Abstract
Background
Progressive cardiac fibrosis is the central aspect of the myocardial involvement in systemic sclerosis (SSc). We hypothesized that circulating biomarkers of the cardiac fibrosis may be useful in the early diagnosis of the cardiac manifestation in this disease. Thus, we investigated the potential correlations between the levels of galectin-3, soluble suppression of tumorigenicity-2 (sST2) and the echocardiographic markers of the myocardial mechanics in SSc patients.
Methods
Forty patients (57.3 ± 13.7 years, 36 female) were investigated. In addition to the conventional echocardiography, tissue Doppler and speckle tracking-derived strain techniques were used to assess the function of both ventricles and atria. To estimate the correlations between galectin-3 and sST2 levels and the echocardiographic variables, partial correlation method was used with age as correcting factor.
Results
In age adjusted analysis galectin-3 level showed significant correlation with left ventricular global longitudinal strain (r = 0.460, p = 0.005); grade of left ventricular diastolic dysfunction (r = 0.394, p = 0.013); septal e’ (r = − 0.369, p = 0.021); septal E/e’ (r = 0.380, p = 0.017) and with the grade of mitral regurgitation (r = 0.323, p = 0.048). No significant correlation was found between sST2 levels and the echocardiographic variables.
Conclusions
Galectin-3 levels, but not sST2 levels show significant correlation with the parameters of the left ventricular systolic and diastolic function. Galectin-3 may be a useful biomarker for the screening and early diagnosis of SSc patients with cardiac involvement.
Keywords: Systemic sclerosis, Myocardial fibrosis, Galectin-3, sST2, Global longitudinal strain, Diastolic dysfunction
Introduction
Systemic sclerosis (SSc) is a connective tissue disease characterized by vascular abnormalities and diffuse fibrosis of the skin and various internal organs [1]. Although its pathogenesis is still not fully elucidated, recurrent ischaemic episodes resulting from coronary microcirculatory abnormalities may be responsible for the myocardial fibrosis [2], which represents the primary myocardial involvement of the disease [3]. Myocardial fibrosis may lead to left ventricular (LV) diastolic dysfunction, which is highly prevalent in SSc [4–6]. Although impaired LV ejection fraction is not common in this disease [7], myocardial fibrosis may eventuate in subclinically impaired LV systolic function, as it was proved by speckle tracking-derived global longitudinal strain (GLS) data [8, 9]. Subclinical right ventricular (RV) dysfunction was also proved, even in SSc patients without manifest pulmonary hypertension, using tissue Doppler or speckle tracking measurements [10–12]. Besides, impaired left (LA) and right atrial (RA) function have recently been reported in SSc, by the help of speckle tracking technique [10, 13–15].
Cardiac involvement implies poor prognosis in SSc [16, 17], thus its early, biomarker-based screening would be crucial. Utility of the well know cardiac biomarker, N-terminal pro–B-type natriuretic peptide (NT-proBNP) has already been proved in SSc [3, 18, 19]. In contrast, the potential clinical value of the circulating biomarkers reflecting myocardial fibrosis is less known in this disease.
Galectin-3 is a beta-galactoside-binding protein member of the lectin family that activates a variety of profibrotic factors, supports fibroblast proliferation and transformation, and mediates collagen production [20]. It plays an important role in cardiac remodelling by enhancing myocardial fibrosis [21]. In addition, it has been shown to be an independent predictor of outcome in heart failure [22, 23].
Soluble suppression of tumorigenicity-2 (sST2) is member of the interleukin-1 receptor family. It enhances myocardial hypertrophy and fibrosis by blocking the favourable influence of IL-33 [24]. Its prognostic value has been reported both in acute and chronic heart failure [24, 25].
Both galectin-3 and sST2 concentrations showed significant correlation with the echocardiographic markers of LV or LA size and function in heart failure patients [26–31]. Besides, in patients with pulmonary arterial hypertension, significant associations were reported between galectin-3 levels and the RV size and function whereas sST2 levels reflected disease severity [32–34].
Thus, in this study we aimed to investigate the potential associations between the levels of galectin-3 and sST2 and the echocardiographic markers of the myocardial mechanics in SSc patients.
Patients and methods
Study population
Our prospective study included 40 consecutive SSc patients diagnosed in the tertiary centre of the Department of Rheumatology and Immunology, University of Pécs. All enrolled cases fulfilled the updated ACR/EULAR classification criteria [35]. Patients with pulmonary arterial hypertension, atrial fibrillation, significant left sided valvular disease or known coronary artery disease were excluded from the study. Baseline clinical, laboratory and spirometry data were collected at enrolment. Duration of the disease was defined as time between the onset of the first non-Raynaud symptom of SSc and the inclusion, in years.
The study complied with the Declaration of Helsinki. The institutional ethics committee approved the study. Written informed consent was obtained from all patients.
Echocardiography
All patients underwent echocardiographic examination performed by a single investigator using Philips EPIQ 7 ultrasound system (Philips Healthcare, Best, The Netherlands). M-mode and 2D data were collected: LV ejection fraction measured by biplane Simpson’s method; basal, mid-cavity, and longitudinal dimensions of the RV corrected for body surface area; tricuspid annular plane systolic excursion (TAPSE); RV fractional area change (RVFAC); maximal and minimal diameters of the inferior vena cava (IVC); collapsibility index of IVC (the percent decrease in the diameter during inspiration). LV mass was calculated according to the Devereux formula and corrected for body surface area (LV mass index). RV wall thickness was measured at end-diastole [36, 37]. Mitral and tricuspid regurgitations were evaluated according to the recent recommendation [38]. In addition to the spectral Doppler parameters of the trans-mitral and trans-tricuspid flow (E, A), myocardial systolic (S), early- (e’) and late- (a’) diastolic velocities were measured from apical four-chamber view at the lateral and septal border of the mitral annulus as well as on the tricuspid annulus using pulsed tissue Doppler imaging. Lateral and septal mitral myocardial velocities were averaged. Mitral and tricuspid E/A and E/e’ ratios were calculated [39]. Systolic pulmonary artery pressure was calculated from tricuspid regurgitation velocity added to the RA pressure. RA pressure (5 to 15 mmHg) was estimated using the diameter and collapsibility index of IVC [37]. Doppler measurements were obtained from ≥3 consecutive beats during end-expiratory apnoea. Elevated RV filling pressure was diagnosed if tricuspid E/e′ > 6 [36]. LV diastolic function was graded according to the current recommendation [39]. In “indeterminate” cases further parameters were also considered for the classification according to the 2020 revision of the recommendation [40].
Strain measurements
For strain analysis, three consecutive heart cycles were recorded digitally. Care was taken to obtain true apical images using standard anatomic landmarks in each view. Foreshortening was avoided. Recordings were processed off-line, using a dedicated software (QLab, Philips Healthcare, Andover, MA, USA). Analysis was performed by a single investigator, blinded to the clinical and conventional echocardiographic data.
To estimate LV GLS, apical four-, three- and two-chamber movies were obtained using 2D echocardiography. The frame rate was set between 50 and 55 frames per second. Regional peak systolic longitudinal strain was determined in all 17 segments. LV GLS was automatically provided by the software as the average of the regional peak systolic longitudinal strain values.
For atrial speckle tracking analysis, atrium-focused apical four- and two-chamber view movies (four-chamber view for RA analysis) were obtained. The frame rate was set between 80 and 90 frames per second. The onset of R-wave on the electrocardiogram was used as zero-reference point of the strain analysis. Reservoir strain was defined as the peak systolic strain, just before mitral valve opening. This was followed by a plateau and a second late peak at the onset of the P-wave indicating the contractile strain. Conduit strain was calculated as the difference between reservoir and contractile strain. Results obtained in apical four- and two-chamber views were averaged for LA strain analysis [41].
