Abstract
Background
Currently inhaled corticosteroids are the main stay in the maintenance treatment of chronic asthma in children. Although inhaled corticosteroids play a crucial role in the management of childhood asthma, the long‐term side effects of inhaled corticosteroids used in the management of chronic asthma in children are not clearly known.
Objectives
The objective of this review is to compare the safety and efficacy of inhaled nedocromil sodium with placebo in the treatment of chronic asthma in children.
Search methods
We searched the Cochrane Airway Group trials register, review articles, and reference lists of articles. We also contacted the drug manufacturer and primary authors for additional citations. We also searched abstracts of major respiratory society meetings. The last search was carried out in November 2009.
Selection criteria
Randomised placebo controlled trials comparing nedocromil sodium to placebo in the treatment of chronic asthma in children (0 to 18 years).
Data collection and analysis
Both authors independently assessed trial quality and extracted data. Study authors were contacted for additional information.
Main results
Fifteen trials (twelve parallel group studies; three crossover trials recruiting 1422 children (837 males and 585 females)) were included. The studies were generally of good methodological quality. Two large long term studies used nedocromil for six months and four to six years and showed conflicting results in symptom free days. Short term studies (duration between 4 weeks to 12 weeks) showed that nedocromil sodium produced some improvement in a number of efficacy measures compared to placebo including FEV1, FVC, FEV1 % predicted, PC20 FEV1, evening PEF and symptom scores. The parent's assessment of efficacy was in favour of nedocromil (odds ratio (OR) 0.5 (95% CI 0.3 to 0.8). Nedocromil sodium has a good safety profile. The only significant side effect observed was unpleasant taste. There was little evidence for a clinically dose response effect and only a few studies recruited participants with severe asthma.
Authors' conclusions
A limited number of small studies have shown that nedocromil is of benefit in improving lung function and some measures of symptoms, but the evidence with regard to the primary outcome of the review was conflicting. Two long‐term trials did not show consistent effects on lung function outcomes, whereas several small short‐term trials have shown benefit in these outcomes. Differing severities at baseline may explain this difference with milder participants experiencing less benefit, although the discrepancy between study findings may also reflect publication bias. Nedocromil sodium is associated with a very good safety profile with no significant short term or long‐ term adverse side effects. Although nedocromil may have advantages over inhaled corticosteroids in terms of side effects, there is a need for head to head trials of nedocromil and inhaled corticosteroids to establish whether asthma control is similar, especially in mild asthma. It is not yet clear where nedocromil should sit in relation to other therapies in the treatment of asthma in children.
Plain language summary
Nedocromil sodium for chronic asthma in children
Nedocromil (or Tilade) is a 'preventer' therapy used to treat chronic asthma in children. It is thought to be safer than inhaled steroids and can be used for the management of mild to moderate asthma. The review of studies including 15 trials and 1422 children found that there were some encouraging results in short term studies when nedocromil was compared on its own with placebo, particularly with regard to lung function tests. However, these results were not confirmed in one large, longer term study of four to six years duration, which did not show significant difference in the primary outcome of symptom free days. This study was conducted in children who had mild asthma. There may be a role for nedocromil in the management of moderate asthma, but it should be assessed in relation to inhaled steroids, whose efficacy is well‐established. This particularly important in symptomatic asthma.
Background
Asthma is a disease of chronic airway inflammation characterised by reversible airway obstruction and increased airway responsiveness (NIH 1997; Warner 1998). It is also defined as "reversible airways obstruction associated with bronchial hyperreactivity, allergic inflammation of the airways and a response to treatment with bronchodilators with regular prophylactic inhaled anti‐inflammatory agents" (Silverman 1997).
Nedocromil sodium is a chromone. It is the disodium salt of a pyranoquinoline dicarboxylic acid developed as an anti‐inflammatory agent in the treatment of asthma (Rainey 1992). A number of clinical trials in adults have shown the efficacy of nedocromil sodium in the management of chronic asthma in adults and a meta‐analysis of all the placebo controlled trials has shown that the drug is an effective treatment for adult asthma (Edwards 1993). However there have been only a few trials looking at the safety and efficacy of this drug in children with asthma.
At present, inhaled nedocromil sodium is one of the controller medications recommended for the treatment of mild persistent asthma along with sodium cromoglycate and inhaled corticosteroids. These recommendations are based on the international guidelines on asthma management, the Global Initiative for Asthma (GINA 1995) and also the international guidelines on diagnosis and management of asthma (NIH 1997). The British asthma guidelines coordinating committee (BTS 1997) recommends nedocromil sodium or sodium cromoglycate as alternatives to corticosteroids at step 2 of the management of chronic asthma in children. A recent Cochrane systematic review concluded that nedocromil sodium used before exercise reduces the severity and duration of exercise induced bronchoconstriction in both children and adults (Spooner 2002).
Although inhaled corticosteroids play a crucial role in the management of childhood asthma, the long‐ term side effects of inhaled corticosteroids used in the management of chronic asthma in children are not clearly known. Pooled effect estimates of fluticasone in children indicate an improvement in Fixed expiratory volume in one second (FEV1) and am Peak expiratory flow (PEF) of around 110 ml and 25.7 L/min respectively when given at a dose 100 mcg/d (Adams 2005). However, there is concern that the adverse effects of long‐ term inhaled corticosteroids in treating children with mild asthma may outweigh the beneficial effects. A recent Cochrane review has shown an effect of inhaled beclomethasone on linear growth in children (Sharek 1999). Nedocromil sodium is associated with very good safety profile with no significant adverse side effects. The common side effect reported with the use of nedocromil is unpleasant taste sensation, which might pose problems with compliance in children (GINA 1995).
Currently inhaled corticosteroids are the main stay in the maintenance treatment of chronic asthma in children. Perhaps the more important question to answer is whether nedocromil sodium can be regarded as an alternative first line anti‐inflammatory treatment to sodium cromoglycate or inhaled corticosteroids in asthmatic children. With this in mind we set out to review the safety and efficacy of nedocromil sodium in the treatment of chronic asthma in children.
Objectives
The objective of this review is to compare the safety and efficacy of inhaled nedocromil sodium with placebo in the treatment of chronic asthma in children.
Methods
Criteria for considering studies for this review
Types of studies
All randomised placebo controlled clinical trials addressing the efficacy of nedocromil sodium in the treatment of chronic asthma in children.
Types of participants
Children aged 0 to 18 years with asthma in all settings (general practice, outpatient departments, emergency departments, hospitalised).
Types of interventions
Inhaled nedocromil sodium, delivered via any device: nebulised, by spinhaler or by metered dose inhaler, with or without holding chamber. Only trials that compared nedocromil sodium with placebo were be included. We also included studies where rescue medications were used as co‐interventions. Studies with combination of medications were excluded.
Types of outcome measures
Primary outcomes
Difference in number of days without asthma symptoms between nedocromil sodium and placebo treatment.
Secondary outcomes
Symptom scores
Indicators of lung function‐ FEV1, PEF
Asthma exacerbations‐ hospital admission rates, days off school, unscheduled doctor visits due to exacerbations
Rescue short acting beta‐2 agonist use
Health related quality of life( HRQOL)
Bronchial hyper responsiveness to histamine and methacholine
Withdrawal rates
Side effects: unpleasant taste sensation, sore throat, headaches, urticaria, angio oedema.
Search methods for identification of studies
Electronic searches
Trials were identified using the Cochrane Airways Group Specialised Register of trials, which is derived from systematic searches of bibliographic databases including the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE, CINAHL, AMED and PsycINFO, and handsearching of respiratory journals and meeting abstracts (please see the Airways Group Module for further details). All records in the Specialised Register coded as 'asthma' were searched using the following terms:
Nedocromil* OR NS OR Tilade
The most recent search was conducted in November 2009.
Searching other resources
We searched the bibliographies of all papers retrieved in full text form and searched the relevant narrative reviews for additional publications. We contacted authors of included studies and asked if they were aware of further studies. We also contacted the UK headquarters of manufacturers of nedocromil sodium to find details of studies sponsored by them, which may have been missed. Finally, we handsearched the proceedings of meetings of the European Respiratory Society, British Thoracic Society and American Thoracic Society for relevant trials.
The search attempted to identify all relevant studies irrespective of language. We translated non‐English papers or relevant data extracted by members of the Cochrane Collaboration.
Data collection and analysis
Selection of studies
Two authors (AVS and MM) scanned the titles and abstracts of all reports identified through the searches . Disagreement as to which papers to include were resolved by consensus. If there was lack of consensus, a third party determined the final decision. Full reports were obtained for trials appearing to meet the inclusion criteria or for which there was insufficient information in the title and abstract to make a clear decision.
Data extraction and management
Both authors independently extracted data using specially designed data extraction forms. The characteristics of the trial participants, interventions and outcomes for the included trials is presented in study tables. We contacted authors or clarification or further information.
One author (AVS) extracted data for each outcome from the published results of included trials. In the case of continuous outcomes such as spirometry:
1. Where outcomes were evaluated at a number of time points, only data from the last time point that could be evaluated was used. 2. Data were extracted from graphical plots when presented in this form; attempt was made to verify such data by contacting authors. 3. If an intention‐to‐treat analysis (ITT) was not used by the investigators, and it was not explicit in the presentation of results how many participants (N) were in each group at the time of last evaluation of that outcome, the appropriate N value for each intervention group was calculated by subtracting the number of participants who withdrew in each intervention group from those randomised to each intervention group.
Authors were written to (by mail, fax and/or electronic mail) on at least two occasions to clarify details of request missing outcome data. An attempt was made to send requests to correct current addresses by searching MEDLINE, EMBASE and hospital World Wide Web (WWW) sites for up‐to‐date contact details. Pantheon pharmaceutical company were approached for data for those trials in which the contact authors did not initially reply or when authors suggested doing so and which had been sponsored by the company.
Assessment of risk of bias in included studies
Methodological quality of all the trials were scored independently by two authors using two sets of criteria: Jadad 1996 and the Cochrane criteria for concealment. Agreement between the authors was assessed by calculating the kappa score.
One author (AVS) made the decision to exclude studies prior to full paper retrieval. Both authors (AVS and MM) independently reviewed the full text papers. Disagreement as to which papers to include was resolved by consensus. Both authors (AVS and MM) independently assessed the methodological quality of each study . The trials were scored using the Cochrane approach: Grade A: adequate allocation concealment Grade B: unclear allocation concealment Grade C: clearly inadequate concealment
The studies were also assessed using a five point scoring instrument (Jadad 1996): a) Was the study described as randomised? (yes = 1 no = 0) b) Was the study described as double blind? (yes =1 no = 0) c) Was there a description of withdrawals and dropouts? (yes = 1 no = 0) d) Was the method of randomisation well described and appropriate? (yes = 1 no = 0) e) Was the method of double blinding well described and appropriate? (yes = 1 no = 0) f) Deduct 1 point if method of randomisation or blinding inappropriate
Inter‐rater agreement was measured using the kappa statistic. Disagreement was resolved by consensus.
Assessment of reporting biases
Visual inspection of funnel plots was undertaken in order to assess whether the pooled effect estimates potentially overestimate the effect of nedocromil. In order to reduce possible publication bias, we have attempted to include results from trials where only a limited number of estimates are available. If the lack of SDs limited the availability of effect estimates which could contribute to a meta‐analysis, we have imputed SDs as an average of those available. This has only been undertaken if there were three or more studies which contributed data.
Data synthesis
A weighted treatment effect across trials was calculated using Cochrane statistical package Review Manager.
For continuous outcomes, we calculated a weighted mean difference (WMD) or a standardised mean difference (SMD) as appropriate. For dichotomous outcomes, we calculated a Relative Risk (RR).
Pooled treatment effects were expressed with 95% confidence intervals (95% CI). We calculated heterogeneity of effect size across pooled studies, with P < 0.05 used as the cut off level for significance (Dersimonian 1986; Van Houwelingen 1995). A fixed‐effect model was used when homogenous treatment effects were present, a random‐effect model if heterogeneity was present. Pooled treatments effects are expressed with their 95% confidence intervals (95% CI).
For continuous outcomes, we calculated a weighted mean difference (WMD) or standardised mean difference (SMD) as appropriate. For binary/dichotomous outcomes, we calculated a relative risk (RR).
A number of a priori conditions were established regarding the comparisons made.
1. The results of parallel and crossover trials were not pooled.
2. It was anticipated that measures of bronchial hyper‐responsiveness (dose of inhalant required to produce a 20% fall in FEV1(PD20 FEV1), concentration of inhalant required to produce a 20% fall in FEV1 (PC20 FEV1)) would often be reported as geometric means. Presentation of results in this way indicates that data has been logarithmically transformed prior to analysis by investigators to take account of a skewed distribution. We only pooled data for such outcomes across studies if the mean and standard deviation of logged values (from which geometric means are derived) could be calculated.
Subgroup analysis and investigation of heterogeneity
asthma severity;
type of delivery;
duration of nedocromil administration ‐ less than four weeks, one to six months, and more than six months;
different doses of nedocromil administration.
Sensitivity analysis
Sensitivity analyses were performed on the basis of methodological quality.
Results
Description of studies
Results of the search
For details of the initial search yield see Table 1. Update searches conducted to November 2008 identified a number of references relevant to CAMP.
1. Search history.
| Search years | Detail |
| All years to October 2004 | Stage 1: Electronic search (nedocromil study) ‐ 205 Abstracts ; Stage 2: Nedocromil Register (NR): Studies in full text: 42 studies; Included studies: 15 studies (12 Parallel group and three crossover studies); Excluded studies: 27 Studies. Studies translated: Two studies were translated from Polish language and one study translated from German language. Agreement between two independent assessments of study quality regarding: randomisation; double‐blinding; withdrawals/dropouts; method of randomisation; method of blinding. There was no disagreement in each of the above categories. |
| October 2004 to November 2006 | One additional excluded study (Koskela 2005) |
| November 2006 to November 2007 | One additional excluded study (Bruce 2006) |
We identified 15 studies for inclusion (41 references). These studies were published between 1990 and 2002. The language of publication of the great majority of the studies was English. No studies were excluded on the basis of language. Twelve studies were in English language. Two studies (Stelmach 2002a; Stelmach 2002b) were translated from Polish and one study (Wonne 1990) was translated from German.
Included studies
The summary of the studies is given in the Characteristics of included studies and described in general terms here.
Overall there was a considerable clinical heterogeneity among the studies. König 1995 and CAMP compared nedocromil with placebo in chronic asthma over a period of six months and four to six years respectively. In three crossover studies (Murray 1993; Spezia 1993; Wonne 1990) assessed the efficacy of a single dose of nedocromil in preventing broncho‐constriction to various agents.
Populations
The studies were conducted in the USA, Italy, Poland, Germany, Netherlands, Belgium, UK, Australia and South Africa. Four studies came from Italy (Businco 1990; Fiocchi 1994; Fiocchi 1997; Spezia 1993), three studies from Poland (Stelmach 2001; Stelmach 2002a; Stelmach 2002b), one study from Germany (Wonne 1990), one study from Australia (Foo 1993), two studies from the USA (König 1995; CAMP), one study from Turkey (Sekerel 1999) and one study from the UK (Murray 1993). One study was a multi‐centre study from UK, Australia and South Africa (Edwards 1999), and one was a multi centre study conducted in Netherlands, Belgium and the UK (van Bever 1996). Four studies were multi‐centre trials (Edwards 1999; König 1995; CAMP; van Bever 1996). Most of the studies were conducted in the secondary care (recruiting participants from out patient clinics). One study (Spezia 1993) was conducted in primary care.
All the studies were conducted in children less than 18 years of age. One study (Businco 1990) included two participants aged 20 and 21 years who had asthma since childhood. The analysis includes data on 1360 children (787 males and 561 females). One study (Wonne 1990) did not report the number of males and females. In most of the studies the participants were children aged more than five years.
One study (Businco 1990) recruited participants with grass pollen asthma. One study (Fiocchi 1997) recruited participants with seasonal hyperreactivity to ultrasonic nebulisation with distilled water (UNDW). One study (König 1995) recruited participants during the viral season, one study (Murray 1993) was done to assess the effect on fog induced bronchoconstriction and one study (Spezia 1993) looked at the efficacy of nedocromil in preventing the bronchoconstriction induced by inhalation of ultrasonically nebulised distilled water (UNDW). One study (Stelmach 2001) recruited participants who were allergic to dust mite.
Design of the studies
Of the 15 studies, 12 studies were randomised placebo controlled parallel group trials. Three studies (Murray 1993;Spezia 1993;Wonne 1990) used a crossover design. Two trials included a third treatment arm (Spezia 1993‐ SCG) and (CAMP‐ budesonide) and one study (Stelmach 2002a) had three more arms in addition to NCS (i.e. triamcinolone acetonide, montelukast and formoterol).
Diagnosis of asthma
In three of the studies (Foo 1993; König 1995; Sekerel 1999) the diagnosis of asthma was based on the ATS 1987 criteria. In three of the studies (Stelmach 2001; Stelmach 2002a; Stelmach 2002b), the diagnosis of asthma was based on the NIH criteria. In two of the studies (Businco 1990; Edwards 1999) the diagnosis of asthma was based on the basis of clinical history and confirmed by 15% reversibility of airway obstruction in response to a bronchodilator. In two studies (Spezia 1993; CAMP) the diagnosis was based on the clinical history and positive methacholine challenge. In four of the studies (;Fiocchi 1994; Fiocchi 1997; Murray 1993; Wonne 1990) there was no clear indication of the criteria upon which a diagnosis of asthma was made and appears to be based only on clinical symptoms.
Severity of asthma
Mild
In three studies (Edwards 1999;Fiocchi 1994;Fiocchi 1997), the investigators stated that the severity of asthma was mild. No information was given regarding the baseline frequency of symptoms in these studies.
Mild to Moderate
In five studies (König 1995;Murray 1993;Sekerel 1999;CAMP;Wonne 1990), the investigators stated that the severity of asthma was mild to moderate. In one study (Sekerel 1999) the severity was based on the ATS criteria. In three studies (König 1995;CAMP;Wonne 1990), the severity was based on clinical symptoms and use of bronchodilators. In one study (Murray 1993), the exact criteria on which the severity was based not mentioned.
Moderate
In three studies (Stelmach 2001;Stelmach 2002a;Stelmach 2002b), the investigators stated that the severity was moderate and this was based on the NIH criteria.
Mild to severe
In four studies (Businco 1990; Foo 1993; Spezia 1993; van Bever 1996), the investigators stated that the severity was mild to severe asthma. In one study (Foo 1993) the severity was based on the ATS criteria. Businco 1990 recruited participants only with grass pollen asthma (seasonal asthma) and symptom free during the rest of the period. The severity was based on clinical symptoms. In one study (Spezia 1993) the severity was based on the clinical symptoms. van Bever 1996 did not indicate the instrument or guideline which determined the severity of asthma.
Intervention
Daily dose of nedocromil sodium
In four studies(Sekerel 1999; Stelmach 2001; CAMP; van Bever 1996), a low dose of nedocromil was used as daily intervention (8 mg/day or less). In two studies (Edwards 1999; Foo 1993), a moderate dose of nedocromil was used as daily intervention (12mg/day). In six studies (Businco 1990; Fiocchi 1994; Fiocchi 1997; Stelmach 2002a; Stelmach 2002b; CAMP), a high dose of nedocromil was used as daily intervention (16 mg/day or more).two studies used a dose of 4 mg twice daily (Sekerel 1999; Stelmach 2001). Two studies (Edwards 1999; Foo 1993) used a dose 4 mg thrice daily. Five studies (Businco 1990; Fiocchi 1994; Fiocchi 1997; Stelmach 2002a; Stelmach 2002b) used 4 mg four times daily. One study (Sekerel 1999) had two arms in the intervention group i.e. 4 mg twice daily and 4 mg four times daily. One study (CAMP) used 8 mg twice daily. Two studies (Spezia 1993; Wonne 1990) used 4 mg once daily. One study (König 1995) used 10 mg three times per day. One study (Murray 1993) used doses between 5 to 10 mg once daily.
Length of intervention period
In four studies (Businco 1990; Stelmach 2001; Stelmach 2002a; Stelmach 2002b) the duration of the intervention period was four weeks. In two studies (Fiocchi 1994; Fiocchi 1997) the duration was six weeks. In two studies (Foo 1993; Sekerel 1999) the duration of intervention was 8 weeks. In two studies (Edwards 1999; van Bever 1996) the duration of intervention was 12 weeks. In one study (König 1995) the duration was 24 weeks. In one study (Wonne 1990) a dose of 4 mg of nedocromil was used once to study the protective effect of nedocromil on cold air hyperinflation challenge. In one study (CAMP) the duration of treatment was four to six years, with data being recorded from the last visit. One study (Spezia 1993) used nedocromil 4 mg once only to study its effect in the prevention of bronchoconstriction induced by ultrasonic nebulised distilled water in asthmatic children. In one study (Murray 1993) nebulised nedocromil 5 and 10 mg were used on consecutive days to study its effect on fog induced bronchoconstriction in asthmatic children.
Delivery device
In eight of the studies a metered dose inhaler (MDI) was used without a spacer to deliver nedocromil sodium. In three of the studies (Edwards 1999; Foo 1993; Spezia 1993), a holding or aerosol chamber was used along with the MDI to deliver the nedocromil sodium. In one study (Fiocchi 1994) the exact delivery device is not stated. In two studies (König 1995; Murray 1993) an ultrasonic nebuliser has been used to deliver the nedocromil sodium solution. In one study the details of inhaler device were not clear (van Bever 1996).
Outcomes assessed
A large number of outcome measures were reported. Nine of the studies reported symptom scores. Nine of the studies reported pulmonary function tests as outcome measures. Two studies (Fiocchi 1994; Murray 1993) did not report of clinical symptom scores as outcome measures. One study reported in abstract form did not contribute data to any outcomes analysed as these were not made available for our review (van Bever 1996).
We did not consider the following outcome measures: 1. Three studies (Stelmach 2001; Stelmach 2002a; Stelmach 2002b) included outcomes related directly to the biological basis of inflammation in asthma. These included eosinophilic blood count, serum eosinophilic cationic protein (Stelmach 2002a), sIL‐2R, IL‐4, sICAM and IgE (Stelmach 2002a) and serum IgE and IL‐4 levels (Stelmach 2002b). These outcomes involve invasive blood sampling and are not generally regarded as outcome measures to assess the clinical efficacy of nedocromil sodium in children. These outcome measures could not be merged because they were different inflammatory markers with often different units. They are potentially interesting data and are discussed in the results. 2. In one study (Murray 1993), nebulised nedocromil sodium was used on two occasions to assess the FEV1 response to fog challenge. 3. In one study (Spezia 1993), single dose of nedocromil was used to assess its efficacy in preventing the bronchoconstriction induced by inhalation of ultrasonically nebulised distilled water (UNDW).
Four of the 15 studies reported on the safety and tolerability of nedocromil sodium. All safety and efficacy measures were considered. Both parent's and clinician's assessment of efficacy were considered. All reported side effects were considered as outcome measures. The common side effects like headache, sore throat and unpleasant taste sensation and other uncommon reported side effects were analysed.
Risk of bias in included studies
Allocation concealment
We were able to ascertain that the methods used to generate allocation sequences were at a low risk of bias in three studies (CAMP; Edwards 1999; Sekerel 1999). For the remaining studies we were unable to ascertain this information.
Jadad Scores
The overall quality of the studies was high. All the 13 studies were randomised and double‐blind. All studies either described drop outs or had none. Using Jadad's five point validity scale, eight studies achieved a score of four or more. In these studies the method double‐blinding was not described. Six studies achieved a score of three and in these studies the method of randomisation and double‐blinding were not described adequately.
Effects of interventions
A structured approach was used in reporting the result (Study design: parallel group or crossover studies; Sub‐groups: all doses, all devices, all durations and all severities; Outcome measurement: FEV1, FVC, PEF etc; Unit of measurement: i.e. absolute, % predicted or change compared to baseline). Within each component sub‐group analysis by dose, length of intervention, device and severity are reported, where the data permit. This was structured in the following way.
A) Comparison 01 considers each outcome according to daily dose of nedocromil used (8 mg/day, 12 mg/day or 16 mg/day or more). B) Comparisons 02 considers each outcome according to the severity of asthma (mild, moderate, severe asthma). C) Comparison 03 considers each outcome according to the type of device used to deliver nedocromil sodium (MDI, MDI plus spacer, nebuliser). D) Comparison 04 considers search outcome according to the duration of intervention (less than one month, one to six months, more than six months). E) Comparison 05 considers long term studies( six months or longer ) versus short term (less than six months) studies. F) In cross over studies (06, 07), the comparison considers each outcome according to the dose of nedocromil used and the delivery device.
The results for these comparisons are discussed in the text of the results for each outcome. In some studies data were not given in the form suitable for meta‐analysis. In one study (Foo 1993), several of the outcomes were estimated based on the mean difference reported and the P values. In two studies (Businco 1990; König 1995), some data have been estimated from the graphs and the P values. Data relating to continuous variables for König 1995 have been imputed based upon the method outlined above. Individual patient data (IPD) were made available for Sekerel 1999.
Primary outcome
Two studies reported data on this outcome (König 1995; CAMP). In CAMP data were available as change from baseline in the average number of episode free days (number per month). In this study 16 mg/day of nedocromil was used via an MDI over a period of four to six years. No significant difference between the two groups was apparent (9.3 versus 9.3 days/month). However, König 1995 reported a significant difference in the % days per month without symptoms (11.8%, P = 0.027).
Secondary outcomes
Spirometry
Change in FEV1 compared baseline (litres): parallel group studies
Five studies compared the change in FEV1 compared to the baseline. SDs were for imputed for König 1995. Two studies with 180 participants (Edwards 1999; Foo 1993) assessed nedocromil 12 mg/day versus placebo over a period of two and three months respectively. There was significant improvement with nedocromil over placebo: WMD 0.1 (95% CI 0.03 to 0.2). Three studies with 852 participants (Businco 1990; König 1995; CAMP) assessed nedocromil 16 mg/day versus placebo over a period of four weeks, 24 weeks and 4.3 years respectively. There was a significant difference between the two groups (Chi‐squared 6.07, df 1, P = 0.01). When all the studies were pooled, there was no significant difference between nedocromil and placebo with fixed‐effect (WMD 0 (95% CI ‐0.02 to 0.1) and random‐effects modelling (WMD 0.1 (95% CI ‐0.02 to 0.2). Further sub‐group analyses based on delivery device and severities showed no significant difference between subgroups. Subgroup analysis based on the duration of treatment did show a significant difference between trials of different durations, (Chi‐squared 10.99, df 2, P = 0.004). The heterogeneity appears to be due to the lack of effect of treatment in the large longer duration study CAMP.
FEV1 (litres): crossover studies
Two studies with 72 participants (Murray 1993; Spezia 1993) assessed the effect of a single dose of nedocromil versus placebo and reported FEV1 (litres). In one study (Murray 1993), there were two treatment arms with different doses (5 mg and 10 mg). A significant treatment effect was apparent in favour of nedocromil (WMD 0.3 (95% CI 0.1 to 0.5)).
FEV1 % predicted: parallel group studies
Studies expressing FEV1 as % predicted were pooled (Sekerel 1999; Stelmach 2001; Stelmach 2002a; Stelmach 2002b). A significant treatment effect in favour of nedocromil was apparent (WMD 14.5% (95% CI 12.9 to 16.1%)). The results from longer duration Sekerel study are negative at high and low dose, which contrasts with the positive results from the Stelmach studies (Chi‐squared 12.28, df 1, P = 0.0005).
Change in FEV1 (% predicted) from baseline: parallel studies
Two studies contributed data to this outcome (Sekerel 1999; CAMP). No significant difference was apparent (‐0.2 (95% CI ‐1.5 to 1.1)).
FEV1 % predicted: crossover studies
One small study compared a single dose of nedocromil with placebo (Spezia 1993) and reported FEV1 as % predicted. No significant difference between the two groups was apparent.
FVC (litres): parallel group studies
One study (Foo 1993) with 111 participants assessed the efficacy of 12 mg/day of nedocromil delivered via a MDI and spacer in mild ‐moderate asthma. There was a significant effect in favour of nedocromil (fixed‐effect 0.2 litres (95%CI 0.06 to 0.38)). The data were estimated based on the mean difference reported in the text of the study and the P values.
Change in FVC (litres) compared to baseline: parallel group studies
Five studies (Businco 1990; Edwards 1999; Foo 1993; König 1995; CAMP) with 1032 participants assessed the change from baseline in FVC (litres). No significant difference between treatment groups was apparent (WMD 0.1 (95% CI ‐0.1 to 0.2)). Subgroup analyses based on duration of treatment showed a significant difference between the groups (Chi‐squared 8.56, df 2, P = 0.01). A short term study (Businco 1990) comprising of 29 participants assessed the efficacy of 16 mg/day of nedocromil, delivered via MDI for a period of four weeks, showed a significant benefit in favour of nedocromil. An other large study (CAMP), with 730 participants assessed the efficacy of nedocromil over more than six month period (four to six years) showed no significant benefit in favour of nedocromil.
Peak expiratory flow rate (PEF)
Change from baseline PEF (L/min): parallel studies
One study (Edwards 1999), with 69 participants assessed the change from baseline in PEF (L/min). No significant difference between the two groups was apparent.
Change from baseline in morning PEF (L/min): parallel studies
Four studies (Businco 1990; Edwards 1999; König 1995; CAMP), with 903 participants assessed the change from baseline in morning PEF (L/min). No significant difference between the two groups was apparent (WMD 15 (95% CI ‐1.8 to 31.7)). Subgroup analyses based on delivery device and duration of treatment showed no significant difference between the two groups. There was a significant difference between subgroups when severity of treatment was considered (Chi‐square 8.72, df 2, P = 0.01). (Note: König 1995 ‐ variance was imputed).
Change from baseline in evening PEF (L/min): parallel studies
Two studies (Edwards 1999; Foo 1993), with 180 participants assessed the change from baseline in evening PEF (L/min). A significant difference in favour of nedocromil was apparent: WMD 11.5 (CI 0.1 to 22.9). (Note: König 1995‐data is not estimable as standard deviation could not be derived).
