Abstract
Objective:
The aim of this study was to compare the clinical efficacy of neoadjuvant chemoradiotherapy (NACRT) combined with postoperative adjuvant XELOX (Oxaliplatin +Capecitabine) chemotherapy and postoperative adjuvant chemotherapy (ACT) with XELOX for local advanced gastric cancer (LAGC).
Methods:
In this prospectively randomized trial, we investigated the effect of NACRT combined with postoperative ACT for LAGC. 60 patients were randomly divided into NACRT group and ACT group, with 30 patients in each group. Patients in NACRT group were given three-dimensional conformal radiotherapy (45 Gy/1.8 Gy/f) accompanied by synchronous XELOX of two cycles, followed by surgery, and then postoperative adjuvant XELOX chemotherapy of four cycles was performed. Patients in ACT group received surgery in advance, and then XELOX chemotherapy of six cycles was given.
Results:
The objective response rate of NACRT was 76.7%. The overall incidence of postoperative complications in NACRT group was not significantly different from that in ACT group (23.1% vs 30.0%, p = 0.560). The 1 year, 2 years, and 3 years progression-free survival (PFS)and overall survival (OS) in NACRT and ACT groups were 80.0% vs 56.7%, 73.3% vs 46.7%, 60.0% vs 33.3%, and 86.7% vs 80.0%, 76.7% vs 66.7%, 63.3% vs 50.0%, respectively. Patients in NACRT group showed a significantly higher R0 resection rate (84.6% vs 56.7%, p = 0.029),lower loco-regional recurrence rate (36.7% vs 11.5%, p = 0.039), longer PFS (p = 0.019) and freedom from locoregional progression(FFLP) (p = 0.004) than patients in ACT group, while there was no difference in OS (p = 0.215) and in toxicity incidence (p > 0.05).
Conclusions:
NACRT combined with postoperative adjuvant XELOX chemotherapy can improve R0 resection rate, reduce loco-regional recurrence, prolong PFS and FFLP without increasing the incidence of postoperative complications in patients with LAGC.
Advances in knowledge:
Compared with postoperative adjuvant chemotherapy, locally advanced gastric cancer patients may benefit from neoadjuvant chemoradiotherapy, and toxicity associated with chemoradiotherapy was tolerant and manageable.
Introduction
Gastric cancer, with an increasing incidence all over the world, has ranked fifth among the most common cancer and emerged as the world’s third leading cause of death from cancer as estimated.1 China exhibits the highest incidence of gastric cancer.2 According to the China Cancer Registry in 2015, about 679,000 people were newly diagnosed and 498,000 people eventually died of gastric cancer.3 In recent years, the overall morbidity and mortality of gastric cancer have shown a downward trend owing to the control of risk factors related to gastric cancer and the development of gastric cancer screening in China. However, gastric cancer still brings a heavy burden and seriously threats the health of Chinese residents due to the large population base and the intensified aging tendency of the population in China.4
Surgery is regarded as a main treatment choice for gastric cancer. However, even after apparent curative resection and lymph node dissection, the loco-regional recurrence rate of about 57% is still surprisingly high,5,6 which is associated with the locally advanced stage at presentation. Because the early symptoms of gastric cancer are hidden and difficult to find, it is already a local progression stage when the symptoms are obvious. Some studies have evaluated the clinical effects of neoadjuvant or adjuvant therapy on locally advanced gastric cancer (LAGC). In INT0116 trial, postoperative chemoradiotherapy (CRT) was verified to better improve local control and overall survival than surgery alone, and was considered standard treatment for LAGC patients who underwent surgery with predominantly D0-1 dissections.7 However, a retrospective analysis reported that CRT failed to significantly lower recurrence rates in patients who following D2 lymph node dissection.8 Compared to postoperative chemotherapy, CRT was also proved unable to significantly decrease recurrence rate after removing D2 lymph node in patients with gastric cancer curative resection, according to the Adjuvant Chemoradiation Therapy in Stomach Cancer (ARTIST) trial.9,10
In order to overcome the limitations of CRT, a series of studies have begun to investigate the therapeutic effect of neoadjuvant chemoradiotherapy (NACRT) on LAGC. A number of small studies have demonstrated the role of NACRT in improving pathological remission rate in resectable gastric cancer.11–13 Nevertheless, the effect of NACRT on resectable LAGC remains uncertain. Therefore, we aimed to explore the clinical efficacy of NACRT in the treatment of resectable LAGC in Chinese patients. Meanwhile, we also observed the surgical resection rate, postoperative complications, recurrence pattern, and toxicity of patients.
Methods and materials
Patient characteristics
From January 2014 to December 2016, 60 patients in total with LAGC were collected and assigned randomly to NACRT group (30 cases) and ACT group (30 cases) by means of random number table.
Inclusion criteria: (1) gastric adenocarcinoma confirmed by pathology; (2) stage T3, T4a and/or N+ confirmed by abdominal enhanced computed tomography (CT), endoscopic ultrasonography and/or PET/CT, in reference to the eighth edition of the cancer staging standard issued by American Joint Committee on Cancer (AJCC); (3) patients were newly diagnosed and had never received radiotherapy, chemotherapy or surgery before on entering; (4) no pleural effusion or ascites, and no metastasis to peritoneum, lung, liver, mediastinal lymph nodes or other distant organs (M1); (5) white blood cell count >3.5 ×109/L, neutrophil count >1.5 ×109/L, platelet count >100 ×109/L, serum creatinine <1.5 mg dl−1, serum bilirubin <2.0 mg dl−1; (6) ECOG performance status was 0 ~ 1, expected survival time was more than half a year; and (7) aged from 32 to 70 years.
Exclusion criteria: (1) unclear clinical stage, or the clear stage did not belong to T3, T4a and/or N+; (2) serious disorders of heart, liver, and kidney function; (3) laboratory tests did not meet the requirements of radiotherapy and chemotherapy; (4) pregnant or having breastfeeding; and (5) suffering from mental illness, or unable to understand protocol and comply to treatment.
Therapies
Patients’ treatment plan in this study was reached to an agreement by a multidisciplinary team (MDT), which consisted of oncologists, surgeons, radiologists, pathologists, and imaging specialists.
