Abstract
Background
In the randomized phase III KEYNOTE-181 study, pembrolizumab prolonged overall survival (OS) compared with chemotherapy as second-line therapy in patients with advanced esophageal cancer and programmed death-ligand 1 (PD-L1) combined positive score (CPS) ≥10. We report a post hoc subgroup analysis of patients with esophageal squamous cell carcinoma (ESCC) enrolled in KEYNOTE-181 in Asia, including patients from the KEYNOTE-181 China extension study.
Patients and methods
Three hundred and forty Asian patients with advanced/metastatic ESCC were enrolled in KEYNOTE-181, including the China cohort. Patients were randomly assigned 1 : 1 to receive pembrolizumab 200 mg every 3 weeks for ≤2 years or investigator's choice of paclitaxel, docetaxel, or irinotecan. OS, progression-free survival, response, and safety were analyzed without formal comparisons. OS was evaluated based on PD-L1 CPS expression level.
Results
In Asian patients with ESCC, median OS was 10.0 months with pembrolizumab and 6.5 months with chemotherapy [hazard ratio (HR), 0.63; 95% CI 0.50-0.80; nominal P < 0.0001]. Median progression-free survival was 2.3 months with pembrolizumab and 3.1 months with chemotherapy (HR, 0.79; 95% CI 0.63-0.99; nominal P = 0.020). Objective response rate was 17.1% with pembrolizumab and 7.1% with chemotherapy; median duration of response was 10.5 months and 7.7 months, respectively. In patients with PD-L1 CPS <1 tumors (pembrolizumab versus chemotherapy), the HR was 0.99 (95% CI 0.56-1.72); the HR (95% CI) for death was better for patients with PD-L1 CPS cut-offs >1 [CPS ≥1, 0.57 (0.44-0.75); CPS ≥5, 0.56 (0.41-0.76); CPS ≥10, 0.53 (0.37-0.75)]. Treatment-related adverse events were reported in 71.8% of patients in the pembrolizumab group and 89.8% in the chemotherapy group; grade 3-5 events were reported in 20.0% and 44.6%, respectively.
Conclusions
Pembrolizumab monotherapy demonstrated promising efficacy in Asian patients with ESCC, with fewer treatment-related adverse events than chemotherapy. PD-L1 CPS ≥1 is an appropriate cut-off and a predictive marker of pembrolizumab efficacy in Asian patients with ESCC.
Key words: esophageal squamous cell carcinoma, PD-1, PD-L1 CPS, pembrolizumab
Highlights
-
•
Second-line pembrolizumab versus chemotherapy was investigated in patients with ESCC enrolled in KEYNOTE-181 in Asia.
-
•
Pembrolizumab monotherapy was safe and effective in patients with advanced ESCC enrolled in KEYNOTE-181 in Asia.
-
•
PD-L1 CPS ≥1 can be used as a predictive marker of pembrolizumab response in patients with ESCC in Asia.
Introduction
Esophageal cancer is the seventh most common cancer diagnosed and the sixth in mortality among all tumor types worldwide.1 Incidence varies by geographic variation, however, with the highest rates in eastern Asia, southern Africa, and eastern Africa.1 The two major histologic subtypes of esophageal cancer are squamous cell carcinoma (SCC) and adenocarcinoma. SCC is most common in Asia and Africa, whereas adenocarcinoma is most common in North America and Europe.1
KEYNOTE-181 was a global, randomized, open-label, phase III study of pembrolizumab compared with chemotherapy in advanced or metastatic esophageal cancer that progressed after one previous therapy.2 Following the completion of enrollment in the global study, patients were enrolled in the KEYNOTE-181 China extension study to further investigate the safety and efficacy of pembrolizumab in the Chinese population. The primary endpoint was overall survival (OS) in patients with programmed death-ligand 1 (PD-L1) combined positive score (CPS) ≥10, in patients with esophageal SCC (ESCC), and in all patients. In the global population at the final analysis, pembrolizumab provided a clinically meaningful survival benefit compared with chemotherapy for patients with PD-L1 CPS ≥10 ESCC [hazard ratio (HR), 0.64; 95% confidence interval (CI) 0.46-0.90] and for patients with ESCC tumors (HR, 0.78; 95% CI 0.63-0.96; P = 0.0095) or PD-L1 CPS ≥10 tumors (HR, 0.69; 95% CI 0.52-0.93; P = 0.0074).2 In a subgroup analysis of OS, a more prominent survival benefit was observed with pembrolizumab compared with chemotherapy in patients enrolled in Asia across all three populations (PD-L1 CPS ≥10, ESCC, and all patients) and a positive trend was observed in patients with PD-L1 CPS <10 in the ESCC population.2
Taken together, these findings suggest that PD-L1 expression may be a predictive marker for pembrolizumab in patients with ESCC. The PD-L1 CPS ≥10 population accounted for 35.4% of the global population, however, and a possible survival trend in favor of pembrolizumab was observed in patients with PD-L1 CPS <10 ESCC.2 Whether there is a more reasonable cut-off value for PD-L1 CPS in this patient population remains unknown. In the ATTRACTION-03 and ESCORT trials, which also evaluated the efficacy of immune checkpoint inhibitors (ICIs) compared with chemotherapy as second-line treatment in global (including Asian) and Chinese populations with ESCC, respectively, the survival benefit was generally similar between patients with PD-L1 expression across various cut-offs.3,4 Only the PD-L1 tumor proportion score, however, was evaluated in these studies. In previous studies, CPS, which evaluates PD-L1 expression on tumor cells and on infiltrating immune cells, was a more reliable and suitable biomarker of response to ICIs in several tumor types.5, 6, 7
In the current analysis, we investigated the clinical characteristics, efficacy, and safety of pembrolizumab compared with chemotherapy in all patients with ESCC enrolled in KEYNOTE-181 in Asia, including those enrolled in the China extension study. We also evaluated the efficacy of pembrolizumab using different PD-L1 CPS expression levels (<1, ≥1, ≥5, and ≥10).
