Abstract
BACKGROUND:
For patients with sentinel lymph node (SLN)-positive cutaneous melanoma, the Second Multicenter Selective Lymphadenectomy trial demonstrated equivalent disease-specific survival (DSS) with active surveillance using nodal ultrasound versus completion lymph node dissection (CLND). Adoption and outcomes of active surveillance in clinical practice and in adjuvant therapy recipients are unknown.
METHODS:
In a retrospective cohort of SLN-positive adults treated at 21 institutions in Australia, Europe, and the United States from June 2017 to November 2019, the authors evaluated the impact of active surveillance and adjuvant therapy on all-site recurrence-free survival (RFS), isolated nodal RFS, distant metastasis-free survival (DMFS), and DSS using Kaplan-Meier curves and Cox proportional hazard models.
RESULTS:
Among 6347 SLN biopsies, 1154 (18%) were positive and had initial negative distant staging. In total, 965 patients (84%) received active surveillance, 189 (16%) underwent CLND. Four hundred thirty-nine patients received adjuvant therapy (surveillance, 38%; CLND, 39%), with the majority (83%) receiving anti-PD-1 immunotherapy. After a median follow-up of 11 months, 220 patients developed recurrent disease (surveillance, 19%; CLND, 22%), and 24 died of melanoma (surveillance, 2%; CLND, 4%). Sixty-eight patients had an isolated nodal rec urrence (surveillance, 6%; CLND, 4%). In patients who received adjuvant treatment without undergoing prior CLND, all isolated nodal recurrences were resectable. On risk-adjusted multivariable analyses, CLND was associated with improved isolated nodal RFS (hazard ratio [HR], 0.36; 95% CI, 0.15-0.88), but not all-site RFS (HR, 0.68; 95% CI, 0.45-1.02). Adjuvant therapy improved all-site RFS (HR, 0.52; 95% CI, 0.47-0.57). DSS and DMFS did not differ by nodal management or adjuvant treatment.
CONCLUSIONS:
Active surveillance has been adopted for most SLN-positive patients. At initial assessment, real-world outcomes align with randomized trial findings, including in adjuvant therapy recipients.
Keywords: active surveillance, cohort studies, cutaneous malignant melanoma, follow-up studies, immunotherapy, lymph node excision, metastatic melanoma, sentinel lymph node
LAY SUMMARY:
• For patients with melanoma of the skin and microscopic spread to lymph nodes, monitoring with ultrasound is an alternative to surgically removing the remaining lymph nodes.
• The authors studied adoption and real-world outcomes of ultrasound monitoring in over 1000 patients treated at 21 centers worldwide, finding that most patients now have ultrasounds instead of surgery.
• Although slightly more patients have cancer return in the lymph nodes with this strategy, typically, it can be removed with delayed surgery.
• Compared with up-front surgery, ultrasound monitoring results in the same overall risk of melanoma coming back at any location or of dying from melanoma.
INTRODUCTION
The management of patients who have melanoma with positive sentinel lymph nodes (SLNs) has changed dramatically over the past decade. Although completion lymph node dissection (CLND) was previously recommended for patients with positive SLNs, 2 large, multi-institutional, randomized controlled trials have recently demonstrated equivalent oncologic outcomes with active nodal surveillance. In lieu of CLND, active surveillance entails serial clinical assessments and nodal basin ultrasounds, reserving therapeutic lymph node dissection for those patients who develop clinically evident nodal disease.1–4 The Second Multicenter Selective Lymphadenectomy trial (MSLT-2), published in 2017, demonstrated no difference in melanoma-specific survival for SLN-positive patients on active surveillance compared with patients who underwent CLND.3 Likewise, the German Dermatologic Cooperative Oncology Group study (DeCOG-SLT) demonstrated no differences in recurrence-free survival (RFS), distant metastasis-free survival (DMFS), or overall survival (OS) for patients managed with active surveillance versus CLND.5,6
Concurrently, landmark trials of immune checkpoint blockade and BRAF/MEK inhibitors have changed the standard of care regarding adjuvant therapy in resected stage III melanoma.7–11 These trials mandated CLND before the initiation of adjuvant therapy for SLN-positive patients and thus do not reflect the experience of patients receiving active nodal basin surveillance and adjuvant therapy. Conversely, the vast majority of participants in the aforementioned studies of active nodal basin surveillance versus CLND received no adjuvant therapy. Consequently, there is no randomized evidence to support the provision of modern adjuvant systemic therapy to patients who receive active nodal surveillance in lieu of CLND.
In the current study, our first objective was to describe the adoption of active surveillance in SLN-positive patients since publication of the MSLT-2 in a large, diverse cohort of international, high-volume melanoma centers, including factors associated with the decision to perform CLND versus active surveillance and fidelity to the ultrasound-based surveillance protocol used in the randomized trials. Second, we sought to compare the early outcomes of patients who undergo active surveillance, with specific attention to those who receive adjuvant systemic therapy.
MATERIALS AND METHODS
Study Design and Support
The study was approved by the Institutional Review Board of Moffitt Cancer Center, which served as the coordinating center. Participating institutions obtained respective institutional review board/ethics approval and performed independent data abstraction, providing data to the coordinating center in compliance with institutional requirements and negotiated data use agreements. All study data had been collected during standard of care evaluation, treatment, and follow-up.
Data Sources and Study Cohort
Included patients were aged ≥18 years, had clinically node-negative cutaneous melanoma without macroscopic satellite or in-transit disease, underwent SLN biopsy between June 1, 2017 and June 30, 2019, and had metastatic disease in at least 1 SLN. Patients were excluded if they had regional or distant metastasis on staging studies before or immediately after positive SLN biopsy. Staging studies were provider-dependent and institution-dependent and consisted of any combination of computed tomography (CT), positron emission tomography (PET), or PET/CT and magnetic resonance imaging (MRI) of the brain. Staging studies that were performed after positive SLN biopsy were completed before a final determination regarding nodal management (surveillance vs CLND), and only those patients who had no evidence of disease were included in the cohort. Additional exclusions were prior or concurrent (second primary) invasive melanoma and insufficient medical records. Participating institutions had preexisting active surveillance protocols for SLN-positive patients who did not undergo CLND. Patients were offered adjuvant therapy at the discretion of treating clinicians. Unlike recent adjuvant therapy trials, patients were not required to undergo CLND before receiving adjuvant treatment.
