Abstract
Negative emotional experiences can be more difficult to forget than neutral ones, a phenomenon termed the “emotional memory effect.” Individual differences in the strength of the emotional memory effect are associated with emotional health. Thus, understanding the neurobiological underpinnings of the emotional memory effect has important implications, especially for individuals at risk for emotional health problems. Although the neural basis of emotional memory effects has been relatively well defined, less is known about how hormonal factors that can modulate emotional memory, such as glucocorticoids, relate to that neural basis. Importantly, probing the role of glucocorticoids in the stress- and emotion-sensitive period of late childhood to adolescence could provide actionable points of intervention. We addressed this gap by testing whether hypothalamic–pituitary–adrenal (HPA) axis activity during a parent–child conflict task at 11 years of age predicted emotional memory and its primary neural circuitry (i.e., amygdala–hippocampus functional connectivity) at 16 years of age in a longitudinal study of 147 girls (104 with complete data). Results showed that lower HPA axis activity predicted stronger emotional memory effects, r(124) = −.236, p < .01, and higher emotional memory-related functional connectivity between the right hippocampus and the right amygdala, β = −.385, p < .001. These findings suggest that late childhood HPA axis activity may modulate the neural circuitry of emotional memory effects in adolescence, which may confer a potential risk trajectory for emotional health among girls.
INTRODUCTION
The ability to recall and relive one's own prior experiences shapes who we are as humans, but sometimes, we wish we could forget information we cannot (Kuntsche, Knibbe, Engels, & Gmel, 2010). Negative experiences can disturb individuals for years (Aldwin, Levenson, & Spiro, 1994), and memories biased toward negative experiences are thought to contribute to the etiology or maintenance of emotion and distress disorders, such as depressive (Beck & Bredemeier, 2016; Gotlib & Joormann, 2010) and traumatic stress disorders (Feduccia & Mithoefer, 2018; Aldwin et al., 1994). Indeed, we remember negative emotional information much better than neutral information (Shields, Dunn, Trainor, & Yonelinas, 2019; Kensinger, 2007)—a phenomenon referred to as the “emotional memory effect” (Schmidt & Saari, 2007). Although a neurobiological basis of the emotional memory effect has been characterized (Yonelinas & Ritchey, 2015), our understanding of the mechanisms and factors that may modulate the emotional memory effect and its neurobiological basis, such as the activity of the stress-responsive hypothalamic–pituitary–adrenal (HPA) axis, is less developed. In this study, we address this gap by examining the association between individual differences in the stress-responsive hormone cortisol in childhood and emotional memory-related functional connectivity between the amygdala and hippocampus, key regions implicated in both stress and memory, in adolescence.
Although a number of theories seek to explain why HPA axis activity might influence emotional memory (for an overview of these theories, see Shields, Sazma, McCullough, & Yonelinas, 2017), nearly all of these are rooted in the theory of consolidation (McGaugh, 2000, 2015; Schwabe, Joëls, Roozendaal, Wolf, & Oitzl, 2012; Cahill & McGaugh, 1998). A central tenet of this theory is that glucocorticoids—hormones, such as cortisol, which are regulated by the HPA axis—improve consolidation of long-term memory in part by modulating communication between the hippocampus and amygdala (McGaugh, 2000). It is therefore plausible from this theoretical foundation to expect that HPA axis activity would modulate both hippocampus–amygdala functional connectivity and emotional memory effects.
The HPA axis facilitates the body's response to stress through the release of the hormone cortisol. Cortisol is postulated to play an important role in responding to threats and shaping behavior to facilitate healthy responding (Shields & Slavich, 2017), although chronically elevated basal levels or unchecked acute response can interfere with the adaptive effects of cortisol (Shields & Slavich, 2017; Silverman & Sternberg, 2012). Evidence shows that cortisol responsivity is highly sensitive to social stressors, particularly those that are personally relevant and uncontrollable (Dickerson & Kemeny, 2004). Moreover, resting levels of cortisol and cortisol reactivity to such stressors increase across the transition from late childhood to adolescence (Hostinar, McQuillan, Mirous, Grant, & Adam, 2014; Gunnar & Quevedo, 2007). A common stressor for children as they shift into adolescence is conflict with parents, which can be a significant source of physiological stress that alters HPA axis activity. For example, increased parent–child conflict across several weeks was related to increases in diurnal cortisol levels in children aged 8–13 years (Kuhlman, Repetti, Reynolds, & Robles, 2016). These observations have significant implications for expanding our understanding of the neural underpinnings of the emotional memory effect. Indeed, much experimental work has shown that cortisol affects or is related to memory in different ways (e.g., Meir Drexler, Merz, Jentsch, & Wolf, 2019; Het, Ramlow, & Wolf, 2005; McGaugh, 2000). For example, administration of cortisol can modulate memory performance (Schwabe et al., 2012; Het et al., 2005), and when considering diurnal cortisol rhythms, greater daily cortisol output has been linked both concurrently and prospectively to memory impairments in adults (Franz et al., 2011; Gomez et al., 2009; Lee et al., 2007; Li et al., 2006; Greendale, Kritz-Silverstein, Seeman, & Barrett-Connor, 2000; Lupien et al., 1994, 1998). Furthermore, the link between HPA axis activity and memory is particularly strong when the information to be learned has a negative emotional valence (e.g., Joëls, Fernandez, & Roozendaal, 2011; Roozendaal, Okuda, de Quervain, & McGaugh, 2006; Van Honk et al., 2003), although this association has not been tested prospectively.
Despite relatively consistent links between HPA axis activity and emotional memory within the context of a manipulation that affects both cortisol and memory (Sazma, Shields, & Yonelinas, 2019; Joëls et al., 2011), the direction of the associations reported between resting or diurnal HPA axis activity and emotional memory are more variable. For example, some studies have found that higher contemporaneous basal or prior HPA axis activity predicts lower emotional memory performance or weaker emotional memory biases (i.e., emotional memory effects; Gutchess, Alves, Paige, Rohleder, & Wolf, 2019; McCullough, Ritchey, Ranganath, & Yonelinas, 2015; Van Honk et al., 2003), whereas other studies have found that higher concurrent basal or prior HPA axis activity predicts higher emotional memory performance or stronger emotional memory effects (Gutchess et al., 2019; Preuß, Schoofs, & Wolf, 2009; Putman, Van Honk, Kessels, Mulder, & Koppeschaar, 2004). To clarify some of these mixed findings, one study to date focused specifically on age as a factor that can influence how HPA axis activity relates to emotional memory effects, finding that older adults showed a negative association, whereas younger adults showed a positive association (Gutchess et al., 2019).
Further consideration of age is warranted for understanding the link between HPA axis function and emotional memory effects because of the likely differential implications of atypical stress sensitivity and emotional memory across development (Andersen & Teicher, 2008). Another notable sample characteristic for consideration is sex. Sex differences in affective responding, HPA axis function and function in threat circuitry underscore the importance of documenting these processes in girls (Bangasser & Valentino, 2014; Cahill, 2012). Importantly, these factors may conjointly be relevant for identifying correlates of emotional memory effects. For example, adolescence is a time of increasing rates of emotional disorders, especially for girls (Guyer, Silk, & Nelson, 2016; Stroud, Papandonatos, Williamson, & Dahl, 2011; Andersen & Teicher, 2008); therefore, identifying predictors of emotional memory processes in adolescent girls has significant implications for understanding both the basic mechanisms of emotional memory as well as ways to protect mental health. No study to date, however, has established whether HPA axis activity assessed earlier in development, in what is a sensitive and vulnerable period for HPA axis development (Jankord et al., 2011; Andersen & Teicher, 2008; McCormick & Mathews, 2007), is associated with later emotional memory or differences in its neural underpinnings in adolescents broadly—or in adolescent girls specifically. This gap in understanding becomes particularly salient when one considers that adolescence is also a time of marked hormonal changes (Casey, Jones, & Hare, 2008) and, for girls, heightened social stress sensitivity (Stroud et al., 2011). Importantly, the amount of stress experienced in interactions with caregivers during the transition to adolescence may set the stage for emotional memory biases that emerge downstream. Therefore, it is critical to identify putative contextually driven biological precursors that, once established, may suggest a mechanistic pathway to manifestations of emotional memory effects.
The neural underpinnings of the emotional memory effect (i.e., a bias toward remembering negative information over more neutral information) has been shown to be largely the result of input from the hippocampus, the amygdala, and communication between these regions (McGaugh, 2015; Yonelinas & Ritchey, 2015; Maroun & Akirav, 2008). The hippocampus, long known to be important for episodic memory (Scoville & Milner, 1957), facilitates emotional memory by binding information together (Yonelinas, 2013)—usually by binding to-be-remembered items with contextual information (Yonelinas, Ranganath, Ekstrom, & Wiltgen, 2019; Diana, Yonelinas, & Ranganath, 2007). The amygdala, in contrast, facilitates the emotional memory effect by “tagging” information as emotional and thereby facilitating its encoding within the hippocampus (Mather, Clewett, Sakaki, & Harley, 2016; Maroun & Akirav, 2008; Akirav & Richter-Levin, 2002) and by binding emotional information to item information sent from the hippocampus (Yonelinas & Ritchey, 2015). Manipulations of glucocorticoids, such as cortisol, influence both the hippocampus and amygdala, as well as their interactions (Schwabe et al., 2012; McGaugh, 2000; Akirav & Richter-Levin, 1999), but in no study to date has prior HPA axis activity been examined in relation to functional connectivity between the hippocampus and amygdala.
The Current Research
This study extended the HPA axis and emotional memory literatures by examining associations between HPA axis activity and emotional memory effects in adolescent girls as well as potential neurobiological mechanisms linking individual differences in HPA axis activity to subsequent negative emotional memory. Specifically, we examined whether cortisol output during a potentially stressful ecologically valid interaction in late childhood predicted both emotional memory effects and event-related functional connectivity between the hippocampus and amygdala during an emotional memory encoding task during adolescence. Drawing on prior work on associations between HPA axis functioning and emotional memory (e.g., Gutchess et al., 2019; Nagamine, Noguchi, Takahashi, Kim, & Matsuoka, 2017), we expected HPA axis activity to relate to emotional memory effects, but we did not have strong a priori predictions about the direction of the association, given that adolescents were not represented in age-specific associations found previously (i.e., that older adults showed a negative whereas younger adults showed a positive association between prior HPA axis activity and emotional memory effects; Gutchess et al., 2019). Moreover, we expected that greater HPA axis activity would be associated with negative emotional memory event-related functional connectivity between the hippocampus and the amygdala because individual differences in HPA axis activity have been linked to individual differences in hippocampus and amygdala structure and function (Algamal et al., 2018; Pagliaccio et al., 2014). Given sparse prior research with functional connectivity between these two regions in relation to HPA axis activity, we did not formulate hypotheses regarding the direction of the association between HPA axis activity and negative emotional memory-related functional connectivity.
