Abstract
BACKGROUND
Within randomized clinical trials (RCTs), coiling of the ruptured aneurysm to prevent rebleeding results in better outcomes than clipping in patients with aneurysmal subarachnoid hemorrhage (aSAH).
OBJECTIVE
To study the association of coiling and clipping with outcome after aSAH in daily clinical practice.
METHODS
In this controlled, nonrandomized study, we compared outcomes after endovascular coiling and neurosurgical clipping of ruptured intracranial aneurysms in an administrative dataset of 7658 aSAH patients (22 tertiary care hospitals from Europe, USA, Australia; 2007-2013). Because the results contradicted those of the randomized trials, findings were further explored in a large clinical dataset from 2 European centers (2006-2016) of 1501 patients.
RESULTS
In the administrative dataset, the crude 14-d case-fatality rate was 6.4% (95% confidence interval [CI] 5.6%-7.2%) after clipping and 8.2% (95% CI 7.4%-9.1%) after coiling. After adjustment for age, sex, and comorbidity/severity, the odds ratio (OR) for 14-d case-fatality after coiling compared to clipping was 1.32 (95% CI 1.10-1.58). In the clinical dataset crude 14-d case fatality rate was 5.7% (95% CI 4.2%-7.8%) for clipping and 9.0% (95% CI 7.3%-11.2%) for coiling. In multivariable logistic regression analysis, the OR for 14-d case-fatality after coiling compared to clipping was 1.7 (95% CI 1.1–2.7), for 90-d case-fatality 1.28 (95% CI 0.91–1.82) and for 90-d poor functional outcome 0.78 (95% CI 0.6–1.01).
CONCLUSION
In clinical practice, coiling after aSAH is associated with higher 14-d case-fatality than clipping and nonsuperior outcomes at 90 d. Both options need to be considered in aSAH patients. Further studies should address the reasons for the discrepancy between current data and those from the RCTs.
Keywords: Aneurysm, Intracranial aneurysm, Subarachnoid hemorrhage, Outcomes research, Epidemiology, Endovascular, Clipping
ABBREVIATIONS
- aSAH
aneurysmal subarachnoid hemorrhage
- CI
confidence interval
- OR
odds ratio
- RCTs
randomized clinical trials
- WFNS
World Federation of Neurological Surgeons
Systematic reviews of randomized clinical trials (RCTs) showed that coiling of the ruptured aneurysm leads to better outcome than clipping in patients with aneurysmal subarachnoid hemorrhage (aSAH). 1,2 Since these trials, coiling has progressively replaced clipping in many centers, 3–5 and is even considered the first line treatment in some centers or countries. 6,7 However, although the internal validity of the trials is good, several factors question the external validity. First, the majority of the patients treated during the study period in the participating centers were not included in the trial. 8 Second, as a result of the trials the numbers of hospitals and physicians performing coiling have increased worldwide, 9–12 which may have led to many new physicians with less experience performing the procedure in hospital settings with less experience than physicians and hospitals that participated within the trials. 13 Third, over the last decade new devices assisting coiling such as balloons or stents came on the market. Use of these devices in coiling procedures is associated with a higher risk of treatment complications than regular coiling. 3,14
These circumstances raise questions about whether outcomes after treatment of aSAH in daily clinical practice, removed from the restrictive nature of clinical trials, differ from the results reported in these clinical trials. To test our hypothesis that also in daily clinical practice coiling is associated with better outcomes than clipping, we studied outcomes first in a large administrative dataset of 3,4,15 an unselected series of patients with aSAH admitted to tertiary care facilities worldwide where both options are available, and thereafter in a large clinical dataset combining data from 2 centers in Europe.
METHODS
Study Design and Patients
Administrative Dataset
For this nonrandomized comparative study, we used the Dr Foster Global Comparators International dataset that consists of anonymized in-hospital administrative medical records data, provided individually by member hospitals from 3 continents, designed for research and quality improvement. The process of reconciling the differing diagnostic coding systems across countries and integration of the medical data into a uniform dataset has been described in a prior report. 16
For this study, we could use data from 10 hospitals in Europe (1 in Belgium, 5 in the United Kingdom, 3 in the Netherlands, and 1 in Norway), 8 in the USA, and 4 in Australia. All hospitals are large, tertiary care hospitals. From these hospitals, we retrieved all patients with aSAH (ICD 9 codes 430 and ICD 10 codes I60.0-9), discharged between January 1, 2007 and December 31, 2013. We extracted data on age, sex, type of aneurysm occlusion (clipping or coiling), comorbidity index, length of stay, and in-hospital case-fatality. Patients treated with flow-diverting stents or WEB-devices were excluded, because these interventions were not consistently coded in the participating hospitals
The results from the analyses of the administrative dataset were opposite to those of the RCTs. Although we adjusted for several potential confounders in this administrative dataset, we had insufficient data to adjust for the clinical condition on admission. Since clinical condition on admission is a pivotal determinant for outcome after aSAH, we decided to test our findings in a clinical dataset that allowed for adjustment for clinical condition on admission, and also for the size and location of the ruptured aneurysm.
