Abstract
Background.
Preventing mother-to-child transmission of human immunodeficiency virus transmission (MTCT) depends on early initiation of antiretroviral therapy (ART). We report the 18-month MTCT risk during the transition from Option A to Option B+ in Zimbabwe, and assess whether ART preconception could eliminate MTCT in breastfeeding populations.
Methods.
In 2013, we consecutively recruited a nationally representative sample of 6051 infants aged 4–12 weeks and their mothers from 151 immunization clinics using a multistage stratified cluster sampling method. We identified 1172 human immunodeficiency virus (HIV)–exposed infants and evaluated them at baseline and every 3 months until the child became HIV-infected, died, or reached age 18 months.
Results.
The cumulative MTCT risk through 18 months postdelivery was 7.0%. Of the HIV-infected mothers, 35.3% started ART preconception, 28.9% during pregnancy, and 9.7% after delivery, and 16.0% received zidovudine during pregnancy. Compared to mothers without antiretroviral drug use, MTCT among those starting ART preconception and during pregnancy was lower by 88% (adjusted hazard ratio [aHR], 0.12; 95% confidence interval [CI], .06–.24) and 75% (aHR, 0.25; 95% CI, .14–.45), respectively. HIV-exposed infants with birth weight <2.5 kg (low birth weight) were 2.6-fold more likely to acquire HIV infection compared to those with birth weight ≥2.5 kg (aHR, 2.57; 95% CI, 1.44–4.59). Controlling for other factors, breastfeeding was not significantly associated with MTCT.
Conclusions.
ART preconception has the highest impact on reducing MTCT, indicating that HIV-infected, reproductive-age women should be prioritized in “treat-all” strategies. HIV-infected mothers without ART use should be identified at the first immunization visit and treatment initiated to reduce postdelivery MTCT. MTCT risk is higher in mothers with low-birth-weight deliveries.
Keywords: Zimbabwe, mother-to-child HIV transmission, birth weight, ART initiation
Globally, there has been dramatic progress in reducing new pediatric human immunodeficiency virus (HIV) infection by 58% from 2000 to 2014 [1]. This achievement is mostly attributable to the successful implementation of national programs on prevention of mother-to-child HIV transmission (PMTCT). Zimbabwe adopted and rapidly implemented the World Health Organization (WHO) PMTCT guidelines, including Option A in 2010 and Option B+ in 2013 [2, 3], to meet the global validation criteria of the elimination of MTCT and achieve zero new pediatric HIV infections [4, 5]. The impact of PMTCT programs depends on the coverage and timing of initiation of triple antiretroviral therapy (ART). We conducted an 18-month observational cohort study of a nationally representative sample of pairs of mothers and HIV-exposed infants (HEIs) aged 4–12 weeks (MHEIPs) to measure the impact of the national PMTCT program during the transition from Option A to just before Option B+ implemented nationwide.
METHODS
We conducted an 18-month prospective observational cohort study from 2013 to 2014. A multistage sampling approach was used to select a nationally representative sample of MHEIPs from public clinics providing routine child immunization from all 10 provinces in Zimbabwe. We described the sampling method to select the study clinics in detail elsewhere [6]. In brief, since the annual coverage of the first infant diphtheria-pertussis-tetanus vaccination (DPT1) in Zimbabwe was >95% in 2010 and 2011, we recruited participants at first routine child immunization clinics [7]. Our sampling frame was 1542 clinics providing routine DPT1 nationwide; we excluded 535 (34.7%) small facilities providing <100 DPT1 annually because of resource and logistical considerations. In each province, we divided the 1007 eligible clinics into 1 of 3 strata (clinics providing 100–140, 141–329, or ≥330 DPT1 annually), then used the simple random selection without replacement method to select 15% of clinics from each stratum. A total of 151 clinics were selected as study sites from 27 strata nationally.
In 2013, from February to August, we spent 6 weeks at each study clinic to consecutively enroll all consenting pairs of caregivers and infants aged 4–12 weeks and not requiring emergency care. A trained nurse conducted face-to-face interviews asking if the biological mother accessed PMTCT services including HIV testing and status, CD4 testing, antiretroviral drug (ARV) use, and infant feeding method. Data from the child health card, antenatal care (ANC) card, and maternal outpatient card were extracted to confirm maternal self-report on HIV status and PMTCT services received. Questions on PMTCT services were omitted if the caregiver was not the child’s biological mother. Infant dried blood spot samples (iDBSs) were taken for HIV testing, first with HIV serology to assess the infant’s HIV exposure status, and then for the presence of HIV DNA to diagnose the HIV infection status of HEIs. All participants were scheduled to return to the clinic for the iDBS test results within 4 weeks after the baseline. During this follow-up visit, no blood samples were collected and all confirmed HEIs and their caregivers were offered enrollment for the 18-month follow-up.
MHEIPs were scheduled for follow-up at 6 months postdelivery, then every 3 months with a window of ±1.5 months until the child was diagnosed with HIV, died, or reached 18 months of age. If a MHEIP missed any study visit, the pair was eligible to return for a later study visit. At each follow-up visit, we interviewed the caregivers using a standardized questionnaire, which was similar to the baseline except for questions on ANC services and maternal HIV status. We collected iDBSs for HIV DNA testing at each study visit except at the final 18-month visit when infant HIV rapid testing was conducted, and results were provided immediately to the mother per recommendations [8]. The infant’s HIV DNA test results were returned to the caregiver within 4 weeks.
Based on our baseline recruitment timing, participating HIV-infected mothers received Option A during pregnancy (ie, HIV-infected pregnant and breastfeeding women with WHO stage III/IV or CD4 count ≤350 cells/μL were eligible for ART; other mothers received zidovudine during pregnancy) [2]. As Option B+ (ie, all HIV-infected pregnant and breastfeeding women are eligible for lifelong ART) started in a few clinics in November 2013 then rolled out to all clinics nationwide by December 2014, ART should have been initiated for participating mothers who received Option A previously regardless of CD4 cell count during the transition from Option A to Option B+ [3].
All participating caregivers provided written consent at the baseline and follow-up enrollment visit. We also obtained verbal consent each time an iDBS specimen was collected. Laboratory-based HIV test results were returned to participants by clinic nurses within 4 weeks. Routine HIV and other care and treatment services were provided per the national guidelines.
Laboratory-Based Human Immunodeficiency Virus Testing
We collected iDBS on Munktell-TFN 5-spot paper and sent to the National Microbiology Reference Laboratory, Harare, for testing for HIV antibodies using an enzyme immunoassay (EIA) (Ani labsystem HIV1/2 Ab EIA 3rd generation, Labsystems Diagnostics Oy, Vantaa, Finland). All antibody-positive samples were confirmed by a second EIA (Enzygnost Anti-HIV1/2 Plus, Siemens Healthcare Diagnostics Products GmbH, Marburg/Germany). Baseline iDBSs with positive EIA results, or from infants with known HIV-infected mothers, were tested for HIV DNA by polymerase chain reaction (PCR) (COBAS Ampli Prep/COBAS TaqMan-CAP/CTM-Qualitative assay, version 1.0, Roche Diagnostics, Branchburg, New Jersey). All first positive or indeterminate results were confirmed by a second PCR test (Amplicor HIV-1 DNA, version 1.5, Roche Diagnostics).
