Abstract
BACKGROUND:
Approximately 30% of children with medulloblastoma (MB) experience recurrence which is usually incurable. This study compared the overall survival (OS) of patients receiving temozolomide (TMZ) and irinotecan with that of patients receiving TMZ, irinotecan and bevacizumab for recurrent MB/CNS primitive neuroectodermal tumor (PNET).
METHODS:
Patients with relapsed/refractory MB or CNS PNET were randomly assigned to receive TMZ (150 mg/m2/day PO on days1-5) and irinotecan (50 mg/m2/day IV on days 1-5) with or without bevacizumab (10 mg/kg IV on days 1 and 15).
RESULTS:
105 patients were eligible and treated on study. Median OS was 13 months in the standard arm and 19 months with the addition of bevacizumab; median EFS was 6 months in the standard arm and 9 months with the addition of bevacizumab. The hazard ratio for death from the stratified relative-risk regression model is 0.63. Overall, 23 patients completed 12 courses of planned protocol therapy, 23% (12/52) in the experimental arm with bevacizumab vs. 21% (11/53) in the standard arm. Toxicity profiles were comparable in both treatment arms. The estimate of the incidence of feasibility events associated with the bevacizumab arm is 3/52=5.8% (95% CI 1.2%-16%). Events included myelosuppression, electrolyte abnormalities, diarrhea and elevated transaminases. One intracranial hemorrhage event was observed in each arm.
CONCLUSION:
The addition of bevacizumab to TMZ/irinotecan significantly reduced the risk of death in children with recurrent MB. The combination was relatively well tolerated in this heavily pre-treated cohort. The 3-drug regimen demonstrated a sufficient risk reduction to warrant further investigation.
Keywords: recurrent medulloblastoma, PNET, bevacizumab, temozolomide, irinotecan
1. INTRODUCTION
Medulloblastoma (MB) is a general classification for what has been proven to be a heterogeneous group of malignant embryonal brain tumors in the posterior fossa. (1–4) Prior to the 2016 WHO reclassification of pediatric brain tumors, the term CNS primitive neuroectodermal tumor (PNET) was used to describe other highly aggressive embryonal tumors. Historically, MB and CNS PNET patients were treated in the same way and often enrolled on the same clinical trials based on prior disease classification systems. While the WHO classification has evolved, data from clinical trials that were initiated prior to 2016 can still yield important information about treating these tumors.
Despite aggressive therapy including surgery and chemotherapy with or without radiation, approximately 30% of children with MB experience recurrence. Curative therapy for recurrent MB remains elusive. Strategies have ranged from palliative care alone to any combination of aggressive surgical resection, re-irradiation and chemotherapy including high-dose chemotherapy with stem-cell rescue. (5,6) While tumor-directed therapy at recurrence seems to improve overall survival compared to palliation alone, long-term survival in most studies remains less than 10%. (7–12) Clearly, improved treatment strategies for recurrent MB are needed, and those regimens with utility in the recurrent setting could be considered for use in newly diagnosed patients.
Temozolomide is an orally administered alkylating agent of the imidazotetrazine derivatives with excellent CNS penetration. Phase II studies have shown variable response rates of 16-47% in children and adolescents with recurrent medulloblastomas or central nervous system primitive neuroectodermal tumor (CNS PNET). (11, 13) Irinotecan is a water-soluble camptothecin derivative that inhibits topoisomerase I (topo I), an enzyme involved in DNA repair, transcription and replication. (14,15) Irinotecan has been shown to have single-agent activity against recurrent medulloblastomas. (16–18) There is demonstrated efficacy of the combination of irinotecan and temozolomide in patients with recurrent MB/CNS PNET. (17) Bevacizumab is a humanized monoclonal neutralizing antibody binding all five isoforms of human vascular endothelial growth factor (VEGF). CNS tumors in general, and MB specifically, are potentially excellent targets for anti-angiogenic therapy given the presence of tumor neo-vascularization and angiogenic profile. (19, 20–25)
In summary, irinotecan and temozolomide have activity against recurrent MB/PNET, the combination has been well tolerated in heavily pre-treated patients, (26,27) and the addition of bevacizumab theoretically may increase the efficacy of chemotherapy. (28–35) Therefore, a phase II (36) trial evaluating the addition of bevacizumab to the combination of irinotecan and temozolomide in MB and CNS PNET of childhood was performed.
2. PATIENTS AND METHODS
Children’s Oncology Group (COG) ACNS0821, approved by the National Cancer Institute (NCI) Central Institutional Review Board (CIRB) and the IRB’s of participating sites, was a randomized Phase II screening trial (36) to compare temozolomide (150 mg/m2 PO for 5 days) with irinotecan (50 mg/m2 IV for 5 days) to temozolomide, irinotecan plus bevacizumab (10 mg/kg IV on Days 1 and 15) in children with recurrent MB or CNS PNET including pineoblastoma. (Figure 1) Each course was repeated every 28 days for up to 12 courses for patients continuing on protocol therapy as long as therapy was tolerated and there was no evidence of further disease progression.
FIGURE 1.
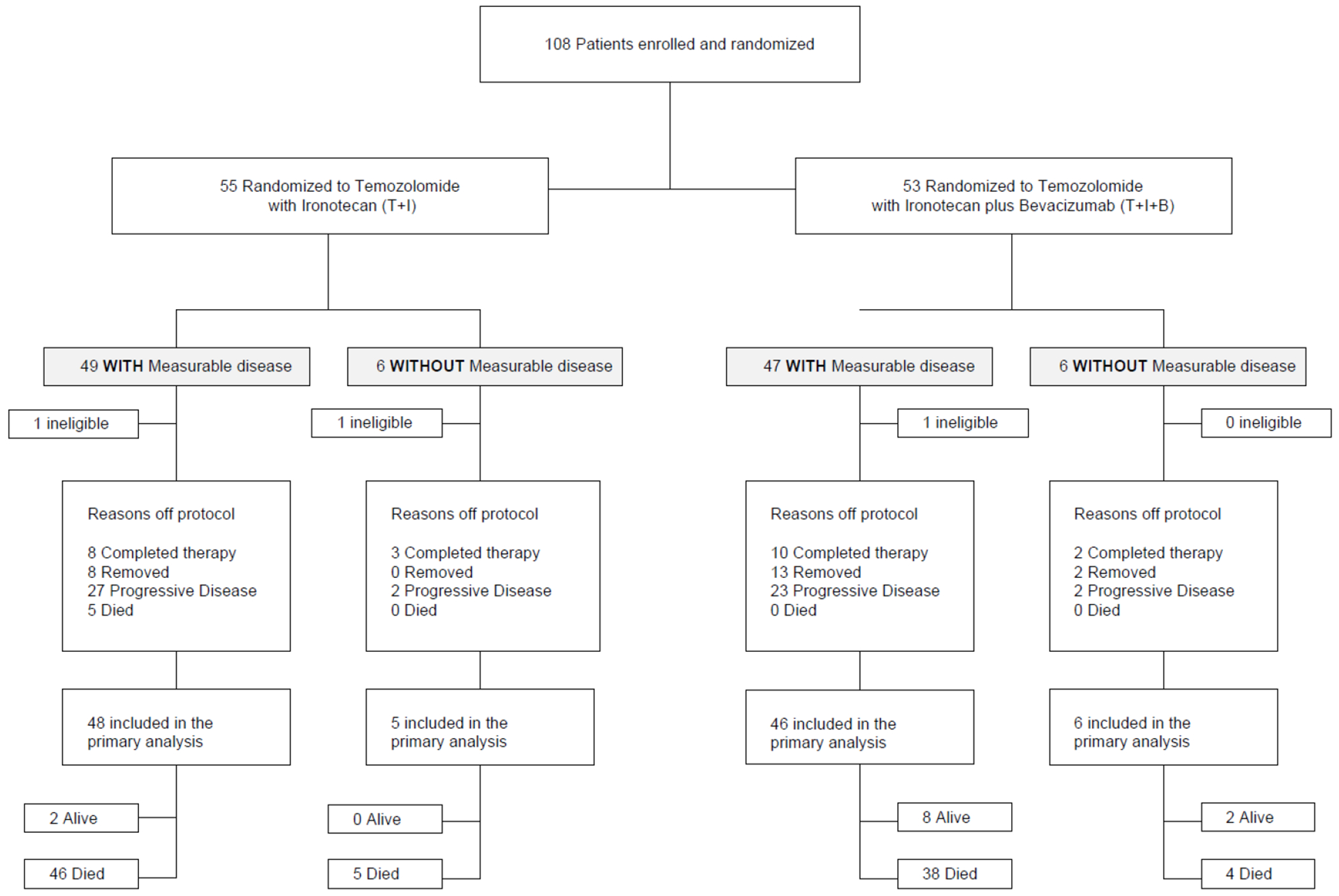
Study participation and flow through the trial.
