Abstract
p-Coumaric acid (PCA) is a phenolic acid that is widely present in numerous plants and human diets. Studies have demonstrated the antioxidant and anti-senescence effects of PCA in different cell types. However, the anti-senescence effects of PCA in nucleus pulposus (NP) cells have remained to be determined. In the present study, reverse transcription-quantitative PCR was used to measure the gene expression of Cyclooxygenase-2 (Cox-2), inducible nitric oxide synthase (iNOS), p53, p16, aggrecan and collagen-2 in NP cells. Immunofluorescence staining was used to evaluate the protein expression of p53, p16 and collagen-2 in NP cells. In addition, cell cycle of NP cells was measured by flow cytometry. β-galactosidase staining were used to investigate the senescence of NP cells. Preliminary results indicated that PCA suppressed ROS-induced senescence in NP cells via both the p16 and p53 pathways. NP cells were pretreated with PCA at a concentration of 10 or 50 µg/ml prior to stimulation with 200 µM hydrogen peroxide (H2O2). Pretreatment with PCA significantly inhibited H2O2-induced cell cycle arrest in a dose-dependent manner. PCA also reduced the gene expression of Cox-2, iNOS, p53 and p16 induced by H2O2. By contrast, aggrecan and collagen-2 expression in NP cells was upregulated after PCA treatment. Furthermore, PCA suppressed H2O2-induced changes in the protein expression of p16, p53 and collagen-2. H2O2 stimulation of NP cells increased senescence-associated β-galactosidase (SA-β-gal) activities, while PCA treatment markedly reversed these SA-β-gal activities. Collectively, the present results indicated that PCA attenuated H2O2-induced oxidative stress and cellular senescence, suggesting a potential therapeutic utility of PCA in intervertebral disc degeneration.
Keywords: p-coumaric acid, senescence, reactive oxygen species, nucleus pulposus cells
Introduction
The incidence of low back pain (LBP) caused by intervertebral disc degeneration (IDD) has exhibited an increasing trend across the world (1,2). It was previously reported that the total number of years lived by individuals with disability caused by low back pain increased by 54% worldwide between 1990 and 2015(1). The condition impairs the quality of life of the affected individuals and imposes substantial socioeconomic and medical costs (1,2). The estimated indirect and direct cost of LBP in the United States can reach 100 billion dollars per year (3,4).
Senescence of the nucleus pulposus (NP) cells is one of the most important features of IDD (5). Collagen-2 is the major component of the extracellular matrix (ECM), which aids in the maintenance of the disc height and responds to external mechanical stress (5,6). NP cells ensure long-term matrix renewal to maintain the homeostasis of ECM. Decreased content of collagen-2 in senescent NP cells has been indicated to eventually lead to IDD (5). Previous studies have reported a positive association of the degree of cellular senescence with the IDD grade (6,7). IDD has long been considered an age-related disease. However, recent studies have documented a trend of occurrence of IDD in increasingly younger individuals (8). This phenomenon may be related to stress-induced premature senescence (SIPS) of NP cells (6).
Production of reactive oxygen species (ROS) is a normal physiological phenomenon in the intervertebral disc due to the oxygen-utilizing metabolism of disc cells (9). ROS is a key factor, which increases survival stress and triggers SIPS at the cellular level (10,11). It has been indicated that excessive ROS may induce senescence and apoptosis of NP cells (10). Antioxidant therapies have also produced good results in the treatment of IDD (12).
p-Coumaric acid (PCA) is a phenolic acid, which is widely distributed in numerous plants, as well as human diets (13,14). Previous studies have demonstrated the antioxidant, cardioprotective, anti-melanogenic, anti-mutagenic, anti-platelet and anti-inflammatory effects of PCA (15-18). In addition, in a recent study, PCA was indicated to attenuate IL-1β-induced inflammatory response and cellular senescence in rat chondrocytes (19). Although potential antioxidant effects have been suggested for PCA, an in-depth investigation of its antioxidant and anti-senescence potential in NP cells has so far not been performed, to the best of our knowledge. Thus, the present study used an in vitro model to investigate the inhibitory effect of PCA on ROS-induced senescence in NP cells.
Materials and methods
Reagents
PCA was purchased from Sigma-Aldrich (Merck KGaA). It was dissolved in DMSO and the final concentration of DMSO in the medium was <0.05%. The same volume of DMSO was added to the control and H2O2 groups in all experiments. The concentration of PCA used in the present study was based on a previous study (19).
Cell isolation and culture
NP tissue was isolated from patients with lumbar fracture who had undergone lumbar interbody fusion surgery. NP tissues used in the present study were obtained from a total of five patients (three male and two female; age, 31.5±6.5 years old). The nucleus pulposus (NP) tissue was collected between January 2020 and December 2020 through surgery from The Second Affiliated Hospital of Zhejiang University School of Medicine (Hangzhou, China). The inclusion criterion was that patients underwent lumbar interbody fusion or discectomy. The exclusion criteria were: Systemic immune diseases; malignant tumor of the spine; or infection of intervertebral disc (IVD). The degeneration grade was evaluated based on modified Pfirrmann grading (20). The degeneration grade was grade 2 in 3 patients and grade 1 in 2 patients. NP tissue was digested with 0.2% collagenase II (Sigma-Aldrich; Merck KGaA) for 4 h at 37˚C and 0.25% trypsin (Sigma-Aldrich; Merck KGaA) for 15 min at 37˚C. NP cells were collected by centrifugation at 300 x g for 5 min at room temperature. Subsequently, NP cells were cultured in high-glucose DMEM (Hyclone; Cytiva) mixed with 10% FBS (Gibco; Thermo Fisher Scientific, Inc.) and antibiotics (100 U/ml penicillin, 100 µg/ml streptomycin; Sigma-Aldrich; Merck KGaA). Cells were cultured at 37˚C with 5% CO2 in fully humidified air.
