Abstract
Objective:
Interest in low-dose radiotherapy (LD-RT) for the symptomatic treatment of nonmalignant conditions, including inflammatory and degenerative disorders of the joints and para-articular soft tissues, has increased substantially in recent years. In the present document, we provide a CT-based contouring atlas to help identify and delineate the most common osteoarticular regions susceptible to LD-RT.
Methods:
The clinical efficacy of LD-RT is supported by a large body of evidence. However, there is no consensus on the parameters for contouring the planning target volume (PTV). Moreover, 3D simulation and planning should be the standard of care even for nonmalignant disorders. For this reason, the present guidelines were prepared to help guide PTV contouring based on CT images, with the same quality criteria for patient immobilization, treatment simulation, planning and delivery as those routinely applied for cancer radiotherapy.
Results:
PTV for radiotherapy requires precise identification of the target areas based on CT and other imaging techniques. Using a series of cases treated at our institution, we have defined the PTVs for each location on the simulation CT to establish the relationship between the image and the anatomical structures to be treated. We also specify the immobilization systems used to ensure treatment accuracy and reproducibility.
Conclusions:
This comprehensive atlas based on CT images may be of value to radiation oncologists who wish to use LD-RT for the symptomatic treatment of degenerative or inflammatory osteoarticular diseases.
Advances in knowledge:
The recommendations and contouring atlas described in this article provide an eminently practical tool for LD-RT in non-malignant conditions, based on the same quality criteria recommended for all modern radiotherapy treatments in Spain.
Background
Degenerative disorders of the joints and para-articular soft tissues are an important challenge for developed countries. It is estimated that nearly one-fourth of the general adult population in high-income countries suffers from osteoarthritis—including 10% of males and 18% of females over age 60. Osteoarthritis is a painful condition that causes stiffness and thus negatively impacts the individual’s ability to perform many activities of daily living. Due to population ageing in the developed world, the prevalence of degenerative osteoarticular diseases continues to rise, leading to increasing disability, which is associated with high social and economic costs. A recent report by the World Health Organisation (WHO) stated that osteoarthritis would be the fourth leading cause of disability by the year 2020.1
Available treatments, both pharmacological and non-pharmacological, have only a limited efficacy that commonly decreases over time. Common conservative measures include weight loss, moderate exercise and physical rehabilitation (local heat, magnetic therapy, shock waves, etc.). Analgesics and non-steroidal anti-inflammatory drugs (NSAIDs), SYSADOAs (symptomatic slow acting drugs for osteoarthritis), corticosteroids, anaesthetics and other local injections, have also been proposed for symptom relief. However, many patients will eventually require prosthetic replacement of the damaged joint, which requires surgery followed by a long rehabilitation process that is often not well tolerated by patients. Moreover, none of the available surgical or pharmacological treatments are without possible side-effects (e.g. gastrointestinal bleeding, kidney disorders, cardiac disorders, etc.), some of which can be severe and even compromise the patient’s life.2 For this reason, many groups have investigated affordable therapeutic alternatives, especially among the elderly in whom comorbidities and polypharmacy are common.3
Radiotherapy is a non-invasive treatment that does not interfere with other treatments commonly offered to elderly patients. The effectiveness of radiotherapy for pain relief and improved functionality was demonstrated decades ago. Although there still exist some concerns about the risk of low-dose radiotherapy (LD-RT) to induce cancer, these fears are largely unfounded. Published data show that the risk of induced cancer is low—ranging from 0.5 to 40 cases per 1000 treatments—and up to three times lower for elderly patients.4–8 In Germany, approximately 10–30% of daily activity at most radiation oncology departments involves radiotherapy for non-malignant conditions.9 Moreover, in recent years, several medical associations have published clinical guidelines and recommendations for the use of radiotherapy for the treatment of non-malignant conditions, including the German Radiation Oncology Society (DEGRO) in Germany and the Royal College of Radiologists (RCR) in the United Kingdom.10–14
Ionizing radiation has long been used to treat cancer and other conditions. Due to technological advances, radiotherapy is constantly improving and becoming safer and more effective. Naturally, treatment planning and delivery has changed as newer technologies become available. While there is a clear emphasis on offering safe and effective radiotherapy for the treatment of cancer, it is important to apply the same criteria to the treatment of non-malignant conditions. In other words, the simulation, planning procedure documentation, radiotherapy administration and subsequent monitoring processes for non-malignant diseases must be applied with the same requirements and quality control as those used in the treatment of cancer.9,10,15,16 Similarly, the volume to be irradiated (planning target volume [PTV]) should be carefully contoured using the planning computed tomography (CT) scan and any other images that are necessary, such as magnetic resonance imaging (MRI) and/or ultrasound, which may help to identify soft tissue involvement.
Although general guidelines are available for the indications and basic contouring recommendations for radiotherapy of benign diseases,17 most of the series published to date did not use simulation techniques based on CT images or three-dimensional (3D) planning. We have developed a CT-based anatomical atlas to fill this gap and to allow radiation oncologists to easily identify the target volume at each location, but always keeping in mind that these recommendations should be taken exclusively as an expression of the usual practice in our department. Our clinical protocols contemplate the use of similar procedures for the simulation, planning and treatment of both oncological pathology and benign diseases. However, we are aware that CT planning could be safely omitted for reasons of radiation protection when referring to the radiation treatment of small joints of the hands, feet, elbows, or heels, since the irradiation region can be adequately defined clinically.
Methods and materials
Joints are complex structures that allow movement between different parts of the body. Diverse tissues and structures such as cartilage, synovial membrane, tendons, fascia, and bursas all play important roles in joint function. Damage to any of these structures can lead to inflammation and/or pain. Depending on the damaged structure, numerous conditions can develop, including arthritis (damage to the articular cartilage), inflammatory conditions such as bursitis (inflammation of the bursa), synovitis (inflammation of the cell layer that lines the tendons), tendonitis (inflamed tendons), and fasciitis (involvement of fascia, especially the plantar fascia).
Target volumes for these disorders should include the entire joint and articular cartilage, the specific joint capsule, the neighbouring bone and/or muscular insertion zone, and the peritendinous bursae and surrounding soft-tissue structures.15
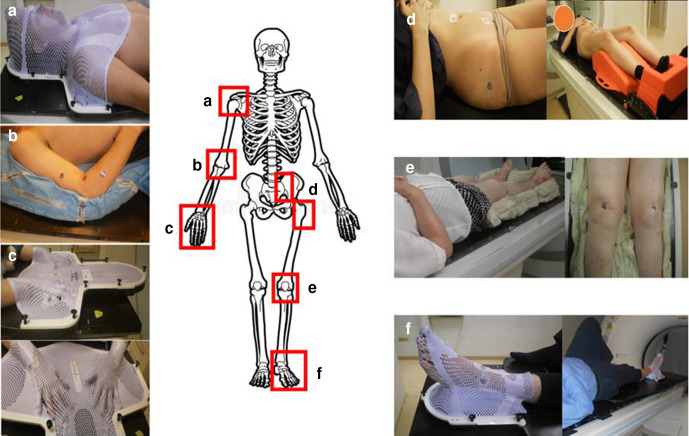
In the present document, we have reviewed the most common osteoarticular diseases treated at our institution and defined the PTV for each condition on a cross-sectional CT scan. Following our department protocols, in all cases, the immobilization procedure for treatment was the same applied in cancer radiotherapy, using vacuum mattresses and custom thermoplastic masks or commercially available immobilizers (Figure 1). For each treatment location, we present a CT study, highlighting in the 3D reconstruction the PTV defined in each CTslice as well as its anatomical correlation on anatomical illustrations from Gray’s Anatomy of the Human Body.18
Figure 1.
Immobilization procedure for LD-RT of shoulder (a), elbow (b), hand (c), pelvis and hip (d), knee (e) and foot and ankle (f).
Table 1 summarizes the proposed PTV definitions for the osteoarticular locations evaluated.
Table 1.