Outcomes
Descriptive end points included the proportion of patients who initiated active surveillance, received adjuvant systemic therapy, and adhered to surveillance. Adherence was defined as having at least 1 ultrasound for every 6-month period of disease-free follow-up, which is a conservative metric based on the every-4-month protocol in MSLT-2 to allow for delays related to scheduling. The primary comparative end point was all-site RFS, which was evaluated between patients who underwent active surveillance versus those who underwent CLND. RFS was defined as the time from SLN biopsy to recurrent melanoma at any site, diagnosed by clinical and/or radiographic findings and confirmed on biopsy when feasible. Although some patients with recurrent disease have gone on to have multiple recurrence events, only the first recurrence event for each patient was included in the primary analysis. Patients were censored for death or at last clinical follow-up. Secondary end points were isolated nodal basin recurrence, defined as an initial recurrence in an SLN-basin without local, in-transit, or distant disease; DMFS, defined as distant metastasis identified during the follow-up period as a site of either first recurrence or subsequent recurrence; and disease-specific survival (DSS), all of which were considered exploratory given the short follow-up duration.
All-site and isolated nodal RFS were compared based on nodal management with active surveillance versus CLND and receipt of adjuvant systemic therapy. BRAF mutational analysis was not required before the initiation of adjuvant systemic therapy, although, ultimately, it was performed in approximately two-thirds of the study cohort. In addition, because a substantial proportion of the cohort had tumor or nodal characteristics that would have excluded them from the MSLT-2 (microsatellitosis, extranodal extension [ENE], >3 positive nodes in trunk or extremity tumors, >6 positive nodes in head/neck tumors), an exploratory analysis was performed in this subgroup.
Statistical Analysis
Descriptive statistics included χ2 independence tests, 2-sample t tests, and Wilcoxon rank-fum tests according to data distributions, with a 2-tailed significance level of 5%. Kaplan-Meier survival curves were created for all-site RFS, isolated nodal basin RFS, and DMFS and were compared by nodal management and adjuvant therapy using log-rank tests stratified by location of treating center (United States, Europe, or Australia). Adjusted analyses were performed for all-site RFS and isolated nodal basin RFS using Cox proportional hazards models, with results reported as hazard ratios (HR) and 95% CIs. Covariates for adjustment were selected a priori and included age, American Joint Committee on Cancer eighth edition cancer staging manual stage, tumor location, total number of positive SLNs, ENE, and largest nodal tumor deposit. Patients who had missing values for the included covariates or outcomes were excluded.
In the Cox models, the proportionality of hazards was verified using the Lin supremum test.12 For variables that did not meet the proportionality of hazards assumption but had a significant association with the outcome on univariate analysis, an interaction between the variable and time was added to account for nonproportional hazards. Variables that violated the proportionality of hazards assumption and those that had a nonsignificant association with the outcome were removed. DSS was evaluated using a cumulative incidence function curve with comparisons using the Gray test and the Fine and Gray method of competing risk assessment.13,14 Statistical analyses were performed using the SAS 9.4 and Stata 15.1 statistical software packages.
RESULTS
There were 6347 SLN biopsies performed at the 21 participating institutions (see Supporting Table 1). Among these, 1165 patients (18%) had at least 1 positive SLN, of whom 11 patients were excluded, yielding a final cohort of 1154 patients. Reasons for exclusion included distant metastases on staging (n = 4), multiple primaries (n = 2), loss to follow-up before a decision regarding CLND versus active surveillance (n = 4), and diffuse benign lymphadenopathy precluding ultrasound surveillance (n = 1). In total, 189 participants (16%) underwent CLND, and the remaining 965 (84%) received active surveillance.
Factors Associated With CLND
CLND was performed more often for younger patients and those who had with head/neck primary sites, greater Breslow thickness, the presence of microsatellites, and BRAF mutation, but not tumor ulceration (Table 1). Nodal features associated with undergoing CLND included more positive SLNs, ENE, and larger nodal deposits. Patients treated in the United States and Europe were more likely to undergo CLND than those treated in Australia. On multivariable analysis, the factors associated with undergoing CLND included head and neck primary, higher numbers of positive SLNs, larger nodal tumor, and location of the treating center (see Supporting Table 2). Documented reasons for CLND were available in 103 of 189 patients, and some had more than 1 rationale. These included patient preference (42 of 103; 41%), surgeon recommendation (19 of 103; 18%), burden of disease based on features of the primary tumor and/or involved SLNs (33 of 103; 32%), inability to participate in active surveillance because of travel constraints (25 of 103; 24%), and for additional prognostic information (14 of 103; 14%).
TABLE 1.
Characteristics of Patients Undergoing Completion Lymph Node Dissection Versus Active Surveillance
| No. of Patients (%) |
|||
|---|---|---|---|
| Characteristic | Surveillance, N = 965 | Dissection, N = 189 | P |
| Location of treating center | |||
| Australia | 174 (18) | 8 (4) | <.01 |
| Europe | 129 (13) | 29 (15) | |
| United States | 662 (69) | 152 (80) | |
| Age: Mean ± SD, y | 59 ± 16 | 57 ± 16 | .11 |
| Men | 590 (61) | 122 (65) | .38 |
| Tumor locationa | |||
| Head/neck | 118 (12) | 39 (21) | <.01 |
| Trunk | 366 (38) | 75 (40) | |
| Upper extremity | 181 (19) | 41 (22) | |
| Lower extremity | 299 (31) | 34 (18) | |
| Breslow thickness, mm | |||
| ≤1.0 | 103 (11) | 22 (12) | .04 |
| >1.0 to 2.0 | 295 (30) | 45 (24) | |
| >2.0 to 4.0 | 325 (34) | 57 (30) | |
| >4.0 | 242 (25) | 65 (34) | |
| Tumor ulcerationa | 377 (40) | 80 (43) | .37 |
| Presence of microsatellitesa | 75 (9) | 25 (13) | .05 |
| No. of positive nodes | |||
| 1 | 755 (78) | 120 (64) | <.01 |
| 2-3 | 200 (21) | 57 (30) | |
| ≥4 | 10 (1) | 12 (6) | |
| Size of SLN metastasis: Median [25th-75th percentile], mma,b | 0.5 [0.0-2.0] | 1.7 [0.1-6.0] | <.01 |
| Extranodal extensiona | 52 (6) | 25 (13) | <.01 |
| AJCC8 stage | |||
| IIIA | 279 (39) | 89 (20) | <.01 |
| IIIB | 221 (23) | 25 (13) | |
| IIIC | 413 (43) | 102 (54) | |
| IIID | 11 (1) | 12 (6) | |
| BRAF mutation status | |||
| Mutant | 280 (46) | 65 (57) | .04 |
| Wild type | 329 (54) | 50 (43) | |
| Adjuvant systemic therapy | 365 (38) | 74 (39) | .75 |
Abbreviations: AJCC8, American Joint Committee on Cancer, eighth edition; SLN, sentinel lymph node.
