Abstract
Neurobiological pain models propose that chronic pain is accompanied by neurofunctional changes that mediate pain processing dysfunctions. In contrast, meta‐analyses of neuroimaging studies in chronic pain conditions have not revealed convergent evidence for robust alterations during experimental pain induction. Against this background, the present neuroimaging meta‐analysis combined three different meta‐analytic approaches with stringent study selection criteria for case–control functional magnetic resonance imaging experiments during acute pain processing with a focus on chronic pain disorders. Convergent neurofunctional dysregulations in chronic pain patients were observed in the left anterior insula cortex. Seed‐based resting‐state functional connectivity based on a large publicly available dataset combined with a meta‐analytic task‐based approach identified the anterior insular region as a key node of an extended bilateral insula‐fronto‐cingular network, resembling the salience network. Moreover, the meta‐analytic decoding showed that this region presents a high probability to be specifically activated during pain‐related processes, although we cannot exclude an involvement in autonomic processes. Together, the present findings indicate that dysregulated left anterior insular activity represents a robust neurofunctional maladaptation and potential treatment target in chronic pain disorders.
Keywords: ABC, ALE, chronic pain, functional magnetic resonance imaging, meta‐analysis
This meta‐analysis shows that chronic pain patients present convergent neurofunctional maladaptations in a cluster located in the anterior insula cortex during the induction of acute pain processing.
1. INTRODUCTION
Chronic pain represents the major cause of disability and suffering across the world population (Goldberg & McGee, 2011; Rice, Smith, & Blyth, 2016).
Although neurobiological mechanisms have been proposed (Tan et al., 2017; Vachon‐Presseau et al., 2016) and a neural signature of chronic pain has been recently determined (Lee et al., 2021), the neuropathophysiological bases of chronic pain remain to be clarified. Several functional magnetic resonance imaging (fMRI) meta‐analyses aimed at determining robust brain functional abnormalities in chronic pain conditions. Early investigations mainly employed the Activation Likelihood Estimation (ALE) algorithm (Eickhoff et al., 2009; Turkeltaub, Eden, Jones, & Zeffiro, 2002), however until 2016 (GingerALE version 2.3.5), this algorithm suffered from drawbacks in the code (Eickhoff, Laird, Fox, Lancaster, & Fox, 2017) that, plausibly, invalidated several of these findings. Two more recent neuroimaging meta‐analyses re‐examined functional alterations in chronic pain patients during experimental pain processing utilizing either the updated version of the GingerALE algorithm (A. Xu et al., 2021) or a different algorithm (LocalALE; Tanasescu, Cottam, Condon, Tench, & Auer, 2016). However, both meta‐analyses did not identify convergent neurofunctional alterations in chronic pain patients. These negative findings stand in strong contrast with both neurobiological pain models (Tan et al., 2017; Vachon‐Presseau et al., 2016) and clinical research demonstrating abnormal pain processing across chronic pain disorders (Chalaye et al., 2012; Goubert et al., 2017; Petersel, Dror, & Cheung, 2011; Staud & Domingo, 2001).
On this background, we performed a meta‐analysis on fMRI studies to determine convergent neurofunctional alterations in chronic pain conditions during acute pain processing. Considering that the negative findings in previous meta‐analyses on chronic pain (Tanasescu et al., 2016; A. Xu et al., 2021) might be related to the high degree of heterogeneity of the selected studies, we adopted very stringent selection criteria to circumscribe this problem. First, in line with the recent meta‐analysis from Xu et al. (A. Xu et al., 2021), we selected only studies directly comparing chronic pain patients and healthy participants. This allowed us to avoid considerable heterogeneity introduced by different experimental settings, such as it occurs employing studies conducted separately in either pain patients or in healthy controls. Second, we focused on well‐defined chronic pain conditions (e.g., fibromyalgia, irritable bowel syndrome, chronic low back pain, and neuropathic pain) and excluded chronic pain conditions due to direct central diseases or insults such as brain tumors or brain trauma, which might be different from other chronic pain disorders in terms of the underlying neurobiological maladaptations.
2. MATERIALS AND METHODS
2.1. Study selection
We followed the PRISMA reporting guidelines (Moher et al., 2009) for the present meta‐analysis (see Figure 1). Public databases of biomedical and life science literature reports, namely Pubmed, Web of Sciences, and Scopus, and the Neurosynth fMRI database (Yarkoni, Poldrack, Nichols, Van Essen, & Wager, 2011), were employed to search for fMRI or positron emission tomography (PET) studies investigating experimental cutaneous and visceral pain in four kinds of chronic pain conditions: neuropathic pain, fibromyalgia, irritable bowel syndrome, and low‐back pain. The following keywords were used to search for articles present in the databases from inception to 26th July 2020:(1) irritable bowel syndrome: (“irritable bowel” OR “functional gastrointestinal”) AND (“functional magnetic resonance” “OR” fMRI “OR “functional” OR “brain activation” OR “neural activity” OR “BOLD” OR “PET”); (2) fibromyalgia (“fibromyalgia” OR “fibrositis”) AND (“functional magnetic resonance” OR “fMRI” OR “functional” OR “brain activation” OR “neural activity” OR “BOLD” OR “PET”). (3) low‐back pain (“low back pain” OR “chronic back pain” OR “chronic low back pain”) AND (“functional magnetic resonance” OR “fMRI” OR “functional” OR “brain activation” OR “neural activity” OR “BOLD” OR “PET”). (4) neuropathic pain (“neuropathic” OR “neuropathy”) AND (“functional magnetic resonance” OR “fMRI” OR “functional” OR “brain activation” OR “neural activity” OR “BOLD” OR “PET”). For each identified article, we collected the title, author names, and date of publication. We merged the results from the different databases and identified and removed all duplicates. A unique identification number was assigned to the remaining articles. The abstracts were carefully read to exclude records investigating other disorders or not investigating brain activity. Then, reviewing the full text, we identified fMRI or PET studies investigating differences in brain activity between individuals suffering from chronic pain and control participants during experimental cutaneous or visceral noxious stimuli. We selected articles reporting between‐group differences at a whole‐brain level as coordinates in the standard Talairach & Tournoux (TAL) (Talairach, 1988) or Montreal Neurological Institute (MNI) (Evans et al., 1993) stereotactic system. Moreover, only studies employing a general linear model approach and random effect analyses (Friston, Holmes, Price, Büchel, & Worsley, 1999) were selected for this meta‐analysis. Studies employing only regions of interest (ROIs) analyses were excluded, as well as studies investigating experimental pain processing only in patients or in control (CTRL) participants, or reporting only resting‐state fMRI/PET results. Moreover, studies reporting brain activity during spontaneous pain in patients were not considered.
FIGURE 1.