Proposed limits of PTV fo r different osteoarticular locations
| SUPERIOR | INFERIOR | MEDIAL | LATERAL | PROXIMAL | DISTAL | |
|---|---|---|---|---|---|---|
|
POST: 1 cm above acromion ANT: coracoid process |
1.5 cm under lesser tubercle | POST: 1.5 cm scapula ANT: 1.5 cm clavicle |
Lateral side-of humeral head, neck and epiphysis and 1 cm of surrounding soft tissues | ||
|
1.5 cm above trochlea and capitellum | 1.5 cm below radial and ulnar tuberosities | 1.5 cm around bone edges into surrounding soft tissues | 1.5 cm around bone edges into surrounding soft tissues | ||
|
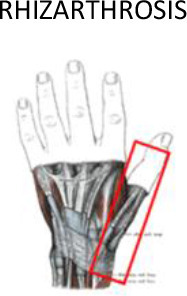
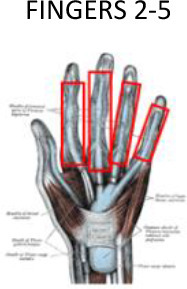
1 cm around of the soft tissues surrounding the bones | 1 cm around of the soft tissues surrounding the bones | Half of the metacarpal bone, the joint with the trapezoid bone and 1 cm through the radial bone | Proximal third of the distal phalanx | ||
|
2 mm inside the dorsal skin | 2 mm inside the ventral skin | 1 cm around of the soft tissues surrounding | 1 cm around of the soft tissues surrounding |
|
|
|
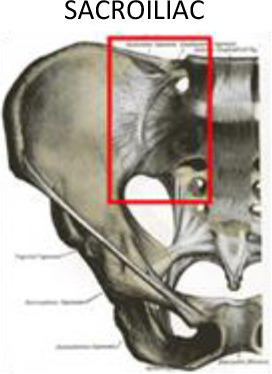
1 cm above sacroiliac joint | 1 cm below sacroiliac joint | 1 cm into sacral bone | 1 cm into iliac bone | ||
|
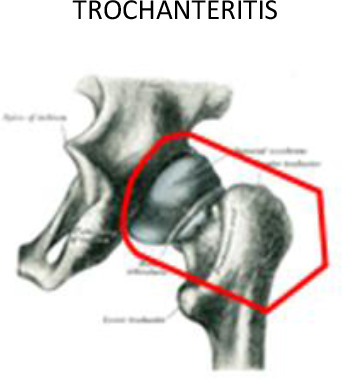
Trochanter, femoral head and neck | Below lesser trochanter | 1 cm around of the soft tissues surrounding | 1 cm around of the soft tissues surrounding | ||
|
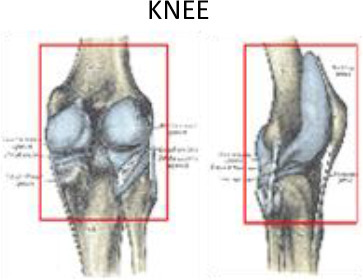
2 cm above femoral condyles | 2 cm below tibial condyles and fibula head | 1 cm around of the soft tissues surrounding | 1 cm around of the soft tissues surrounding | 1 cm above lateral and medial femoral epicondyle | Lateral and medial tibial condyles and fibula head |
|
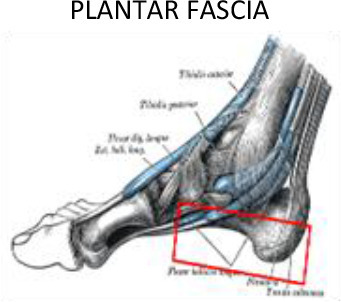
1 cm around of the soft tissues surrounding | 1 cm around of the soft tissues surrounding | Metacarpal-phalanx joint | Calcaneus tuberosity | ||
|
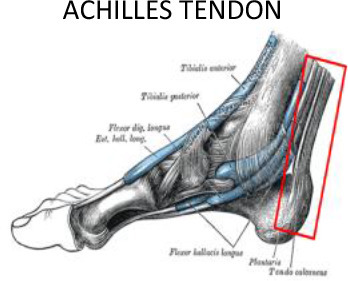
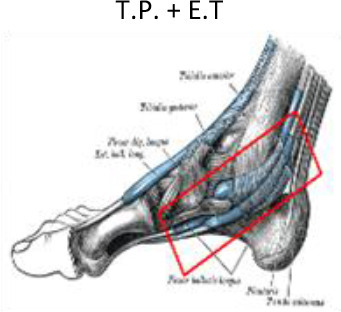
1 cm around the tendon | 1 cm around the tendon | Lateral and medial malleolus | Calcaneus tuberosity, subcutaneous calcaneus bursa, submuscular calcaneus bursa | ||
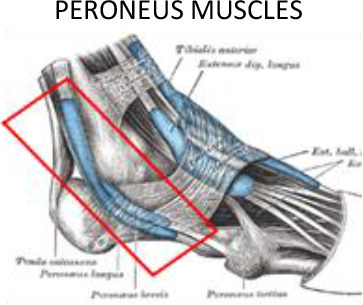
|
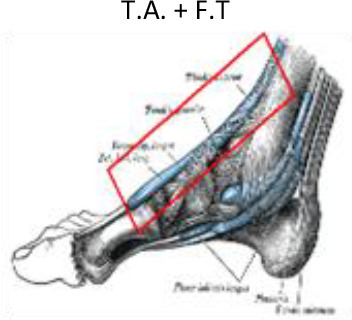
1 cm around the tendon | 1 cm around the tendon | 2 cm above medial malleolus |
|
||
|
1 cm around the tendon | 1 cm around the tendon | 2 cm above lateral malleolus |
|
||
|
1 cm around the tendon | 1 cm around the tendon | 2 cm above lateral malleolus | fifth metatarsal bone, lateral side. |
E.T, Extensor tendons of the toes; F.T, Flexor tendons of the toes; T.A, Tibialis anterior; T.P, Tibilais posterior.
Shoulder
The shoulder includes the acromioclavicular joint between the acromion and the clavicle, and the glenohumeral joint, which involves articulation between the glenoid cavity of the scapula and the humeral head. Shoulder osteoarthritis affects approximately 30% of the elderly population.19
After radiotherapy for benign shoulder conditions, reported improvement rates in terms of reduced pain and increased shoulder mobility range from 37 to 56% immediately after treatment, rising to 73 to 85% during follow-up (up to three years in some studies).20–24 However, it is important to note that the radiation technique applied in most of these studies was either direct apposition of orthovoltage fields or simple two-dimensional (2D) planning with 10 × 10/12 × 12/10 × 15 cm2 or rectangular fields that completely encompassed the shoulder joint with two beam angles (anteroposterior and posteroanterior). Very few of those studies used CT images for simulation.
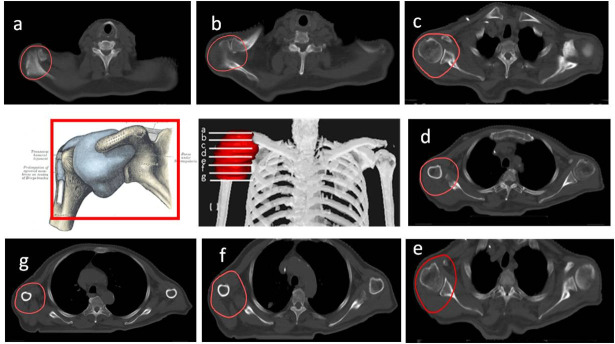
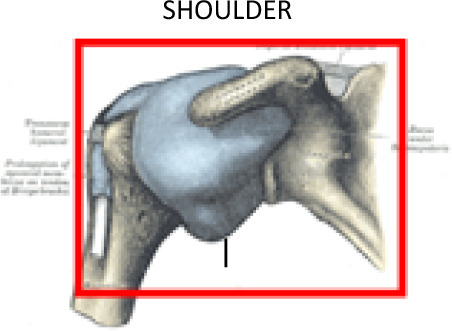
Painful shoulder syndrome is a set of progressive conditions that progress from one to the next, including subacromial impingement, shoulder capsulitis, biceps longus tendinitis or rotator cuff tendinitis, and true degenerative arthrosis of the acromioclavicular or glenohumeral joints. However, in most cases, there are two or more overlapping conditions present at the same time,25 which is why the DEGRO recommends using the same PTV to encompass the entire shoulder joint and adjacent bony and muscular structures (acromion, glenohumeral joint, and the coracoid process; acromioclavicular ligament, coraco-acromial ligament, coraco-humeral ligament, transverse ligament and the superior-middle-inferior glenohumeral ligaments). The synovial bursae located around the shoulder should also be included. These bursae include the following: subacromial-subdeltoid (between the joint capsule and the deltoid muscle), subacromial (between the capsule and the acromion), subcoracoid (between the capsule and the coracoid process of the scapula), coracobrachial (between the subscapularis muscle and the tendon of the coracobrachialis muscle), subscapular (between the capsule and the tendon of the subscapularis muscle), and the supra acromial bursa. The tendons that should be included are those that attach to the muscles (teres minor, infraspinous, subscapular and supraspinous) and to the origin of the deltoid, the long head of the biceps brachii muscle, trapezius insertion, pectoralis minor insertion and short head of the biceps brachii muscle. (Figure 2)17
Figure 2.
CT-based PTV contouring for painful shoulder syndrome (a–h).
