Abstract
A malignant tumor consists of malignant cells as well as a wide array of normal host tissues including stroma, vasculature, and immune infiltrate. The interaction between cancer and these host tissues is critical as these host tissues play a variety of roles in supporting or resisting disease progression. Radiotherapy (RT) has direct effects on malignant cells, but, also, critically important effects on these other components of the tumor microenvironment (TME). Given the growing role of immune checkpoint inhibitors and other immunotherapy strategies, understanding how RT affects the TME, particularly the immune compartment, is essential to advance RT in this new era of cancer therapy. The interactions between RT and the TME are complex, affecting the innate and adaptive arms of the immune system. RT can induce both proinflammatory effects and immune suppressive effects that can either promote or impede antitumor immunity. It is likely that the initial proinflammatory effects of RT eventually lead to rebound immune-suppression as chronic inflammation sets in. The exact kinetics and nature of how RT changes the TME likely depends on timing, dose, fractionation, site irradiated, and tumor type. With increased understanding of the effects of RT on the TME, in the future it is likely that we will be able to personalize RT by varying the dose, site, and timing of intervention to generate the desired response to partner with immunotherapy strategies.
Introduction
The tumor microenvironment (TME) is defined by a continuous and dynamic interaction between the immune system and cancer cells, which are dependent upon immune evasion for survival.1 Recently, immune checkpoint inhibitors have been used to shift the balance of the TME from a state of immunosuppression to a state of immune activation, allowing for a sustained and durable tumor response across multiple disease sites. Increasing use of immune checkpoint blockade has also provided the opportunity for combination with radiotherapy (RT) to produce an abscopal effect, in which radiation to 1 site of metastatic disease may produce a regression in a distant, nonirradiated site.2 However, the mechanisms that underlie the abscopal effect are not well defined, and there have been only limited reported cases of abscopal effect.3 Nonetheless, recent advances in our understanding of the effect of ionizing radiation on the TME have offered new approaches to unleashing the abscopal effect on a broad basis.4 Several ongoing, prospective clinical trials are combining RT and immune checkpoint inhibitors using abscopal response as a surrogate endpoint of efficiency.5 Here, we review the complex interactions between RT and the TME, including both the proinflammatory effects and the immune suppressive effects that can either promote or impede antitumor immunity.
The effect of RT on the TME and immune response is highly dependent upon the fractionation of radiation delivered. While several preclinical studies have demonstrated a synergistic response to combined RT and immunotherapy in a variety of tumor types,2 there has not been a consensus on the optimal dosing of radiation. Initial studies into dose fractionation in breast and colon cancer models found that multiple fractions were superior to a single ablative dose in combination with anti-CTLA4.6 However, other preclinical studies have demonstrated the induction of antitumor T cells with single ablative dosing.7 Given conflicting data from preclinical studies, the effect of radiation doses on the immune response were examined. Low-dose radiation at 2 Gy was shown to stimulate nitric oxide synthase by tumor-associated macrophages and create an immunogenic environment.8 In contrast, higher doses of radiation were shown to promote tumorigenic macrophages9 and cause severe vascular damage, decreasing recruitment of immune cells to the tumor.10 More recent mechanistic studies highlighted the role of radiation in upregulating 3-prime repair endonuclease 1 (TREX1), which digests cytosolic DNA and reduces radiation-induced immunogenicity.11 Radiation doses above 12-18 Gy were shown to highly induce TREX1, as determined by the size of the single dose rather than total dose. Therefore, fractionation and dosage can significantly alter the immune response to radiation on the TME.
The immune response to radiation occurs in 5 distinct phases.12 The initial phase is characterized by the release of damage-associated molecular patterns (DAMPs), which activate the NF-kB pathway, leading to a release of proinflammatory cytokines by innate immune cells and initiation of early inflammation.13 Radiation also induces tumor cells to release chemokines, creating a positive feedback loop for the recruitment of additional immune cells. In the next phase, innate immune cells participate in antigen presentation to T-cells. This stage is enhanced by granulocyte-macrophage colony-stimulating factor (GM-CSF) secretion following radiation to promote dendritic cell differentiation14 and by induction of costimulatory molecules on T-cells following radiation.15,16 Although radiation initially induces an antitumor response, radiation also induces chronic inflammation and rebound immune suppression. In this stage, protumorigenic macrophages are recruited to the tumor in a radiation dose-dependent manner, creating an immunosuppressive TME that supports tumor regrowth and/or resistance. Radiation also induces HIF-a, which induces the expression of PD-L1 in tumor cells and tumor associate macrophages, which results in immune suppression.17 As such, radiation appears to have a temporal effect on the immune response of the TME in which there appears to be a window of anti-tumor response.
In this article we will review the impact of RT on the TME with an emphasis on how RT-induced changes in the microenvironment shape the antitumor efficacy of RT. We will focus heavily on aspects such as non-T cell lymphoid cells and innate cells which have been less comprehensively addressed in other reviews. While any discussion of RT effects on the immune compartment of the TME must necessarily describe effects on tumor vasculature and tumor stroma, we will not separately discuss the tumor vasculature and endothelium but will provide a discussion on the tumor stroma and fibroblasts. We additionally refer the reader to a thorough review by Harrington et al18 for a more detailed examination of these subjects.
Lymphocytes
There is a growing body of data examining the effects of RT on T cells in the TME. These findings have been expertly summarized in a number of recent reviews on the topic such as those from Sharabi et al19 and DeMaria/Formenti et al.4,20 We will provide a brief review here. The effects of RT on T cells in the TME can be broadly divided into 2 overlapping categories: the effects on existing T cell responses and the generation of new T cell responses.
With regards to the augmentation of existing T cell responses, numerous effects have been described. Radiation can sensitize tumor cells to T cell cytotoxicity by increasing expression of major histocompatibility complex (MHC) I21 and Fas.22 Radiation can induce inflammatory cytokines, such as interleukin-1B, tumor necrosis factor-alpha, and interleukin-6, which support the function, expansion, and differentiation of antigen-experienced T cells.23-25 It should be noted that most of the existing cytokine induction data is from analysis of irradiated normal tissues and not the TME. Finally, radiation can induce the homing and infiltration of T cells into the TME.
Two studies from Hammerling et al published roughly a decade apart elegantly demonstrate that RT can normalize tumor vasculature facilitating T cell homing and infiltration into the TME.8,26 Other studies confirm that radiation induces T cell homing and infiltration into the TME27,28 and additional contributing mechanisms, such as radiation induced chemokines29 and vascular adhesion molecules,30 have been described. This induction of tumor infiltrating lymphocytes (TILs) is one of the most commonly discussed immune modulatory effects of RT.
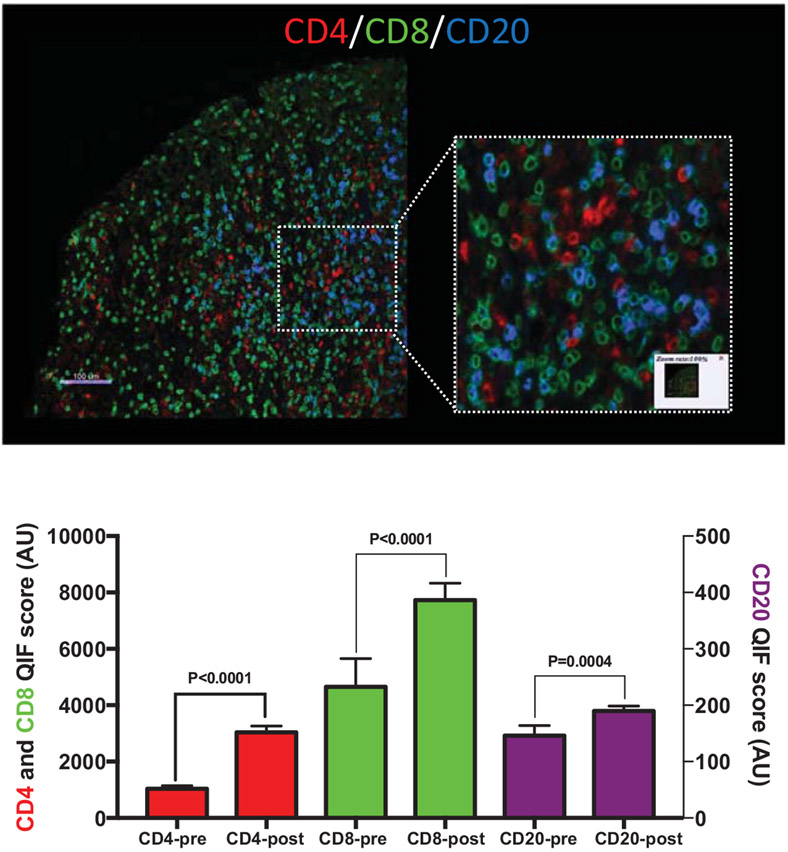
The idea of transforming a “cold” tumor into a “T cell inflamed” tumor is a common rationale for combining RT with immunotherapy. Despite the preclinical data, the clinical data validating this notion are relatively sparse. There are some data that suggest an increase in T-cell proliferation (Ki-67+ T cells) post-RT without demonstrating an overall increase in TILs. An examination of resected oral squamous cell carcinomas revealed that increased proliferation of TILs correlated with preoperative RT.31 A recent study of stereotactic ablative body radiotherapy combined with cytoreductive nephrectomy in patients with metastatic renal cell carcinoma likewise demonstrated an increase in proliferating CD8+ T cells post-RT.32 Several studies have demonstrated increased TILs after neoadjuvant chemoradiotherapy in esophageal,33 cervical34 and rectal cancer.35-37 It is unclear if the increased TILs are a product of RT, chemotherapy, or the combination of the 2. Another study performed in cervical cancer patients reported decreased TILs after RT; it appears these biopsies may have occurred during and immediately after radiation not allowing sufficient time for infiltration by new lymphocytes.38 To address this gap in the literature, we provide here previously unpublished data (courtesy of: Monjazeb AM, Canter RJ, Murphy WM, Schalper KA) examining TILs in a cohort of 29 patients with soft tissue sarcomas treated with standard neo-adjuvant RT (Fig. 1). Comparing the diagnostic core biopsy with the post-RT resection specimen (4-12 weeks post-RT) we see significant increases in CD4+, CD8+, and CD20+ TILs post-RT (Fig. 1).