Some patients had unknown values for tumor location (n = 1), tumor ulceration (n = 19), presence of microsatellites (n = 111), size of SLN metastasis (n = 148), extranodal extension (n = 42), AJCC8 stage (n = 2), BRAF mutation status (n = 430), and adjuvant systemic therapy (n = 3).
These include patients who had isolated tumor cells for which the size of SLN metastasis was reported as 0.0 mm.
Among the patients who underwent CLND, 49 of 189 (26%) had at least 1 positive non-SLN in the completion specimen. CLND resulted in upstaging, according to the American Joint Committee on Cancer eighth edition cancer staging manual, in 14 of 189 cases (7%). Five patients with T2-T3 lesions were upstaged from IIIB to IIIC, and 9 with T4 lesions were upstaged from IIIC to IIID.
Active Surveillance Strategies
For active nodal basin surveillance, 16 of 21 institutions primarily used ultrasound, and 5 used cross-sectional imaging (CT or PET/CT). In patients who received ultrasound surveillance, 58% underwent at least 1 nodal basin ultrasound per 6-month follow-up period (range by treating center, 35%-83%). Among sites that reported all types of imaging performed during follow-up, 89% of patients who underwent active surveillance had at least 1 image per 6-month interval. Patients who received adjuvant therapy were less likely to have adherent ultrasound surveillance (31% vs 69%; P < .01) but had more cross-sectional imaging (47% vs 20%; P < .01).
Disease Recurrence and Methods of Detection
Patients were followed for a median of 11 months (25th to 75th percentile, 6-17 months). During this time, 220 patients (19%) recurred at any site. Modalities by which first recurrences were detected are delineated in Supporting Table 3. Among locoregional-only recurrences, 65% were detectable by patient symptoms and/or clinical examination. Fifty-three percent of distant-only initial recurrences were detected by CT and/or PET alone, whereas 24% were associated with clinical findings (12% based on clinical findings alone, 12% by clinical and imaging findings), 5% were detected by brain MRI only, and the rest were detected by multiple or unknown modalities. In the 68 patients who had isolated nodal basin recurrences, 6% were detected solely on clinical assessment, 21% were detected only by nodal basin ultrasound, and 22% were detected on clinical assessment and/or ultrasound along with another modality. Thirty-four percent of isolated nodal basin recurrences were only detected on CT, PET, and/or PET/CT, whereas the method of detection was not reported for the remaining 18% of isolated nodal recurrences.
The proportion of patients who had a recurrence at any site was comparable between those who received active surveillance (179 of 965 patients; 19%; median follow-up, 10.8 months) and those who underwent CLND (41 of 189 patients; 22%; median follow-up, 13.0 months; P = .31) (Table 2). In unadjusted survival analyses, performance of CLND did not significantly affect all-site RFS (P = .84) or isolated nodal basin RFS (P = .11) (Fig. 1). Recurrence limited to draining nodal basin(s) occurred in 61 of 965 patients (6%) who received active surveillance and in 7 of 189 patients (4%) who underwent CLND (P = .16).
TABLE 2.
Patterns of Initial Recurrence Based on Nodal Management and Receipt of Adjuvant Systemic Therapy
| No. of Patients (%) |
||||
|---|---|---|---|---|
| Nodal Management |
Adjuvant Systemic Therapya |
|||
| Site of Recurrence | Dissection, N = 189 | Surveillance, N = 965 | Yes, N = 439 | No, N = 712 |
| No recurrence | 148 (78) | 786 (81) | 358 (82) | 573 (80) |
| Recurrence | 41 (22) | 179 (19) | 81 (18) | 139 (20) |
| Local-regional onlyb | 11 (6) | 40 (4) | 19 (4) | 32 (5) |
| SLN basin only | 7 (4) | 61 (6) | 24 (5) | 44 (6) |
| Distant only | 16 (8) | 41 (4) | 21 (5) | 36 (5) |
| Multiple sites | 7 (4) | 37 (4) | 17 (4) | 27 (4) |
| Including nodal | 4 (2) | 35 (4) | 14 (3) | 24 (3) |
| Not including nodal | 3 (2) | 2 (<1) | 3 (1) | 3 (1) |
Abbreviation: SLN, sentinel lymph node.
Data regarding adjuvant therapy were not available for 3 participants.
Local-regional includes the primary site and in-transit disease.
Figure 1.
