Abstract
Context
Vascular aging, including endothelial dysfunction secondary to oxidative stress and inflammation, increases the risk for age-associated cardiovascular disease (CVD). Low testosterone in middle-aged/older men is associated with increased CVD risk.
Objective
We hypothesized that low testosterone contributes to age-associated endothelial dysfunction, related in part to greater oxidative stress and inflammation.
Methods
This cross-sectional study included 58 healthy, nonsmoking men categorized as young (N = 20; age 29 ± 4 years; testosterone 500 ± 58 ng/dL), middle-aged/older with higher testosterone (N = 20; age 60 ± 6 years; testosterone 512 ± 115 ng/dL), and middle-aged/older lower testosterone (N = 18; age 59 ± 8 years; testosterone 269 ± 48 ng/dL). Brachial artery flow-mediated dilation (FMDBA) was measured during acute infusion of saline (control) and vitamin C (antioxidant). Markers of oxidative stress (total antioxidant status and oxidized low-density lipoprotein cholesterol), inflammation (interleukin [IL]-6 and C-reactive protein [CRP]), and androgen deficiency symptoms were also examined.
Results
During saline, FMDBA was reduced in middle-aged/older compared with young, regardless of testosterone status (P < 0.001). FMDBA was reduced in middle-aged/older lower testosterone (3.7% ± 2.0%) compared with middle-aged/older higher testosterone (5.7% ± 2.2%; P = 0.021), independent of symptoms. Vitamin C increased FMDBA (to 5.3% ± 1.6%; P = 0.022) in middle-aged/older lower testosterone but had no effect in young (P = 0.992) or middle-aged/older higher testosterone (P = 0.250). FMDBA correlated with serum testosterone (r = 0.45; P < 0.001), IL-6 (r = −0.41; P = 0.002), and CRP (r = −0.28; P = 0.041).
Conclusion
Healthy middle-aged/older men with low testosterone appear to have greater age-associated endothelial dysfunction, related in part to greater oxidative stress and inflammation. These data suggest that low testosterone concentrations may contribute to accelerated vascular aging in men.
Keywords: aging, testosterone, endothelial function, andropause, oxidative stress, inflammation
Serum testosterone declines gradually with age at a rate of ~1% per year after the third decade (1). Data from the Baltimore Longitudinal Study of Aging indicate that 30% of 70-year-old and 50% of 80-year-old men have serum testosterone levels below the normal range for young men (2). However, the clinical significance of age-related declines in testosterone in the absence of symptoms and signs suggestive of androgen deficiency (eg, reduced libido, erectile dysfunction, depressed mood, poor concentration and memory, and/or increased fatigue) is debated. Consistent reports from observational studies suggest an association between low serum testosterone and increased cardiovascular disease (CVD) risk and mortality (3-7). Therefore, a better understanding of the mechanisms by which low serum testosterone, in the absence or presence of symptoms, may lead to increased cardiovascular vulnerability as men age is necessary to inform novel therapeutic strategies in aging men going through andropause (ie, male menopause) to prevent the development of age-associated CVD.
Vascular aging, featuring endothelial dysfunction mediated by oxidative stress and inflammation, is a major risk factor for the development of age-associated CVD (8). Testosterone has reported antioxidant (9, 10) and anti-inflammatory effects (11, 12) and low serum testosterone is associated with endothelial dysfunction (13-16). As such, accelerated vascular aging due to the loss of antioxidant and anti-inflammatory properties of testosterone could explain the greater incidence of CVD and mortality in middle-aged/older men with low testosterone. Whether healthy middle-aged/older men with low serum testosterone have greater age-associated endothelial dysfunction, and whether oxidative stress and inflammation are mechanistically involved has yet to be studied. Additionally, in women, menopausal symptoms are associated with vascular dysfunction across the stages of the menopause transition (17). However, less is known about the physiologic effects of low testosterone, with or without signs and symptoms of androgen deficiency, on cardiovascular health.
Accordingly, the present study tested the hypothesis that middle-aged/older men with low serum testosterone would have greater age-associated endothelial dysfunction, related in part to greater oxidative stress and inflammation. To test this hypothesis the present study assessed macro- (eg, brachial artery flow-mediated dilation [FMDBA]) and micro- (reactive hyperemia index [RHI]) vascular endothelial function during acute systemic saline (control) and vitamin C, and markers of oxidative stress and inflammation in middle-aged/older men with low testosterone compared with younger and middle-aged/older men with higher levels of testosterone. Additionally, we determined whether the presence of symptoms of androgen deficiency modulated the effects of testosterone status.
Methods
Study Design
This was a cross-sectional study that was part of an ongoing registered clinical trial (ClincialTrials.gov identifier NCT02758431). The purpose of this study was to examine the effects of low testosterone on cardiovascular aging in men and, therefore, only men were included. The Colorado Multiple Institution Review Board approved all study protocols and procedures, and they conform to the provisions of the Declaration of Helsinki. All participants provided written and verbal consent prior to participating. All study visits and measurements were performed at the University of Colorado Denver Colorado Clinical and Translational Sciences Institute (CCTSI) Clinical and Translational Research Center (CTRC).
Study Participants
Fifty-eight healthy men of all races/ethnic backgrounds aged 50-75 years (middle-aged/older) and 18-40 years (young) were recruited from the Denver Metropolitan Area. Middle-aged/older men were categorized into 2 groups: higher testosterone (serum testosterone ≥ 13.9 nmol/L [400 ng/dL]) or low testosterone (serum testosterone < 10.4 nmol/L [300 ng/dL]) at screening. All serum testosterone levels were measured in the morning under fasted conditions and were confirmed on the day of the vascular testing (assay described below). Men were included in the study if they met the following criteria: (1) no use of sex hormones for at least 1 year; (2) body mass index (BMI) < 40 kg/m2; (3) nonsmokers; (4) resting blood pressure (BP) < 160/90 mmHg; 5) nondiabetic and fasted plasma glucose < 7.0 mmol/L (126 mg/dL); (6) healthy and free from cardiovascular, cancer, renal, liver, or respiratory disease as assessed by medical history, physical exam, standard blood chemistries (comprehensive metabolic panel, complete blood count, and thyroid stimulating hormone), and electrocardiography at rest and during a graded exercise treadmill test to fatigue; (7) sedentary or recreationally active (< 3 days/week of vigorous aerobic exercise); (8) no use of medications that might influence cardiovascular function including antihypertensive and lipid-lowering medications; and (9) no use of vitamin supplements or anti-inflammatory medications, or willing to stop 1 month prior and throughout the study.
Participant Characteristics
Screening BPs were measured in the morning under fasted conditions (water drinking only) and abstinence of caffeine and exercise. Seated BP was measured via oscillometric assessment (Carescape V100, GE Medical Systems) following ≥ 10 minutes of seated rest. BP was measured in triplicate on both arms and the average of the higher arm is reported here. Total (percent of total mass) body fat was determined using dual x-ray absorptiometry (Hologic Horizon). Hip and waist circumference were measured by a trained technician in triplicate and the average of those measures is reported here. Peak oxygen consumption was determined using an individualized incremental treadmill exercise protocol. A warm-up period was used to determine the walking speed that elicited a heart rate roughly 70% of age-predicted maximum. This speed was maintained during the test while the treadmill grade was increased by 2% every 2 minutes until volitional fatigue. A 12-lead electrocardiogram (Quinton Q4500; Quinton Instruments, Seattle, WA) was used to monitor heart rhythm and rate continuously, while BP was measured during each exercise stage. Cardiorespiratory data were collected at 30-second intervals using a ParvoMedics TrueOne 2400 automated metabolic gas analysis system. The highest oxygen uptake (V̇O2) value achieved was recorded as V̇O2peak. Framingham risk score (FRS) was calculated using a multivariable risk algorithm that has been described previously (18).
Vascular Testing Study Visit
Participants were studied in the supine position after an overnight fast with proper hydration (water only, no caffeine). Participants abstained from exercise for ≥ 20 hours prior to the study visit. Prior to starting the vascular measures, an intravenous catheter was placed into an antecubital vein for the saline and vitamin C infusions and blood sampling (described below). Supine BP levels were measured during saline and vitamin C prior to the vascular assessments.
Microvascular endothelial function
Peripheral artery tonometry was assessed during saline and vitamin C using finger plethysmography (EndoPAT 2000, Itamar Medical) as previously described (19). Briefly, changes in pulse wave amplitude were measured in response to reactive hyperemia induced via forearm ischemia by inflating a BP cuff around the forearm to 250 mmHg that was maintained for 5 minutes. Pneumatic cuffs were placed on the index finger on each hand and recorded changes of finger volume with each pulse wave continuously before, during, and after cuff deflation. The data were analyzed using the automated algorithm built into the EndoPAT software. Reactive hyperemia index (RHI) (20) is the ratio of the average pulse wave amplitude during reactive hyperemia to the average pulse wave amplitude during the baseline period in the occluded arm relative to the same ratio in the nonoccluded arm. The coefficient of variation for RHI measured in 10 individuals in our laboratory is 6.8%.
Macrovascular endothelial function
Brachial artery diameter and blood flow velocity measurements were acquired to assess FMDBA using Doppler ultrasound (Vivid I, GE) with a multifrequency linear-array transducer, as previously described (19, 21, 22). The dilation of the brachial artery in response to the stimulus of forearm ischemia is dependent on the release of vasodilators, predominantly nitric oxide (NO), from the vascular endothelium (23). Briefly, a pediatric cuff was placed around the forearm and images of brachial artery diameter and blood flow velocity were acquired ~3-6 cm above the antecubital fossa. The ultrasound probe was held in place with a stereotactic clamp to ensure the location of the arterial segment remained constant, and the insonation angle was maintained at ≤ 60°. After obtaining concurrent measures of brachial artery diameter and blood flow velocity, forearm occlusion was produced by inflating the cuff to 250 mmHg and occlusion was maintained for 5 minutes. Prior to the release of the cuff, Doppler blood flow velocity was acquired and recorded until ~15 seconds after rapid release of arterial occlusion. B-mode ultrasound brachial artery diameter images were recorded continuously for 2 minutes. Brachial artery diameter and blood flow velocity images were coded by number, blinded to group status, and analyzed using commercially available software (Vascular Research Tools 6, Medical Imaging Applications, LLC) by a trained investigator (K.L.M.). All procedures conformed with recently published guidelines for assessing brachial artery FMD in humans (24). The coefficient of variation of FMDBA (%) from our laboratory is 1.5% (25).