Cell proliferation assay
A commercial kit [Cell Counting Kit-8 (CCK-8); Dojindo Laboratories, Inc.] was used to evaluate the proliferation of NP cells. NP cells were seeded in 96-well plates at a density of 3x103 cells per well. NP cells were pretreated with different concentrations of PCA (10 or 50 µg/ml) for 2 h at 37˚C and then stimulated with 200 µM H2O2, based on a previous study (21), for 2 h at 37˚C in the presence or absence of PCA (10 or 50 µg/ml). After being cultured for 0, 1, 3, 5, 7 or 9 days, the medium was replaced with fresh culture medium, 10 µl CCK-8 solution was added and the cells were incubated for an additional 2 h. The optical density (OD) of each well was measured at 450 nm using a microplate reader (Thermo Fisher Scientific, Inc.). Culture medium only was used as a blank control. Cell proliferation (day x) was calculated using the following equation: Cell proliferation=[OD (day x)-OD (blank)]/[OD (day 0)-OD (blank)].
Cell cycle assay
Cell cycle analysis was performed using a cell cycle measurement kit (Beyotime Institute of Biotechnology). NP cells were seeded at a density of 1x105/well in a six-well plate, pretreated with different concentrations of PCA (10 or 50 µg/ml) for 2 h and then stimulated with 200 µM H2O2 for 2 h in the presence or absence of PCA (10 or 50 µg/ml) at 37˚C. The medium was replaced with fresh culture medium, and cells were cultured for 72 h. Subsequently, the cells were washed with cold PBS and fixed with 70% ethanol at 4˚C overnight. In total, 1x105 cells were resuspended with RNase A solution (100 µl, Sigma-Aldrich; Merck KGaA) and PI (400 µl; Sigma-Aldrich; Merck KGaA) and incubated for 30 min at 37˚C. Finally, the DNA content was detected via flow cytometry (BD LSRFortessa; BD Biosciences) and the percentage of cells in each phase was analyzed by ModFit LT 5.1 (Verity Software House).
Reverse transcription-quantitative (RT-q)PCR assay
After treating the cells with H2O2 and different concentrations of PCA as aforementioned, total RNA of NP cells was isolated using TRIzol® reagent (Invitrogen; Thermo Fisher Scientific, Inc.) following the manufacturer's instructions. RT was performed using the PrimeScript™ II 1st Strand cDNA Synthesis Kit (cat. no. 6210A; Takara Bio, Inc.), using the temperature protocol of 37˚C for 15 min and 85˚C for 5 sec. DNA amplification was performed using SYBR Premix Ex Taq kit (Takara Bio, Inc.) followed by qPCR. The thermocycling conditions were set as follows: Initial denaturation at 95˚C for 3 min; followed by 40 cycles of 95˚C for 30 sec, 55˚C for 20 sec and 72˚C for 20 sec. The primers were designed and checked using BLAST (https://blast.ncbi.nlm.nih.gov/Blast.cgi). Relative gene expression was calculated using the 2-ΔΔCq method and GAPDH as the internal reference (22). The primer sequences are presented in Table I.
Table I.
Primer sequences used for reverse transcription-quantitative PCR.
| Gene | Direction | Primer sequence (5'-3') |
|---|---|---|
| Cox-2 | Forward | ACCCGTGGAGCTCACATTAACTAT |
| Reverse | ATACTGTTCTCCGTACCTTCACCC | |
| iNOS | Forward | CACTTCCAACGCAACATGGG |
| Reverse | CTTTGACCCAGTAGCTGCCA | |
| p53 | Forward | GAGGATTCACAGTCGGAT |
| Reverse | ATCATCTGGAGGAAGAAGTT | |
| p16 | Forward | GCTGCCCAACGCACCGAATA |
| Reverse | ACCACCAGCGTGTCCAGGAA | |
| Aggrecan | Forward | TGAGCGGCAGCACTTTGAC |
| Reverse | TGAGTACAGGAGGCTTGAGG | |
| Collagen-2 | Forward | GAACTGGTGGAGCAGCAAGA |
| Reverse | AGCAGGCGTAGGAAGGTCAT | |
| GAPDH | Forward | CCACCAACTGCTTAGCCCCC |
| Reverse | GCAGTGATGGCATGGACTGTGG |
Cox-2, cyclooxygenase 2; iNOS, inducible nitric oxide synthase.
Immunofluorescence
NP cells were seeded at a density of 3x104/well in a 24-well plate, pretreated with different concentrations of PCA (10 or 50 µg/ml) for 2 h and then stimulated with 200 µM H2O2 for 2 h in the presence or absence of PCA (10 or 50 µg/ml)at 37˚C. The medium was replaced with fresh culture medium, and cells were cultured for 72 h. Subsequently, cells were fixed with 4% paraformaldehyde for 20 min at room temperature, treated with 0.1% Triton X-100 for 10 min and blocked with 5% bovine serum albumin (Beyotime Institute of Biotechnology) for 1 h at room temperature. After washing with PBS three times, NP cells were incubated with primary antibodies against collagen-2 (cat. no. ab34712; 1:100 dilution; Abcam), p53 (cat. no. 2527; 1:100 dilution; Cell Signaling Technology, Inc.) and p16 (cat. no. 18769S; 1:100 dilution; Cell Signaling Technology, Inc.) overnight at 4˚C, before being exposed to Alexa Fluor® 488-conjugated secondary antibodies (cat. no. 4412; 1:1,000 dilution; Cell Signaling Technology, Inc.) for 60 min at room temperature. After washing with PBS three times, NP cells were counterstained with DAPI (cat. no. 40728ES03; 5 mg/ml; 5 min) and phalloidin conjugated with iFluor™ 555 (cat. no. 40737ES75; 1:10,000 dilution; 30 min) at room temperature (Shanghai Yeasen Biotechnology Co., Ltd.). A fluorescence microscope (Olympus Corporation) was used for observation and imaging (x200 magnification). Integrated optical density or positive cell numbers in each image were measured using Image-Pro Plus 6.0 software (Media Cybernetics, Inc.). For every sample, six fields of view were taken before they were averaged.
