Abstract
The aim of this study was to investigate whether a nutritional intervention motivating increased vegetable consumption would be an effective treatment and diet therapy for patients with non-alcoholic fatty liver disease. We examined 15 patients with this disease (5 men and 10 women). During the 6-month intervention period, all participants received a small amount of vegetables twice a month as a nutritional education tool aimed at increasing vegetable consumption. They also received nutritional counseling and underwent ultrasound and blood biochemical examinations at baseline and 3 and 6 months after initiation of the intervention. Moreover, they were requested to submit dietary records for any 2 days. Green, white, and total vegetable intakes were significantly higher at 3 and 6 months than at baseline in 8 patients. These patients had significantly lower alanine aminotransferase and triglyceride concentrations than those whose vegetable intake did not increase. Additionally, green vegetable intake significantly negatively correlated with weight at 3 and 6 months (r = −0.617, p = 0.032 and r = −0.848, p = 0.008, respectively). These results suggest that our nutritional approach effectively increased vegetable consumption in at least some patients with non-alcoholic fatty liver disease, consequently improving their condition.
Keywords: non-alcoholic fatty liver disease, diet therapy, nutritional intervention, obesity, vegetable consumption
Introduction
Non-alcoholic fatty liver disease (NAFLD) is one of the most serious chronic liver diseases. It is not caused by a viral infection, an autoimmune disease, drug use, or excessive alcohol consumption.(1,2) Its pathogenesis involves visceral fat accumulation and insulin resistance and is strongly associated with obesity and type 2 diabetes mellitus (T2DM).(2) The prevalence of NAFLD has increased along with the rise in obesity in developed countries.(3–5) As reported by the Japan Study Group of NAFLD, the prevalence of NAFLD in Japan was 29.7% in 2012.(6) A previous study, which included 31 patients with non-alcoholic steatohepatitis who underwent diet and exercise therapy for 48 weeks, reported that weight loss greater than 7% significantly improves steatosis, ballooning, and liver fibrosis.(7) Therefore, weight reduction by diet and/or exercise therapy is recommended as the treatment modality for NAFLD in the clinical guidelines published by the Japanese Society of Gastroenterology.(8) However, no specific effective diet therapy for NAFLD has been established. A Japanese study showed that patients with NAFLD consume fewer vitamins, minerals, and dietary fiber than the general Japanese population, as well as fewer vegetables that are rich in these nutrients.(9) The consumption of fruits and vegetables is proposed to be important for preventing obesity.(10) A review regarding the diet of choice for NAFLD mentioned that “Vegetables can reduce the overall energy density of the diet and allow consumption of satisfying portions while reducing caloric intake”.(11)
Moreover, the pathogenesis and progression of NAFLD are associated with oxidative stress;(12) thus, the antioxidant effects of vitamins, carotenoids, and some phytochemicals present in vegetables may be effective for NAFLD.(13) In support of this argument, recent studies have shown improved liver function in humans and mice receiving the phytochemicals sulforaphane and β-cryptoxanthin, respectively.(14,15) Although many studies on the intake of “fruits and vegetables” have been reported, few studies have focused only on “vegetables”. Because the intake of fructose, present in fruits, increases hepatic gluconeogenesis and de novo lipogenesis,(16) high consumption of fruits is not recommended for patients with NAFLD. Hence, a diet therapy focusing specifically on vegetable intake should better improve NAFLD than one focusing on both fruits and vegetables.
In our previous efforts, we developed a nutritional intervention program that strongly motivated vegetable intake; after 6 months of practice, we succeeded in increasing vegetable intake in patients with NAFLD.(17) We also found that the program led to weight loss, which is an important factor in improving the condition. However, we have not been able to verify the variation of parameters related to the disease. Therefore, the purpose of this study was to determine whether a practical nutritional approach to NAFLD improved these patients’ condition, and to assess changes in NAFLD-related parameters before and after intervention.
Materials and Methods
Participants
Patients with NAFLD who visited University Hospital, Kyoto Prefectural University of Medicine (Kyoto, Japan), were enrolled. NAFLD was diagnosed by the physician in charge via liver biopsy or transient elastography (Fibroscan®; Echosens, Paris, France). Serum-based indices and interviews were used to confirm the absence of viral infection, autoimmune disease, drug use, and excessive consumption of alcohol. Among the outpatients who visited the department between August 2016 and February 2017, 17 with NAFLD agreed to participate in the study. Two participants were lost to follow-up, resulting in a final sample of 15 participants (5 men and 10 women).
All participants provided written informed consent before study enrollment. This study was approved by the Kyoto Prefectural University Ethics Committee (No. 73) and Kyoto Prefectural University of Medicine Ethics Committee (ERB-C-242).
Nutritional intervention protocol
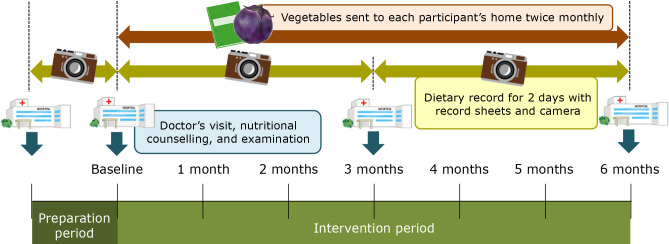
This interventional trial included a 1-month preparation period after consent and a 6-month intervention period (Fig. 1). All participants visited physicians and received nutritional counseling at the start of the intervention (baseline) and subsequently once every 3 months. During the intervention period, vegetables were sent to the participants’ homes approximately twice a month (total: 12 packages per participant), and 6 deliveries were accompanied by a newsletter containing information about vegetables. Each package contained several kinds of vegetables and weighed approximately 1 kg. These vegetables were used as a nutritional educational tool aimed at encouraging the consumption of vegetables.
Fig. 1.
The nutritional intervention protocol used in the present study.
