Abstract
Context
Anti-Mullerian hormone (AMH) was originally described in the context of sexual differentiation in the male fetus but has gained prominence now as a marker of ovarian reserve and fertility in females. In this mini-review, we offer an updated synopsis on AMH and its clinical utility in pediatric patients.
Design and Results
A systematic search was undertaken for studies related to the physiology of AMH, normative data, and clinical role in pediatrics. In males, AMH, secreted by Sertoli cells, is found at high levels prenatally and throughout childhood and declines with progression through puberty to overlap with levels in females. Thus, serum AMH has clinical utility as a marker of testicular tissue in males with differences in sexual development and cryptorchidism and in the evaluation of persistent Mullerian duct syndrome. In females, serum AMH has been used as a predictive marker of ovarian reserve and fertility, but prepubertal and adolescent AMH assessments need to be interpreted cautiously. AMH is also a marker of tumor burden, progression, and recurrence in germ cell tumors of the ovary.
Conclusions
AMH has widespread clinical diagnostic utility in pediatrics but interpretation is often challenging and should be undertaken in the context of not only age and sex but also developmental and pubertal stage of the child. Nonstandardized assays necessitate the need for assay-specific normative data. The recognition of the role of AMH beyond gonadal development and maturation may usher in novel diagnostic and therapeutic applications that would further expand its utility in pediatric care.
Keywords: AMH, MIS, pediatrics, DSD, fertility
Anti-Mullerian hormone (AMH), also known as Mullerian inhibiting substance (MIS), is a hormone produced exclusively in the gonads. Alfred Jost, a pioneering researcher in the field of fetal endocrinology first proposed the existence of the “hormone inhibitrice” in the 1940s when he demonstrated the regression of the “Mullerian ducts” (paramesonephric ducts), anlagen to the uterus, Fallopian tubes, cervix, and upper third of the vagina, in undifferentiated female rabbit embryos following surgical implants of testicular tissue (1,2). Josso et al demonstrated that the Sertoli cells secreted MIS, a glycoprotein (3) that was eventually purified, and coined the term “AMH” now widely in use today, and Donahoe et al synthesized functional recombinant human MIS (4). In the decades that have followed, the sexually dimorphic functions of AMH have not only played a part in the diagnosis of differences (originally “disorders”) in sexual development (DSD) but has found extensive clinical utility in female fertility and reproductive health and its significance continues to evolve with the finding of novel neuroendocrine regulatory actions of AMH (5). In this mini-review, we examine the physiological role of AMH and its clinical utility in pediatric patients.
Search Strategy
We performed a literature review in PubMed limiting to English language articles, with no beginning date and search was last updated in July 2021. The search term was “anti-Mullerian hormone” (MeSH term) comprising all variations of AMH and MIS. We used filters for English language and age (child: birth-18 years), and a total of 599 manuscripts including 41 review articles were identified. We narrowed down the search further with filters for clinical studies and systematic reviews to 70 articles. The reference lists of the original and review articles were then reviewed to ensure completeness.
Physiological Role of AMH
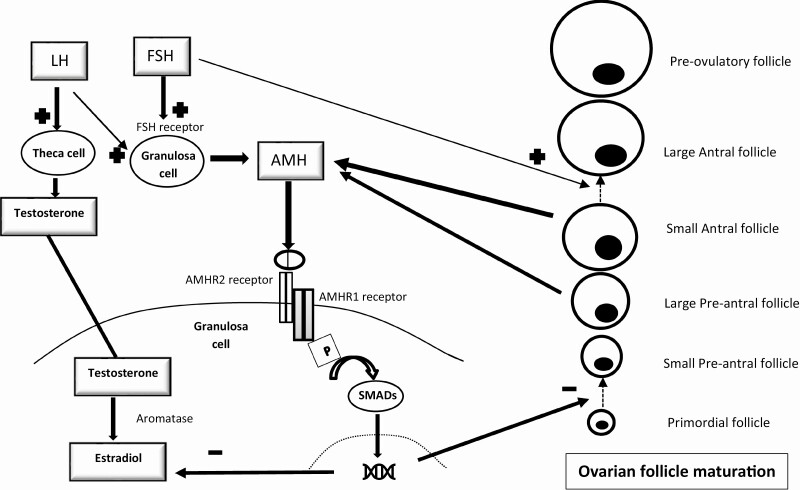
AMH is secreted as a 140kDa dimeric glycoprotein hormone structurally related to transforming growth factor β and inhibin (6). It undergoes proteolytic cleavage and generation of bioactive 25kDa C-terminal dimers that bind to the AMH type 2 receptor (7-13). It is thought that, in a similar manner to other members of the transforming growth factor β family, the ligand bound AMH type 2 receptor phosphorylates the type 1 receptor [also a serine/threonine kinase receptor, that belong to a class of activin-like kinase (ALK2)] inducing signaling through phosphorylation of intracellular Smad proteins, which then translocate to the nucleus and modulate gene transcription (14) (Fig. 1A and B). The AMH gene has been localized to chromosome 19p13.3 (15), and its expression is tightly regulated in Sertoli and granulosa cells specific to sex and developmental stage (fetal, neonatal, pre-and postpubertal) and regulated by SRY with activation of AMH by SOX-9 (16) and subsequent increase in AMH promoter activity by transcription factors steroidogenic factor 1 (SF-1) (17), GATA-4 (18,19), and WT-1 (20) and downregulation by DAX-1 (20). In the testes, high follicle-stimulating (FSH) levels activate AMH secretion (21), but androgens, specifically intratesticular testosterone, downregulates AMH secretion acting through the androgen receptor (Fig. 1A) but requires SF-1 binding to AMH promoter sites (22). In the ovarian granulosa cells, AMH secretion appears to be regulated by transcription factors SF1, FOXL2, FOG2, and GATA-4 and stimulated by FSH and luteinizing hormone (LH) (23-25).
Figure 1.
Regulation of anti-Mullerian hormone (AMH) secretion and signaling. (A) Regulation of AMH secretion in males by developmental stage. (B) Regulation of AMH secretion in females and its role in ovarian follicle maturation. Abbreviation: AR, Androgen receptor.
In the fetal Sertoli cells, AMH expression was seen starting around 8 weeks of gestation (26) during a short window when Mullerian ducts are responsive to its effect (27), leading to irreversible Mullerian duct regression by the ninth week (28). AMH continues to be produced by the fetal testes throughout gestation despite increase in testosterone since Sertoli cells in the fetus do not express androgen receptors (29). Jost proposed that AMH may play a role in the meiotic and mitotic arrest of germ cells (30), and others have suggested roles for AMH in testicular descent, germ cell maturation, and gonadal morphology, but these remain inconclusive (31). After a transient decline postnatally, AMH levels rise in infancy and continue to remain comparatively higher in males until they decline by stages 2 and 3 of puberty as intratesticular testosterone levels start to rise (32). At this point, AMH levels in postpubertal males are comparable and overlap with AMH levels in females considerably although median AMH remains 2- to 3-fold higher compared with females (33). Immunohistochemistry has shown that the expression of AMH type 2 receptor parallels AMH in serum declining after puberty in males (34). A paracrine effect of AMH was also postulated given findings that AMH pathway activation may directly suppress the expression of steroidogenic enzymes in the Leydig cells of the adult testes (35).
In contrast, AMH expression in fetal ovaries was detected around 23 weeks of gestation, with serum AMH detectable postnatally by 26 weeks of gestation (36). AMH expression was highest in the granulosa cells of secondary, preantral, and small antral cells (less than 4 mm in diameter) and significantly declines in larger antral follicles (6-8 mm) (37). Data from rodent studies (38) and immunostaining patterns in human ovaries (37) suggest that AMH may suppress initial recruitment of primordial follicles and also modulate follicular sensitivity to FSH (39), regulating the growth of follicles and the cyclic recruitment of the larger antral follicles. AMH also downregulates the aromatase activity in granulosa cells decreasing estradiol production until follicular selection (40,41). AMH tends to be 35-fold lower in females compared with males in infancy and remains stable through childhood and adolescence (42). Recent longitudinal data suggest a slight rise in AMH by 7 to 9 years with a dip around the ages of 10 to 14 years correlating with the transition through puberty, rising again by age 16 years to peak in young adulthood (43). AMH then declines over the reproductive age proportionate to the decline in the antral follicle pool and decreases rapidly by menopause and, as such, is suggested as a marker of ovarian aging (44).