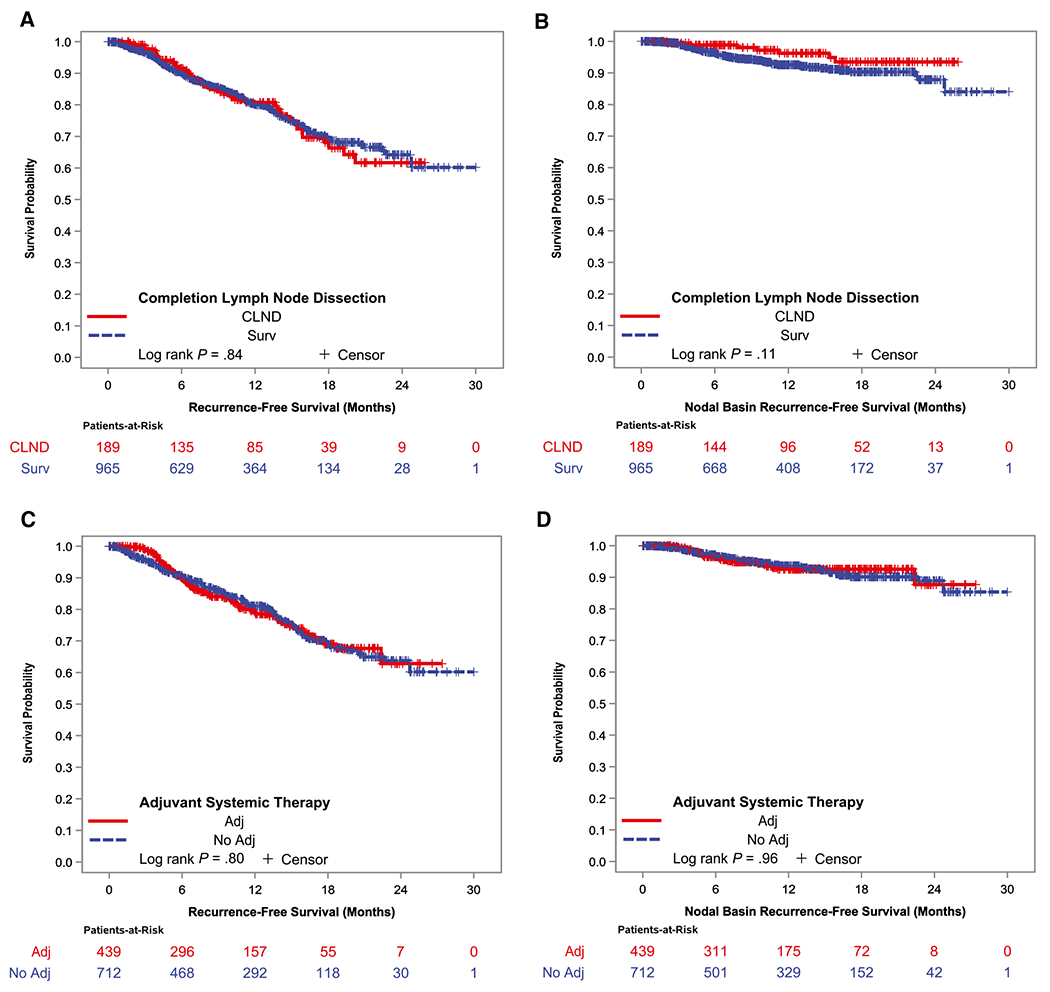
(A) All-site recurrence-free survival and (B) isolated nodal basin recurrence-free survival are illustrated by performance of completion lymph node dissection (CLND) versus active surveillance (Surv) and (C,D) by receipt of adjuvant (adj) systemic therapy. Log-rank tests were stratified by location of the treating center (Australia, Europe, United States). HR indicates hazard ratio.
Among patients receiving active surveillance who had an isolated nodal basin recurrence, 51 of 61 (84%) had undergone nodal resection by the time of data collection. Three patients underwent selective lymph node dissection (ie, removal of only clinically involved nodes rather than clearance of the nodal basin) because of high comorbid disease burden or as part of a trial, whereas most patients underwent formal therapeutic lymph node dissection. Management of the remaining nodal recurrences is unknown or not yet determined; however, to our knowledge, there have been no instances of unresectable recurrence in an SLN basin. Patients with concurrent nodal and other-site recurrences were treated with systemic therapy, and none required nodal surgery for SLN basin-associated symptoms.
Adjuvant Treatment
Seventy-four of 189 patients (39%) who underwent CLND and 365 of 965 patients (38%) who underwent active surveillance received adjuvant systemic therapy. The patients who received adjuvant therapy more often had ulcerated primary tumors, greater Breslow thickness, more positive SLNs, larger nodal tumor deposits, ENE, and higher pathologic stage (Table 3). Patients who received adjuvant therapy were younger and more likely to be treated in the United States and Australia than in Europe.
TABLE 3.
Characteristics of Patients Based on Receipt of Adjuvant Systemic Therapy
| No. of Patients (%) |
|||
|---|---|---|---|
| Characteristic | No Adjuvant Therapy, N = 712 | Adjuvant Therapy, N = 439 | P |
| Location of treating center | |||
| Australia | 88 (12) | 91 (21) | <.01 |
| Europe | 125 (18) | 33 (8) | |
| United States | 499 (70) | 315 (72) | |
| Age: Mean ± SD, y | 59 ± 16 | 57 ± 15 | .02 |
| Men | 432 (61) | 278 (63) | .37 |
| Tumor locationa | |||
| Head/neck | 97 (14) | 59 (14) | .86 |
| Trunk | 266 (37) | 174 (40) | |
| Upper extremity | 141 (20) | 80 (18) | |
| Lower extremity | 208 (29) | 125 (29) | |
| Breslow thickness, mm | |||
| ≤1.0 | 97 (14) | 28 (6) | <.01 |
| >1.0 to 2.0 | 230 (32) | 110 (25) | |
| >2.0 to 4.0 | 225 (32) | 155 (35) | |
| >4.0 | 160 (22) | 146 (33) | |
| Tumor ulcerationa | 242 (34) | 213 (50) | <.01 |
| Presence of microsatellitesa | 59 (10) | 41 (10) | .86 |
| No. of positive nodes | |||
| 1 | 560 (79) | 314 (72) | .02 |
| 2-3 | 142 (20) | 113 (26) | |
| ≥4 | 10 (1) | 12 (3) | |
| Positive nodes ≥1.0 mm | 347 (49) | 282 (64) | <.01 |
| Size of SLN metastasis: Median [25th-75th percentile], mma,b | 0.4 [0.0-2.0]b | 1.2 [0.2-4.0] | <.01 |
| Extranodal extensiona | 33 (5) | 44 (10) | <.01 |
| AJCC8 stagea | |||
| IIIA | 279 (39) | 89 (20) | <.01 |
| IIIB | 147 (21) | 98 (22) | |
| IIIC | 271 (38) | 242 (55) | |
| IIID | 13 (2) | 10 (2) | |
| BRAF mutation status | |||
| Mutant | 200 (48) | 143 (47) | .75 |
| Wild type | 216 (52) | 162 (53) | |
| Underwent CLND | 115 (16) | 74 (17) | .75 |
Abbreviations: AJCC8, American Joint Committee on Cancer, eighth edition; CLND, completion lymph node dissection; SLN, sentinel lymph node.