Patients less than 21 years who had relapsed or become refractory to standard chemotherapy were eligible to enroll. Patients were required to have received at least one and at most two relapses prior to enrollment, and patients with primary refractory disease were eligible. Prior radiation was acceptable but not required. Histologic verification of the malignancy at original diagnosis or at the time of recurrence was required as was clear residual disease. Organ function parameters and bone marrow recovery from prior tumor-directed therapy was required prior to enrollment. At initiation of the trial, residual disease suitable for enrollment was defined as tumor that was measurable in two perpendicular diameters on MRI. There was a subsequent protocol amendment to allow enrollment of patients with diffuse leptomeningeal disease or clear MRI evidence of disease that may not be measurable in two perpendicular diameters. MRI interpretation was performed at each treating institution and reported according to a modified RECIST criteria (37) as per COG guidelines. Central radiology review was not performed. CSF was not used as a response criterion.
Statistical Methods
It is important to note the dramatic evolution of the WHO classification of both MB and CNS PNET since the conception of this trial. (1) All patients were classified according to the histological diagnosis rendered by the institutional pathologist at the time of initial diagnosis into two groups as medulloblastoma or other embryonal CNS tumor. In addition to this classification banked tumor tissue was available for 36 patients. These 36 samples were analyzed, and the molecular subgrouping was determined.
The primary objective was to compare the risk of death between the regimens. Secondary objectives were (1) To assess the response rate for each treatment arm amongst patients who are enrolled with measurable disease, and (2) To estimate the risk for event-free survival (EFS) event across regimens. Feasibility and safety evaluations for each treatment arm were also performed.
Based on prior COG studies, it was estimated that 36 eligible patients would be available for enrollment annually, at a rate of 3 patients per month for 36 months, for a total of approximately 108 eligible patients. Patients were stratified for randomization to each treatment arm according to whether or not they had measurable disease. Stratum 1 patents had measurable disease in two dimensions, while Stratum 2 patients had clear evidence of disease that may not be measurable in two perpendicular diameters (e.g. leptomeningeal disease).
Overall survival and EFS were determined for each patient and compared across regimens using the stratified log-rank test. (38) Overall survival (OS) was defined as time from enrollment to death regardless of cause or date of last patient contact, whichever came first. Patients whose OS follow-up was terminated because of death were considered to have experienced an event; otherwise the patient was censored at last contact. EFS interval was defined as time from enrollment to: (1) disease progression; (2) diagnosis of second malignant neoplasm; (3) death regardless of cause; or (4) date of last contact, whichever came first. Patients whose EFS follow-up was terminated because of reasons (1)-(3) above were considered to have experienced an event; otherwise the patient was considered censored at the conclusion of EFS-time. Kaplan-Meier estimates of the OS and EFS for each of the randomized regimens were constructed. (39)
The study was designed as a screening trial to determine whether there was sufficient evidence to further study the combination of temozolomide, irinotecan and bevacizumab. After accrual and treatment of 108 patients, follow-up was to be continued for six additional months after the last patient was enrolled. A comparison of risk of death between the two regimes was to be conducted and a one-sided p-value of 0.15 or less in favor of the combination of temozolomide, irinotecan and bevacizumab was considered sufficient evidence to further investigate the combination. With this analytic plan the design had 87% power to detect a 40% decrease in the risk of death associated with the bevacizumab containing regimen.
The relative hazard rate (RHR) for death and for EFS-event were estimated from the stratified proportional hazards regression model with randomized treatment assignment as the only covariate and presence of measurable disease as the stratification factor (39). The 95% confidence interval for the RHR was constructed using that proportional hazards regression model. A 2-sided p-value of 0.05 or less was considered significant for comparisons other than the screening comparison conducted as the primary analysis. Heterogeneity of risk of death and EFS-event were assessed by comparing the hazard rates as estimated from each of the strata separately and testing the hypothesis of equal hazard rates using the asymptotic distribution of the coefficients from the proportional hazards regression models. (39) The median follow-up for OS was calculated using the Kaplan-Meier estimate of potential follow-up as proposed by Schemper and Smith (40).
The feasibility of the two regimens was monitored as well. A patient was considered to have experienced a feasibility event if the patient died while receiving protocol therapy and treatment was considered the principal cause of death or the patient was removed from protocol therapy prior to month four because of toxicity. This study utilized the Common Terminology Criteria for Adverse Events (CTCAE) version 4. For the response assessment, MRI scans were required at enrollment and following every 2 courses thereafter.
Only patients enrolled with measurable disease were considered in the evaluation of response rate. One week prior to the third cycle and every other subsequent cycle of therapy, the treating team for each patient evaluated MRI response. The measurements of the longest tumor dimension, and its perpendicular, of all target lesions were determined by changes in size using the longest tumor dimension, and its perpendicular. Either T1 or T2 weighted images were used - whichever gave the best estimate of tumor size as determined by the treating team. The overall response assessment took into account response in both target and non-target lesion, and the appearance of new lesions as detailed in Table 1.
