Abstract
Despite recent advances in minimally invasive pancreatic surgery, laparoscopic pancreaticoduodenectomy (LPD) has not reach a wide diffusion, mainly due to its technical difficulty. Considering its potential benefits, efforts should be made to improve its adoption. Between January 2017 and March 2020, LPD was offered as the primary approach to all the patients with an indication to pancreaticoduodenectomy. The overall cohort was divided into two groups: the early group (EG), including the first 30 cases, and the late group (LG), with the remaining patients. Perioperative data were gathered from a prospectively collected database and retrospectively analyzed, comparing the short-term outcomes of the two groups. In the study period, 52 patients underwent LPD. Among these, 88.4% patients were preoperatively diagnosed with a malignant disease. No difference was found between EG and LG in terms of baseline characteristics, mean operative time, estimated blood loss, and conversion to laparotomy. The overall complication rate was 57.7%, with severe complications occurring in 14 patients (26.9%). Two patients (3.8%) deceased within 90 days from the operation. No difference was found between EG and LG regarding postoperative outcomes. Among oncological patients, 86.7% received an R0 resection, and 13.3% had an R1 resection. The EG and LG did not differ in terms of oncological radicality and number of lymph nodes retrieved. LPD is a reproducible surgical technique that may provide acceptable results in both early and late phase of experience, when performed by surgical team with broad background in laparoscopic surgery.
Keywords: Laparoscopic pancreaticoduodenectomy, Minimally invasive pancreaticoduodenectomy, Pancreatic surgery, Pancreatic cancer
Introduction
During the last decades, the technological and surgical advance has allowed to extend the application of minimally invasive operations in the field of pancreatic surgery. Laparoscopic distal pancreatectomy has already achieved a worldwide acceptance, and it is considered safe and feasible [1, 2]. On the other hand, laparoscopic pancreaticoduodenectomy (LPD) is still questioned to be as safe and reproducible, due to its technical complexity and long operative time; in addition, its oncological efficacy has not yet been demonstrated, compared to the open approach [3, 4]. Although the first LPD was performed more than 20 years ago [5], the adoption rate has been very low [6], especially when compared to other fields of surgical oncology for which laparoscopy is routinely applied [7–9]. The technical complexity, especially related to the dissection close to the vascular structures and to the difficulty of creating multiple anastomoses, represents the main reason limiting the adoption of LPD [10].
Pancreaticoduodenectomy (PD) is still associated with a high number of postoperative complications, both general and pancreas-specific. Ideally, laparoscopy may contribute in reducing such complications thanks to a better visualization, a more careful dissection, and a lower tissue trauma leading to a reduced surgical stress response [11].
LPD may be considered acceptable when performed in high volume centers, with experienced surgeons in both advanced laparoscopic and pancreatic surgery [3]; its application seems associated with improved short-term outcomes in comparison to open PD, without negatively affecting the oncological outcomes [12, 13]. Considering its potential benefits, efforts should be made to improve the diffusion and standardization of LPD technique worldwide. We hereby report our series of LPD with the aim to evaluate its implementation over time by analyzing the differences in postoperative results between a earlier and a most recent phase.
Methods
Patient Population
All consecutive patients who underwent LPD with curative intent for any indications between January 2017 and March 2020, at the division of Surgical Oncology of Niguarda Hospital in Milan, were included in the present analysis. Starting from January 2017, LPD was introduced in our division’s clinical practice as the primary approach for patients with an indication to PD, with the exemption of those presenting with major vascular involvement at preoperative imaging (portal vein, superior mesenteric vein), borderline resectable tumors [14], who received a neoadjuvant treatment or with any anesthesiologic contraindications to pneumoperitoneum.
A previous study assessing the learning curve for LPD concluded that 30 procedures are needed to obtain a stabilization of the postoperative results [15]. In order to evaluate differences related to the growing experience of the surgical team, the overall cohort of patients was divided into two groups: one including the first 30 cases, called the early group (EG), and the other including the remaining patients, called the later group (LG).
All cases were preoperatively discussed in a multidisciplinary board, composed by a surgeon, a pathologist, an endoscopist, a radiologist, and an oncologist.
Data Collection
A database of patients undergoing pancreatic surgery is present at our institution since 2011. Data are prospectively collected by specifically trained personnel and stored in a secured Ethical Committee approved (194–22,042,020) database. Baseline characteristics included the following: patient age, gender, body mass index (BMI), American Society of Anesthesiologists (ASA) score, Charlson comorbidity index [16], preoperative serum CEA and CA 19.9 value, preoperative biliary drainage, type of disease requiring surgery (benign vs malignant). Operative parameters were as follows: date of surgery, operative time, associated procedures, estimated blood loss (EBL), fistula risk score (FRS) [17], conversion rate to laparotomy; conversion occurred when the resection or reconstruction phase needed to be completed by any type of laparotomy. Pathological radicality of resection, pTNM, and pathologic stage were classified according to the American Joint Committee on Cancer (AJCC) staging system 8th edition [18]. Resection margins were defined according to the Royal College of Pathologist; margin status was considered R1 when the distance between the tumor and any resection margins was ≤ 1 mm.
Length of hospital stay (LOS) was defined as the number of nights spent in the hospital from the day of the surgical procedure until discharge that occurred according to the following criteria: independent mobilization, adequate pain control, adequate oral food intake (at least 70% of suggested caloric intake), no need for intravenous fluid infusion, absence of sepsis signs. Postoperative complications were recorded at 90 days and graded according to the Clavien-Dindo (CD) classification [19] and the Comprehensive Complication Index (CCI) [20]. Complications graded ≥ III according to CD classification [19] were considered severe. Specific pancreas-related complications were recorded separately: postoperative pancreatic fistula (POPF), postpancreatectomy hemorrhage (PPH), and delayed gastric emptying (DGE) were defined according to the last International Study Group on Pancreatic Surgery (ISGPS) [21–23]; bile leakage (BL) was defined according to International Study Group of Liver Surgery [24]. Postoperative mortality was calculated as the number of deaths occurring within 90 days from surgery.
Perioperative Management
All patients underwent preoperative complete workup according to their pancreatic disease: pancreatic cystic lesions were managed according to International [25–27] and European guidelines [28]; for the treatment of Pancreatic Neuroendocrine Tumors, international guidelines were followed [29]; malignant epithelial tumors originating from the pancreas, the ampulla, or the extrahepatic biliary tree were staged according to AJCC classification and staging systems for cancer 8th edition [18]. Endoscopic or percutaneous preoperative biliary stenting was considered in case of cholangitis, serum bilirubin level > 15 mg/dL, and jaundice in patients with indication to neoadjuvant treatment.
