Abstract
Background
Radiation-induced myocardial fibrosis increases heart failure (HF) risk and is associated with a restrictive cardiomyopathy phenotype. The myocardial extracellular volume fraction (ECVF) using contrast-enhanced cardiac magnetic resonance (CMR) quantifies the extent of fibrosis which, in severe cases, results in a noncompliant left ventricle (LV) with an inability to augment exercise stroke volume (SV). The peak exercise oxygen pulse (O2Pulse), a noninvasive surrogate for exercise SV, may provide mechanistic insight into cardiac reserve. The relationship between LV ECVF and O2Pulse following thoracic radiotherapy has not been explored.
Methods
Patients who underwent thoracic radiotherapy for chest malignancies with significant incidental heart dose (≥5 Gray (Gy), ≥10% heart) without a pre-cancer treatment history of HF underwent cardiopulmonary exercise testing to determine O2Pulse, contrast-enhanced CMR, and N-terminal pro-brain natriuretic peptide (NTproBNP) measurement. Multivariable-analyses were performed to identify factors associated with O2Pulse normalized for age/gender/anthropometrics.
Results
Thirty patients (median [IQR] age 63 [57–67] years, 18 [60%] female, 2.0 [0.6–3.8] years post-radiotherapy) were included. The peak VO2 was 1376 [1057–1552] mL·min− 1, peak HR = 150 [122–164] bpm, resulting in an O2Pulse of 9.2 [7.5–10.7] mL/beat or 82 (66–96) % of predicted. The ECVF, LV ejection fraction, heart volume receiving ≥10 Gy, and NTproBNP were independently associated with %O2Pulse (P < .001).
Conclusions
In patients with prior radiotherapy heart exposure, %-predicted O2Pulse is inversely associated markers of diffuse fibrosis (ECVF), ventricular wall stress (NTproBNP), radiotherapy heart dose, and positively related to LV function. Increased LV ECVF may reflect a potential etiology of impaired LV SV reserve in patients receiving thoracic radiotherapy for chest malignancies.
Supplementary Information
The online version contains supplementary material available at 10.1186/s40959-021-00127-6.
Keywords: Peak exercise oxygen pulse, Extracellular volume fraction, Radiotherapy, Cardiorespiratory fitness
Introduction
Incidental cardiac radiation exposure following anti-cancer thoracic radiotherapy treatment increases risk of heart failure (HF) in a dose-dependent manner [1] with a predominantly restrictive cardiomyopathy phenotype characterized by diffuse fibrosis within the myocardium [2, 3]. The restrictive cardiomyopathy phenotype following radiotherapy is typically a non-infiltrative disorder with endothelial cell damage resulting in microvascular dysfunction and stimulation of excessive extracellular matrix formation leading to increased myocardial fibrosis [4]. This can lead to a noncompliant left ventricle (LV) that is marked by elevated filling pressures and has limited ability to augment stroke volume (SV) [5].
The peak exercise oxygen pulse, determined with cardiopulmonary exercise testing (CPET), is the quotient of oxygen consumption (VO2) divided by the heart rate (HR) at peak exercise. Through deduction of the Fick equation, the peak oxygen pulse can accurately serve as a noninvasive estimate of the LV SV response to exercise in both healthy subjects and HF patients [6–9]. An additional noninvasive diagnostic strategy, contrast-enhanced cardiac magnetic resonance (CMR) imaging allows tissue characterization, including quantification of the LV extracellular volume fraction (LV ECVF), a surrogate of diffuse myocardial fibrosis [10]. Knowledge of the relationship between LV ECVF and the peak exercise oxygen pulse may provide mechanistic insight into the cardiac reserve of the cancer survivor following thoracic radiotherapy. The purpose of the current study was to examine this relationship in a cross-section of patients with a history of this treatment.
Methods
This study complies with the Declaration of Helsinki, was approved by the Virginia Commonwealth University (VCU) Massey Cancer Center Protocol Review and Monitoring Committee and Institutional Review Board, and all subjects provided informed consent prior to enrollment.
Patients
Patients with a history of external-beam thoracic radiotherapy for the treatment of chest malignancies with significant incidental heart exposure defined as ≥5 Gray (Gy) to ≥10% of the heart volume but were without a pre-cancer treatment history of overt cardiovascular disease (CVD) or HF, were prospectively enrolled. Subjects underwent CPET to determine cardiorespiratory fitness (CRF) including the peak oxygen pulse, N-terminal pro-brain natriuretic peptide (NTproBNP) measurement as a marker of ventricular wall stress [11], and contrast-enhanced CMR imaging. Exclusion criteria consisted of contraindications to CMR with gadolinium-contrast use, moderate or severe renal impairment (glomerular filtration rate < 60 mL/min/1.73 m2), pregnancy, or inability to walk on a treadmill.
Clinical variables analyzed were age, sex, race (Caucasian/African-American), history of established CVD risk factors (Yes/No; hypertension, diabetes mellitus, current cigarette smoking, hypercholesterolemia, obesity [body mass index (BMI) ≥30]), cancer type (breast/ lung or other chest malignancy), prior chemotherapy including type and dose, presence of anemia, and current beta-blocker and/or angiotensin-converting enzyme inhibitor (ACEI)/ angiotensin receptor blockers (ARB) defined per medical record review and patient interview. Due to the likelihood of coexistent chronic obstructive pulmonary disease (COPD) in chest tumor patients the presence of COPD was also considered based upon Global Initiative for Chronic Obstructive Lung Disease criteria from pre-exercise spirometry [12]. Physical activity participation was quantified using a validated questionnaire [13]. Quality of life (QOL) was analyzed using the Functional Assessment of Cancer Therapy-General (FACT-G7) questionnaire [14]. Some individuals in this cohort have previously been partially characterized [15].
Radiotherapy parameters
Radiation dose calculation was performed based on a volumetric computed-tomography (CT) data set obtained during the pre-radiotherapy treatment planning session. A single radiation oncologist (E.W.) performed quantification of total radiation dose and heart volumes exposed. The heart was manually contoured on each CT-slice generating 3-dimensional structures using treatment planning software (Pinnacle, Koninklijke Philips N.V.). After radiation beam definition and target dose calculation, mean cardiac radiation dose (MCRD) was determined for the whole organ volume as well as using dose-volume histograms to generate %volumes (V) of the heart receiving ≥5, 10, 20, 30, 40, and 50 Gray (Gy), respectively.