Some patients had unknown values for receipt of adjuvant therapy (n = 3), tumor location (n = 1), tumor ulceration (n = 19), the presence of microsatellites (n = 111), extranodal extension (n = 42), AJCC8 stage (n = 2), and BRAF mutation status (n = 430).
These include patients who had isolated tumor cells for which the size of SLN metastasis was reported as 0.0 mm.
Single-agent anti–PD-1 immunotherapy was the most common adjuvant regimen (364 of 439 patients; 83%), with nivolumab being most frequently used (332 patients [76%] vs pembrolizumab, 32 patients [7%]). Twenty-one patients (5%) received anti–CTLA-4 or combination immunotherapy; 40 (9%) received BRAF/MEK inhibitor therapy, including 27% of those who ultimately had BRAF-mutated tumors identified; and 14 (3%) received other treatments. Patients remained on adjuvant systemic therapy for a median of 6 months (25th to 75th percentile, 3-10 months), with some still on treatment at the time of data collection and others discontinuing because of toxicity or relapse (see Supporting Fig. 1).
Comparing patients who received adjuvant therapy with those who did not (in whom the median follow-up times were 10.0 and 11.8 months, respectively), unadjusted analyses demonstrated no differences in all-site RFS (P = .80) or isolated nodal basin RFS (P = .96) (Fig. 1). The patterns of recurrence were comparable between patients who received adjuvant therapy and those who did not (Table 2). Specifically, in patients who received adjuvant therapy, those undergoing active surveillance had more isolated nodal basin recurrences (6% vs 1% after CLND; P = .09) but fewer distant recurrences (6% vs 15%; P = .01). There was no difference in all-site RFS between patients with BRAF-mutated and those with wild-type tumors (P = .69).
Multivariable Analysis of All-Site and Isolated Nodal Basin RFS
On risk-adjusted multivariable analysis, undergoing CLND was associated with a 64% reduction in isolated nodal basin recurrence (HR, 0.36; 95% CI, 0.15-0.88), whereas increasing nodal tumor deposit size was associated with worse isolated nodal basin RFS (Table 4). Factors associated with worse all-site RFS included higher stage relative to stage IIIA, head/neck tumor location relative to lower extremity tumor location, and larger nodal tumor deposit (Table 4). The Cox model confirmed that patients who received adjuvant therapy had a high baseline risk of recurrence. However, for each month of follow-up, receipt of adjuvant therapy was associated with a 48% reduction in all-site recurrence (HR, 0.52; 95% CI, 0.47-0.57).
TABLE 4.
Multivariable Analysis of Patient, Tumor, and Treatment Factors Associated With Isolated Nodal Basin Recurrence-Free Survival (RFS) and All-Site RFS in Adult Patients With Cutaneous Melanoma and Positive Sentinel Lymph Nodesa
| Isolated Nodal Basin RFS |
All-Site RFS |
|||
|---|---|---|---|---|
| Variable | HR [95% CI] | P | HR [95% CI] | P |
| No. of recurrence events (% of total cohort) | 68 (6) | 220 (19) | ||
| Age per 1-y increase | 1.01 [0.99-1.03] | .41 | 1.00 [0.99-1.01] | .69 |
| AJCC8 stage | NA | <.01c | ||
| IIIA | NAb | Ref | ||
| IIIB | 1.82 [1.08-3.06]c | |||
| IIIC | 3.38 [2.19-5.23]c | |||
| IIID | 4.88 [2.07-11.49]c | |||
| Tumor location | .64 | .03c | ||
| Lower extremity | Ref | Ref | ||
| Upper extremity | 1.21 [0.54-2.73] | 1.32 [0.84-2.09] | ||
| Trunk | 1.43 [0.73-2.82] | 1.16 [0.79-1.70] | ||
| Head/neck | 0.90 [0.34-2.36] | 1.88 [1.21-2.93]c | ||
| No. positive nodes | .67 | .60 | ||
| 1 | Ref | Ref | ||
| 2-3 | 1.01 [0.53-1.95] | 1.20 [0.85-1.69] | ||
| ≥4 | 1.96 [0.44-8.66] | 1.14 [0.53-2.46] | ||
| Extranodal extension | .26 | .52 | ||
| Absent | Ref | Ref | ||
| Present | 1.67 [0.69-4.07] | 1.17 [0.70-1.94] | ||
| Size of SLN metastasis per 1-mm increase | 1.07 [1.02-1.12]c | <.01c | 1.11 [1.06-1.17]c | <.01c |
| Interaction between size of SLN metastasis and follow-up time | NAd | NA | 0.99 [0.98-1.00] | .03c |
| CLND | .02c | .07 | ||
| No | Ref | Ref | ||
| Yes | 0.36 [0.15-0.88]c | 0.68 [0.45-1.02] | ||
| Adjuvant systemic therapy | ||||
| Receipt at any time | 0.77 [0.43-1.37] | .38 | NAe | NA |
| Risk per mo of follow-up: Interaction between adjuvant systemic therapy and time | NAe | NA | 0.52 [0.47-0.57]c | <.01c |
Abbreviations: AJCC8, American Joint Committee on Cancer, eighth edition; CLND, completion lymph node dissection; HR, hazard ratio; NA, not applicable; Ref, reference category; SLN, sentinel lymph node.
Cox proportional hazard models were performed for outcomes of isolated nodal basin RFS and all-site RFS and were adjusted for the variables listed.
AJCC8 stage was not included in the isolated nodal basin RFS model because of the lack of a univariate association with the outcome and failure to meet proportionality of hazards assumptions.
Values denote statistically significant findings; values <1.00 were associated with decreased recurrence, and values >1.00 were associated with increased recurrence.
Values indicate the interaction term for the size of SLN metastasis and the time required for all-site RFS outcome caused by the nonproportionality of hazards.
The association of adjuvant systemic therapy with all-site RFS is reported with an interaction term for time dependence because of the nonproportionality of hazards.