Clinical Dataset
We combined the databases with prospective data collection from Kuopio University Hospital (KUH) in Kuopio, Finland, and University Medical Center Utrecht (UMCU) in Utrecht, the Netherlands. UMCU provided cases for both (administrative and clinical) datasets. We retrieved all consecutive aSAH patients admitted between January 1, 2006 and December 31, 2015 in whom the aneurysm had been occluded by means of neurosurgical clipping or endovascular coiling. Thus, patients without aneurysm treatment were excluded from the analyses in both datasets. Patients treated with flow-diverting stents or WEB-devices were excluded, because these treatments had not been included in the administrative dataset. Treatment method was selected by multidisciplinary discussion on case-to-case basis in both hospitals. We retrieved data on age, sex, type of aneurysm occlusion (simple coiling, balloon assisted coiling, stent-assisted coiling), clinical condition on admission, location of the ruptured aneurysm, size of the ruptured aneurysm, case-fatality (14 and 90 d), and functional outcome at 90 d. Outcomes were assessed by research nurses or nurse practitioners who were unaware of the research question, because the research question had not been raised at time of outcome assessments.
Statistical Analysis
Administrative Dataset
The primary outcome measurement was case-fatality at 14 d. Since the administrative dataset has no information on clinical condition on admission we identified a list of conditions relevant for comorbidity or being proxy for disease severity for risk adjustment (Table 1).
TABLE 1.
Baseline Characteristics of the 7658 aSAH Patients in the Administrative Dataset Treated by Means of Clipping (n = 3510) or Coiling (n = 4148)
| Australia | Europe | US | ||||
|---|---|---|---|---|---|---|
| Number of hospitals | 4 | 10 | 8 | |||
| Number of patients | 863 | 3979 | 2816 | |||
| Clipping | Coiling | Clipping | Coiling | Clipping | Coiling | |
| n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | |
| 562 (65) | 301 (35) | 1551 (39) | 2428 (61) | 1397 (50) | 1419 (50) | |
| Age (yr) | ||||||
| 0-39 | 14% | 11% | 10% | 12% | 13% | 12% |
| 40-49 | 22% | 25% | 23% | 24% | 25% | 21% |
| 50-59 | 32% | 27% | 30% | 29% | 29% | 30% |
| 60-69 | 18% | 19% | 25% | 21% | 20% | 18% |
| 70+ | 14% | 18% | 11% | 14% | 13% | 19% |
| Female | 63% | 72% | 67% | 67% | 70% | 66% |
| Average number of severity or comorbidity conditions* | 2.0 | 1.7 | 0.5 | 0.6 | 2.2 | 2.3 |
| Length of stay (median days) | 17 | 15 | 18 | 15 | 17 | 15 |
| 14 d case fatality (95% CI) | 12% [10%-15%] | 9% [6%-13%] | 4% [3%-5%] | 7% [6%-8%] | 7% [5%-8%] | 10% [8%-11%] |
*SEVERITY: Cardiac arrhythmias, Fluid and electrolyte disorders; COMORBIDITY: Valvular disease, Rheumatoid arthritis collagen vascular disease, Peptic ulcer disease excluding bleeding, Peripheral vascular disorders, Chronic pulmonary disease, Solid tumour without metastasis, Diabetes complicated, Metastatic cancer, AIDS HIV, Lymphoma, Obesity, Alcohol abuse, Drug abuse; SEVERITY OR COMORBIDITY: Congestive heart failure, Diabetes uncomplicated, Paralysis, Renal failure, Hypertension uncomplicated, Hypertension complicated, Coagulopathy.
Prior to analysis, assuming a 14-d case-fatality of 10% in the surgery group and of 7% in the endovascular group, and a 60% coiling rate versus 40% surgery, we estimated that around 3000 records would be needed to find a statistically significant risk reduction at 0.05. We therefore aimed for a total sample size greater than 3000.
Baseline characteristics (age, sex, co-morbidity/disease severity) and length of stay are presented per continent and treatment type (clipping vs coiling). We calculated crude case-fatality rates with corresponding 95% confidence intervals (CI) at 14 d, split by continent. We calculated odds ratios (OR) with corresponding 95% CI using multiple logistic regression with the enter method to adjust for differences in age and sex, as well as comorbidity and disease severity markers to assess differences between treatment types. We ran one model without region (Australia, Europe, USA) to determine the overall effect size for coiling vs clipping. We ran a second model including region, as well as an interaction term between region and coiling vs clipping, to determine region-specific odds ratios for coiling vs clipping. The study protocol was submitted to and approved by the Scientific and Research Committee of Dr Foster Global Comparators before data analyses were performed.
