Abstract
Background
Hyperhomocysteinemia (HHcy) and metabolic syndrome (MS) are established cardiovascular risk factors of stroke and are frequently associated with hypertension. However, studies on the association between HHcy combined with MS and stroke risk in hypertensive patients were absent.
Material/Methods
In 14 059 selected participants with elevated blood pressure, we assessed the prevalence of the MS and stroke. We defined HHcy as plasma total homocysteine >15 μmol/L. MS was defined according to the Chinese Diabetes Society (CDS) criterion. Multivariable analysis was used to examine the association of HHcy or (and) MS with stroke risk in different models.
Results
The prevalence rates of HHcy and MS were 49.96% and 42.21%, respectively. Patients with stroke had higher plasma total homocysteine levels and a higher prevalence of MS (P<0.001). Multivariable analyses indicated that HHcy and MS are independently associated with higher prevalence of stroke (adjusted-odds ratio (OR): 1.36, 95% CI 1.17 to 1.58, P<0.001; adjusted-OR: 1.68, 95% CI 1.44 to 1.96, P<0.001, respectively). Those with combined HHcy and MS had higher odds of stroke than those with isolated HHcy or MS (adjusted-OR: 1.78, 95% CI 1.47 to 2.15, P<0.001; adjusted-OR: 1.39, 95% CI 1.13 to 1.70, P=0.002, respectively).
Conclusions
HHcy combined with MS was associated with higher prevalence of stroke in Chinese adults with elevated blood pressure.
Keywords: Hyperhomocysteinemia, Metabolic Syndrome, Stroke
Background
Globally, stroke is the second leading cause of death, and its prevalence in adults is 2.7% in the United States [1]. Stroke in Chinese adults has increased over the past 30 years, and the age-standardized prevalence, incidence, and mortality rates are 1114.8/100 000 people, 246.8, and 114.8/100 000 person-years, respectively [2]. There are some modifiable risk factors in the prevention and treatment of stroke, including obesity, hypertension, type 2 diabetes mellitus (T2DM), dyslipidemia, and hyperhomocysteinemia (HHcy) [1–4].
Homocysteine is a sulfurated amino acid that is synthesized during the methionine metabolic cycle, and the pathogenic elevation of the plasma total homocysteine (tHcy) levels above 10–15 μmol/L is defined as HHcy [3,4]. HHcy is an independent risk factor for cardiovascular diseases (CVD) such as hypertension, atherosclerosis, coronary heart disease (CHD), and stroke [5,6] via various pathways, including oxidative stress, endoplasmic reticulum (ER) stress, epigenetic modulation, and protein homocysteinylation [7]. The prevalence of hypertension in the Chinese adult population (≥18 years of age) is 23.2% (about 245 million) [8], and approximately 75% of Chinese patients with hypertension also have HHcy, which is associated with a higher risk of stroke [4].
Metabolic syndrome (MS), which is a global public health problem of great concern, is a cluster of clinical and metabolic factors that significantly increase the risk for T2DM, CHD, and stroke [9]. The interrelated risk factors include central obesity, dyslipidemias, hypertension, hypercoagulable state, and insulin resistance (IR) [10]. According to the International Diabetes Federation (IDF) criteria, the prevalence of MS in the USA is 33–39%, with many being female, while the prevalence of MS in Europe varies widely among countries, ranging from 18% to 30% [11,12]. The estimated prevalence of MS in China is 34.0% according to the revised ATP III criteria and 26.9% by IDF criteria, which is higher in women and older adults [13].
HHcy and MS are both established risk factors of stroke and are frequently associated with hypertension. However, there are no published studies on the combined effect of HHcy and MS on stroke risk in hypertensive patients, a cohort at particularly high risk for stroke [1,2,14]. China bears the huge burden of hypertension and stroke [2]. In this study, we investigated the association of HHcy combined with MS with the prevalence of stroke in Chinese adults with elevated blood pressure.
Material and Methods
Study Population
The China H-type Hypertension Registry Study was a real-world, multicenter, observational study, conducted in March 2018 at Wuyuan, Jiangxi province of China. The enrolled population consisted of hypertensive patients aged 18 years and older. The exclusion criteria included psychological or nervous system impairment resulting in an inability to provide informed consent, unable to participate in long-term followed-up according to the study protocol, or plans to relocate in the near future. The study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the Ethics Committee of the Institute of Biomedicine, Anhui Medical University. Signed informed consent was obtained from all participants [15].
We assessed data on a total of 14 268 individuals. Elevated blood pressure was defined as the 130/85 mmHg threshold [16,17] or having been diagnosed with hypertension and previously started treatment. After excluding those without elevated blood pressure (n=10) or with missing value of blood lipids (n=7), 1 patient with maximum value of Hcy (182.92 μmol/L), and 191 patients with atrial fibrillation that could cause cardiogenic stroke, 14 059 individuals were included in the analysis (Supplementary Figure 1).
Data Collection and Indexes Determination
Data on individuals’ general characteristics (age, sex, and smoking and drinking status), past medical history (hypertension, diabetes, dyslipidemia, cerebral stroke [ischemic stroke or intracerebral hemorrhage]), and medication usage were gathered by professional researchers through a questionnaire survey. Atrial fibrillation was assessed by past medical history and electrocardiograms.
The anthropometric examinations included systolic blood pressure (SBP), diastolic blood pressure (DBP), body height, weight, height, waistline, and hipline. BP was measured, with the participant in a sitting position using the Omron HBP-1300 Professional Portable Blood Pressure Monitor (Kyoto, Japan) on the right arm, which was supported at the heart level. After a 5-min rest period, BP was measured 4 times, and SBP and DBP were calculated as the average of the last 3 readings. Waistline and hipline were measured using an inelastic measuring tape. Body mass index (BMI) was calculated as the weight in kilograms divided by height in meters squared (kg/m2).
The levels of plasma tHcy, fasting blood glucose (FBG), total cholesterol (TC), total triglyceride (TG), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), serum uric acid and creatinine, blood urea nitrogen (BUN), total and direct bilirubin, aspartate aminotransferase (AST), and alanine aminotransferase (ALT) were assessed using biochemical analysis instruments (Beckman Coulter). tHcy was quantified by high-performance liquid chromatography using a fluorescence detector [18]. All laboratory measurements were performed according to the criteria of a standardization and certification program. In our study, HHcy was defined as Hcy >15 μmol/L, in consideration of the fact that the ratio of tHcy levels ≤10 μmol/L was 1.16%. T2DM was defined as FBG levels over 7.0 mmol/L or on appropriate hypoglycemic medication [17]. Dyslipidemia was defined as having 1 of the following features: elevated TG (≥2.3 mmol/L), elevated TC (≥6.2 mmol/L), elevated LDL-C (≥4.1 mmol/L), and reduced HDL-C (<1.0 mmol/L) or on appropriate lipid-lowering medication [19]. The Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation was used to evaluate estimated glomerular filtration rate (eGFR) [20].
Definition of MS
Although the definition of MS is ambiguous, the most popular definition of MS consider ethnicity for assessing waistline, given differences in visceral adipose tissue [16,21]. In our study, MS was defined according to the Chinese Diabetes Society (CDS) criterion [17], as presence of at least 3 of the following 5 criteria: abdominal obesity (waistline ≥90 cm in adult male and 85 cm in adult female subjects), elevated blood pressure (SBP ≥130 mmHg and/or DBP ≥85 mmHg, or on treatment for diagnosed hypertension formerly), hyperglycemia (FBG ≥6.1 mmol/L, or on appropriate hypoglycemic medication), elevated TG (≥1.7 mmol/L, or on appropriate lipid-lowering medication) and reduced HDL-C (<1.03 mmol/L in adult male and <1.30 mmol/L in adult female subjects, or on appropriate lipid-lowering medication). In addition, considering this distinction of sex-specific reduced HDL-C criterion at current popular international guidelines [16], we did not choose the threshold of <1.04 mmol/L for definition of reduced HDL-C according to the CDS criterion in our study [17].
Statistical Analysis
Continuous variables are presented as the mean±standard deviation or the median (IQR), as appropriate, and were compared using the t test or the Mann-Whitney U test, depending on whether the quantitative data were consistent with normal distribution. Categorical variables are presented as count (percentage), and differences between groups were measured by the chi-square test.
Multivariable logistic regression analyses were used to examine the associations of tHcy or (and) MS with prevalence of stroke. Model I was only adjusted for age and sex. Model II (confounder model) screened covariates including age, sex, BMI, heart rate, smoking and drinking status, tHcy, AST, ALT, serum uric acid, eGFR, total and direct bilirubin, abdominal obesity, hyperglycemia, elevated TG, and reduced HDL-C, except for the independent variable itself. We selected these confounders on the basis of a more than 10% association with the outcomes of interest. Supplementary Table 1 show the associations of each confounder with stroke occurrence. We considered the confounder model to be the main model. In addition, the generalized additive model and smooth curve fitting (penalized spline method) were used to visually show the relationship between the subduplicate Hcy levels and stroke probability. In addition, subgroup analysis was executed by stratified and interaction test to investigate the robustness in this association.
