Abstract
The human myocardium contains robust cells that constantly beat from birth to death without being replaced, even when exposed to various environmental stresses. Myocardial robustness is thought to depend primarily on the strength of the reducing power to protect the heart from oxidative stress. Myocardial antioxidant systems are controlled by redox reactions, primarily via the redox reaction of Cys sulfhydryl groups, such as found in thioredoxin and glutathione. However, the specific molecular entities that regulate myocardial reducing power have long been debated. Recently, reactive sulfide species, with excellent electron transfer ability, consisting of a series of multiple sulfur atoms, i.e., Cys persulfide and Cys polysulfides, have been found to play an essential role in maintaining mitochondrial quality and function, as well as myocardial robustness. This review presents the latest findings on the molecular mechanisms underlying mitochondrial energy metabolism and the maintenance of quality control by reactive sulfide species and provides a new insight for the prevention of chronic heart failure.
Keywords: persulfide, reactive sulfide species, electrophile, mitochondrial quality control, cardiac senescence
Introduction
The recent increase in the number of patients with chronic cardiovascular diseases, such as heart failure, is becoming a major problem worldwide. Cellular senescence is observed in cardiovascular tissues as a result of the progression of pathological structural and morphological remodeling,(1–3) and its suppression may lead to preventive and therapeutic strategies for the treatment of chronic cardiovascular diseases.(4)
Hydrogen sulfide (H2S), a colorless and highly toxic gas with a rotten egg odor, has been detected in low concentrations in the body,(5) including in the cardiovascular system.(6,7) It has been reported that treatment with H2S can have various physiological effects, including vascular smooth muscle relaxation,(8) insulin signal suppression,(9) and inflammation.(10) In particular, the anti-aging effect of H2S has attracted attention because it has been reported that H2S can extend the life expectancy of yeast, nematodes, and flies.(11–13) Furthermore, there is increasing evidence that H2S has a protective effect against various diseases associated with cellular senescence, such as cardiac hypertrophy,(14) heart failure,(15) atherosclerosis,(16) and ischemia/reperfusion injury.(17) The biological effects of H2S are thought to be due to the nucleophilicity (or reducing power) similar to the redox-active cysteine thiol groups (Cys-SH, Cys-S−).(5) Actually, we have previously collaborated with Dr. Takaaki Akaike (Tohoku University), and found that hydrogen sulfide anion improves cardiac senescence induced by electrophilic secondary metabolite, 8-nitroguanosine 3',5'-cyclic monophosphate (8-nitro-cGMP).(15) However, we also found that H2S itself is insufficient to react directly with electrophilic chemical substances but requires heavy metals as a catalyst.(15) This indicates that highly nucleophilic substance(s) using H2S as a substrate is formed in cells and mediate the protective action of H2S on organs. Dr. Akaike’s group found that reactive sulfur species (RSS) produced from the H2S biosynthesis pathway are bona fide nucleophilic substances.(18) Cysteine persulfide (Cys-SSH), a RSS, reacts directly with hydrogen peroxide and electrophilic substances for its metabolism and elimination.(19,20) As described below, a RSS is mostly deprotonated under physiological pH conditions and has higher nucleophilicity than H2S (HS−).(21–23) Currently, the role of RSS in life expectancy and tissue homeostasis is being investigated.
In this review, we describe the intracellular mechanism for the production of RSS and the actions of RSS in cellular senescence and cardiac homeostasis.
Cardiac Redox Homeostasis and Antioxidant System
Increasing evidence suggests that disruption of redox homeostasis such as excess oxidative stress play a critical role in the progression of cardiac diseases.(24,25) Redox homeostasis is controlled by the balance between pro-oxidative production and antioxidant defense system. Mitochondria is a major source of reactive oxygen species (ROS) production. To produce a lot of energy for continuous beating, cardiomyocytes possess numerous mitochondria which occupy more than 30% of total cell volume.(26) Therefore, cardiomyocytes continuously produce a lot of ROS as a byproduct of mitochondrial respiration. To adapt these oxidative stress, cardiomyocytes have evolved an elegant antioxidant defense system.(27) For instance, mitochondrial H2O2 is increased in skeletal muscle in mice with both exercise and high-fat high-sucrose diet, whereas it is decreased in heart by increasing expression of thioredoxin reductase (TrxR)-2.(28) Antioxidant system in the heart has been studied by assessing H2O2 removing activities.(29) The respiration-dependent mitochondrial H2O2 removal is believed as the predominant endogenous ROS-eliminating machinery and its rate becomes higher with pyruvate/malate than with succinate. Compared with the capacity of rat liver mitochondria, myocardial mitochondria has higher H2O2-eliminating activity. The heart also has common H2O2-scavenging systems, including Trx/TrxR, peroxiredoxins, glutathione (GSH)/GSH reductase/GSH peroxidase, and catalase.(30) The GSH content is >2-fold higher in liver than heart and skeletal muscle,(31) suggesting that the contribution of each enzyme to antioxidation may differ between organs. The significance of scavenging system ranked by the H2O2-removing rate (%) is estimated: catalase > TrxR > GSH in unenergized heart and GSH > TrxR > catalase in energized heart, and catalase > GSH > TrxR in unenergized and energized liver.(32) However, Two Selenoprotein faimilies, GSH peroxidases and Trx reductases, and Zn2+-containing metallothioneins also act as ROS-scaveinging enzyme in the heart.(33,34) These ROS-eliminating and scavenging systems commonly use sulfur (CysSH) to contribute to ROS removal, and Cys polysulfidation will positively regulate intracellular antioxidant activities.
