Abstract
Context
Daily variation in the thermic effect of food (TEF) is commonly reported and proposed as a contributing factor to weight gain with late eating. However, underlying circadian variability in resting metabolic rate (RMR) is an overlooked factor when calculating TEF associated with eating at different times of the day.
Objective
This work aimed to determine whether methodological approaches to calculating TEF contribute to the reported phenomena of daily variation in TEF.
Methods
Fourteen overweight to obese but otherwise healthy individuals had their resting and postprandial energy expenditure (EE) measured over 15.5 hours at a clinical research unit. TEF was calculated for breakfast, lunch, and dinner using standard methods (above a baseline and premeal RMR measure) and compared to a method incorporating a circadian RMR by which RMR was derived from a sinusoid curve model and TEF was calculated over and above the continuously changing RMR. Main outcome measures were TEF at breakfast, lunch, and dinner calculated by different methods.
Results
Standard methods of calculating TEF above a premeal measured RMR showed that morning TEF (60.8 kcal ± 5.6) (mean ± SEM) was 1.6 times greater than TEF at lunch (36.3 kcal ± 8.4) and 2.4 times greater than dinner TEF (25.2 kcal ± 9.6) (P = .022). However, adjusting for modeled circadian RMR nullified any differences between breakfast (54.1 kcal ± 30.8), lunch (49.5 kcal ± 29.4), and dinner (49.1 kcal ± 25.7) (P = .680).
Conclusion
Differences in TEF between morning and evening can be explained by the underlying circadian resting EE, which is independent of an acute effect of eating.
Keywords: chrononutrition, diet-induced thermogenesis, diurnal, breakfast, energy balance, energy expenditure
Research in murine and human models highlights the importance of circadian rhythms in the regulation of energy metabolism, and the role of circadian disruption in poor health outcomes (1). The mammalian circadian system is composed of the central hypothalamic clock in the suprachiasmatic nuclei and peripheral clocks found throughout the body. The suprachiasmatic nuclei receives photic input from the retina and relays temporal information throughout the brain and peripheral tissues (2). Both central and peripheral rhythms are evident in many key metabolic processes involved in the regulation of energy balance, occurring at the most basic cellular level, through to whole-body energy metabolism (3, 4). Various hormones involved in energy metabolism display circadian oscillations (5), and circadian variations have been observed in human resting metabolic rate (RMR), peaking around 1700 to 1800 hours, and with a trough at approximately 0500 hours (6).
A number of research groups have reported the thermic effect of food (TEF), the postprandial energy expenditure (EE) resulting from digestion and nutrient storage (7), to be greater in the morning compared to the evening, suggesting a strong endogenous circadian effect on TEF (8-11). Richter et al (11) reported TEF being twice as large during the biological morning as compared to the biological evening. The prevailing view is that this daily variation of the TEF response to a meal significantly contributes to a differential energy balance in individuals with evening- compared to morning-predominant energy intake (EI) (12). However, other studies have failed to reproduce TEF variability across the day (13, 14).
TEF is indirectly calculated as the incremental area under the curve over and above a measured RMR, and thus, the assumptions and mathematical method employed strongly influence the value determined. Several methods of measuring TEF are found in the literature. The most widely used method is to measure RMR directly preceding the given test meal, where TEF is calculated as the EE over and above the premeal-measured RMR (9-11). In some instances the fasted RMR measured on waking is used as the basal measure over which the TEF response to all subsequent meals is calculated, irrespective of the time of the meal (8, 14). This latter approach is often used in respiratory chamber studies (14-16). Both of these approaches make the assumption that RMR is constant throughout the entire duration of the TEF measurement. However, it has been demonstrated that RMR is circadian in nature and tracks changes in core body temperature (CBT), with an amplitude of approximately 55.2 kcal/d, a nadir in the early morning (aligning with the CBT nadir), and peak in the late afternoon (aligning with the CBT peak) (6). TEF constitutes a relatively small proportion of total daily EE (TDEE; 3%-10%) and postprandial EE (6%-9% of the energy content of the meal), and as such even small changes in the underlying RMR can lead to large variability in the measurement of TEF (17, 18).
The aim of this study was to demonstrate methodological impacts, specifically the effect of accounting for or neglecting circadian changes in RMR, on the interpretation of morning-evening variation in TEF. This body of work is imperative because inaccurate methods of calculating TEF may greatly overestimate the daily variations in TEF and could hinder understanding the circadian metabolism and interpretation of chrononutrition studies. In this study we show that differential TEF—high morning and low evening—results from failing to account for circadian changes in underlying RMR.