Cardiopulmonary exercise testing
A symptom-limited CPET was administered using a conservative ramping treadmill protocol according to established guidelines [16, 17]. The average value for VO2 obtained during the final 30-s of exercise was used to define peak VO2. The peak exercise respiratory exchange ratio (RER) was defined as the ratio between the peak carbon dioxide production divided by the peak VO2. Predicted peak VO2 was calculated according to the reference equations proposed by Wasserman and colleagues which take into account age, gender, and anthropometric (height, bodyweight) differences [7]. The absolute peak exercise oxygen pulse (milliliters [mL] of O2 per heart beat) was determined by dividing the absolute peak VO2 (mL/minute) by the HR at peak exercise. The predicted peak exercise oxygen pulse was calculated as the quotient of the predicted peak VO2 divided by the age-predicted maximal HR (220 – age). The peak oxygen pulse was normalized as a percent-predicted value (%O2Pulse) to account for subject differences due to age, sex, and anthropometrics. Heart rate (via 12-lead electrocardiography; GE Healthcare, Chicago, IL), blood pressure ([BP], via automated-stress BP monitor; Tango+, Morrisville, NC), and peripheral oxygen saturation ([SpO2]; Nellcor, Minneapolis, MN) were assessed according to standard recommendations [16].
Cardiac magnetic resonance
Resting cardiac magnetic resonance imaging was performed on a Siemens Aera 1.5 Tesla scanner (Siemens Healthcare, Erlangen, Germany). All CMR parameters were interpreted by board-certified cardiovascular radiologists (J.G., L.R-G., F.D.) who were blinded to the results of the remainder of study procedures. Selected MRI sequences were obtained including cardiac dimensions, systolic function, and late-gadolinium enhancement (LGE) post-contrast images. Contrast was administered using 0.2 mmol/kg of intravenous Prohance (Bracco Diagnostics Inc., Monroe Township, NJ). The myocardial volumes; left-ventricular end-diastolic volume (LVEDV), left-ventricular end-systolic volume (LVESV), stroke volume (SV), and cardiac output (CO) were indexed to body surface area. Myocardial tissue composition was quantified through the measurement of native T1 and post-contrast gadolinium-enhanced T1 values using a balanced steady-state free-procession modified Look-Locker inversion recovery pulse sequence. Post-contrast images were obtained at 15-min following contrast administration. Estimation of the ECVF was used to quantify diffuse myocardial injury. The ECVF was determined by the equation [18]:.
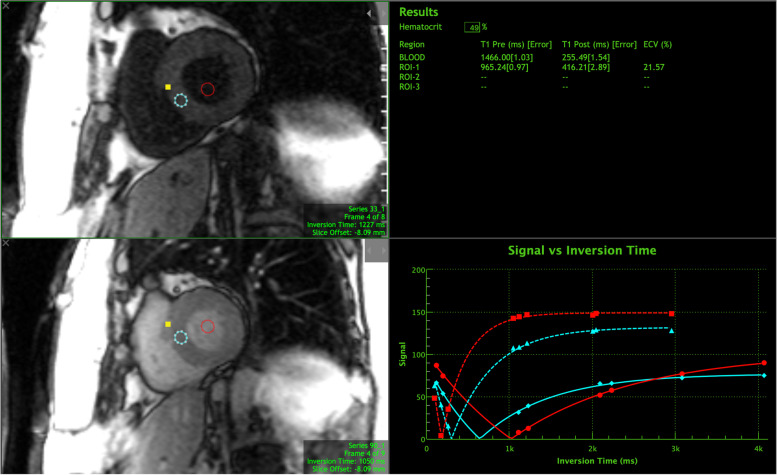
A global ECVF was determined from regions of interest in the septum on short-axis slices obtained at the base, mid, and apex of the LV myocardium. Figure 1 represents an example of an ECVF calculation. Hematocrit used in the ECVF calculations was determined non-invasively from the blood pool using a validated technique [19, 20]. Post-processing was performed using dedicated CMR analysis software (Precession, Heart Imaging Technologies, Durham, NC).
Fig. 1.
Example of myocardial ECVF calculation from native T1, post-contrast T1, and blood pool inversion times. Abbreviations: ROI = region of interest; ECVF = extracellular volume fraction; ms = milliseconds
Statistical analysis
Data are reported as number (%) or median [interquartile range] due to potential non-Gaussian distributions. Chi-square tests were performed to assess differences between nominal variables including Fisher’s exact test for variables with cell count frequencies < 5. Spearman’s rank test was used for univariate analysis of associations between continuous clinical characteristics, radiotherapy, CMR, and CRF variables. The Mann-Whitney U test was performed to compare differences between groups based upon categorical variables and peak oxygen pulse values < 85% or ≥ 85% of predicted [21] and LV ECVF values > or ≤ the median value. A threshold value of < 85% of predicted for peak oxygen pulse values has previously been shown to confer increased risk of cardiac mortality in HF patients [21]. A block multivariate linear regression model was created by first evaluating significant nominal categorical predictors of the %O2Pulse (block-1) followed by inclusion into a stepwise multivariate model combined with significant continuous univariate radiotherapy and CMR variables to determine predictors of %O2Pulse (block-2). All regression beta-coefficients are reported as standardized values. Collinearity diagnostics were performed on significant univariate predictors before entry into the multivariate analysis. Statistical analysis was performed using SPSS v26.0 (IBM Corp, Armonk, NY) with significance set at a P < 0.05.
Results
Patient characteristics
Thirty patients (age 63 [57–67] years, 18 [60%] female, 2.0 [0.6–3.8] years since completion of radiotherapy) underwent evaluation. The peak VO2 was 1376 (1057–1552) mL·min− 1 with a peak HR of 150 (122–164) bpm resulting in a peak exercise O2 pulse of 9.2 (7.5–10.7) mL/beat that was 82 (66–96)% of predicted values. Table 1 provides the detailed clinical characteristics of the cohort including grouped comparisons between those with a peak exercise oxygen pulse < 85% or ≥ 85% of predicted. Sixteen (53%) subjects demonstrated a peak exercise oxygen pulse < 85% of predicted values. When the peak exercise %O2Pulse was evaluated as a continuous variable according to nominal clinical characteristics there were significant differences with respect to smoking status, diabetes, obesity, cancer type, COPD, ACEI/ARB use, and sex. There was not a significant difference between %O2Pulse and race, hypercholesterolemia, hypertension, anemia, beta-blocker use, or prior receipt of chemotherapy.
Table 1.
