Abstract
There is a scarcity of literature available regarding the factors affecting life expectancy in bone metastasis (BM). Our objective is to evaluate the factors affecting life expectancy in adult patients with BM. In this prospective cohort study for over 5 years, 111 adults with BM were included in the analysis. The life expectancy was calculated from the time of diagnosis of BM to death. Statistical analysis was done using the SPSS statistical program. The Pearson chi-square test was used to analyze the significance and life expectancy was represented on the Kaplan Meier curve. The overall median survival time was 9 months. The patients with a primary malignancy detected along with BM had a median survival of 9 months. Those without a known primary at the time of diagnosis survived for a median period of 8 months and those with known primary for 14 months (P-value 0.01). The median survival of patients with BM from the lung, breast, and prostate was 6, 14, and 24 months, respectively (P-value 0.001). Only 22% of patients with extraskeletal metastasis in addition to BM survived more than 6 months (P-value 0.013). Patients with neurological deficits had a median survival of 2 months (P-value 0.0001). There was no statistically significant association between gender and the mode of treatment and survival. There was a significant association between life expectancy and mode of presentation, the primary site of origin, presence of extraskeletal secondary, BM with unknown primary, and symptoms on presentation in patients with BM.
Keywords : Bone metastasis, Life expectancy, Median survival
Introduction
Metastasis is the commonest bone tumors in adults [1]. There is an increasing incidence of bone metastasis (BM) due to the recent progress in the treatment of primary malignancy [2]. Even though malignancies from the breast, lung, prostate, kidney, and thyroid are the most common sites of primary, any tumor can metastasize to the bone [3]. Twenty percent of patients with metastatic carcinoma develop clinically evident bone metastasis [4]. The bone is behind only to the lung and liver in the frequency of the site of metastatic disease [5].
Skeletal metastasis is more common in the axial skeleton than in the appendicular skeleton. Skeletal metastasis is indicative of advanced disease [6, 7]. Pain is the most common presentation. Pathological fractures of long bones, vertebral compression fractures, and neurological deficits are other modes of presentation. Lesions in flat bones can remain asymptomatic for a long time [8–10]. A thorough history and physical examination are essential for diagnosis. A complete hemogram with an erythrocyte sedimentation rate (ESR), serum tumor markers, X-rays of the affected region, and a chest X-ray are the initial investigations [11, 12]. Technetium 99 m with methylene diphosphonate (MDP) bone scan, magnetic resonance imaging (MRI) scan, a computed tomographic scan of the lesion and abdomen, and positron emission tomography (PET) scanning are the usual investigations to identify the primary and characterization of BM [13–16]. The biopsy is the gold standard for the diagnosis of metastatic lesions [17]. Improvement of general health, control of local symptoms, and treatment of primary disease are the goals of treatment. A multidisciplinary approach is often required for the treatment of BM [18].
There are very few studies available in the literature which comprehensively evaluate the life expectancy of a patient with BM. Most studies look into prognosis related to BM from a specific tumor or location [19, 20]. It is very important to know the life expectancy of a patient with skeletal metastasis. It is important for decision making in the treatment. Most patients with skeletal metastasis will be in an advanced stage of the disease. They require palliative therapy in the form of radiation. But some cases may require osteosynthesis or prosthetic replacement. Some require spinal decompression and fixation or even chemotherapy. So life expectancy is one important factor in the choice of treatment modality. We hypothesize that some easily identifiable factors have a role in the determination of the life expectancy of adult patients with bone metastasis. Our objective is to evaluate the relationship of gender, mode of presentation, site of primary, presence of extra-skeletal secondary, bone metastasis with unknown primary, symptoms on presentation and mode of treatment, and life expectancy in adult patients with skeletal metastasis.
Materials and Methods
We conducted a prospective cohort study. Institutional research and ethical committee approval were obtained. Our institution is a tertiary care teaching institution. The study was conducted by the orthopedic department in collaboration with the radiotherapy department. The study period was 5 years. A case of bone metastasis is defined as any bony lesion in adults with or without a detectable primary neoplasm with histopathological confirmation of a skeletal secondary. All patients with skeletal metastasis above the age of 30 years were included in the study. We included only patients who were newly admitted. Those who were not willing to participate in the study were excluded. Patients with primary bone tumors, multiple myeloma, and other hematopoietic malignancies were also excluded.
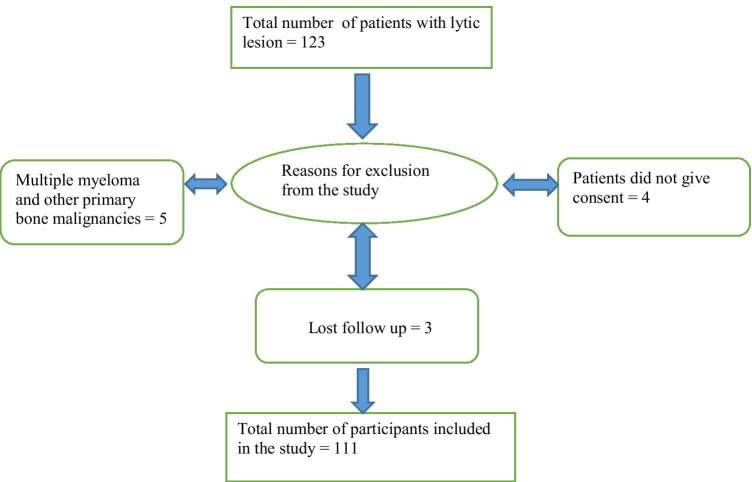
A total of 123 patients with bone lesions were identified. Four patients did not give consent, and 5 patients were excluded because of multiple myeloma and other primary malignancies of the bone. We lost to follow up on 3 patients. So a total of 111 patients were included in our study (Appendix Fig. 7).