METHODS
Participants
Participants were 16-year-old girls participating in the Pittsburgh Girls Study-Emotions (PGS-E), a substudy of the longitudinal Pittsburgh Girls Study (PGS) that has followed 2450 girls from ages 5 to 8 years (Keenan et al., 2010). Both the PGS and PGS-E (Keenan et al., 2010, 2013) recruitment procedures have been described in detail elsewhere; in brief, 50% of the 232 girls constituting the PGS-E sample were recruited at 9 years of age because of screening high for depressive symptoms in the larger PGS study at 8 years of age, whereas the other 50% were recruited as demographic-matched participants who did not screen high for depressive symptoms at 8 years of age. Girls in the PGS study not recruited for the PGS-E study did not have cortisol, fMRI, or emotional memory data, as those tasks were only completed by the PGS-E sample. In comparison to the PGS total sample, the PGS-E sample included a higher number of Black girls (69.8% vs. 52%) and a higher number of families receiving public assistance (43.5% vs. 38.9%). Of the 232 total girls participating in the PGS-E, 147 completed the first of four annual neuroimaging scans at 16 years of age. Of those 147 possible participants, 26 were excluded from fMRI analyses because of issues with their MRI data (19 for excessive motion, five for poor scan quality, and two for gross brain abnormalities); one participant did not complete the recall task; eight participants misunderstood either the encoding or retrieval task, reported making errors in the retrieval task, or reported not having paid attention during at least some portion of the encoding or retrieval tasks; seven participants did not provide sufficient saliva for the cortisol assay in late childhood or did not have detectable cortisol levels in saliva; three participants were excluded from cortisol analyses because of excessively high cortisol levels (>4 SDs from the mean, and no other participants were >3 SDs from the mean); and for three participants, functional connectivity values could not be calculated because of their memory performance in a specific condition. Therefore, 136 girls had usable emotional memory data, 127 had usable cortisol and emotional memory data, and 104 had complete data for all analyses. All girls provided informed assent, and their parents provided informed consent. Participants were monetarily compensated for their time, and all procedures were approved by the study site's Human Research Protection Office. Of the 104 girls with complete data, 65.4% identified as Black, 26.9% identified as White, and 7.7% identified as Multiracial. The girls with complete data did not differ from the broader PGS-E sample in racial distribution, χ2(2) = 0.97, p = .616.
Materials and Procedure
Conflict Resolution Task
The problem-solving task, based on the work of Melby and Conger (2001), was used to create an emotionally arousing and potentially stressful interpersonal interaction when the girls were 9 and 11 years old. The current study focused on the age 11 assessment because of its closer proximity to the age 16 emotional memory assessment and because age 11 marks a transition to adolescence for most individuals; nonetheless, age 9 cortisol values were also analyzed to assess similarity to age 11 values. The problem-solving task has been used widely in samples of youth and their caregivers (Keenan, Hipwell, Hinze, & Babinski, 2009; Scaramella, Conger, Spoth, & Simons, 2002; Melby & Conger, 2001). In this study, the mother and her daughter generated a topic that elicited conflict between them and were instructed to spend 3 min discussing each topic. Before the start of the task and at 20 and 45 min after the end of the task, saliva samples were collected from the girls to assess resultant hormonal activity. Girls were debriefed as to the purpose of the conflict task at the end of study. Although an effort was made to schedule visits between 1:00 p.m. and 4:00 p.m., some visits occurred earlier to accommodate family schedules. The time of day that the study began was significantly related to cortisol levels, p = .013, but controlling for the time of day the study began did not influence any of the results (i.e., all significant associations between cortisol and emotional memory as well as between cortisol and functional connectivity values remained significant, and no nonsignificant associations became significant), nor did time of day moderate any of the results (ps > .495).
Cortisol
To collect saliva samples, an absorbent salivette was applied to the tongue, cheek, and gums for approximately 1 min. Salivettes were placed in a labeled plastic tube and then transferred to a freezer and stored at −20°C until shipped to Salimetrics for batch assay. Samples with sufficient saliva were assayed in duplicate using high-sensitivity unbound cortisol enzyme-linked immunosorbent assays from Salimetrics. Assay sensitivity was .007 μg/dL. Assay coefficients of variation were less than 6.51%. Cortisol output across the three time points was calculated using area under the curve with respect to ground (AUCg), which indexes resting HPA axis function as well as HPA axis responses and can be calculated regardless of whether an increase in cortisol is observed (Pruessner, Kirschbaum, Meinlschmid, & Hellhammer, 2003). Three participants were excluded from cortisol analyses because of excessively high cortisol levels (> 4 SDs from the mean before or after log transforming, and no other participants were > 3 SDs from the mean).
Memory Task
Memory for negative and neutral information was assessed using an adaptation of a faces memory task previously shown to index emotional memory in a clinically meaningful way (Guyer, Choate, Grimm, Pine, & Keenan, 2011; Roberson-Nay et al., 2006; Pine et al., 2004). Each of the faces used as stimuli in the current study came from select databases of emotional faces (Tone, Schmidt, & Davis, n.d.; Ebner, Riediger, & Lindenberger, 2010; Tottenham et al., 2009; Minear & Park, 2004; Nelson, 2004; Lundqvist, Flykt, & Öhman, 1998). Each of the actors was photographed displaying four distinct emotions used in this task (i.e., anger, sadness, happiness, or a neutral expression).
The task had two phases. First, during the incidental encoding phase, which took place during the MRI scan, participants viewed 48 different faces displaying one of four emotions (i.e., anger, sadness, happiness, or a neutral expression) under two different instruction sets, therefore viewing each of the 48 faces twice (with the same emotion on both viewings). Happy faces were unrelated to our a priori analyses, which focused on the difference between negative and neutral memory and related brain activity, and therefore were not considered further. The emotion an actor displayed to each participant during the encoding task was randomly selected, and the randomly selected emotion varied across participants. Participants rated each face using 1 (not at all) to 5 (very much so) on each of the following instruction sets: “How sad does this face make you feel?” and “How wide is the nose?” Each emotional picture was displayed for 2990 msec, no matter how quickly a participant rated each face. Intertrial interval randomly varied from 750 to 1250 msec.
Second, following completion of the scan (approximately 20 min after the encoding task), participants were presented with a surprise memory retrieval task assessing memory for the previously seen faces. In the memory retrieval task, 48 pairs of side-by-side neutral faces were presented to participants on a laptop. Regardless of the emotion displayed by a face to a participant during encoding, each face was presented with a neutral expression at retrieval. In this self-paced task, participants were required to select which of the two faces presented was the face they previously saw during the scan; specifically, they pressed 1 if the recognized face was on the left side of the screen or 2 if the recognized face was on the right side of the screen. Participants were given as much time to make a response as they required, and the task only continued when a response was provided. Mean RTs to select their choice were calculated for each participant for each emotion. This forced-choice recognition paradigm removes response biases from the memory data, entailing that the number of hits (i.e., correct responses) is equivalent to a bias-corrected index of recognition memory. Hits were calculated for each emotion presented during encoding for each participant. Negative memory performance was calculated as the mean of hits to faces presented with angry or sad expressions during encoding, and neutral memory performance was calculated as the sum of hits to faces presented with a neutral expression during encoding. Emotional memory effects were then calculated by subtracting neutral memory performance from negative memory performance (i.e., Negative Hits − Neutral Hits) and used in the analyses.
fMRI Data Acquisition and Preprocessing
MRI data were acquired using a 3.0-T Siemens Tim Trio scanner. The functional scan consisted of 280 contiguous T2-weighted EPI whole-brain functional volumes using a repetition time of 2 sec, an echo time of 28 msec, a flip angle of 90°, 39 slices, a 64 × 64 matrix, a 205-mm field of view, and an acquisition voxel size of 3.2 × 3.2 × 3.1 mm. Additionally, a high-resolution T1-weighted structural image (repetition time = 2.3 sec, echo time = 2.98 msec, flip angle = 9°, 160 slices, field of view = 256 mm, acquisition voxel size = 1.0 × 1.0 × 1.2 mm) was acquired for coregistration and normalization of functional images.
Functional and structural MRI data were preprocessed using the default preprocessing pipeline in CONN toolbox v18.b (Whitfield-Gabrieli & Nieto-Castanon, 2012). This pipeline consists of functional realignment (and unwarp) of functional files, functional centering to (0, 0, 0) coordinates (translation), functional slice-timing correction, functional outlier detection using the artifact detection toolbox (ART; www.nitrc.org/projects/artifact_detect/) for scrubbing, functional segmentation and normalization, structural translation, and structural segmentation and normalization.
ART parameters were set to flag acquisitions with framewise displacement above 0.9 mm or global BOLD signal changes above 5 SDs as potential outliers. Participants (n = 19) with greater than 20% of scans identified as outliers by ART were removed from subsequent analyses. Outlier matrices, three translational and three rotational movement parameters and their first-order temporal derivatives, 10 noise component parameters from the anatomical component-based noise correction procedure (aCompCor; five parameters from white matter and five from cerebrospinal areas), and all effects of conditions (done by default in CONN toolbox to remove illusory correlations) were entered as covariates during first-level analysis.
Functional Connectivity
ROI-to-ROI functional connectivity analyses were conducted using the CONN toolbox v18.b (Whitfield-Gabrieli & Nieto-Castanon, 2012). After preprocessing, a band-pass filter of 0.008–0.09 Hz was applied to the images, and motion was then regressed out. Physiological (including white matter and cerebrospinal fluid) and other noise reduction was then implemented using the component-based noise reduction method, aCompCor, described above (Behzadi, Restom, Liau, & Liu, 2007). The aCompCor approach provides five principal components for both white matter and cerebrospinal fluid to account for variability in their noise across the brain. Whole-brain BOLD signal was not included as a regressor to avoid obtaining incorrect anticorrelations (Murphy, Birn, Handwerker, Jones, & Bandettini, 2009). The aCompCor approach addresses the same concerns as those addressed by regressing out the global signal—in fact, aCompCor accounts for 62% of the variance in the global signal (Yeo, Tandi, & Chee, 2015)—without risk of artificially introducing anticorrelations, thus making anticorrelations interpretable (Whitfield-Gabrieli & Nieto-Castanon, 2012). Moreover, specificity and sensitivity of positive correlations are better using the aCompCor approach than the global signal regression method (Chai, Castañón, Ongür, & Whitfield-Gabrieli, 2012), and aCompCor has been shown to be superior to global signal regression for discerning true group differences in functional connectivity (Shirer, Jiang, Price, Ng, & Greicius, 2015; Saad et al., 2012).
