Abstract
Pulmonary aspiration of gastric contents during elective surgery remains a major cause of airway-related mortality and morbidity. The preoperative fasting times for solids and liquids have been standardized across various anesthesia society guidelines. Enhanced Recovery After Surgery (ERAS) guidelines now advocate liberal clear fluid intake with carbohydrate loading up to 2 h preoperatively. The aim of the study was to assess whether practicing both ASA fasting guidelines and ERAS protocol makes the patients prone to a full stomach. The supine position standard curvilinear ultrasound probe (2–5 MHz) with Sonosite M-Turbo ©system was used to obtain the images. Gastric residual volume (GRV) was derived from the cross-sectional area (CSA) using the Perlas and colleagues model. A total of 102 patients were recruited and analyzed. The mean age and BMI were 50.65 years ± 13.35 years and 22.23 kg/m2 ± 3.7 kg/m2, respectively. A total of four patients (3.92%) had gastric volume > 1.5 ml/kg; out of these four patients, three were female and one was male. We did not observe any case of pulmonary aspiration in any of our patients. In conclusion, even though for elective surgeries, the current fasting guidelines are adequate, these findings cannot be extrapolated to patients with risk factors for high gastric residual volume where further studies need to be performed.
Keywords: Aspiration, ERAS, Fasting, Gastric volume, Ultrasonography
Introduction
Pulmonary aspiration of gastric contents during elective surgery remains a major cause of airway-related mortality and morbidity. According to the National Audit Project (NAP4) by the Royal College of Anesthetists, pulmonary aspiration is the single most common cause of death from airway management incidents [1].
Preoperative fasting continues to be the single most widely used technique to mitigate this risk of pulmonary aspiration [2]. The preoperative fasting times for solids and liquids have been standardized across various anesthesia society guidelines. Enhanced Recovery After Surgery (ERAS) guidelines now advocate liberal clear fluid intake with carbohydrate loading up to 2 h preoperatively [3]. Additionally, there is considerable patient variability in gastric emptying times. A recent study stated that even among patients who adhere to fasting guidelines, up to 4.5% of the patients may present with a full stomach [4].
There has been increasing interest in the application of point-of-care (POC) gastric ultrasonography to identify the “at-risk” stomach in recent times. POC ultrasonography is a safe, simple, non-invasive, and quick method which can have a significant positive impact on outcomes following the intervention [2]. In our institute, we follow the American Society of Anesthesiologists (ASA) fasting guidelines and ERAS protocol of carbohydrate loading 2 h prior to surgery for all patients posted for elective gastrointestinal cancer resection surgeries.
We hypothesized that all patients posted for elective gastrointestinal cancer surgeries who followed ASA fasting guidelines and enrolled under ERAS protocol are having “at-risk” stomachs.
The aim of the study was to assess whether practicing both ASA fasting guidelines and ERAS protocol make the patients prone to a full stomach, having potential risk for pulmonary aspiration, and also emphasize on POC ultrasonography for identifying such cases, thus reducing morbidity.
Methods
This study was undertaken at a tertiary care cancer institute between May 2019 and November 2019 after obtaining approval from the Institutional Ethics Committee. The trial is registered with the Clinical Trial Registry – India (CTRI/2018/11/016479). Inclusion criteria for the study were elective gastrointestinal surgeries, age between 18 and 85 years of both genders. Exclusion criteria were refusal of consent, a patient needing rapid sequence induction, pregnancy, body mass index (BMI) of < 19 or > 40 kg/m2, and large abdominal mass likely to interfere with ultrasound imaging.
All patients who were eligible for the study were fasted according to ASA fasting guidelines. According to local institutional ERAS protocol, 50 g of carbohydrate loading in the form of non-carbonated without pulp drink (appy juice; total volume: 160 ml; every 100 ml containing 15.8 g carbohydrate) was given at night and 2 h prior to the surgery. Patients with a history of diabetes mellitus were given only water in place of carbohydrate loading.
In the operating room, standard ASA monitors were attached and an intravenous line was secured. Patient posted for open surgery was planned for epidural insertion in lateral position for intraoperative and postoperative pain management followed by general anesthesia. Patients posted for laparoscopic surgery were planned for general anesthesia without an epidural.
All of the gastric ultrasounds were performed prior to induction of anesthesia by a single consultant intensivist accredited and experienced (10 years) in advanced critical care ultrasonography. The first ten scans were pilot studies and were cross-checked by a consultant radiologist to ensure accuracy with future scans.