Distant Metastasis-Free and Disease-Specific Survival
One-hundred seven patients (9%) developed distant metastasis as part of a first or subsequent recurrence during follow-up, including 82 (9%) of those who underwent active surveillance and 25 (13%) of those who underwent CLND (P = .040). The proportions of patients who had distant metastasis were comparable based on receipt of adjuvant therapy in unadjusted analyses (40 of 439 patients [9%] who received adjuvant therapy vs 67 of 712 patients [9%] without adjuvant therapy; P = .87). At the median follow-up of 11 months, there were no statistically significant differences in DMFS based on nodal management (P = .17) or adjuvant therapy (P = .94).
At the end of follow-up, 1116 participants were alive. Twenty-four of the 38 deaths were from melanoma, including 7 after CLND (4%) and 17 in the active surveillance cohort (2%; P = .26). Among patients who received adjuvant systemic therapy, there were 9 melanoma-specific deaths (2%), whereas 15 patients (2%) who did not receive adjuvant systemic therapy had died from melanoma by the end of the study (P = .45) (see Supporting Fig. 2).
Patients With MSLT-2 Exclusion Criteria
Fifteen percent of patients (n = 171) had at least 1 reason that they would have been excluded from MSLT-2 (see Supporting Table 4). Although these patients were more likely to undergo CLND (52 of 171 patients; 30%) than those without exclusion criteria (137 of 983 patients; 14%; P < .01), most (70%) received active surveillance. In this group, isolated nodal basin recurrence developed in 12 of 119 patients (10%) undergoing active surveillance and in 3 of 52 patients (6%) who underwent CLND (P = .08). Distant recurrence as the first site of recurrence occurred in 13 of 119 patients (11%) in the active surveillance group and in 14 of 52 patients (27%) in the CLND group (P = .01). By comparison, for patients without exclusion criteria, the rates of isolated nodal recurrence were 6% (49 of 846 patients) in the active surveillance group and 3% (4 of 137 patients) in the CLND group (P = .17), whereas the rates of distant recurrence were 11% (50 of 846 patients) in the active surveillance group and 7% (10 of 137 patients) in the CLND group (P = .53).
DISCUSSION
In this post–MSLT-2 era, international, multi-institutional cohort study of patients with cutaneous melanoma who had positive SLNs, only 16% of patients underwent CLND, demonstrating widespread uptake of active nodal basin surveillance at participating centers. This represents a dramatic change in surgical practice over a period of <3 years.15 At this initial assessment, the findings align with those from the MSLT-2 and DeCOG-SLT trials. Although there were higher rates of isolated nodal recurrence with active surveillance, these were salvageable with therapeutic lymph node dissection, and there was no significant difference in all-site RFS, DMFS, or DSS. A considerably larger proportion of patients undergoing active surveillance in modern clinical practice received adjuvant systemic therapy than in the MSLT-2 or DeCOG-SLT trials. Adjuvant therapy recipients had similar patterns of recurrence, and the rate of isolated nodal basin recurrence remained low, even in those without prior CLND.
Recurrence rates in this study were commensurate with those of the MSLT-2 and DeCOG-SLT trials at comparable points during follow-up. In both randomized trials, approximately one-fifth of patients recurred in the first year of follow-up, compared with 19% at 11 months in our current cohort.3,5 Patterns of recurrence were also similar. Like what was reported in the MSLT-2 and DeCOG-SLT trails, there were higher rates of isolated nodal basin recurrence among patients who did not undergo CLND.
Patients who were selected to undergo CLND in clinical practice had thicker primary tumors and more extensive nodal involvement, including the number of the number of positive nodes, the size of nodal metastasis, and a higher incidence of ENE, so it is not surprising that the rate of positive non-SLNs (26%) was higher than previously published rates.16 Still, this means that the majority of patients who underwent CLND had no additional positive nodes. Furthermore, there were no instances of unresectable sentinel node basin recurrence in the patients who underwent active surveillance. Considering the high risk of disease outside the nodal basin in SLN-positive patients, the ability to salvage isolated nodal recurrences using therapeutic node dissection, and the potential morbidity of lymphadenectomy, the current study reaffirms the value of active nodal basin surveillance to limit subsequent lymph node dissection to the minority of patients who have recurrences limited to the nodal basin.17
Almost 40% of patients who were treated at participating centers received adjuvant systemic therapy. In clinical practice, patients with higher risk primary and nodal features were more likely to receive adjuvant treatment. The finding of no difference in recurrence rates for patients based on receipt of adjuvant therapy on unadjusted analyses, but improvement in RFS in multivariable analyses adjusted for these risk factors, supports the benefit of adjuvant treatment in the higher risk patients in whom it was used. Granted, longer follow-up is needed to confirm these findings because the median RFS in adjuvant immunotherapy trials was approximately 2 years.7–11
In the rapidly changing landscape of advanced melanoma management, this real-world cohort of patients treated from 2017 through 2019 still may not reflect current adjuvant management. Because approvals of anti–PD-1 immunotherapy and BRAF/MEK inhibitors by international regulatory bodies occurred after the initial eligibility period of this study, the finding of adjuvant therapy receipt by 39% of patients may under-represent what is now happening in practice.18,19 Furthermore, the relapse rates reported in this study may be higher than what are now being achieved in SLN-positive melanoma.
Because the aforementioned adjuvant therapy trials mandated CLND, loss of regional nodal basin control in patients undergoing active surveillance who receive adjuvant therapy has been a concern for many providers.20–22 Among our patients who received adjuvant therapy without undergoing prior CLND, 5% have had an isolated nodal recurrence, which is comparable to the rate of isolated nodal recurrence among patients undergoing active surveillance who did not receive adjuvant therapy. Findings from the multivariable analysis demonstrate no impact of CLND on RFS after adjusting for treatment with adjuvant therapy, which indicates that CLND neither helps nor impedes the activity of adjuvant treatment, although longer follow-up is needed to confirm this.
The current study findings do highlight a current dilemma in managing patients undergoing active surveillance who develop isolated nodal recurrence during the adjuvant treatment period. Whether these recurrences represent regional failure because of incomplete clearance of the nodal basin or treatment-resistant disease is an area of active controversy. This has implications for whether the patient resumes the same adjuvant treatment after the nodal recurrence is addressed.