Vascular smooth muscle cell function
Endothelium-independent dilation, an index of vascular smooth muscle cell function, was assessed in a subsample of men (11 young, 8 middle-aged/older low testosterone, 17 middle-aged/older higher testosterone) during the saline infusion only using sublingual nitroglycerin (NTG) (26), an exogenous NO donor. At least 15 minutes after the FMDBA, a baseline recording of brachial artery diameter was recorded. Then, participants received a 0.4 mg dose of sublingual NTG, and brachial artery diameter was recorded continuously from 2 minutes post-NTG until 10 minutes post-NTG. Baseline and peak brachial artery diameter were used to calculate absolute and relative changes in diameter induced by NTG.
Blood Sampling
Fasted plasma concentrations of glucose, insulin, total cholesterol (Roche Diagnostic systems, Indianapolis, IN), and high-density lipoprotein cholesterol (Diagnostic Chemical Ltd, Oxford, CT) were determined using enzymatic/colorimetric methods. Homeostatic model assessment of insulin resistance (HOMA-IR) was calculated as (glucose × insulin)/405 (27). Low-density lipoprotein (LDL) cholesterol was calculated using the Friedewald equation (28). Oxidized LDL was measured using an enzyme-linked immunosorbent plate assay (Alpco Diagnostics, Windham, NH). Serum total antioxidant status (TAS), a measure of overall antioxidant capacity, was measured using an enzymatic kit (Randox Laboratories, Oceanside, CA). Interleukin-6 (IL-6) was measured using an enzyme-linked immunoassay and high-sensitivity C-reactive protein (CRP) was measured using an immunoturbidimetric method. Total serum testosterone, estradiol, and sex hormone binding globulin (SHBG) were measured via chemiluminescence using a Beckman Coulter Access II analyzer. Free testosterone was calculated for each participant from concentrations of serum T, SHBG, and albumin using an online algorithm (www.issam.ch) using the Vermeulen equation (29). All blood samplings occurred on the day of vascular testing and all assays were performed at the University of Colorado Clinical Translational Sciences Institute Clinical Translational Research Center core laboratory.
Vitamin C Infusion Protocol
To investigate the role of systemic oxidative stress as a possible mechanism for endothelial dysfunction, we utilized a well-described experimental model using vitamin C to temporarily and reversibly reduce reactive oxygen species (ROS) as described previously in our laboratory (30-33). Vitamin C is a potent water-soluble antioxidant that can be immediately infused at rates that attain supraphysiological plasma vitamin C concentrations known to reduce the bioavailability of superoxide anions (34). Briefly, supine BP, FMDBA, EndoPAT, and NTG-mediated dilation were obtained after a 20-minute bolus of normal isotonic saline, followed by a “drip” infusion (control condition). Next, BP, FMDBA, and EndoPAT were repeated after a bolus of 100 mL vitamin C (ie, ascorbic acid) solution dosed at 0.06 g vitamin C per kg fat-free mass per 100 mL of normal saline (prepared by a research pharmacist at University of Colorado Hospital). The vitamin C solution was infused 5 mL/min over 20 minutes followed by a “drip” infusion at a rate of 1.7 mL/min. The saline infusion always preceded the vitamin C infusion to avoid any persistent effects of vitamin C. The time between saline and vitamin C measurements was approximately 60 to 90 minutes. The dose of vitamin C has been previously shown to temporarily (reversibly) improve carotid artery stiffness, femoral artery blood flow, FMDBA, and left ventricular diastolic function in older adults (30-33, 35, 36). The difference in FMDBA and RHI during vitamin C vs saline infusion was taken as a measure of the modulation of FMDBA and RHI by oxidative stress.
Androgen Deficiency Symptom Questionnaires
Participants completed the quantitative Androgen Deficiency in the Aging Male (qADAM) questionnaire (37) and the Aging Males’ Symptoms (AMS) scale (38). The qADAM consists of 10 questions with a Likert scale of 1-5, in which 5 representing the absence of a given symptom and 1 representing maximal symptoms, with all questions weighted equally. Summation of responses yields a total qADAM score between 10 and 50, with 10 being the most symptomatic and 50 being least symptomatic (37).
The AMS scale is a commonly used scale for measuring health-related quality of life in aging men (38). The AMS scale consists of 17 questions with a Likert scale ranging from 1-5 with 5 representing the most symptomatic and 1 representing absence of a given symptom. Symptoms are categorized into 3 dimensions: (1) psychological (eg, discouraged, depressed, irritable, anxious, nervous) with a minimum score of 5 and maximum of 25; (2) somato-vegetative (eg, joint/muscle complaints, hot flushes, increased need for sleep, sleep disturbances, impaired general well-being, decrease in muscle strength and decreased energy) with a minimum score of 7 and maximum of 35; and (3) sexual (eg, disturbances of potency, decreased morning erections, decreased libido and sexual activity and beard growth) with a minimum score of 5 and maximum of 25.
Data and Statistical Analysis
To account for differences in resting arterial diameter between groups, allometrically scaled FMD and NTG were analyzed using linear mixed-model analyses with the natural log–transformed diameter change as the dependent variable (ie, lnDpeak – lnDbase), group and infusion as fixed factors, and log–transformed baseline diameter (lnDbase) as the covariate. Significant interactions or main effects were assessed with pairwise comparisons and Fisher’s least-significant difference post hoc test (39).
The statistical approaches reported here were informed by recent guidelines for statistical reporting in cardiovascular research (40). Data were tested for normality using the Shapiro-Wilk normality test. Participant characteristics, sex hormones, inflammatory markers, oxidative stress markers, NTG, and change in FMD from saline to vitamin C infusion were analyzed using a 1-way analysis of variance (ANOVA); in the case of nonnormally distributed data, the Kruskal-Wallis H test was used. FMDBA and RHI were analyzed using a 2-way ANOVA with group and infusion (ie, saline or vitamin C) as factors. In the case of significant main effects, post hoc testing was performed with Tukey’s HSD and paired t tests. Relations between FMDBA, RHI, CVD risk factors, sex hormones, and oxidative stress markers were tested using Pearson product-moment correlations. To examine how CVD risk factors might influence the relation between testosterone and FMDBA, we used partial correlation analysis between testosterone and FMDBA while controlling for glucose, insulin, HOMA-IR, systolic BP (SBP), waist circumference, BMI, percent body fat, and triglycerides. Inflammatory markers were nonnormally distributed so relations with FMD and RHI were examined using Spearman’s rank-order correlations. Because of the potential confounding effects of age on these correlations, correlations that were significant were also examined separately in the pooled sample of middle-aged/older men. To determine whether the effects of low testosterone were independent of symptoms of androgen deficiency, correlations between FMDBA and RHI, and qADAM and AMS scores were also examined in the pooled cohort and within the cohort of middle-aged/older men. Statistical significance was set at P < 0.05 and results are expressed as mean ± SD. Nonnormally distributed data are presented as median (interquartile range). Data analysis was performed with IBM SPSS Statistics version 27.0 and GraphPad Prism version 8.4.1.
Results
Clinical Characteristics
Participant clinical characteristics are reported in Table 1. Middle-aged/older men had higher systolic and diastolic BP, body fat percent, BMI, waist circumference, and hip circumference and lower V̇O2peak compared with young (all P < 0.01); however, there were no differences between the 2 groups of middle-aged/older men. Young men had a lower FRS (1.0 ± 0.3) compared with middle-aged/older men regardless of testosterone status (P < 0.001 for both) and there were no differences between middle-aged/older men with higher (8.9 ± 4.5) or lower (9.1 ± 2.0; P = 0.989 vs higher) testosterone. By design, serum total testosterone and free testosterone were higher in young (total testosterone, P < 0.001; free testosterone, P < 0.001) and middle-aged/older men with higher serum testosterone (total testosterone P < 0.001; free testosterone, P = 0.032) compared with middle-aged/older with low testosterone. Middle-aged/older men with low testosterone had a higher HOMA-IR compared with young (P = 0.022) and middle-aged/older men with higher testosterone (P = 0.040) and higher triglycerides compared with young (P = 0.046). Total and LDL-cholesterols were not statistically different, although there was a trend for higher total and LDL-cholesterols in middle-aged/older men with lower testosterone compared with young (total, P = 0.075; LDL, P = 0.069). There were no differences between groups for HDL-cholesterol (P = 0.719).
Table 1.
