Abstract
Sophisticated network‐based approaches such as structural connectomics may help to detect a biomarker of mild traumatic brain injury (mTBI) in children. This study compared the structural connectome of children with mTBI or mild orthopedic injury (OI) to that of typically developing (TD) children. Children aged 8–16.99 years with mTBI (n = 83) or OI (n = 37) were recruited from the emergency department and completed 3T diffusion MRI 2–20 days postinjury. TD children (n = 39) were recruited from the community and completed diffusion MRI. Graph theory metrics were calculated for the binarized average fractional anisotropy among 90 regions. Multivariable linear regression and linear mixed effects models were used to compare groups, with covariates age, hemisphere, and sex, correcting for multiple comparisons. The two injury groups did not differ on graph theory metrics, but both differed from TD children in global metrics (local network efficiency: TD > OI, mTBI, d = 0.49; clustering coefficient: TD < OI, mTBI, d = 0.49) and regional metrics for the fusiform gyrus (lower degree centrality and nodal efficiency: TD > OI, mTBI, d = 0.80 to 0.96; characteristic path length: TD < OI, mTBI, d = −0.75 to −0.90) and in the superior and middle orbital frontal gyrus, paracentral lobule, insula, and thalamus (clustering coefficient: TD > OI, mTBI, d = 0.66 to 0.68). Both mTBI and OI demonstrated reduced global and regional network efficiency and segregation as compared to TD children. Findings suggest a general effect of childhood injury that could reflect pre‐ and postinjury factors that can alter brain structure. An OI group provides a more conservative comparison group than TD children for structural neuroimaging research in pediatric mTBI.
Keywords: diffusion MRI, graph theory, orthopedic injury, pediatric mild traumatic brain injury, structural connectome
Children with mTBI and OI demonstrated reduced global and regional network efficiency and segregation as compared to TD children. Findings suggest a general effect of childhood injury that could reflect pre‐ and postinjury factors that can alter brain structure. An OI group provides a more conservative comparison group than TD children for pediatric mTBI research.
1. INTRODUCTION
Pediatric mild traumatic brain injury (mTBI) is a major global public health concern that affects millions of children annually (Gilchrist, Thomas, Xu, McGuire, & Coronado, 2011; Ruff et al., 2009). Advanced MRI techniques can detect neuropathology and predict associated outcomes following mTBI in children (Mayer et al., 2018). Diffusion MRI (dMRI) techniques have most commonly been used to study brain structure following mTBI (Schmidt et al., 2018), and can detect white matter microstructural alterations in the absence of radiological evidence of injury (Mayer et al., 2018). Most studies have focused on specific regions or white matter tracts (Mayer et al., 2018). This may limit scientific understanding of outcomes following pediatric mTBI, which typically causes nonspecific postconcussive symptoms and subtle, heterogeneous, and diffuse alterations in brain tissue (Lumba‐Brown et al., 2018; Mayer et al., 2018). Instead, sophisticated network‐based approaches, particularly structural connectomics (i.e., applying graph theory and dMRI techniques to study white matter connections among distributed brain regions), are proving superior in identifying a biomarker of mTBI in adults and pediatric traumatic brain injury (TBI) more generally (i.e., across severities) that is sensitive and specific enough for clinical use (Raji et al., 2020; Rubinov & Sporns, 2010; Yuan, Treble‐Barna, Sohlberg, Harn, & Wade, 2017).
Altered and more diffuse network topology is supported following pediatric mTBI. However, existing research on the structural connectome in pediatric mTBI is not only scarce, but is limited by different diagnostic criteria, small sample sizes, restricted age ranges, biased male to female ratios, and varied methodologies, including different comparison groups (e.g., uninjured, typically developing (TD) children or those with extracranial injury) and network construction approach (Chung, Mannix, Feldman, Grant, & Im, 2019; Königs et al., 2017; Watson, DeMaster, & Ewing‐Cobbs, 2019; Yuan, Wade, & Babcock, 2015). The early effects of pediatric mTBI are particularly understudied. Global (i.e., higher normalized clustering coefficient, small‐worldness, normalized characteristic path length, and modularity and lower global efficiency) and regional (e.g., decreased nodal degree and clustering coefficient in a number of cortical regions) network alterations have been found within 96 hr postinjury in older children with mTBI (n = 23, aged 11–17 year, 91% male) relative to children with orthopedic injury (OI; n = 20; Yuan et al., 2015).
Longitudinal research has provided preliminary evidence of dynamic as opposed to static structural connectome alterations in pediatric mTBI. Chung et al. (2019) found differences acutely (i.e., within 72 hr postinjury; higher transivity and lower network degree) that normalized over time and reduced global efficiency chronically (within 1 year postinjury) in adolescents and young adults (n = 5, aged 11–21 years) with mTBI relative to uninjured, TD and age‐matched peers. Another longitudinal study showed attenuation of early (i.e., at least 4 weeks postinjury) global network differences (lower global network increased; greater normalized characteristic path length decreased) following a 6‐week aerobic training in adolescents with persistent symptoms at least 4 weeks after mTBI (n = 17) relative to uninjured, TD children (Yuan et al., 2017).
Network structure has also been studied in samples of children with mixed severity (mild to severe) pediatric TBI relative to extracranial injury (Königs et al., 2017) and/or uninjured, TD comparison groups (Watson et al., 2019; Yuan, Treble‐Barna, et al., 2017). In one study, global network metrics were altered after 1‐year postinjury in children with complicated mild to severe TBI (n = 17, aged 9–18 years), changed across time postinjury, and correlated with parent and child ratings of executive functioning and sustained attention (Yuan, Treble‐Barna, et al., 2017). Altered global and local network alterations roughly 2 months postinjury in a different sample of children with mild to severe TBI (n = 44, aged 8–15 years) as compared to extracranial injury and especially to uninjured, TD children, which also differed from each other, suggests that abnormalities in network structure were not specific to head injury and that the group with extracranial injury provided a more conservative comparison for studying the effects of pediatric TBI (Watson et al., 2019). This highlights the importance of comparison group selection. However, the results from both of those studies were not specific to pediatric mTBI. The only study to investigate outcomes in mTBI separately from more severe (i.e., moderate to severe) TBI found no differences between children with risk factors (based on the presence of neuroradiological findings) for complicated mTBI (n = 20, aged 8–14 years) and children with extracranial injury at an average of 2.8 years postinjury (Königs et al., 2017).
The current study examined the structural connectome in a larger sample of children with postacute mTBI (n = 83) or mild OI (n = 37) and uninjured, TD (n = 39) children to determine the most appropriate comparison group for neuroimaging research in pediatric mTBI, and also to help increase current understanding of the early neurobiological effects of pediatric mTBI. Reduced global and regional network efficiency and integration were expected to occur in postacute mTBI relative to mild OI and especially compared to TD children (Chung et al., 2019; Watson et al., 2019; Yuan et al., 2015).
2. METHODS
2.1. Study design and procedure
Data were drawn from two pediatric neuroimaging studies conducted on the same MRI scanner at the Alberta Children's Hospital in Calgary, AB between September 2016 and July 2019: the Advancing Concussion Assessment in Pediatrics (A‐CAP) study and a study of typical brain development in childhood and adolescence. Children with mTBI or OI who were between the ages of 8.00–16.99 years were recruited and assessed as part of the A‐CAP study, a large multi‐site study of pediatric mTBI that included a postacute assessment with longitudinal follow‐up (Yeates et al., 2017). For both groups, acute injury signs and symptoms were assessed within 48 hr postinjury at the time of enrollment in the emergency department (ED) at Alberta Children's Hospital. Children with mTBI or OI returned for a postacute assessment targeted for 10 days postinjury that included a 3T MRI scan. The group of TD children was comprised of healthy children who were recruited from the Calgary community as part of a study of typical brain development in childhood and adolescence (Geeraert, Lebel, & Lebel, 2019).
2.2. Standard protocol approvals, registrations, and patient consents
Both studies were conducted with the approval of the conjoint health research ethics board at the University of Calgary, and all participants provided informed assent when appropriate and parents or guardians provided written informed consent in accordance with the Declaration of Helsinki. The relevant methodology for the present study is described in detail below, although both study protocols are published (Geeraert et al., 2019; Yeates et al., 2017).
2.3. Data availability
Anonymized data will be shared by request from any qualified investigator.