Materials and Methods
Study Participants
Participants were overweight to obese (mean body mass index = 31.7 ± 3.0, range, 27.9-36.7) yet otherwise healthy males and females aged 20 to 60 years with a habitual bedtime between 2100 and 2400, getting between 6 to 9 hours sleep per night. Exclusion criteria included current smokers, habitual caffeine intake of more than 300 mg/day, history of circadian or sleep disorders, and current medication use. Recruitment was conducted by the Surrey Clinical Research Facility recruitment staff. Candidate participants were contacted by the volunteer recruitment database via invitation email, in addition social/digital media advertising, local media, and local websites. Interested participants were required to register online or complete a written form, which was reviewed by the clinical research facility recruitment staff to confirm whether the candidate participant was eligible for a screening visit. Eligible participants were provided a participant information sheet and consent form and were invited to attend an in-person screening, which was conducted within 6 weeks of the start of the laboratory study session. Written informed consent was taken prior to conducting any screening procedures. At the screening visit, applicants were required to fill out a number of questionnaires to assess medical history, diet, sleep, chronotype, and mood. This study formed part of a larger trial aimed at addressing circadian influences on energy balance in overweight and obese participants. The study was reviewed and approved by the University of Surrey Ethics Committee (No. UEC/2018/041/FHMS [CRC382]). All participants provided written informed consent to participant before the study.
Study Design
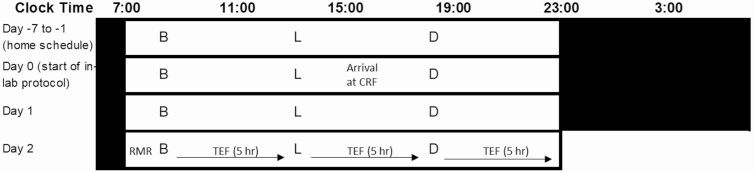
The protocol included a 1-week in-home run-in phase and 1.5 inpatient days in the laboratory to acclimatize to the lab environment before a 15.5-hour test day to measure RMR and TEF following breakfast, lunch, and dinner (Fig. 1). The run-in phase was designed to maximize circadian entrainment before the laboratory protocol and required participants to commit to an agreed on regular 8-hour period for sleep and regular meal times at 1 hour, 6 hours, and 11 hours after waking for breakfast, lunch, and dinner, respectively. Participants’ sleep/wake schedule was aligned so the wake time for all participants was within 15 minutes of their habitual reported wake time. During the run-in phase, participants were required to wear 2 L Actiwatches (Cambridge Neurotechnology) to assess their activity, light exposure, and sleep (CNT Sleep Analysis software, Cambridge Neurotechnology Ltd). At day 0, when participants arrived at the laboratory, the actigraphy data were downloaded and assessed to ensure compliance with the baseline sleep-wake schedule as a condition of admission to the laboratory study. The regular sleep-wake cycle and mealtimes were continued for each participant during the laboratory acclimatization period and test day. During the laboratory stay, lighting was controlled to maintain each individual’s regular circadian rhythms. The 8-hour sleep opportunity in the laboratory was afforded in a single-unit sleep room with light maintained at 0 lux. For the first half of the lights-on, waking period, lighting was at 500 lux, while for the second half of the waking period participants were required to wear orange-tinted glasses designed to block short-wave blue light, which have been shown to be effective in preserving nocturnal increases in melatonin. Throughout the waking periods participants were free to read, watch TV/films, access the internet, or play board games, as long as this did not require postural change or physical exertion. Participants were required to refrain from caffeine for the duration of the study.
Figure 1.
Schematic of the study protocol with an example of a participant waking at 0700 hours and sleep time at 2300 hours. White bars indicate lights on and wakefulness. Black bars indicate lights off and sleep opportunity. B, breakfast; D, dinner; L, lunch. Meals separated by 5 hours. Participants entered the clinical research facility (CRF) day 0 and were provided dinner before an 8-hour sleep opportunity. Day 1 involved a full day of acclimatization with all meals provided before the 15.5-hour test day 2.
Study Diet
Participants were provided controlled meals over the duration of the study. EI was individualized for all participants to maintain energy balance with calorie intake calculated as basal metabolic rate × estimated physical activity level (1.1-1.3). The Mifflin-St Jeor prediction equation based on age, sex, height and weight was used to estimate the basal metabolic rate for each individual (19). Breakfast, lunch, and dinner were isocaloric (33.3% of EI), and the macronutrient profile of the diets was 20% protein, 35% fat, and 45% carbohydrate for each meal. Meals were provided 1 hour after waking, and 5 hours apart for lunch and dinner. Each meal contained a minimum energy content of 550 kcal (2301 kJ), with individual participant energy requirements being met through the addition of prepared milkshakes. Meals were designed using calorie and macronutrient content from an electronic version of McCance and Widdowson’s The Composition of Foods (20), WinDiets software (Professional version, Robert Gordon University, 2017), as well as details from food packaging for brand-specific food items unlisted in the software.