NACRT Group
Patients in NACRT group received neoadjuvant radiation first after LAGC diagnosis. Simultaneous chemotherapy with Oxaliplatin and Capecitabine (XELOX) begun on the first day of radiation therapy for two cycles. Each 3-week cycle comprised of Oxaliplatin of 130 mg/m2 on day one and Capecitabine of 1000 mg/m2 bid on days 1–14. Varian 21EX linear accelerator was used to perform three-dimensional conformal intensity-modulated radiation therapy. The patients were simulated first then scanned with spiral CT who fixed in position by a vacuum-forming mold. The thickness of each scan layer was 5 mm. Images were imported to the radiotherapy planning system (Eclipse v.13.0 software, Varian Medical Systems). The target area and the endangering organs were identified on enhanced CT images. Gross tumor volume (GTV) consists of tumor bed and regional metastatic lymph nodes, while clinical target volume (CTV) comprises of GTV and 5 ~ 10 mm beyond its margin. An additional 5 mm was added to CTV to constitute the planning target volume (PTV). And organs at risk (OAR) included liver, residual stomach, small intestine, colon, kidneys, spinal cord, and heart. The dose-distribution plan was optimized by using dose-volume histograms (DVH). Patients were administered with radiation with spontaneous breath. Irradiation was performed using 6 MV X-ray beam and 45 Gy in total, which was delivered in 25 fractions of 1.8 Gy for five times each week.
Surgery was performed 4 ~ 6 weeks after NACRT completion if the tumor was evaluated as effective. The surgical approach was determined by imaging examinations and intraoperative exploration. Generally, curative total or subtotal gastrectomy with D2 lymph node dissection was performed according to the guidelines of the Japanese Gastric Cancer Association.14 When resection of an adjacent organ was inevitable, a combined resection was performed. For unresectable tumors, partial gastrectomy, gastrojejunostomy, jejunostomy, or simple exploration might be performed according to intraoperative situation.
After surgery, patients in NACRT group received another four cycles of XELOX adjuvant chemotherapy. The dosage and usage of Oxaliplatin and Capecitabine were the same as XELOX in NACRT. Patients continued to undergo second-line chemotherapy with Docetaxel and Cisplatin if the disease progressed and was unresectable after NACRT. If grade IV neutropenia sustained ≥7 days, neutropenic fever or grade IV thrombocytopenia occurred in the first cycle of chemotherapy, the dose of chemotherapy drugs was reduced by 25% for the second-cycle chemotherapy. In addition, the dose of chemotherapy drug also needed to be reduced with non-haematological grade IV toxicities.
ACT Group
Patients in ACT group received surgery first, followed by six cycles of XELOX chemotherapy or postoperative radiotherapy combines with six cycles of XELOX chemotherapy. Patients who have undergone primary D2 lymph node dissection and R0 resection or exploratory surgery (mainly refers to the extensive miliary metastasis of the peritoneum or abdominal organ adhesion that cannot be detected by preoperative imaging, which makes the tumor unable to undergo palliative resection) only received postoperative chemotherapy. In the absence of distant metastasis, postoperative chemoradiotherapy was recommended for patients who received less than a D2 dissection and/or R1 or R2 resection, but the most appropriate treatment method needs to be determined according to the patient’s postoperative physical condition and MDT discussion. The surgical treatment plan and adjuvant chemotherapy regimen for patients in ACT group were the same as the NACRT group.
Assessment of response and toxicity
The efficacy of NACRT, surgical resection rate, postoperative complications, survival rate, recurrence pattern and side-effects of NACRT and postoperative chemotherapy were observed in two groups.
The efficacy of NACRT in NACRT group was evaluated according to the Response Evaluation Criteria in Solid Tumors (RECIST).15
After completing planned adjuvant treatment, all patients in two groups were followed up according to protocol every 3 months during the first year, every 6 months in the second year, and then yearly until 3 years. Follow-up examinations including physical examination, complete blood routine test, serum biochemicals, tumor markers, thoracic, and abdominopelvic tomography and gastroscopy. Local recurrence, metastasis, and death of disease were documented.
Postoperative complications of gastric cancer were classified into five grades: grade I, II, III (IIIa and IIIb), IV (IVa and IVb), and V according to the Clavien-Dindo classification criteria.16 The National Cancer Institute’s Common Terminology Criteria for Adverse Events (CTCAE), v.4.0, were used to evaluated treatment-related toxicities. Late toxicities were defined as symptoms lasting beyond 3 months or first occurring after the completion of NACRT.
Statistical analysis
The primary end point of the study was objective response rate (ORR), progression-free survival (PFS), and overall survival (OS). PFS was described as the intervals between randomization and the recurrence or distant metastases of gastric cancer. OS was defined from random grouping to death or to the final follow-up. Freedom from locoregional progression (FFLP) were defined as the time from the start date of NACRT to the date of death and local progression. Kaplan-Meier method was used to calculate PFS,OS and FFLP, and the log-rank test was used to assess the survival differences between two groups. The clinical general data, postoperative complication rate, recurrence rate and adverse reaction rate between two groups were evaluated by performing χ2 tests. The surgical resection rates between two groups were compared based on binary logistic regression model. Multivariable Cox proportional hazards model was used to evaluate independent prognostic factors affecting OS and PFS of patients in two groups. A P-value of <0.05 was considered statistically significant. Statistical Package for Social Sciences (SPSS) software (version 22.0, Chicago, IL) was used to perform statistical analyses.
Results
Clinical characteristics
Patients’ baseline data and tumor characteristics in NACRT group and ACT group were generally well balanced. Gender, age, ECOG score, pathological type, tumor location, and clinical stage between the two groups showed no significant difference (p > 0.05) (Table 1).
Table 1.
Patients characteristics of NACRT and ACT groups
| Characteristics | NACRT group(n = 30) n(%) | ACT group(n = 30) n(%) | χ2 | P |
|---|---|---|---|---|
| Gender | 1.071 | 0.301 | ||
| male | 18 (60.0) | 14 (46.7) | ||
| female | 12 (40.0) | 16 (53.3) | ||
| Age(years) | 1.714 | 0.190 | ||
| <60 | 15 (50.0) | 20 (66.7) | ||
| ≥60 | 15 (50.0) | 10 (33.3) | ||
| ECOG | 0.278 | 0.598 | ||
| 0 | 17 (56.7) | 19 (63.3) | ||
| 1 | 13 (43.3) | 11 (36.7) | ||
| Pathological type | 1.281 | 0.734 | ||
| well differentiated adenocarcinoma | 10 (33.3) | 9 (30.0) | ||
| middle differentiated adenocarcinoma | 8 (26.7) | 12 (40.0) | ||
| poorly differentiated adenocarcinoma | 8 (26.7) | 6 (20.0) | ||
| mucinous adenocarcinoma | 4 (13.3) | 3 (10.0) | ||
| Tumor location | 1.749 | 0.417 | ||
| gastric body | 13 (43.3) | 18 (60.0) | ||
| pylorus | 9 (30.0) | 7 (23.3) | ||
| cardia | 8 (26.7) | 5 (16.7) | ||
| AJCC Clinical T stage | 2.411 | 0.121 | ||
| T3 | 13 (43.3) | 19 (63.3) | ||
| T4a | 17 (56.7) | 11 (36.7) | ||
| AJCC Clinical N stage | 1.949 | 0.583 | ||
| N0 | 5 (16.7) | 6 (20.0) | ||
| N1 | 8 (26.7) | 12 (40.0) | ||
| N2 | 13 (43.3) | 10 (33.3) | ||
| N3 | 4 (13.3) | 2 (6.7) | ||
| AJCC Clinical Stage | 0.111 | 0.739 | ||
| II | 5 (16.7) | 6 (20.0) | ||
| III | 25 (83.3) | 24 (80.0) |
Efficacy of the NACRT therapy
All patients in NACRT group completed NACRT, and five of these patients (16.7%) had complete remission, 18 patients (60.0%) had partial remission and three patients (10.0%) had stable disease. Four patients (13.3%) progressed due to multiple liver metastases (two cases), extensive abdominal metastasis (one case) and cervical lymph nodes metastasis (one case). Then, the four patients with progression were treated with second-line chemotherapy. The objective response rate (ORR) (complete and partial remission) was 76.7%.