2.4. Participants
2.4.1. Mild TBI group
Children in the mTBI group sustained a blunt head trauma resulting in at least one of the following three criteria, consistent with the World Health Organization (WHO) definition of mTBI: an observed loss of consciousness, a Glasgow Coma Scale score of 13 or 14, or at least one acute sign or symptom of concussion as noted by ED medical personnel on a standard case report form, such as posttraumatic amnesia, focal neurological deficits, vomiting, headache, dizziness, or other mental status changes (Carroll, Cassidy, Holm, Kraus, & Coronado, 2004; Cassidy et al., 2004; Teasdale & Jennett, 1974). Children were excluded if they demonstrated delayed neurological deterioration (i.e., Glasgow Coma Scale <13), required neurosurgical intervention, or had loss of consciousness >30 min or post traumatic amnesia >24 hr.
2.4.2. Mild OI group
Children with OI were included if they sustained an upper or lower extremity fracture, sprain, or strain due to blunt force/physical trauma, associated with an Abbreviated Injury Scale score ≤4 (Committee on Injury Scaling, 1998). Children were excluded from the OI group if they had any head trauma or signs or symptoms of concussion, or any injury requiring surgical intervention or procedural sedation.
Exclusion criteria for both injury groups included any other severe injury that resulted in an Abbreviated Injury Scale score >4; prior concussion within the past 3 months; hypoxia, hypotension, or shock during or following the injury; history of previous TBI requiring hospitalization; premorbid neurological disorder or intellectual disability; injury resulting from nonaccidental trauma; history of severe psychiatric disorder requiring hospitalization; or any MRI contraindications. Additional inclusion/exclusion criteria are described in the published study protocol (Yeates et al., 2017).
2.4.3. TD group
Children in the TD group were recruited from the Calgary community. All had uncomplicated birth histories and were born between 37 and 42 weeks gestational age. Participants were excluded if they had any history of head trauma that required medical attention or hospitalization, severe neurodevelopmental or intellectual disability, neurological or severe psychiatric disorder, or MRI contraindication (Geeraert et al., 2018; Geeraert et al., 2019). Of an initial 53 enrolled participants in the parent TD study, 12 were excluded from this study for having an age outside of the A‐CAP study age range (i.e., <8.00 or >16.99 years), leaving data from 41 children eligible for subsequent analysis (see Figure 1).
FIGURE 1.
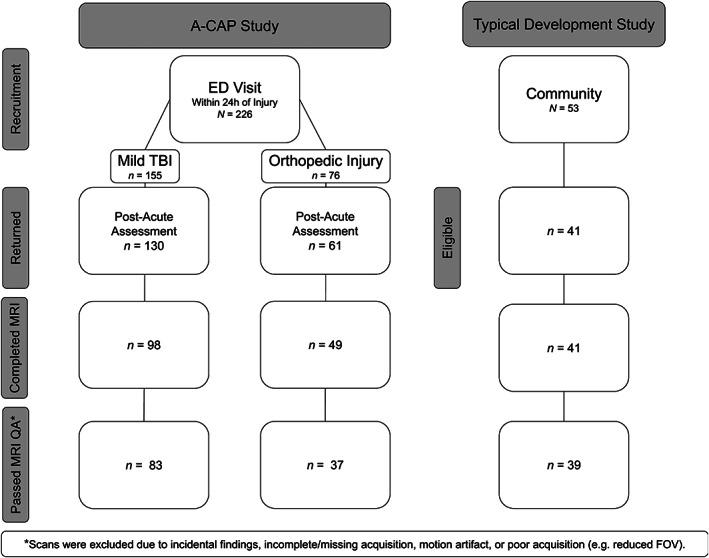
Flow chart summarizing how the final sample was derived. mTBI, mild traumatic brain injury; OI, orthopedic injury; QA, quality assurance; TD, typically developing
2.5. Demographic and injury characteristics
For children with mTBI or OI, parents completed demographic questionnaires and the Health and Behavior Inventory (Ayr, Yeates, Taylor, & Browne, 2009) at the postacute assessment to measure total pre‐ (premorbid) and postinjury symptoms. A standardized reliable change coefficient (z‐score) was calculated to identify whether or not children were symptomatic at the postacute follow‐up relative to premorbid ratings. For TD children, parents completed demographic questionnaires, but symptom ratings were not collected.
2.6. Magnetic resonance imaging
All participants completed a 3T MRI scan without sedation on the same scanner at the Alberta Children's Hospital. Children in the mTBI and OI groups completed MRI 2–20 days postinjury, with most scans (75%) completed 6–10 days postinjury (see Table 1).
TABLE 1.
Summary of global and regional (nodal) graph theory metrics
| Network level | Metric | Abbreviation | Definition | Interpretation | |
|---|---|---|---|---|---|
| Degree | k | Total number of connections among all nodes in the graph. | Network sparsity or density of connectivity among brain regions within the whole network. | ||
| Global metrics | |||||
| Clustering coefficient | Cp | Averaged proportion of each node's neighbors that are also considered to be neighbors. | The overall extent to which proximal network regions are connected or clustered. | ||
| Shortest path length | Lp | Averaged characteristic path length (i.e., most direct connectivity) between all nodes within the graph. | Measures integration efficiency between brain regions within the whole network. | ||
| Small‐worldness |
|
Ratio of standardized a clustering coefficient to standardized path length. | Extent of local clustering among nodes within a network. Higher values indicative of highly specialized and segregated regions for functional specialization that are strongly and efficiently connected for integration. | ||
| Global efficiency | Eg | Averaged efficiency of information transfer among all network nodes; the average inverse shortest path length. | Averaged connectivity among network nodes, with higher values indicating fewer pathways are required to reach to other nodes in the network (i.e., greater efficiency). | ||
| Local efficiency | E local | Averaged efficiency of information transfer between each node in the network and its neighboring nodes. | Averaged connectivity among a given node and all other nodes in the network, with higher values indicating fewer pathways are required to reach to other nodes in the network. | ||
| Regional (nodal) metrics | |||||
| Efficiency | Ne | Efficiency of information transfer of a given node to all other nodes in the graph. | Connectivity among a given node and all other nodes in the network, with higher values indicating fewer pathways are required to reach to other nodes in the network. | ||
| Local efficiency | NLe | Efficiency of information transfer of a given node to proximal or neighboring nodes. | Connectivity among a given node and it's neighboring nodes. | ||
| Clustering coefficient | NCp | Proportion of a given node's neighbors that are also considered to be neighbors. | Measures the overall extent to which nodes are clustered or connected to proximal (local) networks. | ||
| Shortest path length | NLp | Shortest path length among a given node and all other nodes in the graph. | Index of integration efficiency between a given brain region and other brain regions in the (whole) network. | ||
| Betweenness centrality | BC | Frequency that a given node is part of shortest paths to all other (whole) network nodes. | |||
| Degree centrality | Dc | Total edge count for a given node. |
Standardized z‐scores based on randomly generated networks (i.e., N = 1,000).
2.6.1. Image acquisition
MRI data were acquired using a General Electric MR750w 3T scanner with a 32‐channel head coil at the Alberta Children's Hospital in Calgary, AB (GE, Milwaukee, WI). Diffusion‐weighted images were acquired using a spin echo EPI sequence with 5 b = 0 s/mm2 volumes and 30 gradient directions at b = 900 s/mm2, TR/TE = 12,000/88–98 ms, isotropic resolution = 2.2 mm, FOV = 256 × 256 cm, 57 contiguous slices, and scan duration = 7:12 min.
2.6.2. Image processing
Diffusion‐weighted DICOM data were converted into NIfTI format using the dcm2niix tool in MRIcron (publicly available software; https://github.com/rordenlab/dcm2niix), and the bval and bvec files were automatically created from the raw diffusion‐weighted DICOM headers.
2.6.3. Quality assurance
Initial visual review of both image types was conducted to identify and exclude any data with incidental findings (n = 2), scanner artifact such as aliasing or warping (n = 3), data collected without the default scan parameters (n = 10), and any incomplete or partially acquired images (n = 3). The removal of diffusion‐weighted volumes (gradients) with severe motion artifact influenced DTI metrics was conducted for the participants who had 1 bad volume on diffusion‐weighted imaging (n = 68) to account for the effects of motion artifact. Images that had >7 volumes with severe motion artifact (n = 11) were excluded from subsequent analysis.