Dietary surveys
Participants were requested to submit dietary records for any 2 days between each nutritional counseling session. The daily intake amounts of food and nutrients were calculated from the completed records using nutritional analysis software (Excel Eiyokun ver. 6.0; Kenpakusha, Tokyo, Japan). The averages of the values obtained on each of the 2 days were used for the analysis. The intake of 23 nutrients were calculated: energy, protein, fat, carbohydrates, potassium, calcium, magnesium, phosphorus, iron, zinc, vitamins A, B1, B2, B6, B12, niacin, folic acid, vitamin C, saturated fatty acids, mono- and polyunsaturated fatty acids, total dietary fiber, and salt. To calculate intake per food group, foods were categorized into 17 groups: grains and cereals (including rice and noodles), potatoes, sugar, nuts, green vegetables, white vegetables, fruits, mushrooms, algae, pulses, fish and shellfish, meat, eggs, milk, fats and oils, confectionery, and beverages. Energy intake was adjusted per ideal weight, and the intake of all food and nutrients were adjusted per 1,000 kcal. The sum of the intake of green and white vegetables is presented as the total vegetable intake.
Data collection
Patient data (age, medical history, comorbidities, and medications) were retrieved from electronic health records. No participants changed their medications during the intervention period. Participants were asked whether they smoked, had received previous nutritional counseling, and usually cooked for themselves. The number of family members was also recorded, as was their menopause status at baseline (female participants only). Weight and skeletal muscle and body fat percentages were determined using a body composition analyzer (InBody®; InBody Japan, Tokyo, Japan).
The biochemical parameters that were examined included aspartate aminotransferase (AST), alanine aminotransferase (ALT), γ-glutamyl transpeptidase, total bilirubin, triglycerides (TG), total cholesterol, high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol, ferritin, platelets (PLT), fasting blood sugar, hemoglobin A1c, type IV collagen 7S, and Mac-2-binding protein glycosylation isomer.(18) The AST/ALT ratio, which indicates the progression of liver fibrosis,(19) was calculated based on the AST and ALT levels. The fibrosis (FIB)-4 index, which also predicts the progression of liver fibrosis,(20) was calculated as follows:
The controlled attenuation parameter (CAP), which indicates liver fat accumulation, and the liver stiffness measurement (LSM), which indicates fibrosis, were determined using transient elastography. Transient elastography is a diagnostic ultrasonographic technique that noninvasively measures the amount of liver fat accumulation from the skin surface by means of a transducer attached to the tip of an ultrasound probe that measures shear wave velocity. Previous studies have demonstrated its usefulness for measuring these parameters.(21,22)
Statistical analysis
All data are presented as mean ± SD. The Kolmogorov–Smirnov and Shapiro–Wilk tests were used to assess data normality. For comparison of two independent groups, the non-paired t test and Mann–Whitney U test were used for normally and non-normally distributed data, respectively. For comparison of paired samples, the paired t test and Wilcoxon signed-rank test were used for normally and non-normally distributed data, respectively. For comparison of rates in each group, the chi-square test was used. Bivariate correlations were assessed via partial correlation analysis adjusted for age. The significance level was 5% (two-sided test). All statistical analyses were performed using the Statistical Package for Social Sciences, ver. 22.0 (SPSS 22.0; IBM, Armonk, NY).
Results
Table 1 shows the participants’ baseline characteristics. The mean body mass index of all patients exceeded 25.0 kg/m2 (the threshold for obesity in Japan),(23) being 28.0 kg/m2 in male and 27.6 kg/m2 in female patients. The percentage of patients who cooked for themselves was significantly higher among women than among men (p = 0.001).
Table 1.
Characteristics of the participants
| Male (n = 5) | Female (n = 10) | p value | ||
|---|---|---|---|---|
| Age | years | 40.0 ± 13.7 | 59.1 ± 7.4 | 0.032* |
| BMI | kg/m2 | 28.0 ± 4.4 | 27.6 ± 2.8 | 0.858 |
| †Rate of obesity | % (n) | 80 (4) | 90 (9) | 0.591 |
| Comorbidities | ||||
| T2DM | % (n) | 20 (1) | 50 (5) | 0.264 |
| Hypertension | % (n) | 60 (3) | 60 (6) | 1.000 |
| Dyslipidemia | % (n) | 100 (5) | 50 (5) | 0.053 |
| Hyperuricemia | % (n) | 20 (1) | 10 (1) | 0.591 |
| GERD | % (n) | 20 (1) | 10 (1) | 0.591 |
| A history of receiving nutritional councelling | % (n) | 40 (2) | 40 (4) | 1.000 |
| Lifestyle habits | ||||
| Smoking | % (n) | 20 (1) | 30 (3) | 0.680 |
| Number of family members | n | 2.4 ± 1.1 | 2.6 ± 1.2 | 0.759 |
| Cooking for myself | % (n) | 20 (1) | 100 (10) | 0.001* |
| Menopause (only females) | % (n) | — | 80 (8) | — |
Values for age, BMI, and number of family members are presented as mean ± SD. The relationships between sexes were assessed using the non-paired t test for normally distributed data and the Mann–Whitney U test for non-normally distributed data. The rates were compared between the sexes using the chi-square test. †Percentage of patients with a BMI over 25.0 kg/m2. *p<0.05. BMI, body mass index; T2DM, type 2 diabetes mellitus; GERD, gastro-esophageal reflux disease.
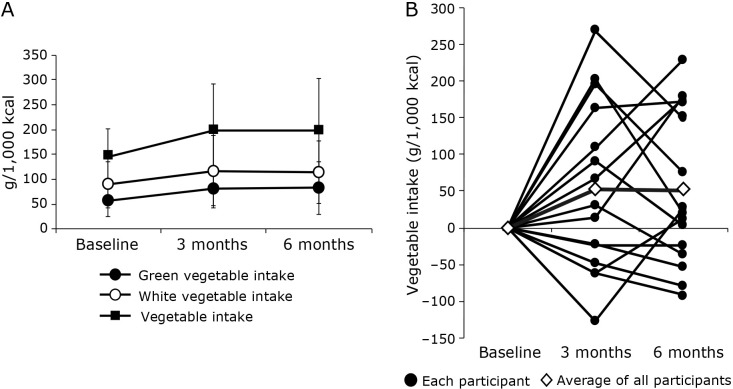
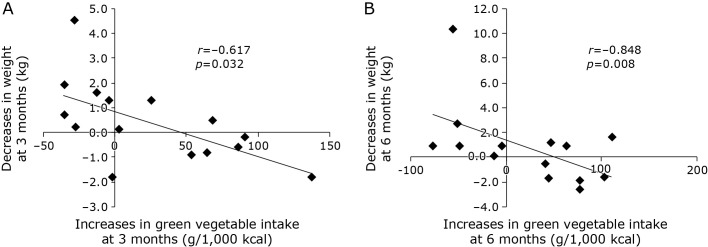
The trends in the patients’ vegetable intake during the intervention period are presented in Fig. 2. Green, white, and total vegetable intakes were higher at 3 and 6 months of intervention than at baseline, although not significantly so (Fig. 2A). Therefore, the comparison of the uptake values for each participant revealed notable differences in their responses to the intervention (Fig. 2B). Moreover, age-adjusted partial correlation analysis was performed to determine whether increased vegetable intake (green, white, and total) correlated with clinical data. As a result, increases in green vegetable intake significantly correlated with decreases in weight at 3 and 6 months (r = −0.617, p = 0.032 and r = −0.848, p = 0.008, respectively; Fig. 3). In terms of white and total vegetable intake, no significant correlations were identified.