Our site of assessment was gastric antrum in the supine position. It is found superficially between the left lobe of the liver anteriorly and the pancreas posteriorly in a sagittal or para-sagittal scanning plane in the epigastrium. Important vascular landmarks, including both the aorta or inferior vena cava (IVC) and either the superior mesenteric artery or vein, can be used to standardize a scanning plane through the antrum (Fig. 1). A standard curvilinear ultrasound probe (2–5 MHz) with Sonosite M-Turbo ©system was used to obtain the images. Gastric residual volume (GRV) was derived from a cross-sectional area (CSA) using the Perlas and colleagues model. Quantitative assessment of GRV is done by measurement of CSA based on the previous study which assumes the antrum to be an elliptical structure, hence requiring measurement of two perpendicular diameters, i.e., anteroposterior (AP) and craniocaudal (CC).
Fig. 1.
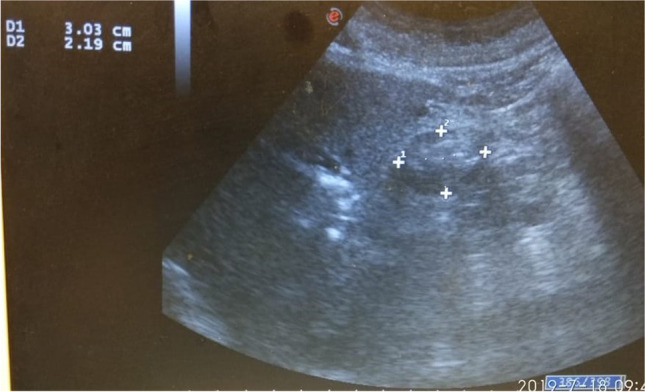
Ultrasonographic measurement of gastric residual volume
Formula of GRV (ml): 27.0 + 14.6 × right lateral CSA(cm2) − 1.28 × age (year).
Despite some controversies, the most accepted upper limit of normal residual gastric secretion in the electively fasted adult is 1.5 ml/kg of actual body weight.
Based on GRV, the patients were divided into two groups, those with GRV < 1.5 ml/kg consistent with baseline gastric secretions and at low risk of pulmonary aspiration. GRV > 1.5 ml/kg in the setting of electively fasted patients is likely to represent delayed gastric emptying and hence would be at higher risk of pulmonary aspiration [5].
After performing gastric ultrasound, anesthesia was induced with injection of fentanyl 2 mcg/kg, injection of propofol 2 mg/kg, and injection of vecuronium 0.1 mg/kg and the trachea was intubated with an appropriate size-cuffed endotracheal tube. Anesthesia was maintained on sevoflurane in oxygen and nitrous oxide. The intermittent bolus of muscle relaxant and epidural infusion on 0.1% levobupivacaine and 2 mcg/ml fentanyl was given as needed.
On the basis of published literature, we identified and selected six risk factors for gastroparesis and these risk factors were studied for GRV and risk of aspiration [6–13]:
diabetes mellitus for > 10 years,
diagnosed case of duodenal ulcer,
post-pylorus preserving pancreaticoduodenectomy surgery < 6 months,
hypothyroid for > 4 months,
diagnosed case of parkinsonism or receiving drugs such as tapentadol or oxycodone, morphine and tricyclic antidepressants such as amitryptiline
Statistical analysis was done using the SPSS version 22 (SPSS Inc., Chicago, IL©). The demographic variables (i.e., age, sex, weight, height, and body mass index) were analyzed using descriptive analysis. Categorical data was presented as counts and percentages. Correlation between gastric volume and risk factors of gastroparesis was compared using Chi-square test, Fisher’s exact test, or Mann–Whitney U formula as appropriate. Statistical significance was considered when p < 0.05 (Table 1).
Table 1.