Surgical results
NACRT group had a significantly higher D2 resection rate than ACT group (88.5% vs 63.3%) (HR:4.439,95% CI: 1.080–18.250, p = 0.039). Significantly more patients received R0 resection in NACRT group than in ACT group (84.6% vs 56.7%) (HR:4.206,95% CI: 1.161–15.234, p = 0.029). Two patients in NACRT group and six patients in ACT group underwent palliative surgery (including partial gastrectomy, jejunostomy and gastrojejunostomy), respectively. One patient in NACRT group and five patients in ACT group initially were assessed as resectable cases, but after receiving an “open and closure” surgery, these patients received XELOX chemotherapy due to the unresolved tumor. All patients who received less than a D2 dissection and/or R1 or R2 resection in ACT group received postoperative chemotherapy due to their postoperative physical condition. Four patients in NACRT group achieved pathologic complete remission after surgery (4/30, 13.3%) (Table 2).
Table 2.
Surgical results of NACRT and ACT groups
| Surgical Resection Rate | NACRT group(n = 26) n(%) | ACT group(n = 30) n(%) | HR(95% CI) | P |
|---|---|---|---|---|
| D2 resection | 23 (88.5) | 19 (63.3) | 4.439 (1.080–18.250) | 0.039 |
| palliative surgery | 2 (7.7) | 6 (20.0) | 0.333 (0.061–1.820) | 0.205 |
| exploratory surgery | 1 (3.8) | 5 (16.7) | 0.200 (0.022–1.837) | 0.155 |
| R0 resection | 22 (84.6) | 17 (56.7) | 4.206 (1.161–15.234) | 0.029 |
| R1 resection | 2 (7.7) | 6 (20.0) | 0.333 (0.061–1.820) | 0.205 |
| R2 resection | 2 (7.7) | 7 (23.3) | 0.274 (0.051–1.458) | 0.129 |
CI, confidence interval; HR, hazard ratio.
Postoperative complications
Surgery-related complications are shown in Table 3. The overall rates of postoperative complications in NACRT group were not significantly different from those in ACT group (23.1% vs 30.0%, p = 0.560). All complications in two groups were grades I, II, IIIa, and IIIb according to the Clavien–Dindo classification method, and most of them were alleviated after drug administration and symptomatic treatment. Neither group had grade IVa, IVb, or V surgery-related complications.
Table 3.
Postoperative complications of NACRT and ACT groups
| Postoperative Complications | NACRT group(n = 26) | ACT group(n = 30) | ||
|---|---|---|---|---|
| Grades I–II | Grades IIIa–IIIb | Grades I–II | Grades IIIa–IIIb | |
| remnant stomach weakness | 2 | 0 | 1 | 1 |
| anastomotic leak | 1 | 0 | 1 | 0 |
| anastomotic bleeding | 0 | 1 | 2 | 0 |
| anastomotic stenosis | 1 | 0 | 0 | 1 |
| intestinal obstruction | 1 | 0 | 2 | 0 |
| wound infection | 0 | 0 | 1 | 0 |
| the overall incidence | 6 (23.1%) | 9 (30.0%) | ||
Survival and patterns of recurrence
All patients in two groups were followed up to December 30, 2019, and no patients were censored. NACRT and ACT group showed a median follow-up period of 42.3 months (ranged 10.5 ~ 71.8 months) and 34.6 months (ranged 8.7 ~ 63.9 months), respectively. When all patients were followed up to the third year, 19 patients in NACRT group and 15 patients in ACT group were still alive. The rest of patients died of loco-regional recurrence, peritoneal metastasis, distant metastasis, or other reasons. The 1 year, 2 years, and 3 years PFS and OS in NACRT and ACT groups were 80.0% vs 56.7%, 73.3% vs 46.7%, 60.0% vs 33.3% and 86.7% vs 80.0%, 76.7% vs 66.7%, 63.3% vs 50.0%, respectively. Patients in NACRT group showed a significantly longer PFS than patients in ACT group (with Log-rank test, p = 0.019, Figure 1), and OS between two groups showed no significant difference (with Log-rank test, p = 0.215, Figure 2).
Figure 1.
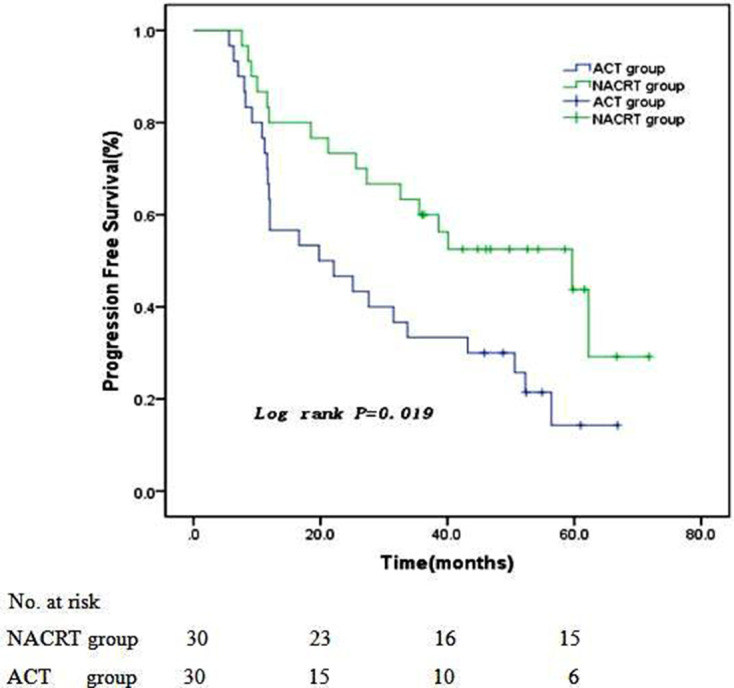
Progression-free survival curves of two groups
Figure 2.