Figure 1.
Tumor infiltrating lymphocytes at baseline and after neoadjuvant radiotherapy in clinical sarcoma specimens. (A) An example of multiplexed immunofluorescence staining for tumor infiltrating CD8+ or CD4+ T cells and CD20+ B cells. (B) Quantification of tumor infiltrating lymphocyte staining before (pre) and after (post) neoadjuvant radiotherapy. Paired baseline biopsy preradiotherapy and tumor resection postneoadjuvant radiotherapy samples from 30 patients treated at UC Davis were used to generate a tissue microarray. Samples were stained with DAPI, anti-CD4, anti-CD8, anti-CD20, and anti-Cytokeratin at the Schalper laboratory.
RT also induces well described changes in the TME that can contribute to the activation, function, and localization of pre-existing T cell responses, but that also have the capacity to prime de novo antitumor T cell responses. RT treated tumor cells undergo immunogenic cell death due to radiation induced expression of danger-associated-molecular-patterns (DAMPs). The release of HMGB1 by irradiated cells can activate Toll-like receptor 4 and induce antigen uptake and cross-presentation by dendritic cells.39 Likewise, translocation of calreticulin to the cell surface of irradiated cells can increase phagocytosis of these cells.40 Finally, DNA from irradiated tumor cells has been shown to be a critical signal, via the c-Gas-STING pathway, for type I interferon production by TME dendritic cells.41 Type I interferon signaling is one of the major immune modulatory signals induced by RT and is central to radiation induced TILs and antitumor effects.42 In fact, it has been suggested that suppression of type I interferon signaling may mediate resistance to checkpoint inhibitors and that mechanism of resistance can be reversed by RT.43 The immune modulatory effects of RT on T-cells in the TME is an active field of study.
Relatively little attention has been given to the effects of RT on other lymphoid cell types. Natural Killer (NK) cells are innate lymphocytes first recognized for their ability to kill cancer cells without prior sensitization or MHC restriction.44 While traditionally considered to play a role in hematologic malignancies growing evidence suggests a role for NK cells in solid tumors as well.45 NK cell activity is mediated by cell surface markers such NKG2D (activating) and killer cell immunoglobulin like receptors (KIRs; activating and inhibitory). The cognate ligands for these receptors are upregulated on cancer cells, virally infected cells, and, in general, as part of the cellular stress response. NK cells also play a critical role in antibody dependent cell cytotoxicity via the CD16 receptor which binds Fc on antibody coated cells. As with other lymphocytes, NK cells may be radiosensitive, and RT has been noted to affect the viability and activity of circulating NK cells.
In vitro work suggests that NK cells are radiosensitive and respond like acutely responding tissues.46 Clinical data from our lab demonstrated a decrease in circulating total and cytotoxic (CD56dimCD16+) NK cells after ablative doses of RT.47 However, some data suggests that NK cells may be more radioresistant than other lymphoid populations.48 In fact, it has been reported that mature NK cells are relatively radioresistant but their precursors are radiosensitive.49 In vitro data suggests that RT, at doses >30 Gy, affects the cytotoxic function of NK cells before death or apoptosis is observed.50-52
Patients in 1 clinical study had no difference in the number of circulating CD56+ cells after fractionated RT but did demonstrate a modest decrease in NK cell activity.53 Other clinical studies corroborate this finding,54 including studies in breast cancer patients after adjuvant chemo-radiotherapy55 and in endometrial cancer patients after RT.56 These effects might be dose dependent since data suggests that low-dose RT can increase the activity of NK cells57 and clinical data from cervical cancer patients demonstrated an increase in the cytotoxic activity of circulating NK cells after RT of the primary tumor, suggesting systemic activation of NK cells.58 Interestingly, in our work described above examining circulating NK cells after ablative RT, despite the decrease in circulating NK cells, we observed a robust increase in TIM3+ NK cells47 which have been reported to be the most functional NK cells.59
RT also has robust effects on the interaction between NK cells and tumor cells in the TME. A number of early studies demonstrated that irradiation targeted cancer cells for NK cell mediated cytotoxicity. Irradiation has been reported to increase the NK cell killing of 3LL Lewis lung carcinoma and MCA105,60 as well as K562 cells.61 Likewise, coculture of human cancer cells with human primary NK cells or the NK-92 cell line yielded no cytotoxicity, but NK cell mediated cytotoxicity was observed when tumor cells were irradiated.62 The authors suggest that radiation induced release of Smac sensitizes cancer cells to granzyme b mediated killing by NK cells. Another study demonstrated the ability of NK cells to eradicate murine 4T1 breast cancer.63 The addition of low-dose chemoirradiation increased plasma levels of NK cell-activating cytokines. NK cell activity, and NK cell mediated elimination of tumors. Thus, RT may not only increase the cytotoxic ability of NK cells but may facilitate their translocation into the TME. Another report also supports this notion, demonstrating that RT induces NK cell migration into tumors in a manner dependent on the CXCL16 chemokine which binds CXCR6 expressed on activated NK cells.64
With regards to the increased NK cell cytotoxic activity observed after RT this is most likely due to the upregulation of cellular stress markers on irradiated tumor cells sensitizing them to NK cell recognition and killing. A seminal study by Raulet et al demonstrated that genotoxic stress can upregulate NK cell ligands on the cell surface of nonmalignant cells in an ataxia-telangiectasia mutated/ataxia-telangiectasia and Rad3 related-dependent manner.65 Studies from our lab have demonstrated that RT upregulates the expression of stress induced NK cell ligands such as MICA/B and Fas on human and murine cancer cells as well as on primary resected human tumors treated with neoadjuvant RT.66 Other studies corroborate the increase of NK cell ligands after RT.67 The upregulation of these ligands on radioresistant stem-like cells surviving after RT may be critical for NK targeting of this surviving fraction and tumor control after RT.66,68
Furthermore, we find that adoptive transfer of NK cells, while having no effect as a single modality, drastically increases the efficacy of RT.66 A recent canine study from our group demonstrated that RT increased NK cell tumor cytotoxicity in a dose dependent manner in vitro.69 Using a patient derived xenograft model with spontaneous canine sarcomas we observed that, after focal tumor irradiation, adoptively transferred NK cells homed to tumors and induced significant antitumor effects. An ensuing pilot clinical trial of NK cell adoptive transfer and focal RT in companion canines with spontaneous osteosarcomas provided preliminary data on the feasibility and efficacy of this approach.
B cells play a critical role both in humoral immunity and antigen presentation and further examination of their role in RT induced immune modulation is warranted. RT has been demonstrated to induce antitumor humoral immunity in prostate cancer patients.70 Two preclinical studies by Sharabi et al and Guha et al have also pointed to B cell activation and humoral immunity as important mediators of the antitumor effects of RT combined with immunotherapy.71,72 Data examining the effects of RT on TME B cells is extremely sparse. Our clinical data above (Fig. 1), however, demonstrates a significant increase in TME B cells post-RT. Clearly more studies are needed addressing the effects of RT on B cell infiltration and function in the TME.
Rebound Immune Suppression
As with of the other body systems, the immune system functions to maintain homeostasis. Too much inflammation can lead to autoimmunity and inflammatory disease whereas immune suppression can lead to overwhelming infection and malignancy. Thus, it stands to reason that every perturbation made will be met with an opposite reaction to maintain balance and homeostasis. We have coined this process “rebound immune suppression”.73 Indeed, the emerging biology of the PD-1/PD-(L)1 axis demonstrates how finely tuned the immune system is and how delicate this balance is. PD-1, although widely considered a marker of exhaustion, is an early activation marker74,75 rapidly upregulated after T cell receptor engagement presumably as a means to temper T cell activity and maintain peripheral tolerance and only mediates exhaustion when engaged by its ligands. Likewise, the inflammatory signals induced by RT often trigger counterregulatory immune suppressive mechanisms which limit the proinflammatory effects of RT. For example, as outlined above, RT induces expression of potent proinflammatory type I and II interferon cytokines. These cytokines, in addition to their above described inflammatory effects, can also upregulate expression of PD-L1.76 Indeed, it has been reported that radiation induces upregulation of PD-L1 in the TME which can mediated resistance to RT.77,78 These findings form the basis for the many trials combining RT with PD-(L)1 checkpoint inhibition.
Indolamine 2,3-dioxygenase (IDO) catalyzes the breakdown of tryptophan to kynurenine and other downstream catabolites and has been implicated as a master orchestrator of immune suppression in the TME. By altering the metabolic landscape of the TME through starvation of tryptophan and induction of immune suppressive catabolites IDO has been implicated in T cell suppression, T reg induction, and induction of immune suppressive myeloid cells (myeloid-derived suppressor cells (MDSCs)) and tumor-associated macrophages (TAMs). Studies from our lab and corroborated by Welsh et al indicate that the inflammatory effects of RT can induce IDO upregulation.73,79 The above studies demonstrate post-RT that IDO expression is associated with increased TAMs or increased MDSCs and that IDO inhibition improves the efficacy of RT and results in reduction of TME immune suppressive elements including Transforming growth factor beta (TGFβ), TAMs, MDSCs, and Tregs. As described below, this same process of rebound immune suppression can be seen with many innate cell types in the TME after RT, which at first may have an inflammatory function but later switch or are replaced by cells with suppressive function.