Indirect Calorimetry Measurements
RMR and TEF were measured using indirect calorimetry (GEM Nutrition). Participants wore a ventilated Perspex hood through which air is drawn at a constant rate into an analyzer that measures the relative concentrations of oxygen and carbon dioxide in inspired and expired air. RMR was measured for 30 minutes, within 10 minutes of waking, to obtain a fasted baseline RMR measure. Postprandial EE was measured for 10 minutes, every half hour, for 5 hours after breakfast, lunch, and dinner, respectively. Thus, measures of EE covered 15.5 hours of the 16-hour waking period. Participants lay in a semi-supine position for the duration of the measurement. RMR was calculated from VO2 and VCO2 using the Elia and Livesay equation (21). RMR was measured on a minute-by-minute basis; the initial 5 minutes of the data were excluded and the RMR calculated from a 15-minute moving average with the lowest coefficient of variation. Postprandial EE from each 10-minute measurement was determined by calculating the consecutive data (minimum of 5 minutes) with the lowest coefficient of variation. Calibrations were carried out before the start of each day and every 2 to 3 hours over the test day or when a drift was noticed. The machines automatically reanalyze room air values of O2 and CO2 at the end of each measure. When these values at the end of a test started to drift outside the expected range (O2: 20.9%-21.0%, CO2: 0.00%-0.08%), the machines were recalibrated.
Calculations
Circadian changes in RMR were predicted by applying SINE equations to model changes in RMR over the day. The curve was based on the findings by Zitting et al (6) indicating an average amplitude of 55 kcal (based on their fitted model), with a nadir aligning with nadir CBT. It is reported that the CBT nadir occurs in normal healthy individuals with no sleep disorders, within about 2 to 3 hours of waking; however, it may differ depending on chronotype and age (22-24). The age for the participants in our study was 45.6 ± 9.6 years (range, 31-57 years) and average wake time was 0721 ± 0036 (range, 0630-0840). We specifically looked at the average of 4 studies using individuals within a similar age range and wake times to our participants to estimate the time interval from nadir CBT to wake time. The age range for the groups in these studies was 18 to 53 years with average wake times between 0748 and 0847 and a difference in CBT minimum to wake time interval of 2 hours 18 minutes to 4 hours 10 minutes, giving an average of 3 hours 7 minutes across the studies within the applicable age groups and chronotypes (22-24). Based on this we used 3 hours as our estimated CBT minimum to wake time interval for all participants.
The sine wave curve was calculated using the standard sine wave formula:
Where Y represents RMR at any given point in time (t), A the amplitude of the curve (55 kcal), B the period (24 hours), X the starting time of the curve, D the new time (therefore (X – D) is the change in time from the midpoint of the curve (t0) in hours, and C the RMR at time 0. Therefore:
The SINE curve starting point (t0), being halfway between the RMR nadir and peak, is 6 hours after the CBT nadir and 3 hours after the measured RMR. Therefore, the measured RMR occurred at a phase of 21 hours and the midpoint (t0) RMR was determined by rearranging the above equation to:
TEF was calculated in 3 ways: 1) as the area under the curve (AUC) above the RMR measured directly before the meal (“premeal RMR approach”); 2) as the AUC above the RMR from the start of the day (“baseline RMR approach”); and 3) as the AUC above the predicted circadian RMR (“circadian RMR approach”). TEF was expressed both in total energy (kilocalories) as well as a percentage of consumed EI.
Statistical Analysis
Statistical analysis was carried out using SPSS V25 (IBM SPSS Statistics for Windows, version 25.0. IBM Corp). Repeated-measures analysis of variance was used to compare breakfast, lunch, and dinner with each of the different approaches. Where significant, pairwise post hoc analysis was completed with Sidak adjustment for multiple comparisons. Results are reported as mean ± SEM and P less than .05 used to determine statistical significance.
Results
Fourteen participants completed the study: 8 male and 6 female. The mean age of the participants was 45.6 years (SD ± 9.6), height 173.2 cm (SD ± 9.2), weight 95.3 kg (SD ± 12.7), and body mass index was 31.7 (SD ± 3.04). The average EI over the day was 2223.4 ± 103.1 kcal (average of 741 ± 34 kcal per meal).