Elbow
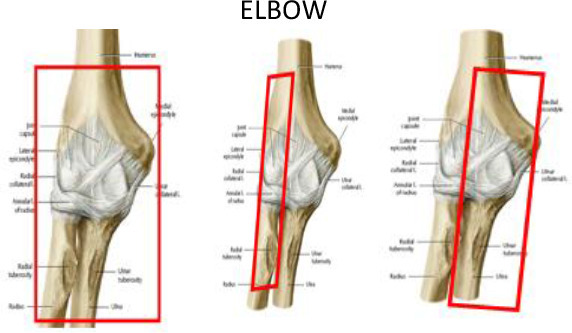
The distal humerus and proximal ulna and radius form the elbow joint connecting the upper arm to the forearm to allow for flexion/extension of the forearm. Chronic pain at the lateral epicondyle of the elbow is known as lateral epicondylitis or “tennis elbow”, while pain at the medial epicondyle is known as epicondylitis or “golfer’s elbow”, both of which are relatively common conditions estimated to affect from 1 to 3% of the population.26 These painful conditions are associated with chronic pathologic changes in tendon origins, although the underlying causes remain unclear. Reported pain relief rates achieved with LD-RT for tennis or golfer’s elbow range from 51 to 98%.27–30 Recently, Hautmann et al30 comprehensively reviewed the available evidence to assess the efficacy of radiation to relieve epicondylitis humeri (EPH)-related pain, finding a mean response rate of 80%. However, as those authors observed, these findings must be interpreted cautiously given the important methodological differences in the studies included in the review, such as differences in radiotherapy techniques and the time period when they were carried out. As in other joint locations, definition of the target volumes for EPH treatment, administered with either orthovoltage or a linear accelerator, was based on the physician’s experience and clinical examination. Generally, a single portal arrangement or two opposing rectangular fields (6 × 8 cm) were used.
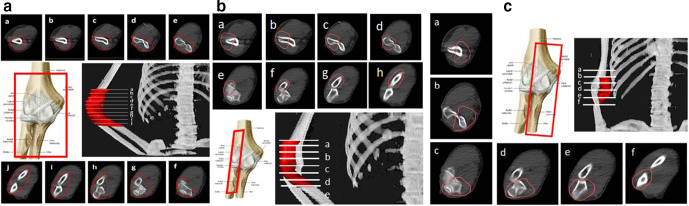
In cases with elbow joint arthrosis, the PTV may include the whole joint and its capsule (2 cm proximally and 3 cm distally). The PTV for radial epicondylitis (tennis elbow syndrome) should include 1–2 cm above the lateral humeral epicondyle, including its trochlea and condyle, the radius head, neck and tuberosity plus 1 cm distally; and for ulnar epicondylitis (golf elbow syndrome), the PTV should include 1–2 cm above the medial epicondyle, the ulna olecranon process, ulna coronoid process and its tuberosity, plus 1 cm distally. In terms of soft tissue, the following should be included: synovial bursae around the olecranon (superficial, subtendinous and intratendinous bursae) or the cubital fossa (bicipitoradial and interosseous bursae), ligaments (articular capsule, and the lateral, collateral, radial collateral, annular, accessory collateral and ulnar collateral ligaments) and tendon insertions in the area.(Figure 3a–c).
Figure 3.
CT-based PTV contouring for painful elbow syndrome (a–j).
Wrist, Hand and Fingers
The hand has numerous joints, including the distal radioulnar joint, radiocarpal joint, midcarpal joint, carpometacarpal joints and thumb and finger joints, which allow mobility of the hand and fingers. Osteoarthritis of the hand and fingers is one of the leading causes of disability worldwide. This condition affects both hand strength and function, thus leading to disability in activities of daily living. The overall prevalence of hand osteoarthritis is estimated to be 43.3%.31,32 LD-RT has been reported to provide pain relief in 56–90% of patients while also achieving a significant improvement in functionality and mobility. LD-RT appears to provide better pain relief in cases with thumb involvement (osteoarthritis at the base of the thumb) compared to cases involving the second to fifth fingers.33–36 In addition to osteoarthritis of the thumb and fingers, other inflammatory disorders likely to benefit from LD-RT are tendonitis and tenosynovitis of the hand and wrist, which can develop secondary to overuse, injury, or degenerative arthritis leading to swelling, pain and functional restriction. Trigger digits (stenosing tenosynovitis of the thumb and fingers), flexor and extensor tenosynovitis, and de Quervain disease are the most common clinical presentations.37
Although none of the studies published to date have precisely defined the treatment volume beyond the use of 6 × 4 cm or 6 × 6 cm fields, Kaltenborn et al33 analyzed the differences in symptomatic response to LD-RT, noting a significant improvement when the target volume was expanded (beyond the thumb saddle joint alone) to include the neighbouring thumb joints or the surrounding joints of the carpal bones.
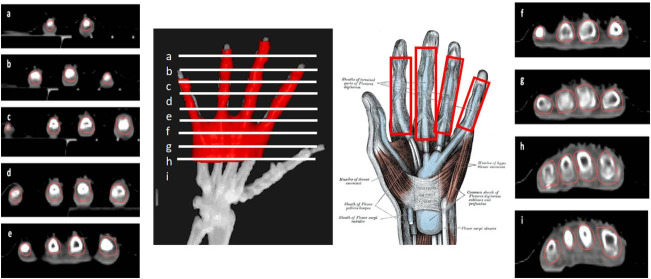
Figures 4–6 illustrate the proposed PTV contouring guidelines according to the specific hand or wrist joint or tendon. For osteoarthritis of the finger joints, the PTV should include the proximal and distal phalanges in the involved joint within 1 cm around the soft tissues in the second to fifth fingers. However, in case of osteoarthritis at the base of the thumb, the proximal limit of the PTV should include half of the metacarpal bone, the joint with the trapezoid bone and continue around 1 cm through the radial bone. To avoid damaging the fingernails, the distal segment of the fingers should be excluded.
Figure 4.
CT-based PTV contouring for second to fifth fingersosteoarthritis (a–i).
Figure 5.
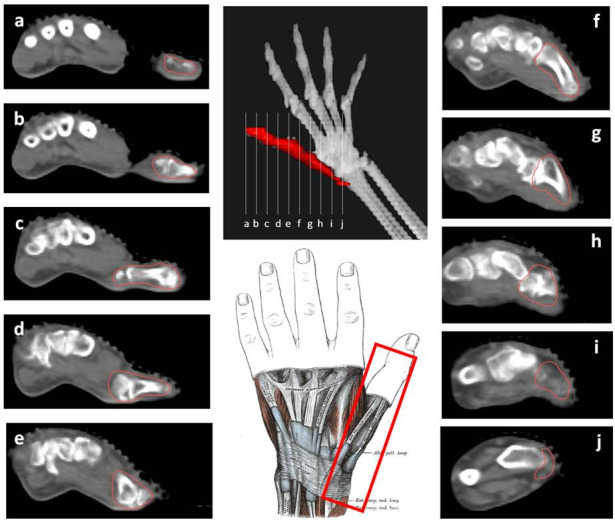
CT-based PTV contouring for thumb osteoarthritis (a–j).
Figure 6.
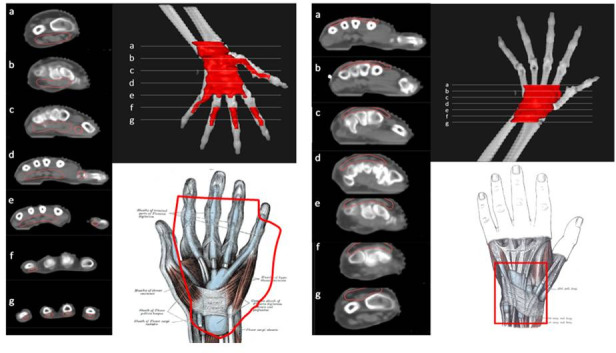
CT-based PTV contouring for flexor tendinitis (left, (a–g) and extensor tendinitis (right, a–g of the hand.
For the treatment of tendonitis and tenosynovitis, the involved tendon should be included from its distal bone insertion point until the beginning of the corresponding muscle fibres. The PTV should be expanded medially and laterally by 0.5–1 cm around the corresponding bones. The synovial sheaths of the involved tendons should be included as follows: (1) the common synovial sheath (ulnar bursa) for tendons of the flexor digitorum for fingers 2 to 5, and radial bursa for the flexor tendon of thumb from the insertion point in the distal phalanx to the ulna and radius heads, including the whole common synovial sheath or (2) the extensor retinaculum that holds tendons of the extensor digitorum in six different but attached compartments.
If the dosimetric distribution is inhomogeneous, a 5–10 mm bolus material can be placed on top of the hand/fingers.
Pelvis and Hip
The proximal area of the legs contains three structures that are especially vulnerable to inflammation and pain: the hip joint, the sacroiliac joint (sacroiliitis) and the trochanter (trochanteric bursitis). Sacroiliitis is a painful condition in which one or both sacroiliac joints become inflamed. This condition if often associated with other conditions, such as ankylosing spondylitis, psoriatic spondylo-arthropathy, pregnancy, reactive arthritis or ligament involvement. In the general population, the prevalence of sacroiliac disorders of any aetiology is estimated at 1.9%.38 Clinically, this disorder manifests as inflammatory low back pain that is exacerbated at night and improves with exercise.
Trochanteric bursitis is defined as the inflammation of any of the bursa around the trochanters, usually the greater trochanter. The pain caused by this condition is also known as meralgia paresthetica, typically occurring when walking up stairs or lying in the lateral position at night. This pain syndrome secondary to trochanteric bursitis affects 15% of females and 6.6% of males.39 The use of LD-RT to treat trochanteric bursitis has been shown to provide overall pain response rates (partial or total relief) ranging from 46–72%.40–42
Coxarthrosis is the consequence of series of events. First, there is a loss of cartilage, then bone spurs form around the joint, and finally, muscle weakness of the extremity appears.