The R statistical package (Te R Foundation; http://www.R-project.org, version 3.4.3) and Empower (R) software (www.empowerstats.com, version 2.2, X&Y Solutions, Inc. Boston, MA) were used for these statistical analyses. A two-tailed P <0.05 was considered to be statistically significant.
Results
Clinical Characteristics
This study analyzed 14 059 adults with elevated blood pressure (age: 63.76±9.35 years, range 27–96 years; male, 47.30%), and the prevalence rates of HHcy and MS were 49.96% and 42.21%, respectively (Table 1). The participants with stroke were more likely to be older, male, smoker; had lower BMI, SBP, DBP, TC, TG, HDL-C, LDL-C, eGFR, and AST, had higher levels of tHcy and serum creatinine, had a higher prevalence of current T2DM, elevated TG, reduced HDL-C, dyslipidemia, and MS, and had a lower prevalence of current abdominal obesity compared to those without stroke (P<0.05).
Table 1.
Clinical characteristics of participants grouped by stroke.
| Characteristics | Total (n=14059) | non-Stroke (n=13099) | Stroke (n=960) | P value |
|---|---|---|---|---|
| General characteristics | ||||
| Age (years) | 63.76±9.35 | 63.68±9.41 | 64.88±8.42 | <0.001 |
| Male, n (%) | 6650 (47.30%) | 6094 (46.52%) | 556 (57.92%) | <0.001 |
| BMI (kg/m2) | 23.62±3.74 | 23.63±3.56 | 23.44±5.67 | 0.010 |
| Waistline (cm) | 83.85±9.85 | 83.86±9.85 | 83.73±9.84 | 0.719 |
| WHR | 0.91±0.16 | 0.91±0.16 | 0.91±0.08 | 0.354 |
| SBP (mmHg) | 148.39±17.85 | 148.63±17.73 | 145.08±19.16 | <0.001 |
| DBP (mmHg) | 88.93±10.75 | 89.08±10.74 | 86.87±10.65 | <0.001 |
| Heart rate (times/min) | 76.55±14.00 | 76.59±14.04 | 76.03±13.50 | 0.221 |
| Smoking status, n (%) | <0.001 | |||
| Never | 8143 (57.93%) | 7670 (58.57%) | 473 (49.27%) | |
| Former smoker | 2275 (16.19%) | 2031 (15.51%) | 244 (25.42%) | |
| Current smoker | 3638 (25.88%) | 3395 (25.92%) | 243 (25.31%) | |
| Drinking status, n (%) | <0.001 | |||
| Never | 9088 (64.66%) | 8467 (64.65%) | 621 (64.75%) | |
| Former drinker | 1913 (13.61%) | 1684 (12.86%) | 229 (23.88%) | |
| Current drinker | 3054 (21.73%) | 2945 (22.49%) | 109 (11.37%) | |
| Blood biochemical tests | ||||
| tHcy (μmol/L) | 14.99 (12.46–19.11) | 14.90 (12.42–18.93) | 16.63 (13.33–21.31) | <0.001 |
| FBG (mmol/L) | 6.18±1.61 | 6.19±1.62 | 6.15±1.53 | 0.410 |
| TC (mmol/L) | 5.16±1.12 | 5.18±1.11 | 4.88±1.22 | <0.001 |
| TG (mmol/L) | 1.47 (1.04–2.16) | 1.47 (1.04–2.17) | 1.41 (1.03–2.03) | 0.013 |
| HDL-C (mmol/L) | 1.57±0.43 | 1.58±0.43 | 1.47±0.39 | <0.001 |
| LDL-C (mmol/L) | 2.98±0.81 | 3.00±0.81 | 2.81±0.90 | <0.001 |
| Serum uric acid (μmol/L) | 6418.62±120.57 | 418.35±120.66 | 422.29±119.40 | 0.297 |
| Serum creatinine (mmol/L) | 66.00 (54.00–81.00) | 65.00 (54.00–81.00) | 72.00 (58.00–89.00) | <0.001 |
| BUN (mmol/L) | 5.45±1.81 | 5.45±1.79 | 5.50±2.06 | 0.481 |
| eGFR (ml/min) | 88.26±20.23 | 88.63±20.11 | 83.27±21.07 | <0.001 |
| Total bilirubin (mmol/L) | 13.30 (10.20–17.40) | 13.30 (10.20–17.40) | 13.00 (9.90–17.10) | 0.083 |
| Direct bilirubin (mmol/L) | 5.10 (4.10–6.50) | 5.10 (4.10–6.50) | 5.20 (4.00–6.50) | 0.929 |
| AST (U/L) | 24.00 (20.00–30.00) | 24.00 (20.00–30.00) | 23.00 (20.00–28.00) | <0.001 |
| ALT (U/L) | 17.00 (12.00–24.00) | 17.00 (12.00–24.00) | 16.00 (12.00–23.00) | 0.088 |
| Medication usage | ||||
| Antihypertensive agents, n (%) | 9080 (64.60%) | 8291 (63.31%) | 789 (82.19%) | <0.001 |
| Hypoglycemic agents, n (%) | 740 (5.26%) | 653 (4.99%) | 87 (9.06%) | <0.001 |
| Lipid-lowering agents, n (%) | 491 (3.49%) | 331 (2.53%) | 160 (16.67%) | <0.001 |
| Antiplatelet agents, n (%) | 527 (3.75%) | 314 (2.40%) | 213 (22.19%) | <0.001 |
| Current diseases | ||||
| Abdominal obesity, n (%) | 5221 (37.14%) | 4902 (37.42%) | 319 (33.23%) | 0.009 |
| Hyperglycemia, n (%) | 5226 (37.17%) | 4865 (37.14%) | 361 (37.60%) | 0.774 |
| T2DM, n (%) | 2569 (18.27%) | 2361 (18.02%) | 208 (21.67%) | 0.005 |
| Dyslipidemia, n (%) | 5293 (37.65%) | 4873 (37.20%) | 420 (43.75%) | <0.001 |
| Elevated TG, n (%) | 5835 (41.50%) | 5379 (41.06%) | 456 (47.50%) | <0.001 |
| Reduced HDL-C, n (%) | 2720 (19.35%) | 2414 (18.43%) | 306 (31.87%) | <0.001 |
| MS, n (%) | 5934 (42.21%) | 5464 (41.71%) | 470 (48.96%) | <0.001 |
| No. of MS components | <0.001 | |||
| One | 4052 (28.82%) | 3796 (28.98%) | 256 (26.67%) | |
| Two | 4073 (28.97%) | 3839 (29.31%) | 234 (24.38%) | |
| Three | 3413 (24.28%) | 3156 (24.09%) | 257 (26.77%) | |
| Four | 1983 (14.10%) | 1825 (13.93%) | 158 (16.46%) | |
| Five | 538 (3.83%) | 483 (3.69%) | 55 (5.73%) |
HHcy – hyperhomocysteinemia; MS – metabolic syndrome; BMI – body mass index; WHR – waist-hip ratio; SBP – systolic blood pressure; DBP – diastolic blood pressure; tHcy – total homocysteine; FBG – fasting blood glucose; TC – total cholesterol; TG – total triglyceride; HDL-C – high-density lipoprotein cholesterol; LDL-C – low-density lipoprotein cholesterol; BUN – blood urea nitrogen; eGFR – estimated glomerular filtration rate; AST – aspartate aminotransferase; ALT – alanine aminotransferase; T2DM – type 2 diabetes mellitus.
Associations Between Plasma tHcy Levels or MS and Cerebral Stroke
The multivariable analyses indicated that plasma tHcy level was positively associated with the prevalence of stroke (adjusted odds ratio [OR] per SD increase: 1.08, 95% CI 1.02 to 1.14, P=0.010; Table 2). Compared to the group without HHcy, participants with HHcy had a higher prevalence of stroke (adjusted-OR: 1.36, 95% CI 1.17 to 1.58, P<0.001). Compared to tHcy concentrations ≤12.46 μmol/L, there were significantly higher prevalence rates of stroke in the third and highest tHcy quartiles (adjusted-OR: 1.37, 95% CI 1.11 to 1.69, P=0.003; adjusted-OR: 1.49, 95% CI 1.19 to 1.85, P <0.001, respectively; P for trend < 0.001). The generalized additive model and penalized spline method were used to find a positive relationship between the subduplicate tHcy levels and stroke probability (Figure 1).