Atrial volume curves were generated by the same software using the endocardial borders created for speckle tracking analysis. The following atrial volumes were obtained: maximal volume (Vmax) at the end of T wave on electrocardiogram, just before the opening of the mitral valve; minimal volume (Vmin) at QRS complex, just at the closure of the mitral valve; and preceding atrial contraction, at the beginning of P wave (Vp). LA volumes obtained in apical four- and two-chamber views were averaged. All volume values were corrected for body surface area (Vmax-, Vmin- and Vp index).
Biomarkers
Blood samples were obtained immediately prior to the echocardiographic studies. Samples were stored at − 80 ∘C until testing.
Analysis of galectin-3 levels was performed using Human Galectin-3 Platinum ELISA kit developed by eBioscience (San Diego, CA, USA).
Analysis of sST2 levels was performed using Presage ST2 assay kit developed by Critical Diagnostics (San Diego, CA, USA).
Plasma concentrations of NT-proBNP were analysed by electrochemiluminescence immunoassay (Elecsys 2010 system, Roche Diagnostics, Mannheim, Germany).
Statistical analysis
Categorical data were expressed as frequencies and percentages; continuous data were expressed as mean ± SD. Mann–Whitney U-test was used for comparisons of variables between two subgroups. Since concentrations of galectin-3, sST2 and NT-proBNP did not show normal distribution, logarithmic transformation was performed. Pearson bivariate method was used to investigate the correlations between ln galectin-3, ln sST2 and the other single variables. As age-related changes in the echocardiographic parameters are well known, in a second step, partial correlation method was applied using age as correcting factor. Partial regression plots were used to visualize these correlations. As creatinine or estimated glomerular filtration rate did not show correlation with galecin-3 or sST2 levels in our study, partial correlations were not adjusted for renal function. A p value of < 0.05 was considered significant. Data were analysed using IBM SPSS 25 statistical software.
Results
A total of 40 SSc patients were enrolled into the study. Detailed clinical data of the patients are reported in Tables 1 and 2.
Table 1.
Baseline characteristics of the systemic sclerosis population (n = 40) and their correlations with galectin-3 and sST2 levels
| Clinical variable | Value | Correlation with ln galectin-3 | Correlations with ln galectin-3, corrected for age | Correlations with ln sST2 | Correlations with ln sST2, corrected for age | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| r | p | r | p | r | p | r | p | |||
| Age (years) | 57.3 ± 13.7 | 0.059 | 0.719 | −0.010 | 0.951 | |||||
| Body surface area (m2) | 1.8 ± 0.2 | 0.206 | 0.202 | 0.206 | 0.208 | 0.063 | 0.700 | 0.063 | 0.704 | |
| Disease duration (years) | 8.0 ± 6.2 | 0.406 | 0.009 | 0.406 | 0.010 | 0.038 | 0.817 | 0.042 | 0.799 | |
| Modified Rodnan skin score | 12.6 ± 9.6 | −0.291 | 0.069 | −0.286 | 0.077 | 0.200 | 0.216 | 0.205 | 0.210 | |
| New York Heart Association functional class | I n (%) | 14 (35%) | 0.179 | 0.269 | 0.171 | 0.297 | −0.021 | 0.899 | −0.018 | 0.912 |
| II n (%) | 16 (40%) | |||||||||
| III n (%) | 10 (25%) | |||||||||
| 6-min walking distance (m) | 397.6 ± 78.3 | −0.107 | 0.516 | −.086 | 0.607 | −0.001 | 0.996 | 0.001 | 0.996 | |
| Modified Borg dyspnoea index | 1.8 ± 1.8 | 0.229 | 0.161 | 0.221 | 0.183 | −0.234 | 0.151 | −0.263 | 0.111 | |
| Forced expiratory volume in 1 s (%) | 92.1 ± 15.5 | −0.320 | 0.044 | −0.334 | 0.032 | −0.067 | 0.682 | −0.066 | 0.689 | |
| Forced vital capacity (%) | 94.9 ± 16.0 | −0.246 | 0.126 | −0.272 | 0.094 | −0.157 | 0.333 | −0.160 | 0.330 | |
| Diffusing capacity of carbon monoxide (%) | 62.7 ± 16.2 | −0.318 | 0.045 | −0.324 | 0.044 | 0.106 | 0.515 | 0.123 | 0.456 | |
| Erythrocyte sedimentation rate (mm/h) | 19.6 ± 13.5 | −0.070 | 0.666 | −0.079 | 0.633 | 0.099 | 0.545 | 0.101 | 0.541 | |
| C-reactive protein (mg/l) | 4.2 ± 6.8 | 0.092 | 0.572 | 0.104 | 0.527 | −0.118 | 0.468 | −0.122 | 0.460 | |
| Creatinine (μmol/l) | 63.8 ± 13.2 | −0.013 | 0.936 | −0.042 | 0.802 | 0.000 | 0.999 | 0.005 | 0.976 | |
| NT-proBNP (pg/ml; ln in correlations) | 177.5 ± 148.6 | 0.094 | 0.566 | 0.081 | 0.623 | −0.129 | 0.426 | −0.131 | 0.425 | |
| Galectin-3 (ng/ml; ln in correlations) | 12.9 ± 4.0 | −0.217 | 0.178 | −0.217 | 0.184 | |||||
| sST2 (ng/ml; ln in correlations) | 28.5 ± 11.3 | −0.217 | 0.178 | −0.217 | 0.184 | |||||
Statistically significant p-values (p < 0.05) are formatted in bold
Table 2.