Electrophile-Mediated Cardiac Premature Senescence
It is believed that there is a close relationship between the aging of individuals and cellular senescence.(35) Senescent cells, which are observed in various tissues according to the degree of aging, secrete high levels of inflammatory cytokines, growth factors, and proteases (known as the senescence-associated secretory phenotype), leading to dysfunction in tissues and individuals.(36) Cells under various types of stress, including the progression of pathological conditions, exhibit a phenotype similar to cellular senescence (premature senescence).(37)
Cardiovascular cells are constantly exposed to hemodynamic load, such as blood flow, so they are more robust than other cell types. However, increased stress caused by diabetes, hypercholesterolemia, and ischemic conditions, triggers premature senescence.(2,15,38,39) Such premature senescence of cardiomyocytes causes decreased cardiac function (heart failure) and arrhythmia, which in turn can cause sudden death.(40)
Telomere shortening, accumulated nucleotide damage, increased oxidative stress, and activation of oncogene products cause cellular senescence.(41) Reactive oxygen species (ROS) and reactive nitrogen species (RNS) undergo a chemical reaction with lipids and nucleic acids in cells, producing various secondary metabolites. These secondary metabolites, such as 8-nitro-cGMP, nitro fatty acids, 15-deoxy-Δ12,14-prostaglandin J2, and 4-hydroxynonenal, often have the ability to accept electrons (electrophiles).(42) In addition to endogenous electrophiles, exogenous environmental pollutants, such as methylmercury (MeHg) and acrolein, also act as electrophilic substances.(43) These electrophiles covalently bind to the cysteine residues in a protein and change the structure and function of the protein.(42,44) We have previously found that 8-nitro-cGMP was generated in the hearts of myocardial infarction model mice and sustained activation of H-Ras through covalent modification of cysteine 184 (S-guanylation) caused myocardial premature senescence.(15) The hydrogen sulfide anion (HS−) is a nucleophile that can react with electrophilic substrates. Therefore, it has been speculated that H2S inhibits cellular senescence through the metabolism and elimination of electrophiles. In fact, we found that the administration of sodium hydrosulfide (NaHS) to myocardial infarction model mice inhibited the S-guanylation of H-Ras and improved cardiac function.(15)
Reactive Sulfur Species (RSS)
The treatment of cells with HS− induces the metabolism of electrophilic substrates.(15) However, recent studies have identified that sulfur intermediates rather than HS− are likely to react with these electrophiles. RSS, such as Cys-SSH and glutathione persulfide (GSSH), have been identified as bona fide nucleophilic intermediates.(18) These persulfides have a sulfane sulfur atom bound to a thiol group. Because of the α-effect, persulfides are more nucleophilic than the corresponding thiols.(21) It has been reported that the pKa value of glutathione persulfide (GSSH) is 6.9 or 5.5, whereas that of GSH is 8.9.(21,22) The pKa value of Cys-SSH is computationally calculated to be 4.3, whereas that of cysteine is 8.3.(23) Therefore, most persulfide is deprotonated under physiological pH conditions and can easily react with electrophiles. GSSH directly reacts with 8-nitro-cGMP, generating non-electrophilic 8-SH-cGMP.(18)
H2S is believed to be produced by cystathionine β-synthase (CBS), cystathionine γ-lyase (CSE), and 3-mercaptopyruvate sulfurtransferase (3-MST) in the trans-sulfuration pathway that mediates the metabolism of sulfur-containing amino acids.(5,8) However, the use of recent advances in mass spectrometry to detect sulfane sulfur atoms has identified that Cys-SSH is produced by CBS and CSE.(18) This sulfane sulfur generated from cystine can be transferred to other thiol groups in peptides, such as GSH to produce GSSH, (trans-sulfidation).(18,43) Cys-SSH and GSSH are ubiquitously present in cells, from yeasts to humans, in micromolar concentrations. For instance, quantitative mass spectrometry analysis identified that GSSH concentration is about 150 μM in brain and 50 μM in heart and liver, which is equivalent to 2–5% of GSH.(18) Recently, cysteinyl-tRNA synthetases (CARSs) have been identified as novel enzymes for Cys-SSH synthesis.(46) CARSs were originally identified as the enzymes that catalyze the ligation of cysteine to tRNA. In mammal, there are two CARS genes: a cytosolic CARS (CARS1) and a mitochondrial isoform of CARS (CARS2). Akaike et al.(46) have reported that CARSs directly catalyze Cys-SSH synthesis from cysteine and produce Cys-SSH bound to cysteinyl-tRNA. This persulfidated cysteinyl-tRNA can be incorporated into nascent polypeptides during the translation step; thus, mediating protein persulfidation (protein-SSH). Protein persulfidation occurs not only via co-translation but also by the post-translation (trans-sulfidation) pathway. Protein persulfidation is important for regulation of the structure and function of the protein.(47)
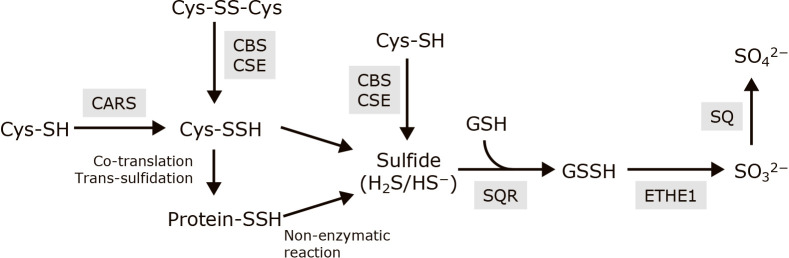
H2S is released from Cys-SSH and protein-SSH by non-enzymatic reaction.(48,49) Sulfide quinone reductase (SQR) catalyzes the oxidation of H2S and generates GSSH through sulfur atom transfer.(50,51) Ethylmalonic encephalopathy protein 1 (ETHE1) catalyzes the oxidation of GSSH, yielding sulfite (SO32−) and GSH. Sulfite is further oxidized to sulfate (SO42−) by sulfite oxidase (Fig. 1).(49)
Fig. 1.