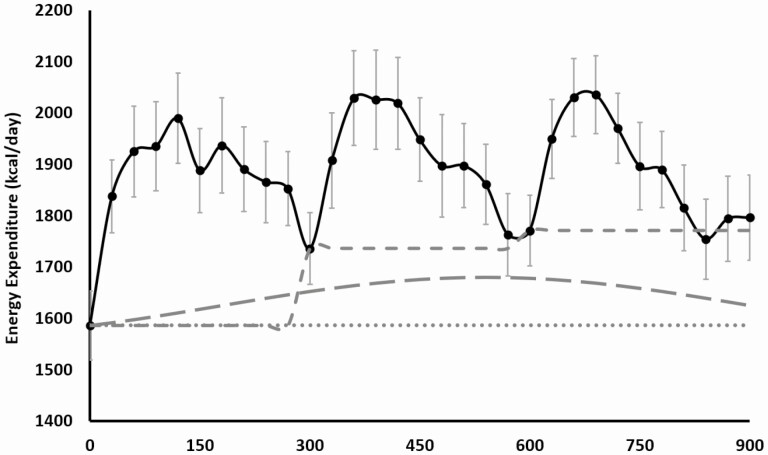
The effects of the different approaches to calculating TEF on the TEF incremental AUC are shown in Fig. 2, and the calculated values are shown in Table 1. We found that when using the premeal RMR approach to calculate TEF, there was an overall significant effect of mealtime, with morning TEF (kcal) 1.6 and 2.4 times greater than TEF at lunch and dinner, respectively. Post hoc analysis showed that breakfast TEF (kcal) was significantly greater than lunch TEF (kcal) (P = .045) and dinner TEF (kcal) (P = .037), with no difference between lunch and dinner TEF (kcal) (P = .749). However, when using either the baseline RMR approach, or the circadian RMR approach, there were no significant effects of mealtime, indicating no differences in TEF between breakfast, lunch, and dinner. This effect was due to the premeal RMR method underestimating the dinner TEF and overestimated the breakfast TEF, compared to the circadian-derived method (see Table 1). While both the baseline RMR approach and circadian RMR approach resulted in no significant differences in TEF between meals, adjusting for the underlying modelled circadian RMR yielded lower values for TEF for all meals compared to the baseline RMR approach (see Table 1). As a percentage of consumed EI, results were nearly identical, with a significant effect of mealtime with the premeal RMR approach (effect of mealtime P = .018; breakfast vs lunch TEF, P = .061; breakfast vs dinner TEF, P = .029; lunch vs dinner TEF, P = .764) and no significant effect of mealtime when calculated using either the baseline RMR approach or the circadian RMR approach.
Figure 2.
Energy expenditure (EE) measured fasting and for 15 hours over a day following 3 test meals provided at breakfast: 1-hour after waking (0 minutes), lunch: 5-hour after breakfast (300 minutes), and dinner: 5-hour after lunch (600 minutes). Mean ± SEM. Solid line, measured EE over the entire day; dotted line, representation of baseline resting metabolic rate (RMR); short dashes, representation of RMR directly before meals; long dashes, representation of circadian model of RMR.
Table 1.
Thermic effect of food calculated with a premeal resting metabolic rate (RMR), baseline RMR of circadian RMR, reported as energy expenditure in kilocalories as well as a percentage of energy intake
| Breakfast | Lunch | Dinner | P | |
|---|---|---|---|---|
| Meal size, kcal | 734.7 ± 28.4 | 729.1 ± 33.3 | 734.7 ± 28.3 | .519 |
| TEF—premeal RMR, kcal | 60.8 ± 5.6a | 36.3 ± 8.4b | 25.2 ± 9.6b | .022 |
| TEF—baseline RMR, kcal | 60.8 ± 5.6 | 67.5 ± 7.9 | 63.7 ± 6.9 | .604 |
| TEF—circadian RMR, kcal | 54.1 ± 5.6 | 49.5 ± 7.9 | 49.1 ± 6.9 | .680 |
| TEF—premeal RMR, %EI | 8.3 ± 0.7a | 5.0 ± 1.2a,b | 3.5 ± 1.3b | .018 |
| TEF—baseline RMR, %EI | 8.3 ± 0.7 | 9.2 ± 0.9 | 8.7 ± 0.9 | .610 |
| TEF—circadian RMR, %EI | 7.4 ± 0.7 | 6.6 ± 0.9 | 6.7 ± 0.9 | .699 |
Mean ± SEM. P based on repeated-measures analysis of variance.
Abbreviations: %EI, percentage of energy intake; RMR, resting metabolic rate; TEF, thermic effect of food.
a ,
b Columns with different letters are significantly different based on post hoc analysis with Sidak adjustment. P less than .05.