A detailed history and physical examination were done in all cases. Examination of the breast, thyroid, abdomen, urogenital system, lung, larynx, and lymph node was routinely done to detect any primary neoplasms. Routine blood investigations, serum calcium, phosphorus, alkaline phosphatase, and tumor markers were done. An ultrasound scan of the abdomen and thyroid was done in indicated patients. The radiogram of the local area and the chest was routine. MRI scans, bone scans, and computerized tomography (CT) scans of the abdomen and chest were included when required especially when the primary is unknown. The diagnosis was confirmed by histo-FNA pathological examination either by fine-needle aspiration cytology (FNAC), trucut biopsy, or by open biopsy. After the diagnosis, surgical management, if any, is done in indicated patients. All patients were then transferred to the radiotherapy department for further treatment. The telephone number and mailing address of patients were collected at the time of admission. We contacted the patients and relatives in between. Routine consultations were done when they were in the hospital and during follow-up. The dates of death of patients who died at their residence were collected using mail or through the telephone. For those who died in the hospital, the dates were collected from the hospital records.
Statistical Analysis
The data were entered and analyzed using Microsoft Excel software. Statistical analysis was done using the SPSS statistical program. The Pearson chi-square test and Fisher exact test were used to analyze the statistical significance. The life expectancy of the patients was analyzed and represented on Kaplan Meier curves. Specifically, we collected data regarding the age, gender, type of detection, site of primary, mode of presentation presence of extra-skeletal metastasis, and mode of treatment for final analysis.
Results
Out of 111 patients, 61% were males with a male to female ratio of 3:2. There were patients between the ages of 31 to 81 years. Most cases were in their sixth decade (36%). Sixty percent of patients presented with pain, and 27% of patients presented with pathological fractures. There were asymptomatic patients with a known primary malignancy (5%). 