Version Changes
Revised. Amendments from Version 1
In response to the helpful comments from the reviewers, we have edited the manuscript to include the following: discussion of daily torpor with hibernation; removed references to aestivation; noted that PACAP/BDNF neurons that express GAD may nevertheless be excitatory; clarified the scenarios in which daily torpor is triggered, and discussed how these triggers may vary between species, strains, and sex; acknowledged that additional humoral effectors likely contribute to the induction of torpor; highlighted that genetic knockout strains might develop compensatory mechanisms that render interpretation of their physiology difficult; added recent publication investigating the relationship between sleep and daily torpor in the mouse (Huang et al., 2022); added discussion of the setpoint theory of thermoregulation including reference to the work by Ramanovsky; acknowledged that TRPM2 is part of a larger system for detecting changes in brain temperature; clarified that neurons in the hypothalamus might be warm-responsive if responding temperature changes in distant regions and removed the term warm-sensing as this implies direct temperature sensitivity; clarified that animals might target maintenance of body fat rather than body mass; clarified that our suggestion that DMH adjusts energy expenditure and PVH adjusts food intake is a generalisation; added reference to work that shows kappa opioid receptors play a role in impaired BAT activation in the context of chronic high fat intake; added vasoconstriction as a key component of daily torpor; added nuance to the discussion of whether indeed a sleep debt accumulates during torpor; added discussion of the distinction between fasting induced reduction in body temperature (within the ‘normal range’) and daily torpor; edited down the discussion of normal thermoregulation; edited the figures to add more detail and to highlight that BDNF / PACAP neurons may be either excitatory or inhibitory; added discussion of Gpr50 knockout mice with respect to leptin and torpor.
Abstract
Torpor is a hypothermic, hypoactive, hypometabolic state entered into by a wide range of animals in response to environmental challenge. This review summarises the current understanding of torpor. We start by describing the characteristics of the wide-ranging physiological adaptations associated with torpor. Next follows a discussion of thermoregulation, control of food intake and energy expenditure, and the interactions of sleep and thermoregulation, with particular emphasis on how those processes pertain to torpor. We move on to review the evidence for the systems that control torpor entry, including both the efferent circulating factors that signal the need for torpor, and the central processes that orchestrate it. Finally, we consider how the putative circuits responsible for torpor induction integrate with the established understanding of thermoregulation under non-torpid conditions and highlight important areas of uncertainty for future studies.
Keywords: Torpor, Metabolism, Homeostasis, Thermoregulation, Hibernation, Energy balance
Introduction
Torpor is a hypothermic, hypometabolic, adaptive response, engaged by a range of animals in order to reduce metabolic demand. Torpor can be brief, in daily heterotherms such as the mouse, or it can be prolonged, in seasonal hibernators. In small animals undergoing torpor, body temperature typically drops to just a few degrees above ambient ( Barnes, 1989). In the hibernating arctic squirrel this can result in core temperatures as low as -2.9°C. Alongside hypothermia are profound reductions in metabolic, heart, and respiratory rates ( Heldmaier et al., 2004). Torpor therefore represents an intriguing deviation from ‘normal’ mammalian physiology in which animals undergo a rapid transition from normothermic active foraging to a state of profound energy conservation. There has been increasing interest in mimicking torpor in the context of critically ill patients ( Cerri, 2017; Stanzani et al., 2020), and for long-distance space travel ( Cerri et al., 2021a). Torpor is a remarkably conserved behaviour seen in all classes of vertebrate life, including the three oldest branches of mammals: monotremes, marsupials, and placentals including primates ( Carey et al., 2003; Heldmaier et al., 2004). Those mammals that engage torpor, do so through activation of ubiquitous genes, rather than a complement of genes that are unique to those torpid species ( Faherty et al., 2016; Srere et al., 1992; Zhao et al., 2010). Hence, this behaviour may represent a fundamental physiological response that has been relatively recently switched off in those animals for which it is not an extant behaviour.
The aim of this review is to provide an overview of the fundamental physiological adaptations associated with torpor, and then to discuss current evidence for how these remarkable adjustments might be achieved. We focus on the physiology of daily torpor in the mouse, primarily because this is the animal that has been most extensively studied. However, where relevant or where data from mice are lacking, hibernating species including hamsters (facultative hibernators), squirrels, bears, and lemurs (obligate hibernators) is included. It is important to note that the extent to which daily torpor and torpor during hibernation are comparable is a matter of debate, and there are clearly significant differences. Torpor bouts during seasonal hibernation are longer, and more profound than daily torpor ( Ruf & Geiser, 2015). Daily torpor is under circadian control and is engaged for example when calorie expenditure is greater than intake ( van der Vinne et al., 2018), regardless of season. In contrast, obligate hibernation occurs at predictable times of the year and is usually preceded by a period of calorie intake greater than expenditure. Facultative hibernators will enter long torpor bouts in response to prolonged periods of cold ambient temperature and short photoperiod days ( Jansky et al., 1984). Key similarities and differences are between daily torpor, facultative hibernation, and seasonal hibernation are summarised in Table 1.
Table 1. Comparison of daily torpor, with facultative, and obligative hibernation.
| Daily torpor | Facultative hibernation | Obligate
hibernation |
|
|---|---|---|---|
| Trigger | Calorie deficit | Photoperiod / ambient
temperature |
Season |
|
Mean minimum temperature reached
( Ruf & Geiser, 2015) |
17°C
(for a 30g animal) |
4°C
(range from -2 to 30°C ) |
|
|
Minimum VO2
( Ruf & Geiser, 2015) |
~0.24 mL O2 g−1 h−1 (~40% of
BMR) |
~0.04 mL O2 g−1 h−1 (~6% of BMR) | |
|
Mean torpor bout duration
( Ruf & Geiser, 2015) |
6–7 hours
(for a 30g animal) |
>120 hours | |
|
Nutritional state (
Carey
et al., 2003;
Huang et al., 2021; Kurtz & Carey, 2007; Ruf & Geiser, 2015) |
Calorie deficit / Weight loss | Reduced body mass (at least
in Syrian golden hamsters) |
Increased
adipose stores |
|
Influence of gonadal function
( Barnes et al., 1988; Jansky et al., 1984; Mzilikazi & Lovegrove, 2002) |
Testosterone inhibits daily torpor
in Saccostomus campestris No known direct effect on gonadal function |
Preceded by gonadal involution, blocked
by testosterone, and followed by gonadal maturation |
|
Discussion
Physiological characteristics of torpor
Due to the large surface area to volume ratio of a mouse, maintenance of normothermia represents a significant energy cost. In mice housed at an ambient temperature of 22°C, over 30% of energy expenditure is directed towards thermogenesis ( Abreu-Vieira et al., 2015). When mice are unable to access sufficient calories to maintain active physiology and normal body temperature, they will enter periods of torpor lasting several hours ( Hudson & Scott, 1979). Stimuli for torpor in mice include acute fasting ( Sunagawa & Takahashi, 2016), a combination of fasting and reduction in the ambient temperature ( Hitrec et al., 2019; Swoap & Gutilla, 2009), food restriction ( van der Vinne et al., 2018), or increase energy costs of foraging ( Schubert et al., 2010). This latter observation supports the hypothesis that it is a relative imbalance of energy supply compared to the demands of maintaining ‘normal’ physiological homeostasis rather than a response to cold and hunger per se, which triggers torpor.
Female mice are more prone to torpor than males, and hence some studies present data from female mice only (for example, Kato et al., 2018; Oelkrug et al., 2010; Swoap & Gutilla, 2009) although males do enter torpor ( Gavrilova et al., 1999; Lo Martire et al., 2018; Solymár et al., 2015; Sunagawa & Takahashi, 2016). Recent work indicates oestrogen receptor expressing neurons in the pre-optic area contribute to the generation of daily torpor in the mouse, and will be discussed in more detail later ( Zhang et al., 2020). Evidence from the pouched mouse, Saccostomus campestris, indicates that testosterone inhibits daily torpor ( Mzilikazi & Lovegrove, 2002). These sex differences might reflect greater adipose tissue reserves in males, or the relatively lower energetic burden that normal activity and reproduction place on male compared to female mice, hence the need to conserve adipose tissue energy reserves may be greater for females. Alternatively, the relative female predisposition to torpor may be due to lower birth weight, since low birth weight may predict higher torpor tendency in female mice irrespective of actual body weight at the time of fasting ( Kato et al., 2018).
Definition, timing, and duration of torpor
The transition to torpor may not be all or nothing: fasted mice exhibit increased variability of both metabolic rate and body temperature, with graded reductions in core body temperature up to full torpor ( Brown & Staples, 2010). Despite the magnitude of the final deviation from normal physiology, there is no consensus on the definition of torpor in mice. Examples include a core body temperature below 34°C proceeded by least fifteen minutes of consecutive decline ( Iliff & Swoap, 2012; Willis, 2007); body temperature below 31°C for at least 30 minutes ( Brown & Staples, 2010); body temperature below 32°C ( Braulke & Heldmaier, 2010); a metabolic rate 25% below expected ( Hudson & Scott, 1979); the duration of a period of monotonic cooling resulting in a reduction in body temperature of at least 5°C followed by a period of monotonic increase up to at least 5°C above the nadir ( Lo Martire et al., 2018); or, deviation from a Bayesian estimate of individual basal metabolic rate or core temperature ( Sunagawa & Takahashi, 2016). These approaches vary in their complexity, as well as their ability to account for individual and/or circadian fluctuations in body temperature or metabolic rate.
Although a reduction in available food might represent an unpredictable environmental stimulus, the timing of torpor entry is under circadian control. Torpor in mice generally occurs during the latter part of the lights off period ( Brown & Staples, 2010; Webb et al., 1982). Timing of torpor entry is primarily under the control the circadian clock, but can be adjusted by the timing or expected timing of food ( van der Vinne et al., 2018), or entrained to food in mice or hamsters lacking endogenous circadian clocks ( Paul et al., 2004; van der Vinne et al., 2018). Duration of torpor in mice is generally inversely proportional to the weight of the mouse ( Hudson & Scott, 1979), and some have suggested that torpor is engaged when food restriction or fasting decreases body weight to approximately 20g ( Solymár et al., 2015), although this experiment was performed only in male mice, and may not be generalizable across strains or sex ( Dikic et al., 2008). Torpor typically persists for between four and six hours, often preceded by shallow, or shorter bouts ( Hudson & Scott, 1979; Webb et al., 1982), although bouts may last 12 hours or more depending on the species or strain studied and the intervention used to induce torpor ( Kato et al., 2018). No single, or absolute, threshold for torpor has been identified, suggesting that many factors likely contribute to generating a permissive state for the emergence of torpor, and that these factors may vary across species, strain, or sex.
Thermoregulation during torpor
During torpor bouts, thermoregulation is not simply suspended: mice maintain active control of their temperature, usually tracking approximately two degrees above ambient temperature, but defending a minimum body temperature of 16 - 19°C ( Hudson & Scott, 1979). Further evidence for the continued - albeit adjusted - thermoregulatory control comes from the observation that the rate of decline in body temperature is lower in torpor than when hypothermia is induced pharmacologically or physically ( Vicent et al., 2017), that is to say, temperature decreases during entry into torpor are controlled. Indeed, a very low ambient temperature may reduce the probability of torpor entry, again indicating ongoing but adjusted thermoregulation ( Sunagawa & Takahashi, 2016). Hypothermia in a torpid animal could be achieved through three distinct mechanisms: increased thermal conductance to the environment; reduction in a ‘setpoint’ target temperature (if indeed, one exists ( Romanovsky, 2007)); or reduced gain in the regulatory feedback system. Hibernating marmots appear to reduce both the gain and the target of the thermoregulatory system ( Florant & Heller, 1977), while daily torpor in mice may predominantly involve reduction in its gain ( Sunagawa & Takahashi, 2016). Whether these observations reflect mechanistic differences between the hypothermia seen during daily torpor and that during torpor in hibernation is not clear.
Cardiovascular function during torpor
The suppression of metabolism associated with torpor allows animals to tolerate profound reductions in cardiac output, respiratory rate, and, presumably, organ perfusion. Heart rates of torpid mice typically reach a nadir of approximately 150 beats per minute (bpm) from resting rates of around 600 bpm ( Swoap & Gutilla, 2009). Hypothermia alone generates a degree of bradycardia, such as is seen clinically during therapeutic hypothermia ( Stær-Jensen et al., 2014). However, heart rates are slower at any given core body temperature during entry into, compared to arousal from torpor ( Swoap & Gutilla, 2009). This suggests dominance of the parasympathetic nervous system during entry ( Zosky, 2002; Zosky & Larcombe, 2003), followed by sympathetic nervous system activation during arousal from torpor, at least in terms of the heart. For a given body temperature, pharmacologically-induced hypothermia induced by CHA injection in mice generates a less profound bradycardia than that seen in torpor, which again supports the hypothesis that heart rate is actively suppressed during torpor entry ( Vicent et al., 2017).
Given that heart rate in torpid mice drops by 75% from resting values, and mean arterial blood pressure is determined by the product of cardiac output vascular resistance, if all other parameters remained the same a 75% drop in mean arterial pressure would be predicted during torpor ( Swoap & Gutilla, 2009). However, systolic, diastolic, and mean arterial pressure drop by only 25–30% during torpor. The maintenance of blood pressure at values only 25–30% below those seen in euthermic mice despite the profound bradycardia suggests significantly increased vascular resistance.
Hence, during torpor there appears to be simultaneous activation of the sympathetic and parasympathetic nervous system with the former driving vasoconstriction and the latter driving bradycardia. Simultaneous activation of both the sympathetic and parasympathetic limbs of the autonomic nervous system has been proposed as a means to optimise cardiac function when pumping blood into a constricted vascular tree ( Paton et al., 2005), and is observed for example in the diving reflex ( Panneton, 2013).
Metabolic rate and respiratory function during torpor
Respiratory function in torpid mice is less well studied, however, in the little pocket mouse Perognathus longimembris (a 7–11 gram rodent from the family Heteromyidae) daily torpor is associated with a reduction in respiratory minute volume to less than 2% of basal levels ( Withers, 1977). Dormice ( Glis glis) exhibit similar characteristics during torpor ( Elvert & Heldmaier, 2005). Both species increase the rate and decrease the depth of ventilation during entry and exit from torpor, a pattern that resembles panting. The purpose of this panting is unclear, but it may serve to increase heat loss during torpor entry, reduce the partial pressure of carbon dioxide in the blood prior to torpor, or expel accumulated carbon dioxide following torpor.
The assessment of acid-base balance and partial pressures of O 2 and CO 2 in hypothermic hibernating animals is complex: the pH of pure water is dependent on temperature, as are the dissociation constants of biological buffers, and the partial pressure of a fixed amount of carbon dioxide ( PCO 2) in a solution decreases with decreasing temperature. One approach for assessing acid-base balance in hypothermic or hibernating animals is to take a sample of blood held in a sealed container, normalise the temperature and then measure the pH. This allows comparison with ‘normal’ values taken at 37° C. Blood taken from hibernating hamsters ( Cricetus cricetus) and warmed to 37°C in a sealed syringe, tends to have a pH between 6.9 and 7.15 compared to euthermic pH of approximately 7.36, and an arterial PCO 2 between 17 and 24 kilopascals (kPa) compared to euthermic values of approximately 6 kPa, indicating a respiratory acidosis. Alternatively, one can measure the dissociation ratio of imidazole groups on proteins (α im): acid conditions reduce the dissociation ratio (as does reduced temperature). Using this measure also indicates an acidic intracellular state during hibernation in blood, brain and muscle of hibernating hamsters ( Malan et al., 1985; Malan, 1988). In contrast to the observation of a respiratory acidosis in hibernating hamsters, hibernating arctic ground squirrels appear to have reduced PCO 2 compared to euthermic ground squirrels and rats ( Ma et al., 2005). This difference may reflect differences in the body temperature of these hibernating species, with greater metabolic suppression in the colder artic ground squirrel leading to less CO 2 production, differences in the physiology of the species, or differences in the method for analysing the blood gases taken from hypothermic animals. The arterial partial pressure of oxygen (PaO2) in hibernating arctic ground squirrels is actually higher than in euthermic animals, suggesting a left shift in the oxygen-haemoglobin dissociation curve, and only dips below normal euthermic values during arousal when metabolic activity is at a peak.
Oxygen consumption decreases to between 0.04 and 0.05ml O2.g -1.hr -1 in torpid pocket mice and dormice, which in the former is less than 1% of levels when housed at an ambient temperature of 10°C ( Elvert & Heldmaier, 2005; Withers, 1977). Larger mammals such as the Alaskan black bear, whose basal metabolic rate is generally lower than that observed in smaller animals, suppress metabolism to 25% of resting levels. Notably, the minimum oxygen consumption seen in hibernating bears is very similar to that seen in smaller mammals, reaching a nadir of 0.06 ml O2.g -1.hr -1 ( Toien et al., 2011). The fact that large seasonal hibernators achieve similar metabolic suppression despite significantly higher body temperature during hibernation compared to small daily heterotherms, indicates that the reduction in metabolic rate is not simply a passive consequence of lowered body temperature, but rather that metabolism is actively suppressed. This hypothesis is further supported by several observations: reductions in heart rate, respiratory rate and oxygen consumption precede decreases in core temperature in all animals studied, and is largely independent of ambient temperature ( Elvert & Heldmaier, 2005; Toien et al., 2011; Withers, 1977). Metabolic rate in torpid dunnarts is several times lower than that seen in a similar sized rat pup rendered hypothermic by exposure to a cold ambient temperature, with reversal of the normal relationship between body warming or cooling and metabolic rate during torpor ( Geiser et al., 2014). Finally, respiration in mitochondria taken from torpid mice is suppressed even when assessed at 37°C ( Brown & Staples, 2010).
While metabolism is clearly suppressed during torpor, there is evidence that it increases immediately prior to torpor entry in the mouse ( Lo Martire et al., 2018), the dormouse ( Elvert & Heldmaier, 2005), and the Djungarian hamster ( Heldmaier et al., 1999). The significance of this is not clear, but it may represent incomplete switching from euthermia to torpor with resultant episodes of shivering, or perhaps there is a need to clear metabolic substrates from the mitochondria prior to torpor in order to suppress metabolism and reduce free radical production during torpor.