Preoperative management consisted in recommending smoking cessation at least 2 weeks before surgery, nutritional support with a mixture containing immuno-nutritional products, intake of clear fluids up to 2 h previous to anesthesia, and intake of solid food up to 6 h before anesthesia; no bowel preparation was administered. The patient received low molecular weight heparin starting the evening before the surgery; the antibiotic prophylaxis was administered 15 min prior to the skin incision. Intermittent pneumatic compression devices were positioned on the lower extremities to prevent thromboembolic events; feet and right shoulder supports were positioned, to allow optimal bed mobilization.
A fistula risk score based on pancreatic parenchymal texture, tumor type, Wirsung diameter, and intraoperative blood loss was assessed [17], at the end of the resection phase.
Surgical Technique
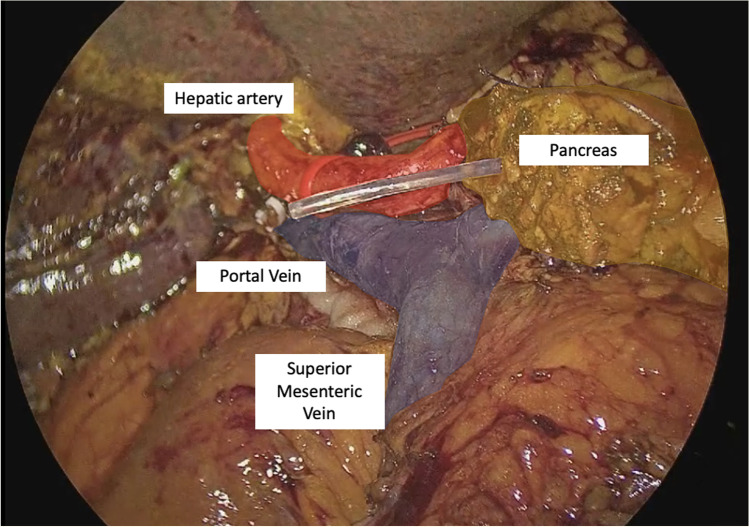
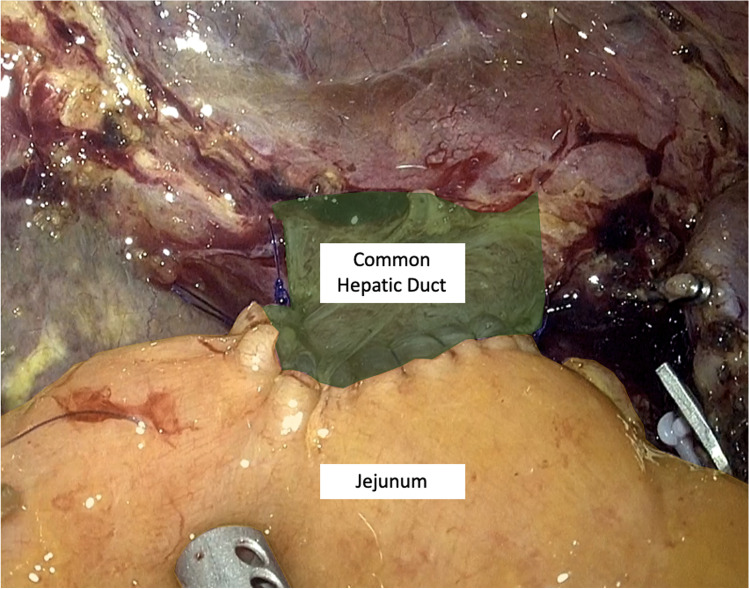
The LPD surgical technique was reported in a previous article [30] and will be described briefly. After the gastrocolic ligament is opened and the right colic flexure is mobilized, an extensive Kocher maneuver is carried out exposing the inferior vena cava and left renal vein; the origin of the superior mesenteric artery (SMA) is identified. The right gastroepiploic vein is sealed at trunk of Henle and the anterior aspect of superior mesenteric vein is cleaned. The second part of the duodenum and the fist jejunal loop are lifted up and pushed toward the left by the assistants’ grasp showing the resection plane along the SMA; a complete dissection of the mesopancreas is performed, moving from a caudal to cephalad fashion parallel to the SMA; the whole lymphovascular tissue between the uncinate process and the anterior-right lateral aspect of the SMA is dissected, sealing with clips the inferior pancreaticoduodenal artery (IPDA). The gastric antrum is resected (Whipple’s procedure) with a 60-mm stapler with purple cartridge (SigniaTM®, Ultra Powered stapling System, Covidien). Lymphadenectomy of stations 8, 9, and 12 is performed, until the gastroduodenal artery is cleared from the lymphatic tissue and sealed. Cholecystectomy and careful dissection of the hepatic pedicle are performed and the common hepatic duct is transected just above the cystic duct. After the retropancreatic tunnel is completed, the pancreas is sectioned with monopolar energy. The first jejunal loop is sectioned approximately 7 cm below the Treitz ligament with 60 mm tristaple Endo Gia (SigniaTM®, Ultra Powered stapling System, Covidien). The total mesopancreas excision (TMpE) is completed and all the tissue between the celiac trunk and the SMA is removed. Surgical specimen is positioned into an endobag and extracted through a Pfannenstiel mini-laparotomy (4–5 cm) (Fig. 1). The 4 K scope is then replaced with a 30° 3D one for the reconstructive phase, performed according to Child; the jejunal loop is transposed supramesocolic through behind the mesenteric root. According to Cattel-Warren, pancreaticojejunal anastomosis is performed with a double layer: the external one, using interrupted non-absorbable 4–0 polypropylene (Prolene®, Ethicon Endosurgery Inc, Cincinnati, OH) with an SH needle, between the sero-muscolar jejunal layer and the pancreatic capsula; the internal one (duct-to-mucosa anastomosis), using six interrupted absorbable 5–0 monofilament sutures (PDS®, Ethicon Endosurgery Inc, Cincinnati, OH), with RB-1 needle (Fig. 2). The end-to-side hepaticojejunostomy is performed using two 5–0 monofilament running sutures (PDS®, Ethicon Endosurgery Inc, Cincinnati, OH) with RB 1 needle (Fig. 3).
Fig. 1.
Surgical field at the end of the resection
Fig. 2.
Pancreato-jejunal anastomosis
Fig. 3.
Hepatico-jejunal anastomosis
The side-to-side gastrojejunostomy is carried out in an antecolic fashion, and it is performed using a 60-mm linear stapler (SigniaTM®, Ultra Powered stapling System, Covidien) with a purple cartridge and two 3–0 running suture are utilized (V-Loc®, Covidien, Inc, Mansfield, MA, USA) to close the hole of the stapler insertion.
All LPD were performed by a single surgeon with a wide experience in both advanced laparoscopic (more than 100 gastrectomies, more than 200 colectomies, more than 50 distal pancreatectomies) and pancreatic (more than 100 pancreatic resections) surgery. The same surgeon also participated in the majority of the cases of OPD as a main surgeon. Other two surgeons participated in the vast majority of cases as the second and the third operator.