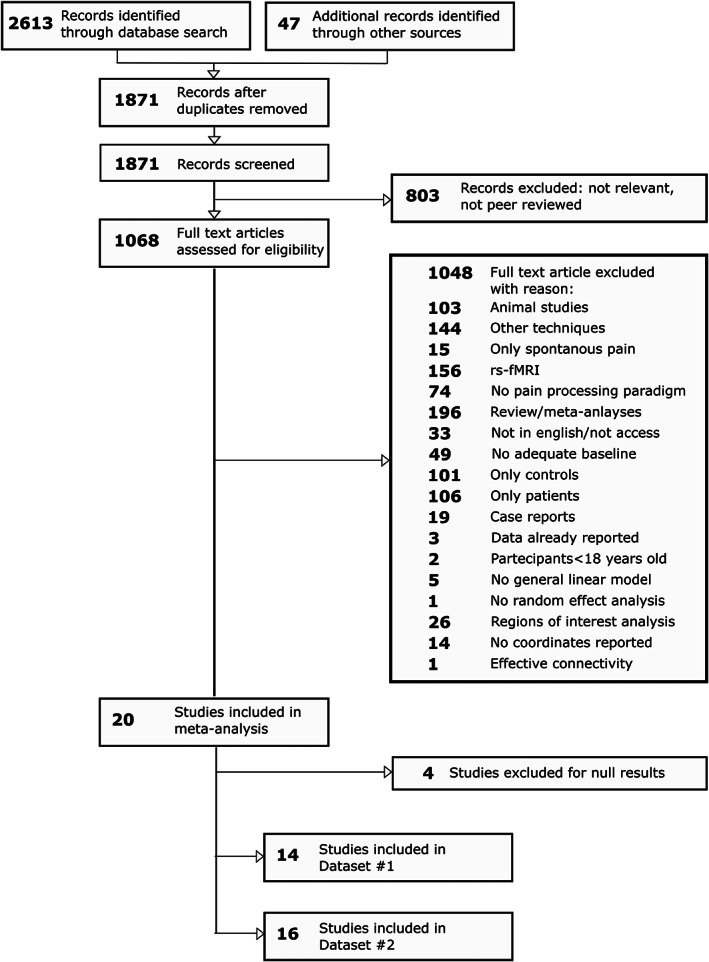
Flowchart of the screening process (PRISMA guideline)
2.2. Data extraction
For each selected article, we extracted the type of chronic pain condition investigated (neuropathic pain, fibromyalgia, irritable bowel syndrome, or low‐back pain), the sample size, and the modality (i.e., visceral or cutaneous stimulation), the type (e.g., mechanical, thermal), and the body site of the administered nociceptive stimulation considering whether it was applied in a region where the chronic pain was experienced or not. Since we were interested in the differences detected during the induction of experimental pain between chronic pain patients and control individuals, we also collected whether the two groups were matched for the same intensity of noxious stimulation or the same intensity of experienced pain. From the selected articles, one of the authors (Stefania Ferraro) manually extracted the coordinates of the significant clusters resulting from the comparison between chronic pain patients and control individuals. Studies reporting no differences between groups were not considered for this meta‐analysis. Standardized space (TAL or MNI) in which the peak coordinates were reported and the relative z‐value or t‐value were also extracted.
2.3. Quality control of the data
To evaluate the presence of possible coordinate extraction errors, three authors (Greta Demichelisc, Anna Nigric, Chiara Pinardic) independently checked the extracted coordinates. When inconsistencies between the coordinates reported in the original study and the extracted coordinates were identified, coordinates were rechecked and corrected.
Then, an automatic diagnostic procedure implemented in NeuRoi and described in C. R. Tench, Tanasescu, Auer, and Constantinescu (2013) was employed. This tool allows determining how much the coordinates of each selected study are commensurate with that of the other studies. By comparing the mean activation likelihood of each study with a random distribution of mean activation likelihood values obtained with the same number of coordinates, the algorithm estimates the proportion of times that the mean estimation likelihood is greater than the random estimation. A small ratio indicates that the estimated mean activation likelihood is similar to the randomized foci and, therefore, that it is necessary to proceed with further verification of the extracted coordinates (Tanasescu et al., 2016; C. R. Tench et al., 2013). When potential errors were detected, coordinates were re‐checked and corrected.
2.4. Meta‐analysis approaches: ginger‐ALE and analysis of brain coordinates (ABC)
There are multiple algorithms for performing CBMA, but each has different empirical parameters and assumptions, and each can produce different results conditional on the assumptions. Therefore, to obtain robust results, two different tools were employed: the GingerALE (Eickhoff et al., 2009) and the Analysis of Brain Coordinates (ABC) C. Tench, Tanasescu, Constantinescu, Auer, and Cottam (2020). Both tools were employed to identify clusters of increased activity in chronic pain patients compared to control participants during experimental pain processing (Dataset #1: only coordinates of increased activity in chronic pain patients compared to control participants). Moreover, ABC was also employed to identify the clusters where chronic pain patients present increased and/or decreased activity in comparison to control participants (Dataset #2: coordinates of increased and/or decreased activity in chronic pain patients in comparison to control participants). We employed ABC to perform this analysis because it allows us to transparently interpret the results providing the data forest plot. Due to the few investigations (n = 8) showing decreased activity in chronic pain patients compared to control participants, we did not perform a separate analysis of these studies.
2.4.1. GingerALE
We employed GingerALE v. 3.0.217, (7). Brains normalized to the MNI space and the TAL space present differences not only in size but also in origin, orientation, and shape (https://imaging.mrc-cbu.cam.ac.uk/imaging/MniTalairach). This implies that the same coordinates in the two spaces do not refer to the same brain regions, posing issues in meta‐analyses in which studies conducted in different coordinate systems need to be analyzed together. To provide results not biased by the employed coordinates system (i.e., MNI or TAL), we conducted the coordinate‐based analyses in both the MNI and the TAL space. As implemented in GingerALE, icbm2tal transformation matrix was used to perform the coordinates conversions from one space to another, producing two different foci formatting (i.e., one in the MNI space, the other in the TAL space). For each selected study, the ALE algorithm, using a conservative mask and based on the number of the participants, computes the Full‐Width Half‐Maximum (FWHM) of the Gaussian kernel to smooth the identified foci and then creates the Modeled Activation Map (Eickhoff et al., 2009). These maps are then combined to obtain one single activation likelihood estimate (ALE) image, and normalized histograms are produced to create a table of p‐values for the ALE scores. A permutation test (number of permutations = 1,000), simulating random data, was used to define the statistical threshold to identify brain areas reported beyond the random chance (Eickhoff et al., 2009). In line with recent recommendations, we thresholded the ALE map at cluster‐level inference threshold (cluster‐level family‐wise error <0.05) and an uncorrected cluster forming threshold of p <.001 (Eickhoff, Bzdok, Laird, Kurth, & Fox, 2012).
2.4.2. Analysis of brain coordinates (ABC)
ABC is a recently developed model‐based method implemented in the NeuRoi image analysis software (https://www.nottingham.ac.uk/research/groups/clinicalneurology/neuroi.aspx), with very few a priori assumptions (C. Tench et al., 2020). This algorithm uses the density of coordinates from independent studies as its statistic and requires only one parameter: the human gray matter volume. Statistical thresholding is performed by requiring a minimum proportion of the studies contributing to a cluster and is generally more conservative than false discovery rate (FDR at [q] = 0.05); however, FDR is used as a limit on how liberal the threshold can get. Importantly, this method, differently from Ginger‐ALE, does not require the empirical choice of Gaussian smoothing kernel to extrapolate coordinates to voxel‐wise activation maps or the randomization of the coordinates in the empirical space to define the statistical threshold. Employing ABC, the meta‐analysis was conducted only in the TAL space (C. Tench et al., 2020).
2.5. Functional characterization of the clusters
We conducted a functional characterization of any cluster that emerged from the ABC on Dataset #2. We took this decision because Dataset #2 presented the largest number of studies and participants and, therefore, an increased statistical power in comparison to Dataset #1. To provide an unbiased functional characterization of clusters, we used Neurosynth, an open‐source platform to synthesize large‐scale fMRI data (http://neurosynth.org; Yarkoni et al., 2011). Before any analyses, the TAL coordinates of the observed cluster were transferred in the MNI space employing the GingerALE algorithm since Neurosynth works in this space. For additional methodological information, see the Supporting Information.