Remarkably, despite the dramatically reduced cardiac output and respiratory rate, hence presumably reduced oxygen delivery, the concentration of blood lactate in hibernating arctic ground squirrels is no different from that seen in euthermic controls ( Ma et al., 2005). This demonstrates the remarkable fine-tuning of metabolic supply and demand during torpor such that in the face of reduced supply there is no overall deficit.
Physiology of mammalian thermoregulation
In order to understand the physiological adaptations of torpor, it is useful to understand the normal control of body temperature. Mammalian thermoregulation presumably evolved to enable control of the core temperature such that cellular and tissue function is optimised to the survival and reproductive benefit of the animal. The considerable energy cost of maintaining a core temperature several degrees above ambient implies homeothermy brings a significant survival advantage, allowing continued activity in the face of diurnal or seasonal reductions in the ambient temperature, and therefore allowing the occupation of a wider range of environmental niches ( Abreu-Vieira et al., 2015).
Despite this investment and its benefits, there are times when mammalian body temperature deviates from the normal range. This deviation can be physiological, such as during fever, sleep, ovulation, stress, or of course, torpor ( Landolt et al., 1995). Additionally, deviations of core temperature can be pathological due to poisoning or environmental challenges. Internal sources of heat in mammals include those that generate heat as a by-product, for example basal metabolism with heat generated as a consequence of pumping ions across membranes, and heat generated by muscles during movement. Additional internal sources of heat include those for which heat production is the primary goal. Examples of this include shivering, and brown adipose thermogenesis where mitochondrial proton flux is uncoupled from adenosine triphosphate (ATP) production in brown adipose tissue (reviewed in ( Nicholls & Rial, 1999)). Mammals can also take steps to reduce heat loss through piloerection, peripheral vasoconstriction, and nest building. Heat defence responses include cutaneous vasodilation with visceral constriction and increased cardiac output to direct blood towards the cooler skin surface, evaporative losses through sweating, panting, and grooming. Despite their mutually antagonistic effects, both the autonomic warm and cold defence responses are under the control of the sympathetic nervous system (reviewed in ( Morrison, 2016b)).
Early models of thermoregulation proposed a temperature target, or set-point, against which incoming signals of core and external temperature were compared, with a homeostatic response generated to drive the core temperature back towards the set-point ( Hammel & Pierce, 1968). More recently, with advances in our understanding of the physiology of thermoregulation, a model of multiple independent mechanisms each with their own threshold has emerged ( Romanovsky, 2007; McAllen et al., 2010).
Afferent thermoregulation signals
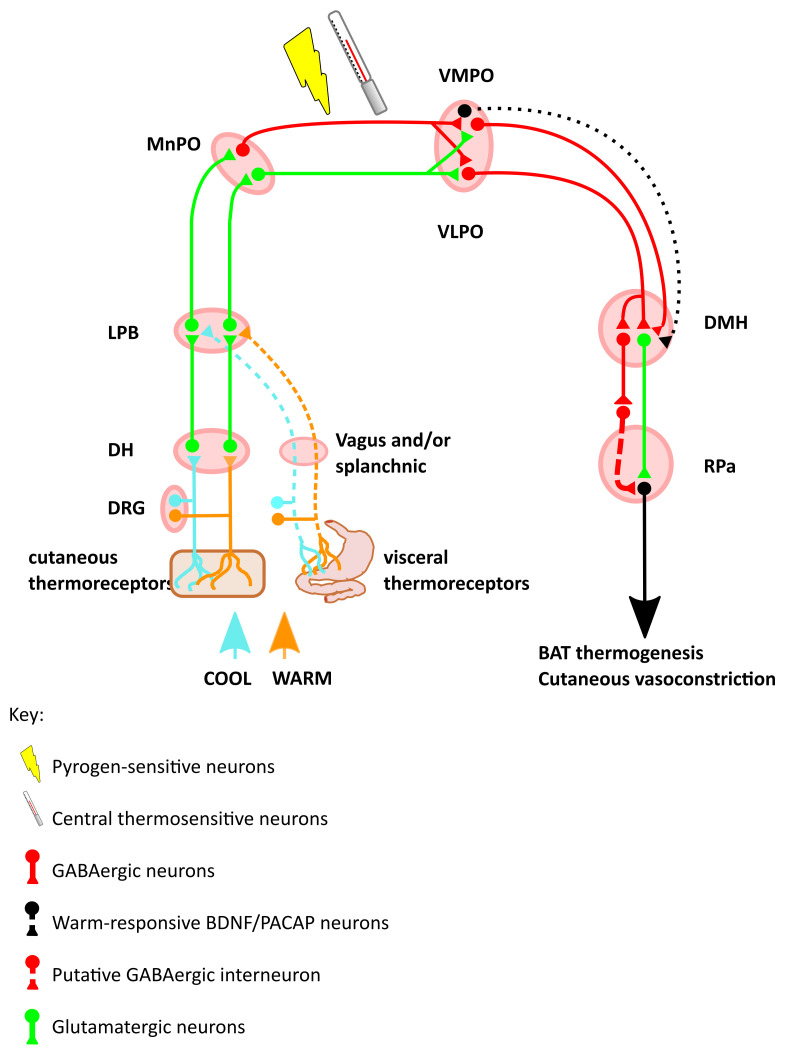
Hot and cold information from the skin flows centrally in parallel streams, both relaying information from the dorsal horn via the parabrachial nucleus to the preoptic area of the hypothalamus (POA). Relatively little is known about the inputs from thermosensitive receptors in the viscera, although it is thought they enter the central nervous system (CNS) via splanchnic and vagal afferents, and follow a similar path through the lateral parabrachial nucleus to the preoptic area ( Madden & Morrison, 2019). The projection that travels via the external lateral and central parts of the lateral parabrachial nucleus (LPBel, LPBc, respectively) responds to skin cooling. Activation of this input to the preoptic area results in increased brown adipose tissue (BAT) thermogenesis, shivering, and increased metabolic and heart rate. In contrast, skin warming induces c-Fos expression and increases firing in POA-projecting neurons in the dorsal part of the lateral parabrachial nucleus (LPBd). Activation of this pathway results in increased heart rate, suppressed BAT sympathetic nerve activity, and cutaneous vasodilatation ( Nakamura & Morrison, 2010). Thermal-sensitive parabrachial neurons predominantly project to the median preoptic nucleus (MnPO) ( Nakamura & Morrison, 2008).
Efferent thermoregulation signals
The POA can be viewed as sitting at the top of a thermoregulatory arc. It integrates information about both the internal and external temperature and contributes to the autonomic response to thermal challenge by modulating BAT thermogenesis, shivering, and vasoconstriction ( Morrison et al., 2012; Morrison, 2016a). Early experiments established that local heating of the POA induced vasodilatation, sweating, and panting responses akin to those seen when heating the entire animal, indicating the existence of intrinsically warm-sensing POA neurons in central thermoregulatory circuits ( Clark et al., 1939; Magoun et al., 1938; Nakayama et al., 1961). More recently, the application of agonists or antagonists to cultured POA neurons that express calcium-sensitive fluorescent dyes established that central temperature-sensing mechanisms are in part mediated by the transient receptor potential M2 channel (TRPM2) ( Song et al., 2016). In summary, the POA receives thermal information from the skin and viscera, as well as directly sensing the local brain temperature, and then uses this information to control down-stream thermoregulation as discussed below.
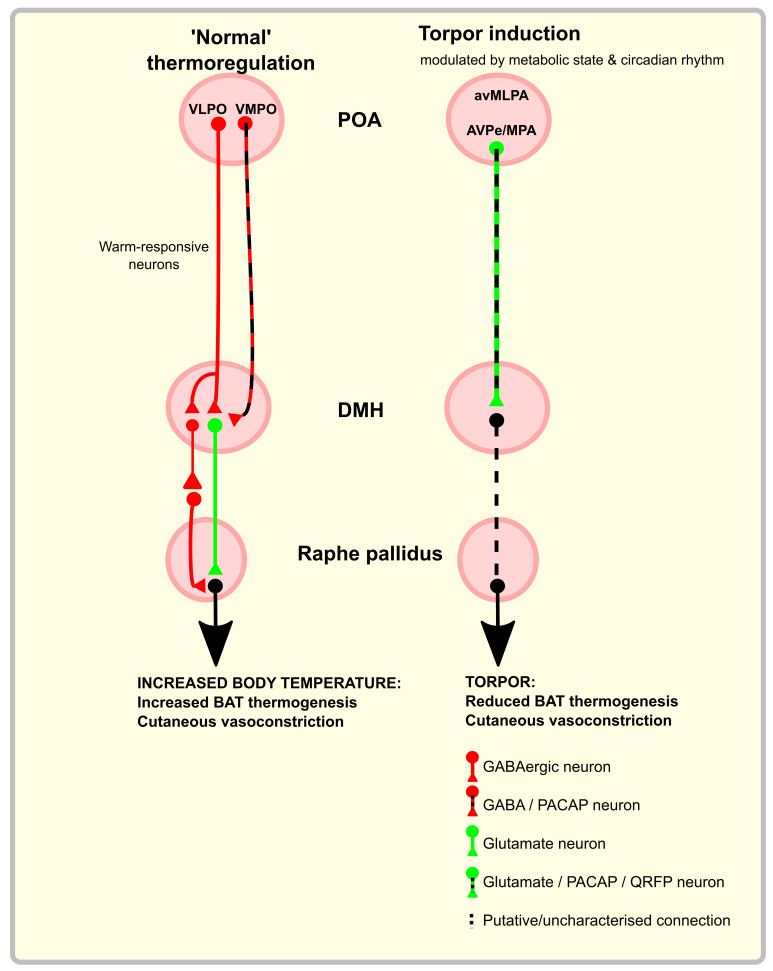
Chemo- or optogenetic excitation of a warm-responsive GABAergic projection from the ventral part of the lateral POA (vLPO) to the dorsomedial hypothalamic nucleus (DMH) suppresses thermogenesis and locomotion, while inhibiting the same projection induces the opposite effects ( Zhao et al., 2017). Data from in-vivo calcium imaging reveals the targets of this projection are both glutamatergic and GABAergic neurons within the DMH, both of which increase their activity in response to low ambient temperature. Chemo- or optogenetic activation of either the GABAergic or the glutamatergic DMH neurons increases core temperature and activity. There is presumably a second inhibitory neuron between the DMH GABAergic neuron and the BAT-activating sympathetic premotor neurons in the raphe pallidus (RPa). This could in principle lie within either the DMH or the RPa or in a relay elsewhere (see Figure 1).
Figure 1. Schematic of the thermal defence circuit.
Inputs include skin surface thermoreceptors, visceral thermoreceptors, intrinsic thermosensitive neurons in the POA, and circulating pyrogens. Warm-sensing neurons increase the inhibitory input from the VMPO and VLPO onto the DMH, which reduces a tonically active thermogenic signal from DMH to RPa. Cool-sensing neurons disinhibit this tonically active DMH to RPa projection, to increase heat production. Abbreviations: DRG, dorsal root ganglion; DH, dorsal horn; LPD, lateral parabrachial nucleus; MnPO, median preoptic area; VLPO, ventrolateral preoptic nucleus; VMPO, ventromedial preoptic nucleus; BDNF, brain-derived neurotrophic factor; PACAP, pituitary adenylate cyclase-activating polypeptide; DMH, dorsomedial hypothalamic nucleus; RPa, raphe pallidus; BAT, brown adipose tissue.
Another projection to the DMH arises from the ventromedial preoptic area (VMPO). These VMPO neurons express brain-derived neurotrophic fact (BDNF) and pituitary adenylate cyclase-activating polypeptide (PACAP) ( Tan et al., 2016). They also express glutamic acid decarboxylase (GAD), and were thought to be inhibitory, although recent evidence suggests they may lack the necessary vesicular transport required for packaging GABA into vesicles, and might in fact be excitatory ( Saper & Machado, 2020). Again, in-vivo calcium imaging reveals that these VMPO neurons are activated by exposure to a warm environment. Opto-activation of the warm-responsive BDNF and PACAP-expressing cell bodies in the VMPO induces a drop in core body temperature, vasodilatation, and preference for a cooler environment. Optoactivation in the DMH of the terminals of these VMPO neurons results in a drop in core body temperature but no vasodilation or cool ambient preference. This implies that the DMH is responsible for the inhibition of BAT thermogenesis, whereas VMPO projections elsewhere generate the vasodilation and behavioural preference. In principle, torpor might be induced by driving activity in some of those same circuits that respond to warm ambient or internal temperatures during homeostatic thermoregulation. Indeed, as will be discussed in more detail later, a PACAP-expressing population of neurons that project from the POA to the DMH has been implicated in torpor induction ( Hrvatin et al., 2020). However, those PACAP neurons with a role in torpor are thought to be predominantly a glutamatergic population, do not appear to respond to warm environments, nor do they appear necessary for homeostatic thermoregulation ( Hrvatin et al., 2020). Hence on the relatively limited available data it appears that the PACAP neurons that have a role in torpor induction might possibly represent a distinct population from those that play a role in homeostatic thermoregulation.
The DMH then sends projections to the rostral raphe nucleus of the medulla that in turn project to, and activate, BAT via the spinal intermediolateral nucleus ( Cao et al., 2004; DiMicco & Zaretsky, 2007). The projection from DMH to Raphe, which eventually targets BAT is involved in thermal defence and also in the thermogenic response to stress ( Kataoka et al., 2014). This implies that either the DMH receives inputs from additional regions beyond the POA thermo-sensitive circuit that mediate the stress response, or else that the POA also responds to stress.
Taken together, this work establishes the principle that the POA sends a projection to the DMH to inhibit thermogenesis. With increasing ambient, hypothalamic, or core temperature, these signals increase to inhibit thermogenesis. Likewise, when internal and/or ambient temperature drops, activity in these POA to DMH projections reduces, disinhibiting the DMH and leading to increased BAT thermogenesis. An additional principle that emerges is that the physiological effects of changes in temperature may be sensed at one level (e.g. the POA), with projections to several downstream sites evoking independent physiological responses. This pattern is seen in the projection from the VMPO to the DMH where activation of this pathway inhibits BAT thermogenesis but does not induce the additional vasodilation or cool environment preference seen when activating the VMPO itself ( Tan et al., 2016). It remains to be established why the DMH contains both excitatory and inhibitory neurons that drive thermogenesis ( Zhao et al., 2017), but a plausible explanation would be that it ensures tight or fail-safe control of this vital homeostatic process.
Thermoregulation, food intake and body mass
There must be at least two processes that link food intake, thermoregulation, and maintenance of adipose tissue stores. The first ensures that as changes in ambient temperature drive changes in energy expenditure, a commensurate adjustment of food intake occurs to maintain fat mass. In this case, energy expenditure and food intake move together in parallel. Hence, cold exposure increases both BAT thermogenesis and food intake while warm exposure reduces both ( Kaiyala et al., 2012; Ravussin et al., 2014; Xiao et al., 2015). The second process ensures that changes in food availability generate compensatory changes in energy expenditure: acute calorie-restriction drives food intake and suppresses body temperature, whereas acute calorie excess increases body temperature and suppresses food intake in humans ( Soare et al., 2011), as well as rodents ( Duffy et al., 1989; Rothwell & Stock, 1997). However, chronic calorie excess may disrupt this homeostatic balance ( Levin et al., 1985; Madden & Morrison, 2016; Sakaguchi et al., 1989)
The following section is not an exhaustive review of the regulation of appetite and food intake, but rather focuses on the mechanisms and anatomical regions in which the control of food intake interacts with thermoregulation and energy expenditure ( Figure 2). For a review of the central control of appetite, see here ( Andermann & Lowell, 2017).
Figure 2. Schematic of the circuits controlling food intake and energy expenditure.
Fasting stimulates the release of ghrelin from the gastrointestinal tract, which acts on arcuate NPY neurons. NPY-expressing neurons in the arcuate project to the PVH to stimulate food intake, and to the DMH to inhibit thermogenesis and reduce energy expenditure. Conversely, leptin, released from WAT in proportion to the amount of stored fat, acts on arcuate POMC neurons. Arcuate POMC neurons project to the PVH, where they inhibit food intake, and to the DMH, where they increase thermogenesis and energy expenditure. Orexin released from the lateral hypothalamus stimulates both food intake and thermogenesis. Sensory signals modulate the activity of arcuate NPY and POMC neurons in anticipation of food intake. At each level in the hypothalamus, the ghrelin-NPY circuits and the leptin-POMC circuits inhibit each other. Abbreviations: NPY, neuropeptide Y; PVH, paraventricular hypothalamic nucleus; DMH, dorsomedial hypothalamic nucleus; WAT, white adipose tissue; POMC, pro-opiomelanocortin;
Amongst several hypothalamic nuclei that contribute to control of food intake, the arcuate nucleus (ARC) is central and contains two distinct but intermingled populations of cells that perform opposing functions. One population responds to leptin, which is released from white adipose tissue (WAT) to signal satiety and replete WAT stores. These neurons express the neuropeptide pro-opiomelanocortin (POMC), which is cleaved into α-melanocyte stimulating hormone (α-MSH), which in turn acts on melanocortin receptors ( Cone, 2005; Dodd et al., 2015). The second population express neuropeptide Y and agouti-related peptide (NPY and AgRP, respectively). This group is activated by ghrelin, a hormone secreted by the gastrointestinal tract during fasting that stimulates food intake ( Hahn et al., 1998; Müller et al., 2015), and is inhibited by leptin. NPY/AgRP neurons, therefore, signal energy deficit and hunger. These two populations are mutually antagonistic: leptin depolarises ARC POMC neurons while hyperpolarising NPY/AgRP neurons, which reduces their inhibitory input onto the POMC population ( Cowley et al., 2001; Myers et al., 2008). Meanwhile, AgRP is a potent antagonist at melanocortin receptors (reviewed here ( Andermann & Lowell, 2017)). Each population and their interactions will be summarised below.