Demographic details and outcomes in all patients
| Variable | Level | GRV < = 1.5 ml/kg (n = 98) | GRV > 1.5 ml/kg (n = 4) | Total (n = 102) | p-value |
|---|---|---|---|---|---|
| Sex | Female | 42 (42.9) | 3 (75.0) | 45 (44.1) | |
| Male | 56 (57.1) | 1 (25.0) | 57 (55.9) | 0.32 | |
| BMI | Mean (SD) | 22.2 (3.7) | 22 (3.8) | 22.2 (3.7) | 0.90 |
| ASA | 1 | 51 (52.0) | 3 (75.0) | 54 (52.9) | 0.65 |
| 2 | 42 (42.9) | 1 (25.0) | 43 (42.2) | ||
| 3 | 5 (5.1) | 0 (0.0) | 5 (4.9) | ||
| Diabetes mellitus > 10 years | No | 90 (91.8) | 4 (100.0) | 94 (92.2) | 1.00 |
| Yes | 8 (8.2) | 0 (0.0) | 8 (7.8) | ||
| Hypothyroidism > 4 months | No | 93 (94.9) | 4 (100.0) | 97 (95.1) | 1.00 |
| Yes | 5 (5.1) | 0 (0.0) | 5 (4.9) | ||
| Morhpine | No | 97 (99.0) | 4 (100.0) | 101 (99.0) | 1.00 |
| Yes | 1 (1.0) | 0 (0.0) | 1 (1.0) |
ASA, American Society of Anesthesiologist; BMI, body mass index; GRV, gastric residual volume
Results
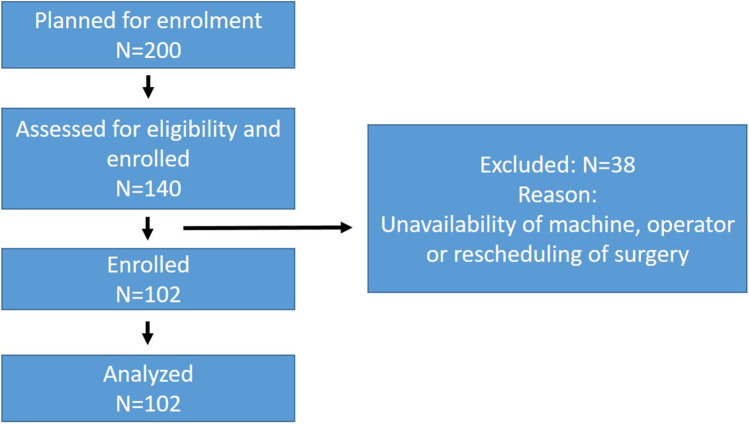
We planned to enroll 200 patients posted for major gastrointestinal cancer surgeries. A total of 140 patients were screened for eligibility and enrolled in the study. Out of 140 patients, 38 were excluded due to unavailability of machine, operator, or rescheduling of surgery (Fig. 2). An interim analysis was conducted on 102 patients, and it was observed that the confidence interval was 96% with (0.8%) when the sample proportion incidence of GRV > 1.5 ml/kg is 4%. Thus, further study and analysis of a sample size of 200 patients was unwarranted as it would not have a significant impact on the result.
Fig. 2.
Consort flowchart
Of 102 patients, 57 were male and 45 were female; the mean age of the study population was 50.65 years ± 13.35 years. The mean BMI of the study population was 22.23 kg/m2 ± 3.7 kg/m2.
Among 102 patients, four patients (3.92%) had gastric volume > 1.5 ml/kg. Of these, three were female and one was male. Patients were either ASA class I or II; none of the patients with GRV > 1.5 ml/kg fell in ASA category III and above. One patient out of these four had a risk factor for delayed gastric emptying (diabetes mellitus). We did not observe any case of pulmonary aspiration in any of our patients. The mean age of patients with GRV > 1.5 ml/kg was 35 years ± 14 years, and this was statistically significant (p < 0.034).
No statistically significant association was found between GRV and sex, BMI, or ASA status of the patient in our study.
Eight patients were identified to have diabetes mellitus of long duration (> 10 years), and one had GRV > 1.5 ml/kg. No statistical association was found between GRV and patients with long-standing diabetes mellitus (p = 1.000).
Among the other risk factors, five patients with hypothyroidism were identified in the study population. None of them had a GRV of > 1.5 ml/kg, and no association was found between hypothyroidism and increased GRV (p = 1.000).
One patient had a history of chronic use of morphine with GRV < 1.5 ml/kg. None of the other identified risk factors were found in the study population.