2.6.4. Structural connectome
ExploreDTI v4.8.6 in MATLAB R2019a was used to calculate the diffusion tensor, conduct whole brain fiber tractography, and compute a connectivity matrix for each participant using the preprocessed diffusion‐weighted images (Leemans, Jeurissen, Sijbers, & Jones, 2009). A deterministic streamline approach was used for whole brain fiber tractography. Fiber tracking was initiated using randomized seed points throughout the brain mask using a 0.10 seed and tractography FA threshold to ensure that fiber pathways terminated in gray matter, 0.5 mm step size, 30° angle threshold, and 50‐500 mm streamline length (Long, Kar, Gibbard, Tortorelli, & Lebel, 2019; Thomas et al., 2014). All tracked streamlines were saved, and the resulting whole brain fiber tractography was extracted and used to compute a connectivity matrix for each participant.
The automated anatomical labeling (AAL‐90) template was used to define 90 nodes from supratentorial brain regions in native (diffusion) space (Long et al., 2019). This was completed in MATLAB R2019a using functions from open‐source software packages, Analysis of Functional Neuroimages (AFNI) v5.3.1, and FMRIB Software Library (FSL) v6.0.0 (Cox, 1996; Jenkinson, Beckmann, Behrens, Woolrich, & Smith, 2012; Smith et al., 2004). FMRIB's Linear Image Registration Tool (FLIRT) was used to register a standardized Montreal Neurological Institute (MNI) template, freely provided by the FSL package (FMRIB58_FA_1mm.nii.gz), and each AAL‐90 atlas brain regions individually to the FA map of each participant in native space (Collins, Neelin, Peters, & Evans, 1994; Jenkinson, 2002). Adjacency matrices were constructed twice, using the average FA of fibers among AAL regions, including passing and terminating fibers, resulting in a 90 × 90 connectivity matrix for each participant in ExploreDTI (Leemans et al., 2009). All connectivity matrices were fully connected.
2.6.5. Network metrics
Graph theoretical metrics were calculated for the binarized connectivity matrix of each participant using the GRaph thEoreTical Network Analysis (GRETNA) toolbox (Wang et al., 2015). Included metrics were global and nodal level of clustering coefficient, shortest path length, small‐worldness, global efficiency, local efficiency, betweenness centrality, and network degree centrality (see Table 1; Bassett & Bullmore, 2006; Bullmore & Sporns, 2009; Humphries & Gurney, 2008; Power, Schlaggar, Lessov‐Schlaggar, & Petersen, 2013; Rubinov & Sporns, 2010). Briefly, clustering coefficient (Cp) is the average proportion of node neighbors that are also considered to be neighbors, reflecting the overall extent to which proximal network regions are connected or clustered. Shortest path length (Lp), the average shortest path length among all nodes within the network, is considered a measure of integration efficiency between two nodes in the network. The ratio between the clustering coefficient (Cp) and shortest path length (Lp), as compared to randomly generated networks, is characterized by sigma (), which is used to index small‐worldness of brain connectivity (i.e., highly specialized and segregated regions for functional specialization that are strongly and efficiently connected for integration). Global efficiency (Eg) measures the efficiency of transferring information from each node to all other nodes in the whole graph, with higher efficiency indicating that fewer pathways are required to reach to other nodes in the network; local efficiency (E local) indexes the efficiency of transferring information from each node and its neighbors. Betweenness centrality (Bc) reflects the frequency that a node is part of average shortest paths; network degree centrality (Dc) is the number of edges (connections) of each node.
2.7. Statistical analyses
Statistical analyses were computed in RStudio v1.1.383 (R v4.0.3; R Core Team, 2017; RStudio Team, 2020). Sample demographic data were analyzed using analysis of variance and chi‐square techniques.
Multiple multivariable regressions were used to investigate group differences on global network metrics, with covariates age and sex included in all models. Permutation testing (k = 1,000, critical p <.05) was conducted for models with a nominally significant (p <.05) effect of group using the “predictmeans” R package to control for multiple comparisons (Long et al., 2019; Luo, Ganesh, & Koolaard, 2021). For the regional (nodal) graph theory metrics, two linear mixed effects models were computed using the “lmerTest” R package to examine differences between the groups, hemispheres, and, in the full factorial model, their interaction, with age and sex as covariates and participant as a random effect. The best fitting model was established for each dependent variable using χ 2 comparison tests of Akaike's Information Criterion and Bayesian Information Criterion (Bates, Mächler, Bolker, & Walker, 2015; Kuznetsova, Brockhoff, & Christensen, 2017). The most parsimonious and best fitting model was used for each dependent variable. The first model was:
and the full factorial model was:
The false discovery rate (FDR) was applied at a critical corrected p <0.05 to correct for multiple comparisons (i.e., 45 models analyzed for bilateral regions; Benjamini & Hochberg, 1995; Kuznetsova et al., 2017; Wang et al., 2015). The BrainNet Viewer toolbox was used to visualize the regional network results (Xia, Wang, & He, 2013).
2.8. Data availability
A dataset with deidentified participant data and a data dictionary will be made available upon reasonable request from any qualified investigator to the corresponding author, subject to a signed data access agreement, with publication.
3. RESULTS
3.1. Sample
A total of 226 children were recruited at Alberta Children's Hospital (Calgary, AB) as part of the larger A‐CAP study. Of the enrolled children with mTBI or OI, 191 (85%) returned for the postacute assessment, of which 147 (65%) children completed MRI (see Figure 1). Orthodontia and scheduling difficulties were the most common reasons that MRI was not completed. The children with mTBI or OI who returned for the postacute assessment did not differ from participants who did not return in terms of sex, race, or age at time of injury, all . Children with mTBI or OI who returned for the postacute assessment and completed the postacute MRI did not differ from participants who returned but did not complete an MRI in terms of preinjury or postacute somatic or cognitive symptoms, race, sex, or age at time of injury, all . After the initial quality review, all remaining diffusion‐weighted scans were manually inspected for motion in accordance with published protocols (Reuter et al., 2015; Roalf et al., 2016; Rosen et al., 2018). Motion artifact was more common in the children with mTBI (β = 0.78, p <.001) and OI (β = 0.73, p <.001) relative to the TD children, but did not differ between the mTBI and OI groups (β = 0.07, p = .717). Figure 1 summarizes how the final sample was derived.
3.2. Sample characteristics
The final sample characteristics and demographic data are presented in Table 2. The mTBI, OI, and TD groups did not differ in terms of Full Scale IQ, race, sex, or age. Time between injury and the postacute MRI scan did not differ between the mTBI and OI groups. Groups did differ in terms of postacute symptoms and mechanism of injury, but not whether the injury was sustained during sport or recreational play.
TABLE 2.
Sample characteristics. Sociodemographic information for the children in the uninjured, typically developing (TD), mild traumatic brain injury (mTBI), and mild orthopedic injury (OI) groups (A), and injury characteristics (B) and postinjury symptom (C) for the children with mTBI or mild OI
| TD | OI | mTBI | |||
|---|---|---|---|---|---|
| n = 39 | n = 37 | n = 83 | p‐value | ||
| A. Sociodemographic characteristics | |||||
| Age [mean (SD) years] | 12.52 (2.34) | 13.07 (2.29) | 12.95 (2.26) | .526 | |
| Full scale IQ [mean (SD)] | 108.58 (14.74) | 108.59 (10.71) | 106.15 (12.43) | .519 | |
| Sex [n (%) male] | 21 (54) | 18 (49) | 53 (64) | .250 | |
| Race [n (%) White] | 22 (58) | 26 (72) | 65 (78) | .058 | |
| Diffusion‐weighted image RMS displacement [mean (SD) mm] | 0.29 (0.09) | 0.28 (0.13) | 0.29 (0.15) | .904 | |
| Density [mean (SD)] | 0.73 (0.05) | 0.72 (0.04) | 0.71 (0.04) | .282 | |
| B. Injury characteristics | |||||
| MRI scan day postinjury [mean (SD)] | 9.41 (3.64) | 8.59 (3.25) | .222 | ||
| Mechanism of injury [n (%)] | .034 | ||||
| Fall | 19 (51.4) | 28 (35.9) | |||
| Bicycle related | 5 (13.5) | 2 (2.6) | |||
| Motor vehicle collision | 0 (0.0) | 1 (1.3) | |||
| Struck object | 6 (16.2) | 23 (29.5) | |||
| Struck person | 5 (13.5) | 23 (29.5) | |||
| Other | 1 (2.7) | 1 (1.3) | |||
| Unknown | 1 (2.7) | 0 (0.0) | |||
| Injured during sport/recreational play [n (%)] | 31 (83.8) | 66 (84.6) | .999 | ||
| Loss of consciousness [n (%) yes] | — | 15 (18.5) | — | ||
| Glasgow coma scale score of 15 [n (%)] | — | 75 (90.4) | — | ||
| Extracranial injury [n (%)] | .017 | ||||
| Fracture | 17 (51.5) | 3 (25.0) | |||
| Sprain/soft tissue injury | 15 (45.5) | 5 (41.7) | |||
| Possible fracture | 1 (3.0) | 1 (8.3) | |||
| Laceration | 0 (0.0) | 3 (25.0) | |||
| C. Postinjury symptoms | |||||
| Total symptoms score [mean (SD)] | 8.00 (7.73) | 19.80 (12.35) | <.001 | ||
| Symptomatic [n (%)] | 2 (5.4) | 37 (45.1) | <.001 | ||
Note: RMS displacement computed for raw images (i.e., 30 volumes) using FSL eddy (Smith et al., 2004 ); Full Scale IQ per the 2‐subtest (i.e., Vocabulary and Matrix Reasoning) version of the Wechsler Abbreviated Scales of Intelligence (Wechsler, 2011); symptomatic children showed significant increase (i.e., z‐score > reliable change index) in postacute as compared with retrospective premorbid symptoms, based on parent Health and Behavior Inventory (Ayr et al., 2009) ratings.