Galectin-3 and sST2 levels in various subgroups of the study population
| Clinical variable | Galectin-3 | sST2 | ||
|---|---|---|---|---|
| Level (ng/ml) | p* | Level (ng/ml) | p* | |
| Female patients (n = 36; 90%) | 13.2 ± 4.1 | 0.123 | 27.3 ± 9.8 | 0.207 |
| Male patients (n = 4; 10%) | 9.6 ± 0.2 | 40.1 ± 18.7 | ||
| Limited cutaneous form (n = 12; 30%) | 12.9 ± 3.1 | 0.805 | 30.1 ± 9.5 | 0.389 |
| Diffuse cutaneous form (n = 28; 70%) | 12.9 ± 4.4 | 27.9 ± 12.1 | ||
| Anti-centromere antibody positive (n = 8; 20%) | 11.3 ± 2.8 | 0.354 | 28.6 ± 9.4 | 0.871 |
| Anti-centromere antibody negative (n = 32; 80%) | 13.0 ± 4.1 | 29.1 ± 11.8 | ||
| Anti-topoisomerase antibody positive (n = 19; 47.5%) | 12.8 ± 4.5 | 0.901 | 27.0 ± 8.6 | 0.989 |
| Anti-topoisomerase antibody negative (n = 21; 52.5%) | 12.8 ± 3.7 | 29.5 ± 13.6 | ||
| Patients with diabetes (n = 2; 5%) | 13.4 ± 6.3 | 0.877 | 36.5 ± 15.2 | 0.400 |
| Patients without diabetes (n = 38; 95%) | 12.8 ± 4.0 | 28.1 ± 11.2 | ||
| Current smokers (n = 3; 7.5%) | 11.5 ± 5.5 | 0.518 | 30.7 ± 27.4 | 0.518 |
| Non-smokers (n = 37; 92.5%) | 13.1 ± 3.8 | 26.9 ± 9.4 | ||
| Hypertension (n = 25; 62.5%) | 13.3 ± 3.9 | 0.581 | 26.9 ± 9.6 | 0.391 |
| No hypertension (n = 15; 37.5%) | 12.2 ± 4.3 | 31.2 ± 13.6 | ||
| Treated with angiotensin convertase enzyme inhibitors (n = 12; 30%) | 14.1 ± 3.6 | 0.218 | 26.6 ± 9.0 | 0.938 |
| Not treated with angiotensin convertase enzyme inhibitors (n = 28; 70%) | 12.3 ± 4.1 | 28.4 ± 12.0 | ||
| Treated with calcium channel blockers (n = 18; 45%) | 12.8 ± 3.6 | 0.925 | 27.1 ± 10.3 | 0.545 |
| Not treated with calcium channel blockers (n = 22; 55%) | 12.9 ± 4.4 | 29.7 ± 12.2 | ||
| Treated with loop diuretics (n = 12; 30%) | 15.5 ± 3.8 | 0.006 | 25.5 ± 7.9 | 0.457 |
| Not treated with loop diuretics (n = 28; 70%) | 11.7 ± 3.6 | 29.8 ± 12.4 | ||
| Treated with mineralocorticoid receptor antagonists (n = 10; 25%) | 13.5 ± 4.3 | 0.569 | 29.7 ± 10.5 | 0.432 |
| Not treated with mineralocorticoid receptor antagonists (n = 30; 75%) | 12.7 ± 4.0 | 28.1 ± 11.7 | ||
| Treated with pentoxifylline (n = 20; 50%) | 12.8 ± 3.6 | 0.758 | 28.4 ± 11.3 | 0.968 |
| Not treated with pentoxifylline (n = 20;50%) | 12.9 ± 4.5 | 28.7 ± 11.6 | ||
* Mann–Whitney U-test. Statistically significant p-values (p < 0.05) are formatted in bold
Standard echocardiographic and tissue Doppler measurements were successfully performed in all patients. GLS, LA atrial strain and RA strain data were successfully obtained in 38, 39 and 37 patients, respectively. Rest of the measurements have failed due to inadequate acoustic window or foreshortening.
Correlations between clinical variables and biomarker levels are reported in Table 1. Galectin-3 and sST2 values did not show correlation with each other or with NT-proBNP levels. Neither galectin-3 nor sST2 levels showed significant correlation with age. Galectin-3 levels showed positive correlation with the duration of SSc, even in age adjusted analysis. Both Forced expiratory volume in 1 s (FEV1) and Diffusing capacity of carbon monoxide (DLCO) showed significant inverse correlation with galectin-3 levels. No significant correlations were found between sST2 levels and clinical variables. Table 2 comprises the galectin-3 and sST2 levels in various subgroups of the study population. Gender-related differences were not significant. In patients requiring loop diuretic treatment significantly higher galectin-3 levels were found.
LV ejection fraction was preserved (≥ 55%) in 39 (97.5%), whereas mildly reduced (45–54%) in 1 (2.5%) patients. LV diastolic function was impaired (Grade I diastolic dysfunction) in 17 (42.5%) patients whereas echocardiographic data suggested elevated LV filling pressure in 12 (30%) patients (all in Grade II). No patients with restrictive pattern (Grade III) were found.
RVFAC < 35%, TAPSE < 16 mm and tricuspid S < 10 cm/s were found in 1 (2.5%), 1 (2.5%) and 4 (10%) patients, respectively. Tricuspid E/e’ suggested elevated RV filling pressure in 13 (32.5%) patients. Elevated pulmonary artery systolic pressure (tricuspid Vmax> 2.8 m/s) was obtained in 11 patients with a maximum of 41 mmHg. Detailed echocardiographic data of the study population as well as correlations between galectin-3 and sST2 levels and the echocardiographic variables are reported in Tables 3 and 4.
Table 3.
Correlations of galectin-3 (ln) and ST2 (ln) with the echocardiographic parameters of the LV and LA size and mechanics in SSc patients
| Parameter | Value | Correlations with ln galectin-3 | Correlations with ln galectin-3, corrected for age |
Correlations with ln ST2 | Correlations with ln ST2, corrected for age |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| r | p | r | p | r | p | r | p | |||
| LV ejection fraction (%) | 60.6 ± 4.6 | −0.273 | 0.089 | −0.267 | 0.100 | 0.082 | 0.614 | 0.082 | 0.618 | |
| LV GLS (%) | −17.5 ± 2.3 | 0.454 | 0.005 | 0.460 | 0.005 | 0.003 | 0.988 | − 0.007 | 0.969 | |
| LV mass index (g/m2) | 93.5 ± 19.7 | 0.009 | 0.958 | −0.039 | 0.814 | −0.147 | 0.367 | −0.184 | 0.262 | |
| Grade of mitral regurgitation | Mild n (%) | 37 (92.5%) | 0.321 | 0.046 | 0.323 | 0.048 | −0.078 | 0.637 | −0.080 | 0.632 |
| Moderate n (%) | 3 (7.5%) | |||||||||
| Severe n (%) | 0 (0%) | |||||||||
| Mitral E (cm/s) | 73.9 ± 15.7 | 0.006 | 0.970 | 0.028 | 0.867 | −0.022 | 0.892 | −0.027 | 0.869 | |
| Mitral A (cm/s) | 72.8 ± 16.9 | 0.320 | 0.044 | 0.334 | 0.038 | 0.132 | 0.417 | 0.156 | 0.342 | |
| Septal S (cm/s) | 7.3 ± 1.2 | −0.224 | 0.166 | − 0.220 | 0.178 | 0.080 | 0.624 | 0.084 | 0.612 | |
| Septal e’ (cm/s) | 7.4 ± 2.0 | −0.306 | 0.055 | −0.369 | 0.021 | −0.088 | 0.591 | −0.131 | 0.426 | |
| Septal a’ (cm/s) | 8.7 ± 1.6 | −0.012 | 0.939 | −0.018 | 0.912 | 0.181 | 0.263 | 0.183 | 0.265 | |
| Septal E/e’ | 10.5 ± 2.8 | 0.375 | 0.017 | 0.380 | 0.017 | 0.002 | 0.989 | 0.006 | 0.970 | |
| Lateral S (cm/s) | 9.2 ± 1.8 | −0.008 | 0.963 | 0.004 | 0.980 | 0.212 | 0.189 | 0.214 | 0.190 | |
| Lateral e’ (cm/s) | 9.2 ± 2.8 | −0.132 | 0.418 | −0.119 | 0.471 | 0.019 | 0.906 | 0.016 | 0.922 | |
| Lateral a’ (cm/s) | 10.6 ± 2.5 | 0.031 | 0.848 | 0.012 | 0.944 | 0.276 | 0.085 | 0.298 | 0.066 | |
| Lateral E/e’ | 8.7 ± 3.0 | 0.109 | 0.502 | 0.106 | 0.522 | −0.063 | 0.699 | − 0.063 | 0.705 | |
| Averaged S (cm/s) | 8.3 ± 1.3 | −0.107 | 0.510 | −0.093 | 0.573 | 0.182 | 0.261 | 0.189 | 0.248 | |
| Averaged e’ (cm/s) | 8.3 ± 2.2 | −0.224 | 0.165 | −0.248 | 0.128 | −0.027 | 0.867 | −0.045 | 0.784 | |
| Averaged a’ (cm/s) | 9.7 ± 1.7 | 0.017 | 0.918 | −0.001 | 0.998 | 0.282 | 0.078 | 0.298 | 0.066 | |
| Averaged E/e’ | 9.3 ± 2.3 | 0.252 | 0.117 | 0.246 | 0.132 | −0.032 | 0.846 | −0.030 | 0.856 | |
| Grade of LV diastolic function | Normal n (%) | 11 (27.5%) | 0.325 | 0.040 | 0.394 | 0.013 | −0.073 | 0.655 | −0.091 | 0.582 |
| Impaired relaxation (Grade I) n (%) | 17 (42.5%) | |||||||||
| Pseudonormal (Grade II) n (%) | 12 (30%) | |||||||||
| LA Vmax index (ml/m2) | 22.8 ± 7.4 | 0.016 | 0.925 | 0.001 | 0.994 | −0.294 | 0.081 | −0.392 | 0.076 | |
| LA Vmin index (ml/m2) | 10.3 ± 5.0 | 0.056 | 0.747 | 0.044 | 0.803 | −0.305 | 0.071 | −0.410 | 0.078 | |
| LA Vp index (ml/m2) | 14.2 ± 6.1 | 0.095 | 0.580 | 0.088 | 0.617 | −0.237 | 0.164 | −0.360 | 0.111 | |
| LA reservoir strain (%) | 43.0 ± 8.7 | −0.148 | 0.383 | −0.154 | 0.370 | −0.045 | 0.790 | −0.005 | 0.978 | |
| LA conduit strain (%) | 23.5 ± 6.9 | −0.117 | 0.491 | −0.137 | 0.426 | −0.066 | 0.700 | 0.005 | 0.979 | |
| LA contractile strain (%) | 19.5 ± 4.8 | −0.102 | 0.549 | −0.106 | 0.538 | 0.012 | 0.945 | −0.014 | 0.936 | |
Statistically significant p-values (p < 0.05) are formatted in bold
Table 4.