Statistical Analysis
We excluded 5 twin infants from the analysis. We defined an HIV-infected mother if she was reported HIV-positive on her ANC card or the child health card or if her infant’s baseline iDBS was positive by HIV EIAs. We defined an HIV-infected child if the iDBS was confirmed positive with DNA PCR or testing positive by 2 rapid HIV tests at 18 months of age.
A combination of retrospective and prospective cohort analysis approach was used to assess whether MTCT occurred perinatally and measured at baseline and at the follow-up visits, respectively. All analyses controlled for the complex survey design (ie, weighting, clustering, and stratification). Domain analyses were performed on subpopulations (ie, HEIs). Sampling weights were calculated as the inverse of the product of the multistage selection probabilities and adjusted for nonresponse.
Cumulative MTCT risks were estimated at a given age of HEI and infant’s death was treated as a competing risk using the stcompet package in Stata (StataCorp LLC, College Station, Texas) [9]. MTCT rate and confidence intervals were estimated for each interval (HEI age ± 1.5 months) using Poisson regression. Person-month was treated as the exposure, and the dependent variable was MTCT. When MTCT was detected, person-month was calculated as the midpoint between last negative and first positive DNA PCR result.
Breastfeeding duration was calculated as the midpoint between the reported date of the last breastfeeding and the study visit when breastfeeding was stopped. For models with categorical predictors, the constant term was suppressed, and all levels of a categorical variable were included in the model. Variance estimation was performed using Stata’s survey jack-knife procedure. Cox regression analysis was used to estimate unadjusted and adjusted hazard ratios (HRs), 95% confidence intervals (CIs), and P values. The initial multivariable model included variables from single variable Cox models, with P < .25 (Wald test) [10]. The initial multivariable model was refit until all variables had P < .05. Interactions between variables in the final model were tested. Maternal age and breastfeeding duration were forced into the initial and final multivariable models.
The study protocol was approved by the Zimbabwe Medical Research Council and the Office of Associate Director of Science, the US Centers for Disease Control and Prevention.
RESULTS
A total of 6809 caregiver–infant pairs underwent screening for eligibility; 6051 (89%) consented to participate in the study (Figure 1). Of those, we identified and enrolled 1172 MHEIPs for the follow-up and excluded 251 (4.1%) pairs from the analysis because the infants’ HIV exposure or infection status could not be confirmed. There was no statistically significant difference between those pairs excluded vs included in the study regarding maternal age, marital status, education level, and the relationship of the caregiver to the infant. By the 18-month visit, we identified 76 MTCT cases, 54 HEIs died, and 827 returned for this visit.
Figure 1.
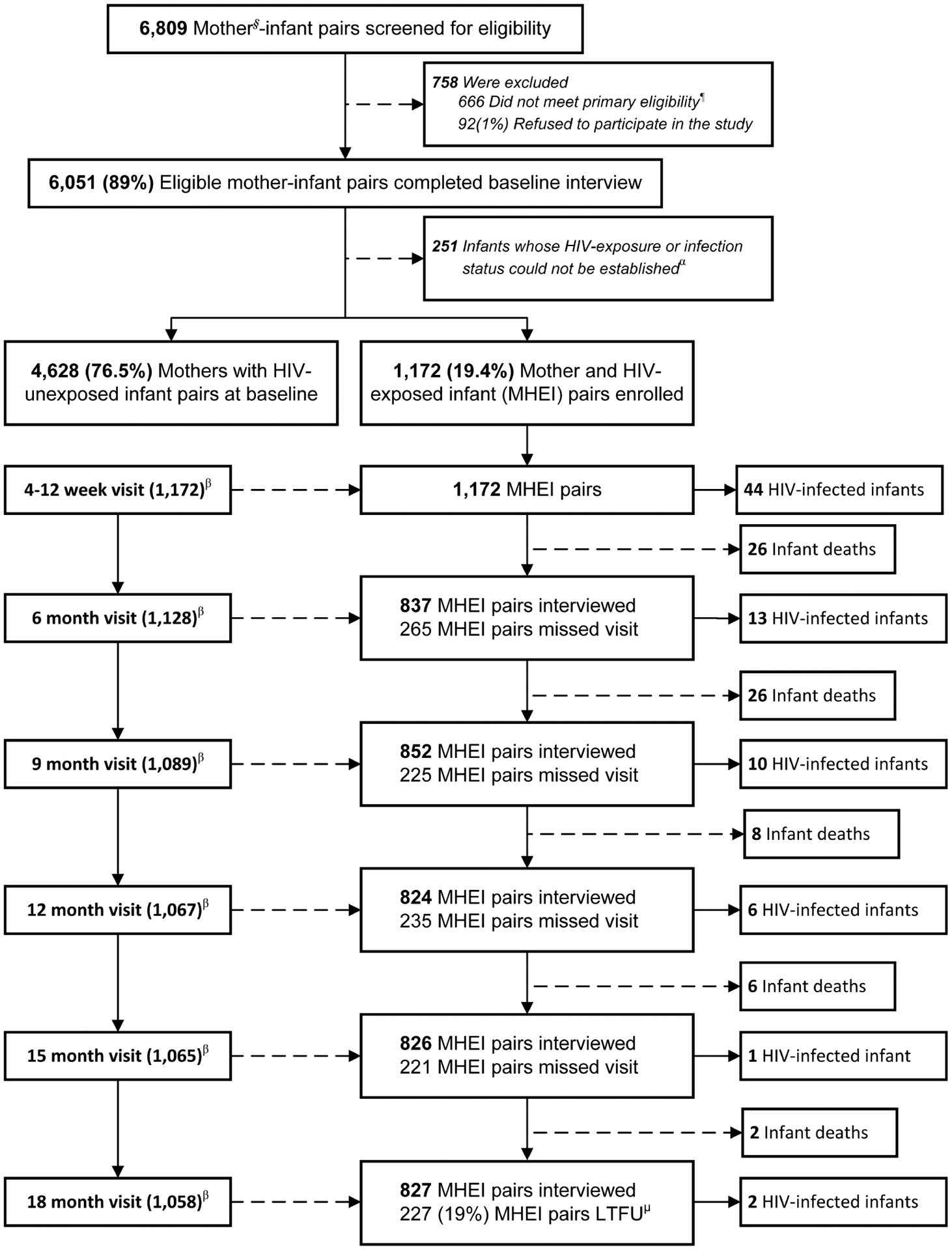
Profile of the impact evaluation of the national prevention of mother-to-child human immunodeficiency virus transmission program from pregnancy throughout 18 months postdelivery during the transition from Option A to Option B+, Zimbabwe, 2013–2014. §Mothers: including 99.5% biological mothers and 5% non-biological mothers who can sign consent forms; ¶Eligibility: infants aged 4–12 weeks and did not need emergency care; α251 infants include 35 refused to provide infant dried-blood-spot sample (iDBS); 200 iDBS missing or rejected; and 16 HEI without DNA PCR result; βEligible for the follow-up visit; μLoss to follow up. Abbreviations: HEI, HIV-exposed infants; HIV, human immunodeficiency virus; LTFU, lost to follow-up; MHEI, mother–HIV-exposed infant pairs; PCR, polymerase chain reaction.