Overall although, the small short term studies showed that nedocromil sodium produced improvement in various lung function measures like FEV1, FVC, FEV1% predicted, histamine PC20 FEV1 and evening PEF compared to placebo, there was no long‐term benefit to be seen in the large study of CAMP.
Asthma symptoms
Daily asthma symptom score: parallel studies
Only four parallel group studies (König 1995; Stelmach 2001; Stelmach 2002a; Stelmach 2002b) reported daily dairy card symptoms score in a form with data suitable for analysis.
In one study (König 1995), daytime asthma (scored 0 to 4), sleeping difficulty due to night time asthma (scored 0 to 2) and cough (0 to 4) were used to score the asthma symptoms. In three studies (Stelmach 2001; Stelmach 2002a; Stelmach 2002b) daytime asthma symptoms were scored (0 = no symptoms to 3 = severe symptoms), along with use of beta 2 agonists (0 = none to 3 = more than three times a day) and nocturnal asthma symptoms (0 = good night, slept well to 3 = bad night, awake most of the night due to symptoms).
In an attempt to standardise scales, effect sizes were expressed using a standardised mean difference (SMD). At each increased dose of nedocromil there was a significantly lower symptom score in the nedocromil treatment group compared to placebo. Nedocromil 8 mg/day: SMD 0.8 (95% CI 0.1 to 1.6). Nedocromil 16 mg/day: SMD 0.5 (95% CI 0.2 to 0.8). When all the studies were pooled there was considerable heterogeneity between the studies (chi square 14.13 df = 2). When pooled overall there was no significant difference in symptom scores between the two groups.
Changes from baseline in symptom score: parallel studies
One parallel study (CAMP), with total of 730 participants assessed the changes from baseline in symptom scores. Although, the scoring was based on the childhood asthma management program (CAMP) the details of the scoring system was not given. In this study 16 mg/day of nedocromil was used via an MDI over a period of four to six years. There was no significant difference between the two groups in changes from baseline in symptom scores (WMD 0.01 (95% CI 0.04 to 0.06)).
Change from baseline in daytime asthma: parallel studies
One parallel study (Edwards 1999), with 69 participants assessed the change from baseline in daytime asthma. In this study 12 mg/day of nedocromil was used via an MDI and spacer over a 12‐ week period. No significant difference between the two groups was apparent, although there was a trend that favoured nedocromil.
Change from baseline in night time asthma: parallel studies
Two parallel studies (Edwards 1999; CAMP), with a total of 799 participants assessed the change from baseline in night‐time asthma. In one study (Edwards 1999) 12 mg/day of nedocromil was used via an MDI and spacer over a 12‐ week period. In the other study (CAMP), 16 mg/day of nedocromil was used via an MDI over a period of four to six years. When the studies were pooled there was no significant difference in change from baseline in night time asthma symptoms between the two groups: WMD 0.02 (95% CI ‐0.1 to 0.2). In one study (Businco 1990), data extracted from graphs and P values were not estimable.
Although three studies (Stelmach 2001; Stelmach 2002b; Stelmach 2002a) using a dose of nedocromil sodium (16 mg/day) given by an MDI over four weeks showed a significant effect on daily asthma symptom score, one study (König 1995) using higher dose of nedocromil (30 mg/day) via a nebuliser for 24 weeks, found no such effect. Similarly other studies (Edwards 1999; CAMP) assessing the change from baseline in daytime asthma and night time asthma showed no effect in favour of nedocromil.
Rescue beta 2 agonist use
Change from baseline in bronchodilator use: parallel studies
There was no significant difference when data from five studies were pooled (‐0.1 puffs/d (95% CI ‐0.2 to 0.1). Sub‐groups analyses based on the delivery device, duration of treatment and severity of asthma showed no significant difference between the two groups.
Asthma exacerbations
CAMP reported no significant difference between nedocromil and placebo in hospitalisations due to asthma (difference of 0.1/100 person year, P = 0.99), but there was a significant reduction in favour of nedocromil in urgent care visits due to asthma (difference of 6/100 person‐year, P = 0.02), and a significantly lower rate of prednisolone courses for acute exacerbations (difference of 20/100 person year, P = 0.01).
No studies assessed days missed from school due to asthma exacerbations. The other reported outcome related to asthma exacerbations was the rate of withdrawal due to asthma exacerbations. There was no significant difference between the two groups in withdrawals due to asthma exacerbations: RR 0.75 (95% CI 0.33 to 1.72). Sub‐group analyses based on the delivery device, duration of treatment and severity of asthma showed no significant difference in the withdrawal rate between the subgroups.
Side effects
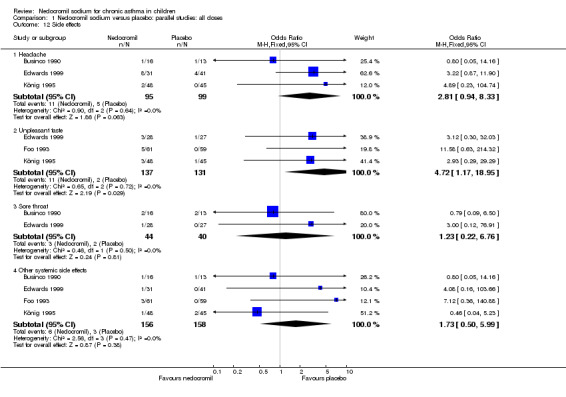
The incidence of sore throat, unpleasant taste, headache, and other systemic side effects were reported in four parallel studies (Businco 1990; Edwards 1999; Foo 1993; König 1995). In two studies (Businco 1990; Edwards 1999), with 84 participants, there was no significant difference in sore throat between nedocromil and the placebo groups (RR 1.23 (95% CI 0.26 to 5.5)). In three studies (Edwards 1999; Foo 1993; König 1995), with 268 participants, there was significant difference in the incidence of unpleasant taste between the two groups with a higher incidence of unpleasant taste in the nedocromil group (RR 4.4 (95% CI 1.15 to 16.88)). In three studies (Edwards 1999; Businco 1990; König 1995), with 194 participants, there was no significant difference in the incidence of headache between the two groups (RR 2.45 (95% CI 0.95 to 6.34)). Four studies (Businco 1990; Edwards 1999; Foo 1993; König 1995), with 314 participants assessed systemic side effects like angio‐neurotic oedema, urticaria, nausea, vomiting, abdominal pain and cough on aerosol inhalation. There was no significance difference between the nedocromil and the placebo groups in pooled systemic side effects (RR 1.71 (95% CI 0.52 to 5.6)).
Long term health outcomes
One study (CAMP) assessed long‐term health outcomes in a parallel study. In this study 16 mg/day of nedocromil was used via an MDI over a period of four to six years. In addition to the nedocromil and placebo arms, there was also budesonide arm in the treatment group. Changes in height (cm), changes in bone density (g/cm2) and change in total score on child depression inventory was assessed as long‐term outcome measures. No significant difference between the two groups was apparent.
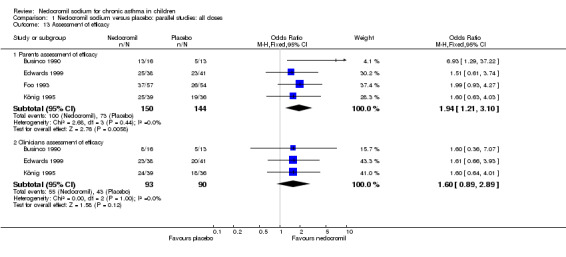
Assessment of efficacy (dichotomised)
Four studies (Businco 1990; Edwards 1999; Foo 1993; König 1995), with a total of 299 participants reported the parent's assessment of efficacy of intervention. There was significant difference between the two groups in favour of nedocromil (OR 2.08 (95% CI 1.30 to 3.33)). Three studies (Businco 1990; Edwards 1999; König 1995), with a total of 183 participants reported the clinician's assessment of efficacy of the intervention. There was no significant difference between the two groups (OR 1.6 (95% CI 0.9 to 2.9)).
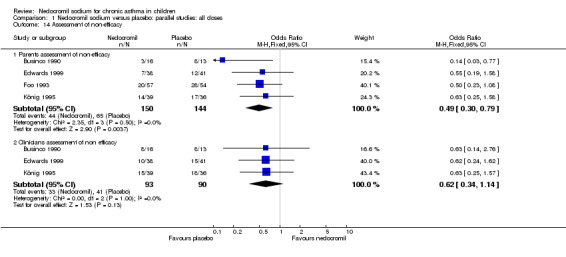
Four studies (Businco 1990; Edwards 1999; Foo 1993; König 1995) reported the parent's assessment of non‐efficacy of the intervention. There was significant difference between the two groups in favour of nedocromil. i.e. the parents felt that the placebo was not effective (OR 0.5 (95% CI 0.3 to 0.8)). Three studies (Businco 1990; Edwards 1999; König 1995) reported the clinician's assessment of non‐efficacy of the intervention. No significant difference between the two groups was apparent.
Bronchial hyper‐responsiveness (BHR)
Bronchial hyper‐responsiveness to a number of pharmacological stimuli was assessed. One small parallel study (Fiocchi 1997), with 20 participants assessed the BHR in response to ultrasonically nebulised distilled water (UNDW). There was no significant difference in the UNDW PC10 FEV1 (log transformed) between the two groups.
One small crossover study (Spezia 1993), with 24 participants assessed the BHR in response to ultrasonically nebulised distilled water (UNDW). This was small study where in a single dose of 4 mg of nedocromil was used with an MDI and spacer. A significant difference in the UNDW PC20 FEV1 (log transformed) was apparent in favour of nedocromil: WMD 5.77(95%CI 1.78 to 9.76). Data from Wonne 1990 was not estimable as standard deviation could not be derived.
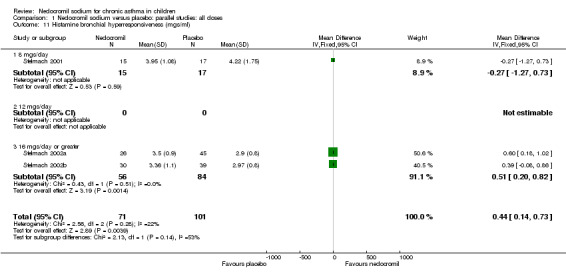
One large study (CAMP), with 730 participants assessed the methacholine hyper responsiveness. There was no significant difference in the methacholine PC20 FEV1 between the two groups. Three parallel studies (Stelmach 2001; Stelmach 2002a; Stelmach 2002b), with a total of 172 participants assessed the histamine responsiveness (PC20 FEV1). In two studies (Stelmach 2002a; Stelmach 2002b), a daily dose of 16 mg of nedocromil was used via an MDI over a four week period. The histamine PC20 FEV1 was significantly greater in participants treated with nedocromil compared to placebo (WMD 0.5 (95% CI 0.2 to 0.8)). In one study (Stelmach 2001), a daily dose of 8 mg of nedocromil was used via an MDI over a four week period. No significant difference between the two groups was apparent. Pooling all the studies showed a significant difference in favour of nedocromil without significant heterogeneity (WMD 0.5 (95% CI 0.2 to 0.7)).
Inflammatory parameters of asthma
One parallel study (Stelmach 2001) which assessed the efficacy of 8 mg/day of nedocromil via an MDI over a four week period, showed a significant difference in percentage of blood eosinophil count in favour of nedocromil sodium (WMD 4.2% (95% CI 1.2 to 7.3)). The same study also showed a significant difference in serum s IL‐ 2R(pg/ml) in favour of nedocromil (WMD 32.1 pg/ml(95% CI 32.07 to 32.13)). In the same study, no significant difference in serum s ICAM (ng/ml) was demonstrated between the two groups. Two studies (Stelmach 2001; Stelmach 2002a), with a total of 103 participants assessed the serum eosinophilic cationic protein (ECP) ng/ml. There was no significant difference between the two groups. Two studies (Stelmach 2001; Stelmach 2002b) with a total of 101 participants assessed the serum IL‐4 (pg/ml) levels. There was significant difference between the two groups in favour of nedocromil group (WMD 0.02 pg/ml (95 CI 0.01 to 0.04)). The same studies also assessed the serum IgE (iu/ml) between the two groups. No significant difference was apparent.
Discussion
This review has assessed the efficacy and side‐effect profile of inhaled nedocromil sodium in the treatment of chronic childhood asthma. Only randomised, placebo controlled trials were included and the overall methodological quality of the studies was good. This review includes fifteen studies of 1422 children (837 males and 585 females). The studies were conducted across eight countries over a 12 year period (1990 to 2002). Three of the studies were multi‐centre trials. Most of the studies were conducted in the secondary care (recruiting participants from outpatient clinics). Only one study was conducted in the primary care setting.
The primary outcome measure of this review was asthma symptom free days. Only one study (CAMP) reported symptom free days as change from baseline in episode free days per month. There was no significant difference demonstrated between nedocromil and the placebo in that study. König 1995 reported a significant effect in the proportion of symptom free days, although it reported no effect on daily asthma symptom score. Similarly studies assessing the change from baseline in daytime asthma, night‐time asthma and episode free days showed no effect in favour of nedocromil (Edwards 1999; CAMP). However three small short term studies (Stelmach 2001; Stelmach 2002a; Stelmach 2002b) using 16 mg/day of nedocromil sodium, reported an improvement in daily asthma symptom score in favour of nedocromil.
Change in FEV1, change in FVC and change in am PEF outcomes are dominated by the effect estimate derived from the large long term study (CAMP). This study reported consistently non‐significant effects in a sample of children mild to moderate asthma. It is of note that the children recruited to the inhaled steroid arm did not appear to derive the same sort of benefit from budesonide as that observed in other studies of children with asthma (Pauwels 2003). The disposition of the participants in CAMP may explain the lack of a response to treatment in terms of lung function outcomes, particularly when the positive response to nedocromil as absolute FEV1% predicted is considered. The asymmetrical funnel plots for these outcomes may well be a function of clinical heterogeneity and the statistical power of CAMP, rather than publication bias (Figure 1; Figure 2; Figure 3).
1.
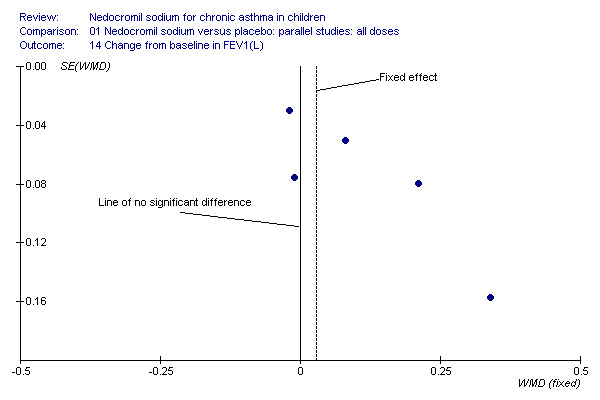
Funnel plot to show that the distribution of effect estimates from the studies shows that there is a propensity of large, imprecise, positive effect estimates in the smaller studies. The non‐significant effect is reported in the large study, suggesting that the fixed‐effect may over‐estimate the true effect for this population.
2.
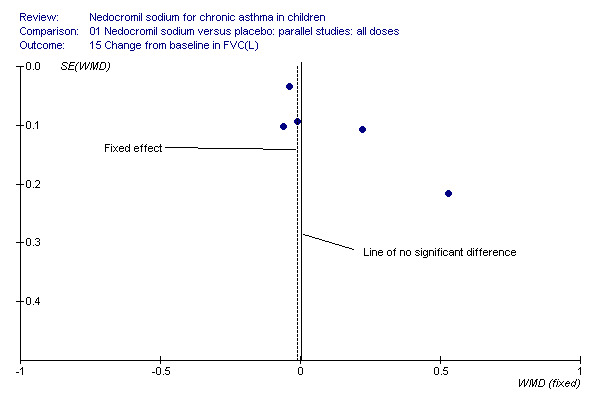
Funnel plot to show that the distribution of effect estimates from the studies shows that there is a propensity of large, imprecise, positive effect estimates in the smaller studies. The non‐significant effect is reported in the large study, suggesting that the fixed‐effect may over‐estimate the true effect for this population.
3.
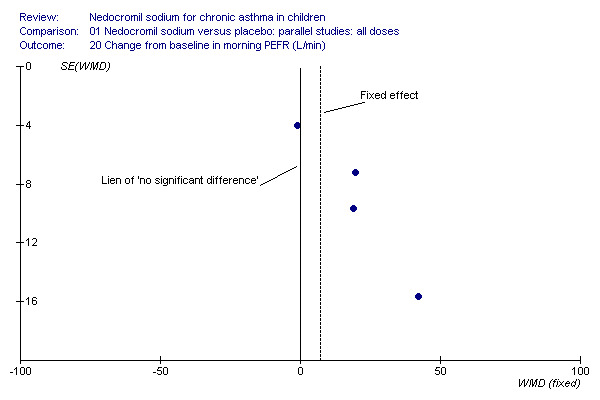
Funnel plot to show that the distribution of effect estimates from the studies shows that there is a propensity of large, imprecise, positive effect estimates in the smaller studies. The non‐significant effect is reported in the large study, suggesting that the fixed‐effect may over‐estimate the true effect for this population.
Bronchial hyper‐responsiveness (BHR), to various pharmacological stimuli was assessed in a few studies. In the large long term study (CAMP), there was no difference between the groups in the methacholine PC20 FEV1. However pooling the three short term studies (Stelmach 2001; Stelmach 2002a; Stelmach 2002b) showed an improvement of 0.44 mg/ml (95% CI 0.14 to 0.73) in histamine PC20 FEV1 in favour of nedocromil sodium.
Overall there were conflicting findings in terms of episode free days, rescue beta‐2 agonist use, in terms of hospitalisations, emergency department attendances and prednisolone courses. It is possible that nedocromil tends to improve day to day persistent asthma symptoms, but is not efficacious enough to prevent an acute attack. There may be underlying reasons for this. It has been shown that peak expiratory flow (PEF) variation is strikingly different during exacerbations compared with day to day poor asthma control (Reddel 1999). This could suggest that a large stimulus to the airways producing a profound drop in PEF is not blocked by a mast‐cell stabilising drug perhaps because other mechanisms are activated.
There was no significant difference in the withdrawal rate due to asthma exacerbations between the two groups. Important outcome measures such as days missed from school due to asthma exacerbations and GP attendance rates were not reported. Withdrawal rates in the context of a clinical trial cannot be considered equivalent to hospital admission rates or GP attendance rates due to exacerbation of asthma, but may be a surrogate marker for these.
Clinician assessment of efficacy and non‐efficacy was not significant, although it was not reported whether the clinician's assessment of efficacy and non‐efficacy was subjective or based on objective criteria. However, nedocromil was significantly more likely to be perceived as effective by parents than placebo. Parental assessment of efficacy and non‐efficacy of treatment although subjective, has major implications for adherence with preventative therapy (Bassler 2004).
Nedocromil sodium has a good safety profile. No other appreciable short term and long‐term side effects were demonstrated. The common side effects reported with the use of nedocromil include sore throat, unpleasant taste, headache and other systemic side effects like angio‐neurotic oedema, urticaria, nausea, vomiting, abdominal pain, cough on aerosol inhalation. The only significant side effect observed due to nedocromil was unpleasant taste. There was no difference between the nedocromil and the placebo groups in the incidence of other side effects (Businco 1990; Edwards 1999; Foo 1993; König 1995). The increased incidence of unpleasant taste could pose a major problem with compliance of nedocromil therapy in children, which is very crucial in effective asthma control.
Only one study (CAMP) reported long term health outcomes in children following nedocromil therapy. Changes in height (cm), changes in bone density (g/cm2) and change in total score on child depression inventory were assessed as long‐ term outcome measures. There was no difference between the nedocromil and the placebo groups. A recent Cochrane review (Sharek 1999) has shown a detrimental effect of inhaled beclomethasone on short term linear growth in children. However in the large long term study (CAMP), although there was a initial reduction in growth velocity with inhaled budesonide group compared to the placebo, there was no change in final stature between the two groups.
Health related quality of life (HRQOL), which was one of the secondary outcome measure was not reported in any of the trials.
There are limited data on the effect of nedocromil sodium on various inflammatory markers in the serum of asthmatic children. Serum eosinophilic blood counts, eosinophilic cationic protein (ECP), sIL ‐2R, IL‐4, sICAM and total IgE are used as serum markers of airway inflammation in asthma (Stelmach 2001). The three studies (Stelmach 2001; Stelmach 2002a; Stelmach 2002b) which, reported inflammatory mediators of asthma showed a significant decrease in the percentage of blood eosinophil count, serum s IL‐2R and serum IL‐4 levels in favour of nedocromil. There was no difference in the serum eosinophilic cationic protein (ECP), serum IgE and serum sICAM levels between the two groups. These inflammatory markers could not be meta‐analysed because of different inflammatory markers and different units in which they are reported. These outcomes involve invasive blood sampling and are not generally regarded as outcome measures to assess the clinical efficacy of nedocromil sodium in children. The anti‐inflammatory properties of nedocromil, opens the possibility of it having a disease modifying effect in the long term, although the results are clearly not consistent between the studies.
In conclusion, this review has shown the large long term studies (König 1995; CAMP) using nedocromil for six months and 4.3 years respectively showed no benefit in favour of nedocromil in asthma symptom free days, lung function tests, BHR to methacholine, broncho‐dilator use, hospitalisations, emergency department attendances, and prednisolone courses. However a number of small short term studies(using nedocromil for four weeks to 12 week period) have shown some improvement in daily asthma symptom scores, lung function tests and BHR to histamine in favour of nedocromil. The reasons for the apparent discrepancy in findings between small short‐term studies and the large long‐term study may be accounted for by severity. This review also has shown that nedocromil sodium is associated with very good safety profile with no significant short term or long‐ term adverse side effects. The only significant side effect observed due to nedocromil was unpleasant taste.
Although nedocromil clearly has advantages over inhaled corticosteroids in regards to side effects, there is a lack of head to head trials of nedocromil versus inhaled corticosteroids to establish if nedocromil has advantages in symptoms, lung function and other outcomes over inhaled steroids especially in mild asthma. It is not yet clear where nedocromil should sit in relation to other therapies in the treatment of asthma in children. Whilst the studies conducted in children with mild asthma do not provide consistent evidence that nedocromil improves symptoms and lung function, the short term studies in more severe participants suggest improvement in these variables. Additional confirmatory studies in more moderately severe children (FEV1 or peak flow % predicted lower than 80% (BTS 2003)) are required. Assessment of any potential clinically relevant effect of nedocromil sodium in the treatment of asthma will be best achieved by a further systematic review assessing trials in which participants have been randomised to nedocromil and inhaled corticosteroids within the same trial such as CAMP. This study showed that continuous daily treatment with inhaled budesonide 400 micrograms/day leads to better control of asthma than nedocromil sodium, and its side effects are limited to a small, transient reduction in growth velocity with no change in final stature between the two groups.
Methodological limitations
1) The possibility of publication bias related to missed published trials occurs in all systematic reviews. A number of strategies have been undertaken in order to minimise the impact of 'missed' negative data sets such as a comprehensive electronic search, imputation, and the inclusion wherever possible of studies not indexed on medical literature databases (van Bever 1996). It is impossible to estimate the impact of missed studies on our findings, but should data become available in the future, substantive amendment will allow us to assess the extent to which additional data affect our findings.
2) A large number of efficacy outcomes were reported, using a range of different scales. This factor, combined with a significant proportion of studies in which numerical data were either not presented with standard deviations around mean scores or in which authors were unable or unwilling to provide such data, reduced the power of the analysis. A wide variety of scales were used to assess the symptoms, and the quality of reporting was poor with several trials failing to provide standard deviation values for mean treatment effects.
3) The majority of studies were conducted in a secondary care setting, with patients attending hospital outpatient clinics for review. In most instances it was not clear how patients were identified as potential recruits (e.g. patients already attending hospital asthma clinics, primary care referrals, advertisement etc. Only one study was carried out in a primary care. Study setting may therefore have biased recruitment towards patients with more symptoms, since they have been largely conducted in secondary care.
4) In some studies data were not given in the form suitable for meta‐analysis. In one study (Foo 1993), several of the outcomes were estimated based on the mean difference reported and the P values. In one study (Businco 1990), the data has been estimated from the graphs and the P values. Imputing missing standard deviations is a compromise for missing data.
5) A significant proportion of outcome data from individual trials could not be included in the meta‐analysis because either standard deviation values for mean effect sizes were not presented in the original citation or no numerical data were presented at all. These missing data will diminish the power of the meta‐analysis.
6) The studies included in this review included children with a spectrum of asthma severity being treated with a range of drug doses delivered by various means (nebuliser, MDI, MDI plus spacer) over a range of treatment periods. These factors should be taken into account when considering the findings of this review.
7) The identification of three ranges for daily nedocromil sodium dose is arbitrary. However the range boundaries were felt to generally reflect dose distinctions made in clinical practice.
8) In three crossover studies the effect of a single dose of nedocromil sodium on bronchial hyper responsiveness (BHR) to various respiratory stimuli has been assessed. The treatment difference between nedocromil and placebo may be of clinical significance, although the level of significant change is difficult to translate into a clinically meaningful measure.
Authors' conclusions
Implications for practice.
A number of small studies have shown that nedocromil sodium improves the airflow limitation, reduces symptoms and reduces bronchial hyper‐responsiveness to various respiratory stimuli. This has not been confirmed in a larger long term study of children with milder asthma. Although nedocromil was shown to be effective, we did not address the relative effects of this drug in relation to inhaled steroids, where the effects of treatment have been well established. Nedocromil sodium has a good safety profile, although there is a lack of head to head trials comparing nedocromil with inhaled corticosteroids to establish relative effects of the drugs on symptoms, lung function, especially in mild asthma where the requirement for inhaled steroids may not be as profound as it is with more severe asthma. The only significant side effect observed was unpleasant taste. Although there were no appreciable short term and long‐term side effects, it is difficult to conclude the long‐term safety of nedocromil sodium in childhood asthma based on only one study. It is not yet clear where nedocromil should sit in relation to other therapies in the treatment of asthma in children.
Implications for research.
1) Consistent methods of reporting symptoms aided by the development of a validated questionnaire (with established thresholds of clinical importance) would greatly assist in the interpretation of symptom changes in response to therapy.
2) More placebo controlled trails are required to assess the true efficacy of nedocromil. These trials should be large enough and of sufficient duration to allow the assessment of economically important outcomes such as hospital admission rates, primary care attendance and days missed from work/school due to asthma exacerbations. Studies should include mild and moderate asthma over longer periods.
3) Improved reporting of primary study methods, particularly details of how patients are allocated to treatment groups in line with CONSORT 2001 recommendations would improve the accuracy of systematic reviews (CONSORT 2001).
4) Assessment of any potential clinically relevant effect of nedocromil sodium in the treatment of asthma will be best achieved by a further systematic review assessing trials in which patients have been randomised to nedocromil and inhaled corticosteroids within the same trial such as the CAMP study (CAMP).
What's new
| Date | Event | Description |
|---|---|---|
| 6 November 2009 | New search has been performed | Literature search re‐run; no new studies identified. An additional three references pertaining to CAMP were identified and added to the study publications |
History
Protocol first published: Issue 1, 2003 Review first published: Issue 3, 2006
| Date | Event | Description |
|---|---|---|
| 5 November 2008 | New search has been performed | Literature search re‐run; no new studies identified. |
| 11 August 2008 | Amended | Converted to new review format. |
| 2 April 2006 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
We would like to thank the Cochrane Airways Group (Toby Lasserson, Liz Arnold and Karen Blackhall) for their help and support in searching the Airways Review Group register, retrieving articles, checking data and other support. We are grateful to Toby Lasserson and Prof RP Skomro for interpretation of foreign language literature. We would also like to thanks Prof M Silverman and Dr J Grigg for their support and advice. We are grateful to Kirsty Loudon for copyediting this review.