Senescence-associated β-galactosidase (SA-β-gal) staining
SA-β-gal staining was performed using the β-galactosidase senescence staining kit by following the manufacturer's instructions (cat. no. C0602; Beyotime Institute of Biotechnology). NP cells were seeded at a density of 1x105/ well in a six-well plate, then washed with PBS and fixed with 4% paraformaldehyde for 15 min at room temperature. Subsequently, NP cells were incubated overnight with the SA-β-gal staining solution at 37˚C. Subsequently, cells with positive staining were observed and counted under an inverted light microscope (x200 magnification, Olympus Corporation). For every sample, six fields of view were taken before they were averaged.
Statistical analysis
All experiments were performed ≥ three times before the results are presented as the mean ± standard deviation. Statistical analysis was performed using one-way analysis of variance followed by Student-Newman-Keuls post-hoc analysis using SPSS 21.0 (IBM Corp.) and GraphPad Prism 9.0 (GraphPad Software, Inc.). P<0.05 was considered to indicate a statistically significant difference.
Results
PCA reduces H2O2-induced anti-proliferative effects and cell cycle arrest in NP cells
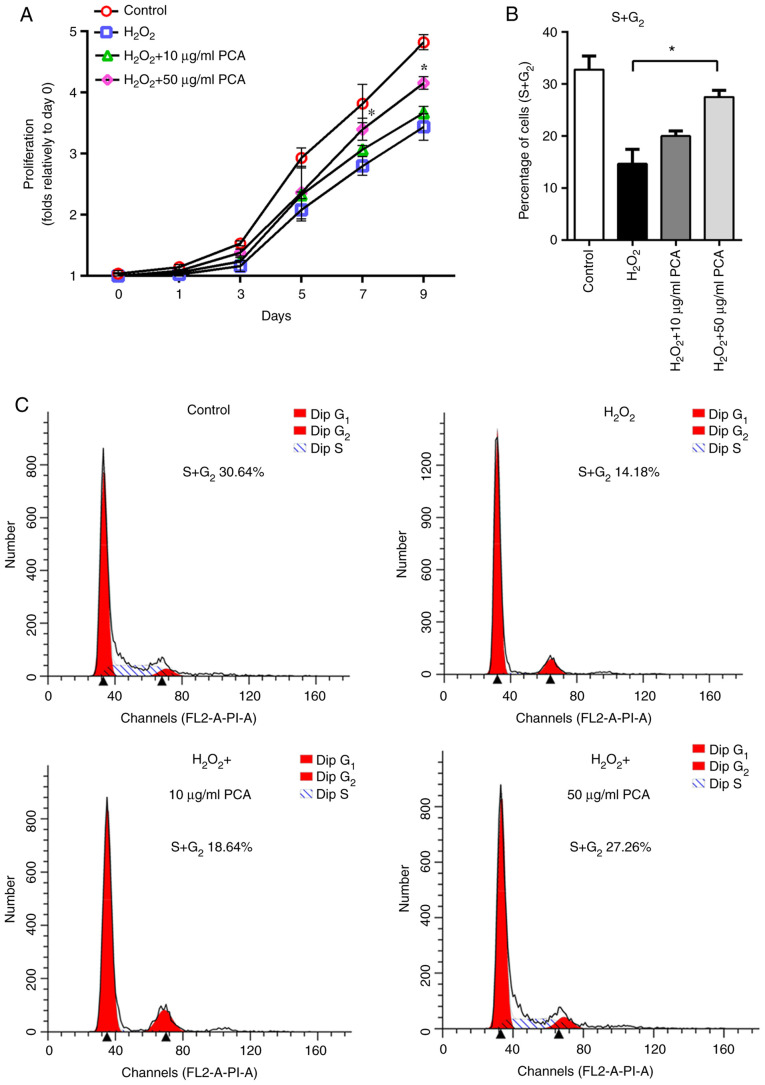
The results of the cell viability assay indicated anti-proliferative effects of H2O2 on NP cells. However, pre-treatment with PCA reduced the anti-proliferative effects of H2O2, where only the 50 µg/ml group exhibited significantly greater proliferative capability on days 7 and 9 compared with that in the H2O2 group (Fig. 1A). To explore whether the ROS-induced anti-proliferative effects were associated with cell cycle arrest, the cell cycle distribution of NP cells was detected using flow cytometry. It was indicated that the percentage of NP cells in S and G2 phases was notably decreased after treatment with H2O2 compared with that in the control group (Fig. 1B and C). Treatment with 50 µg/ml PCA reversed the cell cycle arrest induced by H2O2 (Fig. 1B and C). Furthermore, 10 µg/ml PCA also partly increased the percentage of NP cells in S and G2 phases compared with that in the H2O2 group, although the difference between 10 µg/ml PCA and H2O2 groups in this respect was not statistically significant (Fig. 1B and C).
Figure 1.
PCA reduces the anti-proliferative effect and cell cycle arrest induced by H2O2 in NP cells. NP cells were pretreated with different concentrations of PCA (10 or 50 µg/ml) for 2 h and then stimulated with 200 µM H2O2 for 2 h in the presence or absence of PCA. (A) Cell proliferation rate at days 0, 1, 3, 5, 7 and 9 in different groups. (B) Quantification of cells in S+G2 phase of the cell cycle in different groups. (C) Cell cycle profiles of NP cells in different groups. Values are expressed as the mean ± standard deviation. *P<0.05 vs. H2O2 treatment group. NP, nucleus pulposus; PCA, p-coumaric acid.