The scheme of metabolic pathway of persulfides and sulfides. Enzymes are shown in gray background. Cys-SH, cysteine; Cys-SSH, cysteine persulfide; Cys-SS-Cys, cystine; protein-SSH, protein persulfide; H2S/HS−, hydrogen sulfide/hydrogen sulfide anion; GSH, glutathione; GSSH, glutathione persulfide; SO32−, sulfite; SO42−, sulfate; CARS, cysteinyl-tRNA synthetase; CBS, cystathionine β-synthase; CSE, cystathionine γ-lyase, SQR, sulfide quinone reductase; ETHE1, ethylmalonic encephalopathy protein 1; SO, sulfite oxidase.
RSS and Senescence
Cys-SSH and protein-SSH may be factors that regulate aging and cellular senescence. As described above, Cys-SSH is produced by the enzymatic reactions of CBS and CSE.(18) Several studies have reported that expression of these trans-sulfuration enzymes is closely related with life span.(52) Sulfur-containing amino acid restriction increased the expression of CSE and endogenous H2S production, which is essential for beneficial effects, including longevity and stress resistance, in Caenorhabditis elegans (C. elegans) and mice.(12) CSE expression has been shown to be decreased in aged C. elegans.(53,54) Overexpression of CBS increased the life span,(55) and overexpression of CSE decreased oxidative stress and protected against neurodegenerative disease, in Drosophila melanogaster.(56) Mouse embryonic fibroblasts from CSE knockout mice showed an increase in oxidative stress and an increase in the expression of p53 and p21, resulting in a senescence phenotype.(57) The level of protein persulfidation decreases during aging, whereas the level of irreversibly overoxidized cysteine is increased in aged animals.(58) Cysteine persulfidation protects the cysteine thiol from overoxidation induced by excess ROS, and this process is conserved in various species, including mice and humans.(58) Although these genetic studies speculate the potential relationship between RSS and senescence, it is still unclear why reduced levels of RSS trigger cellular senescence. Additionally, CBS and CSE are multifunctional enzyme that is related to biosynthesis of selenium-containing amino acids as well as sulfur-containing amino acids. Actually selenium deficiency is associated with normal lifespan through suppression of selenoprotein functions.(59,60) However, it is still unclear the relationship between RSS and selenocysteine.
The Critical role of RSS in Mitochondrial Quality Control and Cardiac Homeostasis
Finally, we will describe our recent work regarding the role of RSS in myocardial senescence and cardiac vulnerability, through mitochondrial quality control.
Cardiomyocytes require a lot of energy for continuous contraction and relaxation. Therefore, the quality of the mitochondria in these cells is a determining factor for cardiac robustness.(26) The mitochondrial length and density are thought to be closely related to oxygen consumption.(61) For instance, mitochondrial electron transport chains in skeletal muscle form a dense supercomplex and oxygen consumption is increased.(62) In contrast, an anaerobic metabolic environment, such as found in hyperglycemia and hypoxia, promotes mitochondrial division.(63) Cells accurately regulate systemic energy metabolism by controlling the mitochondrial fission/fusion cycle according to the environment of the cells. Mitochondrial fission is regulated by dynamin-related protein 1 (Drp1), a large GTPase, whereas optic atrophy 1 (Opa1) and mitofusin 1/2 (Mfn1/2), which are also large GTPases, regulate mitochondrial fusion.(64) Drp1 is diffusely distributed in the cytosol and is translocated to the mitochondrial outer membrane when it is bound to GTP (the active form). Drp1 is oligomerized and forms a ring-like structure to divide the mitochondria. Opa1 and Mfn1/2 mediate the fusion of the mitochondrial inner and outer membranes, respectively.(65) Dysfunctional mitochondrial fragments are degraded by mitophagy.(66) Therefore, the proper balance of mitochondrial fission and fusion is indispensable for mitochondrial quality. Aberrant balance of mitochondrial fission and fusion is observed in various diseases, including ischemia-reperfusion injury,(67) cardiomyopathy,(68) amyotrophic lateral sclerosis,(69) Huntington’s disease,(70) and Alzheimer’s disease.(71)
The genetic mutation of mitochondrial regulatory proteins has shown the important role of mitochondrial quality control on cardiac homeostasis. Cardiomyocyte-specific Drp1 deficient mice exhibit a dilated cardiomyopathy-like phenotype. Remarkable mitochondrial fusion and elongation and increased mitophagy and myocardial necrosis have been observed in the cardiomyocytes of these mice.(72) Cardiomyocyte-specific Mfn1/2 double knockout mice showed eccentric cardiac remodeling, including mitochondrial hyperfission, reduced mitophagy, and hypertrophy.(72) Cardiac-specific Drp1/Mfn1/2 triple deficient mice exhibited a concentric hypertrophy-like phenotype with heterogeneous mitochondrial morphology, massive mitochondrial accumulation, and sarcomeric distortion.(73) These studies indicated the importance of proper mitochondrial fission/fusion balance for maintaining cardiac homeostasis.