TABLE 1:
Overall Response Assessment for Target Tumor Measurable in Two Diameters
| Target Lesions | Non-target Lesions | New Lesions | Overall Response |
|---|---|---|---|
| CR | CR | No | CR |
| CR | IR/SD | No | PR |
| PR | CR, IR/SD | No | PR |
| SD | CR, IR/SD | No | SD |
| PD | Any | Yes or No | PD |
| Any | PD | Yes or No | PD |
| Any | Any | Yes | PD |
CR – Complete Response
PR – Partial Response
SD – Stable Disease
PD – Progressive Disease
IR – Incomplete Response
RESULTS
The study was opened in November 2010 and closed to accrual in December 2015. Data current to December 2018 were used for this analysis. One-hundred and eight (108) patients enrolled on this study and 3 were considered ineligible: 2 secondary to organ function requirements, 1 without measurable disease at the time of enrollment prior to the amendment expanding enrollment to include patients with recurrent disease that was not strictly measurable. (Table 2) The best response for patients with measurable disease was a complete response in 17.4% of patients on the three-drug regimen compared to none in the two-drug arm. Progressive disease was the best response for 13% of patients in the three-drug regimen and 33% of patients with measurable disease in the two-drug arm. (Table 3)
TABLE 2:
Baseline Characteristics for all eligible patients
| Characteristic | Categories | Temozolomide + Irinotecan | Temozolomide + Irinotecan + Bevacizumab |
|---|---|---|---|
| Age at Enrollment | Median (Range|IQR1) | 9 (1-21|6-13) | 10 (0-18|7.5-13.5) |
| Number (Percent) | Number (Percent) | ||
| Patient Sex | Male | 33 (62.3) | 36 (69.2) |
| Female | 20 (37.7) | 16 (30.8) | |
| Race | Asian | 2 (3.8) | 2 (3.8) |
| Native Hawaiian or Pacific Islander | 1 (1.9) | 1 (1.9) | |
| Black or African American | 8 (15.1) | 6 (11.5) | |
| White | 39 (73.6) | 41 (78.8) | |
| Not Reported | 3 (5.7) | 2 (3.8) | |
| Ethnicity | Hispanic or Latino | 8 (15.1) | 10 (19.2) |
| Not Hispanic or Latino | 44 (83.0) | 42 (80.8) | |
| Not Reported | 1 (1.9) | 0 (0.0) | |
| Extent of Disease at Enrollment | Measurable Disease | 48 (90.6) | 46 (88.5) |
| Without Measurable Disease | 5 (9.4) | 6 (11.5) | |
| Initial Diagnosis | Medulloblastoma | 44 (83.0) | 41 (78.8) |
| Other Embryonal CNS Tumor | 9 (17.0) | 11 (21.2) | |
| Received Radiation Therapy as a Component of Prior Therapy | Yes | 44 (83.0) | 45 (86.5) |
| No | 9 (17.0) | 7 (13.5) | |
TABLE 3:
Best response for patients with measurable disease.
|
|
|
|
|
|---|---|---|---|
| Temozolomide + Irinotecan |
Temozolomide + Irinotecan - Bevacizumab |
||
| Characteristics |
Categories |
N (%) |
N (%) |
| Best Response | Complete response | 0 (0.0) | 8 (17.4) |
| Non-responder | 2 (4.2) | 1 (2.2) | |
| Progressive disease | 16 (33.3) | 6 (13.0) | |
| Partial response | 16 (33.3) | 14 (30.4) | |
| Stable disease | 14 (29.2) | 17 (37.0) | |
The adverse events reported were within those expected for this patient population and treatment plan. Toxicity profiles were comparable in both treatment arms. (Table 4) A total of 5 patients (4.8% of eligible patients) experienced a feasibility event. The estimate of the incidence of feasibility events associated with the bevacizumab arm is 3/52=5.8% (95% CI 1.2%-16%). Events included myelosuppression, electrolyte abnormalities, diarrhea and elevated transaminases.
TABLE 4:
Grade 3 and Higher Toxicity data for all patients.
| Treatment | |||
|---|---|---|---|
| TEM+IRIN (N=53) | TEM+IRIN+BEVA (N=52) | ||
| % | % | ||
| Organ systems | Toxicity Type | 98.1 | 98.1 |
| None | |||
| Gastrointestinal | Abdominal pain | 3.8 | 1.9 |
| Colitis | 1.9 | ||
| Diarrhea | 18.9 | 28.8 | |
| Mucositis oral | 1.9 | ||
| Nausea | 3.8 | 1.9 | |
| Stomach pain | 1.9 | ||
| Typhlitis | 1.9 | ||
| Vomiting | 9.4 | 1.9 | |
| Investigations | Alanine aminotransferase increased | 5.7 | 5.8 |
| Aspartate aminotransferase increased | 1.9 | ||
| Blood bilirubin increased | 1.9 | ||
| GGT increased | 3.8 | ||
| Lymphocyte count decreased | 11.3 | 19.2 | |
| Neutrophil count decreased | 30.2 | 46.2 | |
| Platelet count decreased | 24.5 | 38.5 | |
| White blood cell decreased | 18.9 | 28.8 | |
| Immune | Allergic reaction | 1.9 | |
| Blood/Lymphatic | Anemia | 13.2 | 13.5 |
| Blood and lymphatic system disorders - Other, specify | 1.9 | ||
| Febrile neutropenia | 7.5 | 9.6 | |
| Metabolism/Nutrition | Anorexia | 5.7 | 3.8 |
| Dehydration | 7.5 | 7.7 | |
| Hypercalcemia | 1.9 | ||
| Hyperuricemia | 1.9 | ||
| Hypoalbuminemia | 5.7 | ||
| Hypocalcemia | 1.9 | ||
| Hypokalemia | 3.8 | 7.7 | |
| Hyponatremia | 5.8 | ||
| Hypophosphatemia | 3.8 | ||
| Infections/Infestations | Catheter related infection | 1.9 | |
| Enterocolitis infectious | 3.8 | 1.9 | |
| Infections and infestations - Other, specify | 3.8 | ||
| Sepsis | 9.4 | 1.9 | |
| Skin infection | 1.9 | ||
| Urinary tract infection | 1.9 | ||
| General/Administration | Fatigue | 1.9 | |
| Fever | 1.9 | ||
| Non-cardiac chest pain | 1.9 | ||
| Vascular | Hypertension | 1.9 | |
| Hypotension | 3.8 | ||
| Psychiatric | Personality change | 1.9 | |
| Suicidal ideation | 1.9 | ||
| Renal/Urinary | Proteinuria | 1.9 | |
| Skin/Subcutaneous | Rash maculo-papular | 1.9 | 1.9 |
| Nervous | Seizure | 1.9 | |
In the TMZ, irinotecan and bevacizumab arm, one patient experienced grade 4 neutropenia, grade 3 hypokalemia and grade 3 thrombocytopenia and was taken off therapy. A second patient experienced possibly related grade 4 hyponatremia, neutropenia, thrombocytopenia, grade 3 diarrhea and fatigue, and was taken off therapy. A third patient experienced grade 4 ALT elevation, grade 4 neutropenia and thrombocytopenia during cycles 1 and 2. Despite protocol-defined dose reductions, the patient experienced grade 4 neutropenia in cycles 3 and 4 and was removed from protocol therapy.
In the TMZ and irinotecan arm, one patient experienced grade 4 ALT, AST and bilirubin elevations, developed sepsis, and died 23 days after enrollment. A second patient experienced grade 4 dehydration secondary to persistent grade 3 vomiting and diarrhea and died of sepsis 26 days after study enrollment. One intracranial hemorrhage event was observed in each arm. One patient experienced a grade 2 intracranial hemorrhage six days after study enrollment on the 3-drug arm, and the event was considered possibly related to protocol therapy by the treating physician. One patient on the 2-drug arm experienced a grade 4 intracranial hemorrhage at the site of the patient’s recurrence of MB, and the event was considered unrelated to protocol therapy by the treating physician.