Clinical Dataset
Baseline characteristics (age, sex, aneurysm location and size, condition on admission according to the World Federation of Neurological Surgeons [WFNS], 17 and treatment type) and outcomes for the clinical dataset are reported. Primary outcome measure was case-fatality at 14 d, secondary outcome measures were case fatality and poor functional outcome (defined as a modified Rankin Scale score of 3-6 (UMCU) or Glasgow Outcome Scale score of 1-3 (KUH)) at 90 d. We calculated crude rates with 95% Cis for case-fatality and for poor functional outcome. Separate logistic regression models were used for 14-d case-fatality, 90-d case fatality, and poor functional outcome at 90 d. Age, sex, treatment modality, location of aneurysm (posterior vs carotid circulation), aneurysm size (diameter), and WFNS were used as variables. We performed subgroup analyses according to sex, age, WFNS, aneurysm size, and aneurysm location. We performed a sensitivity analysis after exclusion of patients who were treated with balloon-, or stent-assisted coiling, for which we used the same logistic regression models as in the main analysis.
Ethical Issues
The study protocol was submitted to and approved by the Scientific and Research Committee of Dr Foster Global Comparators before analyses were performed. IRB of the UMCU decided that no formal approval was needed for this study and the need for patient consent was waived.
We declare that all supporting data are available within the article.
RESULTS
Administrative Dataset
During the study period, 7658 aSAH patients had occlusion of the ruptured aneurysm by means of clipping (n = 3510) or coiling (n = 4148; Table 1). Data for the European countries are combined since there were no important differences in patient characteristics for these countries. The proportion of patients who were clipped decreased during the study period from 51% in 2007 to 38% in 2013 (Figure 1). This decline was seen in all 3 continents.
FIGURE 1.
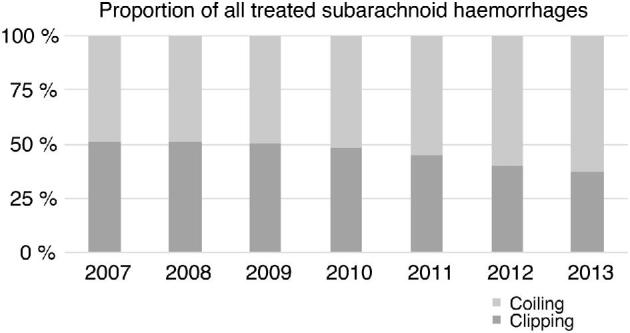
Proportions of clipping and coiling during the study period in the administrative dataset.
The overall crude case fatality rate at 14 d was 6.4% (95% CI, 5.6%-7.2%) after clipping and 8.2% (95% CI, 7.4%-9.1%) after coiling. In Australia, the crude case-fatality was higher after clipping (12.3% [95% CI, 9.6%-15.1%]) than after coiling (9.3% [95% CI, 6.3%-12.6%]), whereas in the other continents the crude case-fatality was higher after coiling than after clipping (Table 1). The high crude fatality rate after clipping in Australia was found in all hospitals (range 10%-16%), with no statistical difference between the hospitals. After adjustment for age, sex, and co-morbidity/disease severity, the OR for case-fatality within 14 d after coiling compared to clipping was 1.32 (95% CI, 1.10-1.58; Table, Supplemental Digital Content 1 ). Continent-specific ORs for case-fatality within 14 d after coiling versus clipping were 0.72 (95% CI, 0.45-1.15) for Australia, 1.77 (95% CI, 1.32-2.38) for Europe, and 1.51 (95% CI, 1.14-1.99) for the USA. The ORs adjusted for comorbidity and disease severity, age category and sex are given in Table, Supplemental Digital Content 1 .
Clinical Dataset
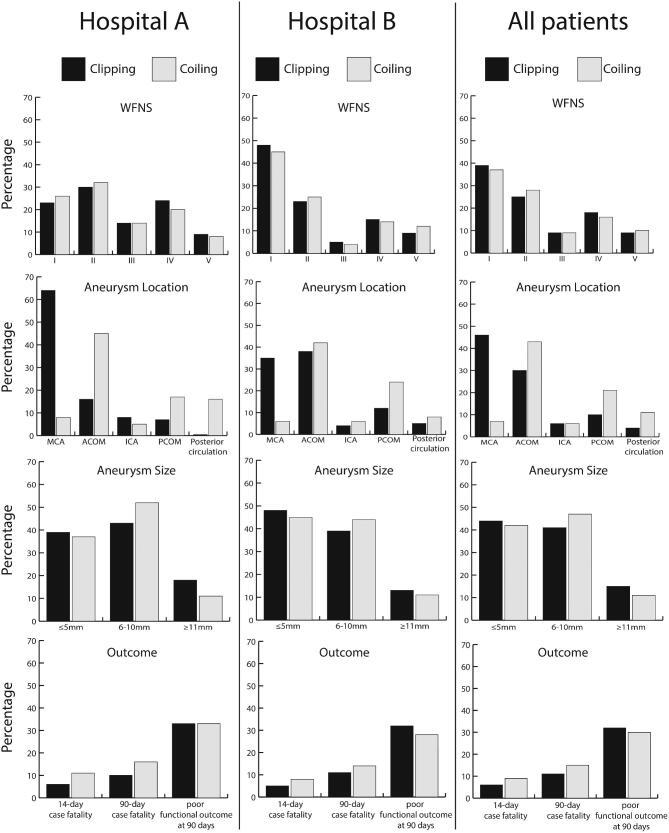
We included 1501 consecutive aSAH patients; 649 treated with clipping and 852 with coiling (Figure 2 and Table, Supplemental Digital Content 2 ). The overall crude 14-d fatality rate was 5.7% (95% CI, 4.2%-7.8%) after clipping and 9.0% (7.3%-11.2%) after coiling. The overall proportions of poor outcome at 90 d were 32.4% (95% CI, 28.8%-36.0%) for clipping and 30.2% (95% CI, 27.1%-33.4%) for coiling. After adjustment for age, sex, clinical condition on admission, location and size of the ruptured aneurysm, the OR for coiling compared to clipping was 1.7 (95% CI, 1.11-2.69) for 14-d case-fatality, 1.28 (95% CI, 0.91-1.82) for 90-d case fatality mortality and 0.78 (95% CI, 0.60-1.01) for poor outcome at 90 d (Table 2).