3.3. Power analysis
A sensitivity power analysis, conducted using G*Power v3.1, indicated that the current sample size (i.e., N = 159) was sufficiently powered (1 − β = .80) to detect small effects (partial R 2 = .10) with 5 predictors at a critical F‐value = 3.09 and α = .05 (Faul, Erdfelder, Buchner, & Lang, 2009).
3.4. Network metrics
The groups did not differ in network sparsity (i.e., degree; range = 0.62–0.83; see Table 2). In the interest of space, only the models with group differences in global and regional (nodal) network metrics that survived correction for multiple comparisons (i.e., permutation p <.05 and FDR corrected p <.05 for global and local metrics, respectively) are presented below.
3.4.1. Global metrics
Results for the multivariable linear regression and descriptive data for group differences in global graph theory metrics are reported in Table 3 and summarized in Figure 2. The mTBI and OI groups did not differ on any of the global graph theory metrics in final models. However, both the mTBI and OI groups demonstrated lower local efficiency (E local) and clustering coefficient (Cp) than the TD group. Across groups, global network metrics were significantly (positively) associated with age, but not sex.
TABLE 3.
Statistical results for the global graph theoretical metrics that differed (permutation p <.05) between the uninjured, typically developing (TD), mild traumatic brain injury (mTBI), and mild orthopedic injury (OI) groups
| 95% confidence interval | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Global metric | Model predictor | F‐value | p‐value a | Group comparison | β | SE | t‐value | Permutation p‐value | Cohen's d | Lower | Upper |
| Clustering coefficient (Cp) | Group | 3.31 | .039 | TD–OI | 1.02e−02 | 4.84e−03 | 2.11 | .045 | 0.49 | 0.03 | 0.94 |
| TD–mTBI | 1.17e−02 | 4.10e−03 | 2.86 | .006 | 0.56 | 0.17 | 0.94 | ||||
| OI–mTBI | 1.50e−03 | 4.19e−03 | 0.36 | .734 | 0.07 | −0.32 | 0.47 | ||||
| Age | 13.42 | <.001 | 2.60e−03 | ||||||||
| Sex | 0.82 | .367 | Female–Male | 3.11e−03 | |||||||
| Local efficiency (Elocal) | Group | 3.31 | .039 | TD–OI | 5.11e−03 | 2.42e−03 | 2.11 | .045 | 0.49 | 0.03 | 0.94 |
| TD–mTBI | 5.86e−03 | 2.05e−03 | 2.86 | .006 | 0.56 | 0.17 | 0.94 | ||||
| OI–mTBI | 7.49e−04 | 2.09e−03 | 0.36 | .733 | 0.07 | −0.32 | 0.47 | ||||
| Age | 13.42 | <.001 | 1.30e−03 | ||||||||
| Sex | 0.82 | .367 | Female–male | 1.56e−03 | |||||||
Unadjusted p‐value reported. Bolded and italicized = permutation p‐value <.05; Underlined = |Cohen's d| ≥ .50 (i.e., ≥ moderate effect size).
FIGURE 2.
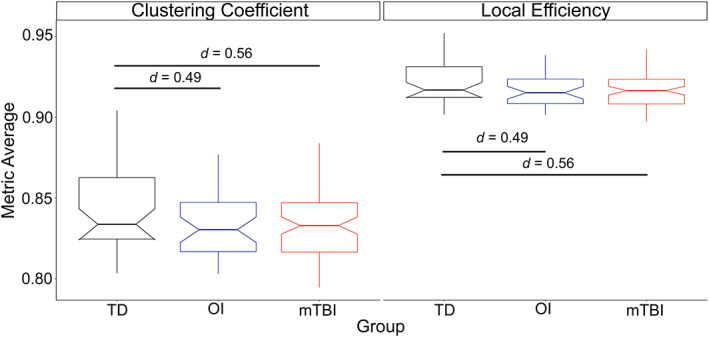
Group differences in global network metrics. Differences were observed between the groups in global graph theory metrics, whereby the uninjured, typically developing (TD) children had significantly greater clustering coefficient (left) and local efficiency (right) than the children with mild traumatic brain injury (mTBI) and orthopedic injury (OI)
3.5. Regional metrics
Results for the linear mixed effects for the regional (nodal) network measures that survived FDR correction are summarized in Table 4 and Figure 3. The mild TBI and OI groups did not differ on any of the nodal graph theory metrics. However, as compared to the TD children, children with mTBI and OI had lower degree centrality (Dc) and nodal efficiency (Ne) and higher characteristic path length (NLp) in the fusiform gyrus, and lower clustering coefficient (NCp; the inverse to nodal efficiency (Ne)] in the superior and medial orbital frontal gyrus, paracentral lobule, insula, and thalamus. Across groups, regional metrics were significantly associated with hemisphere, but not age or sex after correcting for multiple comparisons. The left fusiform gyrus demonstrated higher degree centrality and nodal efficiency with all other nodes in the network, and lower shortest path length as compared with the right fusiform gyrus; and, the clustering coefficient was higher in the left paracentral gyrus and right superior and medial orbital frontal gyrus as compared with the right paracentral gyrus and left superior and medial orbital frontal gyrus.
TABLE 4.