Methods
Study design, patients, and treatment
Full details of the phase III KEYNOTE-181 study (ClinicalTrials.gov, NCT02564263) have been published.2 In brief, eligible patients had histologically confirmed SCC or adenocarcinoma of the esophagus, including human epidermal growth factor receptor 2/neu-negative Siewert type I adenocarcinoma of the esophagogastric junction and documented radiographic or clinical progression on one previous line of standard therapy. Patients were randomly assigned 1 : 1 to receive pembrolizumab 200 mg every 3 weeks or investigator's choice of standard-of-care chemotherapy [paclitaxel (80-100 mg/m2 on days 1, 8, and 15 of each 28-day cycle), docetaxel (75 mg/m2 on day 1 of each 21-day cycle), or irinotecan (180 mg/m2 on day 1 of each 14-day cycle)]. Treatment continued until documented disease progression, unacceptable toxicity, or physician/patient decision to withdraw or after up to 2 years of pembrolizumab. Patients were stratified by histology (ESCC versus adenocarcinoma) and geographic region (Asia versus rest of world). The current analysis focused on all patients with ESCC enrolled in KEYNOTE-181 in Asia, including those enrolled in the China extension study (ClinicalTrials.gov, NCT03933449) (Supplementary Figure S1, available at https://doi.org/10.1016/j.esmoop.2021.100341).
The study protocol and all amendments were approved by the appropriate ethics committee at each center. The study was conducted in accordance with the protocol, its amendments, and standards of Good Clinical Practice. All patients provided written informed consent.
Assessments and outcomes
Tumor responses were assessed using RECIST version 1.1 by central radiology review at week 9 and every 9 weeks thereafter. Adverse events (AEs) were assessed throughout the study and at 30 days after treatment discontinuation (90 days for serious AEs) and were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (version 4.0). Tumor tissue samples were collected for evaluation of PD-L1 using PD-L1 IHC 22C3 pharmDx (Agilent Technologies, Carpinteria, CA) and were scored using CPS [the number of PD-L1-staining cells (tumor cells, lymphocytes, and macrophages) divided by the total number of viable tumor cells, multiplied by 100].
Assessments of the primary efficacy and safety outcomes have been described.2 The current analysis evaluated OS, progression-free survival (PFS), and objective response rate (ORR) per RECIST v1.1 by central review, duration of response (DOR), and safety and tolerability.
Statistical analysis
In the current post hoc analysis, efficacy was evaluated in the intention-to-treat population and safety was evaluated in the as-treated population of Asian patients with ESCC. Data were pooled for patients enrolled in KEYNOTE-181 in Asia and patients enrolled in the KEYNOTE-181 China extension study. Patients were also grouped by PD-L1 CPS expression level (<1, ≥1, ≥5, and ≥10) for OS analysis.
After enrollment in the global KEYNOTE-181 study was completed (N ∼ 600), patients continued to be randomized in a 1 : 1 ratio to pembrolizumab and standard-of-care chemotherapy in the KEYNOTE-181 China extension study until the total sample size of Chinese patients reached ∼120. The extension study will complete after ∼75 deaths have been observed between the two arms in the China cohort and 8 months after the last patient is randomly assigned, assuming the underlying HR = 0.70. With 75 deaths and a true HR of 0.70, the extension study has a >90% chance of observing an HR on OS <1 and an ∼80% chance of observing a point estimate that preserves approximately ≥50% of the empirical risk reduction from the global analysis in the Chinese subpopulation assuming the underlying HR is 0.70, respectively. The above calculations for the consistency evaluation are based on the same assumptions on the median OS and the true HR. OS and PFS were estimated using the nonparametric Kaplan–Meier method, and treatment differences were assessed using a stratified Cox proportional hazards model with the Efron method of handling ties. Nominal P values were computed without multiplicity adjustment.
The data cut-off dates for this analysis were 15 October 2018 (KEYNOTE-181) and 13 February 2019 (KEYNOTE-181 China extension).
Results
Patients
Between 8 December 2015 and 16 June 2017, 628 patients (n = 314, pembrolizumab; n = 314, chemotherapy) were enrolled in KEYNOTE-181; 231 patients with ESCC were enrolled and randomly assigned at Asian sites, including 10 patients in China. In the Asian subgroup of this analysis (n = 221, excluding 10 patients in China), 110 patients were randomly assigned to the pembrolizumab group and 111 to the chemotherapy group. All 110 patients in the pembrolizumab group and 109/111 patients in the chemotherapy group received treatment; most patients discontinued because of progressive disease (n = 90, pembrolizumab; n = 90, chemotherapy) (Supplementary Figure S2A, available at https://doi.org/10.1016/j.esmoop.2021.100341). After enrollment for the global KEYNOTE-181 study was completed, 112 patients (n = 109 with ESCC; n = 3 with adenocarcinoma) were randomly assigned in the KEYNOTE-181 China extension study between 17 June 2017, and 13 June 2018. In the China cohort of this analysis in patients with ESCC (n = 119, including 10 patients in China in the global study), 60 patients were randomly assigned to the pembrolizumab group and 59 to the chemotherapy group. Sixty patients in the pembrolizumab group and 57 patients in the chemotherapy group received treatment; most patients discontinued because of progressive disease (n = 41, pembrolizumab; n = 42, chemotherapy) (Supplementary Figure S2B, available at https://doi.org/10.1016/j.esmoop.2021.100341). The data were pooled, and 340 Asian patients with ESCC were evaluated for this analysis.
