ABSTRACT
Herpesviruses are ubiquitous double-stranded DNA viruses that cause lifelong infections and are associated with a variety of diseases. While they have evolved multiple mechanisms to evade the immune system, they are all recognized by the innate immune system, which can lead to both localized and systemic inflammation. A more recently appreciated mechanism of herpesvirus innate immune activation is through inflammasome signaling. The inflammasome is an intracellular multiprotein complex that, when activated, leads to the release of proinflammatory cytokines, including IL-1β and IL-18, and activation of the inflammatory programed cell death pathway known as pyroptosis. Despite the herpesviruses sharing a similar structure, their mechanisms of inflammasome activation and the consequences of inflammasome activation in cases of virus-associated disease are not uniform. This review will highlight the similarities and differences among herpesviruses with regard to their mechanisms of inflammasome activation and impacts on diseases caused by herpesviruses. Furthermore, it will identify areas where additional studies are warranted to better understand the impact of this important innate immune signaling program on the pathogenesis of these common viruses.
KEYWORDS: inflammasomes, herpesviruses, innate immunity
INTRODUCTION
OVERVIEW OF INFLAMMASOMES
The discovery of inflammasomes as innate immune signaling complexes has transformed our understanding of the innate immune system. Inflammasomes are intracellular multiprotein complexes that form in response to pathogen recognition and/or danger signals. Classical or canonical inflammasomes are composed of three parts: a sensor protein, a multimeric complex of adaptor-apoptosis-associated-speck-like-protein-containing-a-caspase-recruitment domain (ASC), and caspase-1 (1). The formation of this complex leads to caspase-dependent cleavage of the immature (pro-) forms of the proinflammatory cytokines interleukin (IL)-1β and IL-18, and to cleavage and activation of gasdermin D (GSDMD) (2–4). IL-18 and IL-1β are predominantly produced by myeloid cells, such as macrophages and dendritic cells, and mediate immune responses against pathogens and tissue damage (5). IL-1β induction is a vital initial host defense mechanism during viral and bacterial infections (6). IL-18 is structurally similar to IL-1β and its main functions are mediated through the induction of interferon (IFN)-γ secretion from Th1 cells. Together with IL-12, IL-18 leads to Th1 differentiation that activates both adaptive and innate host defense against intracellular bacteria, viruses, and fungi (5, 7, 8).
The formation and activation of some of the canonical inflammasomes can be thought of as a two-step process. The first step, priming, requires activation of the nuclear factor κB (NF-κB) pathway. This is often achieved through the detection of pathogen-associated molecular patterns (PAMPs) by pattern recognition receptors (PRRs) such as Toll-like receptors (TLRs). Translocation of NF-κB into the nucleus leads to the transcription of genes critical to inflammasome signaling, including pro-IL-1β, pro-IL-18, and pro-caspase-1 (9). Notably, several inflammasomes do not necessarily require this first step for activation (10, 11). The second step, activation, requires the sensor protein recognizing its cognate signal. This leads to oligomerization of ASC, assembly of the inflammasome, and caspase-1 cleavage of pro-IL-1β and pro-IL-18 (reference 12, Fig. 1). This activation step can be initiated by multiple sensor proteins that can sense a variety of PAMPs and danger-associated molecular patterns (DAMPs). These sensor proteins are often members of the NOD-like-receptor (NLR) family of proteins, such as NLRP1, NLRC4, and NLPR3, and can respond to a diverse array of stimuli. Absent-in-melanoma-2 (AIM2) is another inflammasome sensor that recognizes and forms inflammasomes in response to dsDNA (13, 14). Specific inflammasomes are thus named by their sensor and different sensors are required for inflammasome activation by different viruses. For example, herpes simplex virus 1 (Human alphaherpesvirus 1, HSV-1/HHV-1) activates the NLRP3 inflammasome (15–17), while cytomegalovirus Human betaherpesvirus 5 (CMV/HHV-5) activates the AIM2 inflammasome (18). After recognition of a cognate PAMP or DAMP, these sensor proteins recruit ASC molecules that undergo oligomerization, followed by the recruitment of pro-caspase-1 to the complex. Pro-caspase-1 then undergoes autolysis to produce active caspase-1 (19). Caspase-1, an IL-1-converting enzyme, then cleaves pro-IL-1β and pro-IL-18 into their mature forms, IL-1β and IL-18, respectively (1).
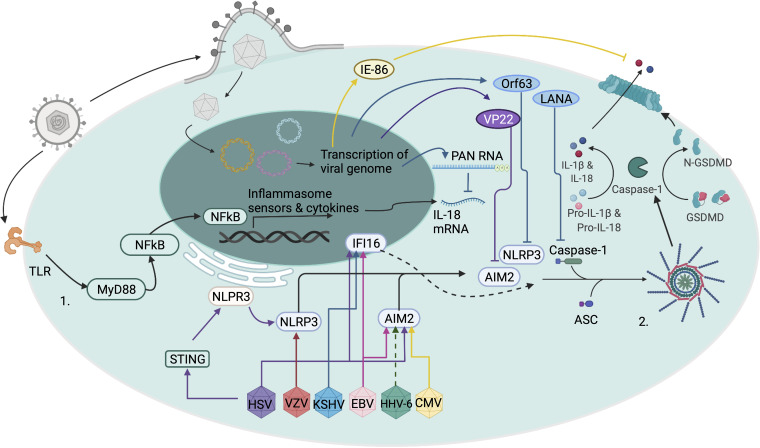
FIG. 1.
Mechanisms of inflammasome activation and regulation by human herpesviruses. The priming signal (step 1) for inflammasome activation is mediated by Toll-like receptors (TLRs) or other pattern recognition receptors. Activation of TLRs by their microbial ligand leads to downstream signaling via MyD88 to NF-κB. NF-κB enters the nucleus as a transcription factor and increases the expression of inflammasome associated genes (9). The activation signal (step 2) for inflammasome activation during herpesvirus infection results from the sensing of different herpesviruses by cytosolic sensors, such as NLRP3 and AIM2. HSV-1/HSV-2 (HSV) can be sensed by NLRP3 and AIM2 in the cytoplasm and by IFI16 in the nucleus (15, 74, 75). However, the HSV-1 protein VP22 inhibits sensing and inflammasome activation by AIM2 (73). Furthermore, NLRP3 inflammasome activation in response to HSV-1 is mediated via STING (76). VZV activates the inflammasome via NLRP3, whereas CMV activates the inflammasome through AIM2 (17, 54). Additionally, the CMV immediate early 86-KD protein (IE-86) inhibits IL-1β release from infected cells (17). It is hypothesized that HHV-6 activates the inflammasome through AIM2, similarly to CMV. EBV infection can lead to inflammasome activation through AIM2 and IFI16 (84–87). KSHV activation of the inflammasome is initiated via IFI16 sensing in the nucleus which then relocates to the cytoplasm (71, 87, 89). The inflammasome response to KSHV can be blunted by the inhibitory activities of KSHV polyadenylated nuclear RNA (PAN RNA), KSHV Orf63 protein, and LANA (90–92). When these sensors receive the activation signal, they oligomerize with ASC and caspase-1 to form the inflammasome and activate caspase-1 (10). Activated caspase-1 cleaves the pro-forms of inflammasome cytokines, IL-18 and IL-1-β, as well as gasdermin-D (GSDMD) (2–4). Cleaved GSDMD forms a pore at the cell surface which allows for the release of IL-18, IL-1β and the influx of ions leading to pyroptotic cell death (22). Figure created with BioRender.com.