Statistical results for the nodal graph theoretical metrics that differed (FDR corrected p <.05) between the uninjured, typically developing (TD) children and the mild traumatic brain injury (mTBI) and orthopedic injury (OI) groups
| 95% confidence interval | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nodal metric | Node | Model predictor | F‐value | p‐value a | Contrast | β | SE | t‐value | Permutation p‐value | Cohen's d | Lower | Upper |
| Degree centrality (Dc) | Fusiform gyrus | Group | 12.71 | <.001 | TD–OI | 6.04 | 1.35 | 4.49 | <.001 | 0.96 | 0.54 | 1.38 |
| TD–mTBI | 5.07 | 1.14 | 4.45 | <.001 | 0.80 | 0.45 | 1.16 | |||||
| OI–mTBI | −9.69e−01 | 1.16 | −0.83 | .999 | −0.15 | −0.52 | 0.21 | |||||
| Hemisphere | 148.99 | <.001 | Left–right | 8.65 | ||||||||
| Age | 0.01 | 0.933 | 0.017 | |||||||||
| Sex | 5.81 | 0.017 | Female–male | 2.31 | ||||||||
| Clustering coefficient (NCp) | Superior orbital gyrus | Group | 5.67 | <.001 | TD–OI | 0.019 | 7.06e−03 | 2.75 | .019 | 0.68 | 0.19 | 1.17 |
| TD–mTBI | 0.019 | 5.98e−03 | 3.17 | <.001 | 0.66 | 0.25 | 1.08 | |||||
| OI–mTBI | −5.18e−04 | 6.11e−03 | −0.08 | .999 | −0.02 | −0.44 | 0.41 | |||||
| Hemisphere | 39.47 | <.001 | Left–right | −2.01e−02 | ||||||||
| Age | 1.58 | .211 | 1.36e−03 | |||||||||
| Sex | <0 .01 | .927 | Female–male | 4.63e−04 | ||||||||
| Middle orbital gyrus | Group | 7.76 | 0.001 | TD–OI | 3.27e−02 | 8.79e−03 | 3.72 | <.001 | 0.95 | 0.44 | 1.45 | |
| TD–mTBI | 2.38e−02 | 7.44e−03 | 3.20 | <.001 | 0.69 | 0.26 | 1.11 | |||||
| OI–mTBI | −8.85e−03 | 7.60e−03 | −1.16 | 1.000 | −0.26 | −0.69 | 0.18 | |||||
| Hemisphere | 53.47 | <.001 | Left–right | −2.83e−02 | ||||||||
| Age | 1.08 | 0.301 | 1.40e−03 | |||||||||
| Sex | 0.33 | 0.566 | Female–male | −3.59e−03 | ||||||||
| Insula | Group | 6.85 | 0.001 | TD–OI | 1.96e−02 | 6.42e−03 | 3.04 | <.001 | 0.78 | 0.27 | 1.29 | |
| TD–mTBI | 1.89e−02 | 5.44e−03 | 3.47 | <.001 | 0.75 | 0.32 | 1.18 | |||||
| OI–mTBI | −6.85e−04 | 5.55e−03 | −0.12 | .999 | −0.03 | −0.47 | 0.41 | |||||
| Hemisphere | 0.04 | 0.834 | Left–right | −5.90e−04 | ||||||||
| Age | 5.70 | 0.018 | 2.35e−03 | |||||||||
| Sex | 5.30 | 0.023 | Female–male | 1.05e−02 | ||||||||
| Thalamus | Group | 7.43 | 0.001 | TD–OI | 2.40e−02 | 8.31e−03 | 2.89 | <.001 | 1.65 | 0.52 | 2.78 | |
| TD–TBI | 2.63e−02 | 7.04e−03 | 3.74 | <.001 | 1.81 | 0.85 | 2.76 | |||||
| OI–TBI | 2.34e−03 | 7.18e−03 | 0.33 | 0.668 | 0.16 | −0.81 | 1.14 | |||||
| Hemisphere | 1.45 | 0.231 | Left–right | −1.96e−03 | ||||||||
| Age | 6.30 | 0.013 | 3.20e−03 | |||||||||
| Sex | 0.18 | 0.670 | Female–male | 2.52e−03 | ||||||||
| Paracentral lobule | Group | 5.80 | <.001 | TD–OI | 1.51e−02 | .03‐e03 | 2.49 | .011 | 0.45 | 0.09 | 0.81 | |
| TD–mTBI | 1.64e−021 | .11‐e03 | 3.21 | .001 | 0.49 | 0.19 | 0.79 | |||||
| OI–mTBI | 1.36e−03 | 5.22‐e03 | 0.26 | .796 | 0.04 | −0.27 | 0.35 | |||||
| Hemisphere | 17.55 | <.001 | Left–right | 1.57e−02 | ||||||||
| Age | 6.38 | .013 | 2.34e−03 | |||||||||
| Sex | 0.07 | .788 | Female–male | 1.16e−03 | ||||||||
| Efficiency (Ne) | Fusiform gyrus | Group | 12.71 | <.001 | TD–OI | 3.39e−02 | 7.56e−03 | 4.49 | <.001 | 0.95 | 0.53 | 1.38 |
| TD–TBI | 2.85e−02 | 6.40e−03 | 4.45 | <.001 | 0.80 | 0.45 | 1.16 | |||||
| OI–TBI | −5.43e−03 | 6.54e−03 | −0.83 | 1.000 | −0.15 | −0.52 | 0.21 | |||||
| Hemisphere | 148.84 | <.001 | Left–right | 4.86e−02 | ||||||||
| Age | 0.01 | 0.935 | 9.43e−05 | |||||||||
| Sex | 5.80 | 0.017 | Female–male | 1.29e−02 | ||||||||
| Shortest path length (NLp) | Fusiform gyrus | Group | 11.91 | <.001 | TD–OI | −4.58e−02 | 1.05e−02 | −4.35 | <.001 | −0.90 | −1.31 | −0.49 |
| TD–TBI | −3.84e−02 | 8.92e−03 | −4.31 | <.001 | −0.75 | −1.10 | −0.41 | |||||
| OI–TBI | 7.41e−03 | 9.11e−03 | 0.81 | 0.437 | 0.15 | −0.21 | 0.50 | |||||
| Hemisphere | 135.35 | <.001 | Left–right | −6.65e−02 | ||||||||
| Age | 0.00 | 0.990 | 2.04e−05 | |||||||||
| Sex | 5.66 | 0.019 | Female–male | −1.78e−02 | ||||||||
Unadjusted p‐value reported. Italicized and bolded = corrected p‐value <.05; Underlined = |Cohen's d| ≥.80 (i.e., ≥ large effect size).
FIGURE 3.
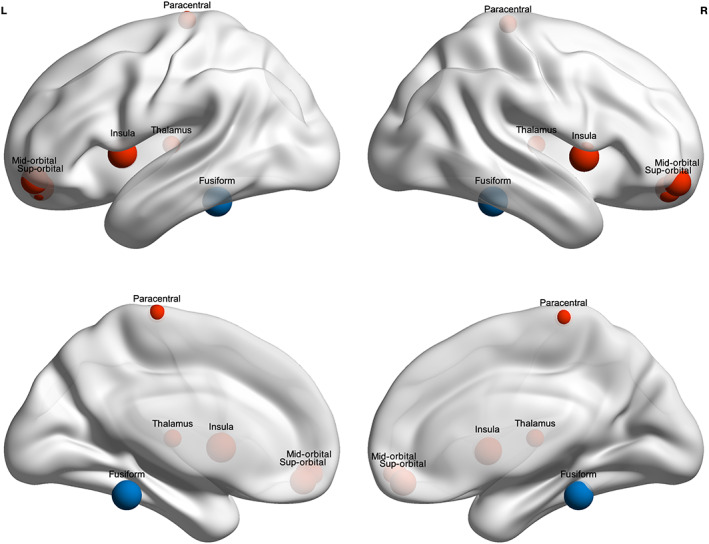
Group differences in nodal (regional) metrics. As compared to the uninjured, typically developng (TD) children, children with mild traumatic brain injury (mTBI) or orthopedic injury (OI) had significantly lower degree centrality (Dc) and nodal efficiency (Ne), but significant higher characteristic path length (NLp) in the fusiform gyrus (shown in blue), and lower nodal clustering coefficient (NCp) in several regions (shown in red). Nodal metrics did not differ between the mTBI and OI groups. Node size corresponds to lowest, absolute Cohen's d value of differences between the TD and injury groups (see Table 4)
3.6. Additional analyses
Results were similar after controlling for group differences in race. For the groups with mTBI and mild OI, MRI scan day postinjury was not associated with global or nodal (regional) graph theory metrics, and did not significantly moderate the (nonsignificant) differences between injury groups.
4. DISCUSSION
We used two comparison groups to delineate the specific effects of mTBI acquired during childhood and adolescence: a TD group and a group with mild OI. Here, features of the postacute mTBI structural connectome were similar at both the global and regional (nodal) levels of brain networks after pediatric mTBI and mild OI, but differed between both injury groups and uninjured, TD children. Thus, global and regional alterations were associated with mild traumatic injury more generally, regardless of whether or not the injury involved the head. Overall, the results provide further evidence that conclusions regarding the effects of mTBI can be influenced by the choice of comparison group (Wilde et al., 2018), and highlight the importance of comparison group selection in neuroimaging studies of pediatric mTBI.
The lack of differences between the mTBI and OI groups indicate that children with OI provided a more conservative comparison group than TD children for studying the early effects of mTBI. This was not altogether surprising given previous structural neuroimaging research in youth and young adults with mTBI and also in children with TBI of mixed severity showing no significant differences in white matter microstructure or the structural connectome among traumatic injury groups, but significant differences between individuals with head or extracranial injury as compared to those with typical development (Watson et al., 2019; Wilde et al., 2018). The current findings could reflect both pre‐ and postinjury factors. Children with mTBI and OI are likely to have similar premorbid psychosocial and neurobehavioral characteristics that increase their risk of injury, for example, hyperactivity and impulsivity (Bruce, Kirkland, & Waschbusch, 2007; Hajek et al., 2010; Loder, Warschausky, Schwartz, Hensinger, & Greenfield, 1995). Extracranial injury results in systemic inflammatory responses that may result in neuroinflammation, obscuring differences between children with mTBI or OI (Sun, McDonald, Brady, O'Brien, & Shultz, 2018). Early postinjury effects of pain and acute post‐traumatic stress could also contribute to these findings (Hajek et al., 2010). Less understood is the possibility of occult or sub‐concussive brain injury in children with OI (Barber Foss et al., 2019; McAllister et al., 2014; Sollmann et al., 2018). Although children with any head trauma or signs or symptoms of concussion were excluded from this OI group, sub‐concussive injuries might influence postacute differences between the children with OI and TD children. This is an important consideration for future research given that most injuries in this sample were sport related.