BOLD time series were first preprocessed and denoised and then spatially normalized to build the time series for each voxel. In line with prior work (Harrison, Burggren, Small, & Bookheimer, 2016), we assessed memory-related functional connectivity using event-related functional connectivity (Rissman, Gazzaley, & D'Esposito, 2004). In particular, we assessed emotional memory-related functional connectivity using a generalized psychophysiological interaction contrast that examined the difference between functional connectivity during the time series involving the encoding of subsequently remembered negative faces (i.e., angry/sad) during both instruction sets and functional connectivity during the time series involving the encoding of subsequently forgotten negative faces during both instruction sets compared with neutral faces in the same conditions (i.e., [Negative Remembered − Negative Forgotten] − [Neutral Remembered − Neutral Forgotten]).
We focused our analyses on connectivity between the hippocampus and the amygdala, as these regions are both responsive to the effects of glucocorticoids, such as cortisol, and are thought to drive emotional memory effects (Yonelinas & Ritchey, 2015; McGaugh, 2000). Masks for the amygdala and hippocampus ROIs were taken from the Harvard–Oxford subcortical atlas included as default ROIs within CONN toolbox. These ROIs in this atlas are probabilistic, entailing that some voxels included in these ROIs are likely but not certainly included in the respective region. To make use of this rich probabilistic data, rather than thresholding the ROIs, we used CONN toolbox to extract the time series of probability-weighted sums from each ROI. For example, if a voxel has a probability of .5 to be included in the ROI, the activity in that voxel was multiplied by .5 and summed together with the remainder of the probability-weighted activity values in that ROI at each scan.
Because of potential differences in laterality and because our functional connectivity analyses were restricted to ROI-to-ROI analyses, we examined hemisphere as a potential moderating factor in relevant analyses. Our a priori plan of analysis was to extract functional connectivity values between each of the four ROIs (left and right hippocampus and amygdala) to examine their associations with HPA axis activity. Connectivity values are Fisher Z-transformed partial correlations.
Analytic Strategy
Functional connectivity values were extracted from CONN, and all analyses were conducted using R, Version 3.6.0. All variables were examined for skew, and variables with significant skew (p < .05 using the D'Agostino test; i.e., HPA axis activity) were log-transformed before analyses. Mixed-model ANOVAs1 were conducted using the lmerTest package, Version 3.1-0, in R. Degrees of freedom for mixed-models and follow-up analyses were estimated using the Satterthwaite approximation and rounded to the nearest integer. Results include presentation of Bayes factors (BF10), calculated using the BayesFactor package, Version 0.9.12-4.2, in R. A Jeffreys-beta prior was used for the prior in all Bayesian analyses presented in this paper. A Bayes factor quantifies the evidence in favor of the data being observed in the model of interest, such that a Bayes factor greater than 1 (e.g., 2.5) indicates that the data were more likely to be observed in the model of interest than in an alternative model (e.g., a Bayes factor of 2.5 indicates that the data are 2.5 times as likely to have occurred given the model of interest than the model it is being tested against, such as a null model), whereas a Bayes factor BF10 of less than 1 indicates evidence against the data occurring under that model. By convention, a Bayes factor BF10 of 3.16 or greater indicates substantial evidence in favor of the data being observed in the model of interest.
RESULTS
Descriptive statistics and correlations for all study variables of interest are presented in Table 1. Of note, participants, on average, recognized more neutral faces (M = 9.80, SD = 1.56) than negative faces (M = 8.57, SD = 1.24) in this paradigm, t(135) = 9.24, p < .001. This is presumably because all faces were presented as neutral within the recognition task, entailing that faces displaying neutral expressions at encoding were presented the same during the recognition task whereas faces displaying negative expressions at encoding were presented with a different emotion (neutral) during the recognition task, thus reducing the influence of familiarity-based processes in assisting with negative face recognition in this paradigm.
Table 1. .
Primary Variable Descriptive Statistics and Correlations
| Variable | Mean (SD) | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
|---|---|---|---|---|---|---|---|---|
| 1. Negative memory accuracy (%) | 71.40 (10.37) | |||||||
| 2. Neutral memory accuracy (%) | 82.01 (13.23) | .43*** | ||||||
| 3. Emotional memory effect (difference) | −10.62 (12.78) | .36*** | −.68*** | |||||
| 4. Cortisol (basal, μg/dL, ln) | −2.66 (0.55) | −.12 | .08 | −.18* | ||||
| 5. Cortisol (20 min post conflict, μg/dL, ln) | −2.87 (0.49) | −.10 | .14 | −.23** | .79*** | |||
| 6. Cortisol (45 min post conflict, μg/dL, ln) | −2.97 (0.43) | −.06 | .17 | −.23** | .67*** | .82*** | ||
| 7. HPA axis activity (AUCg, ln) | 1.22 (0.47) | −.14 | .12 | −.24** | .89*** | .96*** | .86*** | |
| 8. Emotional memory-related right hippocampus–amygdala functional connectivity | −0.01 (0.08) | .06 | −.09 | .14 | −.39*** | −.36*** | −.27** | −.38*** |
n = 147; n = 104 with complete data.
p < .05.
p < .01.
p < .001.
HPA Axis Activity and Emotional Memory
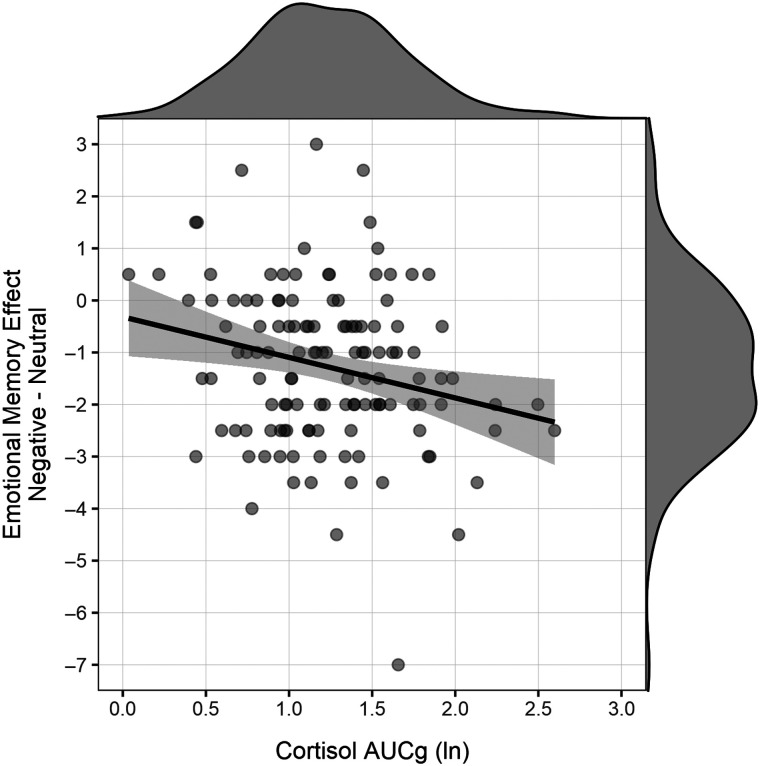
In our primary analyses, we first examined associations between HPA axis activity as measured by AUCg and emotional memory. As expected, we found a significant association between HPA axis activity and the emotional memory effect, r(124) = −.236, p = .008, BF10 = 6.16 (Figure 1), indicating that lower cortisol activity in late childhood was associated with greater emotional memory effects in adolescence. This inverse association with HPA axis activity was driven by an emotional memory effect rather than simply reflecting memory for negative or neutral stimuli individually. In particular, HPA axis activity was not significantly related to hit rates for negative stimuli, r(124) = −.144, p = .108, BF10 = 0.71, or to hit rates for neutral stimuli, r(124) = .116, p = .198, BF10 = 0.46, but the relation of HPA axis activity to memory was significantly different between negative and neutral stimuli, t(126) = 2.73, p = .007. In short, lower HPA axis activity in late childhood prospectively predicted relatively greater emotional memory effects (i.e., greater recall of negative vs. neutral emotional faces) in adolescence.
Figure 1. .
Total HPA axis activity during a conflict resolution task in late childhood was inversely associated with girls' emotional memory biases in adolescence, r = −.236, p = .008 (n = 126). Removing the outlier in emotional memory (i.e., the participant with a value of −7) did not alter this association, r = −.222, p = .013.
HPA Axis Activity and Hippocampus–Amygdala Functional Connectivity
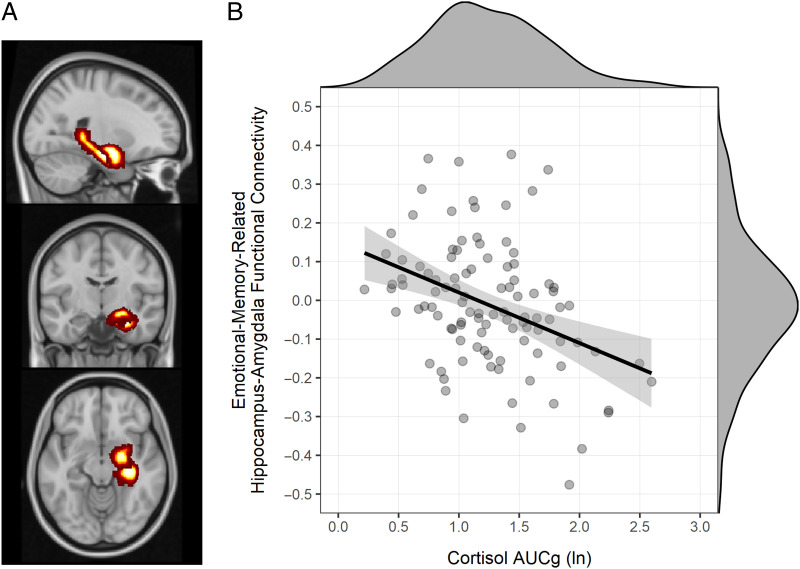
We next examined the association between HPA axis activity and negative emotional memory-related functional connectivity between the hippocampus and amygdala (Figure 2A) in a mixed-model ANOVA with Functional Connectivity as the dependent variable, fixed effects of HPA Axis Activity (centered), Hemisphere (right, left), and the HPA Axis Activity × Hemisphere interaction, and a random intercept per participant (i.e., nesting observations within participants). Although we did not have strong a priori reason to expect hemispheric differences in the association between HPA axis activity and emotional memory-related functional connectivity, we included hemisphere in the model to examine the possibility. In this analysis, we found a marginal HPA Axis Activity Effect, F(1, 104.4) = 2.83, p = .096, that was qualified by a significant HPA Axis Activity × Hemisphere interaction, F(1, 104.4) = 17.17, p < .001. Post hoc analyses revealed that this interaction was driven by a significant cortisol-connectivity association in the right hemisphere, p < .001, which significantly differed (p < .001) from the nonsignificant cortisol–connectivity association in the left hemisphere (p = .096). Therefore, the following analyses focus only on the right hemisphere.