Statistical Analysis
Patients’ characteristics were reported as median and range for continuous data, and as percentages for categorical data. Normal distribution of continuous variables was assessed using Shapiro–Wilk’s test. Quantitative variables were examined by Student’s t-test or Mann–Whitney test, as appropriate. Proportions were compared using Fisher’s exact test or Chi-Square test, as appropriate. All statistics were 2-tailed and statistical significance was accepted when p < 0.05.
All gathered data were recorded on an electronic spreadsheet and analyzed using SPSS 24.0 (IBM, Armonk, NY).
Results
Between January 2017 and March 2020, 82 consecutive patients underwent elective PD at our division. Thirty patients underwent open PD due to clear major vascular involvement at preoperative imaging and/or neoadjuvant treatment (26 patients) and anesthesiologic contraindications to prolonged pneumoperitoneum (4 patients).
Fifty-two patients underwent LPD. Baseline characteristics are reported in Table 1. Eighteen patients received preoperative biliary drainage: 9 for serum bilirubin higher than 15 mg/dl, 4 in order to prevent pancreatitis after endoscopic ampullectomy, 3 for acute cholangitis, 2 because of choledocus stenosis of undetermined origin. In all but 3 of these patients, an endoscopic biliary drainage was used; the remaining underwent a percutaneous transhepatic biliary drainage. The vast majority of patients (46 patients, 88.4%) received indication to LPD for malignant disease, while 6 patients (11.6%) had a benign disease.
Table 1.
Clinical characteristics of patients
| Characteristic | Total (n 52) | Early (n 30) | Late (n 22) | p value |
|---|---|---|---|---|
| Age, median (IQR) | 66.5 (61–74.5) | 65.7 (61.3–73.5) | 69.7 (56.2–76.2) | 0.266 |
| Sex (n), %, male | 25 (48) | 12 (40) | 13 (59.1) | 0.173 |
| BMI, median (IQR) | 25 (23–27.5) | 25.5 (22.4–26.7) | 25.3 (23.6–27.8) | 0.578 |
| ASA score (n), % | 0.555 | |||
| 1 | 5 (9.6) | 3 (10.0) | 2 (9.1) | |
| 2 | 42 (80.8) | 23 (76.7) | 19 (86.4) | |
| 3 | 5 (9.6) | 4 (13.3) | 1 (4.6) | |
| aCCI, median (IQR) | 5 (3.5–5) | 5 (4–5) | 5 (3–6) | 0.579 |
| Performance status (n), % | 0.370 | |||
| 0 | 23 (44.2) | 15 (50.0) | 8 (36.4) | |
| 1 | 25 (48.1) | 12 (40.0) | 13 (59.1) | |
| 2 | 4 (7.6) | 3 (10.0) | 1 (4.6) | |
| Preoperative biliary drainage (n), % | 18 (34.6) | 8 (26.7) | 10 (45.5) | 0.159 |
| Albumine (g/dl), median (IQR) | 3.9 (3.4–4.2) | 4.0 (3.6–4.3) | 3.7 (3.2–3.9) | 0.009 |
| Bilirubin (mg/dl), median (IQR) | 1.5 (0.4–10.6) | 1.1 (0.3–10.3) | 3.9 (0.4–11.6) | 0.257 |
| Ca 19.9 (UI/ml), median (IQR) | 30.3 (11.5–265) | 15.4 (7.5–609.2) | 54.4 (18.1–248) | 0.124 |
| CEA (ng/ml), median (IQR) | 1.9 (1.5–3.1) | 1.8 (1.4–2.9) | 2.2 (1.5–3.2) | 0.747 |
| FRS, median | 6 (3–7) | 6 (4–8) | 5 (2–7) | 0.075 |
| Malignant disease (n), % | 46 (88.4) | 25 (83.3) | 21 (95.5) | 0.636 |
n number, IQR interquartile range, EG early group, LG late group, BMI body mass index, ASA American Society of Anesthesiologists score, aCCI age-adjusted Charlson Comorbidity Index, FRS fistula risk score
No difference was found between EG and LG in terms of baseline characteristics except for preoperative serum albumin level (4 vs 3.7 g/dl in EG and LG, respectively; p = 0.009).
Median operative time was 590 min without differences between the groups (p = 0.341); 2 (3.8%) conversions to laparotomy, both in EG, occurred, due to an intraoperative diagnosis of SMA encasement and to a broad post-ERCP pancreatitis. EBL difference was similar between groups, being 300 ml in EG and 200 ml in LG (p = 0.095).
The overall complication rate was 57.7%, with severe complications occurring in 14 (26.9%) patients. Among these, POPF, BL, and PPH were diagnosed in 8 (15.4%), 5 (9.6%), and 6 (11.5%) patients, respectively.
POPF were treated endoscopically in 2 cases, percutaneously in 3 cases, while 3 cases required reoperation with resection of the pancreatic stump. Grade C PPHs happened in 4 cases, leading to reoperation and intensive care unit admission, while 2 cases were treated with percutaneous embolization. Overall DGE rate was 23.5%, with 5 patients complaining grade B and C (9.6%). Eight patients underwent reoperation (15.4%) due to intra-abdominal hemorrhage in 4 cases, septic shock due to POPF in 2 cases, and intra-abdominal collection and biliary leakage in 1 case, respectively. Two patients (3.8%) deceased within 90 days from the operation, both for consequences of PPH. Median LOS was 14 days, ranging from 10 to 22 days. After discharge, 2 patients were readmitted for infected abdominal collection, 1 for splenic artery pseudoaneurysm, and 1 for urosepsis, with a readmission rate of 8%. Postoperative outcomes are reported in Table 2.
Table 2.
Pathological characteristics of patients
| Characteristic | Total (n 52) | EG (n 30) | LG (n 22) | p value |
|---|---|---|---|---|
| Histology (n), % | 0.151 | |||
| Pancreatic adenocarcinoma | 24 (46.2) | 13 (43.3) | 11 (50.0) | |
| Ampullary cancer | 11 (21.2) | 5 (16.6) | 6 (27.3) | |
| Distal CBD cancer | 6 (11.5) | 3 (10.0) | 3 (13.6) | |
| NET | 3 (5.8) | 3 (10.0) | 0 (0.0) | |
| Metastasis | 1 (1.9) | 1 (3.3) | 0 (0.0) | |
| SPT | 1 (1.9) | 0 (0.0) | 1 (4.5) | |
| IPMN with LGD | 4 (7.7) | 4 (13.3) | 0 (0.0) | |
| Chronic pancreatitis | 1 (1.9) | 0 (0.0) | 1 (4.6) | |
| SCA | 1 (1.9) | 0 (0.0) | 1 (4.6) | |
| Grading* (n),% | 0.229 | |||
| 1 | 6 (13.0) | 3 (12.0) | 3 (14.3) | |
| 2 | 17 (37.0) | 12 (48.0) | 5 (23.8) | |
| 3 | 23 (50.0) | 10 (40.0) | 13 (61.9) | |
| T stage* (n),% | 0.737 | |||
| 1 | 9 (19.6) | 4 (16.0) | 5 (23.8) | |
| 2 | 26 (56.5) | 16 (64.0) | 10 (47.6) | |
| 3 | 10 (21.7) | 4 (16.0) | 6 (28.6) | |
| 4 | 1 (2.2) | 1 (4.0) | 0 (0.0) | |
| N stage* (n),% | 0.956 | |||
| 0 | 23 (50.0) | 12 (48.0) | 11 (52.4) | |
| 1 | 16 (34.8) | 9 (36.0) | 7 (33.3) | |
| 2 | 7 (15.2) | 4 (16.0) | 3 (14.3) | |
| LNs harvested* median (IQR) | 20 (11–27) | 22 (12–28) | 17 (11–27) | 0.269 |
| Radicality* (n),% | 0.684 | |||
| R0 | 40 (86.9) | 21 (84.0) | 19 (90.5) | |
| R1 | 6 (13.1) | 4 (16.0) | 2 (9.5) |
n number, EG early group, LG late group, CBD common bile duct, NET neuroendocrine tumor, SPT solid pseudopapillary tumor, IPMN intraductal papillary mucinous neoplasm, LGD low-grade dysplasia, SCA serous cystadenoma, T tumor, N nodes, LNs lymph nodes
*Calculated on patients with malignant disease (n 46)
No difference was found between EG and LG regarding postoperative outcomes.