Fig. 2.
The trends in the patients’ vegetable intake during the intervention period. (A) Green, white, and total vegetable intakes at baseline and 3 and 6 months for all participants. Values are presented as mean ± SD. Differences in intake between baseline and 3 or 6 months were assessed using the paired t test for normally distributed data and the Wilcoxon signed-rank test for non-normally distributed data. (B) Total vegetable intake at baseline and 3 and 6 months for each participant and the average intake for all participants.
Fig. 3.
Correlation between green vegetable intake and weight at 3 months (A) and 6 months (B) for all participants. Bivariate correlations were analyzed via partial correlation analysis adjusted for age.
Patients were subsequently divided into two groups based on whether their non-adjusted total vegetable intake did or did not show an increase between baseline and 3 months: the “increased” group comprised 8 patients (2 men and 6 women) and the “non-increased” group comprised 7 patients (3 men and 4 women). Table 2 presents a comparison of the characteristics of the two groups. None of the variables examined differed significantly between the groups. Table 3 presents the values for and changes in food and nutrient intakes at baseline, 3 months, and 6 months in the “increased” group. Potassium, vitamin A, vitamin B6, folic acid, green vegetable, and total vegetable intakes were significantly higher, whereas energy intake was significantly lower at 3 and 6 months, respectively, than at baseline. Total dietary fiber and white vegetable intakes were significantly higher at 3 months than at baseline, whereas no significant changes were found in the “non-increased” group (data not shown).
Table 2.
Characteristics of the participants stratified by vegetable intake
| Increased group (n = 8) |
Non-increased group (n = 7) |
p value | ||
|---|---|---|---|---|
| Male/Female | n | 2/6 | 3/4 | 0.464 |
| Age | years | 55.4 ± 9.3 | 49.7 ± 17.0 | 0.955 |
| BMI | kg/m2 | 28.4 ± 4.0 | 27.1 ± 2.1 | 0.478 |
| †Rate of obesity | % (n) | 88 (7) | 86 (6) | |
| Comorbidities | ||||
| T2DM | % (n) | 63 (5) | 14 (1) | 0.057 |
| Hypertension | % (n) | 63 (5) | 57 (4) | 0.833 |
| Dyslipidemia | % (n) | 63 (5) | 71 (5) | 0.573 |
| Hyperuricemia | % (n) | 13 (1) | 14 (1) | 0.733 |
| GERD | % (n) | 0 (0) | 29 (2) | 0.200 |
| A history of receiving nutritional councelling | % (n) | 25 (2) | 57 (4) | 0.205 |
| Lifestyle habits | ||||
| Smoking | % (n) | 38 (3) | 14 (1) | 0.310 |
| Number of family members | n | 2.5 ± 1.4 | 2.6 ± 0.8 | 0.694 |
| Cooking for myself | % (n) | 88 (7) | 57 (4) | 0.185 |
| Menopause (only females) | % (n) | 67 (4) | 100 (4) | 0.197 |
Values for age, BMI, and number of family members are presented as mean ± SD. The relationships between sexes were assessed using the non-paired t test for normally distributed data and the Mann–Whitney U test for non-normally distributed data. The rates were compared between the groups using the chi-square test. †Percentage of patients with a BMI over 25.0 kg/m2. BMI, body mass index; T2DM, type 2 diabetes mellitus; GERD, gastro-esophageal reflux disease.
Table 3.
Values for and changes in food and nutrient intake during the intervention in the “increased” group (n = 8)
| Baseline (n = 8) |
3 months (n = 8) |
6 months (n = 8) |
p value |
Changes |
|||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline vs 3 months |
Baseline vs 6 months |
at 3 months | at 6 months | p value | |||||
| Energy intake (kcal) | 34.1 ± 5.3 | 27.8 ± 6.6 | 30.3 ± 7.2 | 0.015* | 0.025* | −6.3 ± 5.6 | −3.8 ± 3.8 | 0.323 | |
| Protein (g) | 36.1 ± 3.0 | 40.2 ± 6.1 | 39.9 ± 5.8 | 0.073 | 0.121 | 4.2 ± 5.6 | 3.8 ± 6.1 | 0.901 | |
| Fat (g) | 33.1 ± 4.3 | 32.7 ± 6.1 | 33.4 ± 7.2 | 0.867 | 0.852 | −0.4 ± 6.7 | 0.3 ± 4.4 | 0.805 | |
| Carbohydrates (g) | 135.9 ± 9.9 | 134.0 ± 15.2 | 132.8 ± 17.7 | 0.743 | 0.543 | −2.0 ± 16.2 | −3.1 ± 13.9 | 0.877 | |
| Potassium (mg) | 1,132 ± 235 | 1,756 ± 481 | 1,486 ± 345 | 0.012* | 0.012* | 624 ± 516 | 355 ± 281 | 0.221 | |
| Calcium (mg) | 257 ± 147 | 313 ± 104 | 338 ± 120 | 0.331 | 0.221 | 56 ± 152 | 81 ± 171 | 1.000 | |
| Magnesium (mg) | 139 ± 29 | 163 ± 40 | 159 ± 36 | 0.094 | 0.049* | 24 ± 35 | 20 ± 24 | 0.279 | |
