Abstract
Background
Thoracoscopic surgery has greatly alleviated the postoperative pain of patients, but postsurgical acute and chronic pain still exists and needs to be addressed. Indwelling drainage tubes are one of the leading causes of postoperative pain after thoracic surgery. Therefore, the aim of this study was to explore the effects of alternative drainage on acute and chronic pain after video-assisted thoracoscopic surgery (VATS).
Methods
Ninety-two patients undergoing lung wedge resection were selected and randomly assigned to the conventional chest tube (CT) group and the 7-Fr central venous catheter (VC) group. Next, the numeric rating scale (NRS) and pain DETECT questionnaire were applied to evaluate the level and characteristics of postoperative pain.
Results
NRS scores of the VC group during hospitalization were significantly lower than those of the CT group 6 h after surgery, at postoperative day 1, at postoperative day 2, and at the moment of drainage tube removal. Moreover, the number of postoperative salvage analgesics (such as nonsteroidal anti-inflammatory drugs [(NSAIDs]) and postoperative hospitalization days were notably reduced in the VC group compared with the CT group. However, no significant difference was observed in terms of NRS pain scores between the two groups of patients during the follow-up for chronic pain at 3 months and 6 months.
Conclusion
In conclusion, a drainage strategy using a 7-Fr central VC can effectively relieve perioperative pain in selected patients undergoing VATS wedge resection, and this may promote the rapid recovery of such patients after surgery.
Trial registration
ClinicalTrials.gov, NCT03230019. Registered July 23, 2017.
Keywords: Thoracoscopic wedge resection, Chest tube, Double-lumen central venous catheter, Postoperative pain
Background
Favourable pain management after thoracic surgery is of great significance to prevent chronic pain and complications [1–3]. The current incidence of postoperative pain remains high, with evidence suggesting a 59–90% incidence of postoperative pain [4–6], and 11–35% of patients undergoing video-assisted thoracoscopic surgery (VATS) develop chronic pain postoperatively [7–11]. Many factors contribute to the occurrence of postoperative pain; compared with thoracotomy, postoperative pain after VATS is reduced. In addition, intercostal nerve injury, indwelling thoracic drainage tubes and psychosocial factors are also causes of postoperative pain [12–14]. Among them, the importance of chest drains in postoperative pain management is often overlooked, with limited attention focused on this aspect.
In recent years, with the popularization of enhanced recovery after surgery (ERAS), the tubeless strategy [15, 16] has been increasingly favoured by surgeons and patients. Thus, seeking a safe alternative to conventional chest tubes (CTs) or even abolishing drainage tubes has become a mainstream trend for many surgeons in minimally invasive pulmonary surgery. In our previously reported study, we provided evidence demonstrating that the alternative of conventional chest drainage by a 7-Fr double-lumen central venous catheter [17] (CVC) along with a prophylactic air-extraction strategy [18] can be safely applied to patients under lung wedge resection through VATS without causing more postoperative complications.
Intriguingly, we speculated that the alternative conventional chest drainage strategy of 7-Fr double-lumen CVC may reduce postoperative pain after lung wedge resection through VATS in the present study. To verify this hypothesis, we designed a single-centre, prospective, and randomized controlled trial to explore the effects of different drainage methods on acute and chronic pain in patients after lung wedge resection under VATS.
Materials and methods
Ethics statement and registration
This prospective, single-centre, open-label, and randomized controlled trial was approved by the Ethics Committee of Guangdong Provincial People’s Hospital (No. GDREC 2017261H) and registered before patient enrolment at www.clinicaltrials.gov on July 23, 2017 (registration number: NCT03230019). All participants signed written informed consent forms before enrolment in the study.