We found that 20.3% (95% CI, 18.6%–22.0%) of infants aged 4–12 weeks attending immunization clinics were HEIs (Table 1). Of HIV-infected mothers, 94.6% (95% CI, 92.7%–96.4%) reported attending ≥1 ANC, and 99.6% reported knowing their HIV status during pregnancy. Of those mothers who had ≥1 ANC, 20% (95% CI, 17.0%–23.0%) received first ANC before 14 weeks’ gestation. At 4–12 weeks postdelivery, 70% (95% CI, 66.2%–73.8%) and 2.2% (95% CI, 1.4%–3.1%) of HIV-infected mothers reported exclusive breastfeeding and not breastfeeding since giving birth, respectively. By 18 months postdelivery, 57.9% (95% CI, 51.4%–64.3%) reported still breastfeeding.
Table 1.
Coverage of Antenatal Care and Prevention of Mother-to-Child Human Immunodeficiency Virus (HIV) Transmission Services Received by HIV-Infected Mother/HIV-Exposed Infant Pairs During the Transition From Option A to Option B+, Zimbabwe, 2013–2014
| Characteristic and Services | Observed No.a | Weighted, % | (95% CI) | |
|---|---|---|---|---|
| Maternal HIV-prevalence at 4–12 wk postdelivery | 1172 | 20.3 | (18.6–22.0) | |
| Maternal HIV infection status by timing of being diagnosed | At 4–12 wk post-delivery | 64 | 5.4 | (4.1–7.2) |
| During pregnancy | 467 | 39.6 | (35.5–43.8) | |
| Preconception | 641 | 55.0 | (50.5–59.3) | |
| Biological mothers | 1166 | 99.5 | (99.0–99.8) | |
| Attend ≥1 ANC | 1101 | 94.6 | (92.7–96.4) | |
| Knowing HIV status (reported)b | 1130 | 99.6 | (99.2–100.0) | |
| Gestational age at first ANC visit | <14 wk | 187 | 20.0 | (17.0–23.0) |
| 14–27 wk | 523 | 55.7 | (52.4–58.9) | |
| ≥ 28 wk | 230 | 24.3 | (21.0–27.6) | |
| Observed missing value | 232 | 19.8% | ||
| Gestational age at delivery | ≥37 wk | 586 | 67.9 | (58.0–76.5) |
| <37 wk | 274 | 32.1 | (23.5–42.0) | |
| Observed missing value | 312 | 26.6 | ||
| Mode of delivery | Vaginal delivery | 1106 | 94.3 | (92.4–95.8) |
| Assisted delivery | 64 | 5.5 | (4.0–7.4) | |
| Cesarean delivery | 2 | 0.2 | (.0–.7) | |
| Birth weight | <2.5 kg | 133 | 11.9 | (9.9–14.3) |
| ≥2.5 kg | 972 | 88.1 | (85.7–90.1) | |
| Observed missing value | 67 | 5.8 | ||
| CD4 test done | Yes | 1003 | 85.6 | (82.9–88.2) |
| Maternal ART | ART preconception | 415 | 35.3 | (32.0–38.6) |
| ART initiation | ART during pregnancyc | 338 | 28.9 | (25.5–32.3) |
| ART postdeliveryd | 114 | 9.7 | (7.6–11.8) | |
| Zidovudine only | 187 | 16.0 | (13.7–18.3) | |
| No antiretroviral drug | 118 | 10.1 | (7.9–12.3) | |
| Infant nevirapine within 72 h | Yes | 1047 | 96.0 | (94.7–97.2) |
| After birth for a duration of 6 wk | No | 42 | 3.8 | (2.6–5.0) |
| Refused/don’t remember | 2 | 0.2 | (.0–.3) | |
| Feeding practices | ||||
| Birth to 4–12 wk postdelivery | Exclusive breastfeeding | 811 | 70.0 | (66.2–73.8) |
| Mixed breastfeeding | 319 | 27.8 | (23.9–31.7) | |
| No breastfeeding | 26 | 2.2 | (1.4–3.1) | |
| Six mo postdelivery | Exclusive breastfeeding | 367 | 42.8 | (38.6–47.0) |
| Mixed breastfeeding | 453 | 53.3 | (48.9–57.7) | |
| No breastfeeding | 33 | 3.9 | (2.7–5.1) | |
| Nine mo postdelivery | Breastfeeding | 808 | 92.5 | (89.5–95.6) |
| No breastfeeding | 66 | 7.5 | (5.7–9.2) | |
| 12 mo postdelivery | Breastfeeding | 760 | 90.8 | (87.9–92.7) |
| No breastfeeding | 77 | 9.2 | (6.9–11.5) | |
| 15 mo postdelivery | Breastfeeding | 683 | 82.2 | (79.1–85.3) |
| No breastfeeding | 147 | 17.8 | (14.7–20.9) | |
| 18 mo postdelivery | Breastfeeding | 487 | 57.9 | (51.4–64.3) |
| No breastfeeding | 347 | 42.1 | (35.7–48.6) |
Abbreviations: ANC, antenatal care; ART, antiretroviral therapy; CI, confidence interval; HIV, human immunodeficiency virus.
Number of observed participants.
Some mothers knew their HIV-positive status and did not attend ANC.
Of these 338 mothers, 34.3% initially received zidovudine, then ART.
Of these 114 mothers, 61.4% received zidovudine during pregnancy.
Figure 2 indicates the overall cumulative 18-month MTCT of 7.0% with 50% of the risk noted by 6 weeks (3.6%), and another 25% noted by 6 months postdelivery (incremental risk, 1.7%). MTCT rate was significantly higher during the first 3 months postdelivery (13.3 cases/1000 person-months) compared with the subsequent 3 months (5.3 cases/1000 person-months) (Table 2).
Figure 2.
