Abstract
Monarch butterflies (Danaus plexippus) (Lepidoptera Danaidae Danaus plexippus (Linnaeus)) are an iconic species of conservation concern due to declines in the overwintering colonies over the past twenty years. Because of this downward trend in overwintering numbers in both California and Mexico, monarchs are currently considered ‘warranted-but-precluded’ for listing under the Endangered Species Act. Monarchs have a fascinating life history and have become a model system in chemical ecology, migration biology, and host–parasite interactions, but many aspects of monarch biology important for informing conservation practices remain unresolved. In this review, we focus on recent advances using experimental and genetic approaches that inform monarch conservation. In particular, we emphasize three areas of broad importance, which could have an immediate impact on monarch conservation efforts: 1) breeding habitat and host plant use, 2) natural enemies and exotic caterpillar food plants, and 3) the utility of genetic and genomic approaches for understanding monarch biology and informing ongoing conservation efforts. We also suggest future studies in these areas that could improve our understanding of monarch behavior and conservation.
Keywords: conservation, natural enemy, genetics and genomics, exotic milkweed, milkweed preference
Graphical Abstract
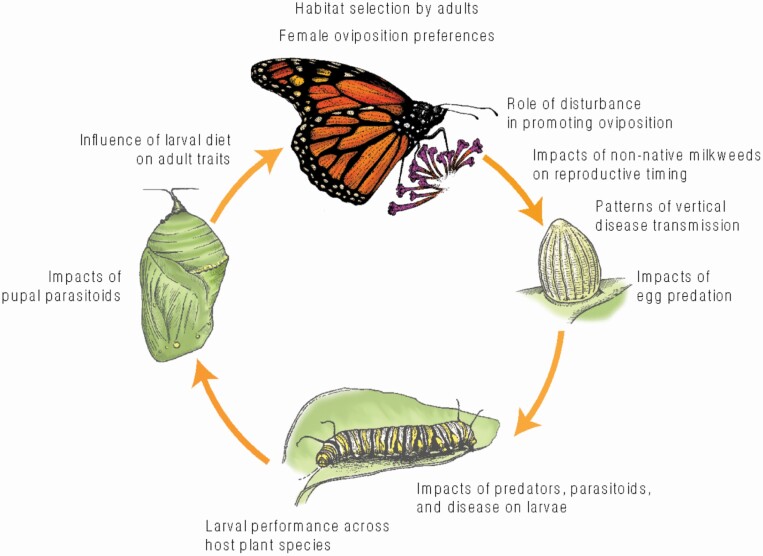
Impacts of specific threats including natural enemies and habitat impacts throughout the monarch life cycle. Life cycle Illustrations by Henry Crawford Adams.
Observational data are a necessary starting point for conservation; researchers, land managers, and citizens need to know where the organism of interest occurs and the timing of its occurrence on the landscape (Haila and Margules 1996). In the case of the monarch butterfly (D. plexippus), well known for its spectacular long-distance migration, bright coloration, and dependence on toxic milkweed plants as larvae, observational studies initiated in the 1960s and 1970s were critical for illuminating fundamental aspects of the monarch’s migratory life cycle (Reichstein et al. 1968; Urquhart and Urquart 1976; Brower 1977; Gustafsson et al. 2015). For example, the monarch’s primary overwintering sites in Mexico were not described to science until 1976 (Urquhart and Urquhart 1976), a breakthrough only made possible through years of tagging and releasing butterflies by Fred and Nora Urquhart (Urquhart and Urquhart 1978). Even today, much of our base knowledge of monarch distribution (Vidal and Rendón-Salinas 2014, Kinkead et al. 2019, Antonsen et al. 2021) and migration patterns (Walton et al. 2005, Gibbs et al. 2006, Badgett and Davis 2015, Davis and Dyer 2015, Inamine et al. 2016) has been synthesized from both survey programs and citizen science efforts based on observational data (Swengel 1995, Howard and Davis 2009, MLMP 2020, NABA 2020, Taylor et al. 2020). Insights from both surveys and citizen science projects—across different regions in North America and during different phases of the monarch’s annual cycle—form the basis of our understanding about broad-scale monarch biology.
Although ongoing observational and survey work is crucial for conservation, some aspects of monarch biology can only be understood using experimental approaches. The literature on experiments involving monarchs is vast, and the goal of this review is not to provide a comprehensive overview of all experiments relevant to monarch conservation; we do not discuss findings of studies that focus on migratory physiology, navigation (Merlin et al. 2009; Reppert et al. 2010; Zhan et al. 2011; Heinze and Reppert 2011, 2012; Guerra et al. 2012, 2013,2014; Heinze et al. 2013; Shlizerman et al. 2016; Reppert and de Roode 2018, Tenger-Trolander et al. 2019, Yang et al. 2019, Nguyen et al. 2021), diapause termination and induction (Herman 1981, Goehring et al. 2004, Green and Kronforst 2019), stress (Pelling et al. 2009, Davis et al. 2018, 2020), or immune response (Lindsey and Altizer 2009, Decker et al. 2021). We note that these studies are important to further understanding of monarch biology and warrant investigation; however, that body of research does not directly inform strategies for monarch conservation that can be applied today. Here we review three lines of research that incorporate experimental approaches to directly inform monarch conservation and habitat restoration efforts: 1) the influence of different milkweed diets and habitat types; 2) monarch interactions with natural enemies and exotic milkweeds; and 3) the application of genetic and genomic approaches to monarchs.
Following the petition to list the monarch under the Endangered Species Act in 2012, a groundswell of habitat establishment and restoration efforts on local, state, and federal levels have been started (Monarch ESA Petition 2014). These projects have focused on adding milkweed plants and nectar sources to the landscape with hopes of boosting monarch numbers, but few account for differences among milkweed species (e.g., plant defenses, differences among habitat types for monarch success, or the impacts of larval host plant on adult traits. Recent work on survival, growth, oviposition, and physiology has revealed the importance of plant host for monarch survivorship and oviposition as well as the influence of larval host plant on adult traits, providing guidance on which milkweeds produce healthy butterflies and therefore may be most beneficial to plant on the landscape.
The monarch butterfly has various natural enemies of which a protozoan parasite, Ophryocystis elektroscirrha (Neogregarinorida: Ophryocystidae), remains the best studied. The environmental persistence of the protozoan poses risk for monarchs in habitats used by multiple generations. Often considered ‘debilitating’, this parasite can have significant impacts on monarch population size, particularly in patches of the exotic milkweed, Asclepias curassavica (Gentianales, Apocynaceae, Asclepias, tropical milkweed). While the vast majority of milkweeds used by monarchs are native to North America, introduced milkweeds are present and empirical work shows that exotic milkweeds vary in their effects on monarch survival and physiology.
Finally, approaches from genetics and genomics have recently been applied to understand aspects of basic monarch biology. Over the past three decades, genetic approaches have become an increasingly important part of conservation decision-making (reviewed in Allendorf et al. 2010), and monarchs are no exception to this pattern. Recent research into monarch butterfly genetics and functional genomics has provided important context for understanding differentiation among populations, describing the underlying basis of the migratory phenomenon, and characterizing naturally occurring variation in ecologically relevant traits.
Monarch Breeding Habitat and Milkweed Use
Habitat loss and land use change have been reported as two of the main threats to the monarch population in the Mexican overwintering colonies and throughout the breeding range (Flockhart et al. 2015, Thogmartin et al. 2017a, Zaya et al. 2017). It is important to note that monarch population size and decline remain a contentious issue with studies both supporting (Espeset et al. 2016, Pleasants et al. 2017, Schultz et al. 2017, Zaya et al. 2017, Stenoien et al. 2018, Pelton et al. 2019) and refuting population decline (Davis 2012, Badgett and Davis 2015, Inamine et al. 2016, Davis 2020). Monarch butterflies are well-known milkweed specialists and will feed on roughly 40 of the 100+ milkweed species across North America (Ackery and Vane-Wright 1984, Malcolm and Brower 1986, Malcolm 1994). Before the widespread adoption of herbicide-resistant crops, the most productive summer breeding areas for monarchs were located in midwestern agricultural fields with Asclepias syriaca (Pleasants 2017, Pleasants and Oberhauser 2013, Stenoien et al. 2018). A. syriaca (Gentianales, Apocynaceae, Asclepias syriaca, common milkweed) was found in over half of Iowa crop fields in 1999, but only in 8% of fields during a follow up survey in 2009 (Hartzler 2010). This decline represents a 97% decline in agricultural milkweed density, in Iowa alone, in a decade (Pleasants 2017) and effective elimination of agricultural milkweed from the monarchs’ historic core breeding range (Wassenaar and Hobson 1998, Flockhart et al. 2015).
Monarchs encounter and utilize a variety of different milkweed species, which vary in toxicity and chemical content, during their multi-generational annual cycle (Malcolm and Brower 1989). Therefore, it is important to understand both how females perceive and use different milkweed species throughout the breeding range as well as larval survival on different species (Agrawal 2017, Pocius et al. 2018b). As both public and private entities move forward with monarch habitat restoration plans from community gardens to large swaths of planted roadsides (Thogmartin et al. 2017b), detailed information is needed about how monarchs use established habitat, which nectar sources and milkweed species are most used by larvae and adults, and how larval host plants impact adult traits, especially those important for migration (e.g., wing morphology and migratory physiology). In the following sections, we detail recent work on larval survival and growth, milkweed oviposition preference, the influence of larval diet on adult traits, and monarchs’ attraction to different habitat types and arrangements with the goal of providing succinct guidance for habitat restoration.
Larval Survival and Growth
Several studies have investigated various aspects of monarch survival from larva to adult over the past four decades, including recent comparative work on multiple milkweed species (Agrawal et al. 2015; Pocius et al. 2017a, b, 2018a; Jones and Agrawal 2019; see Table 1 for milkweed traits). Although A. syriaca is the most abundant plant on the landscape, it is important to understand how larvae respond to a variety of traits, including physical and chemical defenses. Larvae can eat most milkweeds (Malcolm and Brower 1989, Agrawal et al. 2015), though their growth rate, mass, and survivorship can vary widely among milkweed diets (Ladner and Altizer 2005; Yeargan and Allard 2005; Zalucki et al. 2012; Agrawal et al. 2015; Pocius et al. 2017a,b).
Table 1.
Summary of the utility of nine milkweed species examined in Pocius et al. (2017a,b; 2018a,b)
| Milkweed species | Common name | Habitat requirements | U.S. range | Cardenolide content (% dry mass) | Quercetin glycoside content (% dry mass) | Latex exudation upon tissue damage (mg) | Larval survivorship | Oviposition use | Ease of establishment from plugs |
|---|---|---|---|---|---|---|---|---|---|
| A. exaltata | Poke milkweed | Partial shade, woodland edges, upland woods | East of the Mississippi River; Not found in LA, AR, MS, or FL | 0.125 | 0.079 | 0.961 ± 0.193 | High | Medium | Difficult |
| A. hirtella | Tall Green milkweed | Full sun, prairie remnants, fields | Central United States | 0.208 | 0.142 | N/A | Low | Medium | Difficult |
| A. incarnata | Swamp milkweed | Partial to full sun, wetlands, floodplains, marshes | Widespread; Not found in OR, WA, or CA | 0.117 | 0.106 | 0.385 ± 0.066 | High | High | Easy |
| A. speciosa | Showy milkweed | Full sun, roadsides, untilled fields, forest clearings | Widespread west of the Mississippi River. Not found from IL east | 0.227 | 0.102 | 0.819 ± 0.359 | High | Medium | Easy |
| A. sullivantii | Prairie milkweed | Full sun, prairies, roadsides, field edges | Central United States | 0.123 | 0.221 | 4.518 ± 1.458 | High | Medium | Medium |
| A. syriaca | Common milkweed | Full sun, any disturbed areas | Widespread; less common west of the Rocky Mountains | 0.113 | 0.069 | 1.540 ± 0.862 | High | High | Easy |
| A. tuberosa | Butterfly milkweed | Full sun, prairies, open woodlands, | Widespread; Not found in OR, WA, ID, MT, WY, and ND | 0.064 | 0.076 | 0.042 ± 0.099 | High | Low | Easy |
| A. verticillata | Whorled milkweed | Partial to full sun, disturbed areas, roadsides, prairies | Widespread; Not found in OR, WA, CA, NV, ID, UT, or CO. | 0.114 | 0.114 | 0.149 ± 0.030 | High | Low | Easy |
| C. laeve | Honeyvine milkweed | Full sun, disturbed areas, prairies, cities | Widespread East of the Rocky Mountains from NE South | N/A | N/A | N/A | Low | Medium | Easy |
Habitat information is summarized from Kaul et al. 1991 and Eilers and Roosa 1994. Larval survivorship is designated as high if over 60% of larvae reached adulthood; under 60% survival is designated as low. Oviposition use is designated as high if species were in the top third for both laboratory and field oviposition experiments, medium if species were in the second third for both experiments, and low if the species were in the bottom third of egg totals for both experiments. Species are designated as easy to establish if over 60% survived within the demonstration plots from 2015 to 2017 described in Pocius et al. (2018b). Cardenolide content and quercetin glycoside content taken from Agrawal et al. (2009); mean latex exudation upon tissue damage (mg) ± SE taken from Agrawal et al. (2009). Species native ranges compiled from USDA-NRCS (2021).