55% of patients presented with unknown primary. In 41%, there was a known primary. The common sites of the primary malignancy were the lung (38%) followed by the breast (28%), prostate (14%), and thyroid (7%). The spine was the most common site of secondary (47%) followed by the pelvis (18%) and femur in 15%. There was disseminated skeletal metastasis (when more than 2 bones are affected without visceral lesions) in 6% of cases. Isolated lung or liver metastasis was detected along with skeletal lesions in 16 and 14% of patients, respectively. Sixteen percent of patients had more than two sites of extraskeletal involvement and another 12% had disseminated metastasis. Forty-two percent of patients present with isolated skeletal metastasis.
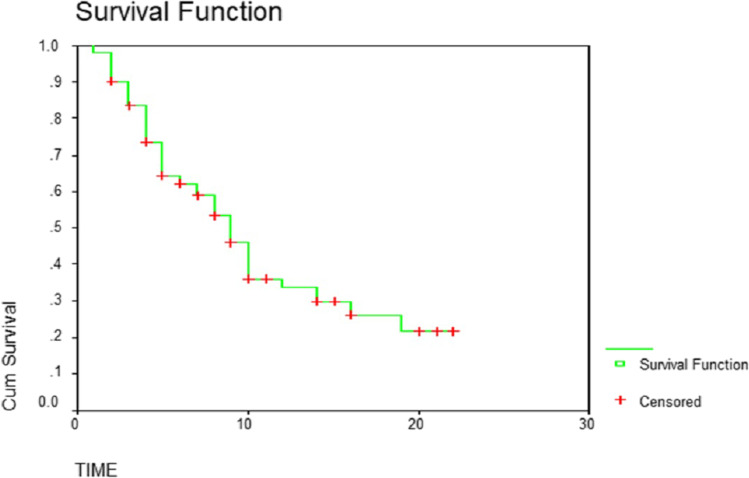
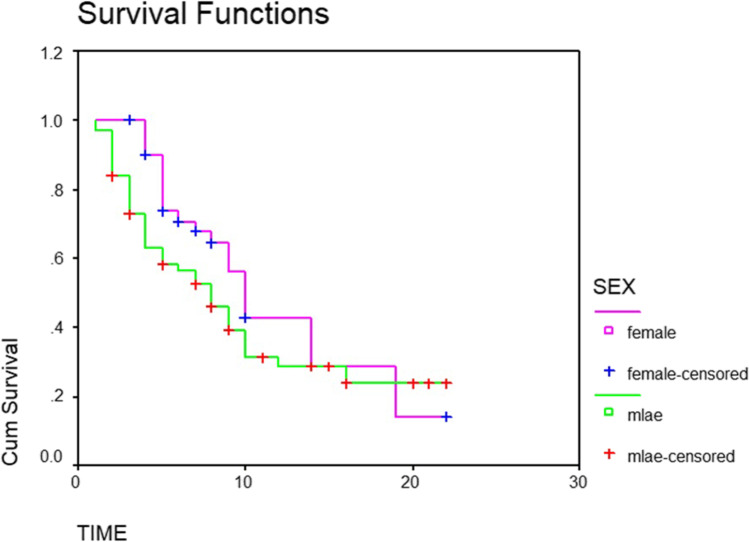
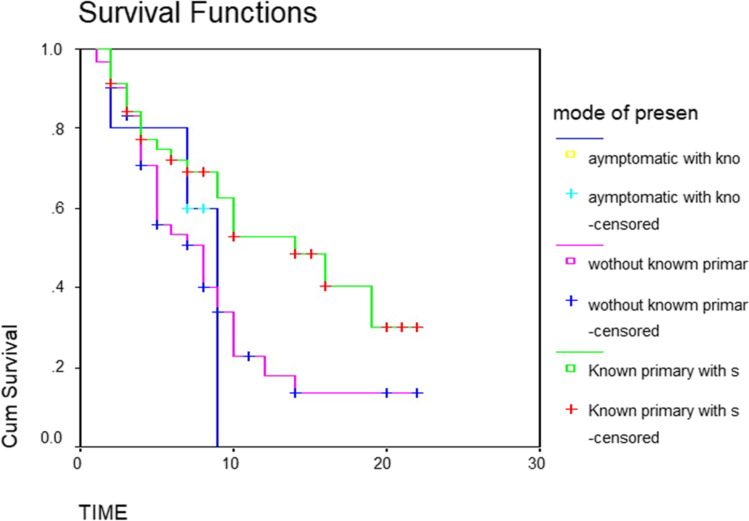
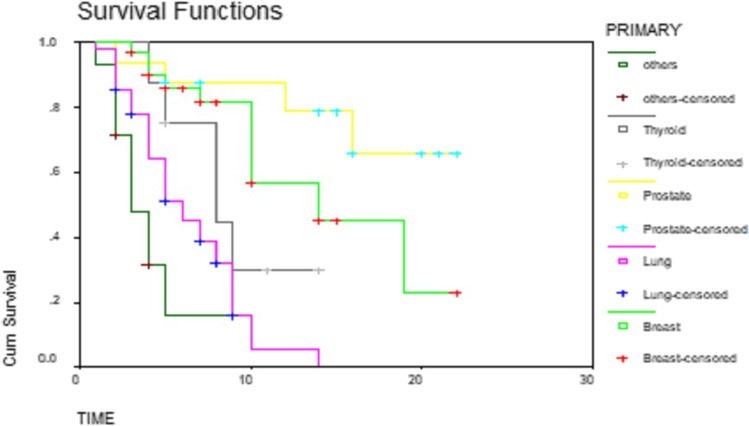
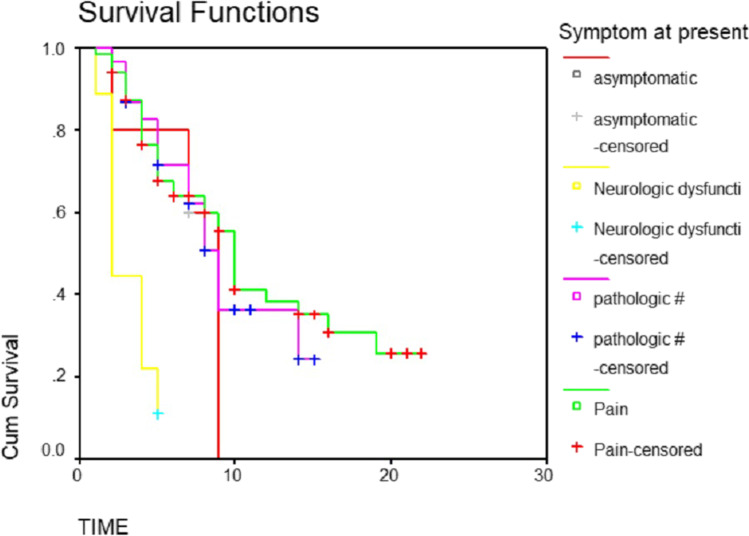
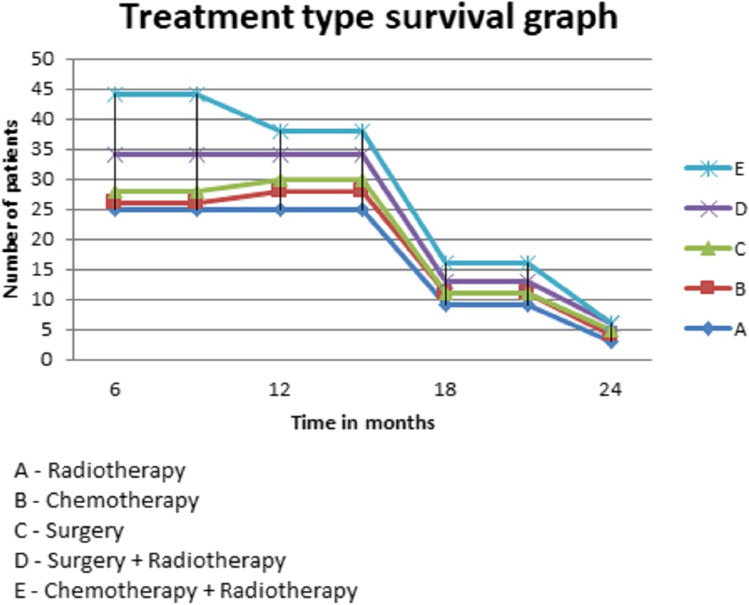
The overall median survival time was 9 months. Fifty percent of patients survived less than 9 months. Thirty-three percent survived between 6 and 12 months. While survival of 13% was between 12 and 18 months, only 4.5% survived up to 24 months (Table 1 and Fig. 1). The median survival for men and women was 8 months and 10 months, respectively (P-value 0.09) (Table 2 and Fig. 2). The patients with a primary malignancy detected along with bone metastasis had a median survival of 9 months, while those without a known primary at the time of diagnosis survived for a median period of 8 months. Patients with a known primary tumor developing a bone metastasis had a long survival rate of 14 months. This was statistically significant with the P-value of 0.03 (Table 3 and Fig. 3). The median survival of bone secondaries from the lung, breast, and prostate was 6, 14, and 24 months, respectively. This was statistically significant with a P-value of 0.0001 (Table 4 and Fig. 4). In metastasis to the spine, if there were neurological deficits at the time of presentation, median survival was 2 months compared to those with back pain (10 months) and pathological fracture (9 months) (P-value 0.0001) (Fig. 5). Only 22% of patients with extra-skeletal metastasis survived more than 6 months compared to 75% of patients with isolated bone lesion had a median survival of 6 months. The P-value is 0.001 (Table 5). There was no statistically significant association between the mode of treatment and survival period (P-value 0.078) (Table 6 and Fig. 6).
