Summary
Anthocyanins play a variety of adaptive roles in both vegetative tissues and reproductive organs of plants. The broad functionality of these compounds requires sophisticated regulation of the anthocyanin biosynthesis pathway to allow proper localization, timing, and optimal intensity of pigment deposition. While it is well-established that the committed steps of anthocyanin biosynthesis are activated by a highly conserved MYB-bHLH-WDR (MBW) protein complex in virtually all flowering plants, anthocyanin repression seems to be achieved by a wide variety of protein and small RNA families that function in different tissue types and in response to different developmental, environmental, and hormonal cues. In this review, we survey recent progress in the identification of anthocyanin repressors and the characterization of their molecular mechanisms. We find that these seemingly very different repression modules act through a remarkably similar logic, the so-called “double negative logic”. Much of the double-negative regulation of anthocyanin production involves signal-induced degradation or sequestration of the repressors from the MBW protein complex. We discuss the functional and evolutionary advantages of this logic design compared with simple or sequential positive regulation. These advantages provide a plausible explanation to why plants have evolved so many anthocyanin repressors.
Keywords: Anthocyanin biosynthesis, developmental cues, double-negative logic, environmental response, hormones, repressor
I. Introduction
Anthocyanins are chemical compounds best known for the red, blue and purple hues that they confer in plants, ranging from the vast diversity of floral colors and patterns, to the vibrancy of autumn foliage. These compounds are members of the flavonoid group of specialized metabolites, which potentially date back to the early land plants (Davies et al., 2020). The presence of anthocyanins in vegetative tissue has been suggested to protect photosynthetic machinery by absorbing excess light, enhance tolerance to drought, cold, high salinity and nutrient deficiency, and defend against herbivory and pathogens (reviewed by Chalker-Scott, 1999; Landi et al., 2015; Davies et al., 2018). Following the emergence of angiosperms, anthocyanins took on a critical role in flowers and fruits for the attraction of pollinators and seed dispersers. The broad functionality of these compounds is due in part to the evolution of sophisticated regulation of the anthocyanin biosynthetic pathway (hereafter referred to as ABP), which allows proper localization, timing, and optimal intensity of pigment deposition in various tissues.
Research on the regulation of anthocyanin biosynthesis led to the molecular cloning of the very first plant transcription factors in the 1980s, including Colorless1 (C1) (Paz-Ares et al., 1987) and Leaf color (Lc) in maize (Ludwig et al., 1989). C1 and Lc encode an R2R3-MYB and a basic helix-loop-helix (bHLH) transcription factor, respectively. Functional evidence of the R2R3-MYB (C1) and bHLH (a paralog of Lc) forming a protein complex to activate ABP genes was reported shortly thereafter (Goff et al., 1992). Subsequent studies in other plant model systems such as snapdragon, petunia, and Arabidopsis, uncovered homologous proteins activating anthocyanin biosynthesis (Goodrich et al., 1992; Quattrocchio et al., 1999; Borevitz et al., 2000; Payne et al., 2000; Spelt et al., 2000; Schwinn et al., 2006). Additionally, studies in these systems uncovered that a third regulatory component, a WD40 repeat (WDR) protein, is also required for the activation of anthocyanin production (de Vetten et al., 1997; Walker et al., 1999), although the precise biochemical function of the WDR protein is still unclear. This MYB-bHLH-WDR regulatory complex (i.e., the “MBW” complex) has since been shown to coordinately activate multiple genes of anthocyanin production (Fig. 1a) in all angiosperms characterized to date and has been addressed in numerous excellent reviews (for example, see Ramsay & Glover, 2005; Grotewold, 2006; Davies et al., 2012; Lloyd et al., 2017).
Figure 1.
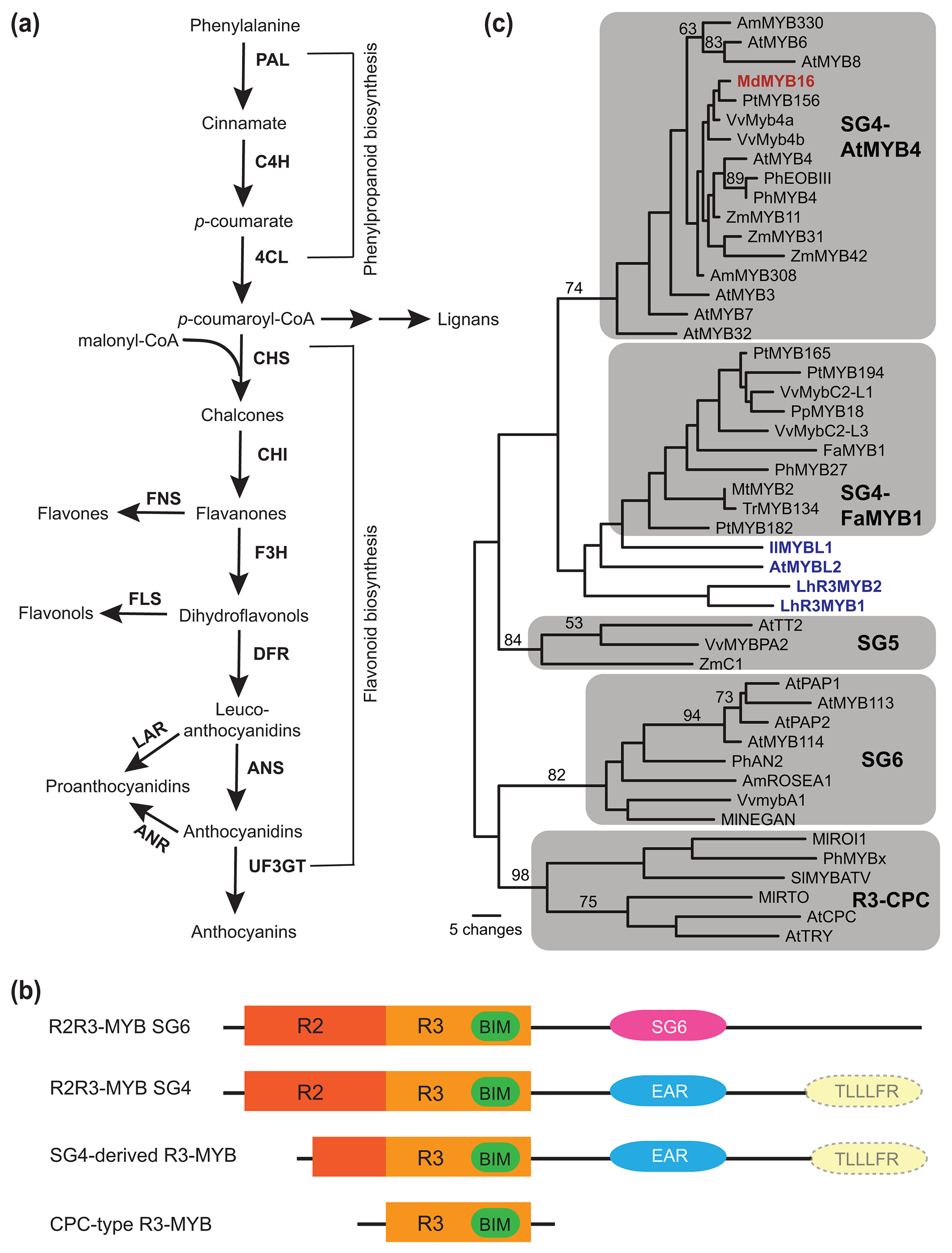
The phenylpropanoid pathway and their MYB repressors. (a) Schematic of the phenylpropanoid pathway leading to the production of anthocyanins and related compounds. Abbreviations: 4CL, 4-coumarate-CoA ligase; ANR, anthocyanidin reductase; ANS, anthocyanidin synthase; C4H, cinnamate 4-hydroxylase; CHI, chalcone isomerase; CHS, chalcone synthase; DFR, dihydroflavonol reductase; F3H, flavanone 3-hydroxylase; FLS, flavonol synthase; FNS, flavone synthase; LAR, leucoanthocyanidin reductase; PAL, phenylalanine ammonia-lyase; UF3GT, flavonoid 3-O-glucosyltransferase. (b) Schematic representation of motifs found in anthocyanin-regulating MYB proteins. Motifs are labeled with the following abbreviations: R2, R2 MYB DNA-binding domain; R3, R3 domain; BIM, bHLH-interacting motif; SG6, subgroup 6-specific motif; EAR, EAR-repression motif; TLLLFR, TLLLFR repression motif. The TLLLFR motif is muted and surrounded by a dashed line to indicate that it is only present in some members. (c) A phylogeny of subgroup 4, 5, 6 R2R3-MYBs and anthocyanin-repressing R3-MYBs, based on the MYB DNA binding domains. The tree was generated using the maximum parsimony method, as implemented in PAUP 4.0 (https://paup.phylosolutions.com), with 200 bootstrap replicates. Bootstrap values greater than 50 are shown along the branches. SG5 and SG6 proteins are activators, whereas the remaining are repressors. MdMYB16 (highlighted in red) clusters with the AtMYB4-type repressors, but it represses anthocyanin biosynthesis. The SG4-derived R3-MYBs are highlighted in blue.
In contrast to this highly conserved core activation complex, a fairly large number of anthocyanin repressors have been identified, mostly in the last decade. These repressors represent a wide variety of protein or small RNA families (Table 1), and the majority of them exert their repressive function in anthocyanin biosynthesis by either repressing the expression of the MBW complex components at the transcriptional level or destabilizing the MBW complex through protein-protein interactions (Figs. 2–4). This raises the question as to why plants have evolved so many repressors acting upon this core activation complex. In this review we will first describe the advances in characterizing the molecular mechanisms of these ABP repressors, which are organized into three broad categories, depending on whether they are primarily regulated by the developmental program, environmental cues, or plant hormones. It should be noted that these divisions do not always have clear boundaries and there could be extensive crosstalk among the three classes of signals (Das et al., 2012a). We will then briefly discuss why plants have evolved this many anthocyanin repressors in light of the logic of gene regulation (Davidson and Levine, 2008; Carroll, 2016), and end the review by defining a set of unanswered questions on anthocyanin repression for future research.
Table 1.
Summary of the 14 different types of anthocyanin repressors or repression modules discussed in this review. Note that this table is meant to provide a quick overview of the repressor diversity, not a comprehensive list of known examples.
| Repressor (module) | Exemplar species | Representative Genes | References |
|---|---|---|---|
| S4 R2R3-MYB | Arabidopsis, strawberry (Fragaria), Petunia, grape (Vitis), poplar (Populus), apple (Malus) | AtMYB4, FaMYB1, PhMYB27, VvMYB2-L1, PtMYB182, MdMYB16 | Jin et al., 2000; Aharoni et al., 2001; Albert et al., 2014; Cavallini et al., 2015; Yoshida et al., 2015; Xu et al., 2017 |
| S4-derived R3-MYB | Arabidopsis, Iochroma, lily (Lilium) | AtMYBL2, IlMYBL1, LhR3MYB1/2 | Dubos et al., 2008; Matsui et al., 2008; Gates et al., 2018; Sakai et al., 2019 |
| CPC-type R3-MYB | Arabidopsis, Petunia, Mimulus, tomato (Solanum), Freesia, grape hyacinth (Muscari) | AtCPC, PhMYBx, MlROI1, MlRTO, SlMYBATV, FhMYBx, MaMYBx | Zhu et al., 2009; Yuan et al., 2013; Albert et al., 2014; Cao et al., 2017; Colandero et al., 2018; Ding et al., 2020; Li et al., 2020; Zhang et al., 2020 |
| SPL | Arabidopsis | AtSPL9 | Gou et al., 2011 |
| miRNA | Arabidopsis, tomato (Solanum) | miR828, miR858 | Rajagopalan et al., 2006; Luo et al., 2012; Jia et al., 2015 |
| HD-ZIP | Arabidopsis, apple (Malus) | AtGLABRA2, MdHB1, AtHAT1 | Wang et al., 2015; Jiang et al., 2017; Zheng et al., 2018 |
| COP1-HY5 | Arabidopsis, eggplant and tomato (Solanum), apple (Malus), pear (Pyrus) | AtCOP1-AtHY5, SmCOP1-SmHY5, SlHY5, MdHY5, MdCOP1, PpCOP1-PpHY5 | Catalá et al., 2011; Li et al., 2012; Maier et al., 2013; Shin et al., 2013; Wang et al., 2016; Jiang et al., 2016; Kim et al., 2017; Park et al., 2017; An et al., 2017; Liu et al., 2018; Tao et al., 2018 |
| LBD | Arabidopsis, grapes (Vitis),apple (Malus) | AtLBD37/38/39, VvLBD39, MdLBD13, MdLOB52 | Rubin et al., 2009; Soubeyrand et al., 2014; Li et al., 2017; Wang, Y et al., 2018b |
| NAC | Arabidopsis, Brassica | AtJUB1, BoNAC019 | Wu et al., 2012; Wang, J et al., 2018 |
| TCP | Arabidopsis | AtTCP15 | Viola et al., 2016 |
| SCFCOI1-JAZ | Arabidopsis, apple (Malus) | AtJAZ1, MdJAZ2 | Shan et al., 2009; Qi et al., 2011; An et al., 2014 |
| SCFTIR1-Aux/IAA-ARF | apple (Malus) | MdARF13 | Wang, Y et al., 2018a |
| D14-SCFD3-D53 | Arabidopsis | AtSMXL6 | Wang et al., 2020 |
| GID1-SCFGID2-DELLA | Arabidopsis | DELLA | Jiang et al., 2007; Li et al., 2014; Qi et al., 2014; Xie et al., 2016 |
Figure 2.
