Abstract
Context
The causal role of endogenous estradiol in cancers other than breast and endometrial cancer remains unclear.
Objective
This Mendelian randomization study assessed the causal associations of endogenous 17β-estradiol (E2), the most potent estrogen, with cancer risk in women.
Methods
As primary genetic instrument, we used a genetic variant in the CYP19A1 gene that is strongly associated with serum E2 levels. Summary statistics genetic data for the association of the E2 variant with breast, endometrial, and ovarian cancer were obtained from large-scale consortia. We additionally estimated the associations of the E2 variant with any and 20 site-specific cancers in 198 825 women of European descent in UK Biobank. Odds ratios (OR) of cancer per 0.01 unit increase in log-transformed serum E2 levels in pmol/L were estimated using the Wald ratio.
Results
Genetic predisposition to higher serum E2 levels was associated with increased risk of estrogen receptor (ER)-positive breast cancer (OR 1.02; 95% CI, 1.01-1.03; P = 2.5 × 10−3), endometrial cancer overall (OR 1.09; 95% CI, 1.06-1.11; P = 7.3 × 10−13), and endometrial cancer of the endometrioid histology subtype (OR 1.10; 95% CI, 1.07-1.13; P = 2.1 × 10−11). There were suggestive associations with breast cancer overall (OR 1.01; 95% CI, 1.00-1.02; P = 0.02), ovarian cancer of the endometrioid subtype (OR 1.05; 95% CI, 1.01-1.10; P = 0.02), and stomach cancer (OR 1.12; 95% CI, 1.00-1.26; P = 0.05), but no significant association with other cancers.
Conclusion
This study supports a role of E2 in the development of ER-positive breast cancer and endometrioid endometrial cancer but found no strong association with other cancers in women.
Keywords: cancer, estrogens, estradiol, Mendelian randomization
Estrogens are a class of steroid hormones with a fundamental role in a wide range of physiological processes, such as menstrual cycle regulation, reproduction, preservation of bone density, and modulation of brain function (1). 17β-estradiol (E2) is the most potent estrogen and has pro-oncogenic effects through increased cell proliferation and decreased apoptosis, mediated primarily by activation of the estrogen receptor (ER) alpha (1). Factors associated with higher lifetime estrogen exposure, such as early menarche, late menopause, and menopausal hormone therapy, are linked to increased risk of cancers of the breast (particularly ER-positive tumors) (2-6), endometrium (7, 8), and ovaries (particularly the endometrioid subtype) (9-11), whereas oral contraceptive use is linked to lower risk of endometrial and ovarian cancer (12). Nevertheless, whether estrogens specifically are largely responsible for the observed associations is not known as reproductive years are also associated with number of ovulations, and hormone therapy may be associated with confounding factors. Furthermore, the increased risk of breast cancer among women taking menopausal hormone therapy is mainly confined to estrogen-progesterone preparations (13), whereas estrogen-only preparations have weak (4) or no (13) association with risk of breast cancer. Although there is ample data on the associations of indirect measures of estrogen exposure and hormone therapy with risk of breast, endometrial, and ovarian cancer, studies on the causal role of endogenous estrogen levels for other cancers are scarce.
Mendelian randomization (MR) is a technique to provide evidence on causal relationships by exploiting genetic variants having a robust association with the exposure as instruments to predict the effect of the exposure on disease risk (14, 15). The advantage of an MR study over conventional observational studies is that confounding is diminished because genetic variants are randomly allocated at conception and thus normally not associated with environmental factors and self-selected behaviors. In addition, reverse causation is avoided because genes cannot be altered by disease status. Here, a 2-sample MR approach was applied to assess the potential causal associations of endogenous E2 levels with any and 20 site-specific cancers in women.
