The involvement of SMG as per nodal stage N0, N1, N2a, N2b, N2c, and N3 was 18%, 0%, 0%, 25%, 56%, and 30% respectively. Our study results were comparable to above study results with higher incidence of level IB and SMG involvement N2 stage disease [25].
There is wide variation in rate of SMG involvement in previous studies ranging from 0.395 to 18% [23, 25]. In a systematic review of 2126 patient from 12 studies, the pooled involvement of SMG was only 1.9% with direct spread from primary site or ECE from LNs. The involvement of intraglandular nodes carcinoma going along the Wharton’s duct was seen only in 0.1% cases [26]. Hence, our results are comparable with above studies suggesting that SMG could be preserved whenever it was unlikely to have direct spread on clinical and radiological assessment. In another systematic review by Dundar Y et al., 2306 patients were analyzed with evaluation of 2792 SMG for metastatic involvement. Overall only 2% cases had SMG involvement. Seventy-four percent cases had direct invasion from primary tumor and 17.2% from adjacent LNs. Only 0.1% cases had intragandular hematogenous metastasis [27]. Several studies have suggested different incidence and modes of SMG involvement in literature [11, 13, 22–24, 28–31] (Table 4).
Table 4.
Distribution and patterns of SMG involvement
| Authors | Total cases | SMG involved | Direct spread | ECS from IB | Metastasis |
|---|---|---|---|---|---|
| Spigel [13] | 196 | 9 | 6 | 3 | 0 |
| Chen [28] | 383 | 7 | 5 | 1 | 1 |
| Razfar [23] | 253 | 1 | 1 | 0 | 0 |
| Byeon [11] | 316 | 2 | 2 | 0 | 0 |
| Basaran [30] | 294 | 13 | 8 | 4 | 1 |
| Okoturo [24] | 229 | 3 | 3 | 0 | 0 |
| Kruse [31] | 171 | 6 | 5 | 1 | 0 |
| Naidu [29] | 69 | 2 | 2 | 0 | 0 |
| N.K.Panda [22] | 163 | 6 | 5 | 2 | 0 |
| Present study | 60 | 6 | 2 | 1 | 0 |
aThree cases had both direct and ECS from IB lymph node
In our study, level IB nodes were positive in 19 cases, out of which 15 cases showed no involvement of submandibular gland in spite of positive level IB nodes. This explains that mere presence of positive level IB nodes does not mean involvement of SMG requiring excision during neck dissection. Similar results were shown by Agarwal et al [14], where level IB were positive in 27 cases (n = 112) but none had SMG involvement.
In several studies, presence of PNI and LVI is considered to be predictors of SMG involvement. In our study, presence of PNI, LVI, and ECE is found to be statistically significant predictor of SMG involvement. Our results are comparable with those of Yao Yu et al. In their study, 18 patients had positive PNI with SMG involvement in nearly half of the cases (p = 0.00032) while ECE was present in 60% patients with SMG involvement. Eighty-percent patients (14/18) had level IB nodal involvement.
Currently, many studies provide promising evidence favoring SMG preservation in neck dissection for oral cancer with the exception of primary tumor in FOM or tongue. Lanzer et al. suggested that in patients with cancer of FOM or tongue, SMG preservation was not desirable due to possibility of possibility of direct invasion of SMG [32].
Although ours was a prospective study, but data were analyzed retrospectively after removal of SMG gland and hence were liable for selection bias. The small sample size and single center study were the other limitations of our study. Hence, more prospective, large-scale studies are needed to evaluate the oncological safety of such procedure.