Table 1.
Cumulative life expectancy: 50% of patients survived less than 6 months, 33% between 6 to 12 months, only 4.5% survived up to 24 months
| Cumulative life expectancy | Number of patients | Percentage |
|---|---|---|
| < 6 months | 55 | 49.5% |
| 6 months to 1 year | 37 | 33.3% |
| 1 to 1.5 years | 14 | 12.6% |
| 1.5 to 2 years | 5 | 4.5% |
| Total | 111 | 100% |
Fig. 1.
Cumulative survival of patients with bone metastasis plotted on a Kaplan Meier curve
Table 2.
Survival based on gender: the median survival for men was 8 months and 10 months for women (P-value 0.09)
| Gender | Life expectancy < 6 months, number and percentage | Life expectancy > 6 months, number and percentage | Total |
|---|---|---|---|
| Male: number and percentage |
38 55.90% |
30 44.10% |
68 100% |
| Female: number and percentage |
17 39.50% |
26 60.50% |
43 100% |
| Total |
55 49.50% |
56 50.50% |
111 100% |
Fig. 2.
Kaplan Meier curve graph showing life expectancy based on gender
Table 3.
Survival based on the mode of detection: patients developing bone metastasis in a known primary tumor survived for maximum time compared to unknown primary (P-value 0.03)
| Type of presentation | Life expectancy < 6 months, number and percentage | Life expectancy > 6 months, number and percentage | Total |
|---|---|---|---|
| Known primary with BM |
16 35.60% |
29 64.40% |
45 100% |
| Unknown primary with BM |
38 62.80% |
23 37.70% |
61 100% |
| Asymptomatic primary detected at the time of BM |
1 20% |
4 80% |
5 100% |
| Total |
55 49.50% |
56 50.50% |
111 100% |
Fig. 3.
Kaplan Meier curve showing survival based on the type of detection
Table 4.
Survival based on the site of primary: maximum survival was for breast secondaries (24 months) and least for lung (6 months) (P-value 0.001)
| Site of primary | Life expectancy < 6 months, number and percentage | Life expectancy > 6 months, number and percentage | Total |
|---|---|---|---|
| Breast |
8 25.80% |
23 74.20% |
31 100% |
| Lung |
26 61.90% |
16 38.10% |
42 100% |
| Prostate |
3 18.80% |
13 81.30% |
16 100% |
| Thyroid |
4 50.00% |
4 50.00% |
8 100% |
| Kidney |
2 100.00% |
2 100% |
|
| Others |
8 100.00% |
8 100% |
|
| Undetected |
4 100.00% |
4 100% |
|
| Total |
55 49.50% |
56 50.50% |
111 100% |
Fig. 4.
The life expectancy based on the site of primary represented on a Kaplan Meier curve
Fig. 5.
Survival based on the mode presentation plotted in a Meier curve
Table 5.
Survival based on associated visceral metastasis: only 22% of patients with extraskeletal metastasis survived for more than 6 months (P-value 0.001)
| Other sites metastasis with BM | Life expectancy < 6 months, number and percentage | Life expectancy > 6 months, number and percentage | Total |
|---|---|---|---|
| No other visceral metastasis |
12 25.50% |
35 74.50% |
47 100% |
| Lung |
10 55.60% |
8 44.40% |
18 100% |
| Liver |
9 56.30% |
7 43.80% |
16 100% |
| Lymph node |
6 75.00% |
2 25.00% |
8 100% |
| Brain |
3 100% |
3 100% |
|
| Others |
1 100% |
1 100% |
|
| Disseminated metastasis |
14 77.80% |
4 22.20% |
18 100% |
| Total |
55 49.50% |
56 50.50% |
111 100% |
Table 6.
Survival based on the mode of treatment: there was no correlation between the mode of treatment and life expectancy (P-value 0.078)
| Mode of treatment | Life expectancy < 1 year, number and percentage | Life expectancy > 1 year, number and percentage | Total |
|---|---|---|---|
| Radiotherapy |
52 75.40% |
17 24.60% |
69 100% |
| Chemotherapy |
7 100% |
7 100% |
|
| Surgery + Fixation |
4 80.00% |
1 20% |
5 100% |
| Surgery + Radiotherapy |
12 100% |
12 100% |
|
| Chemotherapy + Radiotherapy |
17 94.40% |
1 5.60% |
18 100% |
| Total |
92 82.90% |
19 17.10% |
111 100% |
Fig. 6.
Kaplan Meier graph showing the survival of patients with bone metastasis based on treatment
Discussion
In this study, we have evaluated the relationship of gender, type of presentation, site of primary, presence of extra-skeletal secondary, bone metastasis with unknown primary, and mode of treatment and life expectancy in adult patients with skeletal metastasis. The median survival of patients with bone metastasis was 9 months. Only 4.5% of persons survived beyond 24 months, while more than 50% lost their lives within the first 6 months. By the time there is a bone metastasis, the disease will be mostly in the advanced stage. Once a neoplasm has spread to the bone, it can rarely be cured. Whatever treatment we offer can only slow down its growth [21]. Skeletal metastasis can increase the morbidity in cancer patients due to severe pain, pathological fractures, spinal cord compression, and hypercalcemia [22]. We found that out of the 6 factors except for the mode of treatment, all other factors have a significant association with a survival period in skeletal metastasis.