Overall, our understanding of this process of rebound immune suppression needs to increase and it suggests that there may be a window period post-RT where proinflammatory effects dominate presenting an opportunity for immune modulation but after which increasing immune suppression limits the immune response and in-situ vaccination effect. The clinical data from the PACIFIC trial supports this notion as patients who started checkpoint inhibition within 14 days of completing RT appeared to have much better outcomes than patients who started later.80
Innate Immunity
The innate immune system consists of cells that do not express antigen-specific receptors (T cell and B cell receptor) and often serve as the regulators of an immune response controlling the initiation and elimination of an inflammatory response. The innate system is broadly composed of macrophages, NK cells, and DCs. As early detector of cell damage and initiators of inflammation, activation of the innate immune system likely serves as one of the main mechanisms driving the extraordinary efficacy of RT. Evidence of the importance of innate immunity in the response to RT come from studies that demonstrate reduced efficacy for RT in preclinical models of cancer which are deficient in innate immune cells including NK cells,81 macrophages,82,83 and DCs.84 These findings are further supported by numerous observations from patients; 1 study in hepatocellular carcinoma, for example, showed that increased numbers of circulating myeloid cells following RT correlated with poorer responses.85 Thus, given that innate immunity has such an important role in determining the response to RT, multiple groups have explored the mechanisms by which RT interacts with the innate immune system. We discuss the findings from these studies below in the context of the different functions of the innate immune system: initiation of inflammation, activation of the adaptive immune response, and resolution of an immune response.
When cells of the innate immune system detect that there is a problem, for example an infection or tissue damage, they activate a program of inflammation that leads to activation of the adaptive response (T and B cells) that requires maturation of dendritic cells or macrophages into antigen-presenting cells and appropriate expression of MHC molecules and co-stimulatory signals. Interestingly, RT has been shown to upregulate MHC class I and stimulate presentation of unique antigens,72,86,87 as well as costimulatory molecules87,88 by dendritic cells.
The importance of dendritic cell (DC) in mediating the efficacy of RT was shown by Dewan et al, where fractionated RT along with anti-CTLA4 had significant abscopal effects in part through the generation of increased numbers of Batf3 DCs.6 Batf3 dependent DC cells are an important subset of dendritic cells with their ability to efficiently cross-present antigens and regulate tumor growth by enhancing CD8+ T cell migration to the TME and fostering effective T cell response.89,90 Abscopal effects were abolished in the Batf3−/− mice consistent with other observations demonstrating the critical role of Batf3 DC in regulating RT-induced antitumor immune responses.89,91,92 In addition to its effects on DC, RT further contributes to the adaptive immune response by encouraging innate immune cells to establish an inflammatory milieu in irradiated tissue in part through stimulating the release of complement and proinflammatory cytokines and chemokines by innate immune cells.93,94
Several groups have shown that they can improve the response to RT in murine models and early human trials by increasing the growth and differentiation of dendritic cells. One way to increase the number of DC is the cytokine GM-CSF which has been shown to be a crucial pathway for the growth, maturation, and migration of DC.95,96 Several human trials of GM-CSF in melanoma and breast cancer have demonstrated the efficacy of GM-CSF administration alone with improved survival compared to historical controls97,98 and an increase in circulating DC.99 Based on these successful early studies, trials of GM-CSF and RT were initiated. In 1 trial of metastatic patients of various histologies, exogenous administration of GM-CSF with a course of fractionated RT (35 Gy in 10 fractions) found evidence of an abscopal, and hence systemic, anti-tumor immune response in 27% of the patients.96
Another cytokine for DC-specific growth similar to GM-CSF that has been shown to enhance the response to RT is the Fms-like tyrosine kinase 3 ligand (FLT3L).100-102 FLT3L binds and activates FLT3 on hematopoetic progenitors and serves a critical role in steady-state maintenance of DC103 and increased levels of FLT3L during inflammation mobilizes DC.104 Two studies using preclinical models of nonsmall cell lung cancer demonstrated reduced tumor growth, metastases, and improved survival with administration of RT and FLT3L in a T-cell dependent manner.101,102 Preclinical data in a murine model of hepatocellular carcinoma has also shown that the efficacy of RT can be enhanced by augmenting DC function through the use of exogenous IL-12 to help DCs better generate cytotoxic T cells.105
With the recent recognition of the need to alleviate the intrinsic tumor immunosuppression to allow antitumor immunity to progress, much activity has been devoted to targeting the pathways and cells that mediate immunosuppression. Interestingly, many of the cellular targets are innate immune cells such as macrophages. Since RT generates both an antitumor immune response and the corresponding suppressive immune control mechanisms, combinations of RT with agents that target intratumoral immune suppression are thought to allow for an enhanced antitumor immune response following RT. Preclinical models strongly support this notion and clinical data are just emerging that suggests that this strategy may be efficacious in the clinical setting.
One of the most successful regimens targeting intratumoral immunosuppression has been targeting immune suppression with checkpoint inhibitors, which are agents that target the PD-1/PD-L1 and CTLA-4 pathways. Innate immune cells are one of the key sources of signal for the PD-1/PD-L1 pathway with dendritic cells and macrophage serving as one of the primary, nontumor sources of PD-L1 in the TME. Thus, the underlying mechanism of checkpoint blockade likely involves disrupting the effects of innate immune cells on immune response in tumors. To date, a large amount of data has demonstrated the efficacy of checkpoint inhibitors in the preclinical and clinical setting in combination with RT. As several excellent recent reviews have examined the role of combining checkpoint blockade with RT in detail, we will not discuss combinations with checkpoint blockade further here though it should be recognized that including one of these agents as a part of any immune-directed therapeutic regimen will be an important consideration for the foreseeable future.2,106 Likewise, there is a rising interest in immunotherapies that directly activate innate immunity. The rationale and potential of combining innate immune system agonists with RT has also been recently reviewed.107
Beyond checkpoint blockade, macrophages serve as the main source of immunosuppression within the TME following RT. As evidence of the importance of macrophages, various studies have revealed a strong negative correlation between the presence of macrophages and survival in various solid tumors including breast, colon, bladder, and lung cancer.108-110 As described above, macrophages are often associated with resistance to RT and chemotherapy by providing both prosurvival signals and tissue repair functions that protect and/or repair the damage done by these therapies. Various studies have shown that macrophages, the most abundant cells of the TME, are altered by RT to support tumor growth after being damaged and sensing damage resulting from irradiation. For example, Leblond et al found an increase in density of pro-tumor M2 macrophages in the TME post-RT in glioblastoma.111 Kioi et al showed that the RT-recruited macrophages help rectify the damage done by RT by promoting vasculogenesis.112
Given, the protumor role of macrophages following RT, multiple groups have shown that blocking macrophage recruitment via targeting CD11b,83 CCL2113, or CSF-1R,82,114,115 enhances the efficacy of RT in preclinical murine models. In a squamous cell carcinoma model Ahn et al found that administration of a CD11b antibody enhanced the efficacy of RT by blocking myeloid cell recruitment to the tumor site after RT leading to delayed regrowth in part through impaired angiogenesis.83 Other studies have revealed that inhibition of macrophages following RT increases both the antitumor immune response82 and prevents protumor repair mechanisms such as angiogenesis and matrix remodeling.83,112 These studies all demonstrate that targeting macrophages can synergize with RT, however, given the potentially positive role of macrophages in producing cytotoxic antitumor immune responses, other groups have sought to preserve the proinflammatory activation capacity of macrophages while preventing their suppressive differentiation to even further synergize with RT. Interestingly, agents targeting tumor-associated macrophages such as the CCL2 inhibitor carlumab have had limited effect as single agents116 and in fact may only have efficacy when combined with other agents such as RT that perturb the tumor immune microenvironment.82,117
In order to preserve the macrophage capacity to activate antitumor immunity while preventing their differentiation into protumor, immunosuppressive phenotypes, several groups including our own have examined the potential of targeting the pathways that lead to protumor phenotypes in macrophages including IL-4,82 arginase 1,118 TGF-β,119 and Tyro3/Axl/Mer tyrosine kinases120,121 in combination with RT. Targeting macrophage differentiation led to improved antitumor immunity, particularly cytotoxic CD8+ T cells, resulting in dramatically enhanced responses to RT. Though each of these strategies targets a distinct pathway found in myeloid-macrophages, they result in reduction but likely not elimination of immunosuppressive differentiation suggesting that even modest reductions in tumor-associated immunosuppression can have profound effects on therapeutic responsiveness to RT.
Transforming Growth Factor Beta
TGFβ is a multipotent cytokine with both tumor suppressive and tumor promoting properties. In preinvasive disease, such as carcinoma-in-situ, TGFβ acts as a tumor suppressor primarily through its growth inhibitory functions. However, once a tumor becomes invasive, TGFβ is tumor promoting via roles in epithelial to mesenchymal transition,122 angiogenesis,123 tumor cell motility and metastasis,124 cancer-associated fibroblast (CAF) proliferation,125 and immunosuppression.126
TGFβ is produced and released into the TME in its latent form, and can be activated through multiple mechanisms (reviewed in127), including radiation. ProTGFβ is synthesized as a homodimer consisting of the latent-active peptide (LAP) and the active cytokine TGFβ. The LAP is cleaved from the active cytokine in the Golgi by furin-type enzymes, however the homodimeric LAP forms a cage around the dimeric TGFβ preventing its association with cellular receptors. Disulfide bonds form between LAP and the latent-TGFβ-binding protein to form the large latency complex (LLC). The LLC is anchored in the extracellular matrix through covalent binding of the N-terminal region of latent-TGFβ-binding protein to matrix proteins by transglutaminase.