Contrary to the lack of contouring evidence for other joints, several authors describe target volume based on planning CT that includes the trochanteric region and the peritendinous bursae of the great trochanter and femoral neck.
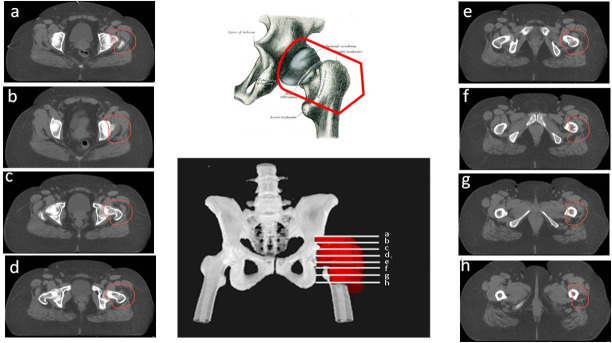
For the treatment of sacroiliitis, the PTV should include the whole joint between the sacral and iliac bones with a margin ≥1 cm on each side, including the sacroiliac ligaments (Figure 7). To treat greater trochanteric pain syndrome, the PTV should include the existing synovial bursae around the greater trochanter of the femur, as follows: the trochanteric bursa (superficial and posterior to the greater trochanter of the femur and subjacent to the iliotibial band), the gluteus medius bursa (between the gluteus medius muscle and the greater trochanter) and the gluteus minimus bursa (located beneath the gluteus minimus tendon at the anterosuperior edge of the greater trochanter) (Figures 7 and 8). In cases with major inflammation, the gluteus femoral bursa should be included caudally, with a final extension up to 7 cm in the craniocaudal direction. In addition, the musculature surrounding the trochanter must be included.
Figure 7.
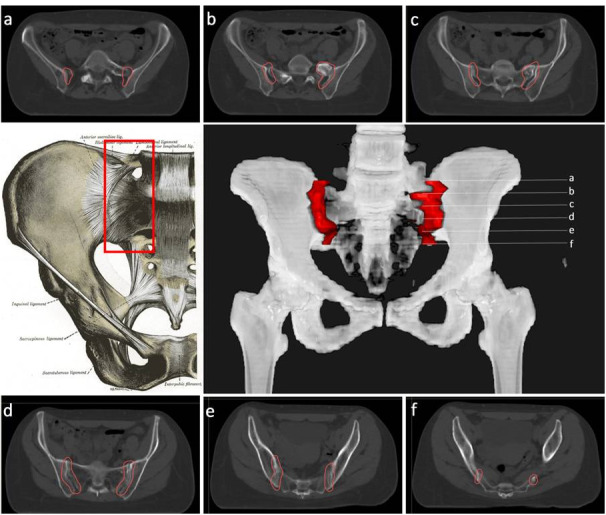
CT-based PTV contouring for sacroiliitis (a–f).
Figure 8.
CT-based PTV contouring trochanteric bursitis (a–h).
For the treatment of coxarthrosis, the PTV might include the whole joint between the iliac bone (acetabulum) and the femoral head, and 1 cm distally through the femoral head (Figure 9).
Figure 9.
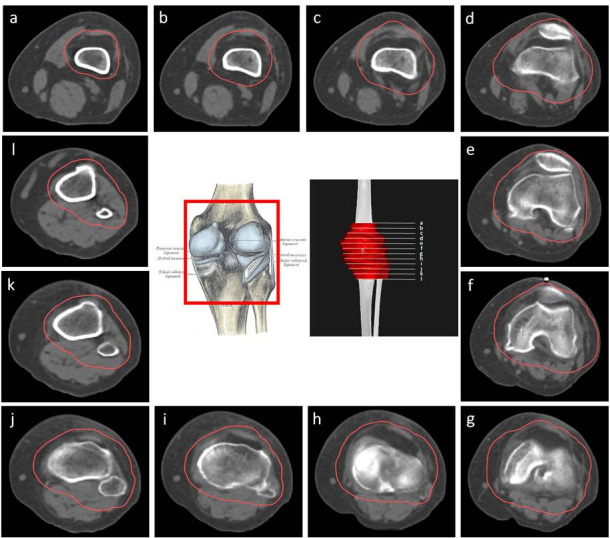
CT-based PTV contouring for knee osteoarthrItis (a–l).
Knee
Knee osteoarthritis is estimated to affect 10% of the population over age 55 and up to 40% of people aged 70–74 years.43,44 Painful gonarthrosis is probably one of the degenerative conditions most refractory to conservative treatment; nevertheless, LD-RT has been shown to achieve pain relief rates ranging from 29 to 49%.45–48 Most published studies have used either direct ventral and dorsal fields, or lateral and medial fields to plan radiotherapy treatments. Mucke et al46 evaluated the role of radiotherapy for the treatment of painful gonarthrosis in a national patterns of care study (2006 to 2008) that included 248 German radiotherapy centres.45 Data were collected on patient accrual, patient numbers, pre-treatment, pain record, treatment indications, RT technique, and target volume concepts. Notably, of the 238 responding centres (96%), only one reported using CT-based 3D planning technique
PTV delineation may include the whole knee joint (lateral and medial supracondylar ridges, capitulum, medial and lateral epicondyles, popliteal fossa, femoral trochlea, lateral and medial tibial condyles, tibial tuberosity, fibula head and the patella bone), the entire synovial capsuleand surrounding soft tissues and musculature, including the main knee bursae (prepatellar, infrapatellar [deep and superficial], suprapatellar, Pes Anserine, semimembranosus (popliteal), and the iliotibial and medial collateral ligament bursae). The collateral (lateral and medial) and cruciate tendons (anterior and posterior) in this area can also be considered for inclusion in the PTV. The tendons included are those located at the insertion at the fibula of the biceps femoris, origin of the peroneus longus, proximal third of the extensor digitorum longus, proximal third of the tibialis anterior, sartorius, semitendinosus, and gracilis insertions, medial head of the gastrocnemius, semimembranous, popliteus, proximal third of the tibialis posterior and origin of the soleus on the fibula head (Figure 10Figure 9).
Figure 10.
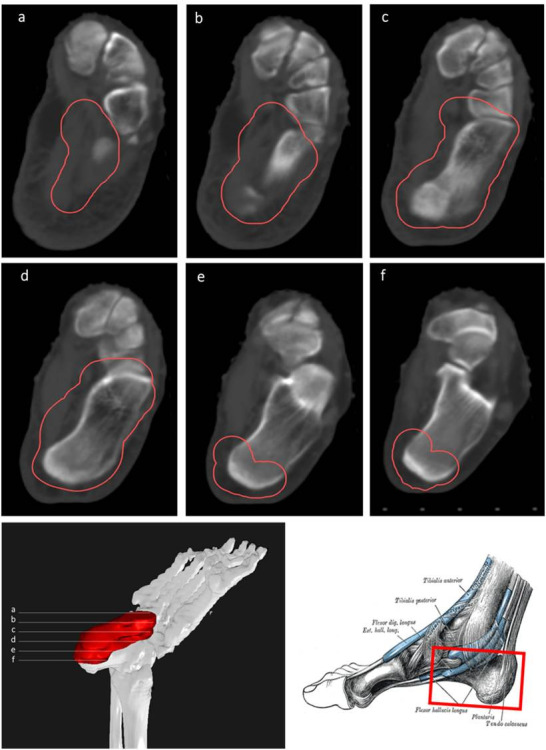
CT-based PTV contouring for plantar fasciitis (a–f).
Foot and ankle
The efficacy of LD-RT to treat calcaneodynia secondary to degenerative irritation of the plantar fascia originating at the medial calcaneal tuberosity of the heel and the surrounding perifascial structures is supported by a large body of evidence (Table 2). This syndrome affects approximately 10% of the general population, with prevalence rates among runners as high as 22%.
Table 2.