Data and analyses
Comparison 1. Nedocromil sodium versus placebo: parallel studies: all doses.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change from baseline in episode free days (no/month) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 16mgs/day | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Percentage of symptom‐free days | 1 | % (Fixed, 95% CI) | Totals not selected | |
| 2.1 16 mgs/day | 1 | % (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 FEV1 (% Predicted ‐ Sekerel LD) | 4 | 195 | Mean Difference (IV, Fixed, 95% CI) | 14.05 [12.45, 15.64] |
| 3.1 8 mgs/day or less | 2 | 55 | Mean Difference (IV, Fixed, 95% CI) | 7.78 [1.73, 13.83] |
| 3.2 12 mgs/day | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.3 16 mgs/day or greater | 2 | 140 | Mean Difference (IV, Fixed, 95% CI) | 14.51 [12.86, 16.17] |
| 4 FEV1 (% Predicted ‐ Sekerel HD) | 4 | 197 | Mean Difference (IV, Fixed, 95% CI) | 14.06 [12.46, 15.65] |
| 4.1 8 mgs/day or less | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | 14.23 [6.58, 21.88] |
| 4.2 12 mgs/day | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.3 16 mgs/day or greater | 3 | 165 | Mean Difference (IV, Fixed, 95% CI) | 14.05 [12.42, 15.68] |
| 5 Daily use of beta 2 agonists(puffs/day ‐ Sekerel LD) | 2 | 43 | Mean Difference (IV, Fixed, 95% CI) | ‐0.96 [‐1.44, ‐0.48] |
| 5.1 8 mgs/day or less | 1 | 23 | Mean Difference (IV, Fixed, 95% CI) | ‐0.94 [‐3.86, 1.98] |
| 5.2 12 mgs/day | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.3 16 mgs/day or greater | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | ‐0.96 [‐1.45, ‐0.47] |
| 6 Daily use of beta 2 agonists (puffs/day ‐ Sekerel HD) | 2 | 45 | Mean Difference (IV, Fixed, 95% CI) | ‐0.94 [‐1.42, ‐0.46] |
| 6.1 8 mgs/day or less | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.2 12 mgs/day | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.3 16 mgs/day or greater | 2 | 45 | Mean Difference (IV, Fixed, 95% CI) | ‐0.94 [‐1.42, ‐0.46] |
| 7 Daily asthma symptom score (Sekerel LD) | 5 | 266 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.44 [‐0.99, 0.10] |
| 7.1 8 mgs/day or less | 2 | 55 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.30 [‐1.36, 0.75] |
| 7.2 12 mgs/day | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.3 16 mgs/day or greater | 3 | 211 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.52 [‐1.26, 0.23] |
| 8 Daily asthma symptom score (Sekerel HD) | 5 | 268 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.46 [‐0.99, 0.07] |
| 8.1 8 mgs/day or less | 1 | 32 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.82 [‐1.55, ‐0.10] |
| 8.2 12 mgs/day | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.3 16 mgs/day or greater | 4 | 236 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.38 [‐1.00, 0.25] |
| 9 Withdrawal due to asthma exacerbations | 5 | 344 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.29, 1.81] |
| 9.1 8 mgs/day or less | 0 | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9.2 12 mgs/day | 2 | 199 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.46 [0.14, 1.50] |
| 9.3 16 mgs/day or greater | 3 | 145 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.65 [0.34, 8.02] |
| 10 Methacholine bronchial hyperresponsiveness | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 10.1 8 mgs/day | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10.2 12 mgs/day | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10.3 16 mgs/day or greater | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 11 Histamine bronchial hyperresponsiveness (mgs/ml) | 3 | 172 | Mean Difference (IV, Fixed, 95% CI) | 0.44 [0.14, 0.73] |
| 11.1 8 mgs/day | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | ‐0.27 [‐1.27, 0.73] |
| 11.2 12 mgs/day | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11.3 16 mgs/day or greater | 2 | 140 | Mean Difference (IV, Fixed, 95% CI) | 0.51 [0.20, 0.82] |
| 12 Side effects | 4 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 12.1 Headache | 3 | 194 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.81 [0.94, 8.33] |
| 12.2 Unpleasant taste | 3 | 268 | Odds Ratio (M‐H, Fixed, 95% CI) | 4.72 [1.17, 18.95] |
| 12.3 Sore throat | 2 | 84 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.22, 6.76] |
| 12.4 Other systemic side effects | 4 | 314 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.73 [0.50, 5.99] |
| 13 Assessment of efficacy | 4 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 13.1 Parents assessment of efficacy | 4 | 294 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.94 [1.21, 3.10] |
| 13.2 Clinicians assessment of efficacy | 3 | 183 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.60 [0.89, 2.89] |
| 14 Assessment of non‐efficacy | 4 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 14.1 Parents assessment of non‐efficacy | 4 | 294 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.30, 0.79] |
| 14.2 Clinicians assessment of non efficacy | 3 | 183 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.34, 1.14] |
| 15 FVC (L) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 15.1 8 mgs/day | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 15.2 12 mgs/day | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 15.3 16 mgs/day | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 16 Bronchial hyperresponsiveness (PD10‐ UNDW) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 16.1 8 mgs /day | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 16.2 12 mgs/day | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 16.3 16 mgs/day | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 17 Change from baseline in day time asthma | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 17.1 12 mgs/day | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 18 Change from baseline in night time asthma | 2 | 799 | Mean Difference (IV, Fixed, 95% CI) | ‐0.02 [‐0.18, 0.14] |
| 18.1 12 mgs/day | 1 | 69 | Mean Difference (IV, Fixed, 95% CI) | ‐0.78 [‐1.77, 0.21] |
| 18.2 16 mgs/day | 1 | 730 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.16, 0.16] |
| 19 Change from baseline in FEV1(L) | 5 | 1032 | Mean Difference (IV, Random, 95% CI) | 0.08 [‐0.02, 0.18] |
| 19.1 12 mgs/day | 2 | 180 | Mean Difference (IV, Random, 95% CI) | 0.13 [0.01, 0.25] |
| 19.2 16 mgs/day | 3 | 852 | Mean Difference (IV, Random, 95% CI) | 0.04 [‐0.10, 0.17] |
| 20 Change from baseline in FVC (L) | 5 | 1032 | Mean Difference (IV, Random, 95% CI) | 0.06 [‐0.08, 0.20] |
| 20.1 12 mgs/day | 2 | 180 | Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.13, 0.32] |
| 20.2 16 mgs/day | 3 | 852 | Mean Difference (IV, Random, 95% CI) | 0.05 [‐0.16, 0.26] |
| 21 Change from baseline in PEF (L/min) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 21.1 12 mgs/day | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 22 Change from baseline in morning PEFR (L/min) | 4 | 903 | Mean Difference (IV, Random, 95% CI) | 14.96 [‐1.74, 31.67] |
| 22.1 12mgs/day | 1 | 69 | Mean Difference (IV, Random, 95% CI) | 19.70 [2.03, 37.37] |
| 22.2 16 mgs/day | 3 | 834 | Mean Difference (IV, Random, 95% CI) | 14.55 [‐8.32, 37.42] |
| 23 Change from baseline in evening PEFR (L/min) | 3 | 255 | Mean Difference (IV, Fixed, 95% CI) | 11.47 [0.08, 22.85] |
| 23.1 12 mgs/day | 2 | 180 | Mean Difference (IV, Fixed, 95% CI) | 11.47 [0.08, 22.85] |
| 23.2 16 mgs/day | 1 | 75 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 24 Change from baseline in bronchodilator use (doses/day ‐ Sekerel LD) | 5 | 925 | Mean Difference (IV, Fixed, 95% CI) | ‐0.08 [‐0.23, 0.06] |
| 24.1 8 mgs/day | 1 | 22 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐2.91, 3.11] |
| 24.2 12 mgs/day | 1 | 69 | Mean Difference (IV, Fixed, 95% CI) | ‐0.8 [‐1.70, 0.10] |
| 24.3 16 mgs/day | 3 | 834 | Mean Difference (IV, Fixed, 95% CI) | ‐0.07 [‐0.21, 0.08] |
| 25 Change from baseline in bronchodilator use (doses/day ‐ Sekerel HD) | 5 | 928 | Mean Difference (IV, Fixed, 95% CI) | ‐0.08 [‐0.23, 0.06] |
| 25.1 12 mgs/day | 1 | 69 | Mean Difference (IV, Fixed, 95% CI) | ‐0.8 [‐1.70, 0.10] |
| 25.2 16 mgs/day | 4 | 859 | Mean Difference (IV, Fixed, 95% CI) | ‐0.06 [‐0.21, 0.08] |
| 26 Changes from baseline in symptom score (Sekerel LD) | 3 | 781 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.03 [‐0.17, 0.12] |
| 26.1 8 mgs/day | 1 | 22 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.84, 0.84] |
| 26.2 16 mgs/day | 2 | 759 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.03 [‐0.17, 0.12] |
| 27 Changes from baseline in symptom score (Sekerel HD) | 3 | 784 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.01 [‐0.16, 0.13] |
| 27.1 16 mgs/day | 3 | 784 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.01 [‐0.16, 0.13] |
| 28 Change in FEV1(% of predicted) from baseline (Szefler pre BD; Sekerel LD) | 2 | 755 | Mean Difference (IV, Fixed, 95% CI) | ‐0.19 [‐1.46, 1.08] |
| 28.1 8 mgs/day | 1 | 25 | Mean Difference (IV, Fixed, 95% CI) | 0.73 [‐2.20, 3.66] |
| 28.2 16 mgs/day | 1 | 730 | Mean Difference (IV, Fixed, 95% CI) | ‐0.4 [‐1.81, 1.01] |
| 29 Change in FEV1(% of predicted) from baseline (Szefler pre BD; Sekerel HD) | 2 | 755 | Mean Difference (IV, Fixed, 95% CI) | 0.38 [‐0.88, 1.65] |
| 29.1 16 mgs/day | 2 | 755 | Mean Difference (IV, Fixed, 95% CI) | 0.38 [‐0.88, 1.65] |
| 30 Morbidity | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 30.1 Urgent care visits due to asthma(no/100 person‐yr) | 1 | 730 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.50, 1.89] |
| 30.2 Hospitalisation due to asthma(no./100 person‐yr) | 1 | 730 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.34 [0.33, 5.42] |
| 30.3 Prednisolone course(no./100 person ‐yr) | 1 | 730 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.86, 1.62] |
| 31 Long term health outcomes | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 31.1 Change in height (cm) | 1 | 730 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.89, 0.69] |
| 31.2 Change in bone density (g/cm2) | 1 | 730 | Mean Difference (IV, Fixed, 95% CI) | ‐0.01 [‐0.02, 0.00] |
| 31.3 Change in total score on child depression inventory | 1 | 730 | Mean Difference (IV, Fixed, 95% CI) | 0.40 [‐0.35, 1.15] |
| 32 Inflammatory parameters of Asthma | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 32.1 Eosinophil count (%) | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | ‐4.23 [‐7.29, ‐1.17] |
| 32.2 Serum Eosinophilic cationic protein (ECP)‐ng/L | 2 | 103 | Mean Difference (IV, Fixed, 95% CI) | 3.03 [‐7.19, 13.24] |
| 32.3 Serum sIL‐2R (pg/ml) | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | 32.10 [32.07, 32.13] |
| 32.4 Serum IL‐4(pg/ml) | 2 | 101 | Mean Difference (IV, Fixed, 95% CI) | ‐0.02 [‐0.04, ‐0.01] |
| 32.5 Serum sICAM (ng/ml) | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | ‐27.23 [‐67.03, 12.57] |
| 32.6 Serum IgE (iu/L) | 2 | 101 | Mean Difference (IV, Fixed, 95% CI) | ‐51.52 [‐139.66, 36.62] |
| 33 Change in FEV1(% of predicted) from baseline (Szefler post BD; Sekerel LD) | 2 | 755 | Mean Difference (IV, Fixed, 95% CI) | ‐0.21 [‐1.64, 1.23] |
| 33.1 8 mgs/day | 1 | 25 | Mean Difference (IV, Fixed, 95% CI) | 0.73 [‐2.20, 3.66] |
| 33.2 16 mgs/day | 1 | 730 | Mean Difference (IV, Fixed, 95% CI) | ‐0.5 [‐2.14, 1.14] |
| 34 Change in FEV1(% of predicted) from baseline (Szefler post BD; Sekerel HD) | 2 | 755 | Mean Difference (IV, Fixed, 95% CI) | 0.52 [‐0.91, 1.95] |
| 34.1 16 mgs/day | 2 | 755 | Mean Difference (IV, Fixed, 95% CI) | 0.52 [‐0.91, 1.95] |
1.1. Analysis.
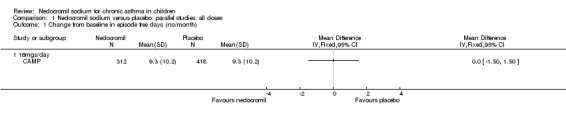
Comparison 1 Nedocromil sodium versus placebo: parallel studies: all doses, Outcome 1 Change from baseline in episode free days (no/month).
1.2. Analysis.

Comparison 1 Nedocromil sodium versus placebo: parallel studies: all doses, Outcome 2 Percentage of symptom‐free days.
1.3. Analysis.
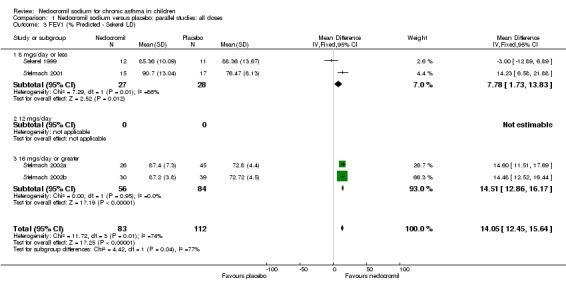
Comparison 1 Nedocromil sodium versus placebo: parallel studies: all doses, Outcome 3 FEV1 (% Predicted ‐ Sekerel LD).
1.4. Analysis.
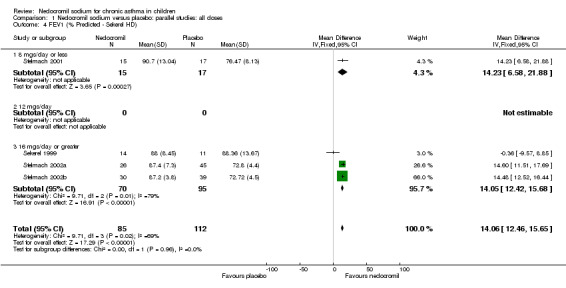
Comparison 1 Nedocromil sodium versus placebo: parallel studies: all doses, Outcome 4 FEV1 (% Predicted ‐ Sekerel HD).
1.5. Analysis.
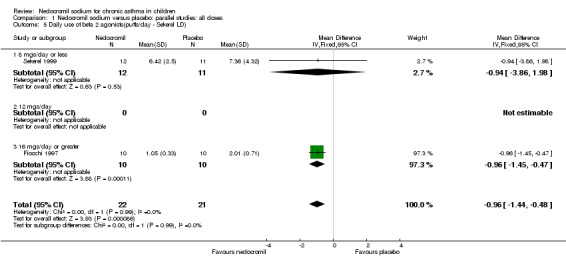
Comparison 1 Nedocromil sodium versus placebo: parallel studies: all doses, Outcome 5 Daily use of beta 2 agonists(puffs/day ‐ Sekerel LD).
1.6. Analysis.
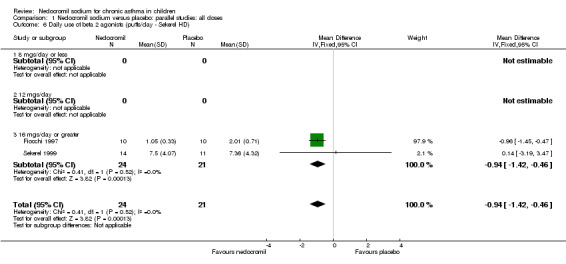
Comparison 1 Nedocromil sodium versus placebo: parallel studies: all doses, Outcome 6 Daily use of beta 2 agonists (puffs/day ‐ Sekerel HD).
1.7. Analysis.
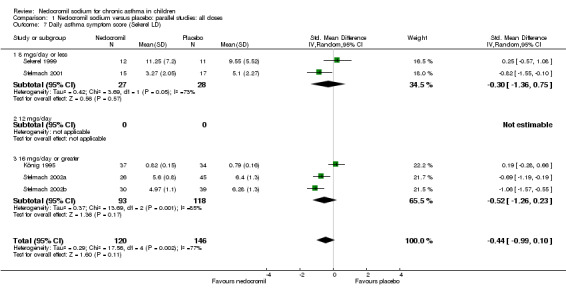
Comparison 1 Nedocromil sodium versus placebo: parallel studies: all doses, Outcome 7 Daily asthma symptom score (Sekerel LD).
1.8. Analysis.
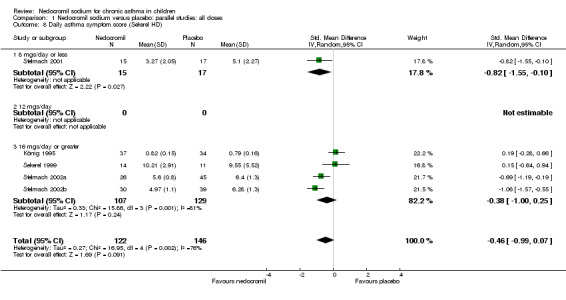
Comparison 1 Nedocromil sodium versus placebo: parallel studies: all doses, Outcome 8 Daily asthma symptom score (Sekerel HD).
1.9. Analysis.
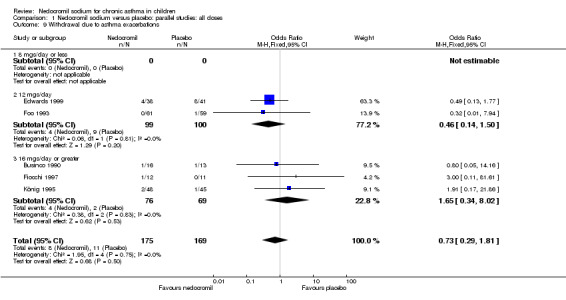
Comparison 1 Nedocromil sodium versus placebo: parallel studies: all doses, Outcome 9 Withdrawal due to asthma exacerbations.
1.10. Analysis.
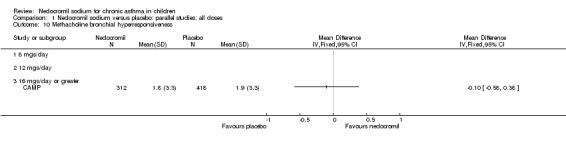
Comparison 1 Nedocromil sodium versus placebo: parallel studies: all doses, Outcome 10 Methacholine bronchial hyperresponsiveness.
1.11. Analysis.
Comparison 1 Nedocromil sodium versus placebo: parallel studies: all doses, Outcome 11 Histamine bronchial hyperresponsiveness (mgs/ml).
1.12. Analysis.
Comparison 1 Nedocromil sodium versus placebo: parallel studies: all doses, Outcome 12 Side effects.
1.13. Analysis.
Comparison 1 Nedocromil sodium versus placebo: parallel studies: all doses, Outcome 13 Assessment of efficacy.
1.14. Analysis.
Comparison 1 Nedocromil sodium versus placebo: parallel studies: all doses, Outcome 14 Assessment of non‐efficacy.
1.15. Analysis.
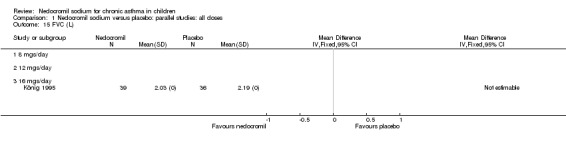
Comparison 1 Nedocromil sodium versus placebo: parallel studies: all doses, Outcome 15 FVC (L).
1.16. Analysis.
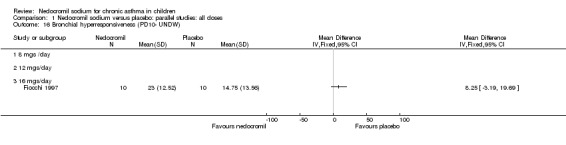
Comparison 1 Nedocromil sodium versus placebo: parallel studies: all doses, Outcome 16 Bronchial hyperresponsiveness (PD10‐ UNDW).
1.17. Analysis.
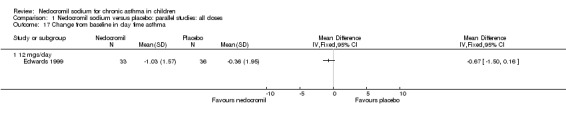
Comparison 1 Nedocromil sodium versus placebo: parallel studies: all doses, Outcome 17 Change from baseline in day time asthma.
1.18. Analysis.
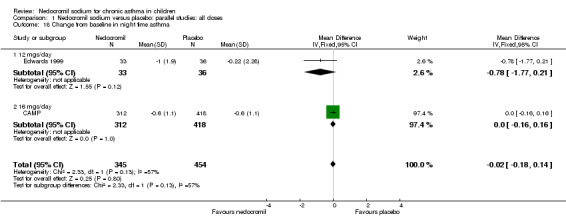
Comparison 1 Nedocromil sodium versus placebo: parallel studies: all doses, Outcome 18 Change from baseline in night time asthma.
1.19. Analysis.
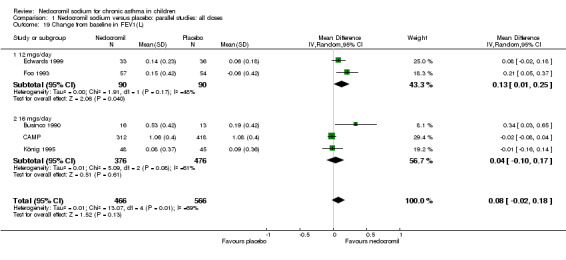
Comparison 1 Nedocromil sodium versus placebo: parallel studies: all doses, Outcome 19 Change from baseline in FEV1(L).
1.20. Analysis.
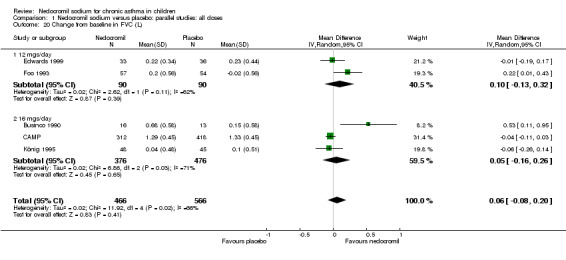
Comparison 1 Nedocromil sodium versus placebo: parallel studies: all doses, Outcome 20 Change from baseline in FVC (L).
1.21. Analysis.
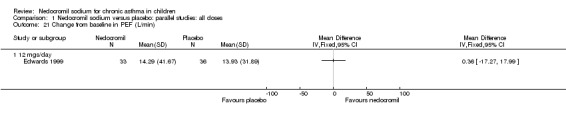
Comparison 1 Nedocromil sodium versus placebo: parallel studies: all doses, Outcome 21 Change from baseline in PEF (L/min).
1.22. Analysis.
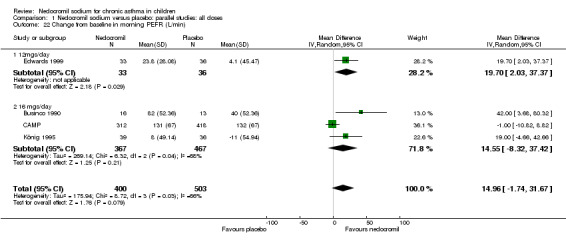
Comparison 1 Nedocromil sodium versus placebo: parallel studies: all doses, Outcome 22 Change from baseline in morning PEFR (L/min).
1.23. Analysis.
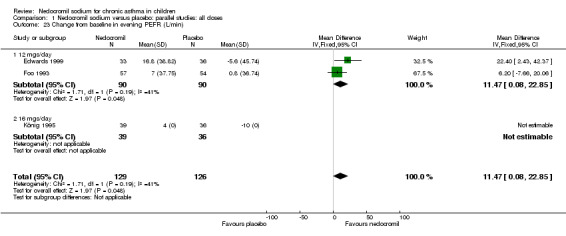
Comparison 1 Nedocromil sodium versus placebo: parallel studies: all doses, Outcome 23 Change from baseline in evening PEFR (L/min).
1.24. Analysis.
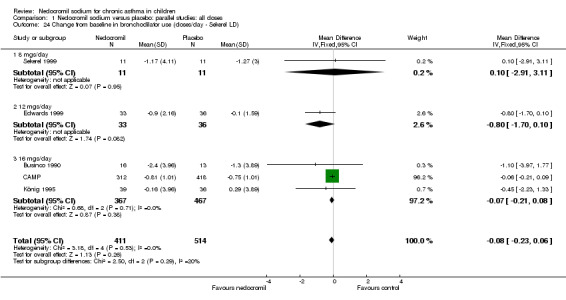
Comparison 1 Nedocromil sodium versus placebo: parallel studies: all doses, Outcome 24 Change from baseline in bronchodilator use (doses/day ‐ Sekerel LD).
1.25. Analysis.
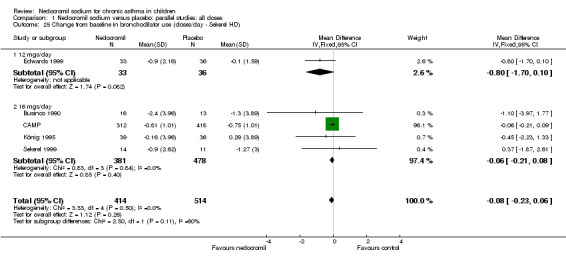
Comparison 1 Nedocromil sodium versus placebo: parallel studies: all doses, Outcome 25 Change from baseline in bronchodilator use (doses/day ‐ Sekerel HD).
1.26. Analysis.
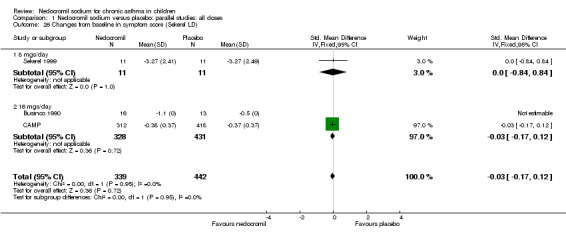
Comparison 1 Nedocromil sodium versus placebo: parallel studies: all doses, Outcome 26 Changes from baseline in symptom score (Sekerel LD).
1.27. Analysis.
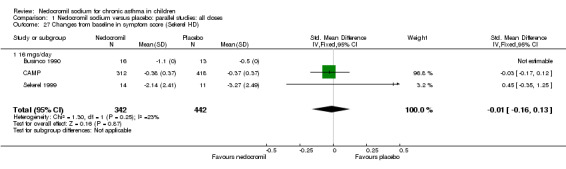
Comparison 1 Nedocromil sodium versus placebo: parallel studies: all doses, Outcome 27 Changes from baseline in symptom score (Sekerel HD).
1.28. Analysis.
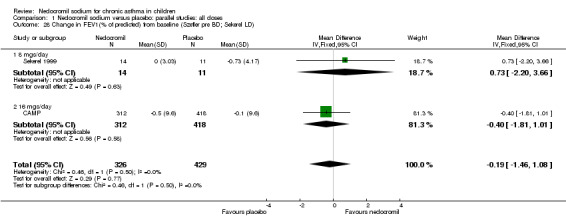
Comparison 1 Nedocromil sodium versus placebo: parallel studies: all doses, Outcome 28 Change in FEV1(% of predicted) from baseline (Szefler pre BD; Sekerel LD).
1.29. Analysis.
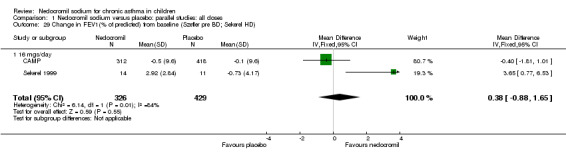
Comparison 1 Nedocromil sodium versus placebo: parallel studies: all doses, Outcome 29 Change in FEV1(% of predicted) from baseline (Szefler pre BD; Sekerel HD).
1.30. Analysis.
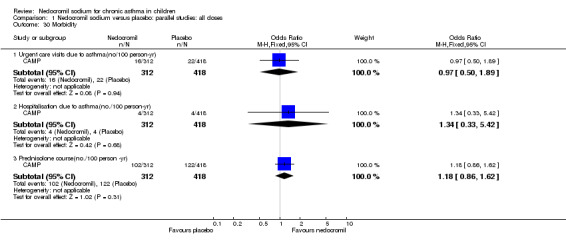
Comparison 1 Nedocromil sodium versus placebo: parallel studies: all doses, Outcome 30 Morbidity.
1.31. Analysis.
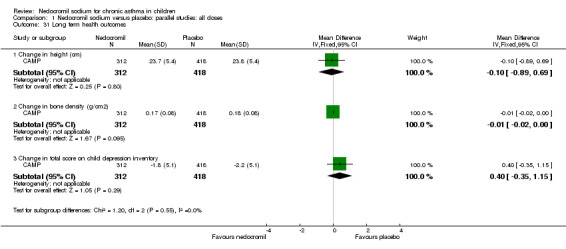
Comparison 1 Nedocromil sodium versus placebo: parallel studies: all doses, Outcome 31 Long term health outcomes.
1.32. Analysis.
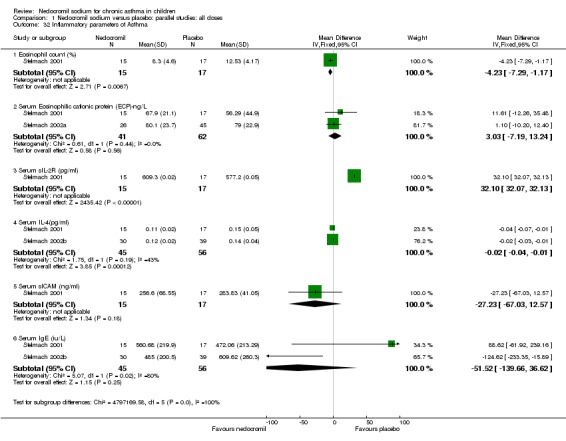
Comparison 1 Nedocromil sodium versus placebo: parallel studies: all doses, Outcome 32 Inflammatory parameters of Asthma.
1.33. Analysis.
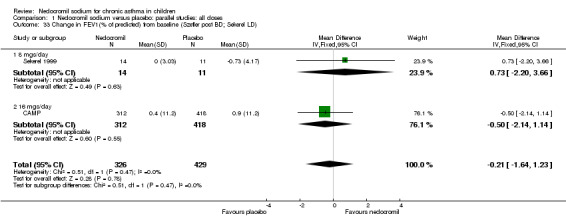
Comparison 1 Nedocromil sodium versus placebo: parallel studies: all doses, Outcome 33 Change in FEV1(% of predicted) from baseline (Szefler post BD; Sekerel LD).
1.34. Analysis.
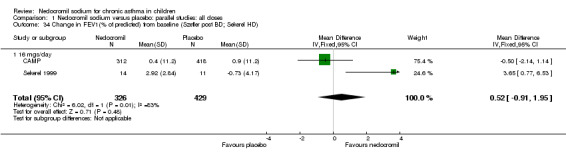
Comparison 1 Nedocromil sodium versus placebo: parallel studies: all doses, Outcome 34 Change in FEV1(% of predicted) from baseline (Szefler post BD; Sekerel HD).