Release of active TGFβ from the LLC can occur through proteolytic cleavage by matrix metalloproteases (ie MMP-2 and MMP-9),127 disruption of the LAP-TGFβ interaction by thrombospondin-1,127 or physical force-dependent activation via unfastening of the “straightjacket” domain by integrin binding and stretching of LAP resulting in conformation change releasing bound TGFβ.128 Radiation-mediated generation of reactive oxygen species can also activate TGFβ by modifying LAP causing disruption of its interaction with, and therefore activation of, TGFβ.129 Elevated levels of TGFβ can be detected within 1 hour of radiation in vivo, related to activation of the LLC already deposited in the extracellular matrix.129 Additionally, increased transcription of pro-TGFβ is induced 3-7 days following radiation, particularly within macrophages and neutrophils (pending publication), consistent with the timing of increased TGFβ during wound healing.130,131
Once activated, dimeric TGFβ binds the heteromeric receptor consisting of 2 copies of the type I receptor (TGFβRI) and 2 copies of the type II receptor (TGFβRII). Binding of TGFβ to its receptor leads to phosphorylation of the serine/threonine kinase, TGFβRI, leading to phosphorylation of the intracellular signaling mediators, Smad2 and Smad3, which are then capable of binding the common Smad, Smad4, leading to nuclear translocation of the Smad complex. Once in the nucleus, Smad3 and Smad4 bind DNA at Smad-binding elements, termed CAGA boxes, and regulate transcription of TGFβ target genes.
TGFβ contributes to an immunosuppressive TME through effects on all immune subsets. TGFβ skews innate immune populations towards phagocytic anti-inflammatory macrophages,132 while inhibiting dendritic cell activation, maturation, and migration,133,134 thereby hampering effective tumor antigen presentation. Furthermore, TGFβ is known to suppress T cell effector function, in part, through Smad-mediated downregulation of the target genes granzyme, perforin, and interferon.126 TGFβ promotes regulatory T cell differentiation, further suppressing effector T cells. TGFβ has also been shown to inhibit central memory T cell differentiation by a noncanonical SMAD and mammalian target of rapamycin independent mechanism.135 These finding are not universal as other studies have suggested that TGFβ is critical for the differentiation and maintenance of memory CD8+ T cells.136 TGFβ can have differing effects on different T cell subpopulations and these nuances should be noted, as depending on the context TGFβ may play a critical role in support of T cells and is not purely immunosuppressive. For example TGFβ has also been demonstrated to play a central role in development, migration, and retention of tissue resident memory T cells in the gut.137,138
TGFβ promotes stromal fibrosis further contributing to immune escape. TGFβ is a critical mediator of wound healing, enhancing fibroblast migration to the site of injury, resulting in deposition of collagen, inhibition of MMPs, and aiding the transition from the inflammatory phase to the proliferative phase.130 Radiation-related adverse events such as pneumonitis and fibrosis may be viewed as impaired wound healing responses, and are associated with elevated levels of serum TGFβ139,140 and polymorphisms in TGFβ.141 Recent data links TGFβ-mediated fibrosis and immunosuppression. Subsets of patients with metastatic cancer, including urothelial and colorectal cancers, who failed to respond to checkpoint blockade and exhibited a T cell excluded phenotype, harbored an elevated stromal/fibroblast TGFβ gene expression signature.142-144 Together, these data suggest that TGFβ promotes a suppressive tumor stroma which may exclude T cell infiltration into tumors rendering them resistant to T cell-directed immunotherapy. We and others have previously shown that blockade of TGFβ signaling improves response to radiation dependent upon CD8 T cells, and synergizes with immune checkpoint blockade.145-149 In addition to the stromal exclusion of T cells, TGFβ may also overpower the antitumor effects of infiltrating immune cells as observed in certain subtypes of breast cancer.150 Based on these data, we conclude that TGFβ contributes to immunosuppression through promotion of fibrosis and collagen deposition leading to exclusion of T cells; T cells that are able to infiltrate the tumor are rendered less effective by TGFβ mediated suppression of T cell cytotoxicity. Combination of radiation, TGFβ inhibition, and immune checkpoint blockade may be more effective than radiation and checkpoint blockade alone.
Cancer Associated Fibroblasts and Fibrosis
Radiation efficacy can be limited by alterations in the tumor stroma. For example, pancreatic ductal adenocarcinoma (PDAC) is an aggressive malignancy with a poor prognosis characterized by a fibrotic stroma and poor immune infiltrate. PDAC is relatively radioresistant with poor drug penetrance and elevated levels of hypoxia limiting the efficacy of chemoradiotherapy.149 An additional benefit of radiation is its ability to expose tumor antigens and create a focal inflammatory response,26,86,151 with efficacy dependent on CD8 T cells,149,152,153 which is also limited in PDAC. CAFs may be the link between these phenomena in PDAC as well as in other cancers. CAFs are a chief source of extracellular matrix fibrotic components, such as collagen, hyaluronic acid, and fibronectin, which result in impaired drug penetration, poor immune cell infiltration, and reduced vascular patency.154 Furthermore, CAFs can secrete cyotkines /chemokines and participate in direct cell to cell interactions that govern the functional fate of innate and adaptive immune cells in the TME.155 CAFs may also play a metabolic role in the TME. Recent studies demonstrate that CAFs may metabolically support malignant cells by secretion of alanine156 but this could also have some positive immune consequences as T cell function has also been shown to rely on extracellular alanine.157
Recent data suggests 4 CAF subtypes in breast cancer, with the immunosuppressive CAF-S1 subtype expressing fibroblast activation protein (FAP).158 PDACs express high levels of FAP compared to normal pancreas.159,160 Given the dependence of high-dose radiation on CD8 T cells, combination radiation with immunotherapy has been attempted to enhance PDAC tumor clearance, but with little success, in part attributed to impaired ability of immune cells to penetrate the fibrotic stroma and interact with tumor cells.118,142,143,149,161 As mentioned above, fibroblast derived TGFβ sequesters immune cells outside of tumors leading to resistance to immune checkpoint blockade.142,143 Mouse models targeting CAFs resulted in improved drug penetrance and CD8 T cell infiltration.162 However, tumor infiltrating T cells have impaired efficacy due in part to upregulation of immune checkpoint ligand expression on CAFs and other stromal cells.163,164 CAFs polarize the tumor immune cells to an immunosuppressive phenotype characterized by M2 macrophages expressing Arginase and regulatory T cells, achieved in part via expression of IL-6, IL-10, CXCL12, and TGFβ.158,165-167 Depletion of CAFs by targeting FAP, resulted in improved antitumor immunity characterized by higher levels of interferon-gamma and tumor necrosis factor-alpha.168 Additionally, in a melanoma tumor model, administration of a vaccine targeting tumor antigen and FAP resulted in tumor clearance as a result of antigen spreading.169 However, combination of radiation, aPD1, and targeting of FAP using a highly selective orally bioavailable FAP small molecule inhibitor, or a vaccine strategy to deplete FAP expressing cells, failed to improve survival in murine models of PDAC, despite increasing CD8 T cell infiltration and polyfunctionality.160 These data suggest additional suppressive pathways or key mediators may be contributing to resistance to radiation and immunotherapy in fibrotic tumors.
Targeting upstream mediators of fibrosis has shown efficacy in combination with immuno- and chemotherapy. Stromal stiffness contributes to tumor progression though mechanical forces signaling through integrins and adhesion kinases resulting in enhanced invasion and growth via PI3K activity.170 One such adhesion kinase, focal adhesion kinase, is elevated in PDAC regulating the fibrotic and immunosuppressive microenvironment. Combination focal adhesion kinase inhibition with gemcitabine and αPD1 led to significantly improved survival in murine PDAC.171 In addition, immunosuppressive subtypes of B cells have been shown to be drivers of fibrosis, and targeting B cells by genetic ablation of IgA+ cells or using BTK inhibitors can attenuate carcinogenesis and induce CD8 T cell-mediated tumor regression,172 as well as improve the efficacy of chemotherapy.173 Interestingly, reprograming CAFs to a less immunosuppressive phenotype shows promise for improving T cell function and response to checkpoint blockade using tumor-microenvironment activated angiotensin receptor blockers.174
Radiation fibrosis is a well-characterized deleterious treatment side effect. Radiation mediated fibrosis occurs via a feed-forward loop resulting in enhanced TGFβ production. Radiation increases lactate dehydrogenase-A activity increasing lactate levels, acidifying the extracellular compartment and activating latent TGFβ.175 This leads to myofibroblast differentiation and excess deposition of extracellular matrix proteins.175 Therefore, CAF differentiation and fibrosis can be driven by radiation, but also contribute to radiation resistance and immunosuppression. Further investigation into preventing fibrosis, either at baseline or following radiation, may result in improved responses to immunotherapy.
Conclusions
Given the expanding scope of immunotherapy strategies in the treatment of cancer, improved characterization of the effects of RT on the TME is needed. The inflammatory and immune modulatory effects of RT have been used to rationalize many clinical trials combining RT and immunotherapy, but, in many cases, without robust empirical data to support these approaches. The equivocal outcomes, to date, of clinical trials combining RT and immunotherapy suggest that more learning is required to optimally combine these modalities. The data reviewed herein clearly demonstrate that radiation can induce profound changes in the TME but the exact nature of these changes requires further investigation.
Radiation can induce inflammatory changes and increase the number and functionality of T cells, NK cells, and antigen-presenting cells through numerous mechanisms including interferon and toll-like receptor signaling in immune cells and inducing immunogenic cell death or upregulation of cellular stress markers and MHC on tumor cells. However, radiation can also induce suppressive immune changes in the TME such as induction of TGFβ, IDO, and PD-L1 with resultant increase of suppressive cells within the TME such as Tregs and TAMs. Overall, it is likely that acutely radiation induces inflammation which can lead to antitumor effects but that over time, in response to the sustained/chronic inflammation in the TME, counterregulatory immune suppressive mechanisms are activated in a process of rebound immune suppression. The time course and extent of each phase of this process are likely to vary based on dose, fractionation, tumor type, and site irradiated. With increased understanding of the effects of RT on the TME, in the future it is likely that we will be able to personalize RT by varying the dose, site, and timing of intervention to generate the desired response to partner with immunotherapy strategies.