FIGURE 2.
Characteristics and outcome of 1501 aSAH patients in the clinical dataset. Hospital A, Kuopio University Hospital; Hospital B, University Medical Center Utrech.
TABLE 2.
Odds Ratios for 14-d Case-Fatality, 90-d Case-Fatality and Poor Outcome at 90 d (mRS 3-6 or GOS 1-3) After aSAH in the Clinical Dataset of 1501 Patients
| 14-d case-fatality | 90-d case-fatality | Poor functional outcome | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | OR | 95% CI | |
| Age | 1.002 | 0.99-1.02 | 1.03 | 1.02-1.04 | 1.04 | 1.03-1.05 |
| Female sex | 1.02 | 0.66-1.58 | 1.09 | 0.76-1.56 | 0.81 | 0.62-1.06 |
| WFNS I | Reference | Reference | Reference | |||
| WFNS II | 1.28 | 0.60-2.77 | 1.53 | 0.87-2.70 | 1.84 | 1.30-2.60 |
| WFNS III | 4.66 | 2.16-10.1 | 4.19 | 2.26-7.75 | 4.26 | 2.75-6.59 |
| WFNS IV | 6.36 | 3.35-12.1 | 6.88 | 4.18-11.30 | 8.61 | 6.02-12.3 |
| WFNS V | 12.94 | 6.68-25.5 | 13.4 | 7.83-22.9 | 15.4 | 9.80-24.1 |
| Aneurysm size | ||||||
| ≤5 mm | Reference | Reference | Reference | |||
| 6-10 mm | 1.36 | 0.87-2.13 | 1.02 | 0.72-1.47 | 1.15 | 0.87-1.51 |
| ≥11 mm | 1.16 | 0.62-2.18 | 1.17 | 0.72-1.91 | 1.72 | 1.17-2.53 |
| Anterior circulation | Reference | Reference | Reference | |||
| Posterior circulation | 0.96 | 0.51-1.78 | 1.08 | 0.65-1.78 | 1.14 | 0.74-1.69 |
| Clipping | Reference | Reference | Reference | |||
| Coiling | 1.73 | 1.11-2.69 | 1.28 | 0.91-1.82 | 0.78 | 0.60-1.01 |
CI = confidence interval, OR = odds ratio, WFNS = World Federation of Neurosurgical Societies subarachnoid hemorrhage grading scale.
Subgroup and Sensitivity Analyses
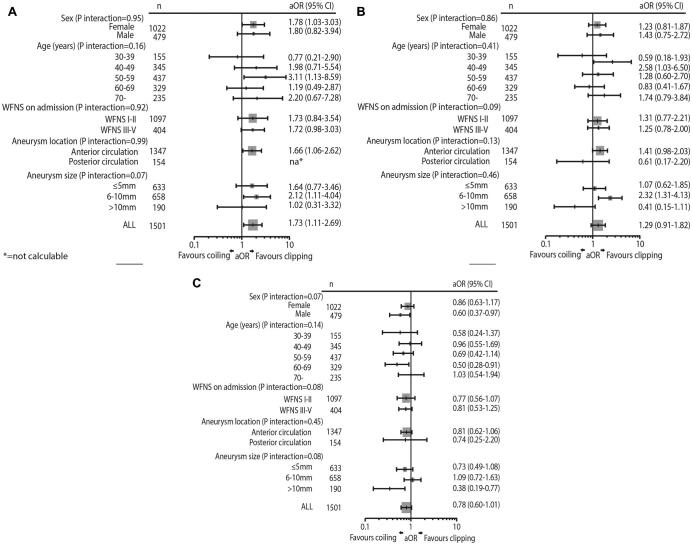
ORs of 14-d case-fatality for subgroups from the multilevel logistic regression model are shown in Figure 3. In a subgroup analysis for aneurysms ≤10 mm, ORs associated with coiling were 1.88 (95% CI, 1.16-3.05) for 14-d case-fatality, 1.57 (95% CI, 1.07-2.32) for 90-d case-fatality and 0.86 (95% CI, 0.65-1.14) for poor functional outcome. For aneurysms ≥11 mm ORs were 1.75 (95% CI, 1.13-2.73) for 14-d case-fatality, 0.42 (95% CI, 0.15-1.13) for 90-d case-fatality and 0.41 (95% CI, 0.19-0.88) for poor functional outcome.