Main findings of studies published since the year 2000 that have evaluated the efficacy of LD-RT for degenerative or inflammatory musculoskeletal disorders
| Author & year | Type of study | N | Total dose (dose per fraction) | MFU (months) |
|---|---|---|---|---|
| Painful shoulder syndrome: periarthritis humeroscapularis | ||||
| Schultze 200413 | Retrospective | 94 | 6 Gy (0,75 Gy) | 4 |
| Niewald 200714 | Retrospective | 141 | 4–8 Gy (0.5–7 Gy) & Gy (1 Gy): 89% | 47 |
| Adamietz 201015 | Retrospective | 102 | 3 Gy (0.5 Gy) x two series | 18 |
| Ott 201416 | Randomized | 312 | 3 Gy (0.5 Gy) (51%) 6 Gy (1 Gy) (49%) | 35 |
| Micke 201817 | Retrospective | 162 | 12 × 0.5 Gy (74%) 6 × 1 Gy (26%) | 42 |
| Painful elbow syndrome: epicondylitis humeri | ||||
| Ott 201419 | Randomized | 216 | 3 Gy (0.5 Gy) (52%) 6 Gy (1 Gy) (48%) | 35 |
| Leszek 201520 | Retrospective | 50 | 6 Gy (1 Gy) | 12 |
| Hautmann 201921 | Retrospective | 138 | 3 Gy (0.5 Gy) (9.4%) 6 Gy (1 Gy) (89.9%) Other (0.8%) | 29 |
| Hautmann 202022 | Retrospective | 99 | Reirradiation: 3 Gy (0.5 Gy) (62.6%) 6 Gy (1 Gy) (37.4%) | 28 |
| Finger joint osteoarthritis | ||||
| Kaltenborn 201625 | Retrospective | 84 | 6 Gy (1 Gy) | 3 |
| Minten 201826 | Randomized | 56 | 6 × 1 Gy vs Sham radiotherapy | 3 |
| Alvarez 202027 | Prospective | 51 (25 rizarthrosis; 26 other finger joint osteoarthritis) | 6 Gy (1 Gy) | 8 |
| Rogers 202028 | Retrospective | 99 | 4 Gy (0.5 Gy) | NE |
| Trochanteric bursitis | ||||
| Valduvieco 201632 | Retrospective | 60 | 10 Gy (1 Gy) | 18.5 |
| Kaltenborn 201733 | Retrospective | 60 | 3 Gy (0.5 Gy) (39%) 6 Gy (1 Gy) (61%) | 18 |
| Micke 201817 | Retrospective | 70 | 12 × 0.5 Gy (94%) 6 × 1 Gy (4%) | 29 |
| Gonarthrosis | ||||
| Glatzel 200237 | Retrospective | 114 | 3–6 Gy (median, 6 Gy) (0.5–1 Gy, median 1 Gy) | 29 |
| Mucke 201038 | Retrospective | 4544 | Total dose: 3–12 Gy (median 6 Gy) Dose/fraction: 0.25–3 Gy (median 1 Gy) | NE |
| Keller 201339 | Retrospective | 1037 | Total dose: 0.5–10 Gy (median 4 Gy) Dose/fraction: 0.5–1.5 Gy (median 1 Gy) | NE |
| Micke 201817 | Retrospective | 139 | 12 × 0.5 Gy (80.6%) 6 × 1 Gy (19.4%) | 19.5 |
| Mahler 201940 | Randomized | 55 | 6 × 1 Gy vs Sham radiotherapy | 3 |
| Plantar fasciitis and Achiles tendinopathy | ||||
| Glatzel 200143 | Retrospective | 141 | 6 Gy (1 Gy) | 30 |
| Schneider 200444 | Retrospective | 62 | 5 Gy (0.25 Gy-1 Gy) | 40 |
| Muecke 200746 | Retrospective | 502 | 5–6 Gy (0.5–1 Gy) | 26 |
| Heyd 200747 | Randomized | 130 | 3 Gy (0.5 Gy) (50%) 6 Gy (1 Gy) (50%) | 6 |
| Hajtmanova 201048 | Retrospective | 323 | 4 Gy (1 Gy) | 3 |
| Niewald 201249 | Randomized | 62 | 0.6 Gy (0.1 Gy) (53%) 6 Gy (1 Gy) (47%) | 12 |
| Hermann 201350 | Retrospective | 250 | 3 Gy (0.5 Gy) (18%) 6 Gy (1 Gy) (72%) | 11 |
| Koca 201451 | Retrospective | 62 | 8 Gy (4 Gy) | 28 |
| Badakhshi 201452 | Retrospective | 171 | 3 Gy (0.5 Gy) | 54 |
| Uysal 201453 | Retrospective | 450 | 8 Gy (4 Gy) | 12 |
| Canyilmaz 201534 | Randomized | 124: 60 RT 64 Injection CE + AN | 6 Gy (1 Gy) vs Injection CE + AN | 12.5 |
| Niewald 201554 | Randomized | 117 | 6 Gy (1 Gyx6) vs 6 Gy (0.5 Gyx12) | 3 |
| Ott 201455 | Randomized | 457 | 3 Gy (0.5 Gy) (46%) 6 Gy (1 Gy) (54%) | 32 |
| Micke 201817 | Retrospective | 286 | 6 Gy (0.5 Gy) (92.6%) 6 Gy (1 Gy) (7.4%) | 34 |
| Ott 201556 | Randomized (Achilles tendinopathy) | 112 | 6 Gy (0.5 Gy) 6 Gy (1 Gy) | 24 |
Plantar fasciitis accounts for 11 to 15% of all foot symptoms in adults.49,50 Several studies have shown that pain relief rates range from 61–98%.51–64 In most of these studies, the target volume was determined by physical examination and conventional 2D-simulation. This volume generally covers the calcaneus with plantar aponeurosis, with an additional margin to include the neighbouring areas. However, heterogenous radiation fields have been reported.53 In our routine clinical practice, we define the field based on CT images encompassing the entire calcaneus, including the insertion points of the plantar fascia and the Achilles tendon. Nonetheless, other groups have suggested a more restrictive approach limited to the painful region with 2 cm margins extending into the neighbouring areas (Figure 10).
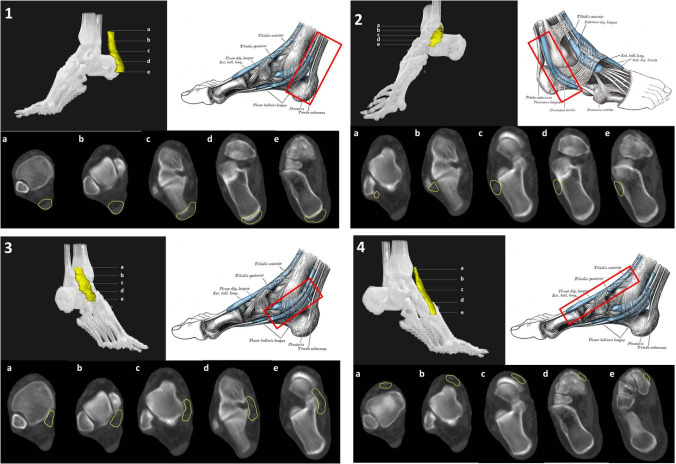
In addition to plantar fascia, LD-RT can provide symptomatic relief of other inflammatory lesions affecting the foot and ankle. The Achilles tendon crosses the ankle at its posterior aspect. Achilles tendinopathy is the most common ankle and heel injury, especially in athletes, with an incidence of 2.35 cases per 1000 persons per year in adults aged 20 to 60 years of age.65 LD-RT provides achillodynia relief in 90–95% of cases.24,66 Tarsal tunnel syndrome involving the foot arch can occur due to trauma, inflammatory diseases, or to the position of the valgus. In this case, the tendons under the retinacula of the flexors (posterior tibial tendon, flexors tendons) should be included in the PTV (Figure 11.1).
Figure 11.
CT-based PTV contouring for Achilles tendinopathy (11.1, a–e); peroneus longus and brevis tenosynovitis (11.2, a–e); tibialis anterior tendinopathy (11.3, a–e); tibialis posterior tendinopathy (11.4, a–e).
In addition to the Achilles tendon, other tendons located in the medial, lateral and anterior aspects of the ankle and foot may also be affected. These tendons include the tibialis posterior tendons and flexor tendons of the toes (flexor hallux longus and digitorum brevis), and the peroneus longus and brevis or tibialis anterior and toe extensor tendons (extensor hallux longus and digitorum brevis). All of these tendons can suffer from overload and inflammation leading to tendonitis, which may benefit from treatment with LD-RT. Although smaller volumes limited to the insertion of the plantar fascia and the calcaneal spur have been proposed by some authors,51–64 we recommend a PTV that encompasses the entire calcaneus and the region of the plantar aponeurosis, including insertion of the plantar fascia and the Achilles tendon (Figure 10). If the dosimetric distribution is not homogenous, a 5–10 mm bolus can be placed at the top of the Achilles tendon. For the treatment of tendonitis or tenosynovitis of the foot and ankle, the PTV should include the tendons and synovial sheaths appropriate to each anatomic location (Figure 11).
Discussion
The main objective of this review is to provide the radiation oncologist, when considered necessary, a set of simple tools to define radiotherapy treatment volumes for degenerative disorders of the joints and para-articular soft tissues based on current 3D planning criteria on CT images.
