Abstract
Daydreaming and creativity have similar cognitive processes and neural basis. However, few empirical studies have examined the relationship between daydreaming and creativity using cognitive neuroscience methods. The present study explored the relationship between different types of daydreaming and creativity and their common neural basis. The behavioral results revealed that positive constructive daydreaming is positively related to creativity, while poor attentional control is negatively related to it. Machine learning framework was adopted to examine the predictive effect of daydreaming‐related brain functional connectivity (FC) on creativity. The results demonstrated that task FCs related to positive constructive daydreaming and task FCs related to poor attentional control both predicted an individual's creativity score successfully. In addition, task FCs combining the positive constructive daydreaming and poor attentional control also had significant predictive effect on creativity score. Furthermore, predictive analysis based on resting‐state FCs showed similar patterns. Both of the subscale‐related FCs and combined FCs had significant predictive effect on creativity score. Further analysis showed the task and the resting‐state FCs both mainly located in the default mode network, central executive network, salience network, and attention network. These results showed that daydreaming was closely related to creativity, as they shared common FC basis.
Keywords: creativity, daydreaming, functional connectivity, prediction
The present study explored the relationship between different types of daydreaming and creativity and their common neural basis. The results showed the common task and the resting‐state functional connectivities both mainly located in the default mode network, central executive network, salience network, and attention network.

1. INTRODUCTION
Daydreaming refers to the occurrence of spontaneous thoughts unrelated to one's current situation (Singer, 1975; Singer & Schonbar, 1961; Smallwood & Schooler, 2006). Everyone experiences daydreaming, and this phenomenon covers 30–50% of our daily waking time (Christoff, Gordon, Smallwood, Smith, & Schooler, 2009; Kane et al., 2007; Killingsworth & Gilbert, 2010; McMillan, Kaufman, & Singer, 2013). As a complex and multifaceted construct, daydreaming has been associated with both adaptive and maladaptive consequences (Fox & Beaty, 2019; McMillan et al., 2013; Ottaviani & Couyoumdjian, 2013). One of its adaptive consequences is people's heightened creativity when their minds wander (Zedelius & Schooler, 2016). The relationship between daydreaming and creativity has long been a lucrative topic for researchers.
Creativity is a complex concept, which is usually defined as the ability to produce novel and useful outputs (Beaty, Benedek, Silvia, & Schacter, 2016; Benedek, Jurisch, Koschutnig, Fink, & Beaty, 2020; Runco & Jaeger, 2012; Sternberg & Lubart, 1996). From the perspective of cognition, daydreaming and creativity have similar cognitive processes. They both have an unintentional generation stage and a deliberate stage (Fox & Beaty, 2019). Daydreaming includes self‐generated thoughts, which is unrelated to the current situation or the ongoing task (Smallwood & Schooler, 2006). This is a relatively spontaneous generation process (Fox & Beaty, 2019). This process is similar to the generation process of creativity, which involves the searching processes through one's memory system to combine remote associations and formulate original ideas (Christensen, Kenett, Cotter, Beaty, & Silvia, 2018; Madore, Thakral, Beaty, Addis, & Schacter, 2019; Sowden, Pringle, & Gabora, 2015). In addition, daydreaming involves metacognitive awareness and intentional guidance process, which is similar to the evaluation process of creativity (Benedek et al., 2020; Christensen et al., 2018; Christoff et al., 2009; Finke, Ward, & Smith, 1992). It is known that participants have the ability to notice that their mind have wandered (Smallwood, Mcspadden, & Schooler, 2007). Such metacognitive awareness might contribute to the regulation of daydreaming directly or indirectly (Schooler et al., 2011). For example, metacognitive awareness might be conducive to the identification of daydreaming and the reengagement of the primary task subsequently (Schooler et al., 2011). During the evaluation process about creativity, people assess the efficacy of their potential creative opinions, select and modify these opinions to meet the goal of a creativity task (Christensen et al., 2018; Madore et al., 2019; Sowden et al., 2015). Recent studies on creativity further propose that creative cognition is modulated by metacontrol state (Zhang, Sjoerds, & Hommel, 2020). Both of daydreaming and creativity involve the top‐down control processes. Hence, we can easily infer that a positive correlation exists between daydreaming and creativity, given their similarities. Psychologists hypothesized that daydreaming may facilitate creativity through the reorganization of existing mental images and the formation of remote and original associations (Shepard, 1978). Numerous experimental studies support this opinion. In an early research, Singer and Schonbar (1961) found the positive correlation between the frequency of daydreaming and creativity. Meta‐analytic showed that taking a break from divergent thinking task or switching to another unrelated task for a period was helpful to the following creativity performance (Sio & Ormerod, 2009). Empirical research demonstrated that engaging in an undemanding task which permits daydreaming was conducive to the performance of a creativity task (Baird et al., 2012).
However, daydreaming is not always positively related to creativity. Some evidence has showed that daydreaming has a negative impact on creativity. Hao, Wu, Runco, and Pina (2015) distinguished high and low daydreaming groups on the basis of daydreaming frequency. The researchers found that high daydreaming group had lower fluency and originality scores during a divergent thinking task in comparing with low daydreaming group. Furthermore, the originality score decreased in the high daydreaming group as the task progressed. However, it remained stable for the low daydreaming group. The researchers believed that this finding was reasonable because of the attentional control process of daydreaming and creativity (Hao et al., 2015). The executive‐control‐failure model posits that daydreaming stems from the failure of executive control (Mcvay & Kane, 2010). Daydreaming is determined by automatically generated thoughts related to mental and environmental cues (Mcvay & Kane, 2010); the executive‐control process plays a role in dealing with this interference. People who experience high amounts of daydreaming are less efficient in keeping attention on the current task than individuals who experience low amounts of daydreaming (McVay & Kane, 2009, 2010). The loss of the attentional focus has an adverse effect on the cognitive process of creativity and plays the dark role in the relationship between daydreaming and creativity. During a creative task, individuals need to focus their attention on idea generation to generate original ideas (Beaty et al., 2016). For instance, Ostafin and Kassman (2012) found that creativity is positively related to mindful awareness, which is adverse to daydreaming. The top‐down executive process helps the inhibition of the interference of unrelated stimuli and ordinary response (Beaty & Silvia, 2012; Benedek, Beaty, et al., 2014; Benedek, Jauk, Sommer, Arendasy, & Neubauer, 2014; Fink, Graif, & Neubauer, 2009; Silvia & Beaty, 2012; Silvia, Beaty, & Nusbaum, 2013; Takeuchi et al., 2012). It also aids in searching and retrieving processes in working memory (Silvia et al., 2013). The opposite needs of controlled and focused thought in daydreaming and creativity lead to the inference that daydreaming is detrimental to creativity.
Recent research asserts that daydreaming varies in styles and different kinds of daydreaming have various effects on creativity (Zedelius & Schooler, 2016). Singer (1975) distinguished three styles of daydreaming: positive constructive daydreaming, which was characterized by planning, pleasant thoughts, vivid and wishful imagery, and curiosity; guilty‐dysphoric daydreaming, which was characterized by obsessive, guilty, and anguished fantasies; and poor attentional control, which was characterized by the inability to focus attention on either the internal thoughts or the external tasks (Singer, 1975). Although studies exploring the relationship among the different kinds of daydreaming and creativity are still rare, some researchers support the view that the relationship between daydreaming and creativity is complex. Zhiyan and Jerome (1997) demonstrated that positive constructive daydreaming was positively associated with an individuals' openness to experience, which was a kind of personality trait also closely related to creativity. Research also links the negative association between narrow focus of attention and creativity. Wegbreit, Suzuki, Grabowecky, Kounios, and Beeman (2012) found that a broad focus task (such as rapid object identification task) led to increased insight performance in the following verbal creativity task; meanwhile, a narrow focus task (such as flanker task) led to predominantly analytic solutions.