2.5.1. Seed‐based functional connectivity
To identify the neural circuit that might be most likely abnormal in patients with chronic pain, we produced a conjunction map of the resting‐state functional connectivity (rs‐FC) map and of the meta‐analytic coactivation map, both seeded in the peak coordinates of clusters of interest and both generated with the Neurosynth function locations. This allowed us to obtain a robust condition‐independent (i.e., independent from “task free” and “task‐based” states) identification of the functional network connected to the cluster of interest (Schnellbächer et al., 2020). See Supporting Information for details.
2.5.2. Decoding
Using NiMARE python packages (https://nimare.readthedocs.io/en/latest/), we decoded the cluster that emerged from the meta‐analysis. The decoding quantifies the probability that a specific term is present given the activity in a specific brain area ([P(term|activation)], or “reverse inference”). Therefore, based on the assumption that terms present in a work are as proxies for cognitive processes, the reverse inference allows to probabilistically identify the cognitive process that might be selectively associated with the activity of a particular region (Poldrack, 2011; Yarkoni et al., 2011).
3. RESULTS
3.1. Study sample characteristic
For Dataset #1 we identified 14 studies reporting increased activity in chronic pain patients in comparison to control participants (for details, see Tables 1 and 2). These studies investigated a total of 263 chronic pain patients (chronic pain patients in each study, mean: 19, median:16, range: 8–48) and 179 control subjects (mean:13, median:11, range:8–21). For Dataset #2, (for details, see Tables 1 and 2), we identified 16 studies reporting increased as well as decreased activity in chronic pain patients relative to controls. This dataset comprised all studies from Dataset #1, as well as two additional investigations reporting only decreased activity. These studies investigated a total of 295 chronic pain patients (mean: 18; median: 16; range:8–48) and 211 control subjects (mean: 13; median:12; range:8–21). From this dataset, we identified 86 total foci. Notably, in both datasets, there was a higher proportion of women (Dataset #1: total chronic pain patients = 263; 222 women, 84% of the sample; Dataset #2: total chronic pain patients: 295; 246 women: 83% of the sample).
TABLE 1.
Studies characteristics
| Studies | Participants (#) | Duration of chronic pain (in months) | Direction of between‐group differences in depression scores | Direction of between‐group differences in anxiety scores | Medications wash‐out (yes/no); time before scanning session | |
|---|---|---|---|---|---|---|
| CP(F/M) | CTRL(F/M) | |||||
| Fibromyalgia | ||||||
| Gracely et al. (2002) | 16(15/1) | 16(15/1) | Not reported | Not reported | Not reported | Yes; 12 hr |
| Pujol et al. (2009) a | 9(9/0) | 9(9/0) | M = 104 | Not reported | Not reported | Yes; 72 hr |
| Jensen et al. (2009) | 16(16/0) | 16(16/0) | Not reported | Not reported | Not reported | Yes; flexible |
| Burgmer et al. (2012) | 17(17/0) | 17(17/0) | >24 | FM > CTRL | FM > CTRL | Yes; 48 hr |
| Kim et al. (2013) | 21(21/0) | 11 (11/0) | Not reported | FM > CTRL | FM = CTRL | Not reported |
| Schreiber et al. (2017) | 38(33/5) | 15(10/5) | Not reported | FM > CTRL | FM > CTRL | No |
| Low‐back pain | ||||||
| Derbyshire et al. (2002) a | 16(12/4) | 16(11/5) | Not reported | Trend LBP > CTRL | LBP > CTRL | Yes; 2 weeks |
| Baliki et al. (2010) | 16(8/8) | 16(8/8) | Not reported | Not reported | Not reported | Not reported |
| Rodriguez‐Raecke et al. (2014) | 19(12/7) | 21(15/6) | >2 | LBP > CTRL | LBP = CTRL | No |
| Irritable bowel syndrome | ||||||
| Verne et al. (2003) a | 9(6/3) | 9(6/3) | >60 | Not reported | Not reported | Not reported |
| Elsenbruch et al. (2010) a | 15(15/0) | 12(12/0) | >12 | IBS = CTRL | IBS > CTRL | Not reported |
| Bouhassira et al. (2010) a | 19(19/0) | 11(11/0) | M = 216 | IBS = CTRL | IBS = CTRL | Yes; 7 days |
| Guleria et al. (2017) a | 20 (0/20) | 10 (0/10) | M = 28 | Not reported | Not reported | Not reported |
| Neuropathic pain | ||||||
| Albuquerque et al. (2006) | 8(8/0) | 8(8/0) | M = 48 | NP = CTRL | NP > CTRL | Yes; >4 half‐lives |
| Vartiainen et al. (2009) a | 8(7/1) | 11(5/6) | 36–240 | Not reported | Not reported | Not reported |
| Neuropathic pain and fibromyalgia | ||||||
| Hampson et al. (2013) a | 48(48/0) | 13(13/0) | Not reported | Not reported | Not reported | Not reported |
| Total participants | 295(246/49) | 211(167/44) | ||||
Studies contributing to the cluster identified in the analysis of Dataset #2 with the coordinate density analysis (ABC). Bibliography in Supporting Information.
Abbreviations: CP, chronic pain patients; CTRL, control individuals; F, female; M, male.
TABLE 2.
Studies characteristics
| Study | Foci (#): CP versus CTRL | Stimulation type | Localization: in/out chronic pain body regions | Matching: perceived pain (P) or stimulus intensity (S) | Perceived pain (P) scores or stimulus intensity (S) | Between‐group differences for perceived pain (P) or stimulus intensity (S) | Employed scale for diagnosis | |
|---|---|---|---|---|---|---|---|---|
| Fibromyalgia | ↑ | ↓ | ||||||
| Gracely et al. (2002) | 13 | 1 | Mechanical press | Out‐L thumb | S | 0.45–4.5 kg/mm2 (S) | CP > CTRL (P) | Am. Coll. Rheum. |
| Pujol et al. (2009) a | 5 | 0 | Mechanical press | Out‐R thumb | P | 70/100 (P) | CP < CTRL (S) | Am. Coll. Rheum. |
| Jensen et al. (2009) | 0 | 2 | Mechanical press | Out‐thumb | P | 50/100 (P) | CP < CTRL (S) | Not reported |
| Burgmer et al. (2012) | 1 | 2 | Mechanical press | Out‐R forearm | S | Incision (S) | CP > CTRL (P) | Am. Coll. Rheum. |
| Kim et al. (2013) | 8 | 0 | Mechanical press | Out‐L thumb | P | 14/21 (P) | CP < CTRL (S) | Not reported |
| Schreiber et al. (2017) | 6 | 0 | Mechanical press | Out‐L calf | P | 40/100 (P) | CP < CTRL (S) | Am. Coll. Rheum. |
| Low‐back pain | ||||||||
| Derbyshire et al. (2002) a | 1 | 3 | Thermal | Out‐R hand | P | 40–60/100 (P) | CP = CTRL (S) | Not reported |
| Baliki et al. (2010) | 0 | 2 | Thermal | In‐back | S | 47°–51°C (S) | CP = CTRL (P) | Not reported |
| Rodriguez‐Raecke et al. (2014) | 1 | 0 | Thermal | Out‐L forearm | S | 48°C (S) | CP = CTRL (P) | Quebec task force |
| Irritable bowel syndrome | ||||||||
| Verne et al. (2003) a | 8 | 2 | Mechanical dist | In‐rectum | S | 55 mmHg (S) | CP > CTRL (P) | Rome III |
| Elsenbruch et al. (2010) a | 2 | 0 | Mechanical dist | In‐rectum | P | 5/6 (P) | CP = CTRL (S) | Rome III |
| Bouhassira et al. (2010) a | 5 | 0 | Mechanical dist | In‐rectum | P | 5/6 (P) | CP < CTRL (S) | Rome III |
| Guleria et al. (2017) a | 4 | 2 | Mechanical dist | In‐rectum | P | Mod‐sev(P) | CP < CTRL (S) | Rome III |
| Neuropathic pain | ||||||||
| Albuquerque et al. (2006) | 6 | 3 | Thermal | In‐R trigeminus | S | 47°‐49° C (S) | CTRL = CTRL | Not reported |
| Vartiainen et al. (2009) a | 3 | 7 | Thermal | Out‐bil. Hand | P | 6–7/10 (P) | CP < CTRL (S) | Not reported |
| Neuropathic pain and fibromyalgia | ||||||||
| Hampson et al. (2013) a | 1 | 0 | Mechanical press | Out‐L thumb | P | 13.5/21 (P) | CP < CTRL (S) | Not reported |
| Total foci | 64 | 22 | ||||||
Studies contributing to the cluster identified in the analysis of Dataset#2 employing the coordinate density analysis (ABC). Foci(#): CP versus CTRL: number of foci of abnormal activity (↑ = increased activity, ↓ = decreased activity) observed in the comparison between chronic pain patients and control participants. Stimulation type: type of painful stimulation applied for inducing pain; Stimulation site (in/out chronic pain body regions): part of the body subjected to painful stimulation and whether this body part was located in or out the regions where chronic pain was experienced; Matching perceived pain (P) or stimulus intensity (S): chronic pain patients and control participants were matched for the perceived pain (P) or for the intensity of painful stimulation (S); Pain scores or Stimulus intensity: scores on VAS or Likert scale for perceived pain (when groups were matched for perceived pain) or intensity of the stimulation (when groups were matched for the intensity of painful stimulation); Between‐group difference for pain (P) or stimulation (S): direction of the behavioral differences in the perceived pain (when groups were matched for the intensity of painful stimulation) or in the intensity of the applied painful stimulation (when groups were matched for perceived pain). Bibliography in Supporting Information.