Figure 2. .
Emotional memory-related functional connectivity between the right hippocampus and the right amygdala in adolescent girls (A) was inversely associated with total HPA axis activity during a conflict resolution task in late childhood (B), r = −.383, p < .001 (n = 104).
We next examined the longitudinal association between prior HPA axis activity and subsequent emotional memory-related (i.e., [Negative Remembered − Negative Forgotten] − [Neutral Remembered − Neutral Forgotten]) functional connectivity between the right hippocampus and the right amygdala. These analyses showed that HPA axis activity in late childhood was inversely associated with emotional memory-related functional connectivity between the right hippocampus and the right amygdala in adolescence, β = −.383, p < .001, BF10 = 342.28 (Figure 2B), explaining 14.6% of the variance (R2 = .146) in emotional memory-related functional connectivity. Secondary analyses using age 9 cortisol AUCg values produced similar albeit weaker results. Age 9 cortisol AUCg was an inverse predictor of emotional memory effects, r(124) = −.234, p = .008, and a marginally inverse predictor of emotional-memory-related hippocampus-amygdala functional connectivity, r(99) = −.177, p = .076.
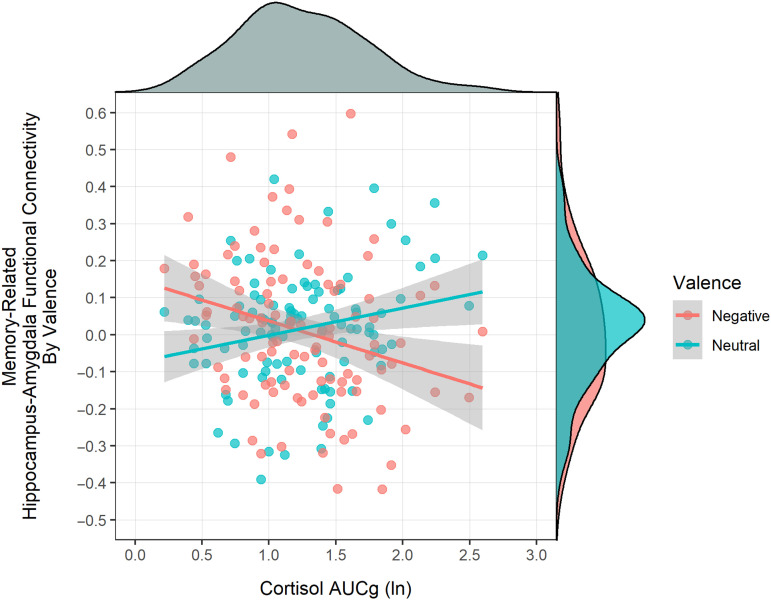
We probed this association further by examining whether HPA axis activity in late childhood predicted adolescent memory-related hippocampus–amygdala functional connectivity by valence. In this model, as expected, a significant Cortisol × Valence interaction emerged, F(1, 104.0) = 19.89, p < .001 (Figure 3). At lower levels of HPA axis activity in late childhood (mean − 1 SD), negative stimuli showed significant adolescent memory-related functional connectivity between the hippocampus and amygdala (M = 0.064, SE = 0.024), p = .004, whereas neutral stimuli did not (M = −0.019, SE = 0.024), p = .212, a difference that was statistically significant, t(104) = 2.95, p = .004. As HPA axis activity increased, however, memory-related functional connectivity between the hippocampus and amygdala decreased for negative stimuli, β = −.270, p = .006, BF10 = 6.78, whereas it increased for neutral stimuli, β = .230, p = .019, BF10 = 2.55. This divergent memory-related association showed that, at the mean level of HPA axis activity, memory-related hippocampus–amygdala functional connectivity was nonsignificant and positive for both negative stimuli (M = 0.010, SE = 0.017), p = .274, and neutral stimuli (M = 0.016, SE = 0.017), p = .170, and the difference between negative and neutral stimuli was no longer significant, t(104) = −0.30, p = .767. In summary, adolescent memory-related functional connectivity between the hippocampus and amygdala distinguished between negative and neutral stimuli. This effect was stronger when encoding emotional stimuli than neutral stimuli when late childhood HPA axis activity was low; however, as late childhood HPA axis activity increased, adolescent memory-related functional connectivity between the hippocampus and amygdala was no longer greater for negative stimuli than neutral stimuli.
Figure 3. .
Association of HPA axis activity with memory-related hippocampus–amygdala functional connectivity by valence. HPA axis activity was positively related to memory-related hippocampus–amygdala functional connectivity for neutral stimuli, whereas HPA axis activity was inversely related to memory-related hippocampus–amygdala functional connectivity for negative stimuli. HPA axis activity and valence showed a crossover dissociation in predicting memory-related functional connectivity.
Sensitivity Analyses
We next reran the above analyses controlling for potentially important covariates (i.e., time of day at cortisol sample collection, body mass index, depression symptoms at 16 years of age assessed by the Adolescent Symptom Inventory–Fourth Edition [Gadow, 2015], family receipt of public assistance at 9 years of age, verbal intelligence assessed using the Wechsler Intelligence Scale for Children, 3rd Edition [WISC-III; Wechsler, 1991], and age 11 pubertal status assessed with the Petersen Physical Development Scale [Petersen, Crockett, Richards, & Boxer, 1988]). Controlling for these covariates did not alter any of the above results. In brief, childhood HPA axis activity remained a significant predictor of adolescent emotional memory effects, β = −.239, p = .018, still interacted with hemisphere to predict emotional memory-related hippocampus–amygdala functional connectivity, F(1, 194.0) = 14.83, p < .001, and remained a significant inverse predictor of right hemisphere emotional memory-related hippocampus–amygdala functional connectivity, β = −.422, p < .001.
Supplemental Analyses
Although our primary analyses of interest related to emotional memory-related functional connectivity between the hippocampus and amygdala, we analyzed emotional memory-related neural activity in each of these regions independently as well. There was a significant HPA Axis Activity × Memory × Valence interaction, F(1, 1290.0) = 4.77, p = .029, but no interaction with ROI or hemisphere. Probing this, greater HPA axis activity predicted less BOLD activity during viewing of subsequently forgotten neutral faces, B = −0.012, but greater BOLD activity during viewing of subsequently remembered neutral faces, B = 0.037; the difference between these slopes was significant, t(1290.0) = 1.69, p = .047. No other slope (subsequently remembered negative: B = 0.005; subsequently forgotten negative: B = .032) significantly differed from one another (ps > .074).
Additionally, although we had chosen to use cortisol AUCg a priori, results from analyses using the individual cortisol samples (i.e., instead of using AUCg as the predictor, using Sample 1 [baseline], Sample 2 [20 min postconflict], or Sample 3 [45 min postconflict]—at 9 or 11 years of age) led to the same conclusions. All age 11 cortisol samples were inverse predictors both of emotional memory effects (Sample 1: r = −.179, p = .045; Sample 2: r = −.229, p = .010; Sample 3: r = −.230, p = .010), and of emotional-memory-related hippocampus-amygdala functional connectivity (Sample 1: r = −.386, p < .001; Sample 2: r = −.362, p < .001; Sample 3: r = −.271, p = .005). Controlling for the covariates led to similar conclusions. Similarly, all individual cortisol samples at age 9 were inverse predictors both of emotional memory effects (Sample 1: r = −.224, p = .012; Sample 2: r = −.226, p = .011; Sample 3: r = −.205, p = .021), and of emotional-memory-related hippocampus-amygdala functional connectivity (Sample 1: r = −.195, p = .050; Sample 2: r = −.263, p = .008; Sample 3: r = −.252, p = .011), including when controlling for the covariates.
Finally, we analyzed event-related hippocampus–amygdala functional connectivity at different levels of memory and emotion in order to determine the role of functional connectivity between these regions in our task. These analyses indicated significant hippocampus-amygdala functional connectivity in the left and right hemispheres during this task (ps < .001), and, in the right hemisphere alone, a significant memory effect (gPPI remembered: p = .040; forgotten: p = .720; contrast: p = .031), but no interaction between memory and valence (p = .650). Subsequent analyses determined the lack of interaction was due to neutral items producing memory-related functional connectivity between these regions in a similar magnitude to negative items. Perhaps faces are not truly neutral, or perhaps all faces being presented as neutral at test can explain this finding. In any case, HPA axis activity predicted the degree to which both negative and neutral valence items conferred memory-related functional connectivity between these two regions (Figure 3). Therefore, although there was no significant emotional-memory-related functional connectivity between these regions on average, their functional connectivity did play a role in relations among the HPA axis and emotional memory performance.
DISCUSSION
Despite the well-established effects and clinical relevance of negative emotional memory, little is known about how factors that can modulate negative emotional memory, such as cortisol levels, relate to negative emotional memory effects and their neural basis—especially in adolescents. We addressed this gap in this study by assessing the extent to which HPA axis activity during a common stressor in late childhood prospectively predicted negative emotional memory effects and negative emotional memory-related functional connectivity between the hippocampus and amygdala in adolescence. The results showed that HPA axis activity in late childhood was inversely related to emotional memory in adolescence. Moreover, HPA axis activity was inversely associated with memory-related functional connectivity between the right hippocampus and the right amygdala during encoding of negative stimuli, whereas it was positively associated with memory-related functional connectivity between the right hippocampus and the right amygdala during encoding of neutral stimuli. In other words, at low levels of prior HPA axis activity, memory-related hippocampus–amygdala functional connectivity was greater when encoding negative stimuli than when encoding neutral stimuli, but as HPA axis activity levels increased, this negative emotion advantage disappeared. These results therefore could be taken to suggest that childhood HPA axis activity in response to stressful experiences in late childhood may alter emotional memory-related hippocampus–amygdala functional connectivity and thereby emotional memory effects. In addition, these results support the theory of consolidation, as this theory predicts that glucocorticoids such as cortisol play a critical role in modulating hippocampus–amygdala activity and communication in ways that facilitate negative emotional memory (e.g., McGaugh, 2000).