Numerous studies have explored the brain basis underlying daydreaming and creativity separately. Neural research highlighted the role of the default mode network (DMN) and the executive network (EN) and the limbic system in daydreaming (Golchert et al., 2017). Using both thought sampling and brain imaging methods, Mason et al. (2007) found that daydreaming during visuospatial working‐memory tasks was related to the recruitment of regions in the DMN. In addition, self‐reported daydream frequency was correlated with the activity of regions in the DMN. Recent studies further revealed that daydreaming was also represented in the dynamic functional connectivities (FCs) of the DMN on a faster time scale (Kucyi, 2018). In addition to DMN activation, daydreaming is associated with EN recruitment (Christoff et al., 2009). Christoff et al. (2009) used experience sampling to measure daydreaming during a concurrent task and observed a parallel recruitment of the DMN and the EN. Mooneyham et al. (2017) examined the dynamic FC state of brain regions within the DMN, the EN, and the salience network (SN) when participants were engaging in a sustained attention task. They found that the FC state associated with daydreaming exhibited positive FC among several key brain regions across all three networks. Golchert et al. (2017) combined both structure and functional data and conducted a multimodal approach to explore the brain cortical organization that underlies individual differences in daydreaming. They found that higher reports of daydreaming were associated with the structure and FC of regions in the DMN, the EN, and the limbic system. Similar to the research on the neural basis of daydreaming, a wide variety of neuroimaging studies about creativity have been conducted, and have revealed several key brain areas which have been implicated in creative tasks (Abraham, Beudt, Ott, & Cramon, 2012; Dietrich & Kanso, 2010; Fink et al., 2009; Huang, Fan, & Luo, 2015; Huang, Zhao, Zhou, & Luo, 2019; Sun, Liu, et al., 2019). Meta‐analysis studies of task‐based fMRI revealed that the posterior parietal cortex, the precuneus, the lateral prefrontal cortex, the temporal cortex, and the anterior cingulate cortex were activated in fMRI tasks involved in creativity‐related processes (Gonen‐Yaacovi et al., 2013; Pidgeon et al., 2016; Wu et al., 2015). Recent neuroscientific investigations tend to discuss the neural basis underlying creativity through brain functional networks (Abraham, 2014; Beaty et al., 2016; Jung, Mead, Carrasco, & Flores, 2013; Mok, 2014). Researchers have made an agreement that during the cognitive process of creativity the DMN devotes to the generation of novel ideas and the EN is involved in the top‐down process to allocation of cognitive resources. And the SN plays a role in modulating the interaction between DMN and EN (Sun et al., 2016). Although some differences exist, overlaps are present between the key brain regions and brain networks of daydreaming and creativity.
Based on above, the present study developed the scientific problem that whether daydreaming and creativity had common cognitive and neural basis. Clarifying this problem will help us to understand the potential mechanism of the interaction between daydreaming and creativity. The aim of the present study is to explore the relationship between different kinds of daydreaming and creativity and the underlying common brain basis. To address the scientific problem and the aim, we adopted machine learning based method in this study. We hypothesized that different kinds of daydreaming would have various correlations with creativity. Daydreaming and creativity shared a common neural basis. In addition, the subscale of daydreaming could predict creativity through brain FCs. Furthermore, the combinations of the FCs related to these subscales could also predict creativity. To examine these hypotheses, we combined behavioral data of daydreaming and creativity and brain FCs data and constructed a regression model based on machine learning framework to predict participants' creativity scores.
2. METHODS
2.1. Participants
This study has two samples. The first sample included 94 participants and all of them completed behavior measures and an fMRI task. The second sample included 158 participants and participants completed the behavior measure and resting‐state fMRI scanning. All of the participants were recruited from Southwest University, China and were right‐handed. These two samples had no overlaps. All participants met the safety criteria of fMRI study with no history of neurological or psychiatric illness. This study was approved by the Brain Imaging Center Institutional Review Board at the Southwest University, China. In accordance with the Declaration of Helsinki (1991), written informed consent was obtained from all participants. Participants were excluded whose head motions were greater than 3 mm maximum translation or 3° rotations or mean frame‐wise displacement (mean FD) >0.2 mm during the fMRI scanning. Six participants in the first sample and sixteen participants in the second sample were excluded because of their excessive head motions. Finally, 88 participants in the first sample and 142 participants in the second sample were included in this study. The average age for the first sample was 21.24 years (range = 18–27, SD = 1.86, 27 males). The average age for the second sample was 20.98 years (range = 18–26, SD = 1.50, 45 males).
2.2. Behavioral measures
Participants completed the behavioral measures of Creative Behavior Inventory (CBI) (Hocevar, 1979, 1980) and Short Imaginal Processes Inventory (SIPI) (Huba, Singer, Aneshensel, & Antrobus, 1982; Huba & Tanaka, 1983). CBI is a self‐report questionnaire that measures creativity which includes 28 items. The CBI asked participants to indicate their participation in various creative activities on a 4‐point scale (0 = never did this; 3 = did this more than five times). SIPI is a self‐report questionnaire that measures daydreaming which includes 45 items. It contains three subscales: positive‐constructive daydreaming (e.g., “Sometimes an answer to a difficult problem will come to me during a daydream.”), guilt and fear‐of‐failure daydreaming (e.g., “In my fantasies, a friend discovers that I have lied.”), and poor attentional control (e.g., “I tend to be easily bored.”). For each item, participants were asked to indicate the extent to which each statement applies to themselves on a five‐point scale (1 = definitely untrue or strongly uncharacteristic of me, 5 = very true or strongly characteristic of me). The reliability for these measures in the present study was acceptable (α CBI = .92, α SIPI = .82) (Dollinger, 2011; Huba & Tanaka, 1983). Furthermore, Pearson correlation was used to explore the relationship between creativity and different kinds of daydreaming. Meanwhile, false discovery rate (FDR) method was used to correct for multiple comparisons.
2.3. fMRI data acquisition
Images were acquired using a 3 T Trio scanner (Siemens Medical Systems). Participants were in supine position and were also instructed to keep still to control the head movement. BOLD images were obtained using an Echo Planar Imaging sequence: repetition time = 2,000 ms; echo time = 30 ms; slices = 32; flip angle = 90°; thickness = 3 mm; resolution matrix = 64 × 64; field of view = 220 × 220 mm2; slice gap = 1 mm; and voxel size = 3.4 × 3.4 × 4 mm3. Resting‐state fMRI collected 242 volumes in total. Meanwhile, task fMRI collected 1,360 volumes in total.
2.4. fMRI task
Alternative uses task (AUT) was used in the scanner. Object characteristics task (OCT) was used as the control task. The AUT asked participants to generate as many original uses as possible for a familiar object in 60 s. The OCT task asked participants to generate the typical characteristics of a familiar object within 60 s. Each task condition had 20 items for both AUT and OCT and every item was presented in a separate block. Before each item, a cue was given about the task type (AUT or OCT), which lasted 2 s. There was a fixation point lasting 4–8 s among the items. During each item, participants were required to press the button when they thought of an idea. They continued to formulate ideas until the end of 60 s.
2.5. Imaging data preprocessing
The preprocessing of task‐based fMRI and resting‐state fMRI data were performed using the Data Processing Assistant for Resting‐State fMRI (http://resting-fmri.sourceforge.net/) (Yan & Zang, 2010) based on SPM8 (Wellcome Department of Imaging Neuroscience, London, UK; www.fil.ion.ucl.ac.uk/spm). The participants whose head motion was more than 3 mm maximum translation or 3.0° rotation or 0.2 mm mean FD were excluded.
For the task‐based fMRI data, the functional imaging data of each participant were slice‐timing corrected and motion corrected first. Thereafter, each participant's functional image was normalized to the Montreal Neurological Institute (MNI) space (EPI template with resampling voxel size = 3 × 3 × 3 mm3). Then, spatial smoothing (6 mm full width at half maximum Gaussian kernel) was conducted to decrease spatial noise.
For the resting‐state data, the first 10 functional volumes were discarded to suppress equilibration effects. The remaining data were slice‐time adjusted, motion corrected, normalized to the MNI space (EPI template, resampling voxel size = 3 × 3 × 3 mm3), spatial smoothed (6 mm full width at half maximum Gaussian kernel), and detrended. Nuisance covariates including the cerebrospinal fluid, white matter signals, global mean signals, and Friston 24‐parameter head motion were regressed out (Friston, Williams, Howard, Frackowiak, & Turner, 1996). Then, band‐pass filter (0.008–0.1 Hz) was performed. Scrubbing procedure was performed to reduce the potential effect of head motion further. Bad time points were deleted with a criterion of any volume with FD > 0.5 mm. The ratio of the remaining time points across all participants was 99%.