Global and regional (nodal) network metrics showed medium sized differences in the first few weeks after mild traumatic injury (i.e., mTBI and OI) compared to the TD group. At the global brain network level, lower clustering coefficient and local efficiency suggest reduced whole brain network efficiency and more segregation of specialized, clustered brain networks in children with traumatic injury. This aligns with limited research in pediatric traumatic injury and mTBI in particular, although the specific global metrics that differ vary across studies (Chung et al., 2019; Königs et al., 2017; Watson et al., 2019; Yuan et al., 2015). Regional (nodal) degree centrality, clustering coefficient, efficiency, and shortest path length differed in several frontal and temporal cortical regions as well as the thalamus, bilaterally, when comparing the two injury groups to the TD group. Although the results were not specific to head injury, frontal and temporal cortices are thought to be especially sensitive to mTBI (Mayer et al., 2018). In addition, those regions are part of frontal‐subcortical and frontal‐parietal brain networks that subserve executive functioning and other higher‐order cognitive processes, and might contribute to commonly reported postconcussive symptoms such as forgetfulness and inattention in pediatric mTBI. However, future research is needed to disentangle whether the lack of significant differences between the mTBI and OI groups reflects premorbid or postinjury factors, or some combination thereof.
Age was robustly associated with global, but was less strongly associated with regional, graph theory metrics across the groups. In typical development, global network changes likely reflect increases in white matter connectivity coupled with gray matter pruning, both of which occur into young adulthood and allow for increasingly efficient information processing and integration (Gogtay et al., 2004; Lebel, Treit, & Beaulieu, 2019; Rubinov & Sporns, 2010). Significant hemispheric differences were also observed across groups for the nodal metrics, suggesting that the left fusiform gyrus and paracentral gyrus and the right orbital frontal regions were more efficient and segregated in relation to all other regions in the network than in the opposite hemisphere. The role of the left hemisphere and orbital prefrontal circuits involved in language processing and executive control that develop with maturation during childhood and into early adulthood may drive the asymmetry observed here (Lebel et al., 2019). However, in contrast to research in TD children (Ingalhalikar et al., 2014), robust sex differences were not found for the graph theory metrics in final models after correction for multiple comparisons.
4.1. Limitations
Binarized FA values were examined, but other matrix construction methods (e.g., weighted FA; streamline count) may yield different conclusions about group differences. The study did not examine longitudinal changes, but only early postinjury effects. Prospective, longitudinal research is needed to clarify how mTBI impacts brain structure across time postinjury. Additional research is needed to establish whether OI and mTBI groups differ when using other advanced neuroimaging modalities (e.g., resting‐state and functional MRI and/or electrophysiology) given that results may be specific to this neuroimaging modality, that is, dMRI. Based on the clinical characteristics (i.e., Glasgow Coma Scale score, loss of consciousness) of this sample, children with mTBI had relatively mild injuries. Future studies that examine more severe injuries within the mTBI spectrum are needed to corroborate the results of the current study.
5. CONCLUSIONS AND FUTURE DIRECTIONS
The current study addressed shortcomings of previous research by investigating the structural connectome in a larger, prospectively recruited sample of children with mTBI as compared to two different comparison groups: children with mild OI and TD children. Overall, results suggest that traumatic injury, regardless of whether it is to the head, is associated with differences in whole brain and regional network alterations in children. Results also suggest that children with OI may provide a more conservative comparison group than TD children for structural neuroimaging research in pediatric mTBI. Longitudinal research is needed to help determine whether the absence of significant differences between children with mTBI or OI reflects pre‐ or postinjury factors, and also to better understand changes in the structural connectome across recovery from both types of injuries. Finally, research that examines the functional correlates (e.g., postconcussive symptoms) of differences in structural brain networks would help further our understanding of the neurobiological outcomes of pediatric mTBI. This may be especially important given initial evidence that graph theory metrics can be influenced by intervention and that those changes may moderate postinjury symptom trajectory (Yuan, Treble‐Barna, et al., 2017).
CONFLICT OF INTEREST
The authors declare no potential conflict of interest.
AUTHOR CONTRIBUTIONS
Ashley L. Ware, PhD: University of Calgary, Calgary, AB, Canada; Site co‐investigator; Designed analyses, processed and analyzed data, drafted manuscript. Keith Owen Yeates, PhD: University of Calgary, Calgary, AB, Canada; Principal investigator; Designed parent study of concussion and orthopedic injury, provided data, designed statistical analyses, edited manuscript. Bryce Geeraert, PhD: University of Calgary, Calgary, AB, Canada; Site co‐investigator; Provided data, designed analyses, edited manuscript. Xiangyu Long, PhD: University of Calgary, Calgary, AB, Canada; Site co‐investigator; Designed processing workflow, edited manuscript. Miriam H. Beauchamp, PhD: University of Montreal & CHU Sainte‐Justine Hospital Research Center, Montreal, Quebec; Parent study site principal investigator; Edited manuscript. William Craig, MDCM: University of Alberta and Stollery Children's Hospital, Edmonton, Alberta; Parent study site principal investigator; Edited manuscript. Quynh Doan, MDCM: University of British Columbia, BC, Canada; Parent study site principal investigator; Edited manuscript. Stephen B. Freedman, MDCM, MSc: University of Calgary, Calgary, AB, Canada; Parent study site principal investigator; Edited manuscript. Bradley G. Goodyear PhD: University of Calgary, Calgary, AB, Canada; Site co‐investigator; Edited manuscript. Roger Zemek, MD: University of Ottawa, Ottawa, Ontario, Canada; Parent study site principal investigator; Edited manuscript. Catherine Lebel, PhD: University of Calgary, Calgary, AB, Canada; Principal investigator; Designed and led parent study of healthy participants, provided data, designed analyses, edited manuscript.
ACKNOWLEDGMENTS
This work is supported by a Canadian Institute of Health Research (CIHR) Foundation Grant (FDN143304), as well as by the Ronald and Irene Ward Chair in Pediatric Brain Injury from the Alberta Children's Hospital Foundation, both awarded to Keith Owen Yeates, the Canada Research Chair program to Catherine Lebel, and the Hotchkiss Brain Institute, Alberta Children's Hospital Research Institute, and the Killam Postdoctoral Fellowship at the University of Calgary to Ashley L. Ware, Bryce Geeraert, and Xiangyu Long. Stephen B. Freedman is supported by the Alberta Children's Hospital Foundation Professorship in Child Health and Wellness. Collection of the typical development data was supported by a grant to Catherine Lebel from the Natural Sciences and Engineering Research Council (NSERC) of Canada.
APPENDIX A.
Pediatric Emergency Research Canada A‐CAP Study team Co‐Investigators
| Name | Location | Role | Contribution |
|---|---|---|---|
| Carolyn Emery, PhD | University of Calgary, Calgary, AB, Canada | Site co‐investigator | Assisted in design of parent study |
| Lianne Tomfohr, PhD | University of Calgary, Calgary, AB, Canada | Site co‐investigator | Assisted in design of parent study |
| Tyler Williamson, PhD | University of Calgary, Calgary, AB, Canada | Site co‐investigator | Assisted in design of parent study |
| Karen Barlow, PhD | University of Calgary, Calgary, AB, Canada | Site co‐investigator | Assisted in design of parent study |
| Francois Bernier, PhD | University of Calgary, Calgary, AB, Canada | Site co‐investigator | Assisted in design of parent study |
| Brian Brooks, PhD | University of Calgary, Calgary, AB, Canada | Site co‐investigator | Assisted in design of parent study |
| Ashley Harris, PhD | University of Calgary, Calgary, AB, Canada | Site co‐investigator | Assisted in design of parent study |
| Ryan Lamont, MD | University of Calgary, Calgary, AB, Canada | Site co‐investigator | Assisted in design of parent study |
| Kathryn Schneider, PhD | University of Calgary, Calgary, AB, Canada | Site co‐investigator | Assisted in design of parent study |
| Jocelyn Gravel, MD | Hospital Ste Justine, University of Montreal, Montreal, Quebec, Canada | Site co‐investigator | Assisted in design of parent study, coordinated recruitment at site |
| Bruce Bjornson, MD | University of British Columbia, BC Children's Hospital, Vancouver, BC, Canada | Site co‐investigator | Assisted in design of parent study, directed imaging at site |
| Nishard Abdeen, PhD | CHEO‐OCTC, University of Ottawa, Ottawa, Ontario, Canada | Site co‐investigator | Assisted in design of parent study, directed imaging at site |
| Christian Beaulieu, PhD | University of Alberta, Edmonton, Alberta, Canada | Site co‐investigator | Assisted in design of parent study, directed imaging at site |
| Kelly Mrklas, PhD | University of Calgary, Calgary, AB, Canada | Site co‐investigator | Assisted in design of parent study. |
| Angelo Mikrogianakis, PhD | University of Calgary, Calgary, AB, Canada | Site co‐investigator | Assisted in design of parent study, coordinated recruitment at site |
| Mathieu Dehaes, MD | University of Montréal and Ste‐Justine Research Center, Montréal, Canada | Site co‐investigator | Assisted in design of parent study, assisted with imaging at site |
| Sylvain Deschenes, PhD | University of Montréal and Ste‐Justine Research Center, Montréal, Canada | Site co‐investigator | Assisted in design of parent study, assisted with imaging at site |
Ware, A. L. , Yeates, K. O. , Geeraert, B. , Long, X. , Beauchamp, M. H. , Craig, W. , Doan, Q. , Freedman, S. B. , Goodyear, B. G. , Zemek, R. , Lebel, C. , & Pediatric Emergency Research Canada A‐CAP Study Team (2022). Structural connectome differences in pediatric mild traumatic brain and orthopedic injury. Human Brain Mapping, 43(3), 1032–1046. 10.1002/hbm.25705
Funding information Natural Sciences and Engineering Research Council (NSERC) of Canada; Killam Postdoctoral Fellowship at the University of Calgary; Alberta Children's Hospital Foundation and Research Institute; Hotchkiss Brain Institute; Canada Research Chair Program; Canadian Institute of Health Research (CIHR) Foundation, Grant/Award Number: FDN143304
DATA AVAILABILITY STATEMENT
Anonymized data will be shared by request from any qualified investigator.