Correlations of galectin-3 (ln) and ST2 (ln) with the echocardiographic parameters of the RV and RA size and mechanics in SSc patients
| Parameter | Value | Correlations with ln galectin-3 | Correlations with ln galectin-3, corrected for age |
Correlations with ln ST2 | Correlations with ln ST2, corrected for age |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| r | p | r | p | r | p | r | p | |||
| Pulmonary artery systolic pressure (mm Hg) | 28.6 ± 7.5 | 0.225 | 0.251 | 0.260 | 0.191 | −0.174 | 0.375 | −0.188 | 0.348 | |
| RV basal diameter index (mm/m2) | 18.3 ± 2.4 | −0.254 | 0.119 | −0.253 | 0.126 | −0.015 | 0.928 | −0.015 | 0.928 | |
| RV mid-cavity diameter index (mm/m2) | 13.0 ± 2.1 | −0.169 | 0.311 | −0.169 | 0.318 | −0.037 | 0.827 | −0.037 | 0.828 | |
| RV longitudinal diameter index (mm/m2) | 30.0 ± 3.6 | −0.011 | 0.948 | −0.011 | 0.948 | −0.342 | 0.135 | −0.341 | 0.148 | |
| Inferior vena cava (mm) | 13.2 ± 3.9 | −0.053 | 0.765 | −0.052 | 0.775 | −0.255 | 0.146 | −0.254 | 0.153 | |
| Collapsibility index (%) | 55.2 ± 10.7 | 0.134 | 0.449 | 0.134 | 0.457 | −0.131 | 0.462 | −0.132 | 0.465 | |
| RV wall thickness (mm) | 5.1 ± 1.2 | −0.091 | 0.597 | −0.106 | 0.545 | −0.051 | 0.766 | −0.086 | 0.622 | |
| RV fractional area change (%) | 47.9 ± 6.8 | −0.138 | 0.423 | −0.142 | 0.415 | −0.001 | 0.997 | 0.013 | 0.943 | |
| Tricuspid annular plane systolic excursion (mm) | 21.4 ± 2.6 | −0.249 | 0.121 | −0.243 | 0.136 | 0.315 | 0.248 | 0.321 | 0.284 | |
| Grade of tricuspid regurgitation | Mild n (%) | 38 (95%) | 0.168 | 0.307 | 0.165 | 0.321 | −0.105 | 0.523 | − 0.105 | 0.530 |
| Moderate n (%) | 2 (5%) | |||||||||
| Severe n (%) | 0 (0%) | |||||||||
| Tricuspid E cm/s | 47.4 ± 8.0 | −0.073 | 0.658 | −0.087 | 0.602 | −0.060 | 0.751 | −0.050 | 0.767 | |
| Tricuspid A cm/s | 40.1 ± 8.5 | 0.371 | 0.020 | 0.372 | 0.022 | −0.160 | 0.330 | −0.162 | 0.331 | |
| Tricuspid S (cm/s) | 12.4 ± 2.0 | −0.170 | 0.293 | −0.169 | 0.305 | 0.363 | 0.221 | 0.363 | 0.227 | |
| Tricuspid e’ (cm/s) | 10.0 ± 2.8 | −0.038 | 0.817 | −0.009 | 0.956 | 0.006 | 0.972 | 0.001 | 0.996 | |
| Tricuspid a’ (cm/s) | 13.1 ± 2.9 | −0.100 | 0.539 | −0.147 | 0.372 | 0.154 | 0.342 | 0.182 | 0.267 | |
| Tricuspid E/e’ ratio | 5.2 ± 1.6 | 0.003 | 0.985 | 0.004 | 0.983 | −0.001 | 0.995 | −0.008 | 0.963 | |
| RA Vmax index (mL/m2) | 19.9 ± 5.8 | −0.013 | 0.947 | 0.017 | 0.929 | −0.048 | 0.800 | −0.101 | 0.602 | |
| RA Vmin index (mL/m2) | 8.1 ± 4.0 | 0.227 | 0.227 | 0.245 | 0.201 | −0.136 | 0.473 | −0.163 | 0.397 | |
| RA Vp index (mL/m2) | 14.3 ± 5.5 | 0.058 | 0.760 | 0.083 | 0.668 | −0.112 | 0.555 | −0.156 | 0.419 | |
| RA reservoir strain (%) | 45.3 ± 9.0 | −0.239 | 0.167 | −0.236 | 0.179 | 0.218 | 0.209 | 0.229 | 0.193 | |
| RA conduit strain (%) | 24.2 ± 6.4 | −0.213 | 0.220 | −0.210 | 0.233 | 0.199 | 0.253 | 0.220 | 0.212 | |
| RA contractile strain (%) | 22.0 ± 6.1 | −0.220 | 0.204 | −0.231 | 0.188 | 0.065 | 0.713 | 0.056 | 0.753 | |
Statistically significant p-values are formatted in bold (p < 0.05)
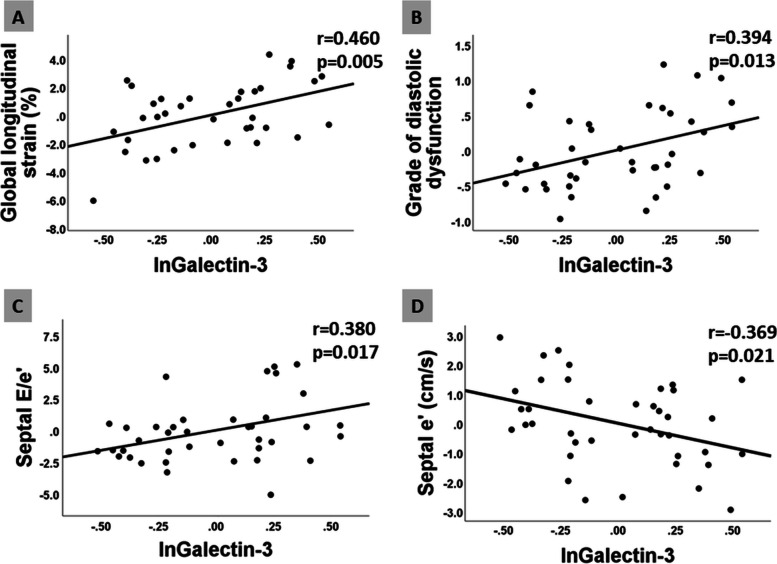
Galectin-3 levels showed significant correlation with GLS (Fig. 1A), with the grade of LV diastolic dysfunction (Fig. 1B), with septal E/e’(Fig. 1C), and with the grade of mitral regurgitation, even in age adjusted analysis. Correlation between galectin-3 level and septal e’ became significant after age correction (Fig. 1D). No significant correlation was found between sST2 values and the echocardiographic variables.
