Abstract
Background
Immunotherapy using inhibitors targeting immune checkpoint programmed cell death protein 1 (PD-1)/programmed death-ligand 1 (PD-L1) is currently the standard of care in patients with advanced non-small-cell lung cancer (NSCLC).
Materials and methods
We carried out a nationwide cohort retrospective study of consecutive patients with advanced, refractory NSCLC who received nivolumab as second to later lines of treatment as part of the expanded access program. Key objectives were to assess the efficacy and safety of nivolumab and the efficacy of first post-nivolumab treatment.
Results
Nine hundred and two patients were enrolled: 317 (35%) with squamous cell carcinoma and 585 (65%) with non-squamous cell carcinoma. Median age was 64 years; there were 630 (70%) men, 795 (88%) smokers, 723 (81%) patients with an Eastern Cooperative Oncology Group (ECOG) performance status (PS) of 0/1, 197 (22%) patients with brain metastases, and 212 (27%) with liver metastases. Best response was partial response for 16.2% and stable disease (SD) for 30.5%. Progression-free survival and overall survival (OS) rates at 2, 3, and 5 years were 8% and 25%, 6% and 16%, and 4% and 10%, respectively. At multivariate analysis, ECOG PS ≥2 [hazard ratio (HR) = 2.13, 95% confidence interval (95% CI) 1.78-2.55, P < 0.001], squamous histology (HR = 1.17, 95% CI 1.01-1.36, P = 0.04), and presence of central nervous system metastases (HR = 1.29, 95% CI 1.08-1.54, P = 0.005) were significantly associated with lower OS. Four hundred and ninety-two patients received at least one treatment after discontinuation of nivolumab, consisting of systemic therapies in 450 (91%). Radiation therapy was delivered to 118 (24%) patients.
Conclusion
The CLINIVO cohort represents the largest real-world evidence cohort with the use of immune checkpoint inhibitor in advanced, metastatic NSCLC after failure of first-line chemotherapy, with long-term follow-up and analysis of subsequent therapies. Our data confirm the efficacy of nivolumab in a cohort larger than that reported in landmark clinical trials and identify prognostic factors, which reinforces the need for accurate selection of patients for treatment with immune checkpoint inhibitors. Our data indicate that oligoprogression is frequent after nivolumab exposure and provide a unique insight into the long-term survival.
Key words: non-small-cell lung cancer, immunotherapy, sequence, real-life evidence, chemotherapy, lung cancer
Highlights
-
•
CLINIVO is the largest real-world evidence cohort with second-line nivolumab.
-
•
Progression-free survival and OS rates at 5 years were 4% and 10%, respectively.
-
•
Oligoprogression after nivolumab is frequent, with eligibility to radiotherapy.
Introduction
Non-small-cell lung cancer (NSCLC) represents the first cause of cancer-related death,1 as a majority of patients are diagnosed with advanced disease, for which historical treatment options have been limited leading to poor outcomes.2 Immunotherapy using inhibitors targeting the immune checkpoint programmed cell death protein 1 (PD-1)/programmed death-ligand 1(PD-L1) is currently the standard of care as first-line treatment,3 both as single agent4 or combined with standard platin-based chemotherapy,5,6 and possibly combined with other immune checkpoint inhibitors targeting cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) in selected patients.7 Historically, anti-PD-1/PD-L1 immune checkpoint inhibitors have been used as second-line treatment, after landmark phase III, randomized trials were reported, demonstrating the benefit as compared to docetaxel chemotherapy.8, 9, 10, 11 As a significant proportion of patients may actually not be eligible for immunotherapy in the first-line setting,12 some patients may still receive immunotherapy as second-line treatment. Meanwhile, long-term follow-up is available from clinical trials assessing immune checkpoint in that setting.13, 14, 15
Nivolumab was the first fully human PD-1 immune checkpoint inhibitor antibody approved in advanced NSCLC. Nivolumab demonstrated a significant objective response rate (ORR) and overall survival (OS) benefit versus docetaxel in pretreated patients with advanced squamous and non-squamous NSCLC, with an ORR of 20% and 19% versus 9% and 12% (P = 0.008 and P = 0.020), respectively, and a median OS of 9.2 and 12.2 months versus 6.0 and 9.4 months [hazard ratio (HR) = 0.59, 96% confidence interval (CI) 0.44-0.79, P < 0.001, and HR = 0.73, 96% CI 0.59-0.89, P = 0.002, respectively].8,9 From the limited cohort of patients treated as part of those trials, long-term follow-up indicates a 5-year progression-free survival (PFS) and OS of 8.0% versus 0%, and 13.4% versus 2.6% for nivolumab versus docetaxel, respectively.13
Besides randomized clinical trials, real-world data represent a major piece of knowledge in the clinical decision making for immunotherapy in NSCLC, providing clinicians with data from special population not enrolled or analyzed in such trials, capturing the actual treatment sequences before and after immunotherapy, and ultimately assessing the reproducibility of results in patients, especially in the long-term setting.16 Here, we report the results of French Cooperative Thoracic Intergroup (IFCT) 1502-CLINIVO, a French nationwide cohort study of consecutive patients with advanced, refractory NSCLC who received nivolumab as second to later lines of treatment, that provide with a unique opportunity to address those objectives.
Materials and methods
Study design
IFCT 1502-CLINIVO study is a retrospective study of patients who received nivolumab as part of the French expanded access program (Autorisation Temporaire d'Utilisation) that took place from January 2015 for squamous, and June 2015 for non-squamous NSCLC, until August 2015. Nivolumab (3 mg/kg every 2 weeks) was available upon physician request after the failure of at least one prior line of platinum-based chemotherapy. A total of 1946 patients were included in this program. The study was approved by the Protocol Assessment Committee of the French Respiratory Medicine Society on 15 June 2016, the Consulting Committee for Information Technology on Health Data on 12 July 2016, and the National Commission on Informatics and Liberties on 28 December 2016. The study was registered in ClinicalTrials.gov database under the ID NCT02933346.