Table 2.
Relationship between plasma tHcy levels or MS and the prevalence of stroke in different models.
| Variables | Event, n (%) | Crude Model | Model I | Model II | |||
|---|---|---|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | OR (95% CI) | P value | ||
| tHcy (μmol/L) | |||||||
| Per SD increase | 960 (6.83%) | 1.18 (1.12, 1.23) | <0.001 | 1.13 (1.07, 1.19) | <0.001 | 1.08 (1.02, 1.14) | 0.010a |
| tHcy (μmol/L) | |||||||
| <15 | 367 (5.22%) | Ref | Ref | Ref | |||
| ≥15 | 593 (8.44%) | 1.68 (1.46, 1.92) | <0.001 | 1.48 (1.28, 1.71) | <0.001 | 1.36 (1.17, 1.58) | <0.001a |
| tHCY tertile (μmol/L) | |||||||
| T1 [5.88, 13.23] | 231 (4.93%) | Ref | Ref | Ref | |||
| T2 [13.24, 17.37] | 307 (6.54%) | 1.35 (1.13, 1.61) | <0.001 | 1.24 (1.04, 1.49) | 0.017 | 1.28 (1.06, 1.53) | 0.009b |
| T3 [17.38, 134.50] | 422 (9.00%) | 1.91 (1.62, 2.25) | <0.001 | 1.62 (1.36, 1.94) | <0.001 | 1.49 (1.23, 1.81) | <0.001b |
| tHcy quartile (μmol/L) | |||||||
| Q1 [5.88, 12.46] | 176 (5.02%) | Ref | Ref | Ref | |||
| Q2 [12.47, 15.00] | 191 (5.41%) | 1.08 (0.88, 1.34) | 0.459 | 1.01 (0.82, 1.25) | 0.922 | 1.04 (0.84, 1.29) | 0.709c |
| Q3 [15.01, 19.11] | 268 (7.60%) | 1.56 (1.28, 1.89) | <0.001 | 1.38 (1.13, 1.69) | 0.002 | 1.37 (1.11, 1.69) | 0.003c |
| Q4 [19.12, 134.50] | 326 (9.27%) | 1.93 (1.60, 2.34) | <0.001 | 1.61 (1.31, 1.97) | <0.001 | 1.49 (1.19, 1.85) | <0.001c |
| P for trend | <0.001 | <0.001 | <0.001 | ||||
| MS | |||||||
| No | 490 (6.03%) | Ref | Ref | Ref | |||
| Yes | 470 (7.92%) | 1.34 (1.18, 1.53) | <0.001 | 1.58 (1.38, 1.81) | <0.001 | 1.68 (1.44, 1.96) | <0.001d |
| No. of MS components | |||||||
| One | 256 (6.32%) | Ref | Ref | Ref | |||
| Two | 234 (5.75%) | 0.90 (0.75, 1.09) | 0.278 | 1.01 (0.84, 1.21) | 0.935 | 1.09 (0.90, 1.32) | 0.394e |
| Three | 257 (7.53%) | 1.21 (1.01, 1.44) | 0.039 | 1.47 (1.22, 1.77) | <0.001 | 1.74 (1.41, 2.13) | <0.001e |
| Four | 158 (7.97%) | 1.28 (1.04, 1.58) | 0.018 | 1.64 (1.32, 2.03) | <0.001 | 1.99 (1.56, 2.54) | <0.001e |
| Five | 55 (10.22%) | 1.69 (1.24, 2.29) | <0.001 | 2.22 (1.62, 3.04) | <0.001 | 2.60 (1.84, 3.67) | <0.001e |
| P for trend | <0.001 | <0.001 | <0.001 | ||||
tHcy – total homocysteine; MS – metabolic syndrome; Ref – reference; OR – odds ratio; CI – confidence interval; SD – standard deviation. Model I adjusted for age and sex. Model II:
adjusted for sex, smoking and drinking status, eGFR, reduced HDL-C and abdominal obesity.
adjusted for a + serum uric acid.
adjusted for b + age and elevated TG.
adjusted for age, sex, BMI, smoking and drinking status, eGFR and tHcy.
adjusted for d + heart rate and serum uric acid.
Figure 1.
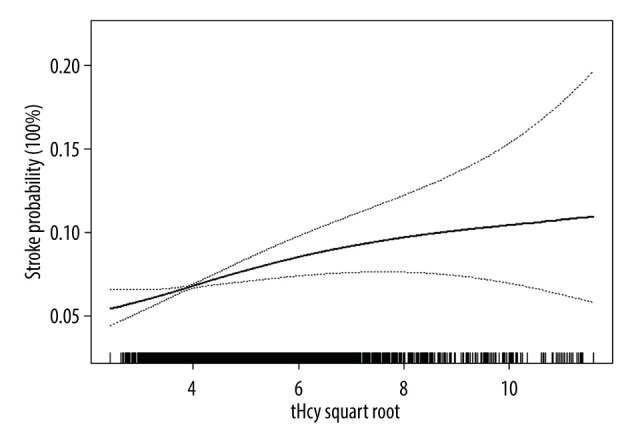
Smooth curve of correlation between the subduplicate tHcy levels and stroke probability. Smooth curve adjusted for sex, smoking and drinking status, eGFR, T2DM, and MS. tHcy – total homocysteine. EmpowerStats software (version 2.2, X&Y Solutions, Inc) was used to create the figure.
Similarly, the multivariable analyses also showed that, compared to the group without MS, participants with MS had a higher prevalence of stroke (adjusted-OR: 1.68, 95% CI 1.44 to 1.96, P<0.001; Table 2). When the group with only 1 component (hypertension) of MS was used as the reference, a statistically significant higher prevalence of stroke was found in the group with 3, 4 or 5 components of MS (adjusted-OR: 1.74, 95% CI 1.41 to 2.13, P<0.001; adjusted-OR: 1.99, 95% CI 1.56 to 2.54, P<0.001; adjusted-OR: 2.60, 95% CI 1.84 to 3.67, P<0.001, respectively; P for trend <0.001; Table 2).
Association Between HHcy Combined with MS and Stroke Risk in Hypertensive Patients
Clinical characteristics of participants grouped by HHcy and MS are presented in Supplementary Table 2. Compared to the control group, the prevalence of stroke was markedly increased in the isolated HHcy group, isolated MS group, and combined HHcy and MS group (adjusted-OR: 1.32, 95% CI 1.07 to 1.62, P=0.008; adjusted-OR: 1.78, 95% CI 1.41 to 2.23, P<0.001; adjusted-OR: 2.46, 95% CI 1.97 to 3.08, P <0.001, respectively; P for trend <0.001; Supplementary Table 3). Individuals with both HHcy and MS had increased odds of stroke than those with only HHcy or only MS (adjusted-OR: 1.78, 95% CI 1.47 to 2.15, P<0.001; adjusted-OR: 1.39, 95% CI 1.13 to 1.70, P=0.002, respectively; Table 3).
Table 3.
Effect size of combined HHcy and MS on the prevalence of stroke in different models compared to its isolated forms.
| Variables | Crude Model | Model I | Model II | |||
|---|---|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | OR (95% CI) | P value | |
| Isolated HHcy | Ref | Ref | Ref | |||
| HHcy+MS | 1.36 (1.15, 1.61) | <0.001 | 1.57 (1.32, 1.87) | <0.001 | 1.78 (1.47, 2.15) | <0.001 |
| Isolated MS | Ref | Ref | Ref | |||
| HHcy+MS | 1.68 (1.39, 2.03) | <0.001 | 1.47 (1.21, 1.78) | <0.001 | 1.39 (1.13, 1.70) | 0.002 |
HHcy – hyperhomocysteinemia; MS – metabolic syndrome; Ref – reference; OR – odds ratio; CI – confidence interval. Model I adjusted for age and sex. Model II adjusted for sex, BMI, smoking and drinking status, eGFR and serum uric acid.
Subgroup Analyses by Potential Effect Modifiers
To explore whether the association between HHcy combined with MS and stroke risk in hypertensive patients was still stable, we conducted subgroup analyses, which suggested that there were no interactions in any of the subgroups, including age (<60 vs ≥60 years), sex (male vs female), smoking habit (no vs yes), drinking habit (no vs yes), BMI (≤24 vs >24 kg/m2), and eGFR (≥60 vs <60 ml/min) (all P for interaction >0.05; Supplementary Figure 2).