Baseline demographic and disease characteristics were generally similar between treatment groups (Table 1). When assessing Asian patients and Chinese patients, characteristics were generally comparable, including PD-L1 status (Table 1). More patients with Eastern Cooperative Oncology Group performance status (ECOG PS) 1 enrolled in the China cohort (87.4%) than in the Asian subgroup (51.6%). Earlier treatment regimens were different; more Chinese patients than Asian patients had previously received taxane (82.4% versus 30.3%), whereas more Asian patients than Chinese patients had previously received fluoropyrimidine (95.5% versus 34.5%).
Table 1.
Baseline demographics and clinicopathological characteristics of Asian patients with ESCC
| Characteristic | Asian subgroup N = 221 |
China cohort N = 119 |
||
|---|---|---|---|---|
| Pembrolizumab n = 110 | Chemotherapy n = 111 | Pembrolizumab n = 60 | Chemotherapy n = 59 | |
| Age, years, median (range) | 66.0 (45-80) | 64.0 (33-84) | 61.5 (45-74) | 59.0 (41-77) |
| Male | 100 (90.9) | 97 (87.4) | 55 (91.7) | 56 (94.9) |
| ECOG PS | ||||
| 0 | 56 (50.9) | 51 (45.9) | 9 (15.0) | 6 (10.2) |
| 1 | 54 (49.1) | 60 (54.1) | 51 (85.0) | 53 (89.8) |
| PD-L1 CPS | ||||
| ≥1 | 89 (80.9) | 90 (81.1) | 50 (83.3) | 51 (86.4) |
| <1 | 19 (17.3) | 21 (18.9) | 8 (13.3) | 7 (11.9) |
| ≥5 | 71 (64.5) | 72 (64.9) | 32 (53.3) | 37 (62.7) |
| <5 | 37 (33.6) | 39 (35.1) | 26 (43.3) | 21 (35.6) |
| ≥10 | 57 (51.8) | 54 (48.6) | 24 (40.0) | 28 (47.5) |
| <10 | 51 (46.4) | 57 (51.4) | 34 (56.7) | 30 (50.8) |
| Nonassessablea | 2 (1.8) | 0 (0) | 2 (3.3) | 1 (1.7) |
| Previous (neo)adjuvant therapy | 13 (11.8) | 15 (13.5) | 11 (18.3) | 12 (20.3) |
| Disease stage | ||||
| Metastatic | 97 (88.2) | 102 (91.9) | 57 (95.0) | 55 (93.2) |
| Locally advanced | 13 (11.8) | 9 (8.1) | 3 (5.0) | 4 (6.8) |
| Previous therapies | ||||
| One previous therapyb | 108 (98.2) | 110 (99.1) | 60 (100) | 59 (100) |
| Previous anthracycline | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Previous fluoropyrimidine | 106 (96.4) | 105 (94.6) | 20 (33.3) | 21 (35.6) |
| Previous taxane | 32 (29.1) | 35 (31.5) | 45 (75.0) | 53 (89.8) |
Data are presented as number (%) unless indicated otherwise. Patients in the chemotherapy group received investigator's choice of paclitaxel, docetaxel, or irinotecan. Patients in the Asian subgroup do not include Chinese patients. Percentages may not total 100 because of rounding.
CPS, combined positive score; ECOG PS, Eastern Cooperative Oncology Group performance status; ESCC, esophageal squamous cell carcinoma; PD-L1, programmed death-ligand 1.
PD-L1 expression could not be evaluated because samples had inadequate numbers of cells or no cells.
Three patients in the Asian subgroup previously received one or two lines of therapy (n = 2, pembrolizumab; n = 1, chemotherapy).