Eligibility criteria
The French expanded access program required patients to fulfill the main inclusion criteria of the landmark clinical trials of nivolumab:8,9 (i) pathological diagnosis of NSCLC; (ii) stage IIIB or IV, or recurrent; (iii) age of 18 years or older; (iv) life expectancy of at least 3 months; (v) Eastern Cooperative Oncology Group (ECOG) performance status (PS) of 0 or 1; (vi) adequate hematologic, hepatic, and renal function. Patients were required to have progression or recurrence during or after at least one systemic platin-based chemotherapy. Exclusion criteria included: (i) treatment with steroids ≥10 mg equivalent prednisone in the last 14 days before the initiation of nivolumab; (ii) human immunodeficiency virus infection or known autoimmune disease, with the exception of residual hypothyroidism due to an autoimmune condition, type 1 diabetes mellitus, or psoriasis not requiring systemic treatment; (iii) symptomatic or active central nervous system (CNS) metastasis; (iv) previous treatment with any immune checkpoint inhibitor; (v) absence of eligibility for an ongoing clinical trial. Eligibility was centrally reviewed as part of the program.
Study endpoints
The key objectives of the study were the following: (i) assessing the efficacy and the safety of nivolumab and (ii) assessing the efficacy of first post-nivolumab treatment. The main endpoints were OS, PFS, and best ORR to nivolumab and first post-nivolumab, and grade ≥2 toxicities of nivolumab. We aimed at identifying predictors for those endpoints. Patients were treated in a real-life setting, but imaging assessment using brain, thorax, and abdomen computed tomography scan carried out every 8 weeks was actually mandatory and reports were centrally reviewed before continuation of nivolumab was allowed. Patient safety was evaluated on the basis of physical examination, blood tests as per local regulations and standards of care, and had also to be centrally reviewed every 8 weeks. Adverse events were graded according to National Cancer Institute Common Terminology Criteria for Adverse Events version 4.0.
Collection of data
Data from the first 902 patients enrolled in the program were collected from medical records at 69 investigator sites, by research study assistants working at the French Thoracic Cancer Intergroup, using a dedicated case report form. Besides study endpoints, a total of 225 variables were actually collected, including the above-mentioned eligibility criteria, smoking history, histology, PD-L1 and routine sequencing results for oncogenic alterations such as EGFR, KRAS mutations and ALK rearrangements, site of metastases at baseline and at time of progression, and treatment received before and after nivolumab treatment.
Statistical analyses
All patients who received at least one injection of nivolumab were included in the statistical analyses. Follow-up was conducted until April 2020. Disease progression and responses were evaluated by Response Evaluation Criteria in Solid Tumours (RECIST) v1.1, by each investigator. PFS and OS were estimated by using the Kaplan–Meier method; median times were reported with 95% CIs. PFS was calculated from the start of nivolumab/first post-nivolumab treatment until any evidence of progressive disease or death, whichever occurred first. OS was calculated from the start of nivolumab/first post-nivolumab treatment until death from any cause or last follow-up. The log-rank test was used for survival comparisons. A proportional hazards regression model was used to test the association of each factor with PFS and OS, and then factors with a P value <0.20 were included in a multivariate model to identify the independent prognostic roles of patient characteristics. HRs and their 95% CIs were reported. Statistical analyses were computed with SAS 9.4 software (SAS Institute Inc., Cary, NC).
Results
Patient population
A total of 902 patients were enrolled in the study (Supplementary Figure S1, available at https://doi.org/10.1016/j.esmoop.2021.100353), including 317 (35%) patients with squamous cell carcinoma and 585 (65%) patients with non-squamous cell carcinoma. Baseline characteristics—at the time of nivolumab initiation—of the 902 patients enrolled in the study are reported in Table 1. Overall, median age was 64 years, and there were 630 (70%) men, 795 (88%) smokers, 723 (80%) ECOG PS 0/1 patients, 197 (22%) patients with brain metastases, and 212 (26%) with liver metastases. Overall, 52 (6%) patients had a history of dysthyroidism and 108 (12%) of diabetes mellitus; 161 (39%) patients had chronic pulmonary obstructive disease (COPD). Eighty-one (9%) patients had received steroids within 2 weeks before nivolumab initiation.
Table 1.
Characteristics of the 902 patients who received nivolumab enrolled in the IFCT 1502-CLINIVO study
| Squamous (n = 317) | Non-squamous (n = 585) | Total (N = 902) | |||
|---|---|---|---|---|---|
| Sex | F | n (%) | 58 (18.3) | 214 (36.6) | 272 (30.2) |
| Age (years) | Median | 66 | 63 | 64 | |
| Range | (36.4-86.6) | (34.1-88.2) | (34.1-88.2) | ||
| Smoking | Never smoker | n (%) | 18 (5.7) | 88 (15.1) | 795 (88.2) |
| Smoker | n (%) | 299 (94.3) | 496 (84.9) | 106 (11.8) | |
| Number of pack-years | Median | 40 | 35 | 40 | |
| Range | (1-130) | (0.3-132) | (0.3-132) | ||
| Number of prior lines | 1 | n (%) | 80 (25.2) | 163 (27.9) | 243 (26.9) |
| 2 | n (%) | 115 (36.3) | 170 (29.1) | 285 (31.6) | |
| 3 | n (%) | 64 (20.2) | 113 (19.3) | 177 (19.6) | |
| 4 | n (%) | 44 (13.9) | 71 (12.1) | 115 (12.7) | |
| >4 | n (%) | 14 (4.4) | 68 (11.6) | 82 (9.1) | |
| Brain metastasis (at initiation of nivolumab) | No | n (%) | 277 (87.4) | 428 (73.2) | 705 (78.2) |
| Yes | n (%) | 40 (12.6) | 157 (26.8) | 197 (21.8) | |
| PD-L1 (IHC) | Negative | n (%) | 18 (85.7) | 32 (57.1) | 50 (64.9) |
| Positive | n (%) | 3 (14.3) | 21 (37.5) | 24 (31.2) | |
| Performance status (initiation of nivolumab) | 0 | n (%) | 63 (20) | 134 (23.2) | 197 (22.1) |
| 1 | n (%) | 195 (61.9) | 331 (57.3) | 526 (58.9) | |
| 2 | n (%) | 49 (15.6) | 99 (17.1) | 148 (16.6) | |
| 3 | n (%) | 6 (1.9) | 13 (2.2) | 19 (2.1) | |
| 4 | n (%) | 2 (0.6) | 1 (0.2) | 3 (0.3) | |
| Missing | n | 2 | 7 | 9 | |
IFCT, French Cooperative Thoracic Intergroup; IHC, immunohistochemistry; PD-L1, programmed death-ligand 1.