Acknowledgments
Grant support provided by UC Davis CCSG 5P30CA093373-15.
Footnotes
Conflicts of interest: Dr. Monjazeb is on the advisory board for and/or receives clinical trial / research support from: Zosano, BMS, A-Z, EMD Serono, Dynavax, Incyte, Merck, Genentech, Transgene.
References
- 1.Sharma P, Hu-Lieskovan S, Wargo JA, et al. : Primary, adaptive, and acquired aesistance to cancer immunotherapy. Cell 168:707–723, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ngwa W, Irabor OC, Schoenfeld JD, et al. : Using immunotherapy to boost the abscopal effect. Nat Rev Cancer 18:313–322, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abuodeh Y, Venkat P, Kim S: Systematic review of case reports on the abscopal effect. Curr Probl Cancer 40:25–37, 2016 [DOI] [PubMed] [Google Scholar]
- 4.Rodriguez-Ruiz ME, Vanpouille-Box C, Melero I, et al. : Immunological mechanisms responsible for radiation-induced abscopal effect. Trends Immunol 39:644–655, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kang J, Demaria S, Formenti S: Current clinical trials testing the combination of immunotherapy with radiotherapy. J Immunother cancer 4:51, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dewan MZ, Galloway AE, Kawashima N, et al. : Fractionated but not single-dose radiotherapy induces an immune-mediated abscopal effect when combined with anti-CTLA-4 antibody. Clin Cancer Res 15:5379–5388, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Filatenkov A, Baker J, Mueller AMS, et al. : Ablative tumor radiation can change the tumor immune cell microenvironment to induce durable complete remissions. Clin Cancer Res 21:3727–3739, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Klug F, Prakash H, Huber PE, et al. : Low-dose irradiation programs macrophage differentiation to an iNOS+/M1 phenotype that orchestrates effective T cell immunotherapy. Cancer Cell 24:589–602, 2013 [DOI] [PubMed] [Google Scholar]
- 9.Tsai C-S, Chen F-H, Wang C-C, et al. : Macrophages from irradiated tumors express higher levels of iNOS, arginase-I and COX-2, and promote tumor growth. Int J Radiat Oncol Biol Phys 68:499–507, 2007 [DOI] [PubMed] [Google Scholar]
- 10.Park HJ, Griffin RJ, Hui S, et al. : Radiation-induced vascular damage in tumors: Implications of vascular damage in ablative hypofractionated radiotherapy (SBRT and SRS). Radiat Res 177:311–327, 2012 [DOI] [PubMed] [Google Scholar]
- 11.Vanpouille-Box C, Alard A, Aryankalayil MJ, et al. : DNA exonuclease Trex1 regulates radiotherapy-induced tumour immunogenicity. Nat Commun 8:15618, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shi X, Shiao SL: The role of macrophage phenotype in regulating the response to radiation therapy. Transl Res 191:64–80, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Richmond A: Nf-kappa B, chemokine gene transcription and tumour growth. Nat Rev Immunol 2:664–674, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gallo PM, Gallucci S: The dendritic cell response to classic, emerging, and homeostatic danger signals. Implications for autoimmunity. Front Immunol 4:138, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Seo A, Ishikawa F, Nakano H, et al. : Enhancement of B7-1 (CD80) expression on B-lymphoma cells by irradiation. Immunology 96:642–648, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vereecque R, Buffenoir G, Gonzalez R, et al. : Gamma-ray irradiation induces B7.1 expression in myeloid leukaemic cells. Br J Haematol 108:825–831, 2000 [DOI] [PubMed] [Google Scholar]
- 17.Noman MZ, Desantis G, Janji B, et al. : PD-L1 is a novel direct target of HIF-1α, and its blockade under hypoxia enhanced MDSC-mediated T cell activation. J Exp Med 211:781–790, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barker HE, Paget JTE, Khan AA, et al. : The tumour microenvironment after radiotherapy: Mechanisms of resistance and recurrence. Nat Rev Cancer 15:409–425, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sharabi AB, Lim M, DeWeese TL, et al. : Radiation and checkpoint blockade immunotherapy: Radiosensitisation and potential mechanisms of synergy. Lancet Oncol 16:e498–e509, 2015 [DOI] [PubMed] [Google Scholar]
- 20.Demaria S, Golden EB, Formenti SC: Role of local radiation therapy in cancer immunotherapy. JAMA Oncol 1:1325–1332, 2015 [DOI] [PubMed] [Google Scholar]
- 21.Reits EA, Hodge JW, Herberts CA, et al. : Radiation modulates the peptide repertoire, enhances MHC class I expression, and induces successful antitumor immunotherapy. J Exp Med 203:1259–1271, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chakraborty M, Abrams SI, Camphausen K, et al. : Irradiation of tumor cells up-regulates Fas and enhances CTL lytic activity and CTL adoptive immunotherapy. J Immunol 170:6338–6347, 2003 [DOI] [PubMed] [Google Scholar]
- 23.Hong JH, Chiang CS, Tsao CY, et al. : Rapid induction of cytokine gene expression in the lung after single and fractionated doses of radiation. Int J Radiat Biol 75:1421–1427, 1999 [DOI] [PubMed] [Google Scholar]
- 24.Liu W, Ding I, Chen K, et al. : Interleukin 1beta (IL1B) signaling is a critical component of radiation-induced skin fibrosis. Radiat Res 165:181–191, 2006 [DOI] [PubMed] [Google Scholar]
- 25.Petit-Frère C, Capulas E, Lyon DA, et al. : Apoptosis and cytokine release induced by ionizing or ultraviolet B radiation in primary and immortalized human keratinocytes. Carcinogenesis 21:1087–1095, 2000 [PubMed] [Google Scholar]
- 26.Ganss R, Ryschich E, Klar E, et al. : Combination of T-cell therapy and trigger of inflammation induces remodeling of the vasculature and tumor eradication. Cancer Res 62:1462–1470, 2002 [PubMed] [Google Scholar]
- 27.Lugade AA, Moran JP, Gerber SA, et al. : Local radiation therapy of B16 melanoma tumors increases the generation of tumor antigen-specific effector cells that traffic to the tumor. J Immunol 174:7516–7523, 2005 [DOI] [PubMed] [Google Scholar]
- 28.Dovedi SJ, Cheadle EJ, Popple AL, et al. : Fractionated radiation therapy stimulates antitumor immunity mediated by both resident and infiltrating polyclonal T-cell populations when combined with PD-1 blockade. Clin Cancer Res 23:5514–5526, 2017 [DOI] [PubMed] [Google Scholar]
- 29.Matsumura S, Wang B, Kawashima N, et al. : Radiation-induced CXCL16 release by breast cancer cells attracts effector T cells. J Immunol 181:3099–3107, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hallahan D, Kuchibhotla J, Wyble C: Cell adhesion molecules mediate radiation-induced leukocyte adhesion to the vascular endothelium. Cancer Res 56:5150–5155, 1996 [PubMed] [Google Scholar]
- 31.Suwa T, Saio M, Umemura N, et al. : Preoperative radiotherapy contributes to induction of proliferative activity of CD8+ tumor-infiltrating T-cells in oral squamous cell carcinoma. Oncol Rep 15:757–763, 2006 [PubMed] [Google Scholar]
- 32.Singh AK, Winslow TB, Kermany MH, et al. : A pilot study of stereotactic body radiation therapy combined with cytoreductive nephrectomy for metastatic renal cell carcinoma. Clin Cancer Res 23:5055–5065, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kelly RJ, Zaidi AH, Smith MA, et al. : The dynamic and transient immune microenvironment in locally advanced esophageal adenocarcinoma post chemoradiation. Ann Surg 268:992–999, 2018 [DOI] [PubMed] [Google Scholar]
- 34.Dorta-Estremera S, Colbert LE, Nookala SS, et al. : Kinetics of intratumoral immune cell activation during chemoradiation for cervical cancer. Int J Radiat Oncol Biol Phys 102:593–600, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lim YJ, Koh J, Kim S, et al. : Chemoradiation-induced alteration of programmed death-ligand 1 and CD8+ tumor-infiltrating lymphocytes identified patients with poor prognosis in rectal cancer: A matched comparison analysis. Int J Radiat Oncol Biol Phys 99:1216–1224, 2017 [DOI] [PubMed] [Google Scholar]
- 36.Teng F, Meng X, Kong L, et al. : Tumor-infiltrating lymphocytes, forkhead box P3, programmed death ligand-1, and cytotoxic T lymphocyte-associated antigen-4 expressions before and after neoadjuvant chemoradiation in rectal cancer. Transl Res. Elsevier Inc. 166:721–732, 2015. e1 [DOI] [PubMed] [Google Scholar]
- 37.Lim SH, Chua W, Cheng C, et al. : Effect of neoadjuvant chemoradiation on tumor-infiltrating/associated lymphocytes in locally advanced rectal cancers. Anticancer Res 34:6505–6513, 2014 [PubMed] [Google Scholar]
- 38.Qinfeng S, Depu W, Xiaofeng Y, et al. : In situ observation of the effects of local irradiation on cytotoxic and regulatory T lymphocytes in cervical cancer tissue. Radiat Res 179:584–589, 2013 [DOI] [PubMed] [Google Scholar]
- 39.Apetoh L, Ghiringhelli F, Tesniere A, et al. : Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med 13:1050–1059, 2007 [DOI] [PubMed] [Google Scholar]
- 40.Obeid M, Panaretakis T, Joza N, et al. : Calreticulin exposure is required for the immunogenicity of gamma-irradiation and UVC light-induced apoptosis. Cell Death Differ 14:1848–1850, 2007 [DOI] [PubMed] [Google Scholar]
- 41.Deng L, Liang H, Xu M, et al. : STING-Dependent Cytosolic DNA Sensing Promotes Radiation-Induced Type I Interferon-Dependent Antitumor Immunity in Immunogenic Tumors, 41. Immunity: Elsevier Inc., 543–852, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lim JYH, Gerber SA, Murphy SP, et al. : Type I interferons induced by radiation therapy mediate recruitment and effector function of CD8(+) T cells. Cancer Immunol Immunother 63:259–271, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang X, Schoenhals JE, Li A, et al. : Suppression of type I IFN signaling in tumors mediates resistance to anti-PD-1 treatment that can be overcome by radiotherapy. Cancer Res 77:839–850, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Herberman RB, Holden HT, Ting CC, et al. : Cell-mediated immunity to leukemia virus- and tumor-associated antigens in mice. Cancer Res 36:615–621, 1976 [PubMed] [Google Scholar]