Overall, 23 patients completed 12 courses of planned protocol therapy, 23% in the experimental arm with bevacizumab vs. 21% in the standard arm. (TABLE 5) The median follow-up for OS was 65 months. The calculation of median potential follow-up takes into account the follow-up time contributed by all eligible patients regardless of the amount of protocol therapy delivered. T+I+B met the screening criterion for reducing the risk of death (1-sided p = 0.01; RHR = 0.63; 95% CI 0.41-0.93; Figure 2A). T+I+B significantly reduced risk for EFS event (p = 0.0059; 95% RHR = 0.57; CI 0.38-0.85; Figure 2B). Median EFS was 6 months in the standard arm and 9 months with the addition of bevacizumab, and median OS was 13 months in the standard arm and 19 months with the addition of bevacizumab. There is no evidence to suggest that the efficacy of T+I+B is different in stratum 1 (measurable disease) when compared with stratum 2 (disease clearly present but not measurable in 2 dimensions) (p = 0.33). Thus, the presence of measurable disease at enrollment did not appear to be related to improved outcome of the bevacizumab arm.
TABLE 5:
Number of patients who continued on protocol therapy delivered on each treatment arm.
| Number of Cycles | T+I | T+I+B | Combined |
|---|---|---|---|
| 1-2 | 53 | 52 | 105 |
| 3-4 | 33 | 41 | 74 |
| 5-6 | 26 | 29 | 55 |
| 7-8 | 18 | 22 | 40 |
| 9-10 | 16 | 15 | 31 |
| 11-12 | 11 | 13 | 14 |
FIGURE 2.
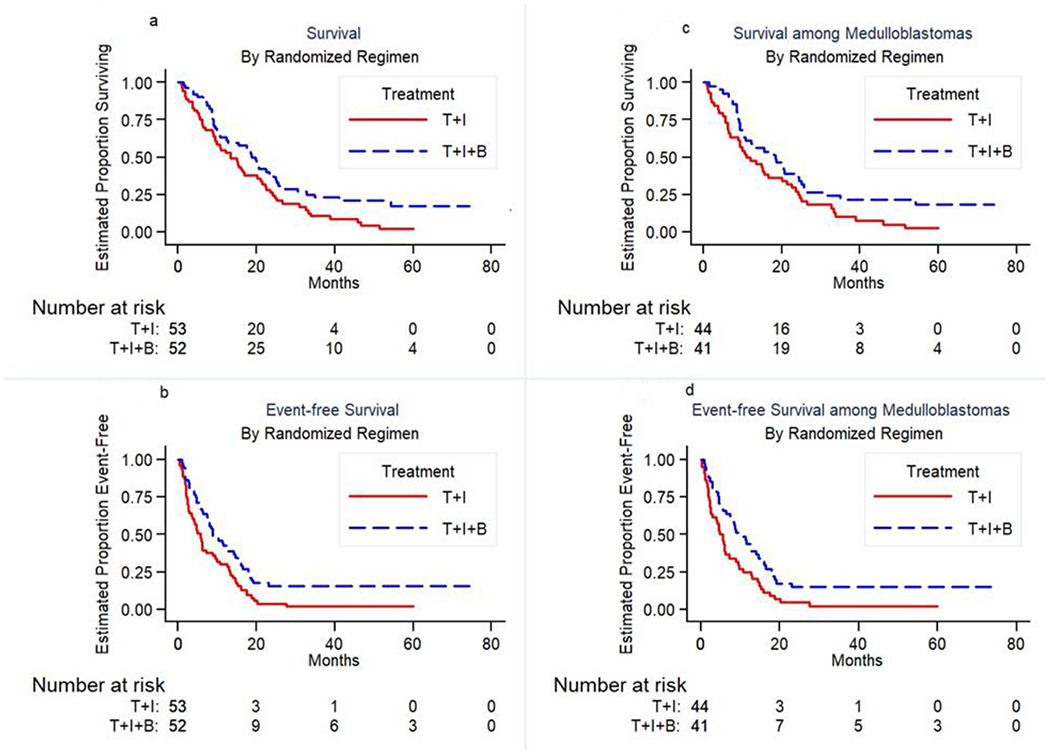
Overall Survival and Event Free Survival for all enrolled patients (figures on the left) and Overall Survival and Event Free Survival for all medulloblastoma patients (figures on the right).
When restricted to the 85 patients with medulloblastoma, T+I+B met the screening criterion for reducing the risk of death (1-sided p = 0.024; RHR = 0.63; 95% CI 0.39-1). T+I+B significantly reduced risk for EFS event (p = 0.0078; 95% RHR = 0.54; CI 0.42-0.69; Figure 2D). Median EFS was 5 months in the standard arm and 10 months with the addition of bevacizumab, and median OS was 11 months in the standard arm and 19 months with the addition of bevacizumab. (Figure 2C) The maximum survival and EFS time was 74.5 months and occurred in a patient with medulloblastoma who had not demonstrated an EFS event at that time of last study follow-up.
Histology was confirmed at each treating center for enrollment, but for 36 of the enrolled patients, tumor tissue was available for further classification through the COG biorepository. (Table 6) There are no apparent significant relationships between molecular grouping and randomized treatment. The relationship between molecular grouping and risk for death is shown in Figure 3. The limited modern classification data for those patients with historically categorized “PNET” tumors is insufficient to support a conclusion regarding differences in efficacy of each regimen for these tumors.
TABLE 6:
Histology and molecular grouping.
| Temozolomide + Irinotecan | Temozolomide + Irinotecan + Bevacizumab | ||
|---|---|---|---|
|
|
|||
| Histology | Molecular Grouping | N (%) | N (%) |
| Medulloblastoma | MB,G3 | 5 (9.4) | 3 (5.8) |
| MB,G4 | 13 (24.5) | 6 (11.5) | |
| MB,SHH | 1 (1.9) | 1 (1.9) | |
| MB,ETMR | 0 (0.0) | 1 (1.9) | |
| PNET | ETMR | 1 (1.9) | 0 (0.0) |
| PB | 3 (5.7) | 2 (3.8) | |
| Unknown* | 30 (56.6) | 39 (75.0) | |
No grouping available
PB=Pineoblastoma
MB, G3= medulloblastoma Group 3
MB, G4= medulloblastoma Group 4
MB, SHH= medulloblastoma Sonic Hedgehog
ETMR=Embryonal tumors with multilayered rosettes
FIGURE 3.