Nivolumab was administered as second, third, fourth line and plus of treatment and beyond for 243 (27%), 285 (32%), and 374 (41%) patients, respectively. PD-L1 expression had been assessed in only 74 (8%) patients. With regard to molecular characterization of non-squamous cell carcinomas, EGFR and KRAS mutations were present in 34 (6%) and 163 (28%) patients, respectively.
Treatment with nivolumab
Best response as per investigator assessment was objective response (OR) for 18.8% (95% CI 16.1% to 21.6%), stable disease (SD) for 35.5% (95% CI 32.1% to 38.9%), and progression disease (PD) for 45.0% (95% CI 41.5% to 48.5%) of patients. Median duration of nivolumab treatment was 2.5 months (range: 0.4-64.7 months), with a median number of nivolumab injections of 6 (range: 1-128); median duration of response was 15.3 months (range: 0.3-62.2 months). After a median follow-up of 57.2 months (95% CI 56.9-57.5 months), 887 (98%) patients had discontinued nivolumab; median PFS was 2.0 months (95% CI 1.9-2.2 months), and median OS from the initiation of nivolumab was 9.7 months (95% CI 9.0-11.1 months) (Figure 1A and B).
Figure 1.
Overall survival (A) and progression-free survival (B) of the 902 patients enrolled in the French Cooperative Thoracic Intergroup (IFCT) 1502-CLINIVO study after initiation of nivolumab.
CI, confidence interval.
At univariate analysis, patients treated with steroid administration within the previous 2 weeks before first nivolumab administration had a significant lower median PFS (1.7 versus 2.1 months, P < 0.01) and OS (5.7 versus 10.3 months, P < 0.001), as well as patients with liver metastases [1.7 versus 2.3 months for PFS (P < 0.001) and 5.1 versus 11.6 months for OS (P < 0.001)]. At multivariate analysis, ECOG PS ≥2 (HR = 2.05, 95% CI 1.69-2.47, P < 0.001), squamous histology (HR = 1.23, 95% CI 1.05-1.45, P = 0.01), presence of CNS metastases (HR = 1.23, 95% CI 1.02-1.48, P = 0.03), presence of liver metastasis (HR = 1.58, 95% CI 1.34-1.88, P < 0.001), and presence of bone metastasis (HR = 1.26, 95% CI 1.07-1.47, P = 0.004) were significantly associated with lower OS (Table 2). Gender, age at initiation of nivolumab, smoking history, and steroid administration within the previous 2 weeks before first nivolumab were not significantly associated with OS. In PS ≥2 patients, median PFS and OS were 1.7 (95% CI 1.5-1.8) months and 3.4 (95% CI 2.7-4.2) months, respectively (Supplementary Figure S2, available at https://doi.org/10.1016/j.esmoop.2021.100353). In patients with CNS metastases, median PFS and OS were 1.8 (95% CI 1.7-1.9) months and 6.8 (95% CI 5.2-8.6) months, respectively (Supplementary Figure S2, available at https://doi.org/10.1016/j.esmoop.2021.100353).
Table 2.
Predictors of overall survival in the 902 patients who received nivolumab enrolled in the IFCT 1502-CLINIVO study
| Cox model for OS |
||||||||
|---|---|---|---|---|---|---|---|---|
| Univariate model |
Multivariate model |
|||||||
| Factors | n | HR | 95% CI | P | HR | 95% CI | P | |
| Gender | Female | 272 | 1.000 | |||||
| Male | 630 | 0.919 | (0.79-1.07) | 0.2780 | ||||
| Age class I | LT70y | 670 | 1.000 | |||||
| GTE70y | 232 | 0.993 | (0.85-1.17) | 0.9347 | ||||
| Smoking | No | 106 | 1.000 | |||||
| Yes | 795 | 0.909 | (0.73-1.13) | 0.3972 | ||||
| PS class | LT2 | 723 | 1.000 | 1.000 | ||||
| GTE2 | 170 | 2.165 | (1.81-2.59) | <0.0001 | 2.048 | (1.69-2.47) | <0.0001 | |
| Histology | NSCC | 585 | 1.000 | 1.000 | ||||
| SCC | 317 | 1.124 | (0.97-1.30) | 0.1187 | 1.234 | (1.05-1.45) | 0.0120 | |
| Brain metastasis | No | 705 | 1.000 | 1.000 | ||||
| Yes | 197 | 1.216 | (1.03-1.44) | 0.0243 | 1.229 | (1.02-1.48) | 0.0278 | |
| Liver metastasis | No | 600 | 1.000 | 1.000 | ||||
| Yes | 212 | 1.640 | (1.39-1.94) | <0.0001 | 1.585 | (1.34-1.88) | <0.0001 | |
| Bone metastasis | No | 508 | 1.000 | 1.000 | ||||
| Yes | 305 | 1.292 | (1.11-1.51) | 0.0010 | 1.257 | (1.07-1.47) | 0.0044 | |
| Steroid administration | No | 817 | 1.000 | 1.000 | ||||
| Yes | 81 | 1.519 | (1.19-1.93) | 0.0006 | 1.175 | (0.91-1.52) | 0.2135 | |
CI, confidence interval; GTE, greater than or equal; HR, hazard ratio; IFCT, French Cooperative Thoracic Intergroup; LT, lower than; NSCC, non-squamous cell carcinoma; OS, overall survival; PS, performance status; SCC, squamous cell carcinoma.
Among the 243 patients treated in a second-line setting, OR to first-line chemotherapy was predictive of longer median OS (HR = 0.45, 95% CI 0.30-0.67, P < 0.001).
In this cohort, the number of patients with known EGFR or PD-L1 status was too small to assess efficacy endpoints in these subsets of patients. The presence of KRAS mutation was not statistically correlated with outcomes.