Inclusion and exclusion criteria
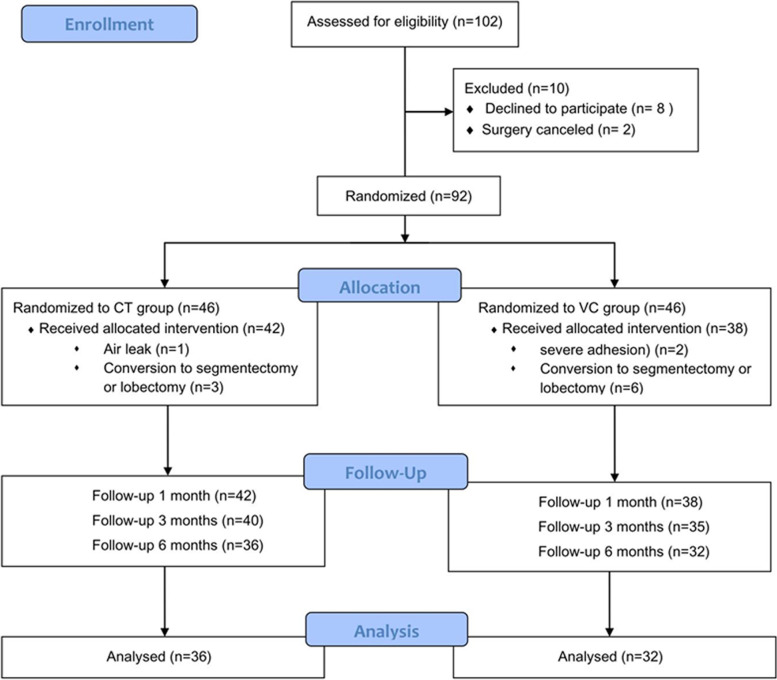
The subjects for the screening included patients who received lung wedge resection through VATS at Guangdong Provincial People’s Hospital, and their age was above 18 years. The preoperative exclusion criteria were as follows: (1) any unstable systemic diseases such as active infection, poorly controlled hypertension within 3 months, diabetes or unstable angina; (2) history of ipsilateral chest surgery; (3) preoperative chest X-ray showing pneumonia or atelectasis; (4) bleeding tendency; (5) administration of anticoagulant drugs; or (6) history of other chronic chest pain. Patients were excluded if they needed to receive segmentectomy or lobectomy, if they showed severe adhesions during the surgery, if they required further exploratory surgery, or if air leaks were detected during leak examinations (Fig. 2).
Fig. 2.
Consolidated Standards of Reporting Trials (CONSORT) flow diagram. CT, routine chest tube group; VC, central venous catheter group
Sample size
The present study aimed to validate that the drainage strategy of the VC group after lung wedge resection through VATS could ameliorate postoperative pain. A previous retrospective study [18] documented that the mean NRS scores of postoperative acute pain in the CT group and VC group were 3.4 and 2.3, respectively, while the standard deviations were 1.1 and 0.8, respectively. The bilateral α was 0.01, and the power was 95%, in which half of the participants were assigned to the CT group and the rest to the VC group. The loss of follow-up and refusal to follow-up was calculated as 20%. Moreover, thirty-eight patients each were included in the CT group and VC group, as quantified by PASS software (version 15.0; NCSS, Kaysville, UT, USA). Hence, the total number of participants was at least 76.
Randomization and blinding
Patients were selected before operation. After surgical incision (before wedge resection), one surgical team member randomly generated codes by using simple random sampling and SAS statistical software (SAS Institute, Cary, NC, USA) to randomly assign patients to the VC group or conventional CT group in a 1:1 ratio. It was easy to identify the grouping of patients during follow-up, and the patients and investigators were not blinded to the group assignment.
Anaesthesia and surgery
For all patients, general anesthesia was induced with midazolam, sufentanil, propofol and cisatracurium, then double lumen endobronchial tube intubation was performed, and maintained with sevoflurane inhalation, remifentanil infusion, and cisatracurium. Non-steroidal drug flurbiprofen axetil 50 mg and antiemetic drugs were given before skin incision. In the post-anaesthesia care unit (PACU), each patient received intravenous analgesia: sufentanil 150 μg, flurbiprofen 300 mg and antiemetic agents with a total of 75 ml and a background dose of 1 ml/h. Rescue analgesics were administered according to the patient’s pain level after returning to the ward. Salvage analgesic measures indicated that patients with mild pain were given nonsteroidal anti-inflammatory drugs (NSAIDs) (such as Celebrex 200 mg and flurbiprofen axetil injection 50 mg), patients with moderate pain were treated with tramadol (50–100 mg), and patients with severe pain were treated with morphine (7–10 mg).