Methods
Genetic Instruments
As the primary genetic instrument for serum E2, we used the single-nucleotide polymorphism (SNP) rs727479 in CYP19A1, which encodes aromatase, an enzyme that converts androgens to estrogens. Aromatase is expressed in the gonads, placenta, adipose tissue, brain, and other tissues. Rs727479 and an SNP in complete linkage disequilibrium with this genetic variant in the CYP19A1 gene (rs7173595) have previously been shown to be strongly associated with serum E2 levels in genome-wide association studies (GWAS) of postmenopausal women (16) and men (17, 18). This SNP was also associated with serum E2 in 25 502 premenopausal European women (<50 years of age and not reporting a hysterectomy or that menopause has occurred) in UK Biobank. We constructed a secondary genetic instrument for serum E2 that consisted of SNPs previously identified to be associated with this hormone in 206 927 men of European ancestry in the UK Biobank (18) and which were also associated with serum E2 at P < 0.05 in 25 502 premenopausal European women in the same cohort. Five SNPs met the criteria for the secondary genetic instrument. Table 1 shows the characteristics of the SNPs used for the primary and secondary genetic instruments for serum E2.
Table 1.
Single-nucleotide polymorphisms used as instrumental variables for serum E2 levels in the primary and secondary genetic instrument
| Association with E2 in mena | Association with E2 in premenopausal womenb | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Instrument | SNP | Chr | Gene | EA | OA | Beta | SE | P value | Beta | SE | P value |
| Primary | rs727479 | 15 | CYP19A1 | A | C | 1.390 | 0.120 | 8.2 × 10-30 | 0.014 | 0.006 | 0.011 |
| Secondary | rs1260326 | 2 | GCKR | C | T | 0.006 | 0.001 | 9.6 × 10-11 | 0.012 | 0.006 | 0.036 |
| Secondary | rs45446698 | 7 | CYP3A7 | T | G | 0.016 | 0.002 | 7.9 × 10-14 | 0.032 | 0.014 | 0.020 |
| Secondary | rs34019140 | 14 | ADAM6 | G | A | 0.012 | 0.001 | 6.9 × 10-42 | 0.011 | 0.006 | 0.043 |
| Secondary | rs7173595c | 15 | CYP19A1 | T | C | 0.016 | 0.001 | 3.6 × 10-72 | 0.014 | 0.006 | 0.012 |
| Secondary | rs727428 | 17 | SHBG | C | T | 0.006 | 0.001 | 1.8 × 10-11 | 0.025 | 0.005 | <0.001 |
Abbreviations: Chr, chromosome; E2, 17β-estradiol; EA, effect allele (ie, the allele associated with higher serum E2 levels); OA, other allele; SE, standard error; SNP, single-nucleotide polymorphism.
aEffects estimates (beta coefficients and standard errors) represent the change in serum E2 in pg/mL from the genome-wide association study by Eriksson et al (17) (primary instrument) and the change in log-transformed E2 in pmol/L from the genome-wide association study by Ruth et al (18) (secondary instrument) per additional effect allele.
bEffects estimates (beta coefficients and standard errors) represent the change in serum E2 in log-transformed pmol/L per additional effect allele in premenopausal women in UK Biobank.
cIn complete linkage disequilibrium with rs727479 (CYP19A1).
Data Sources for Cancer
We obtained summary statistics GWAS data for breast, endometrial, and ovarian cancer from the Breast Cancer Association Consortium (19), a meta-GWAS of endometrial cancer (including data from the Endometrial Cancer Association Consortium, the Epidemiology of Endometrial Cancer Consortium, and UK Biobank) (20), and the Ovarian Cancer Association Consortium (21), respectively. Data from these consortia were extracted from the MR-Base platform (22).
We additionally estimated the associations of the E2-associated SNPs with any and 20 site-specific cancers in 198 825 unrelated women (37 to 73 years of age at the baseline assessment) of European descents in the UK Biobank cohort using logistic regression with adjustment for age and 10 genetic principal components, as described previously (23). Information on cancer outcomes was obtained from the national cancer registry, hospital episode statistics and death certification data, electronic health records, and self-reported information verified by interview with a nurse (Table 2). All analyses were restricted to pre- and postmenopausal women of European ancestry to minimize bias from population stratification.