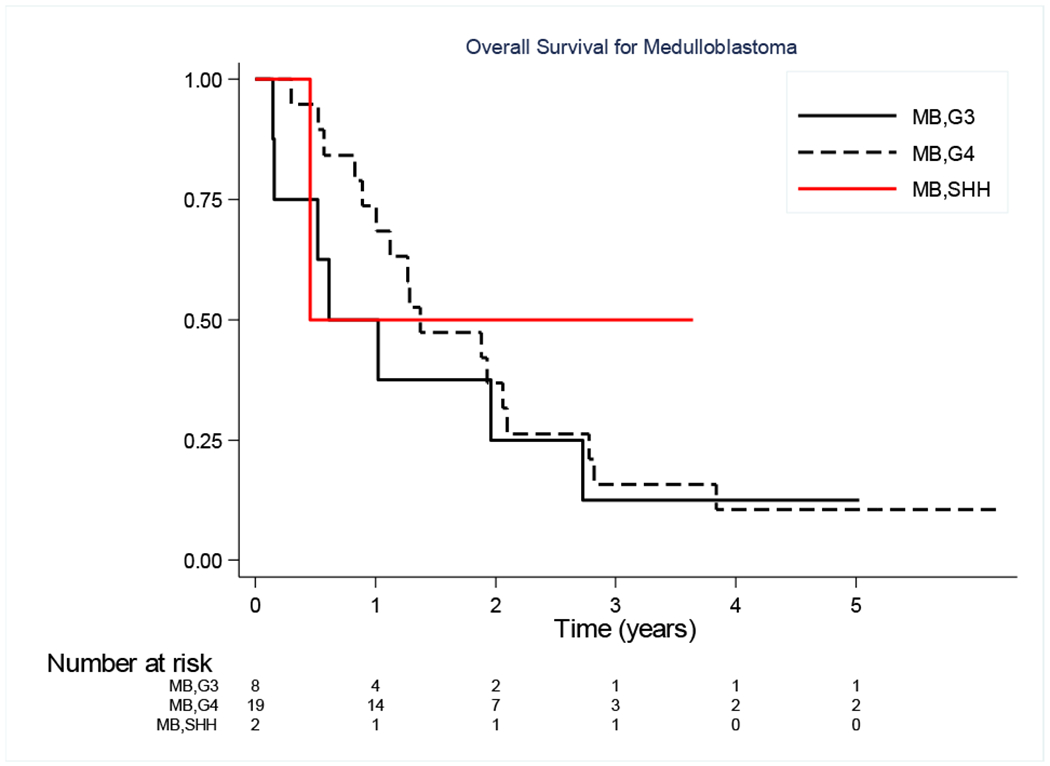
Overall Survival of Medulloblastoma patients for which molecular classification was available.
DISCUSSION
The addition of bevacizumab to TMZ/irinotecan significantly reduced the risk of death and an EFS-event in children with recurrent MB. The combination was relatively well tolerated in this heavily pre-treated cohort. Based on the initial protocol goals, the 3-drug regimen demonstrated a sufficient risk reduction to warrant further investigation.
Bevacizumab was investigated in this group of tumors based on preclinical data demonstrating expression of VEGF and VEGF receptors in MB. (21–23) Since it is well known that interpretation of tumor measurements in response to bevacizumab may be challenging, this study was designed such that the primary objective was to compare the risk of death between the regimens. We report the outcomes of the secondary objectives, response rate and EFS, which also seem improved with bevacizumab. But we emphasize that risk of death was significantly reduced with the addition of bevacizumab as this outcome is not impacted by imaging interpretation.
The understanding of the biology of malignant CNS embryonal tumors has evolved tremendously over the past decade since this protocol was first conceived, and this better understanding drives current concepts in MB clinical trials. (41–43) Medulloblastoma is now known to be a number of molecularly distinct subgroups. Likewise, “PNET” is no longer recognized by the WHO and these are now recognized as distinct tumors based on modern diagnostic techniques/criteria. As such, the relevance of including CNS PNET and pineoblastoma in this study is questionable given today’s tumor classifications. This is a weakness of the study, but this does not diminish the findings that bevacizumab improved outcomes in this patient cohort. For that reason, we provide outcome analysis for MB patients alone. It is likely that the children who had recurrent disease and were enrolled on ACNS0821 had high-risk disease at initial presentation. There were more males enrolled which is consistent with the knowledge that males are more likely to be type 3 and 4 MB (4).
Given the evolution of medulloblastoma and CNS embryonal tumor diagnoses, we analyzed outcomes based on updated tumor classifications for those tumor samples that were further classified. This information is included but we emphasize that the study could not have been designed at the time of initiation to adequately power this analysis to establish any clear conclusions. Of the patients enrolled on ACNS0821, 36 were enrolled on COG biology studies and COG studies for newly diagnosed CNS tumors. Conclusions based on molecular subgrouping are statistically limited as the study was not designed to assess differences in outcome among these subgroups and the number of samples with this diagnostic specificity is small. But based on the data available, there are no apparent significant relationships between MB molecular grouping and randomized treatment. That is, based on the available data, the distribution of tumor subgroups appears balanced between both treatment arms.
It is important to note that while MB subgroup does not appear to change at the time of recurrence, (44) there is substantial genetic divergence of the dominant clone after therapy. (45) As such, the hypothetical actionable targeted therapeutic options for these patient tumors at initial presentation may or may not hold true at the time of their recurrence. Since repeat biopsy is not always in the patient’s best interest, this will remain a challenge in future studies of recurrent CNS tumors.
Prior reports demonstrated some efficacy of these agents alone or in combination, (46–48),but this report is the largest cohort to date. In an Italian multi-institutional phase II trial, Cefalo et al. (49) demonstrated that temozolomide is an active agent in children with recurrent medulloblastoma/primitive neuroectodermal tumor. Patients received TMZ 120 to 200 mg/m2/day x 5 days. The estimated overall survival rates at 6 and 12 months were 42.5% and 17.5%. Nicholson et al (13) showed an estimated response rate for patients with MB treated with TMZ of 16% on Children’s Cancer Group protocol A09701. Grill et al (50), using TMZ and irinotecan, demonstrated an objective response rate during the first 4 cycles of 32.6%, a median duration of response of 27.0 weeks, and a median survival of 16.7 months. In a small cohort of patients receiving TMZ, irinotecan and bevacizumab, Aguilera et al (51) demonstrated a median time to progression of 11 months, a median overall survival of 13 months, with an objective tumor response at 3 months of 67 % (6 PR, 3 SD). Fangusaro et al (52) reported the tolerability of bevacizumab and irinotecan in recurrent pediatric CNS tumor patients. The most common toxicities attributable to BVZ included hypertension (38% of patients), fatigue (30%), epistaxis (24%) and proteinuria (22%); Twenty-two patients (24%) stopped therapy due to toxicity. Unfortunately, the eligibility criteria, treatment and objectives for many studies differ, making clear comparisons challenging. (53)
In conclusion, the addition of bevacizumab to TMZ and irinotecan proved tolerable with significantly improved EFS and OS. With the evolution in our understanding the classification of MB has changed since the inception of this study. Nonetheless, the distribution of MB subgroups appears balanced in this randomized study, and those with recurrent MB would have more likely had high-risk MB subgroup tumors. Thus, despite the inherent limitations which are acknowledged, these results support the further evaluation of TMZ, irinotecan and bevacizumab in high risk MB. Since OS was still unacceptably low for these patients with recurrent disease, this combination could be considered for future upfront trials in patients with high-risk medulloblastoma.
Funding:
Research reported in this publication was supported by the Children’s Oncology Group, the National Cancer Institute of the National Institutes of Health under award number NCTN Operations Center Grant U10CA180886 and NCTN Statistics & Data Center Grant U10CA180899. In addition, Genentech, Inc., also supported this NCI-sponsored trial with drug supply through the cooperative research and development agreement (CRADA) program. Support of COG Study ACNS0821 was also provided by St. Baldrick’s Foundation.
Abbreviations key:
- MB
Medulloblastoma
- OS
Overall Survival
- EFS
Event Free Survival
- TMZ
Temozolomide
- CNS
Central Nervous System
- PNET
primitive neuroectodermal tumor
- MR
Magnetic resonance
- VEGF
vascular endothelial growth factor
- COG
Children’s Oncology Group
- NIH
National Institutes of Health
Footnotes
Presented in abstract form (Neuro-Oncology, Volume 19, Issue suppl_6, 6 November 2017, Pages vi186, https://doi.org/10.1093/neuonc/nox168.753).