2.6. Functional network construction
For the task‐based fMRI data, we used the CONN toolbox (Whitfield‐Gabrieli & Nieto‐Castanon, 2012) to construct FC matrices. For each participant, the preprocessed functional data were submitted to CONN. A component‐based (CompCor) strategy was used to remove the non‐neural sources of confounders. Nuisance covariates such as principle components associated with white matter, cerebrospinal fluid, and head movement parameters were regressed out. The data were temporally filtered with band‐pass filter ranging 0.008–0.1 Hz. We adopted the 264‐region parcellation system as network nodes (Power et al., 2011), which contained 264 regions. The time series of the brain functional imaging signals data were extracted from each voxel within each ROI and averaged. A rectified hemodynamic response function was used in order to account the delay in hemodynamic response by convolving the regressors for every task condition. For each task condition, the scans associated with nonzero effects of the time series were concatenated and weighted by the value of the corresponding time series (Whitfield‐Gabrieli & Nieto‐Castanon, 2012). For the resting‐state data, time series of each voxel within each region in the 264‐region parcellation system was extracted and averaged. Pearson correlation between the time courses of each pair of regions were calculated for both task‐based data and resting‐state data, which resulted in a 264 × 264 connectivity matrix with 34,716 edges for each participant. Then, the matrixes were normalized using the Fisher's z transformation.
2.7. Connectome‐based predictive analysis
Leave‐one‐out cross validation was performed in the task‐based data using relevance vector regression (RVR) to explore the predictive effect of daydreaming‐related FCs on creativity. RVR (Tipping, 2001) is a sparse kernel multivariate regression method that uses Bayesian inference to obtain sparse regression models. Specifically, leave‐one‐out cross validation was performed n times (n represents the number of participants). Each time one participant in the sample was left as a test set, and the rest of the n‐1 participants were used as a training set. The participants in the training set were used to construct the brain FC networks associated with daydreaming. In the training set, feature selection was performed by calculating the relationship between each subscale score of SIPI and the whole‐brain FC using partial correlation. The effects of gender, age, and mean FD were controlled. A common threshold of p < .05 was used to retain significantly correlated functional connections and remove the spurious connections. According to the hypothesis, we first used each subscale‐related FCs to predict creativity. Then, we used the combination of the subscale‐related FCs to predict creativity. According to the results of behavioral analysis, only two subscale scores of daydreaming, positive‐constructive daydreaming and poor attentional control were included in the analysis.
A predictive model was built that fit a linear regression between daydreaming‐related FCs and CBI scores in the training set. The model was then applied to a new participant of the test set in a leave‐one‐out cross validation procedure to obtained the predicted scores of the participant in the test set. The prediction performance of the model was assessed by the Pearson correlation coefficient between the predicted CBI scores and actual measured CBI scores and statistical significance. We conducted permutation tests, which randomly shuffled the label of CBI scores and FC matrixes 1,000 times and reran the prediction procedure each time to form a null distribution of r values representing the relationship between the predicted CBI scores and actual measured CBI scores (permutation test, p < .05). Considering the AUT task and CBI scores are closely related, although the features used in the regression models are related to daydreaming, it is expected that the predictive effect will be significant. So we also used resting‐state fMRI data to further explore the predictive effect. The same calculation process was conducted in the resting‐state data. The data analysis processes are shown in Figure 1.
FIGURE 1.
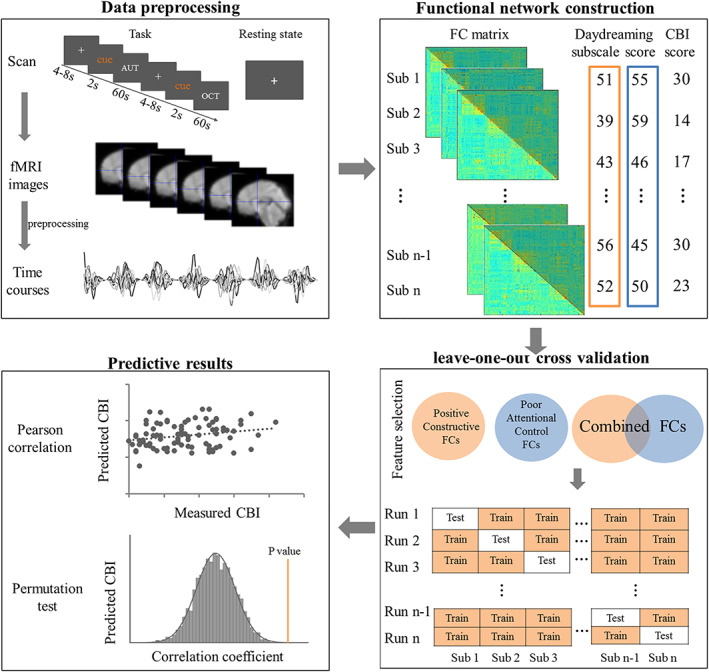
Flowchart of analysis process for predictions of the creativity score using daydreaming‐related brain functional connectivities (FCs). The time series from 264‐region parcellation system were extracted and a 264 × 264 FC matrix was constructed for each participant. Leave‐one‐out cross validation and 1,000 times permutation tests were performed
2.8. Validation analysis
To test the robustness of our findings, we conducted validation analysis. Ten‐fold cross validation was applied to examine the predictive power. Unlike the leave‐one‐out procedure, 10‐fold cross validation randomly divided the sample into a training set (90% of the whole sample) and a test set (10% of the whole sample). The prediction procedure was repeated 100 times given that each time of random division of the whole sample brings about the difference in test sets and training sets (He et al., 2021). The scores of each participant were averaged to obtain the final prediction score.
We also performed correlation analysis between behavioral measures (CBI and subscale scores of daydreaming) and FCs. The effects of gender, age, and mean FD were controlled. The results are shown in Supporting Information (Figure S1).
3. RESULTS
3.1. Behavioral results
The Pearson correlation results of behavioral data are summarized in Table 1. After FDR correction, in both of the two samples, positive‐constructive daydreaming was positively related to CBI scores and poor attentional control was negatively related to CBI scores. These results indicated that the more daydreaming one experienced, the higher the level of creativity one had. Meanwhile, the poorer attentional control ability one had, the lower creativity score one performed. In the following fMRI data analysis, we mainly focused on these two subscales of daydreaming.
TABLE 1.
The correlation between daydreaming and creative behavior score
| PCtask | GFtask | PAtask | CBItask | PCrest | GFrest | PArest | CBIrest | |
|---|---|---|---|---|---|---|---|---|
| PC | — | — | ||||||
| GF | 0.281** | — | 0.203* | — | ||||
| PA | 0.058 | 0.475*** | — | 0.030 | 0.269** | — | ||
| CBI | 0.267** | −0.061 | −0.209* | — | 0.244** | −0.001 | −0.175* | — |
Note: *Corrected p < .05, **corrected p < .01, ***corrected p < .001.
Abbreviations: CBI: Creative Behavior Inventory; GF, guilt and fear‐of‐failure daydreaming; PA, poor attentional control; PC, positive‐constructive daydreaming.
3.2. Results from cross validation
Using leave‐one‐out cross validation in the first sample, we found that two subscales of daydreaming‐related FCs could significantly predict individual CBI scores (see Figure 2). When using positive‐constructive daydreaming‐related FCs (759 FCs), the r value between actual measured and predicted CBI scores was 0.232 (p = .030). The FCs mainly involve nodes in the DMN (e.g., posterior cingulate, region 91, degree = 10; middle temporal gyrus, region 83, degree = 14), along with the task control network (e.g., middle frontal gyrus, region 196, degree = 12; middle frontal gyrus, region 188, degree = 11) and the visual network (VN, e.g., middle occipital gyrus, region 149, degree = 19). When using poor attentional control‐related FCs (796 FCs), the r value between actual measured and predicted CBI scores was 0.286 (p = .007). Meanwhile, the FCs mainly involve nodes in the DMN (e.g., posterior cingulate, region 91, degree = 17; medial frontal gyrus, region 110, degree = 25), along with the task control network (e.g., middle frontal gyrus, region 196, degree = 10; inferior parietal lobule, region 177, degree = 12), SN (e.g., inferior frontal gyrus, region 210, degree = 16; anterior cingulate gyrus, region 216, degree = 16), and sensory/somatomotor hand network (SSH, e.g., precentral gyrus, region 21, degree = 18). The combination of the subscale‐related FCs (1,557 FCs) could also significantly predict individual CBI scores. The r value between actual measured scores and predicted scores was 0.295 (p = .005). The combined FCs mainly related to nodes in the DMN (e.g., posterior cingulate, region 91, degree = 28; medial frontal gyrus, region 110, degree = 27), task control network (e.g., middle frontal gyrus, region 196, degree = 28; inferior parietal lobule, region 177, degree = 16), SN (e.g., inferior frontal gyrus, region 210, degree = 27; anterior cingulate gyrus, region 216, degree = 20), and VN (e.g., middle occipital gyrus, region 149, degree = 24).