FIGURE 3.
Forest plots showing adjusted odds ratios of A, 14-d case-fatality, B, 90-d case-fatality, and C, poor functional outcome at 90 d associated with treatment by coiling from the multilevel logistic regression model in subgroups of the clinical dataset.
In subgroup analysis for patients on good clinical condition on admission (WFNS I-II), ORs were 1.30 (95% CI, 0.58-2.93) for 14-d case-fatality, 0.98 (95% CI, 0.54-1.76) for 90-d case-fatality and 0.68 (95% CI, 0.47-0.97) for poor functional outcome. For the subgroup of patients in intermediate or poor condition on admission (WFNS 3-5), ORs were 1.95 (95% CI, 1.12-3.30) for 14-d case-fatality, 1.53 (95% CI, 1.00-2.35) 90-d case-fatality and 0.90 (0.61-1.32) for poor functional outcome.
In the sensitivity analysis that excluded patients who were treated with balloon-, or stent-assisted coiling, 1343 patients were included. The results were in line with the main analysis: the ORs for coiling were 1.66 (95% CI, 1.05-2.60) for 14-d case-fatality, 1.20 (95% CI, 0.83-1.72) for 90-d case-fatality and 0.76 (95% CI, 0.58-1.01) for poor functional outcome.
DISCUSSION
Key Results
In this large international study, coiling was associated with higher case-fatality at 14 d than clipping, both in the administrative dataset and in the clinical dataset with additional adjustment for clinical factors. At 90 d, coiling was associated with comparable case-fatality and a trend towards a lower proportion of poor functional outcome compared with clipping, but this trend was mainly driven by patients with large aneurysms. In patients with aneurysms ≤10 mm, which constitute 90% of all patients, we found no statistically significant difference in proportions of patients with functional outcome after coiling or clipping.
Our primary outcome measurement was 14-d case-fatality, because we had no data on functional outcome or mortality after discharge in the administrative dataset. In the systematic review on 3 trials comparing coiling and clipping and reporting on 2243 patients, 1 the absolute risk reduction was 1% (95% CI, 1%-3%) for death at 2 mo and 2% (95% CI, 0%-4%) for death at 1 yr. Thus, although the trials were powered to detect a difference in the combined outcome of death or dependency, they also found a reduction in case-fatality after coiling. In our administrative dataset, we had overall case-fatality rates similar to those in the RCTs but data on 3 times as many patients as in the systematic review. In contrast to trials included in the Cochrane review, 1 we found an increased case-fatality rate after coiling both in the administrative and clinical dataset.
A recent cohort study involving more than 4000 patients with ruptured cerebral aneurysms who underwent aneurysm treatment, of whom 63% underwent coiling, between 2009-2013 in the state of New York, found a lower case-fatality rate after clipping than after coiling in unadjusted analyses, but after adjustment no association of treatment method with inpatient case-fatality. 18 Clipping was however associated with a higher rate of discharge to rehabilitation. Thus, as in our study, this study contrasts with the RCTs in that there was no benefit in in-hospital case fatality for coiling, but lower rate of poor outcome assessed as discharge to rehabilitation. However, in the New York study data of condition on admission were not available and an instrumental variable analysis was used in an attempt to control for baseline variation. In our study, encompassing more patients, more centers and more countries and allowing the adjustment for clinical variables, we found an even higher case-fatality after coiling.
Limitations
This study has several weaknesses that may have influenced the results. Our study was not randomized, and in the administrative dataset we had no data on clinical condition on admission or aneurysm location or size. Thus, the higher case-fatality after coiling we found in the administrative dataset might be explained by selection of patients with poor clinical condition or posterior circulation aneurysms to coiling. 19,20 However, in our clinical dataset we found, after adjustment for condition on admission, aneurysm location, and other factors, a higher case-fatality rate after coiling than after clipping. Thus, we feel it is very unlikely that the finding of a higher case-fatality after coiling is explained by selection of patients in poor condition for coiling. Another shortcoming for the administrative dataset is that we could only retrieve data on coiling procedures, but not on other endovascular procedures, such as stenting or WEB devices. Also, the clinical dataset included only 2 European centers, which can be considered a weakness regarding the generalizability. However, these results were in line with those from the international administrative dataset wherein the contributing hospitals were all large academic or teaching facilities, which contributes to the internal validity of our data.
Generalizability
We only included patients in whom the aneurysm has been treated. Thus, the data are not a comparison of strategies with intended coiling or clipping early after admission, but rather a comparison of patients who actually underwent the treatment. Also, due to the nonrandomized nature of the study, conclusions cannot be made regarding causality. The study only shows associations. Moreover, since we studied only coiling as an endovascular procedure, our data cannot be extrapolated to other endovascular procedures, such as different types of stents.