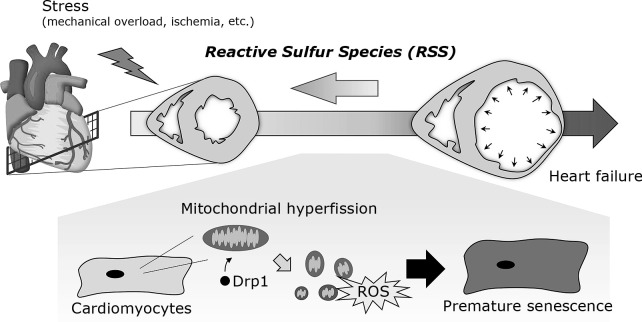
We analyzed the mitochondrial dynamics during cardiac remodeling and found that aberrant mitochondrial hyperfission and Drp1 hyperactivation occurred in cardiomyocytes in the early stage of myocardial infarction in a mice model. Hypoxic stress induced Drp1-mediated mitochondrial hyperfission, leading to myocardial senescence through mitochondrial ROS generation (Fig. 2).(74)
Fig. 2.
Mitochondrial hyperfission-mediated myocardial senescence. When the heart is exposed to chronic stress, such as ischemia and mechanical overload, it gradually undergoes maladaptive remodeling, including premature senescence. These stresses induce Drp1-mediated mitochondrial hyperfission in cardiomyocytes, leading to myocardial premature senescence.
The cellular features of stress-induced premature senescence are similar to those of CARS2-deficient cells. CARS2 knockout cells show severe mitochondrial fragmentation and a decrease in the mitochondrial membrane potential and oxygen consumption rate.(46) In collaboration with the Akaike group, we found that CARS2 mediated Drp1 Cys624 persulfidation. Drp1 activity was negatively regulated by persulfidation.(46) These findings suggested that Drp1 persulfidation by CARS2 affects mitochondrial quality control and energy homeostasis.
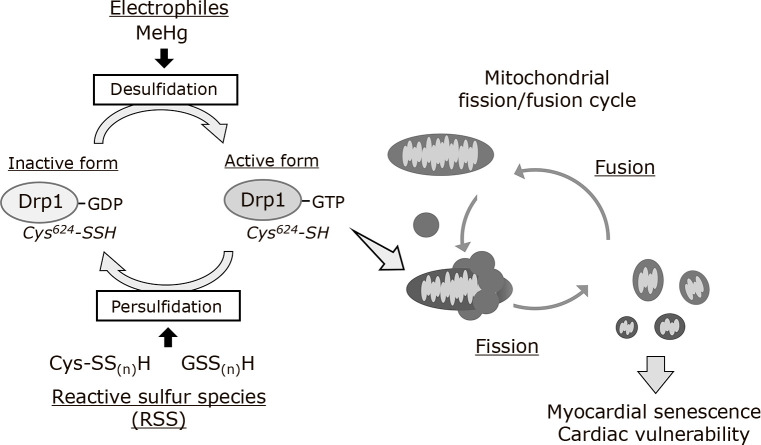
Because Drp1 persulfidation was found to negatively regulate mitochondrial fission, the pathophysiological role of Drp1 desulfidation in cardiomyocytes was investigated. In the environment, there are various electrophilic pollutants that adversely affect human health, including organic mercury, cadmium, aldehydes, and quinones.(43) MeHg is an environmental electrophile that accumulates in fish and marine mammals.(75) MeHg can covalently react with the sulfhydryl group of a protein to form a MeHg-S-protein complex (S-mercuration). This pathological modification disrupts the proper protein function causing neurotoxicity.(76–79) Importantly, epidemiological studies have suggested that MeHg exposure can increase the risk of cardiac dysfunction at a lower concentration than that associated with neurotoxicity.(80–83) Oral exposure of mice to a low dose of MeHg that does not induce neurotoxicity for 1 week did not alter the body weight, urine excretion/dietary intake, or activity of the mice but exacerbated cardiac dysfunction induced by pressure overload.(84) The level of Drp1 Cys624 persulfidation was decreased in MeHg-exposed cardiomyocytes, which was accompanied by a increase in Drp1 activation and mitochondrial hyperfission. Desulfidation of Drp1 and mitochondrial hyperfission because of MeHg exposure were almost completely abolished by the treatment of cardiomyocytes with NaHS, a sulfur substrate required to form RSS. MeHg-induced desulfidation of Drp1 promoted mitochondrial hyperfission by enhancing the interaction of Drp1 with filamin A, an actin-binding protein that acts as a guanine nucleotide exchange factor for Drp1.(74) In addition, administration of NaHS inhibited the hyperfission of myocardial mitochondria and rescued cardiac dysfunction after pressure overload in MeHg-exposed mice.(84) These results suggested that polysulfidation of Drp1 proteins negatively regulates cardiac vulnerability to mechanical stress through maintaining mitochondrial quality (Fig. 3).
Fig. 3.
