Abstract
Context
MSCA1 (mesenchymal stem cell antigen 1) and CD36 (cluster of differentiation 36) have been described as novel adipocyte progenitor markers in adults with a potential relevance for obesity and adipocyte progenitor function.
Objective
With the early manifestation of obesity in children and formation of adipose tissue (AT) dysfunction, children provide the opportunity to characterize the function of MSCA1 and CD36 during physiological AT accumulation and with obesity and related disease.
Methods
We investigated MSCA1 and CD36 expression in adipocytes and stroma vascular fraction (SVF) cells from 133 children of the Leipzig AT Childhood cohort with regard to AT accumulation and biology. In a subsample we analyzed how MSCA1 and CD36 expression is related to adipose progenitor capacities in vitro (ie, proliferation, differentiation and mitochondrial function).
Results
Both MSCA1 and CD36 are differentially expressed in adipocytes and SVF cells of children. MSCA1 expression is positively correlated to obesity-associated AT dysfunction (ie, adipocyte hypertrophy and serum high-sensitivity C-reactive protein), and high SVF MSCA1 expression is associated with increased mitochondrial respiration in vitro. CD36 expression is not associated with AT dysfunction but SVF CD36 expression is downregulated in children with overweight and obesity and shows a positive association with the differentiation capacity of SVF cells ex vivo and in vitro.
Conclusion
Both MSCA1 and CD36 are associated with obesity-related alterations in AT of children. In particular, CD36 expression predicts adipogenic potential of SVF cells, indicating a potential role in the regulation of adipocyte hyperplasia and hypertrophy with obesity development in children.
Keywords: adipocyte progenitor cells, adipose tissue, adipose tissue dysfunction, adipocyte differentiation, children, obesity
Obesity can manifest during early childhood (1,2). The characteristic feature of obesity is an increase in adipose tissue (AT) mass caused by an increase in the number of adipocytes (hyperplasia) and/or an increase in adipocyte size (hypertrophy). This AT accumulation is not pathological per se. However, if adipocyte hypertrophy with inflammation and adipokine disbalance is prevalent, this so-called AT dysfunction drives development of obesity-related comorbidities (3). Hence, assessment of AT composition and function at an early obesity stage is of relevance. It is assumed that during obesity-associated AT expansion, adipocyte progenitor cells play an important role, particularly through differentiation into metabolically active adipocytes (4). Adipocyte progenitor cells are contained within the stroma vascular fraction (SVF) of AT (5,6). Several studies show that the SVF contains multiple subpopulations of adipocyte progenitor cells with different abilities to proliferate and differentiate (7,8). These subpopulations are characterized by the expression of specific cell surface markers, in particular mesenchymal stem cell antigen 1 (MSCA1), also known as tissue nonspecific alkaline phosphatase or alkaline phosphatase, liver/bone/kidney (ALPL), and scavenger receptor cluster of differentiation 36 (CD36) (9-11).
MSCA1 is encoded by the ALPL gene and is expressed in various tissues, including AT, bone marrow, and heart (12). It has been shown that MSCA1 is involved in cellular differentiation processes (13,14), immunomodulatory functions (15,16), and metabolism (17). Recent results from studies in human adults provided evidence that in subcutaneous AT MSCA1 is a surface marker for cells with higher white and brite adipogenic potential compared to other adipocyte progenitor cell subtypes, and both number of MSCA1-positive progenitor cells and magnitude of MSCA1 expression are positively correlated to body mass index (BMI) in adults (9). In addition, a recent study by Sun et al has provided evidence that mitochondrial tissue nonspecific alkaline phosphatase controls thermogenesis via regulation of the futile creatine cycle in AT of mice (18).
Gao et al demonstrated that the MSCA1-positive adipocyte progenitor population can be further subdivided by the presence of CD36 (10). CD36 is expressed in many cell types, including adipocytes, endothelial cells, and hematopoietic cells, such as macrophages and platelets (19). In fact, CD36 is an established marker of adipocyte differentiation and its loss of function in mice is associated with decreased adipocyte differentiation, ectopic lipid accumulation in the liver, and insulin resistance (20). In line with this, it has been demonstrated that CD36-positive cells isolated from human adult subcutaneous AT differentiate more efficiently than CD36-negative cells, suggesting that CD36 is a marker of human adipocyte progenitor cells with high adipogenic potential (10).
These preliminary data suggest that MSCA1 and CD36 might play a role during obesity-related AT accumulation by affecting processes related to AT function. However, most of the studies have been performed in patients with morbid obesity, and the relevance for processes involved in early obesity progression remains poorly understood. In this regard, studies in children are of special value since the functional relevance of adipocyte progenitor markers can be addressed during AT accumulation with normal development and with early development of obesity and related AT dysfunction (21).
Within this study, we assessed an association of MSCA1 and CD36 gene expression with physiological or obesity-related AT accumulation in children. Furthermore, we investigated whether an elevated MSCA1 and CD36 expression is related to functional properties of adipocyte progenitor cells, such as proliferation, differentiation, and mitochondrial function.
Materials and Methods
Samples (Leipzig AT Childhood Cohort)
Subcutaneous AT samples were retrieved from the Leipzig AT Childhood Cohort from 133 Caucasian children (0-18 years) undergoing elective surgery (21). All children were free of diseases or medications that potentially affect AT biology. Children with diabetes, generalized inflammation, malignant diseases, genetic syndromes, or permanently immobilized children were excluded. Written informed consent was obtained from all parents. The study was approved by the local ethics committee (Reg. No. 265-08, 265-08-ff, University of Leipzig; NCT02208141).
BMI data were standardized to age- and sex-specific German reference data and are given as BMI SD score (SDS) (22). A cutoff of 1.28 and 1.88 SDS defined overweight and obesity in children, respectively. Fasting blood samples were obtained prior to surgery. Levels of adiponectin, leptin, high-sensitivity C-reactive protein (hs-CRP), glucose, and insulin were measured by a certified laboratory (Institute of Laboratory Medicine, Clinical Chemistry and Molecular Diagnostics, University of Leipzig, Leipzig, Germany). Homeostasis model assessment-insulin resistance (HOMA IR) was calculated to evaluate insulin resistance (23).
Isolation and Characterization of Adipocytes and Cells of the Stromal Vascular Fraction From Human AT Samples
Immediate isolation of adipocytes and stromal vascular fraction cells from AT after extraction during surgery and subsequent functional analyses was performed as previously described by Landgraf et al (21). The freshly isolated adipocytes were used for in vitro determination of lipolytic capacity or fixed in osmium tetroxide for analysis of adipocyte cell size and number using a Coulter counter (Multisizer III; Beckmann Coulter, Krefeld, Germany) with a 560 µm aperture. Lipolytic activity was normalized to adipocyte number determined by the Coulter counter method and is given as the release of glycerol in ng/mL per 1000 adipocytes.
The in vitro proliferation and differentiation assays of SVF cells were also carried out as previously described (21). To estimate the potential for proliferation of the seeded cells, the cell doubling time was calculated as the fold change from the mean cell numbers on days 8 and 10 relative to day 2. For samples with observed cell proliferation, the growth rate [gr = (ln[N(t)/N(t0)])/t)] and generation time [=ln (2)/gr] were calculated as the mean from the cell numbers on days 6, 8, and 10 in comparison to day 4. The differentiation efficiency was determined with a fluorescence microscope as the percentage of differentiated cells (Nile Red/Hoechst 33342 double-stained cells) from the total number of cells (Hoechst 33342 stained) and as Oil Red O (Sigma, Darmstadt, Germany) absorbance at 540 nm (CLARIOstar, BMG LABTECH, Offenburg, Germany) per well at day 8.
For immunohistochemical analysis, AT samples were fixed in 4% paraformaldehyde, paraffin-embedded, and sectioned, and macrophages were stained using a monoclonal CD68 antibody (1:100; clone PGM-1, M0876 DAKO).