Several explanations may be considered for the discrepant findings between our study and the RCTs. First, although we adjusted for age, sex, comorbidity, clinical condition on admission, and location and size of the ruptured aneurysm in the clinical dataset, it may still be that patient selection with more patients with poor prognosis in the coiled cohort effects the results. Second is that since publication of the RCTs a wider range of aneurysms was treated by coiling compared to the period when the trials were conducted. For these types of aneurysms that were not included in the trials, results may be less favorable after coiling compared to those for types of aneurysms that were included in the trials. A third explanation may be the expertise of the interventionalist. In the RCTs, clipping was done by neurosurgeons, and coiling by radiologists. Currently, clipping is still done only by neurosurgeons, but coiling may be performed not only by radiologists, but also by neurosurgeons or neurologists. Thus, it may not be the technique, but the operator performing the technique that explains the higher case-fatality after coiling. Indeed, a survey (data not shown) in the hospitals participating in the administrative dataset revealed that coiling was done by radiologists, neurosurgeons, and neurologists. Arguing against this potential explanation, a recent study showed that surgeons who perform both surgical and endovascular procedures had no worse outcomes than radiologists for endovascular procedures. 21 Furthermore, in both centers that provided data for the clinical dataset coiling procedures were done by small experienced teams of interventional neuroradiologists. One hospital (UMCU) provided cases for both datasets. However, the administrative dataset included only 305 patients from this hospital, which is too small a number to significantly influence the overall results. Moreover, if this would have influenced the results, it would have led towards similarity in results instead of discrepancies. Fourth, it may be that since the publication of ISAT, physicians have learned to choose the therapies more appropriately, which has improved the outcomes. A recently published SAH outcome prediction calculator indicates that other factors may be more important in predicting SAH outcome than treatment modality. 22 Fifth, in the participating centers only a minority of patients were included in ISAT during the study period, with most treated outside the trial. In our study, we give overview of all treated patients at large institutions throughout the world. Sixth, the apparent trend that SAH increasingly tend to occur from smaller aneurysms 23 that are harder to coil may have worsened the outcomes after endovascular treatment. Last, after aneurysm occlusion the care for patients may be different according to the type of aneurysm treatment. An example of different treatment strategies is that platelet aggregation inhibitors are often prescribed after coiling, but rarely after clipping, 24 which might relate to a higher proportion of patients with hemorrhagic complications, eg, during insertion of removal of external ventricular drains.
CONCLUSION
In this large series of patients admitted to tertiary care facilities worldwide, we found that coiling is associated with higher in-hospital case-fatality, and not with superior outcomes at 90 d. The reasons for the discrepancy between outcomes in current daily practice compared to those from RCTs should be the subject of further studies such as studies with good data to assess the influence on outcome of level of experience of staff performing the endovascular or surgical procedure, the types of aneurysms treated by both methods, and the patient care after aneurysm treatment. For clinical practice, our findings indicate that surgical treatment should remain as a readily available treatment option for ruptured aneurysms worldwide. A timely and proper multidisciplinary discussion where all treatment options are discussed seems the best strategy to decide on how the aneurysm should be treated in patients with aSAH.
Disclosures
Ms Turner is a former employee of Dr Foster Ltd The other authors have no personal, financial, or institutional interest in any of the drugs, materials, or devices described in this article. Dr Meretoja has received speaker's bursaries and consulted for Stryker, Nestle, Boehringer-Ingelheim, and Phagenesis.
Supplementary Material
Acknowledgments
We would like to thank the participating hospitals of the Stroke GOAL of Dr Foster Global Comparators that contributed to this project. Belgium: Dr Robin Lemmens, University Hospitals Leuven. Netherlands: Dr Gabriel Rinkel, University Medical Center Utrecht. United Kingdom: Dr Louise Shaw, Royal United Hospital Bath NHS Trust; Dr Emma Vaux Royal Berkshire NHS Foundation Trust; Dr Marc Randall, Sheffield Teaching Hospitals NHS Foundation Trust. United States: Mary Spencer and Dr Jin-Moo Lee, Barnes-Jewish Hospital; Dr Gudridur (“Peggy”) H. Matzkiw and Jillian Newman, Huntsville Hospital; Dr Natalia Rost, Massachusetts General Hospital; Dr Thomas Hemmen, UC San Diego Health System, Dr Mark Alberts, UTSouthwestern Health system. Norway: Dr Arnstein Tveiten. Finland and Australia: Atte Meretoja, Helsinki University Hospital and Royal Melbourne Hospital. This paper was written on behalf of the Dr Foster Global Comparators network, Dr Foster, in association with the Dr Foster Unit at Imperial College London. We would like to thank the following hospitals for their administrative data which were used as the basis for this study; Alfred Health, Austin Health, Melbourne Health, Monash Health, UZ Leuven, Cambridge University Hospital, Coventry & Warwickshire University Hospital, Imperial College Hospital, Sheffield University Hospital, University College London Hospital, Maastricht UMC + , Leiden UMC, UMC Utrecht, Oslo UH °, Barnes-Jewish Hospital, Huntsville Hospital and UC San Diego Hospital.
Notes
The abstract of this work was presented as a poster at the International Stroke Conference 2018, January 24–26, 2018 in Los Angeles, California.