Leptin, pro-opiomelanocortin, and α-melanocyte stimulating hormone
Leptin is a peptide hormone released by WAT in proportion to the size of the adipose tissue reserve, reflecting long-term food intake and energy stores ( Frederich et al., 1995). In addition to reflecting WAT stores, leptin release is suppressed during periods of fasting in mice ( Swoap et al., 2006) and in humans ( Bergendahl et al., 2000). Leptin receptors are widespread, but of particular relevance here, are found in the ARC, PVH, DMH, POA, and the nucleus of the solitary tract (NTS) ( Myers et al., 2008; Zhang et al., 2011). Leptin release is also inhibited by exposure to a short photoperiod in hamsters ( Freeman et al., 2004), an observation that may account for the observation that humans eat more as winter approaches ( de Castro, 1991).
Physiology of leptin
As a signal of replete WAT stores, leptin inhibits food intake and increases energy expenditure through BAT thermogenesis, growth, and reproductive behaviours ( Frederich et al., 1995; Myers et al., 2008; Schwartz et al., 1996). Exogenous leptin blunts the neuroendocrine consequences of starvation ( Ahima et al., 1996) and suppresses food intake ( Mistry et al., 1997). Leptin deficient ( ob/ob) mice are obese, due to both increased ad-libitum food intake ( Welton et al., 1973) and reduced basal metabolic rate, such that ob/ob mice pair-fed with lean littermates maintain a higher body mass with increased fat stores ( Trayhurn & James, 1978).
In keeping with a role linking energy balance and thermoregulation, leptin-deficient mice show defects in thermoregulation: hypothermic at sub-thermoneutral ambient temperatures and fatally incapable of defending body temperature with acute exposure to low ambient temperatures ( Kaiyala et al., 2015; Trayhurn et al., 1977). In addition to a central deficit in leptin signalling, ob/ob mice show reduced BAT response to electrical or noradrenergic stimulation, indicating a role for peripheral leptin in priming BAT thermogenesis ( Seydoux et al., 1982). Hence, the ob/ob mice are unable to recognise their ample fat stores and adapt as if in a starved state by suppressing thermogenesis and increasing food intake.
Functional anatomy of leptin
Leptin receptors are highly-expressed in the ARC, which is a central hub in the processing of signals regarding the energy state of an animal ( Chen et al., 2015; Cone, 2005). Leptin’s actions here suppress food intake and, when combined with high circulating insulin, increase the sympathetically-mediated browning of WAT ( Dodd et al., 2015). Leptin’s effects in the ARC are mediated by a coordinated increase in the activity of POMC neurons, and reduction in the activity of neurons that co-express NPY and AgRP ( Cowley et al., 2001; Myers et al., 2008). POMC-expressing ARC neurons project to diverse brain regions including PVH, where they release α-MSH, which acts on melanocortin receptors ( Cone, 2005).
In-vivo calcium imaging reveals that the ARC POMC neurons react to both the presence of food and the sensory anticipation of food availability ( Chen et al., 2015). This suggests that leptin-responsive POMC neurons have a role in acute satiety and foraging, in addition to long-term energy balance. Transgenic mice lacking the melanocortin 4 receptor show a similar phenotype to ob/ob mice: obese with increased food intake and suppressed energy expenditure. Selective reintroduction of melanocortin 4 receptors in neurons within the PVH normalises the excess food intake but not the suppressed energy expenditure seen in these mice ( Balthasar et al., 2005).
In addition to running through circuits that modulate food intake, leptin receptors are also expressed in regions involved in thermoregulation: neurons that express leptin receptors are trans-synaptically labelled by pseudorabies virus injected into BAT ( Zhang et al., 2011). This delineates the presence of leptin receptors throughout the efferent thermoregulatory circuit from BAT, up to the RPa, the DMH, and finally, the median POA (MnPO). Leptin receptor-expressing neurons in the DMH produce c-Fos in response to acute cold exposure. Chemogenetic activation of these DMH leptin receptor-expressing neurons stimulates BAT thermogenesis and locomotor activity without affecting food intake, resulting in decreased body weight ( Rezai-Zadeh et al., 2014). Hence there is overlap between the circuits mediating the thermogenic effects of leptin and those mediating the thermogenic effect of acute cold exposure.
Neurons in the POA that express leptin receptors also respond to exposure to a warm environment. Chemogenetic activation of these neurons inhibits feeding and reduces energy expenditure by inhibiting BAT thermogenesis ( Yu et al., 2016). The simultaneous inhibition of feeding and thermogenesis is not usually a role attributed to leptin. Rather, leptin is usually associated with suppression of feeding and stimulation of thermogenesis ( Myers et al., 2008). Hence one might anticipate that the effect of leptin on these neurons would be to inhibit their activity. Indeed, the leptin receptor has been shown to be capable of inhibiting synaptic transmission ( Thompson & Borgland, 2013). These observations were dependent on ambient temperature, which suggests a role for POA leptin receptor-expressing neurons linking the necessary alterations of food intake that must accompany changes in the energy demands of thermogenesis. It would be interesting to establish the effects of leptin at its receptors in these neurons, and to identify their projection targets.
In conclusion, the picture that emerges from the literature is that leptin tends to move energy expenditure and food intake in opposite directions: increased leptin drives weight loss by suppressing food intake and increasing energy expenditure. This suggests that its primary role is to control body mass and / or WAT stores. It may also contribute to suppressing both thermogenesis and food intake following exposure to a warm environment, although this is less well established.
Neuropeptide Y and agouti-related peptide
NPY is one of the most highly expressed neuropeptides found in the brain, where it is commonly co-expressed with AgRP ( Chronwall et al., 1985). It is involved in energy homeostasis ( Bi et al., 2003), circadian rhythms, the stress response, and cognition ( Elmquist et al., 1999). NPY production is widespread, but of particular relevance to energy homeostasis, it is expressed in the ARC, the NTS, the DMH, and the PVH. There are three main NPY receptors in humans and rodents: Y1, Y2, and Y5. Expression of these receptors is also widespread, but densities occur in areas related to homeostasis, thermoregulation, and energy expenditure including: PVH, ARC, lateral hypothalamus, NTS and DMH. As introduced above, NPY and AgRP respond to fasting or WAT store depletion by suppressing energy expenditure and increasing food intake ( Cone, 2005; Reichmann & Holzer, 2016). Hence, NPY/AgRP have opposite and antagonistic functions to leptin.
Physiology of NPY and AgRP
Peripheral administration of ghrelin induces c-Fos expression in, and depolarises, ARC NPY/AgRP neurons ( Chen et al., 2015; Cowley et al., 2003; Wang et al., 2002). These ARC NPY/AgRP neurons also express leptin receptors, the action of which reduces NPY and AgRP expression ( Wang et al., 1997), and causes hyperpolarisation ( Cowley et al., 2001). Hence, NPY and AgRP neurons are activated by low WAT energy stores and hunger. As with POMC neurons, NPY/AgRP neurons are modulated by the sensory anticipation of food, such that in food-restricted mice, presentation of sensory cues indicating food availability reduces firing ( Chen et al., 2015). Adult ablation of NPY/AgRP neurons leads to mice with low body weight, reduced food intake, and increased BAT activity ( Bewick et al., 2005; Gropp et al., 2005; Luquet et al., 2005). Intra-cerebroventricular (ICV) administration of NPY acutely increases food intake and decreases BAT activity. ICV NPY also increases WAT lipoprotein lipase activity (indicating increased lipid deposition), an effect that persists in food-restricted rats, suggesting a direct effect rather than as a consequence of increased metabolic substrate availability ( Billington et al., 1991).
The effects of NPY on both BAT and WAT are mediated by sympathetic innervation, rather than via a circulating factor ( Egawa et al., 1991). NPY’s ability to drive lipoprotein lipase is an interesting observation since one might anticipate that NPY would liberate fat stores in an animal that is hungry. Instead, the activation of lipoprotein lipase suggests that the role of NPY is directed primarily towards replenishing fat stores rather than providing energy for immediate metabolism.
Functional anatomy of NPY and AgRP
Local injections of NPY into discrete hypothalamic nuclei has at times produced contradictory findings. Injection into the ARC induces hypothermia, as might be expected ( Jolicoeur et al., 1995). NPY injection into the PVH may inhibit BAT sympathetic nerve activity ( Egawa et al., 1991), and yet it has also been shown to induce hyperthermia ( Jolicoeur et al., 1995). Likewise, injection into the medial preoptic area (MPA) can increase sympathetic nerve activity ( Egawa et al., 1991), but may also induce hypothermia ( Jolicoeur et al., 1995). This bidirectional response might indicate that high doses of NPY activate autoreceptors in a negative feedback loop to block transmission. This could result in increased BAT thermogenesis. This hypothesis is supported by the observation that ICV injection of NPY at low doses induces hypothermia and at higher doses causes hyperthermia ( Jolicoeur et al., 1995). Inhibition of NPY release by activation of presynaptic Y2 receptors has been observed in-vitro ( King et al., 1999). This is an important consideration with implications for experiments that use agonist injections or opto- or chemogenetics to activate circuits in a potentially supra-physiological manner.
ARC NPY /AgRP neurons project to the DMH and PVH, both of which also contain cell bodies that express NPY ( Chao et al., 2011; Chronwall et al., 1985; Tiesjema et al., 2007; Wang et al., 1997). While both acute and chronic food restriction induce NPY mRNA expression in the ARC, only chronic food-restriction induces NPY mRNA in the DMH ( Bi et al., 2003). Knock-down of DMH NPY expression increases WAT browning, lipolysis, and BAT UCP1. This increased BAT thermogenesis combined with observed increases in locomotor activity is not accompanied by increases in food intake, and therefore results in weight loss ( Chao et al., 2011). The effect of NPY knockdown in the DMH mimics the effect of leptin in this nucleus, with a tendency to affect thermogenesis and energy expenditure more than food intake ( Rezai-Zadeh et al., 2014). The DMH, however, may be capable of increasing food intake via a cholinergic input to the ARC, which increases inhibitory tone on ARC POMC neurons ( Jeong et al., 2017). One might speculate that this projection forms the basis for the link that drives increased food intake when the energetic costs of thermogenesis are high. Opto- and chemogenetic manipulation of ARC NPY/AgRP neurons that project to the PVH indicates that this circuit stimulates feeding via GABAergic input on PVH oxytocin neurons ( Atasoy et al., 2012; Chen et al., 2015).
Food intake and thermoregulation: summary
The ARC, DMH, and PVH form a circuit that integrates information about the past, the present, and the future energy state. These signals are generated at least in part by leptin, ghrelin, and sensory inputs, respectively. Leptin signals long-term energy balance as reflected by WAT stores. Ghrelin signals recent food intake and time since last meal. Sensory inputs signal the approaching likelihood of food ( Chen et al., 2015). The system comprises parallel antagonistic and mutually inhibitory branches: elevated leptin indicates replete energy stores and releases the brakes on energy expenditure while inhibiting further food intake by activating POMC neurons and inhibiting ARGP/NPY neurons; suppressed leptin and/or elevated ghrelin signals depletion of energy stores, driving food intake and suppression of energy expenditure through activation of NPY/AgRP and inhibition of POMC neurons.
At each level neurons that are activated by ghrelin are generally inhibited by leptin and vice versa. A model that emerges from review of the literature is that the ARC to PVH projection responds rapidly to cues regarding acute energy requirements and food availability to drive changes in food intake. On the other hand, the ARC to DMH projection appears predominantly concerned with adjusting energy expenditure so that WAT stores are maintained within a target range. Within this putative framework, the PVH might modulate feeding through the NTS, while the DMH would modulate energy expenditure via the RPa. While this model gives a framework in which to consider control of energy expenditure and food intake, it is an over-simplification: the PVH may also control BAT thermogenesis ( Billington et al., 1994; Shi et al., 2021), and as mentioned above, the DMH may regulate feeding ( Jeong et al., 2017).
Thermoregulation and sleep
In humans intracranial temperature reaches a peak in the period one to two hours prior to the onset of darkness, and drops by approximately 1°C during sleep ( Landolt et al., 1995). The cooling associated with sleep is an active process, in humans driven at least in part by peripheral vasodilation and heat dissipation through sweating ( Kräuchi et al., 2000). Similar alterations in core and brain temperature during sleep are seen in rodents ( Harding et al., 2019; Zhang et al., 2015).
Non rapid-eye-movement (NREM) sleep is characterised by a controlled reduction in body temperature such that while core body temperature reduces, changes in ambient or hypothalamic temperature continue to induce thermoregulatory responses, including sweating ( Geschickter et al., 1966), panting, and shivering ( Parmeggiani & Rabini, 1967). During NREM sleep, the temperature threshold for inducing metabolic heating is reduced, and the slope of the response also reduced compared to wake ( Glotzbach & Heller, 1976). In contrast, rapid-eyemovement (REM) sleep appears to involve total cessation of thermoregulation such that (in small animals at least) body temperature follows changes in ambient temperature, and changes in local hypothalamic temperature do not generate adjustments in metabolic heat production ( Glotzbach & Heller, 1976; Heller & Glotzbach, 1977; Walker et al., 1983). This abandonment of thermoregulation during REM may be a factor limiting the duration of REM epochs in sub-thermoneutral environments ( Heller & Glotzbach, 1977). REM sleep is also characterised by cerebral oxygen consumption similar to that seen during waking, which is in stark contrast with NREM sleep ( Madsen et al., 1991).
The interaction between sleep and body temperature is reciprocal: sleep is associated with a reduction in body temperature, and increased body or hypothalamus temperature or a warm ambient environment promotes sleep ( Bunnell et al., 1988; Kräuchi et al., 1999; Lo Martire et al., 2012; McGinty & Szymusiak, 1990; Romeijn et al., 2012). This relationship has led some to hypothesise that sleep is fundamentally a thermoregulatory homeostatic process ( McGinty & Szymusiak, 1990). The observation that vasodilation and a rapid rate of body cooling is associated with sleep onset appears somewhat at odds with the fact that prior to sleep humans and rodents seek out warmth ( Harding et al., 2018). The reason behind this apparent paradox may lie in the observations that while increased temperature, be that core, brain, or ambient is associated with increased sleep, in particular NREM sleep, it is not the elevated temperature but rather the subsequent high rate of heat loss that seems to be most predictive of sleep initiation.
In humans, increasing peripheral vasodilation associated with a falling core body temperature from its peak in the hours prior to sleep onset predicts latency to sleep ( Kräuchi et al., 1999; Kräuchi et al., 2000). Humans also tend to select a bed time that coincides with the maximum rate of circadian body temperature reduction ( Campbell & Broughton, 1994). A similar observation has been made in mice: reactivating warm-sensing POA neurons induces a drop in core temperature and increased NREM sleep ( Harding et al., 2018). The tendency to seek warm environments prior to sleep may therefore help to activate these sleep-inducing POA neurons.
On the other hand, the high rate of heat loss and relative unresponsiveness of the thermoregulatory system may reflect an undesirable but inevitable side-effect of the process of sleep induction. In this case, seeking out warm environments prior to sleep would be a means to mitigate some of the heat loss that results from those side-effects. For example, if reduced sympathetic tone is required to allow the animal to enter a low vigilance state prior to sleep onset, then a corollary of that might be increased vasodilation and reduced BAT thermogenesis.
Functional anatomy linking sleep and thermoregulation: the preoptic hypothalamus
The POA acts as a critical hub linking thermoregulation and sleep. In mice, recovery sleep following a period of deprivation is associated with a drop in core temperature of between 1.5 and 2°C, and increased delta power, indicating NREM sleep. Chemogenetic reactivation of median and lateral POA neurons that are active during recovery sleep recapitulates both this increased NREM sleep and drop in core body temperature ( Kroeger et al., 2018; Zhang et al., 2015). Lateral POA (LPO) neurons are also the target of the α-2 agonist dexmedetomidine, which induces sedation that mimics recovery sleep.
The population of neurons in the region of the LPO and VLPO that are capable of inducing NREM and hypothermia express galanin ( Kroeger et al., 2018): chemo- or optogenetic stimulation of GABA- and galanin-expressing neurons in the ventrolateral preoptic nucleus (VLPO) induces NREM and a drop in body temperature; knock-out of lateral preoptic (LPO) galanin neurons significantly attenuates the sedation and hypothermia associated with dexmedetomidine administration, and causes a rise in body temperature with disrupted sleep homeostasis ( Ma et al., 2019).
These findings support the hypothesis that reduced sympathetic tone associated with activation of presynaptic α-2 receptors disinhibits sleep- and hypothermia-promoting galanin neurons in the lateral and/or ventrolateral preoptic area. VLPO neurons that are active during sleep project monosynaptically to the tuberomammillary nucleus, which is known to modulate arousal ( Sherin et al., 1996). In a related study, warm sensitive neurons in the region of the MnPO/MPO were reactivated using activity-dependent tagging ( Harding et al., 2018).. While reactivation of a GABAergic subpopulation of these neurons induced NREM sleep without a significant change in body temperature, reactivation of the glutamatergic/nitrergic subpopulation induced both, indicating that they may be part of the circuit that coordinates NREM sleep induction with body temperature reduction.
In all these experiments, the drop in body temperature during chemogenetic-driven sleep was deeper than that seen in natural sleep ( Harding et al., 2018; Kroeger et al., 2018; Zhang et al., 2015). This observation may reflect the somewhat abnormal nature of the stimulation ( Armbruster et al., 2007), or may reflect an additional role for these regions in torpor.
Sleep and adenosine
The role of adenosine in sleep is complex and beyond the scope of this review; presented here is a summary of some key aspects, as they relate to torpor (a more comprehensive review is provided here ( Silvani et al., 2018)). The G protein-coupled adenosine receptors A1R, and A2R are widely expressed throughout the brain. The A1R is inhibitory and generally considered to be neuroprotective through suppression of glutamate release and hyperpolarisation (reviewed here ( Cunha, 2005)), and by modulating cerebral blood flow and metabolic rate ( Blood et al., 2003). In addition to their central effects, adenosine receptors in the cardiovascular system mediate negative inotropic, chronotropic, dromotropic, and anti-adrenergic effects via A1Rs, and vasodilatation via A2Rs (reviewed here ( Shryock & Belardinelli, 1997)). Central activation of A1Rs promotes sleep, hypothermia, sedation, and reduced locomotor activity ( Anderson et al., 1994).
Sleep deprivation increases the homeostatic drive for sleep, and is reflected in elevated time spent in NREM and by increased EEG delta power during subsequent recovery sleep (reviewed here ( Borbély et al., 2016)). Expression of this rebound increase in NREM sleep is dependent on the presence of neuronal A1Rs, via an interaction with glia ( Bjorness et al., 2009; Bjorness et al., 2016), although additional mechanisms may also be capable of providing this function, for example in whole-animal A1R knockouts ( Stenberg et al., 2003). In this way, adenosine links the homeostatic drive for sleep with suppression of metabolic and cardiovascular systems, and induction of NREM sleep.