Efficacy
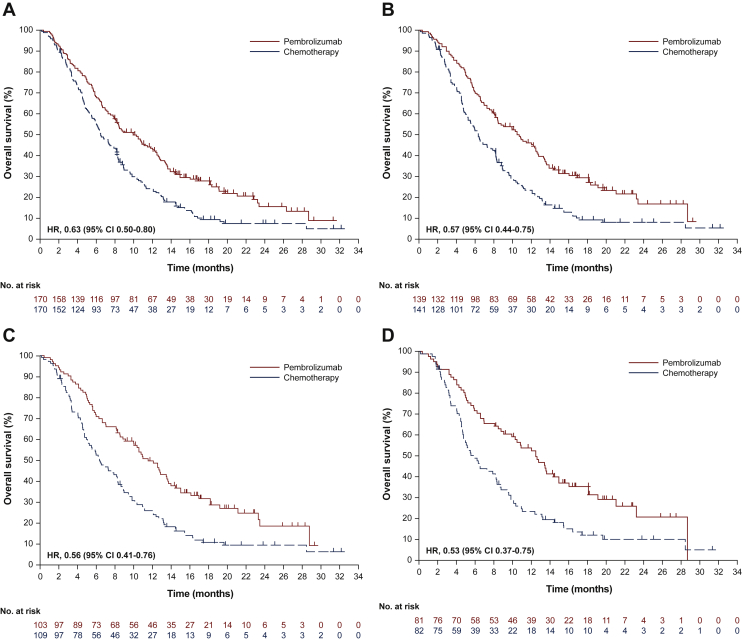
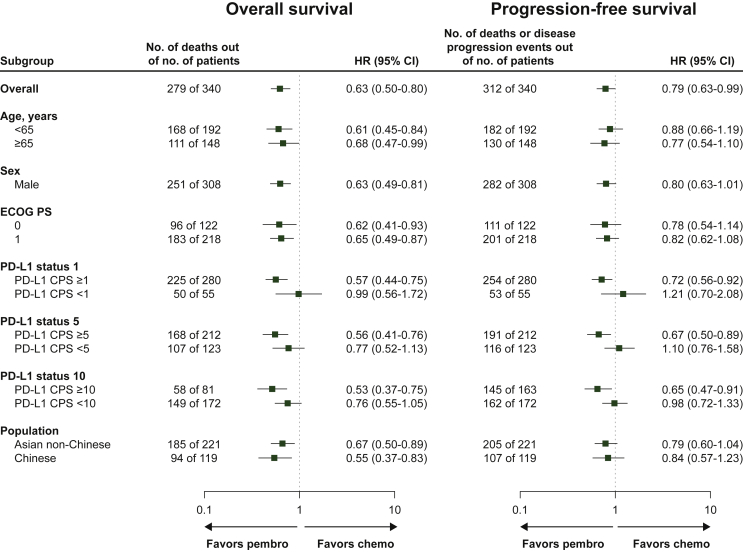
At the data cut-off date (15 October 2018 for Asian non-Chinese patients and 13 February 2019 for Chinese patients), 130/170 patients (76.5%) in the pembrolizumab group and 151/170 patients (88.8%) in the chemotherapy group had died; median OS was 10.0 months (95% CI 8.0-12.2 months) and 6.5 months (95% CI 5.6-8.2 months), respectively (HR, 0.63; 95% CI 0.50-0.80; nominal P < 0.0001) (Figure 1A). The 6-month survival rate was 68.2% in the pembrolizumab group and 55.4% in the chemotherapy group. With the exception of patients with PD-L1 CPS <1 tumors, OS HRs followed a similar trend in patient subgroups, favoring pembrolizumab over chemotherapy (Figure 2).
Figure 1.
Kaplan–Meier estimates of OS in Asian patients with ESCC.
(A) All patients. (B) Patients with PD-L1 CPS ≥1. (C) Patients with PD-L1 CPS ≥5. (D) Patients with PD-L1 CPS ≥10.
CI, confidence interval; CPS, combined positive score; ESCC, esophageal squamous cell carcinoma; HR, hazard ratio; OS, overall survival; PD-L1, programmed death-ligand 1.
Figure 2.
Forest plot analysis of OS and PFS in Asian patients with ESCC.
Chemo, chemotherapy; CI, confidence interval; CPS, combined positive score; ECOG PS, Eastern Cooperative Oncology Group performance status; ESCC, esophageal squamous cell carcinoma; HR, hazard ratio; OS, overall survival; PD-L1, programmed death-ligand 1; pembro, pembrolizumab; PFS, progression-free survival.
At the data cut-off date, 152/170 patients (89.4%) in the pembrolizumab group and 160/170 patients (94.1%) in the chemotherapy group experienced disease progression or died; median PFS was 2.3 months (95% CI 2.1-4.0 months) and 3.1 months (95% CI 2.1-3.9 months), respectively (HR, 0.79; 95% CI 0.63-0.99; nominal P = 0.020). The 6-month PFS rate was 30.8% in the pembrolizumab group and 25.5% in the chemotherapy group. PFS for the patient subgroups is shown in Figure 2.
Among all Asian patients with ESCC, 29/170 patients (17.1%) in the pembrolizumab group and 12/170 patients (7.1%) in the chemotherapy group had an objective response (Table 2). The median DOR was 10.5 months (range, 2.1+ to 18.8+) in the pembrolizumab group and 7.7 months (range, 2.1+ to 16.8+) in the chemotherapy group; 63.2% and 46.0% of patients, respectively, had an extended response lasting ≥9 months from Kaplan–Meier estimates.
Table 2.
Response summary in Asian patients with ESCC
| Best overall response, n (%) | Pembrolizumab n = 170 | Chemotherapy n = 170 |
|---|---|---|
| ORRa | 29 (17.1) | 12 (7.1) |
| CR | 6 (3.5) | 0 (0) |
| PR | 23 (13.5) | 12 (7.1) |
| SD | 52 (30.6) | 66 (38.8) |
| DCRb | 81 (47.6) | 78 (45.9) |
| PD | 76 (44.7) | 62 (36.5) |
| No assessment/nonassessablec | 13 (7.6) | 30 (17.6) |
CR, complete response; DCR, disease control rate; ESCC, esophageal squamous cell carcinoma; ORR, objective response rate; PD, progressive disease; PR, partial response; SD, stable disease.
CR + PR.
CR + PR + SD.