| Phosphorus (mg) | 526 ± 52 | 610 ± 121 | 591 ± 115 | 0.066 | 0.114 | 84 ± 109 | 65 ± 101 | 0.721 | |
| Iron (mg) | 4.0 ± 0.9 | 4.9 ± 0.8 | 5.3 ± 2.9 | 0.072 | 0.123 | 0.9 ± 1.2 | 1.3 ± 2.8 | 0.574 | |
| Zinc (mg) | 4.2 ± 0.6 | 4.9 ± 0.6 | 4.6 ± 0.5 | 0.007* | 0.265 | 0.6 ± 0.5 | 0.4 ± 0.9 | 0.465 | |
| Vitamin A (μgRE) | 177 ± 54 | 361 ± 129 | 353 ± 104 | 0.010* | 0.017* | 183 ± 149 | 175 ± 103 | 0.904 | |
| Vitamin B1 (mg) | 0.55 ± 0.13 | 0.68 ± 0.20 | 0.60 ± 0.21 | 0.140 | 0.475 | 0.12 ± 0.21 | 0.05 ± 0.18 | 0.466 | |
| Vitamin B2 (mg) | 0.52 ± 0.08 | 0.61 ± 0.13 | 0.65 ± 0.19 | 0.014* | 0.059 | 0.09 ± 0.08 | 0.14 ± 0.17 | 0.512 | |
| Niacin (mg) | 8 ± 2 | 11 ± 4 | 10 ± 3 | 0.032* | 0.075 | 3 ± 3 | 2 ± 3 | 0.543 | |
| Vitamin B6 (mg) | 0.56 ± 0.12 | 0.79 ± 0.18 | 0.75 ± 0.21 | 0.003* | 0.006* | 0.23 ± 0.15 | 0.19 ± 0.14 | 0.582 | |
| Vitamin B12 (μg) | 1.8 ± 0.9 | 2.5 ± 1.1 | 3.7 ± 2.8 | 0.123 | 0.123 | 0.7 ± 1.5 | 2.0 ± 3.3 | 0.340 | |
| Folic acid (μg) | 151 ± 48 | 239 ± 30 | 225 ± 83 | 0.003* | 0.032* | 88 ± 55 | 74 ± 79 | 0.442 | |
| Vitamin C (mg) | 53 ± 24 | 80 ± 28 | 84 ± 44 | 0.052 | 0.074 | 27 ± 33 | 32 ± 43 | 0.798 | |
| Saturated fatty acids (g) | 8.8 ± 2.1 | 8.6 ± 2.0 | 9.5 ± 2.4 | 0.912 | 0.406 | −0.1 ± 2.7 | 0.8 ± 2.5 | 0.503 | |
| Mono-unsaturated fatty acids (g) | 11.1 ± 2.0 | 10.7 ± 2.5 | 11.3 ± 3.1 | 0.757 | 0.748 | −0.3 ± 2.8 | 0.2 ± 2.1 | 0.654 | |
| Poly-unsaturated fatty acids (g) | 7.7 ± 1.4 | 6.9 ± 1.5 | 6.3 ± 1.5 | 0.323 | 0.102 | −0.8 ± 2.2 | −1.3 ± 2.0 | 0.629 | |
| Total dietary fiber (g) | 7.4 ± 1.8 | 10.3 ± 1.6 | 8.9 ± 2.4 | 0.015* | 0.105 | 2.8 ± 2.5 | 1.5 ± 2.3 | 0.287 | |
| Salt (g) | 5.3 ± 1.3 | 5.8 ± 1.5 | 6.0 ± 2.1 | 0.380 | 0.237 | 0.5 ± 1.5 | 0.6 ± 1.4 | 0.865 | |
| PFC-P (%E) | 14.4 ± 1.2 | 16.1 ± 2.5 | 15.9 ± 2.3 | 0.073 | 0.121 | 1.7 ± 2.2 | 1.5 ± 2.4 | 0.901 | |
| PFC-F (%E) | 29.8 ± 3.8 | 29.5 ± 5.5 | 30.1 ± 6.5 | 0.867 | 0.851 | −0.4 ± 6.0 | 0.3 ± 4.0 | 0.805 | |
| PFC-C (%E) | 54.4 ± 3.9 | 53.6 ± 6.1 | 53.1 ± 7.1 | 0.745 | 0.543 | −0.8 ± 6.5 | −1.3 ± 5.6 | 0.877 | |
| Grains and cereals (g) | 243 ± 23 | 221 ± 58 | 223 ± 46 | 0.674 | 0.208 | −22 ± 75 | −20 ± 47 | 0.505 | |
| Potatoes (g) | 14 ± 15 | 30 ± 23 | 20 ± 18 | 0.213 | 0.372 | 16 ± 33 | 6 ± 17 | 0.445 | |
| Sugar (g) | 4 ± 3 | 1 ± 1 | 6 ± 9 | 0.025* | 0.889 | −4 ± 4 | 2 ± 7 | 0.105 | |
| Nuts (g) | 3 ± 5 | 1 ± 1 | 2 ± 3 | 0.612 | 0.753 | −2 ± 5 | −1 ± 7 | 0.878 | |
| Total vegetables (g) | 124 ± 47 | 265 ± 68 | 223 ± 83 | 0.002* | 0.022* | 141 ± 80 | 99 ± 96 | 0.359 | |
| Green vegetables (g) | 33 ± 12 | 95 ± 44 | 93 ± 43 | 0.007* | 0.003* | 62 ± 46 | 60 ± 37 | 0.920 | |
| White vegetables (g) | 91 ± 51 | 169 ± 53 | 130 ± 56 | 0.025* | 0.198 | 79 ± 62 | 39 ± 77 | 0.278 | |
| Fruits (g) | 62 ± 34 | 30 ± 37 | 17 ± 23 | 0.123 | 0.036* | −32 ± 53 | −45 ± 46 | 0.620 | |
| Mushrooms (g) | 8 ± 8 | 22 ± 19 | 7 ± 6 | 0.105 | 0.658 | 14 ± 21 | −1 ± 8 | 0.105 | |
| Algae (g) | 5 ± 7 | 3 ± 3 | 5 ± 8 | 0.866 | 0.674 | −2 ± 7 | 1 ± 9 | 0.574 | |
| Pulses (g) | 45 ± 26 | 45 ± 43 | 19 ± 19 | 0.398 | 0.029* | 0 ± 54 | −26 ± 27 | 0.442 | |
| Fish and shellfish (g) | 18 ± 13 | 26 ± 22 | 44 ± 30 | 0.208 | 0.108 | 8 ± 16 | 26 ± 40 | 0.269 | |
| Meat (g) | 46 ± 23 | 57 ± 23 | 46 ± 25 | 0.305 | 0.974 | 11 ± 28 | 0 ± 29 | 0.464 | |
| Eggs (g) | 23 ± 16 | 16 ± 26 | 25 ± 18 | 0.327 | 0.790 | −7 ± 20 | 2 ± 20 | 0.405 | |
| Milk (g) | 52 ± 61 | 46 ± 65 | 75 ± 48 | 0.889 | 0.398 | −6 ± 90 | 23 ± 59 | 0.452 | |
| Oils and fats (g) | 8 ± 5 | 6 ± 4 | 6 ± 2 | 0.362 | 0.485 | −2 ± 7 | −1 ± 6 | 0.800 | |
| Confectionery (g) | 3 ± 7 | 15 ± 31 | 7 ± 13 | 0.686 | 0.345 | 12 ± 33 | 4 ± 13 | 0.505 | |
| Beverages (g) | 77 ± 55 | 120 ± 139 | 77 ± 75 | 0.484 | 0.999 | 42 ± 119 | 0 ± 68 | 0.398 | |
Values are presented as mean ± SD. Values of energy intake are presented per ideal weight, and values of other foods and nutrients are presented per 1,000 kcal. The relationships between baseline and 3 months or 6 months were assessed using the paired t test for normally distributed data and the Wilcoxon signed-rank test for non-normally distributed data. The relationships between changes at 3 and 6 months were assessed using the non-paired t test for normally distributed data and the Mann–Whitney U test for non-normally distributed data. *p<0.05. PFC-P, protein-energy rate; PFC-F, fat-energy rate; PFC-C, carbohydrate rate; total vegetables, the sums of green and white vegetable.