Table 2.
Definitions of site-specific cancer outcomes in the UK Biobank cohort
| Cancer | ICD-9 codes | ICD-10 codes | Self-report (field 20001) | Cancer histology |
|---|---|---|---|---|
| Breast & gynecological cancers | ||||
| Breast cancer | 174, 175, V10.3 | C50, Z85.3 | 1002 | |
| Endometrial/uterine cancer | 179, 182, V10.42, | C54, C55, Z85.42 | 1040 | |
| Cervical cancer | 180, V10.41 | C53, Z85.41 | 1041 | |
| Ovarian cancer | 183.0, 183.2, 183.8, 183.9, V10.43 | C56, C57.0, C57.4, Z85.43 | 1039 | |
| Blood cancers | ||||
| Non-Hodgkin lymphoma | 200, 202.0, 202.1, 202.2, 202.7, V10.71 | C82, C83, C84, C85, C86, C88.0, C88.4, Z85.72 | 1053 | |
| Leukemia | 204, 205, 206, 207, 208, V10.6 | C91, C92, C93, C94.0, C94.2, C94.3, C94.4, C94.8, C95, Z85.6 | 1048, 1055, 1056, 1074 | |
| Multiple myeloma | 203.0, 203.1 | C90.0, C90.1 | 1050 | 9732, 9733 |
| Digestive system cancers | ||||
| Colorectal cancer | 153, 154.0, 154.1, V10.05, V10.06 | C18, C19, C20, Z85.038, Z85.048 | 1020, 1022, 1023 | |
| Pancreatic cancer | 157 | C25, Z85.07 | 1034 | |
| Esophageal cancer | 150, V10.03 | C15, Z85.01 | 1017 | |
| Stomach cancer | 151, V10.04 | C16, Z85.028 | 1018 | |
| Biliary tract cancer | 155.1, 156.0 | C22.1, C23, C24 | 1025 | |
| Liver cancer | 155.0 | C22.0 | 1024 | 8170, 8171, 8172, 8173, 8174, 8175 |
| Urinary tract cancers | ||||
| Bladder cancer | 188, 189.1, 189.2, V10.51, V10.53 | C67, C65, C66, Z85.51, Z85.54, Z85.53 | 1035 | |
| Kidney cancer | 189.0, V10.52 | C64, Z85.528 | 1034 | |
| Other cancers | ||||
| Melanoma | 172, V10.82 | C43, Z85.820 | 1059 | |
| Lung cancer | 162, V10.1 | C33, C34, C39.9, Z85.1 | 1001, 1027, 1028, 1080 | |
| Head and neck cancer | 140, 141, 142, 143, 144, 145, 146, 147, 148, 149, 160, 161, V10.01, V10.02, V10.21, V10.22 | C00, C01, C02, C03, C04, C05, C06, C07, C08, C09, C10, C11, C12, C13, C14, C30, C31, C32, Z85.21, Z85.22, Z85.81 | 1006, 1007, 1009, 1004, 1010, 1011, 1012, 1077, 1078, 1079, 1005, 1015, 1016 | |
| Brain cancer | 191, 192.0, 192.1, 192.2, 192.3, V10.85 | C70, C71, C72.0, C72.3, Z85.841 | 1031, 1032, 1033 | |
| Thyroid cancer | 193, V10.87 | C73, Z85.850 | 1065 |
The Self-report and Cancer histology columns provide the internal UK Biobank codes used to define each outcome (available at https://biobank.ctsu.ox.ac.uk/crystal/coding.cgi?id=3 and https://biobank.ctsu.ox.ac.uk/crystal/coding.cgi?id=38).
Abbreviations: ICD, international classification of diseases.
All studies have been approved by a relevant ethical review board, and participants have provided informed consent. The MR analyses were approved by the Swedish Ethical Review Authority.