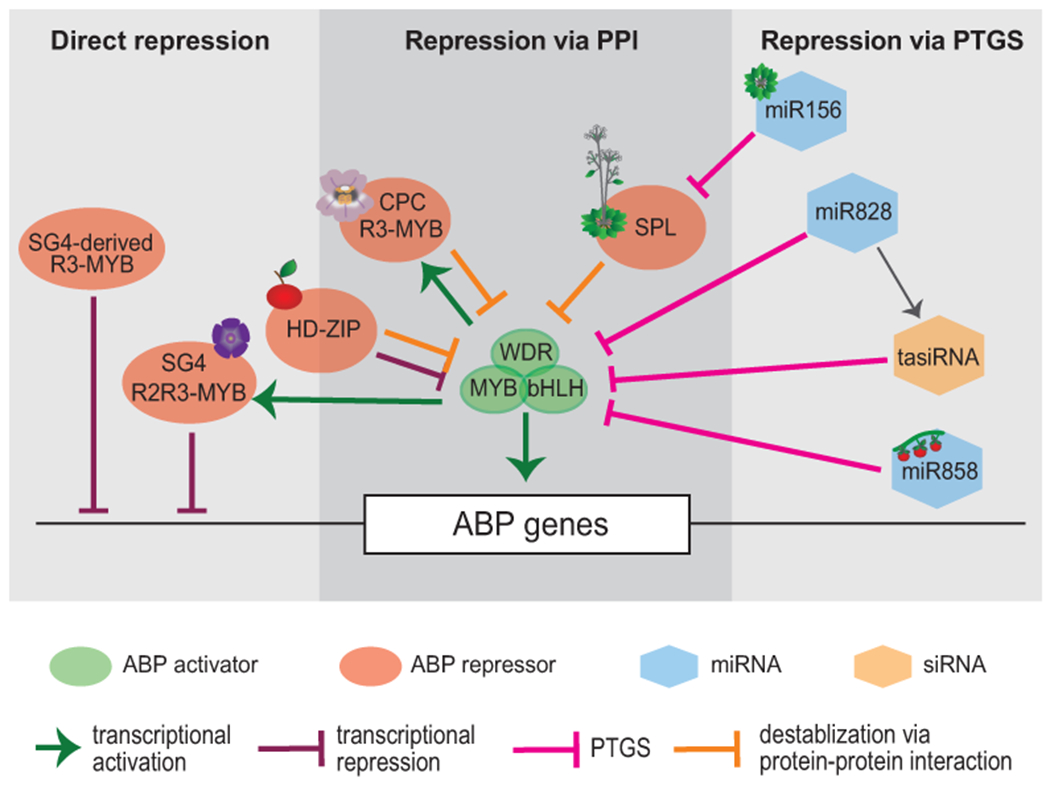
Schematic representation of the interactions between developmental program induced repressors, the MBW activation complex, and ABP genes. The modes of repression are categorized as being direct, via protein-protein interaction (PPI), or via post-transcriptional gene silencing (PTGS). Icons indicate an example system in which those repressors are found, as follows: SG4 R2R3-MYB, petunia; HD-ZIP, apple; CPC R3-MYB, monkeyflower; miR156, juvenile Arabidopsis; SPL, adult Arabidopsis; miR858, tomato.
Figure 4.

Hormone signaling pathways associated with regulation of the MBW activation complex and ABP genes, shown in the absence (top row) and presence (bottom row) of the hormone signal. Red background associated with ABP genes indicates that their transcription has been repressed, while green background is indicative of transcriptional activation. Dashed line around an MBW component indicates that this component is either sequestered by the repressor protein through protein-protein interactions, or transcriptionally repressed in the case of D53, and is thus unavailable for MBW complex formation. Note that gibberellins have mechanisms to both repress (left side of the vertical dashed line) and activate anthocyanin biosynthesis (right side of the vertical dashed line).
II. Developmental cues
1. Subgroup 4 R2R3-MYBs: the active repressors
The R2R3-MYB proteins are particularly common in flowering plants due to a major expansion during early angiosperm evolution and subsequent lineage-specific gene/genome duplication events (Feller et al., 2011). They are often expressed in specific tissues and regulate a variety of plant-specific processes, including specialized metabolism, cellular and organ morphogenesis, and response to environmental stress (reviewed in Dubos et al., 2010; Chezem & Clay, 2016). R2R3-MYBs can be classified into subgroups based on phylogenetic relationships and short signature motifs that are conserved within and unique to each subgroup (Kranz et al., 1998; Dubos et al., 2010). The anthocyanin-activating R2R3-MYBs in most flowering plants belong to subgroup 6, defined by the signature motif “[R/K]PRPRx[F/L]” downstream of the two tandem repeats of the MYB DNA-binding domain (i.e., R2 and R3; Fig. 1b). One exception is the grass family (Poaceae), in which subgroup 6 R2R3-MYBs are absent. Instead, subgroup 5 R2R3-MYBs, which usually activate proanthocyanidin biosynthesis in most angiosperms (Xu et al., 2015), are recruited to activate ABP genes in grasses (e.g., C1 in maize; Paz-Ares et al., 1987). These MYB proteins all have a bHLH-interacting motif in the R3 MYB domain, “[D/E]Lx2[R/K]x3Lx6Lx3R”, enabling physical interactions with the bHLH partners and formation of the MBW complex (Grotewold et al., 2000; Zimmermann et al., 2004; Fig. 1b).
The first R2R3-MYB proteins that repress anthocyanin and/or phenylpropanoid biosynthesis were reported about two decades ago (Tamagnone et al., 1998; Jin et al., 2000; Aharoni et al., 2001). These repressors belong to subgroup 4 and all contain the ethylene-responsive element binding factor-associated amphiphilic repression (EAR) motif (“pdLNL[D/E]L” or “DLNxxP”) in the C-terminal domain (Fig. 1b) (Kranz et al., 1998; Dubos et al., 2010; Chezem & Clay, 2016). In the past decade, numerous subgroup 4 R2R3-MYB repressors have been characterized in a wide range of plant species (reviewed in Chen et al., 2019; Ma & Constabel, 2019). Based on phylogenetic relationships (Fig. 1c), these subgroup 4 MYBs can be further classified into the “FaMYB1-type”, which regulate the flavonoid pathway including committed steps of anthocyanin biosynthesis (see “flavonoid biosynthesis pathway” in Fig. 1a), and the “AtMYB4-type”, which regulate the general phenylpropanoid pathway leading to the production of phenolic acids and lignans (see “phenylpropanoid biosynthesis pathway” in Fig. 1a) (Jun et al., 2015; Yoshida et al., 2015; Chen et al., 2019; Ma & Constabel, 2019). However, there are exceptions to this general trend. For example, the apple (Malus domestica) MdMYB16 falls into the “AtMYB4-type” clade phylogenetically, but it represses anthocyanin biosynthesis functionally (Xu et al., 2017).
Subgroup 4 R2R3-MYBs have been shown to repress the promoter activities of the ABP or general phenylpropanoid pathway genes in many studies (e.g., Jin et al., 2000; Albert et al., 2014; Cavallini et al., 2015; Zhou et al., 2019), but direct binding of these R2R3-MYB repressors to the structural gene promoters was only rarely demonstrated (Fornalé et al., 2010). Subgroup 4 R2R3-MYBs also contain the same bHLH-interacting motif in the R3 MYB domain as the subgroup 6 MYBs, and have frequently been shown to interact with the bHLH component of the MBW complex (Aharoni et al., 2001; Albert et al., 2014; Cavallini et al., 2015; Jun et al., 2015; Xu et al., 2017; Zhou et al., 2019; Huang et al., 2020). However, the biological relevance of this protein-protein interaction is unclear, as deleting the bHLH-interacting motif from MdMYB16 does not seem to affect its repression function, whereas removing the EAR motif completely inactivates the repressor (Xu et al., 2017). In fact, numerous studies where the EAR motif was mutated or deleted have shown the necessity of this motif for the repressive activity of subgroup 4 R2R3-MYBs (Tamagnone et al., 1998; Jin et al., 2000; Aharoni et al., 2001; Albert et al., 2014; Jun et al., 2015; Xu et al., 2017; Zhou et al., 2019), suggesting that these MYBs are active repressors, not passive repressors by merely titrating the bHLH factors from the MBW complex.
It is interesting to note that many of these subgroup 4 R2R3-MYB genes are activated by the MBW complex and show similar expression pattern to the ABP genes, that is, their expression increases as anthocyanin content increases (Albert et al., 2014; Jun et al., 2015; Zhou et al., 2019; Huang et al., 2020). This suggests that the role of these R2R3-MYB repressors is not so much in preventing anthocyanins from being synthesized at all as an “off-switch”, but more in modulating the absolute anthocyanin intensity by serving as a “break”. While the expression of subgroup 4 R2R3-MYB genes is commonly associated with developmental transitions (e.g., the onset of fruit ripening) (Aharoni et al., 2001; Cavallini et al., 2015; Zhou et al., 2019; Huang et al., 2020), it can also be affected by environmental cues in some cases. For example, exposure of Arabidopsis plants to UV-B light down-regulates AtMYB4, leading to de-repression of the cinnamate 4-hydroxylase gene and accumulation of photo-protective sinapate esters (Jin et al., 2000). Similarly, high light conditions repress the expression of PhMYB27 in petunia (Petunia hybrida), resulting in increased anthocyanin accumulation in vegetative tissues (Albert et al., 2014).
2. R3-MYBs derived from Subgroup 4 R2R3-MYBs: lineage-specific origins
AtMYBL2 is a key repressor of anthocyanin biosynthesis in Arabidopsis. AtMYBL2 is phylogenetically related to subgroup 4 R2R3-MYBs (Fig. 1c), contains a fully functional R3 MYB domain that interacts with the bHLH proteins, a degenerate EAR motif downstream of the R3 MYB domain, and a unique repression motif “TLLLFR” at the very end of the C-terminal domain (Fig. 1b) (Dubos et al., 2008; Matsui et al., 2008). However, the R2 MYB domain at the N-terminus is partially truncated, and therefore this protein has been referred to as an “R3-MYB”. Interestingly, some “FaMYB1-type” R2R3-MYBs were recently discovered not only containing the EAR motif, but also the “TLLLFR” motif at the C-terminus (Fig. 1b), including PtMYB182 in Populus (Yoshida et al., 2015) and VvMYBC2-L1 in Vitis vinifera (Cavallini et al., 2015). These observations suggest that AtMYBL2 evolved from a subgroup 4 R2R3-MYB with a “TLLLFR” C-terminus, through truncation at the N-terminus. Deleting the “TLLLFR” motif abolishes the repression function of AtMYBL2, indicating that it is an active repressor, in contrast to the typical CPC-type R3-MYBs that are passive anthocyanin repressors (discussed in the next section). However, the mechanism of “TLLLFR”-mediated repression is completely unknown. AtMYBL2 orthologs have so far only been confirmed in close relatives of Arabidopsis (Song et al., 2018) and are likely restricted to the family Brassicaceae.
Other R3-MYBs derived from subgroup 4 MYBs through N-terminal truncations have also been reported in the past few years, including IlMYBL1 from Iochroma loxense (Gates et al., 2018) and LhR3MYB1/2 from hybrid lilies (Lilium spp.; Sakai et al., 2019). These R3-MYBs contain an EAR motif but not the “TLLLFR” motif, indicating different origins than AtMYBL2, and they appear dispersed on phylogenetic trees in a species-specific fashion (Gates et al., 2018), indicating their independent, lineage-specific origins. Overexpression of IlMYBL1 and LhR3MYB1/2 in tobacco (Nicotiana tabacum) inhibits anthocyanin production, but whether this inhibition requires the EAR motif is yet to be clarified.
3. CPC-type R3-MYBs: fine-tuning pigmentation intensity or spatial patterns
Unlike the lineage-specific R3-MYBs that evolved from subgroup 4 R2R3-MYBs in the recent past, the CPC-type R3-MYBs have a much deeper origin. They form a well-supported, distinct clade (Fig. 1c), predating the divergence between monocots and eudicots (Gates et al., 2018; Li et al., 2020; Zhang et al., 2020). They are encoded by virtually all angiosperm genomes, including CPC and TRY in Arabidopsis (Wada et al., 1997; Schellmann et al., 2002; Zhu et al., 2009), ROI1 and RTO in Mimulus (Yuan et al., 2013; Ding et al., 2020), MYBx in Petunia (Albert et al., 2014), SlMYBATV in tomatoes (Solanum spp.; Cao et al., 2017; Colandero et al., 2018), MaMYBx in grape hyacinth (Muscari spp.; Zhang et al., 2020), and FhMYBx in Freesia hybrida (Li et al., 2020). The CPC-type R3-MYB repressors are very small (80~100 amino acids), containing the highly conserved bHLH-interacting motif but lacking any obvious repressive motifs (Fig. 1b). Therefore, they only exert passive repression by sequestering the bHLH proteins from the anthocyanin-activating MBW complex (Zhu et al., 2009; Albert et al., 2014).
These R3-MYB repressors often function in fine-tuning anthocyanin pigment intensity or spatial patterning. For example, ROI1 underlies a major quantitative trait locus controlling the variation in flower color intensity between closely related Mimulus species (Yuan et al., 2013). Loss-of-function alleles of SlMYBATV, introgressed from the wild tomato species Solanum cheesmaniae, plays an important role in producing fruit anthocyanins in purple tomato cultivars (Cao et al., 2017; Colandero et al., 2018).