PCA reduces ROS and senescence-related gene expression changes in NP cells
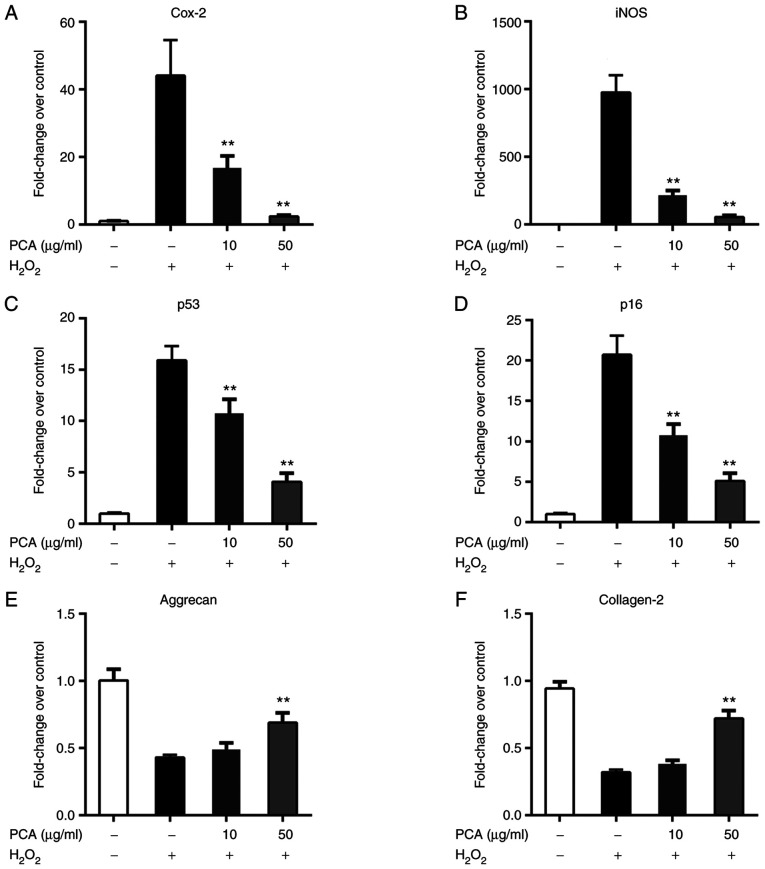
Stimulation with H2O2 led to notable upregulation of the expression of ROS and senescence-related genes in NP cells (Fig. 2A and B). PCA markedly inhibited the mRNA expression of cyclooxygenase 2, inducible nitric oxide synthase, p53 and p16 in a concentration-dependent manner (Fig. 2C and D). Furthermore, H2O2 stimulation also downregulated the expression of aggrecan and collagen-2 (Fig. 2E and F). Only 50 µg/ml PCA treatment was able to counteract the ROS-induced downregulation of collagen-2 and aggrecan (Fig. 2E and F).
Figure 2.
PCA reduces ROS- and senescence-related gene expression changes in NP cells. NP cells were treated with H2O2 and different concentrations of PCA and then cultured for 72 h. Subsequently, ROS- and senescence-related gene expression was measured via reverse transcription-quantitative PCR. (A) Cox-2, (B) iNOS, (C) p53, (D) p16, (E) aggrecan and (F) collagen-2. Values are expressed as the mean ± standard deviation. **P<0.01 vs. H2O2 treatment group. NP, nucleus pulposus; PCA, p-coumaric acid; ROS, reactive oxygen species; Cox-2, cyclooxygenase 2; iNOS, inducible nitric oxide synthase.
PCA reduces p16 and p53 protein expression induced by H2O2 in NP cells
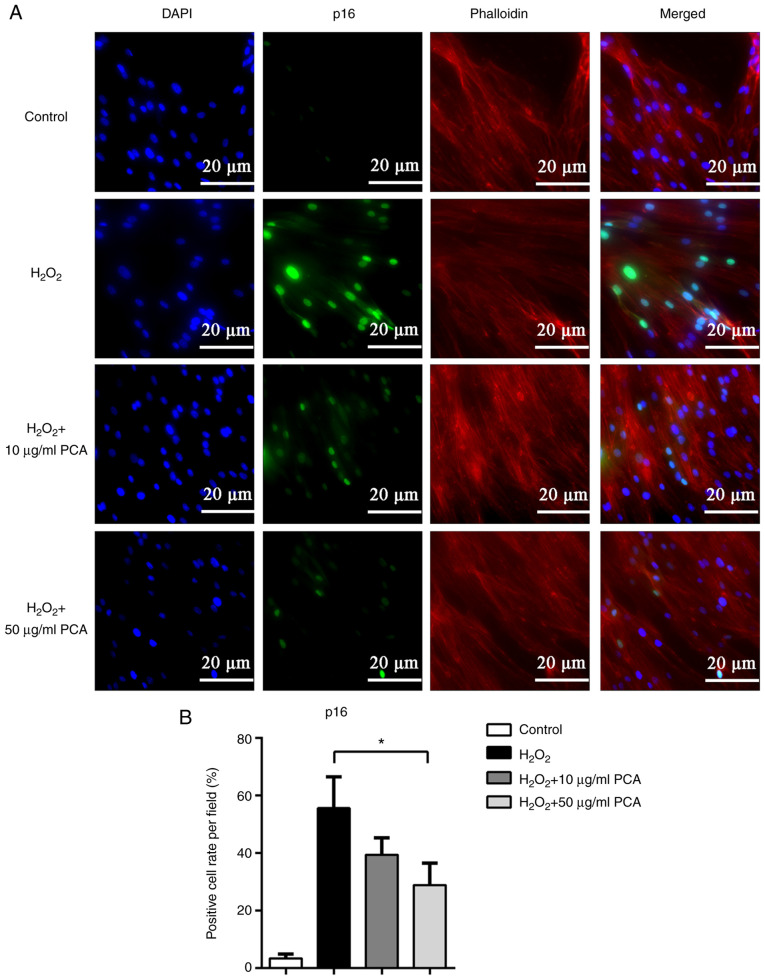
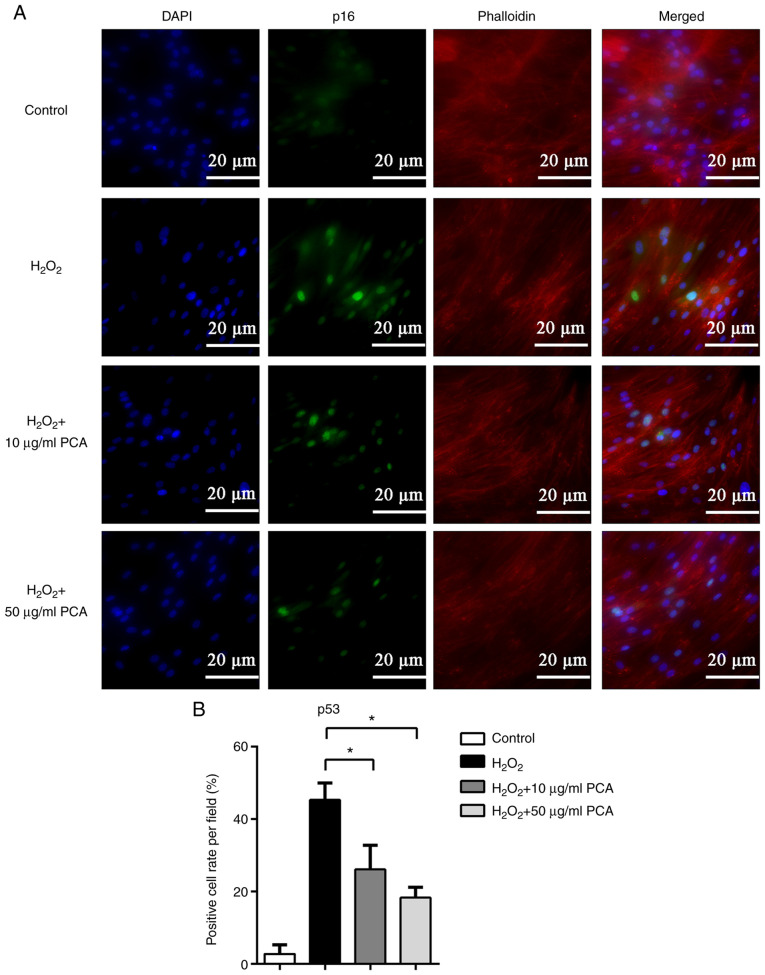
To further investigate the changes in the expression of senescence-related proteins, immunofluorescence staining was used to locate and measure the expression of p16 and p53. Both p16 and p53 were observed to be expressed in the nucleus and the expression of p16 and p53 was markedly upregulated after stimulation with H2O2 (Figs. 3 and 4). PCA treatment markedly reduced p16 and p53 expression in NP cells compared with that in the H2O2 group (Figs. 3 and 4). PCA treatment at a concentration of 10 µg/ml resulted in significantly less p53 expression compared with that in the H2O2 group (Fig. 4).