Caspase-1 associated inflammasomes are often termed canonical inflammasomes, while other caspases form noncanonical inflammasomes. These noncanonical inflammasomes are dependent on caspases-4/5 in humans and caspase-11 in mice and form in response to lipopolysaccharide (LPS) from bacteria (20–22). This review will focus on the caspase-1-dependent canonical inflammasomes.
In addition to cleaving IL-1β and IL-18, caspase-1 also cleaves GSDMD. GSDMD in turn forms pores in the plasma membrane, leading to cell death through pyroptosis and release of IL-1β and IL-18 (23). There is some debate as to whether GSDMD pore formation is a terminal event that necessarily leads to cell death and whether there are other mechanisms of IL-1β and IL-18 release. The nuances of these arguments are beyond the scope of this review.
HERPESVIRUSES ARE COMMON HUMAN PATHOGENS
The human herpesviruses (HHV) are capable of causing a wide range of diseases, from asymptomatic infection to oncogenesis, retinitis, and lethal encephalitis. HHV virions consist of (i) an icosahedral capsid surrounding the dsDNA genome, (ii) a largely unstructured proteinaceous layer called the tegument that surrounds the capsid, and (iii) an outer lipid bilayer envelope studded with glycoproteins. The hallmark of HHVs is their ability to cause lifelong latent infections, and they are subdivided into three subfamilies known as alpha-, beta-, and gammaherpesvirinae (24).
Alphaherpesviruses consist of HSV-1 and HSV-2 (Human alphaherpesvirus 2, HHV-2), which replicate in cells and tissues of many mammalian species, and varicella-zoster virus (Human alphaherpesvirus 3, VZV/HHV-3), which can only replicate in cells of human or simian origin. All three viruses have broad cellular tropism, but become latent in ganglia along the entire neuraxis, from which they can reactivate to cause recurrent viral shedding or disease (24). HSV-1 and HSV-2 are quite prevalent, with more than half the adult population infected with one or both viruses (25–28). VZV is common worldwide, with seroprevalence rates of >90% in most populations (29). HSV-1- and HSV-2-related disease ranges from mild mucocutaneous lesions in the oral and genital mucosa to vision-threatening keratitis and life-threatening encephalitis. VZV is the etiologic agent of varicella (chickenpox) and herpes zoster (shingles) and can cause devastating disease in special populations, including immunocompromised hosts (30).
Betaherpesviruses include CMV, HHV-6A (Human betaherpesvirus 6A), HHV-6B (Human betaherpesvirus 6B), and HHV-7 (Human betaherpesvirus 7), which can establish latent infection in lymphocytes and other hematopoietic cells (31). In the United States, 40 to 60% of individuals are infected with CMV by adulthood, with seroprevalence approaching 100% in some parts of the world (32, 33). Of these, CMV is the most clinically relevant as it is a major cause of neonatal complications and morbidity in immunosuppressed populations (31, 34, 35).
The gammaherpesvirus subfamily includes Epstein-Barr virus (Human gammaherpesvirus 4, EBV/HHV-4) and Kaposi’s sarcoma-associated herpesvirus (Human gammaherpesvirus 8, KSHV/HHV-8). The prevalence of EBV is 70 to 95% in adults worldwide, with infection usually occurring during childhood. EBV persists mostly in memory B cells. Primary infection is often asymptomatic, but can lead to mononucleosis in adolescents and adults. EBV is also associated with a number of malignancies, including nasopharyngeal carcinoma (NPC) and Burkitt’s lymphoma (BL) (24, 30, 36). KSHV seroprevalence is high in sub-Saharan Africa (30% to 50%) and in the Mediterranean region. It is the etiological agent of the most common AIDS-related malignancy, Kaposi’s sarcoma (KS), as well as primary effusion lymphoma (PEL), multicentric Castleman disease (MCD), and KSHV inflammatory cytokine syndrome (KICS), all of which primarily occur in immunocompromised patients (37, 38).
INFLAMMASOME ACTIVATION HAS VARIABLE IMPACT ON DISEASES CAUSED BY HERPESVIRUSES
Viral activation of the inflammasome is common, including by influenza (39), hepatitis C (HCV) (40, 41), HIV (42), and herpesviruses. Data regarding the role of inflammasome activation in the pathogenesis of alphaherpesviruses in humans are limited. However, evidence in murine HSV-1 and HSV-2 infection models supports a central role for inflammasome activation in both control and pathogenesis. IL-1β knockout (KO) mice are significantly more susceptible to lethal HSV-1 encephalitis than wild-type (WT) controls (43). This indicates that the IL-1β produced by monocytes/macrophages early during infection is critical for protection from overwhelming disease (44). Similarly, IL-18 is essential for NK cell activation (45) and protection from both lethal HSV-1 pneumonitis (46, 47) and HSV-2 genital disease (48, 49). Furthermore, IL-18 may help ameliorate eye lesions in herpes stromal keratitis (HSK) (50). In keeping with the hypothesis that the inflammasome plays a protective role in HSV disease, a study using a murine model of HSK found that NLRP3 KO mice had more severe HSK lesion development than WT mice (51).
However, several recent studies have found that inflammasome activation in the context of HSV-1/HSV-2 infection may be detrimental to the host. IL-1β can lead to HSV-1 reactivation in neurons (52). Furthermore, more virulent strains of HSV-1 cause more severe corneal pathology that is associated with increased IL-1β and IL-18 levels (53), and IL-18 contributes to HSV-2 pathology in a genital model of infection (54). Finally, HSV-1 infection of ASC KO and NLRP3 KO mice led to decreased inflammation and mortality compared to WT controls in a model of encephalitis (17). These findings are consistent with those of a study in humans which found that a ratio of high IL-1β in the cerebral spinal fluid to low IL-1 receptor antagonist (IL-1RA) in plasma was associated with a poor outcome in HSV-1 encephalitis (55). The differences between studies showing both protective and pathogenic roles for inflammasome activation during HSV-1 and HSV-2 infection may reflect the distinct effects of inflammasome cytokines at different disease sites, the opposite effects of moderate versus very high levels of inflammasome activation, or other unknown factors. Additionally, there can be non-inflammasome sources of IL-1β and IL-18, making it challenging to consistently draw direct links between inflammasome activation and pathological observations associated with IL-18 and IL-1β in herpes infections (7, 56, 57). Regardless, these data highlight the complex interactions between intracellular signaling pathways and immune response coordination, and underscore the need for additional research on the impact of inflammasome signaling in the context of HSV-1 and HSV-2 infection in both human and animal models.
Very little is known about the impact of inflammasome activation in VZV disease in humans, but an investigation with human skin xenografts in a SCID mouse model of VZV revealed that NLRP3 was induced in cells within VZV lesions, suggesting that VZV-induced inflammasome activation takes place in vivo (58). More recently, a study found that pharmacologically reducing endoplasmic reticulum stress in a rat model of VZV-associated post-herpetic neuralgia (PHN) led to decreased IL-1β and IL-18 release and improved PHN scores, suggesting that long term activation of the inflammasome is associated with more severe VZV-induced PHN (59). This, combined with the results for HSV-1 which demonstrate a detrimental effect of inflammasome activation (particularly in encephalitis), suggest a model where inflammasome activation in the periphery helps control viral replication, but inflammasome signaling in neuronal tissue leads to pathological inflammation that is harmful to the host. Additional studies will need to be done to refine this hypothetical model.