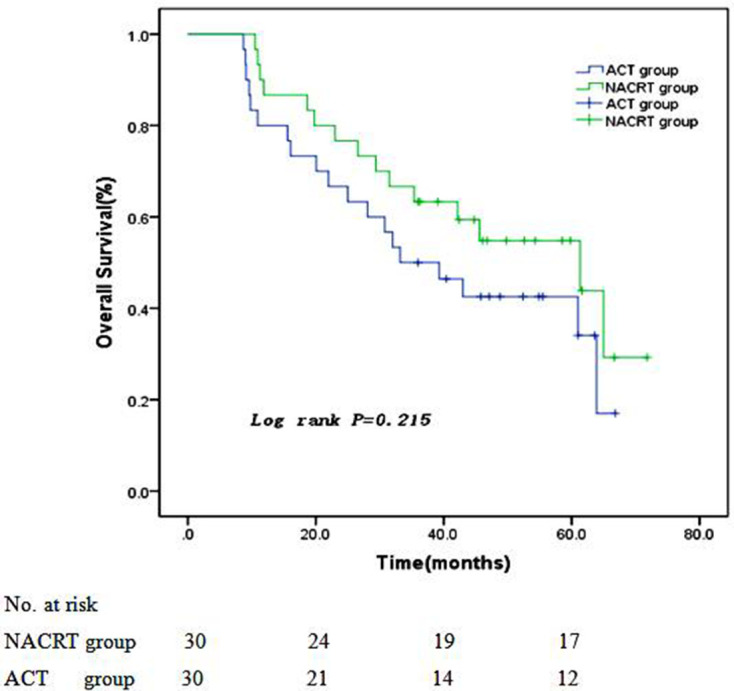
Overall survival curves of two groups
Multivariate analysis revealed that independent parameters predicting improved OS including Clinical T3 stage (HR:0.289,95% CI:0.107–0.782,p = 0.014), well differentiation (HR:0.113,95% CI: 0.023–0.557, p = 0.007), middle differentiation (HR:0.261,95% CI:0.082–0.836, p = 0.024), and R0 resection (HR:0.018,95% CI: 0.003–0.124, P<0.001). Independent parameters predicting high PFS was only R0 resection (HR:0.049, 95% CI: 0.012–0.208, P<0.001).(Table 4)
Table 4.
Univariate and multivariate analyses of risk factors affecting OS and PFS of patients in two groups
| Variables | Affecting OS | Affecting PFS | ||||||
|---|---|---|---|---|---|---|---|---|
| Univariate analysis | Multivariate analysis | Univariate analysis | Multivariate analysis | |||||
| HR(95% CI) | P | HR(95% CI) | P | HR(95% CI) | P | HR(95% CI) | P | |
| Gender | ||||||||
| male | 1.772 (0.898–3.498) | 0.099 | - | - | 1.904 (0.981–3.698) | 0.057 | - | - |
| female | Ref. | Ref. | ||||||
| Age | ||||||||
| <60y | 1.201 (0.624–2.313) | 0.584 | - | - | 0.948 (0.505–1.780) | 0.868 | - | - |
| >60y | Ref. | Ref. | ||||||
| Clinical T stage | ||||||||
| T3 | 0.141 (0.066–0.300) | <0.001 | 0.289 (0.107–0.782) | 0.014 | 0.220 (0.112–0.432) | <0.001 | 0.681 (0.259–1.792) | 0.436 |
| T4a | Ref. | Ref. | Ref. | Ref. | ||||
| Clinical N stage | ||||||||
| N0-N1 | 0.184 (0.086–0.398) | <0.001 | 0.740 (0.236–2.315) | 0.605 | 0.265 (0.135–0.520) | <0.001 | 0.962 (0.338–2.739) | 0.943 |
| N2-N3 | Ref. | Ref. | Ref. | Ref. | ||||
| Differentiation | ||||||||
| well | 0.049 (0.016–0.153) | <0.001 | 0.113 (0.023–0.557) | 0.007 | 0.102 (0.041–0.254) | <0.001 | 0.310 (0.078–1.234) | 0.097 |
| middle | 0.138 (0.058–0.330) | <0.001 | 0.261 (0.082–0.836) | 0.024 | 0.199 (0.090–0.441) | <0.001 | 0.506 (0.160–1.598) | 0.246 |
| poorly | Ref. | Ref. | Ref. | Ref. | ||||
| Surgical approach | ||||||||
| R0 | 0.014 (0.003–0.065) | <0.001 | 0.018 (0.003–0.124) | <0.001 | 0.021 (0.006–0.078) | <0.001 | 0.049 (0.012–0.208) | <0.001 |
| others | Ref. | Ref. | Ref. | Ref. | ||||
Ref, reference.
At the time of analysis, the postoperative treatment failure patterns of patients in the two groups were mainly loco-regional recurrence (residual stomach, anastomosis, and locoregional lymph nodes recurrence), peritoneal metastasis, and distant metastasis. The loco-regional recurrence rate in NACRT group decreased compared with that in ACT group (11.5% vs 36.7%, p = 0.030). Peritoneal (11.5% vs 16.7%, p = 0.584) and distant (7.7% vs 10.0%, p = 0.763) metastasis rates were similar between two groups(Table 5). The 1 year, 2 years, and 3 years FFLP rates were 96.1%,92.3%, and 88.5% in NACRT group and 93.3%, 76.7%, and 63.3% in ACT group, respectively. The FFLP rate of NACRT group was significantly longer than ACT group (with Log-rank test, p = 0.004, Figure 3).
Table 5.
Postoperative treatment failure patterns of NACRT and ACT groups
| Postoperative treatment failure patterns | NACRT group(n = 26) n(%) | ACT group(n = 30) n(%) | χ2 | P |
|---|---|---|---|---|
| loco-regional recurrence | 3 (11.5) | 11 (36.7) | 4.691 | 0.030 |
| residual stomach recurrence | 0 | 2 (6.7) | ||
| anastomosis recurrence | 1 (3.8) | 2 (6.7) | ||
| locoregional lymph nodes recurrence erererecurrence recurrence recurrence | 2 (7.7) | 6 (20.0) | ||
| locoregional lymph nodes and anastomosis recurrence recurrence recurrence |
0 | 1 (3.3) | ||
| peritoneal metastasis | 3 (11.5) | 5 (16.7) | 0.299 | 0.584 |
| distant metastasis | 2 (7.7) | 3 (10.0) | 0.091 | 0.763 |
Figure 3.