Descriptive data
| Group | Young | Middle-aged/older higher testosterone | Middle-aged/older lower testosterone | P value |
|---|---|---|---|---|
| N | 20 | 20 | 18 | |
| Age, y | 29 ± 4 | 60 ± 6* | 59 ± 8* | <0.001 |
| Seated SBP, mmHg | 118 ± 9 | 127 ± 8* | 130 ± 8* | <0.001 |
| Seated DBP, mmHg | 74 ± 5 | 79 ± 8 | 81 ± 8* | 0.006 |
| Heart rate, bpm | 60 ± 11 | 61 ± 10 | 65 ± 13 | 0.430 |
| Body fat, % | 23.7 ± 7.2 | 29.5 ± 4.9* | 31.0 ± 5.6* | <0.001 |
| Height, cm | 180 ± 7 | 178 ± 6 | 177 ± 7 | 0.295 |
| Weight, kg | 78.6 ± 9.0 | 87.4 ± 13.7 | 96.6 ± 17.6* | <0.001 |
| BMI, kg/m2 | 24.2 ± 2.5 | 27.5 ± 3.7* | 29.6 ± 4.8* | <0.001 |
| Waist circumference, cm | 87 ± 7 | 100 ± 9* | 107 ± 13* | <0.001 |
| Hip circumference, cm | 63 ± 5 | 71 ± 7* | 76 ± 8* | <0.001 |
| WHR | 1.37 ± 0.07 | 1.42 ± 0.08 | 1.43 ± 0.14 | 0.163 |
| Testosterone, ng/dL | 500 ± 58 | 512 ± 115 | 269 ± 48*,† | <0.001 |
| Free testosterone, ng/dL | 11.2 ± 3.6 | 8.8 ± 1.5* | 6.7 ± 1.9*,† | <0.001 |
| Estradiol, pg/mL | 31 ± 12 | 47 ± 15* | 37 ± 18 | 0.008 |
| SHBG, nmol/L | 28.4 ± 9.3 | 48.6 ± 14.0* | 26.9 ± 8.7† | <0.001 |
| FSH, mIU/mL | 3.8 ± 1.9 | 5.3 ± 3.1 | 5.0 ± 2.2 | 0.117 |
| LH, mIU/mL | 3.3 ± 1.3 | 3.5 ± 3.1 | 3.7 ± 1.0 | 0.847 |
| Glucose, mg/dL | 87.3 ± 8.7 | 89 ± 6.6 | 95.1 ± 6.6*,† | 0.006 |
| Insulin, uIU/mLa | 3.0 (2.0-4.0) | 3.0 (2.0-4.0) | 6.0 (2.0-8.5) | 0.085 |
| HOMA-IRa | 0.65 (0.20-0.95) | 0.67 (0.22-0.92) | 1.46 (0.20-1.98)* | 0.044 |
| Total cholesterol, mg/dL | 159.7 ± 25.9 | 184.9 ± 49.5 | 186.9 ± 28.5 | 0.045 |
| HDL-cholesterol, mg/dL | 47.4 ± 8.7 | 47.2 ± 8.5 | 45.2 ± 8.9 | 0.719 |
| LDL-cholesterol, mg/dL | 112.3 ± 30.9 | 134.7 ± 46.3 | 139.8 ± 29.9 | 0.057 |
| Triglycerides, mg/dLa | 84.5 (48.0-111.0) | 70.5 (58.3-100.3) | 108.0 (73.0-169.5)* | 0.026 |
| V̇O2peak, mL/min/kg | 43.5 ± 8.6 | 30.7 ± 5.5* | 27.5 ± 5.6* | <0.001 |
| FRS | 1.0 ± 0.3 | 8.9 ± 4.5* | 9.1 ± 2.0* | <0.001 |
| qADAM | 38.5 ± 4.7 | 35.7 ± 4.9 | 37.7 ± 2.9 | 0.133 |
| AMSa | 21 (18-25) | 27 (21-31) | 21 (10-24) | 0.034 |
| Sexual domaina | 5.0 (5.0-6.75) | 6.0 (5.0-8.0) | 7.0 (6.0-10.0)* | 0.003 |
| Psychological domaina | 9.0 (7.3-10.8) | 5.0 (5.0-7.5)* | 6.0 (5.0-8.0)* | <0.001 |
| Somato-vegetative domaina | 9.0 (7.3-10.8) | 9.0 (8.0-11.5) | 11.0 (9.0-16.0) | 0.030 |
Data were examined using one-way ANOVAs and are displayed as mean ± SD except in the case of non-normally distributed data(a).
Abbreviations: BMI, body mass index; DBP, diastolic blood pressure; FRS, Framingham Risk Score; FSH, follicle-stimulating hormone; HDL, high-density lipoprotein; HOMA-IR, homeostatic model assessment for insulin resistance; LDL, low-density lipoprotein; LH, luteinizing hormone; SBP, systolic blood pressure; SHBG, sex hormone binding globulin; VO2peak, maximal aerobic capacity; WHR, waist-to-hip ratio.
aNon-normally distributed data, examined using the Kruskal-Wallis test and displayed as median (interquartile range).
*P < 0.05 vs young, †P < 0.05 vs middle-aged/older higher testosterone.
There were no differences across the groups in qADAM scores (P = 0.133) but middle-aged/older men with lower testosterone had higher total AMS scale scores than young (P = 0.017) and middle-aged/older men with higher testosterone (P = 0.041). Within the 3 AMS domains, middle-aged/older men with lower testosterone had worse scores for the sexual (P = 0.002) and psychological (P = 0.009) domains compared with young. Similarly, middle-aged/older men with higher testosterone had worse scores for the sexual domain compared with young (P = 0.004). There were no differences between groups in the somato-vegetative domains between groups (all P > 0.05).
Oxidative Stress and Inflammatory Markers
Circulating concentrations of inflammatory and oxidative stress markers are presented in Table 2. IL-6 was higher in both groups of middle-aged/older men compared with young (lower testosterone, P < 0.001; higher testosterone, P = 0.001), and CRP was higher in middle-aged/older with low testosterone compared with young (P = 0.002) and middle-aged/older with higher testosterone (P = 0.022). Oxidized LDL (P = 0.903) and TAS (P = 0.164) were similar across groups.
Table 2.
Circulating oxidative stress and inflammatory markers
| Group | Young | Middle-aged/older higher testosterone | Middle-aged/older lower testosterone | P value |
|---|---|---|---|---|
| N | 20 | 20 | 18 | |
| WBC | 5.1 ± 1.3 | 4.9 ± 1.3 | 6.3 ± 2.3† | 0.029 |
| IL-6 (pg/mL)a | 0.76 (0.60-1.10) | 1.40 (1.06-1.77)* | 2.05 (1.46-2.6)* | <0.001 |
| CRP (mg/L)a | 0.52 (0.37-0.70) | 0.84 (0.53-1.77) | 1.61 (1.13-4.52)* , † | <0.001 |
| TAS (mmol/L) | 1.77 ± 0.13 | 1.69 ± 0.11 | 1.83 ± 0.34 | 0.164 |
| Oxidized LDL (U/L) | 70 ± 28 | 73 ± 23 | 72 ± 14 | 0.903 |
Data were examined using one-way ANOVAs and are displayed as mean ± SD except in the case of non-normally distributed data(a)
Abbreviations: CRP, C-reactive protein; IL-6, interleukin-6; LDL, low-density lipoprotein; TAS, total antioxidant status; WBC, white blood count.
* P < 0.05 vs younger. †P < 0.05 vs middle-aged/older higher testosterone.
aNon-normally distributed data, examined using the Kruskal-Wallis test and displayed as median (interquartile range).
Endothelial Function During Saline and Vitamin C
Macrovascular endothelial function
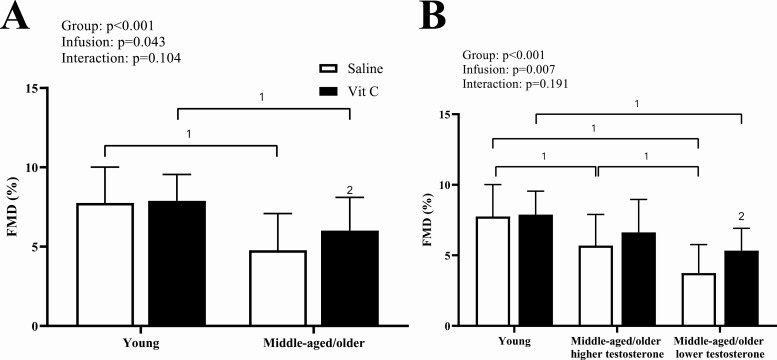
During the saline (control) infusion, middle-aged/older men had lower FMDBA compared with young men regardless of testosterone status (higher testosterone, P = 0.011; lower testosterone, P < 0.001); however, middle-aged/older men with low testosterone had significantly lower FMDBA compared with middle-aged/older men with higher testosterone (P = 0.021; Fig. 1). The results were the same whether the FMDBA was allometrically scaled or expressed as absolute change in mm (P < 0.010 for both; Table 3). There was a significant main effect of the vitamin C infusion on FMDBA (main effect of infusion, P = 0.007; Fig. 1 and Table 3). FMDBA significantly increased in middle-aged/older men with low testosterone to levels that were no longer significantly different from their age-matched peers with higher testosterone (P = 0.151). Vitamin C increased FMDBA in middle-aged/older men with higher testosterone to levels that were no longer significantly different than young men (P = 0.150); however, the within-group improvement was not statistically significant (P = 0.250). As expected, there was no change in FMDBA in young men during the vitamin C infusion (P = 0.992).
Figure 1.
Brachial artery FMD is significantly reduced during the saline (control) infusion (open bars) with middle-aged/older men regardless of testosterone status (A) and vitamin C (black bars) improves brachial artery FMD in the middle-aged/older men, but not young. When middle-aged/older men are separated by testosterone status (B), middle-aged/older men with lower testosterone have reduced brachial artery FMD compared with middle-aged older men with higher testosterone during the saline (control) infusion, and vitamin C increases brachial artery FMD in the middle-aged/older men with lower testosterone alone. 1P < 0.05 between groups; 2indicates P < 0.05 compared with saline infusion. P values represent main effects of 2-way ANOVAs and data are mean ± SD.
Table 3.