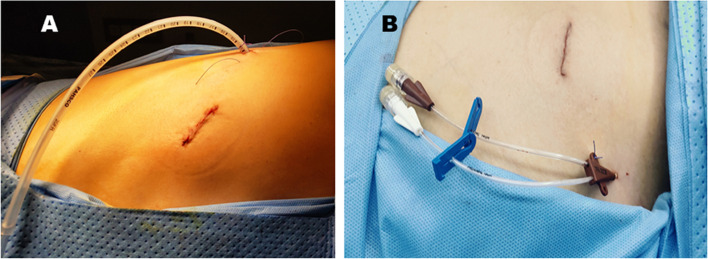
Before skin incision, 2% lidocaine was used for local infiltration anaesthesia along the incision. A three-centimetre-long incision was made between the anterior axillary line and the fourth or fifth intercostal line of the axillary line. Participants were excluded from the study in the case of severe adhesions or necessity of lobectomy due to insufficient surgical margins during the exploratory process. They were subjected to CT drainage, and all wedge resections were performed using a linear cutting stapler (Ethicon, Cincinnati, USA or Medtronic, Minneapolis, USA). After completion of wedge resection, a 20-Fr chest tube was inserted for drainage in the CT group (Fig. 1A), conducting an air-leak test and a water-sealed chest tube bottle was connected. Patients in the VC group were inserted a two-lumen central venous catheter (20 cm × 7 Fr) in the second intercostal space with a puncture needle. While the anaesthesiologist inflated the residual lung, the surgeon sutured the incision and conducted an air tightness test, and the CVC was clamped after air-extraction via injector (Fig. 1B). Patients with air leakage were excluded from the study and received CT drainage. All patients underwent a chest X-ray examination on the first day after surgery. Upon the observation of a large amount of pneumothorax, the CT group were required to strengthen deep breathing and perform coughing exercises or to undergo 8–10-cm H2O suction via the CT. In the scenario of massive pneumothoraxes in the VC group, a syringe was applied to perform a prophylactic air-extraction approximately 3 times a day through the CVC or the reinserted CT. CT/CVC extraction was considered upon indications of blood oxygen saturation (≥) 95%, fully dilated lungs, and no air leakage in the CT group.
Fig. 1.
Procedure showing the position of the routine 20-Fr chest tube and 7-Fr double-lumen central venous catheter. A Insertion of the chest tube. B Insertion of the alternative thoracic drainage catheter
Pain evaluation
Chronic pain was defined as postoperative pain lasting longer than 3 months according to the International Association for the Study of Pain [11], and a numeric rating scale (NRS) was used to assess the postoperative pain level of the patients. A score of 0 indicated no pain, while a score of 10 represented the worst pain. On the basis of the evaluation results, the pain level was divided into 4 grades: no pain (NRS = 0), mild pain (NRS 1–3), moderate pain (NRS 4–6), and severe pain (NRS 7–10). The patient’s pain assessment was conducted in two stages, including the perioperative period and the assessment of chronic pain at 3 and 6 months after surgery. The perioperative evaluation referred to the evaluation during hospitalization and 1 month after the operation. A total of 7 pain evaluations were performed during hospitalization, which was the first 6 h after patients returned to the ward, 8 am and 6 pm on the first day after the surgery, 8 am and 6 pm on the second day after surgery, at the time of extubation and after the doctor issued a discharge from the hospital. In the follow-up of chronic pain, the pain DETECT questionnaire (PD-Q) was used to investigate the characteristics of the pain [19], which included allodynia, insufficiency, hyperalgesia, numbness, tingling, burning pain, and soreness.
Study endpoints
The major endpoint of this study was the level of acute pain after surgery on the first day. The secondary observation indicators mainly included the duration of postoperative intravenous analgesia use, the frequency of postoperative salvage analgesics, the NRS score of extubation, the duration of postoperative drainage, postoperative hospitalization days, the NRS score of postoperative 1 month, and the level and characteristics of chronic pain of patients 3 and 6 months after surgery.
Statistical analysis
Quantitative data are presented as the mean ± standard deviation (mean ± SD). Normally distributed data were compared between two groups using the unpaired t-test, and data with a skewed distribution were compared using the Mann-Whitney U test. Count data are presented as the actual number of cases and percentages, which were processed by the chi-square and Fisher’s exact tests. All the data were analysed by SPSS 25 software (IBM, Armonk, NY, USA), with P < 0.05 indicating statistical significance.
Results
Patient characteristics
From August 2017 to October 2018, a total of 102 patients with proposed wedge resection met the inclusion criteria for this study. Among them, 94 patients signed written informed consent forms, and the surgeries of 2 patients were temporarily cancelled; consequently, 92 patients were randomly assigned to the CT group and VC group. In addition, cases with intraoperative surgery type changes (n = 9), air leakage (n = 1), and severe adhesion (n = 2) were excluded. Finally, 42 patients were included in the CT group, and 38 patients were assigned to the VC group (Fig. 2). The demographic, ASA classification, NRS score baseline and operational details of both groups were balanced, and they are summarized in Table 1. No inpatient deaths or intensive care cases were identified.