The impact of inflammasome activation during CMV infection is also somewhat unclear. A study of CMV viremia in CMV-seronegative (R-) kidney transplant recipients who received a kidney from a seropositive (D+) donor found that IL-18 increased along with other proinflammatory cytokines during viremia, suggesting that CMV activates inflammasomes during acute infection (60). More recently, IL-18 was shown to be associated with more severe CMV disease in solid organ transplant recipients regardless of donor and recipient serostatus (61), and certain polymorphisms in the IL-18 promoter are associated with an increased likelihood of CMV reactivation after stopping prophylaxis in kidney transplant recipients (62). These studies demonstrate that, at least in the immunocompromised population, CMV infection likely leads to inflammasome activation in vivo, associated with CMV-induced pathology, and that IL-18 regulation is related to CMV control.
The other betaherpesvirus that is often studied clinically is HHV-6. Though data on HHV-6 and inflammasome activation are lacking for the immunocompromised population, a small study did identify a potential link between HHV-6 copy number and IL-1β levels in children with febrile seizures. Previous studies showed that HHV-6 DNA is detectable in blood from a minority of patients that suffer febrile seizures (63) and that higher levels of IL-1β were found in the saliva of children with seizures, with HHV-6 copy number and IL-1β in saliva positively correlated (64).
Regarding inflammasome activation in EBV-related diseases, EBV-induced infectious mononucleosis leads to elevated IL-18 levels in plasma and substantial amounts of IL-18 in lymphoid tissues (65, 66). Similarly, IL-1β is elevated in the tonsils of children acutely infected with EBV (67). These early studies showed that acute EBV infection is associated with inflammasome-driven cytokines in vivo. In latent infection, high IL-18 is associated with regression of BL tumors in a murine model (68). Similarly, IL-18 receptor expression is upregulated in EBV-infected BL (69). In EBV-associated NPC, increased inflammasome gene expression was associated with improved survival, and IL-1β inhibited tumor growth in a murine model of the disease (70). Additionally, a recent study demonstrated that inflammasome activation in EBV positive tumor cells leads to lytic replication (71). Therefore, while these studies suggest that inflammasome activation in EBV latency-associated malignancies may be beneficial and lead to decreased tumor burden, it is unclear whether this is a direct effect of inflammasome activation or an indirect effect of lytic replication of the virus.
There is ample evidence of inflammasome activation in diseases caused by KSHV. Proinflammatory cytokines, such as IL-1β, IL-6, and TNF-α, are known to promote the pathogenesis of KSHV-associated diseases (72). IL-1β is elevated in patients with KS and promotes tumorigenesis when added to KS cells in culture. IL-1β also increases resistance to apoptosis, potentially promoting tumor survival (73, 74). MCD flares are also associated with an increase in IL-1β (75), and PEL cells constitutively produce IL-1β (76). Similarly, HIV-infected men with KSHV associated diseases have higher IL-18 and inflammasome activation in monocytes compared to healthy controls (77). Therefore, in contrast to EBV-associated malignancies, it appears that inflammasome activation promotes oncogenesis in KSHV-associated proliferative disorders.
MULTIPLE INFLAMMASOMES ARE TRIGGERED IN RESPONSE TO HERPESVIRUSES
The importance of IL-1β and IL-18 in herpesvirus pathogenesis has long been known, but additional techniques for querying the inflammasome were required to demonstrate that HSV-1 directly activates the inflammasome. During early investigations of the AIM2 inflammasome, it was discovered that HSV-1 activates the inflammasome in macrophages without requiring this dsDNA sensor (78). Subsequently, it was found that a viral protein, VP22, specifically inhibits the AIM2 inflammasome during HSV-1 infection (79). Thus, other sensors have been proposed to function as inflammasome sensors for HSV-1 infection. While there is heterogeneity within the literature, NLRP3 has been consistently found as central to HSV-1 inflammasome activation. This has been demonstrated in keratinocytes (80), human foreskin fibroblasts (HFFs) (16), and macrophages (15). The mechanism by which HSV-1 activates NLRP3 is thought to be mediated through stimulator-of-interferon-genes (STING). STING recruits NLRP3 to the endoplasmic reticulum and attenuates NLRP3 K48- and K63-linked polyubiquitination, thereby promoting inflammasome activation (81). Whether other inflammasomes are activated in response to HSV-1 depends on the specific models of infection studied. The AIM2 inflammasome has been proposed to function in keratinocyte infection (80) and in some mouse models (82). Gamma-interferon-inducible protein 16 (IFI16) sensing of HSV-1 is thought to lead to inflammasome activation in HFFs (16). However, neither AIM2 nor IFI16 are required for inflammasome activation in macrophages (15). These and other inflammasome proteins, including NLRP12, are upregulated during HSV-1 infection in mice, but whether or not they are required for or directly involved in HSV-1-induced inflammasome activation remains unclear (53). Thus, while NLRP3 appears to be the primary inflammasome activated in HSV-1 infection, HSV-1 may activate other inflammasomes in a subset of cell types or tissues.
Apart from the known AIM2-inibitory function of VP22, it is not entirely clear if HSV-1 encodes other inflammasome inhibitory or regulatory elements. ICP0 is thought to attenuate NLRP3 and IFI16 induction in HFFs (16), and there is evidence that some replication-dependent factor inhibits NLRP3 inflammasome activation in macrophages (15). However, the direct impact of inflammasome activation on HSV-1 replication is unclear. As a result, how viral regulation of inflammasome activation affects the viral life cycle and pathogenesis is an open area of investigation.
Few studies have investigated the mechanism of VZV-induced inflammasome activation, but there are data suggesting that it activates the NLRP3 inflammasome in at least three different cell types: primary lung fibroblasts, a human melanoma cell line, and the monocyte/macrophage-like THP-1 cell line, all of which are permissive for VZV replication in vitro (58).
Although it was known that CMV strongly activates innate immune signaling by expressing proinflammatory cytokines and the secretion of IL-1β in multiple cell types (83–85), it was only recently demonstrated that CMV activation of the inflammasome is dependent on AIM2 and enhanced by STING (18, 86). Interestingly, IL-1β inhibits in vitro growth of CMV (87), and CMV immediate early 86-kDa protein (IE-86) inhibits IL-1β release from CMV-infected cells (18). This suggests that regulation of the inflammasome during CMV infection is critical for the viral life cycle and pathogenesis.
Studies on HHV-6 induction of inflammasome signaling in vitro are limited, but HHV-6 infection of peripheral blood mononuclear cells leads to IL-1β production (88). Both HHV-6A and HHV-6B infection of T cells in culture also leads to upregulation of the IL-18 gene (89). Given these findings and what is known about CMV activation of the inflammasome, it is likely that HHV-6 activates the AIM2 inflammasome, but definitive studies are lacking.
EBV infection of both THP-1 cells and primary human monocytes leads to the release of IL-1β, suggesting that EBV is capable of activating inflammasomes in monocytes. EBV infection of these cells leads to upregulation of AIM2, and knockdown of AIM2 attenuates IL-1β release, indicating that EBV activates the AIM2 inflammasome in monocytes (90). However, in B cell infection, IFI16 interacts with the EBV genome and leads to ASC-dependent inflammasome activation (91–93). While not implicated in EBV-induced inflammasome activation, NLRP3 signaling does lead to EBV reactivation from latency in cell culture models (71). Therefore, it is possible that multiple inflammasomes play an important role in EBV replication and cell biology.