- 45.Ames E, Murphy WJ: Advantages and clinical applications of natural killer cells in cancer immunotherapy. Cancer Immunol Immunother 63:21–28, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hietanen T, Pitkänen M, Kapanen M, et al. : Effects of single and fractionated irradiation on natural killer cell populations: Radiobiological characteristics of viability and cytotoxicity in vitro. Anticancer Res 35:5193–5200, 2015 [PubMed] [Google Scholar]
- 47.McGee HM, Daly ME, Azghadi S, et al. : Stereotactic ablative radiation therapy induces systemic differences in peripheral blood immunophenotype dependent on irradiated site. Int J Radiat Oncol Biol Phys 101:1259–1270, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Harrington NP, Chambers KA, Ross WM, et al. : Radiation damage and immune suppression in splenic mononuclear cell populations. Clin Exp Immunol 107:417–424, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hochman PS, Cudkowicz G, Dausset J: Decline of natural killer cell activity in sublethally irradiated mice. J Natl Cancer Inst 61:265–268, 1978 [DOI] [PubMed] [Google Scholar]
- 50.Zarcone D, Tilden AB, Lane VG, et al. : Radiation sensitivity of resting and activated nonspecific cytotoxic cells of T lineage and NK lineage. Blood 73:1615–1621, 1989 [PubMed] [Google Scholar]
- 51.Rana R, Vitale M, Mazzotti G, et al. : Radiosensitivity of human natural killer cells: binding and cytotoxic activities of natural killer cell subsets. Radiat Res 124:96–102, 1990 [PubMed] [Google Scholar]
- 52.Brovall C, Schacter B: Radiation sensitivity of human natural killer cell activity: Control by X-linked genes. J Immunol 126:2236–2239, 1981 [PubMed] [Google Scholar]
- 53.Yamazaki H, Yoshioka Y, Inoue T, et al. : Changes in natural killer cell activity by external radiotherapy and/or brachytherapy. Oncol Rep 9:359–363, 2002 [PubMed] [Google Scholar]
- 54.McGinnes K, Florence J, Penny R: The effect of radiotherapy on the natural killer (NK)-cell activity of cancer patients. J Clin Immunol 7:210–217, 1987 [DOI] [PubMed] [Google Scholar]
- 55.Mozaffari F, Lindemalm C, Choudhury A, et al. : NK-cell and T-cell functions in patients with breast cancer: Effects of surgery and adjuvant chemo- and radiotherapy. Br J Cancer 97:105–111, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mandeville R, Sidrac-Ghali S, Ajdukovic I, et al. : Early inhibition of natural and interferon-activated killers in endometrial cancer patients treated with local radiotherapy. Cancer Detect Prev 10:129–139, 1987 [PubMed] [Google Scholar]
- 57.Sonn CH, Choi JR, Kim T-J, et al. : Augmentation of natural cytotoxicity by chronic low-dose ionizing radiation in murine natural killer cells primed by IL-2. J Radiat Res 53:823–829, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pinel MI, Esteves EB, Rumjanek VM: Increased natural killer cell activity in uterine cervix cancer patients undergoing radiation therapy. Nat Immun 14:216–224, 1995 [PubMed] [Google Scholar]
- 59.Ndhlovu LC, Lopez-Vergès S, Barbour JD, et al. : Tim-3 marks human natural killer cell maturation and suppresses cell-mediated cytotoxicity. Blood 119:3734–3743, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Begovic M, Herberman R, Gorelik E: Increase in immunogenicity and sensitivity to natural cell-mediated cytotoxicity following in vitro exposure of MCA105 tumor cells to ultraviolet radiation. Cancer Res 51:5153–5159, 1991 [PubMed] [Google Scholar]
- 61.Uchida A, Mizutani Y, Nagamuta M, et al. : Effects of X-ray irradiation on natural killer (NK) cell system. I. elevation of sensitivity of tumor cells and lytic function of NK cells. Immunopharmacol Immunotoxicol. 11:507–519, 1989 [DOI] [PubMed] [Google Scholar]
- 62.Yang K-L, Wang Y-S, Chang C-C, et al. : Reciprocal complementation of the tumoricidal effects of radiation and natural killer cells. PLoS One 8:e61797, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.van Gelder M, Vanclée A, van Elssen CHMJ, et al. : Bone marrow produces sufficient alloreactive natural killer (NK) cells in vivo to cure mice from subcutaneously and intravascularly injected 4T1 breast cancer. Breast Cancer Res Treat 161:421–433, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yoon MS, Pham CT, Phan MT, et al. : Irradiation of breast cancer cells enhances CXCL16 ligand expression and induces the migration of natural killer cells expressing the CXCR6 receptor. Cytotherapy 18:1532–1542, 2016 [DOI] [PubMed] [Google Scholar]
- 65.Gasser S, Orsulic S, Brown EJ, et al. : The DNA damage pathway regulates innate immune system ligands of the NKG2D receptor. Nature 436:1186–1190, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ames E, Canter RJ, Grossenbacher SK, et al. : Enhanced targeting of stem-like solid tumor cells with radiation and natural killer cells. Oncoimmunology 4, 2015:e1036212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Heo W, Lee YS, Son CH, et al. : Radiation-induced matrix metalloproteinases limit natural killer cell-mediated anticancer immunity in NCI-H23 lung cancer cells. Mol Med Rep 11:1800–1806, 2015 [DOI] [PubMed] [Google Scholar]
- 68.Ames E, Canter RJ, Grossenbacher SK, et al. : NK cells preferentially target tumor cells with a cancer stem cell phenotype. J Immunol 195:4010–4019, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Canter RJ, Grossenbacher SK, Foltz JA, et al. : Radiotherapy enhances natural killer cell cytotoxicity and localization in pre-clinical canine sarcomas and first-in-dog clinical trial. J Immunother cancer 5:98, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nesslinger NJ, Sahota RA, Stone B, et al. : Standard treatments induce antigen-specific immune responses in prostate cancer. Clin Cancer Res 13:1493–1502, 2007 [DOI] [PubMed] [Google Scholar]
- 71.Zhang H, Liu L, Yu D, et al. : An in situ autologous tumor vaccination with combined radiation therapy and TLR9 agonist therapy. PLoS One 7:e38111, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sharabi AB, Nirschl CJ, Kochel CM, et al. : Stereotactic radiation therapy augments antigen-specific PD-1-mediated antitumor immune responses via cross-presentation of tumor antigen. Cancer Immunol Res 3:345–355, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Monjazeb AM, Kent MS, Grossenbacher SK, et al. : Blocking indolamine-2,3-dioxygenase rebound immune suppression boosts antitumor effects of radio-immunotherapy in murine models and spontaneous canine malignancies. Clin Cancer Res 22:4328–4340, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chemnitz JM, Parry RV, Nichols KE, et al. : SHP-1 and SHP-2 associate with immunoreceptor tyrosine-based switch motif of programmed death 1 upon primary human T cell stimulation, but only receptor ligation prevents T cell activation. J Immunol 173:945–954, 2004 [DOI] [PubMed] [Google Scholar]
- 75.Oestreich KJ, Yoon H, Ahmed R, et al. : NFATc1 regulates PD-1 expression upon T cell activation. J Immunol 181:4832–4839, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Garcia-Diaz A, Shin DS, Moreno BH, et al. : Interferon receptor signaling pathways regulating PD-L1 and PD-L2 expression. Cell Rep 19:1189–1201, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Deng L, Liang H, Burnette B, et al. : Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. J Clin Invest 124:687–695, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dovedi SJ, Adlard AL, Lipowska-Bhalla G, et al. : Acquired resistance to fractionated radiotherapy can be overcome by concurrent PD-L1 blockade. Cancer Res 74:5458–5468, 2014 [DOI] [PubMed] [Google Scholar]
- 79.Li A, Barsoumian HB, Schoenhals JE, et al. : IDO1 inhibition overcomes radiation-induced “rebound immune suppression” by reducing numbers of IDO1-expressing myeloid-derived suppressor cells in the tumor microenvironment. Int J Radiat Oncol Biol Phys 104:903–912, 2019 [DOI] [PubMed] [Google Scholar]
- 80.Antonia SJ, Villegas A, Daniel D, et al. : Durvalumab after chemoradiotherapy in stage III non–small-cell lung cancer. N Engl J Med 377:1919–1929, 2017 [DOI] [PubMed] [Google Scholar]
- 81.Finkel P, Frey B, Mayer F, et al. : The dual role of NK cells in antitumor reactions triggered by ionizing radiation in combination with hyperthermia. Oncoimmunology 5, 2016:e1101206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shiao SL, Ruffell B, DeNardo DG: TH2-Polarized CD4(+) T cells and macrophages limit efficacy of radiotherapy. Cancer Immunol Res 3:518–525, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ahn GO, Tseng D, Liao CH, et al. : Inhibition of Mac-1 (CD11b/CD18) enhances tumor response to radiation by reducing myeloid cell recruitment. Proc Natl Acad Sci U S A 107:8363–8368, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dovedi SJ, Lipowska-Bhalla G, Beers SA, et al. : Antitumor efficacy of radiation plus immunotherapy depends upon dendritic cell activation of effector CD8+ T cells. Cancer Immunol Res 4:621–630, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wang D, An G, Xie S, et al. : The clinical and prognostic significance of CD14(+)HLA-DR(−/low) myeloid-derived suppressor cells in hepatocellular carcinoma patients receiving radiotherapy. Tumour Biol 37:10427–10433, 2016 [DOI] [PubMed] [Google Scholar]