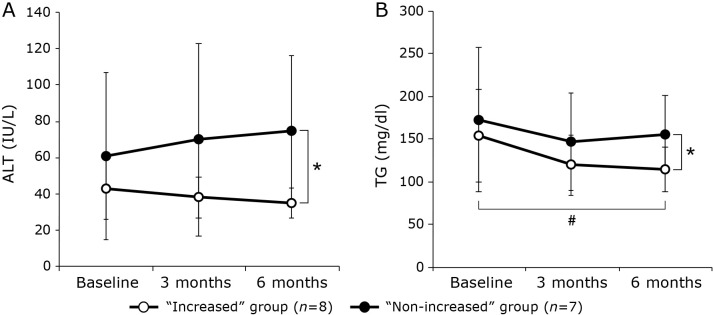
Table 4 presents the examination results at baseline, 3 months, and 6 months. No baseline parameters were found to be significantly different between the “increased” and “non-increased” group. In the “increased” group, TG levels were significantly lower at 6 months than at baseline, and there was a significant increase in HDL-C at 3 months as compared to baseline. The CAP and LSM also decreased in this group, although these changes were not significant. ALT and TG levels were significantly lower in the “increased” than in the “non-increased” group at 6 months (Fig. 4). None of the parameters examined improved significantly in the “non-increased” group.
Table 4.
Comparison of the clinical examination data between baseline and at 3 and 6 months
| Increased group (n = 8) |
Non-increased group (n = 7) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | 3 months | 6 months |
p value |
Baseline | 3 months | 6 months |
p value |
||||
| Baseline vs 3 months |
Baseline vs 6 months |
Baseline vs 3 months |
Baseline vs 6 months |
||||||||
| Weight (kg) | 73.6 ± 15.6 | 73.3 ± 16.1 | 73.5 ± 15.4 | 0.327 | 0.624 | 70.1 ± 11.4 | 71.3 ± 11.1 | 71.7 ± 12.5 | 0.128 | 0.377 | |
| BMI (kg/m2) | 28.4 ± 4.0 | 28.3 ± 4.2 | 28.3 ± 4.1 | 0.327 | 0.779 | 27.1 ± 2.1 | 27.5 ± 2.6 | 28.2 ± 3.5 | 0.237 | 0.387 | |
| Skeletal muscle (kg) | 25.4 ± 6.6 | 25.4 ± 6.9 | 25.5 ± 6.6 | 0.833 | 1.000 | 25.7 ± 6.0 | 26.2 ± 6.2 | 27.5 ± 5.2 | 0.018* | 0.134 | |
| Body fat (%) | 37.1 ± 7.1 | 37.0 ± 7.3 | 36.8 ± 7.9 | 0.400 | 0.575 | 33.6 ± 8.1 | 33.6 ± 8.6 | 33.8 ± 8.9 | 0.865 | 0.511 | |
| AST (IU/L) | 30 ± 12 | 29 ± 11 | 29 ± 11 | 0.916 | 0.944 | 40 ± 25 | 43 ± 24 | 48 ± 26 | 0.072 | 0.397 | |
| ALT (IU/L) | 43 ± 17 | 38 ± 11 | 35 ± 8 | 0.233 | 0.123 | 61 ± 46 | 70 ± 53 | 75 ± 41 | 0.203 | 0.075 | |
| γ-GTP (IU/L) | 52 ± 27 | 47 ± 24 | 45 ± 23 | 0.183 | 0.091 | 54 ± 34 | 52 ± 31 | 89 ± 88 | 0.499 | 0.173 | |
| T-bil (mg/dl) | 0.9 ± 0.3 | 1.2 ± 0.5 | 1.2 ± 0.3 | 0.123 | 0.036* | 0.7 ± 0.1 | 0.8 ± 0.4 | 0.9 ± 0.4 | 0.735 | 0.345 | |
| TG (mg/dl) | 154 ± 54 | 120 ± 35 | 115 ± 26 | 0.123 | 0.050* | 173 ± 85 | 147 ± 57 | 156 ± 45 | 0.237 | 0.708 | |
| T-cho (mg/dl) | 178 ± 21 | 198 ± 29 | 188 ± 27 | 0.310 | 0.398 | 207 ± 36 | 200 ± 51 | 197 ± 47 | 0.397 | 0.177 | |
| HDL-C (mg/dl) | 54 ± 11 | 59 ± 13 | 56 ± 12 | 0.049* | 0.344 | 53 ± 13 | 52 ± 12 | 51 ± 9 | 0.674 | 0.462 | |
| LDL-C (mg/dl) | 106 ± 21 | 129 ± 22 | 115 ± 22 | 0.075 | 0.116 | 130 ± 29 | 128 ± 48 | 125 ± 48 | 0.672 | 0.581 | |
| Ferittin (ng/ml) | 147 ± 85 | 145 ± 69 | 120 ± 55 | 0.345 | 0.116 | 204 ± 255 | 214 ± 259 | 223 ± 253 | 0.345 | 0.345 | |
| PLT (103/μl) | 228 ± 84 | 227 ± 88 | 242 ± 110 | 0.779 | 0.575 | 237 ± 73 | 238 ± 74 | 251 ± 83 | 0.933 | 0.185 | |
| FBS (mg/dl) | 93 ± 5 | 108 ± 19 | 101 ± 7 | 0.025* | 0.012* | 97 ± 19 | 100 ± 8 | 103 ± 15 | 0.237 | 0.075 | |
| HbA1c (%) | 6.0 ± 0.6 | 5.9 ± 0.5 | 6.0 ± 0.5 | 0.407 | 0.607 | 6.2 ± 1.0 | 6.2 ± 1.1 | 6.1 ± 0.9 | 0.480 | 0.386 | |
| Type 4 collagen 7S (ng/ml) | 5.2 ± 1.7 | 5.1 ± 1.4 | 5.2 ± 2.4 | 1.000 | 0.715 | 4.7 ± 0.7 | 5.0 ± 1.0 | 4.7 ± 1.6 | 0.144 | 0.655 | |
| M2BPGi | 1.3 ± 1.2 | 1.2 ± 1.0 | 1.2 ± 1.2 | 0.893 | 0.080 | 0.6 ± 0.2 | 0.8 ± 0.6 | 0.6 ± 0.3 | 0.248 | 0.878 | |
| AST/ALT ratio | 0.7 ± 0.3 | 0.8 ± 0.3 | 0.8 ± 0.2 | 0.018* | 0.036* | 0.7 ± 0.2 | 0.7 ± 0.1 | 0.7 ± 0.1 | 0.310 | 0.450 | |
| FIB-4 index | 1.5 ± 1.3 | 1.6 ± 1.4 | 1.7 ± 1.5 | 0.123 | 0.123 | 1.2 ± 0.6 | 1.3 ± 0.8 | 1.3 ± 1.1 | 0.735 | 0.735 | |
| CAP (dB/m) | 317 ± 65 | 304 ± 52 | 292 ± 39 | 0.575 | 0.208 | 336 ± 40 | 315 ± 36 | 322 ± 76 | 0.063 | 0.623 | |
| LSM (kPa) | 12.9 ± 15.0 | 7.3 ± 3.3 | 8.7 ± 4.9 | 0.674 | 0.612 | 5.3 ± 1.8 | 4.9 ± 2.8 | 5.7 ± 2.1 | 0.237 | 0.591 | |
Values are mean ± SD. The relationships between baseline, 3 months or 6 months were assessed using the paired t test for normally distributed data and the Wilcoxon signed-rank test for non-normally distributed data. *p<0.05. BMI, body mass index; AST, aspartate aminotransferase; ALT, alanine aminotransferase; γ-GTP, γ-glutamyl transpeptidase; T-bil, total bilirubin; TG, triglycerides; T-cho, total cholesterol; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; PLT, platelets; FBS, fasting blood sugar; HbA1c, hemoglobin A1c; M2BPGi, Mac-2 binding protein glycosylation isomer; AAR, AST/ALT ratio; CAP, controlled attenuation parameter; LSM, liver stiffness measurement.