The safety profile of nivolumab is shown in Supplementary Table S1, available at https://doi.org/10.1016/j.esmoop.2021.100353; overall, maximal grade for any adverse event occurring during the first year of treatment was 2/3/4/5 for 216 (24%), 75 (8%), 9 (1%), and 6 (1%) patients, respectively; most frequent events were cutaneous, general, endocrine, and digestive toxicities; >40% of grade >2 toxicities occurred within the first 6 weeks of treatment (Supplementary Figure S3, available at https://doi.org/10.1016/j.esmoop.2021.100353). Late toxicities related to nivolumab—occurring 1 year and beyond after treatment initiation—were observed in 46 (5%) patients, 8 of whom presented with grade 3 endocrine, general, neuromuscular, investigational, metabolism, nervous, and pulmonary events. Nivolumab was discontinued because of toxicity in 84 (9.5%) patients. Overall, 120 patients presented with progression within the first 4 weeks after nivolumab initiation, suggesting occurrence of hyperprogressive disease; the only clinical predictor of such early progression was PS ≥2 (P < 0.001). Of those patients, only 18 received post-nivolumab treatment.
First post-nivolumab treatment
Most frequent sites of disease progression after nivolumab were the following: lung (57% of patients), liver (23% of patients), and brain and bone (18% of patients each); oligoprogressive disease was observed in 193 (35%) patients. Overall, 492 patients received at least one treatment after discontinuation of nivolumab, consisting of systemic therapies in 450 (91%) patients (Table 3). Single-agent chemotherapy was the most frequent option (61%), with gemcitabine (18% of patients), docetaxel (17% of patients), erlotinib (13% of patients), paclitaxel (11% of patients), and vinorelbine (9% of patients). Rechallenge of nivolumab was done in 28 patients. Interestingly, radiation therapy was delivered to 118 (24%) patients, corresponding to 61% of patients with oligoprogressive disease. Access to post-nivolumab treatment was higher in patients with an ECOG PS of 0/1 (90% versus 69%, P < 0.001), patients with non-squamous histology (68% versus 61%, P = 0.02), and patients who received nivolumab as second or third line (63% versus 52%, P = 0.001), but was not different according to gender, age, smoking status, presence of CNS metastases, or disease control with nivolumab.
Table 3.
First post-nivolumab treatment in 492 patients enrolled in the IFCT 1502-CLINIVO study
| Systemic treatment | n = 450 (91.5%) | ||
| Single-agent chemotherapy | n = 273 (61%) | Docetaxel | 77 (17%) |
| Gemcitabine | 79 (18%) | ||
| Paclitacel ± bevacizumab | 50 (11%) | ||
| Vinorelbine | 39 (9%) | ||
| Pemetrexed | 26 (6%) | ||
| Other | 2 (1%) | ||
| Platin-based doublet | n = 47 (10%) | Platin–paclitaxel | 25 (6%) |
| Other | 22 (5%) | ||
| Targeted therapy | n = 78 (17%) | Erlotinib | 59 (13%) |
| Other | 19 (4%) | ||
| Immunotherapy rechallenge | n = 37 (8%) | Nivolumab | 29 (6%) |
| Other | 8 (2%) | ||
| Other/unknown systemic treatment | n = 15 (3%) | ||
| Radiotherapy | n = 118 (24%) | ||
| Surgery | n = 18 (4%) | ||
IFCT, French Cooperative Thoracic Intergroup.
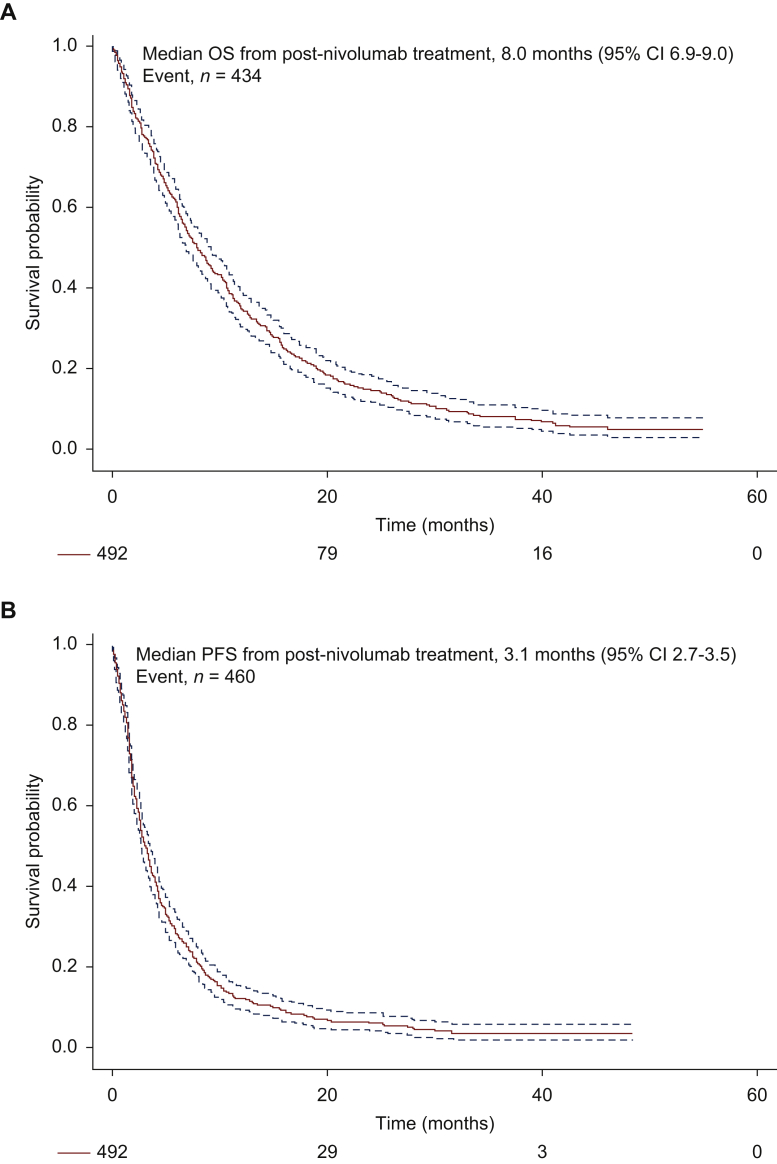
Best response rates to first post-nivolumab treatment was partial response (PR)/SD/PD/not evaluable for 16%/46%/37%/1%, respectively. Median PFS and OS to first post-nivolumab treatment were 3.1 (95% CI 2.7-3.6) months and 8.0 (95% CI 6.9-9.0) months, respectively (Figure 2A and B). In the 28 patients who had nivolumab rechallenge, median duration of treatment was 4.1 months (Supplementary Figure S4, available at https://doi.org/10.1016/j.esmoop.2021.100353). Factors associated with significantly lower OS after initiation of first post-nivolumab treatment were ECOG PS ≥2 (HR = 1.62, 95% CI 1.18-2.22, P = 0.003), squamous histology (HR = 1.38, 95% CI 1.12-1.70, P = 0.002), and presence of CNS metastases (HR = 1.34, 95% CI 1.04-1.72, P = 0.02). Factors associated with significantly higher OS after initiation of first post-nivolumab treatment were response to nivolumab (HR = 0.62, 95% CI 0.41-0.93, P = 0.02) and long-term duration of nivolumab (treatment duration ≥3 months) (HR = 0.68, 95% CI 0.49-0.95, P = 0.02) (Supplementary Table S2, available at https://doi.org/10.1016/j.esmoop.2021.100353); meanwhile, duration of first post-nivolumab treatment was actually not correlated with that of nivolumab treatment (Figure 2C). Subsequent line of treatment did not impact the outcome after initiation of first post-nivolumab treatment.