Abbreviations: Am. Coll. Rheum., American College of Rheumatology; CP, chronic pain patients; CTRL, control individuals; dist, distension; L, left; mod‐sev = from moderate to severe; press, pressure; R, right.
3.2. . Ginger‐ALE results
The ginger‐ALE analyses employing the two different coordinate systems (TAL and MNI space) on Dataset #1 provided convergent results revealing the same two clusters (formed by six studies in total) of higher fMRI activation in chronic pain patients in comparison to control participants. The first cluster was detected in the left anterior insula cortex, and it was centered at [−36.8, 15.2, 3.2] in TAL space and at [−38.7, 17.9, −2.1] in MNI space. When the coordinates obtained in the TAL space [− 36.8, 15.2, 3.2] were converted to MNI space using the algorithm implemented in GingerALE, results resembled the results as by MNI space analysis (i.e., [−38.7, 17.9, −2.1]). In both analyses, five studies contributed to this cluster: all four studies investigating irritable bowel syndrome, and one investigating fibromyalgia. The second cluster of increased activity emerged in both analyses in the left claustrum/anterior insula cortex, and it was centered at [−31.1, 0.8, 9.6] in TAL space and at [−32.4, 3.3, 6.4] in MNI space. Three studies (two of them also contributing to the first cluster) contributed to this second cluster (one study on neuropathic pain, one on irritable bowel syndrome, and one on neuropathic pain and fibromyalgia; see Table 3).
TABLE 3.
Studies and the relative foci expressed as coordinates in the Talairach (TAL) space contributing to the identified clusters employing the Analysis of Brain Coordinates (ABC) and GingerALE on Dataset #1 and Dataset #2
| ABC | GingerALE | Coordinates in TAL space | |||
|---|---|---|---|---|---|
| Dataset #1 | Contributors to cluster #1 | x | y | z | |
| Bouhassira et al. (2010)‐IBS | Bouhassira et al. (2010) | −32.8 | 15.3 | −1.7 | |
| Elsenbruch et al. (2010)‐IBS | Elsenbruch et al. (2010) | −36 | 18 | 1 | |
| Guleria et al. (2017)‐IBS | Guleria et al. (2017) | −40.3 | 11.7 | 7.9 | |
| Verne et al. (2003)‐IBS | Verne et al. (2003) | −35 | 18 | 4 | |
| Pujol et al. (2009)‐FM | Pujol et al. (2009) | −42 | 12 | 5 | |
| Hampson et al. (2013)FM&NP | −34.8 | −1.7 | 10.5 | ||
| Contributors to cluster #2 | |||||
| Hampson et al. (2013) FM&NP | −34.8 | −1.7 | 10.5 | ||
| Verne et al. (2003)‐IBS | −31 | 2 | 10 | ||
| Pujol et al. (2009)‐FM | −27 | 3 | 8 | ||
| Dataset #2 | Contributors to cluster #1 | ||||
| Bouhassira et al. (2010)‐IBS | −32.8 | 15.3 | −1.7 | ||
| Elsenbruch et al. (2010)‐IBS | −36 | 18 | 1 | ||
| Guleria et al. (2017)‐IBS | −40.3 | 11.7 | 7.9 | ||
| Verne et al. (2003)‐IBS | −35 | 18 | 4 | ||
| Pujol et al. (2009)‐FM | −42 | 12 | 5 | ||
| Hampson et al. (2013)FM&NP | −34.8 | −1.7 | 10.5 | ||
| Derbyshire et al. (2002)‐LBP | −31 | 15.4 | 8.3 | ||
| Vartiainen et al. (2009)‐NP | −31 | 14 | 13 | ||
Note: Bibliography in Supporting Information.
Abbreviations: FM, fibromyalagia; IBS, irritable bowel syndrome; LBP, low‐back pain; NP, neuorpathic pain.
3.3. ABC results
ABC results of Dataset #1 revealed a single significant cluster (minimum number of studies = 5) of increased activity in the left anterior insula cortex centered on [−36, 12, 4] TAL space. Notably, the coordinates of this cluster were very similar to the coordinates of the first cluster emerged in the ALE approach [− 36.8, 15.2, 3.2]. Six investigations contributed to this cluster. Specifically, all four studies investigating irritable bowel syndrome, one fibromyalgia, and one neuropathic pain and fibromyalgia contributed to this cluster: five of these also contributed to the respective cluster obtained with GingerALE. The ABC results of Dataset #2 (see Figures 2 and 3) again revealed a cluster of significant functional abnormalities in the left anterior insula (centered in [−34, 14, 6] TAL space; minimum number of studies = 5). As expected, this cluster was formed by all the studies contributing to the cluster obtained with Dataset #1 (see Table 3) and two more additional studies (one on neuropathic pain and one on chronic low‐back pain). Specifically, all four studies investigating irritable bowel syndrome, one investigating fibromyalgia, one neuropathic pain, one neuropathic pain and fibromyalgia, and one low‐back pain contributed to this cluster. Importantly, seven studies contributing to the observed cluster matched the patient and control groups for the intensity of perceived pain and only one study for the intensity of stimulation (one study on irritable bowel syndrome).
FIGURE 2.