FIGURE 2.
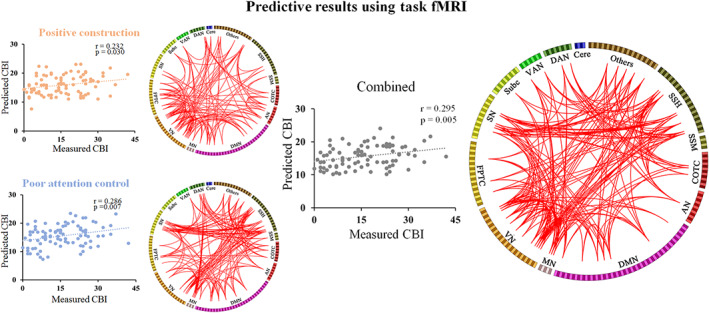
Predictive results using task‐based functional connectivities (FCs). The scatterplots show the correlation between measured and predicted Creative Behavior Inventory (CBI) scores. The circle plot shows FCs that can predict CBI scores. The top 20% regions with the largest number of connections were present for visual presentation. AN, auditory network; Cere, cerebellar; COTC, cingulo‐opercular task control network; DAN, dorsal attention network; DMN, default mode network; FPTC, fronto‐parietal task control network; MN, memory retrieval network; SN, salience network; Subc, subcortical network; SSH, sensory/somatomotor hand network; SSM, sensory/somatomotor mouth network; VAN, ventral attention network; VN, visual network
In the second sample, we further examined the predictive effect using resting‐state data. We found that the subscale of daydreaming‐related FCs (positive‐constructive daydreaming‐related FCs = 973, poor attentional control‐related FCs = 651) could significantly predict individual CBI scores (see Figure 3). The Pearson correlation coefficient between actual and predicted CBI scores was 0.240 (p = .004) and 0.186 (p = .027) when using FCs related to positive‐constructive daydreaming and poor attentional control, respectively. The FCs related to positive‐constructive daydreaming were mainly located in nodes in the DMN (e.g., precuneus, region 89, degree = 19; middle temporal gyrus, region 129, degree = 18), task control network (e.g., middle frontal gyrus, region 196, degree = 14; middle frontal gyrus, region 181, degree = 20), SN (e.g., supplementary motor area, region 213, degree = 29; anterior cingulate gyrus, region 215, degree = 43) and SSH (e.g., postcentral gyrus, region 25, degree = 23). Meanwhile, the FCs related to poor attentional control were mainly located in nodes in the DMN (e.g., posterior cingulate, region 95, degree = 10; medial frontal gyrus, region 110, degree = 13); task control network (e.g., middle frontal gyrus, region 198, degree = 15; middle frontal gyrus, region 175, degree = 19); and attention network (e.g., inferior parietal lobule, region 260, degree = 11; middle temporal gyrus, region 257, degree = 14). The combination of the subscale‐related FCs (1,634 FCs) could also significantly predict individual CBI scores. The r value between actual measured and predicted CBI scores was 0.251 (p = .003). The combined FCs mainly related to nodes in the DMN (e.g., precuneus, region 89, degree = 28; middle temporal gyrus, region 129, degree = 26); task control network (e.g., middle frontal gyrus, region 196, degree = 23; middle frontal gyrus, region 181, degree = 28); SN (e.g., supplementary motor area, region 213, degree = 37; anterior cingulate gyrus, region 215, degree = 45); and attention network (e.g., superior parietal lobule, region 258, degree = 21; superior temporal gyrus, region 240, degree = 31).
FIGURE 3.
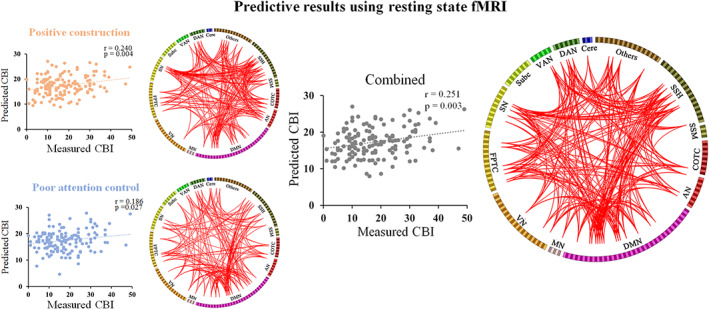
Predictive results using resting‐state functional connectivities (FCs). The scatterplots show the correlation between measured and predicted Creative Behavior Inventory (CBI) scores. The circle plot presents FCs that can predict CBI scores. The top 20% regions with the largest number of connections were present for visual presentation. AN, auditory network; Cere, cerebellar; COTC, cingulo‐opercular task control network; DAN, dorsal attention network; DMN, default mode network; FPTC, fronto‐parietal task control network; MN, memory retrieval network; SN, salience network; Subc, subcortical network; SSH, sensory/somatomotor hand network; SSM, sensory/somatomotor mouth network; VAN, ventral attention network; VN, visual network
We further examined the overlap between task FCs results and resting‐state FCs results. The results are shown in Figure 4.
FIGURE 4.
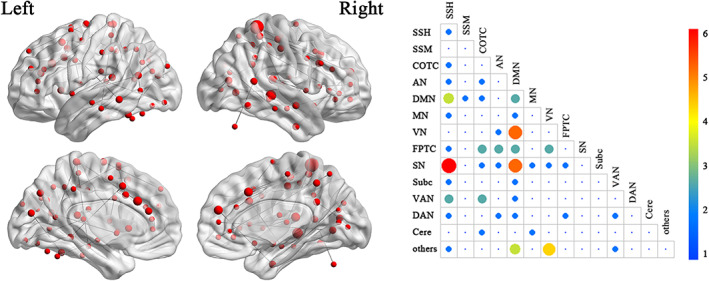
The overlap between task functional connectivities (FCs) results and resting‐state FCs results. The brain map on the left shows the overlap FCs and the node size represents the degree. The right matrix map shows the connection number between brain networks. AN, auditory network; Cere, cerebellar; COTC, cingulo‐opercular task control network; DAN, dorsal attention network; DMN, default mode network; FPTC, fronto‐parietal task control network; MN, memory retrieval network; SN, salience network; Subc, subcortical network; SSH, sensory/somatomotor hand network; SSM, sensory/somatomotor mouth network; VAN, ventral attention network; VN, visual network
3.3. Results from validation analysis
We performed 10‐fold cross validation to examine the predictive power. When using task‐based FCs, positive‐constructive daydreaming‐related FCs (r = .224, p = .036) and poor attentional control‐related FCs (r = .243, p = .022) predicted CBI scores effectively. The combination of the subscale‐related FCs could also significantly predict individual CBI scores (r = .258, p = .015). When using resting‐state FCs, FCs related to positive‐constructive daydreaming (r = .243, p = .004) and poor attentional control (r = .207, p = .013) also predicted CBI scores effectively. Moreover, the combination of the subscale‐related FCs could significantly predict individual CBI scores (r = .246, p = .003). The prediction results were consistent with the findings using leave‐one‐out procedure. These findings suggested that the prediction performance of daydreaming‐related FCs on creativity scores had high reproducibility.
4. DISCUSSION
The purpose of the present study is to explore the relationship between different types of daydreaming and creativity and its neural basis. At the behavioral level, we found that various types of daydreaming had different relationships with creativity. Specifically, positive constructive daydreaming was positively related to creativity, while poor attention was negatively related to creativity. Predictive analysis based on task FCs showed that FCs related to positive constructive daydreaming and FCs related to poor attention both predicted an individual's creativity score effectively. In addition, task‐related FCs combining positive constructive daydreaming and poor attention could also successfully predict an individual's creativity score. Predictive analysis based on resting‐state FCs showed similar patterns. These results showed that daydreaming was closely related to creativity, as they shared common FC basis.