In contrast, multiple studies have indicated that KSHV activates the inflammasome in both epithelial cells and B cells through an IFI16-dependent mechanism (76, 93, 94). KSHV also activates the inflammasome in latency-associated malignancies, including KS and PEL (76). The activation of inflammasomes by KSHV is facilitated and regulated by BRCA1 (93). KSHV also has evolved multiple mechanisms to regulate inflammasome activation. The KSHV protein Orf63 is known to block NLRP1-dependent inflammasome activation, and possibly NLRP3 as well, as a way to promote efficient reactivation from latency (95). The KSHV polyadenylated nuclear RNA (PAN RNA) can modulate the innate immune response by decreasing pro-IL-18 mRNA (96). KSHV latency-associated nuclear antigen (LANA) also has a known caspase-1 cleavage site and functions to blunt IL-1β production (97). Furthermore, there is evidence that release of IFI16 and IL-1β in exosomes is another potential mechanism by which KSHV subverts the host innate response, though the implications of this finding are not yet known (76). Taken together, these studies demonstrate that IFI16 is at the center of KSHV inflammasome biology, yet there is a complex interaction between KSHV and the inflammasome response in viral persistence and pathogenesis which is not fully defined. A summary of various inflammasome activation impacts on herpes virus-associated diseases is provided in Table 1.
TABLE 1.
Impact of inflammasome activation on human herpesvirus-associated diseases
| Virus | Inflammasome activation |
Sensor | Clinical disease | Impact on disease outcome | References | |
|---|---|---|---|---|---|---|
| In vitro | In vivo | |||||
| HSV-1 (HHV-1) and HSV-2 (HHV-2) | Yes | Yes | NLRP3, AIM2, IFI16 | Encephalitis | IL-1β KO worsens disease in mice | 15–17, 43–50, 52–55, 80–82 |
| ↑CSF IL-1β and periphery IL-1RA associated with worse disease in humans | ||||||
| ASC KO and NLRP3 KO decrease inflammation and death in mice | ||||||
| Pneumonitis | ↑IL-18 improves disease outcomes | |||||
| Herpes stromal keratitis (HSK) | ↑IL-18 ameliorates disease | |||||
| HSV-1 reactivation | IL-1β can increase reactivation in neurons | |||||
| Genital disease | Unclear: IL-18 can be protective but also contributes to pathology | |||||
| VZV (HHV-3) | Yes | Yes (in murine models) | NLRP3 | Post-herpetic neuralgia (PHN) | ↓IL-1β and ↓IL-18 decrease disease | 58, 59 |
| CMV (HHV-5) | Yes | Yes | AIM2 | Cytomegalovirus (CMV) in transplant patients | ↑IL-18 associated with severe disease | 18, 60–62, 83–86 |
| HHV-6 | Unknown | Yes | AIM2 (presumed) | Febrile seizures | ↑IL-1β in children with febrile seizures | 63, 64, 88, 89 |
| EBV (HHV-4) | Yes | Yes | AIM2, IFI16 | Burkitt lymphoma (BL) tumors in mice | ↑IL-18 can lead to tumor regression | 65–71, 90–93 |
| Nasopharyngeal carcinoma (NPC) tumor growth in mice | ↑IL-1β inhibits tumor growth | |||||
| KHSV (HHV-8) | Yes | Yes | IFI16 | Kaposi’s sarcoma (KS) | ↑IL-1β can promote tumorigenesis | 72–76, 93, 94 |
| Multicentric Castleman’s Disease (MCD) | ↑IL-1β associated with MCD flares | |||||
| Primary effusion lymphoma (PEL) | IL-1β constitutively expressed by PEL cells | |||||
CONCLUSIONS
The available evidence indicates that the interplay between herpesviruses and inflammasome signaling is central to both the viral life cycle and the pathogenesis of herpesvirus-related diseases. However, it is also clear that both the mechanism of inflammasome activation and the impact of that activation on the host are unique to each herpesvirus and may be unique to the specific cell or tissue that is infected. Therefore, studies of one herpesvirus cannot be generalized to other members of the herpesvirus family and should be interpreted with caution regarding other models of infection for that same virus. Additional investigations into the impact of inflammasome activation on diseases caused by herpesviruses and how herpesviruses activate and regulate inflammasomes are critical. This is particularly true as inflammasome modulators make their way through clinical trials (98). Whether these therapeutics can be used to improve herpesvirus-related diseases or whether they may exacerbate these pathologies is an open question. Given the high prevalence of HHV infections, the scientific community should investigate the effects of inflammasome modulators on herpesvirus-associated disease prior to their wide-spread use.
ACKNOWLEDGMENTS
A.H.K. is supported by the National Institute of Allergy and Infectious Diseases (K08AI156021).
Jaime Friel Blanck, Welch Medical Library, designed the search strategies used in PubMed and Embase to identify the literature cited in this review. We thank Andrea L. Cox for her mentorship, guidance, and input on the manuscript.
A.K. wrote the original draft, A.K. and G.S. created the figures, and A.H.K. developed the concept, provided supervision, and revised the manuscript and figures. All authors contributed to editing of the final manuscript.
None of the authors have any relevant conflicts of interest to disclose.
Contributor Information
Andrew H. Karaba, Email: andrew.karaba@jhmi.edu.