Many larvae do not survive past the first or second instar as they are more vulnerable to both predators (see Natural Enemies below) and plant defenses including cardenolides, milkweed-derived cardiac glycosides sequestered by larvae for their own defense (see Natural Enemies; Reichstein et al. 1968, Duffey and Scudder 1972, Brower and Glazier 1975, Agrawal 2017), and milkweed latex, the white, milky emulsion exuded at point of plant damage (Oyeyele and Zalucki, 1990; Zalucki et al., 1990, 2001a,b). Monarchs have evolved behaviors that circumvent latex exudation including cutting small trenches through leaves (Dussourd 1990, Dussourd and Denno 1991, Zalucki and Brower 1992) or severing leaf petioles to stem latex flow before consuming them (Brewer and Winter 1977, Zalucki and Brower 1992) and incorporate cardenolides into their own tissues for defense (Malcolm and Brower 1989, Jones et al. 2019, Agrawal et al. 2021). Both defenses have been implicated in lowering monarch survival (Zalucki and Brower 1992, Zalucki et al. 2001b). In fact, latex exudation has been identified as the strongest predictor of early instar monarch survival across milkweed species even after controlling for phylogenetic relatedness (Agrawal et al. 2015) but latex exudation does not have a similar effect on later instars.
Taken together, results from studies examining the impact of milkweed species, latex exudation, and cardenolide content suggest that latex is a major source of mortality for early instar monarchs while cardenolide content may slow growth rate in combination with other physical defenses (e.g., latex, trichomes, leaf toughness; Malcolm 1994, Malcolm and Zalucki 1996, Pocius et al. 2017b). Growth may also slow in response to apolar cardenolide conversion in vivo (Agrawal et al. 2021), as monarch preferentially sequester more polar cardenolides (Reichstein et al. 1968, Roeske et al. 1976, Seiber et al. 1980, 1983, Frick and Wink 1995). High cardenolide, high latex milkweeds such as A. humistrata and A. syriaca are associated with slower larval growth, mainly due to latex exudation and latex cardenolide content when compared to low latex, low cardenolide species such as A. incarnata (Zalucki and Malcolm 1999; Zalucki et al. 2001a, b; Pocius et al. 2017a,b). These differences between species remain particularly important for early instar survival in the field (Zalucki and Malcolm 1999, Zalucki et al. 2001b) and additional work is needed to determine the volume, latex cardenolide content, and rate of exudation detrimental to larval survival.
Monarchs are capable of overcoming a variety of milkweed defenses as larvae, reaching adulthood on a wide range of milkweed species (Ladner and Altizer 2005, Robertson et al. 2015, Pocius et al. 2017b). High larval mortality has been correlated with both high cardenolide content and high latex exudation across milkweed species (see Agrawal et al. 2009, Rasmann and Agrawal 2011 for cardenolide content by species and details of monarch resistance to cardenolides), although latex becomes less dangerous as larvae reach later instars. While monarchs successfully develop on a wide variety of milkweeds, we recommend planting native milkweeds such as A. syriaca and A. incarnata (Gentianales, Apocynaceae, Asclepias incarnata, swamp milkweed) with low cardenolide content, lower latex exudation, and consistently high larval survival when possible (Ladner and Altizer 2005, Pocius et al. 2017a, b), but we acknowledge that other species such as A. asperula (Gentianales, Apocynaceae, Asclepias virids, antelope horn milkweed) and A. viridis (Gentianales, Apocynaceae, Asclepias virids, green antelope horn milkweed), both high in cardenolide content, are crucial hosts for spring generation monarchs in Texas and Oklahoma. Future studies must compare the wide variety of native milkweeds in California and Texas, both of which are critical areas for monarch population growth, as monarch hosts. Plant traits such as cardenolide content and trichome density should be assessed in addition to monarch survival on native milkweeds with narrow ranges. Additional work is also needed to understand the mechanisms inhibiting monarch growth and survival on milkweed species beyond A. curassavica with different concentrations and/or combinations of cardenolides.
Oviposition Preference
Female oviposition preference plays a large role in boosting monarch numbers as larval success depends on where they hatch. Monarch oviposition preference is related to plant height, species identity, and foliar chemical concentrations including cardenolides and quercetin glycosides (Oyeyele and Zalucki 1990; Zalucki et al. 1990; Haribal and Renwick 1996, 1998a,b; Agrawal et al. 2021). Prior work focused on the chemical composition of milkweeds (Zalucki et al. 1990; Haribal and Renwick 1996, 1998a,b) and formed the basis of later experiments considering chemical content and composition as a factor in oviposition preference. Specifically, female monarchs rejected high cardenolide hosts (Oyeyele and Zalucki 1990, Zalucki et al. 1990, Haribal and Renwick 1998a) and preferred to deposit eggs on milkweeds with intermediate cardenolide content and relatively high levels of nitrogen (Oyeyele and Zalucki 1990, Zalucki et al. 1990, Agrawal et al. 2021). Females likely rejected these high cardenolide hosts in response to chemical cues determined by alighting on the plant and scratching or drumming the leaf surface (Oyeyele and Zalucki 1990). High cardenolide levels have been linked with low larval survival and slower development rates (Erickson 1973; Zalucki et al. 2001a, b, 2012, see Larval Survival). Alternatively, high quercetin glycoside level located on the leaf surface stimulate oviposition (Agrawal 2017); monarchs respond to these chemicals as part of host plant recognition and females have laid eggs in response to these chemicals without a plant (Haribal and Renwick 1996). When these compounds were isolated monarchs were stimulated to oviposit only when exposed to these compounds at unrealistically high concentrations (Agrawal et al. 2021). Therefore, cardenolide concentration and plant height remain strong predictors of oviposition (Agrawal et al. 2021), although more work needs to investigate the role of flower and nectar cues in oviposition as some results show an impact of nectar cardenolides on monarch oviposition (Jones and Agrawal 2016).
Other research has focused on oviposition between different milkweed species. This work revealed monarch preferences for some milkweed species over others in the field within localized areas in Texas, Florida, and Kentucky. In Texas, eggs were observed on 7 endemic milkweed species, with the highest proportion of eggs per meter of stem recorded on A. latifolia (Gentianales, Apocynaceae, Asclepias latifolia, broadleaf milkweed) (Calvert 1999). In Florida, more eggs were counted on larger A. humistrata (Gentianales, Apocynaceae, Asclepias virids, pinewoods milkweed) plants with intermediate cardenolide content and on A. curassavica compared to A. incarnata in mixed stands (Cohen and Brower 1982, Malcolm and Brower 1986, Zalucki et al. 1990). In Kentucky, monarchs preferred A. syriaca to Cynanchum laeve (Gentianales, Apocynaceae, Cynanchum laeve, honeyvine milkweed) when both plant species inhabited the same field but used either plant in single species stands (Bartholomew and Yeargan 2002); they also preferred taller, broad-leaved milkweed species to shorter, narrow-leafed species in common gardens (Baker and Potter 2018). Importantly, an experimental study revealed that monarchs originating from spatially distant populations (East of the Rocky Mountains vs West of the Rocky Mountains designated by overwintering location) did not display preferences for milkweed species grown in their natal region. Specifically, females captured from Michigan and California choose to deposit higher egg totals on A. incarnata compared to three other species (A. syriaca, A. Fasicularis (Gentianales, Apocynaceae, Asclepias fasicularis, narrowleaf milkweed), and A. Speciosa (Gentianales, Apocynaceae, Asclepias speciosa, showy milkweed)) regardless of their source population (Michigan vs. California; Ladner and Alitizer 2005). Experimental studies in Iowa, New York, and Kentucky have revealed similar monarch oviposition preferences for A. incarnata and A. syriaca over other species. In both field and lab experiments, monarchs laid more eggs on A. incarnata and A. syriaca compared to other milkweed species including A. Exaltata (Gentianales, Apocynaceae, Asclepias exaltata, poke milkweed), A. Hirtella (Gentianales, Apocynaceae, Asclepias hirtella, tall green milkweed), A. Speciosa (Gentianales, Apocynaceae, Asclepias incarnata, swamp milkweed), A. Sullivantii (Gentianales, Apocynaceae, Asclepias sullivantii, prairie milkweed), A. Tuberose (Gentianales, Apocynaceae, Asclepias tuberosa, butterfly milkweed), and A. verticillata (Gentianales, Apocynaceae, Asclepias verticillata, whorled milkweed) (Pocius et al. 2018a, b), and in general taller plants with broad leaves recruited the most eggs across studies (Baker and Potter 2018, Pocius et al. 2018b, Jones and Agrawal 2019). Similarly, wild monarchs readily colonized planted gardens in Kentucky, but laid more eggs on broad-leafed milkweeds when compared to species with other growth forms (Baker and Potter 2018). A mix of available milkweeds may also be important for increasing oviposition, as monarchs laid 2.5 times more eggs when there were multiple species of milkweeds present compared to only one (Pocius et al. 2018a). Across all experiments, monarchs show a willingness to oviposit on all available milkweed species, but prefer species and perhaps individual plants with intermediate cardenolide levels (Agrawal et al. 2021); monarchs display preferences for the wide-ranging A. incarnata and A. syriaca when compared to A. tuberosa, A. fascicularis, A. verticillata, A. speciosa, A. hirtella, and A. sullivantii, and monarchs may favor taller, more apparent milkweeds in the field (Baker and Potter 2018; Pocius et al. 2018a, b; Jones and Agrawal 2019; Agrawal et al. 2021).
Plant age also influences milkweed attractiveness to ovipositing females. Because plant age can impact leaf levels of both water and nitrogen, caterpillars often perform better on younger plants compared to those near senescence (Scriber and Slansky 1981, Slansky 1993). Monarchs clearly prefer to oviposit upon young milkweed stems (Urquahrt 1987, Bergström et al. 1994), but young milkweeds or newly regenerated stems may also harbor fewer natural enemies of young larvae (see Natural enemies). Recent work has shown that targeted mowing and burning produces new growth on existing milkweed plants and increases the number of eggs laid on mowed vs. unmowed plants in roadsides (Knight et al. 2019) and in grasslands (Haan and Landis 2019b) which could be crucial for late-breeding monarchs (Baum and Mueller 2015). These new, tender stems are both attractive to ovipositing females and the disturbance caused by mowing provides 2–4 wk of reduced natural enemy presence potentially allowing young larvae to develop successfully (Haan and Landis 2019a). Disturbance regimes timed for specific latitudes could improve phenological diversity of milkweeds in a variety of habitats, increasing the number of stems used by ovipositing females and reducing pressure from natural enemies (Baum and Mueller 2015, Haan and Landis 2019b). Disturbance should therefore be fully explored as part of a comprehensive monarch conservation strategy.