Statistical Analysis
The associations of serum E2 instrumented by rs727479 in the CYP19A1 gene region with the cancer outcomes were estimated using the Wald ratio method. For the MR analyses of serum E2 instrumented by 5 SNPs, 3 MR methods with different assumptions were applied. These included the multiplicative random-effects inverse variance weighted, weighted median, and MR-Egger methods (24). Effect estimates (beta coefficients and standard errors) for the SNP-E2 associations were obtained from UK Biobank (Table 1). All reported odds ratios (OR) of cancer were scaled per 0.01 unit increase in log-transformed serum E2 levels in pmol/L. Results were deemed statistically significant at the Bonferroni-corrected threshold of P < 0.0025 (P = 0.05/20 site-specific cancers). Associations with a P value between 0.0025 and 0.05 were regarded suggestive. The MR-Base platform (22) and Stata (StataCorp, College Station, Texas) were used for the MR analyses based on data from consortia and UK Biobank, respectively.
Pleiotropy Assessment
The MR-Base platform (22) and PhenoScanner database V2 (25) were utilized to assess pleiotropic associations of the E2-related SNPs with other phenotypes, including potential confounders and mediators (ie, other sex hormones, reproductive factors, body mass index, and smoking).
Results
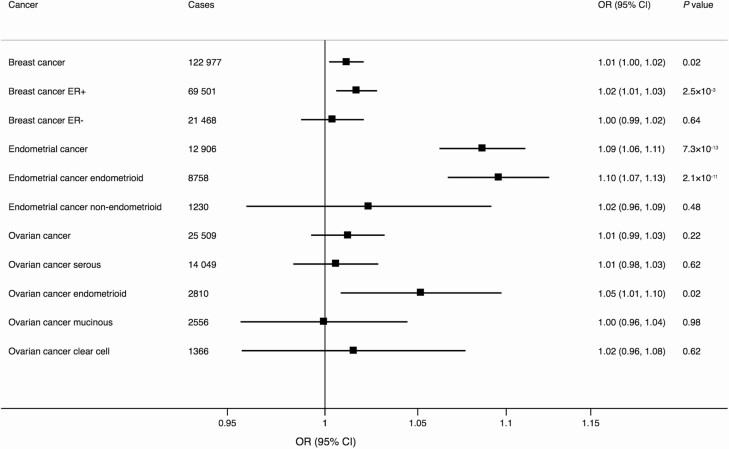
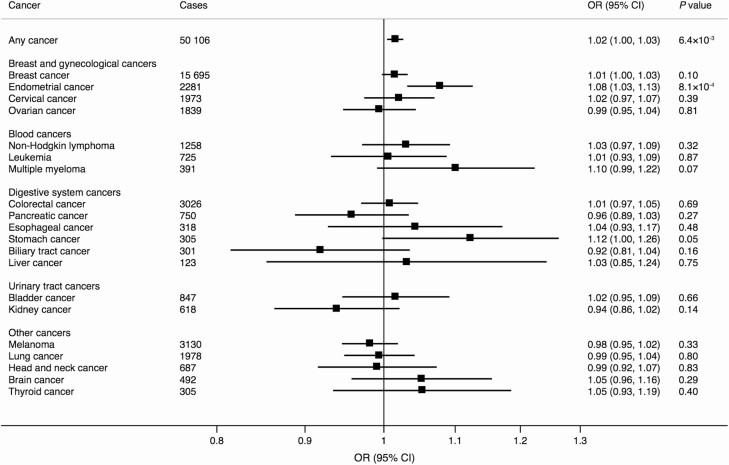
In the analyses based on data from the genetic consortia, genetic predisposition to higher serum E2 levels proxied by rs727479 in the CYP19A1 gene was associated with increased risk of ER-positive breast cancer (OR 1.02; 95% CI, 1.01-1.03; P = 2.5 × 10−3) as well as with endometrial cancer overall (OR 1.09; 95% CI, 1.06-1.11; P = 7.3 × 10−13) and the endometrioid histology subtype (OR 1.10; 95% CI, 1.07-1.13; P = 2.1 × 10−11) (Fig. 1). There were suggestive associations with breast cancer overall (OR 1.01; 95% CI, 1.00-1.02; P = 0.02) and ovarian cancer of the endometrioid subtype (OR 1.05; 95% CI, 1.01-1.10; P = 0.02) (Fig. 1). In UK Biobank, genetic predisposition to higher serum E2 levels was associated with increased risk of any cancer and endometrial cancer, but the association with any cancer did not survive the Bonferroni-corrected significance level (Fig. 2). There was also a suggestive association with stomach cancer (OR 1.12; 95% CI, 1.00-1.26; P = 0.05) but no association with the other site-specific cancers (Fig. 2).