Contributor Information
Stroke GOAL Group, Dr Foster Global Comparators Project, Dr Foster Ltd:
Robin Lemmens, Gabriel Rinkel, Louise Shaw, Emma Vaux, Marc Randall, Mary Spencer, Jin-Moo Lee, Gudridur (“Peggy”) H Matzkiw, Natalia Rost, Thomas Hemmen, Mark Alberts, Arnstein Tveiten, and Atte Meretoja
Supplemental Digital Content 1. Table. Odds ratios for 14-d case fatality from multivariate regression model in the administrative dataset of 7658 patients.
Supplemental Digital Content 2. Table. Baseline characteristics and outcome of 1501 aSAH patients in the clinical dataset.
COMMENT
The authors present a retrospective analysis of an administrative database comparing treatments of aneurysmal subarachnoid hemorrhage with a primary endpoint of 14-day case mortality. Intercontinental data obtained from an administrative dataset of 7658 cases from 22 tertiary care hospitals admitted between 2007–2013 did not include functional outcomes after discharge. These results showed a higher rate of 14-day case-fatality within the coiling cohort, and non-superiority at 90 days.
One-year survival outcomes free of disabilities derived from the randomized clinical trials comparing endovascular versus surgical methods, particularly the International Subarachnoid Aneurysm Trial (ISAT), have raised hypotheses regarding indicated modalities of treatment for this patient population. 1 At 6 years, morbidity outcomes in the Barrow Ruptured Aneurysm Trial, with a poor outcome defined as a modified Rankin Scale (mRS) score > 2, showed no significant difference in outcomes for anterior circulation aneurysms, but identified significantly better outcomes when aneurysms were treated by endovascular means within the vertebrobasilar system. 2 Compared to the equitable dichotimization of coiling and clipping cases in these randomized studies, a modern paradigm of ruptured aneurysm management depicts a higher propensity of endovascularly-managed cases.
Contradicting previous publications regarding reduced case-fatality trends after coiling, the authors of the present study identified a higher tendency for in-hospital case-fatality and 90-day case fatality for patients who were treated with coil embolization. Clinical condition on admission and aneurysm characteristics (location, morphology, size) were not included in the analysis of 83.5% of the sample size. An argument can be made that that the data shortcoming in this administrative dataset was supported with similar results in the clinical dataset, but even these results are not generalizable, as they do not identify a comprehensive picture of the patient's condition. Comorbid conditions are poorly documented within the clinical data set and European subset of patients within the administrative dataset. Comorbid conditions often prohibit the stressors of a craniotomy and thereby influence less invasive means for protection from rerupture. We should be cautious in drawing conclusions from this. Correlation does not indicate causation and treatments were not randomized for this patient cohort.
Brandon Burnsed
Adam Arthur
Memphis, Tennessee
References
- 1. Molyneux A, Kerr R, Stratton I, et al.. International Subarachnoid Aneurysm Trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised trial. Lancet. 2002;360:1267–74. [DOI] [PubMed] [Google Scholar]
- 2. Spetzler RF, McDougall CG, Zabramski JM, et al.. The Barrow Ruptured Aneurysm Trial: 6-year Results. J Neurosurg. 2015;123(3);609– 17. [DOI] [PubMed] [Google Scholar]
Neurosurgery Speaks (Audio Abstracts)
Listen to audio translations of this paper's abstract into select languages by choosing from one of the selections below.
REFERENCES
- 1. van der Schaaf I, Algra A, Wermer Met al.. Endovascular coiling versus neurosurgical clipping for patients with aneurysmal subarachnoid haemorrhage. Cochrane database Syst Rev. 2005; 19(4):CD003085. [DOI] [PubMed] [Google Scholar]
- 2. Falk Delgado A, Andersson T, Falk Delgado A. Clinical outcome after surgical clipping or endovascular coiling for cerebral aneurysms: a pragmatic meta-analysis of randomized and non-randomized trials with short- and long-term follow-up. J NeuroIntervent Surg 2017;9(3):264-277. [DOI] [PubMed] [Google Scholar]
- 3. Hammer A, Steiner A, Kerry Get al.. Treatment of ruptured intracranial aneurysms yesterday and now. PLoS One. 2017;12(3):e0172837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Leake CB, Brinjikji W, Kallmes DF, Cloft HJ. Increasing treatment of ruptured cerebral aneurysms at high-volume centers in the United States. J Neurosurg. 2011;115(6):1179-1183. [DOI] [PubMed] [Google Scholar]
- 5. Stranjalis G, Loufardaki M, Koutsarnakis C, Kalamatianos T. Trends in the Management and Hospital Outcome of Spontaneous Subarachnoid Hemorrhage in the Post-International Subarachnoid Aneurysm Trial Era in Greece: Analysis of 719 Patients During a 13-Year Period. World Neurosurg. 2016;88:327-332. [DOI] [PubMed] [Google Scholar]