Moriah L. Szpara, Pennsylvania State University
Vinayaka R. Prasad, Albert Einstein College of Medicine
REFERENCES
- 1.Evavold CL, Kagan JC. 2019. Inflammasomes: threat-assessment organelles of the innate immune system. Immunity 51:609–624. doi: 10.1016/j.immuni.2019.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shi J, Zhao Y, Wang K, Shi X, Wang Y, Huang H, Zhuang Y, Cai T, Wang F, Shao F. 2015. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature 526:660–665. doi: 10.1038/nature15514. [DOI] [PubMed] [Google Scholar]
- 3.Kayagaki N, Stowe IB, Lee BL, O'Rourke K, Anderson K, Warming S, Cuellar T, Haley B, Roose-Girma M, Phung QT, Liu PS, Lill JR, Li H, Wu J, Kummerfeld S, Zhang J, Lee WP, Snipas SJ, Salvesen GS, Morris LX, Fitzgerald L, Zhang Y, Bertram EM, Goodnow CC, Dixit VM. 2015. Caspase-11 cleaves gasdermin D for non-canonical inflammasome signaling. Nature 526:666–671. doi: 10.1038/nature15541. [DOI] [PubMed] [Google Scholar]
- 4.Kayagaki N, Dixit VM. 2019. Rescue from a fiery death: a therapeutic endeavor. Science 366:688–689. doi: 10.1126/science.aaw1177. [DOI] [PubMed] [Google Scholar]
- 5.Mantovani A, Dinarello CA, Molgora M, Garlanda C. 2019. Interleukin-1 and related cytokines in the regulation of inflammation and immunity. Immunity 50:778–795. doi: 10.1016/j.immuni.2019.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garlanda C, Dinarello CA, Mantovani A. 2013. The interleukin-1 family: back to the future. Immunity 39:1003–1018. doi: 10.1016/j.immuni.2013.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yasuda K, Nakanishi K, Tsutsui H. 2019. Interleukin-18 in health and disease. Int J Mol Sci 20:649. doi: 10.3390/ijms20030649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaplanski G. 2018. Interleukin-18: biological properties and role in disease pathogenesis. Immunol Rev 281:138–153. doi: 10.1111/imr.12616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Broz P, Dixit VM. 2016. Inflammasomes: mechanism of assembly, regulation and signaling. Nat Rev Immunol 16:407–420. doi: 10.1038/nri.2016.58. [DOI] [PubMed] [Google Scholar]
- 10.Man SM, Kanneganti T-D. 2015. Regulation of inflammasome activation. Immunol Rev 265:6–21. doi: 10.1111/imr.12296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duncan JA, Canna SW. 2018. The NLRC4 Inflammasome. Immunol Rev 281:115–123. doi: 10.1111/imr.12607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen I-Y, Ichinohe T. 2015. Response of host inflammasomes to viral infection. Trends Microbiol 23:55–63. doi: 10.1016/j.tim.2014.09.007. [DOI] [PubMed] [Google Scholar]
- 13.Hornung V, Ablasser A, Charrel-Dennis M, Bauernfeind F, Horvath G, Caffrey DR, Latz E, Fitzgerald KA. 2009. AIM2 recognizes cytosolic dsDNA and forms a caspase-1-activating inflammasome with ASC. Nature 458:514–518. doi: 10.1038/nature07725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bauernfried S, Scherr MJ, Pichlmair A, Duderstadt KE, Hornung V. 2021. Human NLRP1 is a sensor for double-stranded RNA. Science 371:eabd0811. doi: 10.1126/science.abd0811. [DOI] [PubMed] [Google Scholar]
- 15.Karaba AH, Figueroa A, Massaccesi G, Botto S, DeFilippis VR, Cox AL. 2020. Herpes simplex virus type 1 inflammasome activation in proinflammatory human macrophages is dependent on NLRP3, ASC, and caspase-1. PLoS One 15:e0229570. doi: 10.1371/journal.pone.0229570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnson KE, Chikoti L, Chandran B. 2013. Herpes simplex virus 1 infection induces activation and subsequent inhibition of the IFI16 and NLRP3 inflammasomes. J Virol 87:5005–5018. doi: 10.1128/JVI.00082-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hayes CK, Wilcox DR, Yang Y, Coleman GK, Brown MA, Longnecker R. 2021. ASC-dependent inflammasomes contribute to immunopathology and mortality in herpes simplex encephalitis. PLoS Pathog 17:e1009285. doi: 10.1371/journal.ppat.1009285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Botto S, Abraham J, Mizuno N, Pryke K, Gall B, Landais I, Streblow DN, Fruh KJ, DeFilippis VR. 2019. Human cytomegalovirus immediate early 86-kDa protein blocks transcription and induces degradation of the immature interleukin-1β protein during virion-mediated activation of the AIM2 inflammasome. mBio 10:e02510-18. doi: 10.1128/mBio.02510-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang Q, Liu R, Yu Q, Bi Y, Liu G. 2019. Metabolic regulation of inflammasomes in inflammation. Immunology 157:95–109. doi: 10.1111/imm.13056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Swanson KV, Junkins RD, Kurkjian CJ, Holley-Guthrie E, Pendse AA, El Morabiti R, Petrucelli A, Barber GN, Benedict CA, Ting JPY. 2017. A noncanonical function of cGAMP in inflammasome priming and activation. J Experimental Medicine 214:3611–3626. doi: 10.1084/jem.20171749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Swanson KV, Deng M, Ting JP-Y. 2019. The NLRP3 inflammasome: molecular activation and regulation to therapeutics. Nat Rev Immunol 19:477–489. doi: 10.1038/s41577-019-0165-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.He Y, Hara H, Nuñez G. 2016. Mechanism and regulation of NLRP3 inflammasome activation. Trends Biochem Sci 41:1012–1021. doi: 10.1016/j.tibs.2016.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lieberman J, Wu H, Kagan JC. 2019. Gasdermin D activity in inflammation and host defense. Sci Immunol 4:eaav1447. doi: 10.1126/sciimmunol.aav1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fields BN, Knipe DM, Howley PM. 2013. Fields virology. Wolters Kluwer Health/Lippincott Williams & Wilkins, Philadelphia, PA. [Google Scholar]
- 25.Looker KJ, Johnston C, Welton NJ, James C, Vickerman P, Turner KME, Boily M-C, Gottlieb SL. 2020. The global and regional burden of genital ulcer disease due to herpes simplex virus: a natural history modeling study. BMJ Glob Health 5:e001875. doi: 10.1136/bmjgh-2019-001875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McQuillan G, Paulose-Ram R. 2018. Prevalence of herpes simplex virus type 1 and type 2 in persons aged 14–49: United States, 2015–2016. NCHS Data Brief 2018:1–8. [PubMed] [Google Scholar]
- 27.Corey L, Schiffer JT. 2010. Herpes simplex virus, p. 1943–1962. In Mandell GL, Bennett JE (ed), Mandell, Douglas, and Bennett’s principles and practice of infectious diseases. Churchill Livingston, Philadelphia, PA. [Google Scholar]
- 28.Koelle DM, Corey L. 2008. Herpes simplex: insights on pathogenesis and possible vaccines. Annu Rev Med 59:381–395. doi: 10.1146/annurev.med.59.061606.095540. [DOI] [PubMed] [Google Scholar]
- 29.Bollaerts K, Riera-Montes M, Heininger U, Hens N, Souverain A, Verstraeten T, Hartwig S. 2017. A systematic review of varicella seroprevalence in European countries before universal childhood immunization: deriving incidence from seroprevalence data. Epidemiol Infect 145:2666–2677. doi: 10.1017/S0950268817001546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bennett JE, Dolin R, Blaser MJ. 2020. Mandell, Douglas, and Bennett’s principles and practice of infectious diseases, 9th ed. Elsevier, Philadelphia, PA. [Google Scholar]