Sleep and torpor
Ground squirrels, pocket mice, and laboratory mice enter torpor through NREM sleep ( Berger, 1984; Heller & Glotzbach, 1977; Huang et al., 2021; Walker et al., 1977). Electroencephalogram (EEG) recordings during torpor display the characteristics of NREM sleep provided brain temperature is above about 25°C. At brain temperatures below 25°C, EEG power is globally reduced but delta waves associated with NREM sleep are discernible. EEG power decreases (and with it, the ability to discern sleep states) with brain temperatures below 20°C, and becomes isoelectric below about 10°C ( Larkin & Heller, 1996; Walker et al., 1981).
Consistent with the observation that low brain temperatures are associated with the loss of NREM EEG pattern, there is evidence that prolonged torpor such as that seen in seasonal hibernators is associated with accumulation of sleep debt. During prolonged seasonal hibernation, arctic ground squirrels periodically arouse to euthermia through NREM sleep. The duration of the post-arousal NREM sleep correlates with the minimum brain temperature reached during the preceding torpor ( Larkin & Heller, 1996; Trachsel et al., 1991). These observations suggest that while torpor at intermediate core temperatures resembles NREM sleep, some of the vital functions of NREM sleep are depressed during torpor at very low body temperature and must be performed at or close to euthermia. However, preventing sleep during interbout arousal by handling or caffeine injection does not result in a rebound increase in percentage time spent in NREM nor increase in the length of the interbout euthermic period in ground squirrels ( Larkin & Heller, 1999). The authors of this study conclude that the increase in NREM sleep observed after torpor may in fact represent some other neurological function rather than correction of a sleep debt as occurs after prolonged wakefulness at euthermia. There are alternative interpretations of these data that are consistent with an accumulated sleep debt during hibernation. For example, it might be the case that whatever restorative process occurs during interbout NREM sleep must occur soon after emergence, or else return to torpor might be prioritised over NREM sleep when that sleep is disturbed. Nevertheless, it is fair to say that the role of interbout arousals, and the associated periods of NREM sleep are debated. In summary, torpor and NREM share several important characteristics including preserved thermoregulatory control despite altered body temperature and reduced energy expenditure. The accumulated evidence suggests that NREM may indeed be a transition state through which torpor is entered. Whether or not torpor is itself an exaggerated NREM state remains to be seen, but as discussed later, there is evidence that neurons in the preoptic area might link the body cooling associated with sleep ( Harding et al., 2019) and torpor ( Hrvatin et al., 2020; Takahashi et al., 2020; Zhang et al., 2020).
Efferent signals triggering torpor entry
The sympathetic nervous system and leptin in torpor
Dopamine beta-hydroxylase (DBH) knock-out mice (DBH-/-) lack norepinephrine and epinephrine, while their heterozygous littermates appear essentially normal. Norepinephrine can be at least partially restored by the administration of L-threo-3,4-dihydroxyphenyserine (DOPS) ( Thomas et al., 1995; Thomas et al., 1998). DBH-/- mice fail to enter torpor after 12 hours of fasting at 20°C ( Swoap et al., 2006). This impairment in torpor can be reversed by the administration of DOPS to restore adrenergic signalling, or by selective activation of beta-3 adrenoceptors. Serum leptin is elevated in both the fed and fasting state in DBH-/- compared to DBH+/- mice. Fasting does not significantly reduce serum leptin in DBH-/- mice, but fasting in combination with administration of DOPS or a beta-3 agonist reduces serum leptin to levels comparable to fasted DBH+/- mice.
The model that emerges from this series of experiments is that activation of beta-3 receptors on WAT suppresses leptin release, which serves as a signal for torpor induction. There are additional studies that support this model. Firstly, DBH-/- mice that also lack leptin signalling (by crossing with ob/ob mice to generate double-mutant mice) regain the ability to enter torpor, albeit displaying unusually early and shallow bouts. The proposal is that in lacking leptin, these modifications bypass the need for sympathetic action on WAT. Once torpid, these double knock-out mice are unsurprisingly slow to rouse given they lack both leptin, which is BAT thermogenic, and norepinephrine, which acts on beta-3 receptors in BAT to stimulate thermogenesis ( Swoap & Weinshenker, 2008). Secondly, exogenous leptin reduces leptin mRNA expression in WAT of DBH+/- mice but does not suppress expression in DBH-/- WAT, indicating that the autoregulation of leptin is dependent on norepinephrine (or perhaps epinephrine) ( Commins et al., 1999). Thirdly, torpor in short photoperiod-adjusted Djungarian hamsters can be blocked by chemical sympathectomy with 6-Hydroxydopamine ( Braulke & Heldmaier, 2010).
This is an appealing model, but there are some caveats:
1. While the torpor bouts generated by administration of DOPS to fasted DBH-/- mice appeared similar to those seen in DBH+/- controls, administration of a beta-3 agonist (CL 316243) produced a hypothermia so profound that the animals did not spontaneously arouse ( Swoap et al., 2006). It is not entirely clear, then, that this was the same as natural torpor.
2. If activation of beta-3 receptors on WAT serves as the first step towards torpor induction, then administration of a beta-3 agonist to wild type mice should increase the probability and or depth of torpor: this has not been reported. If torpor induction really depends upon beta-3 receptor activation in WAT this must be a highly localised process and not via circulating adrenaline, which would otherwise drive BAT thermogenesis.
3. Fasted DBH+/- mice given a selective beta-3 receptor antagonist appear to enter torpor normally, with a rate of decline in core body temperature that is comparable to controls ( Swoap et al., 2006). The difference between this and control torpor bouts appears to be that the beta-3 receptor antagonist caused the torpor bout to be terminated before core temperature reaches a ‘normal’ nadir. This does not fit with the model that beta-3 receptor suppression of WAT leptin release is the initiating trigger for torpor.
4. Given that leptin acts on POMC/α-MSH neurons in the arcuate, blocking this pathway should mimic a drop in leptin and therefore be pro-torpor. However Ay mice, which display ectopic AgRP production and through the antagonist effect of AgRP on melanocortin 4 receptors, impaired α-MSH – melanocortin signalling, in fact show a reduced tendency to torpor ( Gluck et al., 2006), although one cannot exclude compensatory mechanisms at play with genetically modified lines such as this.
Although there is a correlation between the ability to suppress leptin and the ability to enter torpor, a causal nature for this relationship has not been exhaustively demonstrated. An implication of this model is that exogenous leptin should prevent torpor, and that interfering with leptin signalling should induce torpor even in a fed state. These have been difficult to demonstrate and will be discussed in more detail below.
Mice lacking leptin, the ob/ob mice, are prone to deeper and longer torpor bouts than wild type (WT) mice on fasting or food restriction, despite their large adipose tissue stores ( Gavrilova et al., 1999; Himms-Hagen, 1985). However, it is worth pointing out that the ob/ob mouse is not permanently torpid, and neither are A-ZIP/F-1 mice, which have both dramatically reduced WAT and BAT and persistently low leptin levels ( Gavrilova et al., 1999). While A-ZIP/F-1 mice will readily enter torpor on fasting, exogenous leptin administration does not prevent fasting-induced torpor in these mice. In contrast to this, leptin administration to ob/ob mice may block torpor entry. Interpreting the effects of leptin administration to transgenic mice that have adapted to absent leptin signalling is challenging, especially given that the expression of torpor in these mice, even without the additional complexity of adding exogenous leptin, is not the same as torpor seen in WT mice.
Studying the effect of leptin on torpor in WT mice has also produced contradictory findings. One study reports no effect of leptin treatment on core temperature of WT mice during a 24 hour fast ( Gavrilova et al., 1999). In this study leptin administration to male WT mice fasted for 24 hours did not prevent the mild drop in core temperature seen in control fasted mice. However, while the core temperature of both leptin-treated and control male WT mice during 24 hours of fasting did decrease, it remained above 30°C. Therefore, neither control nor leptin treated mice entered torpor, but rather both groups showed a mild suppression of metabolic rate and body temperature in response to fasting. Hence, the extent to which these observations pertain to torpor is not clear
In another study, male WT mice fasted for up to 48 hours showed fasting-induced suppression of metabolism and core temperature, but again not full torpor. Leptin treatment did reduce fasting-induced hypometabolism in these WT mice ( Bechtold et al., 2012). The reasons for these different results are not clear, but may reflect differences in the strain of the mice used, differences in the method for administration of leptin: in the former study ( Gavrilova et al., 1999), leptin was administered via continuous subcutaneous (SC) infusion whereas in the latter study ( Bechtold et al., 2012), leptin was delivered in a single ICV injection. Whatever the reason for these differing results, neither have confirmed that leptin delivery to WT mice prevents full torpor bouts, indeed neither study demonstrated torpor even in control mice fasted for 24-48 hours, which is perhaps surprising.
Leptin treatment in a fasted marsupial mammal ( Sminthopsis macroura) reduces the duration and depth of daily torpor bouts ( Geiser et al., 1998), but again the effect of leptin in this species appears to be predominantly to impair the maintenance rather than the initiation of torpor. It seems that exogenous leptin might reduce the probability of torpor entry in Siberian hamsters although in those leptin-treated hamsters that did enter torpor, the torpor bout depth, duration, and frequency remained comparable to torpor bouts in control hamsters ( Freeman et al., 2004). Comparing hamsters housed under identical conditions that did or did not enter torpor revealed no difference in endogenous serum leptin levels. Likewise, the serum leptin levels were the same in individual animals on days in which the animal did or did not enter torpor. Finally, while animals that entered torpor tended to have low leptin, the lowest levels were recorded in hamsters that did not enter torpor.
Knockout of the orphan receptor Gpr50 (Gpr50 -/-), which is structurally and functionally related to the melatonin receptor ( Reppert et al., 1996) and is expressed in DMH and tanycytes lining the third ventricle, results in a similar phenotype to that seen in ob/ob mice: suppressed dark phase core body temperature and reduced threshold for torpor ( Bechtold et al., 2012). Gpr50 expression is reduced in the DMH of ob/ob and is normalised by leptin replacement. The body temperature of Gpr50 -/- mice does not increase in response to exogenous leptin administration, nor does exogenous leptin block torpor in these mice. Gpr50 -/- mice also have suppressed expression of thyrotropin-releasing hormone (TRH) in the PVH, which is further suppressed by fasting. Administration of a TRH analogue blocked torpor in Gpr50 -/- mice. This evidence suggests that leptin stimulates Gpr50 expression in the DMH, and that this in turn stimulates TRH release in the PVH. Activation of this pathway may provide a mechanism by which leptin reduces the propensity to torpor, and hence a drop in leptin may facilitate torpor.
In summary, the evidence for leptin’s role in torpor garnered from transgenic models varies depending on whether the model used is the primarily leptin-deficient ob/ob line, or the A-ZIP/F-1 line in which absent leptin is secondary to persistently depleted adipose tissue stores. While both models result in low leptin and increased propensity to torpor, only the ob/ob mice are sensitive to leptin replacement. That mice from neither line are in a permanent state of torpor would suggest either adaptive mechanisms appear during development, or else a permissive rather than a sufficient role of low leptin in torpor. Attempts to establish the effects of leptin administration to WT mice have been hampered by the fact that the WT control mice in these experiments were not entering full torpor. That said, converging evidence both from studies specifically examining leptin and torpor, as well as studies looking at the role of leptin under more ‘normal’ physiological settings, indicates that it is likely that high leptin would inhibit torpor and conversely low leptin likely forms at least part of the permissive signal for torpor. Finally, evaluation of the studies to date raises the possibility that the beta-3 adrenoceptor-driven suppression of leptin plays a greater role in maintaining than initiating daily torpor. Consideration of the role of leptin in torpor highlights a crucial distinction between daily torpor, when leptin is low ( Swoap et al., 2006), and seasonal hibernation, when leptin is elevated ( Xing et al., 2015).
NPY, ghrelin, and torpor
Since ghrelin and NPY act as the counterbalance to leptin, signalling hunger and energy deficit (see section 1.3.4), it is reasonable to hypothesise that they contribute to the signal for torpor. Ghrelin injection during a fast in a cool ambient temperature deepens and prolongs torpor bouts in mice but does not induce torpor in the fed state ( Gluck et al., 2006). NPY-/- mice exhibit shallow and aborted torpor bouts, which are not rescued by peripheral ghrelin. This indicates that ghrelin exerts its effects on torpor via NPY neurons.
ICV injection of NPY in cold-acclimated Siberian hamsters (small, heterothermic mammals) reduces core body temperature and can increase the probability of torpor, an effect mediated by Y1 receptors ( Dark & Pelz, 2008). ICV NPY may also inhibit food intake, in proportion to its effects on body temperature or torpor ( Paul et al., 2005). This latter finding is surprising given NPY is usually considered orexigenic), although in these studies food intake was assessed 24 hours after administration of NPY, and might therefore reflect secondary effects rather than direct action of NPY on orexigenic circuits. At some point, in order to enter torpor, the normal response to hunger, which is to forage and increase food intake, is presumably switched to a signal to cease locomotor activity and enter torpor; perhaps this observation reflects that transition. It is also relevant to note that hamsters undergo both fasting-induced torpor, which is triggered by energy deficit at any seasonal time, and short photoperiod-induced torpor, which is seasonal and does not necessarily involve an energy deficit. These distinct torpor phenotypes may involve different regulatory mechanisms ( Cubuk et al., 2017), which could account for the observed effect of NPY on food intake in these animals.
The arcuate is a key locus for NPY signalling, and selective ablation of ARC neurons with monosodium glutamate (MSG), supports a role for this nucleus in torpor. For example, in contrast to controls, ARC-ablated mice do not enter torpor after 24 hours of fasting, although they do show a degree of fasting-induced hypothermia ( Gluck et al., 2006). In Siberian hamsters, ARC ablation impairs short photoperiod-induced torpor, reducing the probability, and slightly reducing the depth and length of torpor bouts. However, torpor was still seen in these hamsters and there was no difference in residual ARC NPY immunoreactivity between ARC-ablated hamsters that did and those that did not enter torpor. Likewise, ARC ablation reduced the probability of torpor in food restricted hamsters but had no effect on the quality or frequency of those torpor bouts in animals in which torpor was seen.
Although NPY receptor antagonists have been shown to prevent NPY-induced hypothermia ( Dark & Pelz, 2008), the same has not been demonstrated for natural torpor. This raises questions about whether the hypothermia seen following NPY injection is torpor, or rather an exaggerated form of the starvation-induced drop in temperature that is seen in non-hibernators ( Billington et al., 1991), although of course the two may lie on a continuum.
In summary, there is evidence indicating roles for ghrelin and NPY within the ARC as signals for the conditions that are associated with torpor. There is also some evidence supporting direct roles in torpor, and a functioning ARC nucleus may be a requisite for the expression of torpor in mice. However, this necessity has not been demonstrated in hamsters, indicating either that alternative mechanisms might exist, capable of bypassing the ARC, or else suggesting that torpor in hamsters and mice is generated through distinct mechanisms. To date, there is no evidence that activity of ARC neurons is sufficient to induce a torpor bout.
Adenosine, orexin, and torpor
Adenosine, which was introduced above, is a natural candidate to link many of the functions associated with torpor ( Silvani et al., 2018). Central infusion of the A1R agonist N 6-cyclohexyladenosine (CHA) into rats exposed to cold ambient temperature generates a state that has many features of torpor, including vagally mediated skipped beats and bradycardia, inhibition of BAT and shivering thermogenesis, and decreased EEG power ( Tupone et al., 2013). Accumulation of adenosine during periods when demands for ATP outstrip supply, and the consequent engagement of a repertoire of responses that limit ATP consumption (reviewed here ( Newby, 1984)), make it an appealing candidate for signalling the drive for torpor.
Prolonged subcutaneous infusion of aminophylline, a non-specific adenosine receptor antagonist, significantly impairs torpor in male mice, resulting in delayed, shallow, and brief torpor bouts. Aminophylline infusion initiated during torpor triggers emergence ( Iliff & Swoap, 2012). In hibernators, the response to adenosine is dependent on the season. For example, central A1R blockade in Syrian hamsters causes arousal during the induction phase of seasonal torpor ( Tamura et al., 2005). In arctic ground squirrels, ICV infusion of CHA induces torpor or a similar state, in a manner that was modulated by the season, and was blocked by central A1R antagonists ( Jinka et al., 2011). Calorie restriction by alternate day feeding suppresses core temperature and respiratory rate in rats, and increases the sensitivity to CHA by increasing the expression of A1Rs in the hypothalamus ( Jinka et al., 2010). Hence, modulation of the central sensitivity to adenosine provides a means for both hibernators and non-hibernators to adjust temperature responses to environmental cues.
Despite the striking similarities between torpor and the physiological response to central A1R activation, there are some features that remain distinct. Changes in heart rate with torpor occur rapidly and display frequent skipped beats, whereas those changes occur over several hours following CHA treatment and involve extension of the inter-beat interval with prolonged asystoles ( Vicent et al., 2017). Temperature changes are slower in natural torpor compared to CHA-driven hypothermia, with no evidence of shivering in the latter. Finally, c-Fos is induced in the liver and heart of mice treated with CHA, but not in natural torpor, indicating calcium influx and potentially signalling cellular stress following CHA treatment. Fasting-induced torpor persists in mice lacking AR1 and AR3 ( Carlin et al., 2017), although as with any germline KO study compensatory mechanisms might be engaged. Future studies using conditional knockout models could provide a more robust demonstration of the role of adenosine signalling in torpor.
Orexinergic neurons may mediate some of the thermoregulatory adaptations seen following central adenosine administration, since orexin -/- mice are less sensitive to the effects of central CHA administration ( Futatsuki et al., 2018). However, these same mice also recover more slowly from the hypothermia induced by CHA, and are prone to deeper, longer, and more frequent torpor bouts than WT controls. In-vivo calcium imaging in this study indicated that orexin neurons are active immediately prior to and after fasting-induced torpor. It is interesting to note that the interaction between orexin and CHA appears to be bidirectional: orexin enhances CHA-induced hypothermia initiation and overcomes it during recovery. Likewise, the effect of orexin on body temperature appears to depend on the sleep/wake cycle: promoting thermogenesis during waking and heat loss during sleep ( Mochizuki et al., 2006).