Figure 3.
PCA reduces p16 protein expression induced by H2O2 in NP cells. NP cells were treated with H2O2 and different concentrations of PCA and then cultured for 72 h. The protein expression of p16 was then investigated via immunofluorescence. (A) Representative fluorescence microscopy images for the different groups (scale bar, 20 µm). (B) Bar graph indicating the p16-positive cell rate per field. Values are expressed as the mean ± standard deviation. *P<0.05. NP, nucleus pulposus; PCA, p-coumaric acid.
Figure 4.
PCA reduces p53 protein expression induced by H2O2 in NP cells. NP cells were treated with H2O2 and different concentrations of PCA and then cultured for 72 h. Subsequently, p53 protein expression was investigated by immunofluorescence. (A) Representative fluorescence microscopy images for the different groups (scale bar, 20 µm). (B) Bar graph indicating the p53-positive cell rate per field. Values are expressed as the mean ± standard deviation. *P<0.05. NP, nucleus pulposus; PCA, p-coumaric acid.
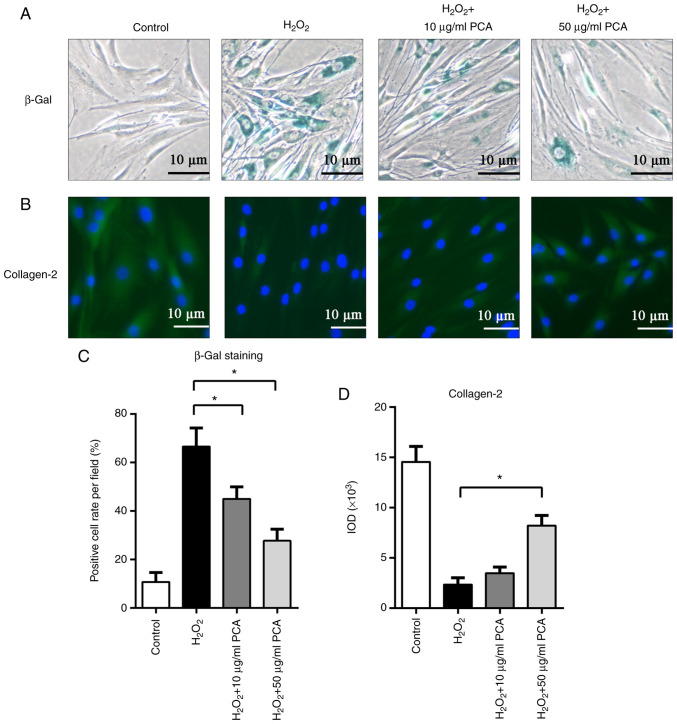
PCA inhibits senescence of NP cells induced by H2O2
As presented in Fig. 5, β-gal staining was used to identify senescent NP cells. After stimulation with H2O2, NP cells exhibited increased β-gal staining. Of note, PCA treatment (10 and 50 µg/ml) significantly reduced β-gal staining after H2O2 treatment (P<0.05; Fig. 5A and C). Furthermore, immunofluorescence staining for collagen-2 also indicated that H2O2 inhibited collagen-2 expression in NP cells, while PCA (50 µg/ml) restored collagen-2 expression after H2O2 stimulation (P<0.05; Fig. 5B and C). However, treatment with 10 µg/ml PCA did not result in any significant difference in collagen-2 expression compared with that in the H2O2 group (P>0.05; Fig. 5B and C).