Clinical Characteristics of the Cohort
| Variables | Entire Cohort N = 30 |
O2 Pulse < 85% n = 16 |
O2 Pulse ≥85% n = 14 |
P-Value |
|---|---|---|---|---|
| Age, y | 63 [57–67] | 61 [54–64] | 64 [58–69] | 0.249 |
| Female | 18 (60%) | 7 (44%) | 10 (71%) | 0.076 |
| Race | 0.107 | |||
| Caucasian | 20 (67%) | 9 (56%) | 5 (36%) | |
| African-American | 10 (33%) | 7 (44%) | 2 (14%) | |
| Body Mass Index, kg/m2 | 27.1 [23.6–30.6] | 24.5 [20.8–27.2] | 30.3 [27.0–31.2] | 0.005 |
| Body Surface Area, m2 | 1.88 [1.73–1.99] | 1.81 [1.71–1.94] | 1.92 [1.79–2.14] | 0.132 |
| COPD | 18 (60%) | 13 (81%) | 5 (36%) | 0.023 |
| FACT-G7 score | 20.0 [15.0–23.5] | 17.5 [14.3–22.3] | 21.0 [18.0–25.0] | 0.041 |
| Cancer type | 0.008 | |||
| Lung or Other* | 20 (67%) | 14 (88%) | 5 (36%) | |
| Breast | 10 (33%) | 2 (13%) | 8 (57%) | |
| Other Malignancies | ||||
| Esophageal | 2 (7%) | |||
| Hodgkin’s Lymphoma | 1 (3%) | |||
| Desmoid Tumor | 1 (3%) | |||
| Castleman’s Disease | 1 (3%) | |||
| Time since Diagnosis, y | 2.6 [1.3–4.2] | 2.8 [1.4–7.0] | 2.4 [0.6–2.8] | 0.398 |
| Time since Radiotherapy, y | 2.0 [0.6–3.8] | 2.2 [0.8–5.8] | 2.2 [0.6–2.8] | 0.423 |
| Time since chemotherapy, y | 1.7 [0.5–2.9] | 1.5 [0.2–4.2] | 1.9 [0.6–2.8] | 0.423 |
| Prior Chemotherapy | 26 (87%) | 14 (88%) | 11 (79%) | 0.617 |
| CVD Risk Factors | ||||
| Hypertension | 17 (57%) | 8 (50%) | 8 (57%) | 0.404 |
| Diabetes Mellitus | 7 (23%) | 1 (6%) | 6 (43%) | 0.019 |
| Hypercholesterolemia | 14 (47%) | 8 (50%) | 6 (43%) | 0.566 |
| Current Smoker | 6 (20%) | 5 (31%) | 1 (7%) | 0.037 |
| Obesity | 10 (33%) | 3 (19%) | 7 (50%) | 0.064 |
| Beta-blocker Use | 5 (17%) | 2 (13%) | 3 (21%) | 0.396 |
| ACEI/ARB Use | 6 (20%) | 2 (13%) | 4 (29%) | 0.423 |
| NTproBNP, pg/mL | 187 [51–310] | 298 [89–445] | 72 [31–200] | 0.013 |
Data are listed as median and [interquartile range] or n (%). *Other malignancies grouped with lung cancer subjects. P-values are differences between groups (< 85% and ≥ 85% predicted peak exercise O2 Pulse)
Abbreviations: y years, COPD chronic obstructive pulmonary disease, FACT-G7 Functional Assessment of Cancer Therapy-General (7-item version), ACEI/ARB angiotensin converting enzyme inhibitor/angiotensin receptor blocker, NTproBNP N-terminal pro-brain natriuretic peptide
Table 2 provides a detailed description of selected CPET variables. Overall, the peak VO2 was moderately-reduced (62% of predicted) compared to normalized values. The peak exercise %O2pulse demonstrated a significant positive correlation with the peak VO2 (r = + 0.78, P < 0.001) and a significant inverse relationship with the cardiac biomarker NTproBNP (r = − 0.51, P = 0.004). The peak %O2Pulse and peak VO2 were positively associated with FACT-G7 scores (r = + 0.39, P = 0.038; r = + 0.40, P = 0.031, respectively) reflecting higher CRF correlated with higher QOL.
Table 2.
Cardiopulmonary Exercise Test Variables
| Variables | Entire Cohort | O2 Pulse < 85% | O2 Pulse ≥85% | P-Value |
|---|---|---|---|---|
| Peak VO2, mL·min− 1 | 1376 [1057–1552] | 1166 [805–1330] | 1575 [1439–1946] | < 0.001 |
| Percent-predicted peak VO2, % | 62 [52–89] | 53 [46–61] | 91 [69–96] | < 0.001 |
| Peak VO2, mL·kg− 1·min− 1 | 16.9 [14.4–20.8] | 16.1 [12.4–18.2] | 19.7 [16.5–22.8] | 0.012 |
| Peak O2 Pulse, mL·beat− 1 | 9.2 [7.5–10.7] | 7.9 [6.9–9.3] | 10.4 [9.3–12.5] | 0.001 |
| %O2Pulse | 82 [66–96] | 68 [55–76] | 98 [93–112] | < 0.001 |
| Peak RER | 1.02 [0.95–1.09] | 0.97 [0.92–1.09] | 1.05 [1.00–1.11] | 0.045 |
| Resting Heart Rate, bpm | 73 [68–86] | 77 [67–86] | 73 [66–76] | 0.449 |
| Exercise Heart Rate, bpm | 150 [122–164] | 136 [115–157] | 151 [141–170] | 0.101 |
| Rest Systolic BP, mmHg | 124 [111–143] | 130 [113–144] | 123 [103–140] | 0.531 |
| Rest Diastolic BP, mmHg | 70 [63–81] | 70 [67–82] | 68 [57–79] | 0.374 |
| Exercise Systolic BP, mmHg | 174 [155–190] | 175 [150–182] | 170 [158–201] | 0.475 |
| Exercise Diastolic BP, mmHg | 70 [70–80] | 72 [70–80] | 70 [63–81] | 0.329 |
| Breathing Reserve, % | 33 [22–46] | 24 [16–44] | 37 [26–49] | 0.156 |
| Peak SpO2, % | 97 [95–99] | 98 [94–100] | 97 [96–99] | 0.682 |
Data are listed as median and (interquartile range). P-values are differences between groups (< 85% and ≥ 85% predicted peak exercise O2 Pulse)
Abbreviations: VO2 oxygen consumption, O2 oxygen, %O2Pulse percent-predicted peak exercise oxygen pulse, RER respiratory exchange ratio, BP blood pressure, SpO2 peripheral oxygen saturation
The peak exercise %O2Pulse was not significantly associated with age, physical activity levels, rest or maximal HR or blood pressures. From a chronicity perspective, there was not a significant association between %O2Pulse achieved and time from cancer diagnosis or completion of chemotherapy or radiotherapy.
Cardiac magnetic resonance imaging
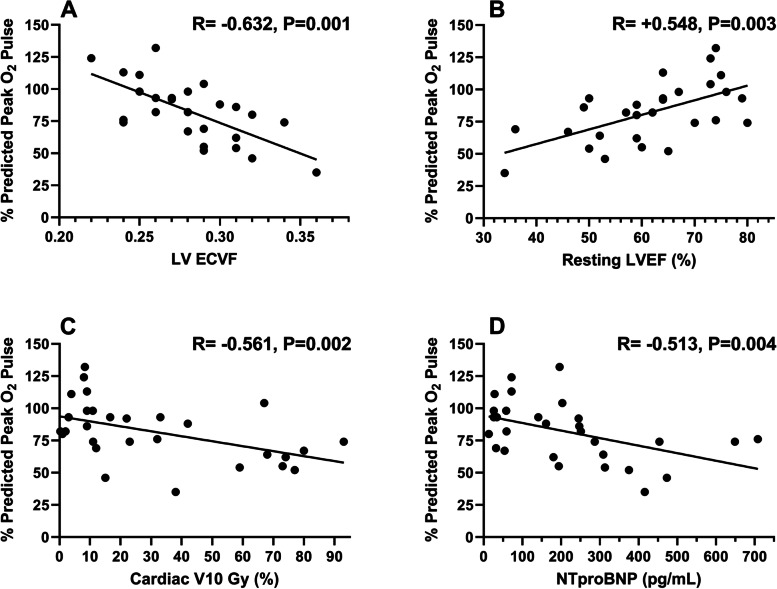
Cardiac magnetic resonance variables of interest are detailed in Table 3. Most patients had left-ventricular ejection fractions (LVEF) within normal range (64 [53–74]%), with nearly half (n = 12 [41%]) of subjects exhibiting qualitative evidence of LGE with post-contrast CMR. Analysis of CMR variables with peak exercise %O2Pulse demonstrated a negative correlation with the composite LV ECVF (r = − 0.63, P = 0.001), a positive correlation with LVEF (r = + 0.55, P = 0.003) (Fig. 2; Panels A,B) and the resting SV index (r = + 0.52, P = 0.011). There was no significant correlation between the %O2Pulse and native T1 (r = − 0.23, P = 0.269), post-contrast T1 (r = − 0.20, P = 0.340), or %LGE burden (r = − 0.03, P = 0.878). Similarly, there was no significant difference between dichotomous qualitative presence of LGE and %O2Pulse (P = 0.99).