A subset of patients in this study who received active surveillance would have been excluded from the MSLT-2 and DeCOG-SLT trials based on ENE, microsatellitosis, and/or the number of positive nodes. In these patients, who had at least 1 exclusion criteria, there was a trend toward fewer isolated nodal recurrences after CLND compared with the patients who underwent active surveillance, but the rate of distant recurrence was significantly higher in the CLND group than in the active surveillance group. This finding may indicate that patients with exclusion criteria have a heightened risk of distant disease when CLND is performed. Alternatively, in this nonrandomized cohort, these findings may represent selection bias toward performance of CLND in high-risk patients, whose true risk cannot be adequately characterized by the presence of any single exclusion criterion. Given the ongoing controversy regarding nodal management for SLN-positive patients who were not represented in prior randomized trials, this merits further study.
At the participating centers, there was significant variation in adherence to the MSLT-2 and DeCOG-SLT surveillance protocols. Some centers did not have access to high-quality nodal basin ultrasound or preferred cross-sectional imaging.23,24 At sites with ultrasound access, slightly less than 60% of patients had a minimum of 6-monthly ultrasounds performed. Although ultrasound has greater sensitivity and specificity for detecting nodal basin recurrence when studied in a research context, findings may be more variable in clinical practice based on who performs the ultrasound and the anatomic site that is being observed. In this cohort, patients receiving adjuvant therapy often had cross-sectional imaging in lieu of nodal basin ultrasound. The added value of nodal basin ultrasound in adjuvant therapy patients who have cross-sectional imaging has not been established and would be studied best in a prospective fashion, with all patients receiving both ultrasound and cross-sectional imaging at designated intervals.23 In addition, there is limited evidence that early detection of recurrence affects long-term oncologic outcomes, so the optimal surveillance strategy for SLN-positive patients remains unknown.4,24–26
The short follow-up duration provides early insight into real-world outcomes of active surveillance in SLN-positive patients but limits the ability to draw firm conclusions. Recognizing that the majority of melanoma recurrences occur within 2 years of diagnosis, subsequent assessments of this cohort are planned. Furthermore, unlike a randomized trial design that balances groups with respect to both measured and unmeasured variables, in this cohort study, we were unable to account for potentially unmeasured variables, which could have affected patient selection for CLND versus active surveillance and receipt of adjuvant therapy. The patients who underwent CLND had more complete nodal staging, which could have influenced adjusted analyses. However, few patients were upstaged by CLND findings and, in many prognostic models, the status of non-SLNs is less relevant that other features of the primary tumor and SLNs.27
Although we have provided some information regarding reasons for undergoing CLND, this is limited by the retrospective study design. We were also unable to determine the specific reasons for patients’ receipt of adjuvant therapy or the rationale for the selected treatment. Also, whereas the inclusion of multiple international institutions increases study generalizability, central review of pathology and imaging was not carried out, and post-relapse treatment was not standardized.
Although the institutions included in this study were diverse in their geographic distribution, all are major melanoma treatment centers. The adoption of active nodal basin surveillance and fidelity to an active surveillance protocol outside these referral centers have not been explored. Furthermore, it is unknown whether similar oncologic outcomes will be achieved in other settings that may be more reflective of melanoma management in nonspecialist centers worldwide.
Conclusion
This real-world cohort study demonstrates a high level of adoption of active surveillance at many of the major melanoma treatment centers throughout the world. Early findings reinforce the conclusions of the MSLT-2 and DeCOG-SLT trials that active surveillance is an effective strategy for SLN-positive patients that limits unnecessary lymph node dissections and their attendant morbidities, although long-term survival data are needed. The use of adjuvant therapy in patients who have or have not undergone CLND yields a similarly low rate of isolated nodal basin recurrences, which are largely salvageable with therapeutic lymph node dissection. Future studies should seek to refine our understanding of which patients who undergo active surveillance benefit most from adjuvant therapy and when, if ever, to perform CLND.
Supplementary Material
FUNDING SUPPORT
This work was supported in part by the Biostatistics and Bioinformatics Shared Resource at the H. Lee Moffitt Cancer Center and Research Institute, a National Cancer Institute-designated comprehensive cancer center (P30-CA076292).
CONFLICT OF INTEREST DISCLOSURES
John F. Thompson reports personal fees from Bristol-Meyers Squibb Australia and Merck Sharp & Dohme Australia and personal fees and travel support from GlaxoSmithKline and Provectus Inc, outside the submitted work. Tina J. Hieken reports grants from Genentech, outside the submitted work. David E. Gyorki reports personal fees from Amgen, outside the submitted work. Alexander van Akkooi reports grants and personal fees from Amgen, Bristol-Myers Squibb, and Novartis; and personal fees from 4SC, Merck-Pfizer, MSD-Merck, and Sanofi, outside the submitted work. Jeffrey M. Farma reports personal fees from Novartis and Delcath, outside the submitted work. Roger Olofsson Bagge reports grants from Astra Zeneca and personal fees from Amgen, BD/Bard, Bristol-Myers Squibb, Merck Sharp & Dohme, Novartis, Roche, and Sanofi Genzyme, outside the submitted work. Georgia Beasley reports grants form Istari Oncology and personal fees from Sanofi-Regeneron, outside the submitted work. Amod A. Sarnaik reports grants from Provectus Inc and grants and personal fees from Iovance Biotherapeutics, outside the submitted work. Vernon K. Sondak reports personal fees from Array, Bristol-Myers Squibb, Genentech/Roche, Merck, Novartis, Regeneron, Replimune, Pfizer, and Polynoma, outside the submitted work. Jonathan S. Zager reports grants from Amgen, Delcath Systems, Philogen, and Provectus; grants and personal fees from Castle Biosciences and Novartis, and personal fees from Array Biopharma, Merck, and Sun Pharma, outside the submitted work. The remaining authors made no disclosures.
Footnotes
Additional supporting information may be found in the online version of this article.