Out of the 111 patients, there were 67 males and 43 females. The male to female ratio was 3:2. Only 38 (55.9%) males and 17 (39.5%) females survived less than 6 months after the detection of skeletal metastasis irrespective of other factors. The p-value was 0.09. The median survival for men and women was 8 months and 10 months, respectively. In an epidemiological study of skeletal metastasis of unknown primary, the sex ratio of patients was 4:2 [23]. There are reports of sex differences in the occurrence of bone metastasis in breast, lung, and prostate cancer. Different factors affecting the bone marrow environment in either sex like the genetic variation that affects sex hormone levels, the direct effect of sex hormones, or natural factors attributed to this difference. Bone metastasis is common in female-leaning genetic variations or hormonal states that feminize the bone marrow. It has been observed that mastectomy and administration of androgen in breast cancer patients reduce bone metastasis [24]. In yet another study, female sex is considered as a protective factor for bone metastasis from lung cancer [25]. The survival rate of unstable spinal metastasis following breast cancer is low [26].
There were three modes of presentation. One group presented with features of bone metastasis and on investigation, primary tumor was detected. In the second type of presentation, we were not able to find out the primary tumor. In the final category, a known primary was present at the time of the diagnosis of skeletal lesions. When we compared the median survival after detection of skeletal metastasis among these groups it was 9, 8, and 14 months, respectively. So if we cannot find out a primary tumor in skeletal metastasis, it is a bad prognostic indicator. If patients with known malignancy develop skeletal metastasis, they will survive more. This was statistically significant. Metastasis of unknown etiology occurs in 3 to 4% of all malignancies. Among them, 10 to 15% occur in the bone [27]. Adenocarcinoma is the main histological type. The Lung, liver, pancreas, and gastrointestinal tract are the commonest sites of occult primary [28]. Bone metastasis with unknown primary has shown a median survival of 3 months [29]. The lung is the commonest primary site from which bone metastasis of unknown origin occurs [30]. We used to do a contrast-enhanced CT scan of the chest and abdomen, bone scan, and clinical examination of the abdomen, genitourinary system, thyroid, and larynx to find out a primary neoplasm. Expectant screening, early detection, and treatment may be the reason for the prolonged survival of a patient of BM with a known primary tumor.
We calculated the survival rate of bone metastasis from different primary sites, and the results were as follows. The median survival of bone secondaries from the lung, breast, and prostate was 6, 14, and 24 months, respectively. These are statistically significant. The incidence of metastatic bone disease from different neoplasms varies. Cancers of the breast (65–75%), prostate (65–75%), thyroid (60%), lung (30–40%), urinary bladder (40%), renal cell carcinoma (20–25%), and melanoma in 14 to 45% of cases can metastasize to the bone in advanced stages. The median survival of bone secondaries from different primaries also varies. The median survival from the diagnosis of bone metastasis is 6 months in melanoma; 6–7 months in the lung; 6–9 months in the bladder; 12 months in renal cell carcinoma; 12–53 months in the prostate; 19–25 months in malignant melanoma; and 48 months in the thyroid [31]. In a population-based cohort study of survival after bone metastasis, patients with lung cancer were showed the lowest 1-year survival, and patients with breast cancer showed the highest survival. For 5 years, 10% of breast cancer patients had survived [32]. Our results also show the lowest survival for patients with lung cancer and the highest for patients with breast cancers. There are other reports also showing long survival following bone secondaries in breast cancers followed by thyroid and prostate and least for lung cancers [33].
Patients with other extra-skeletal metastasis at the time of diagnosis were found to have a low life expectancy. In our cases, isolated bone metastasis was found only in 42% of cases. Isolated lung and liver metastases were detected along with skeletal lesions in 16 and 14% of patients, respectively. Sixteen percent of patients had disseminated metastasis. Only 22% of patients with extra-skeletal metastasis survived more than 6 months compared to 75% of patients with an isolated bone lesion who had a median survival of 6 months. Except for the carcinoma cervix, ovary, and bladder, the risk of mortality increased when bone metastasis is associated with other areas of metastasis [32, 33]. The presence or absence of metastasis to other vital organs is an important prognostic factor in the life expectancy of patients with skeletal metastasis. In a retrospective study, Kuru et al. showed that in patients with breast cancer, the best survival is for those with isolated bone metastasis followed by those with multiple bone lesions and worst for those associated with visceral metastasis [34]. Visceral metastasis is an important prognostic factor in patients with metastasis in the spine [35]. We have also noticed that those skeletal lesions which are detected by X-ray have a lower survival rate. It is probably due to the long time taken for the lesion to appear in an X-ray.
The management of skeletal metastasis is done by the medical oncology and radiotherapy department. The role of an orthopedic surgeon is only when there is an impending or actual pathological fracture. The medical treatment includes preventive care, therapeutic care, care of medical complications, therapy of underlying cancer, and palliation. Radiotherapy (RT) is useful before the occurrence of pathological fracture. If a fracture is present, its role without surgery is limited. The role of radiotherapy is mainly in pain relief and local control of the tumor [36, 37]. There are not many pieces of evidence in the literature about the superiority of one mode of treatment over another concerning life expectancy in bone secondaries. We have 52 cases who were treated with RT only and seven patients with only CT. In 12 patients, we did surgical fixation and RT. Surgical excision and fixation alone were done in four cases and CT and RT were given to 17 patients. On analyzing the life expectancy among these different groups, we found no significant association.