Data Sharing Statement:
Children’s Oncology Group Data Sharing Statement
The Children’s Oncology Group Data Sharing policy describes the release and use of COG individual subject data for use in research projects in accordance with National Clinical Trials Network (NCTN) Program and NCI Community Oncology Research Program (NCORP) Guidelines. Only data expressly released from the oversight of the relevant COG Data and Safety Monitoring Committee (DSMC) are available to be shared. Data sharing will ordinarily be considered only after the primary study manuscript is accepted for publication. For phase 3 studies, individual-level de-identified datasets that would be sufficient to reproduce results provided in a publication containing the primary study analysis can be requested from the NCTN/NCORP Data Archive at https://nctn-data-archive.nci.nih.gov/. Data are available to researchers who wish to analyze the data in secondary studies to enhance the public health benefit of the original work and agree to the terms and conditions of use. For non-phase 3 studies, data are available following the primary publication. An individual-level de-identified dataset containing the variables analyzed in the primary results paper can be expected to be available upon request. Requests for access to COG protocol research data should be sent to: datarequest@childrensoncologygroup.org. Data are available to researchers whose proposed analysis is found by COG to be feasible and of scientific merit and who agree to the terms and conditions of use. For all requests, no other study documents, including the protocol, will be made available and no end date exists for requests. In addition to above, release of data collected in a clinical trial conducted under a binding collaborative agreement between COG or the NCI Cancer Therapy Evaluation Program (CTEP) and a pharmaceutical/biotechnology company must comply with the data sharing terms of the binding collaborative/contractual agreement and must receive the proper approvals.
Conflicts of Interest: None
REFERENCES
- 1.Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D6, Cavenee WK, Ohgaki H, Wiestler OD, Kleihues P, Ellison DW. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol. 2016. Jun;131(6):803–20. doi: 10.1007/s00401-016-1545-1. Epub 2016 May 9. DOI: 10.1007/s00401-016-1545-1 [DOI] [PubMed] [Google Scholar]
- 2.Ramaswamy V, Remke M, Bouffet E, Bailey S, Clifford SC, Doz F, Kool M7, Dufour C, Vassal G, Milde T, Witt O, von Hoff K, Pietsch T, Northcott PA 13, Gajjar A, Robinson GW, Padovani L, André N, Massimino M, Pizer B, Packer R, Rutkowski S, Pfister SM, Taylor MD, Pomeroy SL. Risk stratification of childhood medulloblastoma in the molecular era: the current consensus. Acta Neuropathol. 2016. Jun;131(6):821–31. doi: 10.1007/s00401-016-1569-6. Epub 2016 Apr 4.. DOI: 10.1007/s00401-016-1569-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ramaswamy V, Taylor MD. Medulloblastoma: From Myth to Molecular. J Clin Oncol. 2017. Jul 20;35(21):2355–2363. doi: 10.1200/JCO.2017.72.7842. Epub 2017 Jun 22..DOI: 10.1200/JCO.2017.72.7842 [DOI] [PubMed] [Google Scholar]
- 4.Gajjar A, Bowers DC, Karajannis MA, Leary S, Witt H, Gottardo NG. Pediatric Brain Tumors: Innovative Genomic Information Is Transforming the Diagnostic and Clinical Landscape. J Clin Oncol. 2015. Sep 20;33(27):2986–98. doi: 10.1200/JCO.2014.59.9217. Epub 2015 Aug 24. DOI: 10.1200/JCO.2014.59.9217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gururangan S, Krauser J, Watral MA, et al. Efficacy of high-dose chemotherapy or standard salvage therapy in patients with recurrent medulloblastoma. Neuro-Oncology. 2008;10(5):745–751. doi: 10.1215/15228517-2008-044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Butturini AM, Jacob M, Aguajo J, Vander-Walde NA, Villablanca J, Jubran R, Erdreich-Epstein A, Marachelian A, Dhall G, Finlay JL. High-dose chemotherapy and autologous hematopoietic progenitor cell rescue in children with recurrent medulloblastoma and supratentorial primitive neuroectodermal tumors: the impact of prior radiotherapy on outcome. Cancer. 2009. Jul 1;115(13):2956–63. doi: 10.1002/cncr.24341. [DOI] [PubMed] [Google Scholar]
- 7.Pizer B, Donachie PH, Robinson K, Taylor RE, Michalski A, Punt J, Ellison DW, Picton S. Treatment of recurrent central nervous system primitive neuroectodermal tumours in children and adolescents: results of a Children’s Cancer and Leukaemia Group study. Eur J Cancer. 2011. Jun;47(9):1389–97. doi: 10.1016/j.ejca.2011.03.004. Epub 2011 Apr 5. [DOI] [PubMed] [Google Scholar]
- 8.Sabel M, Fleischhack G, Tippelt S, Gustafsson G, Doz F, Kortmann R, Massimino M, Navajas A, von Hoff K, Rutkowski S, Warmuth-Metz M, Clifford SC, Pietsch T, Pizer B, Lannering B; SIOP-E Brain Tumour Group. Relapse patterns and outcome after relapse in standard risk medulloblastoma: a report from the HIT-SIOP-PNET4 study. J Neurooncol. 2016. Sep;129(3):515–524. doi: 10.1007/s11060-016-2202-1. Epub 2016 Jul 16. DOI: 10.1007/s11060-016-2202-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aref D, Croul S. Medulloblastoma: recurrence and metastasis. CNS Oncol. 2013. Jul;2(4):377–85. doi: 10.2217/cns.13.30. DOI: 10.2217/cns.13.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wetmore C, Herington D, Lin T, Onar-Thomas A, Gajjar A, Merchant TE. Reirradiation of recurrent medulloblastoma: does clinical benefit outweigh risk for toxicity? Cancer. 2014. Dec 1;120(23):3731–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koschmann C, Bloom K, Upadhyaya S, Geyer JR, Leary SE. Survival After Relapse of Medulloblastoma. J Pediatr Hematol Oncol. 2016. May;38(4):269–73. doi: 10.1097/MPH.0000000000000547. DOI: 10.1097/MPH.0000000000000547. [DOI] [PubMed] [Google Scholar]
- 12.Johnston DL, Keene D, Strother D, Taneva M, Lafay-Cousin L, Fryer C, Scheinemann K, Carret AS, Fleming A, Afzal S, Wilson B, Bowes L, Zelcer S, Mpofu C, Silva M, Larouche V, Brossard J, Bouffet E. Survival Following Tumor Recurrence in Children With Medulloblastoma. J Pediatr Hematol Oncol. 2018. Apr;40(3):e159–e163. doi: 10.1097/MPH.0000000000001095. DOI: 10.1097/MPH.0000000000001095 [DOI] [PubMed] [Google Scholar]
- 13.Nicholson HS, Kretschmar CS, Krailo M, et al. : Phase 2 study of temozolomide in children and adolescents with recurrent central nervous system tumors: a report from the Children’s Oncology Group. Cancer 110:1542–50, 2007 [DOI] [PubMed] [Google Scholar]