- 6. Goel G, Gupta V, Chinchure S, Gupta A, Kaur G, Jha AN. A decade after International Subarachnoid Aneurysm Trial: Coiling as a first choice treatment in the management of intracranial aneurysms - Technical feasibility and early management outcomes. Asian J Neurosurg. 2014;9(3):137-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cognard C, Pierot L, Anxionnat R, Ricolfi F. Clarity Study Group, Results of Embolization Used as the First Treatment Choice in a Consecutive Nonselected Population of Ruptured Aneurysms: Clinical Results of the Clarity GDC Study. Neurosurgery. 2011;69(4):837-842. [DOI] [PubMed] [Google Scholar]
- 8. Molyneux A, Kerr R, Stratton Iet al.. International Subarachnoid Aneurysm Trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised trial. Lancet North Am Ed. 2002;360(9342):1267-1274. [DOI] [PubMed] [Google Scholar]
- 9. Brinjikji W, Lanzino G, Rabinstein AA, Kallmes DF, Cloft HJ. Age-related trends in the treatment and outcomes of ruptured cerebral aneurysms: A study of the nationwide inpatient sample 2001–2009. AJNR Am J Neuroradiol 2013;34(5):1022-1027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Walendy V, Strauss C, Rachinger J, Stang A. Treatment of aneurysmal subarachnoid haemorrhage in germany: A nationwide analysis of the years 2005-2009. Neuroepidemiology. 2014;42(2):90-97. [DOI] [PubMed] [Google Scholar]
- 11. Stranjalis G, Loufardaki M, Koutsarnakis C, Kalamatianos T. Trends in the management and hospital outcome of spontaneous subarachnoid hemorrhage in the post-international subarachnoid aneurysm trial era in greece: analysis of 719 patients during a 13-year period. World Neurosurg. 2016;88:327-332. [DOI] [PubMed] [Google Scholar]
- 12. Gnanalingham KK, Apostolopoulos V, Barazi S, O’Neill K. The impact of the international subarachnoid aneurysm trial (ISAT) on the management of aneurysmal subarachnoid haemorrhage in a neurosurgical unit in the UK. Clin Neurol Neurosurg. 2006;108(2):117-123. [DOI] [PubMed] [Google Scholar]
- 13. Brinjikji W, Lanzino G, Kallmes DF, Cloft HJ, Brinjikji W. Cerebral aneurysm treatment is beginning to shift to low volume centers. J NeuroIntervent Surg. 2014;6(5):349-352. [DOI] [PubMed] [Google Scholar]
- 14. Lawson A, Goddard T, Ross S, Tyagi A, Deniz K, Patankar T. Endovascular treatment of cerebral aneurysms using the Woven EndoBridge technique in a single center: preliminary results. J Neurosurg. 2017;126(1):17-28. [DOI] [PubMed] [Google Scholar]
- 15. Stranjalis G, Korfias S, Vemmos KN, Sakas DE. Spontaneous subarachnoid haemorrhage in the era of transition from surgery to embolization. A study of the overall outcome. Br J Neurosurg. 2005;19(5):389-394. [DOI] [PubMed] [Google Scholar]
- 16. Bottle A, Middleton S, Kalkman CJ, Livingston EH, Aylin P. Global comparators project: International comparison of hospital outcomes using administrative data. Health Serv Res. 2013;48(6pt1):2081-2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Teasdale GM, Drake CG, Hunt Wet al.. A universal subarachnoid hemorrhage scale: report of a committee of the World Federation of Neurosurgical Societies. Neurol Neurosurg Psychiatry. 1988;51(11):1457-1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bekelis K, Missios S, Coy S, Rahmani R, Singer RJ, MacKenzie TA. Surgical clipping versus endovascular intervention for the treatment of subarachnoid hemorrhage patients in new york state. PLoS One. 2015;10(9):e0137946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Schievink WI, Wijdicks EFM, Piepgras DG, Chu C-P, O’Fallon WM, Whisnant JP. The poor prognosis of ruptured intracranial aneurysms of the posterior circulation. J Neurosurg. 1995;82(5):791-795. [DOI] [PubMed] [Google Scholar]
- 20. Inagawa T. Site of Ruptured Intracranial Saccular Aneurysms in Patients in Izumo City, Japan. Cerebrovasc Dis. 2010;30(1):72-84. [DOI] [PubMed] [Google Scholar]
- 21. Fennell VS, Martirosyan NL, Palejwala SK, Lemole GM, Dumont TM. Morbidity and mortality of patients with endovascularly treated intracerebral aneurysms: does physician specialty matter? J Neurosurg. 2016;124(1):13-17. [DOI] [PubMed] [Google Scholar]
- 22. Jaja BNR, Saposnik G, Lingsma HFet al.. Development and validation of outcome prediction models for aneurysmal subarachnoid haemorrhage: the SAHIT multinational cohort study. BMJ. 2018;18;360:j5745. [DOI] [PubMed] [Google Scholar]
- 23. Bender MT, Wendt H, Monarch Tet al.. Small aneurysms account for the majority and increasing percentage of aneurysmal subarachnoid hemorrhage: A 25-year, single institution study. Neurosurgery. 2018;83(4):692-699. [DOI] [PubMed] [Google Scholar]
- 24. van den Bergh WM, Algra A, Rinkel GJE. Group MS, Magnesium and aspirin treatment in patients with subarachnoid haemorrhage. J Neurol. 2009;256(2):213-216. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.