Comparison 2. Nedocromil sodium versus placebo: parallel studies: all severities.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Daily asthma symptoms score | 5 | 270 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.44 [‐0.69, ‐0.19] |
| 1.1 Mild | 1 | 75 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.19 [‐0.26, 0.65] |
| 1.2 Mild to moderate | 1 | 23 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.25 [‐0.57, 1.08] |
| 1.3 Moderate | 3 | 172 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.86 [‐1.18, ‐0.54] |
| 1.4 Moderate to severe | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Daily use of beta 2 agonists (puffs/day) | 2 | 43 | Mean Difference (IV, Fixed, 95% CI) | ‐0.96 [‐1.44, ‐0.48] |
| 2.1 Mild | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 Mild to moderate | 2 | 43 | Mean Difference (IV, Fixed, 95% CI) | ‐0.96 [‐1.44, ‐0.48] |
| 2.3 Moderate | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.4 Moderate to severe | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Methacholine hyperresponsiveness | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 Mild | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Mild to Moderate | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 Moderate | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.4 Moderate to severe | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Histamine bronchial hyperresponsiveness | 3 | 172 | Mean Difference (IV, Fixed, 95% CI) | 0.44 [0.14, 0.73] |
| 4.1 Mild | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 Mild to moderate | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.3 Moderate | 3 | 172 | Mean Difference (IV, Fixed, 95% CI) | 0.44 [0.14, 0.73] |
| 4.4 Moderate to severe | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Withdrawal due to asthma exacerbations | 5 | 344 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.29, 1.81] |
| 5.1 Mild | 1 | 79 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.13, 1.77] |
| 5.2 Mild to Moderate | 3 | 236 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.27 [0.28, 5.82] |
| 5.3 Moderate | 0 | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.4 Moderate to severe | 1 | 29 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.8 [0.05, 14.16] |
| 6 Headache | 3 | 194 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.81 [0.94, 8.33] |
| 6.1 Mild | 2 | 165 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.49 [1.05, 11.58] |
| 6.2 Mild to moderate | 0 | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.3 Moderate | 0 | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.4 Moderate to Severe | 1 | 29 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.8 [0.05, 14.16] |
| 7 Parental assessment of efficacy | 4 | 294 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.94 [1.21, 3.10] |
| 7.1 Mild | 2 | 154 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.55 [0.81, 2.97] |
| 7.2 Mild to moderate | 1 | 111 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.99 [0.93, 4.27] |
| 7.3 Moderate | 0 | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7.4 Moderate to Severe | 1 | 29 | Odds Ratio (M‐H, Fixed, 95% CI) | 6.93 [1.29, 37.22] |
| 8 Clinician assessment of efficacy | 3 | 183 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.60 [0.89, 2.89] |
| 8.1 Mild | 2 | 154 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.61 [0.85, 3.04] |
| 8.2 Mild to moderate | 0 | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8.3 Moderate | 0 | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8.4 Moderate to Severe | 1 | 29 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.6 [0.36, 7.07] |
| 9 Change from baseline in FVC (L) | 5 | 1032 | Mean Difference (IV, Random, 95% CI) | 0.06 [‐0.09, 0.21] |
| 9.1 Mild | 2 | 162 | Mean Difference (IV, Random, 95% CI) | ‐0.03 [‐0.17, 0.10] |
| 9.2 Mild‐ moderate | 2 | 841 | Mean Difference (IV, Random, 95% CI) | 0.06 [‐0.22, 0.34] |
| 9.3 Moderate ‐ severe | 1 | 29 | Mean Difference (IV, Random, 95% CI) | 0.53 [0.11, 0.95] |
| 10 Bronchial hyperresponsiveness(PD10‐UNDW) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 10.1 Mild | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10.2 Mild‐moderate | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10.3 Moderate‐severe | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 11 FEV1 (% predicted) | 4 | 195 | Mean Difference (IV, Fixed, 95% CI) | 14.05 [12.45, 15.64] |
| 11.1 Mild | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11.2 Mild‐ moderate | 1 | 23 | Mean Difference (IV, Fixed, 95% CI) | ‐3.0 [‐12.89, 6.89] |
| 11.3 Moderate | 3 | 172 | Mean Difference (IV, Fixed, 95% CI) | 14.50 [12.88, 16.12] |
| 11.4 Moderate‐severe | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12 Change in FEV1 (L) | 5 | 1032 | Mean Difference (IV, Random, 95% CI) | 0.08 [‐0.02, 0.18] |
| 12.1 Mild | 2 | 162 | Mean Difference (IV, Random, 95% CI) | 0.05 [‐0.03, 0.13] |
| 12.2 Mild‐moderate | 2 | 841 | Mean Difference (IV, Random, 95% CI) | 0.08 [‐0.14, 0.31] |
| 12.3 Moderate | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12.4 Moderate‐severe | 1 | 29 | Mean Difference (IV, Random, 95% CI) | 0.34 [0.03, 0.65] |
| 13 Change from baseline in morning PEFR (L/min) | 4 | 903 | Mean Difference (IV, Random, 95% CI) | 14.96 [‐1.74, 31.67] |
| 13.1 Mild | 2 | 144 | Mean Difference (IV, Random, 95% CI) | 19.45 [5.29, 33.61] |
| 13.2 Mild‐moderate | 1 | 730 | Mean Difference (IV, Random, 95% CI) | ‐1.0 [‐10.82, 8.82] |
| 13.3 Moderate | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13.4 Moderate‐severe | 1 | 29 | Mean Difference (IV, Random, 95% CI) | 42.0 [3.68, 80.32] |
| 14 Change from baseline in evening PEFR(L/min) | 3 | 255 | Mean Difference (IV, Fixed, 95% CI) | 11.47 [0.08, 22.85] |
| 14.1 Mild | 2 | 144 | Mean Difference (IV, Fixed, 95% CI) | 22.4 [2.43, 42.37] |
| 14.2 Mild‐moderate | 1 | 111 | Mean Difference (IV, Fixed, 95% CI) | 6.2 [‐7.66, 20.06] |
| 14.3 Moderate | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 14.4 Moderate‐severe | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 15 Change from baseline in daily beta‐agonist use (puffs/day) | 5 | 925 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.25, 0.04] |
| 15.1 Mild | 2 | 144 | Mean Difference (IV, Fixed, 95% CI) | ‐0.73 [‐1.53, 0.08] |
| 15.2 Mild‐moderate | 2 | 752 | Mean Difference (IV, Fixed, 95% CI) | ‐0.06 [‐0.21, 0.09] |
| 15.3 Moderate | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 15.4 Moderate‐severe | 1 | 29 | Mean Difference (IV, Fixed, 95% CI) | ‐1.2 [‐2.29, ‐0.11] |
| 16 Change from baseline in daily symptom scores | 3 | 781 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.03 [‐0.17, 0.12] |
| 16.1 Mild | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 16.2 Mild‐moderate | 2 | 752 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.03 [‐0.17, 0.12] |
| 16.3 Moderate | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 16.4 Moderate‐severe | 1 | 29 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 17 Change in FEV1 (% predicted) | 2 | 755 | Mean Difference (IV, Fixed, 95% CI) | ‐0.19 [‐1.46, 1.08] |
| 17.1 Mild | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 17.2 Mild‐moderate | 2 | 755 | Mean Difference (IV, Fixed, 95% CI) | ‐0.19 [‐1.46, 1.08] |
| 17.3 Moderate | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 17.4 Moderate‐severe | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 18 Unpleasant taste | 3 | 268 | Odds Ratio (M‐H, Fixed, 95% CI) | 4.72 [1.17, 18.95] |
| 18.1 Mild | 2 | 148 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.02 [0.59, 15.54] |
| 18.2 Mild to moderate | 1 | 120 | Odds Ratio (M‐H, Fixed, 95% CI) | 11.58 [0.63, 214.32] |
| 18.3 Moderate | 0 | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 18.4 Moderate to Severe | 0 | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 19 Sore thoat | 2 | 84 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.22, 6.76] |
| 19.1 Mild | 1 | 55 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.12, 76.91] |
| 19.2 Mild to moderate | 0 | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 19.3 Moderate | 0 | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 19.4 Moderate to Severe | 1 | 29 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.09, 6.50] |
| 20 Other systemic effects | 4 | 314 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.73 [0.50, 5.99] |
| 20.1 Mild | 2 | 165 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.19, 6.14] |
| 20.2 Mild to moderate | 1 | 120 | Odds Ratio (M‐H, Fixed, 95% CI) | 7.12 [0.36, 140.88] |
| 20.3 Moderate | 0 | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 20.4 Moderate to Severe | 1 | 29 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.8 [0.05, 14.16] |
| 21 Side effects | 2 | 108 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.32 [0.37, 4.69] |
| 21.1 Mild | 1 | 79 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.43 [0.34, 34.48] |
| 21.2 Mild to moderate | 0 | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 21.3 Moderate | 0 | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 21.4 Moderate to Severe | 1 | 29 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.15, 3.84] |
| 22 Change from baseline in night time asthma | 2 | 799 | Mean Difference (IV, Fixed, 95% CI) | ‐0.02 [‐0.18, 0.14] |
| 22.1 Mild | 1 | 69 | Mean Difference (IV, Fixed, 95% CI) | ‐0.78 [‐1.77, 0.21] |
| 22.2 Mild‐moderate | 1 | 730 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.16, 0.16] |
| 22.3 Moderate | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 22.4 Moderate‐severe | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
2.1. Analysis.
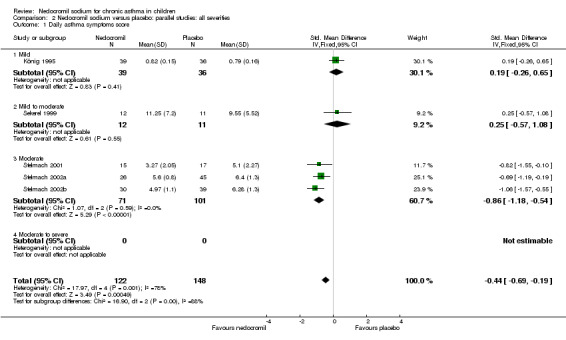
Comparison 2 Nedocromil sodium versus placebo: parallel studies: all severities, Outcome 1 Daily asthma symptoms score.
2.2. Analysis.
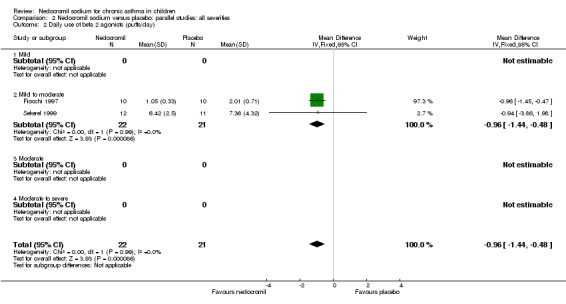
Comparison 2 Nedocromil sodium versus placebo: parallel studies: all severities, Outcome 2 Daily use of beta 2 agonists (puffs/day).
2.3. Analysis.
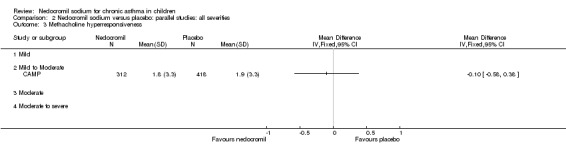
Comparison 2 Nedocromil sodium versus placebo: parallel studies: all severities, Outcome 3 Methacholine hyperresponsiveness.
2.4. Analysis.
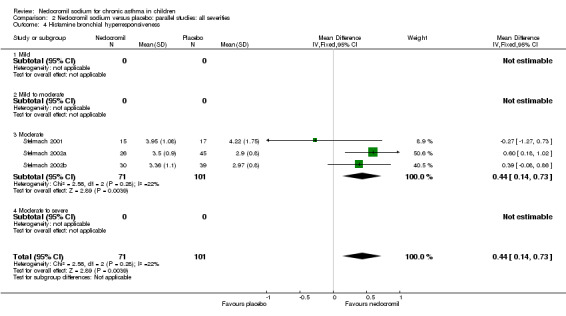
Comparison 2 Nedocromil sodium versus placebo: parallel studies: all severities, Outcome 4 Histamine bronchial hyperresponsiveness.
2.5. Analysis.
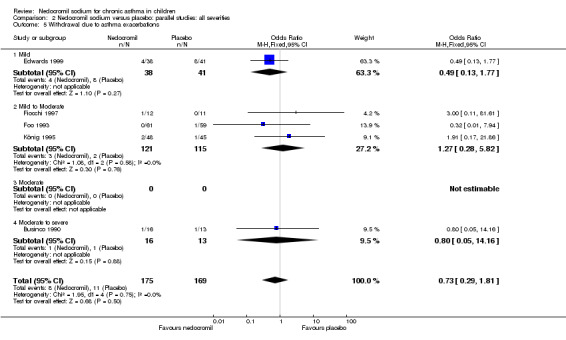
Comparison 2 Nedocromil sodium versus placebo: parallel studies: all severities, Outcome 5 Withdrawal due to asthma exacerbations.
2.6. Analysis.
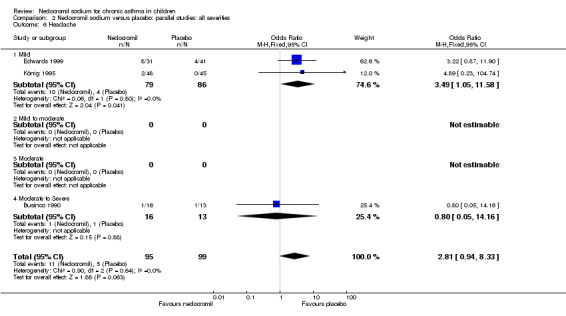
Comparison 2 Nedocromil sodium versus placebo: parallel studies: all severities, Outcome 6 Headache.
2.7. Analysis.
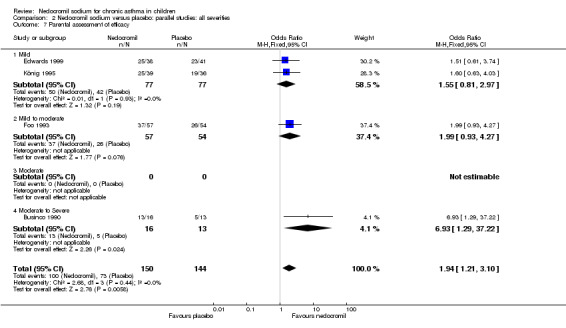
Comparison 2 Nedocromil sodium versus placebo: parallel studies: all severities, Outcome 7 Parental assessment of efficacy.
2.8. Analysis.
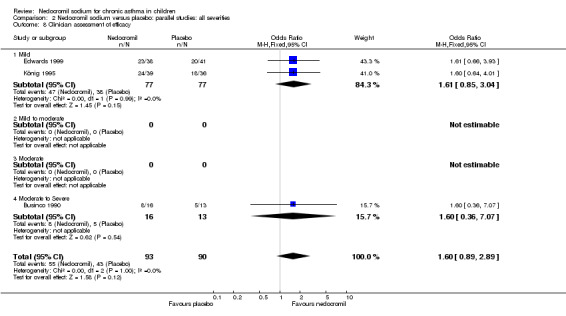
Comparison 2 Nedocromil sodium versus placebo: parallel studies: all severities, Outcome 8 Clinician assessment of efficacy.
2.9. Analysis.
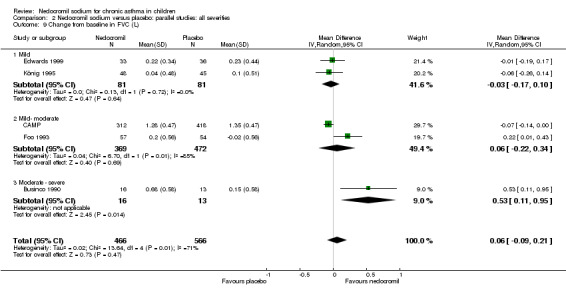
Comparison 2 Nedocromil sodium versus placebo: parallel studies: all severities, Outcome 9 Change from baseline in FVC (L).
2.10. Analysis.
Comparison 2 Nedocromil sodium versus placebo: parallel studies: all severities, Outcome 10 Bronchial hyperresponsiveness(PD10‐UNDW).
2.11. Analysis.
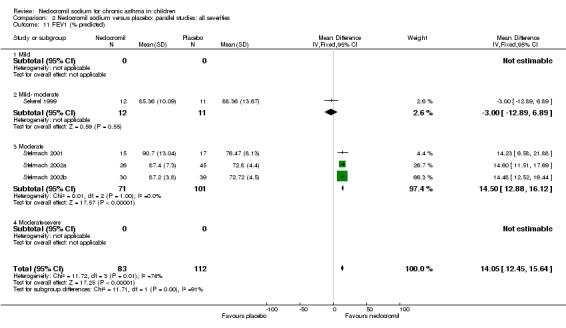
Comparison 2 Nedocromil sodium versus placebo: parallel studies: all severities, Outcome 11 FEV1 (% predicted).
2.12. Analysis.
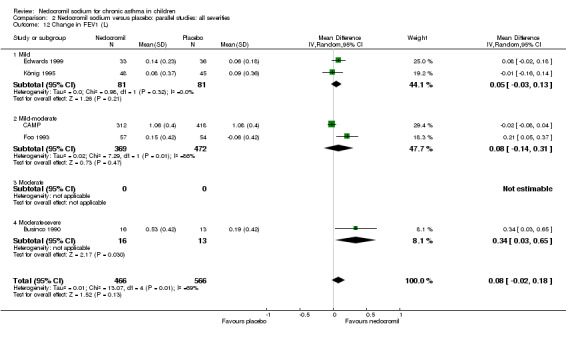
Comparison 2 Nedocromil sodium versus placebo: parallel studies: all severities, Outcome 12 Change in FEV1 (L).
2.13. Analysis.
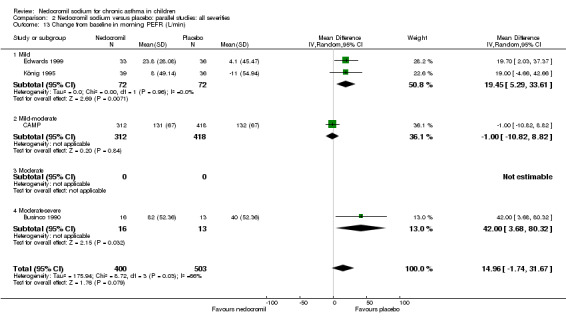
Comparison 2 Nedocromil sodium versus placebo: parallel studies: all severities, Outcome 13 Change from baseline in morning PEFR (L/min).
2.14. Analysis.
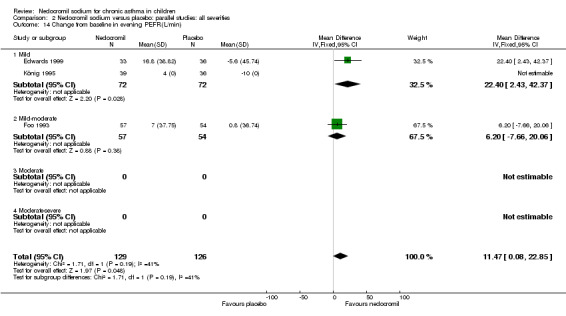
Comparison 2 Nedocromil sodium versus placebo: parallel studies: all severities, Outcome 14 Change from baseline in evening PEFR(L/min).
2.15. Analysis.
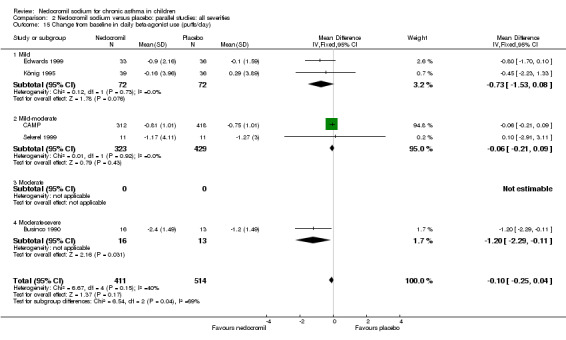
Comparison 2 Nedocromil sodium versus placebo: parallel studies: all severities, Outcome 15 Change from baseline in daily beta‐agonist use (puffs/day).
2.16. Analysis.
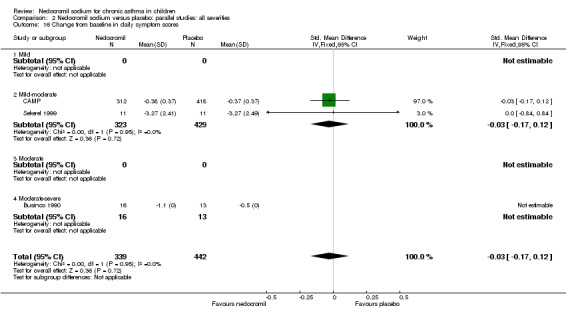
Comparison 2 Nedocromil sodium versus placebo: parallel studies: all severities, Outcome 16 Change from baseline in daily symptom scores.
2.17. Analysis.
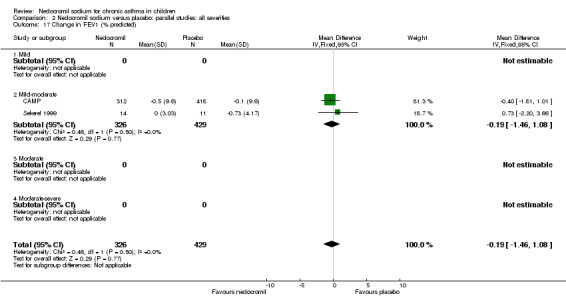
Comparison 2 Nedocromil sodium versus placebo: parallel studies: all severities, Outcome 17 Change in FEV1 (% predicted).
2.18. Analysis.
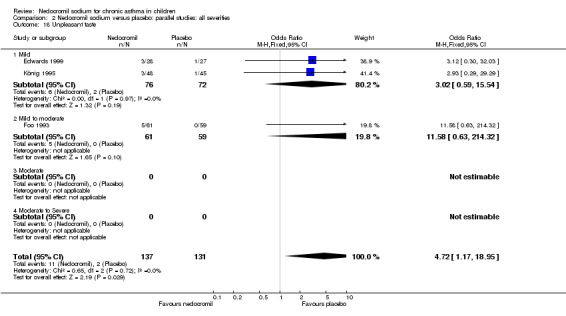
Comparison 2 Nedocromil sodium versus placebo: parallel studies: all severities, Outcome 18 Unpleasant taste.
2.19. Analysis.
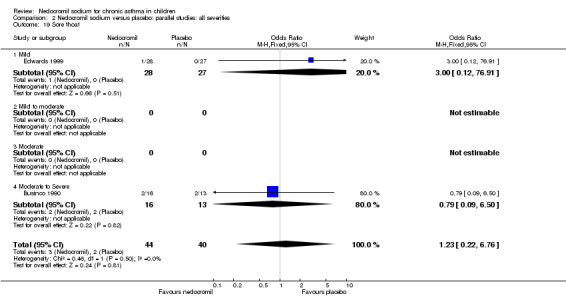
Comparison 2 Nedocromil sodium versus placebo: parallel studies: all severities, Outcome 19 Sore thoat.
2.20. Analysis.
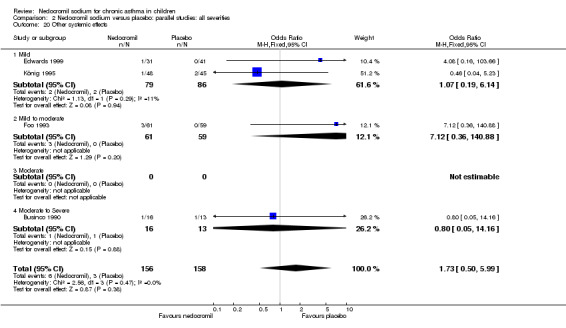
Comparison 2 Nedocromil sodium versus placebo: parallel studies: all severities, Outcome 20 Other systemic effects.
2.21. Analysis.
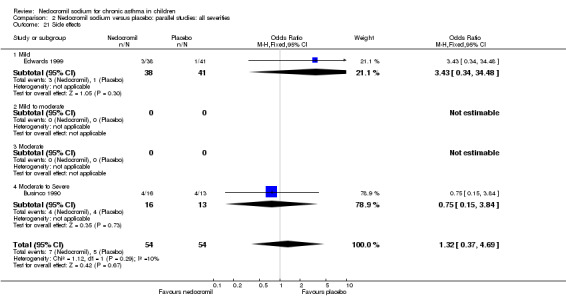
Comparison 2 Nedocromil sodium versus placebo: parallel studies: all severities, Outcome 21 Side effects.
2.22. Analysis.
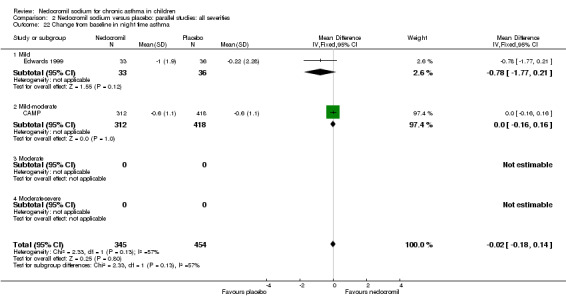
Comparison 2 Nedocromil sodium versus placebo: parallel studies: all severities, Outcome 22 Change from baseline in night time asthma.
Comparison 3. Nedocromil sodium versus placebo: parallel studies: all devices.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Daily asthma symptom score | 5 | 270 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.44 [‐0.99, 0.10] |
| 1.1 MDI | 4 | 195 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.65 [‐1.13, ‐0.17] |
| 1.2 MDI plus Spacer | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.3 Nebuliser | 1 | 75 | Std. Mean Difference (IV, Random, 95% CI) | 0.19 [‐0.26, 0.65] |
| 2 Daily use of beta 2 agonists (puffs/day) | 2 | 43 | Mean Difference (IV, Fixed, 95% CI) | ‐0.96 [‐1.44, ‐0.48] |
| 2.1 MDI | 2 | 43 | Mean Difference (IV, Fixed, 95% CI) | ‐0.96 [‐1.44, ‐0.48] |
| 2.2 MDI plus Spacer | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.3 Nebuliser | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Methacholine hyperresponsiveness | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 MDI | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 MDI plus Spacer | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 Nebuliser | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Histamine hyperresponsiveness | 3 | 172 | Mean Difference (IV, Fixed, 95% CI) | 0.44 [0.14, 0.73] |
| 4.1 MDI | 3 | 172 | Mean Difference (IV, Fixed, 95% CI) | 0.44 [0.14, 0.73] |
| 4.2 MDI plus Spacer | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.3 Nebuliser | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Withdrawal due to asthma exacerbations | 5 | 344 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.29, 1.81] |
| 5.1 MDI | 3 | 172 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.17, 4.63] |
| 5.2 MDI plus Spacer | 1 | 79 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.13, 1.77] |
| 5.3 Nebuliser | 1 | 93 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.91 [0.17, 21.86] |
| 6 Side effects | 2 | 108 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.32 [0.37, 4.69] |
| 6.1 MDI | 1 | 29 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.15, 3.84] |
| 6.2 MDI plus Spacer | 1 | 79 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.43 [0.34, 34.48] |
| 6.3 Nebuliser | 0 | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Parental assessment of efficacy | 4 | 294 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.94 [1.21, 3.10] |
| 7.1 MDI | 2 | 140 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.48 [1.25, 4.92] |
| 7.2 MDI plus Spacer | 1 | 79 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.51 [0.61, 3.74] |
| 7.3 Nebuliser | 1 | 75 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.60 [0.63, 4.03] |
| 8 Clinician assessment of efficacy | 3 | 183 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.60 [0.89, 2.89] |
| 8.1 MDI | 1 | 29 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.6 [0.36, 7.07] |
| 8.2 MDI plus Spacer | 1 | 79 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.61 [0.66, 3.93] |
| 8.3 Nebuliser | 1 | 75 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.6 [0.64, 4.01] |
| 9 Change from baseline in FVC (L) | 5 | 1032 | Mean Difference (IV, Random, 95% CI) | 0.06 [‐0.09, 0.21] |
| 9.1 MDI | 3 | 870 | Mean Difference (IV, Random, 95% CI) | 0.17 [‐0.13, 0.48] |
| 9.2 MDI plus spacer | 1 | 69 | Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.19, 0.17] |
| 9.3 Nebuliser | 1 | 93 | Mean Difference (IV, Random, 95% CI) | ‐0.06 [‐0.26, 0.14] |
| 10 Bronchial hyperesponsiveness | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 10.1 MDI | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10.2 MDI plus spacer | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10.3 Nebuliser | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 11 FEV1 (% predicted) | 4 | 197 | Mean Difference (IV, Fixed, 95% CI) | 14.02 [12.43, 15.62] |
| 11.1 MDI | 4 | 197 | Mean Difference (IV, Fixed, 95% CI) | 14.02 [12.43, 15.62] |
| 11.2 MDI plus Spacer | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11.3 Nebuliser | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12 Change in FEV1 (L) | 5 | 1032 | Mean Difference (IV, Random, 95% CI) | 0.08 [‐0.02, 0.18] |
| 12.1 MDI | 3 | 870 | Mean Difference (IV, Random, 95% CI) | 0.14 [‐0.07, 0.36] |
| 12.2 MDI plus spacer | 1 | 69 | Mean Difference (IV, Random, 95% CI) | 0.08 [‐0.02, 0.18] |
| 12.3 Nebuliser | 1 | 93 | Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.16, 0.14] |
| 13 Change from baseline in Morning PEFR (L/min) | 4 | 903 | Mean Difference (IV, Random, 95% CI) | 14.96 [‐1.74, 31.67] |
| 13.1 MDI | 2 | 759 | Mean Difference (IV, Random, 95% CI) | 16.35 [‐23.00, 57.69] |
| 13.2 MDI plus Spacer | 1 | 69 | Mean Difference (IV, Random, 95% CI) | 19.70 [2.03, 37.37] |
| 13.3 Nebuliser | 1 | 75 | Mean Difference (IV, Random, 95% CI) | 19.0 [‐4.66, 42.66] |
| 14 Change from baseline in Evening PEFR (L/min) | 3 | 255 | Mean Difference (IV, Fixed, 95% CI) | 11.47 [0.08, 22.85] |
| 14.1 MDI | 1 | 111 | Mean Difference (IV, Fixed, 95% CI) | 6.2 [‐7.66, 20.06] |
| 14.2 MDI plus Spacer | 1 | 69 | Mean Difference (IV, Fixed, 95% CI) | 22.4 [2.43, 42.37] |
| 14.3 Nebuliser | 1 | 75 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 15 Change from baseline in daily beta‐agonist use (puffs/day) | 5 | 925 | Mean Difference (IV, Fixed, 95% CI) | ‐0.06 [‐0.20, 0.08] |
| 15.1 MDI | 3 | 781 | Mean Difference (IV, Fixed, 95% CI) | ‐0.08 [‐0.23, 0.07] |
| 15.2 MDI plus spacer | 1 | 69 | Mean Difference (IV, Fixed, 95% CI) | 0.8 [‐0.10, 1.70] |
| 15.3 Nebuliser | 1 | 75 | Mean Difference (IV, Fixed, 95% CI) | ‐0.45 [‐2.23, 1.33] |
| 16 Change from baseline in daily symptom scores | 3 | 781 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.03 [‐0.17, 0.12] |
| 16.1 MDI | 3 | 781 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.03 [‐0.17, 0.12] |
| 17 Change in FEV1 (% predicted) | 2 | 755 | Mean Difference (IV, Fixed, 95% CI) | ‐0.19 [‐1.46, 1.08] |
| 17.1 MDI | 2 | 755 | Mean Difference (IV, Fixed, 95% CI) | ‐0.19 [‐1.46, 1.08] |
| 17.2 MDI plus spacer | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 17.3 Nebuliser | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 18 Other systemic effects | 4 | 314 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.73 [0.50, 5.99] |
| 18.1 MDI | 2 | 149 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.80 [0.43, 18.13] |
| 18.2 MDI + spacer | 1 | 72 | Odds Ratio (M‐H, Fixed, 95% CI) | 4.08 [0.16, 103.66] |
| 18.3 Nebuliser | 1 | 93 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.46 [0.04, 5.23] |
| 19 Sore thoat | 2 | 84 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.22, 6.76] |
| 19.1 MDI | 1 | 29 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.09, 6.50] |
| 19.2 MDI + spacer | 1 | 55 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.12, 76.91] |
| 19.3 Nebuliser | 0 | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 20 Unpleasant taste | 3 | 268 | Odds Ratio (M‐H, Fixed, 95% CI) | 4.72 [1.17, 18.95] |
| 20.1 MDI | 1 | 120 | Odds Ratio (M‐H, Fixed, 95% CI) | 11.58 [0.63, 214.32] |
| 20.2 MDI + spacer | 1 | 55 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.12 [0.30, 32.03] |
| 20.3 Nebuliser | 1 | 93 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.93 [0.29, 29.29] |
| 21 Headache | 3 | 194 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.81 [0.94, 8.33] |
| 21.1 MDI | 1 | 29 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.8 [0.05, 14.16] |
| 21.2 MDI + spacer | 1 | 72 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.22 [0.87, 11.90] |
| 21.3 Nebuliser | 1 | 93 | Odds Ratio (M‐H, Fixed, 95% CI) | 4.89 [0.23, 104.74] |
| 22 Change from baseline in night time asthma | 2 | 799 | Mean Difference (IV, Fixed, 95% CI) | ‐0.02 [‐0.18, 0.14] |
| 22.1 MDI | 1 | 730 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.16, 0.16] |
| 22.2 MDI plus spacer | 1 | 69 | Mean Difference (IV, Fixed, 95% CI) | ‐0.78 [‐1.77, 0.21] |
| 22.3 Nebuliser | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
3.1. Analysis.
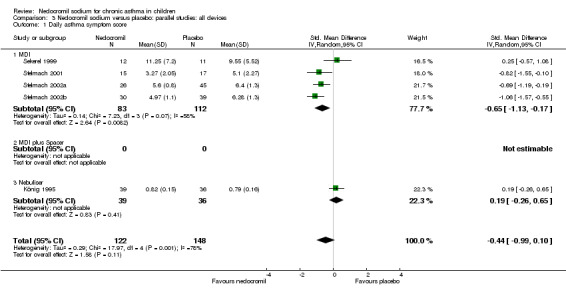
Comparison 3 Nedocromil sodium versus placebo: parallel studies: all devices, Outcome 1 Daily asthma symptom score.