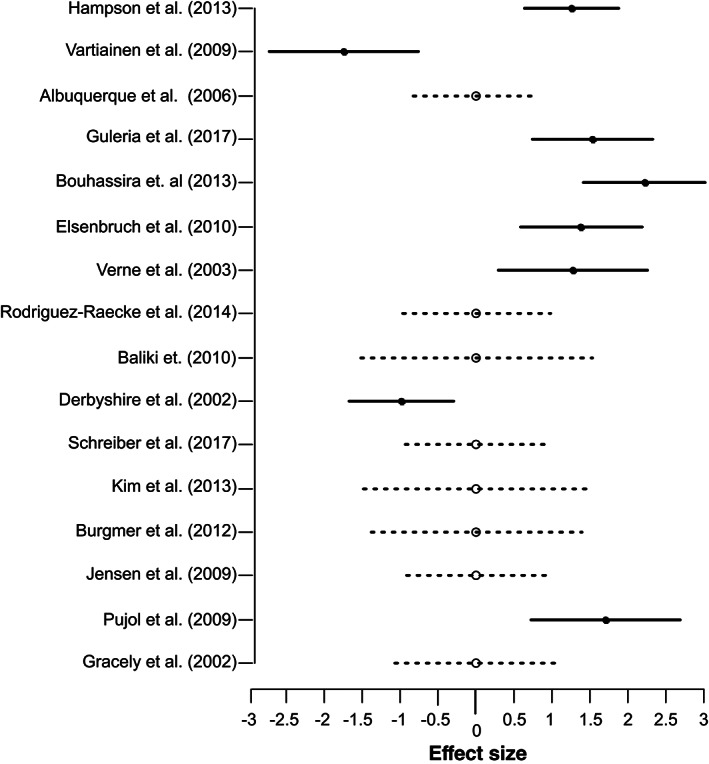
Effect size of the studies contributing to the cluster of abnormality emerged from the coordinate density analysis (ABC) 17 of Dataset#2 (comprising studies showing increased and/or decreased activity in chronic pain patients in comparison to control participants)
FIGURE 3.
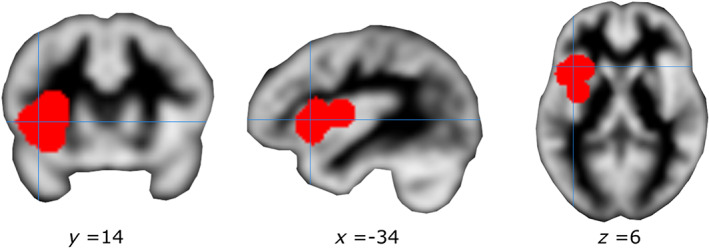
Cluster of convergent activity emerged from the coordinate density analysis (ABC) on Dataset #2. Mean of the coordinates of the cluster in the Talairach space [−34, 14, 6]
3.4. Functional characterization
3.4.1. Seed‐based functional connectivity
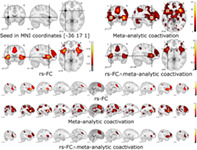
The conjunction map obtained from the rs‐FC map and the meta‐analytic co‐activation map both seeded in the region of altered neural activity in chronic pain patients (ABC on Dataset #2; MNI coordinate: [−36, 17, 1]), revealed a widespread bilateral network (Table 4 and Figure 4). Specifically, three large connected clusters were identified, the first encompassing the right insula lobe and the right inferior and middle frontal gyri, the second encompassing the left insula lobe, the left inferior frontal regions (inferior frontal gyrus, the Rolandic operculum), and the left precentral gyrus, while the third encompassed the right midcingulate cortex, the left anterior cingulate cortex, and the left posterior medial frontal gyrus.
TABLE 4.
Anatomical regions and MNI coordinates of the peaks of the conjunction analysis performed with the minimum statistic approach between the resting‐state functional connectivity (rs‐FC) map and the meta‐analytic coactivation map both seeded in the MNI coordinates [−36 17 1] of the significant cluster emerged from the analysis of brain coordinates (ABC) on Dataset #2
| K E | MNI coord. | Lat. | Anatomical region | |||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Cluster #1 | 3,691 | 42 | 24 | 8 | R | Insula lobe |
| 36 | 50 | 20 | R | Middle frontal gyrus | ||
| 52 | 12 | 24 | R | Inferior frontal gyrus | ||
| Cluster #2 | 2,563 | −36 | 16 | 0 | L | Insula lobe |
| −50 | 10 | 8 | L | Inferior frontal gyrus | ||
| −52 | 6 | 14 | L | Precentral gyrus | ||
| −40 | −4 | 12 | L | Rolandic operculum | ||
| Cluster #3 | 2,439 | 2 | 18 | 44 | R | Posterior‐med. frontal gyrus |
| 2 | 20 | 40 | R | Midcingulate cortex | ||
| 2 | 34 | 24 | L | Anterior cingulate cortex | ||
| 4 | 2 | 62 | R | Posterior‐med. frontal gyrus | ||
| Cluster #4 | 1,399 | −38 | 46 | 18 | L | Middle frontal gyrus |
| −42 | 32 | 20 | L | Inferior frontal gyrus | ||
| Cluster #5 | 852 | −40 | −46 | 42 | L | Inferior parietal lobule |
| −60 | −34 | 36 | L | Supramarginal gyrus | ||
| Cluster #6 | 709 | 38 | −44 | 44 | R | Inferior parietal lobule |
| 52 | −38 | 44 | R | Supramarginal gyrus | ||
| Cluster #7 | 94 | 14 | 10 | 4 | R | Pallidum |
| Cluster #8 | 86 | −12 | 6 | 4 | L | Pallidum |
Note: Regions larger than 50 voxels were automatically labeled with Anatomy toolbox (Eickhoff et al., 2005).
Abbreviations: L, left; Lat., hemisphere; K E , number of voxels; R, right.
FIGURE 4.
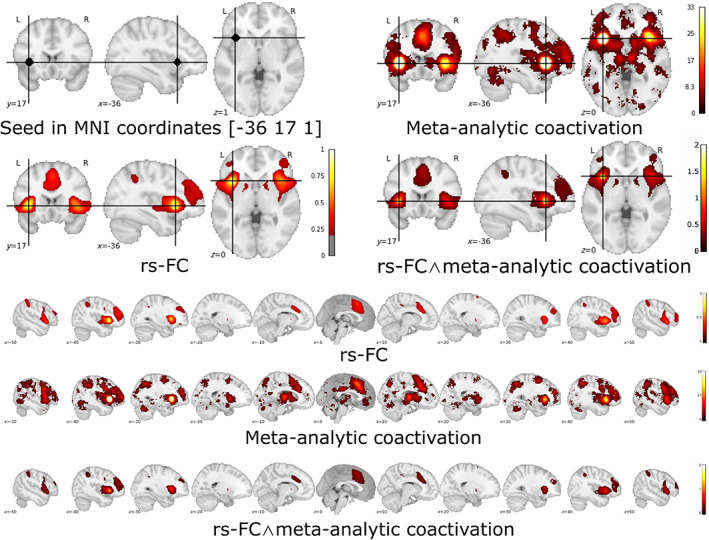
Seed‐based functional connectivity analyses (seed in the mean of the coordinates of the cluster obtained from the coordinate density analysis (ABC) on Dataset #2): resting‐state functional connectivity (rs‐FC) map, meta‐analytic coactivation map, and the relative conjunction map [rs‐FC map ∧ Coactivation map]. Projection on coronal, sagittal and transversal plane, and multislice on the sagittal plane
3.4.2. Decoding
Decoding the functional properties of the identified left anterior insula region showed that among the first 15 terms ordered according to their posterior probability for reverse inference, four terms were related to pain processing (“painful,” “nociceptive,” “noxious,” and” pain”). In contrast, no term was related to interoception (Table 5). The highest posterior probability was reported for the term “painful” (posterior probability = .77), yet also other pain‐related terms (“nociceptive,” “pain,” “noxious”) presented a high posterior probability (p = >.70). Together, these results indicate that this region presents a high probability to be specifically involved in pain processing. The terms “autonomic” and “interoceptive” showed no significant results for the reverse inference (Table 5).
TABLE 5.