REFERENCES
- 1.Wong SL, Morton DL, Thompson JF, et al. Melanoma patients with positive sentinel nodes who did not undergo completion lymphadenectomy: a multi-institutional study. Ann Surg Oncol. 2006;13:809–816. [DOI] [PubMed] [Google Scholar]
- 2.Klemen ND, Han G, Leong SP, et al. Completion lymphadenectomy for a positive sentinel node biopsy in melanoma patients is not associated with a survival benefit. J Surg Oncol. 2019;119:1053–1059. [DOI] [PubMed] [Google Scholar]
- 3.Faries MB, Thompson JF, Cochran AJ, et al. Completion dissection or observation for sentinel-node metastasis in melanoma. N Engl J Med. 2017;376:2211–2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leiter U, Buettner PG, Eigentler TK, Forschner A, Meier F, Garbe C. Is detection of melanoma metastasis during surveillance in an early phase of development associated with a survival benefit? Melanoma Res. 2010;20:240–246. [DOI] [PubMed] [Google Scholar]
- 5.Leiter U, Stadler R, Mauch C, et al. Complete lymph node dissection versus no dissection in patients with sentinel lymph node biopsy positive melanoma (DeCOG-SLT): a multicentre, randomised, phase 3 trial. Lancet Oncol. 2016;17:757–767. [DOI] [PubMed] [Google Scholar]
- 6.Leiter U, Stadler R, Mauch C, et al. Final analysis of DeCOG-SLT trial: no survival benefit for complete lymph node dissection in patients with melanoma with positive sentinel node. J Clin Oncol. 2019;37:3000–3008. [DOI] [PubMed] [Google Scholar]
- 7.Eggermont AM, Chiarion-Sileni V, Grob JJ, et al. Adjuvant ipilimumab versus placebo after complete resection of high-risk stage III melanoma (EORTC 18071): a randomised, double-blind, phase 3 trial. Lancet Oncol. 2015;16:522–530. [DOI] [PubMed] [Google Scholar]
- 8.Eggermont AM, Chiarion-Sileni V, Grob JJ, et al. Prolonged survival in stage III melanoma with ipilimumab adjuvant therapy. N Engl J Med. 2016;375:1845–1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eggermont AMM, Blank CU, Mandala M, et al. Adjuvant pembrolizumab versus placebo in resected stage III melanoma. N Engl J Med. 2018;378:1789–1801. [DOI] [PubMed] [Google Scholar]
- 10.Weber J, Mandala M, Del Vecchio M, et al. Adjuvant nivolumab versus ipilimumab in resected stage III or IV melanoma. N Engl J Med. 2017;377:1824–1835. [DOI] [PubMed] [Google Scholar]
- 11.Long GV, Hauschild A, Santinami M, et al. Adjuvant dabrafenib plus trametinib in stage III BRAF-mutated melanoma. N Engl J Med. 2017;377:1813–1823. [DOI] [PubMed] [Google Scholar]
- 12.Lin DY. MULCOX2: a general computer program for the Cox regression analysis of multivariate failure time data. Comput Methods Programs Biomed. 1993;40:279–293. [DOI] [PubMed] [Google Scholar]
- 13.Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16:1141–1154. [Google Scholar]
- 14.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 15.Herb JN, Dunham LN, Ollila DW, Stitzenberg KB, Meyers MO. Use of completion lymph node dissection for sentinel lymph node-positive melanoma. J Am Coll Surg. 2020;230:515–524. [DOI] [PubMed] [Google Scholar]
- 16.McMasters KM, Wong SL, Edwards MJ, et al. Frequency of nonsentinel lymph node metastasis in melanoma. Ann Surg Oncol. 2002;9:137–141. [DOI] [PubMed] [Google Scholar]
- 17.Coit D The enigma of regional lymph nodes in melanoma. N Engl J Med. 2017;376:2280–2281. [DOI] [PubMed] [Google Scholar]
- 18.Kwak M, Farrow NE, Salama AKS, et al. Updates in adjuvant systemic therapy for melanoma. J Surg Oncol. 2019;119:222–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moyers JT, Chong EG, Mitchell J, Patel A, Jeong ISD, Nagaraj G. Abstract 4338: Immunotherapy in resected stage III melanoma: an analysis of the National Cancer Database. Proceedings: AACR Annual Meeting 2020; June 22-24, 2020; Philadelphia, PA. Cancer Res. 2020;80(16 suppl):4338. [Google Scholar]
- 20.Wong SL, Faries MB, Kennedy EB, et al. Sentinel lymph node biopsy and management of regional lymph nodes in melanoma: American Society of Clinical Oncology and Society of Surgical Oncology clinical practice guideline update. J Clin Oncol. 2018;36:399–413. [DOI] [PubMed] [Google Scholar]
- 21.Hieken TJ, Kane JM 3rd, Wong SL. The role of completion lymph node dissection for sentinel lymph node-positive melanoma. Ann Surg Oncol. 2019;26:1028–1034. [DOI] [PubMed] [Google Scholar]
- 22.Eroglu Z, Babacan N, Grossman K, et al. Retrospective analysis of patients with sentinel lymph node (SNL) positive melanoma (MEL) who received adjuvant nivolumab (NIVO) without completion lymph node dissection (CLND) [abstract]. J Clin Oncol. 2019;37(15 suppl):9590. [Google Scholar]
- 23.Xing Y, Bronstein Y, Ross MI, et al. Contemporary diagnostic imaging modalities for the staging and surveillance of melanoma patients: a meta-analysis. J Natl Cancer Inst. 2011;103:129–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dinnes J, Ferrante di Ruffano L, Takwoingi Y, et al. Ultrasound, CT, MRI, or PET-CT for staging and re-staging of adults with cutaneous melanoma. Cochrane Database Syst Rev. 2019;7:CD012806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Freeman M, Laks S. Surveillance imaging for metastasis in high-risk melanoma: importance in individualized patient care and survivorship. Melanoma Manag. 2019;6:MMT12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leon-Ferre RA, Kottschade LA, Block MS, et al. Association between the use of surveillance PET/CT and the detection of potentially salvageable occult recurrences among patients with resected high-risk melanoma. Melanoma Res. 2017;27:335–341. [DOI] [PubMed] [Google Scholar]
- 27.Verver D, van Klaveren D, van Akkooi ACJ, et al. Risk stratification of sentinel node-positive melanoma patients defines surgical management and adjuvant therapy treatment considerations. Eur J Cancer. 2018;96:25–33. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