Although age-dependent associations between emotional memory effects and HPA axis activity have been found in prior work with older and younger adults (Gutchess et al., 2019), our study is the first to have examined this association in a sample of adolescents. Although our study design did not permit us to examine age-related differences directly, somewhat surprisingly, our results showing a negative association between HPA axis activity and emotional memory effects were more consistent with results in older (mean age = 76.7 years)—not younger (mean age = 19.1 years)—adults (Gutchess et al., 2019). It is unclear why cortisol relates to emotional memory effects in adolescents in the same way as it does in older adults, but it is possible that young adults show a different HPA axis–emotional memory association than both adolescents and older adults because of full maturation without age-related decline in the HPA axis, hippocampus, and amygdala. Alternatively, adolescents exposed to early life stress show low HPA axis activity in early adolescence but high HPA axis activity in later adolescence (VanTieghem, 2020), so it may be the case that low late childhood HPA axis activity serves as a marker for high mid-adolescent HPA axis activity, which would then positively relate to emotional memory in mid-adolescence. The characteristics of our sample, that is, all female, mostly Black, and many raised in low-resourced homes, increase the plausibility of this interpretation given the greater likelihood of their exposure to stress/bias/discrimination experiences from family to structural levels. Speculation aside, our results indicate that preadolescent HPA axis activity is a moderately strong inverse predictor of mid-to-late adolescent emotional memory effects, which may have important implications for psychopathologies related to emotional memory biases.
Although we did not have strong a priori hypotheses regarding potential hemisphere differences, we are not the first to find that functional connectivity between the right hippocampus and the right amygdala may be more important in links between cortisol and emotional memory than functional connectivity between the left hippocampus and the left amygdala. For example, repeated exposure to traumatic memories strengthens functional connectivity between the right but not the left amygdala and hippocampus (Cisler et al., 2014), and another study found an inverse association between cortisol responses to stress and the right hippocampus–amygdala functional connectivity but not the left hippocampus–amygdala functional connectivity (Fan et al., 2015).
Our data suggest that HPA axis activity is intimately linked to communication between the right hippocampus and the right amygdala in the context of emotional memory. Hippocampus–amygdala interactions underpin not only emotional memory (Yonelinas & Ritchey, 2015; Phelps & LeDoux, 2005) but also stress-induced enhancements of memory (Roozendaal, Okuda, Van der Zee, & McGaugh, 2006; McGaugh, 2000; Akirav & Richter-Levin, 1999; though see Shields, McCullough, Ritchey, Ranganath, & Yonelinas, 2019). One intriguing implication of our results, therefore, is that resting or diurnal HPA axis activity may influence the extent to which acute stress modulates emotional memory specifically (e.g., Rohleder, Wolf, Kirschbaum, & Wolf, 2009). This possibility suggests that studies that examine the effects of acute stress on memory and relate those effects to cortisol responses should consider using measures of HPA axis function sensitive to baseline differences (e.g., cortisol AUCg, residualized change scores) rather than simple increases in cortisol.
Although our results are correlational, a discussion of traits associated with low HPA axis activity might help inform interpretation of these results. For example, in children and/or adolescents, low basal cortisol is associated with greater callous-unemotional traits and antisocial behavior (Hawes, Brennan, & Dadds, 2009), greater externalizing behaviors (Shirtcliff, Granger, Booth, & Johnson, 2005), and a variety of personality traits, including higher vulnerability and lower self-discipline (Laceulle, Nederhof, van Aken, & Ormel, 2015). Our finding that low HPA axis activity predicts a relatively greater emotional memory effect adds to this body of literature, indicating that low cortisol in childhood or early adolescence may be a trait marker of emotion and stress disorder vulnerability, which should be tested in future research.
This study has several strengths—including its longitudinal design, a relatively large sample size for an fMRI study, examination of an adolescent sample, use of a parent–child interaction paradigm to elicit stress in an ecologically valid experience common in the daily lives of adolescents, and event-related functional connectivity analyses. Some limitations also are noted. First, other work has found age effects in links between cortisol and emotional memory (Gutchess et al., 2019), and it is unclear whether our results would generalize to a younger or older sample. Relatedly, gonadal hormones can also influence memory and alter the effects of the HPA axis on memory (Shields et al., 2017; Barros, Tufik, & Andersen, 2015), further suggesting that the associations we observed may be restricted to the developmental window we assessed. Second, given that there are sex differences in HPA axis functioning (Timmons, Hamadeh, Devries, & Tarnopolsky, 2005; Kirschbaum, Kudielka, Gaab, Schommer, & Hellhammer, 1999), our focus on adolescent girls may limit the generalizability of our findings to female adolescents. Third, the emotional memory task used presented neutral faces as neutral at both encoding (for analyzing event-related contrasts) and retrieval, whereas it presented negative faces as negative at encoding only (they were presented as neutral at retrieval). Together, these conditions were presumably responsible for the relatively greater memory for neutral faces than negative faces, which is opposite from most findings on emotional memory (e.g., Kensinger, 2007, 2009). Although we have no strong reason to expect that the ways in which cortisol relates to the neural basis of a relative bias toward emotional versus neutral memory would differ with a different memory task, it is possible, and future research should therefore attempt to replicate these results with another emotional memory task. Fourth, the study protocol did not allow much time to acclimate to the lab before the conflict task. Fifth, the number of cortisol samples taken did not permit appropriate characterization of cortisol trajectories over the course of the study. Sixth, contrary to expectations, the problem-solving task did not produce a significant cortisol increase. Although we can only speculate as to why the task failed to elicit a stress response, it is possible that there was an insufficient acclimation period to the laboratory setting before the task or that the task did not engender motivated performance to the degree required to elicit a robust stress response (Dickerson & Kemeny, 2004). Associations between cortisol AUCg and either functional connectivity and emotional memory should therefore be considered presumably more related to baseline HPA axis functioning than reactivity, which is consistent with the baseline cortisol sample relating to functional connectivity and emotional memory in the same way as AUCg or any of the subsequent cortisol measurements. Finally, the sample with complete data may be unrepresentative of the broader sample from which it was drawn, although our analysis of demographics did not suggest that to be the case.
Conclusion
Although individual differences in HPA axis functioning have been linked to emotional memory effects, the neural basis of this link was unclear. We aimed to address this gap by extending the model to include HPA axis functioning in late childhood as a predictor of adolescent emotional memory. We found that HPA axis activity was inversely related to both emotional memory effects and emotional memory-related functional connectivity between the hippocampus and amygdala in girls. These results therefore show that individual differences in HPA axis functioning are important for understanding emotional memory and hippocampus–amygdala function, which suggests that the role of HPA axis activity in emotional memory posited by the theory of consolidation may be evident over many years. More speculatively, these findings could be taken to suggest that individual differences in hormone levels or hormonal responses to common stressors may presage variation in neural connectivity and emotional memory effects, which may result in a trajectory of emotional health problems for those adolescent girls. Although we often wish we could forget negative experiences, the very hormones that help prime our bodies to deal with stress may act on the neural circuitry of those memories to alter our ability to forget them.
Acknowledgments
This research was funded by National Institutes of Health grants R01MH093650 (A. E. G., K. K., E. E. F.), R01MH066167 (K. K.), and R03MH116519 (A. E. G.); a Young Investigator grant from the Brain and Behavior Research Foundation (#26780; V. V.); and a grant from the University of California (UC) Consortium on the Developmental Science of Adolescence awarded from the UC Office of the President, Multicampus Research Programs and Initiatives (grant ID: MRP-17-454825; A. E. G., C. E. H., V. V.).
Reprint requests should be sent to Amanda E. Guyer, Department of Human Ecology, Center for Mind and Brain, University of California Davis, 267 Cousteau Place, Davis, CA 95618, or via e-mail: aeguyer@ucdavis.edu.
Author Contributions
Grant S. Shields: Conceptualization; Formal analysis; Methodology; Visualization; Writing—Original draft; Writing—Review & editing. Camelia E. Hostinar: Conceptualization; Funding acquisition; Writing—Review & editing. Veronika Vilgis: Data curation; Funding acquisition; Writing—Review & editing. Erika E. Forbes: Data curation; Funding acquisition; Investigation; Project administration; Writing—Review & editing. Alison E. Hipwell: Data curation; Funding acquisition; Investigation; Project administration; Writing—Review & editing. Kate Keenan: Conceptualization; Data curation; Funding acquisition; Investigation; Methodology; Project administration; Writing—Review & editing. Amanda E. Guyer: Conceptualization; Data curation; Funding acquisition; Investigation; Methodology; Project administration; Resources; Supervision; Writing—Review & editing.
Funding Information
National Institute of Mental Health (http://dx.doi.org/10.13039/100000025), grant numbers: R01MH066167, R01MH093650, R03MH116519. University of California Office of the President, grant number: MRP-17-454825. Brain and Behavior Research Foundation (http://dx.doi.org/10.13039/100000874), grant number: 26780.
Diversity in Citation Practices
A retrospective analysis of the citations in every article published in this journal from 2010 to 2020 has revealed a persistent pattern of gender imbalance: Although the proportions of authorship teams (categorized by estimated gender identification of first author/last author) publishing in the Journal of Cognitive Neuroscience (JoCN) during this period were M(an)/M = .408, W(oman)/M = .335, M/W = .108, and W/W = .149, the comparable proportions for the articles that these authorship teams cited were M/M = .579, W/M = .243, M/W = .102, and W/W = .076 (Fulvio et al., JoCN, 33:1, pp. 3–7). Consequently, JoCN encourages all authors to consider gender balance explicitly when selecting which articles to cite and gives them the opportunity to report their article's gender citation balance.
Note
That is, anova(lmerTest::lmer()).