The efficacy of LD-RT for symptomatic relief of non-neoplastic degenerative or inflammatory disorders of joints and paraarticular soft tissues has been demonstrated through numerous studies conducted over the past 100 years. However, those studies are highly heterogenous in terms of the number of patients treated, anatomic locations, total doses, fractionation schedules, treatment volumes, irradiation technique, and methods used to assess response. As a result, it is difficult to draw firm conclusions supported by strong evidence. In fact, many of those studies had only a short follow-up, even although the analgesic effects of LD-RT typically increase over time, thus significantly improving long-term efficacy of LD-RT compared to the immediate post-RT results. In addition, a second course of local irradiation is typically necessary from 10 to 12 weeks after the first treatment for degenerative conditions with inflammation of joints and periarticular soft tissues. A patterns of care study carried out in Germany (n = 238 institutions) to assess the role of LD-RT for symptomatic osteoarthritis showed symptomatic pain relief in 79.5% of patients, although nearly 30% of the 4544 patients required a second course of radiotherapy 6–12 weeks after completion of the first treatment.16 Table 1 summarizes the results of studies published in the last 20 years (since the year 2000). In this table, we included only studies with at least 50 patients and adequate data about treatment volumes, total dose and fractionation.20–24,27,30,33–36,40,45–48,51,52,54–64,66 In most of these studies, a key limitation is the lack of a control group, which is important as the placebo effect cannot be ruled out. In this regard, well-designed, randomised controlled trials are needed.
Of the studies that have used modern radiotherapy planning and delivery techniques, the only study to randomise patients was the trial performed by Canylmaz et al62, who randomized 124 patients with plantar fasciitis to receive radiotherapy (6 Gy in six fractions of 1 Gy) or local injections of corticosteroids and anaesthetics. At a median follow-up of 12.5 months, pain relief was significantly higher in the radiotherapy group (68% vs 28%, p < 0.001).
Recently, the results of two well-designed trials were published. Minten et al26 evaluated the efficacy of LD-RT in 56 patients with hand osteoarthritis (finger joint or base of the thumb). Mahler and colleagues48 evaluated 55 patients with knee osteoarthritis. Both studies used an smart randomized, double-blind study design comparing active treatment—LD-RT (6 Gy in fractions of one Gy, three fractions/week) to sham radiotherapy. Reirradiation was not permitted in patients who showed partial response, and the final follow-up was three months after treatment completion. Importantly, neither study found between-group differences (radiotherapy vs sham RT) on any of the parameters evaluated. Despite these negative findings, it is important to underscore the relevant limitations of both studies: (1) the three month follow-up period is insufficient to conclude that LD-RT is not effective; (2) a second RT treatment was not performed when total relief was not achieved; and (3) the patient population had a pain history >5 years before irradiation and the available evidence suggests that it is more difficult to improve pain in patients with long-term pain.16,46,67,68
Even though the available data support LD-RT for symptomatic relief of degenerative and inflammatory diseases of the joints and periarticular soft tissue, many radiation oncologists remain reluctant to consider this approach as a standard therapeutic alternative, even after other therapeutic measures have failed,69 mainly due to the risk of radiation-induced malignancy. However, it is important to consider the following: (1) most evidence on the carcinogenic risks associated with ionising radiation comes from studies on survivors of the atomic bombs, which is obviously completely different from clinical radiotherapy; (2) current radiotherapy techniques allow us to delivery the radiation with a much greater accuracy than was possible with obsolete techniques used in the past; (3) molecular studies have demonstrated that LD-RT, in particular a single dose per fraction of 0.5 Gy, has no harmful effects on healthy non-inflamed joints, and 4) age is a determining factor in carcinogenic risk, which is markedly lower in elderly patients who are the usual candidates for LD-RT.4–7,70 Nevertheless, as a precaution, we do not offer LD-RT to patients under age 40.
Treatment volume definition (PTV) in radiotherapy for non-cancerous conditions requires an appropriate compromise between selecting a volume size that is sufficient to provide benefits while offering maximum protection of surrounding healthy tissues. PTVs should be tailored to the area of interest, but it is essential to keep in mind that excessive restriction of the PTV may decrease treatment effectiveness.25 On the other hand, given the low dose per fraction and low total dose administered in treatments that have an anti-inflammatory intention, no clearly defined constraints are currently available. Consequently, we strongly recommend application of the ALARA (as low as reasonably achievable https://www.cdc.gov/nceh/radiation/alara.html) principle for organs at risk for radiation doses outside the PTV.
To our knowledge, no CT-based imaging atlases are currently available to guide PTV contouring of benign osteoarticular diseases. For this reason, we have developed the present PTV definition atlas for the most common osteoarticular diseases, which will allow for the systematic definition of target volumes in patients who may benefit from low-dose radiotherapy. These recommendations are based on our clinical experience and aim to ensure the application of the same quality standards we use for the treatment of malignant tumours. We strongly believe that the CT-based contouring atlas and recommendations provided here will be of value to radiation oncologists interested in LD-RT for the symptomatic treatment of degenerative or inflammatory disorders of the joints and para-articular soft tissues, although, as we have said in the introduction, in certain cases it may not be necessary if there is sufficient experience and there is a way of clinical delimitation.
Footnotes
Conflicts of interest: All authors have made substantive contributions to the manuscript, read and approved the manuscript, and assume full responsibility for its content. All authors declare no sources of funding or financial interests, nor other conflicts of interest, regarding any part of this manuscript.
Beatriz Alvarez and Angel Montero have contributed equally to this study and should be considered as co-first authors.
Contributor Information
Beatriz Alvarez, Email: balvarez@hmhospitales.com.
Angel Montero, Email: angel.monteroluis@gmail.com.
Ovidio Hernando, Email: ohernando@hmhospitales.com.
Raquel Ciervide, Email: rciervide@hmhospitales.com.
Juan Garcia, Email: jgruizzorrilla@hmhospitales.com.
Mercedes Lopez, Email: mlopez@hmhospitales.com.
Mariola Garcia-Aranda, Email: mgarciaaranda@hmhospitales.com.
Xin Chen, Email: xchen@hmhospitales.com.
Ines Flores, Email: ines.flores.cacho@gmail.com.
Emilio Sanchez, Email: esanchezsaugar@hmhospitales.com.
Jeannette Valero, Email: jvalero@hmhospitales.com.
Alejandro Prado, Email: alejandropb_@hotmail.com.
Rosa Alonso, Email: rmalonso@hmhospitales.com.
Leyre Alonso, Email: lalonso@hmhospitales.com.
Pedro Fernandez-Leton, Email: pfernandezleton@hmhospitales.com.
Carmen Rubio, Email: crubio@hmhospitales.com.
REFERENCES
- 1.Woolf AD, Pfleger B. Burden of major musculoskeletal conditions bull. World Health Organ 2003; 81: 646–56. [PMC free article] [PubMed] [Google Scholar]
- 2.Wieland HA, Michaelis M, Kirschbaum BJ, Rudolphi KA. Osteoarthritis - an untreatable disease? Nat Rev Drug Discov 2005; 4: 331–44. doi: 10.1038/nrd1693 [DOI] [PubMed] [Google Scholar]
- 3.Micke O, Seegenschmiedt MH, Adamietz IA, Kundt G, Fakhrian K, Schaefer U, et al. Low-Dose radiation therapy for benign painful skeletal disorders: the typical treatment for the elderly patient? Int J Radiat Oncol Biol Phys 2017; 98: 958–63. doi: 10.1016/j.ijrobp.2016.12.012 [DOI] [PubMed] [Google Scholar]
- 4.Jansen JTM, Broerse JJ, Zoetelief J, Klein C, Seegenschmiedt HM. Estimation of the carcinogenic risk of radiotherapy of benign diseases from shoulder to heel. RadiotherOncol 2005; 76: 270–7. doi: 10.1016/j.radonc.2005.06.034 [DOI] [PubMed] [Google Scholar]
- 5.McKeown SR, Hatfield P, Prestwich RJD, Shaffer RE, Taylor RE. Radiotherapy for benign disease; assessing the risk of radiation-induced cancer following exposure to intermediate dose radiation. Br J Radiol 2015; 88: 20150405. doi: 10.1259/bjr.20150405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mazonakis M, Lyraraki E, Tzedakis A, Damilakis J. Radiotherapy for non-malignant shoulder syndrome: is there a risk for radiation-induced carcinogenesis? Phys Med 2017; 43: 73–8. doi: 10.1016/j.ejmp.2017.10.021 [DOI] [PubMed] [Google Scholar]
- 7.Deloch L, Rückert M, Fietkau R, Frey B, Gaipl US. Low-Dose radiotherapy has no harmful effects on key cells of healthy non-inflamed joints. Int J Mol Sci 2018; 19: 3197. doi: 10.3390/ijms19103197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Trott K-R, Kamprad F. Estimation of cancer risks from radiotherapy of benign diseases. Strahlenther Onkol 2006; 182: 431–6. doi: 10.1007/s00066-006-1542-8 [DOI] [PubMed] [Google Scholar]
- 9.Seegenschmiedt MH, Micke O, Muecke R, .the German Cooperative Group on Radiotherapy for Non-malignant Diseases (GCG-BD) . Radiotherapy for non-malignant disorders: state of the art and update of the evidence-based practice guidelines. Br J Radiol 2015; 88: 20150080. doi: 10.1259/bjr.20150080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reichl B, Block A, Schäfer U, Bert C, Mueller R, Jung H. DEGRO practical guidelines for radiotherapy of non-malignant disorders: Part I: physical principles, radiobiological mechanisms, and radiogenic risk Strahlenther. Onkol.: Organ der Deutschen Rontgen gesellschaft 2015; 191: 701–9. [DOI] [PubMed] [Google Scholar]
- 11.Ott OJ, Niewald M, Weitmann HD, Jacob I, Adamietz IA, Schaefer U. German Cooperative group on radiotherapy for benign diseases (GCG-BDDEGRO guidelines for the radiotherapy of non-malignant disorders. Strahlenther Onkol 2015; 191: 1–6. [DOI] [PubMed] [Google Scholar]
- 12.Seegenschmiedt MH, Micke O, Niewald M, Mücke R, Eich HT, Kriz J. DEGRO guidelines for the radiotherapy of non-malignant disorders Strahlenther. Onkol 2015; 191: 541–8. [DOI] [PubMed] [Google Scholar]
- 13.Reinartz G, Eich HT, Pohl F. German Cooperative Group on Radiotherapy for Benign Diseases (GCG-BD)DEGRO practical guidelines for the radiotherapy of non-malignant disorders – Part IV Strahlenther. Onkol 2015; 191: 295–302. [DOI] [PubMed] [Google Scholar]
- 14.Royal College of Radiologists .A review of the use of radiotherapy in the UK for the treatment of benign clinical conditions and benign tumors. London: Royal College of Radiologists; 2015. [Google Scholar]
- 15.Micke O, Seegenschmiedt MH. German Working group on radiotherapy in Germany. consensus guidelines for radiation therapy of benign diseases: a multicenter approach in Germany. Int J RadiatOncolBiol Phys 2002; 52: 496–513. [DOI] [PubMed] [Google Scholar]
- 16.Kriz J, Seegenschmiedt HM, Bartels A, et al. Updated strategies in the treatment of benign diseases-a patterns of care study of the German Cooperative group on benign diseases. AdvRadiatOncol 2018; 3: 240–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DEGRO. StrahlentherapiegutartigerErkrankungenFachgruppenspezifischeevidenzbasierte S2e-Leitlinie der DeutschenGesellschaftfürRadioonkologie (DEGRO). Version 2.0 vom 02.11.2018. 2018. Available from: https://www.degro.org/wp-content/uploads/2018/11/S2-Leitlinie-Strahlentherapie-gutartiger-Erkrankungen-update-2018-Endversion.pdf.