Rearing Conditions
Environmental conditions, including temperature, are key to the development of immature monarch stages. Warming spring and summer temperatures (above 12°C) increase the developmental rate (Barker and Herman 1976), although extreme heat is detrimental and even lethal (York and Oberhauser 2002). In particular, the high heat of the southern US states is thought to limit monarch’s ability to reproduce successfully during the summer months, resulting in most monarch generations originating at higher latitudes (Malcolm et al. 1987). On the other hand, cooling fall temperatures slow development and play a role in inducing the migratory state in monarchs, including reproduce diapause (Goehring and Oberhauser 2002), elongated wings (Li et al. 2016), lower flight metabolism (Schroeder et al. 2020), and flight oriented towards the overwintering sites (Zhu et al. 2009). Decreasing daylight is also an important factor in the migratory state induction (Zhu et al. 2009). Cooling temperatures and decreasing daylength in the fall months are more clearly demarked in northern compared to southern latitudes which might explain why the majority of migratory monarchs originate from this region (Hobson et al. 1999, Flockhart et al. 2017). For this reason, the practice of collecting and rearing caterpillars indoors, which can alter both temperature and light regime, has raised concerns. Indeed, fall monarch tagging studies recovered fewer captive reared monarchs than wild counterparts (Malcolm 2018, Pelton 2018). Several recent experiments examined the impact of indoor rearing and found that indoor reared monarchs did not orient south on flight simulator (Tenger-Trolander and Kronforst 2020), but once released some adults did orient southward (Wilcox et al. 2021). Additional work indicates that captive reared monarchs might be weaker than wild reared adults (Davis et al. 2020). Together, these studies suggest that the development and unique physiological state of the fall generation can be easily disrupted.
Impact of Larval Diet on Adult Traits
Although larvae can eat a large variety of milkweed species, there are noted differences in larval mass, growth rate, and survival on different milkweed species (Agrawal et al. 2015, Pocius et al. 2017b). Recent work has also revealed that larval diet impacts adult traits such as wing length, wing shape, wing toughness, body mass, and initial lipid store in larvae reared under summer conditions (Pocius et al. 2017b, Davis and De Roode 2018, Decker et al. 2019, Soule et al. 2020). Adults that fed on A. incarnata and A. syriaca as larvae had more elongated wings, a better shape for sustained migratory flight, than those that fed on A. curassavica or A. speciosa (Soule et al. 2020). Similarly, adults that fed on A. incarnata and A. tuberosa as larvae eclosed with the largest initial lipid stores and high dry mass, although A. curassavica was not included in this study (Pocius et al. 2017b). A. curassavica-fed larvae also emerged with lower wing density as adults whereas those that fed on A. syriaca had higher wing loading, a trait associated with powered flight, further supporting the importance of a native milkweed diet for long-distance migrants (Soule et al. 2020). Larval diet also impacts both adult resting and flight metabolic rate, with those that fed on A. curassavica investing more heavily in flight musculature but incurring the highest energetic cost of flight per unit body mass (Pocius et al. 2021). This was in contrast to larvae that fed on native milkweeds (A. exaltata, A. incarnata, A. sullivantii, A. syriaca, A. speciosa, A. tuberosa, and A. verticillata) and eclosed as relatively small adults with lower energetic costs for both flight and maintenance (Pocius et al. 2021). These differences in metabolic rate could be due to tradeoffs in processing apolar cardenolides contained in A. curassavica (Agrawal et al. 2021) and highlight the lasting impacts of larval diet on adult physiology. More work needs to be done to elucidate the links between the components of the larval diet (phytochemicals, cardenolide content, nitrogen content) and adult metabolism in relation to long distance migratory flights, during which even small metabolic differences could influence migration success.
Monarch Attraction to Restored Habitats and Gardens
Although monarchs are obligate milkweed specialists, the location of milkweed stands within habitats may also influence females’ attraction to milkweed. Recent work has revealed the importance of both larger-scale conservation areas and gardens for both the recruitment and survival of monarchs (Geest et al. 2019). Tachinid fly (Diptera: Tachinidae) parasitism was 25% lower in larvae collected from conservation sites compared to those collected from gardens, but monarch recruitment did not differ by site type (Geest et al. 2019). Additionally, milkweed position and garden composition were important although milkweed density did not impact oviposition (Baker and Potter 2019, Nestle et al. 2020). Oviposition on garden milkweeds was higher when milkweeds were evenly spaced around garden perimeters or when milkweeds were spatially isolated than when milkweeds were interspersed with nectar plants and native grasses (Baker and Potter 2019). Oviposition was 22% higher in more diverse plantings (milkweed plus nectar species) than in milkweed monoculture without increased predation rates of monarch larvae (Nestle et al. 2020). However, it is important to note that high density of immature stages on milkweed can have consequences for transmission of infectious pathogens (Lindsey et al. 2009) and a very high oviposition rate per plant can lead to food limitation for larvae, resulting in unintended consequences (see Natural Enemies). These results suggest that both resource availability for both larvae and adults as well as garden layout are important factors to consider when planning future conservation planting endeavors for monarchs.
In summary, this section provides an overview of the milkweed species monarchs use successfully, the impact of larval host plant identity and rearing conditions on adult traits, and monarch attraction to different habitats. Larvae develop successfully on a wide variety of milkweed species but have the highest survival on plants with intermediate cardenolide content and lower latex exudation, especially in early instars (Zalucki et al. 2001b; Agrawal et al. 2015; Pocius et al. 2017a,b). Monarchs prefer to lay their eggs on young milkweed stems (Haan and Landis 2019a), taller stems with intermediate cardenolide content (Cohen and Brower 1982, Agrawal et al. 2021) and produce the highest egg counts in areas that contain a mix of milkweed species (Pocius et al. 2018a). Together, studies examining oviposition reveal preferences for more apparent plants on the landscape with intermediate cardenolide concentrations. Studies that examine the impact of milkweed diet on adult traits have revealed differences between adults that fed on native milkweeds vs. A. curassavica. While temperatures during development may show stronger effects on adult traits than larval diet (Soule et al. 2020), higher metabolic rate per unit body mass in monarchs that fed on A. curassavica may indicate a higher cost for body maintenance and sustained flight (Pocius et al. 2021). We encourage managers to allow monarchs to develop outside, lessening the chance of temperature interference with their development. In conclusion, we recommend planting native milkweeds on the landscape including, but not limited to A. syriaca and A. incarnata to boost the potential for oviposition, larval survival, and development of healthy adults with wing traits suitable for long distance flight. We also encourage land managers to establish pollinator habitat with milkweeds along the perimeter for maximum monarch utility (Baker and Potter 2018) as well as timing mowing during the early summer between generations, allowing fresh milkweed to sprout for monarch use later in the summer where possible (Haan and Landis 2019a, b).
Natural Enemies and Exotic Caterpillar Food Plants
The monarch butterfly is a prey and a host to numerous natural enemies (Table 2). All life stages are faced with a repertoire of vertebrate and invertebrate predators with monarch eggs and caterpillars being the most vulnerable stages to predation: less than 3% eggs survive to pupation (Nail et al. 2015, Grant et al. 2020). Arthropods have long been recognized as significant sources of mortality, yet the introduced fire ant (Solenopsis invicta (Formicidae, Hymenoptera, Solenopsis invicta)) is particularly concerning (Oberhauser et al. 2015). A study in Texas suggested the introduced fire ant caused 0% survival of eggs and early instar caterpillars (Calvert 1996). It is important to note that ants, including native species, are a common monarch predator and milkweed plants with aphids are more likely to attract ants and increase predation of eggs and caterpillars (Presby 2004). Experimental work in grasslands indicated that mowing in mid-summer can decrease predation risk (Haan and Landis 2019a, b), which might be due in part to both a reduction of the plant’s aphid population, which attract predators, and changes in habitat complexity. This work suggests that mowing could be used as a management strategy to offset high predation rates by ants, yet whether this might work for reduction of the introduced fire ants remains unstudied.
Table 2.
Overview of the array monarch natural enemies, life stages they affect most often, and the main takeaways of recent work on each category of natural enemies
| Natural enemy and stage affected | Example | Main takeaways |
|---|---|---|
| Predators all stages (eggs and early instar stages are most vulnerable) Examples: Mantids such as Iris oratoria Wasp Polistes dominulus Lacewing larvae Chrysoperla rufilabris Fire ants Formica montana |

Photo by J. Dicus |
More than 30 genera of invertebrate predators have been identified. Invertebrate predators likely pose the biggest threat to immature stages. For example, the introduced fire ant causes high mortality (nearing 100%) of eggs and early instar larvae). Some milkweed herbivores ‘accidentally’ consume monarch eggs. Adults are thought to have fewer predators than all other stages, however adults may be particularly vulnerable to bird and mice predation when roosting at overwintering sites. Cardenolide toxin concentrations found in milkweeds can provide some protection against predators whereby monarchs sequester the toxins, causing them to be less palatable to predators. The more ‘toxic’ the milkweed, the greater the protection. For example, paper wasps (Polistes dominulus) were less likely to consume caterpillars raised on more toxic tropical milkweed (A. curassavica) compared to a native swamp milkweed (A. incarnata) or common milkweed (A. syriaca), both of which have a lower concentration and diversity of cardenolide toxins. |
| Parasitoids Late stage larvae and pupae Examples: Parasitic wasp Pteromalus cassotis Tachinid fly Lespesia archippivora |

Photo by AAM |
Parasitoid flies and wasps cause significant mortality to monarch butterfly. Over 15 species of parasitoids have been documented infecting caterpillars and pupa. Adult parasitoid oviposits eggs into caterpillar or fresh pupa. Those eggs develop into larvae and slowly consume the monarch leading to its death. |
| Infectious pathogens all stages Examples: Ophryocystis elektroscirrha (OE) protozoan Nuclear polyhedrosis virus Beauvaria bassiana fungus Pseudomonas bacteria Microsporidian fungus |

Photo by J. Arnold |
While OE is the best-studied parasite of the monarch, multiple other microscopic pathogens can kill monarchs. ‘Black death’ in which the caterpillar or pupa becomes increasingly dark and eventually black is fatal and caused by virus, bacteria, or fungus. |
Natural Enemies
Monarchs’ immature stages are susceptible to infection by various microscopic pathogens. For instance, unexpected mortality of caterpillars in lab experiments (e.g., Yeargan and Allard 2005) is often attributed to the nuclear polyhedrosis virus (Arnott et al. 1968), commonly referred to as ‘black death’ (Table 2). The best known pathogen of the monarch butterfly is a neogregarine protozoan parasite, Ophryocystis elektroscirrha (OE). Generally, the proportion of infected adults in eastern North America is relatively low (<10%), with a somewhat higher proportion in the western United States (De Roode et al. 2008), and a very high infection rate (50–100%) in non-migratory populations (e.g., southern Florida; Altizer et al. 2000, Satterfield et al. 2015, Satterfield et al. 2016). However, OE poses concerns due to its virulence and the overall increase in prevalence over the last few decades (Majewska et al. 2021, under review).
While OE is often broadly described as ‘debilitating’, recent work has estimated its virulence given all of the documented negative effects, including reduced adult lifespan (De Roode et al. 2007), decreased fecundity (Altizer and Oberhauser 1999), lower mating success, and poor flight performance (Bradley and Altizer 2005). Together these effects result in OE reducing monarchs’ population size, although the severity of this impact depends on the proportion of the population that is infected during the breeding season. For instance, when a very high proportion (nearing 100%) of adults are infected, the population is reduced by 50% (Majewska et al. 2019). The high loss of monarchs due to the pathogen calls for efforts to reduce OE spread.