Fig. 4.
Changes in ALT (A) and TG (B) levels during the 6-month intervention period in patients who had an increased vegetable consumption (“increased” group) and those who did not (“non-increased” group). Values are presented as mean ± SD. The relationships between baseline and 3 months or 6 months were assessed using the paired t test for normally distributed data and the Wilcoxon signed-rank test for non-normally distributed data. The relationships between both groups at baseline, 3 months, and 6 months were assessed using the non-paired t test for normally distributed data and the Mann–Whitney U test for non-normally distributed data. *p<0.05 between groups, #p<0.05 vs baseline. ALT, alanine aminotransferase; TG, triglyceride.
Discussion
We examined whether a nutritional intervention method that motivates frequent and active vegetable intake could help improve NAFLD. Similar to a previous study,(17) we found that the intervention increased the intake of vegetables, although no significant difference was observed after the intervention owing to large interindividual variability. Conversely, when the correlation between the change in green vegetable intake and that in body weight was examined, a significantly strong negative correlation was found—this is a notable result due to weight loss being an important factor in improving NAFLD. This may be because an increased intake of vegetables, which has a low energy density, promotes a decreased intake of energy, leading to weight loss. Since previous studies have shown that patients with NAFLD have an extremely low vegetable intake compared to patients without NAFLD, the former may be expected to lose weight and improve their condition with even a small change in dietary behavior.
Due to the large individual differences in vegetable intake in this intervention study, we divided the patients into two groups—those whose vegetable intake increased after the intervention and those whose vegetable intake did not increase—and attempted to examine the effect of an increased vegetable intake on the improvement of NAFLD. The results showed that an increased intake of vegetables resulted in beneficial changes for patients with NAFLD; these include an increased intake of many vitamins, dietary fiber, and potassium, and a decreased intake of energy and fruit. Additionally, there was a significant increase in HDL-C, and a decrease in the ALT and TG concentration. Furthermore, a decreasing trend in the CAP and LSM was observed, although the trend was not significant. Vitamins that are abundant in vegetables, especially vitamins A and C, have antioxidant effects and hence may reduce oxidative stress in NAFLD.(24) In particular, a significant inverse association between vitamin C levels and NAFLD has been reported.(25) Dietary fiber has no clear direct relationship with NAFLD but is known to correlate inversely with obesity and the risk of developing T2DM.(26,27) It may also improve NAFLD by altering the intestinal microbiota and reducing oxidative stress caused by endotoxin inflow to the hepatic portal vein.(28) In a cross-sectional study involving Latino youth, consumption of nutrient-rich vegetables (dark green and deep orange/yellow) significantly reduced visceral fat levels and increased insulin sensitivity.(29) Because green vegetables contain higher amounts of carotenoids and phytochemicals than white vegetables,(30) they are more likely to be effective in improving NAFLD. We note that the software program used for nutritional analysis in this study was based on the Standard Table of Food Composition in Japan, which does not provide accurate intake values for carotenoids and phytochemicals; hence, the results for these variables were not provided here. It was not possible to determine whether the beneficial results were due to weight loss or the direct effects of the vegetable components; however, even a small change in dietary behavior may be useful in improving NAFLD.