RNA Isolation and Gene Expression Analyses
Total RNA was extracted using the RNeasy Mini Kit (Qiagen, Hilden, GER), and a total of 250 ng of RNA was reverse transcribed using Moloney Murine Leukemia Virus Reverse Transcriptase (Thermo Fisher, Waltham, MA, USA) and random hexamer primer (Roche, Basel, Switzerland). The amplification of the complimentary DNA sample by quantitative real-time reverse transcription polymerase chain reaction was performed in a 20 µL reaction mix with 10 µL 2 × quantitative polymerase chain reaction MasterMix (Eurogentec, Seraing, Belgium), 900 nM of each primer, and 200 nM probe. Copy numbers of MSCA1 and CD36 were determined from a standard curve and normalized to the reference genes ACTB (beta actin), HPRT (hypoxanthine guanine phosphoribosyl transferase), and TBP (TATA box binding protein) (24). The following primers and probes were used: CD36 (Hs00169627_m1, Thermo Fisher), MSCA1 (ALPL, Hs01029144_m1, Thermo Fisher), CKB (Hs00176484_m1, Thermo Fisher), ADIPOQ (forward 5´-GGCCGTGATGGCAGAGAT-3´, reverse 5´-CCTTCAGCCCCGGGTACT-3´, probe 5´-FAM-CGATGTCTCCCTTAGGACCAATAAGACCTGG-TAMRA-3´), PPARG (forward 5´-GATCCAGTGG TTGCAGATTACAA-3´, reverse 5´-GAGGGAGTTGG AAGGCTCTTC-3´, probe 5´-FAM-TGACCTGAAACTT CAAGAGTACCAAAGTGCAA-TAMRA-3´), PGC1A (forward 5´-CACCAAACCCACAGAGAACA-3´, reverse 5´-GGGTCATTTGGTGACTCTGG-3´, probe 5´-FAM- CGCAGTCACAACACTTACAAGCCAAAC-TAMRA-3´), PRDM16 (forward 5´-CCAATAGTGAG ATGAACCAAGCAT-3´, reverse 5´-CCGTCCACGAT CTGCATGT-3´, probe 5´-FAM-AACGCGAACAGAG AAACGGGCG-TAMRA-3´), UCP1 (forward 5´-ACGA CACGGTCCAGGAGTTC-3´, reverse 5´-ACCAGCTAAAA TCTTGCTTCCTAAAC-3´, probe 5´-FAM-TCACCGCA GGGAAAGAAACAGCACC-TAMRA-3´), ACTB (forward 5´-CGAGCGCGGCTACAGCTT-3´, reverse 5´-CCTT AATGTCACGCACGATTT-3´, probe 5´-FAM-ACCACC ACGGCCGAGCGG-TAMRA-3´), TBP (forward 5´-TTGTAAACTTGACCTAAAGACCATTGC-3´, reverse 5´-TTCGT GGCTCTCTTATCCTCATG-3´, probe 5´-HEX-AACGCCG AATATAATCCCAAGCGGTTTG-TAMRA-3´), and HPRT (forward 5´-GGCAGTATAATCCAAAGATGGTCAA-3´, reverse 5´-GTCTGGCTTATATCCAACACTTCGT-3´, probe 5´-FAM-CAAGCTTGCTGGTGAAAAGGACCCC-TAMRA-3´).
Small Interfering RNA-mediated Knockdown in Simpson Golabi Behmel Syndrome Cells
Small interfering RNA transfections were performed in Simpson Golabi Behmel Syndrome (SGBS) cells using the Neon Transfection System 100 µL Kit (Thermo Fisher). Electroporation was performed at a cell density of 6 × 106 cells/mL at pulse voltage 1300 V, pulse width 20 ms, pulse number 2. Gene-specific ON-TARGETplus SMARTpool small interfering RNAs and ON-TARGET plus control reagents (Dharmacon, Lafayette, CO, USA) were used at a final concentration of 500 nM. A total of 100 000 transfected SGBS cells per well were seeded in 12-well format and differentiated into mature adipocytes as previously described by Landgraf et al (24).
Mitochondrial Function of Cultivated SVF Cells
For the analysis of mitochondrial function, selected cryopreserved SVF cell samples were cultivated for 4 to 11 passages in culture medium (Gibco Dulbecco’s Modified Eagle Medium/ Nutrient Mixture F-12, 10% fetal calf serum, 100 units/mL penicillin-streptomycin) at 37°C and 5% CO2 before seeding in Seahorse XF24 Cell Culture Microplates (Agilent, Santa Clara, CA, USA) at a density of 60 000 cell/cm². The measurement was performed 48 h later in Assay Medium [XF Base Medium (Agilent), 10 mM glucose (Roth, Karlsruhe, Germany), 2 mM pyruvate (Sigma), 2 mM glutamine (Sigma)] using the Seahorse XF Cell Mito Stress Test Kit (Agilent). The kit components were used in the following concentrations: oligomycin 2 µM, carbonyl cyanide-4 (trifluoromethoxy) phenylhydrazone 3 µM and Rotenone/antimycin A 0.5 µM. Determination of the oxygen consumption rate (OCR) was performed on the Seahorse XFe24 Analyzer (Agilent) together with the Seahorse Wave software and normalized to the cell number as assessed by bicinchoninic acid assay measurement of the amount of protein per well (Thermo Fisher).
Statistical analyses
Data that did not represent a Gaussian distribution were transformed logarithmically before being analyzed. Quantitative variables were analyzed by parametric tests (Student’s t test, analysis of variance, Pearson correlation analysis), whereas for categorical variables a χ² test was performed. For multiple regression analyses, the stepwise forward model was used. Statistical analysis was performed using Statistica 13.3 (StatSoft, Tulsa, OK, USA) and GraphPad Prism 6 (GraphPad Software, Inc, San Diego, CA, USA).
Results
To answer the question whether gene expression of the adipocyte progenitor markers MSCA1 and CD36 is associated with obesity in children or related to functional parameters of adipocyte progenitor cells, we investigated AT samples from 59 lean children and 74 children with overweight and obesity of the Leipzig AT Childhood Cohort (21). The general characteristics of the study participants and samples are presented in Table 1. There were no significant differences in sex distribution and height SDS between lean children and children with overweight or obesity, although lean children were about 3 years younger and, hence, presented at an earlier pubertal stage. As described in previous studies, biological indicators of AT dysfunction, such as adipocyte hypertrophy and hyperplasia, increased AT inflammation, and related alterations in metabolic and inflammatory serum parameters, such as increased HOMA IR and hs-CRP levels, were present in the group of children with overweight or obesity (21). Functional AT parameters, such as proliferation and differentiation of SVF cells, were not different between groups (Table 1).
Table 1.
Characteristics of the Leipzig AT Childhood Cohort (N = 133)
| Lean | Overweight and obese | P | |||||
|---|---|---|---|---|---|---|---|
| n | Mean ± SEM | Range | n | Mean ± SEM | Range | ||
| Anthropometric parameters | |||||||
| Male/female (% male) | 59 | 27/32 (45.8) | 74 | 35/39 (47.3) | 0.860 | ||
| Age, years | 59 | 10.4 ± 0.6 | 1.1 to 18.3 | 74 | 13.3 ± 0.3 | 4.8 to 18.4 | <0.001 |
| Pubertal stage, PH | 50 | 2 ± 0 | 1 to 6 | 64 | 3 ± 0 | 1 to 6 | 0.003 |
| BMI SDS | 59 | 0.07 ± 0.11 | −1.80 to 1.24 | 74 | 2.31 ± 0.07 | 1.32 to 4.34 | <0.001 |
| Height SDS | 51 | −0.04 ± 0.17 | −3.18 to 2.31 | 61 | 0.35 ± 0.14 | −3.16 to 2.37 | 0.076 |
| AT composition | |||||||
| Adipocyte diameter, µm | 32 | 114 ± 2 | 81 to 139 | 44 | 127 ± 2 | 98 to 175 | <0.001 |
| Total adipocyte number × 109a | 21 | 24.7 ± 3.2 | 9.1 to 69.8 | 31 | 52.0 ± 4.8 | 22.7 to 114.4 | <0.001 |
| Isolated adipocytes per g AT × 106a | 32 | 2.3 ± 0.1 | 1.2 to 4.4 | 45 | 1.9 ± 0.8 | 1.0 to 3.1 | 0.003 |
| Isolated SVF cells per g AT × 105a | 27 | 1.0 ± 0.1 | 0.0 to 2.4 | 31 | 1.2 ± 0.1 | 0.1 to 4.1 | 0.841 |
| AT inflammation | |||||||
| Macrophages per 100 adipocytesa | 49 | 9 ± 1 | 0 to 29 | 59 | 16 ± 2 | 0 to 115 | 0.013 |
| CLS per 100 adipocytesa | 49 | 0 ± 0 | 0 to 2 | 59 | 1 ± 0 | 0 to 8 | <0.001 |
| hs-CRP mg/La | 47 | 0.7 ± 0.1 | 0.3 to 3.2 | 65 | 2.1 ± 0.3 | 0.3 to 18.7 | <0.001 |
| TNF-α pg/mL | 44 | 1.96 ± 0.19 | 0.68 to 1.78 | 61 | 2.03 ± 0.14 | 0.66 to 5.77 | 0.698 |
| AT functional parameters | |||||||
| Doubling time of native cells from the SVF, ha | 22 | 142.8 ± 26.6 | 14.5 to 524.3 | 29 | 178.7 ± 34.1 | 17.8 to 735.9 | 0.617 |
| Growth rate of proliferating native SVF cellsa | 21 | 0.016 ± 0.002 | 0.003 to 0.037 | 24 | 0.013 ± 0.001 | 0.004 to 0.030 | 0.363 |
| Generation time of proliferating native SVF cells, ha | 21 | 72.3 ± 12.7 | 18.9 to 205.0 | 24 | 65.7 ± 8.1 | 23.3 to 180.7 | 0.894 |
| Differentiation of cells from the SVF, % | 21 | 28.6 ± 3.6 | 4.0 to 58.2 | 27 | 26.1 ± 3.3 | 0.2 to 73.2 | 0.608 |
| Basal lipolysis in adipocytes | 15 | 0.49 ± 0.05 | 0.20 to 0.83 | 14 | 0.36 ± 0.05 | 0.18 to 0.74 | 0.071 |
| Isoproterenol-stimulated lipolysis in adipocytesa | 15 | 2.07 ± 0.22 | 0.48 to 3.77 | 15 | 1.98 ± 0.34 | 0.37 to 5.08 | 0.569 |
| Adipokines and HOMA IR | |||||||
| Adiponectin, mg/La | 45 | 9.0 ± 1.0 | 1.6 to 43.8 | 64 | 5.7 ± 0.3 | 1.7 to 15.1 | <0.001 |
| Leptin, ng/mLa | 42 | 7.5 ± 1.2 | 0.4 to 28.2 | 65 | 29.9 ± 2.7 | 1.3 to 89.1 | <0.001 |
| HOMA IRa | 48 | 1.5 ± 0.2 | 0.0 to 5.6 | 63 | 3.6 ± 0.3 | 0.3 to 12.7 | <0.001 |
| MSCA1 and CD36 expression in AT | |||||||
| MSCA1 mRNA in SVF cellsa | 59 | 0.162 ± 0.015 | 0.031 to 0.648 | 74 | 0.176 ± 0.015 | 0.041 to 0.593 | 0.509 |
| MSCA1 mRNA in adipocytesa | 59 | 0.114 ± 0.013 | 0.002 to 0.375 | 74 | 0.139 ± 0.009 | 0.022 to 0.363 | 0.104 |
| CD36 mRNA in SVF cellsa | 55 | 0.165 ± 0.010 | 0.053 to 0.292 | 74 | 0.124 ± 0.006 | 0.053 to 0.299 | <0.001 |
| CD36 mRNA in adipocytesa | 59 | 0.233 ± 0.014 | 0.063 to 0.691 | 74 | 0.233 ± 0.012 | 0.058 to 0.600 | 0.975 |
Data are presented as mean with standard error of the mean and range. n indicates the number of subjects. Significant P-values (P < 0.05) are indicated in bold. Statistical significance for sex distribution was examined by χ² test. Statistical significance for differences between lean children and children with overweight and obesity was determined by the Student’s t test. Basal lipolysis is given as glycerol release in (ng/mL)/1000 adipocytes.