Discussion
In our study, we observed that 4 patients had gastric volume > 1.5 ml/kg. One of them had a risk factor for delayed gastric emptying (diabetes mellitus) with a primary diagnosis of carcinoma stomach. The rest of the three patients had a primary diagnosis of carcinoma pancreas and rectum with no associated comorbidities. This estimates the incidence of “at-risk” fasted stomach to be 3.92% in elective surgical patients. Our results are similar to previous studies which estimate the risk to be 4.5% in electively fasted patients [4]. This result is reassuring given that our cohort of patients was allowed liberal intake of fluids with carbohydrate loading preoperatively. Additionally, all of our patients had tumors of the gastrointestinal tract who were allowed liberal fluid intake up to 2 h prior to surgery. Given the low incidence of GRV > 1.5 ml/kg in this group, it can be safely said that primary cancers of the GI tract do not delay gastric emptying. A study done by Sakai et al. in 2006 reported a similar finding with a low incidence (1:4580) of aspiration in patients anesthetized for primary GI pathology [14]. Aspiration pneumonia reported by Mendelson for more than 70 years continues to be a major cause of morbidity and mortality associated with anesthesia. Warner et al. conducted one of the first large-scale retrospective studies to assess the incidence of aspiration associated with anesthesia in 1993. They reported an overall incidence of aspiration to be 1 in 3216; however, in emergency cases, this rose to 1 in 895. In addition to causing significant morbidity from respiratory failure, acute lung injury, and multi-organ failure, the associated mortality is about 20% [15].
An important risk factor for aspiration is gastric volume, determined in large part by gastric emptying. In patients with anticipated delayed gastric emptying times, it may not be possible to rely on preoperative fasting guidelines to avoid aspiration.
Ultrasound has progressively emerged as a useful replacement because it is cheap and can be performed at the bedside. Point-of-care ultrasound (POCUS) is being increasingly used to qualify and quantify gastric residual volume in various perioperative settings. In the case of gastric ultrasound, this is typically a dichotomous question. Is the patient’s stomach “empty” or “full”? Gastric ultrasound has been studied in pregnant and non-pregnant adults, severely obese subjects, elective and non-elective situations, and pediatric patients [2]. Several recent editorials in major anesthesiology journals have called for greater adoption and teaching of gastric POCUS in anesthesia practice [16–18]. Benhamou suggested that this skill should be part of the basic armamentarium of anesthesiologists for daily practice [16]. Meineri et al. reported a POCUS curriculum for anesthesiologists that includes gastric ultrasound along with other more established applications such as lung and cardiac assessment [17].
Current understanding accepts 1.5 ml/kg (actual body weight) as the upper limit of normal for gastric secretion or clear fluid content. This value would approximate to 100–130 ml in the average adult and correlates with the 95th centile for fasted elective surgical (obstetric or non-obstetric) patients. Therefore, in the presence of antral fluid, a volume of more than 1.5 ml/kg is considered as the critical volume threshold of gastric fluid that by itself increases the risk of aspiration [2].
Geriatric patients may be considered at higher risk of regurgitation of gastric content due to a decrease in gastric motility and also increase in chances of hiatal hernia and gastroesophageal reflux. In our study, we found no association between the increase in gastric residual volume and increase in age as the mean age of patients with GRV > 1.5 ml/kg was 35 ± 14.30. A similar result was observed in some studies where they found that as age increases, the gastric volume decreases. One possible explanation for this could be the decrease in gastric secretion by 50% with advancing age. Also, it was observed that the gastric wall is more compliant in older than younger patients [19]. However, Kaydu and Gokcek did a preoperative assessment of ultrasonographic measurement of antral area for gastric content. They found a weak correlation between age and antral cross-sectional area (p < 0.05). As the age increases, the antral area of gastric content increases [20].
Obese patients are considered more likely to possess high volume and low pH (HVLP) gastric contents even after standard preoperative fasting. Out of 102 patients, 4 patients had a BMI of > 30 kg/m2 but the gastric volume was observed to be < 1.5 ml/kg in all of them. A study conducted by Harter et al. [21] assessed the pH and volume of gastric content between obese and lean patients. They found that obesity is associated with a significantly decreased risk of HVLP gastric contents among surgical patients with no history of gastroesophageal pathology after a normal interval of preoperative fasting. However, many studies have shown a positive correlation between BMI and antral cross-sectional area, thus implying that patients with higher BMI were 1.07 times more at risk for aspiration [22].
Diabetes mellitus is one of the risk factors for delayed gastric emptying. In our study group, eight patients had diabetes mellitus for > 10 years. But the gastric volume was observed to be < 1.5 ml/kg in all of them. Some studies also found no association between gastric volume and diabetes [22, 23]. However, Sabry et al. [24] observed that the antral cross-section and predicted gastric volume was higher in diabetic patients as compared to non-diabetic patients in semi-sitting and also in right lateral position and a positive correlation was observed between aspirated volume from the nasogastric tube and antral cross-sectional area in both supine and right lateral position.