Fig. 1.
Partial regression plots demonstrate that in age adjusted analyses galectin-3 (ln) correlates with LV GLS (A); with the grade of LV diastolic dysfunction (B); with septal E/e’(C) and with septal e’ (D)
Discussion
Progressive cardiac fibrosis has been reported as a central aspect of the myocardial involvement in SSc [3]. Thus - although the causative role of galectin-3 and sST2 is not proved in the pathogenesis of SSc - we hypothesized that circulating biomarkers of the cardiac fibrosis may be useful in screening and early diagnosis of the cardiac manifestation in this disease. Therefore, in the present study we aimed to investigate the correlations between the levels of sST2, galectin-3 and the echocardiographic markers of the myocardial mechanics in SSc patients.
ST2 is part of the interleukin (IL)-1 receptor family which may be found on cardiac myocytes and fibroblasts. ST2 exists in two forms. The transmembrane isoform (ST2L) is responsible for the cardioprotective effect of IL-33: IL-33 has antihypertrophic and antifibrotic effects transduced by ST2L. The soluble isoform (sST2), in contrast, eliminates the cardioprotective pathway of the IL-33/ST2L interaction, by binding circulating IL-33 molecules. All clinical conditions that increase wall stress, inflammation and macrophage activation increase sST2 level and may therefore lead to an increase of cardiac fibrosis [42]. sST2 has been reported to be increased in patients with heart failure, myocardial infarction, hypertension, severe obesity, diabetes and pulmonary arterial hypertension [33, 34, 43]. In the PRIDE (ProBNP Investigation of Dyspnea in the Emergency Department) study, sST2 concentrations were higher in patients with acute heart failure and were strongly predictive of mortality at 1 year even when used together with NT-proBNP [44]. sST2 is equally prognostic in heart failure patients with preserved ejection fraction (HFpEF) as it is in those with reduced ejection fraction (HFrEF), although its concentrations are lower in patients with HFpEF [45]. Among dyspnoeic patients with and without acute heart failure, sST2 concentrations were linked to higher LV dimensions and volumes, poorer LV ejection fraction, worse right ventricular function, and higher pulmonary pressures. sST2 levels also showed correlation with the tissue Doppler-derived mitral annular e’ velocity, but not with other indices of LV diastolic function [31]. In other studies, sST2 levels did not correlate significantly with the echocardiographic markers of LV size or function but showed positive correlation with LA volume index [29], or inverse correlation with LA reservoir strain [28].
The behaviour of the IL-33/ST2 pathway has already been widely investigated is SSc. Serum IL-33 levels were elevated in SSc patients compared with healthy individuals, especially in patients with diffuse cutaneous form. IL-33 levels correlated with the extent of skin sclerosis and the severity of pulmonary fibrosis [46]. Correlations between sST2 levels and the myocardial involvement of the disease, however, have not been investigated up to now. In our present study, unlike to the previous findings, we failed to demonstrate any relationship between sST2 levels and the clinical characteristics of the disease or the echocardiographic markers of the myocardial mechanics in SSc patients.
Recent studies suggest that galectin-3 plays key role in the fibrogenesis in different organ systems, such as liver, kidney and lung [20]. In addition, galectin-3 is considered an active contributor to the development of heart failure as mediator of the myocardial fibrosis. Clinically, serum galectin-3 levels are significantly increased in patients with heart failure and are often associated with a greater risk of adverse cardiovascular events [22, 23]. Galectin-3 levels are more markedly associated with outcomes in HFpEF population compared with HFrEF patients [47].
Although the pathogenic role of galectin-3 is not proved in SSc, the potential associations between serum galectin-3 levels and the clinical features of the patients have already been investigated. Taniguchi et al. found that serum galectin-3 levels were significantly lower in the early diffuse cutaneous form of the disease compared with control subjects and showed significant correlation with total skin score. On the other hand, galectin-3 levels showed increase with the course of the disease and were higher in SSc patients with elevated right ventricular systolic pressure than in those without pulmonary hypertension [48]. Recently, Mora et al. reported lower galectin-3 expression in the skin lesions of SSc patients compared with healthy subjects. Nevertheless, relatively higher galectin-3 values were found in SSc patients with pulmonary hypertension or higher modified Rodnan skin score [49]. In addition, galectin-3 has recently been reported as an independent predictor of all-cause and cardiovascular mortality in SSc. Higher levels of galectin-3 were associated with more severe pulmonary involvement and raised inflammatory markers [50]. Our current findings also suggest that galectin-3 is a biomarker of pulmonary fibrosis in SSc. These results are in line with the work of Ho et al. They reported that in the general population elevated galectin-3 concentrations are associated with interstitial lung abnormalities including decreased lung volumes and altered gas exchange [51]. Correlations between galectin-3 levels and the echocardiographic markers of the myocardial mechanics, however, have not been investigated yet in SSc.
LV diastolic dysfunction is frequent in SSc [4–6], as it represents the primary myocardial involvement of the disease [3]. In our present study, galectin-3 levels showed significant correlation not only with the grade of LV diastolic dysfunction, but with two well defined parameters of the LV diastolic function and filling pressure: septal e’ and E/e’. Similar finding was reported by Shah at al., where higher levels of galectin-3 showed clear associations with the Doppler indices of impaired myocardial relaxation and higher filling pressure in patients with heart failure [27]. Likewise, Beltrami et al. reported significant correlation between E/e’ and galectin-3 levels in HFpEF patients [26].
Reduced LV ejection fraction is not common in SSc [7], but myocardial fibrosis may contribute to the subclinical impairment of the LV systolic function. By speckle tracking-derived 2D strain method, reduced GLS values were found in SSc patients compared to healthy subjects [8, 9]. In the study of Cameli et al., GLS showed good correlation with the extent of myocardial fibrosis in LV tissue samples obtained from heart transplantation recipients [52]. In addition, in hypertrophic cardiomyopathy patients, strong correlation was found between LV GLS and the extent of late gadolinium enhancement obtained by contrast-enhanced cardiac MRI [53]. These results suggest an unequivocal correlation between LV GLS and the severity of LV myocardial fibrosis and may explain the significant correlation found between GLS and galectin-3 levels in our study. Similarly to our findings, in the study of Hromádka et al., galectin-3 showed significant correlation with the cardiac MRI-derived parameters of the myocardial fibrosis (extracellular volume, native T1 values) and also with the speckle tracking-derived LV GLS in SSc patients [54].
Speckle tracking technique and, to a lesser extent, tissue Doppler imaging are useful in recognizing early myocardial involvement not only in the LV, but in the other cardiac chambers. Subclinical RV dysfunction was proved in SSc patients using tissue Doppler or speckle tracking measurements [10–12]. Impaired LA and RA function have also been reported in SSc, by the help of speckle tracking technique [10, 13–15]. LA reservoir strain showed significant correlation with the amount of LA wall fibrosis as assessed by cardiac MRI in patients with atrial fibrillation [55], and with the extent of LA interstitial fibrosis in patients with mitral valve disease in histopathologic specimens [56]. RV free wall strain has been reported to correlate with the extent of RV myocardial fibrosis in heart transplantation recipients [57]. Nevertheless, no correlations were found between galectin-3 levels and the tissue Doppler and strain parameters of the RV or LA and RA function in our SSc patients. These data may suggest an uncoupling between galectin-3, myocardial fibrosis, and myocardial function in these chambers, but this phenomenon requires further investigation.