- 31.Fulkerson HL, Nogalski MT, Collins-McMillen D, Yurochko AD. 2021. Overview of human cytomegalovirus pathogenesis. Methods Mol Biol 2244:1–18. doi: 10.1007/978-1-0716-1111-1_1. [DOI] [PubMed] [Google Scholar]
- 32.Griffiths P, Reeves M. 2021. Pathogenesis of human cytomegalovirus in the immunocompromised host. Nat Rev Microbiol 19:759–773. doi: 10.1038/s41579-021-00582-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Griffiths P, Baraniak I, Reeves M. 2015. The pathogenesis of human cytomegalovirus. J Pathol 235:288–297. doi: 10.1002/path.4437. [DOI] [PubMed] [Google Scholar]
- 34.Boeckh M, Geballe AP. 2011. Cytomegalovirus: pathogen, paradigm, and puzzle. J Clin Invest 121:1673–1680. doi: 10.1172/JCI45449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Muller WJ, Jones CA, Koelle DM. 2010. Immunobiology of herpes simplex virus and cytomegalovirus infections of the fetus and newborn. Curr Immunol Rev 6:38–55. doi: 10.2174/157339510790231833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thorley-Lawson DA, Gross A. 2004. Persistence of the Epstein-Barr virus and the origins of associated lymphomas. N Engl J Med 350:1328–1337. doi: 10.1056/NEJMra032015. [DOI] [PubMed] [Google Scholar]
- 37.de Sanjose S, Mbisa G, Perez-Alvarez S, Benavente Y, Sukvirach S, Hieu NT, Shin H-R, Anh PTH, Thomas J, Lazcano E, Matos E, Herrero R, Muñoz N, Molano M, Franceschi S, Whitby D. 2009. Geographic variation in the prevalence of Kaposi sarcoma-associated herpesvirus and risk factors for transmission. J Infect Dis 199:1449–1456. doi: 10.1086/598523. [DOI] [PubMed] [Google Scholar]
- 38.Kawaguchi Y, Mori Y, Kimura H. SpringerLink . 2018. Human herpesviruses, 2018 ed. Springer Singapore, Singapore. [Google Scholar]
- 39.Allen IC, Scull MA, Moore CB, Holl EK, McElvania-TeKippe E, Taxman DJ, Guthrie EH, Pickles RJ, Ting JP-Y. 2009. The NLRP3 inflammasome mediates in vivo innate immunity to influenza A virus through recognition of viral RNA. Immunity 30:556–565. doi: 10.1016/j.immuni.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chattergoon MA, Levine JS, Latanich R, Osburn WO, Thomas DL, Cox AL. 2011. High plasma interleukin-18 levels mark the acute phase of hepatitis C virus infection. J Infect Dis 204:1730–1740. doi: 10.1093/infdis/jir642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Negash AA, Olson RM, Griffin S, Gale M. 2019. Modulation of calcium signaling pathway by hepatitis C virus core protein stimulates NLRP3 inflammasome activation. PLoS Pathog 15:e1007593-24. doi: 10.1371/journal.ppat.1007593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chattergoon MA, Latanich R, Quinn J, Winter ME, Buckheit RW, Blankson JN, Pardoll D, Cox AL. 2014. HIV and HCV activate the inflammasome in monocytes and macrophages via endosomal Toll-like receptors without induction of type 1 interferon. PLoS Pathog 10:e1004082-12. doi: 10.1371/journal.ppat.1004082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sergerie Y, Rivest S, Boivin G. 2007. Tumor necrosis factor-α and interleukin‐1β play a critical role in the resistance against lethal herpes simplex virus encephalitis. J Infect Dis 196:853–860. doi: 10.1086/520094. [DOI] [PubMed] [Google Scholar]
- 44.Lucinda N, Figueiredo MM, Pessoa NL, Santos BSÁdS, Lima GK, Freitas AM, Machado AMV, Kroon EG, Antonelli LRdV, Campos MA. 2017. Dendritic cells, macrophages, NK and CD8+ T lymphocytes play pivotal roles in controlling HSV-1 in the trigeminal ganglia by producing IL1-beta, iNOS and granzyme B. Virol J 14:37. doi: 10.1186/s12985-017-0692-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Barr DP, Belz GT, Reading PC, Wojtasiak M, Whitney PG, Heath WR, Carbone FR, Brooks AG. 2007. A role for plasmacytoid dendritic cells in the rapid IL-18-dependent activation of NK cells following HSV-1 infection. Eur J Immunol 37:1334–1342. doi: 10.1002/eji.200636362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Reading PC, Whitney PG, Barr DP, Wojtasiak M, Mintern JD, Waithman J, Brooks AG. 2007. IL-18, but not IL-12, regulates NK cell activity following intranasal herpes simplex virus type 1 infection. J Immunol 179:3214–3221. doi: 10.4049/jimmunol.179.5.3214. [DOI] [PubMed] [Google Scholar]
- 47.Fujioka N, Akazawa R, Ohashi K, Fujii M, Ikeda M, Kurimoto M. 1999. Interleukin-18 protects mice against acute herpes simplex virus type 1 infection. J Virol 73:2401–2409. doi: 10.1128/JVI.73.3.2401-2409.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Harandi AM, Svennerholm B, Holmgren J, Eriksson K. 2001. Interleukin-12 (IL-12) and IL-18 are important in innate defense against genital herpes simplex virus type 2 infection in mice but are not required for the development of acquired gamma interferon-mediated protective immunity. J Virol 75:6705–6709. doi: 10.1128/JVI.75.14.6705-6709.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee AJ, Chen B, Chew MV, Barra NG, Shenouda MM, Nham T, van Rooijen N, Jordana M, Mossman KL, Schreiber RD, Mack M, Ashkar AA. 2017. Inflammatory monocytes require type I interferon receptor signaling to activate NK cells via IL-18 during a mucosal viral infection. J Exp Med 214:1153–1167. doi: 10.1084/jem.20160880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Varanasi SK, Rajasagi NK, Jaggi U, Rouse BT. 2018. Role of IL-18 induced amphiregulin expression on virus induced ocular lesions. Mucosal Immunol 11:1705–1711. doi: 10.1038/s41385-018-0058-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Giménez F, Bhela S, Dogra P, Harvey L, Varanasi SK, Jaggi U, Rouse BT. 2016. The inflammasome NLRP3 plays a protective role against a viral immunopathological lesion. J Leukoc Biol 99:647–657. doi: 10.1189/jlb.3HI0715-321R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cuddy SR, Schinlever AR, Dochnal S, Seegren PV, Suzich J, Kundu P, Downs TK, Farah M, Desai BN, Boutell C, Cliffe AR. 2020. Neuronal hyperexcitability is a DLK-dependent trigger of herpes simplex virus reactivation that can be induced by IL-1. Elife 9:e58037. doi: 10.7554/eLife.58037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Coulon P-G, Dhanushkodi N, Prakash S, Srivastava R, Roy S, Alomari NI, Nguyen AM, Warsi WR, Ye C, Carlos-Cruz EA, Mai UT, Cruel AC, Ekmekciyan KM, Pearlman E, BenMohamed L. 2019. NLRP3, NLRP12, and IFI16 inflammasomes induction and caspase-1 activation triggered by virulent HSV-1 strains are associated with severe corneal inflammatory herpetic disease. Front Immunol 10:38–19. doi: 10.3389/fimmu.2019.01631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lebratti TJ, Lim YS, Cofie A, Andey PS, Jiang X, Scott JM, Fabbrizi MR, Ozanturk AN, Pham