Table 3.
Cardiac Magnetic Resonance Imaging Parameters
| Variables | Entire Cohort N = 27 |
Peak O2 Pulse < 85% n = 12 |
Peak O2 Pulse ≥85% n = 15 |
P-Value |
|---|---|---|---|---|
| LVEF, % | 64 [53–74] | 59 [50–65] | 67 [62–75] | 0.046 |
| LVEDV Index, mL/m2 | 62 [55–71] | 61 [49–71] | 62 [56–74] | 0.651 |
| LVESV Index, mL/m2 | 25 [18–32] | 24 [18–38] | 25 [15–32] | 0.695 |
| LV SV Index, mL/m2 | 38 [31–44] | 32 [27–42] | 39 [34–45] | 0.044 |
| LV Cardiac Index, L·min−1/m2 | 2.7 [2.2–3.0] | 2.3 [1.8–3.0] | 2.7 [2.6–2.9] | 0.190 |
| Presence of LGE | 12 (44%) | 5 (42%) | 7 (47%) | 0.274 |
| LGE, % | 0 [0–3] | 0 [0–2] | 2 [0–4] | 0.427 |
| Native T1-Global, ms | 1042 [1013–1063] | 1044 [1019–1070] | 1039 [1009–1047] | 0.631 |
| Post-contrast T1-Global, ms | 434 [410–463] | 425 [386–473] | 436 [416–454] | 0.781 |
| LV ECVF-Global, % | 28 [26–31] | 29 [28–32] | 27 [25–29] | 0.036 |
Data are listed as median and [interquartile range] or n (%). P-values are differences between groups (< 85% and ≥ 85% predicted peak exercise O2 Pulse)
Abbreviations: LVEF left-ventricular ejection fraction, LVEDV left-ventricular end-diastolic volume, LVESV left-ventricular end-systolic volume, SV stroke volume, LGE late-gadolinium enhancement, T1 myocardial T1, LV ECVF left-ventricular extracellular volume fraction
Fig. 2.
Independent predictors of peak exercise %O2 pulse. Legend: Panel A - correlation between LV ECVF and %O2 pulse. Panel B - correlation between resting LVEF and %O2 pulse. Panel C - correlation between cardiac V10Gy and %O2 pulse. Panel D shows the inverse correlation between NTproBNP and %O2 pulse. Abbreviations: %O2 pulse = %-predicted oxygen pulse; ECVF = extracellular volume fraction; LVEF = left-ventricular ejection fraction; V10Gy = %volume of the heart receiving ≥10 Gray; NTproBNP=N-terminal pro-brain natriuretic peptide
The global LV ECVF demonstrated significant relationships with LVEF (r = − 0.51, P = 0.006), peak VO2 (r = − 0.42, P = 0.032), cancer type (higher in lung or other chest malignancy vs. breast cancer; P = 0.020) and use of angiotensin blockade (lower in patients on ACE-I/ARB; P = 0.007) (Supplemental Fig. 1). The LV ECVF did not significantly associate with presence of established CVD risk factors (all P > 0.050), MCRD or V5-50Gy heart doses (all P > 0.050), age (P > 0.136), presence of COPD (P = 0.190), or prior chemotherapy use (P = 0.278).
Radiotherapy Dosimetric and chemotherapy variables
The total prescribed radiotherapy dose was 60.0 (48.0–60.4) Gy, MCRD 5.6 (3.7–17.8) Gy, and the %-volume of heart exposed to V5Gy was 39.5 (15.8–80.5)%, V10Gy 19.3 (8.8–67.3)%, V20Gy 7.0 (1.2–35)%, V30Gy 2.5 (0–15)%, V40Gy 1.0 (0–7.8)%, and V50Gy 0 (0–3)%, respectively. There was a negative correlation between the MCRD and the peak %O2Pulse (r = − 0.51, P = 0.005). Additionally, all %-volumes of the heart receiving between V5-V50Gy of radiotherapy demonstrated a negative association with peak %O2Pulse (V5Gy [r = − 0.51, P = 0.004], V10Gy [r = − 0.56, P = 0.002], V20Gy [r = − 0.45, P = 0.014], V30Gy [r = − 0.48, P = 0.009], V40Gy [r = − 0.54, P = 0.003], V50Gy [r = − 0.42, P = 0.023], respectively). Total prescribed radiotherapy dose was not associated with %O2Pulse (r = − 0.06, P = 0.761).
Due to the heterogeneity of chemotherapy use, comparison with peak %O2Pulse was restricted to those chemotherapy agents wherein at least 20% of the cohort received that agent. The doses of the chemotherapy agents analyzed included paclitaxel (r = + 0.42, P = 0.090), carboplatin (r = + 0.38, P = 0.226), cyclophosphamide (r = + 0.12, P = 0.774), and doxorubicin (r = − 0.40, P = 0.313), which were not significantly associated with peak %O2Pulse. Supplemental Table 1 provides the chemotherapy regimens of the cohort.