Serum AMH Measurements
Enzyme-linked immunosorbent assays were first developed to measure AMH in the 1990s (45) but were not adequately sensitive to measure levels especially in females. In 2000, an ultrasensitive assay was developed (46) with a detection limit of 2 ng/mL [Immunotech (IOT), Marseille, France] followed by a more sensitive assay with a detection limit of 6.3 pg/mL [Diagnostic Systems Laboratory (DSL), Webster, TX, USA] (41). Since the calibrators and antibodies were different, the DSL assay results for serum AMH in women was 4.6-fold lower than the IOT assay (47), invalidating comparisons between studies using these 2 different nonstandardized assays. A Gen II assay of AMH (Beckman Coulter, Chaska, MN, USA) has been in use since 2010 (48), and normative data on serum AMH are depicted in Figure 2 (49). Although calibrated to the IOT assay, several studies have raised concerns with regard to the Gen II AMH assay, especially related to sample characteristics and differences in calibration compared with newer immunoassays in the past few years (50). Hence, there is still a need to establish assay-specific normative data in the absence of an international reference standard.
Figure 2.
Reference ranges for serum anti-Mullerian hormone (AMH). Serum AMH in 1027 males (blue circles) and 926 females (red circles) is depicted with blue and red lines depicting (median, +/−2 SD) reference ranges for males and females, respectively. Connecting grey lines represent longitudinal values in infancy. Age in years on the x-axis and serum AMH (pmol/L) measured on Immunotech (IOT) on the logarithmic y-axis. Comparative data for Diagnostic Systems Laboratory (DSL) and Gen II assays were calculated as follows: AMH (IOT) pmol/L = 2.0 × AMH (DSL) μg/L × 7.14 pmol/μg and AMH (IOT) pmol/L = 0.74 × AMH (Gen II) μg/L × 7.14 pmol/μg. This figure is reproduced from Johansen ML, et al (49). Copyright © 2013 Marie Lindhardt Johansen et al. This is an open access article distributed under the Creative Commons Attribution License http://creativecommons.org/licenses/by/3.0/ which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
AMH concentrations are age and sex-specific (49), rise in early infancy starting 1 month after birth peaking around 6 months of age in male infants while they remain low in female infants (Fig. 2). Throughout childhood, AMH concentrations are distinctly higher in males (almost 35-fold) (51), so despite individual variability and a broad normal range, it is easily discriminated from female norms (33) (Fig. 2). In adolescence with progression through puberty, the mean AMH levels decline in males and increase in females with considerable overlap (33,52).
Clinical Utility of AMH
AMH and Differences in Sexual Development
The process of sexual differentiation is a carefully orchestrated process with multiple regulatory elements impacting steps of differentiation. AMH was first described in the context of dysregulated sexual differentiation, and the first clinical utility was in the diagnosis of differences of sexual development. While the presence of Mullerian duct derivatives reflects the lack of AMH secretion during the sixth to tenth weeks of fetal life, the serum AMH measurements after birth reflect not only Sertoli cell function in patients with DSD but also serve as a biomarker of the relative influence of FSH and androgens (53). FSH increases Sertoli cell mass and AMH secretion, but testosterone acting via the androgen receptors in Sertoli cells postnatally suppresses AMH (22,54). So, AMH concentrations are directly correlated to FSH but inversely to testosterone levels in childhood and adolescence, in contrast to prenatal values. AMH concentrations vary by pathology, genetic defect, and developmental stage and are summarized in Table 1. In 46,XY gonadal dysgenesis, the AMH concentrations are proportionate to the presence of testicular tissue, typically being low or undetectable in conditions affecting testicular development (55). In Leydig cell hypoplasia, and androgen synthetic defects, the AMH concentrations are typically normal and may even be high during the minipuberty of infancy due to a lack of the suppressive effect of androgens (55,56). In 5-alpha reductase deficiency, characterized by a high testosterone:dihydrotestosterone ratio, AMH was in the lower range of normal (<−1 SD) since dihydrotestosterone is not required for suppression of AMH secretion (57). In androgen insensitivity syndrome, however, the relatively high gonadotropins and lack of a functional androgen receptor cause AMH levels to be inappropriately high for the degree of testosterone in the serum (56). Recent studies indicate that the hyperestrogenic state in complete AIS may also play a role in AMH elevation (58). A study of the testosterone response to human chorionic gonadotropin (hCG) stimulation in prepubertal patients with 46,XY DSD found that a normal serum AMH had a positive predictive value of 84% for a normal post-hCG testosterone level, but a low AMH was not useful in predicting a suboptimal testosterone response (59). Gonadal dysgenesis and ovotestes were associated with mean AMH concentrations in between levels seen in anorchia and undescended testes (60). In summary, AMH can be a useful tool for assessment of Sertoli cell function in 46,XY DSD and can help distinguish testicular dysgenesis from biosynthetic defects.
Table 1.
AMH and differences in sexual development
| Pathology | Karyotype | Phenotype | Serum AMH | Other markers |
|---|---|---|---|---|
| 46,XY DSD | ||||
| Complete gonadal dysgenesis (Swyer syndrome) | 46,XY | Female external genitalia and presence of Mullerian structures Streak/dysgenetic gonads |
Undetectable | Low testosterone, inhibin B and elevated LH/FSH at puberty |
| Partial gonadal dysgenesis | 46,XY | Genital ambiguity +/− presence of Mullerian structures Dysgenetic testes |
Low and reflective of testicular Sertoli cell mass | Low testosterone, inhibin B and elevated LH/FSH at puberty |
| Persistent Mullerian duct syndrome | 46,XY | Male external genitalia and Persistence of Mullerian structures Transverse testicular ectopia common |
Low (AMH gene mutations) | Normal age appropriate FSH/LH and testosterone. |
| Normal (AMH receptor mutations) male | ||||
| Testicular synthetic defects (including steroidogenic defects and Leydig cell hypoplasia) | 46,XY | Undervirilized male/ambiguous genitalia and absence of Mullerian structures with bilateral testes | Normal in childhood/high for male standard in neonates/puberty; | Low testosterone and normal to high LH based on cause |
| 5-alpha reductase deficiency | 46,XY | Undervirilized male/ambiguous genitalia and absence of Mullerian structures Bilateral testes |
Lower range of normal male | Increased testosterone: dihydrotestosterone ratio |
| Androgen insensitivity | 46,XY | Absence of Mullerian structures generally with Ambiguous genitalia (partial androgen insensitivity) Female external genitalia (complete androgen insensitivity) Bilateral testes |
High (in first year of life and during puberty with FSH stimulation); normal in childhood | Normal to high testosterone |
| Anorchia and testicular regression syndrome | 46,XY | Absence of Mullerian structures and normal male external genitalia Absent testes |
Undetectable | Low testosterone and elevated FSH/LH during puberty |
| 46,XX DSD | ||||
| 46,XX males (SRY translocation, etc) | 46,XX | Male external genitalia or ambiguous genitalia Dysgenetic testes |
Above female reference ranges in childhood and declines at puberty | Low testosterone and elevated FSH/LH at puberty |
| Congenital adrenal hyperplasia, aromatase deficiency etc | 46,XX | Virilized female or ambiguous genitalia. Bilateral ovaries and presence of Mullerian structures. | Normal female range | High testosterone and/or other androgens based on cause |
| Sex chromosome DSD | ||||
| Turner syndrome | 45,X and variable mosaic karyotype | Female external genitalia with presence of Mullerian structures Streak or dysgenetic gonads typically |
Typically, low for female reference range but may be normal and correlates with karyotype/age | Variable but typically low estradiol and elevated LH/FSH at puberty |
| Klinefelter syndrome | 47 XXY | Male external genitalia and absent Mullerian structures Dysgenetic testes |
Normal male reference range but declines after puberty to low levels | Low or low-normal testosterone and elevated FSH/LH at puberty |
| Ovotesticular DSD | 46,XX or | Ambiguous genitalia with +/− persistent unilateral/bilateral Mullerian structures | AMH above female reference standards after birth but decline in childhood | Variable but typically low testosterone/estradiol and elevated FSH/LH at puberty |
| 46,XX/46,XY typically | Ovotestes or unilateral dysgenetic testes/contralateral ovarian tissue | |||
| Mixed gonadal dysgenesis | 45,X/46XY typically | Ambiguous or male external genitalia Asymmetric gonadal dysgenesis, with dysgenetic testes/streak gonads Mullerian structures variable and often absent on testicular side |
AMH above female reference standards after birth typically, but variable and may decline in childhood | Variable, but typically low testosterone for reference male for age |
Abbreviations: AMH, anti-Mullerian hormone; FSH, follicle-stimulating hormone; LH, luteinizing hormone.
In 46,XX DSD, serum AMH above the female reference standards suggest the presence of testicular tissue (as seen in ovotesticular or testicular DSD), which distinguishes these disorders from virilized female infants with high extratesticular androgens (such as congenital adrenal hyperplasia) (55) who have typical female AMH levels.