Cross-talk between the mitochondrial fission/fusion cycle and reactive sulfur-dependent Drp1 activation cycle. Drp1 activity is negatively regulated by persulfidation at the redox-sensitive Cys624 in the basal state. Electrophiles, such as MeHg, induce sulfur deprivation of Drp1. The activation of desulfidated Drp1 is promoted through the interaction with filamin A, a guanine nucleotide exchange factor for Drp1, leading to mitochondrial fission.
Conclusion
For many years, H2S has been believed to be a gaseous signaling mediator, such as nitric oxide (NO) and carbon monoxide (CO), and many papers have been published on this theme. However, with the development of advanced technology for detecting sulfur-containing molecules, it is clear that the active entity is not H2S but RSS.(18) The enzymes responsible for the production and metabolism of RSS have been identified and the biological significance of RSS has been revealed. The relationship between RSS and mitochondrial energy metabolism is important,(46) and our research has shown the influence of RSS in mitochondrial quality control and myocardial senescence-related cardiac diseases.(84) In the future, we would like to develop medical technology that utilizes these properties of RSS.
Author Contributions
AN and MN wrote and edited the manuscript. TT, YK, and KN edited the manuscript and prepared figures.
Acknowledgments
This work was supported by JST, CREST Grant Number JPMJCR2024 (20348438 to MN and AN) and Grants-in-Aid for Scientific Research (KAKENHI) from the Ministry of Education, Culture, Sports, Science and Technology (No. 19K07085 to AN, and 19K22443 and 19H03383 to MN), Innovative Areas (Research in a Proposed Research Area ‘Integrated Bio-metal Science’ (20H05512 to MN), and Mochida Memorial Foundation for Medical and Pharmaceutical Research (No. 6-11 to AN). Victoria Muir, PhD, from Edanz (https://www.jp.edanz.com/ac) edited a draft of this manuscript.
Abbreviations
- CARS
cysteinyl-tRNA synthetase
- CBS
cystathionine β-synthase
- CO
carbon monoxide
- CSE
cystathionine γ-lyase
- Cys-SSH
cysteine persulfide
- Drp1
dynamin-related protein 1
- ETHE1
ethylmalonic encephalopathy protein 1
- GSH
glutathione
- GSSH
glutathione persulfide
- H2S/HS−
hydrogen sulfide/hydrogen sulfide anion
- MeHg
methylmercury
- Mfn1/2
mitofusin 1/2
- 3-MST
3-mercaptopyruvate sulfurtransferase
- 8-nitro-cGMP
8-nitroguanosine 3',5'-cyclic monophosphate
- NO
nitric oxide
- Opa1
optic atrophy 1
- RNS
reactive nitrogen species
- ROS
reactive oxygen species
- RSS
reactive sulfur species
- SO32−
sulfite
- SO42−
sulfate
- SQR
sulfide quinone reductase
- TrxR
thioredoxin reductase
Conflict of Interest
No potential conflicts of interest were disclosed.
References
- 1.Sun R, Zhu B, Xiong K, et al. Senescence as a novel mechanism involved in β-adrenergic receptor mediated cardiac hypertrophy. PLoS One 2017; 12: e0182668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maejima Y, Adachi S, Ito H, Hirao K, Isobe M. Induction of premature senescence in cardiomyocytes by doxorubicin as a novel mechanism of myocardial damage. Aging Cell 2008; 7: 125–136. [DOI] [PubMed] [Google Scholar]
- 3.Boon RA, Iekushi K, Lechner S, et al. MicroRNA-34a regulates cardiac ageing and function. Nature 2013; 495: 107–110. [DOI] [PubMed] [Google Scholar]
- 4.Le Couteur DG, Lakatta EG. A vascular theory of aging. J Gerontol A Biol Sci Med Sci 2010; 65: 1025–1027. [DOI] [PubMed] [Google Scholar]
- 5.Paul BD, Snyder SH. H2S signalling through protein sulfhydration and beyond. Nat Rev Mol Cell Biol 2012; 13: 499–507. [DOI] [PubMed] [Google Scholar]
- 6.Pan LL, Liu XH, Gong QH, Yang HB, Zhu YZ. Role of cystathionine γ-lyase/hydrogen sulfide pathway in cardiovascular disease: a novel therapeutic strategy? Antioxidants Redox Signal 2012; 17: 106–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu YH, Lu M, Hu LF, Wong PT, Webb GD, Bian JS. Hydrogen sulfide in the mammalian cardiovascular system. Antioxidants Redox Signal 2012; 17: 141–185. [DOI] [PubMed] [Google Scholar]
- 8.Bhatia M. Hydrogen sulfide as a vasodilator. IUBMB Life 2005; 57: 603–606. [DOI] [PubMed] [Google Scholar]
- 9.Zhang H, Huang Y, Chen S, et al. Hydrogen sulfide regulates insulin secretion and insulin resistance in diabetes mellitus, a new promising target for diabetes mellitus treatment? A review. J Adv Res 2020; 27: 19–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Szabõ C. Hydrogen sulphide and its therapeutic potential. Nat Rev Drug Discov 2007; 6: 917–935. [DOI] [PubMed] [Google Scholar]