Notably, some of these R3-MYBs contain a “WxM” motif that allows for intercellular movement (Kurata et al., 2005), and their expression can be activated by the MBW complex; the translated R3-MYB products in turn inhibit the function of the MBW complex (Albert et al., 2014; Ding et al., 2020). These properties fit the classical two-component, reaction-diffusion model in generating periodic patterns in biological objects (Turing, 1952; Kondo & Miura, 2010; Meinhardt, 2012). An R2R3-MYB/R3-MYB protein pair has been implicated in spatial patterning of Arabidopsis trichome and root hair development (Pesch & Hulskamp, 2004; Benítz et al., 2011), and a similar model was hypothesized to explain anthocyanin spot formation in flower petals (Davies et al., 2012). Evidence supporting this model was recently realized in Mimulus flowers: the R3-MYB gene RTO is activated by a self-activating subgroup 6 R2R3-MYB, and the translated RTO protein then diffuses from the source cell to neighboring cells and inhibits the activity of the R2R3-MYB activator along the diffusion path. Computer simulations and transgenic perturbations demonstrated that the RTO repressor and its corresponding R2R3-MYB activator indeed constitute such a simple two-component, reaction-diffusion system underlying the dispersed anthocyanin spots (Ding et al., 2020). Given that both the subgroup 6 R2R3-MYB activators and CPC-type R3-MYB repressors are widespread throughout flowering plants, it is tempting to speculate that these activator-repressor MYB pairs are also responsible for the formation of dispersed anthocyanin spots or stripes in many other angiosperm species (Davies et al., 2012).
4. The miR156-SPL module: molecular basis of juvenile reddening in seedlings
Many plant species exhibit a remarkable phenomenon called “juvenile reddening” — anthocyanins are accumulated in the young seedlings, but not in the vegetative tissues at the adult stage (Chalker-Scott, 1999). This phenomenon has been known for decades, but the molecular mechanism remained completely unknown until a link between the miR156-SPL module and anthocyanin regulation provided a plausible explanation (Gou et al., 2011). The miR156-SPL module regulates juvenile-to-adult phase transition in plants (Wu & Poethig, 2006; Xu et al., 2016). In Arabidopsis, miR156 is highly expressed at juvenile stage, silencing its target SQUAMOSA PROMOTER BINDING PROTEIN-LIKE (SPL) genes, including AtSPL9. AtSPL9 inhibits anthocyanin production through physically interacting with the anthocyanin-activating R2R3-MYB AtPAP1 (Gou et al., 2011) and destabilizing the MBW complex (Fig. 2). Young seedlings of Arabidopsis accumulate anthocyanins at the hypocotyl-cotyledon junction in accordance with high expression level of miR156 and low expression level of AtSPL9. The age-dependent decline of miR156 expression allows AtSPL9 to accumulate, which represses anthocyanin production in the vegetative tissues during the adult stage. Overexpression of miR156 in both Arabidopsis and poplar (Populus alba × P. tremula) led to ectopic anthocyanin accumulation in stems of adult plants (Gou et al., 2011; Y.Wang et al., 2020), indicating that the age-dependent regulation of anthocyanin biosynthesis by the miR156-SPL module is conserved across species.
It should be clarified that “juvenile reddening in seedlings” discussed above refers to red pigmentation displayed by juvenile plants, as opposed to the phenomenon known as “juvenile red fading”, wherein young leaves on adult plants are red upon emergence and turn green as leaf ages (e.g., Hughes et al., 2007; Deng et al., 2020). While “juvenile red fading” in sweetpotato (Ipomoea batatas) leaves was correlated with expression dynamics of multiple MYB activators and repressors (Deng et al., 2020), whether these expression changes during leaf maturation is related to the miR156-SPL module is unclear.
5. miR858 and miR828-TAS4: double-edged swords targeting MYB regulators
miR828 and miR858 have been characterized in numerous plant species and shown to target multiple MYB genes, including both anthocyanin-activating and anthocyanin-repressing MYBs (Rajagopalan et al., 2006; Luo et al., 2012; Yang et al., 2013; Jia et al., 2015; Wang et al., 2016; Bonar et al., 2018; Tirumalai et al., 2019). The cleavage sites of both miR828 and miR858 locate in the highly conserved R3-domain. Sometimes these two miRNAs target the same MYB gene, with the miR858 cleavage site just 12-bp upstream of the miR828 site (Xia et al., 2012; Jia et al., 2015).
In Arabidopsis miR828 directly targets the anthocyanin activator AtMYB113, a closely related paralog of AtPAP1 and AtPAP2. Moreover, miR828 also targets the non-coding TAS4 transcript and triggers the production of secondary, phased siRNAs. One of these siRNAs, TAS4-siR81(−), further silences AtMYB113, AtPAP1, and AtPAP2 (Rajagopalan et al., 2006; Luo et al., 2012; Yang et al., 2013). Consequently, miR828 overexpression in Arabidopsis led to inhibition of anthocyanin production. However, in potatoes and grapes, miR828 and TAS4-siR81(−) activate anthocyanin biosynthesis by targeting MYB repressors, and the accumulation of these sRNAs is associated with anthocyanin-rich varieties and tissues (i.e., potato tuber, grape skin) (Bonar et al., 2018; Tirumalai et al., 2019). miR858 positively regulates anthocyanin biosynthesis in Arabidopsis seedlings by inhibiting the anthocyanin repressor AtMYBL2 (Wang et al., 2016). By contrast, miR858 negatively regulates anthocyanin biosynthesis in tomato by inhibiting the expression of SlMYB7-like. Blockage of miR858 function by ectopic expression of small tandem target mimic of miR858 elevated anthocyanin accumulation in tomato seedlings (Jia et al., 2015).
Clearly, both miR828 and miR858 are double-edged swords in anthocyanin regulation. Whether they exert their anthocyanin activation or repression function depends on which target MYB genes are co-expressed with them.
6. HD-ZIP proteins: just an oddity?
The Arabidopsis homeodomain-leucine zipper class IV (HD-ZIP-IV) transcription factor GLABRA2 (GL2) has been long known as a central regulator in trichome and root hair development (Rerie et al., 1994; Schiefelbein, 2003). It was only recently found to also regulate anthocyanin biosynthesis (Wang et al., 2015). GL2 is a transcriptional repressor that directly binds the TAAATGTT/A L1 box in the promoters of the anthocyanin activators AtPAP2 and AtMYB113, repressing their expression at the transcriptional level and consequently inhibiting anthocyanin biosynthesis. Unlike the repressor types described thus far, which have conserved repressive function in anthocyanin biosynthesis across multiple plant systems, anthocyanin repression by GL2-like HD-ZIP proteins has been reported only in Arabidopsis to date, making one wonder whether it is just an oddity or if similar function in other plants has yet to be discovered.
More recently, an HD-ZIP class II member in Arabidopsis, AtHAT1, was found to repress anthocyanin biosynthesis both through destabilization of the MBW complex via interaction with AtPAP1, and by direct repression of the ABP genes through EAR motif-mediated recruitment of the TOPLESS corepressor (Zheng et al., 2019). However, the observations that AtHAT1 only interacts with AtPAP1 but not its paralog AtPAP2, and that AtPAP1 only interacts with AtHAT1 but not its paralogs AtHAT2 or AtHAT3, raise the question whether the AtHAT1-AtPAP1 interaction and its role in anthocyanin repression are peculiar to Arabidopsis.
The apple HD-ZIP class-I protein MdHB1 has been shown to indirectly repress anthocyanin biosynthesis (Jiang et al., 2017). Virus-induced gene silencing of MdHB1 in apple fruit leads to enhanced red coloration and ABP gene expression, whereas transient overexpression of MdHB1 results in reduced anthocyanin content. MdHB1 does not directly bind promoters of ABP genes, but physically sequesters the MBW components in the cytoplasm (Jiang et al., 2017). Similar to AtGL2 and AtHAT1, the anthocyanin-repression function of MdHB1 had been implicated in only one study so far. However, since overexpression of this apple gene was shown to also represses anthocyanin production and ABP gene expression in tobacco (Nicotiana tabacum) (Jiang et al., 2017), the function of MdHB1 in anthocyanin repression may be conserved in other plants.
III. Environmental signals
1. The COP1-HY5 module: light and temperature response
Like most other aspects of plant growth and development, anthocyanin accumulation generally requires light. One of the most vivid demonstrations of this comes from bagged fruits. For example, eggplants can grow to normal size in light-impermeable paper bags, with pure white peels, but undergo massive anthocyanin accumulation just a few days after debagging (Jiang et al., 2016). It is widely accepted that the RING-finger protein CONSTITUTIVE PHOTOMORPHOGENIC1 (COP1) is a central switch of light-responsive developmental and physiological processes, through destabilizing ELONGATED HYPOCOTYL5 (HY5) and various other transcriptional regulators (reviewed in Gangappa & Botto, 2016).
COP1 represses anthocyanin biosynthesis in the dark via both HY5 independent and HY5 dependent mechanisms. In both apple and Arabidopsis, COP1 physically interacts with the anthocyanin-activating, subgroup 6 R2R3-MYBs in the nucleus, leading to their ubiquitination and subsequent proteasome-mediated degradation in the dark (Maier et al., 2013). Light exposure triggers re-localization of COP1 from the nucleus to the cytoplasm, leading to stabilization of the R2R3-MYBs in the nucleus and anthocyanin biosynthesis (Maier et al., 2013). COP1 also represses anthocyanin production in the dark by interacting with and degrading nuclear-localized HY5. In Arabidopsis, HY5 positively regulates anthocyanin biosynthesis by at least three mechanisms (Fig. 2): (i) HY5 directly activates PAP1 and the ABP genes by binding their promoters (Shin et al., 2013); (ii) HY5 activates miR858a, which targets the anthocyanin repressor MYBL2 (Wang et al., 2016); (iii) HY5 directly binds the promoter of MYBL2 and represses its expression through histone modifications (Wang et al., 2016). HY5 is stabilized in the nucleus after light-induced re-localization of COP1 to the cytoplasm, thereby inducing anthocyanin production. The role of this COP1-HY5 module in light-responsive anthocyanin regulation appears to be highly conserved among other species characterized to date, including eggplant (Jiang et al., 2016), tomato (Liu et al., 2018), apple (An et al., 2017), and pear (Tao et al., 2018). Despite this conserved functionality in light-responsive anthocyanin accumulation, exceptions for light-dependence do exist. For example, some potato and sweetpotato varieties produce anthocyanin-rich underground tubers (Liu et al., 2019; Wei et al., 2020), but how the regulation of anthocyanin biosynthesis in these tubers is decoupled from the COP1-HY5 signaling pathway remains an intriguing question.
The same COP1-HY5 module is also reported to regulate anthocyanin production in response to ambient temperature in Arabidopsis (Catalá et al., 2011; Kim et al., 2017). Warm temperatures trigger the import of COP1 to the nucleus (Park et al., 2017), which destabilizes HY5 and represses anthocyanin biosynthesis (Kim et al., 2017). By contrast, cold temperatures deplete COP1 from the nucleus, resulting in HY5 stabilization and increase in anthocyanin production (Catalá et al., 2011). Given that anthocyanin induction by cold temperatures (e.g., Levya et al., 1995) and repression by high ambient temperatures (e.g., Nakatsuka et al., 2019) are observed in many plant species, it will be interesting to test the role of the COP1-HY5 module in the temperature response of anthocyanin biosynthesis in other plants.
2. The LBD proteins and nitrogen deficiency
Nitrogen deficiency has been long known to induce “purpling” in plants (Lawanson et al., 1972), potentially due to stimulation of phenylpropanoid biosynthesis known to occur in response to shifts in carbon-nitrogen balance (Fritz et al. 2006). Three paralogs of the LATERAL ORGAN BOUNDARY DOMAIN (LBD) gene family in Arabidopsis, LBD37, LBD38, and LBD39, encode proteins that repress a variety of nitrogen-responsive roles, including anthocyanin biosynthesis (Rubin et al., 2009). When nitrogen availability is suitable for growth and development, LBD37/38/39 proteins repress the anthocyanin-activating R2R3-MYB genes PAP1 and PAP2, as well as other genes involved in nitrate uptake and assimilation (Rubin et al., 2009). When nitrogen is depleted, the AtLBD37/38/39 are turned off and anthocyanin biosynthesis is de-repressed. Similar nitrate-dependent anthocyanin regulation has been attributed to homologs of AtLBD37/38/39 in other systems, including VvLBD39 during fruit ripening of grapevine berries (Soubeyrand et al., 2014), MdLBD13 in Royal Gala apple (Li et al., 2017), and MdLOB52 in Fuji apples (Y. Wang et al., 2018b). As was observed in Arabidopsis (Rubin et al., 2009), overexpression of MdLBD13 and MdLOB52 appear to repress anthocyanins through downregulation of both the R2R3-MYB and bHLH activators (Li et al., 2017; Y. Wang et al., 2018b). Although the exact functional mechanism of these LBD proteins is yet to be elucidated, a high-throughput yeast two-hybrid screen in Arabidopsis revealed that AtLBD37 directly interacts with the co-repressor TOPLESS (Causier et al., 2012), suggesting a potential role of histone modification in the repression of anthocyanin biosynthesis by AtLBD37/38/39.
3. The NAC family transcription factors and oxidative stress
The NAC family transcription factors regulate numerous developmental processes, and a few members have been shown to negatively regulate anthocyanin biosynthesis in Arabidopsis and Brassica in response to oxidative stress. The first, JUNGBRUNNEN1 (JUB1) in Arabidopsis, is induced by hydrogen peroxide accumulation following oxidative stress or high salinity, and leads to delayed senescence (Wu et al., 2012). Overexpression of JUB1 led to reduced expression of PAP1 and PAP2, while knockdown led to significant upregulation of PAP2, indicating that JUB1 is a repressor of these ABP activation components (Wu et al., 2012).