Abbreviations: AT, adipose tissue; BMI, body mass index; CD36, cluster of differentiation 36; HOMA IR, homeostatic model assessment-insulin resistance; hs-CRP, high-sensitivity C-reactive protein; mRNA, messenger RNA; MSCA1, mesenchymal stem cell antigen 1; SDS standard deviation score; SEM, standard error of the mean; SVF, stromal vascular fraction; TNF-α, tumor necrosis factor α;
a Statistical analyses were performed with log-transformed parameters.
MSCA1 and CD36 Messenger RNA Expression Is Age Dependent and Differs Between SVF cells and Adipocytes
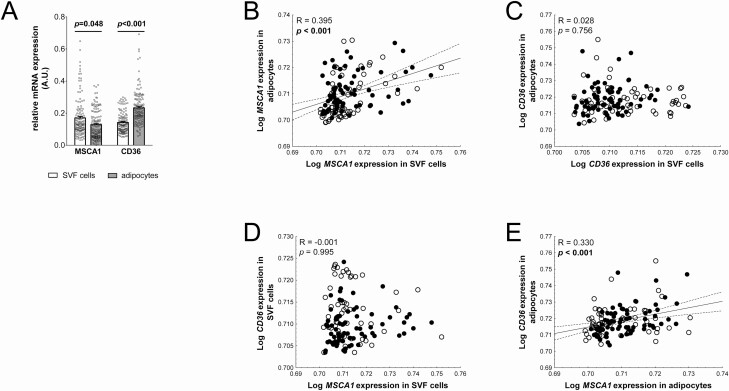
To address potential associations of MSCA1 and CD36 expression with obesity and related parameters in children, we first compared freshly isolated SVF cells and adipocytes. We detected significantly higher MSCA1 expression in SVF cells while CD36 expression was higher in adipocytes (Fig. 1A).
Figure 1.
CD36 (cluster of differentiation 36) and MSCA1 (mesenchymal stem cell antigen 1) are differentially expressed in SVF cells and adipocytes. (A) MSCA1 expression is significantly lower and CD36 expression is significantly higher in adipocytes compared to cells of the stroma vascular fraction (SVF; mean ± standard error of the mean; individual values represented as circles; n = 133). (B) MSCA1 expression in SVF cells is positively associated with adipocyte MSCA1 expression. (C) CD36 expression in SVF cells does not show a correlation with the amount of CD36 messenger RNA in adipocytes. (D) There is no association between MSCA1 and CD36 expression in SVF cells. (E) MSCA1 correlates with CD36 gene expression in adipocytes. Pearson correlation coefficient R and P-value are given in each scatter plot. Significant P-values (P < 0.05) are indicated in bold. Lean children are represented as open circles and children with overweight/obesity, as closed circles.
To assess whether the MSCA1 and CD36 expression in SVF cells might be derived from cell populations with potential for adipocyte differentiation, we correlated gene expression levels in SVF cells with those in mature adipocytes. We observed that higher MSCA1 expression in SVF cells was associated with a higher expression in adipocytes (Fig. 1B). In contrast, we did not find such a relationship for CD36 (Fig. 1C). Some of the adipocyte progenitor populations within the SVF exhibit both cell surface markers, MSCA1 and CD36 (10). Based on this, we further investigated whether in SVF samples the expression of MSCA1 is related to CD36, indicating the presence of such progenitor cell populations in AT of children. However, we did not detect a correlation between MSCA1 and CD36 expression in SVF cells (Fig. 1D), whereas in mature adipocytes, the expression levels of MSCA1 and CD36 correlated positively (Fig. 1E).
We next analyzed whether MSCA1 and CD36 expression in adipocyte progenitor cells is associated with obesity-related parameters already in children. To exclude a potential bias due to the influence of sex and developmental parameters, we first performed multiple regression analyses in the subcohort of lean children. MSCA1 expression significantly depended on the age of the children. Similarly, CD36 expression in SVF cells was related to age, whereas CD36 expression in adipocytes was not affected by any of the investigated variables (Table 2). When we compared expression levels between lean children and children with overweight and obesity, we did not observe a significant difference for MSCA1 neither in SVF cells nor in adipocytes. In contrast, we found significantly increased CD36 expression in SVF cells of lean children, whereas expression in adipocytes did not differ (Table 1). In quantitative analyses, MSCA1 showed a weak positive association with BMI SDS for expression in SVF cells (R = 0.201; P = 0.021) and adipocytes (R = 0.224; P = 0.009), which did, however, not withstand adjustment for age (SVF cells: Radjusted = 0.144, Padjusted = 0.138; adipocytes: Radjusted = 0.129, Padjusted = 0.184). In contrast, gene expression of CD36 in SVF cells negatively correlated with BMI SDS (R = −0.360; P < 0.001) independently from age of the children (Radjusted = −0.259; Padjusted = 0.008). There was no significant correlation for adipocyte CD36 expression and BMI SDS (R = 0.079; P = 0.366).
Table 2.
Multiple regression analyses for anthropometric parameters in lean children of the Leipzig AT Childhood Cohort
| Dependent variable | Independent variable | Step | Parameter | ΔR² | β ± SEM | P | n |
|---|---|---|---|---|---|---|---|
| MSCA1 mRNA in native SVF cells (R²=0.086; P = 0.114) | Age, sex, height SDS | 1 2 |
Age height SDS |
0.064 0.023 |
0.263 ± 0.138 −0.151 ± 0.138 |
0.063 0.281 |
51 |
| MSCA1 mRNA in adipocytes (R²=0.282; P < 0.001) | Age, sex, height SDS | 1 2 |
Age height SDS |
0.242 0.040 |
0.507 ± 0.123 −0.201 ± 0.123 |
<0.001
0.108 |
51 |
| CD36 mRNA in native SVF cells (R²=0.174; P = 0.004) | Age, sex, height SDS | 1 | Age | 0.174 | −0.417 ± 0.135 | 0.004 | 47 |
| CD36 mRNA in adipocytes | Age, sex, height SDS | ns | 51 |
Significant P-values (P < 0.05) are indicated in bold.
Abbreviations: CD36, cluster of differentiation 36; mRNA, messenger RNA; MSCA1, mesenchymal stem cell antigen 1; ns, not significant; SDS, SD score; SVF, stroma vascular fraction.
In summary, MSCA1 and CD36 are differentially expressed in SVF cells and adipocytes, and in particular, CD36 expression in SVF cells decreases with obesity in children.