We found that positive constructive daydreaming was positively related to CBI scores which meant that the more daydreaming one experienced, the higher the level of creativity one had. This finding is consistent with previous studies. Many influential scientists, such as Newton and Einstein, claimed that they had their moments of inspiration while relinquishing the effort to solve the problem they were working on (Baird et al., 2012). Empirical research used an incubation paradigm and found that engaging in an undemanding task during unusual uses task that maximized daydreaming brought about improvements in creativity performance (Baird et al., 2012). Positive constructive daydreaming on behalf of the adaptive nature of daydreaming and is one of the main styles of daydreaming (McMillan et al., 2013). Furthermore, positive constructive daydreaming is associated with personality trait such as openness to experience and curiosity, which is also closely related to creativity (Zhiyan & Jerome, 1997). Some researchers hold the opinion that positive constructive daydreaming benefits creativity through enhanced cognitive flexibility (Zedelius & Schooler, 2016). Although specific empirical research about the relationship between daydreaming and creative thinking is still lacking, recent opinions have claimed that daydreaming and creativity share similar cognitive mechanisms especially in self‐generated thoughts and deliberate stage (Fox & Beaty, 2019). Our results further support such claim.
We also found that the poor attentional control of daydreaming was negatively related to CBI scores which meant that higher scores in poor attentional control was related to lower scores in creativity performance. Daydreaming is characterized by a decoupling of attention from the current task toward unrelated concerns. Several studies have linked daydreaming to poor performance in tasks about sustained attention. For example, daydreaming during reading task results in slow reading speed and prolonging fixation duration (Foulsham, Farley, & Kingstone, 2013). The damaged attention process of daydreaming can lead to serious and destructive consequences such as traffic accidents or scholastic failure (Galera et al., 2012; Smallwood, Fishman, & Schooler, 2007). The poor attentional control of daydreaming may have negative effect on the creativity process. Hao et al. (2015) found that for the high daydreaming group, during a 20‐min creative production task, the originality of ideas decreased as time passes. But the originality score of the low daydreaming group kept stable. The authors posited that the cognitive control processes related to the generation of creative idea were impaired by daydreaming. Our results further prove a negative correlation between the poor attentional control of daydreaming and creativity.
In addition to the behavioral findings, the present study used machine learning approach to explore the common neural basis underlying daydreaming and creativity. The results revealed that daydreaming‐related FCs could predict creativity effectively, thus indicating that daydreaming and creativity shared common FC basis. Specifically, in both task‐based fMRI data and resting‐state fMRI data, most FCs were related to the DMN. This observation is consistent with the findings of previous studies. Existing research on fMRI suggests that the DMN is activated during daydreaming and individuals' tendency to daydream is correlated with activity in the DMN (Christoff et al., 2009; Kucyi, Salomons, & Davis, 2013; Mason et al., 2007). Recent studies further confirm the role of the DMN in daydreaming using the dynamic FC approach. Kucyi and Davis (2014) used resting‐state data found that daydreaming frequency was positively correlated with dynamic FCs within the subsystem of DMN. The DMN also plays a significant role in the cognitive process of creativity. Previous resting‐state fMRI studies revealed that FCs within the DMN and FCs between the DMN and other brain systems were related to creativity (Beaty et al., 2014; Beaty, Benedek, Kaufman, & Silvia, 2015; Chen et al., 2014; Liu et al., 2015). For example, resting‐state research showed that higher creative score was correlated with stronger FCs in the inferior frontal cortex and the DMN (Beaty et al., 2014). Sun, Liu, et al. (2019) and Sun, Shi, et al. (2019) found that the dynamic FCs within the DMN was positively related to creativity. Task‐based research also highlights the role of the DMN in the creative process. During a task involving divergent thinking, the inferior parietal lobule is positively functional connected to the key regions of the DMN, including the middle temporal gyrus and the precuneus (Sun, Shi, et al., 2019). The DMN has been closely related to the spontaneous generation process of creative thinking, and the functional coupling between the DMN and other brain systems support the creative process collaboratively (Jung et al., 2013).
Besides the DMN, our findings showed that the control system and the attention system also played important parts in the prediction analysis. Previous studies suggest that daydreaming is associated with the central EN (Christoff, Irving, Fox, Spreng, & Andrews‐Hanna, 2016). Brain regions related to executive control, such as the dorsolateral prefrontal cortex and the dorsal anterior cingulate cortex, exhibit consistently activation when individuals are engaging in demanding tasks (Duncan & Owen, 2000; Smith & Jonides, 1999). Task‐based fMRI studies have demonstrated that in addition to the activation of the DMN, daydreaming is also related to the recruitment of the central EN (Christoff et al., 2009). Furthermore, daydreaming is related to the attention system. Meta‐analysis found common activation of the posterior inferior parietal lobule during tasks such as daydreaming, personal goal processing, and episodic future thinking (Stawarczyk & D'Argembeau, 2015). Researchers also surmise that the posterior inferior parietal lobule supports bottom‐up attentional processes (Cabeza, Ciaramelli, & Moscovitch, 2012; Ciaramelli, Grady, & Moscovitch, 2008; Stawarczyk & D'Argembeau, 2015). The central EN and the attention network are also related to the cognitive process of creativity. The central EN modifies and directs self‐generated thoughts to satisfy the specific goals of a task (Beaty et al., 2016). The attention network and ENs are coupled to support the production of creative ideas (Beaty et al., 2015). This is also supported by the finding that the dynamic FCs of the attention network and the DMN are related to the individual difference of creativity (Sun, Liu, et al., 2019). The results of the present study further confirm the recruitment of the attention system and the control system in the cognitive process of daydreaming and creativity.
The predictive analysis also emphasized the role of SN in daydreaming and creativity. The hub brain regions of the SN locate in the anterior insula and the anterior cingulate cortex. Christoff et al. (2016) proposed a neural model for spontaneous thought in daydreaming. They posited that the SN, together with the attention network and the DMN, could exert automatic constraints on the output of the medial temporal lobe and sensorimotor regions to limit the fluctuation of thought. Recent studies have shown that during daydreaming, the brain regions within the DMN, EN, and SN manifest a dynamic FC pattern (Mooneyham et al., 2017). In the process of creativity, the SN modulates the interplay between the DMN and the central EN (Jung et al., 2013). Our results are consistent with these studies and further link daydreaming and creativity through the FC of the SN.
Besides the DMN, EN, and SN, the present study also found that the common brain basis of daydreaming and creativity was related to networks such as SSH and VN. These primary sensory/somatomotor networks are typically not involved in daydreaming and creativity. But in this study, these networks were functional connected to DMN and SN. It is possible that both of the daydreaming and creativity involve dealing with information from external sensory input (Pisapia, Bacci, Parrott, & Melcher, 2016; Schooler et al., 2011). The external sensory information is inputted from the sensory/somatomotor networks and is subsequently processed by DMN and SN.
In our findings, positive constructive daydreaming and poor attentional control had opposite relationship with creativity. The opposite correlations have effect on the location of FCs and the correlation of these FCs and behavioral measures. FCs positively correlated with positive constructive daydreaming may be also positively correlated with creativity, while FCs positively correlated with poor attentional control may be negatively correlated with creativity. But it is worth noting that both positive constructive daydreaming and poor attentional control are used to select FCs, and the prediction analysis showed that these selected FCs are effective in predicting creativity. Therefore, there is a positive correlation between the predicted value and the measured value. Notably, the prediction model of task‐based fMRI and resting‐state fMRI showed a similar pattern. The common FC pattern of daydreaming and creativity was similar in task and resting state. This outcome is consistent with our previous study, which revealed that the FCs between the subsystem of DMN and frontal–parietal network during divergent thinking task are positively correlated with those FCs during resting state (Shi et al., 2018). Our findings further suggest that both in creativity task and resting state, daydreaming and creativity share common FCs basis. We also found that the FCs related to positive constructive daydreaming and the FCs related to poor attentional control predicted creativity effectively. When combining the FCs related to positive constructive daydreaming and the FCs related to poor attentional control, the predictive power was higher, thus representing better predictive effect. These results demonstrated the importance of combining different types of daydreaming when predicting creativity.
This study has some limitations. One limitation is that only adults are used. Although research about adults is also meaningful, research involving children and adolescents can bring insight into the common development trajectory of daydreaming and creativity. Another limitation is the research design. Daydreaming and creativity are measured separately in the present study. Future research should use fMRI task that involves both the cognitive process of daydreaming and creativity to further explore the common neural basis underling daydreaming and creativity.