OE is predominantly transmitted during egg laying through spores that are ingested by caterpillars (McLaughlin and Myers 1970, De Roode et al. 2009). Extensive lab and field experiments show that besides mother to offspring transmission (vertical), OE is also transmitted by two additional routes: (1) environmental transmission where unrelated infected monarchs that scatter spores onto milkweed, which can persist for weeks on the plant (Satterfield et al. 2017), and by (2) adult transfer, where contacts such as the mating of infected and healthy monarchs leads to transfer of spores, which are then passed to the offspring (Altizer et al. 2004, De Roode et al. 2009, Majewska et al. 2019). Given that a single spore can cause infection in monarchs and that spores can persist on milkweed for extended periods of time, milkweed sites that are crowded by monarchs and used by multiple generations for oviposition pose a risk of infection for the butterfly. This might occur when patches of milkweed are isolated in the landscape due to habitat fragmentation or planted in habitats where naturally occurring milkweed is generally scarce (e.g., southeastern United States).
Exotic Caterpillar Food Plants
Monarchs oviposit and successfully develop on a variety of native and exotic milkweeds (Ackery and Vane-Wright 1984, Malcolm and Brower 1986, Malcolm 1994), although several species of invasive swallow-wort, namely Cynanchum louisea and Cynanchum rossicum, are not suitable as caterpillar food plants for monarch development and cause 100% larval mortality (DiTommaso and Losey 2003). Exotic milkweed species, those not native to North America, have been introduced via the horticulture trade and are widely available in the United States. While most exotic milkweeds are suitable hosts and allowed monarchs to colonize and persist in new regions of the world (e.g., Azores), the full breadth of impacts of planting exotic milkweeds need to be considered, especially in the context of disrupting monarch migration.
One exotic milkweed species which has received considerable attention is tropical milkweed, A. curassavica, due to its widespread planting and link to high infection rates in non-migratory monarch populations in the southern latitudes in the United States (see section on disease; (Satterfield et al. 2015, Majewska et al. 2019)). Field experiments have shown that tropical milkweed is highly attractive to monarch females resulting in high oviposition rates (Majewska et al. 2018), presumably due to the high cardenolide content that provides some protection from predators and parasites. However, the increased density of monarchs on milkweed results in an increased risk of infection (Lindsey et al. 2009). Indeed, experimental gardens containing tropical milkweed in coastal Georgia show a high density of eggs and caterpillars and high transmission rates of OE infection (Majewska et al. 2019). Further, the tropical milkweed gardens have continual adult, egg and caterpillar presence into the winter months (Majewska et al. 2019), causing a buildup of OE spores on the plants and resulting in 100% infection rates.
Observations of winter-breeding monarchs have raised concern about the impact of tropical milkweed on monarchs’ migration in the fall months. Because tropical milkweed does not enter dormancy in the fall, continual breeding activity is possible until a freeze event severely damages the plant. Indeed, experimental work indicates that tropical milkweed promotes the reproductive state in the fall months. In particular, when monarch caterpillars were reared in fall-like conditions on tropical milkweed, emerging adults were more likely to emerge reproductive than those reared on native milkweed (Majewska and Altizer 2019). Further, migrating adults exposed to tropical milkweed in the fall show considerable risk of halting diapause and becoming reproductive (Majewska and Altizer 2019). With evidence pointing towards impact of tropical milkweed on monarch migration, cutting the milkweed back in the fall has been recommended. However, the population level impact of this management strategy remains to be examined.
In short, the monarch is the prey of numerous predators and host of various parasitic organisms. Although not considered problematic for monarchs, the well-studied protozoan parasite is in fact virulent and might play a larger role in population size than previously thought. In addition to the natural enemies, the monarch is challenged by introduced milkweed species, of which few have significant effects on caterpillar survival and infection dynamics.
Genetics and Genomics in Monarch Conservation
Genetic variation provides the raw material upon which natural selection acts, and the ability for species and populations to adapt to changes in their environment depends on levels of standing genetic diversity. Therefore, efforts to preserve genetic diversity have become a central paradigm in conservation biology (e.g., Allendorf et al. 2010). Monarch conservation has traditionally focused on census-based and observational methods for determining population numbers, population trends through time, and movement patterns during migration, but more recently, genetic and genomic approaches have been applied to monarchs to shed light on important aspects of their biology.
Genetics and genomics have contributed to our understanding of monarchs in two broad ways. First, population genetic and genomic studies have revealed how monarch populations in North America and around the world are related to one another, as well as highlighting genes likely involved in migration (Zhan et al. 2014). Second, controlled rearing experiments have revealed patterns of quantitative genetic variation in ecologically important traits. In part due to their unique migration biology, monarchs have recently become a model system in ecological genomics (see Reppert and de Roode 2018, Merlin et al. 2020 for recent reviews), with multiple genome assemblies (Zhan et al. 2011, Zhan and Reppert 2013), including recent chromosome-level assemblies (Gu et al. 2019, Ranz et al. 2020). An array of tools for gene editing has also been developed for monarchs, allowing for novel gene knockout-based approaches to studying migration (e.g., Markert et al. 2016, Iiams et al. 2019) and wing patterning (Mazo-Vargas et al. 2017).
Population Genetic and Genomic Approaches Applied to Monarchs
Monarchs were among the first species in which patterns of genetic variation were documented: Eanes and Koehn (1978) used allozyme markers and found that monarchs sampled from locations throughout North America show little evidence for geographical differentiation. Subsequent studies have corroborated this pattern and strongly suggest that monarchs across all of their migratory North American range—including populations in eastern and western North America—represent a single genetically indistinguishable population (Shephard et al. 2002, Lyons et al. 2012, Talla et al. 2020). This genetic panmixia likely results from random mating at overwintering sites and during their spring return migration into the southern United States, as well as gene flow between eastern and western monarchs.
The lack of detectable population structure within North American monarchs has a number of important conservation implications. Unlike many other migratory species such as Chinook (Oncorhynchus tshawytscha) and steelhead salmon (O. mykiss)—where species-wide genetic diversity is partitioned across many spatially subdivided populations (Waples et al. 2004, Prince et al. 2017)—genetic diversity in North American monarchs is shared evenly among the tens of millions of butterflies that comprise the whole population. This continent-scale lack of genetic differentiation suggests that monarchs originating from disparate locations throughout North America are functionally equivalent and that conservation efforts that increase monarch numbers in one location are broadly beneficial to monarch numbers in other locations as well. The lack of genetic differentiation between eastern and western monarchs is also important for contextualizing the conservation significance of the recent precipitous declines in numbers of overwintering western monarchs (Espeset et al. 2016, Schultz et al. 2017, Pelton et al. 2019). Rates of gene flow between eastern and western monarchs are high enough to prevent any strong genomic signature of divergent natural selection from developing, despite differences in their migratory behavior (Talla et al. 2020; reviewed in Freedman et al. 2021).
In accordance with the lack of overall population structure in North America, studies that have estimated metrics of genome wide diversity in monarchs generally indicate a very large migratory population, with an estimated effective population size of >2 × 107 (Zhan et al. 2014). These estimates are consistent with a robust monarch population harboring high levels of genetic diversity. High levels of genetic diversity are generally considered to be beneficial and contribute to adaptive capacity (but see Teixeira and Huber 2021). However, high levels of heterozygosity in migratory North American monarchs can also mask the presence of rare deleterious recessive alleles, which may help to explain why North American monarchs are susceptible to strong inbreeding depression (Mongue et al. 2016).
By contrast, monarch populations in locations outside of North America show clear genetic differences from their North American ancestors (Lyons et al. 2012, Pierce et al. 2014, Zhan et al. 2014, reviewed in Pierce et al. 2015). Non-migratory monarchs also have reduced genetic diversity and effective population sizes (Zhan et al. 2014), and the genetic differences between migrants and non-migrants are generally accompanied by phenotypic differences (e.g., wing size and shape) associated with loss of seasonal migration. Thus, non-migratory monarch populations outside of North America may not possess genetic variants associated with seasonal migration and therefore may not be adequate stand-ins for their migratory ancestors in conservation efforts aimed at preserving functional genetic variation.
Finally, another advantage of studying genomic diversity in monarchs is that it can enable estimates of changes in population size across various time scales. Demographic models using allele frequency data from contemporary specimens have been used to estimate historical population fluctuations over evolutionary time scales and suggest a recent population expansion around 20, 000 yr ago (e.g., Zhan et al. 2014, 2017), coinciding with the end of the last glacial maximum in North America. More recently, ongoing research has compared genomic diversity from present-day versus historical monarch specimens to test whether published accounts of declines in overwintering monarchs in Mexico (Brower et al. 2012, Semmens et al. 2016) are corroborated by reductions in genomic diversity over the same time frame (Talla and de Roode, in prep). Together, demographic inferences from genomic data can provide important context for understanding changes in monarch population size over both macro-evolutionary and contemporary timescales.
Functional Genetic Diversity in Monarchs
Even in the absence of direct DNA sequencing, experimental approaches can still provide valuable information about functional genetic variation in natural populations. This is most commonly accomplished through quantitative genetic approaches, particularly controlled experiments with breeding designs (e.g., comparisons among half-sib or full-sib families) that enable estimates of genetic variation from levels of measured phenotypic variation. Monarchs are well-suited for these approaches: larvae are comparatively easy to rear in large numbers under controlled conditions, and virgin females can be hand-mated with males to make targeted crosses (e.g., Solensky and Oberhauser 2009).
Studies that rear the offspring of wild-caught monarchs under controlled conditions have provided important context about levels of standing variation for ecologically important traits, even when this is not an explicit aim of the experiment. These studies most commonly involve using either wild-caught gravid females or wild-collected caterpillars reared to adults and then used to make crosses. Some of the traits assayed using experiments that explicitly track effects of family of origin include wing morphology (Altizer and Davis 2010), oviposition preference and host plant performance (Ladner and Altizer 2005, Freedman et al. 2020), induction of reproductive diapause (Freedman et al. 2018), and resistance and tolerance to OE (De Roode and Altizer 2010, Sternberg et al. 2013; see Table 3). An example of an insight gained through controlled rearing experiments is that monarch maternal families differ substantially in their wing morphology even when reared under identical environmental conditions (Altizer and Davis 2010), suggesting that varying selection pressures across the migratory life cycle may maintain variation in wing morphological traits. Rearing approaches are most informative about levels of standing genetic variation when they include the F1 progeny of wild-caught monarchs, though approaches using lab-reared colonies may still be ecologically informative if they are regularly supplemented with genetic variants from wild, outbred monarchs.
Table 3.
Example of studies that have used rearing designs that explicitly recorded family-level variation in ecologically important monarch traits. Monarch family refers to all offspring from one female butterfly
| Study | Trait(s) assayed | Finding |
|---|---|---|
| Altizer and Davis (2010) | Forewing size, forewing shape | Substantial variation among families; substantial variation among populations (eastern, western, South Florida) corresponding to migratory status |
| Ladner and Altizer (2005) | Larval performance across host plants, oviposition preference | Substantial variation among families for oviposition preference and performance across hosts |
| Freedman et al. (2020) | Larval performance across host plants | Substantial variation in family quality, but little family-level variation in performance rank order across host species |
| Freedman et al. (2018) | Induction of reproductive arrest | Substantial family-level variation in post-eclosion reproductive development in monarchs from Australia |
| Davis et al. (2005) | Larval and adult melanism | Substantial variation among families and between populations (eastern, western, South Florida) |
| De Roode and Altizer (2010) | Resistance to OE | Strong host family effects for level of parasite virulence |
| Sternberg et al. (2013) | Tolerance to OE | Substantial variation among families and between populations (eastern, western, South Florida, Hawaii) |
Overall, recent research has shown that North American monarchs comprise a single genetically panmictic population with high levels of genetic diversity. This genetic diversity is also apparent from experimental rearing studies, which consistently find substantial phenotypic variation among monarch families, offspring derived from the same adult female butterfly, in ecologically important traits. An important future direction will be identifying the genetic basis of traits associated with migration (e.g., diapause induction, lipid metabolism, wing morphology, parasite resistance) and measuring changes in the frequency of the underlying alleles through time. For example, under climate warming scenarios that predict a longer growing season for milkweed, natural selection may favor genetic variants associated with earlier spring return migration and/or increased voltinism in monarchs. A priori knowledge of the genetic variants underlying these traits provides the opportunity to directly measure evolution in action.