Captures patients for whom no post-baseline assessments were carried out because of death, withdrawal of consent, loss to follow-up, or start of new anticancer therapy and patients who had ≥1 post-baseline tumor assessment, none of which were evaluable for response determination (e.g. not all target lesions were captured).
In an exploratory analysis, we evaluated OS among patients with various PD-L1 CPS expression levels. Of the 55 patients with PD-L1 CPS <1, 24/27 (88.9%) in the pembrolizumab group and 26/28 (92.9%) in the chemotherapy group had died; median OS was 6.6 months (95% CI, 2.8-11.8 months) and 7.4 months (95% CI, 6.0-8.9 months), respectively (HR, 0.99; 95% CI 0.56-1.72) (Supplementary Figure S3, available at https://doi.org/10.1016/j.esmoop.2021.100341). Of the 280 patients with CPS ≥1, 103/139 patients (74.1%) in the pembrolizumab group and 124/141 patients (87.9%) in the chemotherapy group had died; median OS was 10.5 months (95% CI 8.2-12.6 months) and 6.3 months (95% CI 5.2-8.1 months), respectively (HR, 0.57; 95% CI 0.44-0.75) (Figure 1B).
We also evaluated OS for patients with PD-L1 using CPS cut-offs of 5 and 10. Of 216 patients with CPS ≥5, 74/103 patients (71.8%) in the pembrolizumab group and 96/109 patients (88.1%) in the chemotherapy group died; median OS was 11.5 months (95% CI 9.1-13.6 months) and 6.3 months (95% CI 5.1-8.3 months), respectively (HR, 0.56; 95% CI 0.41-0.76) (Figure 1C). Of 163 patients with CPS ≥10, 56/81 patients (69.1%) in the pembrolizumab group and 71/82 patients (86.6%) in the chemotherapy group died; median OS was 12.5 months (95% CI 9.1-14.9 months) and 6.0 months (95% CI 4.7-8.2 months), respectively (HR, 0.53; 95% CI 0.37-0.75) (Figure 1D).
Safety
Most Asian patients with ESCC experienced at least one AE (95.9%, pembrolizumab; 96.4%, chemotherapy) (Table 3). Treatment-related AEs were reported in 122/170 patients (71.8%) in the pembrolizumab group and 149/166 patients (89.8%) in the chemotherapy group; grade 3-5 events were reported in 34/170 patients (20.0%) and 74/166 patients (44.6%), respectively. The most common any-grade treatment-related AEs (≥15%) were hypothyroidism (16.5%) in the pembrolizumab group and decreased white blood cell count (39.2%), decreased neutrophil count (30.7%), alopecia (28.3%), anemia (25.3%), peripheral sensory neuropathy (22.3%), decreased appetite (17.5%), fatigue (16.3%), diarrhea (15.7%), and nausea (15.1%) in the chemotherapy group (Supplementary Table S1, available at https://doi.org/10.1016/j.esmoop.2021.100341). The most common grade 3-5 treatment-related AEs were diarrhea and pneumonitis (n = 2 each; 1.2%) in the pembrolizumab group and decreased white blood cell count (n = 35; 21.1%) and decreased neutrophil count (n = 29; 17.5%) in the chemotherapy group (Supplementary Table S1, available at https://doi.org/10.1016/j.esmoop.2021.100341).
Table 3.
AE summary in Asian patients with ESCC
| Event, n (%) | Pembrolizumab n = 170 | Chemotherapy n = 166 |
|---|---|---|
| ≥1 AE | 163 (95.9) | 160 (96.4) |
| Grade 3-5 | 85 (50.0) | 102 (61.4) |
| Led to discontinuation | 20 (11.8) | 22 (13.3) |
| Serious | 58 (34.1) | 64 (38.6) |
| Serious and led to discontinuation | 17 (10.0) | 13 (7.8) |
| Led to death | 13 (7.6) | 13 (7.8) |
| ≥1 Treatment-related AE | 122 (71.8) | 149 (89.8) |
| Grade 3-5 | 34 (20.0) | 74 (44.6) |
| Led to discontinuation | 13 (7.6) | 13 (7.8) |
| Serious | 29 (17.1) | 33 (19.9) |
| Serious and led to discontinuation | 10 (5.9) | 5 (3.0) |
| Led to deatha | 3 (1.8) | 2 (1.2) |
AE, adverse event; ESCC, esophageal squamous cell carcinoma.
Grade 5 treatment-related AEs were pneumonitis (n = 2) and cardiopulmonary failure (n = 1) in the pembrolizumab group and decreased neutrophil count (n = 1), decreased white blood cell count (n = 1), and pneumonia aspiration (n = 1) in the chemotherapy group.
Fifty-two of 170 patients (30.6%) in the pembrolizumab group and 9/166 patients (5.4%) in the chemotherapy group experienced an immune-mediated AE or an infusion reaction. The most common immune-mediated AEs in the pembrolizumab group were hypothyroidism (n = 29; 17.1%) and pneumonitis (n = 9; 5.3%); infusion-related reactions were reported in four patients (2.4%) (Supplementary Table S2, available at https://doi.org/10.1016/j.esmoop.2021.100341).
Discussion
In this analysis of patients with ESCC enrolled in the KEYNOTE-181 study in Asia, pembrolizumab was found to numerically improve OS and to have a favorable safety profile compared with chemotherapy as second-line treatment. PD-L1 CPS ≥1 was a suitable cut-off value and a predictive marker for pembrolizumab efficacy in this patient population.