MSCA1 but Not CD36 Expression Is Associated With Parameters of AT (Dys)function in Children
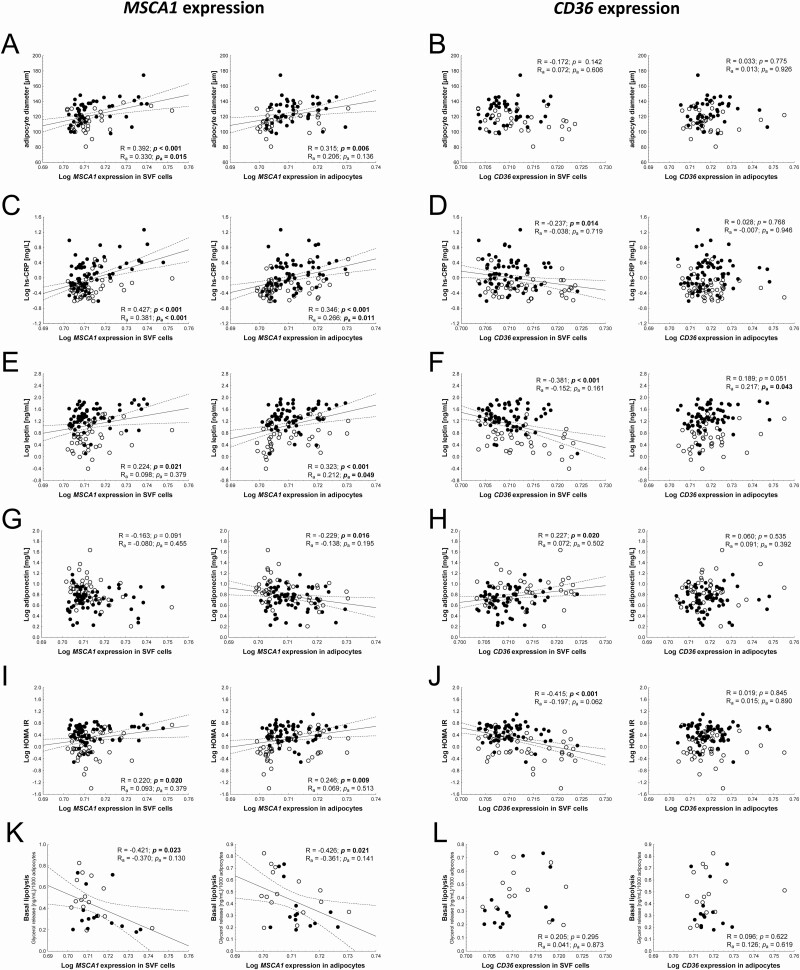
We next investigated the interrelation of the messenger RNA (mRNA) expression of both genes and obesity-related parameters including AT composition, AT inflammation, and AT function, serum adipokine levels, and HOMA IR as a marker for insulin resistance. For parameters related to AT composition, we observed a positive relationship of MSCA1 but not CD36 expression and adipocyte size (Fig. 2A and 2B) and a negative correlation with the number of adipocytes per gram AT (SVF MSCA1: R = −0.228, P = 0.046; adipocyte MSCA1: R = −0.226, P = 0.048). However, only the correlation between adipocyte size and MSCA1 expression in SVF cells remained significant after adjustment for the covariates age and BMI SDS (Table 3). Interestingly, only CD36 expression in SVF cells showed a significant and BMI-dependent association with total adipocyte number as a measure of hyperplasia with lower CD36 levels being associated with a higher total adipocyte number (R = −0.408, P = 0.003), which was dependent on BMI SDS and age of the children (Table 3). Although MSCA1 expression in SVF cells is related to adipocyte hypertrophy and this is associated with AT inflammation (21), we did not observe correlations of SVF MSCA1 expression with macrophage infiltration, crown-like structure (CLS) formation, and tumor necrosis factor α serum levels (Table 3) or TNFA expression in AT (data not shown), while there was a significant correlation with hs-CRP serum levels, which we detected similarly for adipocyte MSCA1 expression (Table 3, Fig. 2C). For CD36 SVF expression and hs-CRP serum levels, we observed a negative relationship (Fig. 2D), which was secondary to BMI-SDS and age of children (Table 3). Accordingly, MSCA1 expression is increased with adipocyte hypertrophy and AT inflammation in children, which could not be observed for CD36.
Figure 2.
MSCA1 (mesenchymal stem cell antigen 1) expression correlates with adipocyte size and parameters of adipose tissue (dys)function. (A) High MSCA1 expression in stroma vascular fraction (SVF) cells and adipocytes is associated with larger adipocytes in adipose tissue, while (B) CD36 (cluster of differentiation 36) expression is not correlated to adipocyte size. (C) The low-grade system inflammation marker high-sensitivity C-reactive protein (hs-CRP) is increased with increasing MSCA1 expression levels. (D) CD36 expression in SVF cells shows a negative association with hs-CRP. (E) MSCA1 expression in both SVF cells and adipocytes is positively associated with leptin serum levels whereas (F) CD36 expression in SVF cells shows a negative correlation. (G) MSCA1 expression in adipocytes shows a negative correlation with adiponectin serum levels whereas there is no correlation for expression in SVF cells. (H) In contrast, a positive association exists between CD36 expression in SVF cells and adiponectin serum levels but not with CD36 expression in adipocytes. (I) Similar to leptin, the homeostasis model assessment-insulin resistance shows a positive correlation with MSCA1 expression and (J) a negative association with CD36 expression in SVF cells. (K) MSCA1 expression is negatively associated with basal lipolytic activity of adipocytes (L) but shows no correlation with CD36 expression. Pearson correlation coefficient are given unadjusted (R and P-value) and adjusted (Ra and Pa-value) in each scatter plot. Significant P-values (P < 0.05) are indicated in bold. Lean children are represented as open circles and children with overweight/obesity, as closed circles.
Table 3.
Age- and BMI SD score–adjusted correlation analyses between MSCA1/CD36 and obesity-related adipose tissue and serum parameters
| MSCA1 mRNA in SVF cellsa | MSCA1 mRNA in adipocytesa | CD36 mRNA in SVF cellsa | CD36 mRNA in adipocytesa | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Radj | Pa | n | Radj | Padj | n | Radj | Padj | n | Radj | Padj | |
| AT composition | ||||||||||||
| Adipocyte diameter, µm | 76 | 0.330 | 0.004 | 76 | 0.206 | 0.079 | 74 | 0.072 | 0.549 | 76 | 0.013 | 0.913 |
| Total adipocyte number × 109a | 52 | −0.223 | 0.119 | 52 | −0.133 | 0.356 | 50 | −0.129 | 0.389 | 52 | 0.345 | 0.014 |
| Isolated adipocytes per g AT × 106a | 77 | −0.157 | 0.177 | 77 | −0.138 | 0.239 | 75 | 0.039 | 0.742 | 77 | 0.217 | 0.061 |
| Isolated SVF cells per g AT × 105a | 57 | 0.047 | 0.735 | 57 | 0.228 | 0.094 | 56 | −0.202 | 0.14 | 57 | 0.014 | 0.919 |
| AT inflammation | ||||||||||||
| Macrophages per 100 adipocytesa | 108 | 0.137 | 0.161 | 108 | 0.100 | 0.310 | 105 | −0.119 | 0.232 | 108 | −0.070 | 0.476 |
| CLSa | 108 | 0.032 | 0.745 | 108 | 0.174 | 0.075 | 105 | −0.005 | 0.959 | 108 | −0.081 | 0.407 |
| hs-CRP, mg/La | 112 | 0.381 | <0.001 | 112 | 0.266 | 0.005 | 108 | −0.038 | 0.698 | 112 | −0.007 | 0.941 |
| TNF-α, pg/mLa | 105 | 0.056 | 0.797 | 105 | −0.064 | 0.521 | 101 | 0.114 | 0.262 | 105 | 0.114 | 0.251 |
| Adipokines and HOMA IR | ||||||||||||
| Adiponectin, mg/La | 109 | −0.080 | 0.414 | 109 | −0.138 | 0.157 | 105 | 0.072 | 0.472 | 109 | 0.091 | 0.349 |
| Leptin, ng/mLa | 107 | 0.098 | 0.319 | 107 | 0.212 | 0.030 | 104 | −0.152 | 0.128 | 107 | 0.217 | 0.026 |
| HOMA IRa | 111 | 0.093 | 0.335 | 111 | 0.069 | 0.474 | 107 | −0.197 | 0.045 | 111 | 0.015 | 0.879 |
| AT functional parameters | ||||||||||||
| Doubling time of native cells from the SVF, ha | 51 | 0.133 | 0.362 | 51 | 0.035 | 0.811 | 50 | 0.207 | 0.159 | 51 | −0.037 | 0.802 |
| Growth rate of proliferating native SVF cellsa | 45 | −0.061 | 0.699 | 45 | −0.301 | 0.050 | 44 | −0.089 | 0.576 | 45 | 0.038 | 0.808 |
| Generation time of proliferating native SVF | 45 | 0.014 | 0.932 | 45 | 0.336 | 0.028 | 44 | 0.024 | 0.879 | 45 | −0.098 | 0.533 |
| Differentiation of native cells from the SVF, % | 48 | −0.079 | 0.601 | 48 | −0.255 | 0.088 | 47 | 0.177 | 0.244 | 48 | 0.025 | 0.870 |
| Basal lipolysis of adipocytes | 29 | −0.370 | 0.057 | 29 | −0.361 | 0.064 | 28 | 0.041 | 0.844 | 29 | 0.126 | 0.532 |
| Isoproterenol-stimulated lipolysis of adipocytes | 30 | −0.417 | 0.027 | 30 | −0.069 | 0.727 | 29 | −0.003 | 0.990 | 30 | 0.182 | 0.354 |
R indicates the Pearson correlation coefficient. Significant P-values are indicated in bold. Radj, and Padj analyses are adjusted for age and BMI SDS.
Abbreviations: AT, adipose tissue; BMI, body mass index; CLS, crown-like structure; HOMA IR, homeostatic model assessment-insulin resistance; hs-CRP, high-sensitivity C-reactive protein; SVF, stromal vascular fraction; TNF-α, tumor necrosis factor α; SDS, SD score.
a Statistical analysis was performed for log-transformed parameters.