CTN, Clemens RA, Artyomov M, Dinauer MC, Shin H. 2021. A sustained type I IFN-neutrophil-IL-18 axis drives pathology during mucosal viral infection. Elife 10:e65762. doi: 10.7554/eLife.65762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Michael BD, Griffiths MJ, Granerod J, Brown D, Keir G, Wnęk G, Cox DJ, Vidyasagar R, Borrow R, Parkes LM, Solomon T. 2016. The interleukin-1 balance during encephalitis is associated with clinical severity, blood-brain barrier permeability, neuroimaging changes, and disease outcome. J Infect Dis 213:1651–1660. doi: 10.1093/infdis/jiv771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chan AH, Schroder K. 2019. Inflammasome signaling and regulation of interleukin-1 family cytokines. J Exp Med 217:e20190314. doi: 10.1084/jem.20190314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dinarello CA. 2018. Overview of the IL-1 family in innate inflammation and acquired immunity. Immunol Rev 281:8–27. doi: 10.1111/imr.12621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nour AM, Reichelt M, Ku C-C, Ho M-Y, Heineman TC, Arvin AM. 2011. Varicella-zoster virus infection triggers formation of an interleukin-1β (IL-1β)-processing inflammasome complex. J Biol Chem 286:17921–17933. doi: 10.1074/jbc.M110.210575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhu Y, Zhang S, Wu Y, Wang J. 2021. P2X7 receptor antagonist BBG inhibits endoplasmic reticulum stress and pyroptosis to alleviate postherpetic neuralgia. Mol Cell Biochem 476:3461–3468. doi: 10.1007/s11010-021-04169-3. [DOI] [PubMed] [Google Scholar]
- 60.van de Berg PJ, Heutinck KM, Raabe R, Minnee RC, Young SL, van Donselaar-van der Pant KA, Bemelman FJ, van Lier RA, ten Berge IJ. 2010. Human cytomegalovirus induces systemic immune activation characterized by a type 1 cytokine signature. J Infect Dis 202:690–699. doi: 10.1086/655472. [DOI] [PubMed] [Google Scholar]
- 61.Karaba AH, Figueroa A, Werbel WA, Dioverti MV, Steinke SM, Ray SC, Cox AL, Avery RK. 2021. Interleukin-18 and tumor necrosis factor-α are elevated in solid organ transplant recipients with possible cytomegalovirus end-organ disease. Transpl Infect Dis 23:e13682. doi: 10.1111/tid.13682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pérez-Flores I, Santiago JL, Fernández-Pérez C, Urcelay E, Moreno de la Higuera MÁ, Romero NC, Cubillo BR, Sánchez-Fructuoso AI. 2019. Impacts of interleukin-18 polymorphisms on the incidence of delayed-onset cytomegalovirus infection in a cohort of kidney transplant recipients. Open Forum Infect Dis 6:ofz325. doi: 10.1093/ofid/ofz325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Epstein LG, Shinnar S, Hesdorffer DC, Nordli DR, Hamidullah A, Benn EKT, Pellock JM, Frank LM, Lewis DV, Moshe SL, Shinnar RC, Sun S, the FEBSTAT study team . 2012. Human herpesvirus 6 and 7 in febrile status epilepticus: the FEBSTAT study. Epilepsia 53:1481–1488. doi: 10.1111/j.1528-1167.2012.03542.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bartolini L, Piras E, Sullivan K, Gillen S, Bumbut A, Lin C-TM, Leibovitch EC, Graves JS, Waubant EL, Chamberlain JM, Gaillard WD, Jacobson S. 2018. Detection of HHV-6 and EBV and cytokine levels in saliva from children with seizures: results of a multi-center cross-sectional study. Front Neurol 9:834. doi: 10.3389/fneur.2018.00834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Setsuda J, Teruya-Feldstein J, Harris NL, Ferry JA, Sorbara L, Gupta G, Jaffe ES, Tosato G. 1999. Interleukin-18, interferon-γ, IP-10, and Mig expression in Epstein-Barr virus-induced infectious mononucleosis and posttransplant lymphoproliferative disease. Am J Pathol 155:257–265. doi: 10.1016/s0002-9440(10)65119-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.van de Veerdonk FL, Wever PC, Hermans MHA, Fijnheer R, Joosten LAB, van der Meer JWM, Netea MG, Schneeberger PM. 2012. IL-18 serum concentration is markedly elevated in acute EBV infection and can serve as a marker for disease severity. J Infect Dis 206:197–201. doi: 10.1093/infdis/jis335. [DOI] [PubMed] [Google Scholar]
- 67.Foss H-D, Herbst H, Hummel M, Araujo I, Latza U, Rancsò C, Dallenbach F, Stein H. 1994. Patterns of Cytokine Gene Expression in Infectious Mononucleosis. Blood 83:707–712. doi: 10.1182/blood.V83.3.707.707. [DOI] [PubMed] [Google Scholar]
- 68.Yao L, Setsuda J, Sgadari C, Cherney B, Tosato G. 2001. Interleukin-18 expression induced by Epstein-Barr virus-infected cells. J Leukoc Biol 69:779–784. [PubMed] [Google Scholar]
- 69.Pagès F, Galon J, Karaschuk G, Dudziak D, Camus M, Lazar V, Camilleri-Broët S, Lagorce-Pagès C, Lebel-Binay S, Laux G, Fridman W-H, Henglein B. 2005. Epstein-Barr virus nuclear antigen 2 induces interleukin-18 receptor expression in B cells. Blood 105:1632–1639. doi: 10.1182/blood-2004-08-3196. [DOI] [PubMed] [Google Scholar]
- 70.Chen L-C, Wang L-J, Tsang N-M, Ojcius DM, Chen C-C, OuYang C-N, Hsueh C, Liang Y, Chang K-P, Chen C-C, Chang Y-S. 2012. Tumor inflammasome-derived IL-1β recruits neutrophils and improves local recurrence-free survival in EBV-induced nasopharyngeal carcinoma. EMBO Mol Med 4:1276–1293. doi: 10.1002/emmm.201201569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Burton EM, Goldbach-Mansky R, Bhaduri-McIntosh S. 2020. A promiscuous inflammasome sparks replication of a common tumor virus. Proc Natl Acad Sci USA 117:1722–1730. doi: 10.1073/pnas.1919133117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jones KD, Aoki Y, Chang Y, Moore PS, Yarchoan R, Tosato G. 1999. Involvement of interleukin-10 (IL-10) and viral IL-6 in the spontaneous growth of Kaposi’s sarcoma herpesvirus-associated infected primary effusion lymphoma cells. Blood 94:2871–2879. doi: 10.1182/blood.V94.8.2871.420k25_2871_2879. [DOI] [PubMed] [Google Scholar]
- 73.Simonart T, van Vooren J-P. 2002. Interleukin-1β increases the BCL-2/BAX ratio in Kaposi’s sarcoma cells. Cytokine 19:259–266. doi: 10.1006/cyto.2002.1964. [DOI] [PubMed] [Google Scholar]
- 74.Samaniego F, Markham PD, Gendelman R, Gallo RC, Ensoli B. 1997. Inflammatory cytokines induce endothelial cells to produce and release basic fibroblast growth factor and to promote Kaposi’s sarcoma-like lesions in nude mice. J Immunol 158:1887–1894. [PubMed] [Google Scholar]
- 75.Polizzotto MN, Uldrick TS, Wang V, Aleman K, Wyvill KM, Marshall V, Pittaluga S, O'Mahony D, Whitby D, Tosato G, Steinberg SM, Little RF, Yarchoan R. 2013. Human and viral interleukin-6 and other cytokines in Kaposi sarcoma herpesvirus-associated multicentric Castleman disease. Blood 122:4189–4198. doi: 10.1182/blood-2013-08-519959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Singh VV, Kerur N, Bottero V, Dutta S, Chakraborty S, Ansari MA, Paudel N, Chikoti L, Chandran B. 2013. Kaposi’s sarcoma-associated herpesvirus latency in endothelial and B Cells activates gamma interferon-inducible protein 16-mediated inflammasomes. J Virol 87:4417–4431. doi: 10.1128/JVI.03282-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ramaswami R, Lage S, Lurain K, Rocco J, Manion M, Yarchoan R, Sereti I. 2021. Inflammasome activation in patients with KSHV-associated disorders. In Brain connections, malignancies, and tumor viruses. Conference on Retroviruses and Opportunistic Infections (CROI). Virtual. [Google Scholar]