- 86.Reits Ea, Hodge JW, Herberts Ca, et al. : Radiation modulates the peptide repertoire, enhances MHC class I expression, and induces successful antitumor immunotherapy. J Exp Med 203:1259–1271, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sharma A, Bode B, Wenger RH, et al. : Gamma-radiation promotes immunological recognition of cancer cells through increased expression of cancer-testis antigens in vitro and in vivo. PLoS One 6:e28217, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kroon P, Gadiot J, Peeters M, et al. : Concomitant targeting of programmed death-1 (PD-1) and CD137 improves the efficacy of radiotherapy in a mouse model of human BRAFV600-mutant melanoma. Cancer Immunol Immunother 65:753–763, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Desai P, Tahiliani V, Abboud G, et al. : Batf3-dependent dendritic cells promote optimal CD8 T cell responses against respiratory poxvirus infection. J Virol 92, 2018. e00495–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Spranger S, Dai D, Horton B, et al. : Tumor-residing Batf3 dendritic cells are required for effector T cell trafficking and adoptive T cell therapy. Cancer Cell 31:711–723, 2017. e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hildner K, Edelson BT, Purtha WE, et al. : Batf3 deficiency reveals a critical role for CD8alpha+ dendritic cells in cytotoxic T cell immunity. Science 322:1097–1100, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Fuertes MB, Kacha AK, Kline J, et al. : Host type I IFN signals are required for antitumor CD8+ T cell responses through CD8{alpha}+ dendritic cells. J Exp Med 208:2005–2016, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Cameron RB, Spiess PJ, Rosenberg SA: Synergistic antitumor activity of tumor-infiltrating lymphocytes, interleukin 2, and local tumor irradiation. Studies on the mechanism of action. J Exp Med. 171:249–263, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Spiotto M, Fu YX, Weichselbaum RR: The intersection of radiotherapy and immunotherapy: mechanisms and clinical implications. Sci Immunol 1, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ushach I, Zlotnik A: Biological role of granulocyte macrophage colony-stimulating factor (GM-CSF) and macrophage colony-stimulating factor (M-CSF) on cells of the myeloid lineage. J Leukoc Biol 100:481–489, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Golden EB, Chhabra A, Chachoua A, et al. : Local radiotherapy and granulocyte-macrophage colony-stimulating factor to generate abscopal responses in patients with metastatic solid tumours: A proof-of-principle trial. Lancet Oncol 16:795–803, 2015 [DOI] [PubMed] [Google Scholar]
- 97.Pinedo HM, Buter J, Luykx-de Bakker SA, et al. : Extended neoadjuvant chemotherapy in locally advanced breast cancer combined with GM-CSF: Effect on tumour-draining lymph node dendritic cells. Eur J Cancer 39:1061–1067, 2003 [DOI] [PubMed] [Google Scholar]
- 98.Spitler LE, Grossbard ML, Ernstoff MS, et al. : Adjuvant therapy of stage III and IV malignant melanoma using granulocyte-macrophage colony-stimulating factor. J Clin Oncol 18:1614–1621, 2000 [DOI] [PubMed] [Google Scholar]
- 99.Daud AI, Mirza N, Lenox B, et al. : Phenotypic and functional analysis of dendritic cells and clinical outcome in patients with high-risk melanoma treated with adjuvant granulocyte macrophage colony-stimulating factor. J Clin Oncol 26:3235–3241, 2008 [DOI] [PubMed] [Google Scholar]
- 100.Demaria S, Ng B, Devitt ML, et al. : Ionizing radiation inhibition of distant untreated tumors (abscopal effect) is immune mediated. Int J Radiat Oncol Biol Phys 58:862–870, 2004 [DOI] [PubMed] [Google Scholar]
- 101.Chakravarty PK, Guha C, Alfieri A, et al. : Flt3L therapy following localized tumor irradiation generates long-term protective immune response in metastatic lung cancer: Its implication in designing a vaccination strategy. Oncology 70:245–254, 2006 [DOI] [PubMed] [Google Scholar]
- 102.Chakravarty PK, Alfieri A, Thomas EK, et al. : Flt3-ligand administration after radiation therapy prolongs survival in a murine model of metastatic lung cancer. Cancer Res 59:6028–6032, 1999 [PubMed] [Google Scholar]
- 103.Shortman K, Naik SH: Steady-state and inflammatory dendritic-cell development. Nat Rev Immunol 7:19–30, 2007 [DOI] [PubMed] [Google Scholar]
- 104.Guermonprez P, Helft J, Claser C, et al. : Inflammatory Flt3l is essential to mobilize dendritic cells and for T cell responses during Plasmodium infection. Nat Med 19:730–738, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wu CJ, Tsai YT, Lee IJ, et al. : Combination of radiation and interleukin 12 eradicates large orthotopic hepatocellular carcinoma through immunomodulation of tumor microenvironment. Oncoimmunology 7, 2018:e1477459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hwang WL, Pike LRG, Royce TJ, et al. : Safety of combining radiotherapy with immune-checkpoint inhibition. Nat Rev Clin Oncol 15:477–494, 2018 [DOI] [PubMed] [Google Scholar]
- 107.Baird JR, Monjazeb AM, Shah O, et al. : Stimulating innate immunity to enhance radiation therapy-induced tumor control. Int J Radiat Oncol Biol Phys 99:362–373, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Shankaran V, Ikeda H, Bruce AT, et al. : IFNgamma and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature 410:1107–1111, 2001 [DOI] [PubMed] [Google Scholar]
- 109.Steidl C, Lee T, Shah SP, et al. : Tumor-associated macrophages and survival in classic Hodgkin’s lymphoma. N Engl J Med 362:875–885, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hanada T, Nakagawa M, Emoto A, et al. : Prognostic value of tumor-associated macrophage count in human bladder cancer. Int J Urol 7:263–269, 2000 [DOI] [PubMed] [Google Scholar]
- 111.Leblond MM, Peres EA, Helaine C, et al. : M2 macrophages are more resistant than M1 macrophages following radiation therapy in the context of glioblastoma. Oncotarget 8:72597–72612, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kioi M, Vogel H, Schultz G, et al. : Inhibition of vasculogenesis, but not angiogenesis, prevents the recurrence of glioblastoma after irradiation in mice. J Clin Invest 120:694–705, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kalbasi A, Komar C, Tooker GM, et al. : Tumor-derived CCL2 mediates resistance to radiotherapy in pancreatic ductal adenocarcinoma. Clin Cancer Res 23:137–148, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.DeNardo DG, Brennan DJ, Rexhepaj E, et al. : Leukocyte complexity predicts breast cancer survival and functionally regulates response to chemotherapy. Cancer Discov 1:54–67, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Stafford JH, Hirai T, Deng L, et al. : Colony stimulating factor 1 receptor inhibition delays recurrence of glioblastoma after radiation by altering myeloid cell recruitment and polarization. Neuro Oncol 18:797–806, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Pienta KJ, Machiels JP, Schrijvers D, et al. : Phase 2 study of carlumab (CNTO 888), a human monoclonal antibody against CC-chemokine ligand 2 (CCL2), in metastatic castration-resistant prostate cancer. Invest New Drugs 31:760–768, 2013 [DOI] [PubMed] [Google Scholar]
- 117.Quail DF, Bowman RL, Akkari L, et al. : The tumor microenvironment underlies acquired resistance to CSF-1R inhibition in gliomas. Science 352, 2016. aad3018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Crittenden MR, Savage T, Cottam B, et al. : Expression of arginase I in myeloid cells limits control of residual disease after radiation therapy of tumors in mice. Radiat Res 182:182–190, 2014 [DOI] [PubMed] [Google Scholar]
- 119.Bouquet F, Pal A, Pilones KA, et al. : TGFbeta1 inhibition increases the radiosensitivity of breast cancer cells in vitro and promotes tumor control by radiation in vivo. Clin Cancer Res 17:6754–6765, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Crittenden MR, Baird J, Friedman D, et al. : Mertk on tumor macrophages is a therapeutic target to prevent tumor recurrence following radiation therapy. Oncotarget 7:78653–78666, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Aguilera TA, Rafat M, Castellini L, et al. : Reprogramming the immunological microenvironment through radiation and targeting Axl. Nat Commun 7:13898, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.David CJ, Huang YH, Chen M, et al. : TGF-β tumor suppression through a lethal EMT. Cell 164:1015–1030, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sounni NE, Dehne K, van Kempen L, et al. : Stromal regulation of vessel stability by MMP14 and TGFbeta. Dis Model Mech 3:317–332, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Friedl P, Alexander S: Cancer invasion and the microenvironment: Plasticity and reciprocity. Cell. Elsevier Inc. 147:992–1009, 2011 [DOI] [PubMed] [Google Scholar]
- 125.Calon A, Espinet E, Palomo-Ponce S, et al. : Dependency of colorectal cancer on a TGF-β-Driven program in stromal cells for metastasis initiation. Cancer Cell 22:571–584, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Thomas Da, Massagué J: TGF-beta directly targets cytotoxic T cell functions during tumor evasion of immune surveillance. Cancer Cell 8:369–380, 2005 [DOI] [PubMed] [Google Scholar]
- 127.Annes JP, Munger JS, Rifkin DB: Making sense of latent TGFbeta activation. J Cell Sci 116:217–224, 2003 [DOI] [PubMed] [Google Scholar]