Conclusion
This study demonstrates that although squamous cell carcinoma of oral cavity had low potential to metastasize to SMG, high-risk factors for SMG involvement included site of primary tumor, higher nodal burden in level IB, presence of LVI and PNI, and presence of ECE in neck nodes. In the absence of these high-risk factors, SMG preservation with complete nodal clearance in level IB is a promising technique for reducing future complications in patients undergoing neck dissection wherever feasible. Decision to excise the SMG may be done during surgery depending on intraoperative findings and frozen section if necessary. If there was no need to expose large oral cavity tumors through the submandibular triangle, or when there was no direct extension of primary tumor into neck or direct involvement of SMG by regional lymph nodes in, it may be safe to preserve the submandibular gland. Patients undergoing neck dissection with preservation of submandibular gland may need more intensive follow-up to ensure oncological safety of the procedure.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.DiNardo LJ. Lymphatics of the submandibular space: an anatomic, clinical, and pathologic study with applications to floor-of-mouth carcinoma. Laryngoscope. 1998;108(2):206–214. doi: 10.1097/00005537-199802000-00009. [DOI] [PubMed] [Google Scholar]
- 2.Dawes C. Factors influencing salivary flow rate and composition. In: Edgar WE, O’Mullane DM, editors. Saliva and oral health. 2. London: Brithsh Dental Association; 1996. pp. 27–41. [Google Scholar]
- 3.Scheyer LH. Source of resting total mixed saliva of man. J Appl Physiol. 1956;9:79–81. doi: 10.1152/jappl.1956.9.1.79. [DOI] [PubMed] [Google Scholar]
- 4.Jacob RF, Weber RS, King GE. Whole salivary flow rates following submandibular gland resection. Head Neck. 1996;18(3):242–247. doi: 10.1002/(SICI)1097-0347. [DOI] [PubMed] [Google Scholar]
- 5.Dünne AA, Folz BJ, Kuropkat C, et al. Extent of surgical intervention in case of N0 neck in head and neck cancer patients: an analysis of data collection of 39 hospitals. Eur Arch Otorhinolaryngol. 2004;261:295–303. doi: 10.1007/s00405-003-0680-1. [DOI] [PubMed] [Google Scholar]
- 6.Jansma J, Vissink A, Spijkervet FK, Roodenburg JL, Panders AK, Vermey A, Szabó BG, Johannes’s-Gravenmade E. Protocol for the prevention and treatment of oral sequelae resulting from head and neck radiation therapy. Cancer. 1992;70(8):2171–80. doi: 10.1002/1097-0142(19921015)70:8<2171::AID-CNCR2820700827>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 7.Mossman K, Shatzman A, Chencharick J. Long-term effects of radiotherapy on taste and salivary function in man. Int J Radiat Oncol Biol Phys. 1982;8(6):991–7. doi: 10.1016/0360-3016(82)90166-3. [DOI] [PubMed] [Google Scholar]
- 8.Fox PC, van der Ven PF, Sonies BC, Weiffenbach JM, Baum BJ. Xerostomia: evaluation of a symptom with increasing significance. J Am Dent Assoc. 1985;110(4):519–25. doi: 10.14219/jada.archive.1985.0384. [DOI] [PubMed] [Google Scholar]
- 9.Frank RM, Herdly J, Philippe E. Acquired dental defects and salivary gland lesions after irradiation for carcinoma. J Am Dent Assoc. 1965;70:868–83. doi: 10.14219/jada.archive.1965.0220. [DOI] [PubMed] [Google Scholar]
- 10.Berini-Aytes L, Gay-Escoda C. Morbidity associated with removal of the submandibular gland. J Craniomaxillofac Surg. 1992;20(5):216–219. doi: 10.1016/s1010-5182(05)80318-x. [DOI] [PubMed] [Google Scholar]
- 11.Byeon HK, Lim YC, Koo BS, Choi EC. Metastasis to the submandibular gland in oral cavity squamous cell carcinomas: pathologic analysis. Acta Otolaryngol. 2009;129(1):96–100. doi: 10.1080/00016480802032801. [DOI] [PubMed] [Google Scholar]
- 12.Ebrahim AK, Loock JW, Afrogheh A, Hille J. Is it oncologically safe to leave the ipsilateral submandibular gland during neck dissection for head and neck squamous cell carcinoma? J Laryngol Otol. 2011;125(8):837–840. doi: 10.1017/S0022215111001095. [DOI] [PubMed] [Google Scholar]
- 13.Spiegel JH, Brys AK, Bhakti A, Singer MI. Metastasis to the submandibular gland in head and neck carcinomas. Head Neck. 2004;26(12):1064–1068. doi: 10.1002/hed.20109. [DOI] [PubMed] [Google Scholar]
- 14.Agarwal G, Nagpure PS, Chavan SS. Questionable necessity for removing submandibular gland in neck dissection in squamous cell carcinoma of oral cavity. Indian J Otolaryngol Head Neck Surg. 2016;68(3):314–316. doi: 10.1007/s12070-016-0966-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kruse A, Grätz KW. Evaluation of metastases in the submandibular gland in head and neck malignancy. J Craniofac Surg. 2009;20(6):2024–2027. doi: 10.1097/SCS.0b013e3181be87a3. [DOI] [PubMed] [Google Scholar]