Table 1.
The demographic and clinical characteristics of the two groups
| CT group (n = 42) | VC group (n = 38) | P value | |
|---|---|---|---|
| Age, years | 54.7 ± 11.6 | 53.6 ± 9.2 | 0.646 |
| Sex | 0.644 | ||
| Female | 28 | 23 | |
| Male | 14 | 15 | |
| BMI (kg/m2) | 23.1 ± 2.4 | 22.3 ± 2.7 | 0.169 |
| Classification of ASA | 1.000 | ||
| I | 39 | 35 | |
| II | 3 | 3 | |
| Surgical incision length, cm | 3.6 ± 0.5 | 3.5 ± 0.6 | 0.713 |
| Surgery time, median (25th–75th percentiles), minutes | 60 (50–88) | 60 (50–89) | 0. 927 |
| Resection length, median (25th–75th percentiles), mm | 135 (120–180) | 135 (120–180) | 0. 636 |
Data are presented as the mean ± SD, median (range)
CT Chest Tube, VC Venous Catheter, BMI Body Mass Index, ASA American Society of Anesthesiologists
Primary outcomes
The results of the primary outcome mainly focused on NRS scores of hospitalization. NRS scores of the VC group were considerably lower than those of the CT group at 6 h after surgery (2.3 ± 0.9 vs. 2.9 ± 0.8, P = 0.001), postoperative day 1 (2.6 ± 0.9 vs. 2.8 ± 0.8, P = 0.026), postoperative day 2 (2.2 ± 0.8 vs. 2.7 ± 0.7, P = 0.009), and CT removal (2.8 ± 0.7 vs. 3.3 ± 0.7, P = 0.003).
Moreover, the frequency of postoperative rescue analgesics NSAIDs (2.0 ± 1.5 vs. 3.0 ± 2.0, P = 0.023) and postoperative hospitalization days (2.7 ± 1.4 vs. 3.2 ± 1.2, P = 0.001) were remarkably reduced in the VC group compared with the CT group. No statistically significant difference was observed regarding the pain level of the two groups of patients at discharge (1.3 ± 0.7 vs. 1.5 ± 0.6, P = 0.267) (Table 2).
Table 2.
Patients’ perioperative outcomes
| CT group (n = 42) | VC group (n = 38) | 95% Confidence interval | P value | |
|---|---|---|---|---|
| Postoperative 6 h (NRS) | 2.9 ± 0.8 | 2.3 ± 0.9 | – | 0.001 |
| POD1 (NRS) | 2.8 ± 0.8 | 2.6 ± 0.9 | – | 0.026 |
| POD1 NRS ≥3 | 19 | 11 | ||
| POD2 (NRS) | 2.7 ± 0.7 | 2.2 ± 0.8 | −0.75 to − 0.11 | 0.009 |
| POD2 NRS ≥3 | 18 | 8 | ||
| Drainage tube removal (NRS) | 3.3 ± 0.7 | 2.8 ± 0.7 | – | 0.003 |
| Discharge (NRS) | 1.5 ± 0.6 | 1.3 ± 0.7 | −0.44 to 0.12 | 0.267 |
| Intravenous analgesia days | 1.3 ± 0.6 | 1.1 ± 0.4 | −0.41 to 0.02 | 0.069 |
| Sufentanil (μg) | 54.6 ± 18.8 | 60.4 ± 26.6 | −19.61 to 0.74 | 0.069 |
| Flurbiprofen (mg) | 109.0 ± 37.5 | 128.0 ± 53.2 | −39.2 to 1.49 | 0.069 |
| Salvage analgesics* | ||||
| NSAIDs | 3.0 ± 2.0 | 2.0 ± 1.5 | −1.72 to −0.13 | 0.023 |
| Opioid | 0.5 (0–1) | 1.0 (1–2) | – | 0.545 |
| chest tube/catheter removal (days) | 1.7 ± 0.7 | 1.7 ± 0.9 | – | 0.416 |
| Length of stay (days) | 3.2 ± 1.2 | 2.7 ± 1.4 | – | 0.001 |
| POM1 (NRS) | 1.1 ± 0.5 | 0.9 ± 0.5 | – | 0.182 |
*Frequency of salvage analgesics
Data are presented as the mean ± SD, median (range)
POD Postoperative Day, NRS Numeric Rating Scale, NSAIDs Nonsteroidal Antiinflammatory Drugs, POM Postoperative Month
It seems that the postoperative average NRS score was less than 3 in both groups. In fact, the proportion of patients with NRS scores≥3 on the first day and the second day (Table 2) in the CT group was higher than that in the VC group (44% vs 25%), so the demand for salvage analgesia NSAIDs in the CT group was greater than that in the VC group. However, there was no significant difference in terms of pain levels and characteristics between the two groups, including the frequency of postoperative salvage opioid use (P = 0.545), days of postoperative venous analgesia (1.1 ± 0.4 vs. 1.3 ± 0.6, P = 0.069).