There is a lack of evidence in the literature regarding the factors affecting life expectancy in patients with skeletal metastasis. Most of the studies conducted in the past were regarding the prognosis of bone secondaries in various cancers especially in the lung and breast. In a study conducted by Sugiura H et al., to analyze the life expectancy in patients with bone secondaries and lung cancer, they found that solitary bone metastasis in the axial skeleton without pathological fracture, females with adenocarcinoma, and early treatment had good prognosis [38]. The survival of non-small cell lung cancer with skeletal metastasis was 9.5 months [33]. In another study to find out the role of the season in the overall survival of patients with bone metastasis undergoing radiotherapy fail to find out any significant association [39]. A systematic review to assess the prognostic value of the factors involved in the revised Tokuhashi Score found that all significant factors must be considered before treatment of spinal metastatic lesions. In a review of bone metastasis in the carcinoma cervix, it has been found that bone metastasis occurs infrequently in the carcinoma cervix. It can shorten the life span and RT has only a palliative role [40]. The life expectancy of patients with lung cancer and bone metastasis is usually less than 6 months and treatment using zoledronic acid is effective in reducing pain [41]. A life expectancy of more than 6 months is needed for the healing of the pathological fracture. In this study, pathological fracture due to renal cell carcinoma and breast cancer united while pathological fracture from lung cancer did not unite [42]. Increased levels of Parathyroid hormone-related protein in patients with hypercalcemia of malignancy indicate advanced tumors and reduced life expectancy [43]. Skeletal metastasis in thyroid cancer usually occurs commonly in patients over 45 years and is multi-centric. The survival is better in patients with no non-osseous metastasis and lesions concentrated radioactive iodine [44]. Bone metastasis, metachronous metastasis, and extra-osseous metastasis were found to be bad prognostic factors in gastric cancers [45]. In head and neck tumors, non-nasopharyngeal tumors with BM have a high risk for pathological fractures, spinal cord compression, and hypercalcemia compared to nasopharyngeal carcinomas. In both varieties, life expectancy is reduced after BM [46]. About 3 to 7% of colorectal cancer metastasizes to bone. Rectal cancers with lymph node metastasis or lung metastasis at the time of surgery are risk factors for BM which is associated with a bad prognosis [47]. Most of the studies related to life expectancy or prognosis of bone metastasis deal with a specific tumor or tumors of a region. In this study, we tried to assess the relationship between some common and easily identifiable factors with the survival of patients with skeletal metastasis. We think it is very simple. This can help the treating surgeon to find out the average expected life expectancy using these factors. This can help them to determine the prognosis of patients and thereby helping them to take decisions on further treatments.
Our study has certain limitations. As it was done in a tertiary care referral hospital, so there is an element of referral bias that is possible. So mortality and complications can be higher than expected. Most of our patients belong to the middle or lower income group, so in most patients, the detection of bone secondaries could have been delayed due to financial constraints. We have not taken into consideration the confounding factors like histology of the tumor and variation of treatment. And lastly, our sample size was small. So we think that further studies with a larger sample size are required for external validation of our results.
Conclusion
Life expectancy assessment in skeletal metastasis is important for management purposes. There was a significant association between life expectancy and type of detection of bone secondaries, site of primary, presence of extraskeletal secondary, bone metastasis with unknown primary, and mode of presentation in patients with skeletal metastasis. There was no significant association between gender, mode of treatment, and life expectancy. We think that further studies with a larger sample size are required for external validation of our results.
Appendix
Fig. 7.
Flow chart showing the selection of participants included in the study
Author Contribution
The corresponding author conceived of the presented idea. He and the second author together design the methodology, collected the data, analyzed the data, and made conclusion. We together wrote the primary draft and later revised and edited it to its present form. The third and fourth authors helped in the statistical analysis and editing and rewriting of the final manuscript.
Declarations
Conflict of Interest
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Balaji Zacharia, Email: balaji.zacharia@gmail.com.
Jerin Joy, Email: drjerry.81@gmail.com.
Dhiyaneswaran Subramaniam, Email: dhiyane.mmc@gmail.com.
Puneeth Katapadi Pai, Email: puneethpaik@gmail.com.
References
- 1.Rose C, Kagan A. The final report of the expert panel for the Radiation Oncology Bone Metastasis Work Group of the American College of Radiology. Int J Radiat Oncol Biol Phys. 1998;40(5):1117–24. doi: 10.1016/s0360-3016(97)00952-8. [DOI] [PubMed] [Google Scholar]