At pathological examination, 40 patients were diagnosed with adenocarcinoma, originating from the pancreas, the ampulla, and the distal common bile duct in 24, 11, and 5 patients, respectively. Among 46 patients who underwent LPD for malignancy, 86.9% received an R0 resection, while 13.3% had an R1 resection with the positive margin being the posterior one in all the cases [31]. No cases of R2 resection were reported. Mean number of lymph nodes harvested was 20. The EG and LG did not differ in terms of either surgical radicality and number of lymph nodes retrieved. Pathological outcomes are reported in Table 3.
Table 3.
Perioperative outcomes
| Characteristic | Total (n 52) | EG (n 30) | LG (n 22) | p value |
|---|---|---|---|---|
| Operative time, median (IQR), min | 590 (537–615) | 581.5 (519–601) | 597.5 (559–660) | 0.341 |
| Conversion (n), % | 2 (3.8) | 2 (6.7) | 0 (0.0) | 0.502 |
| Estimated blood loss, mean, ml | 255 (180–395) | 300 (207.5–400) | 200 (130–362.5) | 0.095 |
| Complication (n), % | 30 (57.7) | 15 (50) | 15 (68.2) | 0.259 |
| Clavien-Dindo | 0.434 | |||
| 1 | 0 (0.0) | 0(0.0) | 0 (0.0) | |
| 2 | 14 (26.9) | 3 (10.0) | 11 (50.0) | |
| 3a | 7 (13.5) | 3 (10.0) | 4 (18.2) | |
| 3b | 2 (3.8) | 1 (3.3) | 1 (4.5) | |
| 4a | 4 (7.7) | 3 (10.0) | 1 (4.5) | |
| 4b | 1 (1.9) | 1 (3.3) | 0 (0.0) | |
| Severe complication (n), % | 14 (26.9) | 9 (30) | 5 (22.7) | 0.753 |
| CCI: median (IQR) | 20.9 (0–33.5) | 10.5 (0–33.5) | 20.9 (0–33.5) | 0.365 |
| mean (SD) | 21.4 (24.1) | 22.1 (28.9) | 20.6 (16.2) | |
| Reoperation (n) % | 8 (15.4) | 7 (23.3) | 1 (4.6) | 0.064 |
| POPF (n), % | 8 (15.4) | 6 (20) | 2 (9.1) | 0.429 |
| DGE (n), % | 12 (23.1) | 5 (16.7) | 7 (31.8) | 0.318 |
| BL (n) % | 5 (9.6) | 2 (6.7) | 3 (13.6) | 0.639 |
| PPH (n) % | 6 (11.5) | 4 (13.3) | 2 (9.1) | 0.636 |
| Length of stay, median (IQR) | 14.5 (10–22) | 14 (10–28) | 15 (9–21) | 0.573 |
| Readmission (n) % | 4 (8.0) | 1 (3.6) | 3 (13.6) | 0.308 |
| Mortality (n) % | 2 (3.8) | 2 (6.7) | 0 (0.0) | 0.502 |
n number, EG early group, LG late group, CCI comprehensive comorbidity index, POPF postoperative pancreatic fistula, DGE delayed gastric emptying, BL biliary leakage, PPH postpancreatectomy hemorrhage
*Calculated on the number of oncological patients
Discussion
We reported the short-term outcomes of a case-series of consecutive LPD from a single institution, comparing the earlier and the latter phase of the series. To the best of our knowledge, this represents one of the largest series on LPD yet published from an European setting [32–34].
Although the first LPD was performed more than 20 years ago [5], it was adopted by few, due to its technical complexity. The high complication rate of PD may heavily affect the postoperative recovery and minimize the potential advantages of laparoscopy. This, together with the paucity of long-term data, has brought to a lack of international consensus about the safety, the feasibility, and efficacy of LPD.
Our data show that in this series, acceptable intra- and postoperative results were achieved even during the initial period of the experience. Median operative time, conversion rate, severe postoperative complications, and median LOS did not differ between the early and late phase. According to a growing evidence demonstrating a strong association between surgical expertise, hospital volume, and outcomes [35–37], this was probably due to a wide experience in both laparoscopic oncologic surgery and open pancreatic surgery by the operating team, in a tertiary referral European center, with an overall caseload of more than 50 pancreatic resections/years. Furthermore, other authors [38] suggested that the first phase of the LPD learning curve can require more than 60 cases to achieve a reduction in postoperative complications and conversions; our results can be influenced by the learning curve cut-off at 30 cases. On the other hand, in our series, a trend of reduction in EBL and reoperation rate, although not statistically significant, was found in favor of LG, suggesting a growing experience in the management of intra- and postoperative accidents and a likely learning curve effect. Our results do not challenge the concept of learning-curve-related implementation, but consistently with the European association for endoscopic surgery consensus statement [39] suggest that LPD can be safely performed by experienced surgeons, skilled in advanced laparoscopy, during their early learning curve.
Selection criteria are considered of critical importance, especially when approaching a novel surgical technique. Since its introduction in 2017, the laparoscopic approach was offered in our center to all the patients who were candidate to PD, with the only exclusion criteria being borderline resectable tumors [14], neoadjuvant treatment, and anesthesiological contraindications to laparoscopy. Patients with a BMI within normal range, who are affected by duodenal, small ampullary, or distal biliary tract tumors and without previous upper-mesocolic abdominal surgeries, are considered ideal candidates for LPD, especially in the early phase of a learning curve. We did not considered BMI as a contraindication to the laparoscopic approach, as obese patients may benefit even more from a minimally invasive approach [40, 41]. The median values of BMI, ASA score, and age in our patients were quite higher than those reported in the literature, reflecting the idea that these patients could particularly advantage from a minimally invasive approach [42]. Since 2017, LPD accounts for 65% of our PD, reaching up to 80% when considering only the last year of practice. Our broad selection criteria, in addition to the extremely low conversion rate in our series (3.8%), may explain the longer median operative time compared to other series [42, 43].