Vascular data
| Variables | Infusion | Group | Infusion | Interaction | |||
|---|---|---|---|---|---|---|---|
| Group | Younger | Middle-aged/older higher testosterone | Middle-aged/older lower testosterone | ||||
| N | 20 | 20 | 18 | ||||
| Baseline brachial artery diameter, mm | Saline | 4.1 ± 0.5 | 4.7 ± 0.6 | 5.0 ± 0.5 | <0.001 | 0.253 | 0.065 |
| Vit C | 4.2 ± 0.5 | 4.7 ± 0.5 | 4.9 ± 0.6 | ||||
| Brachial artery diameter change, mm | Saline | 0.32 ± 0.06 | 0.26 ± 0.10 | 0.19 ± 0.09 | <0.001 | 0.007 | 0.139 |
| Vit C | 0.32 ± 0.07 | 0.31 ± 0.10 | 0.26 ± 0.07 | ||||
| Allometrically scaled FMD, % | Saline | 6.8 ± 2.7 | 5.8 ± 1.8 | 4.1 ± 2.6 | 0.003 | 0.005 | 0.113 |
| Vit C | 7.4 ± 2.2 | 7.0 ± 2.0 | 5.9 ± 2.1 | ||||
| RHI | Saline | 1.76 ± 0.48 | 2.19 ± 0.57 | 2.14 ± 0.40 | 0.086 | 0.480 | 0.272 |
| Vit C | 1.88 ± 0.51 | 2.07 ± 0.51 | 1.97 ± 0.45 | ||||
| lnRHI | Saline | 0.53 ± 0.26 | 0.75 ± 0.25 | 0.74 ± 0.18 | 0.052 | 0.544 | 0.248 |
| Vit C | 0.60 ± 0.26 | 0.69 ± 0.26 | 0.66 ± 0.19 | ||||
Data examined using 2-way ANOVAs and displayed as mean ± SD.
Abbreviations: FMD, flow-mediated dilation; lnRHI, natural log–transformed reactive hyperemia index; RHI, reactive hyperemia index.
Microvascular endothelial function
There were no differences observed in RHI during the saline infusion across the groups (P = 0.086), nor did RHI change during the vitamin C infusion (P = 0.480). Expressing the data as the natural log–transformed RHI (lnRHI; Table 3) did not change these findings (group, P = 0.052; vitamin C infusion, P = 0.544).
Vascular smooth muscle cell function
NTG-induced dilation of the brachial artery was not different across groups (young = 22.6 ± 5.8; middle-aged/older higher testosterone = 19.5 ± 5.2; middle-aged/older lower testosterone = 17.5 ± 6.0 %; P = 0.143). Allometric scaling did not change these findings (P = 0.969; data not shown).
Correlates of testosterone concentrations and endothelial function
FMDBA had significant inverse relations with systolic BP, BMI, waist circumference, and blood glucose, as well as the inflammatory markers IL-6 and CRP (Table 4). In addition, FMDBA was positively associated with total and free testosterone and VO2peak. In contrast, RHI was inversely correlated with free testosterone and positively correlated with FRS and IL-6 but was not correlated with any specific CVD risk factor, sex hormone, or inflammatory or oxidative stress marker. Neither FMDBA nor RHI were related to qADAM questionnaire scores or AMS scale scores.
Table 4.
Correlations of CVD risk factors, sex hormone concentrations, and inflammatory and oxidative stress markers with brachial artery FMD and RHI in the pooled cohort
| Variables | Brachial artery FMD | RHI | Total testosterone | Free testosterone | ||||
|---|---|---|---|---|---|---|---|---|
| r | P | r | P | r | P | r | P | |
| Age, yr | -0.47 | <0.001 | 0.40 | 0.002 | -0.22 | 0.097 | -0.54 | <0.001 |
| Seated SBP, mmHg | -0.44 | <0.001 | 0.11 | 0.432 | -0.24 | 0.069 | -0.38 | 0.003 |
| BMI, kg/m2 | -0.33 | 0.012 | 0.25 | 0.068 | -0.39 | 0.002 | -0.33 | 0.012 |
| Total body fat, % | -0.22 | 0.100 | 0.17 | 0.221 | -0.30 | 0.020 | -0.30 | 0.019 |
| Waist circumference, cm | -0.40 | 0.002 | 0.17 | 0.209 | -0.33 | 0.010 | -0.33 | 0.011 |
| WHR | -0.22 | 0.102 | 0.01 | 0.999 | 0.142 | 0.282 | 0.02 | 0.872 |
| Total cholesterol, mg/dL | 0.02 | 0.890 | 0.03 | 0.813 | -0.02 | 0.883 | -0.12 | 0.348 |
| LDL-cholesterol, mg/dL | 0.03 | 0.827 | 0.05 | 0.733 | -0.4 | 0.782 | -0.15 | 0.250 |
| Triglycerides, mg/dLa | -0.05 | 0.773 | 0.05 | 0.691 | -0.39 | 0.002 | -0.37 | 0.004 |
| Glucose, mg/dL | -0.39 | 0.003 | 0.09 | 0.493 | -0.23 | 0.075 | -0.22 | 0.090 |
| Insulin, uIU/mLa | -0.10 | 0.463 | 0.01 | 0.948 | -0.26 | 0.046 | -0.05 | 0.702 |
| HOMA-IRa | -0.18 | 0.176 | 0.04 | 0.759 | -0.28 | 0.035 | -0.08 | 0.563 |
| Total testosterone, ng/dL | 0.45 | <0.001 | -0.15 | 0.272 | 1 | - | 0.72 | <0.001 |
| Free testosterone, ng/dL | 0.40 | 0.002 | -0.36 | 0.006 | 0.72 | <0.001 | 1 | - |
| Estradiol, pg/mL | -0.11 | 0.394 | 0.07 | 0.601 | 0.39 | 0.002 | 0.01 | 0.937 |
| SHBG, nmol/L | 0.11 | 0.401 | 0.18 | 0.176 | 0.56 | <0.001 | -0.16 | 0.234 |
| LH, mIU/mL | 0.09 | 0.489 | 0.20 | 0.143 | 0.06 | 0.681 | -0.11 | 0.417 |
| FSH, mIU/mL | -0.6 | 0.663 | 0.29 | 0.034 | -0.24 | 0.069 | -0.28 | 0.034 |
| V̇O2peak, mL/min/kg | 0.38 | 0.005 | -0.24 | 0.078 | 0.37 | 0.004 | -0.48 | <0.001 |
| WBC | -0.14 | 0.309 | -0.09 | 0.515 | -0.30 | 0.022 | -0.11 | 0.426 |
| IL-6, pg/mLa | -0.50 | <0.001 | 0.27 | 0.047 | -0.44 | 0.001 | -0.53 | <0.001 |
| CRP, mg/La | -0.38 | 0.004 | 0.07 | 0.607 | -0.44 | <0.001 | -0.44 | <0.001 |
| TAS, mmol/L | -0.03 | 0.821 | -0.20 | 0.143 | -0.19 | 0.159 | 0.08 | 0.563 |
| Oxidized LDL-cholesterol, U/L | 0.13 | 0.332 | -0.07 | 0.592 | 0.19 | 0.165 | 0.10 | 0.469 |
| FRS | -0.34 | 0.010 | 0.47 | >0.001 | -0.21 | 0.116 | -0.54 | <0.001 |
| qADAM score | 0.02 | 0.357 | -0.06 | 0.660 | 0.27 | 0.042 | 0.19 | 0.152 |
| AMS scorea | -0.14 | 0.316 | 0.34 | 0.013 | -0.30 | 0.027 | -0.26 | 0.058 |
Bold font indicates significant correlation.
Abbreviations: AMS, aging male symptom scale; BMI, body mass index; CRP, C-reactive protein; FMD, flow-mediated dilation; FRS, Framingham Risk Score; FSH, follicle-stimulating hormone; HOMA-IR, homeostatic model assessment of insulin resistance; IL-6, interleukin-6; LDL, low-density lipoprotein; LH, luteinizing hormone; qADAM, quantitative androgen deficiency in the again male questionnaire; SBP, systolic blood pressure; SHBG, sex hormone binding globulin; TAS, total antioxidant status; WHR, waist-hip ratio; WBC, white blood count.
anon-parametric data analyzed using Spearman correlations.
Correlations were also examined among middle-aged/older participants only, as age can contribute to these associations (Table 5). In the pooled cohort of middle-aged/older participants, FMDBA was associated with total testosterone and SHBG, and tended to be correlated with fasted glucose but no other sex hormone, CVD risk factor, symptom questionnaire score, or marker of inflammation was statistically significant. Partial correlation analysis between FMDBA and testosterone were performed and adjusted for CVD risk factors that correlated with testosterone. Controlling for fasted glucose (r = 0.419; P = 0.010), insulin (r = 0.488; P = 0.002), HOMA-IR (r = 0.479; P = 0.003), triglycerides (r = 0.506; P = 0.001), BMI (r = 0.505; P = 0.001), percent body fat (r = 0.492; P = 0.002), systolic BP (r = 0.448; P = 0.005), or waist circumference (r = 0.497; P = 0.002) did not change the correlation between testosterone and FMDBA. When controlling for testosterone, the correlation between FMDBA and glucose was no longer statistically significant (r = −0.226; P = 0.178). The positive correlation between RHI and FRS remained significant; however, there were no other significant correlations with RHI in pooled middle-aged/older men.
Table 5.