Next, we assessed serum parameters indicative for AT dysfunction and early signs of metabolic disease. Mirroring the associations with BMI SDS, we found a positive correlation of SVF as well as adipocyte MSCA1 expression and leptin serum levels (Fig. 2E, Table 3). For SVF CD36 expression, we found a negative association with leptin (Fig. 2F). Furthermore, we observed a significant and BMI-independent positive correlation of CD36 expression in adipocytes and serum leptin levels (Table 3). Expectedly, adiponectin serum levels were significantly associated with MSCA1 (Fig. 2G) and CD36 (Fig. 2H) expression levels with correlations showing opposite directions compared to leptin. Similar results were obtained for the association between adiponectin (ADIPOQ) expression in AT with MSCA1 (SVF: R = −0.235, P = 0.035; adipocytes: R = −0.397, P < 0.001) and CD36 (SVF: R = 0.496, P < 0.001; adipocytes: R = 0.092, P = 0.414). In line with this, HOMA IR showed a positive correlation with MSCA1 mRNA levels in SVF cells and adipocytes as well as a negative correlation with CD36 mRNA in SVF cells but not in adipocytes (Fig. 2I and J). However, none of the associations with adiponectin and only the association of SVF CD36 expression and HOMA IR withstood adjusting to BMI SDS and age of children (Table 3). In summary, CD36 expression in SVF cells is not only associated with obesity in children but also with early signs of metabolic disease.
Finally, we investigated a potential relationship of MSCA1 and CD36 expression in AT and functional AT parameters (ie, proliferation and differentiation of native SVF cells in vitro as well basal and isoproterenol stimulated lipolytic activity of adipocytes). In this regard, only adipocyte MSCA1 expression was related to functional characteristics of native SVF cells in quantitative analyses. In particular, we detected a significant and age and BMI SDS independent association with growth rate and generation time in vitro (Table 3). Furthermore, we detected an inverse and BMI-dependent correlation of MSCA1 expression with basal lipolysis as well as a BMI-independent correlation of SVF MSCA1 expression with isoproterenol stimulated lipolysis (Fig. 2K, Table 3). There was no association between CD36 expression in AT and lipolytic function of isolated adipocytes (Fig. 2L, Table 3). Taken together, MSCA1 but not CD36 expression is related to parameters of AT function.
CD36 mRNA Expression Correlates With Adipocyte Progenitor Cell Differentiation In Vitro
To analyze a possible relationship between MSCA1 and CD36 gene expression and adipogenesis in vitro in more detail, we made use of 23 cryopreserved SVF samples derived from 7 lean and 16 children with overweight or obesity.
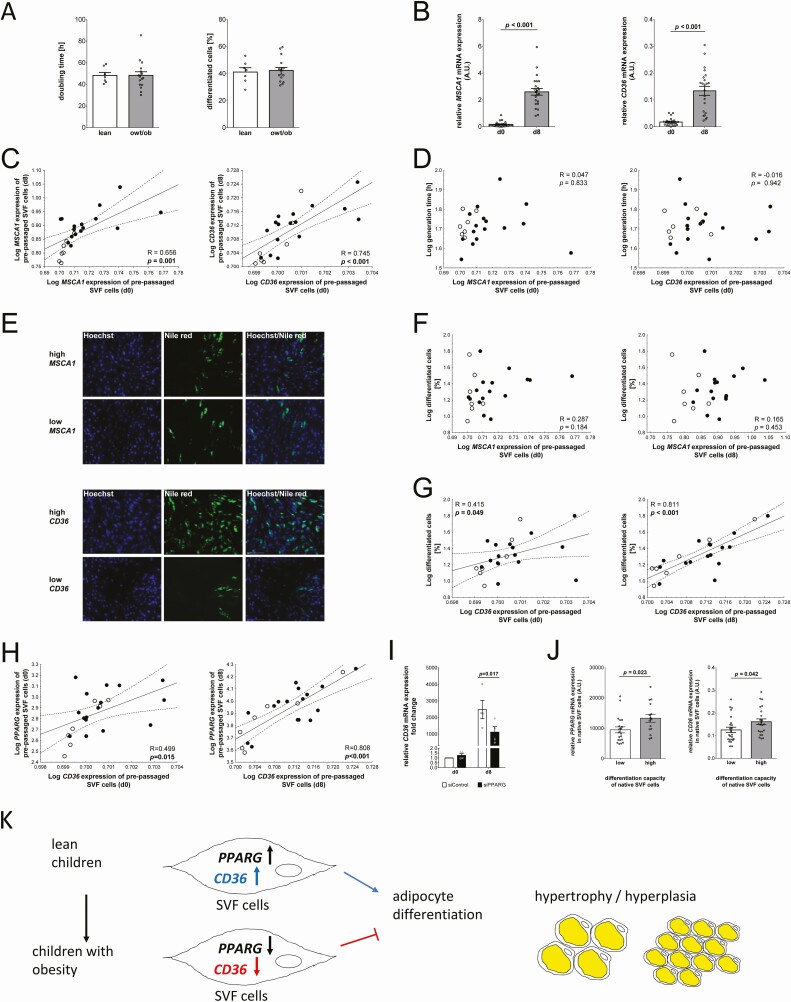
First, we investigated the in vitro proliferation and differentiation potential of the prepassaged SVF cells. Our data showed no difference in the proliferation rate or the ability of the cells to differentiate into mature adipocytes between lean children and children with overweight or obesity (Fig. 3A).
Figure 3.
CD36 (cluster of differentiation 36) gene expression is related to differentiation capacity of stroma vascular fraction (SVF) cells in vitro. (A) Body weight groups are not indicative for in vitro proliferation or differentiation capacity of prepassaged SVF cells. Quantitative data of n = 23 children samples are presented as mean ± standard error of the mean (SEM). (B) MSCA1 (mesenchymal stem cell antigen 1) and CD36 messenger RNA (mRNA) levels of prepassaged SVF cells increase during adipocyte differentiation (bar plots show quantitative data of n = 23 SVF samples; mean ± SEM). (C) SVF mRNA level of MSCA1 and CD36 before differentiation correlate with gene expression after differentiation. (D) There is no association between MSCA1/CD36 expression in prepassaged SVF cells and generation time of proliferating cells. (E) Cells were grouped for high and low MSCA1 or CD36 expression at baseline (d0) and adipocyte differentiation was analyzed. Representative images of differentiated SVF cells (d8) after Hoechst and Nile Red staining (scale bar 200 µm). MSCA1 gene expression is not related to (F) the percentage of differentiated cells while (G) high CD36 expression is associated with higher percentages of differentiated cells. (H) In accordance with this, there is a positive correlation between PPARG expression and CD36 expression, before and after differentiation into mature adipocytes. (I) Small interfering RNA knockdown of PPARG in Simpson Golabi Behmel Syndrome cells results in decreased CD36 expression. (J) High differentiation capacity of native SVF cells (grouped according to the median of the samples) is associated with higher PPARG and CD36 mRNA expression in native SVF cells (bar plots show quantitative data of n = 47 SVF samples; mean ± SEM). (K) Schematic overview of the proposed role of CD36 mRNA in adipose tissue of children. Pearson correlation coefficient R and P-value are given in each scatter plot. Significant P-values (P < 0.05) are indicated in bold. Lean children are represented as open circles and children with overweight/obesity (owt/ob), as closed circles. Abbreviation: PPARG, peroxisome proliferator-activated receptor gamma.
When we compared mRNA expression during the differentiation process, we observed an increase in MSCA1 and CD36 expression (Fig. 3B), and for both, mRNA expression before differentiation correlated with expression after differentiation (Fig. 3C). Next, we studied whether MSCA1 and CD36 expression in native SVF cell samples might be predictive for their in vitro proliferation and/or differentiation capacity after several passages of cell culture. There was no correlation between the native SVF expression of MSCA1 (R = 0.309, P = 0.152) or CD36 (R = 0.324, P = 0.141) and generation time as a parameter for cell proliferation. Similarly, differentiation potential of prepassaged SVF cells did not show an association with the native expression of MSCA1 (R = −0.244, P = 0.263) or CD36 (R = 0.218, P = 0.329). Considering the changing composition of cell types during cultivation of SVF cells in vitro, we further investigated the relationship between in vitro proliferation and differentiation potential of the prepassaged cells with their intrinsic MSCA1/CD36 gene expression levels. We did not detect a correlation of MSCA1 or CD36 expression levels in undifferentiated prepassaged SVF cells and their in vitro proliferation rate (Fig. 3D). Furthermore, we did not observe a correlation between MSCA1 gene expression and in vitro differentiation efficiency measured by the percentage of differentiated cells (Fig. 3E and 3F) or the Oil Red O absorbance on day 8 of adipocyte differentiation (R = −0.183, P = 0.402). In contrast, we observed that in vitro differentiation capacity of SVF cells was increased in cells with higher CD36 mRNA levels already before adipogenic induction as well as in differentiated cells (CD36 mRNA and Oil Red O absorbance on day 8: R = 0.605, P = 0.002) (Fig. 3E and 3G). Since PPARG is the master regulator of adipogenesis, and since there is evidence that CD36 expression is activated by peroxisome proliferator-activated receptor γ (25), we assessed a potential association of these genes. We observed that there is a significant positive correlation between the expression of CD36 and PPARG, in prepassaged SVF cells before and after adipocyte differentiation (Fig. 3H), which was even more pronounced in freshly isolated native SVF cells (R = 0.732, P < 0.001). For MSCA1, we did not observe any association with PPARG, neither in native SVF (R = −0.096, P = 0.390) cells nor in prepassaged SVF cells before (R = 0.352, P = 0.099) and after (R = 0.221, P = 0.311) differentiation, which is line with the observation that there is no association of MSCA1 with the in vitro adipogenic potential of SVF cells. To examine the relationship between CD36 and PPARG in more detail, we performed small interfering RNA-mediated knockdown experiments in SGBS cells and confirmed downregulation of CD36 expression after knockdown of PPARG (Fig. 3I). To confirm this finding, we went back into the analyses of native SVF cells of the Leipzig AT Childhood cohort and grouped the samples into high and low differentiation for comparison of PPARG and CD36 expression. Indeed, we observed that both PPARG and CD36 expression was elevated in native SVF cells with high differentiation capacity (Fig. 3J), hence indicating that CD36 expression and associated regulation of adipocyte differentiation might be linked to processes of adipocyte hypertrophy/hyperplasia in AT with obesity development in children (Fig. 3K).