Figure 2.
Overall survival (OS) (A) and progression-free survival (PFS) (B) of the 492 patients enrolled in the French Cooperative Thoracic Intergroup (IFCT) 1502-CLINIVO study from the initiation of first post-nivolumab treatment. (C) Duration of nivolumab treatment (red line) and first post-nivolumab treatment (blue line).
CI, confidence interval.
Long-term survival
In our cohort, PFS and OS rates at 2, 3, and 5 years were 8% and 25%, 6% and 16%, and 4% and 10%, respectively (Figure 1). No significant predictor of OS ≥5 years was identified.
Discussion
The CLINIVO cohort represents the largest real-world evidence cohort with the use of nivolumab or actually any other immune checkpoint inhibitor in advanced, metastatic NSCLC after the failure of first-line chemotherapy, with long-term follow-up and analysis of subsequent therapies. Taken together, our results in a cohort of 902 patients (i) confirm the efficacy of nivolumab in a cohort of patients larger than that reported in landmark clinical trials; (ii) identify prognostic factors—PS ≥2 and presence of brain metastases—that were not part of the inclusion of those trials, which reinforces the need for accurate selection of patients for treatment with immune checkpoint inhibitors; (iii) indicate that oligoprogression is frequent after nivolumab exposure; and (iv) provide a unique insight into the long-term survival of those patients.
In our cohort of 317 patients with squamous cell carcinomas and 585 patients with non-squamous cell carcinomas, ORR was 16% and OS was 9.7 months, which confirms the efficacy of nivolumab in chemotherapy-refractory NSCLC; these figures are in line with those reported in the second-line trials CheckMate-017—for squamous cell carcinomas—and CheckMate-057—for non-squamous cell carcinomas—of 20% and 9.2 months, and 19% and 12.2 months, respectively, despite patients in our cohort being treated in a more advanced setting—75% of patients had received two lines of previous treatment.8,9,13 In addition, selection of patients in the CLINIVO study was less stringent regarding clinical characteristics, as 18% of patients had a PS ≥2 and 13% had brain metastases, which were exclusion criteria in those trials, as well as in the vast majority of clinical trials assessing immune checkpoint inhibitors, these characteristics were part of the recommended enrollment criteria for requesting the expanded access program, but physicians actually treated patients ultimately not fulfilling those, based on the analysis of medical records. Interestingly, our multivariate analysis indicates that these patients had a significantly worse outcome. Most patients who presented with early disease progression within the first 4 weeks were those with PS ≥2. Dedicated trials are needed to further assess the opportunities in such clinical situations; CheckMate-817 is a multi-cohort, open-label phase IIIb/IV study investigating the safety and efficacy of flat-dose nivolumab plus weight-based low-dose ipilimumab in advanced NSCLC, conducted in patients with ECOG PS of 2 or asymptomatic untreated brain metastases, hepatic or renal impairment, or human immunodeficiency virus; reported results show a more limited efficacy of immunotherapy in those patients.17 Other trials are ongoing. Meanwhile, some clinical features previously reported to impact the outcome with immune checkpoint inhibitors were not confirmed in our study, including gender, liver metastases, steroid administration before treatment, COPD, or digestive tract disease.18, 19, 20, 21, 22 As in the CheckMate-817 trial, these characteristics were not associated with a worse safety profile in our study.17 Ultimately, biomarkers were not systematically tested at the time CLINIVO was initiated to allow further analyses.
Finally, we did not confirm any association between early occurrence of side-effects and treatment outcomes, while, as previously reported,18, 19, 20, 21, 22, 23 these occurred within the first 6 weeks of treatment for the majority of patients.
A major lesson learned from our study is the unique analysis of progression patterns and treatment strategies beyond nivolumab. Our data indicate that oligoprogressive disease was observed in 193 (35%) patients, which may lead to a pragmatic decision based on the experience with oncogene-addicted NSCLC for which local ablative treatment is delivered, while continuation of the ongoing line of systematic therapy may be decided, with significant survival benefit;24 such strategy was previously reported with immune checkpoint inhibitors in NSCLC,25 and has been integrated as an option at disease progression in the design of most clinical trials as well. Our study in a large cohort of consecutive patients indicates that a large subset of patients—24% received radiotherapy as first post-nivolumab treatment—may benefit from such strategy.