Another NAC family member, BoNAC019 (homologous to AtNAC019) from Brassica oleracea, was similarly found to be upregulated in response to numerous abiotic stress factors, and overexpression of this gene led to reduced anthocyanin production during drought stress (J. Wang et al., 2018). The authors hypothesized that this transcription factor may act through members of a cytochrome P450 (CYP) monooxygenase gene family, CYP707A, which were shown to be upregulated in BoNAC019 overexpression lines (J. Wang et al., 2018) and have previously been associated with negative regulation of anthocyanin production in sweet cherries during drought conditions (Li et al., 2015).
Therefore, while it is clear that NAC transcription factors are capable of repressing anthocyanin production under certain conditions, the molecular mechanisms of these repressions are largely unknown. It is not clear whether these NAC transcription factors represent a few peculiar cases in Arabidopsis and close relatives, or if they play a more general role in anthocyanin repression among other plants.
4. The TCP proteins and redox-dependent anthocyanin modulation
Members of the TCP protein family are best known for their roles in plant growth and development, including leaf shape and curvature, branch patterning, and floral symmetry (reviewed in Nicolas & Cubas, 2016). However, recent work in Arabidopsis has identified a class I type TCP transcription factor, TCP15, that represses anthocyanin biosynthesis by negatively regulating the expression of both ABP genes (e.g., CHS) and the MBW components PAP1/2 (Viola et al., 2016). Since expression of the chimeric TCP15 protein with the repressive EAR motif fused at the C-terminus leads to higher rather than lower anthocyanin levels, the endogenous TCP15 is probably a transcriptional activator that represses anthocyanin production by activating a yet-to-be-identified anthocyanin repressor. Interestingly, the accumulation of ROS following extended periods of high-light stress was found to result in the oxidation of a conserved cysteine residue in TCP15 and inactivation of its DNA-binding capability. Consequently, the anthocyanin-repressing effect of TCP15 overexpression can be overridden by prolonged high-light exposure (Viola et al., 2016), leading the authors to propose that TCP15 prevents anthocyanin production during short bursts of high-light stress, but is reliably disabled to allow ABP activation during extended high-light exposure. Curiously, a class II TCP protein, TCP3, was shown to positively regulate anthocyanin biosynthesis in Arabidopsis by interacting with the R2R3-MYBs and enhancing the MBW stability (Li & Zachgo, 2013). However, whether high-light stress or ROS accumulation attenuates the positive role of TCP3 in anthocyanin regulation is unknown.
IV. Plant Hormones
Hormones affect almost every aspect of plant development and physiology, including anthocyanin biosynthesis. The signaling pathway from hormone perception to anthocyanin activation or inhibition is relatively clear for jasmonate, auxin, strigolactone, and gibberellic acid (Fig. 4). As will be elaborated below, the basic logic of the signaling mechanisms for these four different hormones is remarkably similar. They all involve a Skp1/Cullin/F-box (SCF) E3 ubiquitin ligase complex that targets a protein substrate for poly-ubiquitination and degradation by the 26S proteasome. What distinguishes each signaling pathway are the specific hormone receptors and the targets of the F-box protein (Fig. 4). Other plant hormones, including ethylene, abscisic acid, cytokinins, are also known to affect anthocyanin biosynthesis (Loreti et al., 2008; Das et al., 2012a). However, whether they have a positive or negative effect on anthocyanin accumulation seems to vary from species to species (Loreti et al., 2008), and the signaling mechanisms that relay the hormone perception to anthocyanin biosynthesis are still poorly understood for these hormones.
1. The SCFCOI1-JAZ module and jasmonate response
Jasmonates (e.g., jasmonic acid (JA) and methyl jasmonate (MeJA)) are the sweet-smelling hormones released by plants undergoing environmental challenges, such as herbivory. The bioactive jasmonyl-L-isoleucine (JA-Ile) conjugate mediates the physical interaction between jasmonate zim-domain (JAZ) proteins and the F-box protein CORONATINE-INSENSITIVE PROTEINI (COI1), a component of the Skp1/Cullin/F-box (SCFCOI1) complex that flags JAZ proteins for ubiquitination and subsequent degradation (reviewed in Pauwels & Goossens, 2011).
Among all plant hormones, the effect of jasmonates on anthocyanin biosynthesis is the least contentious: exogenous application of MeJA induces anthocyanin production in various plants (e.g., Tamari et al., 1995; Shan et al., 2009). In Arabidopsis, this is because the JAZ proteins interfere with the anthocyanin-activating MBW complex by directly binding the R2R3-MYB and the bHLH components. MeJA application triggers the degradation of the JAZ proteins and thus the release of the R2R3-MYB and the bHLH proteins to reform the anthocyanin-activating MBW complex (Fig. 4) (Shan et al., 2009; Qi et al., 2011). The same mechanism has been shown to regulate anthocyanin biosynthesis in response to MeJA in apple (An et al., 2014), and presumably is conserved among other plants as well.
2. The SCFTIR1-Aux/IAA-ARF module and auxin response
Early experiments in Brassica found that exogenous application of the auxin indole acetic acid (IAA) inhibits anthocyanin in a dose-dependent manner (Kang & Burg, 1973). Later studies in various species, mostly using callus culture, showed the same general trend that high auxin concentration inhibits anthocyanin biosynthesis (Makunga et al., 1997; Jeong et al., 2004; Zhou et al., 2008; Liu et al., 2014; Ji et al., 2015; Y. Wang et al., 2018a; but see Mori et al., 1994). A study of red-fleshed apple calli (Y. Wang et al., 2018a) revealed that an Auxin Response Factor, MdARF13, represses anthocyanin biosynthesis both by directly binding the promoter of the ABP gene MdDFR and repressing its transcription, and by physically interacting with the subgroup 6 R2R3-MYB activator MdMYB10 and destabilizing the MBW complex. Under low auxin concentration, the auxin/indole-3-acetic acid (Aux/IAA) repressor MdIAA121 interacts with MdARF13 and prevents it from binding the MdDFR promoter or interacting with MdMYB10. Exogenous auxin application causes MdIAA121 degradation by the 26S proteasome (Y. Wang et al., 2018a), presumably through mediating SCFTIR1-Aux/IAA interactions, and subsequently MdARF13 is released from Aux/IAA to exert its repressive function on anthocyanin biosynthesis (Fig. 4). While callus culture provides a convenient platform to study hormone response, whether this paradigmatic model of auxin response also explains anthocyanin response to auxin in whole plants remains to be tested.
3. The D14-SCFD3-D53 module and strigolactone response
Strigolactones (SLs) are carotenoid-derived plant hormones that have been shown to enhance anthocyanin accumulation (L. Wang et al., 2020). The SL signaling pathway was not characterized until very recently. With the presence of SL, the receptor protein DWARF14 (D14) undergoes a conformational change to facilitate its interaction with the F-box protein D3, a component of the SCFD3 complex, and the DWARF53 (D53) repressor, leading to ubiquitination and degradation of D53 (Jiang et al., 2013; Zhou et al., 2013; Yao et al., 2016). The homolog of D53 in Arabidopsis, AtSMXL6, was recently shown to repress transcription of AtPAP1 by directly binding to its promoter (L. Wang et al., 2020). Hence, SLs trigger degradation of D53/AtSMXL6, de-repressing the subgroup 6 R2R3-MYB genes and activate anthocyanin biosynthesis (Fig. 4).
4. The GID1-SCFGID2-DELLA module and GA response
Gibberellic acids (GA) have been reported to have both enhancing (Weiss et al., 1995; Hosokawa, 1999) and inhibiting effects (Kim et al., 2006; Jiang et al., 2007; Xie et al., 2016) on anthocyanin biosynthesis, depending on species or tissue types. In plant cells, GA is perceived by the receptor GID1 (GA-INSENSITIVE DWARF1). GA perception promotes the interaction between GID1 and the DELLA proteins, triggering a conformational change that enhances the binding affinity of the GID1-DELLA complex to the SCFSLY1/GID2 complex and leads to the proteasome-mediated degradation of DELLA (reviewed in Davière et al., 2008).
In Arabidopsis, the DELLA proteins are positive regulators of anthocyanin biosynthesis, as they directly interact and sequester the AtMYBL2 and AtJAZ repressors, resulting in higher MBW complex activities (Xie et al., 2016). GA degrades DELLA, and hence negatively regulates anthocyanin biosynthesis. Conversely, in another study it was shown that the DELLA proteins can also directly interact with the R2R3-MYB and bHLH components of the MBW complex, thereby destabilizing the activation complex (Qi et al., 2014). As such, DELLA proteins can theoretically be negative regulators of anthocyanin biosynthesis and GA can potentially enhance anthocyanin production. This offers a plausible explanation to the seemingly contradicting observations on the effect of GA in different species: perhaps it is the different DELLA-interacting proteins in different species or tissue types that determine whether GA has an enhancing or inhibiting effect on anthocyanin biosynthesis (Fig. 4).
Notably, both phosphate-deficiency and sucrose induced anthocyanin accumulation in Arabidopsis seem to depend on the GA-DELLA pathway (Jiang et al., 2007; Li et al., 2014). Phosphate starvation was shown to reduce the concentration of bioactive GA, leading to the accumulation of DELLA proteins and enhanced anthocyanin production (Jiang et al., 2007). Sucrose was shown to specifically inhibit the GA-mediated degradation of DELLA proteins, also leading to the stabilization of DELLA proteins and enhanced anthocyanin accumulation (Li et al., 2014).
V. Crosstalk between modules
Thus far we have described the repression of anthocyanin biosynthesis as a series of linear regulatory pathways. However, some modules have the capacity to respond to multiple discrete signals, making them individual components of larger networks. For example, the miR156-SPL module, which we have classified here as being developmentally regulated due to its primary role in vegetative-phase transitioning (Wu & Poethig, 2006; Xu et al., 2016), can also crosstalk with the GA signaling pathway through DELLA-SPL protein-protein interactions, allowing plants to integrate the developmental program with hormonal cues (Curaba et al., 2014). In addition, stress conditions such as drought and high salinity have been shown to trigger anthocyanin production through upregulation of miR156 in Arabidopsis (Cui et al., 2014), and drought alone was shown to have the same impact in alfalfa (Medicago sativa) (Feyissa et al., 2019), indicating that the miR156-SPL module can also integrate developmental signals with exogenous environmental cues.
Another well-studied signaling hub is the HY5-COP1 module, which is responsible for transmitting signals from photoreceptors to the key players of photomorphogenesis. As described in the HY5-COP1 module section, HY5 activates the transcription of some of the MBW components and ABP genes under light or at cool temperatures but is degraded by COP1 in the dark or under warm temperatures. COP1 has been shown to also destabilize MYC2 (Chico et al., 2014), a moderator of crosstalk between GA and jasmonate signaling (Wild et al., 2012). Thus, the HY5-COP1 module can integrate light, temperature, and hormone signaling in anthocyanin regulation. Since the absorption of light is also critical for anthocyanin-induction by ethylene (Craker et al., 1973), cytokinin (Deikman et al., 1995), and abscisic acid (Nagira et al., 2006), it would not be surprising if the HY5-COP1 module turns out to also play an important role in the regulation of anthocyanin biosynthesis related to these hormones.
Other anthocyanin repressors similarly respond to multiple signals (e.g., subgroup 4 R2R3-MYB, LBD, NAC), although the molecular mechanisms governing these responses remain unknown. In short, crosstalk between these repression modules might be extensive, and elucidation of the molecular nature of these interactions will be a fertile research area over the coming years.
VI. Why are there so many repressors of anthocyanin biosynthesis?
In this review, we have described more than a dozen different types of repressors (Table 1; Figs. 2–4). Most of these repressors were discovered in the past decade, and great progress has been made towards characterizing their molecular mechanisms in anthocyanin regulation. No doubt additional repressors will be uncovered in the coming years (e.g., the ones that mediate anthocyanin response to abscisic acids or cytokinins). But why have plants evolved this many different repressors or repression modules for anthocyanin regulation?
Anthocyanins have broad functionality in both vegetative and reproductive organs and thus, it is advantageous for plants to evolve mechanisms to regulate when, where, and how much anthocyanin is produced in response to a myriad of developmental, environmental, and hormonal cues. It makes sense that plants needed to evolve multiple regulators upstream of the MBW complex that can be independently triggered by different stimuli. These distinct regulatory modules enable plants to avoid unnecessary anthocyanin production due to pleiotropy, and meanwhile maintain some degree of freedom and evolvability. But why repressors? In other words, why did plants not evolve a similarly large number of independent activators of the MBW complex that can respond to the various internal and external stimuli? To address this question, we need to first examine the logic of regulation among these repression modules.
Despite that these repressors represent a wide variety of protein or sRNA families, the logic of their molecular function in anthocyanin regulation is remarkably similar. The majority of them act through the so-called “double negative logic” (Davidson & Levine, 2008; Carroll, 2016): an input signal, be it developmental, environmental, or hormonal, represses/degrades a repressor of the anthocyanin-activating complex, leading to the activation of anthocyanin biosynthesis as the output (Fig. 5a). For example, in the miR156-SPL module, the age-dependent miR156 production serves as the signal. This signal represses the SPL repressor, thereby de-repressing the MBW activation complex and anthocyanin biosynthesis; in the SCFCOI1-JAZ module, JA is the input signal, which degrades the JAZ repressor, de-repressing the MBW complex and activating anthocyanin production. Even the few repressors that do not exactly fit the double negative logic operate through a slight variant of this logic design (Fig. 5b–d). For example, some of the subgroup 4 R2R3-MYBs and the CPC-type R3-MYBs not only repress the MBW complex, but themselves are also activated by the MBW complex (Albert et al., 2014; Zhou et al., 2019; Ding et al., 2020; Huang et al., 2020), adding a feedback onto the double negative logic (Fig. 5b). Such a negative feedback loop can prevent overshoot of the MBW activity and is particularly suitable to maintain homeostasis of anthocyanin level in certain tissue types (e.g., ripening fruit or opening flower).