In summary, adenosine represents a candidate signal for torpor initiation but, once again, must be designated as ‘contributing’ or ‘permissive’ and not a necessary and sufficient master switch. One might expect orexin to reduce the likelihood of torpor, and to assist in arousals, and while this role is supported by the observation of increased torpor depth and duration in orexin -/- mice, the role in WT mice or other species is not clear. There is currently no accepted explanation for the apparent bidirectional effects of orexin on body temperature and following CHA administration.
Torpor and endogenous opioids
The endogenous opioid system contributes to pain modulation, reward, the stress response, and several autonomic functions including digestion, arousal, and control of heart and respiratory rate (reviewed here ( Benarroch, 2012)). It comprises three groups of peptide transmitters: β-endorphin, enkephalins, and dynorphins, which act predominantly but not exclusively at µ-, δ-, and κ- opioid receptors, respectively. Of note, β-endorphin is produced in POMC neurons, by an alternate cleaving of the precursor POMC. Early investigations into the thermoregulatory effects of intracerebral β-endorphin injection reported that the effects depended on both the location of the injection and the dose used. For example, injection into the POA, anterior hypothalamus (AH), periaqueductal grey (PAG), nucleus accumbens (NAcc), reliably produced an initial hypothermia, with core temperature dropping by approximately 1°C ( Tseng et al., 1980). This was generally followed by a period of hyperthermia, except when high doses were injected into the NAcc, where high doses appeared to produce a sustained hypothermia. A similar effect is seen following administration of morphine to rats at increasing doses (reviewed here ( Rawls & Benamar, 2011)). These biphasic responses might be the result of time- and dose- dependent activation of different opioid receptor classes. Studying the effects of various opioid receptor-specific agonists and antagonists in rats and mice suggests that activation of κ- or δ-opioid receptors results in hypothermia, whereas the µ-opioid receptor mediates hyperthermia. The hypothesis that κ-opioid receptor activation suppresses BAT thermogenesis is supported by the observation that activation of these receptors in the MnPO may drive the impaired BAT response to cold exposure observed in obese rats ( Conceição et al., 2021).
Some have argued for the existence of a ‘hibernation induction trigger’ (HIT) that circulates in blood of seasonal hibernators, and can be transfused from a hibernating individual into a non-hibernating individual with the effect of inducing hibernation ( Dawe & Spurrier, 1969), although this is controversial ( Wang et al., 1988). The apparent induction of hibernation via HIT transfusion is impaired by infusion of µ or κ agonists, whereas infusion of the δ agonist DADLE ([D-Ala, D-Leu]-Enkephalin) appeared to mimic the effects of HIT infusion by inducing hibernation in summer-active ground squirrels. It has therefore been argued that natural hibernation generates a circulating δ-receptor agonist that is capable of triggering hibernation ( Oeltgen et al., 1988).
Less controversial observations of the role of the endogenous opioid system in torpor derive from experiments infusing agonists or antagonists either locally or ICV in hibernating hamsters. Arousal from the maintenance but not the induction phase of torpor can be triggered by ICV naloxonazine (a µ1 opioid receptor antagonist) in hibernating Syrian hamsters ( Tamura et al., 2005). Thus, maintained suppression of body temperature may depend on POMC neurons in the ARC that project to regions including DMH, AH, posterior hypothalamus (PH), and ventromedial hypothalamus (VMH) ( Tamura et al., 2012).
In summary, there is conflicting evidence from these experiments. In rats, and non-torpid mice, the evidence suggests that δ-opioid receptor activation induces hypothermia whereas µ-opioid receptors induce hyperthermia. One might therefore expect δ-opioid receptor activation to be involved in inducing or maintaining torpor. Experiments using HIT infusion to induce torpor or a torpor-like state in ground squirrels support this model, with a role for δ-opioid receptor activation in torpor induction, while µ- and κ-opioid receptors appear to inhibit torpor entry. In contrast to this, and out of keeping with the findings in rats and non-torpid mice, in Syrian hamster undergoing seasonal hibernation, the evidence would suggest that POMC neurons in the ARC activate µ-opioid receptors in several hypothalamic areas to maintain low body temperature in seasonal hibernation. It is difficult to draw any synergy from these findings: it is possible that different opioid receptors are involved in both promoting and inhibiting torpor, perhaps as part of a system that prevents excessively long or deep torpor bouts. Alternatively, it is worth considering whether the doses of agonists and antagonists used resulted in non-specific activation of several opioid receptor subtypes. It would be interesting to test the effects of modulating endogenous opioid pathways in mice undergoing daily torpor, as the data above only describes effects on seasonal hibernators or euthermic mice and rats.
Neural control of torpor
In the 13-lined ground squirrel, a seasonal hibernator, in-situ hybridisation (ISH) reveals distinct patterns of c-fos expression during different phases of the hibernation cycle ( Bratincsák et al., 2007). Entrance into torpor is associated with increased c-fos mRNA in the ventrolateral part of the MPA, whereas arousal from torpor is associated with increased expression in the ventromedial part of the MPA. In awake animals during interbout arousals, the ARC and dorsolateral hypothalamus were active. The SCN and reticular thalamus were active throughout all stages of torpor, areas involved in circadian rhythm generation and inhibition of motor activity, respectively. In torpid mice the combination of c-Fos immunohistochemistry and retrograde tracer expression identifies a group of neurons in the DMH that project to the RPa, which are specifically activated during torpor ( Hitrec et al., 2019). It is anticipated that activating this pathway would inhibit thermogenesis by reducing the output from RPa to BAT, and indeed pharmacological inhibition of the rostral ventromedial medulla (a region that includes the RPa) induces a torpor-like state in the rat ( Cerri et al., 2013).
Three recent publications significantly furthered our understanding of the neural control of torpor entry, all converging on the preoptic area of the mouse hypothalamus as a key region, potentially containing neurons that represent a torpor ‘master switch’. One of these studies ( Hrvatin et al., 2020) used activity-dependent recombination (‘TRAPing’, ( Allen et al., 2017; DeNardo et al., 2019)) to selectively express designer receptors exclusively activated by designer drugs (DREADDs ( Alexander et al., 2009)) in neurons that were active during daily torpor in the mouse. Chemogenetic reactivation of neurons within the anterior and ventral portions of the medial and lateral preoptic area (avMLPA) generated a profound hypothermia and reduction in locomotor activity that appeared to mimic torpor.
These torpor-TRAPed avMLPA neurons project to several regions likely to be involved in torpor including the dorsomedial hypothalamus. Further experiments suggested that within the population of TRAPed preoptic area neurons, a subset of glutamatergic, PACAP expressing neurons generate the drop in temperature and activity observed during both natural and synthetic torpor. Blocking synaptic transmission in either glutamatergic or PACAP expressing neurons within the avMLPA impaired the expression of natural fasting-induced torpor.
The second paper ( Takahashi et al., 2020) used a different approach but came to similar conclusions. They targeted expression of excitatory DREADDs to hypothalamic neuropeptide pyroglutamylated RFamide peptide (Qrfp) neurons. This neuropeptide was previously implicated in the modulation of food intake, adrenal activity, and anxiety, but not torpor ( Okamoto et al., 2016; Takayasu et al., 2006). Chemoactivation of Qrfp-expressing neurons in the medial preoptic area (MPA) and the anteroventral periventricular nucleus (AVPe, together termed AVPe/MPA) induced a long-lasting torpor-like hypothermic state in mice, with suppressed core temperature, oxygen consumption, heart rate, respiratory rate, and locomotor activity (termed QIH, for Q-neuron-induced hypothermia and hypometabolism).
QIH was recapitulated by selective optogenetic activation of the terminals of AVPe/MPA Qrfp neurons in the DMH. It was predominantly dependent on glutamatergic transmission within the Qrfp neurons population, although GABAergic Qrfp neurons appear to contribute to a smaller extent. Blocking synaptic transmission in Qrfp neurons impaired normal fasting-induced torpor resulting in a more gradual reduction in body temperature on fasting, and reduced the normal diurnal fluctuation in body temperature.
Hence, the authors identified a population of Qrfp-expressing neurons whose cell bodies lie in the preoptic area, which appear to have a role in generating the usual rapid decrease in core temperature associated with torpor induction, and whose terminals project to the DMH. Activation of this Qrfp neuron projection from the AVPe/MPA to the DMH generates a torpor-like state in mice. RNA in-situ hybridisation revealed that approximately 80% of Qrfp neurons also express PACAP, indicating significant overlap with the torpor-induing neurons identified by Hrvatin et al.
It is worth noting that Takahashi et al. did not demonstrate that the Qrfp neurons were active during torpor. Indeed, there were some differences between natural torpor and QIH induced by Takahashi et al., which appear to relate to whether the mouse is attempting to lose heat to the environment. During natural torpor, the mouse adopts a hunched posture consistent with attempts to conserve heat, irrespective of the ambient temperature. During QIH at high ambient temperature, the mouse adopts an extended posture, consistent with attempts to lose heat. In addition, at 21°C ambient temperature, QIH is associated with an initial increase in tail surface temperature, indicating vasodilatation. In contrast, natural torpor may be associated with increased total peripheral resistance, which suggests at least on the whole-body scale, vasoconstriction ( Swoap & Gutilla, 2009). Hence, there remains a question regarding the degree of overlap between QIH and natural torpor.
Furthermore, Takahashi et al. did not confirm that the Qrfp neurons projecting from POA to DMH were glutamatergic or GABAergic. This is an important question, since the observed hypothermia might, for example, be the result of activating the established POA to DMH GABAergic projection that is involved in warm-sensing and thermoregulatory homeostasis ( Tan et al., 2016), rather than a specific torpor-inducing pathway.
Finally, Zhang et al. ( Zhang et al., 2020) demonstrated a population of oestrogen-sensitive neurons within the medial preoptic area whose activity increases during fasting-induced torpor in the mouse. Selective ablation of these neurons using Cre-dependent Caspase 3 expression impaired fasting induced torpor in these mice, suggesting a role in natural torpor. Chemogenetic activation of these neurons generated hypothermia and bradycardia as well as suppressed locomotor activity and oxygen consumption: all features of natural daily torpor in the mouse. However, they too noted peripheral tail vasodilatation associated with this DREADD-driven torpor-like state. Importantly, these oestrogen sensitive MPA neurons did not appear to respond to increased ambient temperature, indicating that they are not part of the thermal defence circuit.
Towards a torpor circuit
The data presented in the studies of Hrvatin, Takahashi, and Zhang represent significant advances in our understanding of torpor. From this data, a model that emerges is that glutamatergic neurons in the preoptic area, which express PACAP and/or Qrfp, and perhaps oestrogen receptors, generate torpor. The preoptic area is well-placed for the role attributed to it in this model. It is a key site involved in thermoregulation and energy balance, receiving information regarding the external environmental temperature as well as directly sensing hypothalamic temperature ( Song et al., 2016), this information is then used to modulate BAT thermogenesis ( Tan et al., 2016; Zhao et al., 2017).
Warm-sensing glutamatergic POA neurons also play a role in coordinating the parallel decrease in core temperature observed with the onset of NREM sleep ( Harding et al., 2018). NREM sleep has several characteristics in common with torpor: it is a hypoactive, hypometabolic, bradycardic state, with maintained thermoregulation despite a reduced core body temperature ( Glotzbach & Heller, 1976; Heller & Glotzbach, 1977; Kräuchi, 2007; Schwimmer et al., 2010). Supposing that torpor is induced by the same circuit that links reduced body temperature with NREM sleep onset, then the distinction between the two states might rest upon the degree to which these POA glutamatergic neurons are activated. This could be either in terms of firing frequency, or duration. Hence, glutamatergic neurons in the medial preoptic area might represent a common circuit that links NREM sleep onset, the core body temperature alterations associated with sleep, and torpor. In keeping with this hypothesis, Takahashi et al. ( Takahashi et al., 2020) observed disrupted diurnal temperature variation in mice in which POA Qrfp neurotransmission was blocked. It would be very interesting to establish the degree of overlap between the POA glutamatergic neurons that drive NREM sleep and cooling as identified by Harding et al. ( Harding et al., 2018), and the torpor-inducing neurons identified by Hrvatin et al., and Takahashi et al.
In order to contribute to torpor induction, POA circuits with a role in thermoregulation and sleep induction within the POA would need to also receive information regarding the nutritional status of the animal. Such information might come from circulating leptin, receptors for which are indeed found on hypothermia-inducing glutamatergic neurons in the POA ( Yu et al., 2016).
The dorsomedial hypothalamus is also well-placed to contribute to torpor, and a projection from the POA to DMH is capable of driving reduced body temperature ( Takahashi et al., 2020; Tan et al., 2016; Zhao et al., 2017). As well as playing a role in thermoregulation ( Jeong et al., 2015; Liedtke, 2017; Zhao et al., 2017), the DMH also adjusts circadian rhythms based on the timing of food availability ( Gooley et al., 2006). One might speculate that the dorsomedial hypothalamus integrates information about the availability and timing of food in order to optimise the timing of torpor, which is known to be under circadian control but can be adjusted according to food availability ( van der Vinne et al., 2018).
This proposal that POA to DMH projections may be involved in torpor induction differs from the more established model of thermoregulation in several interesting ways ( Saper & Machado, 2020). Current understanding of homeostatic thermoregulation proposes that a predominantly GABAergic warm-sensing projection from the POA synapses on both GABAergic and glutamatergic neurons in the DMH ( Tan et al., 2016; Zhao et al., 2017). Activation of either the glutamatergic or the GABAergic neurons in the DMH drives thermogenesis ( Zhao et al., 2017). Hence, core temperature is determined by the balance between, on the one hand, the activity of DMH glutamatergic and GABAergic neurons, both of which drive thermogenesis, and on the other hand, the inhibitory input from the POA GABAergic neurons, which suppresses this thermogenic activity in the DMH ( Figure 1). In contrast, the POA to DMH projection that has been implicated in torpor appears to involve predominantly glutamatergic neurons, which express PACAP and / or Qrfp ( Hrvatin et al., 2020; Takahashi et al., 2020) ( Figure 3). Activation of this presumably excitatory POA to DMH pathway may contribute to the hypothermia associated with torpor ( Takahashi et al., 2020). The nature of the DMH neurons targeted by this projection is unknown, but they appear to have antagonistic effects to the populations of glutamatergic and GABAergic DMH neurons implicated in homeostatic thermoregulation ( Zhao et al., 2017). That is to say, previously identified DMH neurons - be they glutamatergic or GABAergic – are thought to drive thermogenesis ( Zhao et al., 2017), whereas the population targeted by the excitatory PACAP / Qrfp projection from the POA appear to induce hypothermia and torpor. One possibility is that these hypothermia-inducing DMH neurons are cholinergic ( Jeong et al., 2015), although the limited evidence suggests cholinergic DMH neurons are not active during torpor ( Hitrec et al., 2019).
Figure 3. Schematic comparing thermoregulation with torpor induction pathways.
The current thermoregulatory model (left) proposes predominantly GABAergic projections from POA to DMH. These GABAergic projections are activated by skin, viscera, or CNS warming and pyrogens. DMH contains both glutamatergic and GABAergic neurons, the activation of which causes increased BAT thermogenesis, vasoconstriction, and increased core temperature. The location of the second GABAergic neuron in the relay from DMH to raphe pallidus has not been established. The emerging model for torpor induction (right) suggests glutamatergic / PACAP / QRFP neurons project from POA to DMH. Similar to the effects of activating the GABAergic POA to DMH, activating this excitatory POA to DMH pathway reduces body temperature, and in this case, induces torpor. The nature of the DMH neurons that are activated by the excitatory POA to DMH projection remains unknown. Abbreviations: GABA, gamma-aminobutyric acid; POA, preoptic area; DMH, dorsomedial hypothalamic area; CNS, central nervous system; BAT, brown adipose tissue; PACAP, Pituitary adenylate-cyclase-activating polypeptide; QRFP, pyroglutamylated RFamide peptide (QRFP).
Conclusions
The field is gaining momentum as new techniques, particularly in the field of systems neuroscience, allow manipulation of the activity of specific hypothalamic neurons. There are, however, several outstanding questions. The lack of an agreed definition of torpor is problematic for the field. While a simple threshold of core temperature has been used, this approach fails to account for the circadian fluctuations in body temperature and fails to distinguish torpor from pathological hypothermia induced by disturbances to normal thermoregulation. This approach also ignores the many additional characteristics of torpor such as active cardiorespiratory suppression, suppression of mitochondrial oxygen consumption, and behavioural changes.
We propose that there are three key features of torpor, which might serve as a pragmatic proxy for identifying torpor experimentally: reduced body temperature with ongoing active thermoregulation such that a minimum (albeit adjusted) core temperature is defended; controlled cardiovascular depression such that a characteristic heart rate versus body temperature hysteresis curve is observed ( Vicent et al., 2017); and vasoconstriction ( Swoap & Gutilla, 2009), distinguishes interventions that activate warm defence (with reduced body temperature and and vasodilatation) from interventions that trigger torpor
Another outstanding question is whether daily torpor and seasonal hibernation are manifestations of the same process. While some groups argue that these are mechanistically distinct ( Sunagawa & Takahashi, 2016), this is not widely agreed. It is also unclear whether there exists a torpor ‘master switch’ capable of triggering the full spectrum of associated physiological adaptations, or whether several parallel regions detect the signal for torpor entry and independently trigger components of the response. While current evidence converges on the POA playing a key role ( Hrvatin et al., 2020; Takahashi et al., 2020; Zhang et al., 2020), these studies have tended to focus on body temperature as a proxy for the more complex adaptations associated with natural torpor. Aspects of torpor such as posture have not been recapitulated by these chemo- or optoactivation studies. While vasodilatation has been observed in the tail during chemoactivation of POA neurons ( Takahashi et al., 2020; Zhang et al., 2020), but it is not clear whether this is a feature of natural torpor. Indeed this represents an important outstanding question: do animals actively lose heat early in the torpor bout? If the POA does indeed contain the torpor master switch, whether a projection from here to the DMH ( Takahashi et al., 2020) is sufficient for inducing all aspects of torpor is also unknown, and represents an important question.