With regard to switching to subsequent line of treatment after nivolumab, most patients received single-agent chemotherapy; while some reports in small cohorts of patients suggested a potentially higher efficacy of chemotherapy after exposure to immune checkpoint inhibitors,26 our data are more mitigated in this regard, as ORR, PFS, and OS—12%, 3.1 months, and 8.0 months, respectively—are in line with expectations of efficacy of late-line therapies in NSCLC.27 Only 28 patients in our study had nivolumab rechallenge, for a median duration of 4.1 months, which is similar to reported data.15
As immune checkpoint inhibitors are now part of first-line treatment, as single agent or in combination with chemotherapy,3, 4, 5, 6, 7 these findings regarding the patterns of disease progression, post-immunotherapy strategies, and rechallenge require to be revisited, to assess whether our results are still applicable; limited data have been made available so far. The understanding of the immunological spatial heterogeneity is also a key in this regard.
CLINIVO provides for the first time long-term outcomes with nivolumab in a real-world setting; PFS and OS rates at 5 years were 4% and 10%, respectively, indicating that 40% of alive patients had no event related to their disease, and may then be considered as cured. From the long-term data from landmark second-line clinical trials, 5-year PFS is 8% and OS ranges between 13% and 16% in unselected patients;13, 14, 15,28 these figures were higher in the KEYNOTE-010 trial with pembrolizumab, which excluded PD-L1-negative patients.15 Long-term OS is much higher with first-line immune checkpoint inhibitors targeting PD-1 or PD-L1, alone or in combination with CTLA-4 inhibitors or chemotherapy, ranging between 20% and 30%;29,30 the most significant predictor may actually be PD-L1 status, which was not assessable in our study. Ultimately, the chance of cure, which may be defined as no event related to the disease and discontinuation of any anticancer treatment, may be achieved in up to half of those patients.
Acknowledgements
The CLINIVO contributors, listed here, collaborated in this project and provided data for one patient or more (not included in the list of authors): Dr Jean-Loup Mouysset, Aix-en-Provence, Polyclinique du Parc Rambot, France; Dr Stéphanie Martinez, Aix-en-Provence, CH, France; Dr Claire Poulet, Amiens, CHU, France; Dr Philippe Romand, Annemasse, CH, France; Dr Laure Belmont, Argenteuil, CH, France; Dr Nicolas Cloarec, Avignon, CH, France; Dr Sophie Schneider, Bayonne, CH, France; Dr Fethi Khanjari, Blois, CH, France; Dr Boris Duchemann, Bobigny, Hôpital Avicenne, France; Dr Rémi Veillon, Bordeaux, CHU, France; Dr Jeannick Madelaine, Caen, CHU, France; Dr Marie Coudurier, Chambéry, CH, France; Dr Laetitia Rajpar, Chartres, CH, France; Pr Jacques Margery, Clamart, Hôpital Percy/Armées, France; Dr Pascale Dubray-Longeras, Clermont-Ferrand, Centre Jean Perrin, France; Dr Lionel Moreau, Colmar, CH, France; Dr Pierre Mourlanette, Cornebarrieu, Clinique des Cèdres, France; Dr Laure Gautier-Felizot, Dax, CH, France; Pr Denis Moro-Sibilot, Grenoble, CHU, France; Dr Cécile Leyronnas, Grenoble, Institut Daniel Hollard, France; Dr Florence Parent, Hôpital du Kremlin-Bicêtre, France; Dr Acya Bizieux Thaminy, La Roche-Sur-Yon, CH, France; Dr Samir Abdiche, Libourne, CH, France; Pr Alexis Cortot, Lille, Hôpital Calmette, CHU, France; Dr Thomas Egenod, Limoges, CHU, France; Dr Elodie Vandeix, Limoges, Clinique Chénieux, France; Dr Virginie Avrillon, Lyon, CRLCC, France; Dr Marie Darrason, Lyon, HCL, France; Dr Cyril Foa, Marseille, Hôpital Privé Clairval, France; Dr Stéphane Raymond, Metz, Hôpital Robert Schuman, France; Dr Jérôme Dauba, Mont-de-Marsan, CH, France; Dr Stefano Kim, Montbéliard, CH, France; Dr Didier Debieuvre, Mulhouse, GHRMSA, France; Dr Fabien Brocard, Nancy, Polyclinique Gentilly, France; Dr Hélène Senellart, Nantes, CRLCC, France; Dr Stéphanie Bordenave-Caffre, Nantes, Hôpital Laennec, France; Dr Josiane Otto, Nice, CRLCC, France; Dr Sylvie Van Hulst, Nîmes, CHU, France; Dr Adrien Dixmier, Orléans, CHR, France; Dr Elizabeth Fabre, Paris, HEGP, France; Dr Ludovic Doucet, Paris, Saint-Louis, France; Dr Aldo Renault, Pau, CHG, France; Dr Cécile Durand-Matringe, Pontoise, CH, France; Dr Daniel Coëtmeur, Saint-Brieuc, CHG, France; Dr Carole Helissey, Saint-Mandé, HIA Begin, France; Pr Pierre Fournel, Saint-Priest-en-Jarez, ICL, France; Dr Henri Berard, Toulon, Sainte-Anne Hia, France; Dr Eric Pichon, Tours, CHU, France; Dr Ulrike Lerolle, Trelazé, Clinique Saint-Joseph, France; Dr Annie Wdowik, Vannes, Hôpital Bretagne Atlantique, France; Dr Cécile Dujon, Versailles, CH, France; Dr Luc Odier, Villefranche-sur-Saône, CH, France.
Funding
This work was supported by the French Cooperative Thoracic Intergroup (IFCT) and unrestricted grants from BMS, France and French League Against Cancer (no grant number). The funding sources had no role in study design, data collection, data analysis, data interpretation, or preparation of this manuscript.