Figure 5.

Schematic representation of common genetic logic observed in the regulation of anthocyanin biosynthesis. (a) double negative logic, (b) a feedback loop incorporated into the double negative logic, (c) double negative regulation of a primary and secondary activator, and (d) triple negative logic of the auxin signaling pathway. Green lines represent activation and red lines represent repression. Abbreviations are as follows: A, primary activator; A’, secondary activator; R, repressor.
The double negative logic was first discovered by Jacob and Monod in their classical work on bacterial enzyme induction (i.e., the E. coli Lac operon; Jacob & Monod, 1961) and has since been found to be a general “rule” in the regulation of many biological processes (Carroll, 2016). What is the functional and evolutionary advantage of this logic design compared with simple or sequential positive regulation (e.g., a signal directly turns on the MBW complex or an upstream activator of the MBW complex)?
There are at least two major advantages. First, a simple or sequential positive logic design will require each of the MBW genes to gain numerous cis-regulatory elements or enhancers in their promoter regions so that they can be independently activated by the upstream activators in response to the myriad developmental, environmental, and hormonal cues, which is evolutionarily challenging to achieve. The double negative logic relieves the necessity to dedicate a separate cis-regulatory element or enhancer to each signal, as a distinct repressive module can evolve to achieve spatiotemporal control under each stimulus, with the MBW complex itself remaining the same. Second, much of the double-negative regulation of anthocyanin production involves degradation or sequestering of the repressors from the MBW protein complex. De-repression of the MBW complex at the protein level enables plants to respond more rapidly than they would under simple or sequential positive logic designs, especially to environmental or hormonal signals, since no de novo transcription and protein translation are required (Fig. 3 and 4).
Figure 3.

Schematic representation of the interactions between repressors that primarily respond to environmental signals, the MBW activation complex, and the ABP genes. Icons indicate an example system in which those repressors are found, or an environmental trigger, as follows: Arabidopsis plant associated with TCP and MYBL2 indicates a representative organism in which these proteins have been reported; thermometers associated with COP1 and HY5 indicate that they are expressed in response to hot and cold temperatures, respectively; greyscale sun associated with COP1 indicates expression in the dark, while bright sun associated with HY5 indicates light-dependent expression; bright sun associated with TCP indicates prevention of anthocyanin production during short bursts of high-light, with TCP inactivation occurring in response to extended periods of high light exposure; downward arrow with chemical structure of nitrate indicates that LBD proteins are repressed during low nitrate availability, which in turn activates anthocyanin production and “purpling”; star with “ROS” associated with NAC indicates expression of JUB1 in response to reactive oxygen species. Question marks indicate that the molecular mechanism of repression is not known in detail.
These two advantages suggest that the double negative logic might be widely employed in the regulation of phenotypic traits that respond rapidly to environmental perturbations, but less prominent in traits that require developmental robustness. Indeed, the double negative logic also operates in the regulation of biosynthesis of other classes of specialized metabolites. For example, glucosinolate biosynthesis in Arabidopsis is activated by the subgroup 12 R2R3-MYBs, which interact with the bHLH protein AtMYC2. JAZ proteins repress glucosinolate biosynthesis by interacting with AtMYC2 and inhibiting the formation of the functional MYB-bHLH complex. JA induces the degradation of the JAZ repressors and activates glucosinolate biosynthesis in a similar way as in promoting anthocyanin biosynthesis (Mitreiter & Gigolashvili, 2020). Another example is the regulation of camalexin biosynthesis. Under normal conditions, the Arabidopsis WRKY transcription factor AtWRKY33 exists in an inactive protein complex with the MAP kinase 4 (MPK4) and its substrate MKS1. In response to pathogen infection, MPK4 is activated to phosphorylate MKS1, leading to the release of the MKS1-WRKY33 complex from MPK4 and subsequent activation of camalexin biosynthesis (Qiu et al., 2008). By contrast, the regulatory networks for developmentally robust traits (i.e., resilient to environmental perturbations) such as secondary cell wall synthesis (Taylor-Teeples et al., 2015), stomata patterning (Herrmann & Torii, 2020), and leaf adaxial-abaxial identity (Kuhlemeier & Timmermans, 2016), are dominated by feed-forward loops or/and negative feedback loops instead of the double negative logic. It is also interesting to note that although the double negative logic can be readily identified in the regulatory networks of various specialized metabolites (Patra et al., 2013; Chezem & Clay, 2016; Mitreiter & Gigolashvili, 2020), this logic design does not predominate in those networks as it does in anthocyanin biosynthesis. This is perhaps not surprising. Given the breadth of functionality of these pigments, and the need to regulate their localization, timing, and intensity across different tissue types and multiple life cycle stages of a plant, the anthocyanin biosynthetic pathway naturally evolved into one of the biochemical pathways that can be induced by probably the largest number of external stimuli. The double negative logic provided a convenient solution to achieve this evolutionary fate.
In addition to the numerous repressors discussed in this review, a few activators of anthocyanin biosynthesis other than MBW components have been discovered in recent years, including AtHY5 and AtTCP3 mentioned earlier as well as NAC transcription factors (Zhou et al., 2015; Wei et al., 2020). These anthocyanin activators mostly act upstream of the MBW components or physically interact with the MBW complex to enhance its function. While HY5 abundance in the nucleus is regulated by a double negative logic (Fig. 5c), how the NAC and TCP activators respond to various stimuli and whether these activators themselves are regulated by a double negative logic will be interesting questions to address.
VII. Unanswered questions about anthocyanin repression and future perspectives
Although the pace of identifying new anthocyanin repressors and characterizing their molecular mechanisms has been quite remarkable in the past decade, there are still many unanswered questions. These knowledge gaps present exciting opportunities for future research. Here we present a list of questions that we think will lead to fruitful investigations:
To what degree are the anthocyanin-repression modules conserved across the land plant phylogeny? On one end of the spectrum, the HD-ZIP, NAC, and class I TCP proteins have been shown to repress anthocyanin biosynthesis in only one species (Arabidopsis or apple) so far. Functional characterization of the orthologs of these genes in other plant species are badly needed. On the other end of the spectrum, the subgroup 4 R2R3-MYBs and CPC-type R3-MYBs have been shown to repress anthocyanin biosynthesis in all angiosperms characterized to date. However, even for these MYB repressors, their regulatory roles in the biosynthesis of anthocyanins or other flavonoids in non-angiosperm species (e.g., gymnosperms, ferns, lycophytes, and bryophytes) remain poorly understood (Davies et al., 2020). Comparative functional analyses in early-diverged land plant lineages will be of great value towards understanding the origin and evolution of these repressors. Recent functional studies of bryophyte and gymnosperm WDR proteins and R2R3-MYB activators (Nemesio-Gorriz et al., 2017; Albert et al., 2018; Airoldi et al., 2019) provide excellent examples in this research area.
What is the exact mechanism of repression by the EAR motif and the TLLLFR motif found in subgroup 4 R2R3-MYBs and their R3-MYB derivatives? Do both motifs interact with TOPLESS-like co-repressors like what was shown for the EAR motif in the HD-ZIP protein AtHAT1 (Zheng et al., 2019)?
What is the functional significance of the physical interaction between the subgroup 4 MYBs and the bHLH proteins? Theoretically, overexpressing a truncated version of the R2R3-MYB without the EAR motif should titrate the bHLH proteins from the MBW complex, thereby negatively affecting anthocyanin biosynthesis, but experimental observations contradict this prediction (Aharoni et al., 2001; Jun et al., 2015; Xu et al., 2017). Do the subgroup 4 MYBs and their R3-MYB derivatives (e.g., AtMYBL2) require this interaction to exert their repressive function?
Is the two-component, activator-inhibitor system, consisting of a subgroup 6 R2R3-MYB activator and a CPC-type R3-MYB, a common feature underlying the dispersed anthocyanin spots or stripes observed in many angiosperm species?
What are the molecular mechanisms through which the LBD, NAC, and class I TCP proteins repress the expression of MBW and/or ABP genes?
Does the COP1-HY5 module regulate thermal response of anthocyanin production in other systems beyond Arabidopsis?
How are the various environmental stimuli (e.g., UV-B, high light, nitrogen deficiency, oxidative stress) relayed to the repressor genes (e.g., subgroup 4 R2R3-MYBs, LBD, NAC) to affect their expression?
How commonly are non-coding RNAs other than miR156, miR828, or miR858 employed to regulate anthocyanin biosynthesis? Liu et al. (2017) reported a novel miRNA, which targets chalcone isomerase (CHI) during fruit ripening of Litchi chinensis. Zhang et al. (2018) showed that two long non-coding RNAs, LNC1 and LNC2, act as endogenous target mimics for miR156a and miR828a and participate in regulation of fruit ripening in sea buckthorn (Hippophae rhamnoides L.). Although these novel non-coding RNAs most likely represent lineage-specific peculiarities, they could have very interesting evolutionary consequences (e.g., Bradley et al., 2017).
To what extent do DNA methylation and histone modification play a role in anthocyanin repression? Methylation of the promoters of ABP genes and/or their regulatory genes has been shown to repress anthocyanin production in various apple, peach, and pear cultivars (Telias et al., 2011; Wang et al., 2013; El-Sharkawy et al., 2015; Zhu et al., 2020). In addition, anthocyanin production was found to be repressed in Arabidopsis through incorporation of a conserved histone H2 variant (H2A.Z) into nucleosomes associated with several ABP genes, which causes decreased H3K4me3 and ABP gene expression (Cai et al., 2019). The extent to which these epigenetic marks are employed to fine-tune anthocyanin biosynthesis is an exciting area that certainly warrants further investigations.
What is the role of plant hormones ABA, cytokinins, ethylene and brassinosteroids (Nagira et al., 2006; and see review by Das et al., 2012b) in anthocyanin regulation, and what are their molecular mechanisms? It would not be surprising if some of these responses involve new repressors, or if they prove to be additional components of currently known modules. Furthermore, how do these hormone signaling pathways crosstalk with each other and with other developmentally or environmentally regulated modules?
Are there additional layers of feedback regulation on top of the basic double negative logic design for the various repressors represented in Fig. 5a? Can we design novel genetic circuits including both the double negative logic and various feedback loops through synthetic biology to reach fine-scale control of the timing, localization, and optimal intensity of anthocyanin accumulation in horticultural and crop plants?
Answers to these questions will not only contribute new knowledge to the molecular mechanisms of anthocyanin regulation, but also enhance our understanding of the developmental mechanisms of pattern formation (Davies et al., 2012; Ding et al., 2020) and the genetic bases of phenotypic evolution (Sobel & Streisfeld, 2013). Furthermore, research on anthocyanin repression (or de-repression) is likely to have significant translational impact, as there is tremendous commercial interest in the regulation of anthocyanin biosynthesis due to the known benefits of anthocyanins to human health (e.g., Butelli et al., 2008) and increasing demand for novel coloration in the food and floriculture industries.
Acknowledgements
We would like to thank Drs. Foen Peng, Mei Liang, and Baoqing Ding, and two anonymous reviewers for their constructive comments on earlier versions of the manuscript. We apologize to colleagues whose work could not be cited here due to space constraints. Work on anthocyanin regulation in the Yuan lab is supported by a grant from the National Institutes of Health (R01GM131055).