REFERENCES
- Akirav, I., & Richter-Levin, G. (1999). Biphasic modulation of hippocampal plasticity by behavioral stress and basolateral amygdala stimulation in the rat. Journal of Neuroscience, 19, 10530–10535. DOI: https://doi.org/10.1523/JNEUROSCI.19-23-10530.1999, PMID: 10575049, PMCID: PMC6782400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akirav, I., & Richter-Levin, G. (2002). Mechanisms of amygdala modulation of hippocampal plasticity. Journal of Neuroscience, 22, 9912–9921. DOI: https://doi.org/10.1523/JNEUROSCI.22-22-09912.2002, PMID: 12427848, PMCID: PMC6757810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldwin, C. M., Levenson, M. R., & Spiro, A. (1994). Vulnerability and resilience to combat exposure: Can stress have lifelong effects? Psychology and Aging, 9, 34–44. DOI: https://doi.org/10.1037/0882-7974.9.1.34, PMID: 8185866 [DOI] [PubMed] [Google Scholar]
- Algamal, M., Ojo, J. O., Lungmus, C. P., Muza, P., Cammarata, C., Owens, M. J., et al. (2018). Chronic hippocampal abnormalities and blunted HPA axis in an animal model of repeated unpredictable stress. Frontiers in Behavioral Neuroscience, 12, 150. DOI: https://doi.org/10.3389/fnbeh.2018.00150, PMID: 30079015, PMCID: PMC6062757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen, S. L., & Teicher, M. H. (2008). Stress, sensitive periods and maturational events in adolescent depression. Trends in Neurosciences, 31, 183–191. DOI: https://doi.org/10.1016/j.tins.2008.01.004, PMID: 18329735 [DOI] [PubMed] [Google Scholar]
- Bangasser, D. A., & Valentino, R. J. (2014). Sex differences in stress-related psychiatric disorders: Neurobiological perspectives. Frontiers in Neuroendocrinology, 35, 303–319. DOI: https://doi.org/10.1016/j.yfrne.2014.03.008, PMID: 24726661, PMCID: PMC4087049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barros, L. A., Tufik, S., & Andersen, M. L. (2015). The role of progesterone in memory: An overview of three decades. Neuroscience and Biobehavioral Reviews, 49, 193–204. DOI: https://doi.org/10.1016/j.neubiorev.2014.11.015, PMID: 25434881 [DOI] [PubMed] [Google Scholar]
- Beck, A. T., & Bredemeier, K. (2016). A unified model of depression: Integrating clinical, cognitive, biological, and evolutionary perspectives. Clinical Psychological Science, 4, 596–619. DOI: 10.1177/2167702616628523 [DOI] [Google Scholar]
- Behzadi, Y., Restom, K., Liau, J., & Liu, T. T. (2007). A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. Neuroimage, 37, 90–101. DOI: https://doi.org/10.1016/j.neuroimage.2007.04.042, PMID: 17560126, PMCID: PMC2214855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill, L. (2012). A half-truth is a whole lie: On the necessity of investigating sex influences on the brain. Endocrinology, 153, 2541–2543. DOI: https://doi.org/10.1210/en.2011-2167, PMID: 22492307, PMCID: PMC3359593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill, L., & McGaugh, J. L. (1998). Mechanisms of emotional arousal and lasting declarative memory. Trends in Neurosciences, 21, 294–299. DOI: https://doi.org/10.1016/S0166-2236(97)01214-9, PMID: 9683321 [DOI] [PubMed] [Google Scholar]
- Casey, B. J., Jones, R. M., & Hare, T. A. (2008). The adolescent brain. Annals of the New York Academy of Sciences, 1124, 111–126. DOI: https://doi.org/10.1196/annals.1440.010, PMID: 18400927, PMCID: PMC2475802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai, X. J., Castañón, A. N., Ongür, D., & Whitfield-Gabrieli, S. (2012). Anticorrelations in resting state networks without global signal regression. Neuroimage, 59, 1420–1428. DOI: https://doi.org/10.1016/j.neuroimage.2011.08.048, PMID: 21889994, PMCID: PMC3230748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisler, J. M., Steele, J. S., Lenow, J. K., Smitherman, S., Everett, B., Messias, E., et al. (2014). Functional reorganization of neural networks during repeated exposure to the traumatic memory in posttraumatic stress disorder: An exploratory fMRI study. Journal of Psychiatric Research, 48, 47–55. DOI: https://doi.org/10.1016/j.jpsychires.2013.09.013, PMID: 24139810, PMCID: PMC4019667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diana, R. A., Yonelinas, A. P., & Ranganath, C. (2007). Imaging recollection and familiarity in the medial temporal lobe: A three-component model. Trends in Cognitive Sciences, 11, 379–386. DOI: https://doi.org/10.1016/j.tics.2007.08.001, PMID: 17707683 [DOI] [PubMed] [Google Scholar]
- Dickerson, S. S., & Kemeny, M. E. (2004). Acute stressors and cortisol responses: A theoretical integration and synthesis of laboratory research. Psychological Bulletin, 130, 355–391. DOI: https://doi.org/10.1037/0033-2909.130.3.355, PMID: 15122924 [DOI] [PubMed] [Google Scholar]
- Ebner, N. C., Riediger, M., & Lindenberger, U. (2010). FACES—A database of facial expressions in young, middle-aged, and older women and men: Development and validation. Behavior Research Methods, 42, 351–362. DOI: https://doi.org/10.3758/BRM.42.1.351, PMID: 20160315 [DOI] [PubMed] [Google Scholar]
- Fan, Y., Pestke, K., Feeser, M., Aust, S., Pruessner, J. C., Böker, H., et al. (2015). Amygdala–hippocampal connectivity changes during acute psychosocial stress: Joint effect of early life stress and oxytocin. Neuropsychopharmacology, 40, 2736–2744. DOI: https://doi.org/10.1038/npp.2015.123, PMID: 25924202, PMCID: PMC4864649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feduccia, A. A., & Mithoefer, M. C. (2018). MDMA-assisted psychotherapy for PTSD: Are memory reconsolidation and fear extinction underlying mechanisms? Progress in Neuropsychopharmacology & Biological Psychiatry, 84, 221–228. DOI: https://doi.org/10.1016/j.pnpbp.2018.03.003, PMID: 29524515 [DOI] [PubMed] [Google Scholar]
- Franz, C. E., O'Brien, R. C., Hauger, R. L., Mendoza, S. P., Panizzon, M. S., Prom-Wormley, E., et al. (2011). Cross-sectional and 35-year longitudinal assessment of salivary cortisol and cognitive functioning: The Vietnam era twin study of aging. Psychoneuroendocrinology, 36, 1040–1052. DOI: https://doi.org/10.1016/j.psyneuen.2011.01.002, PMID: 21295410, PMCID: PMC3130089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadow, K. D. (2015). The Symptom Inventories: An annotated bibliography. Stony Brook, NY: Checkmate Plus. [Google Scholar]
- Gomez, R. G., Posener, J. A., Keller, J., DeBattista, C., Solvason, B., & Schatzberg, A. F. (2009). Effects of major depression diagnosis and cortisol levels on indices of neurocognitive function. Psychoneuroendocrinology, 34, 1012–1018. DOI: https://doi.org/10.1016/j.psyneuen.2009.01.017, PMID: 19261389 [DOI] [PubMed] [Google Scholar]
- Gotlib, I. H., & Joormann, J. (2010). Cognition and depression: Current status and future directions. Annual Review of Clinical Psychology, 6, 285–312. DOI: https://doi.org/10.1146/annurev.clinpsy.121208.131305, PMID: 20192795, PMCID: PMC2845726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greendale, G. A., Kritz-Silverstein, D., Seeman, T., & Barrett-Connor, E. (2000). Higher basal cortisol predicts verbal memory loss in postmenopausal women: Rancho Bernardo study. Journal of the American Geriatrics Society, 48, 1655–1658. DOI: https://doi.org/10.1111/j.1532-5415.2000.tb03878.x, PMID: 11129757 [DOI] [PubMed] [Google Scholar]
- Gunnar, M., & Quevedo, K. (2007). The neurobiology of stress and development. Annual Review of Psychology, 58, 145–173. DOI: https://doi.org/10.1146/annurev.psych.58.110405.085605, PMID: 16903808 [DOI] [PubMed] [Google Scholar]
- Gutchess, A., Alves, A. N., Paige, L. E., Rohleder, N., & Wolf, J. M. (2019). Age differences in the relationship between cortisol and emotional memory. Psychology and Aging, 34, 655–664. DOI: https://doi.org/10.1037/pag0000367, PMID: 31180698, PMCID: PMC6682424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyer, A. E., Choate, V. R., Grimm, K. J., Pine, D. S., & Keenan, K. (2011). Emerging depression is associated with face memory deficits in adolescent girls. Journal of the American Academy of Child and Adolescent Psychiatry, 50, 180–190. DOI: https://doi.org/10.1016/j.jaac.2010.11.008, PMID: 21241955, PMCID: PMC3072062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyer, A. E., Silk, J. S., & Nelson, E. E. (2016). The neurobiology of the emotional adolescent: From the inside out. Neuroscience and Biobehavioral Reviews, 70, 74–85. DOI: https://doi.org/10.1016/j.neubiorev.2016.07.037, PMID: 27506384, PMCID: PMC5074886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison, T. M., Burggren, A. C., Small, G. W., & Bookheimer, S. Y. (2016). Altered memory-related functional connectivity of the anterior and posterior hippocampus in older adults at increased genetic risk for Alzheimer's disease. Human Brain Mapping, 37, 366–380. DOI: https://doi.org/10.1002/hbm.23036, PMID: 26503161, PMCID: PMC4715627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawes, D. J., Brennan, J., & Dadds, M. R. (2009). Cortisol, callous-unemotional traits, and pathways to antisocial behavior. Current Opinion in Psychiatry, 22, 357–362. DOI: https://doi.org/10.1097/YCO.0b013e32832bfa6d, PMID: 19455037 [DOI] [PubMed] [Google Scholar]
- Het, S., Ramlow, G., & Wolf, O. T. (2005). A meta-analytic review of the effects of acute cortisol administration on human memory. Psychoneuroendocrinology, 30, 771–784. DOI: https://doi.org/10.1016/j.psyneuen.2005.03.005, PMID: 15919583 [DOI] [PubMed] [Google Scholar]
- Hostinar, C. E., McQuillan, M. T., Mirous, H. J., Grant, K. E., & Adam, E. K. (2014). Cortisol responses to a group public speaking task for adolescents: Variations by age, gender, and race. Psychoneuroendocrinology, 50, 155–166. DOI: https://doi.org/10.1016/j.psyneuen.2014.08.015, PMID: 25218656, PMCID: PMC4253051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankord, R., Solomon, M. B., Albertz, J., Flak, J. N., Zhang, R., & Herman, J. P. (2011). Stress vulnerability during adolescent development in rats. Endocrinology, 152, 629–638. DOI: https://doi.org/10.1210/en.2010-0658, PMID: 21106877, PMCID: PMC3037163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joëls, M., Fernandez, G., & Roozendaal, B. (2011). Stress and emotional memory: A matter of timing. Trends in Cognitive Sciences, 15, 280–288. DOI: https://doi.org/10.1016/j.tics.2011.04.004, PMID: 21571575 [DOI] [PubMed] [Google Scholar]