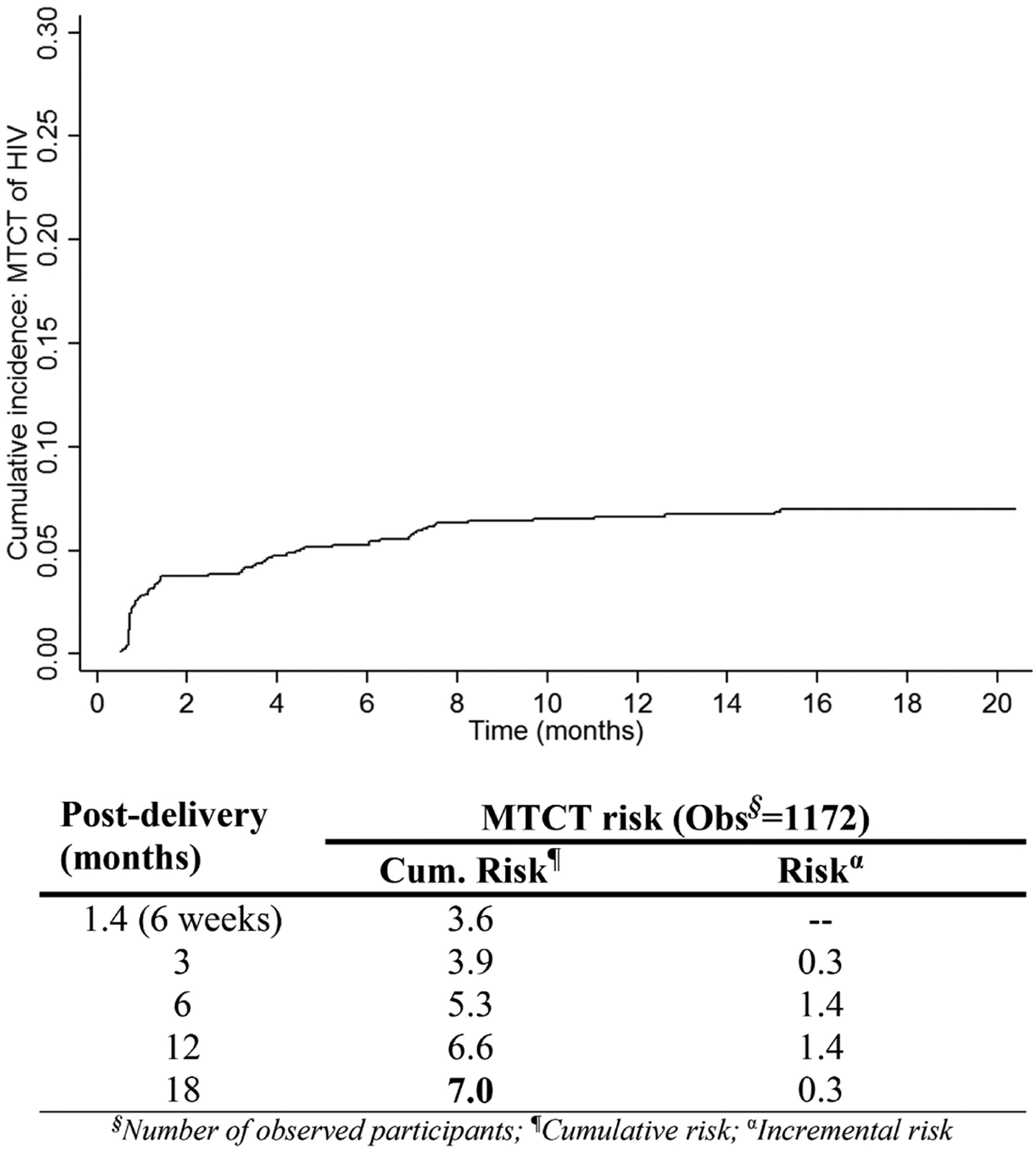
Weighted cumulative risk (%) and rate of mother-to-child transmission (MTCT) of human immunodeficiency virus (HIV) from pregnancy throughout 18 months postdelivery during the transition from Option A to Option B+, Zimbabwe, 2013–2014.
Table 2.
Weighted Mother-to-Child Transmission Rates per 1000 Person-months Measured Throughout 18 Months Postdelivery by Timing of Antiretroviral Therapy Initiation, Zimbabwe, 2013–2014
| Postdelivery (Window)a | Overall (N = 1172) | Timing of ART Initiation | ||||
|---|---|---|---|---|---|---|
| ART Preconception (n = 415) | ART During Pregnancy (n = 338) | ART After Birth (n = 114) | ZDV (n = 187) | No ARV (n = 118) | ||
| 3 mo (0–3.4 mo) | 13.3 (9.8–18.0) | 3.9 (1.4–10.4) | 12.0 (6.8–21.3) | 14.7 (4.40–48.92) | 22.1 (11.6–42.3) | 50.5 (28.8–88.4) |
| 6 mo (3.5–74 mo) | 5.3 (3.3–8.6) | 2.5 (1.0–6.2) | 2.6 (.7–10.4) | 4.85 (.74–31.57) | 17.7 (8.2–38.5) | 12.4 (5.3–28.9) |
| 9 mo (7.5–10.4 mo) | 1.4 (.5–4.6) | 1.9 (.3–12.4) | 0 (.0–6.7) | 0 (.0–.71) | 3.2 (.0–394 × 1015) | 5.5 (.0–13 × 1015) |
| 12 mo (10.5–13.4 mo) | 0.9 (.1–5.2) | 1.1 (.0–181 × 108) | 0 (.0–6.7) | 0 (.0–.72) | 0.0 (.0–.71) | 6.7 (.0–205 × 1015) |
| 15 mo (13.5–16.4 mo) | 0.8 (.1–5.8) | 0 (.0–602 × 1011) | 1.2 (.0–6.2) | 0 (.0–.70) | 3.8 (.0–434 × 1015) | 0.0 (.0–.7) |
| 18 mo (16.5–19.4 mo) | 0 (.0–1.9) | 0 (.0–6.9) | 0 (.0–6.9) | 0 (.0–.74) | 0.0 (.0–.8) | 0.0 (.0–.7) |
Data are presented as transmission rate per 100 person-months (95% confidence interval) unless otherwise indicated. Among mothers starting ART during pregnancy and after giving birth, 34.3% and 61.4%, respectively, started with ZDV first.
Abbreviations: ART, antiretroviral therapy; ARV, antiretroviral drug; n, observed number of HIV-exposed infant–mother pairs; ZDV, zidovudine.
We assigned a window of ±1.5 months using exact age of the HIV-exposed infant.