Baseline characteristics of Asian patients included in the current analysis were generally similar to those of the global population.2 In addition, characteristics were comparable between the Asian subgroup and the Chinese cohort except for ECOG PS and the type of previous chemotherapy received (Table 1). Although more patients in the China cohort than in the Asian subgroup had ECOG PS 1 (87.4% versus 51.6%), survival outcomes in the pooled analysis were comparable between patients with ECOG PS 0 and 1 (Figure 2). Another difference in baseline characteristics between the Asian subgroup and the China cohort was the type of chemotherapy previously received; more Chinese patients received a taxane (82.4% versus 30.3%) whereas more Asian (non-Chinese) patients received a fluoropyrimidine (95.5% versus 34.5%) (Table 1). This difference was likely due to clinical practice preference and guideline recommendations between China and the rest of the world. Although fluoropyrimidine is considered a standard first-line treatment choice in Western countries and Japan,8,9 paclitaxel is more commonly used in China.10 These treatment choices affected the regimens chosen as second-line treatment, with irinotecan often chosen for Chinese patients.10
Subgroup analysis of OS found a positive trend favoring pembrolizumab across all subgroups evaluated, especially in patients with PD-L1-positive tumors (CPS ≥1, CPS ≥5, and CPS ≥10) (Figure 2). A survival benefit was also observed in patients regardless of age, baseline ECOG PS, and region [Asian (non-Chinese) or Chinese] (Figure 2). Although the survival benefit was more prominent in Chinese patients (HR, 0.55; 95% CI 0.37-0.83), pembrolizumab did show a survival benefit compared with chemotherapy in all Asian patients with ESCC (Asian subgroup combined with Chinese cohort) with an HR of 0.63 (95% CI 0.50-0.80; nominal P < 0.0001).
Emerging global data from phase III trials has demonstrated the benefit of ICIs when used in combination with chemotherapy for ESCC in the first-line setting. In KEYNOTE-590, pembrolizumab plus chemotherapy versus chemotherapy improved OS (HR, 0.72; 95% CI 0.60-0.88; P = 0.0006) and PFS (HR, 0.65; 95% CI 0.54-0.70; P < 0.0001) in patients with ESCC11 and data from the CheckMate 648 study showed superior OS with nivolumab plus chemotherapy versus chemotherapy (HR, 0.74; 99.1%, 0.58-0.96; P = 0.0021).12
PD-L1 expression was comparable between Asian (non-Chinese) patients and Chinese patients, and PD-L1 CPS ≥10 was seen in 50.2% and 43.7% of patients, respectively (Table 1). In the global KEYNOTE-181 study, PD-L1 CPS ≥10 was seen in 35.4% of the enrolled population.2 Although there was a statistically significant survival benefit with pembrolizumab compared with chemotherapy in the global PD-L1 CPS ≥10 population (HR, 0.69; 95% CI 0.52-0.93; P = 0.0074), a trend for prolonged survival was also observed in patients with PD-L1 CPS <10 ESCC (HR, 0.88; 95 CI 0.66-1.16); this positive trend in OS was not observed in non-Asian patients regardless of subgroup.2 To further explore a proper cut-off value of PD-L1 CPS in the Asian population with ESCC, we analyzed OS for various PD-L1 CPS expression levels (<1, ≥1, ≥5, and ≥10): PD-L1 CPS <1 was seen in 16.2% of them, and OS was similar between those who received pembrolizumab and those who received chemotherapy (HR, 0.99; 95% CI 0.56-1.72). Although the survival benefit was slightly more favorable in the PD-L1 CPS ≥10 population (HR, 0.53; 95% CI 0.37-0.75), a substantial OS improvement was still observed with pembrolizumab compared with chemotherapy in the PD-L1 CPS ≥1 population (HR, 0.57; 95% CI 0.44-0.75). Given the lack of second-line treatment options in patients with ESCC, PD-L1 CPS ≥1 should be a reasonable cut-off value for Asian patients with ESCC.
The difference in the survival benefits of pembrolizumab between Asian and non-Asian patients with ESCC might be explained by the intertumor and geographic heterogeneity of ESCC. Risk factors for esophageal cancer differ among geographic regions. In Western countries, the most common risk factors for ESCC include smoking tobacco and alcohol consumption,13 whereas in high incidence areas of China, neither smoking nor drinking alcohol was found to be a significant risk factor for ESCC.14 In Asia, a common risk factor for ESCC includes consumption of hot beverages,15 which could cause thermal damage to the esophageal mucosa. In addition, eating foods containing N-nitroso compounds is a common risk factor in high incidence areas of China.16 Such different risk factors may contribute to the heterogeneity of ESCC. Genetic differences have also been observed in ESCC, including cross-population studies comparing genetic changes between Asian and Caucasian patients with ESCC. In The Cancer Genomic Database, TP53, EP300, and NFE2L2 showed higher mutational frequencies in Asian patients than in Caucasian patients.17 In another study, COL11A1 had higher mutation frequency, greater methylation, and lower protein expression in Caucasian patients.18 The difference in gene mutation frequencies might be related to epidemiology, risk factors, and gene loci associated with susceptibility to ESCC between different racial populations. These findings indicate that the cut-off value of PD-L1 CPS may be different for patients from different geographic regions.