Peak oxygen pulse & LV ECVF threshold comparisons
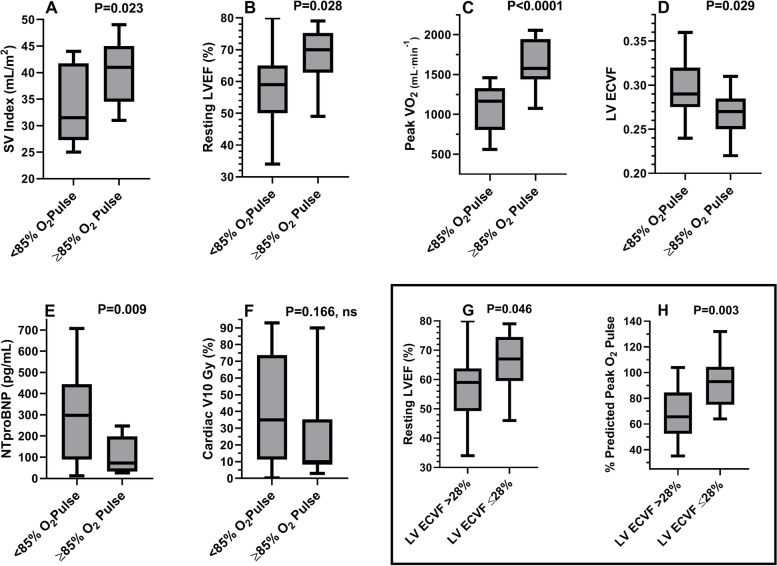
When dichotomized based upon a peak oxygen pulse < or ≥ 85% of predicted values, a known prognostic threshold [21], there were significant group differences between the SV index, LVEF, peak VO2, LV ECVF, and NTproBNP (Fig. 3; Tables 1-3). Furthermore, separation of the LV ECVF at the median value (≤ or > 28%) revealed a significant difference between the LVEF and %O2Pulse (Fig. 3; Panels G, H). Supplemental Table 2 provides a comparison of groups separated by the LV ECVF ≤28% or > 28% of the median value.
Fig. 3.
Differences between groups based upon percent-predicted peak exercise O2 pulse or median LV ECVF. Legend: Box and whisker plots (Panels A-F) demonstrating median (horizontal line within rectangular box), interquartile range, whiskers, and range between groups based upon %O2 pulse (< or ≥ 85%). Panel A: Lower SVI in those with peak O2 pulse < 85% of predicted. Panel B: Lower LVEF in those with a peak O2 pulse < 85% of predicted. Panel C: Lower peak VO2 in those with a peak O2 pulse < 85% of predicted. Panel D: Higher LV ECVF in those with a peak O2 pulse < 85% of predicted. Panel E: Higher NTproBNP levels in those with a peak O2 pulse < 85% of predicted. Panel F: Trend towards higher V10Gy in those with a peak O2 pulse < 85% of predicted. Panel G: Lower resting LVEF in those with LV ECVF values >median. Panel H: Lower peak %O2 pulse values in those with LV ECVF >median. Abbreviations: SVI = stroke volume index; O2 = oxygen; LVEF = left-ventricular ejection fraction; VO2 = oxygen consumption; ECVF = extracellular volume fraction; NTproBNP=N-terminal pro-brain natriuretic peptide; V10Gy = %volume of heart receiving ≥10 Gray
Multivariate analysis model
In the multivariate model, significant nominal-level clinical predictors of %O2Pulse analyzed in block-1 included: smoking status, diabetes, obesity, sex, cancer type, ACEI/ARB use, and COPD. Cancer type was the only nominal variable retained (β = .644, P < 0.001) in block-1 of the multivariate model with an adjusted-R2 of 0.394.
When evaluating radiotherapy dose parameters, collinearity diagnostics revealed significant multicollinearity between the MCRD and V5-V50Gy doses. Due to this finding, the cardiac V10Gy was the only radiotherapy variable entered into the multivariate model as it had the highest univariate relationship with %O2Pulse.
In block-2 of the multivariate model, cancer type (block-1) was added to the significant univariate continuous predictors: LV ECVF, LVEF, cardiac V10Gy, and NTproBNP into a stepwise analysis. The LV ECVF (β = −.281, P = 0.049), LVEF (β = .455, P = 0.002), cardiac V10Gy (β = −.330, P = 0.006), and NTproBNP (β = −.319, P = 0.013) were retained in the model as independent predictors of %O2Pulse while cancer type was removed from the model. The overall-model fit demonstrated an adjusted-R2 of 0.732.
Discussion
In this cross-sectional analysis of patients with significant incidental heart exposure following thoracic radiotherapy without an established history of CVD or HF, we found peak exercise %O2Pulse (a normalized surrogate of exercise LV SV response) was inversely associated with a CMR-derived marker of diffuse myocardial fibrosis (LV ECVF), ventricular wall stress (NTproBNP), cardiac radiation dose, and positively related to cardiac function (LVEF). This demonstrates a potential mechanistic relationship between the effects of thoracic radiotherapy on myocardial function and tissue composition (evidenced by increased LV ECVF, decreased LVEF, elevated NTproBNP) and its resulting influence on cardiac reserve measured via peak exercise %O2Pulse. Although the adverse effects of thoracic radiotherapy on late CVD/HF risk are well-known optimal methods for screening to detect subclinical cardiac dysfunction and the role of advanced imaging or exercise testing remain understudied [22, 23].
We recently observed reductions in peak VO2 following thoracic radiotherapy driven primarily by impaired diastolic reserve [15, 24]. Increased interstitial myocardial fibrosis that can occur following cancer treatment can impair LV systolic and diastolic function [25]. Work has demonstrated elevated LV ECVF in anthracycline-treated patients (29% received chest radiation) that was negatively associated with diastolic function with lower diastolic function in those with concomitant reduced systolic function [26]. Takagi and colleagues prospectively evaluated changes in global LV function and myocardial tissue characterization in 24 esophageal cancer patients following chemo-radiation therapy which demonstrated early changes in LV ECVF and native T1 (increased 0.5-years after) followed by late (1.5-years after) adverse changes in the SV index [27]. Although CRF was not evaluated it highlights the ability of LV ECVF to detect early subclinical changes that translate into later functional decline (reduced SV). In childhood cancer survivors that previously underwent anthracycline-based chemotherapy regimens (17% received chest irradiation), Tham et al. demonstrated an inverse relationship between LV ECVF and peak VO2, however, oxygen pulse was not reported [28]. Similarly, Duca et al. showed an inverse association between LV ECVF and functional status (6-min walk distance, New York Heart Association class) and stroke volume in patients with HF and preserved ejection fraction (HFpEF) [29]. In a prospective study of the ability to differentiate hypertensive heart disease and HFpEF, Mordi et al. demonstrated LV ECVF was an excellent discriminator between hypertension and HFpEF (area under the curve = 0.88) and was inversely associated with peak VO2 [30].
The peak oxygen pulse is an expression of peak VO2 that by its nature corrects for HR and when further corrected for age, sex, and anthropometrics can differentiate cardiac from noncardiac causes of exercise intolerance [31, 32]. The findings of the current study have implications, as the peak O2 pulse is associated with risk for sudden cardiac death, fatal coronary heart disease, CVD, and all-cause mortality [33–35]. Moreover, the peak %O2Pulse provides additive predictive accuracy to intermediate-range peak VO2 (10–14 mL·kg− 1·min− 1) for mortality risk assessment in HF patients [21]; some reports have even suggested superiority of the peak O2 pulse over peak VO2 for predicting clinical HF events particularly when it’s normalized for body mass [36]. Although not reporting oxygen pulse, in a retrospective analysis of patient with cancer therapy-induced HF (CTHF) (44% received chest radiotherapy) compared with non-cancer therapy HF (NCTHF), CTHF patients demonstrated a distinct profile of higher LVEF and worse LV diastolic and systolic (global longitudinal strain) function that was associated with a lower peak VO2 and higher incidence of the composite endpoint (all-cause mortality, heart transplant or LV assist device implant) [23]. Furthermore, in an adjusted multivariate analysis (including peak VO2, VE/VCO2 slope) CTHF was associated with a higher risk of death and the composite endpoint.