Conclusion
Although research into monarch biology and behavior is ongoing, we propose key questions that will help to advance understanding of monarch populations and interactions with other plants and insects to help inform best conservation practices (Table 4). First, a better understanding of the contributions of larval diet and environmental conditions to migratory potential and ability are needed to better understand the nutritional and habitat requirements of the developing migratory generation. Second, the incorporation of targeted disturbance regimes must be evaluated in restored habitats across monarch breeding sites to provide insight into the interactions between timing and frequency of disturbance with monarchs, other milkweed specialists, and natural enemies across different habitat types. Third, studies examining monarchs’ interaction with and attraction to exotic milkweeds including chemical differences compared to native species throughout the growing season would help conservation managers choose milkweed varieties that boost monarch migratory potential. Fourth, additional DNA sequencing data across multiple years would be useful for understanding how, if at all, population genetic diversity has changed in North American monarchs through time. These efforts would be especially helpful to better understand the historic fluctuations in average overwintering numbers. Together, results of these studies would add much needed depth to current conservation plans.
Table 4.
Outstanding monarch research questions that would directly inform ongoing conservation efforts
| Question | Research need |
|---|---|
| Does larval diet influence mating success and egg production (both larval and adult diet)? Diet effect on milkweed preference- generational esp. during the summer Impact of including targeted disturbance into conservation plans |
Assessment of nutritional quality and diet toxicity on adult traits that influence mating behavior, success, and egg production. Comparison of larval and adult milkweed preferences based on natal diet and plant quality Examination of timing and frequency of disturbance and the interactions between monarchs, enemies, and other milkweed specialists in different habitat types (grasslands, roadsides, right of ways, managed gardens) |
| What chemical features of exotic milkweeds decrease odds of reproductive dormancy in fall generation monarchs? Have natal origins and the prevalence of winter breeding changed in western North American monarchs? |
Comparing changes in nutritional quality and cardenolide content of native vs. exotic milkweeds from late summer to autumn (1) Comparison of overwintering monarch stable isotopes across years to see if natal origins have changed (e.g., Flockhart et al. 2017) (2) Comparison of cardenolide fingerprints across years to see if A. curassavica fingerprint has become more common through time. |
| Have monarch overwintering numbers in Mexico declined since collections there began? | Comparison of polymorphism data from DNA sequences of historical versus contemporary specimens to see whether there is an appreciable change in genetic diversity |
Supplementary Material
Acknowledgments
We thank Kelsey Fisher for helping to organize the Butterflies in Peril Symposium at EntSoc 2109 that led to this collection as well as our fellow symposium presenters. The authors would also like to thank Nathan Haan for conversations that led to preparing this review as well as Venkat Talla and Jaap de Roode for sharing the results of as-yet-unpublished research. The authors also thank Larry Hurd, Josh Lancette, and Lisa Junker for inviting submissions for this paper collection. AAM was supported by the National Institute of Health/National Institute of General Medical Sciences K-12 Postdoctoral Fellowship Project Number:5K12GM000680-19.
References Cited
- Ackery, P. R., and Vane-Wright R. I.. . 1984. Milkweed butterflies, their cladistics and biology, being an account of the natural history of the Danainae, a subfamily of the Lepidoptera, Nymphalidae. Br. Mus. (Nat. Hist.). 1. [Google Scholar]
- Agrawal, A. A. 2017. Monarchs and milkweed: a migrating butterfly, a poisonous plant, and their remarkable story of coevolution. Princeton University Press, Princeton, NJ. [Google Scholar]
- Agrawal, A. A., Salminen J. P., and Fishbein M.. . 2009. Phylogenetic trends in phenolic metabolism of milkweeds (Asclepias): evidence for escalation. Evolution 63: 663–673. [DOI] [PubMed] [Google Scholar]
- Agrawal, A. A., Ali J. G., Rasmann S., and Fishbein M.. . 2015. Macroevolutionary trends in the defense of milkweeds against monarchs, pp.47–59. InMonarchs in a changing world: biology and conservation of an iconic butterfly. Cornell University Press, Ithaca, New York, USA. [Google Scholar]
- Agrawal, A. A., Böröczky K., Haribal M., Hastings A. P., White R. A., Jiang R. W., and Duplais C.. . 2021. Cardenolides, toxicity, and the costs of sequestration in the coevolutionary interaction between monarchs and milkweeds. Proc. Nat. Acad. Sci. 118: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allendorf, F. W., Hohenlohe P. A., and Luikart G.. . 2010. Genomics and the future of conservation genetics. Nat. Rev. Genet. 11: 697–709. [DOI] [PubMed] [Google Scholar]
- Altizer, S., and Davis A. K.. . 2010. Populations of Monarch butterflies with different migratory behaviors show divergence in wing morphology. Evolution 64: 1018–1028. [DOI] [PubMed] [Google Scholar]
- Altizer, S. M., and Oberhauser K. S.. . 1999. Effects of the protozoan parasite ophryocystis elektroscirrha on the fitness of monarch butterflies (Danaus plexippus). J. Invertebr. Pathol. 74: 76–88. [DOI] [PubMed] [Google Scholar]
- Altizer, S., Oberhauser K. S., and Brower L. P.. . 2000. Associations between host migration and the prevalence of a protozoan parasite in natural populations of adult monarch butterflies. Ecol. Entomol. 25: 125–139. [Google Scholar]
- Altizer, S., Oberhauser K. S., and Geurts K. A.. . 2004. Transmission of the protozoan parasite, Ophryocystis elektroscirrha, in monarch butterfly populations: implications for prevalence and population-level impacts, pp. 203–218. InOberhauser K. S. and Solensky M. (eds.), The monarch butterfly: biology and conservation. Cornell University Press, Ithaca, NY. [Google Scholar]
- Antonsen, A. K., Kral-O’Brien K. C., Hovick T. J., Limb R. F., Geaumont B. A., and Harmon J. P.. . 2021. Intra-annual spatiotemporal dynamics of the monarch butterfly (Lepidoptera: Danaidae), regal fritillary (Lepidoptera: Heliconiinae), and their floral resources in North Dakota, United States. Ann. Entomol. Soc. Am. saab013. doi: 10.1093/aesa/saab013 [DOI] [Google Scholar]
- Arnott, H. J., Smith K. M., and Fullilove S. L.. . 1968. Ultrastructure of a cytoplasmic polyhedrosis virus affecting the monarch butterfly, Danaus plexippus. I. Development of virus and normal polyhedra in the larva. J. Ultrastruct. Res. 24: 479–507. [DOI] [PubMed] [Google Scholar]
- Badgett, G., and Davis A. K.. . 2015. Population trends of monarchs at a northern monitoring site: analyses of 19 years of fall migration counts at Peninsula Point, MI. Ann. Entomol. Soc. Am. 108: 700–706. [Google Scholar]
- Barker, J. F., and Herman W. S.. . 1976. Effect of photoperiod and temperature on reproduction of the monarch butterfly, Danaus plexippus. J. Insect Physiol. 22: 1565–1568. [DOI] [PubMed] [Google Scholar]
- Baker, A. M. and Potter D. A.. . 2018. Colonization and usage of eight milkweed (Asclepias) species by monarch butterflies and bees in urban garden settings. J. Insect Conserv. 22: 405–418. [Google Scholar]
- Baker, A. M. and Potter D. A.. . 2019. Configuration and location of small urban gardens affect colonization by monarch butterflies. Front. Ecol. Evol. 7: 474. [Google Scholar]
- Bartholomew, C., and Yeargan K.. . 2002. Phenology of milkweed (Asclepiadaceae) growth and monarch (Lepidoptera: Nymphalidae) reproduction in Kentucky and ovipositional preference between common and honeyvine milkweed. J. Kansas Entomol. Soc. 74: 211–220. [Google Scholar]
- Baum, K. A., and Mueller E. K.. . 2015. Grassland and roadside management practices affect milkweed abundance and opportunities for monarch recruitment. Monarchs in a changing world: Biology and conservation of an iconic butterfly, pp. 197–202. [Google Scholar]
- Bergström, G., Rothschild M., Groth I., and Crighton C.. . 1994. Oviposition by butterflies on young leaves: investigation of leaf volatiles. Chemoecology 5: 147–158. [Google Scholar]
- Bradley, C. A., and Altizer S.. . 2005. Parasites hinder monarch butterfly flight: Implications for disease spread in migratory hosts. Ecol. Lett. 8: 290–300. [Google Scholar]
- Brewer, J., and Winter D.. . 1977. Short-lived phenomena. News of the Lepidopterists’ Society 1977: 7. [Google Scholar]
- Brower, L. P. 1977. Monarch migration. Nat. Hist. 84: 40–53. [Google Scholar]
- Brower, L. P., and Glazier S. C.. . 1975. Localization of heart poisons in the monarch butterfly. Science 188: 19–25. [DOI] [PubMed] [Google Scholar]
- Brower, L. P., Taylor O. R., Williams E. H., Slayback D. A., Zubieta R. R., and Ramirez M. I.. . 2012. Decline of monarch butterflies overwintering in Mexico: is the migratory phenomenon at risk?. Insect Conserv. Div. 5: 95–100. [Google Scholar]
- Calvert, W. H. 1999. Patterns in the spatial and temporal use of Texas milkweeds(Asclepiadaceae) by the monarch butterfly(Danaus plexippus L.) during fall, 1996. J. Lepidop. Soc. 53: 37–44. [Google Scholar]
- Cohen, J. A., and Brower L. P.. . 1982. Oviposition and larval success of wild monarch butterflies (Lepidoptera: Danaidae) in relation to host plant size and cardenolide concentration. J. Kansas Entomol. Soc. 343–348. [Google Scholar]
- Davis, A. K. 2012. Are migratory monarchs really declining in eastern North America? Examining evidence from two fall census programs. Insect Conserv. Divers. 5: 101–105. [Google Scholar]
- Davis, A. 2020. A review of published and unpublished findings from 20 long-term monitoring studies of eastern monarch butterflies: the population was never in danger, despite recent winter colony declines. Preprint.