In the current analysis, treatment with pembrolizumab resulted in fewer grade 3-5 treatment-related AEs than chemotherapy (Table 3). The incidence of serious AEs and serious treatment-related AEs, however, was similar between the two treatment groups. The most common immune-mediated AEs with pembrolizumab were endocrine disorders (hypothyroidism) and respiratory, thoracic, and mediastinal disorders (pneumonitis) (Supplementary Table S2, available at https://doi.org/10.1016/j.esmoop.2021.100341). Hepatobiliary disorders and immune system disorders were relatively rare. These safety results are similar to those of the global KEYNOTE-181 study.2 Overall, second-line pembrolizumab was well tolerated and toxicity was acceptable in Asian patients with ESCC.
This analysis has several limitations. First, it is a subgroup analysis of a global study that enrolled a new set of patients in the extension study who were not included in the global study. Although inclusion and exclusion criteria and treatment regimens for the KEYNOTE-181 China extension study were the same as for the global KEYNOTE-181 study, bias could not be completely avoided. Second, there was a difference in the chemotherapy regimen used in the Asian (non-Chinese) subgroup and the Chinese cohort that could have affected the HR of pembrolizumab compared with that of chemotherapy. Third, we did not collect information regarding subsequent treatment, which also could have affected the analysis of survival benefit.
In conclusion, pembrolizumab demonstrated a survival benefit compared with chemotherapy as second-line treatment of Asian patients with ESCC. Second-line pembrolizumab was also better tolerated and was associated with less toxicity than chemotherapy. PD-L1 CPS ≥1 is a reasonable cut-off value and can be used as an indication of Asian patients with ESCC likely to respond to pembrolizumab as second-line treatment.
Acknowledgements
The authors thank the patients and their families and caregivers and all primary investigators and site personnel for participating in the study. The authors also thank Prof. Lin Shen. Medical writing and/or editorial assistance were provided by Holly C. Cappelli, PhD, CMPP, of ApotheCom (Yardley, PA). This assistance was funded by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA.
Funding
This study was supported by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA. The funder participated in the study design, data analysis and interpretation, and manuscript writing and maintained the study database. All authors had full access to the data and had final responsibility for the decision to submit for publication.
Disclosure
YC, SQ, SL, ZL, YC, YF, YS, XY, XY, WL, TL, XL, JZ, YB, CB, KG, H-MP, LB, J-WY, JC have declared no conflicts of interest.
C-HH reports honoraria and fees for consulting and lecture from Bristol Myers Squibb, Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA, Ono Pharmaceutical, and Roche; fees for consulting from Merck Serono; and grants from AstraZeneca and BeiGene.
S-BK reports grants (to institution) from DongKook Pharm Co., Novartis, and Sanofi Genzyme; and personal fees (advisory) for Dae Hwa Pharma Co. Ltd, Daiichi-Sankyo, ISU Abxis.
TK reports grants and personal fees from Astellas Amgen BioPharma, Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA, and Ono Pharmaceutical; grants from Shionogi and Taiho Pharmaceutical; and personal fees from Astellas Pharma, Bristol Myers Squibb, and Oncolys BioPharma.
S-HL reports personal fees from AstraZeneca, Bristol Myers Squibb, Merck Sharp & Dohme Corp., and Roche; and grants from Merck Sharp & Dohme Corp., KM reports research funding for Amgen, Daiichi-Sankyo, Merck Serono, Merck Sharp & Dohme Corp., Ono, Parexel International, Pfizer, Sanofi, Solasia Pharma, and Taiho; personal fees (consulting fee and/or honorarium) for Amgen, AstraZeneca, Bayer, Bristol Myers Squibb, Chugai, Eli Lilly, Ono, Sanofi, Taiho, and Takeda.
TD reports grants from AbbVie, Boehringer Ingelheim, Bristol Myers Squibb, Daiichi-Sankyo, Eisai, Janssen, Lilly, Merck Serono, Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA, Novartis, Pfizer, Quintile (IQVIA), Sumitomo Dainippon, and Taiho; and personal fees from AbbVie, Amgen, Astellas Pharma, Bayer, Boehringer Ingelheim, Bristol Myers Squibb, Chugai Pharma, Daiichi Sankyo, Janssen, Merck Sharp & Dohme Corp., Novartis, Oncolys BioPharma, Ono Pharmaceutical, Otsuka Pharma, Rakuten Medical, Sumitomo Dainippon, Taiho, and Takeda.
YC is an employee of MSD China, Shanghai, China.
WL is an employee of MSD China, Shanghai, China.
Data sharing
Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA (MSD) is committed to providing qualified scientific researchers access to anonymized data and clinical study reports from the company's clinical trials for the purpose of conducting legitimate scientific research. MSD is also obligated to protect the rights and privacy of trial participants and, as such, has a procedure in place for evaluating and fulfilling requests for sharing company clinical trial data with qualified external scientific researchers. The MSD data sharing website (available at: http://engagezone.msd.com/ds_documentation.php) outlines the process and requirements for submitting a data request. Applications will be promptly assessed for completeness and policy compliance. Feasible requests will be reviewed by a committee of MSD subject matter experts to assess the scientific validity of the request and the qualifications of the requestors. In line with data privacy legislation, submitters of approved requests must enter into a standard data-sharing agreement with MSD before data access is granted. Data will be made available for request after product approval in the US and EU or after product development is discontinued. There are circumstances that may prevent MSD from sharing requested data, including country or region-specific regulations. If the request is declined, it will be communicated to the investigator. Access to genetic or exploratory biomarker data requires a detailed, hypothesis-driven statistical analysis plan that is collaboratively developed by the requestor and MSD subject matter experts; after approval of the statistical analysis plan and execution of a data-sharing agreement, MSD will either perform the proposed analyses and share the results with the requestor or will construct biomarker covariates and add them to a file with clinical data that is uploaded to an analysis portal so that the requestor can perform the proposed analyses.