- 11.Miller DL, Roth MB. Hydrogen sulfide increases thermotolerance and lifespan in Caenorhabditis elegans. Proc Natl Acad Sci U S A 2007; 104: 20618–20622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hine C, Harputlugil E, Zhang Y, et al. Endogenous hydrogen sulfide production is essential for dietary restriction benefits. Cell 2015; 160: 132–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ng LT, Ng LF, Tang RMY, et al. Lifespan and healthspan benefits of exogenous H2S in C. elegans are independent from effects downstream of eat-2 mutation. NPJ Aging Mech Dis 2020; 6: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shao M, Zhuo C, Jiang R, et al. Protective effect of hydrogen sulphide against myocardial hypertrophy in mice. Oncotarget 2017; 8: 22344–22352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nishida M, Sawa T, Kitajima N, et al. Hydrogen sulfide anion regulates redox signaling via electrophile sulfhydration. Nat Chem Biol 2012; 8: 714–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang ZJ, Wu J, Guo W, Zhu YZ. Atherosclerosis and the hydrogen sulfide signaling pathway-therapeutic approaches to disease prevention. Cell Physiol Biochem 2017; 42: 859–875. [DOI] [PubMed] [Google Scholar]
- 17.Wu D, Wang J, Li H, Xue M, Ji A, Li Y. Role of hydrogen sulfide in ischemia-reperfusion injury. Oxid Med Cell Longev 2015; 2015: 186908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ida T, Sawa T, Ihara H, et al. Reactive cysteine persulfides and S-polythiolation regulate oxidative stress and redox signaling. Proc Natl Acad Sci U S A 2014; 111: 7606–7611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fujii S, Sawa T, Motohashi H, Akaike T. Persulfide synthases that are functionally coupled with translation mediate sulfur respiration in mammalian cells. Br J Pharmacol 2019; 176: 607–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kasamatsu S, Ihara H. Regulation of redox signaling by reactive sulfur species. J Clin Biochem Nutr 2021; 68: 111–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Edwards JO, Pearson RG. The factors determining nucleophilic reactivities. J Am Chem Soc 1962; 84: 16–24. [Google Scholar]
- 22.Benchoam D, Semelak JA, Cuevasanta E, et al. Acidity and nucleophilic reactivity of glutathione persulfide. J Biol Chem 2020; 295: 15466–15481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cuevasanta E, Lange M, Bonanata J, et al. Reaction of hydrogen sulfide with disulfide and sulfenic acid to form the strongly nucleophilic persulfide. J Biol Chem 2015; 290: 26866–26880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dhalla NS, Das PK, Sharma GP. Subcellular basis of cardiac contractile failure. J Mol Cell Cardiol 1978; 10: 363–385. [DOI] [PubMed] [Google Scholar]
- 25.Dhalla NS, Temsah RM, Netticadan T. Role of oxidative stress in cardiovascular diseases. J Hypertens 2000; 18: 655–673. [DOI] [PubMed] [Google Scholar]
- 26.Piquereau J, Caffin F, Novotova M, et al. Mitochondrial dynamics in the adult cardiomyocytes: which roles for a highly specialized cell? Front Physiol 2013; 4: 102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bartz RR, Suliman HB, Piantadosi CA. Redox mechanisms of cardiomyocyte mitochondrial protection. Front Physiol 2015; 6: 291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fisher-Wellman KH, Mattox TA, Thayne K, et al. Novel role for thioredoxin reductase-2 in mitochondrial redox adaptations to obesogenic diet and exercise in heart and skeletal muscle. J Physiol 2013; 591: 3471–3486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Venditti P, Napolitano G, Di Meo S. Role of enzymatic and non-enzymatic processes in H2O2 removal by rat liver and heart mitochondria. J Bioenerg Biomembr 2014; 46: 83–91. [DOI] [PubMed] [Google Scholar]
- 30.Nishida M, Nishimura A, Matsunaga T, Motohashi H, Kasamatsu S, Akaike T. Redox regulation of electrophilic signaling by reactive persulfides in cardiac cells. Free Radic Biol Med 2017; 109: 132–140. [DOI] [PubMed] [Google Scholar]
- 31.Carfagna S, Napolitano G, Barone D, Pinto G, Pollio A, Venditti P. Dietary supplementation with the microalga Galdieria sulphuraria (Rhodophyta) reduces prolonged exercise-induced oxidative stress in rat tissues. Oxid Med Cell Longev 2015; 2015: 732090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kamunde C, Sharaf M, MacDonald N. H2O2 metabolism in liver and heart mitochondria: low emitting-high scavenging and high emitting-low scavenging systems. Free Radic Biol Med 2018; 124: 135–148. [DOI] [PubMed] [Google Scholar]
- 33.Gul M, Demircan B, Taysi S, et al. Effects of endurance training and acute exhaustive exercise on antioxidant defense mechanisms in rat heart. Comp Biochem Physiol A Mol Integr Physiol 2006; 143: 239–245. [DOI] [PubMed] [Google Scholar]
- 34.Xue M, Joo YA, Li S, et al. Metallothionein protects the heart against myocardial infarction via the mTORC2/FoxO3a/Bim pathway. Antioxid Redox Signal 2019; 31: 403–419. [DOI] [PubMed] [Google Scholar]
- 35.Childs BG, Durik M, Baker DJ, van Deursen JM. Cellular senescence in aging and age-related disease: from mechanisms to therapy. Nat Med 2015; 21: 1424–1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van Deursen JM. The role of senescent cells in ageing. Nature 2014; 509: 439–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.de Magalhães JP, Passos JF. Stress, cell senescence and organismal ageing. Mech Ageing Dev 2018; 170: 2–9. [DOI] [PubMed] [Google Scholar]