- Keenan, K., Hipwell, A., Babinski, D., Bortner, J., Henneberger, A., Hinze, A., et al. (2013). Examining the developmental interface of cortisol and depression symptoms in young adolescent girls. Psychoneuroendocrinology, 38, 2291–2299. DOI: https://doi.org/10.1016/j.psyneuen.2013.04.017, PMID: 23726646, PMCID: PMC3776001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keenan, K., Hipwell, A., Chung, T., Stepp, S., Stouthamer-Loeber, M., Loeber, R., et al. (2010). The Pittsburgh Girls Study: Overview and initial findings. Journal of Clinical Child and Adolescent Psychology, 39, 506–521. DOI: https://doi.org/10.1080/15374416.2010.486320, PMID: 20589562, PMCID: PMC2946599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keenan, K., Hipwell, A., Hinze, A., & Babinski, D. (2009). Equanimity to excess: Inhibiting the expression of negative emotion is associated with depression symptoms in girls. Journal of Abnormal Child Psychology, 37, 739–747. DOI: https://doi.org/10.1007/s10802-009-9301-9, PMID: 19184401, PMCID: PMC2744501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kensinger, E. A. (2007). Negative emotion enhances memory accuracy: Behavioral and neuroimaging evidence. Current Directions in Psychological Science, 16, 213–218. DOI: 10.1111/j.1467-8721.2007.00506.x [DOI] [Google Scholar]
- Kensinger, E. A. (2009). Remembering the details: Effects of emotion. Emotion Review, 1, 99–113. DOI: https://doi.org/10.1177/1754073908100432, PMID: 19421427, PMCID: PMC2676782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschbaum, C., Kudielka, B. M., Gaab, J., Schommer, N. C., & Hellhammer, D. H. (1999). Impact of gender, menstrual cycle phase, and oral contraceptives on the activity of the hypothalamus-pituitary-adrenal axis. Psychosomatic Medicine, 61, 154–162. DOI: https://doi.org/10.1097/00006842-199903000-00006, PMID: 10204967 [DOI] [PubMed] [Google Scholar]
- Kuhlman, K. R., Repetti, R. L., Reynolds, B. M., & Robles, T. F. (2016). Change in parent–child conflict and the HPA-axis: Where should we be looking and for how long? Psychoneuroendocrinology, 68, 74–81. DOI: https://doi.org/10.1016/j.psyneuen.2016.02.029, PMID: 26963373, PMCID: PMC5403246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuntsche, E., Knibbe, R., Engels, R., & Gmel, G. (2010). Being drunk to have fun or to forget problems? Identifying enhancement and coping drinkers among risky drinking adolescents. European Journal of Psychological Assessment, 26, 46–54. DOI: 10.1027/1015-5759/a000007 [DOI] [Google Scholar]
- Laceulle, O. M., Nederhof, E., van Aken, M. A. G., & Ormel, J. (2015). Adolescent personality: Associations with basal, awakening, and stress-induced cortisol responses. Journal of Personality, 83, 262–273. DOI: https://doi.org/10.1111/jopy.12101, PMID: 24730365 [DOI] [PubMed] [Google Scholar]
- Lee, B. K., Glass, T. A., McAtee, M. J., Wand, G. S., Bandeen-Roche, K., Bolla, K. I., et al. (2007). Associations of salivary cortisol with cognitive function in the Baltimore memory study. Archives of General Psychiatry, 64, 810–818. DOI: https://doi.org/10.1001/archpsyc.64.7.810, PMID: 17606815 [DOI] [PubMed] [Google Scholar]
- Li, G., Cherrier, M. M., Tsuang, D. W., Petrie, E. C., Colasurdo, E. A., Craft, S., et al. (2006). Salivary cortisol and memory function in human aging. Neurobiology of Aging, 27, 1705–1714. DOI: https://doi.org/10.1016/j.neurobiolaging.2005.09.031, PMID: 16274857 [DOI] [PubMed] [Google Scholar]
- Lundqvist, D., Flykt, A., & Öhman, A. (1998). The Karolinska Directed Emotional Faces (KDEF) [CD-ROM]. Stockholm: Department of Clinical Neuroscience, Karolinska Institute. [Google Scholar]
- Lupien, S. J., de Leon, M., de Santi, S., Convit, A., Tarshish, C., Nair, N. P. V., et al. (1998). Cortisol levels during human aging predict hippocampal atrophy and memory deficits. Nature Neuroscience, 1, 69–73. DOI: https://doi.org/10.1038/271, PMID: 10195112 [DOI] [PubMed] [Google Scholar]
- Lupien, S. J., Lecours, A. R., Lussier, I., Schwartz, G., Nair, N. P. V., & Meaney, M. J. (1994). Basal cortisol levels and cognitive deficits in human aging. Journal of Neuroscience, 14, 2893–2903. DOI: https://doi.org/10.1523/jneurosci.14-05-02893.1994, PMID: 8182446, PMCID: PMC6577490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maroun, M., & Akirav, I. (2008). Arousal and stress effects on consolidation and reconsolidation of recognition memory. Neuropsychopharmacology, 33, 394–405. DOI: https://doi.org/10.1038/sj.npp.1301401, PMID: 17429409 [DOI] [PubMed] [Google Scholar]
- Mather, M., Clewett, D., Sakaki, M., & Harley, C. W. (2016). Norepinephrine ignites local hotspots of neuronal excitation: How arousal amplifies selectivity in perception and memory. Behavioral and Brain Sciences, 39, e200. DOI: https://doi.org/10.1017/S0140525X15000667, PMID: 26126507, PMCID: PMC5830137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick, C. M., & Mathews, I. Z. (2007). HPA function in adolescence: Role of sex hormones in its regulation and the enduring consequences of exposure to stressors. Pharmacology, Biochemistry, and Behavior, 86, 220–233. DOI: https://doi.org/10.1016/j.pbb.2006.07.012, PMID: 16901532 [DOI] [PubMed] [Google Scholar]
- McCullough, A. M., Ritchey, M., Ranganath, C., & Yonelinas, A. P. (2015). Differential effects of stress-induced cortisol responses on recollection and familiarity-based recognition memory. Neurobiology of Learning and Memory, 123, 1–10. DOI: https://doi.org/10.1016/j.nlm.2015.04.007, PMID: 25930175, PMCID: PMC4530068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGaugh, J. L. (2000). Memory—A century of consolidation. Science, 287, 248–251. DOI: https://doi.org/10.1126/science.287.5451.248, PMID: 10634773 [DOI] [PubMed] [Google Scholar]
- McGaugh, J. L. (2015). Consolidating memories. Annual Review of Psychology, 66, 1–24. DOI: https://doi.org/10.1146/annurev-psych-010814-014954, PMID: 25559113 [DOI] [PubMed] [Google Scholar]
- Meir Drexler, S., Merz, C. J., Jentsch, V. L., & Wolf, O. T. (2019). How stress and glucocorticoids timing-dependently affect extinction and relapse. Neuroscience and Biobehavioral Reviews, 98, 145–153. DOI: https://doi.org/10.1016/j.neubiorev.2018.12.029, PMID: 30594494 [DOI] [PubMed] [Google Scholar]
- Melby, J. N., & Conger, R. D. (2001). The Iowa Family Interaction Rating Scales: Instrument summary. In Kerig P. & Lindahl K. (Eds.), Family observational coding systems: Resources for systematic research (pp. 33–58). Mahwah, NJ: Erlbaum. [Google Scholar]
- Minear, M., & Park, D. C. (2004). A lifespan database of adult facial stimuli. Behavior Research Methods, Instruments, and Computers, 36, 630–633. DOI: https://doi.org/10.3758/BF03206543, PMID: 15641408 [DOI] [PubMed] [Google Scholar]
- Murphy, K., Birn, R. M., Handwerker, D. A., Jones, T. B., & Bandettini, P. A. (2009). The impact of global signal regression on resting state correlations: Are anti-correlated networks introduced? Neuroimage, 44, 893–905. DOI: https://doi.org/10.1016/j.neuroimage.2008.09.036, PMID: 18976716, PMCID: PMC2750906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagamine, M., Noguchi, H., Takahashi, N., Kim, Y., & Matsuoka, Y. (2017). Effect of cortisol diurnal rhythm on emotional memory in healthy young adults. Scientific Reports, 7, 10158. DOI: https://doi.org/10.1038/s41598-017-10002-z, PMID: 28860606, PMCID: PMC5579256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson, E. E. (2004). Adolescent and adult facial expressions stimuli database. Bethesda, MD: Section on Development and Affective Neuroscience, National Institute of Mental Health. [Google Scholar]
- Pagliaccio, D., Luby, J. L., Bogdan, R., Agrawal, A., Gaffrey, M. S., Belden, A. C., et al. (2014). Stress-system genes and life stress predict cortisol levels and amygdala and hippocampal volumes in children. Neuropsychopharmacology, 39, 1245–1253. DOI: https://doi.org/10.1038/npp.2013.327, PMID: 24304824, PMCID: PMC3957120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen, A. C., Crockett, L., Richards, M., & Boxer, A. (1988). A self-report measure of pubertal status: Reliability, validity, and initial norms. Journal of Youth and Adolescence, 17, 117–133. DOI: https://doi.org/10.1007/BF01537962, PMID: 24277579 [DOI] [PubMed] [Google Scholar]
- Phelps, E. A., & LeDoux, J. E. (2005). Contributions of the amygdala to emotion processing: From animal models to human behavior. Neuron, 48, 175–187. DOI: https://doi.org/10.1016/j.neuron.2005.09.025, PMID: 16242399 [DOI] [PubMed] [Google Scholar]
- Pine, D. S., Lissek, S., Klein, R. G., Mannuzza, S., Moulton, J. L., Guardino, M., et al. (2004). Face-memory and emotion: Associations with major depression in children and adolescents. Journal of Child Psychology and Psychiatry, 45, 1199–1208. DOI: https://doi.org/10.1111/j.1469-7610.2004.00311.x, PMID: 15335340 [DOI] [PubMed] [Google Scholar]
- Preuß, D., Schoofs, D., & Wolf, O. T. (2009). Associations between endogenous cortisol levels and emotional memory in young women: Influence of encoding instructions. Stress, 12, 379–387. DOI: https://doi.org/10.1080/10253890802524592, PMID: 19006010 [DOI] [PubMed] [Google Scholar]
- Pruessner, J. C., Kirschbaum, C., Meinlschmid, G., & Hellhammer, D. H. (2003). Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology, 28, 916–931. DOI: https://doi.org/10.1016/S0306-4530(02)00108-7, PMID: 12892658 [DOI] [PubMed] [Google Scholar]