Reverse inference metrics [P(term|activation)]: p‐value, z‐score, and posterior probability of the first 15 terms (TERM) and for specific terms (post‐hoc TERM) obtained from the decoding of the 6 mm spherical region of interest (ROI) centered in the mean of the coordinates forming the cluster obtained with Analysis of Brain Coordinates (ABC) from Dataset #2 (left anterior insula cortex; mean of the coordinates forming the cluster in MNI space: [−36,17,1])
| TERMS | Reverse inference metrics | ||
|---|---|---|---|
| p‐value | z‐score | Post. prob. | |
| Painful | .0000 | 7.7138 | .7687 |
| Choose | .0000 | 4.6392 | .7592 |
| Negative feed. | .0003 | 3.6112 | .7269 |
| Sensation | .0001 | 4.0265 | .7211 |
| Distress | .0003 | 3.6329 | .7209 |
| Nociceptive | .0009 | 3.3282 | .7200 |
| Articulatory | .0003 | 3.5852 | .7186 |
| Noxious | .0005 | 3.4916 | .7139 |
| Stop signal | .0005 | 3.4868 | .7074 |
| Signal task | .0008 | 3.3560 | .7070 |
| Stop | .0000 | 4.1914 | .7049 |
| Pain | .0000 | 7.3244 | .7037 |
| Congruency | .0020 | 3.0845 | .6994 |
| Intense | .0033 | 2.9371 | .6984 |
| Regulatory | .0007 | 3.7330 | .6960 |
| Post‐hoc TERMS | |||
| Autonomic | .1074 | 1.6095 | .6152 |
| Interoceptive | .2952 | 1.0466 | .5945 |
Abbreviations: post. prob., posterior probability.
4. DISCUSSION
The present study employed two different CBMA approaches (GingerALE and ABC) to determine whether chronic pain disorders are characterized by neurofunctional dysregulations during experimental pain induction. In contrast to two recent neuroimaging meta‐analyses, which, surprisingly, did not reveal convergent functional alterations in chronic pain disorders (Tanasescu et al., 2016; A. Xu et al., 2021), the present study shows robust evidence for altered neurofunctional processing in the left anterior insular cortex of chronic pain patients during experimental pain induction. Notably, functional abnormalities in the left anterior insula cortex were robustly confirmed on Dataset #1 with both ALE and ABC approaches, although with subtle differences, and on Dataset #2 with ABC. Remarkably, the application of ABC on Dataset #2 indicated that the dysfunction of this region in chronic pain patients might be present as either an increased or a decreased activity in comparison to control individuals. Functional characterization of the identified left insula region employing an unbiased approach (combining a meta‐analytic co‐activation map and an intrinsic connectivity approach) revealed that this area represents a core node in a functionally integrated network encompassing the bilateral insula cortex, and the anterior cingulate and midcingulate cortex. Moreover, via reverse inference (Poldrack, 2006, 2011), we demonstrated that this anterior insula region has a high probability of being specifically involved in pain processing, with rather unspecific involvement in related processes of interoception.
4.1. Anterior insula cortex
The sensory information coming from the posterior insula cortex, part of the spino‐thalamic cortical pathway, and the affective and autonomic inputs, coming from the spino‐parabrachial‐amygdalar pathway, are integrated in the anterior insula cortex, possibly creating the experience of pain (Bastuji, Frot, Perchet, Hagiwara, & Garcia‐Larrea, 2018; Frot, Faillenot, & Mauguière, 2014). Notably, almost all the studies (7 out of 8) contributing to the convergent cluster of functional abnormality compared patients and control participants for the experience (perceived) of pain and not for the intensity of the nociceptive stimulation suggesting, therefore, an abnormal experience of pain. Moreover, the studies contributing to the identified cluster induced experimental nociception in as well outside of body regions affected by chronic pain, supporting the hypothesis of a generalized dysfunction of acute pain processing in chronic patients, as suggested by previous clinical studies (Chalaye et al., 2012; Goubert et al., 2017; Petersel et al., 2011; Staud & Domingo, 2001).
Notably, the anterior insula cortex plays a complex role in processes directly or indirectly related to the acute and chronic pain experience, including pain empathy (Fallon, Roberts, & Stancak, 2020; X. Xu et al., 2020; Zhou et al., 2020), interoception and salience processing (Li et al., 2018; Yao et al., 2018) as well as emotional experience (Gogolla, 2017).To further disentangle the specific role of the anterior insula region that exhibited neurofunctional dysregulations in chronic pain, a series of independent and meta‐analytic functional characterization strategies was utilized. First, we characterized the corresponding functional network of this region, which consequently may be affected in chronic pain. Determining the co‐activation and intrinsic connectivity networks of the identified region in the left anterior insula cortex revealed that it represents a core node of a bilateral network encompassing anterior insular and adjacent ventrolateral prefrontal regions as well as anterior cingulate and midcingulate regions. This network clearly resembles the rs‐fMRI “salience” network, anchored in the anterior insula and the midcingulate cortex (Seeley et al., 2007), which plays an important role in the regulation of interoceptive versus external guided attention (Xin et al., 2021; Yao et al., 2018) and the guidance of flexible behavior (Menon & Uddin, 2010; Seeley et al., 2007). Abnormalities in this network have been repeatedly observed in chronic pain (Cauda et al., 2009; Hemington, Wu, Kucyi, Inman, & Davis, 2016; Wu, Inman, & Davis, 2013) and neuropsychiatric conditions, including depression (Manoliu et al., 2014; Shao et al., 2018), anxiety (Geng, Li, Chen, Li, & Gu, 2016), or addiction (Klugah‐Brown et al., 2020, 2021) which are commonly found to be co‐morbid in chronic pain (Bair, Robinson, Katon, & Kroenke, 2003; Finan & Smith, 2013). These results, therefore, support the hypothesis that chronic pain patients might present an impairment of the salience network and, possibly, of its main functions. Second, functional decoding via a meta‐analytic database (Yarkoni et al., 2011) revealed a high probability for this region to be specifically involved in pain‐related processes but not in autonomic regulation. However, we cannot exclude that the observed convergent abnormalities in the left anterior insula cortex during pain processing in chronic pain conditions are related to the altered activity of the autonomic system, often reported as abnormal in several chronic pain conditions (Yeater et al., 2021). This is also supported by the evidence that all the studies investigating irritable bowel syndrome with visceral pain, known to induce robust modulation of the autonomic system (Cervero & Laird, 1999), contributed to the significance of the observed cluster. Although our results are more compatible with the hypothesis that the observed region is probabilistically more involved in pain processing than in the modulation of the autonomic system, it is important to recognize that this platform contains more than 500 studies containing the terms “pain,” while 117 conatining the term the “autonomic,” leading to the possibility that the results are biased in favor of pain processing studies.
4.2. Previous meta‐analysis
Early neuroimaging meta‐analyses performed on chronic pain suffered from a bias toward false‐positive results since they employed previous ALE versions (Eickhoff et al., 2017). However, two robust recent meta‐analyses conducted with LocaleALE (Tanasescu et al., 2016) and with the GingerALE updated version (A. Xu et al., 2021) did not determine robust neurofunctional differences between chronic pain patients and control individuals during experimental pain induction. The null findings in the previous studies (Tanasescu et al., 2016; A. Xu et al., 2021) might be explained by study selection criteria. As for the work of Tanasescu et al. (2016), we employed only studies investigating differences between chronic pain patients and control participants during the same experiment. This allowed us to avoid considerable heterogeneity introduced by different experimental settings, such as it occurs employing studies conducted separately in either pain patients or in healthy controls. Moreover, in respect to Tanasescu et al. (2016) who investigated only cutaneous pain fMRI studies, we additionally included investigations on irritable bowel syndrome, which employed acute visceral pain. As for the work of A. Xu et al. (2021), we only selected studies investigating well‐recognized entities of chronic pain conditions (irritable bowel syndrome, neuropathic pain, fibromyalgia, and low back pain), and we excluded pain conditions related to direct central insults (e.g., traumatic brain injury, brain tumors, and chemotherapy) and studies investigating episodic migraine.