References
- Airoldi CA, Hearn TJ, Brockington SF, Webb AAR, Glover BJ. 2019. TTG1 proteins regulate circadian activity as well as epidermal cell fate and pigmentation. Nature Plants 5:1145–1153. [DOI] [PubMed] [Google Scholar]
- Aharoni A, De Vos CHR, Wein M, Sun Z, Greco R, Kroon A, Mol JNM, O’Connell AP. 2001. The strawberry FaMYB1 transcription factor suppresses anthoycanin and flavonol accumulation in transgenic tobacco. The Plant Journal 38:319–332. [DOI] [PubMed] [Google Scholar]
- Albert NW, Davies KM, Lewis DH, Zhang H, Montefiori M, Brendolise C, Boase MR, Ngo H, Jameson PE, Schwinn KE. 2014. A conserved network of transcriptional activators and repressors regulates anthocyanin pigmentation in eudicots. The Plant Cell 26:962–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert NW, Thrimawithana AH, McGhie TK, Clayton WA, Deroles SC, Schwinn KE, Bowman JL, Jordan BR, Davies KM. 2018. Genetic analysis of the liverwort Marchantia polymorpha reveals that R2R3MYB activation of flavonoid production in response to abiotic stress is an ancient character in land plants. New Phytologist 218:554–566. [DOI] [PubMed] [Google Scholar]
- An J-P, Qu F-J, Yao J-F, Wang X-N, You C-X, Wang X-F, Hao Y-J. 2017. The bZIP transcription factor MdHY5 regulates anthocyanin accumulation and nitrate assimilation in apple. Horticulture Research 4:17023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An X-H, Tian Y, Chen K-Q, Liu X-J, Liu D-D, Xie X-B, Cheng C-G, Cong P-H, Hao Y-J. 2014. MdMYB9 and MdMYB11 are involved in the regulation of the JA-induced biosynthesis of anthocyanin and proanthocyanidin in apples. Plant and Cell Physiology 56:650–662. [DOI] [PubMed] [Google Scholar]
- Benítez M, Monk NAM, Alvarez-Buylla ER. 2011. Epidermal patterning in Arabidopsis: models make a difference. Journal of Experimental Zoology 316:241–253. [DOI] [PubMed] [Google Scholar]
- Bonar N, Liney M, Zhang R, Austin C, Dessoly J, Davidson D, Stephens J, McDougall G, Taylor M, Bryan GJ, et al. 2018. Potato miR828 is associated with purple tuber skin and flesh color. Frontiers in Plant Science 9:1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borevitz JO, Xia YJ, Blount J, Dixon RA, Lamb C. 2000. Activation tagging identifies a conserved MYB regulator of phenylpropanoid biosynthesis. Plant Cell 12:2383–2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley D, Xu P, Mohorianu I-I, Whibley A, Field D, Tavares H, Couchman M, Copsey L, Carpenter R, Li M, et al. 2017. Evolution of flower color pattern through selection on regulatory small RNAs. Science 358:925–928. [DOI] [PubMed] [Google Scholar]
- Butelli E, Titta L, Giorgio M, Mock H-P, Matros A, Peterek S, Schijlen EGWM, Hall RD, Bovy AG, Luo J, et al. 2008. Enrichment of tomato fruit with health-promoting anthocyanins by expression of select transcription factors. Nature Biotechnology 26:1301–1308. [DOI] [PubMed] [Google Scholar]
- Cai H, Zhang M, Chai M, He Q, Huang X, Zhao L, Qin Y. 2019. Epigenetic regulation of anthocyanin biosynthesis by an antagonistic interaction between H2A.Z and H3K4me3. New Phytologist 221:295–308. [DOI] [PubMed] [Google Scholar]
- Cao X, Qiu Z, Wang X, Giang TV, Liu X, Wang J, Wang X, Gao J, Guo Y, Du Y, et al. 2017. A putative R3 MYB repressor is the candidate gene underlying atroviolacium, a locus for anthocyanin pigmentation in tomato fruit. Journal of Experimental Botany 68:5745–5758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll SB. 2016. The Serengeti Rules: The Quest to Discover How Life Works and Why it Matters. Princeton University Press. [Google Scholar]
- Catalá R, Medina J, Salinas J. 2011. Integration of low temperature and light signaling during cold acclimation response in Arabidopsis. Proceedings of the National Academy of Sciences 108:16474–16480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Causier B, Ashworth M, Guo W, Davies B. 2012. The TOPLESS interactome: a framework for gene repression in Arabidopsis. Plant Physiology 158:423–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavallini E, Matus JT, Finezzo L, Zenoni S, Loyola R, Guzzo F, Schlechter R, Ageorges A, Arce-Johnson P, Tornielli GB. 2015. The phenylpropanoid pathway is controlled at different branches by a set of R2R3-MYB C2 repressors in grapevine. Plant Physiology 167:1448–1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalker-Scott L 1999. Environmental significance of anthocyanins in plant stress responses. Photochemistry and Photobiology 70:1–9. [Google Scholar]
- Chen L, Hu B, Qin Y, Hu G, Zhao J. 2019. Advance of the negative regulation of anthocyanin biosynthesis by MYB transcription factors. Plant Physiology and Biochemistry 136:178–187. [DOI] [PubMed] [Google Scholar]
- Chezem WR, Clay NK. 2016. Regulation of plant secondary metabolism and associated specialized cell development by MYBs and bHLHs. Phytochemistry 131:26–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chico J-M, Fernández-Barbero G, Chini A, Fernández-Calvo P, Díez-Díez M, Solano R. 2014. Repression of jasmonate-dependent defenses by shade involves differential regulation of protein stability of MYC transcription factors and their JAZ repressors in Arabidopsis. The Plant Cell 26:1967–1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colandero S, Perata P, Gonzali S. 2018. The atroviolacea gene encodes an R3-MYB protein repressing anthocyanin synthesis in tomato plants. Frontiers in Plant Science 9:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craker LE, Wetherbee PJ. 1973. Ethylene, light, and anthocyanin synthesis. Plant physiology 51: 436–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui LG, Shan JX, Shi M, Gao JP, Lin HX. 2014. The miR156‐SPL9‐DFR pathway coordinates the relationship between development and abiotic stress tolerance in plants. The Plant Journal 80:1108–1117. [DOI] [PubMed] [Google Scholar]
- Curaba J, Singh MB, Bhalla PL. 2014. miRNAs in the crosstalk between phytohormone signalling pathways. Journal of Experimental Botany 65:1425–1438. [DOI] [PubMed] [Google Scholar]
- Das PK, Shin DH, Choi DH, Choi S-B, Yoo S-D, Choi G, Park Y-I. 2012a. Cytokinins enhance sugar-induced anthocyanin biosynthesis in Arabidopsis. Molecules and Cells 34:93–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das PK, Shin DH, Choi S-B, Park Y-I. 2012b. Sugar-hormone cross-talk in anthocyanin biosynthesis. Molecules and Cells 34:501–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson EH, Levine MS. 2008. Properties of developmental gene regulatory networks. Proceedings of the National Academy of Sciences 105:20063–20066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davière J-M, De Lucas M, Prat S. 2008. Transcriptional factor interaction: a central step in DELLA function. Current Opinion in Genetics & Development 18:295–303. [DOI] [PubMed] [Google Scholar]
- Davies KM, Albert NW, Schwinn KE. 2012. From landing lights to mimicry: the molecular regulation of flower colouration and mechanisms for pigmentation patterning. Functional Plant Biology 39:619–638. [DOI] [PubMed] [Google Scholar]
- Davies KM, Albert NW, Zhou Y, Schwinn KE. 2018. Functions of flavonoid and betalain pigments in abiotic stress tolerance in plants. Annual Plant Reviews online 1:21–62. [Google Scholar]
- Davies KM, Jibran R, Zhou Y, Albert NW, Brummell DA, Jordan BR, Bowman JL, Schwinn KE. 2020. The evolution of flavonoid biosynthesis: A bryophyte perspective. Frontiers in Plant Science 11:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deikman J, Hammer PE. 1995. Induction of anthocyanin accumulation by cytokinins in Arabidopsis thaliana. Plant Physiology 108:47–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng J, Wu D, Shi J, Balfour K, Wang H, Zhu G, Liu Y, Wang J, Zhu Z. 2020. Multiple MYB activators and repressors collaboratively regulate the juvenile red fading in leaves of sweetpotato. Frontiers in Plant Science 11:941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vetten N, Quattrocchio F, Mol J, Koes R. 1997. The an11 locus controlling flower pigmentation in petunia encodes a novel WD-repeat protein conserved in yeast, plants, and animals. Genes & development 11:1422–1434. [DOI] [PubMed] [Google Scholar]
- Ding B, Patterson EL, Holalu S, Li J, Johnson GA, Stanley LE, Greenlee AB, Peng F, Bradshaw HD Jr., Blinov ML, et al. 2020. Two MYB proteins in a self-organizing activator-inhibitor system produce spotted pigmentation patterns. Current Biology 30:802–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubos C, Le Gourrierec J, Baudry A, Huep G, Lanet E, Debeaujon I, Routaboul J-M, Alboresi A, Weisshaar B, Lepiniec L. 2008. MYBL2 is a new regulator of flavonoid biosynthesis in Arabidopsis thaliana. The Plant Journal 55:940–953. [DOI] [PubMed] [Google Scholar]
- Dubos C, Stracke R, Grotewold E, Weisshaar B, Martin C, Lepiniec L. 2010. MYB transcription factors in Arabidopsis. Trends in Plant Science 15:573–581. [DOI] [PubMed] [Google Scholar]
- El-Sharkawy I, Liang D, Xu K. 2015. Transcriptome analysis of an apple (Malus x domestica) yellow fruit somatic mutation identifies a gene network module highly associated with anthocyanin and epigenetic regulation. Journal of Experimental Botany 66: 7359–7376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feller A, Machemer K, Braun EL, Grotewold E. 2011. Evolutionary and comparative analysis of MYB and bHLH plant transcription factors. The Plant Journal 66:94–116. [DOI] [PubMed] [Google Scholar]
- Feyissa BA, Arshad M, Gruber MY, Kohalmi SE, Hannoufa A. 2019. The interplay between miR156/SPL13 and DFR/WD40–1 regulate drought tolerance in alfalfa. BMC Plant Biology 19:434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornalé S, Shi X, Chai C, Encina A, Irar S, Capellades M, Fuguet E, Torres J-L, Rovira P, Puigdomènech P, et al. 2010. ZmMYB31 directly represses maize lignin genes and redirects the phenylpropanoid metabolic flux. The Plant Journal 64:633–644. [DOI] [PubMed] [Google Scholar]
- Fritz C, Palacios‐Rojas N, Feil R, Stitt M. 2006. Regulation of secondary metabolism by the carbon–nitrogen status in tobacco: nitrate inhibits large sectors of phenylpropanoid metabolism. The Plant Journal 46:533–548. [DOI] [PubMed] [Google Scholar]
- Gangappa SN, Botto JF. 2016. The multifaceted roles of HY5 in plant growth and development. Molecular Plant 9:1353–1365. [DOI] [PubMed] [Google Scholar]
- Gates DJ, Olson BJ, Clemente TE, Smith SD. 2018. A novel R3 MYB transcriptional repressor associated with the loss of floral pigmentation in Iochroma. New Phytologist 217:1346–1356. [DOI] [PubMed] [Google Scholar]
- Goff SA, Cone KC, Chandler VL. 1992. Functional analysis of the transcriptional activator encoded by the maize B gene: evidence for a direct functional interaction between two classes of regulatory proteins. Genes and Development 6:864–875. [DOI] [PubMed] [Google Scholar]
- Goodrich J, Carpenter R, Coen E. 1992. A common gene regulates pigmentation pattern in diverse plant species. Cell 68:955–964. [DOI] [PubMed] [Google Scholar]
- Gou J-Y, Felippes FF, Liu C-J, Weigel D, Wang J-W. 2011. Negative regulation of anthocyanin biosynthesis in Arabidopsis by a miR156-targeted SPL transcription factor. The Plant Cell 23:1512–1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grotewold E, Sainz MB, Tagliani L, Hernandez JM, Bowen B, Chandler VL. 2000. Identification of the residues in the Myb domain of maize C1 that specify the interaction with the bHLH cofactor R. Proceedings of the National Academy of Sciences 97:13579–13584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grotewold E 2006. The genetics and biochemistry of floral pigments. Annual Review of Plant Biology 57:761–780. [DOI] [PubMed] [Google Scholar]
- Herrmann A, Torii KU. 2020. Shouting out loud: signaling modules in the regulation of stomatal development. Plant Physiology. doi: 10.1093/plphys/kiaa061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosokawa K 1999. Cell layer-specific accumulation of anthocyanins in response to gibberellic acid in tepals of Hyacinthus orientalis. Bioscience, Biotechnology, and Biochemistry 63:930–931. [DOI] [PubMed] [Google Scholar]
- Huang D, Tang Z, Fu J, Yuan Y, Deng X, Xu Q. 2020. CsMYB3 and CsRuby1 form an ‘activator-and-repressor’ loop for the regulation of anthocyanin biosynthesis in citrus. Plant and Cell Physiology 61:318–330. [DOI] [PubMed] [Google Scholar]
- Hughes NM, Morley CB, Smith WK. 2007. Coordination of anthocyanin decline and photosynthetic maturation in juvenile leaves of three deciduous tree species. New Phytologist 175:675–685. [DOI] [PubMed] [Google Scholar]
- Jacob F, Monod J. 1961. Genetic regulatory mechanisms in the synthesis of proteins. Journal of Molecular Biology 3:318–356. [DOI] [PubMed] [Google Scholar]
- Jeong ST, Goto-Yamamoto N, Kobayashi S, Esaka M. 2004. Effects of plant hormones and shading on the accumulation of anthocyanins and the expression of anthocyanin biosynthetic genes in grape berry skins. Plant Science 167:247–252. [Google Scholar]