Figure 1.
Associations of serum E2 levels instrumented by rs727479 in the CYP19A1 gene region with breast, endometrial, and ovarian cancer and their subtypes based on data from consortia. The odds ratios are scaled per 0.01 unit increase in log-transformed serum E2 levels in pmol/L. The number of controls is 108 979 in the endometrial cancer meta-GWAS, 105 974 in the Breast Cancer Association Consortium, and 40 941 in the Ovarian Cancer Association Consortium. Abbreviations: E2, 17β-estradiol; ER+, estrogen receptor positive; ER-, estrogen receptor negative; OR, odds ratio.
Figure 2.
Associations of serum E2 levels instrumented by rs727479 in the CYP19A1 gene region with any and 20 site-specific cancers in the UK Biobank cohort. The odds ratios are scaled per 0.01 unit increase in log-transformed serum E2 levels in pmol/L. Abbreviations: E2, 17β-estradiol; OR, odds ratio.
MR analyses of serum E2 instrumented by 5 SNPs showed no significant association with breast, endometrial, or ovarian cancer and their subtypes based on consortia data and 3 different MR methods (all P values > 0.05). Given the lack of association of this 5-SNP instrument with the positive control outcomes ER-positive breast cancer and endometrial cancer, we did not proceed with the corresponding analyses for 20 site-specific cancers using UK Biobank data, as these variants did not seem to be valid instruments for E2.
All SNPs but the ADAM6 variant were associated with serum testosterone in men and women combined in UK Biobank. The CYP19A1 and GCKR variants were further associated with fasting insulin, and the GCKR variant associated with body mass index. The CYP3A7 variant was additionally associated with dehydroepiandrosterone sulfate, whereas the SHBG variant had further associations with dihydrotestosterone and body fat percentage.
Discussion
This is the first MR investigation of the potential causal role of endogenous E2 levels for any cancer and a broad range of site-specific cancers. Our findings based on a genetic variant in the CYP19A1 gene provide support that elevated serum E2 levels are causally linked to higher risk of ER-positive breast cancer and endometrial cancer, particularly of the endometrioid histology, suggesting a role of E2 in hormone-sensitive cancers. We found suggestive evidence that higher serum E2 levels may increase the risk of endometrioid ovarian cancer and stomach cancer. Serum E2 levels were not significantly associated with any other site-specific cancer but showed a suggestive positive association with risk of any cancer.
Our findings based on the CYP19A1 variant corroborate the results of a pooled analysis of 4998 endometrial cancer cases and 8285 controls from 10 studies in the Epidemiology of Endometrial Cancer Consortium (26) as well as a study based on 6608 endometrial cancer cases and 37 925 controls from 4 studies (16). In those studies, each additional rs727479 A allele was associated with an 8% (26) and 15% (16) higher odds of endometrial cancer. Rs727479 and a correlated SNP (rs749292) in the CYP19A1 gene region have also been reported to be associated with an increased risk of ovarian cancer in a small case-control study (367 cases and 602 controls) from Hawaii (27). Although serum E2 was not associated with ovarian cancer overall in the present analysis, our results suggested a positive association between serum E2 and the endometrioid subtype of ovarian cancer. CYP19A1 gene polymorphisms, including rs727479 and rs3764221, have also been associated with an increased risk of lung cancer in small case-control studies (28, 29). We found no support for a positive association between serum E2 and lung cancer in our MR study. Data on E2-raising gene polymorphisms in relation to other cancers are scarce.