Finally, although we are beginning to identify some of the neuronal circuits responsible for torpor entry, the mechanism that transduces the signal from deplete energy stores to the POA and wider central nervous system remains unknown. Possible candidates include leptin, ghrelin, NPY, and adenosine, but a necessary and sufficient role for any of these signals has not been demonstrated. Furthermore, we do not yet know whether a single mechanism induces and maintains torpor, with arousal occurring when that mechanism ceases to be engaged, or on the other hand, whether separate processes govern these different stages of torpor.
Better understanding of the characteristics or torpor and the neural and endocrine mechanisms that control it may pave the way to mimicking this intriguing physiological state in humans, with potentially profound applications in medicine ( Aslami & Juffermans, 2010; Lee, 2008; Stanzani et al., 2020) and long distance space travel ( Cerri et al., 2021b).
Data availability
No data are associated with this article.
Funding Statement
This work was supported by Wellcome [211029 <a href=https://doi.org/10.35802/211029>https://doi.org/10.35802/211029</a>].
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[version 2; peer review: 2 approved, 1 approved with reservations, 1 not approved]
References
- Abreu-Vieira G, Xiao C, Gavrilova O, et al. : Integration of body temperature into the analysis of energy expenditure in the mouse. Mol Metab. Elsevier GmbH,2015;4(6):461–470. 10.1016/j.molmet.2015.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahima RS, Prabakaran D, Mantzoros C, et al. : Role of leptin in the neuroendocrine response to fasting. Nature. 1996;382(6588):250–252. 10.1038/382250a0 [DOI] [PubMed] [Google Scholar]
- Alexander GM, Rogan SC, Abbas AI, et al. : Remote Control of Neuronal Activity in Transgenic Mice Expressing Evolved G Protein-Coupled Receptors. Neuron. 2009;63(1):27–39. 10.1016/j.neuron.2009.06.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen WE, DeNardo LA, Chen MZ, et al. : Thirst-associated preoptic neurons encode an aversive motivational drive. Science. 2017;357(6356):1149–1155. 10.1126/science.aan6747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andermann ML, Lowell BB: Toward a Wiring Diagram Understanding of Appetite Control. Neuron. 2017;95(4):757–778. 10.1016/j.neuron.2017.06.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson R, Sheehan MJ, Strong P: Characterization of the adenosine receptors mediating hypothermia in the conscious mouse. Br J Pharmacol. 1994;113(4):1386–1390. 10.1111/j.1476-5381.1994.tb17151.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armbruster BN, Li X, Pausch MH, et al. : Evolving the lock to fit the key to create a family of G protein-coupled receptors potently activated by an inert ligand. Proc Natl Acad Sci U S A. 2007;104(12):5163–5168. 10.1073/pnas.0700293104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aslami H, Juffermans NP: Induction of a hypometabolic state during critical illness - a new concept in the ICU? Neth J Med. 2010;68(5):190–198. [PubMed] [Google Scholar]
- Atasoy D, Betley JN, Su HH, et al. : Deconstruction of a neural circuit for hunger. Nature. 2012;488(7410):172–177. 10.1038/nature11270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balthasar N, Dalgaard LT, Lee CE, et al. : Divergence of melanocortin pathways in the control of food intake and energy expenditure. Cell. 2005;123(3):493–505. 10.1016/j.cell.2005.08.035 [DOI] [PubMed] [Google Scholar]
- Barnes BM, Kretzmann M, Zucker I, et al. : Plasma androgen and gonadotropin levels during hibernation and testicular maturation in golden-mantled ground squirrels. Biol Reprod. 1988;38(3):616–622. 10.1095/biolreprod38.3.616 [DOI] [PubMed] [Google Scholar]
- Barnes BM: Freeze avoidance in a mammal: body temperatures below 0 degree C in an Arctic hibernator. Science. 1989;244(4912):1593–1595. 10.1126/science.2740905 [DOI] [PubMed] [Google Scholar]
- Bechtold DA, Sidibe A, Saer BR, et al. : A role for the melatonin-related receptor GPR50 in leptin signaling, adaptive thermogenesis, and torpor. Curr Biol. 2012;22(1):70–77. 10.1016/j.cub.2011.11.043 [DOI] [PubMed] [Google Scholar]
- Benarroch EE: Endogenous opioid systems: Current concepts and clinical correlations. Neurology. 2012;79(8):807–814. 10.1212/WNL.0b013e3182662098 [DOI] [PubMed] [Google Scholar]
- Bergendahl M, Iranmanesh A, Evans WS, et al. : Short-term fasting selectively suppresses leptin pulse mass and 24-hour rhythmic leptin release in healthy midluteal phase women without disturbing leptin pulse frequency or its entropy control (pattern orderliness). J Clin Endocrinol Metab. 2000;85(1):207–213. 10.1210/jcem.85.1.6325 [DOI] [PubMed] [Google Scholar]
- Berger RJ: Slow wave sleep, shallow torpor and hibernation: homologous states of diminished metabolism and body temperature. Biol Psychol. 1984;19(3–4):305–326. 10.1016/0301-0511(84)90045-0 [DOI] [PubMed] [Google Scholar]
- Bewick GA, Gardiner JV, Dhillo WS, et al. : Post-embryonic ablation of AgRP neurons in mice leads to a lean, hypophagic phenotype. FASEB J. 2005;19(12):1680–1682. 10.1096/fj.04-3434fje [DOI] [PubMed] [Google Scholar]
- Bi S, Robinson BM, Moran TH: Acute food deprivation and chronic food restriction differentially affect hypothalamic NPY mRNA expression. Am J Physiol Regul Integr Comp Physiol. 2003;285(5):1030–1036. 10.1152/ajpregu.00734.2002 [DOI] [PubMed] [Google Scholar]
- Billington CJ, Briggs JE, Grace M, et al. : Effects of intracerebroventricular injection of neuropeptide Y on energy metabolism. Am J Physiol. 1991;260(2 Pt 2):R321–7. 10.1152/ajpregu.1991.260.2.R321 [DOI] [PubMed] [Google Scholar]
- Billington CJ, Briggs JE, Harker S, et al. : Neuropeptide Y in hypothalamic paraventricular nucleus: A center coordinating energy metabolism. Am J Physiol. 1994;266(6 Pt 2):R1765–70. 10.1152/ajpregu.1994.266.6.R1765 [DOI] [PubMed] [Google Scholar]
- Bjorness TE, Kelly CL, Gao T, et al. : Control and function of the homeostatic sleep response by adenosine A 1 receptors. J Neurosci. 2009;29(5):1267–1276. 10.1523/JNEUROSCI.2942-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorness TE, Dale N, Mettlach G, et al. : An adenosine-mediated glial-neuronal circuit for homeostatic sleep. J Neurosci. 2016;36(13):3709–3721. 10.1523/JNEUROSCI.3906-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blood AB, Hunter CJ, Power GG: Adenosine mediates decreased cerebral metabolic rate and increased cerebral blood flow during acute moderate hypoxia in the near-term fetal sheep. J Physiol. 2003;553(Pt 3):935–945. 10.1113/jphysiol.2003.047928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borbély AA, Daan S, Wirz-Justice A, et al. : The two-process model of sleep regulation: A reappraisal. J Sleep Res. 2016;25(2):131–143. 10.1111/jsr.12371 [DOI] [PubMed] [Google Scholar]
- Bratincsák A, McMullen D, Miyake S, et al. : Spatial and temporal activation of brain regions in hibernation: c-fos expression during the hibernation bout in thirteen-lined ground squirrel. J Comp Neurol. 2007;505(4):443–458. 10.1002/cne.21507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braulke LJ, Heldmaier G: Torpor and ultradian rhythms require an intact signalling of the sympathetic nervous system. Cryobiology. Elsevier Inc.,2010;60(2):198–203. 10.1016/j.cryobiol.2009.11.001 [DOI] [PubMed] [Google Scholar]
- Brown JC, Staples JF: Mitochondrial metabolism during fasting-induced daily torpor in mice. Biochim Biophys Acta. Elsevier B.V.,2010;1797(4):476–486. 10.1016/j.bbabio.2010.01.009 [DOI] [PubMed] [Google Scholar]
- Bunnell DE, Agnew JA, Horvath SM, et al. : Passive body heating and sleep: Influence of proximity to sleep. Sleep. 1988;11(2):210–219. 10.1093/sleep/11.2.210 [DOI] [PubMed] [Google Scholar]
- Campbell SS, Broughton RJ: Rapid Decline in Body Temperature Before Sleep: Fluffing the Physiological Pillow? Chronobiol Int. 1994;11(2):126–131. 10.3109/07420529409055899 [DOI] [PubMed] [Google Scholar]
- Cao WH, Fan W, Morrison SF: Medullary pathways mediating specific sympathetic responses to activation of dorsomedial hypothalamus. Neuroscience. 2004;126(1):229–240. 10.1016/j.neuroscience.2004.03.013 [DOI] [PubMed] [Google Scholar]
- Carey HV, Andrews MT, Martin SL: Mammalian Hibernation: Cellular and Molecular Responses to Depressed Metabolism and Low Temperature. Physiol Rev. 2003;83(4):1153–1181. 10.1152/physrev.00008.2003 [DOI] [PubMed] [Google Scholar]
- Carlin JL, Jain S, Gizewski E, et al. : Hypothermia in mouse is caused by adenosine A 1 and A 3 receptor agonists and AMP via three distinct mechanisms. Neuropharmacology. 2017;114:101–113. 10.1016/j.neuropharm.2016.11.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerri M, Mastrotto M, Tupone D, et al. : The inhibition of neurons in the central nervous pathways for thermoregulatory cold defense induces a suspended animation state in the rat. J Neurosci. 2013;33(7):2984–2993. 10.1523/JNEUROSCI.3596-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerri M: The Central Control of Energy Expenditure: Exploiting Torpor for Medical Applications. Annu Rev Physiol. 2017;79(1):167–186. 10.1146/annurev-physiol-022516-034133 [DOI] [PubMed] [Google Scholar]
- Cerri M, Hitrec T, Luppi M, et al. : Be cool to be far: Exploiting hibernation for space exploration. Neurosci Biobehav Rev. Elsevier Ltd,2021a;128:218–232. 10.1016/j.neubiorev.2021.03.037 [DOI] [PubMed] [Google Scholar]
- Cerri M, Hitrec T, Luppi M, et al. : Be cool to be far: Exploiting hibernation for space exploration. Neurosci Biobehav Rev. Elsevier Ltd,2021b;128:218–232. 10.1016/j.neubiorev.2021.03.037 [DOI] [PubMed] [Google Scholar]
- Chao PT, Yang L, Aja S, et al. : Knockdown of NPY Expression in the Dorsomedial Hypothalamus Promotes Development of Brown Adipocytes and Prevents Diet-Induced Obesity. Cell Metab. 2011;13(5):573–583. 10.1016/j.cmet.2011.02.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Lin YC, Kuo TW, et al. : Sensory Detection of Food Rapidly Modulates Arcuate Feeding Circuits. Cell. Elsevier Inc.,2015;160(5):829–841. 10.1016/j.cell.2015.01.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chronwall BM, DiMaggio DA, Massari VJ, et al. : The anatomy of neuropeptide-y-containing neurons in rat brain. Neuroscience. 1985;15(4):1159–1181. 10.1016/0306-4522(85)90260-x [DOI] [PubMed] [Google Scholar]
- Clark G, Magoun HW, Ranson SW: HYPOTHALAMIC REGULATION OF BODY TEMPERATURE. J Neurophysiol. 1939;2(1):61–80. 10.1152/jn.1939.2.1.61 [DOI] [Google Scholar]
- Commins SP, Marsh DJ, Thomas SA, et al. : Norepinephrine is required for leptin effects on gene expression in brown and white adipose tissue. Endocrinology. 1999;140(10):4772–4778. 10.1210/endo.140.10.7043 [DOI] [PubMed] [Google Scholar]
- Conceição EPS, Reynolds CA, Morrison SF, et al. : Activation of Transient Receptor Potential Vanilloid 1 Channels in the Nucleus of the Solitary Tract and Activation of Dynorphin Input to the Median Preoptic Nucleus Contribute to Impaired BAT Thermogenesis in Diet-Induced Obesity. eNeuro. 2021;8(2): ENEURO.0048-21.2021. 10.1523/ENEURO.0048-21.2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cone RD: Anatomy and regulation of the central melanocortin system. Nat Neurosci. 2005;8(5):571–578. 10.1038/nn1455 [DOI] [PubMed] [Google Scholar]
- Cowley MA, Smart JL, Rubinstein M, et al. : Leptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleus. Nature. 2001;411(6836):480–484. 10.1038/35078085 [DOI] [PubMed] [Google Scholar]
- Cowley MA, Smith RG, Diano S, et al. : The Distribution and Mechanism of Action of Ghrelin in the CNS Demonstrates a Novel Hypothalamic Circuit Regulating Energy Homeostasis. Neuron. 2003;37(4):649–661. 10.1016/s0896-6273(03)00063-1 [DOI] [PubMed] [Google Scholar]
- Cubuk C, Markowsky H, Herwig A: Hypothalamic control systems show differential gene expression during spontaneous daily torpor and fasting-induced torpor in the Djungarian hamster ( Phodopus sungorus). PLoS One. 2017;12(10):e0186299. 10.1371/journal.pone.0186299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha RA: Neuroprotection by adenosine in the brain: From A(1) receptor activation to A (2A) receptor blockade. Purinergic Signal. 2005;1(2):111–134. 10.1007/s11302-005-0649-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dark J, Pelz KM: NPY Y1 receptor antagonist prevents NPY-induced torpor-like hypothermia in cold-acclimated Siberian hamsters. Am J Physiol Regul Integr Comp Physiol. 2008;294(1):R236–245. 10.1152/ajpregu.00587.2007 [DOI] [PubMed] [Google Scholar]
- Dawe AR, Spurrier WA: Hibernation induced in ground squirrels by blood transfusion. Science. 1969;163(3864):298–299. 10.1126/science.163.3864.298 [DOI] [PubMed] [Google Scholar]
- de Castro JM: Seasonal rhythms of human nutrient intake and meal pattern. Physiol Behav. 1991;50(1):243–248. 10.1016/0031-9384(91)90527-u [DOI] [PubMed] [Google Scholar]
- DeNardo LA, Liu CD, Allen WE, et al. : Temporal evolution of cortical ensembles promoting remote memory retrieval. Nat Neurosci. 2019;22(3):460–469. 10.1038/s41593-018-0318-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dikic D, Heldmaeir G, Meyer CW: Induced torpor in difference strains of laboratory mice.In Lovegrove, B. and McKechnie, A. E. (eds) Hypometabolism in animals: hibernation, torpor and cryobiology. University of KwaZulu-Natal,2008;224–230. Reference Source [Google Scholar]
- DiMicco JA, Zaretsky DV: The dorsomedial hypothalamus: A new player in thermoregulation. Am J Physiol Regul Integr Comp Physiol. 2007;292(1):R47–63. 10.1152/ajpregu.00498.2006 [DOI] [PubMed] [Google Scholar]
- Dodd GT, Decherf S, Loh K, et al. : Leptin and Insulin Act on POMC Neurons to Promote the Browning of White Fat. Cell. 2015;160(1–2):88–104. 10.1016/j.cell.2014.12.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy PH, Feuers RJ, Leakey JA, et al. : Effect of chronic caloric restriction on physiological variables related to energy metabolism in the male Fischer 344 rat. Mech Ageing Dev. 1989;48(2):117–133. 10.1016/0047-6374(89)90044-4 [DOI] [PubMed] [Google Scholar]
- Egawa M, Yoshimatsu H, Bray GA: Neuropeptide Y suppresses sympathetic activity to interscapular brown adipose tissue in rats. Am J Physiol. 1991;260(2 Pt 2):R328–34. 10.1152/ajpregu.1991.260.2.R328 [DOI] [PubMed] [Google Scholar]
- Elmquist JK, Elias CF, Saper CB: From lesions to leptin: Hypothalamic control of food intake and body weight. Neuron. 1999;22(2):221–232. 10.1016/s0896-6273(00)81084-3 [DOI] [PubMed] [Google Scholar]
- Elvert R, Heldmaier G: Cardiorespiratory and metabolic reactions during entrance into torpor in dormice, Glis glis. J Exp Biol. 2005;208(Pt 7):1373–1383. 10.1242/jeb.01546 [DOI] [PubMed] [Google Scholar]
- Faherty SL, Villanueva-Cañas JL, Klopfer PH, et al. : Gene Expression Profiling in the Hibernating Primate, Cheirogaleus Medius. Genome Biol Evol. Oxford University Press, 2016;8(8):2413–26. 10.1093/gbe/evw163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florant GL, Heller HC: CNS regulation of body temperature in euthermic and hibernating marmots (Marmota flaviventris). Am J Physiol. 1977;232(5):R203–8. 10.1152/ajpregu.1977.232.5.R203 [DOI] [PubMed] [Google Scholar]
- Frederich RC, Hamann A, Anderson S, et al. : Leptin levels reflect body lipid content in mice: Evidence for diet-induced resistance to leptin action. Nat Med. 1995;1(12):1311–1314. 10.1038/nm1295-1311 [DOI] [PubMed] [Google Scholar]
- Freeman DA, Lewis DA, Kauffman AS, et al. : Reduced leptin concentrations are permissive for display of torpor in Siberian hamsters. Am J Physiol Regul Integr Comp Physiol. 2004;87(1):97–103. 10.1152/ajpregu.00716.2003 [DOI] [PubMed] [Google Scholar]
- Futatsuki T, Yamashita A, Ikbar KN, et al. : Involvement of orexin neurons in fasting- and central adenosine-induced hypothermia. Sci Rep. Springer US.2018;8(1):2717. 10.1038/s41598-018-21252-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavrilova O, Leon LR, Marcus-Samuels B, et al. : Torpor in mice is induced by both leptin-dependent and -independent mechanisms. Proc Natl Acad Sci U S A. 1999;96(25):14623–14628. 10.1073/pnas.96.25.14623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiser F, Currie SE, O'Shea KA, et al. : Torpor and hypothermia: reversed hysteresis of metabolic rate and body temperature. Am J Physiol Regul Integr Comp Physiol. 2014;307(11):R1324–R1329. 10.1152/ajpregu.00214.2014 [DOI] [PubMed] [Google Scholar]