3.2. Analysis.
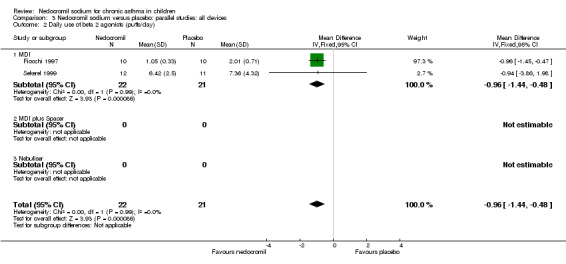
Comparison 3 Nedocromil sodium versus placebo: parallel studies: all devices, Outcome 2 Daily use of beta 2 agonists (puffs/day).
3.3. Analysis.
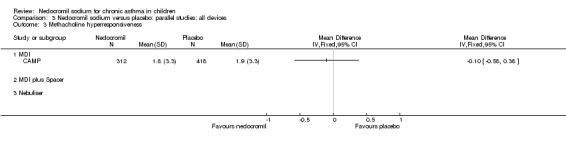
Comparison 3 Nedocromil sodium versus placebo: parallel studies: all devices, Outcome 3 Methacholine hyperresponsiveness.
3.4. Analysis.
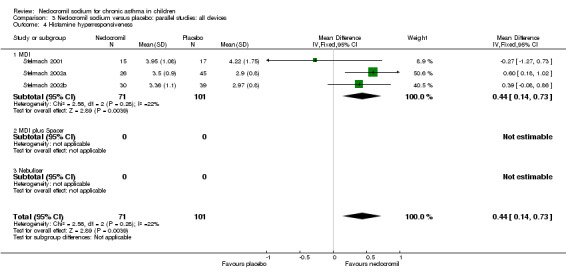
Comparison 3 Nedocromil sodium versus placebo: parallel studies: all devices, Outcome 4 Histamine hyperresponsiveness.
3.5. Analysis.
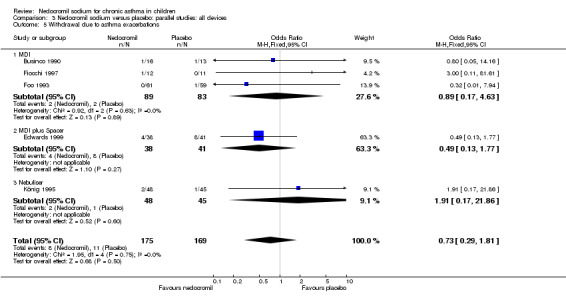
Comparison 3 Nedocromil sodium versus placebo: parallel studies: all devices, Outcome 5 Withdrawal due to asthma exacerbations.
3.6. Analysis.
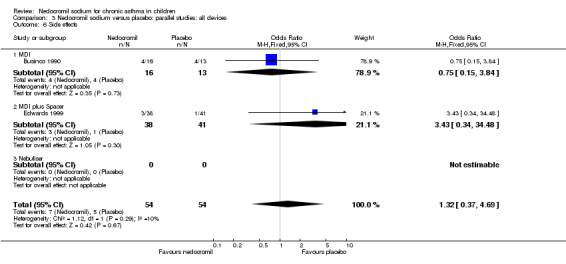
Comparison 3 Nedocromil sodium versus placebo: parallel studies: all devices, Outcome 6 Side effects.
3.7. Analysis.
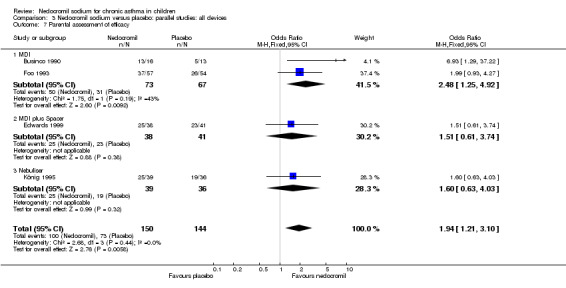
Comparison 3 Nedocromil sodium versus placebo: parallel studies: all devices, Outcome 7 Parental assessment of efficacy.
3.8. Analysis.
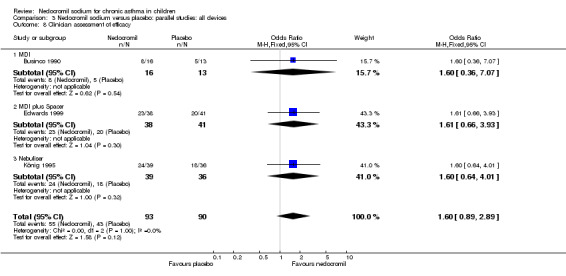
Comparison 3 Nedocromil sodium versus placebo: parallel studies: all devices, Outcome 8 Clinician assessment of efficacy.
3.9. Analysis.
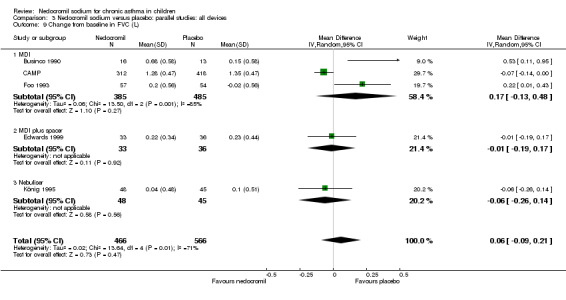
Comparison 3 Nedocromil sodium versus placebo: parallel studies: all devices, Outcome 9 Change from baseline in FVC (L).
3.10. Analysis.
Comparison 3 Nedocromil sodium versus placebo: parallel studies: all devices, Outcome 10 Bronchial hyperesponsiveness.
3.11. Analysis.
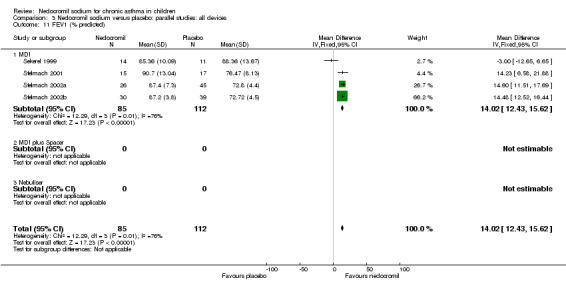
Comparison 3 Nedocromil sodium versus placebo: parallel studies: all devices, Outcome 11 FEV1 (% predicted).
3.12. Analysis.
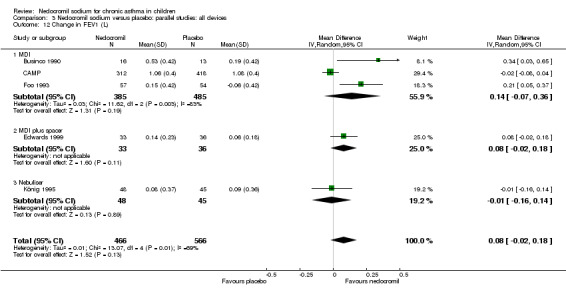
Comparison 3 Nedocromil sodium versus placebo: parallel studies: all devices, Outcome 12 Change in FEV1 (L).
3.13. Analysis.
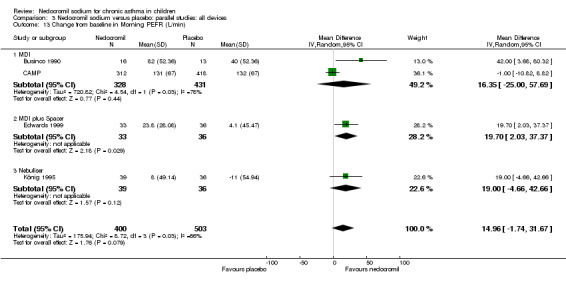
Comparison 3 Nedocromil sodium versus placebo: parallel studies: all devices, Outcome 13 Change from baseline in Morning PEFR (L/min).
3.14. Analysis.
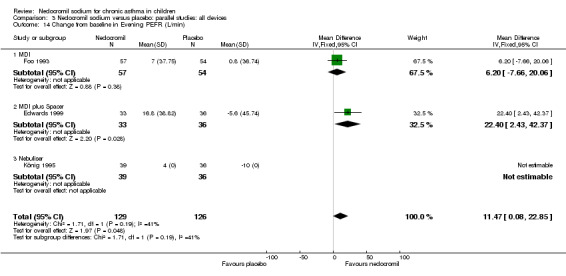
Comparison 3 Nedocromil sodium versus placebo: parallel studies: all devices, Outcome 14 Change from baseline in Evening PEFR (L/min).
3.15. Analysis.
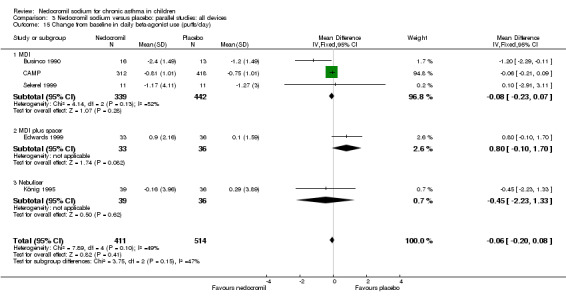
Comparison 3 Nedocromil sodium versus placebo: parallel studies: all devices, Outcome 15 Change from baseline in daily beta‐agonist use (puffs/day).
3.16. Analysis.
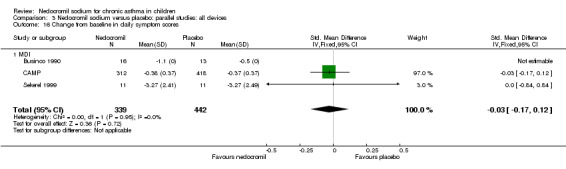
Comparison 3 Nedocromil sodium versus placebo: parallel studies: all devices, Outcome 16 Change from baseline in daily symptom scores.
3.17. Analysis.
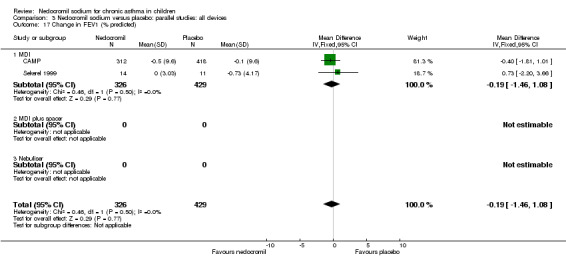
Comparison 3 Nedocromil sodium versus placebo: parallel studies: all devices, Outcome 17 Change in FEV1 (% predicted).
3.18. Analysis.
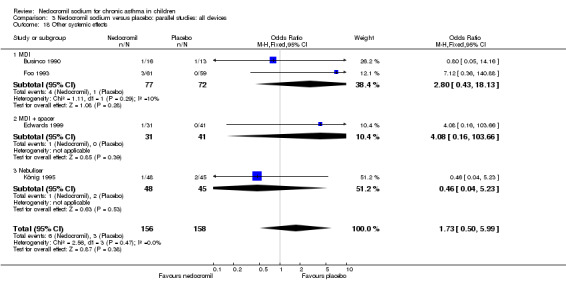
Comparison 3 Nedocromil sodium versus placebo: parallel studies: all devices, Outcome 18 Other systemic effects.
3.19. Analysis.
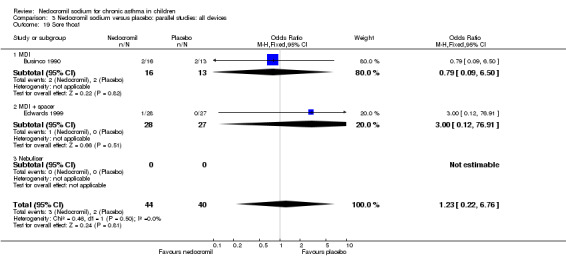
Comparison 3 Nedocromil sodium versus placebo: parallel studies: all devices, Outcome 19 Sore thoat.
3.20. Analysis.
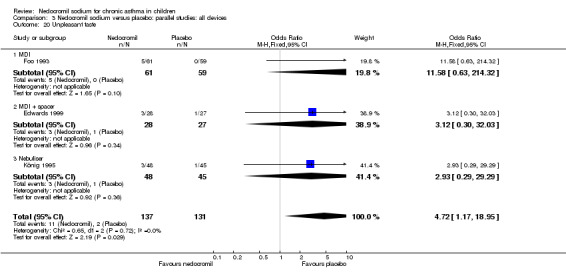
Comparison 3 Nedocromil sodium versus placebo: parallel studies: all devices, Outcome 20 Unpleasant taste.
3.21. Analysis.
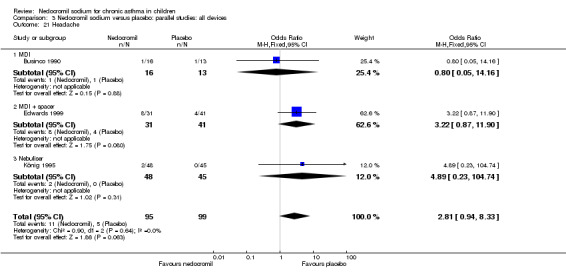
Comparison 3 Nedocromil sodium versus placebo: parallel studies: all devices, Outcome 21 Headache.
3.22. Analysis.
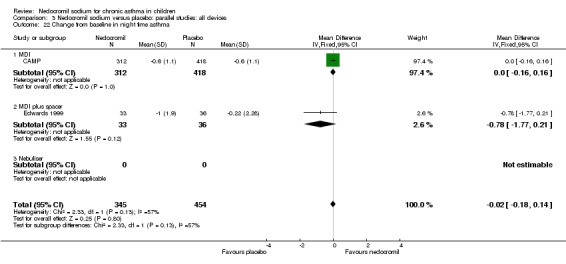
Comparison 3 Nedocromil sodium versus placebo: parallel studies: all devices, Outcome 22 Change from baseline in night time asthma.
Comparison 4. Nedocromil sodium versus placebo: parallel studies: all study durations.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Daily asthma symptom score | 5 | 270 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.44 [‐0.99, 0.10] |
| 1.1 1‐4 weeks | 3 | 172 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.86 [‐1.18, ‐0.54] |
| 1.2 1‐5 months | 1 | 23 | Std. Mean Difference (IV, Random, 95% CI) | 0.25 [‐0.57, 1.08] |
| 1.3 6 months or longer | 1 | 75 | Std. Mean Difference (IV, Random, 95% CI) | 0.19 [‐0.26, 0.65] |
| 2 Daily use of beta2 agonists | 2 | 43 | Mean Difference (IV, Fixed, 95% CI) | ‐0.96 [‐1.44, ‐0.48] |
| 2.1 1‐4 weeks | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 1‐5 months | 2 | 43 | Mean Difference (IV, Fixed, 95% CI) | ‐0.96 [‐1.44, ‐0.48] |
| 2.3 6 months or longer | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Methacholine hyperresponsiveness | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 1‐4 weeks | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 1‐5 months | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 6 months or longer | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Histamine hyperresponsiveness | 3 | 172 | Mean Difference (IV, Fixed, 95% CI) | 0.44 [0.14, 0.73] |
| 4.1 1‐4 weeks | 3 | 172 | Mean Difference (IV, Fixed, 95% CI) | 0.44 [0.14, 0.73] |
| 4.2 1‐5 months | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.3 6 months or longer | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Withdrawal due to asthma exacerbations | 4 | 224 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.30, 2.07] |
| 5.1 1‐4 weeks | 1 | 29 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.8 [0.05, 14.16] |
| 5.2 1‐5 months | 2 | 102 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.20, 2.03] |
| 5.3 6 months or longer | 1 | 93 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.91 [0.17, 21.86] |
| 6 Side effects | 2 | 108 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.32 [0.37, 4.69] |
| 6.1 1‐4 weeks | 1 | 29 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.15, 3.84] |
| 6.2 1‐4 months | 1 | 79 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.43 [0.34, 34.48] |
| 6.3 6 months or longer | 0 | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Parental assessment of efficacy | 4 | 294 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.94 [1.21, 3.10] |
| 7.1 1‐4 weeks | 1 | 29 | Odds Ratio (M‐H, Fixed, 95% CI) | 6.93 [1.29, 37.22] |
| 7.2 1‐4 months | 2 | 190 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.77 [0.99, 3.18] |
| 7.3 6 months or longer | 1 | 75 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.60 [0.63, 4.03] |
| 8 Clinician assessment of efficacy | 3 | 183 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.60 [0.89, 2.89] |
| 8.1 1‐4 weeks | 1 | 29 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.6 [0.36, 7.07] |
| 8.2 1‐4 months | 1 | 79 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.61 [0.66, 3.93] |
| 8.3 6 months or longer | 1 | 75 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.6 [0.64, 4.01] |
| 9 Change from baseline in FVC (L) | 5 | 1032 | Mean Difference (IV, Random, 95% CI) | 0.06 [‐0.09, 0.21] |
| 9.1 1‐4 weeks | 1 | 29 | Mean Difference (IV, Random, 95% CI) | 0.53 [0.11, 0.95] |
| 9.2 1‐5 months | 2 | 180 | Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.13, 0.32] |
| 9.3 6 months or greater | 2 | 823 | Mean Difference (IV, Random, 95% CI) | ‐0.07 [‐0.13, ‐0.00] |
| 10 Bronchial hyperesponsiveness (PD10‐UNDW) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 10.1 1‐4 weeks | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10.2 1‐5 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10.3 6 months or greater | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 11 FEV1(% Predicted) | 4 | 197 | Mean Difference (IV, Fixed, 95% CI) | 14.02 [12.43, 15.62] |
| 11.1 1‐4 weeks | 3 | 172 | Mean Difference (IV, Fixed, 95% CI) | 14.50 [12.88, 16.12] |
| 11.2 1‐5 months | 1 | 25 | Mean Difference (IV, Fixed, 95% CI) | ‐3.0 [‐12.65, 6.65] |
| 11.3 6 months or longer | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12 Change in FEV1 (% predicted) | 2 | 755 | Mean Difference (IV, Fixed, 95% CI) | ‐0.19 [‐1.46, 1.08] |
| 12.1 1‐4 weeks | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12.2 1‐5 months | 1 | 25 | Mean Difference (IV, Fixed, 95% CI) | 0.73 [‐2.20, 3.66] |
| 12.3 6 months or longer | 1 | 730 | Mean Difference (IV, Fixed, 95% CI) | ‐0.4 [‐1.81, 1.01] |
| 13 Change in FEV1 (L) | 5 | 1032 | Mean Difference (IV, Random, 95% CI) | 0.08 [‐0.02, 0.18] |
| 13.1 1‐4 weeks | 1 | 29 | Mean Difference (IV, Random, 95% CI) | 0.34 [0.03, 0.65] |
| 13.2 1‐5 months | 2 | 180 | Mean Difference (IV, Random, 95% CI) | 0.13 [0.01, 0.25] |
| 13.3 6 months or greater | 2 | 823 | Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.07, 0.04] |
| 14 Change from baseline in morning PEFR (L/min) | 4 | 903 | Mean Difference (IV, Random, 95% CI) | 14.96 [‐1.74, 31.67] |
| 14.1 1‐4 weeks | 1 | 29 | Mean Difference (IV, Random, 95% CI) | 42.0 [3.68, 80.32] |
| 14.2 1‐5 months | 1 | 69 | Mean Difference (IV, Random, 95% CI) | 19.70 [2.03, 37.37] |
| 14.3 6 months or longer | 2 | 805 | Mean Difference (IV, Random, 95% CI) | 5.98 [‐12.70, 24.67] |
| 15 Change from baseline evening PEFR (L/min) | 3 | 209 | Mean Difference (IV, Fixed, 95% CI) | 11.47 [0.08, 22.85] |
| 15.1 1‐4 weeks | 1 | 29 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 15.2 1‐5 months | 2 | 180 | Mean Difference (IV, Fixed, 95% CI) | 11.47 [0.08, 22.85] |
| 15.3 6 months or longer | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 16 Change in daily beta‐agonist use | 5 | 925 | Mean Difference (IV, Random, 95% CI) | ‐0.11 [‐0.72, 0.51] |
| 16.1 1‐4 weeks | 1 | 29 | Mean Difference (IV, Random, 95% CI) | ‐1.2 [‐2.29, ‐0.11] |
| 16.2 1‐5 months | 2 | 91 | Mean Difference (IV, Random, 95% CI) | 0.74 [‐0.12, 1.61] |
| 16.3 6 months or longer | 2 | 805 | Mean Difference (IV, Random, 95% CI) | ‐0.06 [‐0.21, 0.08] |
| 17 Change from baseline in daily symptom scores | 3 | 781 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.03 [‐0.17, 0.12] |
| 17.1 1‐4 weeks | 1 | 29 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 17.2 1‐5 months | 1 | 22 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.84, 0.84] |
| 17.3 6 months or longer | 1 | 730 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.03 [‐0.17, 0.12] |
| 18 Other systemic effects | 4 | 314 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.73 [0.50, 5.99] |
| 18.1 1‐4 weeks | 1 | 29 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.8 [0.05, 14.16] |
| 18.2 1‐5 months | 2 | 192 | Odds Ratio (M‐H, Fixed, 95% CI) | 5.72 [0.64, 50.69] |
| 18.3 6 months or greater | 1 | 93 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.46 [0.04, 5.23] |
| 19 Sore thoat | 2 | 84 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.22, 6.76] |
| 19.1 1‐4 weeks | 1 | 29 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.09, 6.50] |
| 19.2 1‐5 months | 1 | 55 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.12, 76.91] |
| 19.3 6 months or greater | 0 | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 20 Unpleasant taste | 3 | 268 | Odds Ratio (M‐H, Fixed, 95% CI) | 4.72 [1.17, 18.95] |
| 20.1 1‐4 weeks | 0 | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 20.2 1‐5 months | 2 | 175 | Odds Ratio (M‐H, Fixed, 95% CI) | 5.98 [1.02, 35.04] |
| 20.3 6 months or greater | 1 | 93 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.93 [0.29, 29.29] |
| 21 Headache | 3 | 194 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.81 [0.94, 8.33] |
| 21.1 1‐4 weeks | 1 | 29 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.8 [0.05, 14.16] |
| 21.2 1‐5 months | 1 | 72 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.22 [0.87, 11.90] |
| 21.3 6 months or greater | 1 | 93 | Odds Ratio (M‐H, Fixed, 95% CI) | 4.89 [0.23, 104.74] |
| 22 Change from baseline in night time asthma | 2 | 799 | Mean Difference (IV, Fixed, 95% CI) | ‐0.02 [‐0.18, 0.14] |
| 22.1 1‐4 weeks | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 22.2 1‐5 months | 1 | 69 | Mean Difference (IV, Fixed, 95% CI) | ‐0.78 [‐1.77, 0.21] |
| 22.3 6 months or longer | 1 | 730 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.16, 0.16] |
4.1. Analysis.
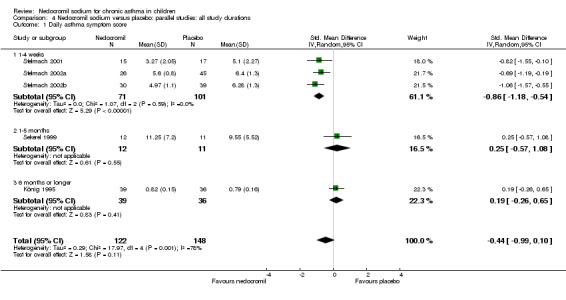
Comparison 4 Nedocromil sodium versus placebo: parallel studies: all study durations, Outcome 1 Daily asthma symptom score.
4.2. Analysis.
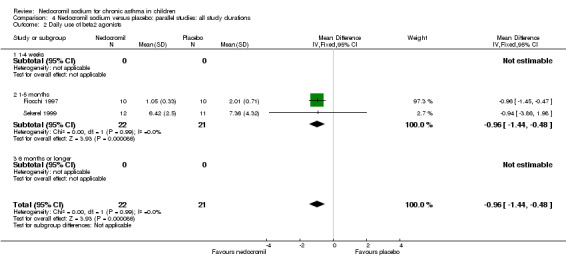
Comparison 4 Nedocromil sodium versus placebo: parallel studies: all study durations, Outcome 2 Daily use of beta2 agonists.
4.3. Analysis.
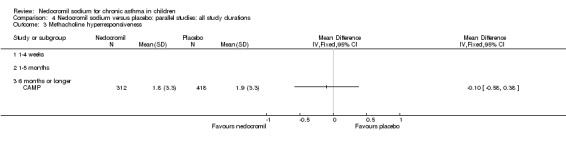
Comparison 4 Nedocromil sodium versus placebo: parallel studies: all study durations, Outcome 3 Methacholine hyperresponsiveness.
4.4. Analysis.
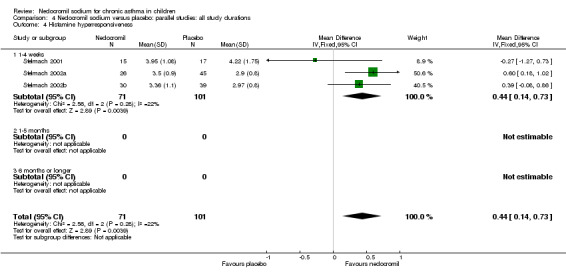
Comparison 4 Nedocromil sodium versus placebo: parallel studies: all study durations, Outcome 4 Histamine hyperresponsiveness.
4.5. Analysis.
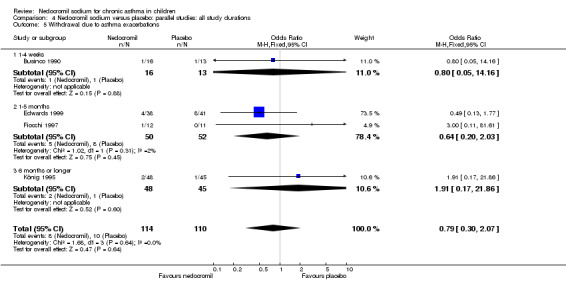
Comparison 4 Nedocromil sodium versus placebo: parallel studies: all study durations, Outcome 5 Withdrawal due to asthma exacerbations.
4.6. Analysis.
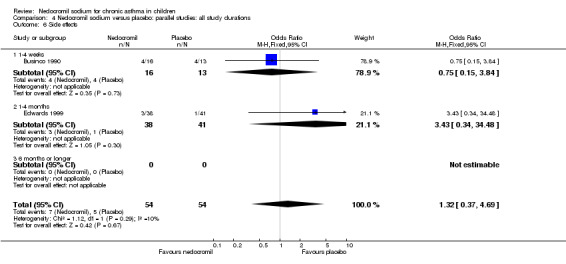
Comparison 4 Nedocromil sodium versus placebo: parallel studies: all study durations, Outcome 6 Side effects.
4.7. Analysis.
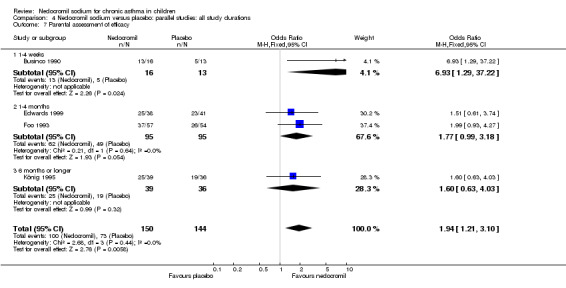
Comparison 4 Nedocromil sodium versus placebo: parallel studies: all study durations, Outcome 7 Parental assessment of efficacy.