Discussion
The findings of this research demonstrate that HHcy and MS are independently and positively associated with the prevalence of cerebral stroke in Chinese adults with elevated blood pressure. Homocysteine is a non-essential, possibly cytotoxic amino acid and HHcy caused by its metabolic imbalance is an independent risk factor for various vascular diseases [7,14], especially stroke [5,6,22,23]. Many possible mechanisms linking HHcy with atherogenesis have been suggested, including pro-thrombotic and pro-inflammatory effects, endothelial dysfunction, and smooth muscle cell proliferation [24]. Celermajer et al [25] found that HHcy was associated with impaired endothelial function of systemic arteries and decreased nitric oxide bioavailability in hyperhomocystinuria children. Cheng et al [26] found that HHcy impaired endothelium-dependent micro-vasculature relaxation to acetylcholine in mouse small mesenteric artery via oxidation of SKCa/IKCa. Intermediate HHcy potentiated diabetes impaired endothelium-derived hyperpolarizing factor (EDHF)-induced microvasculature relaxation via hydrogen sulfide (H2S)-downregulation and IKCa tyrosine nitration in T2DM/HHcy mice [27]. Recent research showed HHcy can cause mitochondrial dysfunction and systemic tissue damage, mostly by inhibiting mitochondrial complex I gene expression, which leads to impaired electron transfer, complex assembly, and redox homeostasis dysregulation in human disease and experimental models [28]. Therefore, we speculate that HHcy can cause intracranial vascular endothelial dysfunction and tissue damage through oxidative stress and mitochondrial dysfunction, which can lead to a stroke.
MS is associated with significantly higher risk of CVD such as CHD, stroke, and peripheral arterial diseases [9,16]. Similar to previous reports, we also found that the participants with MS were inclined to be female (non-MS vs MS: 45.2% vs 63.0%) and above the age of 50 years due to disappearance of estrogen protection after menopause [13,29], and had a higher prevalence of formerly cerebral stroke and current T2DM [9] (Supplementaty Table 1). Moreover, MS is independently associated with higher prevalence of stroke.
The molecular mechanisms underlying IR, MS, and CVD are intricate and confusing. IR is triggered by cellular inflammation, lipotoxicity, mitochondrial dysfunction, and endoplasmic stress, which lead to insulin signal transduction disorder, dysregulation of genes, and modifications of regulatory proteins [16]. Central obesity, with disturbances in lipid metabolism with deposition of fat in the waist and internal organs, is also linked to IR and CVD [30]. Dyslipidemia characterized by elevated TG and lowered HDL-C increased the risk for CVD in obese children with MS [31,32]. Vascular IR is a potential link between metabolic and CVD. In IR, the effect of insulin on endothelial cells is minimized with reduced nitric oxide production, making endothelial cells more susceptible to oxidized lipid-induced injury and atherogenesis [33]. In patients with IR, the process of lipolysis within adipose tissue is accelerated, resulting in increased free fatty acid release into the portal circulation. Moreover, macrophage infiltration and polarization (increased ratio of M1 to M2) of adipose tissue, altered immune response, and imbalance in the synthesis of pro- and anti-inflammatory cytokines affect the activity of insulin in the liver and muscles [34,35].
Previous study has found that patients with MS and HHcy or smoking are at particularly high cardiovascular risk compared with those with MS but with normal tHcy levels [36]. There was an interaction reported between HHcy and MS in individuals at risk for atherosclerosis [36]. In our research, those individuals with combined HHcy and MS had higher odds of stroke than those with only HHcy or only MS in Chinese adults with elevated blood pressure, thus showing an association between HHcy combined with MS and stroke risk in hypertensive patients.
Targeted stroke prevention should include healthy lifestyle improvement and treatment of MS and HHcy in an individualized and comprehensive way [37]. Among Chinese hypertensive adults without a history of CVD but with low baseline folate levels, folic acid supplementation significantly reduced the risk of first stroke [38], and elevated total cholesterol levels modified this benefit [39]. The reduction of stroke was proportional to the reduction of tHcy [40], and the reduction of stroke was greater in participants with HHcy [41] or elevated LDL-C [39]. Notably, folic acid therapy was beneficial among those with impaired renal function [42]. Although B vitamin therapy might reduce the stroke risk, this benefit might have been obscured in early trials by increased risk of CVD in participants with renal failure receiving high-dose cyanocobalamin [43]. In countries where folate fortification is in place, vitamin B12 deficiency is the main nutritional determinant of HHcy [44,45]. The potential benefits of methylcobalamin supplementation with folic acid therapy to lower plasma tHcy levels in populations at high risk of stroke warrant further investigation [43].
However, in the Insulin Resistance Intervention after Stroke (IRIS) randomized clinical trial, pioglitazone reduced new-onset diabetes by half and reduced stroke or myocardial infarction by 24% over 5 years among patients with IR [46], particularly in those with good adherence [47]. Pioglitazone also significantly lowered BP and TG and raised HDL-C, indicated that the benefits from this drug outweigh the risks of fracture and fluid retention [47]. Therefore, treatment with pioglitazone in patients with MS needs to be reconsidered given the large effects of pioglitazone in prediabetes [48].
This study had several limitations. The hypertensive population at Wuyuan had relatively higher and wider tHcy concentrations (median: 14.99 μmol/L), which may have led to differences in odds ratios of tHcy on the prevalence of stroke compared to other studies [49,50]. Additionally, we cannot draw any causal relationship between HHcy, MS, and stroke considering the cross-sectional design. Moreover, the subtype of stroke was not exactly classified due to the lack of imaging evidence originally. Finally, we did not measure the levels of glycated hemoglobin and insulin, which could have better evaluated the glycometabolism of our hypertensive patients.
Conclusions
HHcy and MS are independently associated with higher prevalence of stroke in Chinese adults with elevated blood pressure. Those with combined HHcy and MS had increased odds of stroke than those with only HHcy or only MS. We found an association between HHcy combined with MS and stroke risk in hypertensive patients.
Supplementary Data
Data flow chart of participants in our analysis. tHcy – total homocysteine; HHcy – hyperhomocysteinemia; MS – metabolic syndrome. EmpowerStats software (version 2.2, X&Y Solutions, Inc) was used to create the figure.
Supplementary Table 1.
Associations of covariates with stroke occurrence.
| Covariates | OR (95% CI) | P value |
|---|---|---|
| Age (years) | 1.01 (1.01, 1.02) | <0.001 |
| Sex | ||
| Male | Ref | |
| Female | 0.63 (0.55, 0.72) | <0.001 |
| BMI (kg/m2) | 0.99 (0.97, 1.00) | 0.124 |
| Heart rate (times/min) | 1.00 (0.99, 1.00) | 0.227 |
| Smoking status, n (%) | ||
| Never | Ref | |
| Former smoker | 1.95 (1.66, 2.29) | <0.001 |
| Current smoker | 1.16 (0.99, 1.36) | 0.068 |
| Drinking status, n (%) | ||
| Never | Ref | |
| Former drinker | 1.85 (1.58, 2.18) | <0.001 |
| Current drinker | 0.50 (0.41, 0.62) | <0.001 |
| tHcy (μmol/L) | 1.01 (1.01, 1.02) | <0.001 |
| Serum uric acid (mmol/L) | 1.00 (1.00, 1.00) | 0.329 |
| eGFR (ml/min) | 0.99 (0.99, 0.99) | <0.001 |
| Total bilirubin (mmol/L) | 0.99 (0.98, 1.00) | 0.255 |
| Direct bilirubin (mmol/L) | 1.00 (0.98, 1.03) | 0.900 |
| AST (U/L) | 1.00 (1.00, 1.00) | 0.372 |
| ALT (U/L) | 1.00 (1.00, 1.01) | 0.183 |
| Abdominal obesity, n (%) | ||
| No | Ref | 0.010 |
| Yes | 0.83 (0.72, 0.96) | |
| Hyperglycemia, n (%) | ||
| No | Ref | |
| Yes | 1.02 (0.89, 1.17) | 0.774 |
| Elevated TG, n (%) | ||
| No | Ref | |
| Yes | 1.30 (1.14, 1.48) | <0.001 |
| Reduced HDL-C, n (%) | ||
| No | Ref | |
| Yes | 2.07 (1.80, 2.39) | <0.001 |
BMI – body mass index; tHcy – total homocysteine; TC – total cholesterol; HDL-C – high-density lipoprotein cholesterol; eGFR – estimated glomerular filtration rate; AST – aspartate aminotransferase; ALT – alanine aminotransferase; T2DM – type 2 diabetes mellitus; Ref – reference; OR – odds ratio; CI – confidence interval. Values are regression coefficients [OR (95% CI)] from univariate regression models and reflect differences in stroke occur per unit change of each covariate and for different categories of each covariate as compared to the reference group.
Supplementary Table 2.