- 14.Slichenmyer WJ, Rowinsky EK, Donehower RC, et al. : The current status of camptothecin analogues as antitumor agents. J Natl Cancer Inst 85:271–91, 1993 [DOI] [PubMed] [Google Scholar]
- 15.Burris HA 3rd, Fields SM: Topoisomerase I inhibitors. An overview of the camptothecin analogs. Hematol Oncol Clin North Am 8:333–55, 1994 [PubMed] [Google Scholar]
- 16.Vassal G, Doz F, Frappaz D, et al. : A phase I study of irinotecan as a 3-week schedule in children with refractory or recurrent solid tumors. J Clin Oncol 21:3844–52, 2003 [DOI] [PubMed] [Google Scholar]
- 17.Turner CD, Gururangan S, Eastwood J, et al. : Phase II study of irinotecan (CPT-11) in children with high-risk malignant brain tumors: the Duke experience. Neuro Oncol 4:102–8, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bomgaars LR, Bernstein M, Krailo M, et al. : Phase II trial of irinotecan in children with refractory solid tumors: a Children’s Oncology Group Study. J Clin Oncol 25:4622–7, 2007. [DOI] [PubMed] [Google Scholar]
- 19.Grill J, Geoerger B, Gesner L, Perek D, Leblond P, Cañete A, Aerts I, Madero L, de Toledo Codina JS, Verlooy J, Estlin E, Cisar L, Breazna A, Dorman A, Bailey S, Nicolin G, Grundy RG, Hargrave D; European Consortium Innovative Therapies for Children with Cancer (ITCC) and the European Society for Paediatric Oncology (SIOPE) brain tumor group. Phase II study of irinotecan in combination with temozolomide (TEMIRI) in children with recurrent or refractory medulloblastoma: a joint ITCC and SIOPE brain tumor study. Neuro Oncol. 2013. Sep;15(9):1236–43. doi: 10.1093/neuonc/not097. Epub 2013 Jul 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li VW, Folkerth RD, Watanabe H, et al. : Microvessel count and cerebrospinal fluid basic fibroblast growth factor in children with brain tumours. Lancet 344:82–6, 1994 [DOI] [PubMed] [Google Scholar]
- 21.Huang H, Held-Feindt J, Buhl R, et al. : Expression of VEGF and its receptors in different brain tumors. Neurol Res 27:371–7, 2005 [DOI] [PubMed] [Google Scholar]
- 22.Slongo ML, Molena B, Brunati AM, et al. : Functional VEGF and VEGF receptors are expressed in human medulloblastomas. Neuro Oncol 9:384–92, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Glade-Bender J, Kandel JJ, Yamashiro DJ: VEGF blocking therapy in the treatment of cancer. Expert Opin Biol Ther 3:263–76, 2003 [DOI] [PubMed] [Google Scholar]
- 24.Glade Bender JL, Adamson PC, Reid JM, Xu L, Baruchel S, Shaked Y, Kerbel RS, Cooney-Qualter EM, Stempak D, Chen HX, Nelson MD, Krailo MD, Ingle AM, Blaney SM, Kandel JJ, Yamashiro DJ; Children’s Oncology Group Study. Phase I trial and pharmacokinetic study of bevacizumab in pediatric patients with refractory solid tumors: a Children’s Oncology Group Study. J Clin Oncol. 2008. Jan 20;26(3):399–405. doi: 10.1200/JCO.2007.11.9230. [DOI] [PubMed] [Google Scholar]
- 25.Huber H, Eggert A, Janss AJ, et al. : Angiogenic profile of childhood primitive neuroectodermal brain tumours/medulloblastomas. Eur J Cancer 37:2064–72, 2001 [DOI] [PubMed] [Google Scholar]
- 26.Wagner LM, McAllister N, Goldsby RE, et al. : Temozolomide and intravenous irinotecan for treatment of advanced Ewing sarcoma. Pediatr Blood Cancer 48:132–9, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Kushner BH, Kramer K, Modak S, et al. : Irinotecan plus temozolomide for relapsed or refractory neuroblastoma. J Clin Oncol 24:5271–6, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Blagosklonny MV: How Avastin potentiates chemotherapeutic drugs: action and reaction in antiangiogenic therapy. Cancer Biol Ther 4:1307–10, 2005 [DOI] [PubMed] [Google Scholar]
- 29.Jain RK: Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science 307:58–62, 2005 [DOI] [PubMed] [Google Scholar]
- 30.Jain RK, Tong RT, Munn LL: Effect of vascular normalization by antiangiogenic therapy on interstitial hypertension, peritumor edema, and lymphatic metastasis: insights from a mathematical model. Cancer Res 67:2729–35, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fukumura D, Jain RK: Tumor microvasculature and microenvironment: targets for anti-angiogenesis and normalization. Microvasc Res 74:72–84, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tong RT, Boucher Y, Kozin SV, et al. : Vascular normalization by vascular endothelial growth factor receptor 2 blockade induces a pressure gradient across the vasculature and improves drug penetration in tumors. Cancer Res 64:3731–6, 2004 [DOI] [PubMed] [Google Scholar]
- 33.Jain RK: Normalizing tumor vasculature with anti-angiogenic therapy: a new paradigm for combination therapy. Nat Med 7:987–9, 2001 [DOI] [PubMed] [Google Scholar]
- 34.Dickson PV, Hamner JB, Sims TL, et al. : Bevacizumab-induced transient remodeling of the vasculature in neuroblastoma xenografts results in improved delivery and efficacy of systemically administered chemotherapy. Clin Cancer Res 13:3942–50, 2007 [DOI] [PubMed] [Google Scholar]
- 35.Wildiers H, Guetens G, De Boeck G, et al. : Effect of antivascular endothelial growth factor treatment on the intratumoral uptake of CPT-11. Br J Cancer 88:1979–86, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rubinstein LV, Korn EL, Freidlin B, et al. : Design issues of randomized phase II trials and a proposal for phase II screening trials. J Clin Oncol 23:7199–206, 2005. [DOI] [PubMed] [Google Scholar]
- 37.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000. Feb 2;92(3):205–16. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 38.Kalbfleisch JD and Prentice RL. The statistical analysis of failure time data. John Wiley and Sons, New York, 2002. [Google Scholar]
- 39.Kaplan EL and Meier P. Nonparametric estimation from incomplete observations. J Amer Statist Assoc 53, 1958, 457–481. [Google Scholar]
- 40.Schemper M and Smith TL. A note on quantifying follow-up in studies of failure time. Controlled Clinical Trials 17, 343–346, 1996. [DOI] [PubMed] [Google Scholar]