- 16.Dhiwakar M, Ronen O, Malone J, Rao K, Bell S, Phillips R, Shevlin B, Robbins KT. Feasibility of submandibular gland preservation in neck dissection: a prospective anatomic-pathologic study. Head Neck. 2011;33(5):603–609. doi: 10.1002/hed.21499. [DOI] [PubMed] [Google Scholar]
- 17.Cain AJ, Goodlad J, Denholm SW. Metachronous bilateral submandibular gland metastases from carcinoma of the breast. J Laryngol Otol. 2001;115(8):683–684. doi: 10.1258/0022215011908649. [DOI] [PubMed] [Google Scholar]
- 18.Moudouni SM, Tligui M, Doublet JD, Haab F, Gattegno B, Thibault P. Late metastasis of renal cell carcinoma to the submaxillary gland 10 years after radical nephrectomy. Int J Urol. 2006;13(4):431–432. doi: 10.1111/j.1442-2042.2006.01318.x. [DOI] [PubMed] [Google Scholar]
- 19.Vessecchia G, Di Palma S, Giardini R. Submandibular gland metastasis of breast carcinoma: a case report and review of the literature. Vichows Archiv A Pathol Anat. 1995;427:349–351. doi: 10.1007/BF00203404. [DOI] [PubMed] [Google Scholar]
- 20.Lim YC, Kim JW, Koh YW, Kim K, Kim HJ, Kim KM, Choi EC. Perivascular–submandibular lymph node metastasis in squamous cell carcinoma of the tongue and floor of mouth. Eur J Surg Oncol (EJSO) 2004;30(6):692–698. doi: 10.1016/j.ejso.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 21.Subramaniam N, Balasubramanian D, Reddy R, Rathod P, Murthy S, Vidhyadharan S, Thankappan K, Iyer S. Determinants of level Ib involvement in oral squamous cell carcinoma and implications for submandibular gland-sparing neck dissection. Int J Oral Maxillofac Surg. 2018;47(12):1507–1510. doi: 10.1016/j.ijom.2017.11.019. [DOI] [PubMed] [Google Scholar]
- 22.Panda NK, Patro SK, Bakshi J, Verma RK, Das A, Chatterjee D. Metastasis to submandibular glands in oral cavity cancers: can we preserve the gland safely? Auris Nasus Larynx. 2015;42(4):322–325. doi: 10.1016/j.anl.2015.02.006. [DOI] [PubMed] [Google Scholar]
- 23.Razfar A, Walvekar RR, Melkane A, Johnson JT, Myers EN. Incidence and patterns of regional metastasis in early oral squamous cell cancers: feasibility of submandibular gland preservation. Head Neck. 2009;31:1619–1623. doi: 10.1002/hed.21129. [DOI] [PubMed] [Google Scholar]
- 24.Okoturo EM, Trivedi NP, Kekatpure V, Gangoli A, Shetkar GS, Mohan M, Kuriakose MA. A retrospective evaluation of submandibular gland involvement in oral cavity cancers: a case for gland preservation. Int J Oral Maxillofac Surg. 2012;41(11):1383–1386. doi: 10.1016/j.ijom.2012.07.016. [DOI] [PubMed] [Google Scholar]
- 25.Yu Y, Daly ME, Farwell DG, Luu Q, Gandour-Edwards R, Donald PJ, Chen AM. Level IB nodal involvement in oropharyngeal carcinoma: implications for submandibular gland-sparing intensity-modulated radiotherapy. Laryngoscope. 2015;125(3):608–14. doi: 10.1002/lary.24907. [DOI] [PubMed] [Google Scholar]
- 26.Yang S, Wang X, Su JZ, Yu GY. Rate of submandibular gland involvement in oral squamous cell carcinoma. J Oral Maxillofac Surg. 2019;77(5):1000–1008. doi: 10.1016/j.joms.2018.12.011. [DOI] [PubMed] [Google Scholar]
- 27.Dundar Y, Mandle Q, Raza SN, Lin H-S, Cramer J, Hotaling JM. Submandibular gland invasion by oral cavity cancers: a systematic review. Otolaryngol Head Neck Surg. 2019;161(2):227–234. doi: 10.1177/0194599819838475. [DOI] [PubMed] [Google Scholar]
- 28.Chen TC, Lou PJ, Ko JY, Yang TL, Lo WC, Hu YL, Wang CP. Feasibility of preservation of the submandibular gland during neck dissection in patients with early-stage oral cancer. Ann Surg Oncol. 2011;18(2):497–504. doi: 10.1245/s10434-010-1294-7. [DOI] [PubMed] [Google Scholar]
- 29.Naidu TK, Naidoo SK, Ramdial PK. Oral cavity squamous cell carcinoma metastasis to the submandibular gland. J Laryngol Otol. 2012;126(3):279–284. doi: 10.1017/S0022215111002660. [DOI] [PubMed] [Google Scholar]
- 30.Basaran B, Ulusan M, Orhan KS, Gunes S, Suoglu Y. Is it necessary to remove submandibular glands in squamous cell carcinomas of the oral cavity? Acta Otorhinolaryngol Ital. 2013;33(2):88. [PMC free article] [PubMed] [Google Scholar]
- 31.Kruse A, Grätz KW. Evaluation of metastases in the submandibular gland in head and neck malignancy. J Craniofac Surg. 2009;20(6):2024–2027. doi: 10.1097/SCS.0b013e3181be87a3. [DOI] [PubMed] [Google Scholar]
- 32.Lanzer M, Gander T, Lübbers HT, Metzler P, Bredell M, Reinisch S. Preservation of ipsilateral submandibular gland is ill advised in cancer of the floor of the mouth or tongue. Laryngoscope. 2014;124(9):2070–2074. doi: 10.1002/lary.24672. [DOI] [PubMed] [Google Scholar]