Correlations of CVD risk factors, sex hormone concentrations, and inflammatory markers with brachial artery FMD and RHI in middle-aged/older men only
| Variables | Brachial Artery FMD (%) | RHI | T | Free T | ||||
|---|---|---|---|---|---|---|---|---|
| r | P | r | P | r | P | r | P | |
| Age, yr | 0.122 | 0.465 | 0.32 | 0.053 | -0.00 | 0.997 | -0.16 | 0.340 |
| Seated SBP, mmHg | -0.18 | 0.275 | 0.04 | 0.810 | -0.17 | 0.303 | -0.21 | 0.210 |
| BMI, kg/m2 | -0.03 | 0.840 | 0.13 | 0.444 | -0.45 | 0.005 | -0.27 | 0.094 |
| Total body fat, % | -0.01 | 0.962 | 0.09 | 0.615 | -0.34 | 0.034 | -0.27 | 0.094 |
| Waist circumference, cm | -0.01 | 0.953 | 0.01 | 0.958 | -0.36 | 0.026 | 0.18 | 0.268 |
| WHR | -0.05 | 0.764 | -0.17 | 0.317 | 0.18 | 0.285 | 0.11 | 0.511 |
| Total cholesterol, mg/dL | 0.23 | 0.186 | -0.11 | 0.518 | 0.03 | 0.857 | 0.10 | 0.537 |
| LDL-cholesterol, mg/dL | 0.22 | 0.196 | -0.08 | 0.638 | 0.01 | 0.959 | 0.071 | 0.669 |
| Triglycerides, mg/dLa | -0.05 | 0.788 | -0.12 | 0.483 | -0.47 | 0.003 | -0.31 | 0.057 |
| Glucose, mg/dL | -0.32 | 0.054 | -0.08 | 0.646 | -0.29 | 0.078 | -0.15 | 0.352 |
| Insulin, uIU/mLa | 0.03 | 0.864 | 0.02 | 0.928 | -0.49 | 0.001 | -0.22 | 0.180 |
| HOMA-IRa | -0.05 | 0.778 | 0.01 | 0.945 | -0.51 | 0.001 | 0.22 | 0.172 |
| Total testosterone, ng/dL | 0.43 | 0.007 | -0.13 | 0.429 | 1 | - | 0.76 | <0.001 |
| Free testosterone, ng/dL | 0.31 | 0.062 | -0.20 | 0.227 | 0.76 | <0.001 | 1 | - |
| Estradiol, pg/mL | 0.21 | 0.201 | -0.04 | 0.817 | 0.63 | <0.001 | 0.45 | 0.005 |
| SHBG, nmol/L | 0.47 | 0.003 | 0.05 | 0.739 | 0.851 | <0.001 | 0.32 | 0.044 |
| LH, mIU/mL | 0.28 | 0.094 | 0.22 | 0.215 | -0.04 | 0.820 | -0.12 | 0.494 |
| FSH, mIU/mL | 0.09 | 0.617 | 0.25 | 0.144 | -0.19 | 0.255 | -0.31 | 0.065 |
| V̇O2peak, mL/min/kg | -0.05 | 0.777 | -0.20 | 0.241 | 0.38 | 0.019 | 0.36 | 0.030 |
| WBC | -0.09 | 0.586 | -0.10 | 0.547 | -0.45 | 0.004 | -0.29 | 0.076 |
| IL-6, pg/mLa | -0.17 | 0.308 | 0.07 | 0.689 | -0.45 | 0.004 | -0.41 | 0.012 |
| CRP, mg/La | -0.19 | 0.274 | -0.07 | 0.692 | -0.52 | 0.001 | -0.45 | 0.004 |
| TAS, mmol/L | -0.30 | 0.068 | -0.24 | 0.161 | -0.29 | 0.081 | -0.23 | 0.162 |
| Oxidized LDL-cholesterol (U/L) | 0.37 | 0.022 | -0.21 | 0.210 | 0.10 | 0.563 | 0.09 | 0.613 |
| FRS | 0.17 | 0.296 | 0.38 | 0.019 | -0.15 | 0.359 | -0.31 | 0.061 |
| qADAM score | 0.07 | 0.963 | 0.01 | 0.600 | 0.36 | 0.029 | 0.43 | 0.008 |
| AMS scorea | -0.05 | 0.761 | 0.23 | 0.182 | -0.42 | 0.011 | -0.46 | 0.004 |
Bold font indicates significant correlation.
Abbreviations: AMS, aging male symptom scale; BMI, body mass index; CRP, C-reactive protein; FMD, flow-mediated dilation; FRS, Framingham Risk Score; FHS, follicle stimulating hormone; HOMA-IR, homeostatic model assessment of insulin resistance; IL-6, interleukin-6; LDL, low-density lipoprotein; LH, luteinizing hormone; qADAM, quantitative androgen deficiency in the again male questionnaire; SBP, systolic blood pressure; SHBG, sex hormone binding globulin; TAS, total antioxidant status; WHR, waist-hip ratio; WBC, white blood count.
anon-parametric data analyzed using Spearman correlations.
Discussion
The novel findings of the present study are that middle-aged/older men with lower testosterone have evidence of “accelerated” vascular aging, as indicated by a greater age-associated endothelial dysfunction of large conduit arteries compared with their age-matched peers. The greater macrovascular endothelial dysfunction in middle-aged/older men with chronically low testosterone was independent of CVD risk factors or symptoms of androgen deficiency. Moreover, our findings also indicate that increased systemic oxidative stress and inflammation are mechanistically linked to the greater age-associated endothelial dysfunction in middle-aged/older men with lower testosterone.
Vascular Aging in Men
The vascular endothelium is a single layer of cells that is critical for homeostasis. Damage to endothelial cells leads to profound macro- and microvascular dysfunction, favoring a vasoconstrictive, pro-inflammatory, pro-coagulative, and proliferative phenotype (41). Accordingly, endothelial dysfunction is considered the initial step in the pathogenesis of atherosclerosis (42). Consistent with previous vascular aging studies (19, 43, 44), we found that FMDBA, a noninvasive assessment of endothelium-dependent vasodilation mediated in part by the pro-vasodilatory and anti-atherogenic molecule NO (45), was ~40% lower in our pooled population of healthy middle-aged/older men compared with young men during the control (saline) infusion.
Cross-sectional studies of men over a broad age range and disease status (eg, preexisting CVD, liver and renal disease, diabetes) have reported that lower levels of total and free testosterone are independently associated with reduced FMDBA and RHI (13, 15, 46); however, to our knowledge the impact of gonadal aging and declines in testosterone on age-associated endothelial dysfunction has not been considered in prior vascular aging studies conducted in healthy men. In the present study, healthy middle-aged/older men with low testosterone (<10.4 nmol/L [300 ng/dL]) had worse age-associated endothelial dysfunction than age-matched men with higher testosterone levels (≥13.9 nmol/L [400 ng/dL]). The reduced FMDBA was moderately correlated with lower total and free testosterone levels in the pooled cohort and restricting the analyses to middle-aged/older men to control for age did not change the correlation between total testosterone and FMDBA. Importantly, the difference in FMDBA (~2%) between middle-aged/older men with low testosterone compared with age-matched men with higher testosterone is clinically relevant, as reductions in FMD of 1% are associated with a ~9% to 13% increase in CVD risk (47, 48). These data indicate that declines in testosterone with aging may lead to an acceleration in vascular aging in healthy men even in the absence of clinical disease. Moreover, the greater age-associated endothelial dysfunction in men with lower testosterone may explain documented associations between low testosterone and increased CVD risk (3-7).
In the present study, microvascular endothelial function measured via peripheral arterial tonometry (ie, EndoPAT) tended to be higher in middle-aged/older men compared with young regardless of testosterone levels. This observation is in contrast to Corrigan et al who reported reduced RHI in men aged 18-82 years with testosterone levels <12 nmol/L (350 ng/dL) compared with men with higher levels (≥12.1 nmol/L [350 ng/dL]) (46). Moreover, there was no correlation between RHI and serum total testosterone levels in our pooled cohort or within the cohort of middle-aged/older men, also in contrast to Corrigan et al, who reported a positive, albeit modest, correlation with total and free testosterone (46). The reasons for the discordant findings are not completely clear but could be related to study population; whereas our participants were extremely healthy, nonsmokers, and nonmedicated, Corrigan et al included participants with hypertension, diabetes, and hyperlipidemia, as well as tobacco use (46). Moreover, we categorized participants by age groups and only included men with low testosterone in the middle-aged/older men, whereas Corrigan et al included men across the lifespan and categorized men according to testosterone levels regardless of age (46).
Our finding showing no association between macrovascular and microvascular function, as assessed using peripheral arterial tonometry, is consistent with recently published data from our laboratory in healthy men (19) and with observations from a large cohort study of middle-aged/older adults (49), but in contrast to studies demonstrating associations between FMD and RHI (50, 51). The contrast in findings may be explained by differences in study population (eg, healthy men vs inclusion of both sexes and those with preexisting CVD) (50, 51) and/or methodology (eg, lower vs upper arm occlusion to induce reactive hyperemia) (51). Additionally, because the EndoPAT uses automated analysis of a fixed time frame during hyperemia to calculate RHI, and the fact that peak dilation responses are delayed in older compared with younger adults, it is possible that using an inappropriate time frame obscures differences that exist between groups (52).
Finally, consistent with previous aging studies (44, 53-55), NTG-mediated vasodilation was not statistically different between the groups of young and middle-aged/older men, indicating preserved vascular smooth muscle cell function. Although the difference in NTG-mediated vasodilation between the groups of middle-aged/older men was not statistically significant, the values were lower in the men with low testosterone consistent with observations in men with end-stage kidney disease classified as androgen deficient (total testosterone < 10.4 nmol/L [300 ng/dL]) who had moderate reductions in NTG-mediated vasodilation compared with men not classified as androgen deficient (56). Because not all men underwent the NTG-mediated vasodilation assessment in the present study, it is possible that with a larger sample size this magnitude of difference, if sustained, would be statistically significant.
Role of Oxidative Stress and Inflammation as Mechanisms Underlying Age-Associated Endothelial Dysfunction in Men With Low Testosterone
Oxidative stress, characterized by excessive ROS production relative to antioxidant defense capacity, and chronic-low grade inflammation are 2 interconnected mechanisms that contribute to reduced NO bioavailability and endothelial-dependent vasodilation with aging in men (44, 57, 58). Mechanistic insight on the role of oxidative stress in impairing endothelial function can be assessed by infusing supraphysiological levels of the potent antioxidant vitamin C. This common experimental approach temporarily and reversibly reduces superoxide anions and other ROS (30-33). Using this approach, vitamin C infusion has been shown to acutely rescue macro- (FMDBA) and micro- (forearm blood flow response to acetylcholine) vascular endothelial function in older men but has no effect in young men (44, 58), indicating oxidative stress as a primary mechanism underlying endothelial dysfunction with aging in men.