In the Women’s Health Initiative trial, there was suggestive evidence that estrogen plus progestin treatment might reduce the risk of colorectal cancer (6). The present MR study did not support an association between genetically predicted serum E2 and colorectal cancer risk in women. Whether estrogens or progesterone play a role in the prevention of colorectal cancer merits further study.
Our finding of a suggestive association between the CYP19A1 gene variant and stomach cancer contrasts with observational studies which have shown that menopausal hormone therapy is associated with a lower risk of stomach cancer (30, 31). Furthermore, a nationwide cohort study of men with a diagnosis of prostate cancer found evidence of a reduced risk of stomach cancer in a male cohort exposed to estrogen (32). Given these inconsistent results and the weak evidence for causation in this investigation, it is possible that the suggestive association observed represents a chance finding.
The principal advantage of this study is the MR design, which reduced potential bias from confounders and reverse causality. Another important strength is that we evaluated the associations between serum E2 levels and a broad range of cancers of which most cancers have not previously been examined in relation to genetically predicted serum E2 levels. A limitation is that our analyses merely included women of European ancestry, thereby restricting the generalizability of our results to other populations. Another shortcoming is that the precision was low in the analyses of cancers with a limited number of cases (fewer than 1000 cases) and therefore we may have overlooked weak associations. Finally, higher genetically predicted E2 levels were associated with lower serum testosterone. Given that genetically predicted serum testosterone is positively associated with breast and endometrial cancer risk (18), the risk estimates for the associations between genetically predicted serum E2 and these cancers may be attenuated. Other serum E2-associated SNPs used in the secondary instrument were also associated with serum testosterone as well as with dehydroepiandrosterone sulfate, dihydrotestosterone, or body mass index. Thus, the lack of associations of serum E2 proxied by the secondary genetic instrument consisting of 5 genetic variants may be related to pleiotropy.
In conclusion, these MR findings support a causal role of endogenous E2 levels in ER-positive breast cancer and endometrioid endometrial cancer. Nevertheless, we found no evidence of a strong association of endogenous E2 levels with a broad range of other site-specific cancers in women.
Acknowledgments
The authors thank the participants and investigators of the UK Biobank and investigators of all studies that contributed data to the Breast Cancer Association Consortium, the Endometrial Cancer Association Consortium, the Epidemiology of Endometrial Cancer Consortium, and the Ovarian Cancer Association Consortium. Analyses of UK Biobank data were conducted under application 29202. Data from the UK Biobank resource are accessible upon application (https://www.ukbiobank.ac.uk/).
Financial Support: S.C.L. is supported by research grants from the Swedish Research Council (Vetenskapsrådet 2019-00977), the Swedish Research Council for Health, Working Life and Welfare (Forte, 2018-00123), and the Swedish Heart-Lung Foundation (Hjärt-Lungfonden, 20190247). S.K. is supported by United Kingdom Research and Innovation Future Leaders Fellowship (MR/T043202/1). S.B. is supported by Sir Henry Dale Fellowship jointly funded by the Wellcome Trust and the Royal Society (204623/Z/16/Z).
Author Contributions: S.C.L. contributed to the conception and design of the study, the data analyses, interpretation of the results, and drafting of the manuscript. S.B. contributed to the conception and design of the study, the data analyses, interpretation of the results and critical revision of the manuscript. All authors contributed to the interpretation of the results and critical revision of the manuscript.
Glossary
Abbreviations
- E2
17β-estradiol
- ER
estrogen receptor
- GWAS
genome-wide association studies
- MR
Mendelian randomization
- OR
odds ratio
- SNP
single-nucleotide polymorphism
Additional Information
Disclosures: The authors have no conflict of interest to declare.