Maternal ARV coverage was 80.2% during pregnancy including 16.0% only receiving zidovudine and 35.3% starting ART preconception (Table 1). After giving birth, another 9.7% of mothers started ART; 96.0% of HEIs received nevirapine within 72 hours for the first 6 weeks postpartum. During the first 3 months postdelivery, the lowest MTCT rate was among those mothers starting ART preconception (3.9 cases/1000 person-months), followed by those starting ART during pregnancy (12.0 cases/1000 person-months) and those receiving zidovudine during pregnancy (22.1 cases/1000 person-months) (Table 2). The MTCT risk measured at 6 weeks postpartum was 1.2% among mothers starting ART preconception, and was 2-fold higher (2.4%) in those starting ART during pregnancy (Figure 3). Similarly, the postdelivery MTCT risk measured cumulatively from 6 weeks through 18 months was 1.8% and 2.7% in mothers starting ART preconception and starting ART during pregnancy, respectively. Of mothers starting ART during pregnancy, the MTCT rate was reduced from 2.6 to almost zero cases/1000 person-months from 6 to 12 months postdelivery (Table 2). Meanwhile, the rate was decreased from 2.5 to 1.1 cases/1000 person-months among mothers started ART preconception.
Figure 3.
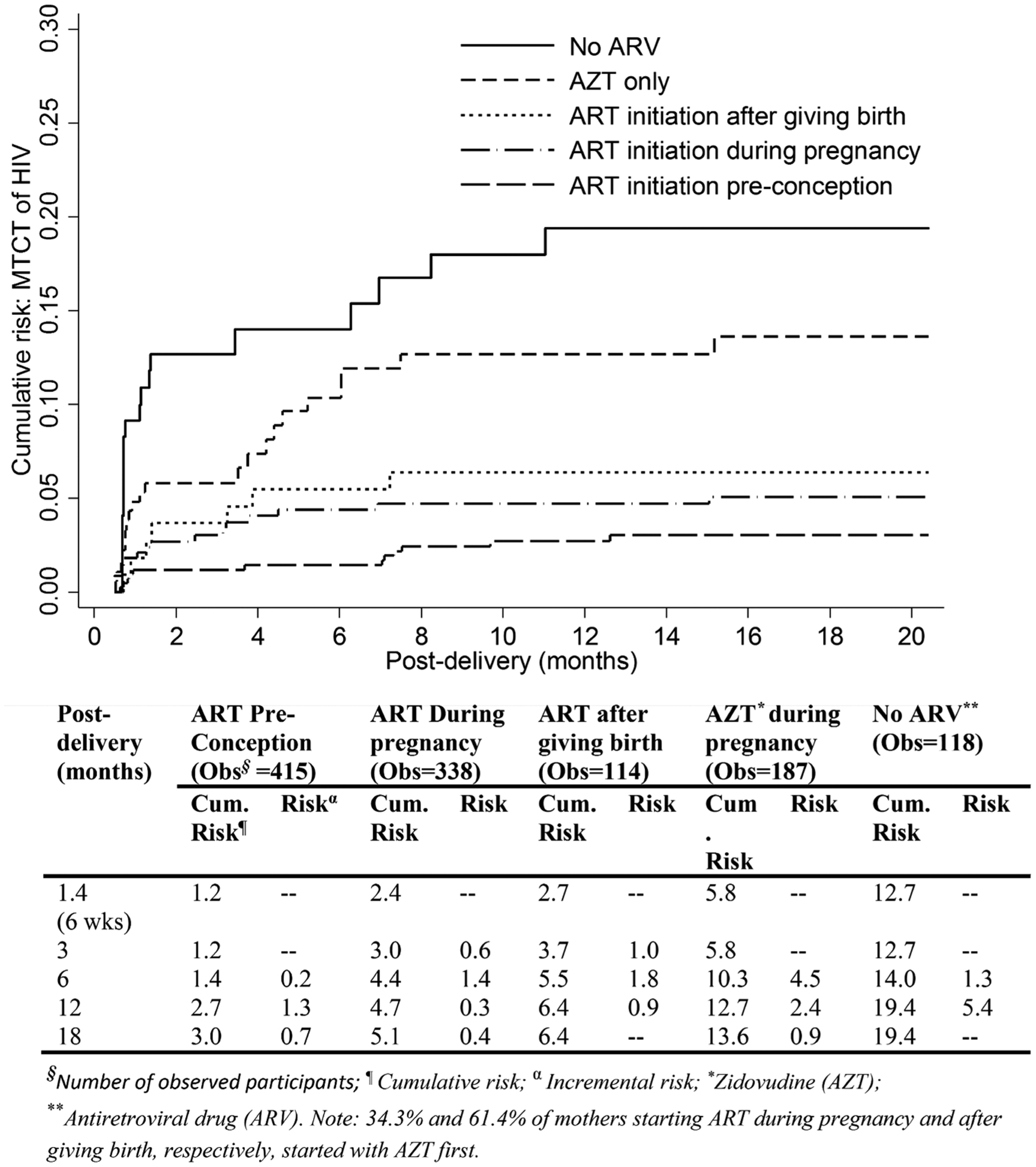
Weighted cumulative risk (%) of mother-to-child transmission of human immunodeficiency virus (HIV) measured during 18 months postdelivery by timing of HIV antiretroviral therapy initiation during the transition from Option A to Option B+, Zimbabwe, 2013–2014. Abbreviations: ART, antiretroviral therapy; ARV, antiretroviral drug; HIV, human immunodeficiency virus; MTCT, mother-to-child transmission.
The MTCT risks measured at 6 weeks and 18 months among HIV-infected mothers with birth-weight delivery <2.5 kg (low birth weight [LBW]) were >2-fold higher than those with birth-weight delivery ≥2.5 kg (6.7% vs 3.0% and 13.5% vs 5.9%, respectively) (Figure 4).
Figure 4.

Weighted cumulative risk (%) of mother-to-child transmission (MTCT) of human immunodeficiency virus (HIV) by infant birth weight during the transition from Option A to Option B+, Zimbabwe, 2013–2014.
In multivariable analysis, mothers starting ART preconception and during pregnancy significantly reduced the risk of MTCT throughout 18 months postdelivery by 88% (adjusted HR [aHR], 0.12 [95% CI, .06–.24]) and 58% (aHR, 0.25 [95% CI, .14–.45]), respectively, compared to mothers without ARVs (Table 2). Mothers starting ART postdelivery had a 33% reduced MTCT risk compared to mothers without ARV (aHR, 0.67 [95% CI, .36–1.27]). Adjusting for other factors including maternal ART initiation timing, HEIs with LBW were 2.6-fold (aHR, 2.57 [95% CI, 1.44–4.59]) more likely to acquire HIV from their mothers compared to those with LBW. Our data also show that mothers aged ≤19 years and duration of breastfeeding did not significantly modify MTCT risk after controlling for the timing of maternal ART initiation and birth weight of HEIs. No statistically significant interaction between HEI birth weight and ARV initiation timing was found.