A reduction in motor activity of the stomach, small intestine, and colon has been reported in hypothyroid patients, causing a significant reduction in gastric emptying. There were five patients among our total study group who were hypothyroid for more than 4 months, and GRV was observed to < 1.5 ml/kg. Although a study conducted by Yaylali et al. determined that there was delayed esophageal emptying and the esophageal transit time was extended (50.5 s) in a hypothyroid patient [9].
Kaufman et al. analyzed the role of opiate receptors in the regulation of colonic transit. It was observed that morphine caused slower filling at 2 h, with increased retention at 44 h as compared with saline [12]. In our study, one patient had a history of chronic use of morphine with a gastric volume of < 1.5 ml/kg. The number of patients on chronic opioid medicines in our sample size is insufficient to make a statistical inference.
Other risk factors for delayed gastric emptying are duodenal ulcer, post-pylorus preserving pancreaticoduodenectomy, Parkinson’s disease, and patient on drugs such as tapentadol, oxycodone, and amitriptyline. Many reports have determined a positive correlation between the above-mentioned risk factors and delayed gastric emptying [7, 8, 10, 11, 13].
There were some limitations in this study. We did not measure the CSA in the right lateral position which is most sensitive for picking up gastric volume. Further research is required in larger groups or groups with specific risk factors to determine any significant association with gastric residual volume. The current fasting guidelines are adequate for elective surgeries, but the group with risk factors for gastroparesis needs to be studied to find a significant association with increased gastric residual volume.
Conclusion
Ultrasound assessment of preoperative gastric volume is an effective screening tool in patients and can become a standard of care, especially in patients prone to gastroparesis, as it would certainly help to determine individual risk and subsequent prophylactic strategy. Even though for elective surgeries the current fasting guidelines are adequate, these findings cannot be extrapolated to patients with risk factors for high gastric residual volume where further studies need to be performed.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Cook TM, Woodall N, Frerk C, Fourth National Audit Project Major complications of airway management in the UK: results of the Fourth National Audit Project of the Royal College of Anaesthetists and the Difficult Airway Society Part 1: anaesthesia. Br J Anaesth. 2011;106(5):617–31. doi: 10.1093/bja/aer058. [DOI] [PubMed] [Google Scholar]
- 2.El-Boghdadly K, Wojcikiewicz T, Perlas A. Perioperative point-of-care gastric ultrasound. BJA Educ. 2019;19(7):219–226. doi: 10.1016/j.bjae.2019.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Melnyk M, Casey RG, Black P, Koupparis AJ. Enhanced recovery after surgery (ERAS) protocols: time to change practice? Can UrolAssoc J. 2011;5:342–348. doi: 10.5489/cuaj.11002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Van de Putte P, Vernieuwe L, Jerjir A, Verschueren L, Tacken M, Perlas A. When fasted is not empty: a retrospective cohort study of gastric content in fasted surgical patients. Br J Anaesth. 2017;118(3):363–371. doi: 10.1093/bja/aew435. [DOI] [PubMed] [Google Scholar]
- 5.Doctor JR, Chandan P, Shetty N, Gala K, Ranganathan P. Ultrasound-guided assessment of gastric residual volume in patients receiving three types of clear fluids: a randomised blinded study. Indian J Anaesth. 2021;65:289–294. doi: 10.4103/ija.IJA_1291_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Krishnan B, Babu S, Walker J, Walker AB, Pappachan JM. Gastrointestinal complications of diabetes mellitus. World J Diabetes. 2013;4(3):51–63. doi: 10.4239/wjd.v4.i3.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kerrigan DD, Read NW, Houghton LA, Taylor ME, Johnson AG. Disturbed gastroduodenal motility in patients with active and healed duodenal ulceration. Gastroenterology. 1991;100(4):892–900. doi: 10.1016/0016-5085(91)90261-i. [DOI] [PubMed] [Google Scholar]
- 8.Tanaka M. Gastroparesis after a pylorus-preserving pancreatoduodenectomy. Surg Today. 2005;35(5):345–350. doi: 10.1007/s00595-004-2961-8. [DOI] [PubMed] [Google Scholar]