4.8. Analysis.
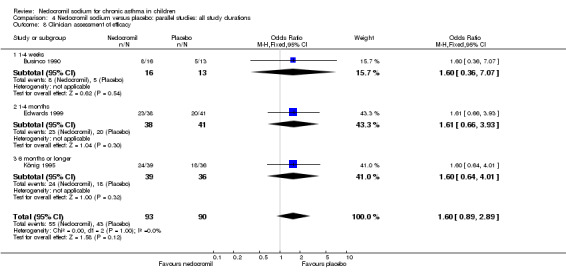
Comparison 4 Nedocromil sodium versus placebo: parallel studies: all study durations, Outcome 8 Clinician assessment of efficacy.
4.9. Analysis.
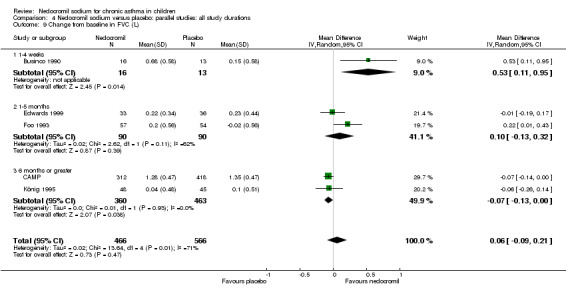
Comparison 4 Nedocromil sodium versus placebo: parallel studies: all study durations, Outcome 9 Change from baseline in FVC (L).
4.10. Analysis.
Comparison 4 Nedocromil sodium versus placebo: parallel studies: all study durations, Outcome 10 Bronchial hyperesponsiveness (PD10‐UNDW).
4.11. Analysis.
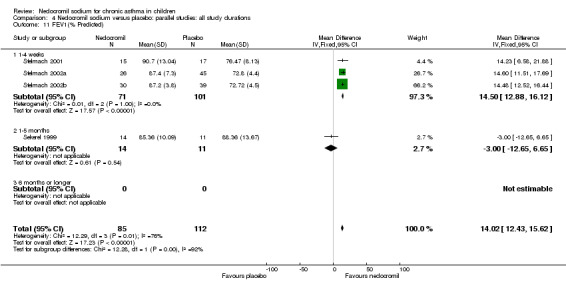
Comparison 4 Nedocromil sodium versus placebo: parallel studies: all study durations, Outcome 11 FEV1(% Predicted).
4.12. Analysis.
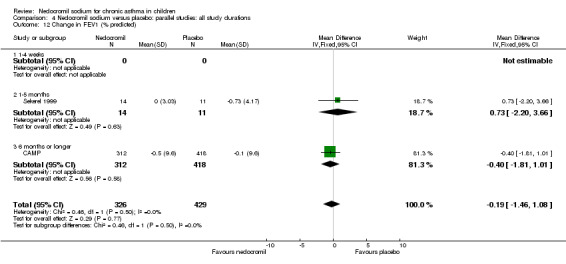
Comparison 4 Nedocromil sodium versus placebo: parallel studies: all study durations, Outcome 12 Change in FEV1 (% predicted).
4.13. Analysis.
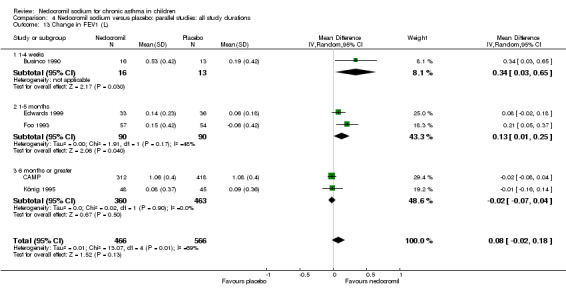
Comparison 4 Nedocromil sodium versus placebo: parallel studies: all study durations, Outcome 13 Change in FEV1 (L).
4.14. Analysis.
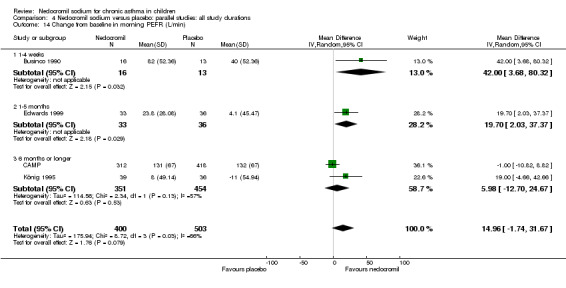
Comparison 4 Nedocromil sodium versus placebo: parallel studies: all study durations, Outcome 14 Change from baseline in morning PEFR (L/min).
4.15. Analysis.
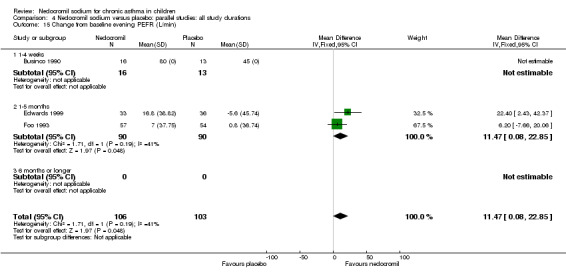
Comparison 4 Nedocromil sodium versus placebo: parallel studies: all study durations, Outcome 15 Change from baseline evening PEFR (L/min).
4.16. Analysis.
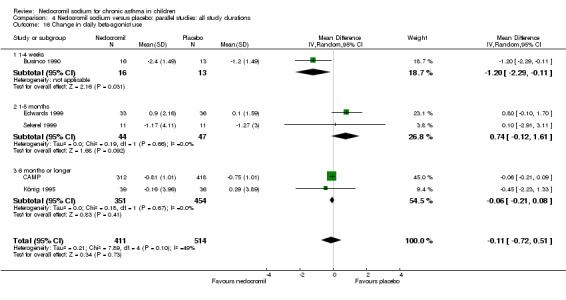
Comparison 4 Nedocromil sodium versus placebo: parallel studies: all study durations, Outcome 16 Change in daily beta‐agonist use.
4.17. Analysis.
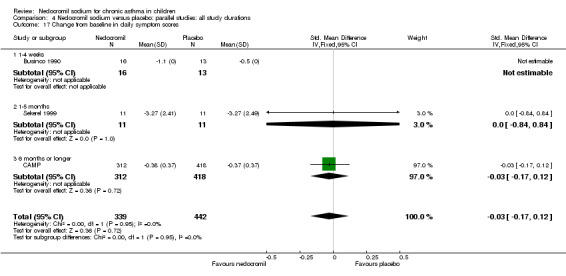
Comparison 4 Nedocromil sodium versus placebo: parallel studies: all study durations, Outcome 17 Change from baseline in daily symptom scores.
4.18. Analysis.
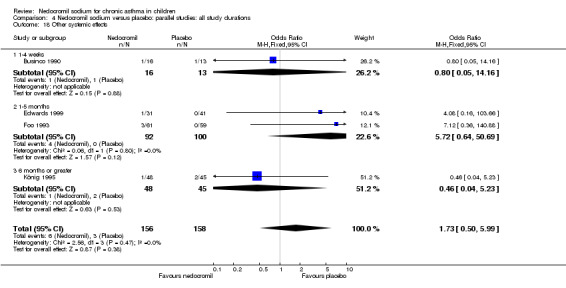
Comparison 4 Nedocromil sodium versus placebo: parallel studies: all study durations, Outcome 18 Other systemic effects.
4.19. Analysis.
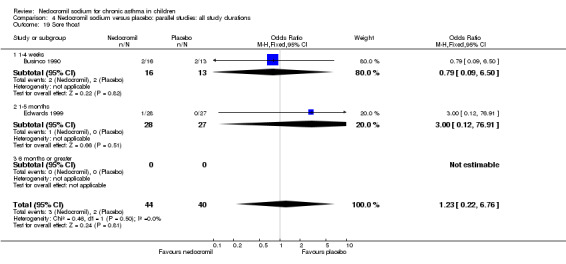
Comparison 4 Nedocromil sodium versus placebo: parallel studies: all study durations, Outcome 19 Sore thoat.
4.20. Analysis.
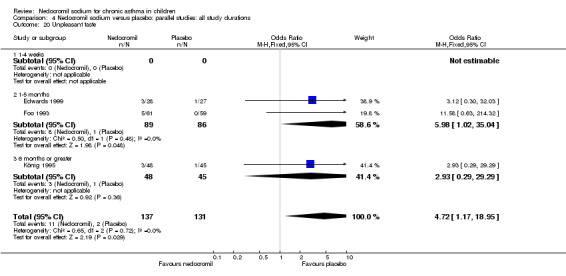
Comparison 4 Nedocromil sodium versus placebo: parallel studies: all study durations, Outcome 20 Unpleasant taste.
4.21. Analysis.
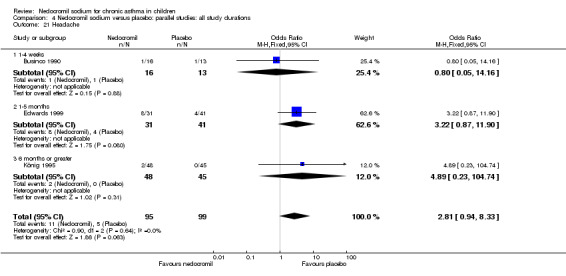
Comparison 4 Nedocromil sodium versus placebo: parallel studies: all study durations, Outcome 21 Headache.
4.22. Analysis.
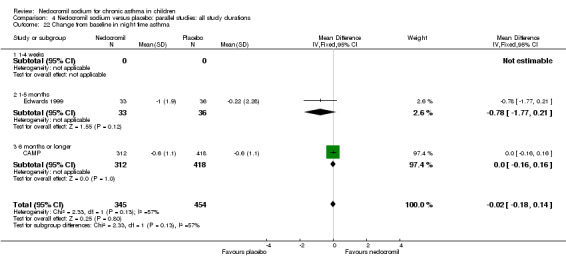
Comparison 4 Nedocromil sodium versus placebo: parallel studies: all study durations, Outcome 22 Change from baseline in night time asthma.
Comparison 5. Nedocromil versus placebo: short term versus long term studies.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Daily asthma symptom score | 5 | 266 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.44 [‐0.99, 0.10] |
| 1.1 Long term studies (6 months or longer) | 1 | 71 | Std. Mean Difference (IV, Random, 95% CI) | 0.19 [‐0.28, 0.66] |
| 1.2 Short term studies( less than 6 months) | 4 | 195 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.65 [‐1.13, ‐0.17] |
| 2 Daily use of beta2 agonists | 2 | 43 | Mean Difference (IV, Fixed, 95% CI) | ‐0.96 [‐1.44, ‐0.48] |
| 2.1 Long term studies (6 months or longer) | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 Short term studies (less than 6 months) | 2 | 43 | Mean Difference (IV, Fixed, 95% CI) | ‐0.96 [‐1.44, ‐0.48] |
| 3 Histamine hyperresponsiveness | 3 | 172 | Mean Difference (IV, Fixed, 95% CI) | 0.44 [0.14, 0.73] |
| 3.1 Long‐term studies (6 months or longer) | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 Short term studies( less than 6 months) | 3 | 172 | Mean Difference (IV, Fixed, 95% CI) | 0.44 [0.14, 0.73] |
| 4 Methacholine hyperresponsiveness | 0 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 1‐4 weeks | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 1‐5 months | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.3 6 months or longer | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Withdrawal due to asthma exacerbations | 4 | 224 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.30, 2.07] |
| 5.1 Long term studies (6 months or longer) | 1 | 93 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.91 [0.17, 21.86] |
| 5.2 Short term studies (less than 6 months) | 3 | 131 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.23, 1.92] |
| 6 Side effects | 2 | 108 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.32 [0.37, 4.69] |
| 6.1 Long term studies (6 months or longer) | 0 | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.2 Short term studies (less than 6 months) | 2 | 108 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.32 [0.37, 4.69] |
| 7 Parental assessment of efficacy | 4 | 294 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.94 [1.21, 3.10] |
| 7.1 Long term studies (6 months or longer) | 1 | 75 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.60 [0.63, 4.03] |
| 7.2 Short term studies (less than 6 months) | 3 | 219 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.07 [1.20, 3.57] |
| 8 Clinician assessment of efficacy | 3 | 183 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.60 [0.89, 2.89] |
| 8.1 Long term studies (6 months or longer) | 1 | 75 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.6 [0.64, 4.01] |
| 8.2 Short term studies (less than 6 months) | 2 | 108 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.61 [0.75, 3.46] |
| 9 Change from baseline in FVC (L) | 5 | 1032 | Mean Difference (IV, Fixed, 95% CI) | ‐0.01 [‐0.07, 0.05] |
| 9.1 Long term studies (6 months or longer) | 2 | 823 | Mean Difference (IV, Fixed, 95% CI) | ‐0.04 [‐0.10, 0.02] |
| 9.2 Short term studies( less than 6 months) | 3 | 209 | Mean Difference (IV, Fixed, 95% CI) | 0.13 [0.00, 0.26] |
| 10 Bronchial hyperesponsiveness (PD10‐UNDW) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 10.1 1‐4 weeks | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10.2 1‐5 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10.3 6 months or greater | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 11 FEV1(% Predicted) | 4 | 197 | Mean Difference (IV, Fixed, 95% CI) | 14.02 [12.43, 15.62] |
| 11.1 Long term studies (6 months or longer) | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11.2 Short term studies( less than 6 months) | 4 | 197 | Mean Difference (IV, Fixed, 95% CI) | 14.02 [12.43, 15.62] |
| 12 Change in FEV1 (% predicted) | 2 | 755 | Mean Difference (IV, Fixed, 95% CI) | ‐0.19 [‐1.46, 1.08] |
| 12.1 Long term studies (6 months or longer) | 1 | 730 | Mean Difference (IV, Fixed, 95% CI) | ‐0.4 [‐1.81, 1.01] |
| 12.2 Short term studies( less than 6 months) | 1 | 25 | Mean Difference (IV, Fixed, 95% CI) | 0.73 [‐2.20, 3.66] |
| 13 Change from baseline in FEV1(L) | 5 | 1356 | Mean Difference (IV, Fixed, 95% CI) | 0.03 [‐0.01, 0.08] |
| 13.1 Long term studies(6 months or longer) | 2 | 823 | Mean Difference (IV, Fixed, 95% CI) | ‐0.02 [‐0.07, 0.04] |
| 13.2 Short term studies( less than 6 months) | 3 | 533 | Mean Difference (IV, Fixed, 95% CI) | 0.12 [0.05, 0.19] |
| 14 Change from baseline in morning PEFR (L/min) | 4 | 903 | Mean Difference (IV, Random, 95% CI) | 14.96 [‐1.74, 31.67] |
| 14.1 Long term studies( 6 months or longer) | 2 | 805 | Mean Difference (IV, Random, 95% CI) | 5.98 [‐12.70, 24.67] |
| 14.2 Short term studies (less than 6 months) | 2 | 98 | Mean Difference (IV, Random, 95% CI) | 24.10 [6.70, 41.50] |
| 15 Change from baseline evening PEFR (L/min) | 3 | 255 | Mean Difference (IV, Fixed, 95% CI) | 11.47 [0.08, 22.85] |
| 15.1 Long term studies( 6 months or longer) | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 15.2 Short term studies (less than 6 months) | 3 | 255 | Mean Difference (IV, Fixed, 95% CI) | 11.47 [0.08, 22.85] |
| 16 Change from baseline in brochodilator use (doses/day) | 5 | 925 | Mean Difference (IV, Random, 95% CI) | ‐0.11 [‐0.72, 0.51] |
| 16.1 Long term studies (6 months or longer) | 2 | 805 | Mean Difference (IV, Random, 95% CI) | ‐0.06 [‐0.21, 0.08] |
| 16.2 Short term studies (less than 6 months) | 3 | 120 | Mean Difference (IV, Random, 95% CI) | ‐0.12 [‐1.68, 1.44] |
| 17 Change from baseline in asthma symptom score | 2 | 752 | Mean Difference (IV, Fixed, 95% CI) | ‐0.01 [‐0.06, 0.04] |
| 17.1 Long term studies (6 months or longer) | 1 | 730 | Mean Difference (IV, Fixed, 95% CI) | ‐0.01 [‐0.06, 0.04] |
| 17.2 Short term studies( less than 6 months) | 1 | 22 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐2.05, 2.05] |
| 18 Daily asthma symptom score | 5 | 270 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.44 [‐0.99, 0.10] |
| 18.1 Long term studies (6 months or longer) | 1 | 75 | Std. Mean Difference (IV, Random, 95% CI) | 0.19 [‐0.26, 0.65] |
| 18.2 Short term studies (less than 6 months) | 4 | 195 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.65 [‐1.13, ‐0.17] |
| 19 Change from baseline in night time asthma | 2 | 799 | Mean Difference (IV, Fixed, 95% CI) | ‐0.02 [‐0.18, 0.14] |
| 19.1 Long term studies( 6 months or longer) | 1 | 730 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.16, 0.16] |
| 19.2 Short term studies( less than 6 months) | 1 | 69 | Mean Difference (IV, Fixed, 95% CI) | ‐0.78 [‐1.77, 0.21] |
| 20 Headache | 3 | 194 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.81 [0.94, 8.33] |
| 20.1 Long term studies (6 months or longer) | 1 | 93 | Odds Ratio (M‐H, Fixed, 95% CI) | 4.89 [0.23, 104.74] |
| 20.2 Short term studies (less than 6 months) | 2 | 101 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.52 [0.78, 8.15] |
| 21 Unpleasant taste | 3 | 268 | Odds Ratio (M‐H, Fixed, 95% CI) | 4.72 [1.17, 18.95] |
| 21.1 Long term studies (6 months or longer) | 1 | 93 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.93 [0.29, 29.29] |
| 21.2 Short term studies (less than 6 months) | 2 | 175 | Odds Ratio (M‐H, Fixed, 95% CI) | 5.98 [1.02, 35.04] |
| 22 Sore thoat | 2 | 84 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.22, 6.76] |
| 22.1 Long term studies (6 months or longer) | 0 | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 22.2 Short term studies (less than 6 months) | 2 | 84 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.22, 6.76] |
| 23 Other systemic effects | 4 | 314 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.73 [0.50, 5.99] |
| 23.1 Long term studies (6 months or longer) | 1 | 93 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.46 [0.04, 5.23] |
| 23.2 Short term studies (less than 6 months) | 3 | 221 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.08 [0.61, 15.51] |
5.1. Analysis.
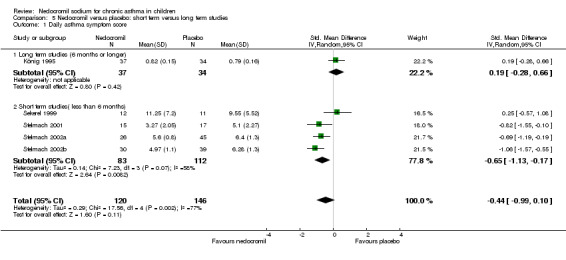
Comparison 5 Nedocromil versus placebo: short term versus long term studies, Outcome 1 Daily asthma symptom score.
5.2. Analysis.
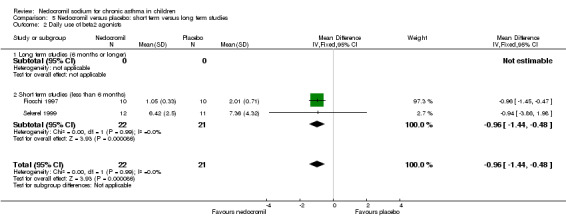
Comparison 5 Nedocromil versus placebo: short term versus long term studies, Outcome 2 Daily use of beta2 agonists.
5.3. Analysis.
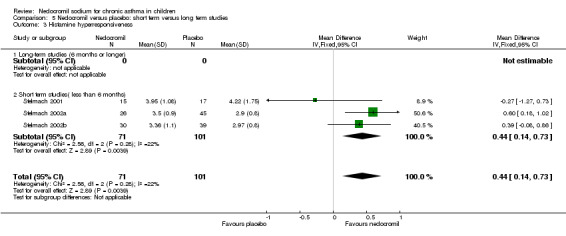
Comparison 5 Nedocromil versus placebo: short term versus long term studies, Outcome 3 Histamine hyperresponsiveness.
5.5. Analysis.
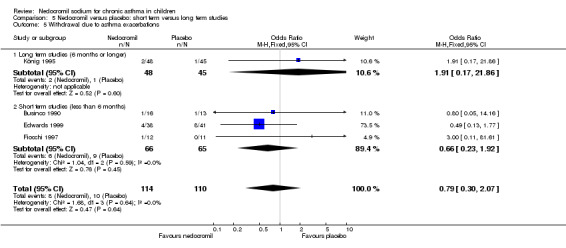
Comparison 5 Nedocromil versus placebo: short term versus long term studies, Outcome 5 Withdrawal due to asthma exacerbations.
5.6. Analysis.
Comparison 5 Nedocromil versus placebo: short term versus long term studies, Outcome 6 Side effects.
5.7. Analysis.
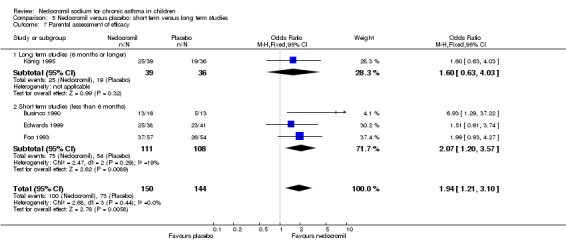
Comparison 5 Nedocromil versus placebo: short term versus long term studies, Outcome 7 Parental assessment of efficacy.
5.8. Analysis.
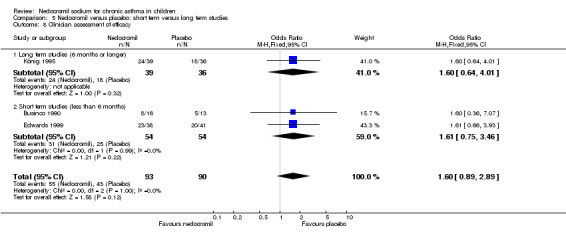
Comparison 5 Nedocromil versus placebo: short term versus long term studies, Outcome 8 Clinician assessment of efficacy.
5.9. Analysis.
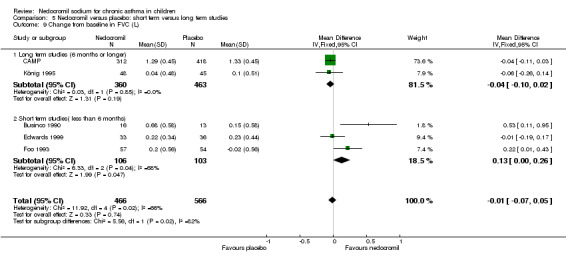
Comparison 5 Nedocromil versus placebo: short term versus long term studies, Outcome 9 Change from baseline in FVC (L).
5.10. Analysis.
Comparison 5 Nedocromil versus placebo: short term versus long term studies, Outcome 10 Bronchial hyperesponsiveness (PD10‐UNDW).
5.11. Analysis.
Comparison 5 Nedocromil versus placebo: short term versus long term studies, Outcome 11 FEV1(% Predicted).
5.12. Analysis.
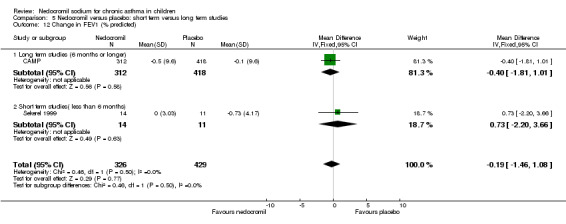
Comparison 5 Nedocromil versus placebo: short term versus long term studies, Outcome 12 Change in FEV1 (% predicted).
5.13. Analysis.
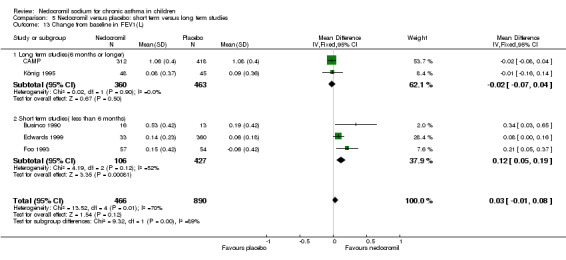
Comparison 5 Nedocromil versus placebo: short term versus long term studies, Outcome 13 Change from baseline in FEV1(L).
5.14. Analysis.
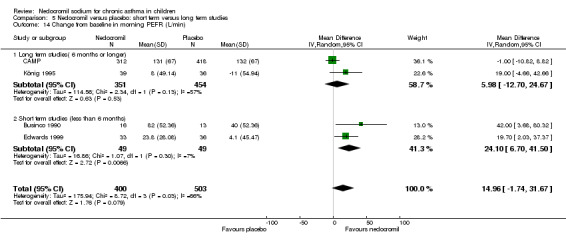
Comparison 5 Nedocromil versus placebo: short term versus long term studies, Outcome 14 Change from baseline in morning PEFR (L/min).
5.15. Analysis.
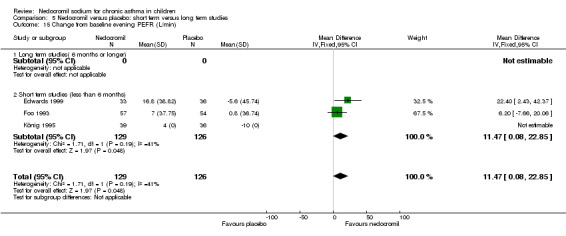
Comparison 5 Nedocromil versus placebo: short term versus long term studies, Outcome 15 Change from baseline evening PEFR (L/min).
5.16. Analysis.
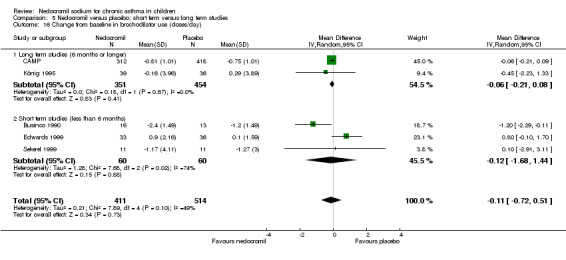
Comparison 5 Nedocromil versus placebo: short term versus long term studies, Outcome 16 Change from baseline in brochodilator use (doses/day).
5.17. Analysis.
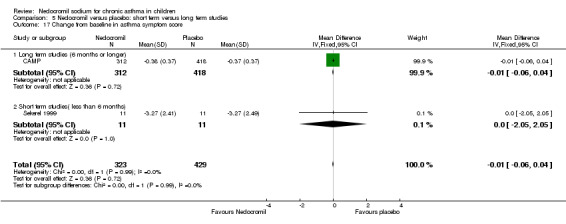
Comparison 5 Nedocromil versus placebo: short term versus long term studies, Outcome 17 Change from baseline in asthma symptom score.
5.18. Analysis.
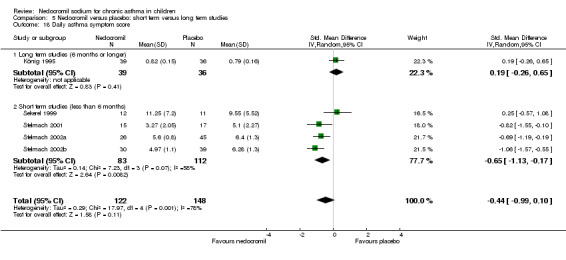
Comparison 5 Nedocromil versus placebo: short term versus long term studies, Outcome 18 Daily asthma symptom score.
5.19. Analysis.
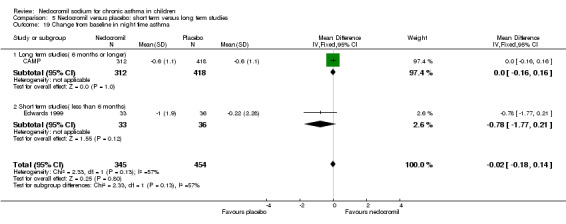
Comparison 5 Nedocromil versus placebo: short term versus long term studies, Outcome 19 Change from baseline in night time asthma.
5.20. Analysis.
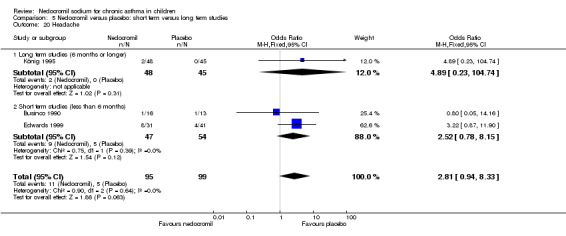
Comparison 5 Nedocromil versus placebo: short term versus long term studies, Outcome 20 Headache.
5.21. Analysis.
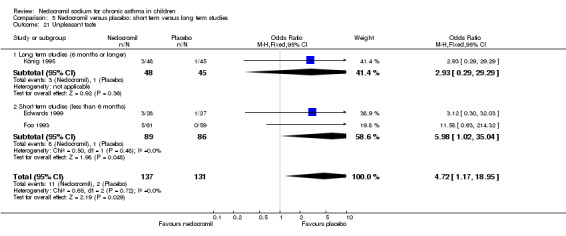
Comparison 5 Nedocromil versus placebo: short term versus long term studies, Outcome 21 Unpleasant taste.
5.22. Analysis.
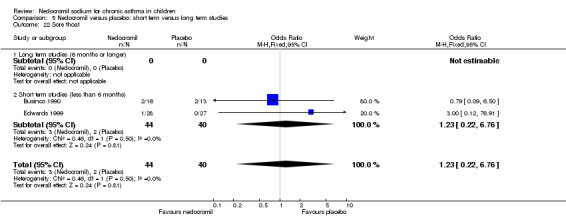
Comparison 5 Nedocromil versus placebo: short term versus long term studies, Outcome 22 Sore thoat.