DISCUSSION
We found that as of December 2014, before Option B+ was implemented nationwide in January 2015, Zimbabwe attained a national cumulative 18-month MTCT rate of 7.0%. This finding is consistent with other cross-sectional surveys conducted in 5 provinces in Zimbabwe, which estimated the cumulative MTCT risk at 9–18 months of approximately 6.7% in 2014 [11, 12]. However, Goga et al reported South Africa’s national cumulative 18-month MTCT of 4.3% also during the transition (2012–2014) from Option A to Option B+ as of August 2014 [13]. The higher MTCT in Zimbabwe is likely attributed to the lower coverage of ARV/ART use during pregnancy (80.0% in Zimbabwe vs 91.3% in South Africa) because both countries achieved ≥95% of women receiving ≥1 ANC visits; and 95% knew their HIV status during pregnancy per the validation criteria for the elimination of MTCT [5, 14]. With Option B+ implemented nationwide since January 2015, the coverage of ART during pregnancy increased to >90%; therefore, the cumulative 18-month MTCT risk could be decreased to <5% by 2017.
Among mothers starting ART preconception, we found the incremental MTCT risk after 6 weeks’ age increased from 0.2% to 1.3% between the 6-month to 12-month postdelivery visits. Ngoma et al reported that the cumulative risk from 6 weeks through 18 months was double the early MTCT (1.4% vs 2.7%) among breastfeeding mothers starting ART between 14 and 30 weeks’ gestation [15]. The increasing risk during postdelivery in the Zambia study and ours could be that many mothers did not adhere to ART after giving birth, resulting in viremia and, subsequently, increased MTCT. These findings underline the importance of taking ART to maintain durable viral suppression during breastfeeding. Compared to mothers receiving Option A (maternal zidovudine during pregnancy and infant nevirapine), the cumulative MTCT measured from 6 weeks to 6 months postdelivery among mothers starting ART postdelivery was lower (1.8% vs 4.5%), and similar to what was shown in clinical trials (1%–5%) in which all mothers started ART at 6 weeks postdelivery [16–18]. Because breastfeeding is a norm in most developing countries, it is important to identify HIV-infected mothers not on ART at the infants’ first immunization visit to initiate treatment and reduce postdelivery MTCT.
MTCT detected at 6 weeks postdelivery was 1.2% among mothers starting ART preconception and 2.4% in those starting during pregnancy. Hoffman et al from South Africa reported a MTCT measured at 4–6 weeks postpartum of 0.7% among mothers starting ART preconception and 5.7% in those starting during pregnancy [19]. In France, Mandelbrot et al also reported a 6-week MTCT rate of 0.2% in mothers starting ART preconception [20]. The lower risk among those starting ART preconception in both studies is likely due to a higher proportion of mothers with viral suppression during pregnancy. The 6-week MTCTs of 2.2% and 1.4% were also reported by Mandelbrot et al [20] and Ngoma et al [15], respectively, among mothers starting ART between 14 and 30 weeks of gestation (the second and third trimester). In our study, though 95% of HIV-infected mothers received ANC, only 20% of these reported a first ANC visit within 14 gestational weeks; we suspect that the majority of ART initiations were between 14 and 30 weeks of gestation. These findings also suggest that the lower MTCT (1.4%) among mothers starting ART between 14 and 30 weeks of gestation reported by Ngoma et al is likely due to a higher proportion of viral suppression than that among mothers in France (2.2%) or in the present study (2.4%). Townsend et al (United Kingdom and Ireland) reported that each additional week of maternal ART during pregnancy was associated with the 0.5% MTCT measured at 6 weeks [21]. This finding again confirmed that the 6-week MTCT could be as low as 0.5% if ART were initiated early (eg, during the first trimester) to ensure good viral control.
We found that LBW deliveries were associated with MTCT. Our data show that HEIs with LBW were at a 2.6-fold higher risk of acquiring maternal HIV than those with birth weight ≥2.5 kg. Some studies have also demonstrated that ART was associated with LBW delivery [22, 23]. However, our data show that the interaction between these 2 factors was not statistically significant. It is likely that 80% of HIV-infected mothers who received the first ANC visit after 14 weeks’ gestation could have been ill, including acquiring other opportunistic infections or possibly transmitting HIV to their infants in utero before starting ART or zidovudine. These conditions may affect fetal growth. Additionally, European studies reported that use of ART (with or without protease inhibitors) in pregnancy was associated with an approximately 2-fold increase in preterm delivery [24–35]. Although we could not directly measure the association of LBW and preterm delivery because 26% of the mothers did not remember their gestation age at delivery, preterm delivery may likely contribute to the number of LBW deliveries in our study. Our data show that the MTCT risk during postdelivery was also higher in mothers with LBW deliveries. It is likely that these mothers may acquire other infections or not adhere to ART postdelivery; therefore, viral load may increase despite the timing of ART initiation and increase MTCT risk. Additionally, these LBW HEIs may be more susceptible to acquiring HIV. Singh et al reported the possibility of increased susceptibility to infections in LBW newborns at term [36, 37]. Arboleya et al reported that deficiency or delay in the establishment of normal microbiota function seems to be present in preterm infants [38]. We also think that the higher MTCT risk may reflect gut immaturity among LBW babies [39].
Our study design may not be optimal for determining the impact of ART timing on birth outcomes because we recruited 4- to 12-week-old infants and their caregivers attending immunization clinics providing ≥100 DPT1/year. Although the coverage of the DPT1 was >95% since 2010, and the retention was 78.2%, we acknowledge that our estimates may not reflect information from 100% of MHEIPs in Zimbabwe. Our findings did not account for those infants who attended the excluded clinics or died before 4 weeks of age; however, we cannot predict the direction of these biases. As only HEIs born to HIV-infected mothers who acquired HIV before or during pregnancy were recruited to the study, we may underestimate the 18-month MTCT because some mothers may acquire HIV during the breastfeeding period. Our data on ARV/ART and breastfeeding practice were based on self-report; consequently, we are not able to accurately report if women received first- or second-line ART, or the timing of ART initiation by gestational age.
CONCLUSIONS
Elimination of MTCT is achievable in limited-resource settings where breastfeeding is a norm. ART preconception has the highest impact on reducing MTCT; therefore, HIV-infected women of reproductive age should be prioritized in the “treat-all” approach to eliminating MTCT. It is important to identify HIV-infected mothers not on ART at the infants’ first immunization visit to initiate ART and reduce postdelivery MTCT. Importantly, MTCT risk is higher in mothers with LBW deliveries; thus, intensified maternal VL monitoring and support could reduce viremia and MTCT. Last, with high coverage of maternal ART and infant prophylaxis, breastfeeding may not increase MTCT risk and should not be discouraged. Table 3
Table 3.