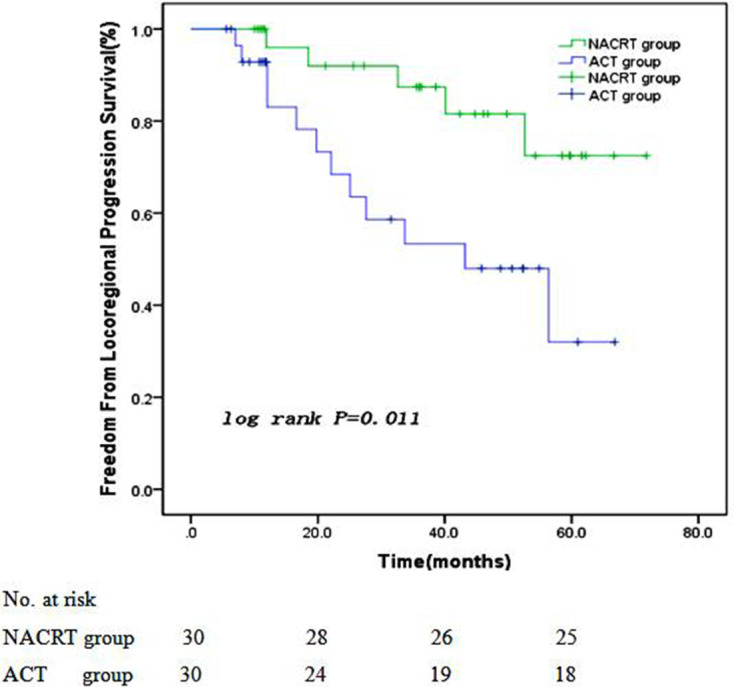
Freedom from locoregional progression survival curves of two groups
Toxicities
All patients in two groups completed NACRT and postoperative ACT, and their adverse events were mainly myelosuppression, gastrointestinal reactions and hand-foot syndrome. Other toxicities including liver and renal dysfunctions were observed in a few cases of each group.
The toxicities associated with NACRT in NACRT group were summarized in Table 6. The major NACRT-related toxicities were grades 1, 2, and 3, including nausea, vomiting, diarrhea, leukopenia, anemia, thrombocytopenia, hand-foot syndrome, which were relieved after symptomatic treatment with drugs. Two patients (6.7%) with pyloric tumor vomited for nearly 1 month after completion of the radiotherapy, which is considered to be related to pyloric mucosal edema caused by radiotherapy, but the symptom was cured after surgery. NACRT-related toxicity greater than Grade 3 and late radiation-induced toxicity did not occur.
Table 6.
Toxicities associated with neoadjuvant chemoradiotherapy of NACRT group
| Adverse Events | Grade1 | Grade 2 | Grade 3 | Grade 4 | Grade 5 | Overall n(%) |
|---|---|---|---|---|---|---|
| Nausea | 10 | 4 | 5 | 0 | 0 | 19 (63.3) |
| Vomiting | 3 | 4 | 2 | 0 | 0 | 9 (30.0) |
| Diarrhea | 2 | 6 | 2 | 0 | 0 | 10 (33.3) |
| Leukopenia | 6 | 9 | 4 | 0 | 0 | 19 (63.3) |
| Anemia | 7 | 5 | 5 | 0 | 0 | 17 (56.7) |
| thrombocytopenia | 3 | 2 | 2 | 0 | 0 | 7 (23.3) |
| hand-foot syndrome | 6 | 8 | 2 | 0 | 0 | 16 (53.3) |
| liver dysfunction | 2 | 1 | 0 | 0 | 0 | 3 (10.0) |
| renal dysfunction | 1 | 0 | 0 | 0 | 0 | 1 (3.3) |
Grades 1, 2, and 3 toxicities were common during postoperative ACT, and neither group developed life-threatening side-effects (Grade 4 to 5). Three patients (10.0%)in NACRT group (Grade 1) and 2 patients (6.7%) in ACT group (Grade 2) suffered from late hand-foot syndrome and successfully cured by proper drug administration. The incidence of all toxic reactions associated with postoperative ACT between the two groups showed no significant difference (p > 0.05). As shown in Table 7.
Table 7.
Toxicities associated with postoperative adjuvant chemotherapy of NACRT and ACT groups
| Adverse Events | NACRT group (n = 30) | ACT group (n = 30) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Grade1 | Grade 2 | Grade 3 | Grade 4 | Grade 5 | Overall n(%) n% | Grade 1 | Grade 2 | Grade 3 | Grade 4 | Grade 5 | Overall n(%) | |
| nausea | 6 | 4 | 2 | 0 | 0 | 12 (40.0) | 5 | 6 | 3 | 0 | 0 | 14 (46.7) |
| vomiting | 3 | 2 | 1 | 0 | 0 | 6 (20.0) | 2 | 5 | 2 | 0 | 0 | 9 (30.0) |
| diarrhea | 2 | 4 | 2 | 0 | 0 | 8 (26.7) | 4 | 3 | 2 | 0 | 0 | 7 (23.3) |
| leukopenia | 6 | 5 | 4 | 0 | 0 | 15 (50.0) | 8 | 4 | 3 | 0 | 0 | 15 (50.0) |
| anaemia | 5 | 3 | 3 | 0 | 0 | 11 (36.7) | 5 | 6 | 2 | 0 | 0 | 13 (43.3) |
| thrombocytopenia | 3 | 1 | 2 | 0 | 0 | 6 (20.0) | 5 | 2 | 1 | 0 | 0 | 8 (26.7) |
| hand-foot syndrome | 6 | 4 | 2 | 0 | 0 | 12 (40.0) | 3 | 7 | 1 | 0 | 0 | 11 (36.7) |
| liver dysfunction | 2 | 2 | 0 | 0 | 0 | 4 (13.3) | 3 | 2 | 0 | 0 | 0 | 5 (16.7) |
| renal dysfunction | 1 | 1 | 0 | 0 | 0 | 2 (6.7) | 3 | 0 | 0 | 0 | 0 | 3 (10.0) |
Discussion
Most gastric cancer patients in China are locally advanced and difficult to remove surgically. Even after radical surgery, high local recurrence rates at tumor bed, regional lymph nodes and diffuse peritoneal involvement all seriously affect patients’ survival.17 Therefore, the key to prolonging survival is to improve the rate of R0 surgical resection, reduce the rate of local recurrence and distant metastasis. We investigated the effect of NACRT combined with postoperative adjuvant XELOX chemotherapy among patients with LAGC, which demonstrated that NACRT was a feasible treatment strategy and toxicity associated with chemoradiotherapy was tolerant and manageable, without mortality or delay of surgery, as Leong et al18 reported.