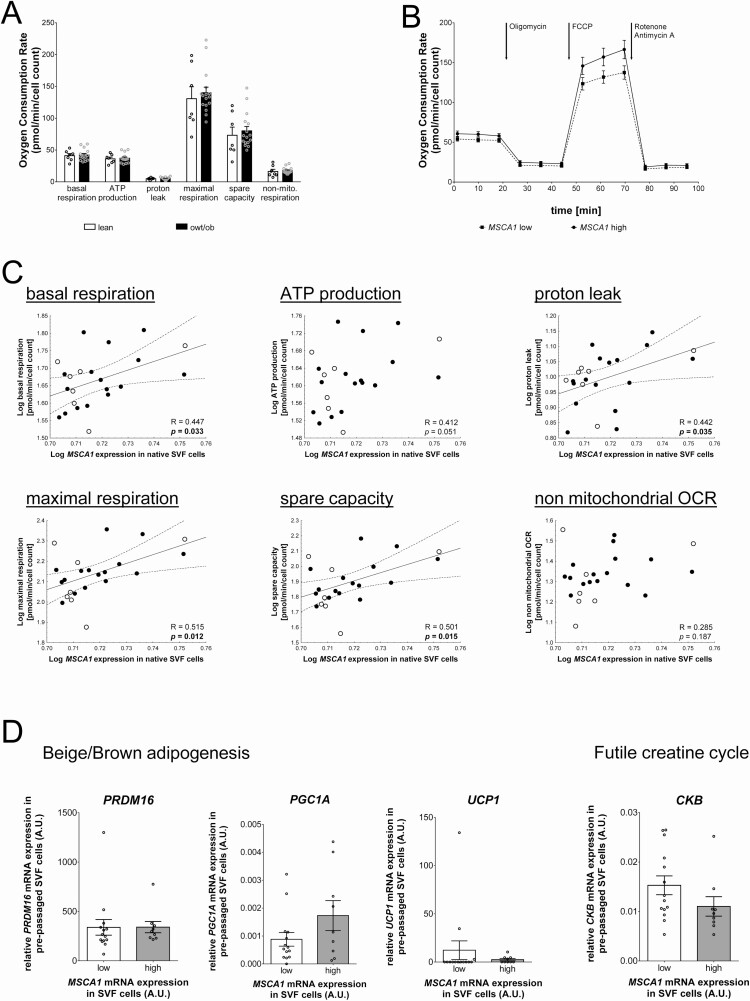
MSCA1 mRNA Levels in Native SVF Cells Are Correlated With Parameters of Mitochondrial Respiration
Particularly, MSCA1-positive progenitor cells are characterized by a higher mitochondrial content and show an increased potential for adipogenesis in beige adipocytes (9). Therefore, we additionally measured functional parameters of the mitochondrial respiratory chain in undifferentiated prepassaged SVF cells using Seahorse technology. We did not observe any differences in the metabolic activity of the mitochondria between lean children and children with overweight and obesity (Fig. 4A). We then investigated whether mitochondrial function is associated with CD36 and MSCA1 gene expression in SVF cells and stratified samples into low-and high-expressing examples according to mean expression in native or prepassaged SVF cells, respectively. We did not detect an association between parameters of the mitochondrial respiratory chain and CD36 expression, neither in native nor in prepassaged SVF cells (Table 4). MSCA1 expression in prepassaged SVF cells did also not show a correlation with mitochondrial activity. In contrast, when samples were stratified for MSCA1 expression in native SVF cells, we observed that the mean OCR of cells with high MSCA1 expression was elevated compared to cells with low expression (Fig. 4B, Table 4). In addition, high MSCA1 expression was associated with higher OCR values for maximum respiration and the corresponding spare capacity as well as increased nonmitochondrial OCR (Table 4). In line with this, in quantitative analyses, we detected a significant positive correlation between MSCA1 expression levels in native SVF cells and several parameters of mitochondrial function, such as basal respiration, proton leak, maximal respiration, and spare capacity (Fig. 4C). To look at this relationship in more detail, we performed gene expression analysis of marker genes characterizing beige/brown adipogenesis (PRDM16, PGC1A, UCP1) as well as CKB as a regulator of the futile creatine cycle, which has been recently implicated in MSCA1-mediated regulation of thermogenic activity in AT of mice (18), in the prepassaged SVF cells in which mitochondrial activity was measured. We did not detect a clear association of MSCA1 expression with expression of the analyzed regulators of beige and brown adipocyte function or the futile creatine cycle. However, there was a nominal significant tendency toward a correlation of high MSCA1 expression in native SVF cells with increased PGC1A expression (P = 0.059) and with decreased CKB expression (P = 0.075). In summary, our findings indicate a potential role of MSCA1 in beige adipogenesis in children.
Figure 4.
MSCA1 (mesenchymal stem cell antigen 1) expression in native SVF cells is associated with cellular respiration in vitro. (A) The OCR of the investigated specific parameters of the mitochondrial respiratory chain did not differ between pre-passaged SVF cells of lean children and children with overweight/obesity (owt/ob). Bar plots show quantitative data of n = 23 children (mean ± SEM). (B) High MSCA1 expression in native SVF cells is associated with a higher OCR in pre-passaged cells. Graph depicts the averaged seahorse measurement (mean ± SEM) of n = 9 SVF cell samples with high compared to n = 14 samples with low MSCA1 expression. (C) MSCA1 expression in native SVF cells correlates with specific parameters of the mitochondrial respiratory chain, ie, basal respiration, proton leak, maximal respiration and spare capacity. (D) The gene expression of marker genes for beige/brown adipogenesis (PRDM16, PGC1A, UCP1) and the regulator of the futile creatine cycle CKB showed no significant correlation with MSCA1 expression in native SVF cells. Pearson correlation coefficient R and p value are given in each scatter plot. Significant p values (P < 0.05) are indicated in bold. SVF samples from lean children are represented as open circles and children with overweight/obesity as closed circles. Abbreviations: ATP, adenosine triphosphate; CKB, creatine kinase B; FCCP, carbonyl cyanide-4 (trifluoromethoxy) phenylhydrazone; OCR, oxygen consumption rate; PGC1A, Peroxisome proliferator-activated receptor gamma coactivator 1-alpha; PRDM16, PR domain containing 16; UCP1, uncoupling protein 1.
Table 4.
Correlation analyses between functional parameters of mitochondrial respiration and CD36 or MSCA1 gene expression in SVF cells
| MSCA1 expression | ||||||
|---|---|---|---|---|---|---|
| Native SVF cells | Prepassaged SVF cells | |||||
| Functional parameters of mitochondrial respiration chain | Low (≤0.228) n = 14 |
High (>0.228) n = 9 |
P | Low (≤0.180) n = 15 |
High (>0.180) n = 8 |
P |
| Basal respiration, pmol/min/cell count | 39.3 ± 2.1 | 48.6 ± 3.6 | 0.060 | 42.0 ± 2.3 | 41.9 ± 2.9 | 0.978 |
| ATP production, pmol/min/cell count | 34.7 ± 1.8 | 40.3 ± 2.1 | 0.057 | 37.1 ± 1.9 | 36.5 ± 2.4 | 0.835 |
| Proton leak, pmol/min/cell count | 4.7 ± 0.4 | 5.8 ± 0.8 | 0.187 | 4.9 ± 0.5 | 5.5 ± 0.7 | 0.530 |
| Maximal respiration, pmol/min/cell count | 120.5 ± 7.9 | 163.4 ± 12.8 | 0.006 | 140.0 ± 12.0 | 132.2 ± 7.0 | 0.657 |
| Spare capacity, pmol/min/cell count | 65.9 ± 5.5 | 96.9 ± 10.5 | 0.009 | 80.6 ± 8.8 | 73.4 ± 6.5 | 0.585 |
| Non-mitochondrial OCR, pmol/min/cell count | 15.3 ± 1.5 | 20.4 ± 1.9 | 0.044 | 17.4 ± 1.7 | 16.9 ± 1.8 | 0.856 |
| CD36 expression | ||||||
| Native SVF cells | Prepassaged SVF cells | |||||
| Functional parameters of mitochondrial respiration chain | Low(≤0.120) n = 14 |
High (>0.120) n = 9 |
P | Low (≤0.018) n = 13 |
High (>0.018) n = 10 |
P |
| Basal respiration, pmol/min/cell count | 43.8 ± 2.7 | 39.2 ± 1.2 | 0.219 | 42.2 ± 2.6 | 41.7 ± 2.4 | 0.891 |
| ATP production, pmol/min/cell count | 38.3 ± 2.3 | 34.7 ± 1.1 | 0.230 | 37.2 ± 2.2 | 36.6 ± 1.9 | 0.844 |
| Proton leak, pmol/min/cell count | 5.4 ± 0.6 | 4.6 ± 0.5 | 0.301 | 5.1 ± 0.5 | 5.1 ± 0.7 | 0.915 |
| Maximal respiration, pmol/min/cell count | 146.1 ± 12.2 | 123.7 ± 6.8 | 0.183 | 143.9 ± 11.8 | 128.7 ± 10.5 | 0.366 |
| Spare capacity, pmol/min/cell count | 84.6 ± 9.1 | 67.9 ± 5.6 | 0.189 | 84.3 ± 8.9 | 69.9 ± 7.6 | 0.251 |
| Nonmitochondrial OCR, pmol/min/cell count | 17.7 ± 1.5 | 16.5 ± 2.3 | 0.645 | 17.4 ± 1.6 | 17.1 ± 2.2 | 0.927 |
Significant P-values (P < 0.05) are indicated in bold. Gene expression of n = 23 samples is grouped into high (>mean expression) and low (≤mean expression).