EBL varies between different series reported in the literature, ranging from 74 to 1106 ml; it depends on patient’s characteristics, type of tumors, and experience of the surgical equipe [42]. Our technique consists of a particular dissection of retroportal lamina using a modified artery-first approach: this is achieved firstly by “downward,” with a clear visualization of the resection plane along the SMA and an easier and reproducible identification of IPDA; then, once the IPDA is sectioned, by “upperward,” with the section of pancreatico-duodenal veins and completing the TMpE. This approach allowed us to maintain an average EBL of 250 ml; this may be due by an easier recognition of the correct dissection plane along the SMA.
Despite the enormous progress made in the field of pancreatic surgery in the recent years, demonstrated by the decrease in mortality rate, PD is still burdened by relevant postoperative morbidity. In this scenario, minimally invasive surgery has raised great expectations as a tool to improve postoperative outcomes, both general and pancreas specific; on the other hand, the premature closure of LEOPARD-2 trial [44] because of the high severe postoperative complications and mortality rates in patients undergoing LPD caused serious concerns.
A great heterogeneity in the postoperative complication rate, ranging from 26 to 74% [42, 45], is reported in the literature. In our series, the overall complication rate was 57.7%; this may depend by the broad selection criteria and the postoperative complication time range of 90 days. However, severe complications occurred in only 26.9% of cases with 8 patients (15.4%) needing reoperation. This is consistent with the previous literature [46] and with a propensity score matched analysis recently published by our group [47], demonstrating no difference in terms of 90 days postoperative morbidity and mortality between LPD and OPD.
The most frequently reported postoperative pancreas-specific complications was DGE (23.5%), followed by POPF (18%) and PPH (8%) [22]. Despite not being a life-threatening condition, DGE is a troublesome complication that can lead to an impaired recovery and prolonged LOS. Although we use a number of precautions in order to prevent DGE, such as a 60 mm gastro-jejunal anastomosis, antecolic jejunal positioning, and splitting of the greater omentum, 23.1% of patients in our series experienced a DGE, being a slightly higher rate than what reported in literature [43]. However, only 5 of these (9.5%) were a grade B or C DGE.
Fashioning the pancreatic anastomosis is one of the most challenging phases of PD; this procedure represents the ideal field of application of minimally invasive surgery for two main reasons: the possibility of using a 3D scope with enhanced vision and depth perception, and careful, gentle, and precise tissue manipulation. In our series POPF happened in 15% of patients; this data is consistent with what reported in the literature and should be considered in the light of the average fistula risk score [48], being 5 in our series. In all cases, we used a double layer interrupted suture. We do not routinely place a stent in the Wirsung duct because of no evidence of reduced risk of POPF after stent placement and high risk of early stent occlusion with consequent pancreatitis of the stump [49–52].
A recently published paper [53] provided a benchmark value of 7% for severe PPH after OPD. In our series, they exceed this value, occurring in 11.5% of patients and represented the main cause of reoperation and mortality. This could be partially due to the learning curve effect; carefully dissection of meso pancreatic vessels, with adequate ligation using clips of IPDA, may help to reduce PPH by limiting the sub-advential arterial dissection and using hemostatic matrix on the right aspect of SMA/SMV.
Reduced postoperative pain and early mobilization are well-recognized advantages of laparoscopic surgery, pathing the way to a faster recovery. A recent review [42, 43] reported a median LOS of 21, 13, and 9.4 days in European, Asiatic, and US series, respectively; this variability seems attributable not only to the rate and severity of postoperative complications but also to cultural and organizational burdens. In our series from a south-european high volume center, median LOS was 14 days, and it was associated with a quite low readmission rate (8%) compared to what reported in the literature, with readmission rates ranging from 5 to 30% [43].
Perioperative wound-related complications and incisional hernias are frequent adverse events after major open surgery, especially in high-risk patients [25]. Consistently to previous reports, in our series of patients, laparoscopy seemed associated with some clear benefits in terms of wound-related complications (seroma, hematoma, skin or fascial disruption, chronic wounds, skin necrosis, and cellulitis) as none of the patients experienced them; at the beginning of experience, 3.85% asymptomatic incisional hernia occurred. At that time, we used a supraumbilical incision for specimen extraction; no incisional hernia occurred since we changed to a sovrapubic mini-laparotomy.
A critical aspect of LPD is oncological radicality. In line with other series [52, 54, 55], considering only malignant cases, the mean number of harvested lymph nodes was 20 and the rate of negative resection margins was 86.9%, with no R2 resections. Performing TMpE is recommended because it can help reducing the incidence of R + resections improving the posterior margin clearance and the number of harvested LNs, compared to a standard pancreaticoduodenectomy [25, 26].
The small sample size, the monocentric setting, and the retrospective analysis represent the main limitations of this study and can partially explain some apparently contradictory results such as the longer operative time and postoperative stay, the higher bile leak and readmission rate, and the greater number of overall complications in the LG. Our criteria excluded patients with vascular infiltration, poor general conditions, and previous neoadjuvant therapy, introducing a clear selection bias. In addition, PD is an unpredictable surgery, with severe complications potentially occurring even after 52nd case, limiting the generizability of the results. Nevertheless, this study adds value to a still lacking field of evidence such as LPD, especially in Western countries, demonstrating the feasibility and the good short-term outcomes of LPD, even in the early phase of the experience, highlighting the usefulness of technique standardization.