In oncological surgery patients, an abnormal exercise oxygen pulse response has been associated with increased mortality, neoadjuvant treatments, and was a strong independent predictor of post-operative cardiopulmonary complications even surpassing peak VO2 [37–39]. Collectively, these findings suggest a potential role of CPET including measurement of oxygen pulse in the risk stratification and determination of the causes of exercise intolerance in cancer survivors including those receiving thoracic radiotherapy. To the best of our knowledge, we are not aware of any prior studies that have examined the association between the peak exercise oxygen pulse and LV ECVF.
Study limitations
The limitations of this study are its small-sample size, single-center, and cross-sectional nature, limiting the findings to hypothesis generating rather than definitively establishing causality and require further exploration. Another limitation is that evaluation of the oxygen pulse is complex, as it depends upon many factors such as fitness, body mass, and is also significantly influenced by arterial oxygen supply and peripheral oxygen extraction. However, in the current study normalization of the oxygen pulse reported as a %-predicted value accounted for the influences of age, sex, and anthropometrics. Furthermore, arterial oxygen supply (quantified by SpO2), presence of anemia, or COPD were not significant predictors of %O2Pulse in the current study. Unfortunately, the influence of peripheral oxygen extraction was not directly assessed in the current study. Future studies should explore the relationships of radiotherapy dose to specific cardiac substructures with %O2Pulse and LV ECVF for a more precise mechanistic understanding of radiotherapy-related cardiac dysfunction [40]. Lastly, the synthetic hematocrit used for the derivation of LV ECVF was analyzed using an automated method based on the linear relationship between the longitudinal T1 relaxation properties of the blood and blood hematocrit versus direct assessment.
Conclusions
A multimodality noninvasive assessment, including myocardial tissue composition (using contrast-enhanced CMR to derive LV ECVF) and assessment of cardiac reserve (using CPET to determine the normalized peak exercise %O2Pulse) may provide mechanistic insight into the causes of exercise intolerance in the cancer survivor following thoracic radiotherapy, suggesting that an expansion of myocardial LV ECV may be contributing to the inability to increase LVSV during exercise leading to reductions in exercise capacity. Although requiring further study, our findings indicate reductions in %O2Pulse may represent subclinical reductions in stroke volume that is associated with elevations in LV ECVF. This may allow early detection of radiotherapy-related myocardial dysfunction prior to the onset of overt HF thus opening the door to prophylactic therapeutic interventions.
Supplementary Information
Acknowledgements
The authors would like to thank all of the patients and staff in the VCU Massey Cancer Center, VCU Clinical Research Unit, and VCU Pauley Heart Center who participated in this work.
Abbreviations
- CMR
Cardiac magnetic resonance imaging
- COPD
Chronic obstructive pulmonary disease
- CPET
Cardiopulmonary exercise test
- CRF
Cardiorespiratory fitness
- CVD
Cardiovascular disease
- CT
Computed tomography
- ECVF
Extracellular volume fraction
- FACT-G7
Functional Assessment of Cancer Therapy-General
- Gy
Gray units
- HF
Heart failure
- HR
Heart rate
- LGE
Late-gadolinium enhancement
- LVEDV
Left-ventricular end-diastolic volume
- LVEF
Left-ventricular ejection fraction
- LVESV
Left-ventricular end-systolic volume
- MCRD
Mean cardiac radiation dose
- NTproBNP
N-terminal pro-brain natriuretic peptide
- O2
Oxygen
- %O2Pulse
Percent-predicted peak oxygen pulse
- RER
Respiratory exchange ratio
- SpO2
Peripheral oxygen saturation
- SV
Stroke volume
- T1
Time constant representing the recovery of longitudinal magnetization
- V
Volume
- VO2
Oxygen consumption
Authors’ contributions
All authors have approved this manuscript for publication. JDG, LRG, and FD had a role in CMR acquisition, CMR analysis, and manuscript writing; JHJ had a role in interpretation of data and manuscript writing; AR had a role in radiotherapy data acquisition, radiotherapy data analysis, and manuscript writing; CRT, SC, LFB, DK had a role in acquisition of data, and manuscript writing; RKE, RG, BVT had a role in study conception, design, and analysis and interpretation of data, and manuscript writing; WGH had a role in manuscript design and revisions; JMC, EW (radiotherapy data acquisition, radiotherapy data analysis), AA had a role in study conception, design, acquisition of data, analysis and interpretation of data, and manuscript writing.
Funding
This work was supported by a Virginia Commonwealth University Massey Cancer Center Pilot Project grant #P30CA016059K. This study was also supported by a Center for Clinical and Translational Research grant #1UL1TR002649 from the National Center for Advancing Translational Science to Virginia Commonwealth University, Richmond, Virginia, United States.
Availability of data and materials
The dataset analyzed during the current study are available from the corresponding author upon reasonable request.
Declarations
Ethics approval and consent to participate
This research involved human subjects and was performed in accordance with the Declaration of Helsinki. All participants provided informed consent. This study was approved by the Institutional Review Board of Virginia Commonwealth University.
Consent for publication
Not applicable.