Discussion
Our data show that the daily mealtime effect of TEF is abolished when TEF is calculated using a method that accounts for circadian RMR. Numerous metabolic processes exhibit daily variability, in particular glucose tolerance, lipid metabolism, gastric emptying and intestinal motility, and nutrient absorption (4, 25-27). In addition, a number of studies have described circadian influences in weight management, with greater weight loss reported when more calories were consumed earlier rather than later in the day (12, 28). These findings have provoked countless studies targeting meal timing and daily energy distribution as a potential strategy for weight management. The TEF has been proposed as one of the underlying mechanisms responsible for driving greater weight loss with morning-predominant EI based on the results of a few studies reporting greater TEF in the morning compared to the evening after consumption of identical meals (9-11). Our results challenge the prevailing view that daily variations in TEF contribute to differential weight loss with morning-predominant eating, and weight gain with large evening EIs. The difference of approximately 35 kcal in our participants between breakfast and dinner TEF, calculated using the standard premeal RMR approach, was negated after adjusting for modeled circadian RMR, suggesting mathematical error may contribute to the apparent differences in morning vs evening TEF.
Research supporting the notion of diurnal variation in TEF dates back to 1993, when Romon et al (8) demonstrated a diminishing TEF response across the day, with a significantly greater TEF following a standardized test meal (544 ± 37 kcal) consumed in the morning (0900; TEF 15.9 ± 1.6% of consumed EI [mean ± SD]) compared to the same meal consumed in the afternoon (1700; TEF 13.5 ± 1.8% of consumed EI) or night (0100; TEF 10.9 ± 2.2% of consumed EI). Their results were based on calculating the TEF for all meals as the additional EE above a baseline RMR measure taken at 0530 to 0600, which assumes that RMR is constant across the day. Since this research, diurnal variations in TEF have been demonstrated in several other studies. Morris et al (9) reported that TEF in response to a set meal providing 33% of daily energy requirements was 44% lower in the evening (2000) compared to TEF in the morning (0800) (0.13 ± 0.01 kcal/min vs 0.24 ± 0.02 kcal/min). However, it is important to note they measured only early TEF (2-hour postprandial) in their study. Bo and colleagues (10) reported a significantly lower postprandial EE following an evening (2000) compared to morning (0800) meal (1168-kcal meals) in young, lean participants. In a recent, rigorously controlled intervention, Richter et al (11) reported that the TEF in response to breakfast was as much as 2.5 times greater after breakfast compared to dinner following both high-energy (> 1000 kcal [69% of TDEE]) and low-energy (> 250 kcal [11% of TDEE]) meals.
Cumulatively these results have been drawn on to support theories that suggest lower evening TEF is a potential contributor to energy imbalance leading greater conversion of caloric intake into stored energy in the evening (9-11). We now propose that methodological inaccuracies may largely explain these findings. Many of these studies make a number of key assumptions. First, RMR is constant throughout the day and, specifically, constant throughout the postprandial measurement period. Second, RMR measured preceding a test meal is not inflated from carryover of TEF from previous meals. Given, however, that the TEF response has been suggested to last 6 hours or more and as much as 10 hours after large meals (~ 1000 kcal) (29-31), the only RMR measure unaffected by prior EI may be the initial fasted prebreakfast measure, due to the overnight fast during sleep. Third, the assumption that the postprandial TEF profile is consistent, and therefore short incomplete measures of TEF may be used to interpret the entire TEF response. However, this is unlikely given that there are diurnal variations in the rate of gastric emptying with slower gastric emptying in the evening (27), and therefore likely a lower peak and longer TEF response in the evening.
Here we challenge the evidence from prior studies based on the aforementioned assumptions. Both in the Morris et al (9) and Bo et al (10) studies, postprandial EE was measured for only a very short assessment window (Morris et al, 2 × 24-minute measures at 30 minutes and 90 minutes postprandial; Bo et al, 60-minute measure between 120 and 150 minutes postprandial). This would not capture the entire TEF response. Richter and colleagues (11) calculated the TEF as the difference between the measured premeal RMR and postprandial RMR, and thus the breakfast measure was an RMR baseline after a 14-hour overnight fast, whereas the predinner RMR (5 hours post lunch) could have yielded a carryover effect from lunch, resulting in a seemingly higher RMR. Additionally, and of critical influence on the results, all these studies used an RMR measured directly before the meal and assumed a constant RMR throughout the postprandial measurement. Notably, the paper by Richter et al (11) reports a negative TEF response following dinner in their large breakfast/small dinner study arm, a result that is not only implausible, but impossible. Essentially, this would suggest eating a meal is the cause of a reduction in EE. Instead, a superficially inflated premeal RMR from prior meal consumption and/or decreases in RMR throughout the measurement arising from circadian variation in RMR are likely to have contributed to this result. Despite acknowledgement of the limitations of various methods by many authors (eg, Romon et al [8] acknowledge their measurement of TEF included both the true TEF and circadian variation in RMR), circadian variation in RMR has continued to be overlooked, and TEF is consistently calculated as EE above a premeal RMR assuming no circadian variability in RMR.