Data Availability
Some or all datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References
- 1. Liang J, Shang Y. Estrogen and cancer. Annu Rev Physiol. 2013;75:225-240. [DOI] [PubMed] [Google Scholar]
- 2. Burgess S, Thompson DJ, Rees JMB, Day FR, Perry JR, Ong KK. Dissecting causal pathways using Mendelian randomization with summarized genetic data: application to age at menarche and risk of breast cancer. Genetics. 2017;207(2):481-487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Collaborative Group on Hormonal Factors in Breast Cancer. Menarche, menopause, and breast cancer risk: Individual participant meta-analysis, including 118 964 women with breast cancer from 117 epidemiological studies. Lancet Oncol. 2012;13:1141-1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Collaborative Group on Hormonal Factors in Breast Cancer. Type and timing of menopausal hormone therapy and breast cancer risk: individual participant meta-analysis of the worldwide epidemiological evidence. Lancet. 2019;394:1159-1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Endogenous Hormones and Breast Cancer Collaborative Group, Key TJ, Appleby PN, et al. Sex hormones and risk of breast cancer in premenopausal women: a collaborative reanalysis of individual participant data from seven prospective studies. Lancet Oncol. 2013;14:1009-1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rossouw JE, Anderson GL, Prentice RL, et al. ; Writing Group for the Women’s Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the women’s health initiative randomized controlled trial. JAMA. 2002;288(3):321-333. [DOI] [PubMed] [Google Scholar]
- 7. Raglan O, Kalliala I, Markozannes G, et al. Risk factors for endometrial cancer: an umbrella review of the literature. Int J Cancer. 2019;145(7):1719-1730. [DOI] [PubMed] [Google Scholar]
- 8. Felix AS, Yang HP, Bell DW, Sherman ME. Epidemiology of endometrial carcinoma: etiologic importance of hormonal and metabolic influences. Adv Exp Med Biol. 2017;943:3-46. [DOI] [PubMed] [Google Scholar]
- 9. Yang H, Dai H, Li L, et al. Age at menarche and epithelial ovarian cancer risk: a meta-analysis and Mendelian randomization study. Cancer Med. 2019;8(8):4012-4022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yarmolinsky J, Relton CL, Lophatananon A, et al. Appraising the role of previously reported risk factors in epithelial ovarian cancer risk: a Mendelian randomization analysis. PLoS Med. 2019;16(8):e1002893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Collaborative Group on Epidemiological Studies of Ovarian Cancer, Beral V, Gaitskell K, et al. Menopausal hormone use and ovarian cancer risk: individual participant meta-analysis of 52 epidemiological studies. Lancet. 2015;385:1835-1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Karlsson T, Johansson T, Höglund J, Ek WE, Johansson Å. Time-dependent effects of oral contraceptive use on breast, ovarian, and endometrial cancers. Cancer Res. 2021;81(4):1153-1162. [DOI] [PubMed] [Google Scholar]
- 13. Chlebowski RT, Rohan TE, Manson JE, et al. Breast cancer after use of estrogen plus progestin and estrogen alone: analyses of data from 2 women’s health initiative randomized clinical trials. JAMA Oncol. 2015;1(3):296-305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Davies NM, Holmes MV, Davey Smith G. Reading Mendelian randomisation studies: a guide, glossary, and checklist for clinicians. BMJ. 2018;362:k601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Burgess S, Thompson SG.. Mendelian Randomization: Methods for Using Genetic Variants in Causal Estimation. Chapman and Hall: CRC Press; 2015. [Google Scholar]