- 41.Leary SES, Olson JM. The Molecular Classification of Medulloblastoma: Driving the next generation clinical trials. Current Opinion in Pediatrics. 2012;24(1):33–39. doi: 10.1097/MOP.0b013e32834ec106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gajjar A, Pfister SM, Taylor MD, Gilbertson RJ. Molecular Insights into Pediatric Brain Tumors Have the Potential to Transform Therapy. Clinical cancer research : an official journal of the American Association for Cancer Research. 2014;20(22):5630–5640. doi: 10.1158/1078-0432.CCR-14-0833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Khatua S, Song A, Sridhar DC, Mack SC. Childhood Medulloblastoma: Current Therapies, Emerging Molecular Landscape and Newer Therapeutic Insights. Curr Neuropharmacol. 2017. Nov 28. doi: 10.2174/1570159X15666171129111324. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ramaswamy V, Remke M, Bouffet E, Faria CC, Perreault S, Cho YJ, Shih DJ, Luu B, Dubuc AM, Northcott PA, Schüller U, Gururangan S, McLendon R, Bigner D, Fouladi M, Ligon KL, Pomeroy SL, Dunn S, Triscott J, Jabado N, Fontebasso A, Jones DT, Kool M, Karajannis MA, Gardner SL, Zagzag D, Nunes S, Pimentel J, Mora J, Lipp E, Walter AW, Ryzhova M, Zheludkova O, Kumirova E, Alshami J, Croul SE, Rutka JT, Hawkins C, Tabori U, Codispoti KE, Packer RJ, Pfister SM, Korshunov A, Taylor MD. Recurrence patterns across medulloblastoma subgroups: an integrated clinical and molecular analysis. Lancet Oncol. 2013. Nov;14(12):1200–7. doi: 10.1016/S1470-2045(13)70449-2. Epub 2013 Oct 17. Erratum in: Lancet Oncol. 2014 Apr;15(4):e154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Morrissy AS, Garzia L, Shih DJ, Zuyderduyn S, Huang X, Skowron P, Remke M, Cavalli FM, Ramaswamy V, Lindsay PE, Jelveh S, Donovan LK, Wang X, Luu B, Zayne K, Li Y, Mayoh C, Thiessen N, Mercier E, Mungall KL, Ma Y, Tse K, Zeng T, Shumansky K, Roth AJ, Shah S, Farooq H, Kijima N, Holgado BL, Lee JJ, Matan-Lithwick S, Liu J, Mack SC, Manno A, Michealraj KA, Nor C, Peacock J, Qin L, Reimand J, Rolider A, Thompson YY, Wu X, Pugh T, Ally A, Bilenky M, Butterfield YS, Carlsen R, Cheng Y, Chuah E, Corbett RD, Dhalla N, He A, Lee D, Li HI, Long W, Mayo M, Plettner P, Qian JQ, Schein JE, Tam A, Wong T, Birol I, Zhao Y, Faria CC, Pimentel J, Nunes S, Shalaby T, Grotzer M, Pollack IF, Hamilton RL, Li XN, Bendel AE, Fults DW, Walter AW, Kumabe T, Tominaga T, Collins VP, Cho YJ, Hoffman C, Lyden D, Wisoff JH, Garvin JH Jr, Stearns DS, Massimi L, Schüller U, Sterba J, Zitterbart K, Puget S, Ayrault O, Dunn SE, Tirapelli DP, Carlotti CG, Wheeler H, Hallahan AR, Ingram W, MacDonald TJ, Olson JJ, Van Meir EG, Lee JY, Wang KC, Kim SK, Cho BK, Pietsch T, Fleischhack G, Tippelt S, Ra YS, Bailey S, Lindsey JC, Clifford SC, Eberhart CG, Cooper MK, Packer RJ, Massimino M, Garre ML, Bartels U, Tabori U, Hawkins CE, Dirks P, Bouffet E, Rutka JT, Wechsler-Reya RJ, Weiss WA, Collier LS, Dupuy AJ, Korshunov A, Jones DT, Kool M, Northcott PA, Pfister SM, Largaespada DA, Mungall AJ, Moore RA, Jabado N, Bader GD, Jones SJ, Malkin D, Marra MA, Taylor MD. Divergent clonal selection dominates medulloblastoma at recurrence. Nature. 2016. Jan 21;529(7586):351–7. doi: 10.1038/nature16478. Epub 2016 Jan 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bonney PA, Santucci JA, Maurer AJ, Sughrue ME, McNall-Knapp RY, Battiste JD. Dramatic response to temozolomide, irinotecan, and bevacizumab or recurrent medulloblastoma with widespread osseous metastases. J Clin Neurosci. 2016 Apr;26:161–3. doi: 10.1016/j.jocn.2015.10.022. Epub 2016 Jan 14. [DOI] [PubMed] [Google Scholar]
- 47.Le Teuff G, Castaneda-Heredia A, Dufour C, Jaspan T, Calmon R, Devos A, McHugh K, Leblond P, Frappaz D, Aerts I, Zwaan CM, Ducassou S, Chastagner P, Verschuur A, Corradini N, Casanova M, Rubie H, Riccardi R, Le Deley MC, Vassal G, Geoerger B; European consortium Innovative Therapies for Children with Cancer (ITCC). Phase II study of temozolomide and topotecan (TOTEM) in children with relapsed or refractory extracranial and central nervous system tumors including medulloblastoma with post hoc Bayesian analysis: A European ITCC study. Pediatr Blood Cancer. 2020. Jan;67(1):e28032. doi: 10.1002/pbc.28032. Epub 2019 Oct 8. [DOI] [PubMed] [Google Scholar]
- 48.Peyrl A, Chocholous M, Kieran MW, Azizi AA, Prucker C, Czech T, Dieckmann K, Schmook MT, Haberler C, Leiss U, Slavc I. Antiangiogenic metronomic therapy for children with recurrent embryonal brain tumors. Pediatr Blood Cancer. 2012. Sep;59(3):511–7. doi: 10.1002/pbc.24006. Epub 2011 Dec 6. [DOI] [PubMed] [Google Scholar]
- 49.Cefalo G, Massimino M, Ruggiero A, Barone G, Ridola V, Spreafico F, Potepan P, Abate ME, Mascarin M, Garrè ML, Perilongo G, Madon E, Colosimo C, Riccardi R. Temozolomide is an active agent in children with recurrent medulloblastoma/primitive neuroectodermal tumor: an Italian multi-institutional phase II trial. Neuro Oncol. 2014. May;16(5):748–53. doi: 10.1093/neuonc/not320. Epub 2014 Jan 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Grill J, Geoerger B, Gesner L, Perek D, Leblond P, Cañete A, Aerts I, Madero L, de Toledo Codina JS, Verlooy J, Estlin E, Cisar L, Breazna A, Dorman A, Bailey S, Nicolin G, Grundy RG, Hargrave D; European Consortium Innovative Therapies for Children with Cancer (ITCC) and the European Society for Paediatric Oncology (SIOPE) brain tumor group. Phase II study of irinotecan in combination with temozolomide (TEMIRI) in children with recurrent or refractory medulloblastoma: a joint ITCC and SIOPE brain tumor study. Neuro Oncol. 2013. Sep;15(9):1236–43. doi: 10.1093/neuonc/not097. Epub 2013 Jul 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Aguilera D, Mazewski C, Fangusaro J, MacDonald TJ, McNall-Knapp RY, Hayes LL, Kim S, Castellino RC. Response to bevacizumab, irinotecan, and temozolomide in children with relapsed medulloblastoma: a multi-institutional experience. Childs Nerv Syst. 2013. Apr;29(4):589–96. doi: 10.1007/s00381-012-2013-4. Epub 2013 Jan 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fangusaro J(1), Gururangan S, Poussaint TY, McLendon RE, Onar-Thomas A, Warren KE, Wu S, Packer RJ, Banerjee A, Gilbertson RJ, Jakacki R, Gajjar A, Goldman S, Pollack IF, Friedman HS, Boyett JM, Kun LE, Fouladi M. Bevacizumab (BVZ)-associated toxicities in children with recurrent central nervous system tumors treated with BVZ and irinotecan (CPT-11): a Pediatric Brain Tumor Consortium Study (PBTC-022). Cancer. 2013. Dec 1;119(23):4180–7. doi: 10.1002/cncr.28343. Epub 2013 Sep 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bautista F, Fioravantti V, de Rojas T, Carceller F, Madero L, Lassaletta A, Moreno L. Medulloblastoma in children and adolescents: a systematic review of contemporary phase I and II clinical trials and biology update. Cancer Med. 2017. Nov;6(11):2606–2624. doi: 10.1002/cam4.1171. Epub 2017 Oct 4. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]