Predictors of Mother-to-Child Human Immunodeficiency Virus Transmission During Pregnancy Through 18 Months Postdelivery During the Transition From Option A to Option B+, Zimbabwe, 2013–2014
| Risk Factor (n = 1172) | Unadjusted Weighted | Adjusted Weighteda | ||
|---|---|---|---|---|
| Hazard Ratio | (95% CI) | Hazard Ratio | (95% CI) | |
| Maternal ageb,c | ||||
| ≤19 y | 2.16 | (1.02–4.58) | 1.33 | (.57–3.07) |
| >19 y | Ref | |||
| Highest educational level | ||||
| Grades 1–7 | 0.90 | (.20–4.00) | ||
| Grades 8+ | 0.63 | (.14–2.83) | ||
| No education | Ref | |||
| No. of pregnanciesb | 0.89 | (.73–1.07) | ||
| No. of live children | 0.91 | (.75–1.02) | ||
| No. of ANC visits | 0.90 | (.73–1.1) | ||
| Timing of maternal HIV infection diagnosed | ||||
| At 4–12 wk postdelivery | 5.1 | (2.4–10.7) | ||
| During pregnancy | 1.7 | (1.1–2.7) | ||
| Preconception | Ref | |||
| Reported CD4 during pregnancy | ||||
| ≤350 cells/μL | 1.49 | (.72–3.09) | ||
| >350 cells/μL | Ref | … | ||
| Living in the same household with a tuberculosis patient during pregnancy | ||||
| No | 1.34 | (.44–4.09) | ||
| Yes | Ref | |||
| Mode of delivery | ||||
| Caesarean/assisted delivery | 0.67 | (.22–2.05) | ||
| Vaginal | Ref | |||
| Timing of ART initiationb | ||||
| ART preconception | 0.13 | (.07–.25) | 0.12 | (.06–.24) |
| ART during pregnancy | 0.22 | (.13–.39) | 0.25 | (.14–.45) |
| ART after giving birth | 0.28 | (.11–.76) | 0.26 | (.10–.70) |
| ZDV only | 0.61 | (.35–1.08) | 0.67 | (.36–1.27) |
| No ARV | Ref | … | Ref | … |
| Infant nevirapineb | ||||
| Yes | 1.74 | (.74–4.10) | ||
| No | Ref | |||
| Duration of breastfeedingb,c | ||||
| 30 d incremental | 1.33 | (.75–2.37) | 1.41 | (.75–2.65) |
| Infant’s birth weightb | ||||
| <2.5 kg | 2.38 | (1.39–4.09) | 2.57 | (1.44–4.59) |
| ≥2.5 kg | Ref | |||
Abbreviations: ANC, antenatal care; ART, antiretroviral therapy; ARV, antiretroviral drug; CI, confidence interval; HIV, human immunodeficiency virus; n, observed number of HIV-exposed infant–mother pairs; ZDV, zidovudine.
Findings from the final multivariable analysis (all variables had P < .05); interaction between “timing of ART initiation” and “infant’s birth weight” was not statistically significant in the final model.
Variables having P < .25 (Wald test) and were included in the initial multivariable analysis.
Variables were forced into the model based on previous knowledge.
Acknowledgments.
We thank Dr Lynne Mofenson; Dr Surbhi Modi; the participants (mother-infant pairs); the study team including Tendai Shamu, Peter Gumbo, Chipo Chimamise, Fannuel Wamambo, Nicol Redzo, Vasco Chikwasha, and Elizabeth Gonese; data collectors; and leadership of the study districts, provinces, and Ministry of Health in Zimbabwe. We also thank Mobenzi for their support in developing and managing our data collections using the web-based real-time platform.
Financial support.
This evaluation was primarily supported by the President’s Emergency Plan for AIDS Relief (PEPFAR) under a cooperative agreement (number U2GGH00315-01) between the CDC and the University of Zimbabwe through the Department of Community Medicine. CDC assisted with protocol development, managing the study, data analysis, and manuscript writing.
Footnotes
Publisher's Disclaimer: Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention (CDC).
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Joint United Nations Programme on HIV/AIDS. How AIDS changed everything—MDG6: 15 years, 15 lessons of hope from the AIDS response. Geneva, Switzerland: UNAIDS, 2015. [Google Scholar]
- 2.World Health Organization. Antiretroviral drugs for treating pregnant women and preventing HIV infection in infants, 2010. Recommendations for a public health approach. Geneva, Switzerland: WHO, 2010. [PubMed] [Google Scholar]
- 3.World Health Organization. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. Recommendations for a public health approach. 2015. Geneva, Switzerland: WHO, 2013. [PubMed] [Google Scholar]
- 4.Joint United Nations Programme on HIV/AIDS. Global plan towards the elimination of new HIV infections among children by 2015 and keeping their mothers alive. Geneva, Switzerland: UNAIDS, 2011. [Google Scholar]
- 5.World Health Organization. Elimination of mother-to-child transmission (EMTCT) of HIV and syphilis. Global guidance on criteria and processes for validation. Geneva, Switzerland: WHO, 2014. [Google Scholar]
- 6.Wiegert K, Dinh TH, Mushavi A, Mugurungi O, Kilmarx PH. Integration of prevention of mother-to-child transmission of HIV (PMTCT) postpartum services with other HIV care and treatment services within the maternal and child health setting in Zimbabwe, 2012. PLoS One 2014; 9:e98236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.World Health Organization. Measuring the impact of national PMTCT programmes: towards the elimination of new HIV infections among children by 2015 and keeping their mothers alive. A short guide on methods. Geneva, Switzerland: WHO, 2012. [Google Scholar]
- 8.World Health Organization. WHO recommendations on the diagnosis of HIV infection in infants and children. Geneva, Switzerland: WHO, 2010. [PubMed] [Google Scholar]
- 9.Coviello V, Boggess M. Cumulative incidence estimation in the presence of competing risks. Stata Journal 2004:9. [Google Scholar]
- 10.Heeringa S, West BT, Berglund PA. Applied survey data analysis. Boca Raton, FL: Chapman & Hall/CRC, 2010. [Google Scholar]
- 11.McCoy SI, Fahey C, Buzdugan R, et al. Targeting elimination of mother-to-child HIV transmission efforts using geospatial analysis of mother-to-child HIV transmission in Zimbabwe. AIDS 2016; 30:1829–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Buzdugan R, Kang Dufour MS, McCoy SI, et al. Option A improved HIV-free infant survival and mother to child HIV transmission at 9–18 months in Zimbabwe. AIDS 2016; 30:1655–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goga AE, Jackson D, Lombard C, et al. Highest risk of mother to child transmission of HIV or death in the first 6 months postpartum: results from 18 month follow-up of an HIV-exposed national cohort, South Africa. 2016. Available at: http://programme.aids2016.org/Search/Search?search=Goga. Accessed 30 November 2016.