Figure 5.
PCA inhibits senescence of NP cells induced by H2O2. NP cells were treated with H2O2 and different concentrations of PCA and then cultured for 72 h. (A) β-Gal staining of NP cells in the different groups. (B) Immunofluorescence staining for collagen-2 in the different groups (scale bars, 10 µm). Quantification of (C) β-Gal staining and (D) collagen-2 immunofluorescence staining. Values are expressed as the mean ± standard deviation. *P<0.05. NP, nucleus pulposus; PCA, p-coumaric acid; β-Gal, β-galactosidase; IOD, integrated optical density.
Discussion
Cell senescence is considered a key factor in the pathogenesis of IDD. In normal IVDs, NP cells achieve a balance between ECM anabolism and catabolism, thus maintaining long-term matrix renewal (6). Senescent NP cells are characterized by proliferative arrest and physiological function abnormalities. For example, senescent NP cells exhibit decreased ECM synthesis and increased secretion of matrix degradation enzymes, which perturbs the ECM homeostasis (6). Senescent cells also have the ability to exhibit senescence-associated secretory phenotype (SASP) (23). SASP was indicated to contain proinflammatory factors, cytokines, enzymes and other bioactive factors (24). SASP may trigger senescence of the surrounding healthy cells, inducing a vicious cycle (24). Certain SASP components, including matrix-degrading enzymes and proinflammatory factors, were also indicated to be associated with a decrease in ECM (25). Furthermore, the avascularity of IVD limits the immune-mediated clearance of senescent cells (24,26). In a previous study, less senescence-associated changes were observed in the end plate (EP) than in the annulus fibrosus and NP cells (27). This may be attributable to the better blood supply in the EP.
Various signaling pathways have been indicated to be associated with cell senescence and most of these eventually regulate cell senescence via interaction with the p53/p21/retinoblastoma (RB) and p16/RB pathways (28,29). ROS are a key factor that increases cellular survival stress and triggers SIPS, and ROS production was indicated to accelerate the onset and progress of senescence via activation of p21(10). The feedback loop between ROS and p21 is also important in SIPS: ROS production activates p21 expression, while p21 also induces mitochondrial dysfunction and ROS production (10). p21-dependent SIPS is not restricted to proliferative tissues and the brain neurons also acquire intense DNA damage caused by ROS production (30).
p53 also serves a critical role in cellular response to stress (31). p53 mainly functions as a transcription factor, regulating the expression of specific target genes (32). These genes are involved in the regulation of autophagy, DNA damage repair, senescence and apoptosis (33). The p53 level is positively associated with the severity and duration of the stress stimulus and different levels of p53 may have different consequences (34). In a study by Chen et al (21), sublethal doses of H2O2 induced senescent-like growth arrest in human diploid fibroblasts, while higher doses induced apoptosis. At higher doses of H2O2, the p53 levels were two times higher compared with those at sublethal doses of H2O2 (21). In the present study, NP cells were treated with 200 µM H2O2 for 2 h, which has been reported as a sublethal dose (21). The results also indicated that after treatment with H2O2, NP cells exhibited senescent features instead of undergoing apoptosis.
The anti-inflammatory and anti-oxidative effects of PCA have been reported in the context of various diseases, including kidney diseases (35), liver diseases (36) and osteoarthritis (19). The dosage of PCA used in different diseases ranged from 100 to 164 µg/ml (37). Huang et al (19) indicated that PCA attenuated IL-1β-induced inflammatory response and cellular senescence in chondrocytes. The dosage of PCA used in their study was 10, 20 and 40 µg/ml, while 40 µg/ml PCA treatment achieved the best results (19). Considering that the biological characteristics of NP cells are similar to those of chondrocytes, 10 and 50 µg/ml PCA were used in the present study. Treatment with 50 µg/ml PCA significantly inhibited the cellular senescence of NP cells without affecting cell proliferation.
The present study was a preliminary study on PCA and cellular senescence and certain limitations require to be acknowledged. Firstly, the mechanisms by which PCA regulates cellular senescence in NP cells were not explored and additional studies are required to investigate the underlying mechanisms. Furthermore, animal studies are required to verify the therapeutic effect of PCA in vivo.
In conclusion, in the present in vitro experiments, PCA suppressed ROS-induced senescence in NP cells via both the p16 and p53 pathways. These results suggest that PCA may be a potential agent for the treatment of IDD.
Acknowledgements
Not applicable.
Funding Statement
Funding: The present study was supported by a grant from the National Natural Science Foundation of China (grant no. 81802228).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
KS, YL, ZW, KH and ZY designed the study. KS, YL, ZW and KH performed the research. KS and ZY analyzed the data. KS, YL and ZY wrote the manuscript. KS and ZY confirm the authenticity of all the raw data. All authors have read and approved the final manuscript.