5. CONCLUSIONS
This study used machine learning methods and found that daydreaming and creativity shared a common neural basis. The FCs related to positive constructive daydreaming and the FCs related to poor attentional control predicted creativity effectively. The common FCs were mainly related to the DMN, the attention system and the control system. Our research used neuroscience methods to prove the existence of a common cognitive neural mechanism between daydreaming and creativity. Furthermore, this study also expands the existing theories by revealing the multifaceted and complex nature of daydreaming and creativity. Our findings provide insight into the complex relationship between daydreaming and creativity from the perspective of neural basis. Future research can attempt to improve creativity by taking advantage of the positive aspect of daydreaming and avoiding the negative aspect of daydreaming.
CONFLICT OF INTEREST
The authors declare no potential conflict of interests.
ETHICS STATEMENT
This study was approved by the Brain Imaging Center Institutional Review Board at the Southwest University, China.
Supporting information
Appendix S1: Supporting Information
ACKNOWLEDGMENTS
This work was supported by the National Natural Science Foundation of China (31771231 and 32071070), the Planned Project of Chongqing humanities and Social Sciences (2018PY80 and 2019PY51), Natural Science Foundation of Chongqing (cstc2019jcyj‐msxmX0520 and cstc2020jcyj‐msxmX0299), and Fundamental Research Funds for the Central Universities (SWU119007), Chang Jiang Scholars Program, National Outstanding Young People Plan, Chongqing Talent Program. J. S. was supported by the National Natural Science Foundation of China (32100850), the project funded by China Postdoctoral Science Foundation (2019TQ0268 and 2020M673100), Fundamental Research Funds for the Central Universities (SWU2109346), and the Chongqing Special Postdoctoral Science Foundation.
Sun, J. , He, L. , Chen, Q. , Yang, W. , Wei, D. , & Qiu, J. (2022). The bright side and dark side of daydreaming predict creativity together through brain functional connectivity. Human Brain Mapping, 43(3), 902–914. 10.1002/hbm.25693
Funding information China Postdoctoral Science Foundation, Grant/Award Numbers: 2019TQ0268, 2020M673100; Fundamental Research Funds for the Central Universities, Grant/Award Numbers: SWU119007, SWU2109346; National Natural Science Foundation of China, Grant/Award Numbers: 31771231, 32071070, 32100850; Natural Science Foundation of Chongqing, Grant/Award Numbers: cstc2019jcyj‐msxmX0520, cstc2020jcyj‐msxmX0299; Planned Project of Chongqing humanities and Social Sciences, Grant/Award Numbers: 2018PY80, 2019PY51; Chongqing Special Postdoctoral Science Foundation
DATA AVAILABILITY STATEMENT
The data used in this study are available from the corresponding author upon reasonable request.
REFERENCES
- Abraham, A. (2014). Creative thinking as orchestrated by semantic processing vs. cognitive control brain networks. Frontiers in Human Neuroscience, 8, 95. 10.3389/fnhum.2014.00095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abraham, A. , Beudt, S. , Ott, D. V. M. , & Cramon, D. Y. V. (2012). Creative cognition and the brain: Dissociations between frontal, parietal‐temporal and basal ganglia groups. Brain Research, 1482(2), 55–70. 10.1016/j.brainres.2012.09.007 [DOI] [PubMed] [Google Scholar]
- Baird, B. , Smallwood, J. , Mrazek, M. D. , Kam, J. W. Y. , Franklin, M. S. , & Schooler, J. W. (2012). Inspired by distraction: Mind wandering facilitates creative incubation. Psychological Science, 23(10), 1117–1122. 10.1177/0956797612446024 [DOI] [PubMed] [Google Scholar]
- Beaty, R. E. , Benedek, M. , Kaufman, S. B. , & Silvia, P. J. (2015). Default and executive network coupling supports creative idea production. Scientific Reports, 5, 10964. 10.1038/srep10964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaty, R. E. , Benedek, M. , Silvia, P. J. , & Schacter, D. L. (2016). Creative cognition and brain network dynamics. Trends in Cognitive Sciences, 20(2), 87–95. 10.1016/j.tics.2015.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaty, R. E. , Benedek, M. , Wilkins, R. W. , Jauk, E. , Fink, A. , Silvia, P. J. , … Neubauer, A. C. (2014). Creativity and the default network: A functional connectivity analysis of the creative brain at rest. Neuropsychologia, 64, 92–98. 10.1016/j.neuropsychologia.2014.09.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaty, R. E. , & Silvia, P. J. (2012). Why do ideas get more creative across time? An executive interpretation of the serial order effect in divergent thinking tasks. Psychology of Aesthetics, Creativity, and the Arts, 6(4), 309–319. 10.1037/a0029171 [DOI] [Google Scholar]
- Benedek, M. , Beaty, R. , Jauk, E. , Koschutnig, K. , Fink, A. , Silvia, P. J. , … Neubauer, A. C. (2014). Creating metaphors: The neural basis of figurative language production. NeuroImage, 90, 99–106. 10.1016/j.neuroimage.2013.12.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedek, M. , Jauk, E. , Sommer, M. , Arendasy, M. , & Neubauer, A. C. (2014). Intelligence, creativity, and cognitive control: The common and differential involvement of executive functions in intelligence and creativity. Intelligence, 46, 73–83. 10.1016/j.intell.2014.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedek, M. , Jurisch, J. , Koschutnig, K. , Fink, A. , & Beaty, R. E. (2020). Elements of creative thought: Investigating the cognitive and neural correlates of association and bi‐association processes. NeuroImage, 210, 116586. 10.1016/j.neuroimage.2020.116586 [DOI] [PubMed] [Google Scholar]
- Cabeza, R. , Ciaramelli, E. , & Moscovitch, M. (2012). Cognitive contributions of the ventral parietal cortex: An integrative theoretical account. Trends in Cognitive Sciences, 16(6), 338–352. 10.1016/j.tics.2012.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, Q. , Yang, W. , Li, W. , Wei, D. , Li, H. , Lei, Q. , … Qiu, J. (2014). Association of creative achievement with cognitive flexibility by a combined voxel‐based morphometry and resting‐state functional connectivity study. NeuroImage, 102(Pt 2), 474–483. 10.1016/j.neuroimage.2014.08.008 [DOI] [PubMed] [Google Scholar]
- Christensen, A. P. , Kenett, Y. N. , Cotter, K. N. , Beaty, R. E. , & Silvia, P. J. (2018). Remotely close associations: Openness to experience and semantic memory structure. European Journal of Personality, 32(4), 480–492. 10.1002/per.2157 [DOI] [Google Scholar]
- Christoff, K. , Gordon, A. M. , Smallwood, J. , Smith, R. , & Schooler, J. W. (2009). Experience sampling during fMRI reveals default network and executive system contributions to mind wandering. Proceedings of the National Academy of Sciences of the United States of America, 106(21), 8719–8724. 10.1073/pnas.0900234106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christoff, K. , Irving, Z. C. , Fox, K. C. , Spreng, R. N. , & Andrews‐Hanna, J. R. (2016). Mind‐wandering as spontaneous thought: A dynamic framework. Nature Reviews Neuroscience, 17(11), 718–731. 10.1038/nrn.2016.113 [DOI] [PubMed] [Google Scholar]
- Ciaramelli, E. , Grady, C. L. , & Moscovitch, M. (2008). Top‐down and bottom‐up attention to memory: A hypothesis (AtoM) on the role of the posterior parietal cortex in memory retrieval. Neuropsychologia, 46(7), 1828–1851. 10.1016/j.neuropsychologia.2008.03.022 [DOI] [PubMed] [Google Scholar]
- Dietrich, A. , & Kanso, R. (2010). A review of EEG, ERP, and neuroimaging studies of creativity and insight. Psychological Bulletin, 136(5), 822–848. 10.1037/a0019749 [DOI] [PubMed] [Google Scholar]
- Dollinger, S. J. (2011). “Standardized minds” or individuality? Admissions tests and creativity revisited. Psychology of Aesthetics, Creativity, and the Arts, 5(4), 329–341. 10.1037/a0023659 [DOI] [Google Scholar]