- Geiser F, Körtner G, Schmidt I: Leptin increases energy expenditure of a marsupial by inhibition of daily torpor. Am J Physiol. 1998;275(5):1627–1632. 10.1152/ajpregu.1998.275.5.R1627 [DOI] [PubMed] [Google Scholar]
- Geschickter EH, Andrews PA, Bullard RW: Nocturnal body temperature regulation in man: a rationale for sweating in sleep. J Appl Physiol. 1966;21(2):623–630. 10.1152/jappl.1966.21.2.623 [DOI] [PubMed] [Google Scholar]
- Glotzbach SF, Heller HC: Central nervous regulation of body temperature during sleep. Science. 1976;194(4264):537–539. 10.1126/science.973138 [DOI] [PubMed] [Google Scholar]
- Gluck EF, Stephens N, Swoap SJ: Peripheral ghrelin deepens torpor bouts in mice through the arcuate nucleus neuropeptide Y signaling pathway. Am J Physiol Regul Integr Comp Physiol. 2006;291(5):R1303–9. 10.1152/ajpregu.00232.2006 [DOI] [PubMed] [Google Scholar]
- Gooley JJ, Schomer A, Saper CB: The dorsomedial hypothalamic nucleus is critical for the expression of food-entrainable circadian rhythms. Nat Neurosci. 2006;9(3):398–407. 10.1038/nn1651 [DOI] [PubMed] [Google Scholar]
- Gropp E, Shanabrough M, Borok E, et al. : Agouti-related peptide-expressing neurons are mandatory for feeding. Nat Neurosci. 2005;8(10):1289–1291. 10.1038/nn1548 [DOI] [PubMed] [Google Scholar]
- Hahn TM, Breininger JF, Baskin DG, et al. : Coexpression of Agrp and NPY in fasting-activated hypothalamic neurons. Nat Neurosci. 1998;1(4):271–272. 10.1038/1082 [DOI] [PubMed] [Google Scholar]
- Hammel HT, Pierce JB: Regulation of Internal Body Temperature. Annu Rev Physiol. 1968;30(1):641–710. 10.1146/annurev.ph.30.030168.003233 [DOI] [PubMed] [Google Scholar]
- Harding EC, Yu X, Miao A, et al. : A Neuronal Hub Binding Sleep Initiation and Body Cooling in Response to a Warm External Stimulus. Curr Biol. Elsevier Ltd.,2018;28(14):2263–2273.e4. 10.1016/j.cub.2018.05.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding EC, Franks NP, Wisden W: The temperature dependence of sleep. Front Neurosci. 2019;13:336. 10.3389/fnins.2019.00336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldmaier G, Klingenspor M, Werneyer M, et al. : Metabolic adjustments during daily torpor in the Djungarian hamster. Am J Physiol. 1999;276(5). 10.1152/ajpendo.1999.276.5.E896 [DOI] [PubMed] [Google Scholar]
- Heldmaier G, Ortmann S, Elvert R: Natural hypometabolism during hibernation and daily torpor in mammals. Respir Physiol Neurobiol. 2004;141(3):317–329. 10.1016/j.resp.2004.03.014 [DOI] [PubMed] [Google Scholar]
- Heller HC, Glotzbach SF: Thermoregulation during sleep and hibernation. Int Rev Physiol. 1977;15:147–88. [PubMed] [Google Scholar]
- Himms-Hagen J: Food restriction increases torpor and improves brown adipose tissue thermogenesis in ob/ob mice. Am J Physiol. 1985;248(5 Pt 1):E531–9. 10.1152/ajpendo.1985.248.5.E531 [DOI] [PubMed] [Google Scholar]
- Hitrec T, Luppi M, Bastianini S, et al. : Neural control of fasting-induced torpor in mice. Sci Rep. Springer US.2019;9(1):15462. 10.1038/s41598-019-51841-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hrvatin S, Sun S, Wilcox OF, et al. : Neurons that regulate mouse torpor. Nature. Springer US.2020;583(7814):115–121. 10.1038/s41586-020-2387-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YG, Flaherty SJ, Pothecary CA, et al. : The relationship between fasting-induced torpor, sleep, and wakefulness in laboratory mice. Sleep. 2021;44(9):zsab093. 10.1093/sleep/zsab093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson JW, Scott IM: Daily Torpor in the Laboratory Mouse, Mus musculus Var. Albino. Physiol Zool. 1979;52(2):205–218. 10.1086/physzool.52.2.30152564 [DOI] [Google Scholar]
- Iliff BW, Swoap SJ: Central adenosine receptor signaling is necessary for daily torpor in mice. Am J Physiol Regul Integr Comp Physiol. 2012;303(5):R477–84. 10.1152/ajpregu.00081.2012 [DOI] [PubMed] [Google Scholar]
- Jansky L, Haddad D, Kahlerova Z, et al. : Effect of external factors on hibernation of golden hamsters. J Comp Physiol B. 1984;154(4):427–433. Reference Source [Google Scholar]
- Jeong JH, Lee DK, Blouet C, et al. : Cholinergic neurons in the dorsomedial hypothalamus regulate mouse brown adipose tissue metabolism. Mol Metab. Elsevier GmbH,2015;4(6):483–492. 10.1016/j.molmet.2015.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong JH, Lee DK, Jo YH: Cholinergic neurons in the dorsomedial hypothalamus regulate food intake. Mol Metab. Elsevier GmbH,2017;6(3):306–312. 10.1016/j.molmet.2017.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinka TR, Carlson ZA, Moore JT, et al. : Altered thermoregulation via sensitization of A adenosine receptors in dietary-restricted rats. Psychopharmacology (Berl). 2010;209(3):217–224. 10.1007/s00213-010-1778-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinka TR, Tøien O, Drew KL: Season primes the brain in an arctic hibernator to facilitate entrance into torpor mediated by adenosine A(1) receptors. J Neurosci. 2011;31(30):10752–10758. 10.1523/JNEUROSCI.1240-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolicoeur FB, Bouali SM, Fournier A, et al. : Mapping of hypothalamic sites involved in the effects of NPY on body temperature and food intake. Brain Res Bull. 1995;36(2):125–129. 10.1016/0361-9230(94)00176-2 [DOI] [PubMed] [Google Scholar]
- Kaiyala KJ, Morton GJ, Thaler JP, et al. : Acutely decreased thermoregulatory energy expenditure or decreased activity energy expenditure both acutely reduce food intake in mice. PLoS One. 2012;7(8):e41473. 10.1371/journal.pone.0041473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiyala KJ, Ogimoto K, Nelson JT, et al. : Leptin Signaling Is Required for Adaptive Changes in Food Intake, but Not Energy Expenditure, in Response to Different Thermal Conditions. PLoS One. Edited by J. A. Chowen,2015;10(3):e0119391. 10.1371/journal.pone.0119391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kataoka N, Hioki H, Kaneko T, et al. : Psychological stress activates a dorsomedial hypothalamus-medullary raphe circuit driving brown adipose tissue thermogenesis and hyperthermia. Cell Metab. Elsevier Inc.,2014;20(2):346–358. 10.1016/j.cmet.2014.05.018 [DOI] [PubMed] [Google Scholar]
- Kato GA, Sakamoto SH, Eto T, et al. : Individual differences in torpor expression in adult mice are related to relative birth mass. J Exp Biol. 2018;221(Pt 12):jeb171983. 10.1242/jeb.171983 [DOI] [PubMed] [Google Scholar]
- King PJ, Widdowson PS, Doods HN, et al. : Regulation of neuropeptide Y release by neuropeptide Y receptor ligands and calcium channel antagonists in hypothalamic slices. J Neurochem. 1999;73(2):641–646. 10.1046/j.1471-4159.1999.0730641.x [DOI] [PubMed] [Google Scholar]
- Kräuchi K, Cajochen C, Werth E, et al. : Warm feet promote the rapid onset of sleep. Nature. 1999;401(6748):36–37. 10.1038/43366 [DOI] [PubMed] [Google Scholar]
- Kräuchi K, Cajochen C, Werth E, et al. : Functional link between distal vasodilation and sleep-onset latency? Am J Physiol Regul Integr Comp Physiol. 2000;278(3):741–748. 10.1152/ajpregu.2000.278.3.R741 [DOI] [PubMed] [Google Scholar]
- Kräuchi K: The thermophysiological cascade leading to sleep initiation in relation to phase of entrainment. Sleep Med Rev. 2007;11(6):439–451. 10.1016/j.smrv.2007.07.001 [DOI] [PubMed] [Google Scholar]
- Kroeger D, Absi G, Gagliardi C, et al. : Galanin neurons in the ventrolateral preoptic area promote sleep and heat loss in mice. Nat Commun. 2018;9(1):4129. 10.1038/s41467-018-06590-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtz CC, Carey HV: Seasonal changes in the intestinal immune system of hibernating ground squirrels. Dev Comp Immunol. 2007;31(4):415–428. 10.1016/j.dci.2006.07.003 [DOI] [PubMed] [Google Scholar]
- Landolt HP, Moser S, Wieser HG, et al. : Intracranial temperature across 24-hour sleep-wake cycles in humans. Neuroreport. England,1995;6(6):913–917. 10.1097/00001756-199504190-00022 [DOI] [PubMed] [Google Scholar]
- Larkin JE, Heller HC: Temperature sensitivity of sleep homeostasis during hibernation in the golden-mantled ground squirrel. Am J Physiol. 1996;270(4 Pt 2):R777–84. 10.1152/ajpregu.1996.270.4.R777 [DOI] [PubMed] [Google Scholar]
- Larkin JE, Heller HC: Sleep after arousal from hibernation is not homeostatically regulated. Am J Physiol. 1999;276(2):R522–529. 10.1152/ajpregu.1999.276.2.R522 [DOI] [PubMed] [Google Scholar]
- Lee CC: Is human hibernation possible? Annu Rev Med. 2008;59(1):177–186. 10.1146/annurev.med.59.061506.110403 [DOI] [PubMed] [Google Scholar]
- Levin BE, Finnegan M, Triscari J, et al. : Brown adipose and metabolic features of chronic diet-induced obesity. Am J Physiol. 1985;248(6 Pt 2):R717–23. 10.1152/ajpregu.1985.248.6.R717 [DOI] [PubMed] [Google Scholar]
- Liedtke WB: Deconstructing mammalian thermoregulation. Proc Natl Acad Sci U S A. 2017;114(8):1765–1767. 10.1073/pnas.1620579114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo Martire V, Silvani A, Bastianini S, et al. : Effects of Ambient Temperature on Sleep and Cardiovascular Regulation in Mice: The Role of Hypocretin/Orexin Neurons. PLoS One. 2012;7(10):e47032. 10.1371/journal.pone.0047032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo Martire V, Valli A, Bingaman MJ, et al. : Changes in blood glucose as a function of body temperature in laboratory mice: implications for daily torpor. Am J Physiol Endocrinol Metab. 2018;315(4):E662–E670. 10.1152/ajpendo.00201.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luquet S, Perez FA, Hnasko TS, et al. : NPY/AgRP Neurons Are Essential for Feeding in Adult Mice but Can Be Ablated in Neonates. Science. 2005;310(5748):683–685. 10.1126/science.1115524 [DOI] [PubMed] [Google Scholar]
- Ma Y, Miracca G, Yu X, et al. : Galanin Neurons Unite Sleep Homeostasis and α2-Adrenergic Sedation. Curr Biol. Elsevier Ltd.,2019;29(19):3315–3322.e3. 10.1016/j.cub.2019.07.087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma YL, Zhu X, Rivera PM, et al. : Absence of cellular stress in brain after hypoxia induced by arousal from hibernation in Arctic ground squirrels. Am J Physiol Regul Integr Comp Physiol. 2005;289(5):R1297–R1306. 10.1152/ajpregu.00260.2005 [DOI] [PubMed] [Google Scholar]
- Madden CJ, Morrison SF: A high-fat diet impairs cooling-evoked brown adipose tissue activation via a vagal afferent mechanism. Am J Physiol Endocrinol Metab. 2016;311(2):E287–E292. 10.1152/ajpendo.00081.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden CJ, Morrison SF: Central nervous system circuits that control body temperature. Neuroscience Letters. 2019;696:225–232. 10.1016/j.neulet.2018.11.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madsen PL, Schmidt JF, Wildschiødtz G, et al. : Cerebral O2 metabolism and cerebral blood flow in humans during deep and rapid-eye-movement sleep. J Appl Physiol (1985). 1991;70(6):2597–2601. 10.1152/jappl.1991.70.6.2597 [DOI] [PubMed] [Google Scholar]
- Magoun HW, Harrison F, Brobeck JR, et al. : ACTIVATION OF HEAT LOSS MECHANISMS BY LOCAL HEATING OF THE BRAIN. J Neurophysiol. 1938;1(2):101–114. 10.1152/jn.1938.1.2.101 [DOI] [Google Scholar]
- Malan A: pH and hypometabolism in mammalian hibernation. Can J Zool. 1988;66(1):95–98. 10.1139/z88-013 [DOI] [Google Scholar]
- Malan A, Rodeau JL, Daull F: Intracellular pH in hibernation and respiratory acidosis in the European hamster. J Comp Physiol B. 1985;156(2):251–258. 10.1007/BF00695780 [DOI] [PubMed] [Google Scholar]
- McAllen RM, Tanaka M, Ootsuka Y, et al. : Multiple thermoregulatory effectors with independent central controls. Eur J Appl Physiol. 2010;109(1):27–33. 10.1007/s00421-009-1295-z [DOI] [PubMed] [Google Scholar]
- McGinty D, Szymusiak R: Keeping cool: a hypothesis about the mechanisms and functions of slow-wave sleep. Trends Neurosci. 1990;13(12):480–487. 10.1016/0166-2236(90)90081-k [DOI] [PubMed] [Google Scholar]
- Mistry AM, Swick AG, Romsos DR: Leptin Rapidly Lowers Food Intake and Elevates Metabolic Rates in Lean and ob/ob Mice. J Nutr. 1997;127(10):2065–2072. 10.1093/jn/127.10.2065 [DOI] [PubMed] [Google Scholar]
- Mochizuki T, Klerman EB, Sakurai T, et al. : Elevated body temperature during sleep in orexin knockout mice. Am J Physiol Regul Integr Comp Physiol. 2006;291(3):R533–40. 10.1152/ajpregu.00887.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison SF: Central control of body temperature [version 1; peer review: 3 approved]. F1000Res. 2016a;5: F1000 Faculty Rev-880. 10.12688/f1000research.7958.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison SF: Central neural control of thermoregulation and brown adipose tissue. Auton Neurosci. Department of Neurological Surgery, Oregon Health & Science University, Portland, OR 97239, Unites States. Electronic address: morrisos@ohsu.edu.,2016b;196:14–24. 10.1016/j.autneu.2016.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison SF, Madden CJ, Tupone D: Central Control of Brown Adipose Tissue Thermogenesis. Front Endocrinol (Lausanne). 2012;3(5):5. 10.3389/fendo.2012.00005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller TD, Nogueiras R, Andermann ML, et al. : Ghrelin. Mol Metab. 2015;4(6):437–460. 10.1016/j.molmet.2015.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers MG, Cowley MA, Münzberg H: Mechanisms of Leptin Action and Leptin Resistance. Annu Rev Physiol. 2008;70:537–556. 10.1146/annurev.physiol.70.113006.100707 [DOI] [PubMed] [Google Scholar]
- Mzilikazi N, Lovegrove BG: Reproductive activity influences thermoregulation and torpor in pouched mice, Saccostomus campestris. J Comp Physiol B. 2002;172(1):7–16. 10.1007/s003600100221 [DOI] [PubMed] [Google Scholar]
- Nakayama T, Eisenman JS, Hardy JD: Single Unit Activity of Anterior Hypothalamus during Local Heating. Science. 1961;134(3478):560–561. 10.1126/science.134.3478.560 [DOI] [PubMed] [Google Scholar]
- Nakamura K, Morrison SF: A thermosensory pathway that controls body temperature. Nat Neurosci. 2008;11(1):62–71. 10.1038/nn2027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K, Morrison SF: A thermosensory pathway mediating heat-defense responses. Proc Natl Acad Sci U S A. 2010;107(19):8848–8853. 10.1073/pnas.0913358107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newby AC: Adenosine and the concept of 'retaliatory metabolites'. Trends Biochem Sci. 1984;9(2):42–44. 10.1016/0968-0004(84)90176-2 [DOI] [Google Scholar]
- Nicholls DG, Rial E: A history of the first uncoupling protein, UCP1. J Bioenerg Biomembr. 1999;31(5):399–406. 10.1023/a:1005436121005 [DOI] [PubMed] [Google Scholar]
- Oelkrug R, Heldmaier G, Meyer CW: Torpor patterns, arousal rates, and temporal organization of torpor entry in wildtype and UCP1-ablated mice. J Comp Physiol B. 2010;181(1):137–145. 10.1007/s00360-010-0503-9 [DOI] [PubMed] [Google Scholar]
- Oeltgen PR, Nilekani SP, Nuchols PA, et al. : Further studies on opioids and hibernation: Delta opioid receptor ligand selectively induced hibernation in summer-active ground squirrels. Life Sci. 1988;43(19):1565–1574. 10.1016/0024-3205(88)90406-7 [DOI] [PubMed] [Google Scholar]
- Okamoto K, Yamasaki M, Takao K, et al. : QRFP-Deficient mice are hypophagic, lean, hypoactive and exhibit increased anxiety-Like behavior. PLoS One. 2016;11(11):e0164716. 10.1371/journal.pone.0164716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panneton WM: The mammalian diving response: an enigmatic reflex to preserve life? Physiology (Bethesda). American Physiological Society,2013;28(5):284–97. 10.1152/physiol.00020.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parmeggiani PL, Rabini C: Shivering and panting during sleep. Brain Res. 1967;6(4):789–791. 10.1016/0006-8993(67)90139-4 [DOI] [PubMed] [Google Scholar]
- Paton JFR, Boscan P, Pickering AE, et al. : The yin and yang of cardiac autonomic control: Vago-sympathetic interactions revisited. Brain Res Brain Res Rev. 2005;49(3):555–565. 10.1016/j.brainresrev.2005.02.005 [DOI] [PubMed] [Google Scholar]