At present, the option for a reasonable treatment in patients with curable locally advanced gastric cancer is still controversial. The Phase III CLASSIC trial19,20 revealed that compared to surgery alone, postoperative ACT could significantly improve 3-year DFS (74% vs 59%, p < 0.0001) in patients with gastric cancer of stage II or IIIB, who received curative gastrectomy accompanied with removing D2 lymph node, and the two strategies had corresponding estimated 5-year OS rates of 78 and 69%, respectively. These results proved that patients with advanced resectable gastric cancer could benefit from postoperative ACT after D2 lymph node resection. Both INT-01167 and ARTIST trial10 showed that postoperative CRT was strongly recommended for patients who only receive less than D2 lymph node dissection. Since the standard treatment of curable gastric cancer for East Asians, especially Chinese patients, is gastrectomy combined with D2 lymph node dissection, postoperative CRT is not suitable for Chinese gastric cancer patients who receive D2 lymph node dissection.
Due to the limited benefits of postoperative CRT in previous studies studies,7,10 optimization of preoperative treatment strategies is essential. Zhang et al conducted a trial, which includes 370 patients with adenocarcinoma of gastric cardia and randomized them to undergo neoadjuvant RT or surgery alone. The results showed that neoadjuvant RT was associated with survival improvement (30% vs 20%, p = 0.0094) and higher resection rate (89.5% vs 79.4%, p < 0.01).21 The Phase III MAGIC trial,22 which compared neoadjuvant chemotherapy (NAC) with surgery alone, demonstrated that NAC improved PFS and OS in patients with gastric or EGJ adenocarcinoma. This landmark study earliest established NAC’s benefit in gastric cancer survival. However, the efficacy of NACRT on improving survival and prognosis is still controversial, and no randomized controlled trial has reached a definite conclusion. Therefore, the treatment for NACRT in NCCN Guidelines is mainly originated from Phase II/III clinical trials involving patients with esophageal and/or EGJ cancer,23–25 and the efficacy of NACRT in LAGC remains unclear. Except for several small, single-center trials,11,26,27 this study may be the first to evaluate the effect and safety of NACRT in patients with LAGC in China.
Our study showed that NACRT could significantly improve the D2 surgery rate (88.5% vs 63.3%, p = 0.039) and R0 resection rate (84.6% vs 56.7%, p = 0.029) of LAGC compared with postoperative ACT alone, and the R0 resection rate of NACRT group was higher than that in other study.11 R0 resection has been reported to be a predictor for survival.28,29 Also, several studies30,31 discovered that pathological complete response (pCR) rate after NACRT or NAC was an independent prognostic factor of OS. Our study demonstrated that NACRT combined with postoperative ACT showed significant advantages with respect to PFS and a tendency to prolong OS compared to ACT. Moreover, considering all patients in NACRT group had a clinical T3 (43.3%) or T4a (56.7%) tumor, the pCR rate of 13.3% was also high, and the patients with pCR correspondingly had an ideal survival. In our study, the overall incidence of postoperative complications in NACRT group (23.1%) was slightly lower than that in ACT group (30.0%) and a previous study (31.9%),32 and neither group showed treatment-related mortality. Therefore, NACRT seems to have little effect on postoperative complications or mortality, but which needs further verification in subsequent studies.
Oppedijk et al29 reported that local recurrence is the main reason for the failure of gastric cancer surgery. Several studies have reported that NACRT reduces loco-regional recurrence rate.33,34 In this study, we also observed promising loco-regional control. After a median follow-up of 42.3 months, only three cases with loco-regional recurrence occurred in NACRT group. Compared with postoperative ACT alone, NACRT significantly reduced loco-regional recurrence rate from 36.7 to 11.5% (p = 0.030) and prolong the FFLP. The reduced local recurrence rate may be associated with the addition of neoadjuvant radiotherapy. However, the high rate of peritoneal metastasis and distant metastases in two groups served as a problem in our trial, which was also reported by Dong et al35 and Takizawa et al.36 The reason may be related to the advanced stage of some patients or insufficient postoperative ACT cycle. Whether the patients with advanced stage (T4a or N3) continue to receive Capecitabine maintenance chemotherapy after six cycles of XELOX regimen to reduce the rate of peritoneal metastasis and distant metastasis or not requires further researches.
Gastrointestinal toxicities are common in the simultaneous chemoradiotherapy of gastric cancer, and many patients often discontinue treatment owing to intolerance, as reported by INT 0116 study.7 In our study, we found that gastrointestinal and other side-effects associated with neoadjuvant chemoradiotherapy and adjuvant chemotherapy were Grades 1, 2, and 3, and could be tolerated by all patients. This may be related to XELOX chemotherapy regimen. Capecitabine is a novel targeted antitumor drug and is absorbed as a complete molecule in the small intestine to avoid directly releasing 5-Fu, which indirectly reduces gastrointestinal side-effects. In addition, Oxaliplatin can increase thymidine phosphorylase (TP enzyme) expression in tumor cells, and Capecitabine can be converted into 5-Fu by high-concentration TP enzyme and then selectively activated in tumor, which reduces the systemic exposure degree of 5-FU, improves efficacy and reduces toxicity.
Despite this is a prospective randomized controlled study, our study still has some limitations. Firstly, a small sample size may cause the limitations of statistical analysis. In this study, we expect to recruit 93 patients, but when the number of patients enrolled in two groups reaches 60, most of patients with gastric cancer are usually diagnosed at an early stage due to the increasing popularity of gastroscopy, which resulted in a significant reduction in locally advanced gastric cancer patients who met the criteria for enrollment. In the next step, we will extend the follow-up time for all patients to observe whether there is a difference in OS between two groups and we will share the corresponding results in time. Finally, although we have unified the standard operation plan in each center before operation, different levels of surgeons may have a slight difference in the scope of strict D2 lymph node dissection, which may have a certain impact on the accuracy of results.
Despite the above limitations, our results are still encouraging. NACRT can increase R0 resection rate, reduce the loco-regional recurrence rate of locally advanced gastric cancer. NACRT combined with postoperative ACT can prolong patients’ PFS and FFLP. Obviously, this result needs further verification by large sample, prospective, multi center researches to draw a more reliable conclusion.
Conclusions
Local advanced gastric cancer (LAGC) is common in China, but optimal therapy for LAGC is still uncertain. Our result demonstrated that neoadjuvant chemoradiotherapy (NACRT) combined with postoperative adjuvant XELOX chemotherapy can improve R0 resection rate, reduce loco-regional recurrence, prolong PFS and FFLP without increasing the incidence of postoperative complications in patients with LAGC. Therefore, we conclude that NACRT combined with ACT is effective and safety in the treatment of patients with LAGC.
Footnotes
Conflict of interest: The authors declare no conflicts of interest.
Funding: This work was supported by Science and Technology Development plan of Weifang and Zibo of Shandong, P.R. China(2014WS107 and 2017kj010110)
ETHICAL STANDARDS: All individual participants signed informed consent. Ethical committees of the Zibo Central Hospital of Shandong University, Shandong Qianfoshan Hospital of Cheeloo College of Medicine of Shandong University and Shouguang People’s Hospital affiliated to Weifang Medical College approved the treatment plan.