Ethics approval and consent to participate
Institutional Review Board approval for the present study was provided by the Ethics Committee of The Second Affiliated Hospital, Zhejiang University School of Medicine (Hangzhou, China; approval no. 2020-1184). Written informed consent was obtained from each donor.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Hartvigsen J, Hancock MJ, Kongsted A, Louw Q, Ferreira ML, Genevay S, Hoy D, Karppinen J, Pransky G, Sieper J, et al. What low back pain is and why we need to pay attention. Lancet. 2018;391:2356–2367. doi: 10.1016/S0140-6736(18)30480-X. [DOI] [PubMed] [Google Scholar]
- 2.Gille O, Bouloussa H, Mazas S, Vergari C, Challier V, Vital JM, Coudert P, Ghailane S. A new classification system for degenerative spondylolisthesis of the lumbar spine. Eur Spine J. 2017;26:3096–3105. doi: 10.1007/s00586-017-5275-4. [DOI] [PubMed] [Google Scholar]
- 3.Vos T, Flaxman AD, Naghavi M, Lozano R, Michaud C, Ezzati M, Shibuya K, Salomon JA, Abdalla S, Aboyans V, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990-2010: A systematic analysis for the global burden of disease study 2010. Lancet. 2012;380:2163–2196. doi: 10.1016/S0140-6736(12)61729-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andersson GB. Epidemiological features of chronic low-back pain. Lancet. 1999;354:581–585. doi: 10.1016/S0140-6736(99)01312-4. [DOI] [PubMed] [Google Scholar]
- 5.Feng C, Liu H, Yang M, Zhang Y, Huang B, Zhou Y. Disc cell senescence in intervertebral disc degeneration: Causes and molecular pathways. Cell Cycle. 2016;15:1674–1684. doi: 10.1080/15384101.2016.1152433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang Y, Yang B, Wang J, Cheng F, Shi K, Ying L, Wang C, Xia K, Huang X, Gong Z, et al. Cell senescence: A nonnegligible cell state under survival stress in pathology of intervertebral disc degeneration. Oxid Med Cell Longev. 2020;2020(9503562) doi: 10.1155/2020/9503562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tang N, Dong Y, Chen C, Zhao H. Anisodamine maintains the stability of intervertebral disc tissue by inhibiting the senescence of nucleus pulposus cells and degradation of extracellular matrix via interleukin-6/janus kinases/signal transducer and activator of transcription 3 pathway. Front Pharmacol. 2020;11(519172) doi: 10.3389/fphar.2020.519172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Machino M, Yukawa Y, Imagama S, Ito K, Katayama Y, Matsumoto T, Inoue T, Ouchida J, Tomita K, Ishiguro N, Kato F. Age-related and degenerative changes in the osseous anatomy, alignment, and range of motion of the cervical spine: A comparative study of radiographic data from 1016 patients with cervical spondylotic myelopathy and 1230 asymptomatic subjects. Spine (Phila Pa 1976) 2016;41:476–482. doi: 10.1097/BRS.0000000000001237. [DOI] [PubMed] [Google Scholar]
- 9.Lee DC, Adams CS, Albert TJ, Shapiro IM, Evans SM, Koch CJ. In situ oxygen utilization in the rat intervertebral disc. J Anat. 2007;210:294–303. doi: 10.1111/j.1469-7580.2007.00692.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Passos JF, Nelson G, Wang C, Richter T, Simillion C, Proctor CJ, Miwa S, Olijslagers S, Hallinan J, Wipat A, et al. Feedback between p21 and reactive oxygen production is necessary for cell senescence. Mol Syst Biol. 2010;6(347) doi: 10.1038/msb.2010.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deniz GY, Altun S. Evaluation of nickel-induced brain injuries in rats via oxidative stress and apoptosis: Attenuating effects of hyperoside. Turk J Zool. 2020;44:104–113. [Google Scholar]
- 12.Tian Y, Bao Z, Ji Y, Mei X, Yang H. Epigallocatechin-3-gallate protects H2O2-induced nucleus pulposus cell apoptosis and inflammation by inhibiting cGAS/sting/NLRP3 activation. Drug Des Devel Ther. 2020;14:2113–2122. doi: 10.2147/DDDT.S251623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tesanovic K, Pejin B, Sibul F, Matavulj M, Rašeta M, Janjušević L, Karaman M. A comparative overview of antioxidative properties and phenolic profiles of different fungal origins: Fruiting bodies and submerged cultures of Coprinus comatus and Coprinellus truncorum. J Food Sci Technol. 2017;54:430–438. doi: 10.1007/s13197-016-2479-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Janjušević L, Karaman M, Šibul F, Tommonaro G, Iodice C, Jakovljević D, Pejin B. The lignicolous fungus trametes versicolor (L.) Lloyd (1920): A promising natural source of antiradical and AChE inhibitory agents. J Enzyme Inhib Med Chem. 2017;32:355–362. doi: 10.1080/14756366.2016.1252759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.An SM, Koh JS, Boo YC. p-Coumaric acid not only inhibits human tyrosinase activity in vitro but also melanogenesis in cells exposed to UVB. Phytother Res. 2010;24:1175–1180. doi: 10.1002/ptr.3095. [DOI] [PubMed] [Google Scholar]
- 16.Luceri C, Giannini L, Lodovici M, Antonucci E, Abbate R, Masini E, Dolara P. p-Coumaric acid, a common dietary phenol, inhibits platelet activity in vitro and in vivo. Br J Nutr. 2007;97:458–463. doi: 10.1017/S0007114507657882. [DOI] [PubMed] [Google Scholar]