- Paul MJ, Freeman DA, Park JH, et al. : Neuropeptide Y induces torpor-like hypothermia in Siberian hamsters. Brain Res. 2005;1055(1–2):83–92. 10.1016/j.brainres.2005.06.090 [DOI] [PubMed] [Google Scholar]
- Paul MJ, Kauffman AS, Zucker I: Feeding Schedule Controls Circadian Timing of Daily Torpor in SCN-Ablated Siberian Hamsters. J Biol Rhythms. 2004;19(3):226–237. 10.1177/0748730404264337 [DOI] [PubMed] [Google Scholar]
- Ravussin Y, Xiao C, Gavrilova O, et al. : Effect of intermittent cold exposure on brown fat activation, obesity, and energy Homeostasis in mice. PLoS One. 2014;9(1):e85876. 10.1371/journal.pone.0085876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawls SM, Benamar K: Effects of opioids, cannabinoids, and vanilloids on body temperature. Front Biosci (Schol Ed). 2011;3(3):822–845. 10.2741/190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichmann F, Holzer P: Neuropeptide Y: A stressful review. Neuropeptides. Europe PMC Funders Group,2016;55:99–109. 10.1016/j.npep.2015.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reppert SM, Weaver DR, Ebisawa T, et al. : Cloning of a melatonin-related receptor from human pituitary. FEBS Lett. 1996;386(2–3):219–24. 10.1016/0014-5793(96)00437-1 [DOI] [PubMed] [Google Scholar]
- Rezai-Zadeh K, Yu S, Jiang Y, et al. : Leptin receptor neurons in the dorsomedial hypothalamus are key regulators of energy expenditure and body weight, but not food intake. Mol Metab. Elsevier,2014;3(7):681–93. 10.1016/j.molmet.2014.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanovsky AA: Thermoregulation: Some concepts have changed. Functional architecture of the thermoregulatory system. Am J Physiol Regul Integr Comp Physiol. 2007;292(1):R37–46. 10.1152/ajpregu.00668.2006 [DOI] [PubMed] [Google Scholar]
- Romeijn N, Raymann RJEM, Møst E, et al. : Sleep, vigilance, and thermosensitivity. Pflugers Arch. 2012;463(1):169–176. 10.1007/s00424-011-1042-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothwell NJ, Stock MJ: A role for brown adipose tissue in diet-induced thermogenesis. Obes Res. 1997;5(6):650–6. 10.1002/j.1550-8528.1997.tb00591.x [DOI] [PubMed] [Google Scholar]
- Ruf T, Geiser F: Daily torpor and hibernation in birds and mammals. Biol Rev Camb Philos Soc. 2015;90(3):891–926. 10.1111/brv.12137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaguchi T, Arase K, Fisler JS, et al. : Effect of a high-fat diet on firing rate of sympathetic nerves innervating brown adipose tissue in anesthetized rats. Physiol Behav. 1989;45(6):1177–1182. 10.1016/0031-9384(89)90106-6 [DOI] [PubMed] [Google Scholar]
- Saper CB, Machado NLS: Flipping the switch on the body’s thermoregulatory system. Nature. 2020;583(7814):34–35. 10.1038/d41586-020-01600-5 [DOI] [PubMed] [Google Scholar]
- Schubert KA, Boerema AS, Vaanholt LM, et al. : Daily torpor in mice: high foraging costs trigger energy-saving hypothermia. Biol Lett. 2010;6(1):132–135. 10.1098/rsbl.2009.0569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz MW, Peskind E, Raskind M, et al. : Cerebrospinal fluid leptin levels: Relationship to plasma levels and to adiposity in humans. Nat Med. 1996;2(5):589–593. 10.1038/nm0596-589 [DOI] [PubMed] [Google Scholar]
- Schwimmer H, Stauss HM, Abboud F, et al. : Effects of sleep on the cardiovascular and thermoregulatory systems: a possible role for hypocretins. J Appl Physiol (1985). 2010;109(4):1053–1063. 10.1152/japplphysiol.00516.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seydoux J, Assimacopoulos-Jeannet F, Jeanrenaud B, et al. : Alterations of Brown Adipose Tissue in Genetically Obese (ob/ob) Mice. I. Demonstration of Loss of Metabolic Response to Nerve Stimulation and Catecholamines and Its Partial Recovery after Fasting or Cold Adaptation. Endocrinology. 1982;110(2):432–438. 10.1210/endo-110-2-432 [DOI] [PubMed] [Google Scholar]
- Sherin JE, Shiromani PJ, McCarley RW, et al. : Activation of ventrolateral preoptic neurons during sleep. Science. 1996;271(5246):216–9. 10.1126/science.271.5246.216 [DOI] [PubMed] [Google Scholar]
- Shi Z, Bonillas AC, Wong J, et al. : Neuropeptide Y suppresses thermogenic and cardiovascular sympathetic nerve activity via Y1 receptors in the paraventricular nucleus and dorsomedial hypothalamus. J Neuroendocrinol. 2021;33(8):e13006. 10.1111/jne.13006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shryock JC, Belardinelli L: Adenosine and adenosine receptors in the cardiovascular system: Biochemistry, physiology, and pharmacology. Am J Cardiol. 1997;79(12A):2–10. 10.1016/s0002-9149(97)00256-7 [DOI] [PubMed] [Google Scholar]
- Silvani A, Cerri M, Zoccoli G, et al. : Is adenosine action common ground for nrem sleep, torpor, and other hypometabolic states? Physiology (Bethesda). 2018;33(3):182–196. 10.1152/physiol.00007.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soare A, Cangemi R, Omodei D, et al. : Long-term calorie restriction, but not endurance exercise, lowers core body temperature in humans. Aging (Albany NY). 2011;3(4):374–379. 10.18632/aging.100280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solymár M, Pétervári E, Balaskó M, et al. : The onset of daily torpor is regulated by the same low body mass in lean mice and in mice with diet-induced obesity. Temperature (Austin). 2015;2(1):129–134. 10.1080/23328940.2015.1014250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song K, Wang H, Wang GB, et al. : The TRPM2 channel is a hypothalamic heat sensor that limits fever and can drive hypothermia. Science. 2016;353(6306):1393–1398. 10.1126/science.aaf7537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srere HK, Wang LCH, Martin SL: Central role for differential gene expression in mammalian hibernation. Proc Natl Acad Sci U S A. 1992;89(15):7119–7123. 10.1073/pnas.89.15.7119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stær-Jensen H, Sunde K, Olasveengen TM, et al. : Bradycardia during therapeutic hypothermia is associated with good neurologic outcome in comatose survivors of out-of-hospital cardiac arrest. Crit Care Med. 2014;42(11): 2401–2408. 10.1097/CCM.0000000000000515 [DOI] [PubMed] [Google Scholar]
- Stanzani G, Tidswell R, Singer M: Do critical care patients hibernate? Theoretical support for less is more. Intensive Care Med. Springer Berlin Heidelberg,2020;46(3):495–497. 10.1007/s00134-019-05813-9 [DOI] [PubMed] [Google Scholar]
- Stenberg D, Litonius E, Halldner L, et al. : Sleep and its homeostatic regulation in mice lacking the adenosine A 1 receptor. J Sleep Res. 2003;12(4):283–290. 10.1046/j.0962-1105.2003.00367.x [DOI] [PubMed] [Google Scholar]
- Sunagawa GA, Takahashi M: Hypometabolism during Daily Torpor in Mice is Dominated by Reduction in the Sensitivity of the Thermoregulatory System. Sci Rep. 2016;6(1):37011. 10.1038/srep37011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swoap SJ, Gutilla MJ, Liles LC, et al. : The full expression of fasting-induced torpor requires beta 3-adrenergic receptor signaling. J Neurosci. 2006;26(1):241–245. 10.1523/JNEUROSCI.3721-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swoap SJ, Gutilla MJ: Cardiovascular changes during daily torpor in the laboratory mouse. Am J Physiol Regul Integr Comp Physiol. 2009;297(3):R769–74. 10.1152/ajpregu.00131.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swoap SJ, Weinshenker D: Norepinephrine controls both torpor initiation and emergence via distinct mechanisms in the mouse. PLoS One. Edited by A. Bartolomucci.2008;3(12):e4038. 10.1371/journal.pone.0004038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi TM, Sunagawa GA, Soya S, et al. : A discrete neuronal circuit induces a hibernation-like state in rodents. Nature. Springer US,2020;583(7814):109–114. 10.1038/s41586-020-2163-6 [DOI] [PubMed] [Google Scholar]
- Takayasu S, Sakurai T, Iwasaki S, et al. : A neuropeptide ligand of the G protein-coupled receptor GPR103 regulates feeding, behavioral arousal, and blood pressure in mice. Proc Natl Acad Sci U S A. 2006;103(19):7438–7443. 10.1073/pnas.0602371103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura Y, Shintani M, Nakamura A, et al. : Phase-specific central regulatory systems of hibernation in Syrian hamsters. Brain Res. 2005;1045(1–2):88–96. 10.1016/j.brainres.2005.03.029 [DOI] [PubMed] [Google Scholar]
- Tamura Y, Shintani M, Inoue H, et al. : Regulatory mechanism of body temperature in the central nervous system during the maintenance phase of hibernation in Syrian hamsters: Involvement of β-endorphin. Brain Res. Elsevier B.V.,2012;1448:63–70. 10.1016/j.brainres.2012.02.004 [DOI] [PubMed] [Google Scholar]
- Tan CL, Cooke EK, Leib DE, et al. : Warm-Sensitive Neurons that Control Body Temperature. Cell. Elsevier Inc.,2016;167(1):47–59.e15. 10.1016/j.cell.2016.08.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas SA, Marck BT, Palmiter RD, et al. : Restoration of Norepinephrine and Reversal of Phenotypes in Mice Lacking Dopamine β-Hydroxylase. J Neurochem. 1998;70(6):2468–2476. 10.1046/j.1471-4159.1998.70062468.x [DOI] [PubMed] [Google Scholar]
- Thomas SA, Matsumoto AM, Palmiter RD: Noradrenaline is essential for mouse fetal development. Nature. 1995;374(6523):643–646. 10.1038/374643a0 [DOI] [PubMed] [Google Scholar]
- Thompson JL, Borgland SL: Presynaptic leptin action suppresses excitatory synaptic transmission onto ventral tegmental area dopamine neurons. Biol Psychiatry. Elsevier,2013;73(9):860–868. 10.1016/j.biopsych.2012.10.026 [DOI] [PubMed] [Google Scholar]
- Tiesjema B, la Fleur SE, Luijendijk CM, et al. : Viral mediated neuropeptide Y expression in the rat paraventricular nucleus results in obesity. Obesity (Silver Spring). 2007;15(10):2424–35. 10.1038/oby.2007.288 [DOI] [PubMed] [Google Scholar]
- Toien O, Blake J, Edgar DM, et al. : Hibernation in Black Bears: Independence of Metabolic Suppression from Body Temperature. Science. 2011;331(6019):906–909. 10.1126/science.1199435 [DOI] [PubMed] [Google Scholar]
- Trachsel L, Edgar DM, Heller HC: Are ground squirrels sleep deprived during hibernation? Am J Physiol. 1991;260(6 Pt 2):R1123–9. 10.1152/ajpregu.1991.260.6.R1123 [DOI] [PubMed] [Google Scholar]
- Trayhurn P, James WP: Thermoregulation and non-shivering thermogenesis in the genetically obese (ob/ob) mouse. Pflugers Arch. 1978;373(2):189–193. 10.1007/BF00584859 [DOI] [PubMed] [Google Scholar]
- Trayhurn P, Thurlby PL, James WPT: Thermogenic defect in pre-obese ob/ob mice. Nature. 1977;266(5597):60–62. 10.1038/266060a0 [DOI] [PubMed] [Google Scholar]
- Tseng, et al. : Central Sites of Analgesia , Changes in Rats1 Catalepsy and Body instrument. Journal of Pharmacology and Experimental Therapeutics. 1980;214(2):328–332. [PubMed] [Google Scholar]
- Tupone D, Madden CJ, Morrison SF: Central activation of the A1 adenosine receptor (A1AR) induces a hypothermic, torpor-like state in the rat. J Neurosci. 2013;33(36):14512–14525. 10.1523/JNEUROSCI.1980-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicent MA, Borre ED, Swoap SJ: Central activation of the A 1 adenosine receptor in fed mice recapitulates only some of the attributes of daily torpor. J Comp Physiol B. 2017;187(5–6):835–845. 10.1007/s00360-017-1084-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Vinne V, Bingaman MJ, Weaver DR, et al. : Clocks and meals keep mice from being cool. J Exp Biol. 2018;221(Pt 15):jeb179812. 10.1242/jeb.179812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker JM, Glotzbach SF, Glotzbach RJ, et al. : Sleep and hibernation in ground squirrels (Citellus spp): electrophysiological observations. Am J Physiol. 1977;233(5):R213–21. 10.1152/ajpregu.1977.233.5.R213 [DOI] [PubMed] [Google Scholar]
- Walker JM, Haskell EH, Berger RJ, et al. : Hibernation at moderate temperatures: a continuation of slow wave sleep. Experientia. 1981;37(7):726–728. 10.1007/BF01967947 [DOI] [PubMed] [Google Scholar]
- Walker JM, Walker LE, Harris DV, et al. : Cessation of thermoregulation during REM sleep in the pocket mouse. Am J Physiol. 1983;244(1):R114–8. 10.1152/ajpregu.1983.244.1.R114 [DOI] [PubMed] [Google Scholar]
- Wang LC, Belke D, Jourdan ML, et al. : The “hibernation induction trigger”: Specificity and validity of bioassay using the 13-lined ground squirrel. Cryobiology. 1988;25(4):355–362. 10.1016/0011-2240(88)90043-0 [DOI] [PubMed] [Google Scholar]
- Wang L, Saint-Pierre DH, Taché Y: Peripheral ghrelin selectively increases Fos expression in neuropeptide Y – synthesizing neurons in mouse hypothalamic arcuate nucleus. Neurosci Lett. 2002;325(1):47–51. 10.1016/s0304-3940(02)00241-0 [DOI] [PubMed] [Google Scholar]
- Wang Q, Bing C, Al-Barazanji K, et al. : Interactions Between Leptin and Hypothalamic Neuropeptide Y Neurons in the Control of Food Intake and Energy Homeostasis in the Rat. Diabetes. 1997;46(3):335–341. 10.2337/diab.46.3.335 [DOI] [PubMed] [Google Scholar]
- Webb GP, Jagot SA, Jakobson ME: Fasting-induced torpor in Mus musculus and its implications in the use of murine models for human obesity studies. Comp Biochem Physiol A Comp Physiol. 1982;72(1):211–219. 10.1016/0300-9629(82)90035-4 [DOI] [PubMed] [Google Scholar]
- Welton RF, Martin RJ, Baumgardt BR: Effects of Feeding and Exercise Regimens on Adipose Tissue Glycerokinase Activity and Body Composition of Lean and Obese Mice. J Nutr. 1973;103(8):1212–1219. 10.1093/jn/103.8.1212 [DOI] [PubMed] [Google Scholar]
- Willis CKR: An energy-based body temperature threshold between torpor and normothermia for small mammals. Physiol Biochem Zool. 2007;80(6):643–651. 10.1086/521085 [DOI] [PubMed] [Google Scholar]
- Withers PC: Metabolic, respiratory and haematological adjustments of the little pocket mouse to circadian torpor cycles. Respir Physiol. 1977;31(3):295–307. 10.1016/0034-5687(77)90073-1 [DOI] [PubMed] [Google Scholar]
- Xiao C, Goldgof M, Gavrilova O, et al. : Anti-obesity and metabolic efficacy of the β 3-adrenergic agonist, CL316243, in mice at thermoneutrality compared to 22°C. Obesity (Silver Spring). 2015;23(7):1450–1459. 10.1002/oby.21124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing X, Yang M, Wang DH: The expression of leptin, hypothalamic neuropeptides and UCP1 before, during and after fattening in the Daurian ground squirrel ( Spermophilus dauricus). Comp Biochem Physiol A Mol Integr Physiol. Elsevier Inc., 2015;184:105–112. 10.1016/j.cbpa.2015.02.012 [DOI] [PubMed] [Google Scholar]
- Yu S, Qualls-Creekmore E, Rezai-Zadeh K, et al. : Glutamatergic Preoptic Area Neurons That Express Leptin Receptors Drive Temperature-Dependent Body Weight Homeostasis. J Neurosci. 2016;36(18):5034–5046. 10.1523/JNEUROSCI.0213-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Kerman IA, Laque A, et al. : Leptin-receptor-expressing neurons in the dorsomedial hypothalamus and median preoptic area regulate sympathetic brown adipose tissue circuits. J Neurosci. 2011;31(5):1873–1884. 10.1523/JNEUROSCI.3223-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Ferretti V, Güntan I, et al. : Neuronal ensembles sufficient for recovery sleep and the sedative actions of α 2 adrenergic agonists. Nat Neurosci. 2015;18(4):553–561. 10.1038/nn.3957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Reis FMCV, He Y, et al. : Estrogen-sensitive medial preoptic area neurons coordinate torpor in mice. Nat Commun. Springer US2020;11(1):6378. 10.1038/s41467-020-20050-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao S, Shao C, Goropashnaya AV, et al. : Genomic analysis of expressed sequence tags in American black bear Ursus americanus. BMC genomics. 2010;11(1):201. 10.1186/1471-2164-11-201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao ZD, Yang WZ, Gao C, et al. : A hypothalamic circuit that controls body temperature. Proc Natl Acad Sci U S A. 2017;114(8):2042–2047. 10.1073/pnas.1616255114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zosky GR: The parasympathetic nervous system: Its role during torpor in the fat-tailed dunnart (Sminthopsis crassicaudata). J Comp Physiol B. 2002;172(8):677–684. 10.1007/s00360-002-0295-7 [DOI] [PubMed] [Google Scholar]
- Zosky GR, Larcombe AN: The parasympathetic nervous system and its influence on heart rate in torpid western pygmy possums, Cercatetus concinnus (Marsupialia: Burramyidae). Zoology (Jena). 2003;106(2):143–150. 10.1078/0944-2006-00108 [DOI] [PubMed] [Google Scholar]