In conclusion, LPD is a standardizable and reproducible surgical technique. The results of our case series suggest that it is possible to achieve acceptable short-term outcomes even during the learning curve phase. Future studies, based on greater sample size, should aim to assess the learning curve for LPD.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.de Rooij T, van Hilst J, van Santvoort H, Boerma D, van den Boezem P, Daams F, van Dam R, Dejong C, van Duyn E, Dijkgraaf M, van Eijck C, Festen S, Gerhards M, Groot Koerkamp B, de Hingh I, Kazemier G, Klaase J, de Kleine R, van Laarhoven C, Luyer M, Patijn G, Steenvoorde P, Suker M, Abu Hilal M, Busch O, Besselink M; Dutch Pancreatic Cancer Group (2019) Minimally Invasive Versus Open Distal Pancreatectomy (LEOPARD): A Multicenter Patient-blinded Randomized Controlled Trial. Ann Surg 269(1):2–9 [DOI] [PubMed]
- 2.Venkat R, Edil BH, Schulick RD, Lidor AO, Makary MA, Wolfgang CL. Laparoscopic distal pancreatectomy is associated with significantly less overall morbidity compared to the open technique: a systematic review and meta-analysis. Ann Surg. 2012;255:1048–1059. doi: 10.1097/SLA.0b013e318251ee09. [DOI] [PubMed] [Google Scholar]
- 3.Dai R, Turley RS, Blazer DG. Contemporary review of minimally invasive pancreaticoduodenectomy. World J Gastrointest Surg. 2016;8:784–791. doi: 10.4240/wjgs.v8.i12.784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kantor O, Talamonti MS, Sharpe S, Lutfi W, Winchester DJ, Roggin KK, Bentrem DJ, Prinz RA, Baker MS. Laparoscopic pancreaticoduodenectomy for adenocarcinoma provides short-term oncologic outcomes and long-term overall survival rates similar to those for open pancreaticoduodenectomy. Am J Surg. 2017;213:512–515. doi: 10.1016/j.amjsurg.2016.10.030. [DOI] [PubMed] [Google Scholar]
- 5.Gagner M, Palermo M. Laparoscopic Whipple procedure: review of the literature. J Hepatobiliary Pancreat Surg. 2009;16:726–730. doi: 10.1007/s00534-009-0142-2. [DOI] [PubMed] [Google Scholar]
- 6.Merkow J, Paniccia A, Edil BH. Laparoscopic pancreaticoduodenectomy: a descriptive and comparative review. Chin J Cancer Res. 2015;27:368–375. doi: 10.3978/j.issn.1000-9604.2015.06.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ohtani H, Tamamori Y, Arimoto Y, Nishiguchi Y, Maeda K, Hirakawa K. A meta-analysis of the short- and long-term results of randomized controlled trials that compared laparoscopy-assisted and open colectomy for colon cancer. J Cancer. 2012;3:49–57. doi: 10.7150/jca.3621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mariette C, Markar SR, Dabakuyo-Yonli TS, Meunier B, Pezet D, Collet D, D’Journo XB, Brigand C, Perniceni T, Carrère N, Mabrut J-Y, Msika S, Peschaud F, Prudhomme M, Bonnetain F, Piessen G, Fédération de Recherche en Chirurgie (FRENCH) and French Eso-Gastric Tumors (FREGAT) Working Group Hybrid minimally invasive esophagectomy for esophageal cancer. N Engl J Med. 2019;380:152–162. doi: 10.1056/NEJMoa1805101. [DOI] [PubMed] [Google Scholar]
- 9.Biere SSAY, van Berge Henegouwen MI, Maas KW, Bonavina L, Rosman C, Garcia JR, Gisbertz SS, Klinkenbijl JHG, Hollmann MW, de Lange ESM, Bonjer HJ, van der Peet DL, Cuesta MA. Minimally invasive versus open oesophagectomy for patients with oesophageal cancer: a multicentre, open-label, randomised controlled trial. Lancet. 2012;379:1887–1892. doi: 10.1016/S0140-6736(12)60516-9. [DOI] [PubMed] [Google Scholar]
- 10.Khatkov IE, Izrailov RE, Khisamov AA, Tyutyunnik PS, Fingerhut A. Superior mesenteric–portal vein resection during laparoscopic pancreatoduodenectomy. Surg Endosc. 2017;31:1488–1495. doi: 10.1007/s00464-016-5115-3. [DOI] [PubMed] [Google Scholar]
- 11.Mari G, Crippa J, Costanzi A, Mazzola M, Rossi M, Maggioni D. ERAS Protocol Reduces IL-6 secretion in colorectal laparoscopic surgery: results from a randomized clinical trial. Surg Laparosc Endosc Percutan Tech. 2016;26:444–448. doi: 10.1097/SLE.0000000000000324. [DOI] [PubMed] [Google Scholar]
- 12.Asbun HJ, Stauffer JA. Laparoscopic vs open pancreaticoduodenectomy: overall outcomes and severity of complications using the accordion severity grading system. J Am Coll Surg. 2012;215:810–819. doi: 10.1016/j.jamcollsurg.2012.08.006. [DOI] [PubMed] [Google Scholar]
- 13.Nagakawa Y, Hosokawa Y, Sahara Y, Takishita C, Nakajima T, Hijikata Y, Tago T, Kasuya K, Tsuchida A. A novel “artery first” approach allowing safe resection in laparoscopic pancreaticoduodenectomy: the uncinate process first approach. Hepatogastroenterology. 2015;62:1037–1040. [PubMed] [Google Scholar]
- 14.Isaji S, Mizuno S, Windsor JA, Bassi C, Fernández-Del Castillo C, Hackert T, Hayasaki A, Katz MHG, Kim S-W, Kishiwada M, Kitagawa H, Michalski CW, Wolfgang CL. International consensus on definition and criteria of borderline resectable pancreatic ductal adenocarcinoma 2017. Pancreatology. 2018;18:2–11. doi: 10.1016/j.pan.2017.11.011. [DOI] [PubMed] [Google Scholar]
- 15.Nagakawa Y, Nakamura Y, Honda G, Gotoh Y, Ohtsuka T, Ban D, Nakata K, Sahara Y, Velasquez VVDM, Takaori K, Misawa T, Kuroki T, Kawai M, Morikawa T, Yamaue H, Tanabe M, Mou Y, Lee W-J, Shrikhande SV, Conrad C, Han H-S, Tang CN, Palanivelu C, Kooby DA, Asbun HJ, Wakabayashi G, Tsuchida A, Takada T, Yamamoto M, Nakamura M. Learning curve and surgical factors influencing the surgical outcomes during the initial experience with laparoscopic pancreaticoduodenectomy. J Hepatobiliary Pancreat Sci. 2018;25:498–507. doi: 10.1002/jhbp.586. [DOI] [PubMed] [Google Scholar]
- 16.Charlson ME, Pompei P, Ales KL, Ronald MacKenzie C. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 17.Callery MP, Pratt WB, Kent TS, Chaikof EL, Vollmer CM., Jr A prospectively validated clinical risk score accurately predicts pancreatic fistula after pancreatoduodenectomy. J Am Coll Surg. 2013;216:1–14. doi: 10.1016/j.jamcollsurg.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 18.Chun YS, Pawlik TM, Vauthey J-N. 8th Edition of the AJCC Cancer Staging Manual: pancreas and hepatobiliary cancers. Ann Surg Oncol. 2018;25:845–847. doi: 10.1245/s10434-017-6025-x. [DOI] [PubMed] [Google Scholar]