- Putman, P., Van Honk, J., Kessels, R. P. C., Mulder, M., & Koppeschaar, H. P. F. (2004). Salivary cortisol and short and long-term memory for emotional faces in healthy young women. Psychoneuroendocrinology, 29, 953–960. DOI: https://doi.org/10.1016/j.psyneuen.2003.09.001, PMID: 15177712 [DOI] [PubMed] [Google Scholar]
- Rissman, J., Gazzaley, A., & D'Esposito, M. (2004). Measuring functional connectivity during distinct stages of a cognitive task. Neuroimage, 23, 752–763. DOI: https://doi.org/10.1016/j.neuroimage.2004.06.035, PMID: 15488425 [DOI] [PubMed] [Google Scholar]
- Roberson-Nay, R., McClure, E. B., Monk, C. S., Nelson, E. E., Guyer, A. E., Fromm, S. J., et al. (2006). Increased amygdala activity during successful memory encoding in adolescent major depressive disorder: An fMRI study. Biological Psychiatry, 60, 966–973. DOI: https://doi.org/10.1016/j.biopsych.2006.02.018, PMID: 16603133 [DOI] [PubMed] [Google Scholar]
- Rohleder, N., Wolf, J. M., Kirschbaum, C., & Wolf, O. T. (2009). Effects of cortisol on emotional but not on neutral memory are correlated with peripheral glucocorticoid sensitivity of inflammatory cytokine production. International Journal of Psychophysiology, 72, 74–80. DOI: https://doi.org/10.1016/j.ijpsycho.2008.03.010, PMID: 18824040 [DOI] [PubMed] [Google Scholar]
- Roozendaal, B., Okuda, S., de Quervain, D. J.-F., & McGaugh, J. L. (2006). Glucocorticoids interact with emotion-induced noradrenergic activation in influencing different memory functions. Neuroscience, 138, 901–910. DOI: https://doi.org/10.1016/j.neuroscience.2005.07.049, PMID: 16310958 [DOI] [PubMed] [Google Scholar]
- Roozendaal, B., Okuda, S., Van der Zee, E. A., & McGaugh, J. L. (2006). Glucocorticoid enhancement of memory requires arousal-induced noradrenergic activation in the basolateral amygdala. Proceedings of the National Academy of Sciences, U.S.A., 103, 6741–6746. DOI: https://doi.org/10.1073/pnas.0601874103, PMID: 16611726, PMCID: PMC1458951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saad, Z. S., Gotts, S. J., Murphy, K., Chen, G., Jo, H. J., Martin, A., et al. (2012). Trouble at rest: How correlation patterns and group differences become distorted after global signal regression. Brain Connectivity, 2, 25–32. DOI: https://doi.org/10.1089/brain.2012.0080, PMID: 22432927, PMCID: PMC3484684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sazma, M. A., Shields, G. S., & Yonelinas, A. P. (2019). The effects of post-encoding stress and glucocorticoids on episodic memory in humans and rodents. Brain and Cognition, 133, 12–23. DOI: https://doi.org/10.1016/j.bandc.2018.10.005, PMID: 31178013, PMCID: PMC6559250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scaramella, L. V., Conger, R. D., Spoth, R., & Simons, R. L. (2002). Evaluation of a social contextual model of delinquency: A cross-study replication. Child Development, 73, 175–195. DOI: https://doi.org/10.1111/1467-8624.00399, PMID: 14717251 [DOI] [PubMed] [Google Scholar]
- Schmidt, S. R., & Saari, B. (2007). The emotional memory effect: Differential processing or item distinctiveness? Memory & Cognition, 35, 1905–1916. DOI: https://doi.org/10.3758/BF03192924, PMID: 18265607 [DOI] [PubMed] [Google Scholar]
- Schwabe, L., Joëls, M., Roozendaal, B., Wolf, O. T., & Oitzl, M. S. (2012). Stress effects on memory: An update and integration. Neuroscience and Biobehavioral Reviews, 36, 1740–1749. DOI: https://doi.org/10.1016/j.neubiorev.2011.07.002, PMID: 21771612 [DOI] [PubMed] [Google Scholar]
- Scoville, W. B., & Milner, B. (1957). Loss of recent memory after bilateral hippocampal lesions. Journal of Neurology, Neurosurgery, and Psychiatry, 20, 11–21. DOI: https://doi.org/10.1136/jnnp.20.1.11, PMID: 13406589, PMCID: PMC497229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields, G. S., Dunn, T. M., Trainor, B. C., & Yonelinas, A. P. (2019). Determining the biological associates of acute cold pressor post-encoding stress effects on human memory: The role of salivary interleukin-1β. Brain, Behavior, and Immunity, 81, 178–187. DOI: https://doi.org/10.1016/j.bbi.2019.06.011, PMID: 31176727, PMCID: PMC6754786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields, G. S., McCullough, A. M., Ritchey, M., Ranganath, C., & Yonelinas, A. P. (2019). Stress and the medial temporal lobe at rest: Functional connectivity is associated with both memory and cortisol. Psychoneuroendocrinology, 106, 138–146. DOI: https://doi.org/10.1016/j.psyneuen.2019.04.001, PMID: 30981087, PMCID: PMC6615559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields, G. S., Sazma, M. A., McCullough, A. M., & Yonelinas, A. P. (2017). The effects of acute stress on episodic memory: A meta-analysis and integrative review. Psychological Bulletin, 143, 636–675. DOI: https://doi.org/10.1037/bul0000100, PMID: 28368148, PMCID: PMC5436944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields, G. S., & Slavich, G. M. (2017). Lifetime stress exposure and health: A review of contemporary assessment methods and biological mechanisms. Social and Personality Psychology Compass, 11, e12335. DOI: https://doi.org/10.1111/spc3.12335, PMID: 28804509, PMCID: PMC5552071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirer, W. R., Jiang, H., Price, C. M., Ng, B., & Greicius, M. D. (2015). Optimization of rs-fMRI pre-processing for enhanced signal-noise separation, test-retest reliability, and group discrimination. Neuroimage, 117, 67–79. DOI: https://doi.org/10.1016/j.neuroimage.2015.05.015, PMID: 25987368 [DOI] [PubMed] [Google Scholar]
- Shirtcliff, E. A., Granger, D. A., Booth, A., & Johnson, D. (2005). Low salivary cortisol levels and externalizing behavior problems in youth. Development and Psychopathology, 17, 167–184. DOI: https://doi.org/10.1017/S0954579405050091, PMID: 15971765 [DOI] [PubMed] [Google Scholar]
- Silverman, M. N., & Sternberg, E. M. (2012). Glucocorticoid regulation of inflammation and its functional correlates: From HPA axis to glucocorticoid receptor dysfunction. Annals of the New York Academy of Sciences, 1261, 55–63. DOI: https://doi.org/10.1111/j.1749-6632.2012.06633.x, PMID: 22823394, PMCID: PMC3572859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroud, L. R., Papandonatos, G. D., Williamson, D. E., & Dahl, R. E. (2011). Sex differences in cortisol response to corticotropin releasing hormone challenge over puberty: Pittsburgh Pediatric Neurobehavioral Studies. Psychoneuroendocrinology, 36, 1226–1238. DOI: https://doi.org/10.1016/j.psyneuen.2011.02.017, PMID: 21489699, PMCID: PMC3270708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmons, B. W., Hamadeh, M. J., Devries, M. C., & Tarnopolsky, M. A. (2005). Influence of gender, menstrual phase, and oral contraceptive use on immunological changes in response to prolonged cycling. Journal of Applied Physiology, 99, 979–985. DOI: https://doi.org/10.1152/japplphysiol.00171.2005, PMID: 15879167 [DOI] [PubMed] [Google Scholar]
- Tone, E. B., Schmidt, S. M., & Davis, J. S. (n.d.). The Georgia State University Diverse Faces Stimulus Set. [Google Scholar]
- Tottenham, N., Tanaka, J. W., Leon, A. C., McCarry, T., Nurse, M., Hare, T. A., et al. (2009). The NimStim set of facial expressions: Judgments from untrained research participants. Psychiatry Research, 168, 242–249. DOI: https://doi.org/10.1016/j.psychres.2008.05.006, PMID: 19564050, PMCID: PMC3474329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Honk, J., Kessels, R. P. C., Putman, P., Jager, G., Koppeschaar, H. P. F., & Postma, A. (2003). Attentionally modulated effects of cortisol and mood on memory for emotional faces in healthy young males. Psychoneuroendocrinology, 28, 941–948. DOI: https://doi.org/10.1016/S0306-4530(02)00116-6, PMID: 12892660 [DOI] [PubMed] [Google Scholar]
- VanTieghem, M. R. (2020). Longitudinal changes in amygdala, hippocampus, and cortisol development following early caregiving adversity (Doctoral dissertation). Columbia University. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuchinich, S., Angelelli, J., & Gatherum, A. (1996). Context and development in family problem solving with preadolescent children. Child Development, 67, 1276–1288. DOI: https://doi.org/10.1111/j.1467-8624.1996.tb01795.x, PMID: 8706521 [PubMed] [Google Scholar]
- Wechsler, D. (1991). Wechsler intelligence scale for children: 3rd edition manual. San Antonio, TX: Psychological Corporation. [Google Scholar]
- Whitfield-Gabrieli, S., & Nieto-Castanon, A. (2012). Conn: A functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connectivity, 2, 125–141. DOI: https://doi.org/10.1089/brain.2012.0073, PMID: 22642651 [DOI] [PubMed] [Google Scholar]
- Yeo, B. T. T., Tandi, J., & Chee, M. W. L. (2015). Functional connectivity during rested wakefulness predicts vulnerability to sleep deprivation. Neuroimage, 111, 147–158. DOI: https://doi.org/10.1016/j.neuroimage.2015.02.018, PMID: 25700949 [DOI] [PubMed] [Google Scholar]
- Yonelinas, A. P. (2013). The hippocampus supports high-resolution binding in the service of perception, working memory and long-term memory. Behavioural Brain Research, 254, 34–44. DOI: https://doi.org/10.1016/j.bbr.2013.05.030, PMID: 23721964, PMCID: PMC3773061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonelinas, A. P., Ranganath, C., Ekstrom, A. D., & Wiltgen, B. J. (2019). A contextual binding theory of episodic memory: Systems consolidation reconsidered. Nature Reviews Neuroscience, 20, 364–375. DOI: https://doi.org/10.1038/s41583-019-0150-4, PMID: 30872808, PMCID: PMC7233541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonelinas, A. P., & Ritchey, M. (2015). The slow forgetting of emotional episodic memories: An emotional binding account. Trends in Cognitive Sciences, 19, 259–267. DOI: https://doi.org/10.1016/j.tics.2015.02.009, PMID: 25836045, PMCID: PMC4414918 [DOI] [PMC free article] [PubMed] [Google Scholar]