AMH in Klinefelter syndrome was found to be within the normal reference range during minipuberty of infancy and throughout childhood (61). The decline in AMH with puberty was delayed in adolescents with Klinefelter syndrome although pubertal onset is still on time (62). This has been attributed to a downregulation of AMH in these affected individuals (63), as well as relatively low testosterone concentrations coupled with Sertoli cell destruction and lack of meiotic germ cells. AMH levels in adults with Klinefelter syndrome were lower than healthy males and attributed to the testicular tissue and Sertoli cell destruction seen in these individuals (62).
Isolated absence of AMH effect with normal testicular Leydig cell function is seen in persistent Mullerian duct syndrome (PMDS), a rare autosomal recessive disorder caused by loss of function mutations in the AMH gene (AMH deficiency) or the AMH type 2 receptor gene, AMHR2 (AMH receptor insensitivity) detectable in nearly 88% of affected individuals (64). PMDS is characterized by completely virilized male external genitalia in 46,XY males and variable persistence of Mullerian structures, presenting with the following phenotypes: (1) bilateral cryptorchidism and testes in the pelvis attached to Fallopian tubes/uterus, (2) unilateral cryptorchidism associated with a testis in the inguinal canal attached to the herniated uterus/Fallopian tube (hernia uteri inguinalis), or (3) transverse testicular ectopia (unique to PMDS, with bilateral testes and Mullerian structures herniated into a single processus vaginalis) (64). AMH gene mutations with a few exceptions, are associated with an unstable protein and, hence, very low or undetectable serum AMH in prepubertal male patients compared to a normal-for-age AMH in AMH receptor defects (65). The AMH concentrations are not elevated in prepubertal males with AMH receptor defects causing PMDS since the testosterone and FSH concentrations are normal in these patients. There is no reported clinical phenotype in females with AMH gene or AMHR2 receptor mutations although there is some speculation for possible early menopause in these individuals based on animal studies (64).
AMH and Cryptorchidism
In infants with male external genitalia and cryptorchidism, the detection of AMH secreted from the Sertoli cells can help differentiate between absent gonads (anorchia with undetectable AMH) and undescended testicles with intact Sertoli cell function. In patients with cryptorchidism without microphallus or genital ambiguity, AMH demonstrated 98% sensitivity and 91% specificity for the identification of testicular tissue (60,66). AMH was low or absent in nearly half of the patients with isolated cryptorchidism, and the number increased to 61% in the presence of cryptorchidism and microphallus (67). In addition, a low AMH was seen in 74% with bilateral nonpalpable gonads and 35% of those with gonads palpable in the inguinal region reflective of diminished Sertoli cell function (67). Additional studies have also shown lower AMH (and inhibin B values) compared to normative data in 2-year-old male children with cryptorchidism, suggesting a functional defect of Sertoli cells in this condition (68). In a large cohort of males with cryptorchidism, AMH was shown to be significantly lower in those with bilateral cryptorchidism compared with unilateral cryptorchidism and controls; abnormally low AMH (<3%) was seen in 36.5% of patients with bilateral cryptorchidism between the ages of 6 months and 2 years (69). In pooled data of the studies by Lee et al (60), normal AMH was 100% predictive of the presence of testes while a measurable but low value was predictive 86% of the time, with an unmeasurable AMH being predictive of absent testicular tissue (anorchia) 94% of the time. One exception to this would be patients with AMH-negative PMDS due to AMH gene mutations, who also have undetectable AMH but nondescent of testes due to the presence of Mullerian-derived structures affecting descent. A pelvic ultrasound to detect Mullerian structures can distinguish this condition from anorchia due to testicular regression syndrome where Mullerian derivatives are absent (53).
AMH and Pubertal Disorders
Low AMH is also characteristic of prepubertal males with hypogonadotropic (central) hypogonadism who have diminished Sertoli cell number and function due to FSH deficiency and has been shown to be low in hypogonadotropic hypogonadism due to multiple pituitary hormone deficiency in infants (70). Inhibin B is often a useful adjunct that parallels AMH secretion as well, and basal inhibin B had a higher discriminatory value for distinguishing hypogonadotropic hypogonadism from constitutional delay of growth and puberty (71). During puberty, AMH is low for Tanner stage (lack of FSH effect) and high for age (lack of testosterone effect) (72). Further, treatment with combined recombinant FSH and hCG lowers AMH during pubertal induction in hypogonadotropic hypogonadism, but testosterone therapy for pubertal induction does not appear to lower AMH (73). This has been attributed to the LH-driven rise in intratesticular testosterone, which suppresses AMH and overshadows the stimulating effect of FSH on AMH during combination therapy, whereas intratesticular testosterone levels are not adequately raised by exogenous testosterone therapy alone (73,74).
In isolated Leydig cell disorders, AMH was normal or high (increased FSH effect but lack of testosterone effect) (75). Precocious puberty in males including central precocious puberty and gonadotropin-independent forms such as familial male limited precocious puberty were associated with low AMH secretion for age due to rise in intratesticular testosterone (76). Gonadotropin-releasing hormone (GnRH) analogue therapy normalizes AMH to prepubertal values, suggesting that the Sertoli cell maturation in early puberty may be reversible and AMH could potentially serve as an additional tool for diagnosis of precocious puberty and treatment efficacy (77), although not of routine clinical utility compared to LH/testosterone.
In females, AMH was normal in most patients with acquired multiple pituitary hormone deficiency but low in severe congenital hypopituitarism reflective of gonadotropin deficiency (78). AMH has been shown to be lower in premature thelarche in females ages 1 to 3 years and negatively correlated with FSH compared with age-matched controls (79). In girls with precocious puberty, AMH levels did not differ from healthy age-matched controls at baseline but were suppressed compared to the pretreatment levels with GnRH analogue therapy and returned to pretreatment levels 6 months after discontinuation of treatment (80). The authors speculated that the suppression of AMH was consistent with gonadotropin-dependence of AMH secretion, but the normal AMH levels and negative correlation with FSH at baseline were due to an individual set point for the pituitary-gonadal feedback loop (80). Regardless, there does not appear to be any clinical utility to routine monitoring of AMH in the management of precocious puberty in female children.
AMH as a Marker of Granulosa Cell Tumors
Granulosa cell tumors (GCTs), the most common subtype of ovarian sex cord-stromal tumors, represent 2% to 5% of all ovarian cancers (81). GCTs are divided histologically into juvenile GCTs, occurring primarily in children and young adults, and adult GCTs, occurring typically in adult women. Signs of excessive estrogen secretion such as precocious puberty in a prepubertal child or menstrual irregularities including hypermenorrhea in a postmenarchal adolescent in the presence of an adnexal mass can lead to the diagnosis of GCT. However, making an accurate preoperative diagnosis remains difficult. These tumors secrete estrogen, inhibin B, and AMH. An elevation in AMH level has been reported in both juvenile and adult GCTs (82-84). AMH can be used as a marker of treatment efficacy and tumor progression and recurrence and correlates with tumor mass as determined by radiology or pathology (84-86). In a recent meta-analysis evaluating the performance of AMH in the diagnosis of GCT, the pooled sensitivity was reported as 89% with a pooled specificity of 93% (87). However, negative testing does not rule out GCTs, and there are reported cases of discrepancy between inhibin B and AMH levels in patients with progressive GCT (88). Given the possibility of false negatives, measuring both AMH and inhibin may improve the detection of GCT.
AMH and Polycystic Ovary Syndrome
Polycystic ovarian syndrome (PCOS) is the most common cause of chronic anovulation and hyperandrogenism in young women, with a prevalence of 8% to 13% (89,90). The International Guideline for the Assessment and Management of PCOS endorses the Rotterdam PCOS diagnostic criteria in adults (2 of oligo- or anovulation, clinical and/or biochemical hyperandrogenism, or polycystic ovaries on ultrasound), after exclusion of related disorders. In adolescents, both oligoanovulation and hyperandrogenism are required, with ultrasound not recommended for diagnosis (91). Serum AMH levels are consistently higher in women with PCOS (92,93). Abnormally slow growth of primary follicles results in an elevated number of AMH-producing cells. There also appears to be an increased production of AMH per follicle as evidenced by a mean AMH level 4× higher in granulosa cells from ovulatory PCOS and 75× higher in granulosa cells of anovulatory PCOS (94). Adolescent daughters of women affected by PCOS have higher AMH levels compared to girls with obesity (95,96). Higher AMH levels in adolescent daughters of women affected by PCOS is associated with menstrual cycle irregularities and higher ovarian volumes, suggesting differences in ovarian folliculogenesis in adolescents at risk of developing PCOS (97). Currently, there is no consensus on an AMH threshold for the diagnosis of PCOS. AMH levels have been reported to be a more reliable marker of polycystic ovarian morphology (PCOM) than follicle number with an AMH threshold of 35 pmol/L (5ng/mL) suggested as a possible inclusion in the diagnostic criteria of PCOS (98). Some authors have proposed increased AMH levels as an adjunct in the diagnosis of PCOS in adolescents and reported that an AMH level > 3.4ng/mL best distinguishes adolescents with PCOS from controls (99). The International Guideline for the Assessment and Management of PCOS does not recommend the use of serum AMH as an alternative to the detection of PCOM or as a single test for the diagnosis of PCOS. However, there is mention that with improved standardization of assays and established cutoff levels or thresholds based on large-scale validation in populations of different ages and ethnicities, AMH assays would be more accurate in the detection of PCOM (91).