Clinical characteristics of participants grouped by HHcy and MS.
| Characteristics | Control (n=3910) | Isolated HHcy (n=4215) | Isolated MS (n=3125) | HHcy and MS (n=2809) | P value |
|---|---|---|---|---|---|
| General characteristics | |||||
| Age (years) | 62.62±8.95 | 67.29±9.20 | 60.22±8.49 | 64.00±9.18 | <0.001 |
| Male, n (%) | 1619 (41.41%) | 2837 (67.31%) | 886 (28.35%) | 1308 (46.56%) | <0.001 |
| BMI (kg/m2) | 22.56±3.23 | 21.99±3.74 | 25.66±3.24 | 25.25±3.18 | <0.001 |
| Waistline (cm) | 79.88±8.55 | 79.58±8.40 | 89.44±8.37 | 89.56±9.12 | <0.001 |
| WHR | 0.89±0.07 | 0.89±0.26 | 0.94±0.06 | 0.95±0.08 | <0.001 |
| SBP (mmHg) | 148.15±16.82 | 148.47±18.86 | 148.46±17.03 | 148.52±18.56 | 0.999 |
| DBP (mmHg) | 88.86±10.33 | 87.81±11.21 | 90.36±10.12 | 89.11±11.08 | <0.001 |
| Heart rate (times/min) | 74.76±13.82 | 75.18±14.07 | 78.41±13.00 | 79.05±14.60 | <0.001 |
| Smoking status, n (%) | <0.001 | ||||
| Never | 2470 (63.17%) | 1740 (41.29%) | 2273 (72.76%) | 1660 (59.12%) | |
| Former smoker | 512 (13.09%) | 928 (22.02%) | 348 (11.14%) | 487 (17.34%) | |
| Current smoker | 928 (23.73%) | 1546 (36.69%) | 503 (16.10%) | 661 (23.54%) | |
| Drinking status, n (%) | <0.001 | ||||
| Never | 2549 (65.19%) | 2315 (54.95%) | 2340 (74.90%) | 1884 (67.09%) | |
| Former drinker | 460 (11.76%) | 751 (17.83%) | 317 (10.15%) | 385 (13.71%) | |
| Current drinker | 901 (23.04%) | 1147 (27.23%) | 467 (14.95%) | 539 (19.20%) | |
| Blood biochemical tests | |||||
| tHcy (μmol/L) | 12.55 (11.35–13.69) | 19.33 (16.77–24.35) | 12.37 (11.20–13.56) | 18.75 (16.51–23.26) | <0.001 |
| FBG (mmol/L) | 5.70±1.00 | 5.76±1.06 | 6.78±2.06 | 6.82±1.95 | <0.001 |
| TC (mmol/L) | 5.06±0.98 | 5.06±1.02 | 5.22±1.20 | 5.37±1.30 | <0.001 |
| TG (mmol/L) | 1.18 (0.90–1.52) | 1.15 (0.89–1.47) | 2.17 (1.70–2.93) | 2.16 (1.71–2.97) | <0.001 |
| HDL-C (mmol/L) | 1.68±0.40 | 1.69±0.44 | 1.39±0.36 | 1.43±0.41 | <0.001 |
| LDL-C (mmol/L) | 2.84±0.72 | 2.87±0.76 | 3.11±0.85 | 3.22±0.91 | <0.001 |
| Serum uric acid (μmol/L) | 371.34±102.59 | 441.38±121.07 | 402.35±108.85 | 468.37±127.64 | <0.001 |
| Serum creatinine (mmol/L) | 59.00 (49.00–70.00) | 76.00 (63.00–94.00) | 58.00 (49.00–69.00) | 75.00 (61.00–92.00) | <0.001 |
| BUN (mmol/L) | 5.27±1.44 | 5.88±2.13 | 4.97±1.38 | 5.60±1.98 | <0.001 |
| eGFR (ml/min) | 96.34±14.33 | 80.17±21.43 | 96.50±15.10 | 80.00±22.05 | <0.001 |
| Total bilirubin (mmol/L) | 12.80 (9.90–16.40) | 13.70 (10.60–18.10) | 12.90 (9.80–16.80) | 13.80 (10.60–18.00) | <0.001 |
| Direct bilirubin (mmol/L) | 5.00 (4.00–6.30) | 5.40 (4.30–7.00) | 4.80 (3.80–6.10) | 5.20 (4.10–6.60) | <0.001 |
| AST (U/L) | 23.00 (20.00–28.00) | 25.00 (21.00–30.00) | 24.00 (20.00–30.00) | 25.00 (20.00–31.00) | <0.001 |
| ALT (U/L) | 15.00 (12.00–21.00) | 15.00 (11.00–21.00) | 20.00 (14.00–28.00) | 19.00 (14.00–28.00) | <0.001 |
| Past medical history | |||||
| Cerebral stroke, n (%) | 175 (4.48%) | 315 (7.47%) | 192 (6.14%) | 278 (9.90%) | <0.001 |
| Antihypertensive agents, n (%) | 2269 (58.03%) | 2783 (66.04%) | 2028 (64.92%) | 2000 (71.23%) | <0.001 |
| Hypoglycemic agents, n (%) | 71 (1.82%) | 66 (1.57%) | 319 (10.21%) | 284 (10.11%) | <0.001 |
| Lipid-lowering agents, n (%) | 0 (0.00%) | 0 (0.00%) | 241 (7.71%) | 250 (8.90%) | <0.001 |
| Antiplatelet agents, n (%) | 56 (1.43%) | 113 (2.68%) | 168 (5.38%) | 190 (6.76%) | <0.001 |
| Current diseases | |||||
| Abdominal obesity, n (%) | 637 (16.29%) | 511 (12.12%) | 2205 (70.56%) | 1868 (66.50%) | <0.001 |
| Hyperglycemia, n (%) | 651 (16.65%) | 849 (20.14%) | 1864 (59.65%) | 1862 (66.29%) | <0.001 |
| T2DM, n (%) | 256 (6.55%) | 302 (7.16%) | 1013 (32.42%) | 998 (35.53%) | <0.001 |
| Dyslipidemia, n (%) | 651 (16.65%) | 849 (20.14%) | 1864 (59.65%) | 1862 (66.29%) | <0.001 |
| Elevated TG, n (%) | 548 (14.02%) | 532 (12.62%) | 2481 (79.39%) | 2274 (80.95%) | <0.001 |
| Reduced HDL-C, n (%) | 175 (4.48%) | 171 (4.06%) | 1333 (42.66%) | 1041 (37.06%) | <0.001 |
HHcy – hyperhomocysteinemia; MS – metabolic syndrome; BMI – body mass index; WHR – waist-hip ratio; SBP – systolic blood pressure; DBP – diastolic blood pressure; tHcy – total homocysteine; FBG – fasting blood glucose; TC – total cholesterol; TG – total triglyceride; HDL-C – high-density lipoprotein cholesterol; LDL-C – low-density lipoprotein cholesterol; BUN – blood urea nitrogen; eGFR – estimated glomerular filtration rate; AST – aspartate aminotransferase; ALT – alanine aminotransferase; T2DM – type 2 diabetes mellitus.
Supplementary Table 3.
Effect size of HHcy and MS on cerebral stroke in different models.
| Variables | Event, n (%) | Crude Model | Model I | Model II | |||
|---|---|---|---|---|---|---|---|
| OR (95% CI) | P-value | OR (95% CI) | P-value | OR (95% CI) | P-value | ||
| Control | 175 (4.48%) | Ref | Ref | Ref | |||
| Isolated HHcy | 315 (7.47%) | 1.72 (1.43, 2.08) | <0.001 | 1.44 (1.19, 1.76) | <0.001 | 1.32 (1.07, 1.62) | 0.008 |
| Isolated MS | 192 (6.14%) | 1.40 (1.13, 1.72) | 0.002 | 1.54 (1.25, 1.91) | <0.001 | 1.78 (1.41, 2.23) | <0.001 |
| HHcy+MS | 278 (9.90%) | 2.34 (1.93, 2.85) | <0.001 | 2.26 (1.86, 2.75) | <0.001 | 2.46 (1.97, 3.08) | <0.001 |
| P for trend | <0.001 | <0.001 | <0.001 | ||||
HHcy – hyperhomocysteinemia; MS – metabolic syndrome; Ref – reference; OR – odds ratio; CI – confidence interval. Model I adjusted for age and sex. Model II adjusted for sex, BMI, smoking and drinking status, eGFR, and serum uric acid.
Effect size of HHcy and MS on stroke in prespecified and exploratory subgroups. Ref – reference; OR – odds ratio; CI – confidence interval, MS – metabolic syndrome; HHcy – hyperhomocysteinemia; BMI – body mass index; eGFR – estimated glomerular filtration rate; UA – serum uric acid (mmol/L). Each stratification adjusted for age, sex, BMI, smoking and drinking status eGFR, and serum uric acid except the subgroup variable. EmpowerStats software (version 2.2, X&Y Solutions, Inc) was used to create the figure.