Limitations of the study
Some limitations of our study need to be acknowledged. First, in the lack of healthy control group, we could not define the cut-of value between normal and elevated serum galectin-3 or sST2 levels. Recent data suggest, however, that in the general population normal plasma concentration of galectin-3 is < 11.0 ng/ml [58], whereas the mean normal values of sST2 for males and for females are 24.9 ng/ml and 16.9 ng/ml, respectively [59].
Circulating levels of the tested fibrosis markers may not reflect the histologically proven cardiac fibrosis. Thus, circulating biomarker levels require careful interpretation in relation to myocardial involvement [60].
RV strain may better reflect the subclinical impairment of the RV systolic function than our traditional and tissue Doppler parameters. Nevertheless, in the lack of appropriate analytical software, RV strain analysis was not performed in our study.
Conclusion
In SSc patients, galectin-3 levels show significant correlation with the parameters of LV diastolic function and with GLS, a parameter reflecting the subclinical impairment of LV systolic function. Our results suggest that galectin-3 may be a useful and simple biomarker for the screening and early identification of SSc patients with cardiac involvement. Our data does not support the use of sST2 for the same purpose.
Acknowledgements
None.
Abbreviations
- GLS
Global longitudinal strain
- HFpEF
Heart failure with preserved ejection fraction
- HFrEF
Heart failure with reduced ejection fraction
- LA
Left atrial
- LV
Left ventricular
- RA
Right atrial
- RV
Right ventricular
- SSc
Systemic sclerosis
- sST2
Soluble suppression of tumorigenicity-2
Authors’ contributions
VV and RF analysed and interpreted the data and drafted the manuscript. MTF performed the laboratory analyses and interpreted the data. AN, AP, MH helped in literature analysis, contributed to discussion of the results. LC and AK reviewed the manuscript. All authors read and approved the final manuscript.
Funding
None.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The study complied with the Declaration of Helsinki. The institutional ethics committee approved the study. Written informed consent was obtained from all patients.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Gabrielli A, Avvedimento EV, Krieg T. Scleroderma. N Engl J Med. 2009;360(19):1989–2003. doi: 10.1056/NEJMra0806188. [DOI] [PubMed] [Google Scholar]
- 2.Pintér T, Faludi R, Magyari B, Vorobcsuk A, Kumanovics G, Minier T, et al. Mechanism of coronary flow reserve reduction in systemic sclerosis: insight from intracoronary pressure wire studies. Rheumatology (Oxford) 2011;50(4):781–788. doi: 10.1093/rheumatology/keq402. [DOI] [PubMed] [Google Scholar]
- 3.Kahan A, Coghlan G, McLaughlin V. Cardiac complications of systemic sclerosis. Rheumatology (Oxford) 2009;48 Suppl 3:iii45–iii48. doi: 10.1093/rheumatology/kep110. [DOI] [PubMed] [Google Scholar]
- 4.Hinchcliff M, Desai CS, Varga J, Shah SJ. Prevalence, prognosis, and factors associated with left ventricular diastolic dysfunction in systemic sclerosis. Clin Exp Rheumatol. 2012;30(2 Suppl 71):S30–S37. [PMC free article] [PubMed] [Google Scholar]
- 5.Faludi R, Költő G, Bartos B, Csima G, Czirják L, Komócsi A. Five-year follow-up of left ventricular diastolic function in systemic sclerosis patients: determinants of mortality and disease progression. Semin Arthritis Rheum. 2014;44(2):220–227. doi: 10.1016/j.semarthrit.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 6.Tennøe AH, Murbræch K, Andreassen JC, Fretheim H, Garen T, Gude E, et al. Left ventricular diastolic dysfunction predicts mortality in patients with systemic sclerosis. J Am Coll Cardiol. 2018;72(15):1804–1813. doi: 10.1016/j.jacc.2018.07.068. [DOI] [PubMed] [Google Scholar]
- 7.Allanore Y, Meune C, Vonk MC, Airo P, Hachulla E, Caramaschi P, et al. Prevalence and factors associated with left ventricular dysfunction in the EULAR scleroderma trial and research group (EUSTAR) database of patients with systemic sclerosis. Ann Rheum Dis. 2010;69(1):218–221. doi: 10.1136/ard.2008.103382. [DOI] [PubMed] [Google Scholar]
- 8.Spethmann S, Dreger H, Schattke S, Riemekasten G, Borges AC, Baumann G, et al. Two-dimensional speckle tracking of the left ventricle in patients with systemic sclerosis for an early detection of myocardial involvement. Eur Heart J Cardiovasc Imaging. 2012;13(10):863–870. doi: 10.1093/ehjci/jes047. [DOI] [PubMed] [Google Scholar]
- 9.Tennøe AH, Murbræch K, Andreassen JC, Fretheim H, Midtvedt Ø, Garen T, et al. Systolic dysfunction in systemic sclerosis: prevalence and prognostic implications. ACR Open Rheumatol. 2019;1(4):258–266. doi: 10.1002/acr2.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Durmus E, Sunbul M, Tigen K, Kivrak T, Ozen G, Sari I, et al. Right ventricular and atrial functions in systemic sclerosis patients without pulmonary hypertension. Speckle-tracking echocardiographic study. Herz. 2015;40(4):709–715. doi: 10.1007/s00059-014-4113-2. [DOI] [PubMed] [Google Scholar]
- 11.Mukherjee M, Chung SE, Ton VK, Tedford RJ, Hummers LK, Wigley FM, et al. Unique abnormalities in right ventricular longitudinal strain in systemic sclerosis patients. Circ Cardiovasc Imaging. 2016;9:e003792. doi: 10.1161/CIRCIMAGING.115.003792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lindqvist P, Caidahl K, Neuman-Andersen G, Ozolins C, Rantapää-Dahlqvist S, Waldenström A, et al. Disturbed right ventricular diastolic function in patients with systemic sclerosis: a Doppler tissue imaging study. Chest. 2005;128(2):755–763. doi: 10.1378/chest.128.2.755. [DOI] [PubMed] [Google Scholar]
- 13.Ágoston G, Gargani L, Miglioranza MH, Caputo M, Badano LP, Moreo A, et al. Left atrial dysfunction detected by speckle tracking in patients with systemic sclerosis. Cardiovasc Ultrasound. 2014;12:30. doi: 10.1186/1476-7120-12-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Porpáczy A, Nógrádi Á, Kehl D, Strenner M, Minier T, Czirják L, et al. Impairment of left atrial mechanics is an early sign of myocardial involvement in systemic sclerosis. J Card Fail. 2018;24(4):234–242. doi: 10.1016/j.cardfail.2018.02.012. [DOI] [PubMed] [Google Scholar]
- 15.Nógrádi Á, Porpáczy A, Porcsa L, Minier T, Czirják L, Komócsi A, et al. Relation of right atrial mechanics to functional capacity in patients with systemic sclerosis. Am J Cardiol. 2018;122(7):1249–1254. doi: 10.1016/j.amjcard.2018.06.021. [DOI] [PubMed] [Google Scholar]