- 78.Rathinam VAK, Jiang Z, Waggoner SN, Sharma S, Cole LE, Waggoner L, Vanaja SK, Monks BG, Ganesan S, Latz E, Hornung V, Vogel SN, Szomolanyi-Tsuda E, Fitzgerald KA. 2010. The AIM2 inflammasome is essential for host defense against cytosolic bacteria and DNA viruses. Nat Immunol 11:395–402. doi: 10.1038/ni.1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Maruzuru Y, Ichinohe T, Sato R, Miyake K, Okano T, Suzuki T, Koshiba T, Koyanagi N, Tsuda S, Watanabe M, Arii J, Kato A, Kawaguchi Y. 2018. Herpes simplex virus 1 VP22 inhibits AIM2-dependent inflammasome activation to enable efficient viral replication. Cell Host Microbe 23:254–265.e7. doi: 10.1016/j.chom.2017.12.014. [DOI] [PubMed] [Google Scholar]
- 80.Strittmatter GE, Sand J, Sauter M, Seyffert M, Steigerwald R, Fraefel C, Smola S, French LE, Beer H-D. 2016. IFN-γ primes keratinocytes for HSV-1-induced inflammasome activation. J Invest Dermatol 136:610–620. doi: 10.1016/j.jid.2015.12.022. [DOI] [PubMed] [Google Scholar]
- 81.Wang W, Hu D, Wu C, Feng Y, Li A, Liu W, Wang Y, Chen K, Tian M, Xiao F, Zhang Q, Shereen MA, Chen W, Pan P, Wan P, Wu K, Wu J. 2020. STING promotes NLRP3 localization in ER and facilitates NLRP3 deubiquitination to activate the inflammasome upon HSV-1 infection. PLoS Pathog 16:e1008335. doi: 10.1371/journal.ppat.1008335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lee S, Karki R, Wang Y, Nguyen LN, Kalathur RC, Kanneganti T-D. 2021. AIM2 forms a complex with pyrin and ZBP1 to drive PANoptosis and host defence. Nature 597:415–419. doi: 10.1038/s41586-021-03875-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Dengler TJ, Raftery MJ, Werle M, Zimmermann R, Schönrich G. 2000. Cytomegalovirus infection of vascular cells induces expression of pro-inflammatory adhesion molecules by paracrine action of secreted interleukin-1beta. Transplantation 69:1160–1168. doi: 10.1097/00007890-200003270-00022. [DOI] [PubMed] [Google Scholar]
- 84.Bayer C, Varani S, Wang L, Walther P, Zhou S, Straschewski S, Bachem M, Söderberg-Naucler C, Mertens T, Frascaroli G. 2013. Human cytomegalovirus infection of M1 and M2 macrophages triggers inflammation and autologous T-Cell proliferation. J Virol 87:67–79. doi: 10.1128/JVI.01585-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Moses AV, Garnett HM. 1990. The effect of human cytomegalovirus on the production and biologic action of interleukin-l. J Infect Dis 162:381–388. doi: 10.1093/infdis/162.2.381. [DOI] [PubMed] [Google Scholar]
- 86.Huang Y, Liu L, Ma D, Liao Y, Lu Y, Huang H, Qin W, Liu X, Fang F. 2017. Human cytomegalovirus triggers the assembly of AIM2 inflammasome in THP-1-derived macrophages. J Med Virol 89:2188–2195. doi: 10.1002/jmv.24846. [DOI] [PubMed] [Google Scholar]
- 87.Iwata M, Vieira J, Byrne M, Horton H, Torok-Storb B. 1999. Interleukin-1 (IL-1) inhibits growth of cytomegalovirus in human marrow stromal cells: inhibition Is Reversed Upon removal of IL-1. Blood 94:572–578. doi: 10.1182/blood.V94.2.572.414k18_572_578. [DOI] [PubMed] [Google Scholar]
- 88.Flamand L, Gosselin J, D'Addario M, Hiscott J, Ablashi DV, Gallo RC, Menezes J. 1991. Human herpesvirus 6 induces interleukin-1 beta and tumor necrosis factor alpha, but not interleukin-6, in peripheral blood mononuclear cell cultures. J Virol 65:5105–5110. doi: 10.1128/JVI.65.9.5105-5110.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mayne M, Cheadle C, Soldan SS, Cermelli C, Yamano Y, Akhyani N, Nagel JE, Taub DD, Becker KG, Jacobson S. 2001. Gene expression profile of herpesvirus-infected T cells obtained using immunomicroarrays: induction of proinflammatory mechanisms. J Virol 75:11641–11650. doi: 10.1128/JVI.75.23.11641-11650.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Torii Y, Kawada J, Murata T, Yoshiyama H, Kimura H, Ito Y. 2017. Epstein-Barr virus infection-induced inflammasome activation in human monocytes. PLoS One 12:e0175053. doi: 10.1371/journal.pone.0175053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ansari MA, Singh VV, Dutta S, Veettil MV, Dutta D, Chikoti L, Lu J, Everly D, Chandran B. 2013. Constitutive interferon-inducible protein 16-inflammasome activation during Epstein-Barr virus latency I, II, and III in B and epithelial cells. J Virol 87:8606–8623. doi: 10.1128/JVI.00805-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ansari MA, Dutta S, Veettil MV, Dutta D, Iqbal J, Kumar B, Roy A, Chikoti L, Singh VV, Chandran B. 2015. Herpesvirus genome recognition induced acetylation of nuclear IFI16 is essential for its cytoplasmic translocation, inflammasome and IFN-β responses. PLoS Pathog 11:e1005019-40. doi: 10.1371/journal.ppat.1005019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Dutta D, Dutta S, Veettil MV, Roy A, Ansari MA, Iqbal J, Chikoti L, Kumar B, Johnson KE, Chandran B. 2015. BRCA1 regulates IFI16 mediated nuclear innate sensing of herpes viral DNA and subsequent induction of the innate inflammasome and interferon-β responses. PLoS Pathog 11:e1005030. doi: 10.1371/journal.ppat.1005030. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 94.Kerur N, Veettil MV, Sharma-Walia N, Bottero V, Sadagopan S, Otageri P, Chandran B. 2011. IFI16 acts as a nuclear pathogen sensor to induce the inflammasome in response to Kaposi sarcoma-associated herpesvirus infection. Cell Host Microbe 9:363–375. doi: 10.1016/j.chom.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gregory SM, Davis BK, West JA, Taxman DJ, Matsuzawa S, Reed JC, Ting JPY, Damania B. 2011. Discovery of a viral NLR homolog that inhibits the inflammasome. Science 331:330–334. doi: 10.1126/science.1199478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Rossetto CC, Pari GS. 2011. Kaposi’s sarcoma-associated herpesvirus noncoding polyadenylated nuclear RNA interacts with virus- and host cell-encoded proteins and suppresses expression of genes involved in immune modulation. J Virol 85:13290–13297. doi: 10.1128/JVI.05886-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Davis DA, Naiman NE, Wang V, Shrestha P, Haque M, Hu D, Anagho HA, Carey RF, Davidoff KS, Yarchoan R. 2015. Identification of caspase cleavage sites in KSHV latency-associated nuclear antigen and their effects on caspase-related host defense responses. PLoS Pathog 11:e1005064. doi: 10.1371/journal.ppat.1005064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gorman R. 2021. Pharma looks to inflammasome inhibitors as all-around therapies. The Scientist Magazine. LabX Media Group, Wilmington, DE. [Google Scholar]