Footnotes
Conflict of interest: None declared
Department and Institution Where Work Was Done
The Department of Cardiovascular Medicine, the Second Affiliated Hospital of Nanchang University, Nanchang, Jiangxi, China.
Ethics Approval and Consent to Participate
The study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the Ethics Committee of Institute of Biomedicine, Anhui Medical University. All participants provided written informed consent.
Declaration of Figures’ Authenticity
All figures submitted have been created by the authors, who confirm that the images are original with no duplication and have not been previously published in whole or in part.
Financial support: This work was supported by the National Natural Science Foundation of China (81760049), the Innovation-Driven “5511” Project of Jiangxi Province (20165BCD41005), the National Key R&D Program of China (No. 2018YFC1312902), and the Key project of Education Department of Jiangxi Province (GJJ170013)
References
- 1.Virani SS, Alonso A, Benjamin EJ, et al. Heart Disease and Stroke Statistics – 2020 update: A Report from the American Heart Association. Circulation. 2020;141:139–596. doi: 10.1161/CIR.0000000000000757. [DOI] [PubMed] [Google Scholar]
- 2.Wang W, Jiang B, Sun H, et al. Prevalence, incidence, and mortality of stroke in China: Results from a nationwide population-based survey of 480 687 adults. Circulation. 2017;135:759–71. doi: 10.1161/CIRCULATIONAHA.116.025250. [DOI] [PubMed] [Google Scholar]
- 3.Ji C, Kaplowitz N. Hyperhomocysteinemia, endoplasmic reticulum stress, and alcoholic liver injury. World J Gastroenterol. 2004;10:1699–708. doi: 10.3748/wjg.v10.i12.1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Qin X, Huo Y. H-Type hypertension, stroke and diabetes in China: Opportunities for primary prevention. J Diabetes. 2016;8:38–40. doi: 10.1111/1753-0407.12333. [DOI] [PubMed] [Google Scholar]
- 5.Gungor L, Polat M, Ozberk MB, et al. Which ischemic stroke subtype is associated with hyperhomocysteinemia? J Stroke Cerebrovasc Dis. 2018;27:1921–29. doi: 10.1016/j.jstrokecerebrovasdis.2018.02.033. [DOI] [PubMed] [Google Scholar]
- 6.Larsson SC, Traylor M, Markus HS. Homocysteine and small vessel stroke: A mendelian randomization analysis. Ann Neurol. 2019;85:495–501. doi: 10.1002/ana.25440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fu Y, Wang X, Kong W. Hyperhomocysteinaemia and vascular injury: Advances in mechanisms and drug targets. Br J Pharmacol. 2018;175:1173–89. doi: 10.1111/bph.13988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang Z, Chen Z, Zhang L, et al. Status of hypertension in China: Results from the China hypertension survey, 2012–2015. Circulation. 2018;137:2344–56. doi: 10.1161/CIRCULATIONAHA.117.032380. [DOI] [PubMed] [Google Scholar]
- 9.Alberti KG, Zimmet P, Shaw J. The metabolic syndrome – a new worldwide definition. Lancet. 2005;366:1059–62. doi: 10.1016/S0140-6736(05)67402-8. [DOI] [PubMed] [Google Scholar]
- 10.Weiss R, Bremer AA, Lustig RH. What is metabolic syndrome, and why are children getting it? Ann N Y Acad Sci. 2013;1281:123–40. doi: 10.1111/nyas.12030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aguilar M, Bhuket T, Torres S, et al. Prevalence of the metabolic syndrome in the United States, 2003–2012. JAMA. 2015;313:1973–74. doi: 10.1001/jama.2015.4260. [DOI] [PubMed] [Google Scholar]
- 12.O’Neill S, O’Driscoll L. Metabolic syndrome: A closer look at the growing epidemic and its associated pathologies. Obes Rev. 2015;16:1–12. doi: 10.1111/obr.12229. [DOI] [PubMed] [Google Scholar]
- 13.Li W, Song F, Wang X, et al. Prevalence of metabolic syndrome among middle-aged and elderly adults in China: Current status and temporal trends. Ann Med. 2018;50:345–53. doi: 10.1080/07853890.2018.1464202. [DOI] [PubMed] [Google Scholar]
- 14.Catena C, Colussi G, Nait F, et al. Elevated homocysteine levels are associated with the metabolic syndrome and cardiovascular events in hypertensive patients. Am J Hypertens. 2015;28:943–50. doi: 10.1093/ajh/hpu248. [DOI] [PubMed] [Google Scholar]
- 15.Li M, Zhan A, Huang X, et al. Positive association between triglyceride glucose index and arterial stiffness in hypertensive patients: The China H-type Hypertension Registry Study. Cardiovasc Diabetol. 2020;19:139. doi: 10.1186/s12933-020-01124-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zafar U, Khaliq S, Ahmad HU, et al. Metabolic syndrome: An update on diagnostic criteria, pathogenesis, and genetic links. Hormones. 2018;17:299–313. doi: 10.1007/s42000-018-0051-3. [DOI] [PubMed] [Google Scholar]
- 17.Jia W, Weng J, Zhu D, et al. Standards of medical care for type 2 diabetes in China 2019. Diabetes Metab Res Rev. 2019;35:e3158. doi: 10.1002/dmrr.3158. [DOI] [PubMed] [Google Scholar]
- 18.Minniti G, Piana A, Armani U, et al. Determination of plasma and serum homocysteine by high-performance liquid chromatography with fluorescence detection. J Chromatogr A. 1998;828:401–5. doi: 10.1016/s0021-9673(98)00812-7. [DOI] [PubMed] [Google Scholar]
- 19.Joint Committee for Developing Chinese guidelines on Prevention and Treatment of Dyslipidemia in Adults. [Chinese guidelines on prevention and treatment of dyslipidemia in adults]. Zhonghua Xin Xue Guan Bing Za Zhi. 2007;35(5):390–419. [in Chinese] [PubMed] [Google Scholar]
- 20.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–12. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lear SA, Gasevic D. Ethnicity and metabolic syndrome: Implications for assessment, management and prevention. Nutrients. 2019;12:15. doi: 10.3390/nu12010015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hu DY, Xu XP. [Prevention of stroke relies on valid control “H” type hypertension]. Zhonghua Xin Xue Guan Bing Za Zhi. 2008;47:976–77. [in Chinese] [PubMed] [Google Scholar]
- 23.Zhang Q, Qiu DX, Fu RL, et al. H-type hypertension and C reactive protein in recurrence of ischemic stroke. Int J Environ Res Public Health. 2016;13:477. doi: 10.3390/ijerph13050477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hayden MR, Tyagi SC. Homocysteine and reactive oxygen species in metabolic syndrome, type 2 diabetes mellitus, and atheroscleropathy: The pleiotropic effects of folate supplementation. Nutr J. 2004;3:4. doi: 10.1186/1475-2891-3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Celermajer DS, Sorensen K, Ryalls M, et al. Impaired endothelial function occurs in the systemic arteries of children with homozygous homocystinuria but not in their heterozygous parents. J Am Coll Cardiol. 1993;22:854–58. doi: 10.1016/0735-1097(93)90203-d. [DOI] [PubMed] [Google Scholar]
- 26.Cheng Z, Jiang X, Kruger WD, et al. Hyperhomocysteinemia impairs endothelium-derived hyperpolarizing factor-mediated vasorelaxation in transgenic cystathionine beta synthase-deficient mice. Blood. 2011;118:1998–2006. doi: 10.1182/blood-2011-01-333310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cheng Z, Shen X, Jiang X, et al. Hyperhomocysteinemia potentiates diabetes-impaired EDHF-induced vascular relaxation: Role of insufficient hydrogen sulfide. Redox Biol. 2018;16:215–25. doi: 10.1016/j.redox.2018.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cueto R, Zhang L, Shan HM, et al. Identification of homocysteine-suppressive mitochondrial ETC complex genes and tissue expression profile – novel hypothesis establishment. Redox Biol. 2018;17:70–88. doi: 10.1016/j.redox.2018.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pucci G, Alcidi R, Tap L, et al. Sex- and gender-related prevalence, cardiovascular risk and therapeutic approach in metabolic syndrome: A review of the literature. Pharmacol Res Pharmacol Res. 2017;120:34–42. doi: 10.1016/j.phrs.2017.03.008. [DOI] [PubMed] [Google Scholar]