Fuli Wang and Aizhong Qu have contributed equally to this study and should be considered as co-first authors.
Contributor Information
Fuli Wang, Email: 1262223640@qq.com.
Aizhong Qu, Email: 530468799@qq.com.
Yinping Sun, Email: 3124693492@qq.com.
Jifeng Zhang, Email: 670961117@qq.com.
Benzun Wei, Email: 836240762@qq.com.
Yong Cui, Email: 891742192@qq.com.
Xiao Liu, Email: 1191603545@qq.com.
Wei Tian, Email: 1121269661@qq.com.
Yan Li, Email: 187197905@qq.com.
REFERENCES
- 1.Fan M, Li G, Shen L, Zhang H, Liang L, Zhang Z. Identification of patients with lymph node metastasis from gastric cancer who may benefit from adjuvant chemoradiotherapy after D2 dissection--do N3 patients benefit from additional radiation? Br J Radiol 2016; 89: 20150758. doi: 10.1259/bjr.20150758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015; 136: E359–86. doi: 10.1002/ijc.29210 [DOI] [PubMed] [Google Scholar]
- 3.Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016; 66: 115–32. doi: 10.3322/caac.21338 [DOI] [PubMed] [Google Scholar]
- 4.Zuo TT, Zheng RS, Zeng HM, Zhang SW, Chen WQ. Epidemiology of stomach cancer in China. Chin J Clin Oncol 2017; 44: 52–8. [Google Scholar]
- 5.Yang W, Hu R, Li G-C, Zhou M-L, Wang Y, Shen L-J, et al. Survival outcomes and patterns of failure after D2 dissection and adjuvant chemoradiotherapy for locally advanced gastric cancer: a retrospective study. Br J Radiol 2018; 91: 20170594. doi: 10.1259/bjr.20170594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tanaka O, Sugiyama A, Omatsu T, Tawada M, Makita C, Matsuo M. Hemostatic radiotherapy for inoperable gastric cancer: a pilot study. Br J Radiol 2020; 93: 20190958. doi: 10.1259/bjr.20190958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smalley SR, Benedetti JK, Haller DG, Hundahl SA, Estes NC, Ajani JA, et al. Updated analysis of SWOG-directed intergroup study 0116: a phase III trial of adjuvant radiochemotherapy versus observation after curative gastric cancer resection. J Clin Oncol 2012; 30: 2327–33. doi: 10.1200/JCO.2011.36.7136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dikken JL, Jansen EPM, Cats A, Bakker B, Hartgrink HH, Kranenbarg EM-K, et al. Impact of the extent of surgery and postoperative chemoradiotherapy on recurrence patterns in gastric cancer. J Clin Oncol 2010; 28: 2430–6. doi: 10.1200/JCO.2009.26.9654 [DOI] [PubMed] [Google Scholar]
- 9.Lee J, Lim DH, Kim S, Park SH, Park JO, Park YS, et al. Phase III trial comparing capecitabine plus cisplatin versus capecitabine plus cisplatin with concurrent capecitabine radiotherapy in completely resected gastric cancer with D2 lymph node dissection: the artist trial. J Clin Oncol 2012; 30: 268–73. doi: 10.1200/JCO.2011.39.1953 [DOI] [PubMed] [Google Scholar]
- 10.Park SH, Sohn TS, Lee J, Lim DH, Hong ME, Kim K-M, et al. Phase III trial to compare adjuvant chemotherapy with capecitabine and cisplatin versus concurrent chemoradiotherapy in gastric cancer: final report of the adjuvant chemoradiotherapy in stomach tumors trial, including survival and subset analyses. J Clin Oncol 2015; 33: 3130–6. doi: 10.1200/JCO.2014.58.3930 [DOI] [PubMed] [Google Scholar]
- 11.Trip AK, Poppema BJ, van Berge Henegouwen MI, Siemerink E, Beukema JC, Verheij M, et al. Preoperative chemoradiotherapy in locally advanced gastric cancer, a phase I/II feasibility and efficacy study. Radiother Oncol 2014; 112: 284–8. doi: 10.1016/j.radonc.2014.05.003 [DOI] [PubMed] [Google Scholar]
- 12.Kwong MLM, Denham L, Selleck MJ, Kim C, Kunihira K, Kubba R, et al. Response to neoadjuvant treatment is influenced by grade in gastric cancer. Am Surg 2019; 85: 1419–22PMID. doi: 10.1177/000313481908501241 [DOI] [PubMed] [Google Scholar]
- 13.Martin-Romano P, Solans BP, Cano D, Subtil JC, Chopitea A, Arbea L, et al. Neoadjuvant therapy for locally advanced gastric cancer patients. A population pharmacodynamic modeling. PLoS One 2019; 14: e0215970. doi: 10.1371/journal.pone.0215970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Japanese Gastric Cancer Association . Japanese gastric cancer treatment guidelines 2014 (VER. 4. Gastric Cancer 2017; 20: 1–19. doi: 10.1007/s10120-016-0622-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1. Eur J Cancer 2009; 45: 228–47. doi: 10.1016/j.ejca.2008.10.026 [DOI] [PubMed] [Google Scholar]
- 16.Yoon PD, Chalasani V, Woo HH. Use of Clavien-Dindo classification in reporting and grading complications after urological surgical procedures: analysis of 2010 to 2012. J Urol 2013; 190: 1271–4. doi: 10.1016/j.juro.2013.04.025 [DOI] [PubMed] [Google Scholar]
- 17.Cats A, Jansen EPM, van Grieken NCT, Sikorska K, Lind P, Nordsmark M, et al. Chemotherapy versus chemoradiotherapy after surgery and preoperative chemotherapy for resectable gastric cancer (critics): an international, open-label, randomised phase 3 trial. Lancet Oncol 2018; 19: 616–28. doi: 10.1016/S1470-2045(18)30132-3 [DOI] [PubMed] [Google Scholar]
- 18.Leong T, Smithers BM, Haustermans K, Michael M, Gebski V, Miller D, et al. TOPGEAR: a randomized, phase III trial of perioperative ECF chemotherapy with or without preoperative chemoradiation for resectable gastric cancer: interim results from an international, intergroup trial of the AGITG, TROG, EORTC and Cctg. Ann Surg Oncol 2017; 24: 2252–8. doi: 10.1245/s10434-017-5830-6 [DOI] [PubMed] [Google Scholar]