- Davis, A. K. and Dyer L. A.. . 2015. Long-term trends in eastern North American monarch butterflies: a collection of studies focusing on spring, summer, and fall dynamics. Ann. Entomol. Soc. Am. 108: 661–663. [Google Scholar]
- Davis, A. K. and de Roode J. C.. . 2018. Effects of the parasite, Ophryocystis elektroscirrha, on wing characteristics important for migration in the monarch butterfly. Anim. Migr. 5: 84–93. [Google Scholar]
- Davis, A. K., Farrey B. D., and Altizer S.. . 2005. Variation in thermally induced melanism in monarch butterflies (Lepidoptera: Nymphalidae) from three North American populations. J. Thermal Biol. 30: 410–421. [Google Scholar]
- Davis, A. K., Smith F. M., and Ballew A. M.. . 2020. A poor substitute for the real thing: captive-reared monarch butterflies are weaker, paler and have less elongated wings than wild migrants. Biol. Lett. 16: 20190922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decker, L. E., Soule A. J., de Roode J. C., and Hunter M. D.. . 2019. Phytochemical changes in milkweed induced by elevated CO2 alter wing morphology but not toxin sequestration in monarch butterflies. Funct. Ecol. 33: 411–421. [Google Scholar]
- Decker, L. E., Jeffrey C. S., Ochsenrider K. M., Potts A. S., de Roode J. C., Smilanich A. M., and Hunter M. D.. . 2021. Elevated atmospheric concentrations of CO2 increase endogenous immune function in a specialist herbivore. J. Anim. Ecol. 90: 628–640. [DOI] [PubMed] [Google Scholar]
- DiTommaso, A., and Losey J. E.. . 2003. Oviposition preference and larval performance of monarch butterflies (Danaus plexippus) on two invasive swallow-wort species. Entomol. Exp. Appl. 108: 205–209. [Google Scholar]
- Dussourd, D. E. 1990. The vein drain; or how insects outsmart plants. Nat. Hist. 90: 44–49. [Google Scholar]
- Dussourd, D. E., and Denno R. F.. . 1991. Deactivation of plant defense: correspondence between insect behavior and secretory canal architecture. Ecology 72: 1383–1396. [Google Scholar]
- Duffey, S. S., and Scudder G. G. E.. 1972. Cardiac glycosides in North American Asclepiadaceae, a basis for unpalatability in brightly coloured Hemiptera and Coleoptera. J. Insect Physiol. 18: 63–78. [Google Scholar]
- Eanes, W. F., and Koehn R. K.. . 1978. An analysis of genetic structure in the monarch butterfly, Danaus plexippus L. Evolution 32: 784–797. [DOI] [PubMed] [Google Scholar]
- Eilers, L. J., and Roosa D. M.. . 1994. The vascular plants of Iowa: an annotated checklist and natural history. University of Iowa Press. [Google Scholar]
- Erickson, J. M. 1973. The utilization of various Asclepias species by larvae of the monarch butterfly, Danaus plexippus. Psyche 80: 230–244. [Google Scholar]
- Espeset, A. E., Harrison J. G., Shapiro A. M., Nice C. C., Thorne J. H., Waetjen D. P., Fordyce J. A., and Forister M. L.. . 2016. Understanding a migratory species in a changing world: climatic effects and demographic declines in the western monarch revealed by four decades of intensive monitoring. Oecologia 181: 819–830. [DOI] [PubMed] [Google Scholar]
- Flockhart, D. T., Pichancourt J. B., Norris D. R., and Martin T. G.. . 2015. Unravelling the annual cycle in a migratory animal: breeding-season habitat loss drives population declines of monarch butterflies. J. Anim. Ecol. 84: 155–165. [DOI] [PubMed] [Google Scholar]
- Flockhart, D. T. T., Brower L. P., Ramirez M. I., Hobson K. A., Wassenaar L. I., Altizer S., and Norris D. R.. . 2017. Regional climate on the breeding grounds predicts variation in the natal origin of monarch butterflies overwintering in Mexico over 38 years. Glob. Chang. Biol. 23: 2565–2576. [DOI] [PubMed] [Google Scholar]
- Freedman, M. G., Dingle H., Tabuloc C. A., Chiu J. C., Yang L. H., and Zalucki M. P.. . 2018. Non-migratory monarch butterflies, Danaus plexippus (L.), retain developmental plasticity and a navigational mechanism associated with migration. Biol. J. Linn. Soc. Lond. 123: 265–278. [Google Scholar]
- Freedman, M. G., Jason C., Ramírez S. R., and Strauss S. Y.. . 2020. Host plant adaptation during contemporary range expansion in the monarch butterfly. Evolution 74: 377–391. [DOI] [PubMed] [Google Scholar]
- Freedman, M., De Roode J., Foriste M., Kronforst M., Pierce A., Schultz C., Taylor O., and Crone E.. . 2021. Are eastern and western monarch butterflies distinct populations? A review of evidence for ecological, phenotypic, and genetic differentiation and implications for conservation. Conserv. Sci. Pract. e432. [Google Scholar]
- Frick, C., and Wink M.. . 1995. Uptake and sequestration of ouabain and other cardiac glycosides in Danaus plexippus (Lepidoptera: Danaidae): Evidence for a carrier-mediated process. J. Chem. Ecol. 21: 557–575. [DOI] [PubMed] [Google Scholar]
- Geest, E. A., Wolfenbarger L. L., and McCarty J. P.. . 2019. Recruitment, survival, and parasitism of monarch butterflies (Danaus plexippus) in milkweed gardens and conservation areas. J. Insect Conserv. 23: 211–224. [Google Scholar]
- Gibbs, D., Walton R., Brower L. P., and Davis A. K.. . 2006. Monarch butterfly (Lepidoptera: Nymphalidae) migration monitoring at Chincoteague, Virginia and Cape May, New Jersey: a comparison of long-term trends. J. Kansas Entomol. Soc. 79: 156–164. [Google Scholar]
- Goehring, L., and Oberhauser K. S.. . 2002. Effects of photoperiod, temperature, and host plant age on induction of reproductive diapause and development time in Danaus plexippus. Ecol. Entomol. 27: 674–685. [Google Scholar]
- Goehring, L., and Oberhauser K. S.. . 2004. Environmental factors influencing postdiapause reproductive development in monarch butterflies, pp. 187–198. InOberhauser K. S. and Solensky M. (eds.), The monarch butterfly. Biology and conservation. Cornell University Press, Ithaca, NY. [Google Scholar]
- Grant, T. J., Flockhart D. T., Blader T. R., Hellmich R. L., Pitman G. M., Tyner S., Norris D. R., and Bradbury S. P.. . 2020. Estimating arthropod survival probability from field counts: a case study with monarch butterflies. Ecosphere 11: e03082. [Google Scholar]
- Green, D. A., 2nd, and Kronforst M. R.. 2019. Monarch butterflies use an environmentally sensitive, internal timer to control overwintering dynamics. Mol. Ecol. 28: 3642–3655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu, L., Reilly P. F., Lewis J. J., Reed R. D., Andolfatto P., and Walters J. R.. . 2019. Dichotomy of dosage compensation along the Neo Z chromosome of the monarch butterfly. Curr. Biol. 29: 4071–4077.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerra, P. A., and Reppert S. M.. . 2013. Coldness triggers northward flight in remigrant monarch butterflies. Curr. Biol. 23: 419–423. [DOI] [PubMed] [Google Scholar]
- Guerra, P. A., Merlin C., Gegear R. J., and Reppert S. M.. . 2012. Discordant timing between antennae disrupts sun compass orientation in migratory monarch butterflies. Nat. Commun. 3: 958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerra, P. A., Gegear R. J., and Reppert S. M.. . 2014. A magnetic compass aids monarch butterfly migration. Nat. Commun. 5: 4164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafsson, K. M., Agrawal A. A., Lewenstein B. V., and Wolf S. A.. . 2015. The monarch butterfly through time and space: the social construction of an icon. BioScience 65: 612–622. [Google Scholar]
- Haan, N. L., and Landis D. A.. . 2019a. Grassland disturbance increases monarch butterfly oviposition and decreases arthropod predator abundance. Biol. Conserv. 233: 185–192. [Google Scholar]
- Haan, N. L. and Landis D. A.. . 2019b. The importance of shifting disturbance regimes in monarch butterfly decline and recovery. Front. Ecol. Evol. 7: 191. [Google Scholar]
- Haila, Y., and Margules C. R.. . 1996. Survey research in conservation biology. Ecography 19: 323–331. [Google Scholar]
- Haribal, M., and Renwick J. A.. . 1996. Oviposition stimulants for the monarch butterfly: flavonol glycosides from Asclepias curassavica. Phytochemistry 41: 139–144. [DOI] [PubMed] [Google Scholar]
- Haribal, M., and Renwick J. A. A.. 1998a. Differential postalightment oviposition behavior of monarch butterflies on Asclepias species. J. Insect Behav. 11: 507–538. [Google Scholar]
- Haribal, M., and Renwick J. A. A.. 1998b. Identification and distribution of oviposition stimulants for monarch butterflies in hosts and nonhosts. J. Chem. Ecol. 24: 891–904. [Google Scholar]
- Hartzler, R. G. 2010. Reduction in common milkweed (Asclepias syriaca) occurrence in Iowa cropland from 1999 to 2009. Crop Prot. 29: 1542–1544. [Google Scholar]
- Heinze, S., and Reppert S. M.. . 2011. Sun compass integration of skylight cues in migratory monarch butterflies. Neuron 69: 345–358. [DOI] [PubMed] [Google Scholar]
- Heinze, S., and Reppert S. M.. . 2012. Anatomical basis of sun compass navigation I: the general layout of the monarch butterfly brain. J. Comp. Neurol. 520: 1599–1628. [DOI] [PubMed] [Google Scholar]
- Heinze, S., Florman J., Asokaraj S., El Jundi B., and Reppert S. M.. . 2013. Anatomical basis of sun compass navigation II: the neuronal composition of the central complex of the monarch butterfly. J. Comp. Neurol. 521: 267–298. [DOI] [PubMed] [Google Scholar]
- Herman, W. S. 1981. Studies on the adult reproductive diapause of the monarch butterfly, Danaus plexippus. Biol. Bull. 160: 89–106. [Google Scholar]
- Hobson, K. A., Wassenaar L. I., and Taylor O. R.. . 1999. Stable isotopes (δD and δ13C) are geographic indicators of natal origins of monarch butterflies in eastern North America. Oecologia 120: 397–404. [DOI] [PubMed] [Google Scholar]
- Howard, E., and Davis A. K.. . 2009. The fall migration flyways of monarch butterflies in eastern North America revealed by citizen scientists. J. Insect Conserv. 13: 279–286. [Google Scholar]
- Iiams, S. E., Lugena A. B., Zhang Y., Hayden A. N., and Merlin C.. . 2019. Photoperiodic and clock regulation of the vitamin A pathway in the brain mediates seasonal responsiveness in the monarch butterfly. Proc. Natl. Acad. Sci. USA 116: 25214–25221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inamine, H., Ellner S. P., Springer J. P., and Agrawal A. A.. . 2016. Linking the continental migratory cycle of the monarch butterfly to understand its population decline. Oikos 125: 1081–1091. [Google Scholar]
- Jones, P. L., and Agrawal A. A.. . 2016. Consequences of toxic secondary compounds in nectar for mutualist bees and antagonist butterflies. Ecology 97: 2570–2579. [DOI] [PubMed] [Google Scholar]
- Jones, P. L., and Agrawal A. A.. . 2019. Beyond preference and performance: host plant selection by monarch butterflies, Danaus plexippus. Oikos 128: 1092–1102. [Google Scholar]
- Kaul, R. B., Rolfsmeier S. B., and Esch J. J.. . 1991. The distribution and reproductive phenology of the milkweeds (Asclepiadaceae: Asclepias and Cynanchum) in Nebraska.