It is likely that the previous studies of vascular aging using vitamin C infusions included older men with varying levels of testosterone. To our knowledge, the present study was the first to characterize the magnitude of oxidative stress suppression on endothelial function in middle-aged/older men categorized according to testosterone status. In doing so, we showed that the FMDBA response to the acute vitamin C infusion was dramatically different between men with low vs higher testosterone. Specifically, although acute vitamin C infusion increased FMDBA by ~15.8% in the middle-aged/older men with higher testosterone to levels that were no longer statistically different than young men, the effect of the vitamin C compared with the saline infusion (control) was not statistically significant. In contrast, acute vitamin C infusion significantly increased FMDBA (compared with saline) by ~43% in the middle-aged/older men with low testosterone to levels that were no longer statistically different than their age-matched peers with higher testosterone, but still significantly lower than young men. Collectively, these data suggest that oxidative stress may play a greater role on macrovascular endothelial dysfunction in middle-aged/older men with low testosterone than in men with higher testosterone levels.
The reasons why oxidative stress appears to be a greater contributing factor to age-associated endothelial dysfunction in middle-aged/older men with low testosterone are unclear. Preclinical data show that testosterone deficiency is associated with increased ROS (ie, lipid peroxidation and nitrotyrosine) and reduced antioxidant defense systems (59, 60) and that testosterone treatment increases the activities of the antioxidants catalase and superoxide dismutase and ameliorates a pro-oxidant state (9, 61). Although we did not observe differences in circulating markers of antioxidant capacity (eg, TAS) or oxidative stress (eg, oxidized LDL-cholesterol) across groups, plasma biomarkers may not accurately reflect levels of oxidative stress and antioxidants observed at the local vascular level. Additionally, it is possible that other sources of ROS and/or antioxidant enzyme concentrations that we did not measure (eg, manganese or copper-zinc superoxide dismutase, catalase) (62) were higher and/or lower, respectively, in men with lower testosterone. Future studies should examine how low testosterone impacts redox balance at the local vascular level.
Low testosterone concentrations are associated with higher inflammatory markers in older men (11, 12). Additionally, IL-6 and TNFα are increased following induced androgen deficiency with a gonadotropin releasing hormone agonist and an aromatase inhibitor in young men (63). These data suggest that testosterone has anti-inflammatory effects and thus, could mitigate the effects of inflammation on vascular aging. Consistent with this, in the present study, middle-aged/older men with low testosterone had higher levels of IL-6, CRP, and white blood cells (WBC) suggesting a higher inflammatory state than age-matched men with higher testosterone levels. Additionally, these markers of inflammation were all moderately correlated with total and free testosterone, with lower levels of testosterone associated with higher levels of inflammatory markers. Moreover, IL-6 and CRP were inversely correlated with FMDBA during control (saline) conditions, consistent with prior observations showing inverse correlations between circulating markers of inflammation and FMDBA in older adults (64-67). Collectively, these data suggest that the greater age-associated endothelial dysfunction in middle-aged/older men with low testosterone may be related to a greater state of inflammation.
Role of CVD Risk Factors and Androgen Deficiency Symptoms Contributing to Endothelial Dysfunction in Men With Low Testosterone
In general, low testosterone is associated with an unfavorable CVD risk factor profile (ie, central obesity, hypertension, insulin resistance/diabetes, dyslipidemia), that can increase oxidative stress and inflammation. Thus, it is possible that the greater age-associated endothelial dysfunction in middle-aged/older men with low testosterone could be related to a greater CVD risk factor burden. In the present study, despite middle-aged/older men with lower testosterone having higher fasted blood glucose, HOMA-IR, and triglycerides compared with young men, and higher fasted glucose than age-matched men, they had a low mean FRS (<10%) that was not statistically different than middle-aged/older men with higher testosterone. Moreover, although systolic BP, BMI, fasted glucose, and FRS were all inversely correlated with FMDBA in the pooled cohort, only fasted glucose remained correlated with FMDBA, albeit this was not statistically significant (P = 0.056), in the pooled cohort of middle-aged/older men. The correlation between testosterone and FMDBA remained unchanged when controlling for glucose with partial correlation analysis, whereas the correlation between glucose and FMDBA was diminished after controlling for testosterone concentrations. Further partial correlation analyses controlling for other CVD risk factors (ie, BP, body composition, triglycerides, insulin, and HOMA-IR) also did not affect the relation between testosterone and FMDBA. Thus, these data suggest that the greater age-associated endothelial dysfunction in men with low testosterone is not necessarily due to an adverse CVD risk factor profile.
The declines in testosterone experienced by aging men are commonly accompanied by signs and symptoms including reduced libido, loss of body and facial hair, poor concentration and memory, and/or increased fatigue (68). It has been suggested that these symptoms may reflect the degree of androgen deficiency, and there is considerable debate regarding the clinical significance of age-related declines in testosterone in the absence of symptoms. Therefore, in the present study we examined whether the presence of self-reported signs and symptoms could explain the greater endothelial dysfunction in middle-aged/older men with low testosterone. There were no differences between the groups of middle-aged/older men in self-reported symptoms as measured by the qADAM and the AMS scale questionnaires, nor were there significant correlations between indices of endothelial function and the severity of symptoms. These data indicate that testosterone deficiency, rather than clinical hypogonadism per se, is associated with impairments in endothelial function in healthy middle-aged/older men.
Limitations and Experimental Considerations
The present study is not without limitations. We only enrolled men who were healthy, and the results may not be generalizable to men with preexisting CVD, diabetes, smokers, or individuals taking medications. Additionally, because we excluded young men with total testosterone levels <13.9 nmol/L (400 ng/dL) and middle-aged/older men with testosterone levels between 10.4 and 13.8 nmol/L (300-399 ng/dL), we cannot determine whether low testosterone has the same effect on endothelial function in young men, or whether endothelial function is impaired in men with testosterone levels between 10.4 and 13.8 nmol/L (300-399 ng/dL). Future studies should examine whether there is a “threshold” level of testosterone at which endothelial dysfunction occurs. Although we were able to disassociate the effects of low testosterone on endothelial function from CVD risk factors and symptoms of androgen deficiency, the cross-sectional design of this study prohibits us from drawing conclusions regarding cause and effect. Moreover, we are unable to determine whether the reduced FMDBA in men with low testosterone was related to some other lifestyle factor (eg, diet, physical activity).
ROS and inflammatory cytokines also increase vasoconstrictors (eg, endothelin 1) (69) and modulate sympathetic alpha-adrenergic vasoconstriction (70), increasing vascular smooth muscle cell vasoconstrictor tone. Therefore, it is possible that these factors may have contributed to the greater endothelial dysfunction in middle-aged/older men with low testosterone. More studies are needed to fully elucidate these potential mechanisms.
The saline infusion was always performed prior to the vitamin C infusion to avoid persistent effects of vitamin C. Therefore, it is possible that there is an order effect in the present study; however, tests were separated by approximately 90 minutes and it is unlikely that an order effect would influence the outcomes of these tests differentially between groups.
It is possible that the higher baseline brachial artery diameter in the lower testosterone group could explain their lower FMDBA (39). However, using allometric scaling to adjust for differences in baseline diameter did not affect our findings.
Additionally, manufacturer recommendations for the EndoPAT recommend using upper, rather than lower arm occlusion. We chose to use lower arm occlusion to maintain a consistent cuff placement, and therefore area of ischemia, as the FMDBA. It is possible that placing the cuff around the lower arm may have contributed to disparate finding between the FMDBA and EndoPAT measures, however a previous report comparing the RHI response to lower and upper arm occlusion indicated no differences in the response (71).
Finally, the aim of this study was to examine the role of low testosterone on age-associated endothelial function in men; therefore, only males were included in this study. These findings are not generalizable to women, and conditions in women in which testosterone concentrations are altered (eg, polycystic ovary syndrome) have been examined previously (72).
Clinical Significance and Conclusions
Hypogonadism is a state of androgen deficiency that has an adverse impact on physical function and quality of life in aging men (73). Additionally, more than 100 studies demonstrate that low serum testosterone is associated with increased all-cause and CVD mortality (74). Estimates are that, over a 20-year period, testosterone deficiency is projected to be involved in the development of ~1.3 million new cases of CVD and other diseases (eg, diabetes, osteoporosis), and may be directly responsible for $190 to $525 billion in USA health care expenditures (75). In 2015, an international expert consensus conference on testosterone deficiency and its treatment concluded that testosterone deficiency is a well-established medical condition that is a global public health concern (76). Yet, studies suggesting potential increased CVD risk with testosterone therapy have heightened caution surrounding the use of testosterone in older men (77, 78). A better understanding of how low testosterone may adversely impact cardiovascular health is important for developing effective treatments and intervention strategies to mitigate the effects of androgen deficiency. The findings from the present study suggest that testosterone deficiency may accelerate vascular aging in healthy men, due in part to increased oxidative stress and inflammation. Moreover, the present study adds to the mounting evidence that normal physiological levels of testosterone may be beneficial to cardiovascular health by attenuating the age-related decline in endothelial function, possibly by mitigating the effects of oxidative stress and inflammation. Future studies should investigate whether maintaining testosterone levels above a certain level and/or implementing other therapeutic strategies (eg, nutraceuticals) that mitigate oxidative stress and inflammation can slow down or reverse the vascular aging process in men.
Acknowledgments
We thank the nursing, core laboratory, bionutrition, information systems and administrative staff of the Clinical and Translational Research Center and the Energy Balance Core of the Nutrition and Obesity Research Center for their support of the study. We also are grateful to the members of our research group who helped with the initiation of the study and carried out day-to-day activities for the project. Finally, we thank the men who volunteered to participate in the study for their time and effort.
Financial Support: This research was supported by National Institutes of Health R01AG049762, National Institutes of Health U54AG062319, National Institutes of Health T32AG000279, Colorado Clinical and Translational Sciences Institute UL1 TR001082, Colorado Nutrition and Obesity Research Center P30 DK048520, and Eastern Colorado GRECC.