4.3. Limitations
The main limitation of this meta‐analysis is the relatively low number of included studies, a consequence of the strict selection criteria. This does not allow to further investigate possible differences among specific chronic pain conditions, which in principle could be sustained by different functional neurosignatures. Despite this drawback, the robustness of our findings is supported by similar results obtained with two different approaches (GingeALE and ABC).
Moreover, we stress the presence of some unknown source of heterogeneity within the selected studies, evidenced by the fact that in Dataset #2, two studies reporting decreased activity in the left anterior insula cortex also contributed, with 6 studies reporting increased activity, to the identified cluster. Notably, the overrepresentation of women (83% of chronic pain patients) in the selected studies makes it difficult to generalize the present findings to men.
To conclude, we show that chronic pain patients present a robust meta‐analytic evidence of a dysregulation of the left anterior insula during acute pain processing.
The functional connectivity investigation of this region supports the hypothesis of abnormalities in the salience network and of its main functions, while the characteristics of the studies contributing to the result suggest an abnormal and generalized experience of pain in chronic pain patients.
CONFLICT OF INTEREST
The authors declare no potential conflict of interest.
Supporting information
Appendix S1. Supporting Information
Ferraro, S. , Klugah‐Brown, B. , Tench, C. R. , Yao, S. , Nigri, A. , Demichelis, G. , Pinardi, C. , Bruzzone, M. G. , & Becker, B. (2022). Dysregulated anterior insula reactivity as robust functional biomarker for chronic pain—Meta‐analytic evidence from neuroimaging studies. Human Brain Mapping, 43(3), 998–1010. 10.1002/hbm.25702
Funding information National Key Research and Development Program of China, Grant/Award Number: 2018YFA0701400; National Natural Science Foundation of China, Grant/Award Number: NSFC31700998
DATA AVAILABILITY STATEMENT
Coordinates and space of normalization (TAL or MNI) of the identified results of each selected study are reported in Supporting Information.
REFERENCES
- Bair, M. J. , Robinson, R. L. , Katon, W. , & Kroenke, K. (2003). Depression and pain comorbidity: A literature review. Archives of Internal Medicine, 163(20), 2433–2445. [DOI] [PubMed] [Google Scholar]
- Bastuji, H. , Frot, M. , Perchet, C. , Hagiwara, K. , & Garcia‐Larrea, L. (2018). Convergence of sensory and limbic noxious input into the anterior insula and the emergence of pain from nociception. Scientific Reports, 8(1), 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cauda, F. , Micon, B. M. , Sacco, K. , Duca, S. , D'Agata, F. , Geminiani, G. , & Canavero, S. (2009). Disrupted intrinsic functional connectivity in the vegetative state. Journal of Neurology, Neurosurgery and Psychiatry, 80(4), 429–431. 10.1136/jnnp.2007.142349 [DOI] [PubMed] [Google Scholar]
- Cervero, F. , & Laird, J. M. A. (1999). Visceral pain. The Lancet, 353(9170), 2145–2148. [DOI] [PubMed] [Google Scholar]
- Chalaye, P. , Goffaux, P. , Bourgault, P. , Lafrenaye, S. , Devroede, G. , Watier, A. , & Marchand, S. (2012). Comparing pain modulation and autonomic responses in fibromyalgia and irritable bowel syndrome patients. The Clinical Journal of Pain, 28(6), 519–526. [DOI] [PubMed] [Google Scholar]
- Eickhoff, S. B. , Bzdok, D. , Laird, A. R. , Kurth, F. , & Fox, P. T. (2012). Activation likelihood estimation meta‐analysis revisited. NeuroImage, 59(3), 2349–2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff, S. B. , Laird, A. R. , Fox, P. M. , Lancaster, J. L. , & Fox, P. T. (2017). Implementation errors in the GingerALE software: Description and recommendations. Human Brain Mapping, 38, 7–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff, S. B. , Laird, A. R. , Grefkes, C. , Wang, L. E. , Zilles, K. , & Fox, P. T. (2009). Coordinate‐based activation likelihood estimation meta‐analysis of neuroimaging data: A random‐effects approach based on empirical estimates of spatial uncertainty. Human Brain Mapping, 30(9), 2907–2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff, S. B. , Stephan, K. E. , Mohlberg, H. , Grefkes, C. , Fink, G. R. , Amunts, K. , & Zilles, K. (2005). A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. Neuroimage, 25(4), 1325–1335. [DOI] [PubMed] [Google Scholar]
- Evans, A. C. , Collins, D. L. , Mills, S. R. , Brown, E. D. , Kelly, R. L. , & Peters, T. M. (1993). 3D statistical neuroanatomical models from 305 MRI volumes. IEEE Conference Record Nuclear Science Symposium and Medical Imaging Conference. Orlando, Florida. pp. 1813–1817.
- Fallon, N. , Roberts, C. , & Stancak, A. (2020). Shared and distinct functional networks for empathy and pain processing: A systematic review and meta‐analysis of fMRI studies. Social Cognitive and Affective Neuroscience, 15(7), 709–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finan, P. H. , & Smith, M. T. (2013). The comorbidity of insomnia, chronic pain, and depression: Dopamine as a putative mechanism. Sleep Medicine Reviews, 17(3), 173–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston, K. J. , Holmes, A. P. , Price, C. J. , Büchel, C. , & Worsley, K. J. (1999). Multisubject fMRI studies and conjunction analyses. NeuroImage, 10(4), 385–396. [DOI] [PubMed] [Google Scholar]
- Frot, M. , Faillenot, I. , & Mauguière, F. (2014). Processing of nociceptive input from posterior to anterior insula in humans. Human Brain Mapping, 35(11), 5486–5499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng, H. , Li, X. , Chen, J. , Li, X. , & Gu, R. (2016). Decreased intra‐and inter‐salience network functional connectivity is related to trait anxiety in adolescents. Frontiers in Behavioral Neuroscience, 9, 350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogolla, N. (2017). The insular cortex. Current Biology, 27(12), R580–R586. [DOI] [PubMed] [Google Scholar]
- Goldberg, D. S. , & McGee, S. J. (2011). Pain as a global public health priority. BMC Public Health, 11(1), 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goubert, D. , Danneels, L. , Graven‐Nielsen, T. , Descheemaeker, F. , Coppieters, I. , & Meeus, M. (2017). Differences in pain processing between patients with chronic low back pain, recurrent low back pain and fibromyalgia. Pain Physician, 20(4), 307–318. [PubMed] [Google Scholar]
- Hemington, K. S. , Wu, Q. , Kucyi, A. , Inman, R. D. , & Davis, K. D. (2016). Abnormal cross‐network functional connectivity in chronic pain and its association with clinical symptoms. Brain Structure and Function, 221(8), 4203–4219. [DOI] [PubMed] [Google Scholar]
- Klugah‐Brown, B. , Di, X. , Zweerings, J. , Mathiak, K. , Becker, B. , & Biswal, B. (2020). Common and separable neural alterations in substance use disorders: A coordinate‐based meta‐analyses of functional neuroimaging studies in humans. Human Brain Mapping, 41(16), 4459–4477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klugah‐Brown, B. , Zhou, X. , Pradhan, B. K. , Zweerings, J. , Mathiak, K. , Biswal, B. , & Becker, B. (2021). Common neurofunctional dysregulations characterize obsessive—compulsive, substance use, and gaming disorders—An activation likelihood meta‐analysis of functional imaging studies. Addiction Biology, 26, e12997. [DOI] [PubMed] [Google Scholar]