- 16.Komócsi A, Vorobcsuk A, Faludi R, Pintér T, Lenkey Z, Költő G, et al. The impact of cardiopulmonary manifestations on the mortality of SSc: a systematic review and meta-analysis of observational studies. Rheumatology (Oxford) 2012;51(6):1027–1036. doi: 10.1093/rheumatology/ker357. [DOI] [PubMed] [Google Scholar]
- 17.Költő G, Faludi R, Aradi D, Bartos B, Kumánovics G, Minier T, et al. Impact of cardiac involvement on the risk of mortality among patients with systemic sclerosis: a 5-year follow-up of a single-center cohort. Clin Rheumatol. 2014;33(2):197–205. doi: 10.1007/s10067-013-2358-4. [DOI] [PubMed] [Google Scholar]
- 18.Költő G, Vuolteenaho O, Szokodi I, Faludi R, Tornyos A, Ruskoaho H, et al. Prognostic value of N-terminal natriuretic peptides in systemic sclerosis: a single centre study. Clin Exp Rheumatol. 2014;32(6 Suppl 86):S-75–S-81. [PubMed] [Google Scholar]
- 19.Allanore Y, Komócsi A, Vettori S, Hachulla E, Hunzelmann N, Distler J, et al. N-terminal pro-brain natriuretic peptide is a strong predictor of mortality in systemic sclerosis. Int J Cardiol. 2016;223:385–389. doi: 10.1016/j.ijcard.2016.08.246. [DOI] [PubMed] [Google Scholar]
- 20.Li LC, Li J, Gao J. Functions of galectin-3 and its role in fibrotic diseases. J Pharmacol Exp Ther. 2014;351(2):336–343. doi: 10.1124/jpet.114.218370. [DOI] [PubMed] [Google Scholar]
- 21.Rieth AJ, Jung C, Gall H, Rolf A, Mitrovic V, Hamm CW, et al. Association of galectin-3 with changes in left ventricular function in recent-onset dilated cardiomyopathy. Biomarkers. 2019;24(7):652–658. doi: 10.1080/1354750X.2019.1642959. [DOI] [PubMed] [Google Scholar]
- 22.Zhong X, Qian X, Chen G, Song X. The role of galectin-3 in heart failure and cardiovascular disease. Clin Exp Pharmacol Physiol. 2019;46(3):197–203. doi: 10.1111/1440-1681.13048. [DOI] [PubMed] [Google Scholar]
- 23.Ho JE, Liu C, Lyass A, Courchesne P, Pencina MJ, Vasan RS, et al. Galectin-3, a marker of cardiac fibrosis, predicts incident heart failure in the community. J Am Coll Cardiol. 2012;60(14):1249–1256. doi: 10.1016/j.jacc.2012.04.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Boer RA, Daniels LB, Maisel AS, Januzzi JL., Jr State of the art: newer biomarkers in heart failure. Eur J Heart Fail. 2015;17(6):559–569. doi: 10.1002/ejhf.273. [DOI] [PubMed] [Google Scholar]
- 25.Shah KB, Kop WJ, Christenson RH, Diercks DB, Henderson S, Hanson K, et al. Prognostic utility of ST2 in patients with acute dyspnea and preserved left ventricular ejection fraction. Clin Chem. 2011;57(6):874–882. doi: 10.1373/clinchem.2010.159277. [DOI] [PubMed] [Google Scholar]
- 26.Beltrami M, Ruocco G, Dastidar AG, Franci B, Lucani B, Aloia E, et al. Additional value of Galectin-3 to BNP in acute heart failure patients with preserved ejection fraction. Clin Chim Acta. 2016;457:99–105. doi: 10.1016/j.cca.2016.04.007. [DOI] [PubMed] [Google Scholar]
- 27.Shah RV, Chen-Tournoux AA, Picard MH, van Kimmenade RR, Januzzi JL. Galectin-3, cardiac structure and function, and long-term mortality in patients with acutely decompensated heart failure. Eur J Heart Fail. 2010;12(8):826–832. doi: 10.1093/eurjhf/hfq091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nagy AI, Hage C, Merkely B, Donal E, Daubert JC, Linde C, et al. Left atrial rather than left ventricular impaired mechanics are associated with the pro-fibrotic ST2 marker and outcomes in heart failure with preserved ejection fraction. J Intern Med. 2018;283(4):380–391. doi: 10.1111/joim.12723. [DOI] [PubMed] [Google Scholar]
- 29.Najjar E, Faxén UL, Hage C, Donal E, Daubert JC, Linde C, et al. ST2 in heart failure with preserved and reduced ejection fraction. Scand Cardiovasc J. 2019;53(1):21–27. doi: 10.1080/14017431.2019.1583363. [DOI] [PubMed] [Google Scholar]
- 30.Zile MR, Jhund PS, Baicu CF, Claggett BL, Pieske B, Voors AA, et al. Plasma biomarkers reflecting profibrotic processes in heart failure with a preserved ejection fraction: Data from the prospective comparison of ARNI with ARB on management of heart failure with preserved ejection fraction study. Circ Heart Fail. 2016;9:e002551. [DOI] [PMC free article] [PubMed]
- 31.Shah RV, Chen-Tournoux AA, Picard MH, van Kimmenade RR, Januzzi JL. Serum levels of the interleukin-1 receptor family member ST2, cardiac structure and function, and long-term mortality in patients with acute dyspnea. Circ Heart Fail. 2009;2(4):311–319. doi: 10.1161/CIRCHEARTFAILURE.108.833707. [DOI] [PubMed] [Google Scholar]
- 32.Fenster BE, Lasalvia L, Schroeder JD, Smyser J, Silveira LJ, Buckner JK, et al. Galectin-3 levels are associated with right ventricular functional and morphologic changes in pulmonary arterial hypertension. Heart Vessel. 2016;31(6):939–946. doi: 10.1007/s00380-015-0691-z. [DOI] [PubMed] [Google Scholar]
- 33.Pratama RS, Hartopo AB, Anggrahini DW, Dewanto VC, Dinarti LK. Serum soluble suppression of tumorigenicity-2 level associates with severity of pulmonary hypertension associated with uncorrected atrial septal defect. Pulm Circ. 2020;10(2):2045894020915832. doi: 10.1177/2045894020915832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Geenen LW, Baggen VJM, Kauling RM, Koudstaal T, Boomars KA, Boersma E, et al. The prognostic value of soluble ST2 in adults with pulmonary hypertension. J Clin Med. 2019;8(10):1517. doi: 10.3390/jcm8101517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van den Hoogen F, Khanna D, Fransen J, Johnson SR, Baron M, Tyndall A, et al. 2013 classification criteria for systemic sclerosis: an American college of rheumatology/European league against rheumatism collaborative initiative. Ann Rheum Dis. 2013;72(11):1747–1755. doi: 10.1136/annrheumdis-2013-204424. [DOI] [PubMed] [Google Scholar]
- 36.Rudski LG, Lai WW, Afilalo J, Hua L, Handschumacher MD, Chandrasekaran K, et al. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr. 2010;23(7):685–713. doi: 10.1016/j.echo.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 37.Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2015;16(3):233–270. doi: 10.1093/ehjci/jev014. [DOI] [PubMed] [Google Scholar]
- 38.Zoghbi WA, Adams D, Bonow RO, Enriquez-Sarano M, Foster E, Grayburn PA, et al. Recommendations for noninvasive evaluation of native valvular regurgitation: a report from the American Society of Echocardiography developed in collaboration with the Society for Cardiovascular Magnetic Resonance. J Am Soc Echocardiogr. 2017;30(4):303–371. doi: 10.1016/j.echo.2017.01.007. [DOI] [PubMed] [Google Scholar]
- 39.Nagueh SF, Smiseth OA, Appleton CP, Byrd BF, 3rd, Dokainish H, Edvardsen T, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2016;29(4):277–314. doi: 10.1016/j.echo.2016.01.011. [DOI] [PubMed] [Google Scholar]