- Duncan, J. , & Owen, A. M. (2000). Common regions of the human frontal lobe recruited by diverse cognitive demands. Trends in Neurosciences, 23(10), 475–483. 10.1016/s0166-2236(00)01633-7 [DOI] [PubMed] [Google Scholar]
- Fink, A. , Graif, B. , & Neubauer, A. C. (2009). Brain correlates underlying creative thinking: EEG alpha activity in professional vs. novice dancers. NeuroImage, 46(3), 854–862. 10.1016/j.neuroimage.2009.02.036 [DOI] [PubMed] [Google Scholar]
- Finke, R. A. , Ward, T. B. , & Smith, S. M. (1992). Creative cognition: Theory, research, and applications. Cambridge, MA: MIT Press. [Google Scholar]
- Foulsham, T. , Farley, J. , & Kingstone, A. (2013). Mind wandering in sentence Reading: Decoupling the link between mind and eye. Canadian Journal of Experimental Psychology‐Revue Canadienne De Psychologie Experimentale, 67(1), 51–59. 10.1037/a0030217 [DOI] [PubMed] [Google Scholar]
- Fox, K. C. R. , & Beaty, R. E. (2019). Mind‐wandering as creative thinking: Neural, psychological, and theoretical considerations. Current Opinion in Behavioral Sciences, 27, 123–130. 10.1016/j.cobeha.2018.10.009 [DOI] [Google Scholar]
- Friston, K. J. , Williams, S. , Howard, R. , Frackowiak, R. S. , & Turner, R. (1996). Movement‐related effects in fMRI time‐series. Magnetic Resonance in Medicine, 35(3), 346–355. 10.1002/mrm.1910350312 [DOI] [PubMed] [Google Scholar]
- Galera, C. , Orriols, L. , M'Bailara, K. , Laborey, M. , Contrand, B. , Ribereau‐Gayon, R. , … Lagarde, E. (2012). Mind wandering and driving: Responsibility case‐control study. BMJ‐British Medical Journal, 345, e8105. 10.1136/bmj.e8105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golchert, J. , Smallwood, J. , Jefferies, E. , Seli, P. , Huntenburg, J. M. , Liem, F. , … Margulies, D. S. (2017). Individual variation in intentionality in the mind‐wandering state is reflected in the integration of the default‐mode, fronto‐parietal, and limbic networks. NeuroImage, 146, 226–235. 10.1016/j.neuroimage.2016.11.025 [DOI] [PubMed] [Google Scholar]
- Gonen‐Yaacovi, G. , de Souza, L. C. , Levy, R. , Urbanski, M. , Josse, G. , & Volle, E. (2013). Rostral and caudal prefrontal contribution to creativity: A meta‐analysis of functional imaging data. Frontiers in Human Neuroscience, 7, 465. 10.3389/fnhum.2013.00465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao, N. , Wu, M. X. , Runco, M. A. , & Pina, J. (2015). More mind wandering, fewer original ideas: Be not distracted during creative idea generation. Acta Psychologica, 161, 110–116. 10.1016/j.actpsy.2015.09.001 [DOI] [PubMed] [Google Scholar]
- He, L. , Wei, D. , Yang, F. , Zhang, J. , Cheng, W. , Feng, J. , … Qiu, J. (2021). Functional connectome prediction of anxiety related to the COVID‐19 pandemic. The American Journal of Psychiatry, 178(6), 530–540. 10.1176/appi.ajp.2020.20070979 [DOI] [PubMed] [Google Scholar]
- Hocevar, D. (1979). The development of the Creative Behavior Inventory (CBI). In Paper Presented at the Annual Meeting of the Rocky Mountain Psychological Association (ERIC Document Reproduction Service No. Ed. 170 350) .
- Hocevar, D. (1980). Intelligence, divergent thinking, and creativity. Intelligence, 4(1), 25–40. [Google Scholar]
- Huang, F. R. , Fan, J. , & Luo, J. (2015). The neural basis of novelty and appropriateness in processing of creative chunk decomposition. NeuroImage, 113, 122–132. 10.1016/j.neuroimage.2015.03.030 [DOI] [PubMed] [Google Scholar]
- Huang, F. R. , Zhao, Q. B. , Zhou, Z. J. , & Luo, J. (2019). People got lost in solving a set of similar problems. NeuroImage, 186, 192–199. 10.1016/j.neuroimage.2018.10.063 [DOI] [PubMed] [Google Scholar]
- Huba, G. J. , Singer, J. L. , Aneshensel, C. S. , & Antrobus, J. S. (1982). Manual for the short imaginal processes inventory. Port Huron, MI: Research Psychologist Press. [Google Scholar]
- Huba, G. J. , & Tanaka, J. S. (1983). Confirmatory evidence for three daydreaming factors in the short Imaginal processes inventory. Imagination, Cognition and Personality, 3(2), 139–147. 10.2190/VUMW-3JWN-YWQT-BBCM [DOI] [Google Scholar]
- Jung, R. E. , Mead, B. S. , Carrasco, J. , & Flores, R. A. (2013). The structure of creative cognition in the human brain. Frontiers in Human Neuroscience, 7, 330. 10.3389/fnhum.2013.00330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane, M. J. , Brown, L. H. , McVay, J. C. , Silvia, P. J. , Myin‐Germeys, I. , & Kwapil, T. R. (2007). For whom the mind wanders, and when—An experience‐sampling study of working memory and executive control in daily life. Psychological Science, 18(7), 614–621. 10.1111/j.1467-9280.2007.01948.x [DOI] [PubMed] [Google Scholar]
- Killingsworth, M. A. , & Gilbert, D. T. (2010). A wandering mind is an unhappy mind. Science, 330(6006), 932–932. 10.1126/science.1192439 [DOI] [PubMed] [Google Scholar]
- Kucyi, A. (2018). Just a thought: How mind‐wandering is represented in dynamic brain connectivity. NeuroImage, 180, 505–514. 10.1016/j.neuroimage.2017.07.001 [DOI] [PubMed] [Google Scholar]
- Kucyi, A. , & Davis, K. D. (2014). Dynamic functional connectivity of the default mode network tracks daydreaming. NeuroImage, 100, 471–480. 10.1016/j.neuroimage.2014.06.044 [DOI] [PubMed] [Google Scholar]
- Kucyi, A. , Salomons, T. V. , & Davis, K. D. (2013). Mind wandering away from pain dynamically engages antinociceptive and default mode brain networks. Proceedings of the National Academy of Sciences of the United States of America, 110(46), 18692–18697. 10.1073/pnas.1312902110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, S. , Erkkinen, M. G. , Healey, M. L. , Xu, Y. , Swett, K. E. , Chow, H. M. , & Braun, A. R. (2015). Brain activity and connectivity during poetry composition: Toward a multidimensional model of the creative process. Human Brain Mapping, 36(9), 3351–3372. 10.1002/hbm.22849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madore, K. P. , Thakral, P. P. , Beaty, R. E. , Addis, D. R. , & Schacter, D. L. (2019). Neural mechanisms of episodic retrieval support divergent creative thinking. Cerebral Cortex, 29(1), 150–166. 10.1093/cercor/bhx312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason, M. F. , Norton, M. I. , Van Horn, J. D. , Wegner, D. M. , Grafton, S. T. , & Macrae, C. N. (2007). Wandering minds: The default network and stimulus‐independent thought. Science, 315(5810), 393–395. 10.1126/science.1131295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMillan, R. L. , Kaufman, S. B. , & Singer, J. L. (2013). Ode to positive constructive daydreaming. Frontiers in Psychology, 4, 626. 10.3389/fpsyg.2013.00626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McVay, J. C. , & Kane, M. J. (2009). Conducting the train of thought: Working memory capacity, goal neglect, and mind wandering in an executive‐control task. Journal of Experimental Psychology‐Learning Memory and Cognition, 35(1), 196–204. 10.1037/a0014104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mcvay, J. C. , & Kane, M. J. (2010). Does mind wandering reflect executive function or executive failure? Comment on Smallwood and Schooler (2006) and Watkins (2008). Psychological Bulletin, 136(2), 188–197. 10.1037/a0018298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mok, L. W. (2014). The interplay between spontaneous and controlled processing in creative cognition. Frontiers in Human Neuroscience, 8, 663. 10.3389/fnhum.2014.00663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooneyham, B. W. , Mrazek, M. D. , Mrazek, A. J. , Mrazek, K. L. , Phillips, D. T. , & Schooler, J. W. (2017). States of mind: Characterizing the neural bases of focus and mind‐wandering through dynamic functional connectivity. Journal of Cognitive Neuroscience, 29(3), 495–506. 10.1162/jocn_a_01066 [DOI] [PubMed] [Google Scholar]