REFERENCES
- Ayr, L. K. , Yeates, K. O. , Taylor, H. G. , & Browne, M. (2009). Dimensions of postconcussive symptoms in children with mild traumatic brain injuries. Journal of the International Neuropsychological Society, 15, 19–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber Foss, K. D. , Yuan, W. , Diekfuss, J. A. , Leach, J. , Meehan, W. , DiCesare, C. A. , … Myer, G. D. (2019). Relative head impact exposure and brain white matter alterations after a single season of competitive football: A pilot comparison of youth versus high school football. Clinical Journal of Sport Medicine, 29, 442–450. [DOI] [PubMed] [Google Scholar]
- Bassett, D. S. , & Bullmore, E. (2006). Small‐world brain networks. The Neuroscientist, 12, 512–523. [DOI] [PubMed] [Google Scholar]
- Bates, D. , Mächler, M. , Bolker, B. , & Walker, S. (2015). Fitting linear mixed‐effects models using lme4. Journal of Statistical Software, 67, 1–48. [Google Scholar]
- Benjamini, Y. , & Hochberg, Y. (1995). Controlling the false discovery rate: A practical and powerful approach to multiple testing. Journal of the Royal Statistical Society: Series B, 57(1), 289–300. [Google Scholar]
- Bruce, B. , Kirkland, S. , & Waschbusch, D. (2007). The relationship between childhood behaviour disorders and unintentional injury events. Paediatrics & Child Health, 12, 749–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullmore, E. , & Sporns, O. (2009). Complex brain networks: Graph theoretical analysis of structural and functional systems. Nature Reviews Neuroscience, 10, 186–198. [DOI] [PubMed] [Google Scholar]
- Carroll, L. , Cassidy, J. D. , Holm, L. , Kraus, J. , & Coronado, V. (2004). Methodological issues and research recommendations for mild traumatic brain injury: The who collaborating centre task force on mild traumatic brain injury. Journal of Rehabilitation Medicine, 36, 113–125. [DOI] [PubMed] [Google Scholar]
- Cassidy, J. D. , Carroll, L. J. , Peloso, P. M. , Borg, J. , von Holst, H. , Holm, L. , … WHO Collaborating Centre Task Force on Mild Traumatic Brain Injury . (2004). Incidence, risk factors and prevention of mild traumatic brain injury: Results of the WHO collaborating Centre task force on mild traumatic brain injury. Journal of Rehabilitation Medicine, Suppl.43, 28–60. [DOI] [PubMed] [Google Scholar]
- Chung, A. W. , Mannix, R. , Feldman, H. A. , Grant, P. E. , & Im, K. (2019). Longitudinal structural connectomic and rich‐club analysis in adolescent mTBI reveals persistent, distributed brain alterations acutely through to one year post‐injury. Scientific Reports, 9, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins, D. L. , Neelin, P. , Peters, T. M. , & Evans, A. C. (1994). Automatic 3D intersubject registration of MR volumetric data in standardized Talairach space. Journal of Computer Assisted Tomography, 18, 192–205. [PubMed] [Google Scholar]
- Cox, R. W. (1996). AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research, 29, 162–173. [DOI] [PubMed] [Google Scholar]
- Faul, F. , Erdfelder, E. , Buchner, A. , & Lang, A.‐G. (2009). Statistical power analyses using G*Power 3.1: Tests for correlation and regression analyses. Behavior Research Methods, 41, 1149–1160. [DOI] [PubMed] [Google Scholar]
- Geeraert, B. L. , Lebel, R. M. , & Lebel, C. (2019). A multiparametric analysis of white matter maturation during late childhood and adolescence. Human Brain Mapping, 40, 4345–4356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geeraert, B. L. , Lebel, R. M. , Mah, A. C. , Deoni, S. C. , Alsop, D. C. , Varma, G. , & Lebel, C. (2018). A comparison of inhomogeneous magnetization transfer, myelin volume fraction, and diffusion tensor imaging measures in healthy children. NeuroImage, 182, 343–350. [DOI] [PubMed] [Google Scholar]
- Gilchrist, J. , Thomas, K. , Xu, L. , McGuire, L. C. , & Coronado, V. G. (2011). Nonfatal traumatic brain injuries related to sports and recreation activities among persons aged ≤19 years—United States, 2001–2009. MMWR, 60, 1337–1342. [PubMed] [Google Scholar]
- Gogtay, N. , Giedd, J. N. , Lusk, L. , Hayashi, K. M. , Greenstein, D. , Vaituzis, A. C. , … Thompson, P. M. (2004). Dynamic mapping of human cortical development during childhood through early adulthood. Proceedings of the National Academy of Sciences, 101, 8174–8179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenspan, L. , McLellan, B A. , & Greig, H. (1985). Abbreviated injury scale and injury severity score. The Journal of Trauma: Injury, Infection, and Critical Care, 25(1), 60–64. [DOI] [PubMed] [Google Scholar]
- Hajek, C. A. , Yeates, K. O. , Gerry Taylor, H. , Bangert, B. , Dietrich, A. , Nuss, K. E. , … Wright, M. (2010). Relationships among post‐concussive symptoms and symptoms of PTSD in children following mild traumatic brain injury. Brain Injury, 24, 100–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphries, M. D. , & Gurney, K. (2008). Network ‘small‐world‐ness’: A quantitative method for determining canonical network equivalence. PLoS One, 3, e0002051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingalhalikar, M. , Smith, A. , Parker, D. , Satterthwaite, T. D. , Elliott, M. A. , Ruparel, K. , … Verma, R. (2014). Sex differences in the structural connectome of the human brain. Proceedings of the National Academy of Sciences, 111, 823–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson, M. (2002). Improved optimization for the robust and accurate linear registration and motion correction of brain images. NeuroImage, 17, 825–841. [DOI] [PubMed] [Google Scholar]
- Jenkinson, M. , Beckmann, C. F. , Behrens, T. E. J. , Woolrich, M. W. , & Smith S. M. (2012). FSL. NeuroImage, 62(2), 782–790. [DOI] [PubMed] [Google Scholar]
- Königs, M. , van Heurn, L. W. E. , Bakx, R. , Vermeulen, R. J. , Goslings, J. C. , Poll‐The, B. T. , … Pouwels, P. J. W. (2017). The structural connectome of children with traumatic brain injury: The connectome after pediatric TBI. Human Brain Mapping, 38, 3603–3614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuznetsova, A. , Brockhoff, P. B. , Christensen, R. H. B. (2017). lmertest package: Tests in linear mixed effects models. Journal of Statistical Software, 82(13), 1–26. [Google Scholar]
- Lebel, C. , Treit, S. , & Beaulieu, C. (2019). A review of diffusion MRI of typical white matter development from early childhood to young adulthood. NMR in Biomedicine, 32, e3778. [DOI] [PubMed] [Google Scholar]
- Leemans, A. , Jeurissen, B. , Sijbers, J. & Jones, D. (2009). ExploreDTI: A graphical toolbox for processing, analyzing, and visualizing diffusion MR data. Proceedings 17th Scientific Meeting, International Society for Magnetic Resonance in Medicine. 17, 3537. http://www.exploredti.com/ref/ExploreDTI_ISMRM_2009.pdf
- Loder, R. T. , Warschausky, S. , Schwartz, E. M. , Hensinger, R. N. , & Greenfield, M. L. (1995). The psychosocial characteristics of children with fractures. Journal of Pediatric Orthopaedics, 15(1), 41–46. [DOI] [PubMed] [Google Scholar]
- Long, X. , Kar, P. , Gibbard, B. , Tortorelli, C. , & Lebel, C. (2019). The brain's functional connectome in young children with prenatal alcohol exposure. NeuroImage: Clinical, 24, 102082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumba‐Brown, A. , Yeates, K. O. , Sarmiento, K. , Breiding, M. J. , Haegerich, T. M. , Gioia, G. A. , … Timmons, S. D. (2018). Diagnosis and management of mild traumatic brain injury in children: A systematic review. JAMA Pediatrics, 172, e182847. [DOI] [PubMed] [Google Scholar]