- 40.Oh JK, Miranda WR, Bird JG, Kane GC, Nagueh SF. The 2016 diastolic function guideline: is it already time to revisit or revise them? JACC Cardiovasc Imaging. 2020;13(1 Pt 2):327–335. doi: 10.1016/j.jcmg.2019.12.004. [DOI] [PubMed] [Google Scholar]
- 41.Badano LP, Kolias TJ, Muraru D, Abraham TP, Aurigemma G, Edvardsen T, et al. Standardization of left atrial, right ventricular, and right atrial deformation imaging using two-dimensional speckle tracking echocardiography: a consensus document of the EACVI/ASE/industry task force to standardize deformation imaging. Eur Heart J Cardiovasc Imaging. 2018;19(6):591–600. doi: 10.1093/ehjci/jey042. [DOI] [PubMed] [Google Scholar]
- 42.Sarhene M, Wang Y, Wei J, Huang Y, Li M, Li L, et al. Biomarkers in heart failure: the past, current and future. Heart Fail Rev. 2019;24(6):867–903. doi: 10.1007/s10741-019-09807-z. [DOI] [PubMed] [Google Scholar]
- 43.Celic V, Majstorovic A, Pencic-Popovic B, Sljivic A, Lopez-Andres N, Roy I, et al. Soluble ST2 levels and left ventricular structure and function in patients with metabolic syndrome. Ann Lab Med. 2016;36(6):542–549. doi: 10.3343/alm.2016.36.6.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Januzzi JL, Jr, Peacock WF, Maisel AS, Chae CU, Jesse RL, Baggish AL, et al. Measurement of the interleukin family member ST2 in patients with acute dyspnea: results from the PRIDE (pro-brain natriuretic peptide investigation of dyspnea in the emergency department) study. J Am Coll Cardiol. 2007;50(7):607–613. doi: 10.1016/j.jacc.2007.05.014. [DOI] [PubMed] [Google Scholar]
- 45.Manzano-Fernández S, Mueller T, Pascual-Figal D, Truong QA, Januzzi JL. Usefulness of soluble concentrations of interleukin family member ST2 as predictor of mortality in patients with acutely decompensated heart failure relative to left ventricular ejection fraction. Am J Cardiol. 2011;107(2):259–267. doi: 10.1016/j.amjcard.2010.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yanaba K, Yoshizaki A, Asano Y, Kadono T, Sato S. Serum IL-33 levels are raised in patients with systemic sclerosis: association with extent of skin sclerosis and severity of pulmonary fibrosis. Clin Rheumatol. 2011;30(6):825–830. doi: 10.1007/s10067-011-1686-5. [DOI] [PubMed] [Google Scholar]
- 47.de Boer RA, Lok DJ, Jaarsma T, van der Meer P, Voors AA, Hillege HL, et al. Predictive value of plasma galectin-3 levels in heart failure with reduced and preserved ejection fraction. Ann Med. 2011;43(1):60–68. doi: 10.3109/07853890.2010.538080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Taniguchi T, Asano Y, Akamata K, Noda S, Masui Y, Yamada D, et al. Serum levels of galectin-3: possible association with fibrosis, aberrant angiogenesis, and immune activation in patients with systemic sclerosis. J Rheumatol. 2012;39(3):539–544. doi: 10.3899/jrheum.110755. [DOI] [PubMed] [Google Scholar]
- 49.Mora GF, Zubieta MR. Galectin-1 and Galectin-3 expression in lesional skin of patients with systemic sclerosis-association with disease severity. J Clin Rheumatol. 2020 Jun 4. Doi: 10.1097/RHU.0000000000001367. Online ahead of print. [DOI] [PubMed]
- 50.Faludi R, Nagy G, Tőkés-Füzesi M, Kovács K, Czirják L, Komócsi A. Galectin-3 is an independent predictor of survival in systemic sclerosis. Int J Cardiol. 2017;233:118–124. doi: 10.1016/j.ijcard.2016.12.140. [DOI] [PubMed] [Google Scholar]
- 51.Ho JE, Gao W, Levy D, Santhanakrishnan R, Araki T, Rosas IO, et al. Galectin-3 is associated with restrictive lung disease and interstitial lung abnormalities. Am J Respir Crit Care Med. 2016;194(1):77–83. doi: 10.1164/rccm.201509-1753OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cameli M, Mondillo S, Righini FM, Lisi M, Dokollari A, Lindqvist P, et al. Left ventricular deformation and myocardial fibrosis in patients with advanced heart failure requiring transplantation. J Card Fail. 2016;22(11):901–907. doi: 10.1016/j.cardfail.2016.02.012. [DOI] [PubMed] [Google Scholar]
- 53.Saito M, Okayama H, Yoshii T, Higashi H, Morioka H, Hiasa G, et al. Clinical significance of global two-dimensional strain as a surrogate parameter of myocardial fibrosis and cardiac events in patients with hypertrophic cardiomyopathy. Eur Heart J Cardiovasc Imaging. 2012;13(7):617–623. doi: 10.1093/ejechocard/jer318. [DOI] [PubMed] [Google Scholar]
- 54.Hromádka M, Seidlerová J, Suchý D, Rajdl D, Lhotský J, Ludvík J, et al. Myocardial fibrosis detected by magnetic resonance in systemic sclerosis patients - relationship with biochemical and echocardiography parameters. Int J Cardiol. 2017;249:448–453. doi: 10.1016/j.ijcard.2017.08.072. [DOI] [PubMed] [Google Scholar]
- 55.Kuppahally SS, Akoum N, Burgon NS, Badger TJ, Kholmovski EG, Vijayakumar S, et al. Left atrial strain and strain rate in patients with paroxysmal and persistent atrial fibrillation: relationship to left atrial structural remodeling detected by delayed-enhancement MRI. Circ Cardiovasc Imaging. 2010;3(3):231–239. doi: 10.1161/CIRCIMAGING.109.865683. [DOI] [PubMed] [Google Scholar]
- 56.Her AY, Choi EY, Shim CY, Song BW, Lee S, Ha JW, et al. Prediction of left atrial fibrosis with speckle tracking echocardiography in mitral valve disease: a comparative study with histopathology. Korean Circ J. 2012;42(5):311–318. doi: 10.4070/kcj.2012.42.5.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lisi M, Cameli M, Righini FM, Malandrino A, Tacchini D, Focardi M, et al. RV longitudinal deformation correlates with myocardial fibrosis in patients with end-stage heart failure. JACC Cardiovasc Imaging. 2015;8(5):514–522. doi: 10.1016/j.jcmg.2014.12.026. [DOI] [PubMed] [Google Scholar]
- 58.McCullough PA. Practical experience using galectin-3 in heart failure. Clin Chem Lab Med. 2014;52(10):1425–1431. doi: 10.1515/cclm-2014-0278. [DOI] [PubMed] [Google Scholar]
- 59.Lu J, Snider JV, Grenache DG. Establishment of reference intervals for soluble ST2 from a United States population. Clin Chim Acta. 2010;411(21–22):1825–1826. doi: 10.1016/j.cca.2010.07.014. [DOI] [PubMed] [Google Scholar]
- 60.de Boer RA, De Keulenaer G, Bauersachs J, Brutsaert D, Cleland JG, Diez J, et al. Towards better definition, quantification and treatment of fibrosis in heart failure. A scientific roadmap by the Committee of Translational Research of the heart failure association (HFA) of the European Society of Cardiology. Eur J Heart Fail. 2019;21(3):272–285. doi: 10.1002/ejhf.1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.