Recently, Zitting et al (6) examined the effects of circadian phase on RMR, independent of behavioral cycles and food intake, and demonstrated that fasting RMR varies according to a circadian rhythm. RMR values were lowest at a time in the late biological night corresponding with the nadir in the circadian rhythm of CBT; ~ 0500), and highest approximately 12 hours later in the late biological afternoon/evening, corresponding to the peak in CBT. This magnitude of change in RMR from nadir to peak, corresponding to approximately 129 kcal/day, will therefore influence calculations of the TEF response to meals at different times of day. In our study, calculating the TEF in the traditional manner, that is, EE above premeal RMR, resulted in a 58% decrease in the TEF from breakfast to dinner, consistent with the wider literature indicating a significant diurnal difference in TEF between morning and evening when calculating the TEF as EE above the premeal RMR (8-11). However, after adjusting for a modeled circadian RMR, TEF values were no longer significantly different for breakfast, lunch, or dinner. Our calculations support the predictions of Melanson and Chen (32), highlighting the methodological issues in current methods of TEF calculation that overinflate the morning vs evening difference. However, while Melanson and Chen propose calculation of TEF as above the baseline, fasted RMR, our data indicate this may still overinflate the value of TEF, albeit abolishing any apparent daily variation. Our findings indicate that the actual TEF response to meals across the day has minimal, if any, circadian variation and the magnitude of effect of TEF at different times of day instead, primarily or wholly, reflects circadian changing values in underlying RMR.
A potential limitation of this study is the duration of the TEF measurement. Although the large majority of studies measure TEF for only 5 to 6 hours (33), the TEF can continue for substantially longer than this. The duration of the TEF response is largely related to meal size and, while TEF may be complete within approximately 5 hours for smaller meals (~ 250 kcal), with larger meals of more than 500 kcal (such as in our own study), the TEF response may last upward of 6 hours (30, 31). Therefore, when assessing TEF in response to a second or third meal later in the day, it is possible that the premeal RMR is not a true reflection of RMR. As such, within our study the premeal RMR measured before lunch and dinner would have likely been elevated by a combination of circadian variability in RMR as well as residual TEF from the prior meal and in turn, resulted in underestimating the lunch and dinner TEF with the premeal RMR method. However, using an estimated circadian RMR overcomes this shortfall and eliminates using an RMR measured premeal, which encompasses carryover TEF from previous meals, as well as removes assumptions about RMR being constant both throughout the day and during the postprandial TEF measurements. Additionally, while 5-hour measures may not capture the entire TEF response, previous studies have indicated that 5-hour measurements will capture as much as 91% to 96% of the TEF response across meals averaging 576 to 945 kcal (7, 29). It is therefore unlikely, with meals in our study averaging 741 kcal, that large amounts of the TEF response were missed.
We also recognize that our methods included a number of assumptions: 1) the amplitude of daily RMR rhythm identified by Zitting et al (6) (55-kcal amplitude in a population group of 50:50 male/female participants) was applicable to our own population group, and, 2) the timing of an individual’s RMR nadir aligns with their CBT nadir, which is approximately 3 hours before an individual’s average wake time (22, 23, 34). In the study by Zitting and colleagues (6), the mean age of the participants was 57.6 years (SD ± 7.24). Given the known age-related decline in RMR (15, 31) and the slightly younger participants in our study (mean age, 45.6 ± 9.6 years), it is possible that the amplitude in circadian variation of RMR may have been larger than the 55 kcal/day reported by Zitting et al (6). In additional, the time interval between nadir CBT and wake time is shown to reduce with age (24); therefore, ideally future studies will individually assess CBT to assign more individualized times for RMR nadir.
Differentiation between the TEF and RMR can be challenging given that energy metabolism is continuous and nutrients are consistently being stored, remobilized, and transformed at various energetic costs. Regardless of circadian variability in the TEF, it would be negligent to overlook the potential for earlier eating as a mechanism to improve a large array of other metabolic health components, including glucose regulation and postprandial lipid metabolism. Nonetheless, further research is essential before we attribute late-night eating to a specific cause of weight gain due to lower-evening TEF, or a particular energetic advantage to early EI due to higher morning TEF. Our data suggest that the magnitude of difference between morning and evening TEF is trivial, and our modeling approach that accounts for circadian RMR removes the artifact of differences in diurnal TEF. In conclusion, we suggest that diurnal variations in TEF are created from a spurious methodological flaw and, as a result, the TEF has limited influence on body weight management.
Acknowledgments
We would like to thank Barbara Fielding, Adam Collins, Hayriye Biyikoglu, Alice Brealy, and Paul Jefcoate as well as all the staff at the Surrey Clinical Research Facility for their assistance in running this study. We would also like to thank Graham Horgan from Biomathematics and Statistics Scotland, for input on the modeling and statistical analysis.