- 9.Yaylali O, Kirac S, Yilmaz M, Akin F, Yuksel D, Demirkan N, Akdag B. Does hypothyroidism affect gastrointestinal motility? Gastroenterol Res Pract. 2009;2009:529802. doi: 10.1155/2009/529802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goetze O, Wieczorek J, Mueller T, Przuntek H, Schmidt WE, Woitalla D. Impaired gastric emptying of a solid test meal in patients with Parkinson’s disease using 13C-sodium octanoate breath test. Neurosci Lett. 2005;375(3):170–173. doi: 10.1016/j.neulet.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 11.Jeong ID, Camilleri M, Shin A, Iturrino J, Boldingh A, Busciglio I, Burton D, Ryks M, Rhoten D, Zinsmeister AR. A randomised, placebo-controlled trial comparing the effects of tapentadol and oxycodone on gastrointestinal and colonic transit in healthy humans. Aliment Pharmacol Ther. 2012;35(9):1088–1096. doi: 10.1111/j.1365-2036.2012.05040.x. [DOI] [PubMed] [Google Scholar]
- 12.Kaufman PN, Krevsky B, Malmud LS, Maurer AH, Somers MB, Siegel JA, Fisher RS. Role of opiate receptors in the regulation of colonic transit. Gastroenterology. 1998;94(6):1351–1356. doi: 10.1016/0016-5085(88)90673-7. [DOI] [PubMed] [Google Scholar]
- 13.Bouras EP, Talley NJ, Camilleri M, Burton DD, Heckman MG, Crook JE, Richelson E. Effects of amitriptyline on gastric sensorimotor function and postprandial symptoms in healthy individuals: a randomized, double-blind, placebo-controlled trial. Am J Gastroenterol. 2008;103(8):2043–2050. doi: 10.1111/j.1572-0241.2008.02021.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sakai T, Planinsic RM, Quinlan JJ, Handley LJ, Kim TY, Hilmi IA. The incidence and outcome of perioperative pulmonary aspiration in a university hospital: a 4-year retrospective analysis. Anesth Analg. 2006;103(4):941–947. doi: 10.1213/01.ane.0000237296.57941.e7. [DOI] [PubMed] [Google Scholar]
- 15.Warner MA, Warner ME, Weber JG. Clinical significance of pulmonary aspiration during the perioperative period. Anesthesiology. 1993;78(1):56–62. doi: 10.1097/00000542-199301000-00010. [DOI] [PubMed] [Google Scholar]
- 16.Benhamou D. Ultrasound assessment of gastric contents in the perioperative period: why is this not part of our daily practice? Br J Anaesth. 2015;114(4):545–548. doi: 10.1093/bja/aeu369. [DOI] [PubMed] [Google Scholar]
- 17.Meineri M, Bryson GL, Arellano R, Skubas N. Core point-of-care ultrasound curriculum: what does every anesthesiologist need to know? Can J Anaesth. 2018;65(4):417–426. doi: 10.1007/s12630-018-1063-9. [DOI] [PubMed] [Google Scholar]
- 18.Lucas DN, Elton CD. Through a glass darkly – ultrasound imaging in obstetric anaesthesia. Anaesthesia. 2016;71(6):617–622. doi: 10.1111/anae.13466. [DOI] [PubMed] [Google Scholar]
- 19.Manchikanti L, Colliver JA, Marrero TC, Roush JR. Assessment of age-related acid aspiration risk factors in pediatric, adult, and geriatric patients. Anesth Analg. 1985;64(1):11–17. doi: 10.1213/00000539-198501000-00003. [DOI] [PubMed] [Google Scholar]
- 20.Kaydu A, Gokcek E. Preoperative assessment of ultrasonographic measurement of antral area for gastric content. Med SciMonit. 2018;24:5542–5548. doi: 10.12659/MSM.908520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harter RL, Kelly WB, Kramer MG, Perez CE, Dzwonczyk RR. A comparison of the volume and pH of gastric contents of obese and lean surgical patients. Anesth Analg. 1998;86(1):147–152. doi: 10.1097/00000539-199801000-00030. [DOI] [PubMed] [Google Scholar]
- 22.Sharma S, Deo AS, Raman P. Effectiveness of standard fasting guidelines as assessed by gastric ultrasound examination: a clinical audit. Indian J Anaesth. 2018;62(10):747–752. doi: 10.4103/ija.IJA_54_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dupont G, Gavory J, Lambert P, Tsekouras N, Barbe N, Presles E, Bouvet L, Molliex S. Ultrasonographic gastric volume before unplanned surgery. Anaesthesia. 2017;72(9):1112–1116. doi: 10.1111/anae.13963. [DOI] [PubMed] [Google Scholar]
- 24.Sabry R, Hasanin A, Refaat S, Abdel Raouf S, Abdallah AS, Helmy N. Evaluation of gastric residual volume in fasting diabetic patients using gastric ultrasound. Acta Anaesthesiol Scand. 2019;63(5):615–619. doi: 10.1111/aas.13315. [DOI] [PubMed] [Google Scholar]