AMH as a Marker of Ovarian Reserve/Fertility
Ovarian reserve, as defined by American Society for Reproductive Medicine, represents the number of oocytes remaining in the ovary or oocyte quantity (oocyte number) (100). Ovarian reserve tests include both ultrasound imaging and biochemical tests. Antral follicle count (AFC), a marker of ovarian reserve, is traditionally performed in the early follicular phase by transvaginal ultrasound, which precludes its use in prepubertal children as well as postmenarchal adolescents who do not tolerate transvaginal imaging. Although transabdominal AFC measurements have been performed in both prepubertal and pubertal children, AMH was only moderately correlated to AFC in premenarchal girls (101). AMH is a sensitive biochemical marker of ovarian reserve and may be more sensitive when compared to early follicular phase FSH and estradiol levels (102). The ideal timing of AMH measurement depends on whether serum AMH levels vary throughout the follicular and luteal phases of the menstrual cycle. Numerous studies have investigated AMH variations during the menstrual cycle, and some have reported only mild intra- and intercycle fluctuations of AMH secretion (103-105), while others have reported a peak AMH level in the mid-follicular phase with a subsequent decrease in the luteal phase (106,107). However, these fluctuations are not large enough to warrant a recommendation of timed AMH measurement on specific menstrual cycle days or phases (107,108). Although AMH has the ability to predict ovarian responsiveness to gonadotropin stimulation and oocyte yield in assisted reproductive technologies, it is a poor predictor of pregnancy and live birth rates (100).
AMH is expressed by granulosa cells of growing follicles after recruitment of primordial follicles from the resting pool and expression increases until the large preantral and small antral follicular stage (109,110) (Fig. 1B). AMH expression is lost in the atretic follicle as well as corpus lutea. With age, AMH decreases, and serum AMH levels correlate strongly with the decrease in size of the antral follicle pool (111,112). There is no marker that can directly measure the number of primordial follicles. However, the number of growing follicles recruited from the primordial follicle pool reflect the number of primordial follicles. As a marker for the number of growing follicles, AMH is used as a proxy for the number of primordial follicles and the quantitative aspect of the ovarian reserve in adults (113). In prepubertal children, however, ovarian follicles remain in a quiescent state after the minipuberty of infancy. AMH is not expressed by primordial or small preantral follicles and, prior to the onset of puberty, may correlate poorly with ovarian reserve in children. In vitro evaluation of ovarian tissue in children undergoing ovarian tissue cryopreservation (OTC) prior to gonadotoxic therapies due to malignant diseases that do not affect ovarian reserve parameter revealed high follicle density despite low AMH levels (114). In addition, studies reporting intervals for AMH in children demonstrated wide variations in AMH in healthy girls ages 1 to 12 years (42,115). Despite these limitations, AMH has been proposed as a potential biomarker of ovarian reserve in childhood to determine possible candidates for fertility preservation and the timing of such interventions in children and adolescents at risk of primary ovarian insufficiency (POI).
Turner syndrome (TS), caused by X-chromosome anomalies with or without mosaicism, is characterized by an increased risk of POI due to accelerated ovarian follicular apoptosis before and/or after puberty (116). In adolescents and young adults with TS, higher AMH levels are associated with spontaneous puberty and ongoing ovarian function (42,115) and negatively correlated with FSH, LH and 45,X karyotype (117). In addition, AMH < 4 pmol/L has been reported as predictive of absent puberty in prepubertal girls and POI in adolescents and adult patients (118). Care should be taken in the generalization of these results, however, as the evaluation of AMH as a predictor of spontaneous puberty was based only on 15 patients before pubertal onset (5 with spontaneous puberty and 10 with puberty induced by hormone replacement therapy). Some investigators have proposed guidelines for performing OTC based on serum AMH levels in prepubertal girls with TS (119). However, the available limited data on OTC in girls <12 years with TS do not allow for meaningful clinical predictions of the feasibility of OTC and ovarian follicular density based on AMH levels alone (120-125).
As the number of childhood cancer survivors increase, attention to long-term adverse health effects outcomes including future fertility has been identified as a major concern of patients and their families (126). Risk factors for POI include age at diagnosis, abdominal/pelvic radiation therapy, and exposure to alkylating agents (127). In a small study of 16 postmenarchal adolescents undergoing chemotherapy for oncology diagnoses (leukemia, lymphoma, and sarcoma), 94% showed a decline in mean AMH levels at 6 months postdiagnosis, but 80% showed at least some recovery of AMH by 18 to 24 months (128). Longitudinal follow-up in female childhood cancer survivors showed that although AMH levels were significantly low compared to age-matched controls, in women with sustained ovarian function (AMH > 1.0µg/L), the decline in AMH is similar to that in the normal population (129). In adolescents requiring treatment with chemotherapy, the predictive value of AMH as it relates to spontaneous pregnancy requires further longitudinal studies.
Future Directions
The clinical utility of AMH has continued to expand with the understanding of sexual differentiation and the elucidation of the molecular basis of AMH action. Originally, AMH expression was thought to be limited to the gonads, but recent studies not only demonstrate AMH and AMHR2 expression in different neurons but also confirm AMH actions at different levels of the hypothalamic-pituitary-gonadal axis to increase the activity of GnRH neurons and the sensitivity of gonadotropes to GnRH signaling, LH pulsatility (130), modulating GnRH/LH/FSH secretion through possibly endocrine (circulating gonadal AMH), or even autocrine effects (5). Loss-of-function heterozygous mutations in AMH and AMHR2 genes were identified in congenital hypogonadotropic hypogonadism, suggesting a possible role of AMH signaling in the development, migration, and function of GnRH neurons (131). Paracrine actions of AMH in adult ovaries regulating follicular recruitment and in adult testes regulating steroidogenesis and Leydig cell/germ cell maturation, as well as a postulated role for AMH in the prenatal reprogramming of neuroendocrine pathways for pathogenesis of PCOS in the offspring, suggest that our understanding of this multifaceted hormone and its clinical utility continues to evolve (5,132).Therapeutic applications of AMH for treating ovarian and endometrial cancer and PCOS, preserving fertility, and delaying ovarian aging (133) hold promise but will require standardization of current assays and development of AMH analogues (134).
Conclusions
AMH is a valuable tool in the care of pediatric patients with diverse conditions affecting gonadal development and function. The interpretation of serum AMH should be informed by assay-specific normative data accounting for age/sex and pubertal stage. In conjunction with gonadotropins and testosterone, AMH can be a useful diagnostic marker of Sertoli cell mass and function in males. Although widely used as a biochemical marker of ovarian reserve in females, the predictive value of AMH in prepubertal females for fertility outcomes needs further study. Assay standardization and widespread availability will further enhance its utility in clinical practice.
Acknowledgments
This figure is reproduced from Johansen ML, et al (49). Copyright © 2013 Marie Lindhardt Johansen et al. This is an open access article distributed under the Creative Commons Attribution License http://creativecommons.org/licenses/by/3.0/ which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Additional Information
Disclosure Summary: The authors have nothing to disclose.
Data Availability
Data sharing is not applicable to this article as no data sets were generated or analyzed during the current study.