It is necessary to understand why some of the patients in our study did not increase their consumption of vegetables during the intervention. Although the percentage of patients who cooked for themselves did not significantly differ between the “increased” and “non-increased” groups, it is possible that cooking frequency differed between the groups. Moreover, the participants might have reacted differently to the intervention depending on their socioeconomic and health conditions; these factors have been shown to influence vegetable consumption.(31) Nevertheless, diet therapy is the most important element in the treatment and prevention of NAFLD. In our study, some parameters did not significantly improve or worsen after the intervention, even in the “increased” group. In addition, our study included only a few participants, and they had various comorbidities, such as T2DM, hypertension, and dyslipidemia. Hence, vegetable intake may improve NAFLD in an indirect manner via mechanisms yet to be revealed. For the vegetable intake non-increase group, it may be necessary to combine approaches other than increasing vegetable intake, such as fish oil intake and exercise intervention, which has been reported to improve insulin resistance and lipid profile.(32)
Additional study limitations are as follows. No control group was enrolled, because this study was conducted to compare and validate results before and after intervention, and it was a trial study with a small number of participants. However, we believe that we were able to demonstrate the benefits of increased vegetable intake by comparing the group that did with the group that did not increase their vegetable consumption. Furthermore, some participants were only diagnosed by transient elastography, without undergoing liver biopsy which is the gold standard. Moreover, the dietary survey was analyzed based on records obtained on any 2 days, which might not accurately reflect habitual dietary intake. Lastly, follow-up after completion of the intervention trial was not possible in this study. Thus, future studies with more participants and long-term follow-ups are required.
In conclusion, we have developed a nutritional intervention protocol that can increase vegetable consumption in patients with NAFLD. The results suggest that increased vegetable consumption may lead to improvement in NAFLD. Our approach is advantageous in that it is simple and safe for patients to implement on a long-term basis. We expect that our intervention will be an effective and unique diet therapy and treatment for patients with NAFLD.
Author Contributions
HS, YK, and SW designed the study. HS, YK, YSu, SW, MT, YSh, KS, and YSa contributed to designing and performing the experiments. HS, YK, and TS analyzed and interpreted the data. HS and YK wrote the manuscript with input from other authors. YSu, SW, WA, YN, and MK conceived the study, researched the data, and reviewed the manuscript. All authors critically reviewed and approved the final version of manuscript.
Acknowledgments
We would like to thank Yuya Seko, M.D., Ph.D. for supporting the performance of this research. This work was supported in part by a Grant of Industry-Academia-Government Collaboration (“Field for Knowledge Integration and Innovation”; No. 16824414) from the Ministry of Agriculture, Forestry and Fisheries of Japan, and by grants-in-aid from the Nakatani Suzuyo Memorial Fund for Nutrition and Dietetics (Tokyo, Japan).
Abbreviations
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- CAP
controlled attenuation parameter
- FIB-4
fibrosis-4
- HDL-C
high-density lipoprotein cholesterol
- LSM
liver stiffness measurement
- NAFLD
non-alcoholic fatty liver disease
- PLT
platelets
- T2DM
type 2 diabetes mellitus
- TG
triglycerides
Conflicts of Interest
YN received a scholarship from EA Pharma. Co. Ltd., collaboration research funding from Fujifilm Medical Co. Ltd., and lecture fees from Mylan EPD Co., Takeda Pharma. Co. Ltd., Mochida Pharma. Co. Ltd., EA Pharma. Co. Ltd., Otsuka Pharma. Co. Ltd., Nippon Kayaku Co. Ltd., and Miyarisan Pharma. Co. Ltd. Our study was partly financed by these funds. These companies had final approval of the manuscript but did not participate in the study design. They had no competing interests.
References
- 1.Schaffner F, Thaler H. Nonalcoholic fatty liver disease. Prog Liver Dis 1986; 8: 283–298. [PubMed] [Google Scholar]
- 2.Vernon G, Baranova A, Younossi ZM. Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther 2011; 34: 274–285. [DOI] [PubMed] [Google Scholar]
- 3.Li J, Zou B, Yeo YH, et al. Prevalence, incidence, and outcome of non-alcoholic fatty liver disease in Asia, 1999–2019: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol 2019; 4: 389–398. [DOI] [PubMed] [Google Scholar]
- 4.Fan JG, Kim SU, Wong VW. New trends on obesity and NAFLD in Asia. J Hepatol 2017; 67: 862–873. [DOI] [PubMed] [Google Scholar]
- 5.Younossi Z, Anstee QM, Marietti M, et al. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol 2018; 15: 11–20. [DOI] [PubMed] [Google Scholar]
- 6.Eguchi Y, Hyogo H, Ono M, et al. Prevalence and associated metabolic factors of nonalcoholic fatty liver disease in the general population from 2009 to 2010 in Japan: a multicenter large retrospective study. J Gastroenterol 2012; 47: 586–595. [DOI] [PubMed] [Google Scholar]
- 7.Promrat K, Kleiner DE, Niemeier HM, et al. Randomized controlled trial testing the effects of weight loss on nonalcoholic steatohepatitis. Hepatology 2010; 51: 121–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.The Japanese Society of Gastroenterology. NAFLD/NASH Clinical Guideline 2014. Tokyo: Nanko-do, Inc., 2014; 50–51. [Google Scholar]
- 9.Tatsumi H, Kobayashi Y, Wada S, Kuwahata M, Sumida Y, Kido Y. An assessment of dietary factors in Japanese non-alcoholic fatty liver disease patients and the relationship with blood parameters. Ann Nutr Metab 2013; 63 (Suppl 1): 301. [Google Scholar]
- 10.Diet, nutrition and the prevention of chronic diseases: report of the joint WHO/FAO expert consultation. WHO Technical Report Series; No. 916. World Health Organization. https://www.who.int/dietphysicalactivity/publications/trs916/en/. Accessed 24 Mar 2021.