- 38.Sussman MA, Anversa P. Myocardial aging and senescence: where have the stem cells gone? Annu Rev Physiol 2004; 66: 29–48. [DOI] [PubMed] [Google Scholar]
- 39.Shimizu I, Minamino T. Cellular senescence in cardiac diseases. J Cardiol 2019; 74: 313–319. [DOI] [PubMed] [Google Scholar]
- 40.Mitry MA, Laurent D, Keith BL, et al. Accelerated cardiomyocyte senescence contributes to late-onset doxorubicin-induced cardiotoxicity. Am J Physiol Cell Physiol 2020; 318: C380–C391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Di Micco R, Krizhanovsky V, Baker D, d′Adda di Fagagna F. Cellular senescence in ageing: from mechanisms to therapeutic opportunities. Nat Rev Mol Cell Biol 2021; 22: 75–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nishida M, Kumagai Y, Ihara H, Fujii S, Motohashi H, Akaike T. Redox signaling regulated by electrophiles and reactive sulfur species. J Clin Biochem Nutr 2016; 58: 91–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kumagai Y, Abiko Y. Environmental electrophiles: protein adducts, modulation of redox signaling, and interaction with persulfides/polysulfides. Chem Res Toxicol 2017; 30: 203–219. [DOI] [PubMed] [Google Scholar]
- 44.Sawa T, Arimoto H, Akaike T. Regulation of redox signaling involving chemical conjugation of protein thiols by nitric oxide and electrophiles. Bioconjug Chem 2010; 21: 1121–1129. [DOI] [PubMed] [Google Scholar]
- 45.Ono K, Akaike T, Sawa T, et al. Redox chemistry and chemical biology of H2S, hydropersulfides, and derived species: implications of their possible biological activity and utility. Free Radic Biol Med 2014; 77: 82–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Akaike T, Ida T, Wei FY, et al. Cysteinyl-tRNA synthetase governs cysteine polysulfidation and mitochondrial bioenergetics. Nat Commun 2017; 8: 1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kasamatsu S, Nishimura A, Morita M, Matsunaga T, Abdul Hamid H, Akaike T. Redox signaling regulated by cysteine persulfide and protein polysulfidation. Molecules 2016; 21: 1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kolluru GK, Shen X, Bir SC, Kevil CG. Hydrogen sulfide chemical biology: pathophysiological roles and detection. Nitric Oxide 2013; 35: 5–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Marutani E, Ichinose F. Emerging pharmacological tools to control hydrogen sulfide signaling in critical illness. Intensive Care Med Exp 2020; 8: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Landry AP, Ballou DP, Banerjee R. H2S oxidation by nanodisc-embedded human sulfide quinone oxidoreductase. J Biol Chem 2017; 292: 11641–11649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Miranda KM, Wink DA. Persulfides and the cellular thiol landscape. Proc Natl Acad Sci U S A 2014; 111: 7505–7506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Orentreich N, Matias JR, DeFelice A, Zimmerman JA. Low methionine ingestion by rats extends life span. J Nutr 1993; 123: 269–274. [DOI] [PubMed] [Google Scholar]
- 53.Walther DM, Kasturi P, Zheng M, et al. Widespread proteome remodeling and aggregation in aging C. elegans. Cell 2015; 161: 919–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Narayan V, Ly T, Pourkarimi E, et al. Deep proteome analysis identifies age-related processes in C. elegans. Cell Syst 2016; 3: 144–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shaposhnikov M, Proshkina E, Koval L, Zemskaya N, Zhavoronkov A, Moskalev A. Overexpression of CBS and CSE genes affects lifespan, stress resistance and locomotor activity in Drosophila melanogaster. Aging (Albany NY). 2018; 10: 3260–3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Snijder PM, adina Baratashvili M, Grzeschik NA, et al. Overexpression of cystathionine γ-lyase suppresses detrimental effects of spinocerebellar ataxia type 3. Mol Med 2016; 21: 758–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yang G, Zhao K, Ju Y, et al. Hydrogen sulfide protects against cellular senescence via S-sulfhydration of keap1 and activation of Nrf2. Antioxidants Redox Signal 2013; 18: 1906–1919. [DOI] [PubMed] [Google Scholar]
- 58.Zivanovic J, Kouroussis E, Kohl JB, et al. Selective persulfide detection reveals evolutionarily conserved antiaging effects of S-sulfhydration. Cell Metab 2019; 30: 1152–1170.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rayman MP, Winther KH, Pastor-Barriuso R, et al. Effect of long-term selenium supplementation on mortality: results from a multiple-dose, randomised controlled trial. Free Radic Biol Med 2018; 127: 46–54. [DOI] [PubMed] [Google Scholar]
- 60.Yim SH, Clish CB, Gladyshev VN. Selenium deficiency is associated with pro-longevity mechanisms. Cell Rep 2019; 27: 2785–2797.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Liesa M, Shirihai OS. Mitochondrial dynamics in the regulation of nutrient utilization and energy expenditure. Cell Metab 2013; 17: 491–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Greggio C, Jha P, Kulkarni SS, et al. Enhanced respiratory chain supercomplex formation in response to exercise in human skeletal muscle. Cell Metab 2017; 25: 301–311. [DOI] [PubMed] [Google Scholar]