References
- 1. Teixeira J, Maheswaran S, Donahoe PK. Müllerian inhibiting substance: an instructive developmental hormone with diagnostic and possible therapeutic applications. Endocr Rev. 2001;22(5):657-674. [DOI] [PubMed] [Google Scholar]
- 2. Jost A. Problems of fetal endocrinology: the gonadal and hypophyseal hormones. Recent Progr Horm Res. 1953;8:379-413. [Google Scholar]
- 3. Josso N. In vitro synthesis of Müllerian-inhibiting hormone by seminiferous tubules isolated from the calf fetal testis. Endocrinology. 1973;93(4):829-834. [DOI] [PubMed] [Google Scholar]
- 4. Ragin RC, Donahoe PK, Kenneally MK, Ahmad MF, MacLaughlin DT. Human Müllerian inhibiting substance: enhanced purification imparts biochemical stability and restores antiproliferative effects. Protein Expr Purif. 1992;3(3):236-245. [DOI] [PubMed] [Google Scholar]
- 5. Silva MSB, Giacobini P. New insights into anti-Müllerian hormone role in the hypothalamic-pituitary-gonadal axis and neuroendocrine development. Cell Mol Life Sci. 2021;78(1):1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cate RL, Mattaliano RJ, Hession C, et al. Isolation of the bovine and human genes for Müllerian inhibiting substance and expression of the human gene in animal cells. Cell. 1986;45(5):685-698. [DOI] [PubMed] [Google Scholar]
- 7. Wilson CA, di Clemente N, Ehrenfels C, et al. Mullerian inhibiting substance requires its N-terminal domain for maintenance of biological activity, a novel finding within the transforming growth factor-beta superfamily. Mol Endocrinol. 1993;7(2):247-257. [DOI] [PubMed] [Google Scholar]
- 8. Baarends WM, van Helmond MJ, Post M, et al. A novel member of the transmembrane serine/threonine kinase receptor family is specifically expressed in the gonads and in mesenchymal cells adjacent to the Müllerian duct. Development. 1994;120(1):189-197. [DOI] [PubMed] [Google Scholar]
- 9. di Clemente N, Wilson C, Faure E, et al. Cloning, expression, and alternative splicing of the receptor for anti-Müllerian hormone. Mol Endocrinol. 1994;8(8):1006-1020. [DOI] [PubMed] [Google Scholar]
- 10. Imbeaud S, Faure E, Lamarre I, et al. Insensitivity to anti-Müllerian hormone due to a mutation in the human anti-Müllerian hormone receptor. Nat Genet. 1995;11(4):382-388. [DOI] [PubMed] [Google Scholar]
- 11. Mishina Y, Rey R, Finegold MJ, et al. Genetic analysis of the Müllerian-inhibiting substance signal transduction pathway in mammalian sexual differentiation. Genes Dev. 1996;10(20):2577-2587. [DOI] [PubMed] [Google Scholar]
- 12. Nachtigal MW, Ingraham HA. Bioactivation of Müllerian inhibiting substance during gonadal development by a kex2/subtilisin-like endoprotease. Proc Natl Acad Sci U S A. 1996;93(15):7711-7716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pépin D, Hoang M, Nicolaou F, et al. An albumin leader sequence coupled with a cleavage site modification enhances the yield of recombinant C-terminal Mullerian inhibiting substance. Technology. 2013;1(1):63-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Clarke TR, Hoshiya Y, Yi SE, Liu X, Lyons KM, Donahoe PK. Müllerian inhibiting substance signaling uses a bone morphogenetic protein (BMP)-like pathway mediated by ALK2 and induces SMAD6 expression. Mol Endocrinol. 2001;15(6):946-959. [DOI] [PubMed] [Google Scholar]
- 15. Cohen-Haguenauer O, Picard JY, Mattéi MG, et al. Mapping of the gene for anti-Müllerian hormone to the short arm of human chromosome 19. Cytogenet Cell Genet. 1987;44(1):2-6. [DOI] [PubMed] [Google Scholar]
- 16. De Santa Barbara P, Bonneaud N, Boizet B, et al. Direct interaction of SRY-related protein SOX9 and steroidogenic factor 1 regulates transcription of the human anti-Müllerian hormone gene. Mol Cell Biol. 1998;18(11):6653-6665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Shen WH, Moore CC, Ikeda Y, Parker KL, Ingraham HA. Nuclear receptor steroidogenic factor 1 regulates the Müllerian inhibiting substance gene: a link to the sex determination cascade. Cell. 1994;77(5):651-661. [DOI] [PubMed] [Google Scholar]
- 18. Viger RS, Mertineit C, Trasler JM, Nemer M. Transcription factor GATA-4 is expressed in a sexually dimorphic pattern during mouse gonadal development and is a potent activator of the Müllerian inhibiting substance promoter. Development. 1998;125(14):2665-2675. [DOI] [PubMed] [Google Scholar]
- 19. Watanabe K, Clarke TR, Lane AH, Wang X, Donahoe PK. Endogenous expression of Müllerian inhibiting substance in early postnatal rat sertoli cells requires multiple steroidogenic factor-1 and GATA-4-binding sites. Proc Natl Acad Sci U S A. 2000;97(4):1624-1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nachtigal MW, Hirokawa Y, Enyeart-VanHouten DL, Flanagan JN, Hammer GD, Ingraham HA. Wilms’ tumor 1 and Dax-1 modulate the orphan nuclear receptor SF-1 in sex-specific gene expression. Cell. 1998;93(3):445-454. [DOI] [PubMed] [Google Scholar]
- 21. Lukas-Croisier C, Lasala C, Nicaud J, et al. Follicle-stimulating hormone increases testicular anti-Mullerian hormone (AMH) production through sertoli cell proliferation and a nonclassical cyclic adenosine 5’-monophosphate-mediated activation of the AMH Gene. Mol Endocrinol. 2003;17(4):550-561. [DOI] [PubMed] [Google Scholar]
- 22. Edelsztein NY, Racine C, di Clemente N, Schteingart HF, Rey RA. Androgens downregulate anti-Müllerian hormone promoter activity in the Sertoli cell through the androgen receptor and intact steroidogenic factor 1 sites. Biol Reprod. 2018;99(6):1303-1312. [DOI] [PubMed] [Google Scholar]
- 23. Anttonen M, Ketola I, Parviainen H, Pusa AK, Heikinheimo M. FOG-2 and GATA-4 Are coexpressed in the mouse ovary and can modulate Mullerian-inhibiting substance expression. Biol Reprod. 2003;68(4):1333-1340. [DOI] [PubMed] [Google Scholar]
- 24. Taieb J, Grynberg M, Pierre A, et al. FSH and its second messenger cAMP stimulate the transcription of human anti-Müllerian hormone in cultured granulosa cells. Mol Endocrinol. 2011;25(4):645-655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jin H, Won M, Park SE, Lee S, Park M, Bae J. FOXL2 is an essential activator of SF-1-induced transcriptional regulation of anti-Müllerian hormone in human granulosa cells. PLoS One. 2016;11(7):e0159112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Josso N, Lamarre I, Picard JY, et al. Anti-Müllerian hormone in early human development. Early Hum Dev. 1993;33(2):91-99. [DOI] [PubMed] [Google Scholar]
- 27. Josso N, Picard JY, Tran D. The anti-Müllerian hormone. Birth Defects Orig Artic Ser. 1977;13(2):59-84. [PubMed] [Google Scholar]
- 28. Taguchi O, Cunha GR, Lawrence WD, Robboy SJ. Timing and irreversibility of Müllerian duct inhibition in the embryonic reproductive tract of the human male. Dev Biol. 1984;106(2):394-398. [DOI] [PubMed] [Google Scholar]
- 29. Chemes HE, Rey RA, Nistal M, et al. Physiological androgen insensitivity of the fetal, neonatal, and early infantile testis is explained by the ontogeny of the androgen receptor expression in Sertoli cells. J Clin Endocrinol Metab. 2008;93(11):4408-4412. [DOI] [PubMed] [Google Scholar]
- 30. Jost A. A new look at the mechanisms controlling sex differentiation in mammals. Johns Hopkins Med J. 1972;130(1):38-53. [PubMed] [Google Scholar]
- 31. Lee MM, Donahoe PK. Mullerian inhibiting substance: a gonadal hormone with multiple functions. Endocr Rev. 1993;14(2):152-164. [DOI] [PubMed] [Google Scholar]
- 32. Lee MM, Donahoe PK, Hasegawa T, et al. Mullerian inhibiting substance in humans: normal levels from infancy to adulthood. J Clin Endocrinol Metab. 1996;81(2):571-576. [DOI] [PubMed] [Google Scholar]