- Kinkead, K. E., Harms T. M., Dinsmore S. J., Frese P. W., and Murphy K. T.. . 2019. Design implications for surveys to monitor monarch butterfly population trends. Front. Ecol. Evol. 7: 195. [Google Scholar]
- Knight, S. M., Norris D. R., Derbyshire R., and Flockhart T. D.. . 2019. Strategic mowing of roadside milkweeds increases monarch butterfly oviposition. Glob. Ecol. Conserv. 19: e00678. [Google Scholar]
- Ladner, D. T., and Altizer S.. . 2005. Oviposition preference and larval performance of North American monarch butterflies on four Asclepias species. Entomol. Exp. Appl. 116: 9–20. [Google Scholar]
- Li, Y., Pierce A. A., and de Roode J. C.. . 2016. Variation in forewing size linked to migratory status in monarch butterflies. Anim. Migr. 1: 27–34. [Google Scholar]
- Lindsey, E., and Altizer, S. 2009. Sex differences in immune defenses and response to parasitism in monarch butterflies. Evol. Ecol. 23: 607–620. [Google Scholar]
- Lindsey, E., Mehta M., Dhulipala V., Oberhauser K., and Altizer S.. . 2009. Crowding and disease: effects of host density on response to infection in a butterfly–parasite interaction. Ecol. Entomol. 34: 551–561. [Google Scholar]
- Lyons, J. I., Pierce A. A., Barribeau S. M., Sternberg E. D., Mongue A. J., and De Roode J. C.. . 2012. Lack of genetic differentiation between monarch butterflies with divergent migration destinations. Mol. Ecol. 21: 3433–3444. [DOI] [PubMed] [Google Scholar]
- Majewska, A. A., and Altizer S.. . 2019. Exposure to non-native tropical milkweed promotes reproductive development in migratory monarch butterflies. Insects 10: 253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majewska, A. A., Sims S., Wenger S. J., Davis A. K., and Altizer S.. . 2018. Do characteristics of pollinator‐friendly gardens predict the diversity, abundance, and reproduction of butterflies?. Insect Conserv. Divers. 11: 370–382. [Google Scholar]
- Majewska, A. A., Sims S., Schneider A., Altizer S., and Hall R. J.. . 2019. Multiple transmission routes sustain high prevalence of a virulent parasite in a butterfly host. Proc. Biol. Sci. 286: 20191630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majewska, A. A., Davis A., de Roode J. C., and Altizer S.. . 2021. Long-term parasite trends in natural populations of monarch butterflies. J. Anim. Ecol. [Google Scholar]
- Malcolm, S. B. 1994. Milkweeds, monarch butterflies and the ecological significance of cardenolides. Chemoecology 5: 101–117. [Google Scholar]
- Malcolm, S. B. 2018. Anthropogenic impacts on mortality and population viability of the monarch butterfly. Annu. Rev. Entomol. 63: 277–302. [DOI] [PubMed] [Google Scholar]
- Malcolm, S. B., and Brower L. P.. . 1986. Selective oviposition by monarch butterflies (Danaus plexippus L.) in a mixed stand of Asclepias curassavica L. and A. incarnata L. in South Florida. J. Lepid. Soc. 40: 255–263. [Google Scholar]
- Malcolm, S. B., and Brower L. P.. . 1989. Evolutionary and ecological implications of cardenolide sequestration in the monarch butterfly. Experientia 45: 284–295. [Google Scholar]
- Malcolm, S. B., and Zalucki M. P.. . 1996. Milkweed latex and cardenolide induction may resolve the lethal plant defence paradox, pp. 193–196. InProceedings of the 9th International Symposium on Insect-Plant Relationships. Springer, Dordrecht, The Netherlands. [Google Scholar]
- Malcolm, S. B., Cockrell B. J., and Brower L. P.. . 1987. Monarch butterfly voltinism: effects of temperature constraints at different latitudes. Oikos 77–82. [Google Scholar]
- Markert, M. J., Zhang Y., Enuameh M. S., Reppert S. M., Wolfe S. A., and Merlin C.. . 2016. Genomic access to monarch migration using TALEN and CRISPR/Cas9-dediated targeted mutagenesis. G3 6: 905–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazo-Vargas, A., Concha C., Livraghi L., Massardo D., Wallbank R. W. R., Zhang L., Papador J. D., Martinez-Najera D., Jiggins C. D., Kronforst M. R., et al. 2017. Macroevolutionary shifts of WntA function potentiate butterfly wing-pattern diversity. Proc. Natl. Acad. Sci. USA 114: 10701–10706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin, R., and Myers J.. . 1970. Ophryocystis elektroscirrha sp. n., a neogregarine pathogen of the monarch butterfly Danaus plexippus (L.) and the Florida queen butterfly D. gilippus berenice Cramer. J. Eukaryot. Microbiol. 17:300–305. [Google Scholar]
- Merlin, C., Gegear R. J., and Reppert S. M.. . 2009. Antennal circadian clocks coordinate sun compass orientation in migratory monarch butterflies. Science 325: 1700–1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlin, C., Iiams S. E., and Lugena A. B.. . 2020. monarch butterfly migration moving into the genetic era. Trends Genet. 36: 689–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (MLMP) Monarch Larva Monitoring Project. 2020. (http://www.mlmp.org/). Accessed 20 March 2020.
- Monarch ESA Petition. 2014. Petition to protect the monarch butterfly (Danaus plexippus plexippus) under the endangered species act. (http://www.biologicaldiversity.org/species/invertebrates/pdfs/Monarch_ESA_Petition.pdf) (accessed 17 December 2020). [Google Scholar]
- Mongue, A. J., Tsai M. V., Wayne M. L., and de Roode J. C.. . 2016. Inbreeding depression in monarch butterflies. J. Insect Conserv. 20: 477–483. [Google Scholar]
- (NABA) North American Butterfly Association. 2020. (http://www.naba.org/butter_counts.html). Accessed 17 October 2020.
- Nail, K. R., Stenoien C., and Oberhauser K.. . 2015. Immature monarch survival: effects of site characteristics, density, and time. Ann. Entomol. Soc. Am. 108: 680–690. [Google Scholar]
- Nestle, R., Daniels J. C., and Dale A. G.. . 2020. Mixed-species gardens increase monarch oviposition without increasing top-down predation. Insects 11: 648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen, T. A. T., Beetz M. J., Merlin C., and El Jundi B.. . 2021. Sun compass neurons are tuned to migratory orientation in monarch butterflies. Proc. Biol. Sci. 288: 20202988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberhauser, K. S., Anderson M., Anderson S., Caldwell W., De Anda A., Hunter M., Kaiser M. C., and Solensky M. J.. . 2015. Lacewings, wasps, and flies—oh my: insect enemies take a bite out of monarchs, pp. 71–82. InOberhauser K., Nail K., and Altizer S. (eds.), Monarchs in a changing world: biology and conservation of an iconic butterfly. Cornell University Press, Ithaca, New York. [Google Scholar]
- Oberhauser, K., Elmquist D., Perilla-López J. M., Gebhard I., Lukens L., and Stireman J.. . 2017. Tachinid fly (Diptera: Tachinidae) parasitoids of Danaus plexippus (Lepidoptera: Nymphalidae). Ann. Entomol. Soc. Am. 110: 536–543. [Google Scholar]
- Oyeyele, S. O., and Zalucki M. P.. . 1990. Cardiac glycosides and oviposition by Danaus plexippus on Asclepias fruticosa in south-east Queensland (Australia), with notes on the effect of plant nitrogen-content. Ecol. Entomol. 15: 177–185. [Google Scholar]
- Pelling, A. E., Wilkinson P. R., Stringer R., and Gimzewski J. K.. . 2009. Dynamic mechanical oscillations during metamorphosis of the monarch butterfly. J. Royal Soc. Inter. 6: 29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelton, E. M. 2018. Keep monarchs wild! Why captive rearing isn’t the way to help monarchs. Xerxes Society for Invertebrate Conservation. (https://xerces.org/ 2018/09/11/keep-monarchs-wild/). [Google Scholar]
- Pelton, E. M., Schultz C. B., Jepsen S. J., Black S. H., and Crone E. E.. . 2019. Western monarch population plummets: status, probable causes, and recommended conservation actions. Front. Ecol. Evol. 7: 258. [Google Scholar]
- Pierce, A. A., Zalucki M. P., Bangura M., Udawatta M., Kronforst M. R., Altizer S., Haeger J. F., and de Roode J. C.. . 2014. Serial founder effects and genetic differentiation during worldwide range expansion of monarch butterflies. Proc. Royal Soc. B. Biol. Sci. 281(1797): 20142230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce, A. A., Altizer S., Chamerlain N. L., Kronforst M. R., & de Roode J. C.. . 2015. Unraveling the mysteries of monarch migration and global dispersal through molecular genetic techniques, pp. 257–267. InOberhauser K. S., Nail K. R., and Altizer S. (eds.), Monarchs in a changing world: biology and conservation of an iconic butterfly. Cornell University Press, Ithaca, NY. [Google Scholar]
- Pleasants, J. 2017. Milkweed restoration in the Midwest for monarch butterfly recovery estimates of milkweeds lost, milkweeds remaining and milkweeds that must be added to increase the monarch population. Insect Conserv. Divers. 10: 42–53. [Google Scholar]
- Pleasants, J. M., and Oberhauser K. S.. . 2013. Milkweed loss in agricultural fields because of herbicide use: effect on the monarch butterfly population. Insect Conserv. Divers. 6: 135–144. [Google Scholar]
- Pleasants, J. M., Zalucki M. P., Oberhauser K. S., Brower L. P., Taylor O. R., and Thogmartin W. E.. . 2017. Interpreting surveys to estimate the size of the monarch butterfly population: Pitfalls and prospects. PLoS One 12: e0181245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pocius, V. M., Debinski D. M., Bidne K. G., Hellmich R. L., and Hunter F. K.. . 2017a. Performance of early instar monarch butterflies (Danaus plexippus L.) on nine milkweed species native to Iowa. J. Lepid. Soc. 71: 153–161. [Google Scholar]
- Pocius, V. M., Debinski D. M., Pleasants J. M., Bidne K. G., Hellmich R. L., and Brower L. P.. . 2017b. Milkweed matters: monarch butterfly (Lepidoptera: Nymphalidae) survival and development on Nine Midwestern Milkweed Species. Environ. Entomol. 46: 1098–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pocius, V. M., Debinski D. M., Pleasants J. M., Bidne K. G., and Hellmich R. L.. . 2018a. Monarch butterflies do not place all of their eggs in one basket: oviposition on nine Midwestern milkweed species. Ecosphere 9: e02064. [Google Scholar]
- Pocius, V. M., Pleasants J. M., Debinski D. M., Bidne K. G., Hellmich R. L., Bradbury S. P., and Blodgett S. L.. . 2018b. Monarch butterflies show differential utilization of nine Midwestern milkweed species. Front. Ecol. Evol. 6: 169. [Google Scholar]
- Pocius, V. M., Matulis N. L., Ankoma-Darko O., Curran D. R., Italia J. C., Darbaidze M., Cibotti S., Ray S., McCartney N. B., Schilder R. J., . et al. 2021. Defenses of milkweed host plants impose variable energetic demands on monarch butterfly flight. Commun. Biol. Under Review. [Google Scholar]
- Prince, D. J., O’Rourke S. M., Thompson T. Q., Ali O. A., Lyman H. S., Saglam I. K., Hotaling T. J., Spidle A. P., and Miller M. R.. . 2017. The evolutionary basis of premature migration in Pacific salmon highlights the utility of genomics for informing conservation. Sci. Adv. 3: e1603198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranz, J. M., González P. M., Clifton B. D., and Nazario N. O.. . 2020. A de novo genome assembly, gene annotation, and expression atlas for the monarch butterfly Danaus plexippus. bioRxiv. Preprint. [Google Scholar]
- Rasmann, S., and Agrawal A. A.. . 2011. Latitudinal patterns in plant defense: evolution of cardenolides, their toxicity and induction following herbivory. Ecol. Lett. 14: 476–483. [DOI] [PubMed] [Google Scholar]
- Reichstein, T., von Euw J., Parsons J. A., and Rothschild M.. . 1968. Heart poisons in the monarch butterfly. Some aposematic butterflies obtain protection from cardenolides present in their food plants. Science 161: 861–866. [DOI] [PubMed] [Google Scholar]
- Reppert, S. M., and de Roode J. C.. . 2018. Demystifying monarch butterfly migration. Curr. Biol. 28: R1009–R1022. [DOI] [PubMed] [Google Scholar]
- Reppert, S. M., Gegear R. J., and Merlin C.. . 2010. Navigational mechanisms of migrating monarch butterflies. Trends Neurosci. 33: 399–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson, G. F., Zalucki M. P., and Paine T. D.. . 2015. Larval host choice of the monarch butterfly (Danaus plexippus L.) on four native California desert milkweed species. J. Insect Behav. 28: 582–592. [Google Scholar]