Glossary
Abbreviations
- AMS
Aging Males’ Symptoms
- ANOVA
analysis of variance
- BMI
body mass index
- BP
blood pressure
- CRP
C-reactive protein
- CVD
cardiovascular disease
- FMD
flow-mediated dilation
- FMDBA
brachial artery flow-mediated dilation
- FRS
Framingham risk score
- HOMA-IR
homeostasis model assessment of insulin resistance
- IL-6
interleukin-6
- LDL
low-density lipoprotein
- lnD
natural log–transformed diameter
- NO
nitric oxide
- NTG
nitroglycerin
- qADAM
quantitative Androgen Deficiency in the Aging Male
- RHI
reactive hyperemia index
- ROS
reactive oxygen species
- SBP
systolic blood pressure
- SHBG
sex hormone binding globulin
- TAS
total antioxidant status
- VO2
oxygen uptake
Additional Information
Disclosures: The authors have nothing to disclose.
Data Availability
Datasets generated and analyzed during the current study are not publicly available but are available from the corresponding author upon reasonable request.
References
- 1. Matsumoto AM. Andropause: clinical implications of the decline in serum testosterone levels with aging in men. J Gerontol A Biol Sci Med Sci. 2002;57(2):M76-M99. [DOI] [PubMed] [Google Scholar]
- 2. Harman SM, Metter EJ, Tobin JD, Pearson J, Blackman MR. Longitudinal effects of aging on serum total and free testosterone levels in healthy men. J Clin Endocrinol Metab. 2001;86(2):724-731. [DOI] [PubMed] [Google Scholar]
- 3. Shores MM, Smith NL, Forsberg CW, Anawalt BD, Matsumoto AM. Testosterone treatment and mortality in men with low testosterone levels. J Clin Endocrinol Metab. 2012;97(6):2050-2058. [DOI] [PubMed] [Google Scholar]
- 4. Shores MM, Biggs ML, Arnold AM, et al. Testosterone, dihydrotestosterone, and incident cardiovascular disease and mortality in the cardiovascular health study. J Clin Endocrinol Metab. 2014;99(6):2061-2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Khaw KT, Dowsett M, Folkerd E, et al. Endogenous testosterone and mortality due to all causes, cardiovascular disease, and cancer in men: European prospective investigation into cancer in Norfolk (EPIC-Norfolk) Prospective Population Study. Circulation. 2007;116(23):2694-2701. [DOI] [PubMed] [Google Scholar]
- 6. Hyde Z, Norman PE, Flicker L, et al. Low free testosterone predicts mortality from cardiovascular disease but not other causes: the Health in Men Study. J Clin Endocrinol Metab. 2012;97(1):179-189. [DOI] [PubMed] [Google Scholar]
- 7. Corona G, Rastrelli G, Monami M, et al. Hypogonadism as a risk factor for cardiovascular mortality in men: a meta-analytic study. Eur J Endocrinol. 2011;165(5):687-701. [DOI] [PubMed] [Google Scholar]
- 8. Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: part II: the aging heart in health: links to heart disease. Circulation. 2003;107(2):346-354. [DOI] [PubMed] [Google Scholar]
- 9. Ahlbom E, Prins GS, Ceccatelli S. Testosterone protects cerebellar granule cells from oxidative stress-induced cell death through a receptor mediated mechanism. Brain Res. 2001;892(2):255-262. [DOI] [PubMed] [Google Scholar]
- 10. Mancini A, Leone E, Festa R, et al. Effects of testosterone on antioxidant systems in male secondary hypogonadism. J Androl. 2008;29(6):622-629. [DOI] [PubMed] [Google Scholar]
- 11. Mohamad NV, Wong SK, Wan Hasan WN, et al. The relationship between circulating testosterone and inflammatory cytokines in men. Aging Male. 2019;22(2):129-140. [DOI] [PubMed] [Google Scholar]
- 12. Maggio M, Basaria S, Ceda GP, et al. The relationship between testosterone and molecular markers of inflammation in older men. J Endocrinol Invest. 2005;28(11 Suppl Proceedings):116-119. [PubMed] [Google Scholar]
- 13. Empen K, Lorbeer R, Dörr M, et al. Association of testosterone levels with endothelial function in men: results from a population-based study. Arterioscler Thromb Vasc Biol. 2012;32(2):481-486. [DOI] [PubMed] [Google Scholar]
- 14. Yilmaz MI, Sonmez A, Qureshi AR, et al. Endogenous testosterone, endothelial dysfunction, and cardiovascular events in men with nondialysis chronic kidney disease. Clin J Am Soc Nephrol. 2011;6(7):1617-1625. [DOI] [PubMed] [Google Scholar]
- 15. Akishita M, Hashimoto M, Ohike Y, et al. Low testosterone level is an independent determinant of endothelial dysfunction in men. Hypertens Res. 2007;30(11):1029-1034. [DOI] [PubMed] [Google Scholar]
- 16. Akishita M, Hashimoto M, Ohike Y, et al. Low testosterone level as a predictor of cardiovascular events in Japanese men with coronary risk factors. Atherosclerosis. 2010;210(1):232-236. [DOI] [PubMed] [Google Scholar]
- 17. Hildreth KL, Ozemek C, Kohrt WM, Blatchford PJ, Moreau KL. Vascular dysfunction across the stages of the menopausal transition is associated with menopausal symptoms and quality of life. Menopause. 2018;25(9):1011-1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. D’Agostino RB Sr, Vasan RS, Pencina MJ, et al. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008;117(6):743-753. [DOI] [PubMed] [Google Scholar]
- 19. Babcock MC, DuBose LE, Witten TL, et al. Assessment of macrovascular and microvascular function in aging males. J Appl Physiol (1985). 2021;130(1):96-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shapiro HM, Stromberg DD, Lee DR, Wiederhielm CA. Dynamic pressures in the pial arterial microcirculation. Am J Physiol. 1971;221(1):279-283. [DOI] [PubMed] [Google Scholar]
- 21. Moreau KL, Hildreth KL, Meditz AL, Deane KD, Kohrt WM. Endothelial function is impaired across the stages of the menopause transition in healthy women. J Clin Endocrinol Metab. 2012;97(12):4692-4700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Moreau KL, Meditz A, Deane KD, Kohrt WM. Tetrahydrobiopterin improves endothelial function and decreases arterial stiffness in estrogen-deficient postmenopausal women. Am J Physiol Heart Circ Physiol. 2012;302(5):H1211-H1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Doshi SN, Naka KK, Payne N, et al. Flow-mediated dilatation following wrist and upper arm occlusion in humans: the contribution of nitric oxide. Clin Sci (Lond). 2001;101(6):629-635. [PubMed] [Google Scholar]
- 24. Thijssen DH, Black MA, Pyke KE, et al. Assessment of flow-mediated dilation in humans: a methodological and physiological guideline. Am J Physiol Heart Circ Physiol. 2011;300(1):H2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Moreau KL, Deane KD, Meditz AL, Kohrt WM. Tumor necrosis factor-α inhibition improves endothelial function and decreases arterial stiffness in estrogen-deficient postmenopausal women. Atherosclerosis. 2013;230(2):390-396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. de Montgolfier O, Pinçon A, Pouliot P, et al. High systolic blood pressure induces cerebral microvascular endothelial dysfunction, neurovascular unit damage, and cognitive decline in mice. Hypertension. 2019;73(1):217-228. [DOI] [PubMed] [Google Scholar]
- 27. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412-419. [DOI] [PubMed] [Google Scholar]
- 28. Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18(6):499-502. [PubMed] [Google Scholar]
- 29. Vermeulen A, Verdonck L, Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab. 1999;84(10):3666-3672. [DOI] [PubMed] [Google Scholar]
- 30. Moreau KL, DePaulis AR, Gavin KM, Seals DR. Oxidative stress contributes to chronic leg vasoconstriction in estrogen-deficient postmenopausal women. J Appl Physiol (1985). 2007;102(3):890-895. [DOI] [PubMed] [Google Scholar]
- 31. Moreau KL, Gavin KM, Plum AE, Seals DR. Oxidative stress explains differences in large elastic artery compliance between sedentary and habitually exercising postmenopausal women. Menopause. 2006;13(6):951-958. [DOI] [PubMed] [Google Scholar]
- 32. Moreau KL, Gavin KM, Plum AE, Seals DR. Ascorbic acid selectively improves large elastic artery compliance in postmenopausal women. Hypertension. 2005;45(6):1107-1112. [DOI] [PubMed] [Google Scholar]
- 33. Hildreth KL, Kohrt WM, Moreau KL. Oxidative stress contributes to large elastic arterial stiffening across the stages of the menopausal transition. Menopause. 2014;21(6):624-632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Jackson TS, Xu A, Vita JA, Keaney JF Jr. Ascorbate prevents the interaction of superoxide and nitric oxide only at very high physiological concentrations. Circ Res. 1998;83(9):916-922. [DOI] [PubMed] [Google Scholar]
- 35. Ozemek C, Hildreth KL, Groves DW, Moreau KL. Acute ascorbic acid infusion increases left ventricular diastolic function in postmenopausal women. Maturitas. 2016;92:154-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Moreau KL, Stauffer BL, Kohrt WM, Seals DR. Essential role of estrogen for improvements in vascular endothelial function with endurance exercise in postmenopausal women. J Clin Endocrinol Metab. 2013;98(11):4507-4515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mohamed O, Freundlich RE, Dakik HK, et al. The quantitative ADAM questionnaire: a new tool in quantifying the severity of hypogonadism. Int J Impot Res. 2010;22(1):20-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Heinemann LA, Zimmermann T, Vermeulen A, Thiel C, Hummel W. A new ‘aging males’ symptoms' rating scale. Aging Male. 1999;2(2):105-114. [Google Scholar]