- Ji X-H, Zhang R, Wang N, Yang L, Chen X-S. 2015. Transcriptome profiling reveals auxin suppressed anthocyanin biosynthesis in red-fleshed apple callus (Malus sieversii f. niedzwetzkyana). Plant Cell, Tissue and Organ Culture (PCTOC) 123:389–404. [Google Scholar]
- Jia X, Shen J, Liu H, Li F, Ding N, Gao C, Pattanaik S, Patra B, Li R, Yuan L. 2015. Small tandem target mimic-mediated blockage of microRNA858 induces anthocyanin accumulation in tomato. Planta 242:283–293. [DOI] [PubMed] [Google Scholar]
- Jiang C, Gao X, Liao L, Harberd NP, Fu X. 2007. Phosphate starvation root architecture and anthocyanin accumulation responses are modulated by the gibberellin-DELLA signaling pathway in Arabidopsis. Plant Physiology 145:1460–1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang L, Liu X, Xiong G, Liu H, Chen F, Wang L, Meng X, Liu G, Yu H, Yuan Y, et al. 2013. DWARF 53 acts as a repressor of strigolactone signalling in rice. Nature 504:401–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang M, Ren L, Lian H, Liu Y, Chen H. 2016. Novel insight into the mechanism underlying light-controlled anthocyanin accumulation in eggplant (Solanum melongena L.). Plant Science 249:46–58. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Liu C, Yan D, Wen X, Liu Y, Wang H, Dai J, Zhang Y, Liu Y, Zhou B, et al. 2017. MdHB1 down-regulation activates anthocyanin biosynthesis in the white-fleshed apple cultivar ‘Granny Smith’. Journal of Experimental Botany 68:1055–1069. [DOI] [PubMed] [Google Scholar]
- Jin H, Cominelli E, Bailey P, Parr A, Mehrtens F, Jones J, Tonelli C, Weisshaar B, Martin C. 2000. Transcriptional repression by AtMYB4 controls production of UV‐protecting sunscreens in Arabidopsis. The EMBO journal 19:6150–6161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jun JH, Liu C, Xiao X, Dixon RA. 2015. The transcriptional repressor MYB2 regulates both spatial and temporal patterns of proanthocyanidin and anthocyanin pigmentation in Medicago truncatula. The Plant Cell 27:2860–2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang BG, Burg SP. 1973. Role of ethylene in phytochrome-induced anthocyanin synthesis. Planta 110:227–235. [DOI] [PubMed] [Google Scholar]
- Kim J-S, Lee B-H, Kim S-H, Oh K-H, Cho KY. 2006. Responses to environmental and chemical signals for anthocyanin biosynthesis in non-chlorophyllous corn (Zea mays L.) leaf. Journal of Plant Biology 49:16–25. [Google Scholar]
- Kim S, Hwang G, Lee S, Zhu J-Y, Paik I, Nguyen TT, Kim J, Oh E. 2017. High ambient temperature represses anthoycanin biosynthesis through degradation of HY5. Frontiers in Plant Science 8:1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo S, Miura T. 2010. Reaction-diffusion model as a framework for understanding biological pattern formation. Science 329:1616–1620. [DOI] [PubMed] [Google Scholar]
- Kranz HD, Denekamp M, Greco R, Jin H, Leyva A, Meissner RC, Petroni K, Urzainqui A, Bevan M, Martin C, et al. 1998. Towards functional characterization of the members of the R2R3-MYB gene family from Arabidopsis thaliana. The Plant Journal 16:263–276. [DOI] [PubMed] [Google Scholar]
- Kuhlemeier C, Timmermans MCP. 2016. The Sussex signal: insights into leaf dorsiventrality. Development 143:3230–3237. [DOI] [PubMed] [Google Scholar]
- Kurata T, Isida T, Kawabata-Awai C, Noguchi M, Hattori S, Sano R, Nagasaka R, Tominaga R, Koshino-Kimura Y, Kato T, et al. 2005. Cell-to-cell movement of the CAPRICE protein in Arabidopsis root epidermal cell differentiation. Development 132:5387–5398. [DOI] [PubMed] [Google Scholar]
- Landi M, Tattini M, Gould KS. 2015. Multiple functional roles of anthocyanins in plant-environment interactions. Environmental and Experimental Botany 119:4–17. [Google Scholar]
- Lawanson AO, Akindele BB, Fasalojo PB, Akpe BL. 1972. Time-course of anthocyanin formation during deficiencies of nitrogen, phosphorus, and potassium in seedlings of Zea mays Linn var. E.S. 1. Zeitschrift für Pflanzenphysiologie 66:251–253. [Google Scholar]
- Levya A, Jarillo JA, Salinas J, Martinez-Zapater JM. 1995. Low temperature induces the accumulation of phenylalanine ammonia-lyase and chalcone synthase mRNAs of Arabidopsis thaliana in a light-dependent manner. Plant Physiology 108:39–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H-H, Liu X, An J-P, Hao Y-J, Wang X-F, You C-X. 2017. Cloning and elucidation of the functional role of apple MdLBD13 in anthocyanin biosynthesis and nitrate assimilation. Plant Cell Tissue and Organ Culture 130:47–59. [Google Scholar]
- Li Q, Chen P, Dai S, Sun Y, Yuan B, Kai W, Pei Y, He S, Liang B, Zhang Y, et al. 2015. PacCYP707A2 negatively regulates cherry fruit ripening while PacCYP707A1 mediates drought tolerance. Journal of Experimental Botany 66:3765–3774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Zachgo S. 2013. TCP3 interacts with R2R3-MYB proteins, promotes flavonoid biosynthesis and negatively regulates the auxin response in Arabidopsis thaliana. The Plant Journal 76:901–913. [DOI] [PubMed] [Google Scholar]
- Li Y, Van den Ende W, Rolland F. 2014. Sucrose induction of anthocyanin biosynthesis is mediated by DELLA. Molecular Plant 7:570–572. [DOI] [PubMed] [Google Scholar]
- Li Y, Shan X, Gao R, Han T, Zhang J, Wang Y, Kimani S, Wang L, Gao X. 2020. MYB repressors and MBW activation complex collaborate to fine-tune flower coloration in Freesia hybrida. Communications Biology 3:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C-C, Chi C, Jin L-J, Zhu J, Yu J-Q., Zhou Y-H. 2018. The bZip transcription factor HY5 mediates CRY1a-induced anthocyanin biosynthesis in tomato. Plant, Cell and Environment 41:1762–1775. [DOI] [PubMed] [Google Scholar]
- Liu R, Lai B, Qin Y, Hu G, Zhao J. 2017. Identification of MicroRNAs and their target genes related to the accumulation of anthocyanins in Litchi chinesis by high-throughput sequencing and degradome analysis. Frontiers in Plant Science 7:2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Lin-Wang K, Espley RV, Wang L, Li Y, Liu Z, Zhou P, Zeng L, Zhang X, Zhang J, et al. 2019. StMYB44 negatively regulates anthocyanin biosynthesis at high temperatures in tuber flesh of potato. Journal of Experimental Botany 70:3809–3824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Shi M-Z, Xie D-Y. 2014. Regulation of anthocyanin biosynthesis in Arabidopsis thaliana red pap1-D cells metabolically programmed by auxins. Planta 239:765–781. [DOI] [PubMed] [Google Scholar]
- Lloyd A, Brockman A, Aguirre L, Campbell A, Bean A, Cantero A, Gonzalez A. 2017. Advances in the MYB–bHLH–WD repeat (MBW) pigment regulatory model: Addition of a WRKY factor and co-option of an anthocyanin MYB for betalain regulation. Plant and Cell Physiology 58:1431–1441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loreti E, Povero G, Novi G, Solfanelli C, Alpi A, Perata P. 2008. Gibberellins, jasmonate and abscisic acid modulate the sucrose‐induced expression of anthocyanin biosynthetic genes in Arabidopsis. New Phytologist 179:1004–1016. [DOI] [PubMed] [Google Scholar]
- Ludwig SR, Habera LF, Dellaporta SL, Wessler SR. 1989. Lc, a member of the maize R gene family responsible for tissue-specific anthocyanin production, encodes a protein similar to transcriptional activators and contains the myc-homology region. Proceedings of the National Academy of Sciences 86:7092–7096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Q-J, Mittal A, Jia F, Rock CD. 2012. An autoregulatory feedback loop involving PAP1 and TAS4 in response to sugars in Arabidopsis. Plant Molecular Biology 80:117–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma D, Constabel CP. 2019. MYB repressors as regulators of phenylpropanoid metabolism in plants. Trends in Plant Science 24:275–289. [DOI] [PubMed] [Google Scholar]
- Maier A, Schrader A, Kokkelink L, Falke C, Welter B, Iniesto E, Rubio V, Uhrig JF, Hülskamp M, Hoecker U. 2013. Light and the E3 ubiquitin ligase COP1/SPA control the protein stability of the MYB transcription factors PAP1 and PAP2 involved in anthocyanin accumulation in Arabidopsis. The Plant Journal 74:638–651. [DOI] [PubMed] [Google Scholar]
- Makunga NP, van Staden J, Cress WA. 1997. The effect of light and 2,4-D on anthocyanin production in Oxalis reclinata callus. Plant Growth Regulation 23:153–158. [Google Scholar]
- Matsui K, Umemura Y, Ohme Takagi M. 2008. AtMYBL2, a protein with a single MYB domain, acts as a negative regulator of anthocyanin biosynthesis in Arabidopsis. The Plant Journal 55:954–967. [DOI] [PubMed] [Google Scholar]
- Meinhardt H 2012. Turing’s theory of morphogenesis of 1952 and the subsequent discovery of the crucial role of local self-enhancement and long-range inhibition. Interface Focus 2:407–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitreiter S, Gigolashvili T. 2021. Regulation of glucosinolate biosynthesis. Journal of Experimental Botany 72: 70–91. [DOI] [PubMed] [Google Scholar]
- Mori T, Sakurai M, Seki M, Furusaki S. 1994. Use of auxin and cytokinin to regulate anthocyanin production and composition in suspension cultures of strawberry cell. Journal of the Science of Food and Agriculture 65:271–276. [Google Scholar]
- Nagira Y, Ikegami K, Koshiba T, Ozeki Y. 2006. Effect of ABA upon anthocyanin synthesis in regenerated torenia shoots. Journal of Plant Research 119:137–144. [DOI] [PubMed] [Google Scholar]
- Nakatsuka T, Suzuki T, Harada K, Kobayashi Y, Dohra H, Ohno H. 2019. Floral organ-and temperature-dependent regulation of anthocyanin biosynthesis in Cymbidium hybrid flowers. Plant Science 287:110173. [DOI] [PubMed] [Google Scholar]
- Nemesio-Gorriz M, Blair PB, Dalman K, Hammerbacher A, Arnerup J, Stenlid J, Mukhtar SM, Elfstrand M. 2017. Identification of Norway spruce MYB-bHLH-WDR transcription factor complex members linked to regulation of the flavonoid pathway. Frontiers in Plant Science 8:305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolas M, Cubas P. 2016. TCP factors: new kids on the signaling block. Current Opinion in Plant Biology 33:33–41. [DOI] [PubMed] [Google Scholar]
- Park Y-J, Lee H-J, Ha J-H, Kim JY, Park C-M. 2017. COP1 conveys warm temperature information to hypocotyl thermomorphogenesis. New Phytologist 215:269–280. [DOI] [PubMed] [Google Scholar]
- Patra B, Schluttenhofer C, Wu Y, Pattanaik S, Yuan L. 2013. Transcriptional regulation of secondary metabolite biosynthesis in plants. Biochimica et Biophysica Acta 1829:1236–1247. [DOI] [PubMed] [Google Scholar]
- Pauwels L, Goossens A. 2011. The JAZ proteins: a crucial interface in the jasmonate signaling cascade. The Plant Cell 23:3089–3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne CT, Zhang F, Lloyd AM. 2000. GL3 encodes a bHLH protein that regulates trichome development in Arabidopsis through interaction with GL1 and TTG1. Genetics 156:1349–1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paz-Ares J, Ghosal D, Wienand U, Peterson PA, Saedler H. 1987. The regulatory c1 locus of Zea mays encodes a protein with homology to myb proto-oncogene products and with structural similarities to transcriptional activators. The EMBO journal 6:3553–3558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesch M, Hülskamp M. 2004. Creating a two-dimensional pattern de novo during Arabidopsis trichome and root hair initiation. Current Opinion in Genetics & Development 14:422–427. [DOI] [PubMed] [Google Scholar]
- Qi T, Huang H, Wu D, Yan J, Qi Y, Song S, Xie D. 2014. Arabidopsis DELLA and JAZ proteins bind the WD-repeat/bHLH/MYB complex to modulate gibberellin and jasmonate signaling synergy. The Plant Cell 26:1118–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi T, Song S, Ren Q, Wu D, Huang H, Chen Y, Fan M, Peng W, Ren C, Xie D. 2011. The Jasmonate-ZIM-domain proteins interact with the WD-Repeat/bHLH/MYB complexes to regulate jasmonate-mediated anthocyanin accumulation and trichome initiation in Arabidopsis thaliana. The Plant Cell 23:1795–1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu J-L, Fiil BK, Petersen K, Nielsen HB, Botanga CJ, Thorgrimsen S, Palma K, Suarez-Rodriguez MC, Sandbech-Clausen S, Lichota J, et al. 2008. Arabidopsis MAP kinase 4 regulates gene expression through transcription factor release in the nucleus. The EMBO journal 27:2214–2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quattrocchio F, Wing J, van der Woude K, Souer E, de Vetten N, Mol J, Koes R. 1999. Molecular analysis of the anthocyanin2 gene of petunia and its role in the evolution of flower color. The Plant Cell 11:1433–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajagopalan R, Vaucheret H, Trejo J, Bartel DP. 2006. A diverse and evolutionarily fluid set of microRNAs in Arabidopsis thaliana. Genes and Development 20:3407–3425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsay NA, Glover BJ. 2005. MYB-bHLH-WD40 protein complex and the evolution of cellular diversity. Trends in Plant Science 10:63–70. [DOI] [PubMed] [Google Scholar]
- Rerie WG, Feldmann KA, Marks MD. 1994. The GLABRA2 gene encodes a homeo domain protein required for normal trichome development in Arabidopsis. Genes and Development 8:1388–1399. [DOI] [PubMed] [Google Scholar]