Disclosure
OM reports personal fees from AstraZeneca, Takeda, BMS, MSD, Novartis, and AMGEN. NG reports research/grant support from MSD, AstraZeneca, AbbVie, Amgen, Boehringer Ingelheim, Eli Lilly, Hoffmann-La Roche, Janssen, Merck, MSD, Novartis, Pfizer, Sivan, and Trizell, and consultative services for Bristol Myers Squibb, AstraZeneca, AbbVie, Amgen, Boehringer Ingelheim, Eli Lilly, Hoffmann-La Roche, Janssen, Merck, MSD, Novartis, Pfizer, Sanofi, and Sivan. BB reports grants from AbbVie, Amgen, Aptitude Health, AstraZeneca, BeiGene, Blueprint Medicines, BMS, Boehringer Ingelheim, Celgene, Cergentis, Cristal Therapeutics, Daiichi-Sankyo, Eli Lilly, GSK, Inivata, Janssen, Onxeo, OSE Immunotherapeutics, Pfizer, Roche-Genentech, Sanofi, Takeda, and Tolero Pharmaceuticals.
FB reports personal fees from AstraZeneca, Bayer, Bristol Myers Squibb, Boehringer Ingelheim, Eli Lilly Oncology, ß. Hoffmann-La Roche Ltd, Novartis, Merck, MSD, Pierre Fabre, Pfizer, and Takeda. CA-V reports personal fees and non-financial support from Roche, BMS, MSD, AstraZeneca, AbbVie, Pfizer, and Takeda. SF reports support for attending meetings and/or travel from Boehringer Ingelheim France, BMS, Leo Pharma, Sandoz, and Novartis Pharma SAS. JM reports personal fees from Roche, AstraZeneca, Pierre Fabre, Takeda, BMS, MSD, Hengrui, BLUEPRINT, DAIICHI, and Novartis and grants from Roche, AstraZeneca, Pierre Fabre, and BMS. JC reports consulting fees from AstraZeneca, Boehringer Ingelheim, BMS, Jansen, MSD, Pfizer, Roche, and Takeda. WH reports payment or honoraria for lectures, presentations, speaker's bureaus, manuscript writing, or educational events from BMS and Astellas and support for attending meetings and/or travel from Astellas, Pfizer, and Janssen. DM-S reports grants or contracts from Roche, AstraZeneca, Amgen, AbbVie, Pfizer, Takeda, and Lilly; consulting fees from Roche, AstraZeneca, Amgen, AbbVie, Pfizer, Takeda, and Lilly; payment or honoraria for lectures, presentations, speaker's bureaus, manuscript writing, or educational events from Roche, AstraZeneca, Amgen, AbbVie, Pfizer, Takeda, Lilly, and BMS; and support for attending meetings and/or travel from Roche, AstraZeneca, Amgen, AbbVie, Pfizer, Takeda, Lilly, and BMS. VW reports honoraria from Roche, AstraZeneca, BMS, and MSD and non-financial support from Roche and Pfizer.
PJS reports consulting fees, support for attending meetings and/or travel, payment or honoraria for lectures, presentations, speaker's bureaus, manuscript writing, or educational events from BMS and participated on a data safety monitoring board or advisory board for BMS. The remaining authors have declared no conflicts of interest.
Supplementary data
CONSORT diagram of the IFCT 1502-CLINIVO study.
Overall survival and progression-free survival in 170 patients with ECOG PS ≥2 (A, B) and 197 patients with CNS metastases (C, D).
Timeline of grade ≥2 toxicities from initiation of nivolumab treatment.
Duration of nivolumab treatment in the 28 patients who had a treatment rechallenge with nivolumab.
References
- 1.Siegel R.L., Miller K.D., Fuchs H.E., Jemal A. Cancer Statistics, 2021. CA Cancer J Clin. 2021;71:7–33. doi: 10.3322/caac.21654. [DOI] [PubMed] [Google Scholar]
- 2.Herbst R.S., Heymach J.V., Lippman S.M. Lung cancer. N Engl J Med. 2008;359:1367–1380. doi: 10.1056/NEJMra0802714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Planchard D., Popat S., Kerr K., et al. Metastatic non-small cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2019;30:863–870. doi: 10.1093/annonc/mdy474. [DOI] [PubMed] [Google Scholar]
- 4.Reck M., Rodríguez-Abreu D., Robinson A.G., et al. Updated analysis of KEYNOTE-024: pembrolizumab versus platinum-based chemotherapy for advanced non-small-cell lung cancer with PD-L1 tumor proportion score of 50% or greater. J Clin Oncol. 2019;37:537–546. doi: 10.1200/JCO.18.00149. [DOI] [PubMed] [Google Scholar]
- 5.Gandhi L., Rodríguez-Abreu D., Gadgeel S., et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med. 2018;378:2078–2092. doi: 10.1056/NEJMoa1801005. [DOI] [PubMed] [Google Scholar]
- 6.Paz-Ares L., Luft A., Vicente D., et al. Pembrolizumab plus chemotherapy for squamous non-small-cell lung cancer. N Engl J Med. 2018;379:2040–2051. doi: 10.1056/NEJMoa1810865. [DOI] [PubMed] [Google Scholar]
- 7.Paz-Ares L., Ciuleanu T.E., Cobo M., et al. First-line nivolumab plus ipilimumab combined with two cycles of chemotherapy in patients with non-small-cell lung cancer (CheckMate 9LA): an international, randomised, open-label, phase 3 trial. Lancet Oncol. 2021;22:198–211. doi: 10.1016/S1470-2045(20)30641-0. [DOI] [PubMed] [Google Scholar]
- 8.Brahmer J., Reckamp K.L., Baas P., et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373:123–135. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Borghaei H., Paz-Ares L., Horn L., et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373:1627–1639. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Herbst R.S., Baas P., Kim D.W., et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387:1540–1550. doi: 10.1016/S0140-6736(15)01281-7. [DOI] [PubMed] [Google Scholar]
- 11.Rittmeyer A., Barlesi F., Waterkamp D., et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet. 2017;389:255–265. doi: 10.1016/S0140-6736(16)32517-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Friedlaender A., Banna G.L., Buffoni L., Addeo A. Poor-performance status assessment of patients with non-small cell lung cancer remains vague and blurred in the immunotherapy era. Curr Oncol Rep. 2019;21:107. doi: 10.1007/s11912-019-0852-9. [DOI] [PubMed] [Google Scholar]