- 63.Tanaka T, Nishimura A, Nishiyama K, Goto T, Numaga-Tomita T, Nishida M. Mitochondrial dynamics in exercise physiology. Pflugers Arch 2020; 472: 137–153. [DOI] [PubMed] [Google Scholar]
- 64.Hoppins S, Lackner L, Nunnari J. The machines that divide and fuse mitochondria. Annu Rev Biochem 2007; 76: 751–780. [DOI] [PubMed] [Google Scholar]
- 65.Kraus F, Roy K, Pucadyil TJ, Ryan MT. Function and regulation of the divisome for mitochondrial fission. Nature 2021; 590: 57–66. [DOI] [PubMed] [Google Scholar]
- 66.Palikaras K, Lionaki E, Tavernarakis N. Mechanisms of mitophagy in cellular homeostasis, physiology and pathology. Nat Cell Biol 2018; 20: 1013–1022. [DOI] [PubMed] [Google Scholar]
- 67.Ong SB, Subrayan S, Lim SY, Yellon DM, Davidson SM, Hausenloy DJ. Inhibiting mitochondrial fission protects the heart against ischemia/reperfusion injury. Circulation 2010; 121: 2012–2022. [DOI] [PubMed] [Google Scholar]
- 68.Wai T, García-Prieto J, Baker MJ, et al. Imbalanced OPA1 processing and mitochondrial fragmentation cause heart failure in mice. Science 2015; 350: aad0116. [DOI] [PubMed] [Google Scholar]
- 69.Joshi AU, Saw NL, Vogel H, Cunnigham AD, Shamloo M, Mochly-Rosen D. Inhibition of Drp1/Fis1 interaction slows progression of amyotrophic lateral sclerosis. EMBO Mol Med 2018; 10: e8166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Song W, Chen J, Petrilli A, et al. Mutant huntingtin binds the mitochondrial fission GTPase dynamin-related protein-1 and increases its enzymatic activity. Nat Med 2011; 17: 377–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cho DH, Nakamura T, Fang J, et al. S-nitrosylation of Drp1 mediates β-amyloid-related mitochondrial fission and neuronal injury. Science 2009; 324: 102–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Song M, Mihara K, Chen Y, Scorrano L, Dorn GW. Mitochondrial fission and fusion factors reciprocally orchestrate mitophagic culling in mouse hearts and cultured fibroblasts. Cell Metab 2015; 21: 273–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Song M, Franco A, Fleischer JA, Zhang L, Dorn GW 2nd. Abrogating mitochondrial dynamics in mouse hearts accelerates mitochondrial senescence. Cell Metab 2017; 26: 872–883.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nishimura A, Shimauchi T, Tanaka T, et al. Hypoxia-induced interaction of filamin with Drp1 causes mitochondrial hyperfission-associated myocardial senescence. Sci Signal 2018; 11: eaat5185. [DOI] [PubMed] [Google Scholar]
- 75.Fujita M, Iwashima K. Estimation of organic and total mercury in seawater around the Japanese archipelago. Environ Sci Technol 1981; 15: 929–933. [DOI] [PubMed] [Google Scholar]
- 76.Shinyashiki M, Kumagai Y, Nakajima H, et al. Differential changes in rat brain nitric oxide synthase in vivo and in vitro by methylmercury. Brain Res 1998; 798: 147–155. [DOI] [PubMed] [Google Scholar]
- 77.Rabenstein DL, Saetre R. Mercury-based electrochemical detector for liquid chromatography for the detection of glutathione and other sulfur-containing compounds. Anal Chem 1977; 49: 1036–1039. [DOI] [PubMed] [Google Scholar]
- 78.Shinyashiki M, Kumagai Y, Homma-Takeda S, et al. Selective inhibition of the mouse brain Mn-SOD by methylmercury. Environ Toxicol Pharmacol 1996; 2: 359–366. [DOI] [PubMed] [Google Scholar]
- 79.Imura N, Miura K, Inokawa M, Nakada S. Mechanism of methylmercury cytotoxicity: by biochemical and morphological experiments using cultured cells. Toxicology 1980; 17: 241–254. [DOI] [PubMed] [Google Scholar]
- 80.Virtanen JK, Voutilainen S, Rissanen TH, et al. Mercury, fish oils, and risk of acute coronary events and cardiovascular disease, coronary heart disease, and all-cause mortality in men in eastern Finland. Arterioscler Thromb Vasc Biol 2005; 25: 228–233. [DOI] [PubMed] [Google Scholar]
- 81.Salonen JT, Seppänen K, Nyyssönen K, et al. Intake of mercury from fish, lipid peroxidation, and the risk of myocardial infarction and coronary, cardiovascular, and any death in eastern Finnish men. Circulation 1995; 91: 645–655. [DOI] [PubMed] [Google Scholar]
- 82.Guallar E, Sanz-Gallardo MI, van't Veer P, et al. Mercury, fish oils, and the risk of myocardial infarction. N Engl J Med 2002; 347: 1747–1754. [DOI] [PubMed] [Google Scholar]
- 83.Choi AL, Weihe P, Budtz-Jørgensen E, et al. Methylmercury exposure and adverse cardiovascular effects in Faroese whaling men. Environ Health Perspect 2009; 117: 367–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nishimura A, Shimoda K, Tanaka T, et al. Depolysulfidation of Drp1 induced by low-dose methylmercury exposure increases cardiac vulnerability to hemodynamic overload. Sci Signal 2019; 12: eaaw1920. [DOI] [PubMed] [Google Scholar]