- 19.Dindo D, Demartines N, Clavien P-A. Classification of Surgical Complications. Ann Surg. 2004;240:205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clavien P-A, Vetter D, Staiger RD, Slankamenac K, Mehra T, Graf R, Puhan MA. The Comprehensive Complication Index (CCI®): added value and clinical perspectives 3 years “down the line”. Ann Surg. 2017;265:1045–1050. doi: 10.1097/SLA.0000000000002132. [DOI] [PubMed] [Google Scholar]
- 21.Bassi C, Marchegiani G, Dervenis C, Sarr M, Hilal MA, Adham M, Allen P, Andersson R, Asbun HJ, Besselink MG, Conlon K, Del Chiaro M, Falconi M, Fernandez-Cruz L, Castillo CF, Fingerhut A, Friess H, Gouma DJ, Hackert T, Izbicki J, Lillemoe KD, Neoptolemos JP, Olah A, Schulick R, Shrikhande SV, Takada T, Takaori K, Traverso W, Vollmer CR, Wolfgang CL, Yeo CJ, Salvia R, Buchler M. The 2016 update of the International Study Group (ISGPS) definition and grading of postoperative pancreatic fistula: 11 Years After. Surgery. 2017;161:584–591. doi: 10.1016/j.surg.2016.11.014. [DOI] [PubMed] [Google Scholar]
- 22.Wente MN, Veit JA, Bassi C, Dervenis C, Fingerhut A, Gouma DJ, Izbicki JR, Neoptolemos JP, Padbury RT, Sarr MG, Yeo CJ, Büchler MW. Postpancreatectomy hemorrhage (PPH)–An International Study Group of Pancreatic Surgery (ISGPS) definition. Surgery. 2007;142:20–25. doi: 10.1016/j.surg.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 23.Wente MN, Bassi C, Dervenis C, Fingerhut A, Gouma DJ, Izbicki JR, Neoptolemos JP, Padbury RT, Sarr MG, Traverso LW, Yeo CJ, Büchler MW. Delayed gastric emptying (DGE) after pancreatic surgery: a suggested definition by the International Study Group of Pancreatic Surgery (ISGPS) Surgery. 2007;142:761–768. doi: 10.1016/j.surg.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 24.Koch M, Garden OJ, Padbury R, Rahbari NN, Adam R, Capussotti L, Fan ST, Yokoyama Y, Crawford M, Makuuchi M, Christophi C, Banting S, Brooke-Smith M, Usatoff V, Nagino M, Maddern G, Hugh TJ, Vauthey J-N, Greig P, Rees M, Nimura Y, Figueras J, DeMatteo RP, Büchler MW, Weitz J. Bile leakage after hepatobiliary and pancreatic surgery: a definition and grading of severity by the International Study Group of Liver Surgery. Surgery. 2011;149:680–688. doi: 10.1016/j.surg.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 25.Menzo EL, Hinojosa M, Carbonell A, Krpata D, Carter J, Rogers AM. American Society for Metabolic and Bariatric Surgery and American Hernia Society consensus guideline on bariatric surgery and hernia surgery. Surg Obes Relat Dis. 2018;14:1221–1232. doi: 10.1016/j.soard.2018.07.005. [DOI] [PubMed] [Google Scholar]
- 26.Wu W, Wang X, Wu X, Li M, Weng H, Cao Y, Bao R, Su S, Lu J, Gong W, Shi W, Gu J, Wang X, Liu Y, Quan Z, Peng S. Total mesopancreas excision for pancreatic head cancer: analysis of 120 cases. Chin J Cancer Res. 2016;28:423–428. doi: 10.21147/j.issn.1000-9604.2016.04.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tanaka M, Castillo CF, Adsay V, Chari S, Falconi M, Jang J-Y, Kimura W, Levy P, Pitman MB, Max Schmidt C, Shimizu M, Wolfgang CL, Yamaguchi K, Yamao K. International consensus guidelines 2012 for the management of IPMN and MCN of the pancreas. Pancreatology. 2012;12:183–197. doi: 10.1016/j.pan.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 28.European Study Group on Cystic Tumours of the Pancreas European evidence-based guidelines on pancreatic cystic neoplasms. Gut. 2018;67:789–804. doi: 10.1136/gutjnl-2018-316027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Falconi M, Eriksson B, Kaltsas G, Bartsch DK, Capdevila J, Caplin M, Kos-Kudla B, Kwekkeboom D, Rindi G, Klöppel G, Reed N, Kianmanesh R, Jensen RT, all other Vienna Consensus Conference participants ENETS consensus guidelines update for the management of patients with functional pancreatic neuroendocrine tumors and non-functional pancreatic neuroendocrine tumors. Neuroendocrinology. 2016;103:153–171. doi: 10.1159/000443171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mazzola M, Morini L, Crippa J, Maspero M, Zironda A, Giani A, Martini PD, Ferrari G. Totally laparoscopic pancreaticoduodenectomy: technical notes. Chirurgia. 2020;115:385–393. doi: 10.21614/chirurgia.115.3.385. [DOI] [PubMed] [Google Scholar]
- 31.Verbeke CS, Leitch D, Menon KV, McMahon MJ, Guillou PJ, Anthoney A. Redefining the R1 resection in pancreatic cancer. Br J Surg. 2006;93:1232–1237. doi: 10.1002/bjs.5397. [DOI] [PubMed] [Google Scholar]
- 32.Corcione F, Pirozzi F, Cuccurullo D, Piccolboni D, Caracino V, Galante F, Cusano D, Sciuto A. Laparoscopic pancreaticoduodenectomy: experience of 22 cases. Surg Endosc. 2013;27:2131–2136. doi: 10.1007/s00464-012-2728-z. [DOI] [PubMed] [Google Scholar]
- 33.Dulucq JL, Wintringer P, Mahajna A. Laparoscopic pancreaticoduodenectomy for benign and malignant diseases. Surg Endosc. 2006;20:1045–1050. doi: 10.1007/s00464-005-0474-1. [DOI] [PubMed] [Google Scholar]
- 34.Boggi U, Signori S, De Lio N, Perrone VG, Vistoli F, Belluomini M, Cappelli C, Amorese G, Mosca F. Feasibility of robotic pancreaticoduodenectomy. Br J Surg. 2013;100:917–925. doi: 10.1002/bjs.9135. [DOI] [PubMed] [Google Scholar]
- 35.Qin H, Qiu J, Zhao Y, Pan G, Zeng Y. Does minimally-invasive pancreaticoduodenectomy have advantages over its open method? A meta-analysis of retrospective studies. PLoS ONE. 2014;9:e104274. doi: 10.1371/journal.pone.0104274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nassour I, Wang SC, Christie A, Augustine MM, Porembka MR, Yopp AC, Choti MA, Mansour JC, Xie X-J, Polanco PM, Minter RM. Minimally invasive versus open pancreaticoduodenectomy: a propensity-matched study from a national cohort of patients. Ann Surg. 2018;268:151–157. doi: 10.1097/SLA.0000000000002259. [DOI] [PubMed] [Google Scholar]