Abbreviations: ATP, adenosine triphosphate; CD36, cluster of differentiation 36; MSCA1, mesenchymal stem cell antigen 1; OCR, oxygen consumption rate; SVF, stroma vascular fraction.
Discussion
Here we investigated the relevance of the adipocyte progenitor marker genes MSCA1 and CD36 for AT biology during physiological and obesity-related AT accumulation in children and assessed a potential link to functional properties of SVF cells in vitro.
We show that AT mRNA levels of MSCA1 but not CD36 increase with age in lean children. MSCA1 has been shown to be involved in processes related to growth in several tissues, including the bone (26), the brain (27), and the AT (12), where it promotes cellular growth and differentiation. One might hence speculate that MSCA1 plays a role in AT accumulation during childhood potentially by regulating AT function. Supporting this assumption, we observed a positive association of MSCA1 expression and parameters of AT composition, in particular adipocyte size. Interestingly, a similar association between MSCA1 and hypertrophy has been described for bone marrow-derived mesenchymal stromal cells (MSCs) before showing that MSCA1 levels were increased in larger cells and that the MSCA1-positive subpopulation exhibited higher levels of hypertrophic markers (28). In previous studies, we have shown that adipocyte hypertrophy is associated with an increase in macrophage infiltration and the presence of CLS in AT already during childhood (21). Although we could not find a direct relationship between MSCA1 and the number of macrophages within the AT, we observed positive associations with other inflammatory parameters related to hypertrophy in AT, such as hs-CRP serum levels, a marker for systemic low-grade inflammation, and the number of CLS in AT, especially for MSCA1 mRNA in adipocytes. This is in line with results from previous studies that indicate a connection between MSCA1 and inflammatory processes in AT (eg, inflammatory cytokines affecting the expression and activity of MSCA1) (9).
We observed that MSCA1 expression increases during adipogenic differentiation of unsorted prepassaged SVF cells in vitro, and Estève et al described a similar effect for CD45-/CD34+/CD31− selected adipocyte progenitor cells (9). They further showed a positive association between MSCA1 and adipogenic potential of adipocyte progenitor cells (9,29), which we could not confirm in our experimental setup. Unfortunately, we were limited by the often small amount of AT we obtained from children during elective surgeries, which results in the fact that we often do not have sufficient native cells available for cell selection by fluorescence-activated cell sorting. Hence, our results are not entirely comparable to those of Estève et al. In this regard, a previous study has shown that lipid production during adipogenesis in unsorted cells was more similar to that of MSCA1 negative cells, while MSCA1 positive cells showed a higher adipogenic potential (29).
Previous studies in human adults indicated that MSCA1 supports beige adipogenesis (9). Studies in mice indicated that obesity-related diseases, such as diabetes, are associated with a reduced mitochondrial activity in AT (30). In contrast, we did not detect any differences in mitochondrial activity between SVF samples derived from lean children and children with overweight and obesity, which might be due to the fact that children with obesity represent early stages of disease progression and mitochondrial dysfunctions may not have developed yet. However, we could show that a high level of MSCA1 in native SVF cells is associated with higher oxygen consumption rates in mitochondrial respiration. Surprisingly, we could not detect this for intrinsic MSCA1 expression of prepassaged SVF cells. Reason for this might be that gene expression in prepassaged cells may differ from that in native SVF cells due to the altered composition of cell types and associated cell-cell interactions. Furthermore, several studies showed that during the cultivation of SVF cells there is a loss of surface receptor expression, which might reflect differences in gene expression (7,9). Nevertheless, we see that MSCA1 expression tends to be related to PGC1A expression. Since PGC1A is a marker gene for beige/brown adipocytes with higher thermogenic potential and higher mitochondrial content than white adipocytes, this correlation could explain the association between MSCA1 and mitochondrial activity of SVF cells. However, without further experimental investigations, such suggestions remain speculative. Taken together, we provide evidence that MSCA1 expression is related to mitochondrial function of SVF cells in vitro. Future studies investigating a potential link to obesity-related adipocyte hypertrophy and AT dysfunction in children would be of interest, as this may be related to the development of obesity-associated disease.
Gao et al discovered that MSCA1 positive adipocyte progenitor cells separate into further subpopulations characterized by presence or absence of the fatty acid translocase and membrane glycoprotein CD36 (10). Several studies indicated that CD36 plays a role in the regulation of lipid storage and lipolysis (10, 31) and in inflammatory processes in AT (32-34) and that its expression is upregulated in AT of adults with obesity (35, 36). Surprisingly, we detected a downregulation of CD36 expression in native SVF cells with increasing BMI SDS and no correlation in adipocytes. Furthermore, there were no prominent BMI-independent associations of CD36 expression with adipocyte hypertrophy or inflammatory processes in AT of children. In contrast to most previous studies in humans, we measured gene expression in the separated fractions of SVF cells and adipocytes instead of intact AT, which allows a more differentiated insight into processes occurring in AT with development of obesity.
Apart from this, analyses in preadipocytes of mice have shown that CD36 expression increases during adipocyte development once the differentiating cells are able to uptake and store fatty acids (19,37). We observed a similar CD36 regulation during adipocyte differentiation of prepassaged SVF cells of children. Previous studies provided evidence that CD36 promotes adipocyte differentiation in vitro (38) and that the MSCA1+/CD36+ adipocyte progenitor population of the SVF shows an increased capability for white adipogenesis (10). Interestingly, in mature adipocytes of children the expression levels of MSCA1 and CD36 correlated positively. One might speculate that this association reflects the presence of differentiated adipocytes derived from MSCA1+/CD36+ adipose progenitor cells. Furthermore, we show here that the link between CD36 expression levels and the adipogenic potential of SVF cells is already present in children with high CD36 expression being associated with increased adipocyte differentiation. In addition, we could show that CD36 expression in SVF cells of children is closely linked to expression of PPARG, which is the master regulator of adipogenesis, and that SVF cells with high adipogenic potential are characterized by in increased expression of both PPARG and CD36. Based on our data showing an inverse relationship between CD36 expression in SVF cells and obesity, inflammation, and insulin sensitivity, one might speculate that, similar to PPARG, CD36 expression is protective in terms of metabolic health. Furthermore, the fact that SVF CD36 expression is inversely correlated to total adipocyte number might indicate a potential role of CD36 in the regulation of hypertrophic vs hyperplasic processes in AT with early obesity development.
One strength of our study is the analyses of AT samples of children, while most previous studies focusing on obesity-related AT expansion have been performed in adults. Due to the fact that obesity manifests in early childhood and is already associated with changes in AT (21), studies in children might allow better insight into the early processes related to obesity development. Moreover, children present early stages of the disease and the influence of drug treatments or diets on AT biology is reduced compared to adults. However, in our experimental analyses of functional AT parameters, we are limited by the amount of AT, which is dependent on the site and nature of surgery and on the age of children. Because of this subsamples are available for different measures of AT function, which did not allow more mechanistic analyses. Nevertheless, to the best of knowledge the Leipzig AT Childhood Cohort is unique in both sample size and the functional comprehensive characterization of AT biology in children.
In conclusion, we provide evidence that both MSCA1 and CD36 are associated with obesity-related alterations in AT of children as well as functional properties of SVF cells in vitro. While MSCA1 is more related to metabolic and mitochondrial function of AT, CD36 expression predicts adipogenic differentiation potential of SVF cells, indicating that it might play a role in the regulation of adipocyte hyperplasia and hypertrophy with obesity development in children.
Acknowledgments
We thank Antje Berthold for technical assistance.
Financial Support : This work was supported by grants from the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation)–Projektnummer 209933838—SFB 1052 project C05 to A.K. and the Federal Ministry of Education and Research (BMBF), Germany, FKZ: 01EO1501 (IFB Adiposity Diseases), and the ESPE Early Career Scientific Development Grant to M.H.