Secondary outcomes
Postoperative complications included pneumothorax, pleural effusion, chest tube reinsertion and subcutaneous emphysema. There were no differences between the two groups in these outcomes. Pneumothorax occurred in 4 patients (10.5%) in the VC group, and all patients recovered well after air extraction and did not need reinsertion of the CT. One patient (2.5%) in the CT group required CT reinsertion because of pleural effusion, and this patient was discharged on postoperative day 13.
NRS scores of postoperative at 1 month was (0.9 ± 0.5 vs. 1.1 ± 0.5, P = 0.182) in VC group and CT group (Tables 2-3). In the chronic pain follow-up, both groups of patients had different degrees of abnormal feelings, which were manifested as sensory changes related to weather change, mainly including paraesthesia, numbness and tingling (Table 3). The degree of discomfort did not affect sleep and daily activities, and no patient needed long-term pain medication.
Table 3.
Three- and 6-month follow-up for chronic pain assessments
| POM3 | POM6 | |||||
|---|---|---|---|---|---|---|
| CT group (n = 40) | VC group (n = 35) | P value | CT group (n = 36) | VC group (n = 32) | P value | |
| Chronic pain positive (%) | 10 (25) | 7 (20) | 0.783 | 7 (19.4) | 4 (12.5) | 0.521 |
| Pain characteristic | ||||||
| Tingling (%) | 2 (5) | 3 (8.6) | 0.659 | 2 (5.6) | 1 (3.1) | 1.000 |
| Numbness (%) | 3 (7.5) | 2 (5.7) | 1.000 | 3 (8.3) | 1 (3.1) | 0.616 |
| Paraesthesia (%) | 5 (12.5) | 2 (5.7) | 0.438 | 2 (5.6) | 2 (6.3) | 1.000 |
Data are presented as n (%)
POM Postoperative Month
Discussion
Thoracotomy has been reported to cause trauma and severe pain, while surgical trauma has been considerably reduced due to the development of VATS [20]. Moreover, with the popularization of ERAS, more precise management is required for the perioperative period. In addition to minimally invasive surgery and multi-modal analgesia, optimizing the management of various tubes, or even abolishing urethral catheters, postoperative drainage tubes in certain types of surgery could be of great value in promoting rapid recovery after surgery.
The population baseline of the two groups were balanced and same was for surgical procedure, while the postoperative NRS score and hospital stay were decreased in the VC group. This is mainly due to the fact that patients in the alternative drainage group received a 7-Fr central VC, which is small in diameter, greatly reduces the discomfort associated with chest tube. Patients in the VC group undergoing lung wedge resection could even get out of bed on the day of the operation. On the other hand, the VC group patients did not need to carry a chest drainage bottle after surgery, promoting early postoperative activity, which in turn was beneficial for wound healing [21]. In terms of length of stay, chest intubation and pain caused by the CT may have been one of the factors resulting in a prolonged hospital stay in the CT group, together with pneumothorax, pleural effusion, subcutaneous emphysema and other complications. In addition, one patient in the CT group was hospitalized for more than 13 days, and another six patients in the CT group experienced poor wound healing, which may be part of the reason for the significant difference in hospital stay.
With regard to chronic pain, our research results suggested only that there was no difference in the effects of the two kinds of tubes on abnormal skin sensation within half a year after the operation. It is hard to conclude that thoracic drainage tubes have nothing to do with postoperative chronic pain. We need more conclusive evidence to explain the connection between CTs and postoperative chronic pain.