Financial Support: This study was funded by the Medical Research Council (grant No. MR/P012205/1, The Big Breakfast Study). A.M.J. and P.J.M. acknowledge funding support from the Scottish Government, Rural and Environment Science and Analytical Services Division.
Glossary
Abbreviations
- AUC
area under the curve
- CBT
core body temperature
- EE
energy expenditure
- EI
Energy intake
- RMR
resting metabolic rate
- TEF
thermic effect of food
Additional Information
Disclosures: J.D.J. has collaborated with Nestlé and has previously undertaken consultancy work for Kellogg’s. The other authors have nothing to disclose.
Data Availability
Some or all data sets generated during and/or analyzed during the present study are not publicly available but are available from the corresponding author on reasonable request. Restrictions apply to the availability of some or all data generated or analyzed during this study to preserve patient confidentiality or because they were used under license. The corresponding author will on request detail the restrictions and any conditions under which access to some data may be provided.
References
- 1. Johnston JD, Ordovás JM, Scheer FA, Turek FW. Circadian rhythms, metabolism, and chrononutrition in rodents and humans. Adv Nutr. 2016;7(2):399-406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Reppert SM, Weaver DR. Coordination of circadian timing in mammals. Nature. 2002;418(6901):935-941. [DOI] [PubMed] [Google Scholar]
- 3. Bass J. Circadian topology of metabolism. Nature. 2012;491(7424):348-356. [DOI] [PubMed] [Google Scholar]
- 4. Asher G, Sassone-Corsi P. Time for food: the intimate interplay between nutrition, metabolism, and the circadian clock. Cell. 2015;161(1):84-92. [DOI] [PubMed] [Google Scholar]
- 5. Ruddick-Collins LC, Morgan PJ, Johnstone AM. Mealtime: a circadian disruptor and determinant of energy balance? J Neuroendocrinol. 2020;32(7):e12886. [DOI] [PubMed] [Google Scholar]
- 6. Zitting KM, Vujovic N, Yuan RK, et al. . Human resting energy expenditure varies with circadian phase. Curr Biol. 2018;28(22):3685-3690.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ruddick-Collins LC, King NA, Byrne NM, Wood RE. Methodological considerations for meal-induced thermogenesis: measurement duration and reproducibility. Br J Nutr. 2013;110(11):1978-1986. [DOI] [PubMed] [Google Scholar]
- 8. Romon M, Edme JL, Boulenguez C, Lescroart JL, Frimat P. Circadian variation of diet-induced thermogenesis. Am J Clin Nutr. 1993;57(4):476-480. [DOI] [PubMed] [Google Scholar]
- 9. Morris CJ, Garcia JI, Myers S, Yang JN, Trienekens N, Scheer FAJL. The human circadian system has a dominating role in causing the morning/evening difference in diet-induced thermogenesis. Obesity (Silver Spring). 2015;23(10):2053-2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bo S, Fadda M, Castiglione A, et al. . Is the timing of caloric intake associated with variation in diet-induced thermogenesis and in the metabolic pattern? A randomized cross-over study. Int J Obes (Lond). 2015;39(12):1689-1695. [DOI] [PubMed] [Google Scholar]
- 11. Richter J, Herzog N, Janka S, Baumann T, Kistenmacher A, Oltmanns KM. Twice as high diet-induced thermogenesis after breakfast vs dinner on high-calorie as well as low-calorie meals. J Clin Endocrinol Metab. 2020;105(3):e211-e221. [DOI] [PubMed] [Google Scholar]
- 12. Jakubowicz D, Barnea M, Wainstein J, Froy O. High caloric intake at breakfast vs. dinner differentially influences weight loss of overweight and obese women. Obesity (Silver Spring). 2013;21(12):2504-2512. [DOI] [PubMed] [Google Scholar]
- 13. Weststrate JA, Weys PJ, Poortvliet EJ, Deurenberg P, Hautvast JG. Diurnal variation in postabsorptive resting metabolic rate and diet-induced thermogenesis. Am J Clin Nutr. 1989;50(5):908-914. [DOI] [PubMed] [Google Scholar]
- 14. Ravussin E, Beyl RA, Poggiogalle E, Hsia DS, Peterson CM. Early time-restricted feeding reduces appetite and increases fat oxidation but does not affect energy expenditure in humans. Obesity (Silver Spring). 2019;27(8):1244-1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Westerterp KR. Diet induced thermogenesis. Nutr Metab (Lond). 2004;1(5):5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Westerterp KR, Wilson SA, Rolland V. Diet induced thermogenesis measured over 24h in a respiration chamber: effect of diet composition. Int J Obes Relat Metab Disord. 1999;23(3):287-292. [DOI] [PubMed] [Google Scholar]