Competing interests
AA has served as a consultant for Astra Zeneca, Janssen, Merck, Novartis, Olatec, and Serpin pharma. BVT has served as a consultant for Novartis and Serpin Pharma. EW reports financial relationships through grants with Varian Medical Systems and the National Institutes of Health. SC is supported by a Career Development Award #19CDA34660318 from the American Heart Association. The remaining authors have nothing to disclose in regard to this study and no conflicts of interest exist for all listed authors.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Saiki H, Petersen IA, Scott CG, Bailey KR, Dunlay SM, Finley RR, Ruddy KJ, Yan E, Redfield MM. Risk of heart failure with preserved ejection fraction in older women after contemporary radiotherapy for breast Cancer. Circulation. 2017;135(15):1388–1396. doi: 10.1161/CIRCULATIONAHA.116.025434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Filopei J, Frishman W. Radiation-induced heart disease. Cardiol Rev. 2012;20(4):184–188. doi: 10.1097/CRD.0b013e3182431c23. [DOI] [PubMed] [Google Scholar]
- 3.Veinot JP, Edwards WD. Pathology of radiation-induced heart disease: a surgical and autopsy study of 27 cases. Hum Pathol. 1996;27(8):766–773. doi: 10.1016/S0046-8177(96)90447-5. [DOI] [PubMed] [Google Scholar]
- 4.Yarnold J, Brotons MC. Pathogenetic mechanisms in radiation fibrosis. Radiother Oncol. 2010;97(1):149–161. doi: 10.1016/j.radonc.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 5.Pereira NL, Grogan M, Dec GW. Spectrum of restrictive and infiltrative cardiomyopathies: part 1 of a 2-part series. J Am Coll Cardiol. 2018;71(10):1130–1148. doi: 10.1016/j.jacc.2018.01.016. [DOI] [PubMed] [Google Scholar]
- 6.Whipp BJ, Higgenbotham MB, Cobb FC. Estimating exercise stroke volume from asymptotic oxygen pulse in humans. J Appl Physiol. 1996;81(6):2674–2679. doi: 10.1152/jappl.1996.81.6.2674. [DOI] [PubMed] [Google Scholar]
- 7.Wasserman K, Hansen JE, Sue DY, Stringer WW, Whipp BJ. Principles of exercise testing and interpretation : including pathophysiology and clinical applications. 4. Philadelphia: Lippincott Williams & Wilkins; 2005. [Google Scholar]
- 8.Agostoni PG, Wasserman K, Perego GB, Guazzi M, Cattadori G, Palermo P, Lauri G, Marenzi G. Non-invasive measurement of stroke volume during exercise in heart failure patients. Clin Sci (Lond) 2000;98(5):545–551. doi: 10.1042/CS19990237. [DOI] [PubMed] [Google Scholar]
- 9.Accalai E, Vignati C, Salvioni E, Pezzuto B, Contini M, Cadeddu C, Meloni L, Agostoni P. Non-invasive estimation of stroke volume during exercise from oxygen in heart failure patients. Eur J Prev Cardiol. 2021;28(3):280–286. doi: 10.1177/2047487320920755. [DOI] [PubMed] [Google Scholar]
- 10.Kellman P, Wilson JR, Xue H, Ugander M, Arai AE. Extracellular volume fraction mapping in the myocardium, part 1: evaluation of an automated method. J Cardiovasc Magn Reson. 2012;14:63. doi: 10.1186/1532-429X-14-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chow SL, Maisel AS, Anand I, Bozkurt B, De Boer RA, Felker GM, Fonarow GC, Greenberg B, Januzzi JL, Kiernan MS, Liu PP, Wang TJ, Yancy CW, Zile MR. American Heart Association clinical pharmacology Committee of the Council on clinical cardiology, council on basic cardiovascular sciences, council on cardiovascular disease in the young, council on cardiovascular and stroke nursing, council on cardiopulmonary critical care perioperative and resuscitation, council on epidemiology and prevention, council on functional genomics and translational biology, council on quality of care, and outcomes research. Role of biomarkers for the prevention, assessment, and management of heart failure: a scientific statement from the American Heart Association. Circulation. 2017;135(22):e1054–e1091. doi: 10.1161/CIR.0000000000000490. [DOI] [PubMed] [Google Scholar]
- 12.Vestbo J, Hurd SS, Agustí AG, Jones PW, Vogelmeier C, Anzueto A, Barnes PJ, Fabbri LM, Martinez FJ, Nishimura M, Stockley RA, Sin DD, Rodriguez-Roisin R. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease GOLD executive summary. Am J Respir Crit Care Med. 2013;187(4):347–365. doi: 10.1164/rccm.201204-0596PP. [DOI] [PubMed] [Google Scholar]
- 13.Craig C, Marshall A, Sjostrom M, Bauman A, Booth M, Ainsworth B, Pratt M, Ekelund U, Yngve A, Sallis J, Oja P. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sport Exerc. 2003;35(8):1381–1395. doi: 10.1249/01.MSS.0000078924.61453.FB. [DOI] [PubMed] [Google Scholar]
- 14.Yanez B, Pearman T, Lis CG, Beaumont JL, Cella D. The FACT-G7: a rapid version of the functional assessment of cancer therapy-general (FACT-G) for monitoring symptoms and concerns in oncology practice and research. Ann Oncol. 2013;24(4):1073–1078. doi: 10.1093/annonc/mds539. [DOI] [PubMed] [Google Scholar]
- 15.Canada JM, Trankle CR, Carbone S, Buckley LF, Medina de Chazal H, Billingsley H, Evans RK, Garten R, Van Tassell BW, Kadariya D, Mauro A, Toldo S, Mezzaroma E, Arena R, Hundley WG, Grizzard JD, Weiss E, Abbate A. Determinants of cardiorespiratory fitness following thoracic radiotherapy in lung or breast Cancer survivors. Am J Cardiol. 2020;125(6):988–996. doi: 10.1016/j.amjcard.2019.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fletcher GF, Ades PA, Kligfield P, Arena R, Balady GJ, Bittner VA, Coke LA, Fleg JL, Forman DE, Gerber TC, Gulati M, Madan K, Rhodes J, Thompson PD, Williams MA. Exercise standards for testing and training: a scientific statement from the American heart association. Circulation. 2013;128(8):873–934. doi: 10.1161/CIR.0b013e31829b5b44. [DOI] [PubMed] [Google Scholar]
- 17.Arena R, Humphrey R, Peberdy MA, Madigan M. Predicting peak oxygen consumption during a conservative ramping protocol: implications for the heart failure population. J Cardiopulm Rehabil. 2003;23(3):183–189. doi: 10.1097/00008483-200305000-00004. [DOI] [PubMed] [Google Scholar]
- 18.Ugander M, Oki AJ, Hsu L-YY, Kellman P, Greiser A, Aletras AH, Sibley CT, Chen MY, Patricia Bandettini W, Arai AE, Bandettini WP, Arai AE. Extracellular volume imaging by magnetic resonance imaging provides insights into overt and sub-clinical myocardial pathology. Eur Heart J. 2012;33(10):1268–1278. doi: 10.1093/eurheartj/ehr481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Treibel TA, Fontana M, Maestrini V, Castelletti S, Rosmini S, Simpson J, Nasis A, Bhuva AN, Bulluck H, Abdel-Gadir A, White SK, Manisty C, Spottiswoode BS, Wong TC, Piechnik SK, Kellman P, Robson MD, Schelbert EB, Moon JC. Automatic measurement of the myocardial Interstitium: synthetic extracellular volume quantification without hematocrit sampling. J Am Coll Cardiol Img. 2016;9(1):54–63. doi: 10.1016/j.jcmg.2015.11.008. [DOI] [PubMed] [Google Scholar]