- 18.Gray H. Anatomy of the human body. 20th ed. Philadelphia: Lea & Febiger, 1918; 2000. www.bartleby.com/107/. [Google Scholar]
- 19.Chillemi C, Franceschini V. Shoulder osteoarthritis. Arthritis 2013; 2013: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schultze J, Schlichting G, Galalae R, Koltze H, Kimmig B. Results of radiation therapy in periarthritishumeroscapularis. Rontgenpraxis; Zeitschrift fur radiologischeTechnik 2004; 55: 160–4. [PubMed] [Google Scholar]
- 21.Niewald M, Fleckenstein J, Naumann S, Ruebe C. Long-Term results of radiotherapy for periarthritis of the shoulder: a retrospective evaluation. RadiationOncology 2007; 2: 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Adamietz B, Schulz-Wendtland R, Alibek S, Uder M, Sauer R, Ott O, et al. Calcifying Tendonitis of the shoulder joint. Strahlentherapie und Onkologie 2010; 186: 18–23. [DOI] [PubMed] [Google Scholar]
- 23.Ott OJ, Hertel S, Gaipl US, Frey B, Schmidt M, Fietkau R. The Erlangen dose optimization trial for radiotherapy of benign painful shoulder syndrome. Strahlentherapie und Onkologie 2014; 190: 394. [DOI] [PubMed] [Google Scholar]
- 24.Micke O, Ugrak E, Bartmann S, et al. Radiotherapy for calcaneodynia, achillodynia, painful gonarthrosis, bursitis trochanterica, and painful shoulder syndrome - Early and late results of a prospective clinical quality assessment. RadiatOncol 2018; 13: 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pardo V, Manuel J. Hombro doloroso E incapacidad temporal. El retorno al trabajo tras larga Baja POR hombro doloroso: causalidad del trabajo en El hombro doloroso. Medicina y SeguridaddelTrabajo 2016; 62: 337–59. [Google Scholar]
- 26.Struijs PA, Kerkhoffs GM, Assendelft WJ, Van Dijk CN. Conservative treatment of lateral epicondylitis: brace versus physical therapy or a combination of both-a randomized clinical trial. Am J Sports Med 2004; 32: 462‐–9. [DOI] [PubMed] [Google Scholar]
- 27.Ott OJ, Hertel S, Gaipl US, Frey B, Schmidt M, Fietkau R. The Erlangen dose optimization trial for low-dose radiotherapy of benign painful elbow syndrome. Long-term results StrahlentherOnkol 2014; 190: 293‐–7. [DOI] [PubMed] [Google Scholar]
- 28.Leszek M, Grygutis I, Zając P, Gierlach G, Spindel J. An evaluation of radiotherapy effectiveness for epicondylitis humeri (Eph. OrtopTraumatolRehabil 2015; 17: 471–9. [DOI] [PubMed] [Google Scholar]
- 29.Hautmann MG, Beyer LP, Süß C, et al. Radiotherapy of epicondylitis humeri: analysis of 138 elbows treated with a linear accelerator. StrahlentherOnkol 2019; 195: 343–51. [DOI] [PubMed] [Google Scholar]
- 30.Hautmann MG, Beyer LP, Hipp M, et al. Re-irradiation for humeral epicondylitis: Retrospective analysis of 99 elbows. RebestrahlungbeiEpicondylitishumeri :Retrospektive Analyse von 99 Ellenbogen. StrahlentherOnkol 2020; 196: 262–9. [DOI] [PubMed] [Google Scholar]
- 31.Pereira D, Peleteiro B, Araújo J, Branco J, Santos RA, Ramos E. The effect of osteoarthritis definition on prevalence and incidence estimates: a systematic review. Osteoarthritis and Cartilage 19: 1270–85. [DOI] [PubMed] [Google Scholar]
- 32.Hunter DJ, Bierma-Zeinstra S. Osteoarthritis. Lancet 2019; 393: 1745–59. doi: 10.1016/S0140-6736(19)30417-9 [DOI] [PubMed] [Google Scholar]
- 33.Kaltenborn A, Bulling E, Nitsche M, Carl UM, Hermann RM. The field size matters: low dose external beam radiotherapy for thumb carpometacarpal osteoarthritis. Strahlentherapie und Onkologie 2016; 192: 582–8. [DOI] [PubMed] [Google Scholar]
- 34.Minten MJM, Leseman-Hoogenboom MM, Kloppenburg M, et al. Lack of beneficial effects of low-dose radiation therapy on hand osteoarthritis symptoms and inflammation: a randomised, blinded, sham-controlled trial. Osteoarthritis Cartilage 2018; 26: 1283–90. [DOI] [PubMed] [Google Scholar]
- 35.Álvarez B, Montero A, Aramburu F, Calvo E, Ángel de la Casa M, Valero J. Radio therapy for ostheoarticular degenerative disorders: Whennothingelse works. Osteoarthritis and Cartilage Open 2020; 1: 100016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rogers S, Eberle B, Vogt DR, Meier E, Moser L, Gomez Ordoñez S, et al. Prospective evaluation of changes in pain levels, quality of life and functionality after low dose radiotherapy for epicondylitis, plantar fasciitis, and finger osteoarthritis. Front Med 2020; 7: 195: 195. doi: 10.3389/fmed.2020.00195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Blood TD, Morrell NT, Weiss A-PC. Tenosynovitis of the hand and wrist: a critical analysis review. JBJS Rev 2016; 429 03 2016. doi: 10.2106/JBJS.RVW.O.00061 [DOI] [PubMed] [Google Scholar]
- 38.Braun J, Bollow M, Remlinger G, Eggens U, Rudwaleit M, Distler A, et al. Prevalence of spondylarthropathies in HLA-B27 positive and negative blood donors. Arthritis Rheum 1998; 41: 58–67. doi: [DOI] [PubMed] [Google Scholar]
- 39.Segal NA, Felson DT, Torner JC, Zhu Y, Curtis JR, Niu J, et al. Greater trochanteric pain syndrome: epidemiology and associated factors. Arch Phys Med Rehabil 2007; 88: 988–92. doi: 10.1016/j.apmr.2007.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Valduvieco I, Biete A, Moreno LA, Gallart X, Rovirosa A, Saez J, et al. Is anti-inflammatory radiotherapy an effective treatment in trochanteritis? Br J Radiol 2017; 90: 20160520. doi: 10.1259/bjr.20160520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kaltenborn A, Carl UM, Hinsche T, Nitsche M, Hermann RM. Low-dose external beam radiotherapy for greater trochanteric pain syndrome : Target volume definition and treatment outcome. Strahlenther Onkol 2017; 193: 260–8. doi: 10.1007/s00066-016-1071-z [DOI] [PubMed] [Google Scholar]
- 42.Biete A, Valduvieco I, Arenas M, Holub K. Low-Dose radiotherapy as an effective treatment of trochanteric bursitis in elderly patients. Ann Radiat Ther Oncol 2017; 1: 1003. [Google Scholar]
- 43.Michael JW-P, Schlüter-Brust KU, Eysel P. The epidemiology, etiology, diagnosis, and treatment of osteoarthritis of the knee. Dtsch Arztebl Int 2010; 107: 152–62. doi: 10.3238/arztebl.2010.0152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Maria BVJ, Gloria VS, Carlos RLJ. Gonarthrosis prevalence in the elderly, its associated factors and degrees of disability. Biomed. Pharmacol. J. 2014; 7: 411–5. doi: 10.13005/bpj/505 [DOI] [Google Scholar]
- 45.Glatzel M, Frohlich D, Kraub A, Basecke S. Results of radiotherapy for gonarthrosis. Benign News 2002; 3: 9–11. [Google Scholar]