- 17. Granata GP, Brandon LJ. The thermic effect of food and obesity: discrepant results and methodological variations. Nutr Rev. 2002;60(8):223-233. [DOI] [PubMed] [Google Scholar]
- 18. Weststrate JA. Resting metabolic rate and diet-induced thermogenesis: a methodological reappraisal. Am J Clin Nutr. 1993;58(5):592-601. [DOI] [PubMed] [Google Scholar]
- 19. Mifflin MD, St Jeor ST, Hill LA, Scott BJ, Daugherty SA, Koh YO. A new predictive equation for resting energy expenditure in healthy individuals. Am J Clin Nutr. 1990;51(2):241-247. [DOI] [PubMed] [Google Scholar]
- 20. Finglas P, Roe M, Pinchen H, et al. . McCance and Widdowson’s The Composition of Foods. 7th Summary Edition. Royal Society of Chemistry; 2014. [Google Scholar]
- 21. Elia M, Livesey G. Energy expenditure and fuel selection in biological systems: the theory and practice of calculations based on indirect calorimetry and tracer methods. World Rev Nutr Diet. 1992;70:68-131. [DOI] [PubMed] [Google Scholar]
- 22. Baehr EK, Revelle W, Eastman CI. Individual differences in the phase and amplitude of the human circadian temperature rhythm: with an emphasis on morningness-eveningness. J Sleep Res. 2000;9(2):117-127. [DOI] [PubMed] [Google Scholar]
- 23. Ozaki S, Uchiyama M, Shirakawa S, Okawa M. Prolonged interval from body temperature nadir to sleep offset in patients with delayed sleep phase syndrome. Sleep. 1996;19(1):36-40. [PubMed] [Google Scholar]
- 24. Duffy JF, Dijk DJ, Klerman EB, Czeisler CA. Later endogenous circadian temperature nadir relative to an earlier wake time in older people. Am J Physiol. 1998;275(5 Pt 2):R1478-R1487. [DOI] [PubMed] [Google Scholar]
- 25. Bass J, Takahashi JS. Circadian integration of metabolism and energetics. Science. 2010;330(6009):1349-1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wehrens SMT, Christou S, Isherwood C, et al. . Meal timing regulates the human circadian system. Curr Biol. 2017;27(12):1768-1775.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Goo RH, Moore JG, Greenberg E, Alazraki NP. Circadian variation in gastric emptying of meals in humans. Gastroenterology. 1987;93(3):515-518. [DOI] [PubMed] [Google Scholar]
- 28. Garaulet M, Gómez-Abellán P, Alburquerque-Béjar JJ, Lee YC, Ordovás JM, Scheer FAJL. Timing of food intake predicts weight loss effectiveness. Int J Obes (Lond). 2013;37(4):604-611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Reed GW, Hill JO. Measuring the thermic effect of food. Am J Clin Nutr. 1996;63(2):164-169. [DOI] [PubMed] [Google Scholar]
- 30. Melanson KJ, Saltzman E, Russell R, Roberts SB. Postabsorptive and postprandial energy expenditure and substrate oxidation do not change during the menstrual cycle in young women. J Nutr. 1996;126(10):2531-2538. [DOI] [PubMed] [Google Scholar]
- 31. Melanson KJ, Saltzman E, Vinken AG, Russell R, Roberts SB. The effects of age on postprandial thermogenesis at four graded energetic challenges: findings in young and older women. J Gerontol A Biol Sci Med Sci. 1998;53(6):B409-B414. [DOI] [PubMed] [Google Scholar]
- 32. Melanson E, Chen K. Letter to the editor: “Twice as high diet-induced thermogenesis after breakfast vs dinner on high-calorie as well as low-calorie meals.” J Clin Endocrinol Metab. 2020;105:e2673-e2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Quatela A, Callister R, Patterson A, MacDonald-Wicks L. The energy content and composition of meals consumed after an overnight fast and their effects on diet induced thermogenesis: a systematic review, meta-analyses and meta-regressions. Nutrients. 2016;8(11):670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lack L, Bailey M, Lovato N, Wright H. Chronotype differences in circadian rhythms of temperature, melatonin, and sleepiness as measured in a modified constant routine protocol. Nat Sci Sleep. 2009;1:1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Some or all data sets generated during and/or analyzed during the present study are not publicly available but are available from the corresponding author on reasonable request. Restrictions apply to the availability of some or all data generated or analyzed during this study to preserve patient confidentiality or because they were used under license. The corresponding author will on request detail the restrictions and any conditions under which access to some data may be provided.