- 20.Kammerlander AA, Duca F, Binder C, Aschauer S, Zotter-Tufaro C, Koschutnik M, Marzluf BA, Bonderman D, Mascherbauer J. Extracellular volume quantification by cardiac magnetic resonance imaging without hematocrit sampling : ready for prime time? Wien Klin Wochenschr. 2018;130(5–6):190–196. doi: 10.1007/s00508-017-1267-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oliveira RB, Myers J, Araujo CG, Arena R, Mandic S, Bensimhon D, Abella J, Chase P, Guazzi M, Brubaker P, Moore B, Kitzman D, Peberdy MA. Does peak oxygen pulse complement peak oxygen uptake in risk stratifying patients with heart failure? Am J Cardiol. 2009;104(4):554–558. doi: 10.1016/j.amjcard.2009.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Raghunathan D, Khilji MI, Hassan SA, Yusuf SW. Radiation-induced cardiovascular disease. Curr Atheroscler Rep. 2017;19(5):22. doi: 10.1007/s11883-017-0658-x. [DOI] [PubMed] [Google Scholar]
- 23.Nadruz W, Jr, West E, Sengeløv M, Grove GLGL, Santos M, Groarke JDJD, Forman DEDE, Claggett B, Skali H, Nohria A, Shah AAMM. Cardiovascular phenotype and prognosis of patients with heart failure induced by cancer therapy. Heart. 2019;105(1):34–41. doi: 10.1136/heartjnl-2018-313234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Canada JM, Thomas GK, Trankle CR, Carbone S, Billingsley H, Van Tassell BW, Evans RK, Garten R, Weiss E, Abbate A. Increased C-reactive protein is associated with the severity of thoracic radiotherapy-induced cardiomyopathy. Cardio-Oncology. 2020;6(1):2. doi: 10.1186/s40959-020-0058-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jordan JH, Todd RM, Vasu S, Hundley WG. Cardiovascular magnetic resonance in the oncology patient. J Am Coll Cardiol Img. 2018;11(8):1150–1172. doi: 10.1016/j.jcmg.2018.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Neilan TG, Coelho-Filho OR, Shah RV, Feng JH, Pena-Herrera D, Mandry D, Pierre-Mongeon F, Heydari B, Francis SA, Moslehi J, Kwong RY, Jerosch-Herold M. Myocardial extracellular volume by cardiac magnetic resonance imaging in patients treated with anthracycline-based chemotherapy. Am J Cardiol. 2013;111(5):717–722. doi: 10.1016/j.amjcard.2012.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Takagi H, Ota H, Umezawa R, Kimura T, Kadoya N, Higuchi S, Sun W, Nakajima Y, Saito M, Komori Y, Jingu K, Takase K. Left ventricular T1 mapping during chemotherapy-radiation therapy: serial assessment of participants with esophageal Cancer. Radiology. 2018;289(2):347–354. doi: 10.1148/radiol.2018172076. [DOI] [PubMed] [Google Scholar]
- 28.Tham EB, Haykowsky MJ, Chow K, Spavor M, Kaneko S, Khoo NS, Pagano JJ, Mackie AS, Thompson RB. Diffuse myocardial fibrosis by T1-mapping in children with subclinical anthracycline cardiotoxicity: relationship to exercise capacity, cumulative dose and remodeling. J Cardiovasc Magn Reson. 2013;15:48. doi: 10.1186/1532-429X-15-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Duca F, Kammerlander AA, Zotter-Tufaro C, Aschauer S, Schwaiger ML, Marzluf BA, et al. Interstitial fibrosis, functional status, and outcomes in heart failure with preserved ejection fraction: insights from a prospective cardiac magnetic resonance imaging study. Circ Cardiovasc Imaging. 2016;9(12):e005277. [DOI] [PubMed]
- 30.Mordi IR, Singh S, Rudd A, Srinivasan J, Frenneaux M, Tzemos N, Dawson DK. Comprehensive echocardiographic and cardiac magnetic resonance evaluation differentiates among heart failure with preserved ejection fraction patients, hypertensive patients, and healthy control subjects. J Am Coll Cardiol Img. 2018;11(4):577–585. doi: 10.1016/j.jcmg.2017.05.022. [DOI] [PubMed] [Google Scholar]
- 31.Ahmad MN, Yusuf SH, Ullah R, Ahmad MM, Ellis MK, Yousaf H, Paterick TE, Ammar KA. Multivariate criteria Most accurately distinguish cardiac from noncardiac causes of dyspnea. Texas Hear Inst J. 2015;42(6):514–521. doi: 10.14503/THIJ-14-4373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Klainman E, Fink G, Lebzelter J, Krelbaumm T, Kramer MR. The relationship between left ventricular function assessed by multigated radionuclide test and cardiopulmonary exercise test in patients with ischemic heart disease. Chest. 2002;121(3):841–845. doi: 10.1378/chest.121.3.841. [DOI] [PubMed] [Google Scholar]
- 33.Laukkanen JA, Kurl S, Salonen JT, Lakka TA, Rauramaa R. Peak oxygen pulse during exercise as a predictor for coronary heart disease and all cause death. Heart. 2006;92(9):1219–1224. doi: 10.1136/hrt.2005.077487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Laukkanen JA, Araujo CGS, Kurl S, Khan H, Jae SY, Guazzi M, Kunutsor SK. Relative peak exercise oxygen pulse is related to sudden cardiac death, cardiovascular and all-cause mortality in middle-aged men. Eur J Prev Cardiol. 2018;25(7):772–782. doi: 10.1177/2047487318761679. [DOI] [PubMed] [Google Scholar]
- 35.Oliveira RB, Myers J, Araújo CGS, Abella J, Mandic S, Froelicher V. Maximal exercise oxygen pulse as a predictor of mortality among male veterans referred for exercise testing. Eur J Prev Cardiol. 2009;16(3):358–364. doi: 10.1097/HJR.0b013e3283292fe8. [DOI] [PubMed] [Google Scholar]
- 36.Lavie CJ, Milani RV, Mehra MR. Peak exercise oxygen pulse and prognosis in chronic heart failure. Am J Cardiol. 2004;93(5):588–593. doi: 10.1016/j.amjcard.2003.11.023. [DOI] [PubMed] [Google Scholar]
- 37.Mann J, Williams M, Wilson J, Yates D, Harrison A, Doherty P, Davies S. Exercise-induced myocardial dysfunction detected by cardiopulmonary exercise testing is associated with increased risk of mortality in major oncological colorectal surgery. Br J Anaesth. 2020;124(4):473–479. doi: 10.1016/j.bja.2019.12.043. [DOI] [PubMed] [Google Scholar]
- 38.Fresard I, Licker M, Adler D, Lovis A, Robert J, Karenovics W, Diaper J, Janssens J-P, Triponez F, Lador F, Rochat T, Espinosa V, Bhatia C, Kayser B, Bridevaux P-O. Preoperative peak oxygen uptake in lung Cancer subjects with neoadjuvant chemotherapy: a cross-sectional study. Respir Care. 2016;61(8):1059–1066. doi: 10.4187/respcare.04299. [DOI] [PubMed] [Google Scholar]
- 39.Campione A, Terzi A, Bobbio M, Rosso GL, Scardovi AB, Feola M. Oxygen pulse as a predictor of cardiopulmonary events in lung resection. Asian Cardiovasc Thorac Ann. 2010;18(2):147–152. doi: 10.1177/0218492310361792. [DOI] [PubMed] [Google Scholar]
- 40.Bergom C, Bradley JA, Ng AK, Samson P, Robinson C, Lopez-Mattei J, Mitchell JD. Past, present, and future of radiation-induced cardiotoxicity: refinements in targeting, surveillance, and risk stratification. JACC CardioOncology. 2021;3(3):343–359. doi: 10.1016/j.jaccao.2021.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The dataset analyzed during the current study are available from the corresponding author upon reasonable request.