- 33. Yates AP, Jopling HM, Burgoyne NJ, Hayden K, Chaloner CM, Tetlow L. Paediatric reference intervals for plasma anti-Müllerian hormone: comparison of data from the roche elecsys assay and the beckman coulter access assay using the same cohort of samples. Ann Clin Biochem. 2019;56(5):536-547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sansone A, Isidori AM, Kliesch S, Schlatt S. Immunohistochemical characterization of the anti-Müllerian hormone receptor type 2 (AMHR-2) in human testes. Endocrine. 2020;68(1):215-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ingraham HA, Hirokawa Y, Roberts LM, et al. Autocrine and paracrine Müllerian inhibiting substance hormone signaling in reproduction. Recent Prog Horm Res. 2000;55:53-67; discussion 67. [PubMed] [Google Scholar]
- 36. Kuiri-Hänninen T, Kallio S, Seuri R, et al. Postnatal developmental changes in the pituitary-ovarian axis in preterm and term infant girls. J Clin Endocrinol Metab. 2011;96(11):3432-3439. [DOI] [PubMed] [Google Scholar]
- 37. Weenen C, Laven JS, Von Bergh AR, et al. Anti-Müllerian hormone expression pattern in the human ovary: potential implications for initial and cyclic follicle recruitment. Mol Hum Reprod. 2004;10(2):77-83. [DOI] [PubMed] [Google Scholar]
- 38. Durlinger AL, Gruijters MJ, Kramer P, et al. Anti-Müllerian hormone inhibits initiation of primordial follicle growth in the mouse ovary. Endocrinology. 2002;143(3):1076-1084. [DOI] [PubMed] [Google Scholar]
- 39. Durlinger AL, Gruijters MJ, Kramer P, et al. Anti-Müllerian hormone attenuates the effects of FSH on follicle development in the mouse ovary. Endocrinology. 2001;142(11):4891-4899. [DOI] [PubMed] [Google Scholar]
- 40. Vigier B, Forest MG, Eychenne B, et al. Anti-Müllerian hormone produces endocrine sex reversal of fetal ovaries. Proc Natl Acad Sci U S A. 1989;86(10):3684-3688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Dewailly D, Andersen CY, Balen A, et al. The physiology and clinical utility of anti-Mullerian hormone in women. Hum Reprod Update. 2014;20(3):370-385. [DOI] [PubMed] [Google Scholar]
- 42. Hagen CP, Aksglaede L, Sørensen K, et al. Serum levels of anti-Müllerian hormone as a marker of ovarian function in 926 healthy females from birth to adulthood and in 172 Turner syndrome patients. J Clin Endocrinol Metab. 2010;95(11):5003-5010. [DOI] [PubMed] [Google Scholar]
- 43. Smith MB, Ho J, Ma L, et al. Longitudinal antimüllerian hormone and its correlation with pubertal milestones. F S Rep. 2021;2(2):238-244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. van Rooij IA, Broekmans FJ, Scheffer GJ, et al. Serum antimullerian hormone levels best reflect the reproductive decline with age in normal women with proven fertility: a longitudinal study. Fertil Steril. 2005;83(4):979-987. [DOI] [PubMed] [Google Scholar]
- 45. Josso N, Legeai L, Forest MG, Chaussain JL, Brauner R. An enzyme linked immunoassay for anti-Müllerian hormone: a new tool for the evaluation of testicular function in infants and children. J Clin Endocrinol Metab. 1990;70(1):23-27. [DOI] [PubMed] [Google Scholar]
- 46. Long WQ, Ranchin V, Pautier P, et al. Detection of minimal levels of serum anti-Müllerian hormone during follow-up of patients with ovarian granulosa cell tumor by means of a highly sensitive enzyme-linked immunosorbent assay. J Clin Endocrinol Metab. 2000;85(2):540-544. [DOI] [PubMed] [Google Scholar]
- 47. Fréour T, Mirallié S, Bach-Ngohou K, Denis M, Barrière P, Masson D. Measurement of serum anti-Müllerian hormone by Beckman Coulter ELISA and DSL ELISA: comparison and relevance in assisted reproduction technology (ART). Clin Chim Acta 2007;375(1-2):162-164. [DOI] [PubMed] [Google Scholar]
- 48. Kumar A, Kalra B, Patel A, McDavid L, Roudebush WE. Development of a second generation anti-Müllerian hormone (AMH) ELISA. J Immunol Methods. 2010;362(1-2):51-59. [DOI] [PubMed] [Google Scholar]
- 49. Lindhardt Johansen M, Hagen CP, Johannsen TH, et al. Anti-Müllerian hormone and its clinical use in pediatrics with special emphasis on disorders of sex development. Int J Endocrinol. 2013;2013:198698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Li HW, Wong BP, Ip WK, Yeung WS, Ho PC, Ng EH. Comparative evaluation of three new commercial immunoassays for anti-Müllerian hormone measurement. Hum Reprod. 2016;31(12):2796-2802. [DOI] [PubMed] [Google Scholar]
- 51. Hagen CP, Aksglaede L, Sørensen K, Mouritsen A, Juul A. Clinical use of anti-Müllerian hormone (AMH) determinations in patients with disorders of sex development: importance of sex- and age-specific reference ranges. Pediatr Endocrinol Rev. 2011;9(suppl 1):525-528. [PubMed] [Google Scholar]
- 52. Hagen CP, Aksglaede L, Sørensen K, et al. Individual serum levels of anti-Müllerian hormone in healthy girls persist through childhood and adolescence: a longitudinal cohort study. Hum Reprod. 2012;27(3):861-866. [DOI] [PubMed] [Google Scholar]
- 53. Josso N, Rey RA. What does AMH tell us in pediatric disorders of sex development? Front Endocrinol (Lausanne). 2020;11:619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Rey R, Lukas-Croisier C, Lasala C, Bedecarrás P. AMH/MIS: what we know already about the gene, the protein and its regulation. Mol Cell Endocrinol. 2003;211(1-2):21-31. [DOI] [PubMed] [Google Scholar]
- 55. Rey RA, Belville C, Nihoul-Fékété C, et al. Evaluation of gonadal function in 107 intersex patients by means of serum antimüllerian hormone measurement. J Clin Endocrinol Metab. 1999;84(2):627-631. [DOI] [PubMed] [Google Scholar]
- 56. Rey R, Mebarki F, Forest MG, et al. Anti-Müllerian hormone in children with androgen insensitivity. J Clin Endocrinol Metab. 1994;79(4):960-964. [DOI] [PubMed] [Google Scholar]
- 57. Stuchi-Perez EG, Hackel C, Oliveira LE, et al. Diagnosis of 5alpha-reductase type 2 deficiency: contribution of anti-Müllerian hormone evaluation. J Pediatr Endocrinol Metab. 2005;18(12):1383-1389. [DOI] [PubMed] [Google Scholar]
- 58. Valeri C, Lovaisa MM, Racine C, et al. Molecular mechanisms underlying AMH elevation in hyperoestrogenic states in males. Sci Rep. 2020;10(1):15062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Lucas-Herald AK, Kyriakou A, Alimussina M, et al. Serum anti-Müllerian hormone in the prediction of response to hCG stimulation in children with DSD. J Clin Endocrinol Metab. 2020;105(5):1608-1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Lee MM, Misra M, Donahoe PK, MacLaughlin DT. MIS/AMH in the assessment of cryptorchidism and intersex conditions. Mol Cell Endocrinol. 2003;211(1-2):91-98. [DOI] [PubMed] [Google Scholar]
- 61. Bastida MG, Rey RA, Bergadá I, et al. Establishment of testicular endocrine function impairment during childhood and puberty in boys with Klinefelter syndrome. Clin Endocrinol (Oxf). 2007;67(6):863-870. [DOI] [PubMed] [Google Scholar]
- 62. Aksglaede L, Christiansen P, Sørensen K, et al. Serum concentrations of anti-Müllerian hormone (AMH) in 95 patients with Klinefelter syndrome with or without cryptorchidism. Acta Paediatr. 2011;100(6):839-845. [DOI] [PubMed] [Google Scholar]
- 63. Wikström AM, Hoei-Hansen CE, Dunkel L, Rajpert-De Meyts E. Immunoexpression of androgen receptor and nine markers of maturation in the testes of adolescent boys with Klinefelter syndrome: evidence for degeneration of germ cells at the onset of meiosis. J Clin Endocrinol Metab. 2007;92(2):714-719. [DOI] [PubMed] [Google Scholar]
- 64. Picard JY, Cate RL, Racine C, Josso N. The persistent Müllerian duct syndrome: an update based upon a personal experience of 157 cases. Sex Dev. 2017;11(3):109-125. [DOI] [PubMed] [Google Scholar]
- 65. Josso N, Rey RA, Picard JY. Anti-Müllerian hormone: a valuable addition to the toolbox of the pediatric endocrinologist. Int J Endocrinol. 2013;2013:674105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Lee MM, Donahoe PK, Silverman BL, et al. Measurements of serum Müllerian inhibiting substance in the evaluation of children with nonpalpable gonads. N Engl J Med. 1997;336(21):1480-1486. [DOI] [PubMed] [Google Scholar]