- Lee, J.‐J. , Kim, H. J. , Čeko, M. , Park, B. , Lee, S. A. , Park, H. , … Woo, C.‐W. (2021). A neuroimaging biomarker for sustained experimental and clinical pain. Nature Medicine, 27(1), 174–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, J. , Xu, L. , Zheng, X. , Fu, M. , Zhou, F. , Xu, X. , … Becker, B. (2018). Common and dissociable contributions of alexithymia and autism to domain‐specific interoceptive dysregulations—a dimensional neuroimaging approach. BioRxiv, 432971. [DOI] [PubMed]
- Manoliu, A. , Meng, C. , Brandl, F. , Doll, A. , Tahmasian, M. , Scherr, M. , … Sorg, C. (2014). Insular dysfunction within the salience network is associated with severity of symptoms and aberrant inter‐network connectivity in major depressive disorder. Frontiers in Human Neuroscience, 7, 930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon, V. , & Uddin, L. Q. (2010). Saliency, switching, attention and control: A network model of insula function. Brain Structure and Function, 214(5–6), 655–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher, D. , Liberati, A. , Tetzlaff, J. , Altman, D. G. , & The PRISMA Group . (2009). Preferred reporting items for systematic reviews and meta‐analyses: The PRISMA statement. PLoS Medicine, 6(7), e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersel, D. L. , Dror, V. , & Cheung, R. (2011). Central amplification and fibromyalgia: Disorder of pain processing. Journal of Neuroscience Research, 89(1), 29–34. [DOI] [PubMed] [Google Scholar]
- Poldrack, R. A. (2006). Can cognitive processes be inferred from neuroimaging data? Trends in Cognitive Sciences, 10(2), 59–63. [DOI] [PubMed] [Google Scholar]
- Poldrack, R. A. (2011). Inferring mental states from neuroimaging data: From reverse inference to large‐scale decoding. Neuron, 72(5), 692–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice, A. S. C. , Smith, B. H. , & Blyth, F. M. (2016). Pain and the global burden of disease. Pain, 157(4), 791–796. [DOI] [PubMed] [Google Scholar]
- Schnellbächer, G. J. , Hoffstaedter, F. , Eickhoff, S. B. , Caspers, S. , Nickl‐Jockschat, T. , Fox, P. T. , … Dogan, I. (2020). Functional characterization of atrophy patterns related to cognitive impairment. Frontiers in Neurology, 11, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley, W. W. , Menon, V. , Schatzberg, A. F. , Keller, J. , Glover, G. H. , Kenna, H. , … Greicius, M. D. (2007). Dissociable intrinsic connectivity networks for salience processing and executive control. The Journal of Neuroscience, 27(9), 2349–2356. 10.1523/JNEUROSCI.5587-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao, J. , Meng, C. , Tahmasian, M. , Brandl, F. , Yang, Q. , Luo, G. , … Sorg, C. (2018). Common and distinct changes of default mode and salience network in schizophrenia and major depression. Brain Imaging and Behavior, 12(6), 1708–1719. [DOI] [PubMed] [Google Scholar]
- Staud, R. , & Domingo, M. (2001). Evidence for abnormal pain processing in fibromyalgia syndrome. Pain Medicine, 2(3), 208–215. [DOI] [PubMed] [Google Scholar]
- Talairach, J. , & Tournoux, P. (1988). 3‐dimensional proportional system. An approach to cerebral imaging. In Co‐planar stereotaxic atlas of the human brain. New York, NY : Thieme Medical Publishers. [Google Scholar]
- Tan, L. L. , Pelzer, P. , Heinl, C. , Tang, W. , Gangadharan, V. , Flor, H. , … Kuner, R. (2017). A pathway from midcingulate cortex to posterior insula gates nociceptive hypersensitivity. Nature Neuroscience, 20(11), 1591–1601. [DOI] [PubMed] [Google Scholar]
- Tanasescu, R. , Cottam, W. J. , Condon, L. , Tench, C. R. , & Auer, D. P. (2016). Functional reorganisation in chronic pain and neural correlates of pain sensitisation: A coordinate based meta‐analysis of 266 cutaneous pain fMRI studies. Neuroscience & Biobehavioral Reviews, 68, 120–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tench, C. , Tanasescu, R. , Constantinescu, C. S. , Auer, D. P. , & Cottam, W. (2020). Coordinate density analysis of neuroimaging studies. BioRxiv .
- Tench, C. R. , Tanasescu, R. , Auer, D. P. , & Constantinescu, C. S. (2013). Coordinate based meta‐analysis of functional neuroimaging data; false discovery control and diagnostics. PLoS One, 8(7), e70143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkeltaub, P. E. , Eden, G. F. , Jones, K. M. , & Zeffiro, T. A. (2002). Meta‐analysis of the functional neuroanatomy of single‐word reading: Method and validation. NeuroImage, 16(3), 765–780. [DOI] [PubMed] [Google Scholar]
- Vachon‐Presseau, E. , Tétreault, P. , Petre, B. , Huang, L. , Berger, S. E. , Torbey, S. , … Apkarian, A. V. (2016). Corticolimbic anatomical characteristics predetermine risk for chronic pain. Brain, 139(7), 1958–1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, Q. , Inman, R. D. , & Davis, K. D. (2013). Neuropathic pain in ankylosing spondylitis: A psychophysics and brain imaging study. Arthritis and Rheumatism, 65(6), 1494–1503. [DOI] [PubMed] [Google Scholar]
- Xin, F. , Zhou, F. , Zhou, X. , Ma, X. , Geng, Y. , Zhao, W. , … Becker, B. (2021). Oxytocin modulates the intrinsic dynamics between attention‐related large‐scale networks. Cerebral Cortex, 31(3), 1848–1860. [DOI] [PubMed] [Google Scholar]
- Xu, A. , Larsen, B. , Henn, A. , Baller, E. B. , Scott, J. C. , Sharma, V. , … Satterthwaite, T. D. (2021). Brain responses to noxious stimuli in patients with chronic pain: A systematic review and meta‐analysis. JAMA Network Open, 4(1), e2032236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, X. , Dai, J. , Liu, C. , Chen, Y. , Xin, F. , Zhou, F. , … Becker, B. (2020). Common and disorder‐specific neurofunctional markers of dysregulated empathic reactivity in major depression and generalized anxiety disorder. Psychotherapy and Psychosomatics, 89(2), 114–116. [DOI] [PubMed] [Google Scholar]
- Yao, S. , Becker, B. , Zhao, W. , Zhao, Z. , Kou, J. , Ma, X. , … Kendrick, K. M. (2018). Oxytocin modulates attention switching between interoceptive signals and external social cues. Neuropsychopharmacology, 43(2), 294–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarkoni, T. , Poldrack, R. A. , Nichols, T. E. , Van Essen, D. C. , & Wager, T. D. (2011). Large‐scale automated synthesis of human functional neuroimaging data. Nature Methods, 8(8), 665–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeater, T. D. , Clark, D. J. , Hoyos, L. , Valdes‐Hernandez, P. A. , Peraza, J. A. , Allen, K. D. , & Cruz‐Almeida, Y. (2021). Chronic pain is associated with reduced sympathetic nervous system reactivity during simple and complex walking tasks: Potential cerebral mechanisms. Chronic Stress, 5, 24705470211030270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, F. , Li, J. , Zhao, W. , Xu, L. , Zheng, X. , Fu, M. , … Becker, B. (2020). Empathic pain evoked by sensory and emotional‐communicative cues share common and process‐specific neural representations. eLife, 9, e56929. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Supporting Information
Data Availability Statement
Coordinates and space of normalization (TAL or MNI) of the identified results of each selected study are reported in Supporting Information.