- 14.Goga AE, Dinh TH, Jackson DJ, et al. ; South Africa PMTCT Evaluation (SAPMCTE) Team. Population-level effectiveness of PMTCT Option A on early mother-to-child (MTCT) transmission of HIV in South Africa: implications for eliminating MTCT. J Glob Health 2016; 6:020405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ngoma MS, Misir A, Mutale W, et al. Efficacy of WHO recommendation for continued breastfeeding and maternal cART for prevention of perinatal and postnatal HIV transmission in Zambia. J Int AIDS Soc 2015; 18:19352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chasela CS, Hudgens MG, Jamieson DJ, et al. ; BAN Study Group. Maternal or infant antiretroviral drugs to reduce HIV-1 transmission. N Engl J Med 2010; 362:2271–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kumwenda NI, Hoover DR, Mofenson LM, et al. Extended antiretroviral prophylaxis to reduce breast-milk HIV-1 transmission. N Engl J Med 2008; 359:119–29. [DOI] [PubMed] [Google Scholar]
- 18.SWEN Study Team. Extended-dose nevirapine to 6 weeks of age for infants to prevent HIV transmission via breastfeeding in Ethiopia, India, and Uganda: an analysis of three randomised controlled trials. Lancet 2008; 372:300–13. [DOI] [PubMed] [Google Scholar]
- 19.Hoffman RM, Black V, Technau K, et al. Effects of highly active antiretroviral therapy duration and regimen on risk for mother-to-child transmission of HIV in Johannesburg, South Africa. J Acquir Immune Defic Syndr 2010; 54:35–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mandelbrot L, Tubiana R, Le Chenadec J, et al. ; ANRS-EPF Study Group. No perinatal HIV-1 transmission from women with effective antiretroviral therapy starting before conception. Clin Infect Dis 2015; 61:1715–25. [DOI] [PubMed] [Google Scholar]
- 21.Townsend CL, Byrne L, Cortina-Borja M, et al. Earlier initiation of ART and further decline in mother-to-child HIV transmission rates, 2000–2011. AIDS 2014; 28:1049–57. [DOI] [PubMed] [Google Scholar]
- 22.Njom Nlend AE, Nga Motazé A, Moyo Tetang S, Zeudja C, Ngantcha M, Tejiokem M. Preterm birth and low birth weight after in utero exposure to antiretrovirals initiated during pregnancy in Yaoundé, Cameroon. PLoS One 2016; 11:e0150565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tookey PA, Thorne C, van Wyk J, Norton M. Maternal and foetal outcomes among 4118 women with HIV infection treated with lopinavir/ritonavir during pregnancy: analysis of population-based surveillance data from the national study of HIV in pregnancy and childhood in the United Kingdom and Ireland. BMC Infect Dis 2016; 16:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lorenzi P, Spicher VM, Laubereau B, et al. Antiretroviral therapies in pregnancy: maternal, fetal and neonatal effects. Swiss HIV Cohort Study, the Swiss Collaborative HIV and Pregnancy Study, and the Swiss Neonatal HIV Study. AIDS 1998; 12:F241–7. [DOI] [PubMed] [Google Scholar]
- 25.European Collaborative Study, Swiss Mother and Child HIV Cohort Study. Combination antiretroviral therapy and duration of pregnancy. AIDS 2000; 14:2913–20. [DOI] [PubMed] [Google Scholar]
- 26.European Collaborative Study. Exposure to antiretroviral therapy in utero or early life: the health of uninfected children born to HIV-infected women. J Acquir Immune Defic Syndr 2003; 32:380–7. [DOI] [PubMed] [Google Scholar]
- 27.Thorne C, Patel D, Newell ML. Increased risk of adverse pregnancy outcomes in HIV-infected women treated with highly active antiretroviral therapy in Europe. AIDS 2004; 18:2337–9. [DOI] [PubMed] [Google Scholar]
- 28.Martin F, Taylor GP. Increased rates of preterm delivery are associated with the initiation of highly active antiretrovial therapy during pregnancy: a single-center cohort study. J Infect Dis 2007; 196:558–61. [DOI] [PubMed] [Google Scholar]
- 29.Ravizza M, Martinelli P, Bucceri A, et al. ; Italian Group on Surveillance on Antiretroviral Treatment in Pregnancy. Treatment with protease inhibitors and coinfection with hepatitis C virus are independent predictors of preterm delivery in HIV-infected pregnant women. J Infect Dis 2007; 195:913–4; author reply 916–7. [DOI] [PubMed] [Google Scholar]
- 30.Townsend CL, Cortina-Borja M, Peckham CS, Tookey PA. Antiretroviral therapy and premature delivery in diagnosed HIV-infected women in the United Kingdom and Ireland. AIDS 2007; 21:1019–26. [DOI] [PubMed] [Google Scholar]
- 31.Grosch-Woerner I, Puch K, Maier RF, et al. ; Multicenter Interdisciplinary Study Group Germany/Austria. Increased rate of prematurity associated with antenatal antiretroviral therapy in a German/Austrian cohort of HIV-1-infected women. HIV Med 2008; 9:6–13. [DOI] [PubMed] [Google Scholar]
- 32.Rudin C, Spaenhauer A, Keiser O, et al. ; Swiss HIV Cohort Study; Swiss Mother and Child HIV Cohort Study. Antiretroviral therapy during pregnancy and premature birth: analysis of Swiss data. HIV Med 2011; 12:228–35. [DOI] [PubMed] [Google Scholar]
- 33.Lopez M, Figueras F, Hernandez S, et al. Association of HIV infection with spontaneous and iatrogenic preterm delivery: effect of HAART. AIDS 2012; 26:37–43. [DOI] [PubMed] [Google Scholar]
- 34.Sibiude J, Warszawski J, Tubiana R, et al. Premature delivery in HIV-infected women starting protease inhibitor therapy during pregnancy: role of the ritonavir boost? Clin Infect Dis 2012; 54:1348–60. [DOI] [PubMed] [Google Scholar]
- 35.European Collaborative Study. Exposure to antiretroviral therapy in utero or early life: the health of uninfected children born to HIV-infected women. J Acquir Immune Defic Syndr 2003; 32:380–7. [DOI] [PubMed] [Google Scholar]
- 36.Singh VV, Chauhan SK, Rai R, Kumar A, Singh SM, Rai G. Decreased pattern recognition receptor signaling, interferon-signature, and bactericidal/permeability-increasing protein gene expression in cord blood of term low birth weight human newborns. PLoS One 2013; 8:e62845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Singh VV, Chauhan SK, Rai R, Kumar A, Rai G. Decreased toll-like receptor-4/myeloid differentiation factor 88 response leads to defective interleukin-1β production in term low birth weight newborns. Pediatr Infect Dis J 2014; 33:1270–6. [DOI] [PubMed] [Google Scholar]
- 38.Arboleya S, Sánchez B, Solís G, et al. Impact of prematurity and perinatal antibiotics on the developing intestinal microbiota: a functional inference study. Int J Mol Sci 2016; 17:649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Groer MW, Luciano AA, Dishaw LJ, Ashmeade TL, Miller E, Gilbert JA. Development of the preterm infant gut microbiome: a research priority. Microbiome 2014; 2:38. [DOI] [PMC free article] [PubMed] [Google Scholar]