- 67. Misra M, MacLaughlin DT, Donahoe PK, Lee MM. Measurement of Mullerian inhibiting substance facilitates management of boys with microphallus and cryptorchidism. J Clin Endocrinol Metab. 2002;87(8):3598-3602. [DOI] [PubMed] [Google Scholar]
- 68. Hamdi SM, Almont T, Galinier P, Mieusset R, Thonneau P. Altered secretion of Sertoli cells hormones in 2-year-old prepubertal cryptorchid boys: a cross-sectional study. Andrology. 2017;5(4):783-789. [DOI] [PubMed] [Google Scholar]
- 69. Grinspon RP, Gottlieb S, Bedecarrás P, Rey RA. Anti-Müllerian hormone and testicular function in prepubertal boys with cryptorchidism. Front Endocrinol (Lausanne). 2018;9:182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Braslavsky D, Grinspon RP, Ballerini MG, et al. Hypogonadotropic hypogonadism in infants with congenital hypopituitarism: a challenge to diagnose at an early stage. Horm Res Paediatr. 2015;84(5):289-297. [DOI] [PubMed] [Google Scholar]
- 71. Coutant R, Biette-Demeneix E, Bouvattier C, et al. Baseline inhibin B and anti-Mullerian hormone measurements for diagnosis of hypogonadotropic hypogonadism (HH) in boys with delayed puberty. J Clin Endocrinol Metab. 2010;95(12):5225-5232. [DOI] [PubMed] [Google Scholar]
- 72. Grinspon RP, Rey RA. Anti-Müllerian hormone and Sertoli cell function in paediatric male hypogonadism. Horm Res Paediatr. 2010;73(2):81-92. [DOI] [PubMed] [Google Scholar]
- 73. Young J, Chanson P, Salenave S, et al. Testicular anti-Mullerian hormone secretion is stimulated by recombinant human FSH in patients with congenital hypogonadotropic hypogonadism. J Clin Endocrinol Metab. 2005;90(2):724-728. [DOI] [PubMed] [Google Scholar]
- 74. Young J, Rey R, Couzinet B, Chanson P, Josso N, Schaison G. Antimüllerian hormone in patients with hypogonadotropic hypogonadism. J Clin Endocrinol Metab. 1999;84(8):2696-2699. [DOI] [PubMed] [Google Scholar]
- 75. Grinspon RP, Rey RA. New perspectives in the diagnosis of pediatric male hypogonadism: the importance of AMH as a Sertoli cell marker. Arq Bras Endocrinol Metabol. 2011;55(8):512-519. [DOI] [PubMed] [Google Scholar]
- 76. Rey R, Lordereau-Richard I, Carel JC, et al. Anti-Mülerian hormone and testosterone serum levels are inversely during normal and precocious pubertal development. J Clin Endocrinol Metab. 1993;77(5):1220-1226. [DOI] [PubMed] [Google Scholar]
- 77. Grinspon RP, Andreone L, Bedecarrás P, et al. Male central precocious puberty: serum profile of anti-Müllerian hormone and inhibin b before, during, and after treatment with GnRH analogue. Int J Endocrinol. 2013;2013:823064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Deubzer B, Weber K, Lawrenz B, Schweizer R, Binder G. Anti-Mullerian hormone deficiency in girls with congenital multiple pituitary hormone deficiency. J Clin Endocrinol Metab. 2014;99(6):1045. [DOI] [PubMed] [Google Scholar]
- 79. Muratoğlu Şahin N, Bayramoğlu E, Nursun Özcan H, et al. Antimüllerian hormone levels of infants with premature thelarche. J Clin Res Pediatr Endocrinol. 2019;11(3):287-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Hagen CP, Sørensen K, Anderson RA, Juul A. Serum levels of antimüllerian hormone in early maturing girls before, during, and after suppression with GnRH agonist. Fertil Steril. 2012;98(5):1326-1330. [DOI] [PubMed] [Google Scholar]
- 81. Pectasides D, Pectasides E, Psyrri A. Granulosa cell tumor of the ovary. Cancer Treat Rev. 2008;34(1):1-12. [DOI] [PubMed] [Google Scholar]
- 82. Gustafson ML, Lee MM, Scully RE, et al. Müllerian inhibiting substance as a marker for ovarian sex-cord tumor. N Engl J Med. 1992;326(7):466-471. [DOI] [PubMed] [Google Scholar]
- 83. Rey RA, Lhommé C, Marcillac I, et al. Antimüllerian hormone as a serum marker of granulosa cell tumors of the ovary: comparative study with serum alpha-inhibin and estradiol. Am J Obstet Gynecol. 1996;174(3):958-965. [DOI] [PubMed] [Google Scholar]
- 84. Lane AH, Lee MM, Fuller AF Jr, Kehas DJ, Donahoe PK, MacLaughlin DT. Diagnostic utility of Müllerian inhibiting substance determination in patients with primary and recurrent granulosa cell tumors. Gynecol Oncol. 1999;73(1):51-55. [DOI] [PubMed] [Google Scholar]
- 85. Gustafson ML, Lee MM, Asmundson L, MacLaughlin DT, Donahoe PK. Müllerian inhibiting substance in the diagnosis and management of intersex and gonadal abnormalities. J Pediatr Surg. 1993;28(3):439-444. [DOI] [PubMed] [Google Scholar]
- 86. Chang HL, Pahlavan N, Halpern EF, MacLaughlin DT. Serum Müllerian inhibiting substance/anti-Müllerian hormone levels in patients with adult granulosa cell tumors directly correlate with aggregate tumor mass as determined by pathology or radiology. Gynecol Oncol. 2009;114(1):57-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Chen S, Yang B, Fan J. Diagnostic value of anti-Mullerian hormone in ovarian granulosa cell tumor: a meta-analysis. Eur J Obstet Gynecol Reprod Biol. 2020;253:266-272. [DOI] [PubMed] [Google Scholar]
- 88. Petraglia F, Luisi S, Pautier P, et al. Inhibin B is the major form of inhibin/activin family secreted by granulosa cell tumors. J Clin Endocrinol Metab. 1998;83(3):1029-1032. [DOI] [PubMed] [Google Scholar]
- 89. Teede H, Deeks A, Moran L. Polycystic ovary syndrome: a complex condition with psychological, reproductive and metabolic manifestations that impacts on health across the lifespan. BMC Med. 2010;8:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Azziz R, Carmina E, Chen Z, et al. Polycystic ovary syndrome. Nat Rev Dis Primers. 2016;2:16057. [DOI] [PubMed] [Google Scholar]
- 91. Teede HJ, Misso ML, Costello MF, et al. ; International PCOS Network . Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Hum Reprod. 2018;33(9):1602-1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. La Marca A, Pati M, Orvieto R, Stabile G, Carducci Artenisio A, Volpe A. Serum anti-Müllerian hormone levels in women with secondary amenorrhea. Fertil Steril. 2006;85(5):1547-1549. [DOI] [PubMed] [Google Scholar]
- 93. Pigny P, Merlen E, Robert Y, et al. Elevated serum level of anti-Mullerian hormone in patients with polycystic ovary syndrome: relationship to the ovarian follicle excess and to the follicular arrest. J Clin Endocrinol Metab. 2003;88(12):5957-5962. [DOI] [PubMed] [Google Scholar]
- 94. Pellatt L, Hanna L, Brincat M, et al. Granulosa cell production of anti-Müllerian hormone is increased in polycystic ovaries. J Clin Endocrinol Metab. 2007;92(1):240-245. [DOI] [PubMed] [Google Scholar]
- 95. Sir-Petermann T, Ladrón de Guevara A, Codner E, et al. Relationship between anti-Müllerian hormone (AMH) and insulin levels during different tanner stages in daughters of women with polycystic ovary syndrome. Reprod Sci. 2012;19(4):383-390. [DOI] [PubMed] [Google Scholar]
- 96. Torchen LC, Legro RS, Dunaif A. Distinctive reproductive phenotypes in peripubertal girls at risk for polycystic ovary syndrome. J Clin Endocrinol Metab. 2019;104(8):3355-3361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Olszanecka-Glinianowicz M, Zachurzok A, Drosdzol-Cop A, et al. Circulating anti-Müllerian hormone levels in daughters of women with and without polycystic ovary syndrome. Horm Res Paediatr. 2016;85(6):372-378. [DOI] [PubMed] [Google Scholar]
- 98. Dewailly D, Gronier H, Poncelet E, et al. Diagnosis of polycystic ovary syndrome (PCOS): revisiting the threshold values of follicle count on ultrasound and of the serum AMH level for the definition of polycystic ovaries. Hum Reprod. 2011;26(11):3123-3129. [DOI] [PubMed] [Google Scholar]
- 99. Sopher AB, Grigoriev G, Laura D, et al. Anti-Mullerian hormone may be a useful adjunct in the diagnosis of polycystic ovary syndrome in nonobese adolescents. J Pediatr Endocrinol Metab. 2014;27(11-12):1175-1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Practice Committee of the American Society for Reproductive Medicine. Testing and interpreting measures of ovarian reserve: a committee opinion. Fertil Steril. 2020;114(6):1151-1157. [DOI] [PubMed] [Google Scholar]