5.23. Analysis.
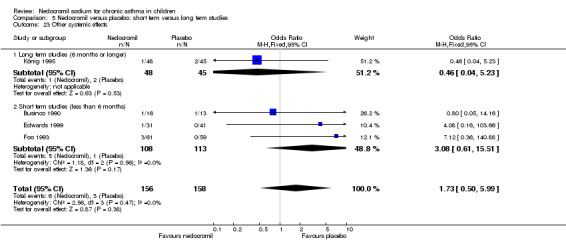
Comparison 5 Nedocromil versus placebo: short term versus long term studies, Outcome 23 Other systemic effects.
Comparison 6. Nedocromil sodium versus placebo: crossover studies: different doses.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 FEV1 (Murray 1993 low dose) | 3 | 72 | Litres (Fixed, 95% CI) | 0.23 [‐0.03, 0.50] |
| 1.1 4 mgs once | 2 | 48 | Litres (Fixed, 95% CI) | 0.05 [‐0.31, 0.41] |
| 1.2 5 mgs once | 1 | 24 | Litres (Fixed, 95% CI) | 0.46 [0.06, 0.86] |
| 1.3 10 mgs once | 0 | 0 | Litres (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 FEV1 (Murray 1993 high dose) | 3 | 72 | Litres (Fixed, 95% CI) | 0.23 [‐0.04, 0.50] |
| 2.1 4 mgs once | 2 | 48 | Litres (Fixed, 95% CI) | 0.05 [‐0.31, 0.41] |
| 2.2 5 mgs once | 0 | 0 | Litres (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.3 10 mgs once | 1 | 24 | Litres (Fixed, 95% CI) | 0.45 [0.05, 0.85] |
| 3 FEV1 predicted | 1 | Litres (Fixed, 95% CI) | Totals not selected | |
| 3.1 4 mgs once | 1 | Litres (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 5 mgs once | 0 | Litres (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 10 mgs once | 0 | Litres (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Bronchial hyperresponsiveness | 2 | 48 | Litres (Fixed, 95% CI) | 5.77 [1.78, 9.76] |
| 4.1 4 mgs once | 2 | 48 | Litres (Fixed, 95% CI) | 5.77 [1.78, 9.76] |
| 4.2 5 mgs once | 0 | 0 | Litres (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.3 10 mgs once | 0 | 0 | Litres (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
6.1. Analysis.
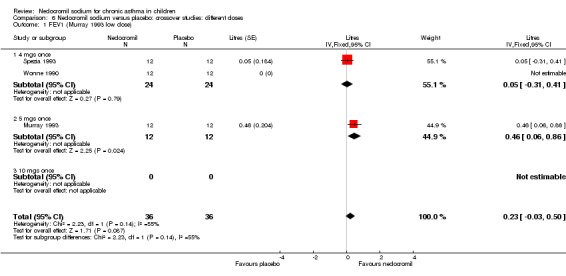
Comparison 6 Nedocromil sodium versus placebo: crossover studies: different doses, Outcome 1 FEV1 (Murray 1993 low dose).
6.2. Analysis.
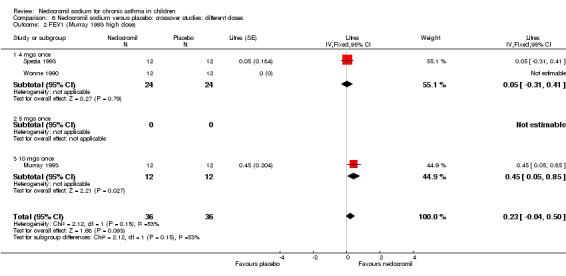
Comparison 6 Nedocromil sodium versus placebo: crossover studies: different doses, Outcome 2 FEV1 (Murray 1993 high dose).
6.3. Analysis.
Comparison 6 Nedocromil sodium versus placebo: crossover studies: different doses, Outcome 3 FEV1 predicted.
6.4. Analysis.
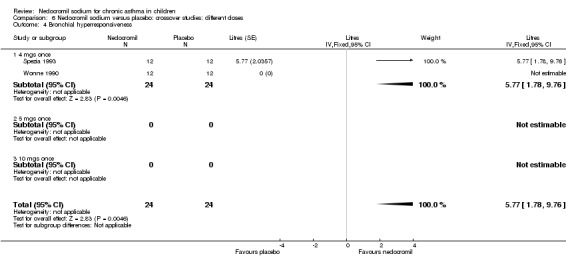
Comparison 6 Nedocromil sodium versus placebo: crossover studies: different doses, Outcome 4 Bronchial hyperresponsiveness.
Comparison 7. Nedocromil versus placebo: crossover studies: different devices.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 FEV1 (Murray 1993 low dose) | 2 | 48 | Litres (Fixed, 95% CI) | 0.23 [‐0.03, 0.50] |
| 1.1 MDI | 0 | 0 | Litres (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 MDI plus Spacer | 1 | 24 | Litres (Fixed, 95% CI) | 0.05 [‐0.31, 0.41] |
| 1.3 Nebuliser | 1 | 24 | Litres (Fixed, 95% CI) | 0.46 [0.06, 0.86] |
| 2 FEV1 (Murray 1993 high dose) | 2 | 48 | Litres (Fixed, 95% CI) | 0.23 [‐0.04, 0.50] |
| 2.1 MDI | 0 | 0 | Litres (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 MDI plus Spacer | 1 | 24 | Litres (Fixed, 95% CI) | 0.05 [‐0.31, 0.41] |
| 2.3 Nebuliser | 1 | 24 | Litres (Fixed, 95% CI) | 0.45 [0.05, 0.85] |
| 3 Bronchial hyperresponsiveness | 1 | Litres (Fixed, 95% CI) | Totals not selected | |
| 3.1 MDI | 0 | Litres (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 MDI plus Spacer | 1 | Litres (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 Nebuliser | 0 | Litres (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
7.1. Analysis.
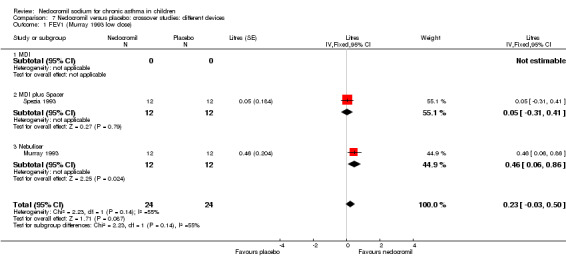
Comparison 7 Nedocromil versus placebo: crossover studies: different devices, Outcome 1 FEV1 (Murray 1993 low dose).
7.2. Analysis.
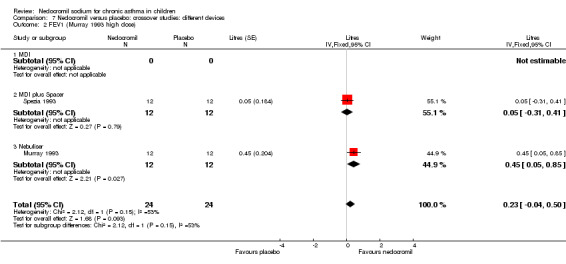
Comparison 7 Nedocromil versus placebo: crossover studies: different devices, Outcome 2 FEV1 (Murray 1993 high dose).
7.3. Analysis.
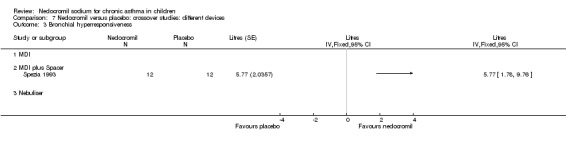
Comparison 7 Nedocromil versus placebo: crossover studies: different devices, Outcome 3 Bronchial hyperresponsiveness.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Businco 1990.
| Methods | Setting: outpatients, university teaching hospital, Italy Design: double blind placebo controlled parallel group trial Randomisation: according to a code Allocation concealment: unclear Exclusion criteria: stated Withdrawals: 2 participants withdrew because of treatment failure Baseline characteristics: comparable except for the nedocromil arm with 15 males and 1 females. Jadad score: 3 | |
| Participants | Total No: 31 children with seasonal asthma. 29 completed the study (NCS 16, Placebo 13) Male/female: 24/7 Age range: 4‐21 years (mean 11 years) Inclusion criteria: 1) children suffering from seasonal asthma, the diagnosis based on the positive history, positive skin tests and or/specific IgE to grass pollen, and confirmed by 15% reversibility of airway obstruction in response to bronchodilator Exclusion criteria: 1) patients who had respiratory infection in the previous 6 weeks; 2) patients receiving SCg, ketotifen and oral/inhaled steroids in the 4 weeks before the baseline period | |
| Interventions | Nedocromil: 4 mg 4 times/day for 4 weeks Placebo: matching placebo Delivery device: Metered dose inhaler Length of intervention period: 1 week baseline, followed by 4 weeks of treatment | |
| Outcomes | 1. Pulmonary function tests (FVC,FEV1,PEFR) 2) Symptom scores(1‐5) 3) Tolerance 4) patients on the NCS group reported headache,sore throat,itching and 4 patients in the placebo group reported abdominal pain,headache,sore throat | |
| Notes | This study was done in children with grass pollen asthma and the study group had two patients aged 20 and 21 years who had asthma since childhood. No reply from author regarding the method of randomisation | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | Information not available |
CAMP.
| Methods | Setting: Multi‐centre (8 centres) study in USA Design: Randomised, placebo controlled parallel group trial Randomisation: Permuted‐blocks randomisation with stratification according to clinic Allocation concealment: encrypted after computer generated schedule generated. Withdrawal/dropouts: accounted for Baseline characteristics: Comparable Jadad score: 5 | |
| Participants | Total no: 1041 children with mild‐moderate asthma, with 3 arms, budesonide group (311), nedocromil group (312) and placebo (418)
Age group: 5‐12 yrs age
Males/females:
Nedocromil: F/M
106/206
Placebo:F/M:
184/234
Inclusion criteria.
1) Children with mild ‐moderate with asthma, as defined by the presence of symptoms or by the use daily medication for asthma.
2) 20% decrease in FEV1 following methacholine challenge. Exclusion criteria: 1) any other clinically significant conditions |
|
| Interventions | Nedocromil: 8 mg twice daily
Placebo: matching placebo
Delivery device: MDI
Length of intervention period: 4‐6 yrs Children used albuterol for asthma symptoms |
|
| Outcomes | 1) Spirometry twice yearly 2) Methacholine challenge yearly 3) Symptom score‐ dairy card measures‐ Use of beta 2 agonists for asthma symptoms, use of prednisolone, absence from school due to asthma, visits to the hospital, severe attacks 4) Physical growth, height ,weight,bone densitometry and skeletal maturation 5) Psychological development 6) Examination for cataracts | |
| Notes | This is a multi‐centre study done in USA looking at the long term effects of nedocromil or budesonide in children with asthma | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | 'A schedule of treatment assignments was prepared for each clinic, encrypted, and installed on the clinic’s CAMP data system. The assignment for a patient was revealed only after all required baseline data were collected, recorded, keyed, and checked for conformance with the eligibility criteria via computer program.' |
Edwards 1999.
| Methods | Setting: multicentre trial done in UK, Australia and South Africa Design: double‐blind placebo controlled parallel group trial Randomisation: yes, methods not stated Allocation concealment: stated Masking: yes Exclusion criteria: stated Withdrawals: stated. out of 142 children , 63 children were withdrawn due failure to meet entry criteria (18), criteria of asthma symptom severity (15) or reversibility (9), developed uncontrolled asthma(2), took disallowed treatment(2) and non trial related reasons(17) Baseline characteristics: comparable Jadad score: 4 | |
| Participants | Total no: 142, 63 withdrew, 79 entered final study Age group: 6‐12 years (Mean 8.8 years) Males/Females: 46 boys and 33 girls Inclusion criteria: Patients with recent or current episode of asthma and with two documented episodes of asthma in the last 6 months. Asthma was defined as airways obstruction that is reversible by at least 15% or an increase in FEV1 of > 140 ml following inhalation of two puffs of bronchodilator administered by MDI Exclusion criteria: 1) Patients with renal, hepatic, cardiovascular or chronic respiratory disorders other than asthma. 2) Patients currently receiving treatment with steroids, SCG 3) Patients who have been treated with inhaled SCG during the previous month or inhaled corticosteroids in the previous 3 months. | |
| Interventions | Nedocromil: 4 mgs , three times/day Placebo: Liquified gas propellants and excipients Delivery Device:Fisonair spacer with MDI WITH 750 ml spacer Length of intervention period: 2 week baseline period followed by 12 weeks of intervention | |
| Outcomes | Symptom scores: severity of daytime and night‐time asthma symptoms, Morning and evening PEFR, number of doses of rescue bronchodilators Pulmonary Function tests( FEV1, FVC and PEF Parents assessment of efficacy (score 1‐5) Safety and tolerability | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | Investigators unaware as to order of treatment group assignment |
Fiocchi 1994.
| Methods | Setting: teaching hospital, Milan, Italy, outpatients Design: randomised placebo controlled, parallel group, double blind study Randomisation: yes but details not stated Allocation concealment: not clear Masking: not clear Exclusion criteria: stated Withdrawals: stated Baseline characteristics: comparable Jadad score: 3 | |
| Participants | Total no: 14 children with 2 dropouts in the placebo group Age group: 7‐17 years Males/Females: 9 males and 5 females Inclusion criteria:1) Patients with bronchial asthma; 2) Skin tests and RAST negative to perennial allergens; 3) seasonal bronchial hyper reactivity to ultrasonic nebulised distilled water between Oct and March months Exclusion criteria: 1) Negative challenge with UNDW between Oct and march months 2) positive challenge to UNDW between Aug and September months 3) Positive skin tests for perennial allergens 4) intake of theophylline,Beta 2 agonists, cromones, corticosteroids, anti histamines 6 hours before testing. | |
| Interventions | Nedocromil: 4 mgs 4 times/day for 42 days (6 weeks) Placebo: yes, type not stated Delivery Device: not stated Length of intervention period: 42 days (6 weeks) | |
| Outcomes | 1) Bronchial reactivity to challenge with UNDW on Day ‐7, day 0,day 1, day 7,day 14, day 28 and day 42. Vital capacity, FEV1, PEF and FEF25‐75 | |
| Notes | The study was done to evaluate whether nedocromil benefits children showing seasonal hyperreactivity to ultrasonic nebulisation of distilled water(UNDW). The outcome measures were mainly pulmonary function tests. There is no data on clinical symptoms and need for rescue bronchodilators. The type of device used to deliver nedocromil/placebo not mentioned. No reply from authors to clarify the clinical data, method of randomisation or type of device used. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | Information not available |
Fiocchi 1997.
| Methods | Setting: teaching hospital, Milan, Italy Design: double‐blind, placebo‐controlled, parallel group trial Randomisation: yes Allocation concealment: not clear Base line characteristics: Comparable between the two groups Withdrawals: stated Exclusion criteria: stated Jadad score: 3 | |
| Participants | Total no: 23 participants with mild atopic asthma, 2 participants were excluded from the treatment group because of acute asthma and respiratory tract infection, one participant excluded from the placebo group because of poor compliance. Finally 10 participants in each group
Age group: 7‐13 yrs
Sex: 2 males and 8 females
Inclusion criteria: 1) Reversible airway obstruction , improvement of FEV1 > 15% following salbutamol
2. Negative skin prick tests to common allergens Exclusion criteria: 1. Patients with symptomatic airway obstruction in the previous month 2. Patients who received inhaled or systemic steroids |
|
| Interventions | Nedocromil: 4 mgs 4 times a day for 6 weeks Placebo: Excipient used/no further details available Delivery device: MDI Length of intervention period: 6 weeks | |
| Outcomes | Outcome measures: 1. Symptom scores 2. Bronchial hyper reactivity to ultrasonic nebulised distilled water(PD10)‐ The cumulative doses of delivered nebulised water producing a 10% fall in FEV1 3. Bronchodilator use 4. Base line lung function | |
| Notes | This study was done to assess the effect of nedocromil sodium on bronchial hyper reactivity in children with mild non atopic asthma | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | Information not available |
Foo 1993.
| Methods | Setting: out patient clinics, Melbourne, Australia Design: double‐blind, randomised, placebo controlled, parallel group trial Randomisation: yes, randomised block Allocation concealment: not clear Masking: double blind Exclusion criteria: stated Withdrawals: stated, 9 withdrew, 4 in the NCS and 5 in the placebo group Baseline characteristics: comparable Jadad score: 4 | |
| Participants | Total no: 120( NCS 61, Placebo 59), 3 patients withdrew because of side effects Age group: 6‐19 years Males/Females: 82 males, 38 females Inclusion criteria: 1) ATS criteria for diagnosis of asthma; 2) ability to use mini PEFR meter and a MDI via a nebuhaler aerosol holding chamber adequately; 3) have a measurable histamine responsiveness, with a provocative dose of histamine causing a 20% fall in FEV1( PD 20 FEV1) of 4 micromol or less Exclusion criteria: 1) patient with renal,hepatic or cardiovascular disease 2) smokers 3) patients on SCG in the last 3 months | |
| Interventions | Nedocromil: 4 mg three times /day Placebo: identical aerosol Delivery Device: MDI inhaler via nebuhaler aerosol holding chamber Length of intervention period; 2 week baseline followed by 8 week treatment period | |
| Outcomes | 1) Symptom control‐ cough,morning tightness, daytime symptoms, night time symptoms( score 0‐4), mean PEF, and PEFV for each week 2) Pulmonary function tests, histamine responsiveness PD 20 FEV1 3) Pathology tests‐ Full blood count,serum biochemistry and liver function tests | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | Information not available |
König 1995.
| Methods | Setting: multi centre trial, USA Design: double‐blind, placebo controlled group parallel group trial Randomisation: stated Allocation concealment: not clear Masking: double blind Exclusion criteria: stated Withdrawals: stated, 18 children withdrew, 9 from each treatment group mainly due to protocol violations Baseline characteristics: comparable, but however NCS group had more females(23 vs 12) Jadad score: 3 | |
| Participants | Total no: 93 children with mild‐moderate asthma Age group: 6‐12 years(mean 8.9 yrs) Males/Females: 58 males and 35 females Inclusion criteria: 1) mild‐mod asthma defined by history and PFT's and a positive methacholine response; 2) history of 2 viral infections in the last year but none in the 6 weeks preceding the study; 3) The diagnosis of asthma based on reversibility of airway obstruction, defined by 15% improvement in FEV1 after beta 2 agonist aerosol and a positive methacholine challenge test defined as a 20% drop in FEV1. Exclusion criteria: not clearly stated | |
| Interventions | Nedocromil: 2 ml of 0.5% nebuliser solution(10 mg) administered by power operated nebuliser, 3 times /day Placebo: matching placebo Delivery Device: ultrasonic nebulizer Length of intervention period: 24 weeks | |
| Outcomes | 1) asthma symptoms: daytime asthma, cough, asthma score and sleep disturbance 2) use of rescue bronchodilators 3) daily PEFR 4) clinic PFT's 5) response to methacholine challenge 6) clinical assessment of asthma severity 7) global opinion of treatment effectiveness 8)Symptomatic respiratory infections 9) safety of nedocromil sodium | |
| Notes | This study was done to evaluate the effect of nedocromil sodium on childhood asthma during the viral season. Immediate release theophylline preparations were allowed on an as needed basis for acute symptoms. Short courses of oral steroids were allowed for asthma exacerbations. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | Information not available |
Murray 1993.
| Methods | Setting: hospital based Design: double blind randomised controlled cross over trial Randomisation: yes, but details not clear Allocation concealment: not clear Masking: not clear Exclusion criteria: negative fog challenge Withdrawals: stated, 1 withdrew because of acute asthmatic attack. 3 patients excluded from the study because of not meeting the study criteria of positive fog challenge and hence excluded from further analysis Baseline characteristics: Jadad score: 3 | |
| Participants | Total no: 12 participants, 1 withdrew because of an acute asthmatic attack, 3 excluded from further analysis because they did not meet the study criteria for positive fog challenge Age group: 7‐12 years Males/females: 10 males and 2 females Inclusion criteria: 1) positive fog challenge with a more than 20% drop in FEV1 Exclusion criteria: patients on inhaled and oral steroids | |
| Interventions | Nedocromil: 0.25% and 0.50% nedocromil nebuliser solutions (2 ml) Placebo: carrier solution without any active drug Delivery device: nebuliser, details not clear Length of intervention period: three visits in 10 days | |
| Outcomes | 1) FEV1 response to fog challenge | |
| Notes | This study was done to assess the efficacy of nebulised nedocromil sodium in fog induced bronchoconstriction in children with mild ‐mod asthma. This is small study of 8 participants looking at FEV1 response to fog. The authors did not reply regarding the details of the randomisation and concealment. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | Information not available |
Sekerel 1999.
| Methods | Setting: outpatient clinics Design: randomised, double blind, placebo controlled, parallel group trial Randomisation: yes Allocation concealment: yes, adequate Masking: yes Exclusion criteria: stated Withdrawals: stated, 4 children were withdrawn before randomisation because of non‐compliance, another 4 patients were lost to follow up after randomisation Baseline characteristics: comparable Jadad score: 4 | |
| Participants | Total no: 46 asthmatics with mild to moderate persistent asthma, turkey, total of 38 completed the study , 11 were treated with placebo, 13 with BD and 14 with QID doses of nedocromil Age group: 7‐14 years( mean 9.74 years) Males/females: 18 boys and 20 girls Inclusion criteria: 1) all patients meeting the ATS criteria for asthma and demonstrating a 15% change in FEV1 response to bronchodilator in the last 6 months; 2) No emergency visits or hospitalisations and they had not used inhaled or oral steroids within the previous month. Exclusion criteria: 1) patients with seasonal allergic asthma and a FEV1 lower than 65% of predicted value were excluded from the study. | |
| Interventions | Nedocromil: nedocromil 4 mgs twice daily (13), 4 times daily (14) Placebo: identical placebo Delivery device: MDI Length of intervention period: 2 week run in period followed by 8 weeks of intervention | |
| Outcomes | 1) Daily dairy of symptoms: day and night asthma symptoms, cough severity (score 0‐3) 2) PEFR measurements morning and evening 3) Rescue beta‐agonists 4) Pulmonary function tests‐ FEV1 5) Methacholine challenge (PC 20) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | Investigators unaware as to order of treatment group assignment |
Spezia 1993.
| Methods | Setting: Verona, Italy. Patients were guests of residential house, in the Italian alps, in an allergen free environment. Design: double‐blind, randomised, placebo controlled trial, cross over trial Randomisation: yes, details not given Allocation concealment: not clear Masking: not clear Exclusion criteria: Withdrawals: no withdrawals Baseline characteristics: comparable Jadad score: 3 | |
| Participants | Total no: 12 children, with mild to severe asthma Age group: 6‐13 yrs of age Males/females: 7 males and 5 females Inclusion criteria: 1) diagnosis of asthma established on the basis of clinical history and bronchial hyper responsiveness to methacholine challenge 2) Positive skin prick tests to one of the aero‐allergens 3) no asthma symptoms at the time of the study Exclusion criteria: not mentioned | |
| Interventions | Nedocromil: 4 mgs on four days Placebo: yes Delivery device: MDI with a 700 ml holding chamber( fisonair) Length of intervention period: daily for 4 days | |
| Outcomes | 1) Pulmonary function tests‐ FEV1 before and after treatment 2) PD 20‐ UNDW values at base line and after treatment (the cumulative dose of delivered nebulised water producing a 20% fall in FEV1) | |
| Notes | This study was done to assess the efficacy of nedocromil in preventing the bronchoconstriction induced by inhalation of ultrasonically nebulised distilled water(UNDW). There was a Sodium cromoglycate arm in the study. Only the results of the NCS and Placebo arm were compared and included for analysis. The clinical data comparison available. The authors were contacted for details of randomisation, concealment and clinical data with no reply | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | Information not available |
Stelmach 2001.
| Methods | Setting: out patients of the university teaching hospital in Poland Design:double‐blind randomised, placebo controlled parallel group trial Randomisation: yes, computer generated allocation schedule Allocation concealment: not clear Masking: Exclusion criteria: patients on oral or inhaled steroids, or leukotriene antagonists Withdrawals: Baseline characteristics: comparable Withdrawals: Baseline characteristics: Jadad score: 4 | |
| Participants | Total no: 39 Children with moderate atopic asthma, 7 patients withdrew Age group: 9‐16 yrs Males/females: 17 males, 15 females Inclusion criteria 1) Moderate atopic asthma and sensitive to house dust mite 2) Atopy measured by skin prick tests and Serum IgE 3) Patients using using only short acting beta ‐2 agonists for 4 weeks prior to the study Exclusion criteria: 1) Patients on oral or inhaled steroids or leukotriene antagonists | |
| Interventions | Nedocromil: 2 puffs 2 times daily, 2mg/puff Placebo: identical Delivery device: inhaler,MDI Length of intervention: 8 Weeks, 4 weeks run in followed by 4 weeks of intervention | |
| Outcomes | 1) Asthma symptom score 2) Lung function ‐FEV1 3) Histamine provocation test FEV1(PC20H) 4) Inflammatory markers‐Eosiniphil blood count, ECP, sIL‐2R,IL‐4, sICAM, and IgE | |
| Notes | This study was done to assess the effect of nedocromil sodium on clinical and inflammatory parameters of asthma in children allergic to dust mite | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | Information not available |
Stelmach 2002a.
| Methods | Setting: Tertiary paediatric allergy clinics in Zgierz, Poland Design: Double‐blind randomised, placebo controlled, parallel group trial Randomisation: yes Allocation concealment: not clear Masking: Exclusion criteria: not specified Withdrawals/dropouts: mentioned Jadad score; 4 | |
| Participants | Total no: 154 children (including 5 arms) ‐ nedocromil and placebo 100 Age group: 9‐17 yrs Males/females Nedocromil boys 14/26 Placebo: boys 20/40 Inclusion criteria: 1) Asthma defined by clinical symptoms and reversibility with salbutamol 2) Severeity of asthma defined in reference 26(NIH) criteria 3) Asthma stability for 6 months 4) All subjects had atopy with a positive 3mm reaction on skin testing 4) inhaled steroids stopped during the 4‐week run in period Exclusion criteria: not mentioned | |
| Interventions | Nedocromil: 4mg 4 times /day
Placebo: identical Delivery device: MDI inhaler Length of intervention period: 4 weeks plus 4 weeks run in period |
|
| Outcomes | 1) Clinical parameters of asthma‐symptom score 2) Histamine challenge: PC20H 3) FEV1 4) Eosinophil blood count, serum eosinophilic cationic protein (ECP) | |
| Notes | Polish study translated to English. This study was done assess the effect of triamcinolone acetonide, montelukast, nedocromil sodium,formoterol on eosinophil blood counts,serum ECP levels and clinical parameters in children with moderate atopic asthma. Only the nedocromil arm and placebo arm were used for analysis of the data. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | Information not available |
Stelmach 2002b.
| Methods | Setting: tertiary paediatric allergy clinic in Zgierz, Poland Design: double‐blind placebo controlled parallel group trial Randomisation: yes Allocation concealment: not clear Withdrawals: mentioned Exclusion criteria Jadad score:4 | |
| Participants | Total no: 81
69 children completed the study Age group: 9‐17 years Males/females 17/39 males in the placebo group, 14/30 males in the treatment group Inclusion criteria: 1) sthma defined by clinical symptoms and reversibility with salbutamol 2) severity of asthma defined in reference 26(NIH) criteria 3) Asthma stability for 6 months 4) all subjects had atopy with a positive 3mm reaction on skin testing 4) inhaled steroids stopped during the 4‐week run in period Exclusion criteria: not mentioned |
|
| Interventions | Nedocromil: 4mg 4 times daily for 4 weeks Placebo: type not mentioned Delivery: MDI inhaler Length of intervention: 4 weeks run in period followed by 4 weeks of intervention | |
| Outcomes | 1) Symptom score 2) FEV1 3) PC20H 4) Serum IgE and IL‐4 levels before and after 4 weeks of treatment | |
| Notes | Polish study translated to English. This study was done to assess the effect of nedocromil sodium on serum levels of IL‐4 nad IgE, CLinical symptoms and bronchial hyper reactivity(BHR) in children with moderate atopic asthma. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | Information not available |
van Bever 1996.
| Methods | Setting: out patient dept, University teaching hospital, Belgium, Netherlands and France, UK Design: double‐blind randomised, parallel group trial Randomisation: yes, details not clear Allocation concealment: not clear, although the aerosol described as identical Masking: Exclusion criteria: no exclusion criteria explained Withdrawals: Baseline characteristics: reported as normal Jadad score: 3 | |
| Participants | Total no: 74 children
Moderate (80%) and severe (11%) asthma No other details provided. |
|
| Interventions | Tilade 2mg 4 x daily versus placebo Inhaler device: not clear Length of intervention period: 12 weeks | |
| Outcomes | Morning and evening peak flow; diurnal and nocturnal symptoms; ß agonist use | |
| Notes | Unpublished conference abstract. No outcome data could be used. No response from trialists | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | Information not available |
Wonne 1990.
| Methods | Setting: Out patient dept, University teaching hospital, Germany Design: Double‐blind randomised, crossover study Randomisation: yes, details not clear Allocation concealment: not clear, although the aerosol described as identical Masking: Exclusion criteria: no exclusion criteria explained Withdrawals: Baseline characteristics: reported as normal Jadad score: 3 | |
| Participants | Total no: 12 Children with mild to mod asthma controlled with beta‐2 agonists alone Age group: 9‐12 yrs Males/females not reported Inclusion criteria: 1. children 9‐12 yrs age; 2. Mild ‐mod asthma controlled with beta ‐2 agonist alone,to have been taken at the least by the preceding evening. Exclusion criteria: no exclusion criteria explained | |
| Interventions | Nedocromil 4 mg Placebo: identical Delivery device: inhaler (MDI) Length of intervention period: 2 days | |
| Outcomes | 1) SRaw, cold air wave in per cent of AGW (PD 100 s Raw %AGW) | |
| Notes | German study translated to English This study was done to assess the protective effect of nedocromil sodium on cold air hyperinflation challenge in children suffering from chronic bronchial asthma | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | Information not available |
ATS: American Thoracic Society; BDP: beclomethasone dipropionate; BHR: bronchial hyper‐responsiveness; DPI: dry powder inhaler; ECP: eosinophil cationic protein; FEV1: forced expired volume in one second; FEF25‐75: forced expiratory flow at 25 to 75% of FVC; FEF25: maximal expiratory flow at 25% of FVC; FEF50: maximal expiratory flow at 50% of FVC; FEF 75: maximal expiratory flow at 75% of FVC; FVC: forced vital capacity; GINA: Global Initiative for Asthma; MDI: metered dose inhaler; NCS: Nedocromil sodium; PC100 SRaw: concentration of inhalant necessary to increase SRaw by 100%; PC20 FEV1: concentration of inhalant required to produce a 20% fall in FEV1; PD100 Raw: dose of inhalant necessary to increase Raw by 100%; PD20 FEV1: dose of inhalant required to produce a 20% fall in FEV1; PEFR: peak expiratory flow rate; PV75 SRaw: provocative ventilation necessary to increase SRaw by 75%; Raw: airway resistance; SRaw: specific airway resistance; TLC: total lung capacity; UNDW: Ultrasonic nebulisation of distilled water;
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Armenio 1993 | Patients were on combination therapy |
| Bel 1990 | Age group 17‐63 years Only one 17 year old patient in the treatment arm No children in the Placebo arm |
| Bergmann 1989 | Data same as Bergmann 1990 No separate paediatric data available |
| Bergmann 1989a | Article in German language Data same as Bergmann 1990 No separate paediatric data available |
| Bergmann 1990 | Age group 12‐78 years No separate paediatric data available Author requested for raw data on paediatric patients with no reply |
| Bianco 1989 | Age group 12‐67 years No separate paediatric data Authors requested for paediatric data with no reply |
| Boos 1989 | Article in German language Age group 12‐65 years Youngest patient in the included group is 19 years (Placebo group) |
| Bruce 2006 | Study conducted in adults (mean age 33 years) |
| Chan 2001 | Not an RCT Single arm open labelled non‐comparative trial |
| Clancy 1994 | Age group 15‐74 years No separate paediatric data available Author requested for paediatric data with no reply |
| Corrias 1990 | Not a randomised controlled trial Despite the study being described as double‐blind, no allocation method is reported. A randomised design not adopted in this study Study translated from Italian language |
| Creticos 1995 | Adult study Age group 18‐66 years |
| Cua‐ Lim 1986 | Age group 16‐60 years No separate paediatric data available Authors requested for paediatric data with no reply |
| del Ponte 1997 | Not an RCT |
| Edwards 1992 | Over view analysis of all RCTs in adults |
| Fink 1994 | Age group 18‐70 years Adult study |
| Fyans 1986 | Age group 17‐74 years No children in the treatment group |
| Greco 1986 | Age group 14‐56 years No separate paediatric data available Authors requested for paediatric data with no reply |
| Joos 1989 | Age group 18‐61 years Adult study |
| Koskela 2005 | Adult study |
| Marin 1996 | Adult study |
| Oggiano 1995 | Not an RCT Control group non‐asthmatic children |
| Paananen 1986 | Age group 16‐70 years in the treatment group No children in the placebo group |
| Rebuck 1990 | Age group 18‐72 years Adult study |
| Ruffin 1986 | Age group 14‐70 years No separate paediatric data available Authors requested paediatric data with no reply |
| Ruffin 1987 | Age group 17‐68 years in the treatment group No children in the placebo group |
| Van As 1986 | No separate paediatric data Participants were on combination therapy with oral theophylline and inhaled beta 2 agonists during the treatment period. |
| Wells 1992 | Age group 16‐74 years No separate Paediatric data available Authors requested for paediatric data |
| Youngchaiyud 1986 | Age group 17‐41 years( mean age 24 years) Only 10 participants in the study |
Contributions of authors
AS: protocol initiation, study assessment, data entry, write‐up MM: protocol development, study assessment, write‐up
Sources of support
Internal sources
Leicester Royal Infirmary, University Hospitals of Leicester NHS trust, Leicester, UK.