- 2.Shimada H, Setoguchi T, Nakamura S, Yokouchi M, Ishidou Y, Tominaga H, et al. Evaluation of prognostic scoring systems for bone metastases using single-center data. Mol Clin Oncol. 2015;3(6):1361–70. doi: 10.3892/mco.2015.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mundy GR. Metastasis to bone: causes, consequences, and therapeutic opportunities. Nat Rev Cancer. 2002;2(8):584–93. doi: 10.1038/nrc867. [DOI] [PubMed] [Google Scholar]
- 4.Buckwalter JA, Brandser EA. Metastatic disease of the skeleton. Am Fam Physician. 1997;55(5):1761–1768. [PubMed] [Google Scholar]
- 5.Piccioli A, Maccauro G, Spinelli MS, Biagini R, Rossi B. Bone metastases of unknown origin: epidemiology and principles of management. J Orthopaed Traumatol. 2015;16(2):81–6. doi: 10.1007/s10195-015-0344-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Allen-Mersh TG (1997) Cancer: Principles and practice of oncology. 5th ed. V. T. Devita Jr, S. Hellman and S. A. Rosenberg (eds). 286 × 220 mm. Pp. 3312. Illustrated. 1996. Philadelphia, Pennsylvania: Lippincott-Raven. £160. Br J Surg. 84(7):1036–1036. from:10.1002/bjs.1800840754
- 7.Khan MN, Sharfuzzaman A, Mostafa MG. Spinal cord compression as initial presentation of metastatic occult follicular thyroid carcinoma. Journal of Neurosciences in Rural Practice. 2014;5(02):155–9. doi: 10.4103/0976-3147.131661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hage WD, Aboulafia AJ, Aboulafia DM. Incidence, location, and diagnostic evaluation of metastatic bone disease. Orthopedic Clinics of North America. 2000;31(4):515–28. doi: 10.1016/s0030-5898(05)70171-1. [DOI] [PubMed] [Google Scholar]
- 9.Wagner G. Frequency of pain in patients with cancer. Recent Results Cancer Res. 1984;89:64–71. doi: 10.1007/978-3-642-82028-1_7. [DOI] [PubMed] [Google Scholar]
- 10.Stamatopoulos T, Shankar GM, Shin JH (2020) Operative treatment of pathologic compression fractures of the spine. Vertebral Compression Fractures in Osteoporotic and Pathologic Bone 153–182. 10.1007/978-3-030-33861-9_16
- 11.Ortiz A, Lin S-H. Osteolytic and osteoblastic bone metastases: two extremes of the same spectrum? Recent Results Cancer Res. 2012;192:225–233. doi: 10.1007/978-3-642-21892-7_11. [DOI] [PubMed] [Google Scholar]
- 12.Bates SE. Clinical applications of serum tumor markers. Ann Intern Med. 1991;115(8):623. doi: 10.7326/0003-4819-115-8-62. [DOI] [PubMed] [Google Scholar]
- 13.Rosenthal D. Radiologic diagnosis of bone metastases. Cancer. 1997;80(8 suppl 15):1595–607. doi: 10.1002/(sici)1097-0142(19971015)80:83.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 14.Hanna SL, Fletcher BD, Fairclough DL, Jenkins JH, Le AH. Magnetic resonance imaging of disseminated bone marrow disease in patients treated for malignancy. Skeletal Radiol. 1991;20(2):79–84. doi: 10.1007/bf00193815. [DOI] [PubMed] [Google Scholar]
- 15.Yang H-L, Liu T, Wang X-M, Xu Y, Deng S-M. Diagnosis of bone metastases: a meta-analysis comparing 18FDG PET, CT, MRI, and bone scintigraphy. Eur Radiol. 2011;21(12):2604–17. doi: 10.1007/s00330-011-2221-4. [DOI] [PubMed] [Google Scholar]
- 16.Hamaoka T, Madewell JE, Podoloff DA, Hortobagyi GN, Ueno NT. Bone imaging in metastatic breast cancer. JCO. 2004;22(14):2942–53. doi: 10.1200/jco.2004.08.181. [DOI] [PubMed] [Google Scholar]
- 17.Traina F, Errani C, Toscano A, Pungetti C, Fabbri D, Mazzotti A, et al. Current concepts in the biopsy of musculoskeletal tumors. J Bone Joint Surg Am. 2015;97(2):e7. doi: 10.2106/jbjs.n.00661. [DOI] [PubMed] [Google Scholar]
- 18.Mori M, Brus C, Bhatia A, Nishihori T, Blum R. A multidisciplinary approach for bone metastases. J Pain Symptom Manag. 2010;39(2):346–7. doi: 10.1016/j.jpainsymman.2009.11.266. [DOI] [Google Scholar]
- 19.Willeumier JJ, van der Linden YM, van der Wal CWPG, Jutte PC, van der Velden JM, Smolle MA, van der Zwaal P, Koper P, Bakri L, de Pree I, Leithner A, Fiocco M, Dijkstra PDS. An easy-to-use prognostic model for survival estimation for patients with symptomatic long bone metastases. J Bone Joint Surg Am. 2018;100:196–204. doi: 10.2106/JBJS.16.01514. [DOI] [PubMed] [Google Scholar]
- 20.Bollen L, van der Linden YM, Pondaag W, Fiocco M, Pattynama BP, Marijnen CA, Nelissen RG, Peul WC, Dijkstra PD. Prognostic factors associated with survival in patients with symptomatic spinal bone metastases: a retrospective cohort study of 1,043 patients. Neuro Oncol. 2014;16:991–998. doi: 10.1093/neuonc/not318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Macedo F, Ladeira K, Pinho F, Saraiva N, Bonito N, Pinto L, et al. Bone metastases: an overview. Oncol Rev. 2017;11(1):321. doi: 10.4081/oncol.2017.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cecchini MG, Wetterwald A, van der Pluijm G, Thalmann GN. Molecular and biological mechanisms of bone metastasis. EAU Updat Ser. 2005;3(4):214–26. doi: 10.1016/j.euus.2005.09.006. [DOI] [Google Scholar]
- 23.Rougraff BT, Kneisl JS, Simon MA. Skeletal metastases of unknown origin. A prospective study of a diagnostic strategy. J Bone Joint Surg Am. 1993;75(9):1276–81. doi: 10.2106/00004623-199309000-00003. [DOI] [PubMed] [Google Scholar]