- 30.Cornier MA, Dabelea D, Hernandez TL, et al. The metabolic syndrome. Endocr Rev. 2008;29:777–822. doi: 10.1210/er.2008-0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Urbina EM, Khoury PR, McCoy CE, et al. Triglyceride to HDL-C ratio and increased arterial stiffness in children, adolescents, and young adults. Pediatrics. 2013;131:e1082–90. doi: 10.1542/peds.2012-1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Burns SF, Lee SJ, Arslanian SA. Surrogate lipid markers for small dense low-density lipoprotein particles in overweight youth. J Pediatr. 2012;161:991–96. doi: 10.1016/j.jpeds.2012.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schulman IH, Zhou MS. Vascular insulin resistance: A potential link between cardiovascular and metabolic diseases. Curr Hypertens Rep. 2009;11:48–55. doi: 10.1007/s11906-009-0010-0. [DOI] [PubMed] [Google Scholar]
- 34.Gustafson B, Hammarstedt A, Andersson CX, et al. Inflamed adipose tissue: A culprit underlying the metabolic syndrome and atherosclerosis. Arterioscler Thromb Vasc Biol. 2007;27:2276–83. doi: 10.1161/ATVBAHA.107.147835. [DOI] [PubMed] [Google Scholar]
- 35.Heilbronn LK, Campbell LV. Adipose tissue macrophages, low grade inflammation and insulin resistance in human obesity. Curr Pharm Des. 2008;14:1225–30. doi: 10.2174/138161208784246153. [DOI] [PubMed] [Google Scholar]
- 36.Azarpazhooh MR, Andalibi MSS, Hackam DG, et al. Interaction of smoking, hyperhomocysteinemia, and metabolic syndrome with carotid atherosclerosis: A cross-sectional study in 972 non-diabetic patients. Nutrition. 2020;79–80:110874. doi: 10.1016/j.nut.2020.110874. [DOI] [PubMed] [Google Scholar]
- 37.Pandian JD, Gall SL, Kate MP, et al. Prevention of stroke: A global perspective. Lancet. 2018;392:1269–78. doi: 10.1016/S0140-6736(18)31269-8. [DOI] [PubMed] [Google Scholar]
- 38.Huo Y, Li J, Qin X, et al. Efficacy of folic acid therapy in primary prevention of stroke among adults with hypertension in China: The CSPPT randomized clinical trial. JAMA. 2015;313:1325–35. doi: 10.1001/jama.2015.2274. [DOI] [PubMed] [Google Scholar]
- 39.Qin X, Li J, Spence JD, et al. Folic acid therapy reduces the first stroke risk associated with hypercholesterolemia among hypertensive patients. Stroke. 2016;47:2805–12. doi: 10.1161/STROKEAHA.116.014578. [DOI] [PubMed] [Google Scholar]
- 40.Huang X, Li Y, Li P, et al. Association between percent decline in serum total homocysteine and risk of first stroke. Neurology. 2017;89:2101–741. doi: 10.1212/WNL.0000000000004648. [DOI] [PubMed] [Google Scholar]
- 41.Kong X, Huang X, Zhao M, et al. Platelet count affects efficacy of folic acid in preventing first stroke. J Am Coll Cardiol. 2018;71:2136–46. doi: 10.1016/j.jacc.2018.02.072. [DOI] [PubMed] [Google Scholar]
- 42.Xu X, Qin X, Li Y, et al. Efficacy of folic acid therapy on the progression of chronic kidney disease: The renal substudy of the China Stroke Primary Prevention Trial. JAMA Intern Med. 2016;176:1443–50. doi: 10.1001/jamainternmed.2016.4687. [DOI] [PubMed] [Google Scholar]
- 43.Spence JD, Yi Q, Hankey GJ. B vitamins in stroke prevention: Time to reconsider. Lancet Neurol. 2017;16:750–60. doi: 10.1016/S1474-4422(17)30180-1. [DOI] [PubMed] [Google Scholar]
- 44.Robertson J, Iemolo F, Stabler SP, et al. Vitamin B12, homocysteine and carotid plaque in the era of folic acid fortification of enriched cereal grain products. CMAJ. 2005;172:1569–73. doi: 10.1503/cmaj.045055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mathiesen EB, Johnsen SH, Wilsgaard T, et al. Carotid plaque area and intima-media thickness in prediction of first-ever ischemic stroke: A 10-year follow-up of 6584 men and women: the Tromsø Study. Stroke. 2011;42:972–78. doi: 10.1161/STROKEAHA.110.589754. [DOI] [PubMed] [Google Scholar]
- 46.Kernan WN, Viscoli CM, Furie KL, et al. Pioglitazone after ischemic stroke or transient ischemic attack. N Engl J Med. 2016;374:1321–31. doi: 10.1056/NEJMoa1506930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Spence JD, Viscoli CM, Inzucchi SE, et al. Pioglitazone therapy in patients with stroke and prediabetes: A post hoc analysis of the IRIS randomized clinical trial. JAMA Neurol. 2019;76:526–35. doi: 10.1001/jamaneurol.2019.0079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pantoni L. Potential new horizons for the prevention of cerebrovascular diseases and dementia. JAMA Neurol. 2019;76:521–22. doi: 10.1001/jamaneurol.2018.4406. [DOI] [PubMed] [Google Scholar]
- 49.Wang CY, Chen ZW, Zhang T, et al. Elevated plasma homocysteine level is associated with ischemic stroke in Chinese hypertensive patients. Eur J Intern Med. 2014;25:538–44. doi: 10.1016/j.ejim.2014.04.011. [DOI] [PubMed] [Google Scholar]
- 50.Pang H, Han B, Fu Q, et al. Association between homocysteine and conventional predisposing factors on risk of stroke in patients with hypertension. Sci Rep. 2018;8:3900. doi: 10.1038/s41598-018-22260-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data flow chart of participants in our analysis. tHcy – total homocysteine; HHcy – hyperhomocysteinemia; MS – metabolic syndrome. EmpowerStats software (version 2.2, X&Y Solutions, Inc) was used to create the figure.
Supplementary Table 1.
Associations of covariates with stroke occurrence.
| Covariates | OR (95% CI) | P value |
|---|---|---|
| Age (years) | 1.01 (1.01, 1.02) | <0.001 |
| Sex | ||
| Male | Ref | |
| Female | 0.63 (0.55, 0.72) | <0.001 |
| BMI (kg/m2) | 0.99 (0.97, 1.00) | 0.124 |
| Heart rate (times/min) | 1.00 (0.99, 1.00) | 0.227 |
| Smoking status, n (%) | ||
| Never | Ref | |
| Former smoker | 1.95 (1.66, 2.29) | <0.001 |
| Current smoker | 1.16 (0.99, 1.36) | 0.068 |
| Drinking status, n (%) | ||
| Never | Ref | |
| Former drinker | 1.85 (1.58, 2.18) | <0.001 |
| Current drinker | 0.50 (0.41, 0.62) | <0.001 |
| tHcy (μmol/L) | 1.01 (1.01, 1.02) | <0.001 |
| Serum uric acid (mmol/L) | 1.00 (1.00, 1.00) | 0.329 |
| eGFR (ml/min) | 0.99 (0.99, 0.99) | <0.001 |
| Total bilirubin (mmol/L) | 0.99 (0.98, 1.00) | 0.255 |
| Direct bilirubin (mmol/L) | 1.00 (0.98, 1.03) | 0.900 |
| AST (U/L) | 1.00 (1.00, 1.00) | 0.372 |
| ALT (U/L) | 1.00 (1.00, 1.01) | 0.183 |
| Abdominal obesity, n (%) | ||
| No | Ref | 0.010 |
| Yes | 0.83 (0.72, 0.96) | |
| Hyperglycemia, n (%) | ||
| No | Ref | |
| Yes | 1.02 (0.89, 1.17) | 0.774 |
| Elevated TG, n (%) | ||
| No | Ref | |
| Yes | 1.30 (1.14, 1.48) | <0.001 |
| Reduced HDL-C, n (%) | ||
| No | Ref | |
| Yes | 2.07 (1.80, 2.39) | <0.001 |
BMI – body mass index; tHcy – total homocysteine; TC – total cholesterol; HDL-C – high-density lipoprotein cholesterol; eGFR – estimated glomerular filtration rate; AST – aspartate aminotransferase; ALT – alanine aminotransferase; T2DM – type 2 diabetes mellitus; Ref – reference; OR – odds ratio; CI – confidence interval. Values are regression coefficients [OR (95% CI)] from univariate regression models and reflect differences in stroke occur per unit change of each covariate and for different categories of each covariate as compared to the reference group.
Supplementary Table 2.