- Roeske, C., Seiber J., Brower L., Moffitt C.. . 1976. Milkweed cardenolides and their comparative processing by monarch butterflies (Danaus plexippus L.), pp. 93–167. InWallace J. W., Mansell R. L. (eds.), Biochemical interaction between plants and insects. Springer, Boston, MA. [Google Scholar]
- de Roode, J. C., and Altizer S.. . 2010. Host-parasite genetic interactions and virulence-transmission relationships in natural populations of monarch butterflies. Evolution 64: 502–514. [DOI] [PubMed] [Google Scholar]
- de Roode, J. C., Gold L. R., and Altizer S.. . 2007. Virulence determinants in a natural butterfly-parasite system. Parasitology 134: 657–668. [DOI] [PubMed] [Google Scholar]
- de Roode, J. C., Yates A. J., and Altizer S.. . 2008. Virulence-transmission trade-offs and population divergence in virulence in a naturally occurring butterfly parasite. Proc. Natl. Acad. Sci. USA 105: 7489–7494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Roode, J. C., Chi J., Rarick R. M., and Altizer S.. . 2009. Strength in numbers: high parasite burdens increase transmission of a protozoan parasite of monarch butterflies (Danaus plexippus). Oecologia 161: 67–75. [DOI] [PubMed] [Google Scholar]
- Satterfield, D. A., Maerz J. C., and Altizer S.. . 2015. Loss of migratory behaviour increases infection risk for a butterfly host. Proc. Biol. Sci. 282: 20141734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satterfield, D. A., Villablanca F. X., Maerz J. C., and Altizer S.. . 2016. Migratory monarchs wintering in California experience low infection risk compared to monarchs breeding year-round on non-native milkweed. Integr. Comp. Biol. 56: 343–352. [DOI] [PubMed] [Google Scholar]
- Satterfield, D. A., Altizer S., Williams M. K., and Hall R. J.. . 2017. Environmental persistence influences infection dynamics for a butterfly pathogen. PLoS One 12: e0169982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder, H., Majewska A., and Altizer S.. . 2020. Monarch butterflies reared under autumn-like conditions have more efficient flight and lower post-flight metabolism. Ecol. Entomol. 45: 562–572. [Google Scholar]
- Schultz, C. B., Brown L. M., Pelton E., and Crone E. E.. . 2017. Citizen science monitoring demonstrates dramatic declines of monarch butterflies in western North America. Biol. Conserv. 214: 343–346. [Google Scholar]
- Scriber, J. M., and Slansky F.. . 1981. The nutritional ecology of immature insects. Ann. Rev. Entomol. 26: 183–211. [Google Scholar]
- Seiber, J. N., Tuskes P. M., Brower L. P., and Nelson C. J.. . 1980. Pharmacodynamics of some individual milkweed cardenolides fed to larvae of the monarch butterfly (Danaus plexippus L.). J. Chem. Ecol. 6: 321–339. [Google Scholar]
- Seiber, J. N., Lee S. M., and Benson J.. . 1983. Cardiac glycosides (cardenolides) in species of Asclepias (Asclepiadaceae), pp. 43–83. InKeeler R. F., Tu A. T. (eds.), Handbook of natural toxins. Marcel Dekker,New York, NY. [Google Scholar]
- Semmens, B. X., Semmens D. J., Thogmartin W. E., Wiederholt R., López-Hoffman L., Diffendorfer J. E., Pleasants J. M., Oberhauser K. S., and Taylor O. R.. . 2016. Quasi-extinction risk and population targets for the Eastern, migratory population of monarch butterflies (Danaus plexippus). Sci. Rep. 6: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shephard, J. M., Hughes J. M., and Zalucki M. P.. . 2002. Genetic differentiation between Australian and North American populations of the monarch butterfly Danaus plexippus (L.) (Lepidoptera: Nymphalidae): an exploration using allozyme electrophoresis. Biol. J. Linn. Soc. Lond. 75: 437–452. [Google Scholar]
- Shlizerman, E., Phillips-Portillo J., Forger D. B., and Reppert S. M.. . 2016. Neural integration underlying a time-compensated sun compass in the migratory monarch butterfly. Cell Rep. 15: 683–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slansky, F. 1993. Nutritional ecology: the fundamental quest for nutrients, pp. 29–91. InStamp N. E. and Casey T. M. (ed.), Caterpillars: ecological and evolutionary constraints on foraging. Chapman and Hall, New York, NY. [Google Scholar]
- Solensky, M. J., and Oberhauser K. S.. . 2009. Sperm precedence in monarch butterflies (Danaus plexippus). Behav. Ecol. 20: 328–334. [Google Scholar]
- Soule, A. J., Decker L. E., and Hunter M. D.. . 2020. Effects of diet and temperature on monarch butterfly wing morphology and flight ability. J. Insect Conserv. 24: 961–975. [Google Scholar]
- Stenoien, C., Nail K. R., Zalucki J. M., Parry H., Oberhauser K. S., and Zalucki M. P.. . 2018. Monarchs in decline: a collateral landscape-level effect of modern agriculture. Insect Sci. 25: 528–541. [DOI] [PubMed] [Google Scholar]
- Sternberg, E. D., Li H., Wang R., Gowler C., and de Roode J. C.. . 2013. Patterns of host-parasite adaptation in three populations of monarch butterflies infected with a naturally occurring protozoan disease: virulence, resistance, and tolerance. Am. Nat. 182: E235–E248. [DOI] [PubMed] [Google Scholar]
- Swengel, A. B. 1995. Population fluctuations of the monarch (Danaus plexippus) in the 4th of July butterfly count 1977–1994. American Midland Naturalist, University of Notre Dame, South Bend, Indiana, pp. 205–214. [Google Scholar]
- Talla, V., Pierce A. A., Adams K. L., de Man T. J. B., Nallu S., Villablanca F. X., Kronforst M. R., and de Roode J. C.. . 2020. Genomic evidence for gene flow between monarchs with divergent migratory phenotypes and flight performance. Mol. Ecol. 29: 2567–2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor, O. R., Pleasants J. M., Grundel R., Pecoraro S. D., J. P.Lovett, Jr., and Ryan A.. . 2020. Evaluating the migration mortality hypothesis using monarch tagging data. Front. Ecol. Evol. 8: 264. [Google Scholar]
- Teixeira, J. C., and Huber C. D.. . 2021. The inflated significance of neutral genetic diversity in conservation genetics. Proc. Natl. Acad. Sci. 118: e2015096118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenger-Trolander, A., and Kronforst M. R.. . 2020. Migration behaviour of commercial monarchs reared outdoors and wild-derived monarchs reared indoors. Proc. Biol. Sci. 287: 20201326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenger-Trolander, A., Lu W., Noyes M., and Kronforst M. R.. . 2019. Contemporary loss of migration in monarch butterflies. Proc. Nat. Acad. Sci. 116: 14671–14676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thogmartin, W. E., López-Hoffman L., Rohweder J., Diffendorfer J., Drum R., Semmens D., Black S., Caldwell I., Cotter D., Drobney P., and Jackson L. L.. . 2017a. Restoring monarch butterfly habitat in the Midwestern US: ‘all hands on deck’. Environ. Res. Lett. 12: 074005. [Google Scholar]
- Thogmartin, W. E., Wiederholt R., Oberhauser K., Drum R. G., Diffendorfer J. E., Altizer S., Taylor O. R., Pleasants J., Semmens D., Semmens B., . et al. 2017b. Monarch butterfly population decline in North America: identifying the threatening processes. R. Soc. Open Sci. 4: 170760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urquhart, F. A. 1987. The monarch butterfly: international traveler. Nelson-Hall, Chicago, IL. [Google Scholar]
- Urquhart, F. A., and Urquhart N. R.. . 1976. The overwintering site of the eastern population of the monarch butterfly (Danaus p. plexippus; Danaidae) in southern Mexico. J. Lepid. Soc. 30: 153–158. [Google Scholar]
- Urquhart, F. A., and Urquhart N. R.. . 1978. Autumnal migration routes of the eastern population of the monarch butterfly (Danaus p. plexippus L.; Danaidae; Lepidoptera) in North America to the overwintering site in the Neovolcanic Plateau of Mexico. Can. J. Zool. 56: 1759–1764. [Google Scholar]
- USDA, NRCS. 2021. The PLANTS database. National Plant Data Team, Greensboro, NC. (http://plants.usda.gov). Accessed 17 May 2021. [Google Scholar]
- Vidal, O., and Rendón-Salinas E.. . 2014. Dynamics and trends of overwintering colonies of the monarch butterfly in Mexico. Biol. Conserv. 180: 165–175. [Google Scholar]
- Walton, R. K., Brower L. P., and Davis A. K.. . 2005. Long-term monitoring and fall migration patterns of the Monarch butterfly in Cape May, New Jersey. Ann. Entomol. Soc. Am. 98: 682–689. [Google Scholar]
- Waples, R. S., Teel D. J., Myers J. M., and Marshall A. R.. . 2004. Life-history divergence in Chinook salmon: historic contingency and parallel evolution. Evolution 58: 386–403. [PubMed] [Google Scholar]
- Wassenaar, L. I., and Hobson A.. . 1998. Natal origins of migratory monarch butterflies at wintering colonies in Mexico: new isotopic evidence. Proc. Natl. Acad. Sci. USA 95: 15436–15439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox, A. A. E., Newman A. E. M., Raine N. E., Mitchell G. W., and Norris D. R.. . 2021. Captive-reared migratory monarch butterflies show natural orientation when released in the wild. Conserv. Physiol. 9: coab032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, M., Hsiao R., Carichner G., Ernst K., Lim J., Green D. A. II, Lee I., Blaauw D., and Kim H. S.. . 2019. Migrating monarch butterfly localization using multi-sensor fusion neural networks. arXiv preprint. arXiv: 1912.06907. [Google Scholar]
- Yeargan, K. V., and Allard C. M.. . 2005. Comparison of common milkweed and honeyvine milkweed (Asclepiadaceae) as host plants for monarch larvae (Lepidoptera: Nymphalidae). J. Kansas Entomol. Soc. 78: 247–251. [Google Scholar]
- York, H. A. and Oberhauser K. S.. . 2002. Effects of duration and timing of heat stress on monarch butterfly (Danaus plexippus) (Lepidoptera: Nymphalidae) development. J. Kansas Entomol. Soc. 75: 290–298. [Google Scholar]
- Zalucki, M. P., and Brower L. P.. . 1992. Survival of first instar larvae of Danaus plexippus (Lepidoptera: Danainae) in relation to cardiac glycoside and latex content of Asclepias humistrata (Asclepiadaceae). Chemoecology 3: 81–93. [Google Scholar]
- Zalucki, M. P., and Malcolm S. B.. . 1999. Plant latex and first-instar monarch larval growth and survival on three North American milkweed species. J. Chem. Ecol. 25: 1827–1842. [Google Scholar]
- Zalucki, M. P., Brower L. P., and Malcolm S. B.. . 1990. Oviposition by Danaus plexippus in relation to cardenolide content of three Asclepias species in the southeastern USA. Ecol. Entomol. 15: 231–240. [Google Scholar]
- Zalucki, M. P., Brower L. P., and Alonso A.. . 2001a. Detrimental effects of latex and cardiac glycosides on survival and growth of first-instar monarch butterfly larvae Danaus plexippus feeding on the sandhill milkweed Asclepias humistrata. Ecol. Entomol. 26: 212–224. [Google Scholar]
- Zalucki, M. P., Malcolm S. B., Paine T. D., Hanlon C. C., Brower L. P., and Clarke A. R.. . 2001b. It’s the first bites that count: survival of first-instar monarchs on milkweeds. Austral Ecol. 26: 547–555. [Google Scholar]
- Zalucki, M. P., Malcolm S. B., Hanlon C. C., and Paine T. D.. . 2012. First-instar monarch larval growth and survival on milkweeds in southern California: effects of latex, leaf hairs and cardenolides. Chemoecology 22: 75–88. [Google Scholar]
- Zaya, D. N., Pearse I. S., and Spyreas G.. . 2017. Long-term trends in Midwestern milkweed abundances and their relevance to monarch butterfly declines. BioScience 67: 343–356. [Google Scholar]
- Zhan, S., and Reppert S. M.. . 2013. MonarchBase: the monarch butterfly genome database. Nucleic Acids Res. 41: D758–D763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan, S., Merlin C., Boore J. L., and Reppert S. M.. . 2011. The monarch butterfly genome yields insights into long-distance migration. Cell 147: 1171–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan, S., Zhang W., Niitepõld K., Hsu J., Haeger J. F., Zalucki M. P., Altizer S., de Roode J. C., Reppert S. M., and Kronforst M. R.. . 2014. The genetics of monarch butterfly migration and warning colouration. Nature 514: 317–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu, H., Gegear R. J., Casselman A., Kanginakudru S., and Reppert S. M.. . 2009. Defining behavioral and molecular differences between summer and migratory monarch butterflies. BMC Biol. 7: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.