- 24.Farach-Carson MC, Sue-Hwa L, Theresa N, Robert LS. Sex differences and bone metastases of breast, lung, and prostate cancers: do bone homing cancers favor feminized bone marrow? Front Oncol. 2017;7:163. doi: 10.3389/fonc.2017.00163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ma W, Peltzer K, Qi L, Xu G, Liu Z, Wang J, et al. Female sex is associated with a lower risk of bone metastases and favorable prognosis in non-sex-specific cancers. BMC Cancer. 2019;19(1):1001. doi: 10.1186/s12885-019-6168-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Foerster R, Bruckner T, Bostel T, Schlampp I, Debus J, Rief H (2015) Prognostic factors for survival of women with unstable spinal bone metastases from breast cancer. Radiat Oncol 10 (1) 10.1186/s13014-015-0458-9 [DOI] [PMC free article] [PubMed]
- 27.Nottebaert M, Exner GU, von Hochstetter AR, Schreiber A. Metastatic bone disease from occult carcinoma: a profile. Int Orthop. 1989;13(2):119–23. doi: 10.1007/bf00266372. [DOI] [PubMed] [Google Scholar]
- 28.Airoldi G (2012) Cancer of unknown primary origin: utility and futility in clinical practice. Ital J Med. 10.4081/itjm.2012.315
- 29.Hemminki K, Riihimäki M, Sundquist K, Hemminki A. Site-specific survival rates for cancer of unknown primary according to the location of metastases. Int J Cancer. 2013;133(1):182–9. doi: 10.1002/ijc.27988. [DOI] [PubMed] [Google Scholar]
- 30.Vandecandelaere M, Flipo R-M, Cortet B, Catanzariti L, Duquesnoy B, Delcambre B. Bone metastases revealing primary tumors. Comparison of two series separated by 30 years. Joint Bone Spine. 2004;71(3):224–9. doi: 10.1016/s1297-319x(03)00123-4. [DOI] [PubMed] [Google Scholar]
- 31.Selvaggi G, Scagliotti GV. Management of bone metastases in cancer: a review. Crit Rev Oncol Hematol. 2005;56(3):365–78. doi: 10.1016/j.critrevonc.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 32.Svensson E, Christiansen CF, Ulrichsen SP, Rørth MR, Sørensen HT. Survival after bone metastasis by primary cancer type: a Danish population-based cohort study. BMJ Open. 2017;7(9):e016022. doi: 10.1136/bmjopen-2017-016022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Singh V, Haseeb A, Alkubaisi A. Incidence and outcome of bone metastatic disease at University Malaya Medical Centre. Smedj. 2014;55(10):539–46. doi: 10.11622/smedj.2014138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gulcelik MA, Gulcelik NE, Kuru B, Camlibel M, Alagol H. Prognostic factors determining survival in differentiated thyroid cancer. J Surg Oncol. 2007;96(7):598–604. doi: 10.1002/jso.20845. [DOI] [PubMed] [Google Scholar]
- 35.Lun D, Chen N, Feng J et al (2020) Visceral metastasis: a prognostic factor of survival in patients with spinal metastases. Orthop Surg 12(2):552–560. 10.1111/os.12657 [DOI] [PMC free article] [PubMed]
- 36.Chow E, Zeng L, Salvo N, Dennis K, Tsao M, Lutz S. Update on the systematic review of palliative radiotherapy trials for bone metastases. Clinical Oncology. 2012;24(2):112–24. doi: 10.1016/j.clon.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 37.Ford JA, Jones R, Elders A, Mulatero C, Royle P, Sharma P, et al. Denosumab for treatment of bone metastases secondary to solid tumors: systematic review and network meta-analysis. Eur J Cancer. 2013;49(2):416–30. doi: 10.1016/j.ejca.2012.07.016. [DOI] [PubMed] [Google Scholar]
- 38.Sugiura H, Yamada K, Sugiura T, Hida T, Mitsudomi T. Predictors of survival in patients with bone metastasis of lung cancer. Clin Orthop Relat Res. 2008;466(3):729–36. doi: 10.1007/s11999-007-0051-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cheng G, Gu C, Song Z (2016) Impact of metastasis site for survival of patients with advanced thymic epithelial tumors. Transl Cancer Res 5(5):546–551. 10.21037/tcr.2016.09.35
- 40.Ratanatharathorn V, Powers WE, Steverson N, Han I, Ahmad K, Grimm J. Bone metastasis from cervical cancer. Cancer. 1994;73(9):2372–9. doi: 10.1002/1097-0142(19940501)73:93.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 41.Katakami N. Lung cancer with bone metastasis. Cancer & Chemotherapy. 2006;8(33):1049–1053. [PubMed] [Google Scholar]
- 42.GAINOR BJ, BUCHERT P. Fracture healing in metastatic bone disease. Clinical Orthopaedics and Related Research [Internet]. 1983 [cited 2020 Apr 10];NA;(178):297???302. from: 10.1097/00003086-198309000-00041 [PubMed]
- 43.Pecherstorfer M, Schilling T, Blind E, Zimmer-Roth I, Baumgartner G, Ziegler R, et al. Parathyroid hormone-related protein and life expectancy in hypercalcemic cancer patients. J Clin Endocrinol Metab. 1994;78(5):1268–70. doi: 10.1210/jcem.78.5.8175989. [DOI] [PubMed] [Google Scholar]
- 44.Pittas AG, Adler M, Fazzari M, Tickoo S, Rosai J, Larson SM, et al. Bone metastases from thyroid carcinoma: clinical characteristics and prognostic variables in one hundred forty-six patients. Thyroid. 2000;10(3):261–8. doi: 10.1089/thy.2000.10.261. [DOI] [PubMed] [Google Scholar]
- 45.Mikami J, Kimura Y, Makari Y, Fujita J, Kishimoto T, Sawada G, et al (2017) Clinical outcomes and prognostic factors for gastric cancer patients with bone metastasis. World J Surg Onc 15 (1). 10.1186/s12957-016-1091-2 [DOI] [PMC free article] [PubMed]
- 46.Grisanti S, Bianchi S, Locati LD, Triggiani L, Vecchio S, Bonetta A, et al. Bone metastases from head and neck malignancies: prognostic factors and skeletal-related events. PLoS ONE. 2019;14(3):e0213934. doi: 10.1371/journal.pone.0213934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Christensen TD, Jensen SG, Larsen FO, Nielsen DL. Systematic review: incidence, risk factors, survival, and treatment of bone metastases from colorectal cancer. J Bone Oncol. 2018;13:97–105. doi: 10.1016/j.jbo.2018.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]