External sources
No sources of support supplied
Declarations of interest
None known.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Businco 1990 {published data only}
- Businco L, Cantani A, Fazio A, Bernardini L. A double‐blind, placebo‐controlled study to assess the efficacy of nedocromil sodium in the management of childhood grass pollen asthma. Clinical and experimental allergy 1990;20(6):683‐8. [DOI] [PubMed] [Google Scholar]
CAMP {published data only}
- Annett RD, Aylward EH, Lapidus J, Bender BG, DuHamel T. Neurocognitive functioning in children with mild and moderate asthma in the childhood asthma management program. The Childhood Asthma Management Program CAMP Research Group. Journal of Allergy & Clinical Immunology 2000;105(4):717‐24. [DOI] [PubMed] [Google Scholar]
- Annett RD, Bender BG, Lapidus J, Duhamel TR, Lincoln A. Predicting children's quality of life in an asthma clinical trial: what do children's reports tell us?. Journal of Pediatrics 2001;139(6):854‐61. [DOI] [PubMed] [Google Scholar]
- Annett RD, Stansbury K, Kelly HW, Strunk RC. Association of hypothalamic‐pituitary‐adrenal axis function with neuropsychological performance in children with mild/moderate asthma. Child Neuropsychology 2005;11(4):333‐48. [DOI] [PubMed] [Google Scholar]
- Anonymous. Design and implementation of a patient education center for the Childhood Asthma Management Program. Childhood Asthma Management Program Research Group. Annals of Allergy, Asthma, & Immunology 1998;81(6):571‐81. [PubMed] [Google Scholar]
- Anonymous. Long‐term effects of budesonide or nedocromil in children with asthma. The Childhood Asthma Management Program Research Group. New England Journal of Medicine 2000;343(15):1054‐63. [DOI] [PubMed] [Google Scholar]
- Anonymous. Recruitment of participants in the childhood Asthma Management Program CAMP. I. Description of methods: Childhood Asthma Management Program Research Group. Journal of Asthma 1999;36(3):217‐37. [PubMed] [Google Scholar]
- Anonymous. The Childhood Asthma Management Program CAMP: design, rationale, and methods. Childhood Asthma Management Program Research Group. Controlled Clinical Trials 1999;20(1):91‐120. [PubMed] [Google Scholar]
- Bacharier LB, Raissy HH, Wilson L, McWilliams B, Strunk RC, Kelly HW. Long‐term effect of budesonide on hypothalamic‐pituitary‐adrenal axis function in children with mild to moderate asthma. Pediatrics 2004;113(6 I):1693‐9. [DOI] [PubMed] [Google Scholar]
- Bender BG, Ellison MC, Gleason M, Murphy JR, Sundstrom DA, Szefler SJ. Minimizing attrition in a long‐term clinical trial of pediatric asthma. Annals of Allergy, Asthma, & Immunology 2003;91(2):168‐76. [DOI] [PubMed] [Google Scholar]
- Bender BG, Annett RD, Ikle D, DuHamel TR, Rand C, Strunk RC. Relationship between disease and psychological adaptation in children in the Childhood Asthma Management Program and their families. CAMP Research Group. Archives of Pediatrics & Adolescent Medicine 2000;154(7):706‐13. [DOI] [PubMed] [Google Scholar]
- CAMP Study Group. Childhood asthma management program (CAMP) phases I and II. Clinicaltrials.Gov 2003. [URL: http://www.clinicaltrials.gov/ct/show/NCT00000575]
- Covar RA, Spahn JD, Martin RJ, Silkoff PE, Sundstrom DA, Murphy J, et al. Safety and application of induced sputum analysis in childhood asthma. Journal of Allergy & Clinical Immunology 2004;114(3):575‐82. [DOI] [PubMed] [Google Scholar]
- Covar RA, Spahn JD, Murphy JR, Szefler SJ. Progression of asthma measured by lung function in the childhood asthma management program. American Journal of Respiratory & Critical Care Medicine 2004;170(3):234‐41. [DOI] [PubMed] [Google Scholar]
- Covar RA, Spahn JD, Murphy JR, Szefler SJ, Childhood Asthma Management Program Research Group. Progression of asthma measured by lung function in the childhood asthma management program. American Journal of Respiratory & Critical Care Medicine 2004;170(3):234‐41. [DOI] [PubMed] [Google Scholar]
- Covar RA, Szefler SJ, Martin RJ, Sundstrom DA, Silkoff PE, Murphy J, et al. Relations between exhaled nitric oxide and measures of disease activity among children with mild‐to‐moderate asthma. Journal of Pediatrics 2003;142(5):469‐75. [DOI] [PubMed] [Google Scholar]
- Kelly HW, Natta ML, Covar RA, Tonascia J, Green RP, Strunk RC, et al. Effect of long‐term corticosteroid use on bone mineral density in children: a prospective longitudinal assessment in the Childhood Asthma Management Program (CAMP) study. Pediatrics 2008;122(1):e53‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raby BA, Steen K, Celedon JC, Litonjua AA, Lange C, Weiss ST. Paternal history of asthma and airway responsiveness in children with asthma. American Journal of Respiratory & Critical Care Medicine 2005;172(5):552‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro GG, DuHamel TR, Wighton TG, Chinn T, Warren Bierman C, Altman LC, et al. The childhood asthma management program (CAMP): Design, rationale, and methods. Controlled Clinical Trials 1999;20(1):91‐120. [PubMed] [Google Scholar]
- Sharma S, Litonjua AA, Tantisira KG, Fuhlbrigge AL, Szefler SJ, Strunk RC, et al. Clinical predictors and outcomes of consistent bronchodilator response in the childhood asthma management program. Journal of Allergy & Clinical Immunology 2008;122(5):921‐8 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman E K, Kwiatkowski D J, Sylvia J S, Lazarus R, Drazen J M, Lange C, et al. Family‐based association analysis of beta2‐adrenergic receptor polymorphisms in the childhood asthma management program. Journal of Allergy & Clinical Immunology 2003;112(5):870‐6. [DOI] [PubMed] [Google Scholar]
- Slaughter JC, Lumley T, Sheppard L, Koenig JQ, Shapiro GG. Effects of ambient air pollution on symptom severity and medication use in children with asthma. Annals of Allergy, Asthma, & Immunology 2003;91(4):346‐53. [DOI] [PubMed] [Google Scholar]
- Strunk RC, Bender B, Young DA, Sagel S, Glynn E, Caesar M, et al. Predictors of protocol adherence in a pediatric asthma clinical trial. Journal of Allergy & Clinical Immunology 2002;110(4):596‐602. [DOI] [PubMed] [Google Scholar]
- Strunk RC, Sternberg AL, Bacharier LB, Szefler SJ. Nocturnal awakening caused by asthma in children with mild‐to‐moderate asthma in the childhood asthma management program. Journal of Allergy & Clinical Immunology 2002;110(3):395‐403. [DOI] [PubMed] [Google Scholar]
- Strunk RC, Sternberg AL, Szefler SJ, Zeiger RS, Bender B, Tonascia J, et al. Long‐term budesonide or nedocromil treatment, once discontinued, does not alter the course of mild to moderate asthma in children and adolescents. The Journal of Pediatrics 2009;154(5):682‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tantisira KG, Colvin R, Tonascia J, Strunk RC, Weiss ST, Fuhlbrigge AL, et al. Airway responsiveness in mild to moderate childhood asthma: sex influences on the natural history. American Journal of Respiratory & Critical Care Medicine 2008;178(4):325‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tantisira KG, Fuhlbrigge AL, Tonascia J, Natta M, Zeiger RS, Strunk RC, et al. Bronchodilation and bronchoconstriction: predictors of future lung function in childhood asthma. Journal of Allergy & Clinical Immunology 2006;117(6):1264‐71. [DOI] [PubMed] [Google Scholar]
- Tantisira KG, Lake S, Szefler S, Strunk R, Fulbrigge AL, Weiss ST. Baseline pulmonary function predictors of longitudinal lung function in the childhood asthma management program (CAMP): a treatment stratified response [Abstract]. American Thoracic Society 100th International Conference, Orlando, May 21‐26. 2004:B26, Poster 401.
- Tantisira KG, Litonjua AA, Weiss ST, Fuhlbrigge AL, Childhood Asthma Management Program Research Group. Association of body mass with pulmonary function in the Childhood Asthma Management Program (CAMP). Thorax 2003;58(12):1036‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss ST, Horner A, Shapiro G, Sternberg AL, Childhood Asthma Management Program (CAMP) Research Group. The prevalence of environmental exposure to perceived asthma triggers in children with mild‐to‐moderate asthma: data from the Childhood Asthma Management Program CAMP. Journal of Allergy & Clinical Immunology 2001;107(4):634‐40. [DOI] [PubMed] [Google Scholar]
- Zeiger R S, Dawson C, Weiss S. Relationships between duration of asthma and asthma severity among children in the Childhood Asthma Management Program CAMP. Journal of Allergy & Clinical Immunology 1999;103(3 Pt 1):376‐87. [DOI] [PubMed] [Google Scholar]
Edwards 1999 {published data only}
- Edwards AM, Lyons J, Weinberg E, Weinberg F, Gillies JD, Reid G, et al. Early use of inhaled nedocromil sodium in children following an acute episode of asthma. Thorax 1999;54(4):308‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fiocchi 1994 {published data only}
- Fiocchi A, Signoroni P, Bruni P, Galeone M, DeCet E, Bogacki S. Effect of nedocromil sodium on aspecific bronchial hyperreactivity in asthmatic children. Mediators of inflammation 1994;3:S43‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fiocchi 1997 {published data only}
- Fiocchi A, Riva E, Santini I, Bernardo I, Sala M, Mirri GP. Effect of nedocromil sodium on bronchial hyperreactivity in children with non‐atopic asthma. Annals of Allergy, Asthma and Immunology 1997;79(6):503‐6. [DOI] [PubMed] [Google Scholar]
Foo 1993 {published data only}
- Foo AL, Lanteri CJ, Burton PR, Sly PD. The effect of nedocromil sodium on histamine responsiveness in clinically stable asthmatic children. Journal of Asthma 1993;30(5):381‐90. [DOI] [PubMed] [Google Scholar]
König 1995 {published data only}
- König P, Eigen H, Ellis MH, Ellis E, Blake K, Geller D, et al. Th effect of nedocromil sodium on childhood asthma during viral season. American Journal of Respiratory and Critical Care Medicine 1995;152(6):1879‐86. [DOI] [PubMed] [Google Scholar]
Murray 1993 {published data only}
- Murray M, Kinnear G, Milner AD. Prevention of fog induced bronchoconstriction in asthmatic children by nedocromil sodium nebulizer solutions. Respiratory Medicine 1993;87:65‐7. [DOI] [PubMed] [Google Scholar]
Sekerel 1999 {published data only}
- Sekerel BE, Saraclar Y, Etikan I, Kalayci O. Comparison of two different dose regimens of nedocromil sodium with placebo in the management of childhood asthma. Journal of Investigational Allergology and Clinical Immunology 1999;9(5):293‐8. [PubMed] [Google Scholar]
Spezia 1993 {published data only}
- Spezia E, Col G, Richelli C, Boner AL. Nedocromil versus sodium cromoglycate pressurised aerosol in the prevention of bronchoconstriction induced by ultrasonic nebulised distilled water in asthmatic children. Pediatric Pulmonology 1993;16:243‐7. [DOI] [PubMed] [Google Scholar]
Stelmach 2001 {published data only}
- Stelmach I, Jerynska J, Brzozowska A, Kuna P. Double‐blind, randomised, placebo‐controlled trial of effect of nedocromil sodium on clinical and inflammatory parameters of asthma in children allergic to dust mite. Allergy 2001;56(6):518‐24. [DOI] [PubMed] [Google Scholar]
Stelmach 2002a {published data only}
- Stelmach I, Grzelewski T, Stelmach W, Majak P, Jerzynska J, Gorski P, et al. Effect of triamcinolone acetonide, montelukast, nedocromil sodium, formoterol on eosinophil blood counts,ECP serum levels and clinical parameters in children with asthma. Polski Merkuriusz Lekarski 2002;12(68):99‐103. [PubMed] [Google Scholar]
Stelmach 2002b {published data only}
- Stelmach I, Grzelewski T, Stelmach W, Majak P, Jerzynska J, Gorski P, et al. The effect of nedocromil sodium on levels of IL‐4 and IgE in serum of children with bronchial asthma. Polski Merkuriusz Lekarski 2002;12(69):214‐7. [PubMed] [Google Scholar]
van Bever 1996 {published data only}
- Bever HP, Razzouk H, Potkamp J. Nedocromil sodium in childhood asthma [Abstract]. European Respiratory Journal 1996;9(Suppl 23):229s, abstract no. P1432. [Google Scholar]
Wonne 1990 {published data only}
- Wonne R, Monkhoff M, Ahrens P, Hofmann D. The study of protective action of nedocromil sodium with bronchial cold‐air provocation in children with bronchial asthma. Pneumologie 1990;44(10):1193‐5. [PubMed] [Google Scholar]
References to studies excluded from this review
Armenio 1993 {published data only}
- Armenio L, Baldini G, Bardare M, Boner A, Burgio R, Cavagni G, et al. Double blind, placebo controlled study of nedocromil sodium in asthma. Archives of Disease in Childhood 1993;68:193‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bel 1990 {published data only}
- Bel EH, Timmers MC, Hermans J, Dijkman JH, Sterk PJ. The long term effects of nedocromil sodium and Beclomethasone propionate responsiveness to methacholine in non‐atopic asthmatic subjects. The American Review of Respiratory Disease 1990;141:21‐8. [DOI] [PubMed] [Google Scholar]
Bergmann 1989 {published data only}
- Bergmann KCh, Bauer CP, Overlack A. A placebo‐controlled, blind comparison of nedocromil sodium and beclomethasone propionate in bronchial asthma. Current Medical Research and Opinion 1989;11(8):533‐42. [DOI] [PubMed] [Google Scholar]
Bergmann 1989a {published data only}
- Bergmann KCh, Bauer CP, Overlack A. Nedocromil sodium and beclomethasone dipropionate in asthma therapy. A placebo group comparative study. Atemw‐Lungenkrkh.Jahrgang 1989;15(5):193‐9. [Google Scholar]
Bergmann 1990 {published data only}
- Bergmann KCh, Bauer CP, Overlack A. A Placebo controlled blinded comparison of nedocromil sodium and beclomethasone propionate in bronchial asthma. Lung 1990;168(Suppl):230‐9. [DOI] [PubMed] [Google Scholar]
Bianco 1989 {published data only}
- Bianco S, Bono N, Grassi V, Orefice U. Effectiveness of nedocromil sodium versus placebo as additions to routine maintenance therapy: a multicentre, double blind, group comparative trial. Respiration 1989;56:204‐11. [DOI] [PubMed] [Google Scholar]
Boos 1989 {published data only}
- Boos R, Kampen C, Weede W, Beck W. Nedocromil sodium therapy in asthma patients. Therapeutic effect in addition to treatment with oral theophylline and inhaled bronchodilator agents. Fortschritte der Medizin 1989;107:712‐6. [PubMed] [Google Scholar]
Bruce 2006 {published data only}
- Bruce CT, Zhao D, Yates DH, Thomas PS. AMP challenge induces a decrease in FENO in asthmatic subjects modulated by nedocromil. European Journal of Clinical Investigation 2006;36(12):899‐905. [DOI] [PubMed] [Google Scholar]
Chan 2001 {published data only}
- Chan PW, Debruyne JA. Inhaled nedocromil sodium for persistent cough in children. The Medical Journal of Malaysia 2001;56:408‐13. [PubMed] [Google Scholar]
Clancy 1994 {published data only}
- Clancy L, Keogan S. Treatment of nocturnal asthma with nedocromil sodium. Thorax 1994;49:1225‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Corrias 1990 {published data only}
- Corrias A, Minelli R, Pelosi U, Loi M, Pazzola P, Cubboni G, et al. Nedocromil in the therapy of chronic asthma in children. Ped Med Chir (Med surg Ped) 1990;12:157‐60. [PubMed] [Google Scholar]
Creticos 1995 {published data only}
- Creticos P, Burk J, Smith L, Comp R, Norman P, Findlay S. The use of twice daily nedocromil sodium in the treatment of asthma. Journal of Allergy and Clinical Immunology 1995;95:829‐36. [DOI] [PubMed] [Google Scholar]
Cua‐ Lim 1986 {published data only}
- Cua‐Lim F, Agbayani F, Lachica D. A double‐blind comparative trial of nedocromil sodium and placebo in the management of bronchial asthma in patients in routinely using oral bronchodilators. European Journal of Respiratory Diseases 1986;69:306‐10. [PubMed] [Google Scholar]
del Ponte 1997 {published data only}
- Ponte A, Marinari S, Lella M, D'intino D, Martino L, Benedetto F. Outcomes of nedocromil long‐term treatment in childhood bronchial asthma [Abstract]. Monatsschrift fur Kinderheilkunde 1997;145:322. [Google Scholar]
Edwards 1992 {published data only}
- Edwards AM, Stevens MT. The clinical efficacy of inhaled nedocromil sodium (Tilade) in the treatment of asthma. European Journal of Respiratory Diseases 1992;6:35‐41. [PubMed] [Google Scholar]
Fink 1994 {published data only}
- Fink JN, Forman S, Silvers WS, Soifer MM, Tashkin DP, Wilson AF. A double blind study of the efficacy of nedocromil sodium in the management of asthma in patients using high doses of bronchodilators. Journal of Allergy and Clinical Immunology 1994;94:473‐81. [DOI] [PubMed] [Google Scholar]
Fyans 1986 {published data only}
- Fyans PG, Chatterjee PC, Chatterjee SS. A trial comparing nedocromil sodium (Tilade) and placebo in the management of bronchial asthma. Clinical Allergy 1986;16:505‐11. [DOI] [PubMed] [Google Scholar]
Greco 1986 {published data only}
- Greco DB, Brum Negreiros E, Chaieb JA, Ferreira‐ Lima P, Crice J. A multi centre double blind group comparative trial of two dose levels of nedocromil sodium and placebo in the management of perennial extrinsic asthma. European Journal of Respiratory Diseases 1986;69:323‐6. [PubMed] [Google Scholar]
Joos 1989 {published data only}
- Joos GF, Pauwels RA, Straeton ME. The effect of nedocromil sodium on the bronchoconstrictor effect of neurokinin A in subjects with asthma. The Journal of Allergy and Clinical Immunology 1989;83:663‐8. [DOI] [PubMed] [Google Scholar]
Koskela 2005 {published data only}
- Koskela HO, Martens R, Brannan JD, Anderson SD, Leuppi J, Chan HK. Dissociation in the effect of nedocromil on mannitol‐induced cough or bronchoconstriction in asthmatic subjects. Respirology 2005;10(4):442‐8. [DOI] [PubMed] [Google Scholar]
Marin 1996 {published data only}
- Marin JM, Carrizo SJ, Garcia R, Ejea MV. Effects of nedocromil sodium in steroid resistant asthma: A randomised controlled trial. Journal of Allergy and Clinical Immunology 1996;97:602‐10. [DOI] [PubMed] [Google Scholar]
Oggiano 1995 {published data only}
- Oggiano N, Kantar A, Bruni S, Cutrona FM, Fabbrizi E, Piccinini R, et al. Effect of inhaled nedocromil sodium therapy on blood gas changes in children challenged with ultrasonically nebulised distilled water. Minerva Pediatrica 1995;47:427‐31. [PubMed] [Google Scholar]
Paananen 1986 {published data only}
- Paananen M, Karakorpi T, Kreus KE. Withdrawal of inhaled corticosteroid under cover of nedocromil sodium. European Journal of Respiratory Diseases 1986;69:330‐5. [PubMed] [Google Scholar]
Rebuck 1990 {published data only}
- Rebuck AS, Kesten S, Boulet LP, Cartier A, Cockcroft D, Gruber J, et al. A 3‐ month evaluation of the efficacy of nedocromil sodium in asthma: A randomised, double‐blind, placebo‐controlled trial of nedocromil sodium conducted by a Canadian multicenter study group. Journal of Allergy and Clinical Immunology 1990;85:612‐7. [DOI] [PubMed] [Google Scholar]
Ruffin 1986 {published data only}
- Ruffin R, Alpers JH, Kroemer DK, Rubinfeld AR, Pain MCF, Czarny D, et al. A 4 ‐week Australian study of nedocromil sodium in asthmatic patients. European Journal of Respiratory Diseases 1986;69:336‐9. [PubMed] [Google Scholar]
Ruffin 1987 {published data only}
- Ruffin R, Alpers JH, Rubinfeld AR, Pain MCF, Czarny D, Bowes G. The efficacy of nedocromil sodium (Tilade) in asthma. Australian and New Zealand Journal of Medicine 1987;17:557‐61. [DOI] [PubMed] [Google Scholar]
Van As 1986 {published data only}
- As A, Chick TW, Bodman SF, Storms WW, Nathan RA, Selner JC, et al. A group comparative study of the safety and efficacy of nedocromil sodium (Tilade) in reversible airways disease: a preliminary report. European Journal of Respiratory Diseases 1986;69:143‐8. [PubMed] [Google Scholar]
Wells 1992 {published data only}
- Wells A, Drennan C, Holst P, Jones D, Rea H, Thornley P. A multicentre double‐blind cross‐over trial comparing Tilade (Nedocromil sodium) at 2 dosage frequencies and placebo in the management of bronchial asthma [Abstract]. Australia & New Zealand Journal of Medicine 1990;20(3 suppl 1):541. [Google Scholar]
- Wells A, Drennan C, Holst P, Jones D, Rea H, Thornley P. Comparison of nedocromil sodium at two dosage frequencies with placebo in the management of chronic asthma. Respiratory Medicine 1992;86:311‐6. [DOI] [PubMed] [Google Scholar]
Youngchaiyud 1986 {published data only}
- Youngchaiyud P, Lee TB. Effect of nedocromil sodium on the immediate response to antigen challenge in asthmatic patients. Clinical Allergy 1986;16:129‐34. [DOI] [PubMed] [Google Scholar]
Additional references
Adams 2005
- Adams NP, Bestall JC, Lasserson TJ, Jones PW. Fluticasone versus placebo for chronic asthma in adults and children. Cochrane Database of Systematic Reviews 2005, Issue 4. [Art. No.: CD003135. DOI: 10.1002/14651858.CD003135.pub3] [DOI] [PubMed] [Google Scholar]
Bassler 2004
- Bassler D, Mitra A, Ducharme FM, Forster J, Schwarzer G. Ketotifen alone or as additional medication for long‐term control of asthma and wheeze in children. Cochrane Database of Systematic Reviews 2004, Issue 1. [Art. No.: CD001384. DOI: 10.1002/14651858.CD001384.pub2.] [DOI] [PMC free article] [PubMed] [Google Scholar]
BTS 1997
- British Thoracic society. The British guidelines on asthma management: 1995 review and position statement. Thorax 1997; Vol. 52, issue Suppl 1:S 1‐21.
BTS 2003
- British Thoracic Society. British Guidelines on Asthma Management. Thorax 2003; Vol. 58, issue Suppl 1.
CONSORT 2001
- Moher D, Schulz KF, Altman DG, CONSORT GROUP (Consolidated Standards of Reporting Trials). The CONSORT statement: revised recommendations for improving the quality of reports of parallel‐group randomized trials. Annals of Internal Medicine 2001;134:657‐62. [DOI] [PubMed] [Google Scholar]
Dersimonian 1986
- Dersimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7:177‐88. [DOI] [PubMed] [Google Scholar]
Edwards 1993
- Edwards AM, Stevens MT. Clinical efficacy of inhaled nedocromil sodium (Tilade) in the treatment of asthma. European Respiratory Journal 1993;6:35‐41. [PubMed] [Google Scholar]
GINA 1995
- National Asthma Education and Prevention Program. Global initiative for asthma management and prevention NHBLI /WHO workshop report. National Institute of Bethesda, MD 1995. NIH publication No: 95‐3659.
Jadad 1996
- Jadad AR, Moor A, Carroll D, Jenkinson C, Reynolds JM, Gavaghan DJ, et al. Assessing the quality of reports of randomised clinical trials: is blinding necessary?. Controlled Clinical Trials 1996;17:1‐12. [DOI] [PubMed] [Google Scholar]
NIH 1997
- National Asthma Education and Prevention Program. Expert panel report 2: Guidelines for the diagnosis and management of asthma: National Heart, Lung and Blood institute. Bethesda, 1997. NIH publication 97‐4051.
Pauwels 2003
- Pauwels RA, Pedersen S, Busse WW, Tan WC, Chen YZ, Ohlsson SV, et al. Early intervention with budesonide in mild persistent asthma: a randomised, double‐blind trial. Lancet 2003;361(9363):1071‐6. [DOI] [PubMed] [Google Scholar]
Rainey 1992
- Rainey DK. Evidence for the anti‐inflammatory activity of nedocromil sodium. Clinical and Experimental Allergy 1992;22:976‐9. [DOI] [PubMed] [Google Scholar]
Reddel 1999
- Reddel H, Ware S, Marks G, Salome C, Jenkins C, Woolcock A. Differences between asthma exacerbations and poor asthma control. The Lancet 1999;353(9150):364‐9. [DOI] [PubMed] [Google Scholar]
Sharek 1999
- Sharek PJ, Bergman DA. Beclomethasone for asthma in children: effects on linear. Cochrane Database of Systematic Reviews 1999, Issue 3. [Art. No.: CD001282. DOI: 10.1002/14651858.CD001282.] [DOI] [PMC free article] [PubMed] [Google Scholar]
Silverman 1997
- Silverman M, Wilson N. Asthma‐time for a change of name?. Archives of Disease in Childhood 1997;77(7):62‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Spooner 2002
- Spooner CH, Saunders LD, Rowe BH. Nedocromil sodium for preventing exercise induced bronchoconstriction. Cochrane Database of Systematic Reviews 2002, Issue 1. [Art. No.: CD001183. DOI: 10.1002/14651858.CD001183.] [DOI] [PubMed] [Google Scholar]
Van Houwelingen 1995
- Houwelingen HC. Meta‐analysis; methods, limitations and applications. Biocybernetics Biomed Eng 1995;15:53‐61. [Google Scholar]
Warner 1998
- Warner JO, Naspitz CK. Third international Pediatric Consensus Statement on the Management of Childhood Asthma. Pediatric Pulmonology 1998;25:1‐17. [DOI] [PubMed] [Google Scholar]