- Ostafin, B. D. , & Kassman, K. T. (2012). Stepping out of history: Mindfulness improves insight problem solving. Consciousness and Cognition, 21(2), 1031–1036. 10.1016/j.concog.2012.02.014 [DOI] [PubMed] [Google Scholar]
- Ottaviani, C. , & Couyoumdjian, A. (2013). Pros and cons of a wandering mind: A prospective study. Frontiers in Psychology, 4, 524. 10.3389/fpsyg.2013.00524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pidgeon, L. M. , Grealy, M. , Duffy, A. H. B. , Hay, L. , McTeague, C. , Vuletic, T. , … Gilbert, S. J. (2016). Functional neuroimaging of visual creativity: A systematic review and meta‐analysis. Brain and Behavior, 6(10), e00540. 10.1002/brb3.540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisapia, N. D. , Bacci, F. , Parrott, D. , & Melcher, D. (2016). Brain networks for visual creativity: A functional connectivity study of planning a visual artwork. Scientific Reports, 6(1), 39185. 10.1038/srep39185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power, J. D. , Cohen, A. L. , Nelson, S. M. , Wig, G. S. , Barnes, K. A. , Church, J. A. , … Petersen, S. E. (2011). Functional network organization of the human brain. Neuron, 72(4), 665–678. 10.1016/j.neuron.2011.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Runco, M. A. , & Jaeger, G. J. (2012). The standard definition of creativity. Creativity Research Journal, 24(1), 92–96. 10.1080/10400419.2012.650092 [DOI] [Google Scholar]
- Schooler, J. W. , Smallwood, J. , Christoff, K. , Handy, T. C. , Reichle, E. D. , & Sayette, M. A. (2011). Meta‐awareness, perceptual decoupling and the wandering mind. Trends in Cognitive Sciences, 15(7), 319–326. [DOI] [PubMed] [Google Scholar]
- Shepard, R. N. (1978). The mental image. American Psychologist, 33(2), 125–137. [Google Scholar]
- Shi, L. , Sun, J. , Xia, Y. , Ren, Z. , Chen, Q. , Wei, D. , … Qiu, J. (2018). Large‐scale brain network connectivity underlying creativity in resting‐state and task fMRI: Cooperation between default network and frontal‐parietal network. Biological Psychology, 135, 102–111. 10.1016/j.biopsycho.2018.03.005 [DOI] [PubMed] [Google Scholar]
- Silvia, P. J. , & Beaty, R. E. (2012). Making creative metaphors: The importance of fluid intelligence for creative thought. Intelligence, 40(4), 343–351. 10.1016/j.intell.2012.02.005 [DOI] [Google Scholar]
- Silvia, P. J. , Beaty, R. E. , & Nusbaum, E. C. (2013). Verbal fluency and creativity: General and specific contributions of broad retrieval ability (Gr) factors to divergent thinking. Intelligence, 41(5), 328–340. 10.1016/j.intell.2013.05.004 [DOI] [Google Scholar]
- Singer, J. L. (1975). The inner world of daydreaming. New York, NY: Harper Row. [Google Scholar]
- Singer, J. L. , & Schonbar, R. A. (1961). Correlates of daydreaming—A dimension of self‐awareness. Journal of Consulting Psychology, 25(1), 1–6. 10.1037/h0048906 [DOI] [Google Scholar]
- Sio, U. N. , & Ormerod, T. C. (2009). Does incubation enhance problem solving? A meta‐analytic review. Psychological Bulletin, 135(1), 94–120. 10.1037/a0014212 [DOI] [PubMed] [Google Scholar]
- Smallwood, J. , Fishman, D. J. , & Schooler, J. W. (2007). Counting the cost of an absent mind: Mind wandering as an underrecognized influence on educational performance. Psychonomic Bulletin & Review, 14(2), 230–236. 10.3758/bf03194057 [DOI] [PubMed] [Google Scholar]
- Smallwood, J. , Mcspadden, M. , & Schooler, J. W. (2007). The lights are on but no one's home: Meta‐awareness and the decoupling of attention when the mind wanders. Psychonomic Bulletin & Review, 14(3), 527–533. [DOI] [PubMed] [Google Scholar]
- Smallwood, J. , & Schooler, J. W. (2006). The restless mind. Psychological Bulletin, 132(6), 946–958. 10.1037/0033-2909.132.6.946 [DOI] [PubMed] [Google Scholar]
- Smith, E. E. , & Jonides, J. (1999). Storage and executive processes in the frontal lobes. Science, 283(5408), 1657–1661. 10.1126/science.283.5408.1657 [DOI] [PubMed] [Google Scholar]
- Sowden, P. T. , Pringle, A. , & Gabora, L. (2015). The shifting sands of creative thinking: Connections to dual‐process theory. Thinking & Reasoning, 21(1), 40–60. 10.1080/13546783.2014.885464 [DOI] [Google Scholar]
- Stawarczyk, D. , & D'Argembeau, A. (2015). Neural correlates of personal goal processing during episodic future thinking and mind‐wandering: An ALE meta‐analysis. Human Brain Mapping, 36(8), 2928–2947. 10.1002/hbm.22818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternberg, R. J. , & Lubart, T. I. (1996). Investing in creativity. Psychological Inquiry, 4(3), 229–232. 10.1207/s15327965pli0403_16 [DOI] [Google Scholar]
- Sun, J. , Chen, Q. , Zhang, Q. , Li, Y. , Li, H. , Wei, D. , … Qiu, J. (2016). Training your brain to be more creative: Brain functional and structural changes induced by divergent thinking training. Human Brain Mapping, 37(10), 3375–3387. 10.1002/hbm.23246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, J. , Liu, Z. , Rolls, E. T. , Chen, Q. , Yao, Y. , Yang, W. , … Jiang, Q. (2019). Verbal creativity correlates with the temporal variability of brain networks during the resting state. Cerebral Cortex, 29(3), 1047–1058. 10.1093/cercor/bhy010 [DOI] [PubMed] [Google Scholar]
- Sun, J. , Shi, L. , Chen, Q. , Yang, W. , Wei, D. , Zhang, J. , … Qiu, J. (2019). Openness to experience and psychophysiological interaction patterns during divergent thinking. Brain Imaging and Behavior, 13, 1580–1589. 10.1007/s11682-018-9965-2 [DOI] [PubMed] [Google Scholar]
- Takeuchi, H. , Taki, Y. , Hashizume, H. , Sassa, Y. , Nagase, T. , Nouchi, R. , & Kawashima, R. (2012). The association between resting functional connectivity and creativity. Cerebral Cortex, 22(12), 2921–2929. 10.1093/cercor/bhr371 [DOI] [PubMed] [Google Scholar]
- Tipping, M. E. (2001). Sparse Bayesian learning and the relevance vector machine. Journal of Machine Learning Research, 1(3), 211–244. 10.1162/15324430152748236 [DOI] [Google Scholar]
- Wegbreit, E. , Suzuki, S. , Grabowecky, M. , Kounios, J. , & Beeman, M. (2012). Visual attention modulates insight versus analytic solving of verbal problems. The Journal of Problem Solving, 4(2), 94–115. 10.7771/1932-6246.1127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield‐Gabrieli, S. , & Nieto‐Castanon, A. (2012). Conn: A functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connectivity, 2(3), 125–141. 10.1089/brain.2012.0073 [DOI] [PubMed] [Google Scholar]
- Wu, X. , Yang, W. , Tong, D. , Sun, J. , Chen, Q. , Wei, D. , … Qiu, J. (2015). A meta‐analysis of neuroimaging studies on divergent thinking using activation likelihood estimation. Human Brain Mapping, 36(7), 2703–2718. 10.1002/hbm.22801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan, C.‐G. , & Zang, Y.‐F. (2010). DPARSF: A MATLAB toolbox for "pipeline" data analysis of resting‐state fMRI. Frontiers in Systems Neuroscience, 4, 13. 10.3389/fnsys.2010.00013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zedelius, C. M. , & Schooler, J. W. (2016). The richness of inner experience: Relating styles of daydreaming to creative processes. Frontiers in Psychology, 6, 2063. 10.3389/fpsyg.2015.02063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, W. , Sjoerds, Z. , & Hommel, B. (2020). Metacontrol of human creativity: The neurocognitive mechanisms of convergent and divergent thinking. NeuroImage, 210, 116572. 10.1016/j.neuroimage.2020.116572 [DOI] [PubMed] [Google Scholar]
- Zhiyan, T. , & Jerome, L. S. (1997). Daydreaming styles, emotionality and the big five personality dimensions. Imagination, Cognition and Personality, 16(4), 399–414. 10.2190/ATEH-96EV-EXYX-2ADB [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1: Supporting Information
Data Availability Statement
The data used in this study are available from the corresponding author upon reasonable request.