- 16. Thompson DJ, O’Mara TA, Glubb DM, et al. ; Australian National Endometrial Cancer Study Group (ANECS); National Study of Endometrial Cancer Genetics Group (NSECG); for RENDOCAS; AOCS Group. CYP19A1 fine-mapping and Mendelian randomization: estradiol is causal for endometrial cancer. Endocr Relat Cancer. 2016;23(2):77-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Eriksson AL, Perry JRB, Coviello AD, et al. Genetic determinants of circulating estrogen levels and evidence of a causal effect of estradiol on bone density in men. J Clin Endocrinol Metab. 2018;103(3):991-1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ruth KS, Day FR, Tyrrell J, et al. ; Endometrial Cancer Association Consortium. Using human genetics to understand the disease impacts of testosterone in men and women. Nat Med. 2020;26(2):252-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Michailidou K, Lindström S, Dennis J, et al. ; NBCS Collaborators; ABCTB Investigators; ConFab/AOCS Investigators. Association analysis identifies 65 new breast cancer risk loci. Nature. 2017;551(7678):92-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. O’Mara TA, Glubb DM, Amant F, et al. Identification of nine new susceptibility loci for endometrial cancer. Nat Commun. 2018;9(1):3166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Phelan CM, Kuchenbaecker KB, Tyrer JP, et al. ; AOCS study group; EMBRACE Study; GEMO Study Collaborators; HEBON Study; KConFab Investigators; OPAL study group. Identification of 12 new susceptibility loci for different histotypes of epithelial ovarian cancer. Nat Genet. 2017;49(5):680-691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hemani G, Zheng J, Elsworth B, et al. The MR-Base platform supports systematic causal inference across the human phenome. Elife. 2018;7:e34408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Larsson SC, Carter P, Kar S, et al. Smoking, alcohol consumption, and cancer: a Mendelian randomisation study in UK Biobank and international genetic consortia participants. PLoS Med. 2020;17(7):e1003178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Burgess S, Bowden J, Fall T, Ingelsson E, Thompson SG. Sensitivity analyses for robust causal inference from Mendelian randomization analyses with multiple genetic variants. Epidemiology. 2017;28(1):30-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kamat MA, Blackshaw JA, Young R, et al. PhenoScanner V2: an expanded tool for searching human genotype-phenotype associations. Bioinformatics. 2019;35(22):4851-4853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Setiawan VW, Doherty JA, Shu XO, et al. Two estrogen-related variants in CYP19A1 and endometrial cancer risk: a pooled analysis in the epidemiology of endometrial cancer consortium. Cancer Epidemiol Biomarkers Prev. 2009;18(1):242-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Goodman MT, Lurie G, Thompson PJ, McDuffie KE, Carney ME. Association of 2 common single-nucleotide polymorphisms in the CYP19A1 locus and ovarian cancer risk. Endocr Relat Cancer. 2008;15(4):1055-1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhang J, Yin Y, Niu XM, et al. CYP19A1 gene polymorphisms and risk of lung cancer. J Int Med Res. 2013;41(3):735-742. [DOI] [PubMed] [Google Scholar]
- 29. Kohno T, Kakinuma R, Iwasaki M, et al. Association of CYP19A1 polymorphisms with risks for atypical adenomatous hyperplasia and bronchioloalveolar carcinoma in the lungs. Carcinogenesis. 2010;31(10):1794-1799. [DOI] [PubMed] [Google Scholar]
- 30. Brusselaers N, Maret-Ouda J, Konings P, El-Serag HB, Lagergren J. Menopausal hormone therapy and the risk of esophageal and gastric cancer. Int J Cancer. 2017;140(7):1693-1699. [DOI] [PubMed] [Google Scholar]
- 31. Green J, Czanner G, Reeves G, et al. Menopausal hormone therapy and risk of gastrointestinal cancer: nested case-control study within a prospective cohort, and meta-analysis. Int J Cancer. 2012;130(10):2387-2396. [DOI] [PubMed] [Google Scholar]
- 32. Lindblad M, Ye W, Rubio C, Lagergren J. Estrogen and risk of gastric cancer: a protective effect in a nationwide cohort study of patients with prostate cancer in Sweden. Cancer Epidemiol Biomarkers Prev. 2004;13(12):2203-2207. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Some or all datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.