- 46.Mücke R, Seegenschmiedt MH, Heyd R, Schäfer U, Prott F-J, Glatzel M, et al. Radiotherapy in painful gonarthrosis. Results of a national patterns-of-care study. Strahlenther Onkol 2010; 186: 7–17. doi: 10.1007/s00066-009-1995-7 [DOI] [PubMed] [Google Scholar]
- 47.Keller S, Müller K, Kortmann R-D, Wolf U, Hildebrandt G, Liebmann A, et al. Efficacy of low-dose radiotherapy in painful gonarthritis: experiences from a retrospective East German bicenter study. Radiat Oncol 2013; 8: 29. doi: 10.1186/1748-717X-8-29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mahler EAM, Minten MJ, Leseman-Hoogenboom MM, Poortmans PMP, Leer JWH, Boks SS, et al. Effectiveness of low-dose radiation therapy on symptoms in patients with knee osteoarthritis: a randomised, double-blinded, sham-controlled trial. Ann Rheum Dis 2019; 78: 83–90. doi: 10.1136/annrheumdis-2018-214104 [DOI] [PubMed] [Google Scholar]
- 49.Buchanan BK, Kushner D. Plantar fasciitis. In: StatPearls. Treasure Island (FL: StatPearls Publishing; 2020. https://www.ncbi.nlm.nih.gov/books/NBK431073/. [PubMed] [Google Scholar]
- 50.Buchbinder R. Clinical practice. plantar fasciitis. N Engl J Med 2004; 350: 2159–66. doi: 10.1056/NEJMcp032745 [DOI] [PubMed] [Google Scholar]
- 51.Glatzel M, Bäsecke S, Krauß A, Fröhlich D. Radiotherapy of the painful plantar heel Spur. Benig News 2001; 2: 18–19. [Google Scholar]
- 52.Schneider O, Stückle CA, Bosch E, Gott C, Adamietz IA. Effectiveness and prognostic factors of radiotherapy for painful plantar heel spurs. Strahlentherapie und Onkologie 2004; 180: 502–9. [DOI] [PubMed] [Google Scholar]
- 53.Micke O, Seegenschmiedt MH. German Cooperative Group on Radiotherapy for Benign Diseases. Radiotherapy in painful heel spurs (plantar fasciitis)-results of a national patterns of care study. Int J Radiat Oncol Biol Phys 2004; 58: 828–43. [DOI] [PubMed] [Google Scholar]
- 54.Heyd R, Tselis N, Ackermann H, Röddiger SJ, Zamboglou N. Radiation therapy for painful heel spurs. Strahlentherapie und Onkologie 2007; 183: 3–9. [DOI] [PubMed] [Google Scholar]
- 55.Hajtmanová E, Kinclová I, Kostková L, Hajtman A, Péc M. Low-Dose radiotherapy in the treatment of plantar fasciitis. Klin Onkol 2010; 23: 104–10. [PubMed] [Google Scholar]
- 56.Hermann RM, Meyer A, Becker A, Schneider M, Reible M, Carl UM, et al. Effect of field size and length of plantar Spur on treatment outcome in radiation therapy of plantar fasciitis: the bigger the better? Int J Radiat Oncol Biol Phys 2013; 87: 1122–8. doi: 10.1016/j.ijrobp.2013.08.042 [DOI] [PubMed] [Google Scholar]
- 57.Muecke R, Micke O, Reichl B, Heyder R, Prott F-J, Seegenschmiedt MH, et al. Demographic, clinical and treatment related predictors for event-free probability following low-dose radiotherapy for painful heel spurs - a retrospective multicenter study of 502 patients. Acta Oncol 2007; 46: 239–46. doi: 10.1080/02841860600731935 [DOI] [PubMed] [Google Scholar]
- 58.Niewald M, Seegenschmiedt MH, Micke O, Graeber S, Muecke R, Schaefer V, et al. Randomized, multicenter trial on the effect of radiation therapy on plantar fasciitis (painful heel Spur) comparing a standard dose with a very low dose: mature results after 12 months' follow-up. Int J Radiat Oncol Biol Phys 2012; 84: e455–62. doi: 10.1016/j.ijrobp.2012.06.022 [DOI] [PubMed] [Google Scholar]
- 59.Koca T, Aydın A, Sezen D, Başaran H, Karaca S. Painful plantar heel Spur treatment with Co-60 teletherapy: factors influencing treatment outcome. Springerplus 2014; 3: 21. doi: 10.1186/2193-1801-3-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Badakhshi H, Buadch V. Low dose radiotherapy for plantar fasciitis. treatment outcome of 171 patients. Foot 2014; 24: 172–5. doi: 10.1016/j.foot.2014.07.005 [DOI] [PubMed] [Google Scholar]
- 61.Uysal B, Beyzadeoglu M, Sager O, Demıral S, Gamsız H, Dıncoglan F, et al. Role of radiotherapy in the management of heel Spur. Eur J Orthop Surg Traumatol 2015; 25: 387–9. doi: 10.1007/s00590-014-1482-4 [DOI] [PubMed] [Google Scholar]
- 62.Canyilmaz E, Canyilmaz F, Aynaci O, et al. Prospective randomized comparison of the effectiveness of radiation therapy and local steroid injection for the treatment of plantar fasciitis. Int J RadiatOncolBiol Phys 2015; 92: 659–66. [DOI] [PubMed] [Google Scholar]
- 63.Niewald M, Holtmann H, Prokein B, Hautmann MG, Rösler H-P, Graeber S, et al. Randomized multicenter follow-up trial on the effect of radiotherapy on painful heel Spur (plantar fasciitis) comparing two fractionation schedules with uniform total dose: first results after three months' follow-up. Radiat Oncol 2015; 10: 174. doi: 10.1186/s13014-015-0471-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ott OJ, Jeremias C, Gaipl US, Frey B, Schmidt M, Fietkau R. Radiotherapy for benign calcaneodynia. Strahlentherapie und Onkologie 2014; 190: 671–5. [DOI] [PubMed] [Google Scholar]
- 65.de Jonge S, van den Berg C, de Vos RJ, van der Heide HJL, Weir A, Verhaar JAN, et al. Incidence of midportion Achilles tendinopathy in the general population. Br J Sports Med 2011; 45: 1026–8. doi: 10.1136/bjsports-2011-090342 [DOI] [PubMed] [Google Scholar]
- 66.Ott OJ, Jeremias C, Gaipl US, Frey B, Schmidt M, Fietkau R. Radiotherapy for benign achillodynia. long-term results of the Erlangen dose optimization trial. Strahlenther Onkol 2015; 191: 979–84. doi: 10.1007/s00066-015-0893-4 [DOI] [PubMed] [Google Scholar]
- 67.Ott OJ, Micke O, Mücke R, Niewald M, Rödel F, Schäfer U, et al. Low-Dose radiotherapy: mayday, mayday. we've been hit! Strahlenther Onkol 2019; 195: 285–8. doi: 10.1007/s00066-018-1412-1 [DOI] [PubMed] [Google Scholar]
- 68.Montero A, Sabater S, Rödel F, Gaipl US, Ott OJ, Seegenschmiedt MH, Arenas M, et al. Is it time to redefine the role of low-dose radiotherapy for benign disease? Ann Rheum Dis 2020; 79: e34. doi: 10.1136/annrheumdis-2018-214873 [DOI] [PubMed] [Google Scholar]
- 69.Park S-H, Lee JE, Radiotherapy LJE. Radiotherapy, a new treatment option for non-malignant disorders: radiobiological mechanisms, clinical applications, and radiation risk. J Rheum Dis 2017; 24: 74–84. doi: 10.4078/jrd.2017.24.2.74 [DOI] [Google Scholar]
- 70.Grant EJ, Brenner A, Sugiyama H, Sakata R, Sadakane A, Utada M, et al. Solid cancer incidence among the life span study of atomic bomb survivors: 1958-2009. Radiat Res 2017; 187: 513–37. doi: 10.1667/RR14492.1 [DOI] [PMC free article] [PubMed] [Google Scholar]