- 13.Borghaei H., Gettinger S., Vokes E.E., et al. Five-year outcomes from the randomized, phase III trials CheckMate 017 and 057: nivolumab versus docetaxel in previously treated non-small-cell lung cancer. J Clin Oncol. 2021;39:723–733. doi: 10.1200/JCO.20.01605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mazieres J., Rittmeyer A., Gadgeel S., et al. Atezolizumab versus docetaxel in pretreated patients with NSCLC: final results from the randomized phase 2 POPLAR and phase 3 OAK clinical trials. J Thorac Oncol. 2021;16:140–150. doi: 10.1016/j.jtho.2020.09.022. [DOI] [PubMed] [Google Scholar]
- 15.Herbst R., Garon E., Kim D., et al. FP13.01—5-year survival update from KEYNOTE-010: pembrolizumab versus docetaxel in previously treated, PD-L1–positive advanced NSCLC. J Thorac Oncol. 2021;16:S223–S224. doi: 10.1016/j.jtho.2021.05.001. [DOI] [PubMed] [Google Scholar]
- 16.Pasello G., Pavan A., Attili I., et al. Real world data in the era of immune checkpoint inhibitors (ICIs): increasing evidence and future applications in lung cancer. Cancer Treat Rev. 2020;87:102031. doi: 10.1016/j.ctrv.2020.102031. [DOI] [PubMed] [Google Scholar]
- 17.Barlesi F., Audigier-Valette C., Felip E., et al. OA04.02 CheckMate 817: first-line nivolumab + ipilimumab in patients with ECOG PS 2 and other special populations with advanced NSCLC. J Thorac Oncol. 2019;14:S214–S215. [Google Scholar]
- 18.Grossi F., Genova C., Crinò L., et al. Real-life results from the overall population and key subgroups within the Italian cohort of nivolumab expanded access program in non-squamous non-small cell lung cancer. Eur J Cancer. 2019;123:72–80. doi: 10.1016/j.ejca.2019.09.011. [DOI] [PubMed] [Google Scholar]
- 19.Garassino M.C., Gelibter A.J., Grossi F., et al. Italian nivolumab expanded access program in nonsquamous non-small cell lung cancer patients: results in never-smokers and EGFR-mutant patients. J Thorac Oncol. 2018;13:1146–1155. doi: 10.1016/j.jtho.2018.04.025. [DOI] [PubMed] [Google Scholar]
- 20.Khozin S., Abernethy A.P., Nussbaum N.C., et al. Characteristics of real-world metastatic non-small cell lung cancer patients treated with nivolumab and pembrolizumab during the year following approval. Oncologist. 2018;23:328–336. doi: 10.1634/theoncologist.2017-0353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Duma N., Abdel-Ghani A., Yadav S., et al. Sex differences in tolerability to anti-programmed cell death protein 1 therapy in patients with metastatic melanoma and non-small cell lung cancer: are we all equal? Oncologist. 2019;24:e1148–e1155. doi: 10.1634/theoncologist.2019-0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mountzios G., de Toma A., Economopoulou P., et al. Steroid use independently predicts for poor outcomes in patients with advanced NSCLC and high PD-L1 expression receiving first-line pembrolizumab monotherapy. Clin Lung Cancer. 2021;22:e180–e192. doi: 10.1016/j.cllc.2020.09.017. [DOI] [PubMed] [Google Scholar]
- 23.Baldini E., Lunghi A., Cortesi E., et al. Immune-related adverse events correlate with clinical outcomes in NSCLC patients treated with nivolumab: the Italian NSCLC expanded access program. Lung Cancer. 2020;140:59–64. doi: 10.1016/j.lungcan.2019.12.014. [DOI] [PubMed] [Google Scholar]
- 24.Lim J.U. Management of oligometastasis and oligoprogression in patients with epidermal growth factor receptor mutation-positive NSCLC in the era of third-generation tyrosine kinase inhibitors. Clin Lung Cancer. 2021;22:e786–e792. doi: 10.1016/j.cllc.2021.03.004. [DOI] [PubMed] [Google Scholar]
- 25.Gettinger S.N., Wurtz A., Goldberg S.B., et al. Clinical features and management of acquired resistance to PD-1 axis inhibitors in 26 patients with advanced non-small cell lung cancer. J Thorac Oncol. 2018;13:831–839. doi: 10.1016/j.jtho.2018.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dwary A.D., Master S., Patel A., et al. Excellent response to chemotherapy post immunotherapy. Oncotarget. 2017;8:91795–91802. doi: 10.18632/oncotarget.20030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McKay C., Burke T., Cao X., Abernethy A.P., Carbone D.P. Treatment patterns for advanced non-small-cell lung cancer after platinum-containing therapy in U.S. Community Oncology Clinical Practice. Clin Lung Cancer. 2016;17:449–460.e7. doi: 10.1016/j.cllc.2016.03.008. [DOI] [PubMed] [Google Scholar]
- 28.Antonia S.J., Borghaei H., Ramalingam S.S., et al. Four-year survival with nivolumab in patients with previously treated advanced non-small-cell lung cancer: a pooled analysis. Lancet Oncol. 2019;20:1395–1408. doi: 10.1016/S1470-2045(19)30407-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reck M., Rodríguez-Abreu D., Robinson A.G., et al. Five-year outcomes with pembrolizumab versus chemotherapy for metastatic non-small-cell lung cancer with PD-L1 tumor proportion score ≥50. J Clin Oncol. 2021;39:2339–2349. doi: 10.1200/JCO.21.00174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Paz-Ares L.G., Ciuleanu T.E., Lee J.S., et al. Nivolumab (NIVO) plus ipilimumab (IPI) versus chemotherapy (chemo) as first-line (1L) treatment for advanced non-small cell lung cancer (NSCLC): 4-year update from CheckMate 227. J Clin Oncol. 2021;39(suppl 15):9016. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
CONSORT diagram of the IFCT 1502-CLINIVO study.
Overall survival and progression-free survival in 170 patients with ECOG PS ≥2 (A, B) and 197 patients with CNS metastases (C, D).
Timeline of grade ≥2 toxicities from initiation of nivolumab treatment.
Duration of nivolumab treatment in the 28 patients who had a treatment rechallenge with nivolumab.