We acknowledge that the present study has limitations and that its restrictive inclusion criteria may impede the universality and applicability of the results. First, the diameter of the catheter is small, the drainage capacity for pleural effusion is limited, and the tube is easily blocked. To prevent pneumothorax and massive pleural effusion after surgery, postoperative X-ray examination and dynamic observation of the patients’ reactions were very important, especially at the beginning of the study. Second, our investigation focused on the data and observations at a single research centre, and the sample size was relatively limited. Last, to directly explain the contribution of CTs to postoperative acute and chronic pain, it is necessary to further explore the difference between patients without CTs and the control group of patients in a follow-up study.
Conclusion
In conclusion, a drainage strategy using a 7-Fr central VC can effectively relieve perioperative pain in selected patients undergoing VATS wedge resection, and this may be beneficial to the rapid recovery of such patients after surgery.
Acknowledgements
Not applicable.
Abbreviations
- VATS
Video-Assisted Thoracic Surgery
- CVC
central venous catheter
- NRS
Numeric Rating Scale
- BMI
Body Mass Index
- ASA
American Society of Anaesthesiologists
- PACU
Post-anaesthesia Care Unit
- NSAIDs
Nonsteroidal Anti-inflammatory Drugs
- ERAS
Enhanced Recovery after Surgery
- BIS
Bispectral Index
Authors’ contributions
JXC and SCW contributed to the conception and design of the work. JTZ and WZZ conducted the patient recruitment, randomization, and allocation. NLD and JM performed the anaesthesia. GYZ analysed the data and prepared the figures and tables. SCW and JXC wrote the manuscript. All authors revised the manuscript and approved the final version to be published.
Funding
This work was supported by grant from Guangdong Provincial People’s Hospital, Guangdong Academy of Medical Sciences (Grant NO.8197131428).
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The study was approved by the Ethics Committee of Guangdong Provincial People’s Hospital (No. GDREC 2017261H) and registered on July 23, 2017 (No. NCT03230019). It strictly abided by Declaration of Helsinki principles and CONSORT guidelines. All participants signed their written informed consent before enrolment in the study.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Watanabe S, Imai K, Kimura T, Saito Y, Takashima S, Matsuzaki I, et al. Effect of lidocaine cream analgesia for chest drain tube removal after video-assisted thoracoscopic surgery for lung cancer: a randomized clinical trial. Reg Anesth Pain Med. 2019;45:16–21. doi: 10.1136/rapm-2019-100760. [DOI] [PubMed] [Google Scholar]
- 2.Kehlet H, Jensen TS, Woolf CJ. Persistent postsurgical pain: risk factors and prevention. Lancet. 2006;367:1618–1625. doi: 10.1016/S0140-6736(06)68700-X. [DOI] [PubMed] [Google Scholar]
- 3.Katz J, Jackson M, Kavanagh BP, Sandler AN. Acute pain after thoracic surgery predicts long-term post-thoracotomy pain. Clin J Pain. 1996;12:50–55. doi: 10.1097/00002508-199603000-00009. [DOI] [PubMed] [Google Scholar]
- 4.Takenaka S, Saeki A, Sukenaga N, Ueki R, Kariya N, Tatara T, et al. Acute and chronic neuropathic pain profiles after video-assisted thoracic surgery. Medicine. 2020;99:e19629. doi: 10.1097/MD.0000000000019629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bayman EO, Parekh KR, Keech J, Larson N, Vander WM, Brennan TJ. Preoperative patient expectations of postoperative pain are associated with moderate to severe acute pain after VATS. Pain Med. 2019;20:543–554. doi: 10.1093/pm/pny096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang H, Li S, Liang N, Liu W, Liu H, Liu H. Postoperative pain experiences in Chinese adult patients after thoracotomy and video-assisted thoracic surgery. J Clin Nurs. 2017;26:2744–2754. doi: 10.1111/jocn.13789. [DOI] [PubMed] [Google Scholar]