- 101. Cooper A, Parker A, Lambert-Messerlian G, et al. Should we be utilizing transabdominal antral follicle count (AFC) ovarian reserve screens in prepubertal and pubertal girls. J Pediatr Adolesc Gynecol. 2011;24(2):e51. [Google Scholar]
- 102. de Vet A, Laven JS, de Jong FH, Themmen AP, Fauser BC. Antimüllerian hormone serum levels: a putative marker for ovarian aging. Fertil Steril. 2002;77(2):357-362. [DOI] [PubMed] [Google Scholar]
- 103. La Marca A, Sighinolfi G, Radi D, et al. Anti-Mullerian hormone (AMH) as a predictive marker in assisted reproductive technology (ART). Hum Reprod Update. 2010;16(2):113-130. [DOI] [PubMed] [Google Scholar]
- 104. La Marca A, Grisendi V, Griesinger G. How much does AMH really vary in normal women? Int J Endocrinol. 2013;2013:959487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Hehenkamp WJ, Looman CW, Themmen AP, de Jong FH, Te Velde ER, Broekmans FJ. Anti-Müllerian hormone levels in the spontaneous menstrual cycle do not show substantial fluctuation. J Clin Endocrinol Metab. 2006;91(10):4057-4063. [DOI] [PubMed] [Google Scholar]
- 106. Cook CL, Siow Y, Taylor S, Fallat ME. Serum Müllerian-inhibiting substance levels during normal menstrual cycles. Fertil Steril. 2000;73(4):859-861. [DOI] [PubMed] [Google Scholar]
- 107. Gorkem U, Togrul C. Is there a need to alter the timing of anti-Müllerian hormone measurement during the menstrual cycle? Geburtshilfe Frauenheilkd. 2019;79(7):731-737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Kissell KA, Danaher MR, Schisterman EF, et al. Biological variability in serum anti-Müllerian hormone throughout the menstrual cycle in ovulatory and sporadic anovulatory cycles in eumenorrheic women. Hum Reprod. 2014;29(8):1764-1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Durlinger AL, Visser JA, Themmen AP. Regulation of ovarian function: the role of anti-Müllerian hormone. Reproduction. 2002;124(5):601-609. [DOI] [PubMed] [Google Scholar]
- 110. Tal R, Seifer DB. Ovarian reserve testing: a user’s guide. Am J Obstet Gynecol. 2017;217(2):129-140. [DOI] [PubMed] [Google Scholar]
- 111. van Rooij IA, Broekmans FJ, te Velde ER, et al. Serum anti-Müllerian hormone levels: a novel measure of ovarian reserve. Hum Reprod. 2002;17(12):3065-3071. [DOI] [PubMed] [Google Scholar]
- 112. Fanchin R, Schonäuer LM, Righini C, Guibourdenche J, Frydman R, Taieb J. Serum anti-Müllerian hormone is more strongly related to ovarian follicular status than serum inhibin B, estradiol, FSH and LH on day 3. Hum Reprod. 2003;18(2):323-327. [DOI] [PubMed] [Google Scholar]
- 113. Moolhuijsen LME, Visser JA. Anti-Müllerian hormone and ovarian reserve: update on assessing ovarian function. J Clin Endocrinol Metab. 2020;105(11):3361-3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. von Wolff M, Roumet M, Stute P, Liebenthron J. Serum anti-Mullerian hormone (AMH) concentration has limited prognostic value for density of primordial and primary follicles, questioning it as an accurate parameter for the ovarian reserve. Maturitas. 2020;134:34-40. [DOI] [PubMed] [Google Scholar]
- 115. Jopling H, Yates A, Burgoyne N, Hayden K, Chaloner C, Tetlow L. Paediatric Anti-Müllerian hormone measurement: male and female reference intervals established using the automated beckman coulter access AMH assay. Endocrinol Diabetes Metab. 2018;1(4):e00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Modi DN, Sane S, Bhartiya D. Accelerated germ cell apoptosis in sex chromosome aneuploid fetal human gonads. Mol Hum Reprod. 2003;9(4):219-225. [DOI] [PubMed] [Google Scholar]
- 117. Hamza RT, Mira MF, Hamed AI, Ezzat T, Sallam MT. Anti-Müllerian hormone levels in patients with turner syndrome: relation to karyotype, spontaneous puberty, and replacement therapy. Am J Med Genet A. 2018;176(9):1929-1934. [DOI] [PubMed] [Google Scholar]
- 118. Lunding SA, Aksglaede L, Anderson RA, et al. AMH as predictor of premature ovarian insufficiency: a longitudinal study of 120 Turner syndrome patients. J Clin Endocrinol Metab. 2015;100(7):1030. [DOI] [PubMed] [Google Scholar]
- 119. Oktay K, Bedoschi G, Berkowitz K, et al. Fertility preservation in women with turner syndrome: a comprehensive review and practical guidelines. J Pediatr Adolesc Gynecol. 2016;29(5):409-416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Borgström B, Birgit B, Hreinsson J, et al. Fertility preservation in girls with turner syndrome: prognostic signs of the presence of ovarian follicles. J Clin Endocrinol Metab. 2009;94(1):74-80. [DOI] [PubMed] [Google Scholar]
- 121. Huang JY, Tulandi T, Holzer H, et al. Cryopreservation of ovarian tissue and in vitro matured oocytes in a female with mosaic turner syndrome: case report. Hum Reprod. 2008;23(2):336-339. [DOI] [PubMed] [Google Scholar]
- 122. Balen AH, Harris SE, Chambers EL, Picton HM. Conservation of fertility and oocyte genetics in a young woman with mosaic Turner syndrome. BJOG. 2010;117(2):238-242. [DOI] [PubMed] [Google Scholar]
- 123. Jensen AK, Rechnitzer C, Macklon KT, et al. Cryopreservation of ovarian tissue for fertility preservation in a large cohort of young girls: focus on pubertal development. Hum Reprod. 2017;32(1):154-164. [DOI] [PubMed] [Google Scholar]
- 124. Mamsen LS, Charkiewicz K, Anderson RA, et al. Characterization of follicles in girls and young women with Turner syndrome who underwent ovarian tissue cryopreservation. Fertil Steril. 2019;111(6):1217-1225.e3. [DOI] [PubMed] [Google Scholar]
- 125. Peek R, Schleedoorn M, Smeets D, et al. Ovarian follicles of young patients with Turner’s syndrome contain normal oocytes but monosomic 45,X granulosa cells. Hum Reprod. 2019;34(9):1686-1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Langeveld NE, Stam H, Grootenhuis MA, Last BF. Quality of life in young adult survivors of childhood cancer. Support Care Cancer. 2002;10(8):579-600. [DOI] [PubMed] [Google Scholar]
- 127. Chemaitilly W, Mertens AC, Mitby P, et al. Acute ovarian failure in the childhood cancer survivor study. J Clin Endocrinol Metab. 2006;91(5):1723-1728. [DOI] [PubMed] [Google Scholar]
- 128. Gupta AA, Lee Chong A, Deveault C, et al. Anti-Müllerian hormone in female adolescent cancer patients before, during, and after completion of therapy: a pilot feasibility study. J Pediatr Adolesc Gynecol. 2016;29(6):599-603. [DOI] [PubMed] [Google Scholar]
- 129. van der Kooi AL, van den Heuvel-Eibrink MM, van Noortwijk A, et al. Longitudinal follow-up in female childhood cancer survivors: no signs of accelerated ovarian function loss. Hum Reprod. 2017;32(1):193-200. [DOI] [PubMed] [Google Scholar]
- 130. Cimino I, Casoni F, Liu X, et al. Novel role for anti-Müllerian hormone in the regulation of GnRH neuron excitability and hormone secretion. Nat Commun. 2016;7:10055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Malone SA, Papadakis GE, Messina A, et al. Defective AMH signaling disrupts GnRH neuron development and function and contributes to hypogonadotropic hypogonadism. Elife. 2019;8:e47198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Barbotin AL, Peigné M, Malone SA, Giacobini P. Emerging roles of anti-Müllerian hormone in hypothalamic-pituitary function. Neuroendocrinology. 2019;109(3):218-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Pépin D, Sabatini ME, Donahoe PK. Müllerian inhibiting substance/anti-Müllerian hormone as a fertility preservation agent. Curr Opin Endocrinol Diabetes Obes. 2018;25(6):399-405. [DOI] [PubMed] [Google Scholar]
- 134. Kushnir VA, Seifer DB, Barad DH, Sen A, Gleicher N. Potential therapeutic applications of human anti-Müllerian hormone (AMH) analogues in reproductive medicine. J Assist Reprod Genet. 2017;34(9):1105-1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no data sets were generated or analyzed during the current study.