- 39. Atkinson G, Batterham AM. Allometric scaling of diameter change in the original flow-mediated dilation protocol. Atherosclerosis. 2013;226(2):425-427. [DOI] [PubMed] [Google Scholar]
- 40. Lindsey ML, Gray GA, Wood SK, Curran-Everett D. Statistical considerations in reporting cardiovascular research. Am J Physiol Heart Circ Physiol. 2018;315(2):H303-H313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Deanfield JE, Halcox JP, Rabelink TJ. Endothelial function and dysfunction: testing and clinical relevance. Circulation. 2007;115(10):1285-1295. [DOI] [PubMed] [Google Scholar]
- 42. Davignon J, Ganz P. Role of endothelial dysfunction in atherosclerosis. Circulation. 2004;109(23 Suppl 1):III27-III32. [DOI] [PubMed] [Google Scholar]
- 43. Pierce GL, Donato AJ, LaRocca TJ, Eskurza I, Silver AE, Seals DR. Habitually exercising older men do not demonstrate age-associated vascular endothelial oxidative stress. Aging Cell. 2011;10(6):1032-1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Eskurza I, Monahan KD, Robinson JA, Seals DR. Effect of acute and chronic ascorbic acid on flow-mediated dilatation with sedentary and physically active human ageing. J Physiol. 2004;556(Pt 1):315-324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Joannides R, Haefeli WE, Linder L, et al. Nitric oxide is responsible for flow-dependent dilatation of human peripheral conduit arteries in vivo. Circulation. 1995;91(5):1314-1319. [DOI] [PubMed] [Google Scholar]
- 46. Corrigan FE 3rd, Al Mheid I, Eapen DJ, et al. Low testosterone in men predicts impaired arterial elasticity and microvascular function. Int J Cardiol. 2015;194:94-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Inaba Y, Chen JA, Bergmann SR. Prediction of future cardiovascular outcomes by flow-mediated vasodilatation of brachial artery: a meta-analysis. Int J Cardiovasc Imaging. 2010;26(6):631-640. [DOI] [PubMed] [Google Scholar]
- 48. Green DJ, Jones H, Thijssen D, Cable NT, Atkinson G. Flow-mediated dilation and cardiovascular event prediction: does nitric oxide matter? Hypertension. 2011;57(3):363-369. [DOI] [PubMed] [Google Scholar]
- 49. Hamburg NM, Palmisano J, Larson MG, et al. Relation of brachial and digital measures of vascular function in the community: the Framingham heart study. Hypertension. 2011;57(3):390-396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Dhindsa M, Sommerlad SM, DeVan AE, et al. Interrelationships among noninvasive measures of postischemic macro- and microvascular reactivity. J Appl Physiol (1985). 2008;105(2):427-432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kuvin JT, Patel AR, Sliney KA, et al. Assessment of peripheral vascular endothelial function with finger arterial pulse wave amplitude. Am Heart J. 2003;146(1):168-174. [DOI] [PubMed] [Google Scholar]
- 52. Black MA, Cable NT, Thijssen DH, Green DJ. Importance of measuring the time course of flow-mediated dilatation in humans. Hypertension. 2008;51(2):203-210. [DOI] [PubMed] [Google Scholar]
- 53. Gates PE, Boucher ML, Silver AE, Monahan KD, Seals DR. Impaired flow-mediated dilation with age is not explained by L-arginine bioavailability or endothelial asymmetric dimethylarginine protein expression. J Appl Physiol (1985). 2007;102(1):63-71. [DOI] [PubMed] [Google Scholar]
- 54. Eskurza I, Kahn ZD, Seals DR. Xanthine oxidase does not contribute to impaired peripheral conduit artery endothelium-dependent dilatation with ageing. J Physiol. 2006;571(Pt 3):661-668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Rossman MJ, Santos-Parker JR, Steward CAC, et al. Chronic supplementation with a mitochondrial antioxidant (MitoQ) improves vascular function in healthy older adults. Hypertension. 2018;71(6):1056-1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Karakitsos D, Patrianakos AP, De Groot E, et al. Androgen deficiency and endothelial dysfunction in men with end-stage kidney disease receiving maintenance hemodialysis. Am J Nephrol. 2006;26(6):536-543. [DOI] [PubMed] [Google Scholar]
- 57. Donato AJ, Eskurza I, Silver AE, et al. Direct evidence of endothelial oxidative stress with aging in humans: relation to impaired endothelium-dependent dilation and upregulation of nuclear factor-kappaB. Circ Res. 2007;100(11):1659-1666. [DOI] [PubMed] [Google Scholar]
- 58. Taddei S, Virdis A, Ghiadoni L, et al. Age-related reduction of NO availability and oxidative stress in humans. Hypertension. 2001;38(2):274-279. [DOI] [PubMed] [Google Scholar]
- 59. Kłapcińska B, Jagsz S, Sadowska-Krepa E, Górski J, Kempa K, Langfort J. Effects of castration and testosterone replacement on the antioxidant defense system in rat left ventricle. J Physiol Sci. 2008;58(3):173-177. [DOI] [PubMed] [Google Scholar]
- 60. Kataoka T, Hotta Y, Maeda Y, Kimura K. Testosterone deficiency causes endothelial dysfunction via elevation of asymmetric dimethylarginine and oxidative stress in castrated rats. J Sex Med. 2017;14(12):1540-1548. [DOI] [PubMed] [Google Scholar]
- 61. Eleawa SM, Sakr HF, Hussein AM, Assiri AS, Bayoumy NM, Alkhateeb M. Effect of testosterone replacement therapy on cardiac performance and oxidative stress in orchidectomized rats. Acta Physiol (Oxf). 2013;209(2):136-147. [DOI] [PubMed] [Google Scholar]
- 62. Schulz E, Anter E, Keaney JF Jr. Oxidative stress, antioxidants, and endothelial function. Curr Med Chem. 2004;11(9):1093-1104. [DOI] [PubMed] [Google Scholar]
- 63. Khosla S, Atkinson EJ, Dunstan CR, O’Fallon WM. Effect of estrogen versus testosterone on circulating osteoprotegerin and other cytokine levels in normal elderly men. J Clin Endocrinol Metab. 2002;87(4):1550-1554. [DOI] [PubMed] [Google Scholar]
- 64. Vita JA, Keaney JF Jr, Larson MG, et al. Brachial artery vasodilator function and systemic inflammation in the Framingham Offspring Study. Circulation. 2004;110(23):3604-3609. [DOI] [PubMed] [Google Scholar]
- 65. Clayton ZS, Hutton DA, Brunt VE, et al. Apigenin restores endothelial function by ameliorating oxidative stress, reverses aortic stiffening, and mitigates vascular inflammation with aging. Am J Physiol Heart Circ Physiol. 2021;321(1):H185-H196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Sindler AL, Fleenor BS, Calvert JW, et al. Nitrite supplementation reverses vascular endothelial dysfunction and large elastic artery stiffness with aging. Aging Cell. 2011;10(3):429-437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Donato AJ, Black AD, Jablonski KL, Gano LB, Seals DR. Aging is associated with greater nuclear NF kappa B, reduced I kappa B alpha, and increased expression of proinflammatory cytokines in vascular endothelial cells of healthy humans. Aging Cell. 2008;7(6):805-812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Bhasin S, Brito JP, Cunningham GR, et al. Testosterone therapy in men with hypogonadism: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2018;103(5):1715-1744. [DOI] [PubMed] [Google Scholar]
- 69. Kähler J, Ewert A, Weckmüller J, et al. Oxidative stress increases endothelin-1 synthesis in human coronary artery smooth muscle cells. J Cardiovasc Pharmacol. 2001;38(1):49-57. [DOI] [PubMed] [Google Scholar]
- 70. Hao L, Nishimura T, Wo H, Fernandez-Patron C. Vascular responses to alpha1-adrenergic receptors in small rat mesenteric arteries depend on mitochondrial reactive oxygen species. Arterioscler Thromb Vasc Biol. 2006;26(4):819-825. [DOI] [PubMed] [Google Scholar]
- 71. Faizi AK, Kornmo DW, Agewall S. Evaluation of endothelial function using finger plethysmography. Clin Physiol Funct Imaging. 2009;29(5):372-375. [DOI] [PubMed] [Google Scholar]
- 72. Wenner MM, Taylor HS, Stachenfeld NS. Androgens influence microvascular dilation in PCOS through ET-A and ET-B receptors. Am J Physiol Endocrinol Metab. 2013;305(7):E818-E825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Tajar A, Huhtaniemi IT, O’Neill TW, et al. ; EMAS Group . Characteristics of androgen deficiency in late-onset hypogonadism: results from the European Male Aging Study (EMAS). J Clin Endocrinol Metab. 2012;97(5):1508-1516. [DOI] [PubMed] [Google Scholar]
- 74. Morgentaler A, Miner MM, Caliber M, Guay AT, Khera M, Traish AM. Testosterone therapy and cardiovascular risk: advances and controversies. Mayo Clin Proc. 2015;90(2):224-251. [DOI] [PubMed] [Google Scholar]
- 75. Moskovic DJ, Araujo AB, Lipshultz LI, Khera M. The 20-year public health impact and direct cost of testosterone deficiency in U.S. men. J Sex Med. 2013;10(2):562-569. [DOI] [PubMed] [Google Scholar]
- 76. Morgentaler A, Zitzmann M, Traish AM, et al. Fundamental concepts regarding testosterone deficiency and treatment: international expert consensus resolutions. Mayo Clin Proc. 2016;91(7):881-896. [DOI] [PubMed] [Google Scholar]
- 77. Vigen R, O’Donnell CI, Barón AE, et al. Association of testosterone therapy with mortality, myocardial infarction, and stroke in men with low testosterone levels. JAMA. 2013;310(17):1829-1836. [DOI] [PubMed] [Google Scholar]
- 78. Basaria S, Coviello AD, Travison TG, et al. Adverse events associated with testosterone administration. N Engl J Med. 2010;363(2):109-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Datasets generated and analyzed during the current study are not publicly available but are available from the corresponding author upon reasonable request.