- 7.Wildgaard K, Ringsted TK, Hansen HJ, Petersen RH, Kehlet H. Persistent postsurgical pain after video-assisted thoracic surgery – an observational study. Acta Anaesth Scand. 2016;60:650–658. doi: 10.1111/aas.12681. [DOI] [PubMed] [Google Scholar]
- 8.WILDGAARD K, RAVN J, NIKOLAJSEN L, JAKOBSEN E, JENSEN TS, KEHLET H. Consequences of persistent pain after lung cancer surgery: a nationwide questionnaire study. Acta Anaesth Scand. 2011;55:60–68. doi: 10.1111/j.1399-6576.2010.02357.x. [DOI] [PubMed] [Google Scholar]
- 9.Shanthanna H, Aboutouk D, Poon E, Cheng J, Finley C, Paul J, et al. A retrospective study of open thoracotomies versus thoracoscopic surgeries for persistent postthoracotomy pain. J Clin Anesth. 2016;35:215–220. doi: 10.1016/j.jclinane.2016.07.029. [DOI] [PubMed] [Google Scholar]
- 10.Kwon ST, Zhao L, Reddy RM, Chang AC, Orringer MB, Brummett CM, et al. Evaluation of acute and chronic pain outcomes after robotic, video-assisted thoracoscopic surgery, or open anatomic pulmonary resection. J Thorac Cardiovasc Surg. 2017;154:652–659. doi: 10.1016/j.jtcvs.2017.02.008. [DOI] [PubMed] [Google Scholar]
- 11.Bayman EO, Parekh KR, Keech J, Selte A, Brennan TJ. A prospective study of chronic pain after thoracic surgery. Anesthesiology. 2017;126:938–951. doi: 10.1097/ALN.0000000000001576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Perkins FM, Kehlet H. Chronic pain as an outcome of surgery. A review of predictive factors. Anesthesiology. 2000;93:1123–1133. doi: 10.1097/00000542-200010000-00038. [DOI] [PubMed] [Google Scholar]
- 13.Kaplowitz J, Papadakos PJ. Acute pain Management for Video-Assisted thoracoscopic Surgery: an update. J Cardiothor Vasc An. 2012;26:312–321. doi: 10.1053/j.jvca.2011.04.010. [DOI] [PubMed] [Google Scholar]
- 14.Ueda K, Hayashi M, Tanaka T, Hamano K. Omitting chest tube drainage after thoracoscopic major lung resection. Eur J Cardio-Thorac. 2013;44:225–229. doi: 10.1093/ejcts/ezs679. [DOI] [PubMed] [Google Scholar]
- 15.Liu CY, Hsu PK, Leong KI, Ting CK, Tsou MY. Is tubeless uniportal video-assisted thoracic surgery for pulmonary wedge resection a safe procedure? Eur J Cardiothorac Surg. 2020;58(Supplement_1):i70–i76. doi: 10.1093/ejcts/ezaa061. [DOI] [PubMed] [Google Scholar]
- 16.Li S, Jiang L, Ang KL, Chen H, Dong Q, Yang H, et al. New tubeless video-assisted thoracoscopic surgery for small pulmonary nodules. Eur J Cardiothorac Surg. 2017;51:689–693. doi: 10.1093/ejcts/ezw364. [DOI] [PubMed] [Google Scholar]
- 17.Zhang J, Dong S, Chu X, Lin S, Yu R, Jiang B, et al. Randomized trial of an improved drainage strategy versus routine chest tube after lung wedge resection. Ann Thorac Surg. 2020;109:1040–1046. doi: 10.1016/j.athoracsur.2019.11.029. [DOI] [PubMed] [Google Scholar]
- 18.Zhang JT, Tang YC, Lin JT, Dong S, Nie Q, Jiang BY, et al. Prophylactic air-extraction strategy after thoracoscopic wedge resection. Thorac Cancer. 2018;9:1406–1412. doi: 10.1111/1759-7714.12850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hirai K, Usuda J. Uniportal video-assisted thoracic surgery reduced the occurrence of post-thoracotomy pain syndrome after lobectomy for lung cancer. J Thorac Dis. 2019;11:3896–3902. doi: 10.21037/jtd.2019.09.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bendixen M, Jorgensen OD, Kronborg C, Andersen C, Licht PB. Postoperative pain and quality of life after lobectomy via video-assisted thoracoscopic surgery or anterolateral thoracotomy for early stage lung cancer: a randomized controlled trial. Lancet Oncol. 2016;17:836–844. doi: 10.1016/S1470-2045(16)00173-X. [DOI] [PubMed] [Google Scholar]
- 21.Rahman NM, Pepperell J, Rehal S, et al. Effect of opioids vs NSAIDs and larger vs smaller chest tube size on pain control and Pleurodesis efficacy among patients with malignant pleural effusion: the TIME1 randomized clinical trial. JAMA. 2015;314:2641–2653. doi: 10.1001/jama.2015.16840. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.