Supplemnetary data
References
- 1.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Kojima T., Shah M.A., Muro K., et al. Randomized phase III keynote-181 study of pembrolizumab versus chemotherapy in advanced esophageal cancer. J Clin Oncol. 2020;38(35):4138–4148. doi: 10.1200/JCO.20.01888. [DOI] [PubMed] [Google Scholar]
- 3.Kato K., Cho B.C., Takahashi M., et al. Nivolumab versus chemotherapy in patients with advanced oesophageal squamous cell carcinoma refractory or intolerant to previous chemotherapy (ATTRACTION-3): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2019;20(11):1506–1517. doi: 10.1016/S1470-2045(19)30626-6. [DOI] [PubMed] [Google Scholar]
- 4.Huang J., Xu J., Chen Y., et al. Camrelizumab versus investigator's choice of chemotherapy as second-line therapy for advanced or metastatic oesophageal squamous cell carcinoma (ESCORT): a multicentre, randomised, open-label, phase 3 study. Lancet Oncol. 2020;21(6):832–842. doi: 10.1016/S1470-2045(20)30110-8. [DOI] [PubMed] [Google Scholar]
- 5.Daud A.I., Wolchok J.D., Robert C., et al. Programmed death-ligand 1 expression and response to the anti-programmed death 1 antibody pembrolizumab in melanoma. J Clin Oncol. 2016;34(34):4102–4109. doi: 10.1200/JCO.2016.67.2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kulangara K., Zhang N., Corigliano E., et al. Clinical utility of the combined positive score for programmed death ligand-1 expression and the approval of pembrolizumab for treatment of gastric cancer. Arch Pathol Lab Med. 2019;143(3):330–337. doi: 10.5858/arpa.2018-0043-OA. [DOI] [PubMed] [Google Scholar]
- 7.Burtness B., Harrington K.J., Greil R., et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a randomised, open-label, phase 3 study. Lancet. 2019;394(10212):1915–1928. doi: 10.1016/S0140-6736(19)32591-7. [DOI] [PubMed] [Google Scholar]
- 8.National Comprehensive Cancer Network NCCN Clinical Practice Guidelines in Oncology. Esophgeal and Esophogogeal Gastric Junction Cancers. Version 4.2020. https://www.nccn.org/store/login/login.aspx?ReturnURL=https://www.nccn.org/professionals/physician_gls/pdf/esophageal.pdf Available at.
- 9.Kitagawa Y., Uno T., Oyama T., et al. Esophageal cancer practice guidelines 2017 edited by the Japan esophageal society: part 2. Esophagus. 2019;16(1):25–43. doi: 10.1007/s10388-018-0642-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.National Health Commission of the People’s Republic of China Chinese guidelines for diagnosis and treatment of esophageal carcinoma 2018 (English version) Chin J Cancer Res. 2019;31(2):223–258. doi: 10.21147/j.issn.1000-9604.2019.02.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sun J.M., Shen L., Manish S., et al. Pembrolizumab plus chemotherapy versus chemotherapy for first-line, advanced esophageal cancer: the randomized, placebo-controlled phase 3 KEYNOTE-590 study. Lancet. 2021;398(10302):759–771. doi: 10.1016/S0140-6736(21)01234-4. [DOI] [PubMed] [Google Scholar]
- 12.Chau I., Doki Y., Ajani J.A., et al. Nivolumab (NIVO) plus ipilimumab (IPI) or NIVO plus chemotherapy (chemo) versus chemo as first-line (1L) treatment for advanced esophageal squamous cell carcinoma (ESCC): first results of the CheckMate 648 study. J Clin Oncol. 2021;39(suppl 18):LBA4001. [Google Scholar]
- 13.Abnet C.C., Arnold M., Wei W.-Q. Epidemiology of esophageal squamous cell carcinoma. Gastroenterology. 2018;154:360–373. doi: 10.1053/j.gastro.2017.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tran G.D., Sun X.D., Abnet C.C., et al. Prospective study of risk factors for esophageal and gastric cancers in the Linxian general population trial cohort in China. Int J Cancer. 2005;113(3):456–463. doi: 10.1002/ijc.20616. [DOI] [PubMed] [Google Scholar]
- 15.Zhang H.-Z., Jin G.-F., Shen H.-B. Epidemiologic differences in esophageal cancer between Asian and Western populations. Chin J Cancer. 2012;31:281–286. doi: 10.5732/cjc.011.10390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lu S.H., Chui S.X., Yang W.X., et al. Relevance of N-nitrosamines to oesophageal cancer in China. IARC Sci Publ. 1991;(105):11–17. [PubMed] [Google Scholar]
- 17.Deng J., Chen H., Zhou D., et al. Comparative genomic analysis of esophageal squamous cell carcinoma between Asian and Caucasian patient populations. Nat Commun. 2017;8(1):1533. doi: 10.1038/s41467-017-01730-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen S., Zhou K., Yang L., et al. Racial differences in esophageal squamous cell carcinoma: incidence and molecular features. Biomed Res Int. 2017:1204082. doi: 10.1155/2017/1204082. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.