- Rubin G, Tohge T, Matsuda F, Saito K, Scheible W-R. 2009. Members of the LBD family of transcription factors repress anthocyanin synthesis and affect additional nitrogen responses in Arabidopsis. The Plant Cell 21:3567–3584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai M, Yamagishi M, Matsuyama K. 2019. Repression of anthocyanin biosynthesis by R3-MYB transcription factors in lily (Lilium spp.). Plant Cell Reports 38:609–622. [DOI] [PubMed] [Google Scholar]
- Schellmann S, Schnittger A, Kirik V, Wada T, Okada K, Beermann A, Thumfahrt J, Jürgens G, Hülskamp M. 2002. TRIPTYCHON and CAPRICE mediate lateral inhibition during trichome and root hair patterning in Arabidopsis. The EMBO journal 21:5036–5046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiefelbein J 2003. Cell-fate specification in the epidermis: a common patterning mechanism in the root and shoot. Current Opinion in Plant Biology 6:74–78. [DOI] [PubMed] [Google Scholar]
- Schwinn KE, Venail J, Shang Y, Mackay S, Alm V, Butelli E, Oyama R, Bailey P, Davies KM, Martin C. 2006. A small family of MYB-regulatory genes controls floral pigmentation intensity and patterning in the genus Antirrhinum. The Plant Cell 18:831–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan X, Zhang Y, Peng W, Wang Z, Xie D. 2009. Molecular mechanism for jasmonate-induction of anthocyanin accumulation in Arabidopsis. Journal of Experimental Botany 60:3849–3860. [DOI] [PubMed] [Google Scholar]
- Shin DH, Choi M, Kim K, Bang G, Cho M, Choi S-B, Choi G, Park Y-I. 2013. HY5 regulates anthocyanin biosynthesis by inducing the transcriptional activation of the MYB75/PAP1 transcription factor in Arabidopsis. FEBS Letters 587:1543–1547. [DOI] [PubMed] [Google Scholar]
- Sobel JM, Streisfeld MA. 2013. Flower color as a model system for studies of plant evo-devo. Frontiers in Plant Science 4:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song H, Yi H, Lee M, Han C-T, Lee J, Kim H, Park J-I, Nou I-S, Kim S-J, Hur Y. 2018. Purple Brassica oleracea var. capitata F. rubra is due to the loss of BoMYBL2–1 expression. BMC Plant Biology 18:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soubeyrand E, Basteau C, Hilbert G, van Leeuwen C, Delrot S, Gomès E. 2014. Nitrogen supply affects anthocyanin biosynthetic and regulatory genes in grapevine cv. Cabernet-Sauvignon berries. Phytochemistry 103:38–49. [DOI] [PubMed] [Google Scholar]
- Spelt C, Quattrocchio F, Mol J, Koes R. 2000. ANTHOCYANIN1 of Petunia encodes a basic helix-loop-helix protein that directly activates transcription of structural anthocyanin genes. The Plant Cell 12:1619–1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamagnone L, Merida A, Stacey N, Plaskitt K, Parr A, Chang C-F, Lynn D, Dow JM, Roberts K, Martin C. 1998. Inhibition of phenolic acid metabolism results in precocious cell death and altered cell morphology in leaves of transgenic tobacco plants. The Plant Cell 10:1801–1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamari G, Borochov A, Atzorn R, Weiss D. 1995. Methyl jasmonate induces pigmentation and flavonoid gene expression in petunia corollas: A possible role in wound response. Physiologia Plantarum 94:45–50. [Google Scholar]
- Tao R, Bai S, Ni J, Yang Q, Zhao Y, Teng Y. 2018. The blue light signal transduction pathway is involved in anthocyanin accumulation in ‘Red Zaosu’ pear. Planta 248:37–48. [DOI] [PubMed] [Google Scholar]
- Taylor-Teeples M, Lin L, de Lucas M, Turco G, Toal TW, Gaudinier A, Young NF, Trabucco GM, Veling MT, Lamothe R, et al. 2015. An Arabidopsis gene regulatory network for secondary cell wall synthesis. Nature 517:571–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telias A, Lin-Wang K, Stevenson DE, Cooney JM, Hellens RP, Allan AC, Hoover EE, Bradeen JM. 2011. Apple skin patterning is associated with differential expression of MYB10. BMC Plant Biology 11: 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tirumalai V, Swetha C, Nair A, Pandit A, Shivaprasad PV. 2019. miR828 and miR858 regulate VvMYB114 to promote anthocyanin and flavonol accumulation in grapes. Journal of Experimental Botany 70:4775–4791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turing AM. 1952. The chemical basis of morphogenesis. Philosophical Transactions of the Royal Society of London B 237:37–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viola IL, Camoirano A, Gonzalez DH. 2016. Redox-dependent modulation of anthocyanin biosynthesis by the TCP transcription factor TCP15 during exposure to high light intensity conditions in Arabidopsis. Plant Physiology 170:74–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada T, Tachibana T, Shimura Y, Okada K. 1997. Epidermal cell differentiation in Arabidopsis determined by a Myb homolog, CPC. Science 277:1113–1116. [DOI] [PubMed] [Google Scholar]
- Walker AR, Davison PA, Bolognesi-Winfield AC, James CM, Srinivasan N, Blundell TL, Esch JJ, Marks MD, Gray JC. 1999. The TRANSPARENT TESTA GLABRA1 locus, which regulates trichome differentiation and anthocyanin biosynthesis in Arabidopsis, encodes a WD40 repeat protein. The Plant Cell 11:1337–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Lian W, Cao Y, Wang X, Wang G, Qi C, Liu L, Qin S, Yuan X, Li X, et al. 2018. Overexpression of BoNAC019, a NAC transcription factor from Brassica oleracea, negatively regulates the dehydration response and anthocyanin biosynthesis in Arabidopsis. Nature Scientific Reports 8:13349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Wang B, Yu H, Guo H, Lin T, Kou L, Wang A, Shao N, Ma H, Xiong G, et al. 2020. Transcriptional regulation of strigolactone signalling in Arabidopsis. Nature 583:277–281. [DOI] [PubMed] [Google Scholar]
- Wang X, Wang X, Hu Q, Dai X, Tian H, Zheng K, Wang X, Mao T, Chen J-G, Wang S. 2015. Characterization of an activation-tagged mutant uncovers a role of GLABRA2 in anthocyanin biosynthesis in Arabidopsis. The Plant Journal 83:300–311. [DOI] [PubMed] [Google Scholar]
- Wang Y, Liu W, Wang X, Yang R, Wu Z, Wang H, Wang L, Hu Z, Guo S, Zhang H, et al. 2020. MiR156 regulates anthocyanin biosynthesis through SPL targets and other microRNAs in poplar. Horticulture Research 7:118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Wang N, Xu H, Jiang S, Fang H, Su M, Zhang Z, Zhang T, Chen X. 2018a. Auxin regulates anthocyanin biosynthesis through the Aux/IAA-ARF signaling pathway in apple. Horticulture Research 5:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Wang N, Xu H, Jiang S, Fang H, Zhang T, Su M, Xu L, Zhang Z, Chen X. 2018b. Nitrogen affects anthocyanin biosynthesis by regulating MdLOB52 downstream of MdARF19 in callus cultures of red-fleshed apple (Malus sieversii f. niedzwetzkyana). Journal of Plant Growth and Regulation 37:719–729. [Google Scholar]
- Wang Y, Wang Y, Song Z, Zhang H. 2016. Repression of MYBL2 by both microRNA858a and HY5 leads to the activation of anthocyanin biosynthetic pathway in Arabidopsis. Molecular Plant 9:1395–1405. [DOI] [PubMed] [Google Scholar]
- Wang Z, Meng D, Wang A, Li T, Jiang S, Cong P-H, Li T. 2013. The methylation of the PcMYB10 promoter is associated with green-skinned sport in Max Red Bartlett Pear. Plant physiology 162: 885–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Z-Z, Hu K-D, Zhao D-L, Tang J, Huang Z-Q, Jin P, Li Y-H, Han Z, Hu L-Y, Yao G-F, et al. 2020. MYB44 competitively inhibits the formation of the MYB340-bHLH2-NAC56 complex to regulate anthocyanin biosynthesis in purple-fleshed sweet potato. BMC Plant Biology 20:258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss D, van der Luit A, Knegt E, Vermeer E, Mol JNM, Kooter JM. 1995. Identification of endogenous gibberellins in petunia flowers. Plant Physiology 107:695–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wild M, Davière J-M, Cheminant S, Regnault T, Baumberger N, Heintz D, Baltz R, Genschik P, Achard P. 2012. The Arabidopsis DELLA RGA-LIKE3 is a direct target of MYC2 and modulates jasmonate signaling responses. The Plant Cell 24: 3307–3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu A, DeviAllu A, Garapati P, Siddiqui H, Dortay H, Zanor M-I, Asensi-Fabado MA, Munné-Bosch S, Antonio C, Tohge T, et al. 2012. JUNGBRUNNEN1, a reactive oxygen species-responsive NAC transcription factor, regulates longevity in Arabidopsis. The Plant Cell 24:482–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G, Poethig RS. 2006. Temporal regulation of shoot development in Arabidopsis thaliana by miR156 and its target SPL3. Development 133:3539–3547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia R, Zhu H, An Y-Q, Beers EP, Liu Z. 2012. Apple miRNAs and tasiRNAs with novel regulatory networks. Genome Biology 13:R47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Y, Tan H, Ma Z, Huang J. 2016. DELLA proteins promote anthocyanin biosynthesis via sequestering MYBL2 and JAZ suppressors of the MYB/bHLH/WD40 complex in Arabidopsis thaliana. Molecular Plant 9:711–721. [DOI] [PubMed] [Google Scholar]
- Xu H, Wang N, Liu J, Qu C, Wang Y, Jiang S, Lu N, Wang D, Zhang Z, Chen X. 2017. The molecular mechanism underlying anthocyanin metabolism in apple using the MdMYB16 and MdbHLH33 genes. Plant Molecular Biology 94:149–165. [DOI] [PubMed] [Google Scholar]
- Xu M, Hu T, Park M-Y, Earley KW, Wu G, Yang L, Poethig RS. 2016. Developmental functions of miR156-regulated SQUAMOSA PROMOTER BINDING PROTEIN-LIKE (SPL) genes in Arabidopsis thaliana. PLOS Genetics 12:e1006263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W, Dubos C, Lepiniec L. 2015. Transcriptional control of flavonoid biosynthesis by MYB–bHLH–WDR complexes. Trends in Plant Science 20:176–185. [DOI] [PubMed] [Google Scholar]
- Yang F, Cai J, Yang Y, Liu Z. 2013. Overexpression of microRNA828 reduces anthocyanin accumulation in Arabidopsis. Plant Cell Tissue and Organ Culture 115:159–167. [Google Scholar]
- Yao R, Ming Z, Yan L, Li S, Wang F, Ma S, Yu C, Yang M, Chen L, Chen L, et al. 2016. DWARF14 is a non-canonical hormone receptor for strigolactone. Nature 536:469–473. [DOI] [PubMed] [Google Scholar]
- Yoshida K, Ma D, Constabel CP. 2015. The MYB182 protein down-regulates proanthocyanidin and anthocyanin biosynthesis in poplar by repressing both structural and regulatory flavonoid genes. Plant Physiology 167:693–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Y-W, Sagawa JM, Young RC, Christensen BJ, Bradshaw HD. 2013. Genetic dissection of a major anthocyanin QTL contributing to pollinator-mediated reproductive isolation between sister species of Mimulus. Genetics 194:255–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G, Chen D, Zhang T, Duan A, Zhang J, He C. 2018. Transcriptomic and functional analyses unveil the role of long non-coding RNAs in anthocyanin biosynthesis during sea buckthorn fruit ripening. DNA Research 25:465–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Gong J, Chen K, Yao W, Zhang B, Wang J, Tian S, Liu H, Wang Y, Liu Y, Du L. 2020. A novel R3 MYB transcriptional repressor, MaMYBx, finely regulates anthocyanin biosynthesis in grape hyacinth. Plant Science 298:110588. [DOI] [PubMed] [Google Scholar]
- Zheng T, Tan W, Yang H, Zhang LE, Li T, Liu B, Zhang D, Lin H. 2019. Regulation of anthocyanin accumulation via MYB75/HAT1/TPL-mediated transcriptional repression. PLOS Genetics 15: e1007993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou F, Lin Q, Zhu L, Ren Y, Zhou K, Shabek N, Wu F, Mao H, Dong W, Gan L, et al. 2013. D14-SCFD3-dependent degradation of D53 regulates strigolactone signalling. Nature 504:406–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H, Lin-Wang K, Wang H, Gu C, Dare AP, Espley RV, He H, Allen AC, Han Y. 2015. Molecular genetics of blood-fleshed peach reveals activation of anthocyanin biosynthesis by NAC transcription factors. The Plant Journal 82:105–121. [DOI] [PubMed] [Google Scholar]
- Zhou H, Lin-Wang K, Wang F, Espley RV, Ren F, Zhao J, Ogutu C, He H, Jiang Q, Allan AC, et al. 2019. Activator-type R2R3-MYB genes induce a repressor-type R2R3-MYB gene to balance anthocyanin and proanthocyanidin accumulation. New Phytologist 221:1919–1934. [DOI] [PubMed] [Google Scholar]
- Zhou L-L, Zeng H-N, Shi M-Z, Xie D-Y. 2008. Development of tobacco callus cultures over expressing Arabidopsis PAP1/MYB75 transcription factor and characterization of anthocyanin biosynthesis. Planta 229:37–51. [DOI] [PubMed] [Google Scholar]
- Zhu H-F, Fitzsimmons K, Khandelwal A, Kranz RG. 2009. CPC, a single-repeat R3 MYB, is a negative regulator of anthocyanin biosynthesis in Arabidopsis. Molecular Plant 2:790–802. [DOI] [PubMed] [Google Scholar]
- Zhu Y-C, Zhang B, Allan AC, Lin-Wang K, Zhao Y, Wang K, Chen K-S, Xu C-J. 2020. DNA demethylation is involved in the regulation of temperature-dependent anthocyanin accumulation in peach. The Plant Journal 102:965–976. [DOI] [PubMed] [Google Scholar]
- Zimmermann IM, Heim MA, Weisshaar B, Uhrig JF. 2004. Comprehensive identification of Arabidopsis thaliana MYB transcription factors interacting with R/B-like BHLH proteins. The Plant Journal 40:22–34. [DOI] [PubMed] [Google Scholar]