Additional Information
Disclosure Summary : The authors have nothing to disclose.
Data Availability
The datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References
- 1. Körner A, Kratzsch J, Gausche R, Schaab M, Erbs S, Kiess W. New predictors of the metabolic syndrome in children–role of adipocytokines. Pediatr Res. 2007;61(6):640-645. [DOI] [PubMed] [Google Scholar]
- 2. Geserick M, Vogel M, Gausche R, et al. . Acceleration of BMI in early childhood and risk of sustained obesity. N Engl J Med. 2018;379(14):1303-1312. [DOI] [PubMed] [Google Scholar]
- 3. Ghaben AL, Scherer PE. Adipogenesis and metabolic health. Nat Rev Mol Cell Biol. 2019;20(4):242-258. [DOI] [PubMed] [Google Scholar]
- 4. Knittle JL, Timmers K, Ginsberg-Fellner F, Brown RE, Katz DP. The growth of adipose tissue in children and adolescents. Cross-sectional and longitudinal studies of adipose cell number and size. J Clin Invest. 1979;63(2):239-246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ng CW, Poznanski WJ, Borowiecki M, Reimer G. Differences in growth in vitro of adipose cells from normal and obese patients. Nature. 1971;231(5303):445. [DOI] [PubMed] [Google Scholar]
- 6. Berry R, Jeffery E, Rodeheffer MS. Weighing in on adipocyte precursors. Cell Metab. 2014;19(1):8-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Maumus M, Peyrafitte JA, D’Angelo R, et al. . Native human adipose stromal cells: localization, morphology and phenotype. Int J Obes. 2011;35(9):1141-1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Li H, Zimmerlin L, Marra KG, Donnenberg VS, Donnenberg AD, Rubin JP. Adipogenic potential of adipose stem cell subpopulations. Plast Reconstr Surg. 2011;128(3):663-672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Estève D, Boulet N, Volat F, et al. . Human white and brite adipogenesis is supported by MSCA1 and is impaired by immune cells. Stem Cells. 2015;33(4):1277-1291. [DOI] [PubMed] [Google Scholar]
- 10. Gao H, Volat F, Sandhow L, et al. . CD36 is a marker of human adipocyte progenitors with pronounced adipogenic and triglyceride accumulation potential. Stem Cells. 2017;35(7):1799-1814. [DOI] [PubMed] [Google Scholar]
- 11. Sobiesiak M, Sivasubramaniyan K, Hermann C, et al. . The mesenchymal stem cell antigen MSCA-1 is identical to tissue non-specific alkaline phosphatase. Stem Cells Dev. 2010;19(5):669-677. [DOI] [PubMed] [Google Scholar]
- 12. Estève D, Galitzky J, Bouloumié A, Fonta C, Buchet R, Magne D. Multiple functions of MSCA-1/TNAP in adult mesenchymal progenitor/stromal cells. Stem Cells Int. 2016;2016:1815982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Battula VL, Treml S, Bareiss PM, et al. . Isolation of functionally distinct mesenchymal stem cell subsets using antibodies against CD56, CD271, and mesenchymal stem cell antigen-1. Haematologica. 2009;94(2):173-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kermer V, Ritter M, Albuquerque B, Leib C, Stanke M, Zimmermann H. Knockdown of tissue nonspecific alkaline phosphatase impairs neural stem cell proliferation and differentiation. Neurosci Lett. 2010;485(3):208-211. [DOI] [PubMed] [Google Scholar]
- 15. Rader BA. Alkaline phosphatase, an unconventional immune protein. Front Immunol. 2017;8:897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ding J, Ghali O, Lencel P, et al. . TNF-alpha and IL-1beta inhibit RUNX2 and collagen expression but increase alkaline phosphatase activity and mineralization in human mesenchymal stem cells. Life Sci. 2009;84(15-16):499-504. [DOI] [PubMed] [Google Scholar]
- 17. Hernández-Mosqueira C, Velez-delValle C, Kuri-Harcuch W. Tissue alkaline phosphatase is involved in lipid metabolism and gene expression and secretion of adipokines in adipocytes. Biochim Biophys Acta. 2015;1850(12):2485-2496. [DOI] [PubMed] [Google Scholar]
- 18. Sun Y, Rahbani JF, Jedrychowski MP, et al. . Mitochondrial TNAP controls thermogenesis by hydrolysis of phosphocreatine. Nature. 2021;593(7860):580-585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Febbraio M, Hajjar DP, Silverstein RL. CD36: a class B scavenger receptor involved in angiogenesis, atherosclerosis, inflammation, and lipid metabolism. J Clin Invest. 2001;108(6):785-791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Vroegrijk IO, van Klinken JB, van Diepen JA, et al. . CD36 is important for adipocyte recruitment and affects lipolysis. Obesity. 2013;21(10):2037-2045. [DOI] [PubMed] [Google Scholar]
- 21. Landgraf K, Rockstroh D, Wagner IV, et al. . Evidence of early alterations in adipose tissue biology and function and its association with obesity-related inflammation and insulin resistance in children. Diabetes. 2015;64(4):1249-1261. [DOI] [PubMed] [Google Scholar]
- 22. Kromeyer-Hauschild K, Wabitsch M, Kunze D, et al. . Perzentilen für den body mass index für das Kindes- und Jugendalter unter Heranziehung verschiedener deutscher Stichproben. [Centiles for body mass index for children and adolescents derived from distinct independent German cohorts]. Monatsschr Kinderheilkd. 2001;149:807-818. [Google Scholar]
- 23. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412-419. [DOI] [PubMed] [Google Scholar]
- 24. Landgraf K, Klöting N, Gericke M, et al. . The obesity-susceptibility gene TMEM18 promotes adipogenesis through activation of PPARG. Cell Rep. 2020;33(3):108295. [DOI] [PubMed] [Google Scholar]
- 25. Yang X, Zhang W, Chen Y, et al. . Activation of peroxisome proliferator-activated receptor γ (PPARγ) and CD36 protein expression: the dual pathophysiological roles of progesterone. J Biol Chem. 2016;291(29):15108-15118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Vimalraj S. Alkaline phosphatase: structure, expression and its function in bone mineralization. Gene. 2020;754:144855. [DOI] [PubMed] [Google Scholar]
- 27. Diaz-Hernandez M, Hernandez F, Miras-Portugal MT, Avila J. TNAP plays a key role in neural differentiation as well as in neurodegenerative disorders. Subcell Biochem. 2015;76:375-385. [DOI] [PubMed] [Google Scholar]
- 28. Kim YH, Yoon DS, Kim HO, Lee JW. Characterization of different subpopulations from bone marrow-derived mesenchymal stromal cells by alkaline phosphatase expression. Stem Cells Dev. 2012;21(16):2958-2968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Marble HD, Sutermaster BA, Kanthilal M, Fonseca VC, Darling EM. Gene expression-based enrichment of live cells from adipose tissue produces subpopulations with improved osteogenic potential. Stem Cell Res Ther. 2014;5(5):145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Medina-Gómez G. Mitochondria and endocrine function of adipose tissue. Best Pract Res Clin Endocrinol Metab. 2012;26(6):791-804. [DOI] [PubMed] [Google Scholar]
- 31. Zhou D, Samovski D, Okunade AL, Stahl PD, Abumrad NA, Su X. CD36 level and trafficking are determinants of lipolysis in adipocytes. FASEB J. 2012;26(11):4733-4742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Barrett L, Dai C, Gamberg J, Gallant M, Grant M. Circulating CD14-CD36+ peripheral blood mononuclear cells constitutively produce interleukin-10. J Leukoc Biol. 2007;82(1):152-160. [DOI] [PubMed] [Google Scholar]
- 33. Seimon TA, Nadolski MJ, Liao X, et al. . Atherogenic lipids and lipoproteins trigger CD36-TLR2-dependent apoptosis in macrophages undergoing endoplasmic reticulum stress. Cell Metab. 2010;12(5):467-482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sheedy FJ, Grebe A, Rayner KJ, et al. . CD36 coordinates NLRP3 inflammasome activation by facilitating intracellular nucleation of soluble ligands into particulate ligands in sterile inflammation. Nat Immunol. 2013;14(8):812-820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bonen A, Tandon NN, Glatz JF, Luiken JJ, Heigenhauser GJ. The fatty acid transporter FAT/CD36 is upregulated in subcutaneous and visceral adipose tissues in human obesity and type 2 diabetes. Int J Obes. 2006;30(6):877-883. [DOI] [PubMed] [Google Scholar]
- 36. Cyr Y, Bissonnette S, Lamantia V, et al. . White adipose tissue surface expression of LDLR and CD36 is associated with risk factors for type 2 diabetes in adults with obesity. Obesity. 2020;28(12):2357-2367. [DOI] [PubMed] [Google Scholar]
- 37. Sfeir Z, Ibrahimi A, Amri E, Grimaldi P, Abumrad N. Regulation of FAT/CD36 gene expression: further evidence in support of a role of the protein in fatty acid binding/transport. Prostaglandins Leukot Essent Fatty Acids. 1997;57(1):17-21. [DOI] [PubMed] [Google Scholar]
- 38. Christiaens V, Van Hul M, Lijnen HR, Scroyen I. CD36 promotes adipocyte differentiation and adipogenesis. Biochim Biophys Acta. 2012;1820(7):949-956. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.