Clinical characteristics of participants grouped by HHcy and MS.
| Characteristics | Control (n=3910) | Isolated HHcy (n=4215) | Isolated MS (n=3125) | HHcy and MS (n=2809) | P value |
|---|---|---|---|---|---|
| General characteristics | |||||
| Age (years) | 62.62±8.95 | 67.29±9.20 | 60.22±8.49 | 64.00±9.18 | <0.001 |
| Male, n (%) | 1619 (41.41%) | 2837 (67.31%) | 886 (28.35%) | 1308 (46.56%) | <0.001 |
| BMI (kg/m2) | 22.56±3.23 | 21.99±3.74 | 25.66±3.24 | 25.25±3.18 | <0.001 |
| Waistline (cm) | 79.88±8.55 | 79.58±8.40 | 89.44±8.37 | 89.56±9.12 | <0.001 |
| WHR | 0.89±0.07 | 0.89±0.26 | 0.94±0.06 | 0.95±0.08 | <0.001 |
| SBP (mmHg) | 148.15±16.82 | 148.47±18.86 | 148.46±17.03 | 148.52±18.56 | 0.999 |
| DBP (mmHg) | 88.86±10.33 | 87.81±11.21 | 90.36±10.12 | 89.11±11.08 | <0.001 |
| Heart rate (times/min) | 74.76±13.82 | 75.18±14.07 | 78.41±13.00 | 79.05±14.60 | <0.001 |
| Smoking status, n (%) | <0.001 | ||||
| Never | 2470 (63.17%) | 1740 (41.29%) | 2273 (72.76%) | 1660 (59.12%) | |
| Former smoker | 512 (13.09%) | 928 (22.02%) | 348 (11.14%) | 487 (17.34%) | |
| Current smoker | 928 (23.73%) | 1546 (36.69%) | 503 (16.10%) | 661 (23.54%) | |
| Drinking status, n (%) | <0.001 | ||||
| Never | 2549 (65.19%) | 2315 (54.95%) | 2340 (74.90%) | 1884 (67.09%) | |
| Former drinker | 460 (11.76%) | 751 (17.83%) | 317 (10.15%) | 385 (13.71%) | |
| Current drinker | 901 (23.04%) | 1147 (27.23%) | 467 (14.95%) | 539 (19.20%) | |
| Blood biochemical tests | |||||
| tHcy (μmol/L) | 12.55 (11.35–13.69) | 19.33 (16.77–24.35) | 12.37 (11.20–13.56) | 18.75 (16.51–23.26) | <0.001 |
| FBG (mmol/L) | 5.70±1.00 | 5.76±1.06 | 6.78±2.06 | 6.82±1.95 | <0.001 |
| TC (mmol/L) | 5.06±0.98 | 5.06±1.02 | 5.22±1.20 | 5.37±1.30 | <0.001 |
| TG (mmol/L) | 1.18 (0.90–1.52) | 1.15 (0.89–1.47) | 2.17 (1.70–2.93) | 2.16 (1.71–2.97) | <0.001 |
| HDL-C (mmol/L) | 1.68±0.40 | 1.69±0.44 | 1.39±0.36 | 1.43±0.41 | <0.001 |
| LDL-C (mmol/L) | 2.84±0.72 | 2.87±0.76 | 3.11±0.85 | 3.22±0.91 | <0.001 |
| Serum uric acid (μmol/L) | 371.34±102.59 | 441.38±121.07 | 402.35±108.85 | 468.37±127.64 | <0.001 |
| Serum creatinine (mmol/L) | 59.00 (49.00–70.00) | 76.00 (63.00–94.00) | 58.00 (49.00–69.00) | 75.00 (61.00–92.00) | <0.001 |
| BUN (mmol/L) | 5.27±1.44 | 5.88±2.13 | 4.97±1.38 | 5.60±1.98 | <0.001 |
| eGFR (ml/min) | 96.34±14.33 | 80.17±21.43 | 96.50±15.10 | 80.00±22.05 | <0.001 |
| Total bilirubin (mmol/L) | 12.80 (9.90–16.40) | 13.70 (10.60–18.10) | 12.90 (9.80–16.80) | 13.80 (10.60–18.00) | <0.001 |
| Direct bilirubin (mmol/L) | 5.00 (4.00–6.30) | 5.40 (4.30–7.00) | 4.80 (3.80–6.10) | 5.20 (4.10–6.60) | <0.001 |
| AST (U/L) | 23.00 (20.00–28.00) | 25.00 (21.00–30.00) | 24.00 (20.00–30.00) | 25.00 (20.00–31.00) | <0.001 |
| ALT (U/L) | 15.00 (12.00–21.00) | 15.00 (11.00–21.00) | 20.00 (14.00–28.00) | 19.00 (14.00–28.00) | <0.001 |
| Past medical history | |||||
| Cerebral stroke, n (%) | 175 (4.48%) | 315 (7.47%) | 192 (6.14%) | 278 (9.90%) | <0.001 |
| Antihypertensive agents, n (%) | 2269 (58.03%) | 2783 (66.04%) | 2028 (64.92%) | 2000 (71.23%) | <0.001 |
| Hypoglycemic agents, n (%) | 71 (1.82%) | 66 (1.57%) | 319 (10.21%) | 284 (10.11%) | <0.001 |
| Lipid-lowering agents, n (%) | 0 (0.00%) | 0 (0.00%) | 241 (7.71%) | 250 (8.90%) | <0.001 |
| Antiplatelet agents, n (%) | 56 (1.43%) | 113 (2.68%) | 168 (5.38%) | 190 (6.76%) | <0.001 |
| Current diseases | |||||
| Abdominal obesity, n (%) | 637 (16.29%) | 511 (12.12%) | 2205 (70.56%) | 1868 (66.50%) | <0.001 |
| Hyperglycemia, n (%) | 651 (16.65%) | 849 (20.14%) | 1864 (59.65%) | 1862 (66.29%) | <0.001 |
| T2DM, n (%) | 256 (6.55%) | 302 (7.16%) | 1013 (32.42%) | 998 (35.53%) | <0.001 |
| Dyslipidemia, n (%) | 651 (16.65%) | 849 (20.14%) | 1864 (59.65%) | 1862 (66.29%) | <0.001 |
| Elevated TG, n (%) | 548 (14.02%) | 532 (12.62%) | 2481 (79.39%) | 2274 (80.95%) | <0.001 |
| Reduced HDL-C, n (%) | 175 (4.48%) | 171 (4.06%) | 1333 (42.66%) | 1041 (37.06%) | <0.001 |
HHcy – hyperhomocysteinemia; MS – metabolic syndrome; BMI – body mass index; WHR – waist-hip ratio; SBP – systolic blood pressure; DBP – diastolic blood pressure; tHcy – total homocysteine; FBG – fasting blood glucose; TC – total cholesterol; TG – total triglyceride; HDL-C – high-density lipoprotein cholesterol; LDL-C – low-density lipoprotein cholesterol; BUN – blood urea nitrogen; eGFR – estimated glomerular filtration rate; AST – aspartate aminotransferase; ALT – alanine aminotransferase; T2DM – type 2 diabetes mellitus.
Supplementary Table 3.
Effect size of HHcy and MS on cerebral stroke in different models.
| Variables | Event, n (%) | Crude Model | Model I | Model II | |||
|---|---|---|---|---|---|---|---|
| OR (95% CI) | P-value | OR (95% CI) | P-value | OR (95% CI) | P-value | ||
| Control | 175 (4.48%) | Ref | Ref | Ref | |||
| Isolated HHcy | 315 (7.47%) | 1.72 (1.43, 2.08) | <0.001 | 1.44 (1.19, 1.76) | <0.001 | 1.32 (1.07, 1.62) | 0.008 |
| Isolated MS | 192 (6.14%) | 1.40 (1.13, 1.72) | 0.002 | 1.54 (1.25, 1.91) | <0.001 | 1.78 (1.41, 2.23) | <0.001 |
| HHcy+MS | 278 (9.90%) | 2.34 (1.93, 2.85) | <0.001 | 2.26 (1.86, 2.75) | <0.001 | 2.46 (1.97, 3.08) | <0.001 |
| P for trend | <0.001 | <0.001 | <0.001 | ||||
HHcy – hyperhomocysteinemia; MS – metabolic syndrome; Ref – reference; OR – odds ratio; CI – confidence interval. Model I adjusted for age and sex. Model II adjusted for sex, BMI, smoking and drinking status, eGFR, and serum uric acid.
Effect size of HHcy and MS on stroke in prespecified and exploratory subgroups. Ref – reference; OR – odds ratio; CI – confidence interval, MS – metabolic syndrome; HHcy – hyperhomocysteinemia; BMI – body mass index; eGFR – estimated glomerular filtration rate; UA – serum uric acid (mmol/L). Each stratification adjusted for age, sex, BMI, smoking and drinking status eGFR, and serum uric acid except the subgroup variable. EmpowerStats software (version 2.2, X&Y Solutions, Inc) was used to create the figure.