- 19.Noh SH, Park SR, Yang H-K, Chung HC, Chung I-J, Kim S-W, et al. Adjuvant capecitabine plus oxaliplatin for gastric cancer after D2 gastrectomy (classic): 5-year follow-up of an open-label, randomised phase 3 trial. Lancet Oncol 2014; 15: 1389–96. doi: 10.1016/S1470-2045(14)70473-5 [DOI] [PubMed] [Google Scholar]
- 20.Bang Y-J, Kim Y-W, Yang H-K, Chung HC, Park Y-K, Lee KH, et al. Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (classic): a phase 3 open-label, randomised controlled trial. Lancet 2012; 379: 315–21. doi: 10.1016/S0140-6736(11)61873-4 [DOI] [PubMed] [Google Scholar]
- 21.Zhang ZX, Gu XZ, Yin WB, Huang GJ, Zhang DW, Zhang RG. Randomized clinical trial on the combination of preoperative irradiation and surgery in the treatment of adenocarcinoma of gastric cardia (AGC)--report on 370 patients. Int J Radiat Oncol Biol Phys 1998; 42: 929–34. doi: 10.1016/S0360-3016(98)00280-6 [DOI] [PubMed] [Google Scholar]
- 22.Cunningham D, Allum WH, Stenning SP, Thompson JN, Van de Velde CJH, Nicolson M, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med 2006; 355: 11–20. doi: 10.1056/NEJMoa055531 [DOI] [PubMed] [Google Scholar]
- 23.van Hagen P, Hulshof MCCM, van Lanschot JJB, Steyerberg EW, van Berge Henegouwen MI, Wijnhoven BPL, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med 2012; 366: 2074–84. doi: 10.1056/NEJMoa1112088 [DOI] [PubMed] [Google Scholar]
- 24.Conroy T, Galais M-P, Raoul J-L, Bouché O, Gourgou-Bourgade S, Douillard J-Y, et al. Definitive chemoradiotherapy with FOLFOX versus fluorouracil and cisplatin in patients with oesophageal cancer (PRODIGE5/ACCORD17): final results of a randomised, phase 2/3 trial. Lancet Oncol 2014; 15: 305–14. doi: 10.1016/S1470-2045(14)70028-2 [DOI] [PubMed] [Google Scholar]
- 25.Bedenne L, Michel P, Bouché O, Milan C, Mariette C, Conroy T, et al. Chemoradiation followed by surgery compared with chemoradiation alone in squamous cancer of the esophagus: FFCD 9102. J Clin Oncol 2007; 25: 1160–8. doi: 10.1200/JCO.2005.04.7118 [DOI] [PubMed] [Google Scholar]
- 26.Rivera F, Galán M, Tabernero J, Cervantes A, Vega-Villegas ME, Gallego J, et al. Phase II trial of preoperative irinotecan-cisplatin followed by concurrent irinotecan-cisplatin and radiotherapy for resectable locally advanced gastric and esophagogastric junction adenocarcinoma. Int J Radiat Oncol Biol Phys 2009; 75: 1430–6. doi: 10.1016/j.ijrobp.2008.12.087 [DOI] [PubMed] [Google Scholar]
- 27.Liu X, Li G, Long Z, Yin J, Zhu X, Sheng W, et al. Phase II trial of preoperative chemoradiation plus perioperative SOX chemotherapy in patients with locally advanced gastric cancer. J Surg Oncol 2018; 117: 692–8. doi: 10.1002/jso.24917 [DOI] [PubMed] [Google Scholar]
- 28.Martín Sánchez M, Pérez Escutia Maria Ángeles, Lora Pablos D, Guardado Gonzales S, Cabezas Mendoza AM, Campos Bonel A, Sánchez MM, , Pablos DL, Gonzales SG, Bonel AC, et al. Adjuvant radiochemotherapy in locally advanced gastric cancer : Treatment results and analysis of possible prognostic factors. Strahlenther Onkol 2017; 193: 1005–13. doi: 10.1007/s00066-017-1173-2 [DOI] [PubMed] [Google Scholar]
- 29.Oppedijk V, van der Gaast A, van Lanschot JJB, van Hagen P, van Os R, van Rij CM, et al. Patterns of recurrence after surgery alone versus preoperative chemoradiotherapy and surgery in the cross trials. J Clin Oncol 2014; 32: 385–91. doi: 10.1200/JCO.2013.51.2186 [DOI] [PubMed] [Google Scholar]
- 30.Wang X-Z, Zeng Z-Y, Ye X, Sun J, Zhang Z-M, Kang W-M. Interpretation of the development of neoadjuvant therapy for gastric cancer based on the vicissitudes of the NCCN guidelines. World J Gastrointest Oncol 2020; 12: 37–5315. doi: 10.4251/wjgo.v12.i1.37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tomasello G, Petrelli F, Ghidini M, Pezzica E, Passalacqua R, Steccanella F, et al. Tumor regression grade and survival after neoadjuvant treatment in gastro-esophageal cancer: a meta-analysis of 17 published studies. Eur J Surg Oncol 2017; 43: 1607–16. doi: 10.1016/j.ejso.2017.03.001 [DOI] [PubMed] [Google Scholar]
- 32.Valenti V, Hernandez-Lizoaín JL, Beorlegui MC, Diaz-Gozalez JA, Regueira FM, Rodriguez JJ, et al. Morbidity, mortality, and pathological response in patients with gastric cancer preoperatively treated with chemotherapy or chemoradiotherapy. J Surg Oncol 2011; 104: 124–9. doi: 10.1002/jso.21947 [DOI] [PubMed] [Google Scholar]
- 33.Kim MS, Lim JS, Hyung WJ, Lee YC, Rha SY, Keum KC, et al. Neoadjuvant chemoradiotherapy followed by D2 gastrectomy in locally advanced gastric cancer. World J Gastroenterol 2015; 21: 2711–8. doi: 10.3748/wjg.v21.i9.2711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hu J-B, Sun X-N, Gu B-X, Wang Q, Hu W-X, JB H, , WX H. Effect of intensity modulated radiotherapy combined with s-1-based chemotherapy in locally advanced gastric cancer patients. Oncol Res Treat 2014; 37(1-2): 11–16. doi: 10.1159/000358164 [DOI] [PubMed] [Google Scholar]
- 35.Dong D, Tang L, Li Z-Y, Fang M-J, Gao J-B, Shan X-H, et al. Development and validation of an individualized nomogram to identify occult peritoneal metastasis in patients with advanced gastric cancer. Ann Oncol 2019; 30: 431–8. doi: 10.1093/annonc/mdz001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Takizawa K, Hatta W, Gotoda T, Kawata N, Nakagawa M, Takahashi A, et al. Recurrence patterns and outcomes of salvage surgery in cases of non-curative endoscopic submucosal dissection without additional radical surgery for early gastric cancer. Digestion 2019; 99: 52–8. doi: 10.1159/000494413 [DOI] [PubMed] [Google Scholar]