- Luo, D. , Ganesh, S. & Koolaard, J. (2021). Predictmeans: Calculate predicted means for linear models. https://CRAN.R-project.org/package=predictmeans
- Mayer, A. R. , Kaushal, M. , Dodd, A. B. , Hanlon, F. M. , Shaff, N. A. , Mannix, R. , … Meier, T. B. (2018). Advanced biomarkers of pediatric mild traumatic brain injury: Progress and perils. Neuroscience & Biobehavioral Reviews, 94, 149–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllister, T. W. , Ford, J. C. , Flashman, L. A. , Maerlender, A. , Greenwald, R. M. , Beckwith, J. G. , … Jain, S. (2014). Effect of head impacts on diffusivity measures in a cohort of collegiate contact sport athletes. Neurology, 82, 63–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power, J. D. , Schlaggar, B. L. , Lessov‐Schlaggar, C. N. , & Petersen, S. E. (2013). Evidence for hubs in human functional brain networks. Neuron, 79, 798–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team . (2017). R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. https://www.R-project.org/ [Google Scholar]
- Raji, C. A. , Wang, M. B. , Nguyen, N. , Owen, J. P. , Palacios, E. M. , Yuh, E. L. , & Mukherjee, P. (2020). Connectome mapping with edge density imaging differentiates pediatric mild traumatic brain injury from typically developing controls: Proof of concept. Pediatric Radiology, 50, 1594–1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter, M. , Tisdall, M. D. , Qureshi, A. , Buckner, R. L. , van der Kouwe, A. J. W. , & Fischl, B. (2015). Head motion during MRI acquisition reduces gray matter volume and thickness estimates. NeuroImage, 107, 107–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roalf, D. R. , Quarmley, M. , Elliott, M. A. , Satterthwaite, T. D. , Vandekar, S. N. , Ruparel, K. , & Gur, R. E. (2016). The impact of quality assurance assessment on diffusion tensor imaging outcomes in a large‐scale population‐based cohort. NeuroImage, 125, 903–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen, A. F. G. , Roalf, D. R. , Ruparel, K. , Blake, J. , Seelaus, K. , Villa, L. P. , … Satterthwaite, T. D. (2018). Quantitative assessment of structural image quality. NeuroImage, 169, 407–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RStudio Team . (2020). RStudio: Integrated development for R. Boston, MA: RStudio, PBC. Retrieved from http://www.rstudio.com/ [Google Scholar]
- Rubinov, M. , & Sporns, O. (2010). Complex network measures of brain connectivity: Uses and interpretations. NeuroImage, 52, 1059–1069. [DOI] [PubMed] [Google Scholar]
- Ruff, R. M. , Iverson, G. L. , Barth, J. T. , Bush, S. S. , Broshek, D. K. , & the NAN Policy and Planning Committee . (2009). Recommendations for diagnosing a mild traumatic brain injury: A National Academy of neuropsychology education paper. Archives of Clinical Neuropsychology, 24, 3–10. [DOI] [PubMed] [Google Scholar]
- Schmidt, J. , Hayward, K. S. , Brown, K. E. , Zwicker, J. G. , Ponsford, J. , van Donkelaar, P. , … Boyd, L. A. (2018). Imaging in pediatric concussion: A systematic review. Pediatrics, 141, e20173406. [DOI] [PubMed] [Google Scholar]
- Smith, S. M. , Jenkinson, M. , Woolrich, M. W. , Beckmann, C. F. , Behrens, T. E. J. , Johansen‐Berg, H. , … Matthews, P. M. (2004). Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage, 23, S208–S219. [DOI] [PubMed] [Google Scholar]
- Sollmann, N. , Echlin, P. S. , Schultz, V. , Viher, P. V. , Lyall, A. E. , Tripodis, Y. , … Koerte, I. K. (2018). Sex differences in white matter alterations following repetitive subconcussive head impacts in collegiate ice hockey players. NeuroImage: Clinical, 17, 642–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, M. , McDonald, S. J. , Brady, R. D. , O'Brien, T. J. , & Shultz, S. R. (2018). The influence of immunological stressors on traumatic brain injury. Brain, Behavior, and Immunity, 69, 618–628. [DOI] [PubMed] [Google Scholar]
- Teasdale, G. , & Jennett, B. (1974). Assessment of coma and impaired consciousness. A practical Scale. Lancet, 2, 81–84. [DOI] [PubMed] [Google Scholar]
- Thomas, C. , Ye, F. Q. , Irfanoglu, M. O. , Modi, P. , Saleem, K. S. , Leopold, D. A. , & Pierpaoli, C. (2014). Anatomical accuracy of brain connections derived from diffusion MRI tractography is inherently limited. Proceedings of the National Academy of Sciences of the United States of America, 111, 16574–16579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, J. , Wang, X. , Xia, M. , Liao, X. , Evans, A. , & He, Y. (2015). GRETNA: A graph theoretical network analysis toolbox for imaging connectomics. Frontiers in Human Neuroscience, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson, C. G. , DeMaster, D. , & Ewing‐Cobbs, L. (2019). Graph theory analysis of DTI tractography in children with traumatic injury. NeuroImage: Clinical, 21, 101673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler, D. (2011). Wechsler abbreviated scale of intelligence–(WASI‐II), Manual (2nd ed.). San Antonio, TX: Pearson. [Google Scholar]
- Wilde, E. A. , Ware, A.L. , Li, X. , Wu, T. C. , McCauley, S. R. , Barnes, A. , … Levin, H. S. (2019). Orthopedic injured versus uninjured comparison groups for neuroimaging research in mild traumatic brain injury. Journal of Neurotrauma, 36(2), 239–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia, M. , Wang, J. , & He, Y. (2013). BrainNet viewer: A network visualization tool for human brain connectomics. PLoS One, 8, e68910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeates, K. O. , Beauchamp, M. , Craig, W. , Doan, Q. , Zemek, R. , Bjornson, B. , … Schneider K. J. (2017). Advancing Concussion Assessment in Pediatrics (A‐CAP): A prospective, concurrent cohort, longitudinal study of mild traumatic brain injury in children: Protocol study. BMJ Open, 7(7), e017012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan, W. , Treble‐Barna, A. , Sohlberg, M. M. , Harn, B. , & Wade, S. L. (2017). Changes in structural connectivity following a cognitive intervention in children with traumatic brain injury. Neurorehabilitation and Neural Repair, 31(2), 190–201. [DOI] [PubMed] [Google Scholar]
- Yuan, W. , Wade, S. L. , & Babcock, L. (2015). Structural connectivity abnormality in children with acute mild traumatic brain injury using graph theoretical analysis. Human Brain Mapping, 36, 779–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan, W. , Wade, S. L. , Quatman‐Yates, C. , Hugentobler, J. A. , Gubanich, P. J. , & Kurowski, B. G. (2017). Structural connectivity related to persistent symptoms after mild TBI in adolescents and response to aerobic training: Preliminary investigation. Journal of Head Trauma Rehabilitation, 32, 378–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data will be shared by request from any qualified investigator.
A dataset with deidentified participant data and a data dictionary will be made available upon reasonable request from any qualified investigator to the corresponding author, subject to a signed data access agreement, with publication.
Anonymized data will be shared by request from any qualified investigator.