- 17.Navaneethan D, Rasool M. p-Coumaric acid, a common dietary polyphenol, protects cadmium chloride-induced nephrotoxicity in rats. Ren Fail. 2014;36:244–251. doi: 10.3109/0886022X.2013.835268. [DOI] [PubMed] [Google Scholar]
- 18.Pragasam SJ, Venkatesan V, Rasool M. Immunomodulatory and anti-inflammatory effect of p-coumaric acid, a common dietary polyphenol on experimental inflammation in rats. Inflammation. 2013;36:169–176. doi: 10.1007/s10753-012-9532-8. [DOI] [PubMed] [Google Scholar]
- 19.Huang X, You Y, Xi Y, Ni B, Chu X, Zhang R, You H. p-Coumaric acid attenuates IL-1β-induced inflammatory responses and cellular senescence in rat chondrocytes. Inflammation. 2020;43:619–628. doi: 10.1007/s10753-019-01142-7. [DOI] [PubMed] [Google Scholar]
- 20.Griffith JF, Wang YX, Antonio GE, Choi KC, Yu A, Ahuja AT, Leung PC. Modified pfirrmann grading system for lumbar intervertebral disc degeneration. Spine (Phila Pa 1976) 2007;32:E708–E712. doi: 10.1097/BRS.0b013e31815a59a0. [DOI] [PubMed] [Google Scholar]
- 21.Chen QM, Liu J, Merrett JB. Apoptosis or senescence-like growth arrest: Influence of cell-cycle position, p53, p21 and bax in H2O2 response of normal human fibroblasts. Biochem J. 2000;347:543–551. doi: 10.1042/0264-6021:3470543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 23.Childs BG, Durik M, Baker DJ, van Deursen JM. Cellular senescence in aging and age-related disease: From mechanisms to therapy. Nat Med. 2015;21:1424–1435. doi: 10.1038/nm.4000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kirkland JL, Tchkonia T. Cellular senescence: A translational perspective. EBioMedicine. 2017;21:21–28. doi: 10.1016/j.ebiom.2017.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kepler CK, Ponnappan RK, Tannoury CA, Risbud MV, Anderson DG. The molecular basis of intervertebral disc degeneration. Spine J. 2013;13:318–330. doi: 10.1016/j.spinee.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 26.de Magalhaes JP, Passos JF. Stress, cell senescence and organismal ageing. Mech Ageing Dev. 2018;170:2–9. doi: 10.1016/j.mad.2017.07.001. [DOI] [PubMed] [Google Scholar]
- 27.Xing QJ, Liang QQ, Bian Q, Ding DF, Cui XJ, Shi Q, Wang YJ. Leg amputation accelerates senescence of rat lumbar intervertebral discs. Spine (Phila Pa 1976) 2010;35:E1253–E1261. doi: 10.1097/BRS.0b013e3181e7d087. [DOI] [PubMed] [Google Scholar]
- 28.Munoz-Espin D, Serrano M. Cellular senescence: From physiology to pathology. Nat Rev Mol Cell Biol. 2014;15:482–496. doi: 10.1038/nrm3823. [DOI] [PubMed] [Google Scholar]
- 29.Chicas A, Wang X, Zhang C, McCurrach M, Zhao Z, Mert O, Dickins RA, Narita M, Zhang M, Lowe SW. Dissecting the unique role of the retinoblastoma tumor suppressor during cellular senescence. Cancer Cell. 2010;17:376–387. doi: 10.1016/j.ccr.2010.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rass U, Ahel I, West SC. Defective DNA repair and neurodegenerative disease. Cell. 2007;130:991–1004. doi: 10.1016/j.cell.2007.08.043. [DOI] [PubMed] [Google Scholar]
- 31.Liu D, Xu Y. p53, oxidative stress, and aging. Antioxid Redox Signal. 2011;15:1669–1678. doi: 10.1089/ars.2010.3644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mijit M, Caracciolo V, Melillo A, Amicarelli F, Giordano A. Role of p53 in the regulation of cellular senescence. Biomolecules. 2020;10(420) doi: 10.3390/biom10030420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vousden KH, Prives C. Blinded by the light: The growing complexity of p53. Cell. 2009;137:413–431. doi: 10.1016/j.cell.2009.04.037. [DOI] [PubMed] [Google Scholar]
- 34.Spallarossa P, Altieri P, Aloi C, Garibaldi S, Barisione C, Ghigliotti G, Fugazza G, Barsotti A, Brunelli C. Doxorubicin induces senescence or apoptosis in rat neonatal cardiomyocytes by regulating the expression levels of the telomere binding factors 1 and 2. Am J Physiol Heart Circ Physiol. 2009;297:H2169–H2181. doi: 10.1152/ajpheart.00068.2009. [DOI] [PubMed] [Google Scholar]
- 35.Rafiee Z, Moaiedi MZ, Gorji AV, Mansouri E. P-coumaric acid mitigates doxorubicin-induced nephrotoxicity through suppression of oxidative stress, inflammation and apoptosis. Arch Med Res. 2020;51:32–40. doi: 10.1016/j.arcmed.2019.12.004. [DOI] [PubMed] [Google Scholar]
- 36.Xie W, Zhang S, Lei F, Ouyang X, Du L. Ananas comosus L. Leaf phenols and p-coumaric acid regulate liver fat metabolism by upregulating CPT-1 expression. Evid Based Complement Alternat Med. 2014;2014(903258) doi: 10.1155/2014/903258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Abazari MF, Nasiri N, Karizi SZ, Nejati F, Haghi-Aminjan H, Norouzi S, Piri P, Estakhr L, Faradonbeh DR, Kohandani M. An updated review of various medicinal applications of p-coumaric acid: From antioxidative and anti-inflammatory properties to effects on cell cycle and proliferation. Mini Rev Med Chem. 2021;21:2187–2201. doi: 10.2174/1389557521666210114163024. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.