- 11.Zelber-Sagi S, Salomone F, Mlynarsky L. The Mediterranean dietary pattern as the diet of choice for non-alcoholic fatty liver disease: evidence and plausible mechanisms. Liver Int 2017; 37: 936–949. [DOI] [PubMed] [Google Scholar]
- 12.Roskams T, Yang SQ, Koteish A, et al. Oxidative stress and oval cell accumulation in mice and humans with alcoholic and nonalcoholic fatty liver disease. Am J Pathol 2003; 163: 1301–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Salomone F, Godos J, Zelber-Sagi S. Natural antioxidants for non-alcoholic fatty liver disease: molecular targets and clinical perspectives. Liver Int 2016; 36: 5–20. [DOI] [PubMed] [Google Scholar]
- 14.Kikuchi Y, Ushida M, Shiozawa H, et al. Sulforaphane-rich broccoli sprout extract improves hepatic abnormalities in male subjects. World J Gastroenterol 2015; 21: 12457–12467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ni Y, Nagashimada M, Zhan L, et al. Prevention and reversal of lipotoxicity-induced hepatic insulin resistance and steatohepatitis in mice by an antioxidant carotenoid, β-cryptoxanthin. Endocrinology 2015; 156: 987–999. [DOI] [PubMed] [Google Scholar]
- 16.Ter Horst KW, Serlie MJ. Fructose consumption, lipogenesis, and non-alcoholic fatty liver disease. Nutrients 2017; 9: 981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hirano S, Kobayashi Y, Sugiyama H, et al. A nutritional invention that strongly promoted vegetable intake for non-alcoholic fatty liver disease modifies the amount of vegetable dishes consumed and the form of food served depending on the duration of the intervention. Jpn J Metab Clin Nutr 2019; 22: 207–215 (in Japanese). [Google Scholar]
- 18.Kuno A, Ikehara Y, Tanaka Y, et al. A serum “sweet-doughnut” protein facilitates fibrosis evaluation and therapy assessment in patients with viral hepatitis. Sci Rep 2013; 3: 1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shimada M, Hashimoto E, Kaneda H, Noguchi S, Hayashi N. Nonalcoholic steatohepatitis: risk factors for liver fibrosis. Hepatol Res 2002; 24: 429–438. [DOI] [PubMed] [Google Scholar]
- 20.Shah AG, Lydecker A, Murray K, et al. Comparison of noninvasive markers of fibrosis in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol 2009; 7: 1104–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sasso M, Beaugrand M, de Ledinghen V, et al. Controlled attenuation parameter (CAP): a novel VCTETM guided ultrasonic attenuation measurement for the evaluation of hepatic steatosis: preliminary study and validation in a cohort of patients with chronic liver disease from various causes. Ultrasound Med Biol 2010; 36: 1825–1835. [DOI] [PubMed] [Google Scholar]
- 22.Yoneda M, Yoneda M, Mawatari H, et al. Noninvasive assessment of liver fibrosis by measurement of stiffness in patients with nonalcoholic fatty liver disease (NAFLD). Dig Liver Dis 2008; 40: 371–378. [DOI] [PubMed] [Google Scholar]
- 23. Examination Committee of Criteria for ‘Obesity Disease’ in Japan, Japan Society for the Study of Obesity. New criteria for ‘obesity disease’ in Japan. Circ J 2002; 66: 987–992. [DOI] [PubMed] [Google Scholar]
- 24.Lee CH, Chan RSM, Wan HYL, et al. Dietary intake of anti-oxidant vitamins A, C, and E is inversely associated with adverse cardiovascular outcomes in Chinese—a 22-years population-based prospective study. Nutrients 2018; 10: 1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wei J, Lei GH, Fu L, Zeng C, Yang T, Peng SF. Association between dietary vitamin C intake and non-alcoholic fatty liver disease: a cross-sectional study among middle-aged and older adults. PLoS One 2016; 11: e0147985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu S, Willett WC, Manson JE, Hu FB, Rosner B, Colditz G. Relation between changes in intakes of dietary fiber and grain products and changes in weight and development of obesity among middle-aged women. Am J Clin Nutr 2003; 78: 920–927. [DOI] [PubMed] [Google Scholar]
- 27.Chen GC, Lv DB, Pang Z, Donq JY, Liu QF. Dietary fiber intake and stroke risk: a meta-analysis of prospective cohort studies. Eur J Clin Nutr 2013; 67: 96–100. [DOI] [PubMed] [Google Scholar]
- 28.Miele L, Valenza V, La Torre G, et al. Increased intestinal permeability and tight junction alteration in nonalcoholic fatty liver disease. Hepatology 2009; 49: 1877–1887. [DOI] [PubMed] [Google Scholar]
- 29.Cook LT, O'Reilly GA, Goran MI, Weigensberg MJ, Spruijt-Metz D, Davis JN. Vegetable consumption linked to decreased visceral and liver fat and improved insulin resistance in overweight Latino youth. J Acad Nutr Diet 2014; 114: 1776–1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Garden-Robinson J. Carotenoids in green vegetables and health aspects. In: Chen C, ed. Pigments in Fruits and Vegetables: Genomics and Dietetics, New York: Springer, 2015; 229–246. [Google Scholar]
- 31.Dehghan M, Akhtar-Danesh N, Merchant AT. Factors associated with fruit and vegetable consumption among adults. J Hum Nutr Diet 2011; 24: 128–134. [DOI] [PubMed] [Google Scholar]
- 32.Hua L, Lei M, Xue S, Li X, Li S, Xie Q. Effect of fish oil supplementation combined with high-intensity interval training in newly diagnosed non-obese type 2 diabetes: a randomized controlled trial. J Clin Biochem Nutr 2020; 66: 146–151. [DOI] [PMC free article] [PubMed] [Google Scholar]