- 37.Torphy RJ, Friedman C, Halpern A, Chapman BC, Ahrendt SS, McCarter MM, Edil BH, Schulick RD, Gleisner A. Comparing short-term and oncologic outcomes of minimally invasive versus open pancreaticoduodenectomy across low and high volume centers. Ann Surg. 2019;270:1147–1155. doi: 10.1097/SLA.0000000000002810. [DOI] [PubMed] [Google Scholar]
- 38.Kim S, Yoon Y-S, Han H-S, Cho JY, Choi Y, Lee B (2021) Evaluation of a single surgeon’s learning curve of laparoscopic pancreaticoduodenectomy: risk-adjusted cumulative summation analysis. Surg Endosc 35:2870–2878 [DOI] [PubMed]
- 39.Edwin B, Sahakyan M, Abu Hilal M, Besselink M, Braga M, Fabre J, Fernandez-Cruz L, Gayet B, Kim SC, Khatkov I, EAES Consensus Conference Study Group Laparoscopic surgery for pancreatic neoplasms: the European association for endoscopic surgery clinical consensus conference. Surg Endosc. 2017;31:2023–2041. doi: 10.1007/s00464-017-5414-3. [DOI] [PubMed] [Google Scholar]
- 40.Shabanzadeh DM, Sørensen LT. Laparoscopic surgery compared with open surgery decreases surgical site infection in obese patients: a systematic review and meta-analysis. Ann Surg. 2012;256:934–945. doi: 10.1097/SLA.0b013e318269a46b. [DOI] [PubMed] [Google Scholar]
- 41.Pasam RT, Esemuede IO, Lee-Kong SA, Kiran RP. The minimally invasive approach is associated with reduced surgical site infections in obese patients undergoing proctectomy. Tech Coloproctol. 2015;19:733–743. doi: 10.1007/s10151-015-1356-8. [DOI] [PubMed] [Google Scholar]
- 42.Boggi U, Amorese G, Vistoli F, Caniglia F, De Lio N, Perrone V, Barbarello L, Belluomini M, Signori S, Mosca F. Laparoscopic pancreaticoduodenectomy: a systematic literature review. Surg Endosc. 2015;29:9–23. doi: 10.1007/s00464-014-3670-z. [DOI] [PubMed] [Google Scholar]
- 43.Kendrick ML, van Hilst J, Boggi U, de Rooij T, Walsh RM, Zeh HJ, Hughes SJ, Nakamura Y, Vollmer CM, Kooby DA, Asbun HJ, Committee MIPRO. Minimally invasive pancreatoduodenectomy. HPB. 2017;19:215–224. doi: 10.1016/j.hpb.2017.01.023. [DOI] [PubMed] [Google Scholar]
- 44.van Hilst J, de Rooij T, Boosscha K, Brinkman D, van Dieren S, Dijkgraaf M, Gerhards M, de Hingh I, Karsten T, Lips D, Luyer M, Busch O, Festen S, Besselink M. Laparoscopic versus open pancreatoduodenectomy for pancreatic or periampullary tumours (LEOPARD-2): a multicentre, patient-blinded, randomised controlled phase 2/3 trial. Lancet Gastroenterol Hepatol. 2019;4:199–207. doi: 10.1016/S2468-1253(19)30004-4. [DOI] [PubMed] [Google Scholar]
- 45.Coppola A, Stauffer JA, Asbun HJ. Laparoscopic pancreatoduodenectomy: current status and future directions. Updates Surg. 2016;68:217–224. doi: 10.1007/s13304-016-0402-z. [DOI] [PubMed] [Google Scholar]
- 46.Wang M, Cai H, Meng L, Cai Y, Wang X, Li Y, Peng B. Minimally invasive pancreaticoduodenectomy: a comprehensive review. Int J Surg. 2016;35:139–146. doi: 10.1016/j.ijsu.2016.09.016. [DOI] [PubMed] [Google Scholar]
- 47.Mazzola M, Giani A, Crippa J, Morini L, Zironda A, Bertoglio CL, De Martini P, Magistro C, Ferratri G. Totally laparoscopic versus open pancreaticoduodenectomy: a propensity score matching analysis of short-term outcomes. Eur J Surg Oncol. 2021;47:674–680. doi: 10.1016/j.ejso.2020.10.036. [DOI] [PubMed] [Google Scholar]
- 48.Miller BC, Christein JD, Behrman SW, Drebin JA, Pratt WB, Callery MP, Vollmer CM. A multi-institutional external validation of the fistula risk score for pancreatoduodenectomy. J Gastrointest Surg. 2014;18:172–180. doi: 10.1007/s11605-013-2337-8. [DOI] [PubMed] [Google Scholar]
- 49.Winter J, Cameron J, Campbell K, Chang D, Riall T, Schulick R, Choti M, Coleman J, Hodgin M, Sauter P. Does pancreatic duct stenting decrease the rate of pancreatic fistula following pancreaticoduodenectomy? Results of a prospective randomized trial. J Gastrointest Surg. 2006;10:1280–1290. doi: 10.1016/j.gassur.2006.07.020. [DOI] [PubMed] [Google Scholar]
- 50.Kamoda Y, Fujino Y, Matsumoto I, Shinzeki M, Sakai T, Kuroda Y. Usefulness of performing a pancreaticojejunostomy with an internal stent after a pancreatoduodenectomy. Surg Today. 2008;38:524–528. doi: 10.1007/s00595-007-3662-x. [DOI] [PubMed] [Google Scholar]
- 51.Tani M, Kawai M, Hirono S, Ina S, Miyazawa M, Shimizu A, Yamaue H. A prospective randomized controlled trial of internal versus external drainage with pancreaticojejunostomy for pancreaticoduodenectomy. Am J Surg. 2010;199:759–764. doi: 10.1016/j.amjsurg.2009.04.017. [DOI] [PubMed] [Google Scholar]
- 52.Sriussadaporn S, Pak-Art R, Sriussadaporn S, Kritayakirana K, Prichayudh S. Pancreaticoduodenectomy with external drainage of the pancreatic remnant. Asian J Surg. 2008;31:167–173. doi: 10.1016/S1015-9584(08)60080-9. [DOI] [PubMed] [Google Scholar]
- 53.Sanchez-Velazquez P, Muller X, Malleo G, Park JS, Hwang HK, Napoli N, Javed A, Inoue Y, Beghdadi N, Kalisvaart M, Vigia E, Walsh C, Lovasik B, et al. Benchmarks in pancreatic surgery: a novel tool for unbiased outcome comparisons. Ann Surg. 2019;270:211–218. doi: 10.1097/SLA.0000000000003223. [DOI] [PubMed] [Google Scholar]
- 54.Dokmak S, Ftériche FS, Aussilhou B, Bensafta Y, Lévy P, Ruszniewski P, Belghiti J, Sauvanet A. Laparoscopic pancreaticoduodenectomy should not be routine for resection of periampullary tumors. J Am Coll Surg. 2015;220:831–838. doi: 10.1016/j.jamcollsurg.2014.12.052. [DOI] [PubMed] [Google Scholar]
- 55.Croome KP, Farnell MB, Que FG, Reid-Lombardo KM, Truty MJ, Nagorney DM, Kendrick ML (2014) Total laparoscopic pancreaticoduodenectomy for pancreatic ductal adenocarcinoma: oncologic advantages over open approaches? Ann Surg 260:633–638 [DOI] [PubMed]