- 128.Shi M, Zhu J, Wang R, et al. : Latent TGF-β structure and activation. Nature 474:343–351, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Barcellos-Hoff MH, Derynck R, Tsang ML, et al. : Transforming growth factor-beta activation in irradiated murine mammary gland. J Clin Invest 93:892–899, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Pakyari M, Farrokhi A, Maharlooei MK, et al. : Critical role of transforming growth factor beta in different phases of wound healing. Adv wound care 2:215–224, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wang X-J, Han G, Owens P, et al. : Role of TGF beta-mediated inflammation in cutaneous wound healing. J Investig dermatology Symp Proc 11:112–117, 2006 [DOI] [PubMed] [Google Scholar]
- 132.Byrne SN, Knox MC, Halliday GM: TGFbeta is responsible for skin tumour infiltration by macrophages enabling the tumours to escape immune destruction. Immunol Cell Biol 86:92–97, 2008 [DOI] [PubMed] [Google Scholar]
- 133.Bekeredjian-Ding I, Schäfer M, Hartmann E, et al. : Tumour-derived prostaglandin E and transforming growth factor-beta synergize to inhibit plasmacytoid dendritic cell-derived interferon-alpha. Immunology 128:439–450, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Weber F, Byrne SN, Le S, et al. : Transforming growth factor-beta1 immobilises dendritic cells within skin tumours and facilitates tumour escape from the immune system. Cancer Immunol Immunother 54:898–906, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Takai S, Schlom J, Tucker J, et al. : Inhibition of TGF-β1 signaling promotes central memory T cell differentiation. J Immunol 191:2299–2307, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ma C, Zhang N: Transforming growth factor-β signaling is constantly shaping memory T-cell population. Proc Natl Acad Sci U S A 112:11013–11017, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Zhang N, Bevan MJ: Transforming growth factor-β signaling controls the formation and maintenance of gut-resident memory T cells by regulating migration and retention. Immunity 39:687–696, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Mani V, Bromley SK, Äijö T, et al. : Migratory DCs activate TGF-β to precondition naïve CD8+ T cells for tissue-resident memory fate. Science 366, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kim JY, Kim YS, Kim YK, et al. : The TGF-β1 dynamics during radiation therapy and its correlation to symptomatic radiation pneumonitis in lung cancer patients. Radiat Oncol 4:1–9, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Anscher MS, Murase T, Prescott DM, et al. : Changes in plasma TGF beta levels during pulmonary radiotherapy as a predictor of the risk of developing radiation pneumonitis. Int J Radiat Oncol Biol Phys 30:671–676, 1994 [DOI] [PubMed] [Google Scholar]
- 141.Shen Z-T, Shen J-S, Ji X-Q, et al. : TGF-β1 rs1982073 polymorphism contributes to radiation pneumonitis in lung cancer patients: A meta-analysis. J Cell Mol Med 20:2405–2409, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Mariathasan S, Turley SJ, Nickles D, et al. : TGFβ attenuates tumour response to PD-L1 blockade by contributing to exclusion of T cells. Nature 554:544–548, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Chakravarthy A, Khan L, Bensler. et al. : TGF-β-associated extracellular matrix genes link cancer-associated fibroblasts to immune evasion and immunotherapy failure. Nat Commun 9:4692, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Tauriello DVF, Palomo-Ponce S, Stork D, et al. : TGFβ drives immune evasion in genetically reconstituted colon cancer metastasis. Nature 554:538–543, 2018 [DOI] [PubMed] [Google Scholar]
- 145.Garrison K, Hahn T, Lee W-C, et al. : The small molecule TGF-β signaling inhibitor SM16 synergizes with agonistic OX40 antibody to suppress established mammary tumors and reduce spontaneous metastasis. Cancer Immunol Immunother 61:511–521, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Tauriello DVF, Palomo-ponce S, Iglesias M, et al. : TGF-beta drives immune evasion in genetically reconstituted colon cancer metastasis. Nat Publ Gr 554:538–543, 2018 [DOI] [PubMed] [Google Scholar]
- 147.Ravi R, Noonan KA, Pham V, et al. : Bifunctional immune checkpoint-targeted antibody-ligand traps that simultaneously disable TGFβ enhance the efficacy of cancer immunotherapy. Nat Commun 9:741, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Holmgaard RB, Schaer DA, Li Y, et al. : Targeting the TGFβ pathway with galunisertib, a TGFβRI small molecule inhibitor, promotes anti-tumor immunity leading to durable, complete responses, as monotherapy and in combination with checkpoint blockade. J Immunother Cancer 6:1–15, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Young KH, Newell P, Cottam B, et al. : TGFβ inhibition prior to hypofractionated radiation enhances efficacy in preclinical models. Cancer Immunol Res 2:1011–1022, 2014 [DOI] [PubMed] [Google Scholar]
- 150.Miller LD, Chou JA, Black MA, et al. : Immunogenic subtypes of breast cancer delineated by gene classifiers of immune responsiveness. Cancer Immunol Res 4:600–610, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Zhang B, Bowerman Na, Salama JK, et al. : Induced sensitization of tumor stroma leads to eradication of established cancer by T cells. J Exp Med 204:49–55, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Lee Y, Auh SL, Wang YYY, et al. : Therapeutic effects of ablative radiation on local tumor require CD8+ T cells: Changing strategies for cancer treatment. Blood 114:589–595, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Gough MJ, Crittenden MR, Sarff M, et al. : Adjuvant therapy with agonistic antibodies to CD134 (OX40) increases local control after surgical or radiation therapy of cancer in mice. J Immunother 33:798–809, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Pereira BA, Vennin C, Papanicolaou M, et al. : CAF subpopulations: A new reservoir of stromal targets in pancreatic cancer. Trends in cancer 5:724–741, 2019 [DOI] [PubMed] [Google Scholar]
- 155.Monteran L, Erez N: The dark side of fibroblasts: Cancer-associated fibroblasts as mediators of immunosuppression in the tumor microenvironment. Front Immunol 10:1835, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Sousa CM, Biancur DE, Wang X, et al. : Pancreatic stellate cells support tumour metabolism through autophagic alanine secretion. Nature 536:479–483, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Ron-Harel N, Ghergurovich JM, Notarangelo G, et al. : T cell activation depends on extracellular alanine. Cell Rep 28:3011–3021, 2019. e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Costa A, Kieffer Y, Scholer-Dahirel A, et al. : Fibroblast heterogeneity and immunosuppressive environment in human breast cancer. Cancer Cell. Elsevier; 33:463–479, 2018. e10 [DOI] [PubMed] [Google Scholar]
- 159.Badea L, Herlea V, Dima SO, et al. : Combined gene expression analysis of whole-tissue and microdissected pancreatic ductal adenocarcinoma identifies genes specifically overexpressed in tumor epithelia. Hepatogastroenterology 55:2016–2027, 2008 [PubMed] [Google Scholar]
- 160.Gunderson AJ, Yamazaki T, McCarty K, et al. : Blockade of fibroblast activation protein in combination with radiation treatment in murine models of pancreatic adenocarcinoma. PLoS One 14:e0211117, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Ene-Obong A, Clear AJ, Watt J, et al. : Activated pancreatic stellate cells sequester CD8+ T cells to reduce their infiltration of the juxtatumoral compartment of pancreatic ductal adenocarcinoma. Gastroenterology 145:1121–1132, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Loeffler M, Krüger JA, Niethammer AG, et al. : Targeting tumor-associated fibroblasts improves cancer chemotherapy by increasing intratumoral drug uptake. J Clin Invest 116:1955–1962, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Nazareth MR, Broderick L, Simpson-Abelson MR, et al. : Characterization of human lung tumor-associated fibroblasts and their ability to modulate the activation of tumor-associated T cells. J Immunol 178:5552–5562, 2007 [DOI] [PubMed] [Google Scholar]
- 164.Zhu Y, Knolhoff BL, Meyer MA, et al. : CSF1/CSF1R blockade reprograms tumor-infiltrating macrophages and improves response to T-cell checkpoint immunotherapy in pancreatic cancer models. Cancer Res 74:5057–5069, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Hawinkels LJ, Paauwe M, Verspaget HW, et al. : Interaction with colon cancer cells hyperactivates TGF-β signaling in cancer-associated fibroblasts. Oncogene 33:97–107, 2014 [DOI] [PubMed] [Google Scholar]
- 166.Liao D, Luo Y, Markowitz D, et al. : Cancer associated fibroblasts promote tumor growth and metastasis by modulating the tumor immune microenvironment in a 4T1 murine breast cancer model. PLoS One 4:e7965, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Mace TA, Ameen Z, Collins A, et al. : Pancreatic cancer-associated stellate cells promote differentiation of myeloid-derived suppressor cells in a STAT3-dependent manner. Cancer Res 73:3007–3018, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Kraman M, Bambrough PJ, Arnold JN, et al. : Suppression of antitumor immunity by stromal cells expressing fibroblast activation protein-alpha. Science 330:827–830, 2010 [DOI] [PubMed] [Google Scholar]
- 169.Gottschalk S, Yu F, Ji M, et al. : A vaccine that co-targets tumor cells and cancer associated fibroblasts results in enhanced antitumor activity by inducing antigen spreading. PLoS One 8:e82658, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Levental KR, Yu H, Kass L, et al. : Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell Elsevier Ltd; 139:891–906, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Jiang H, Hegde S, Knolhoff BL, et al. : Targeting focal adhesion kinase renders pancreatic cancers responsive to checkpoint immunotherapy. Nat Med 22:851–860, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Shalapour S, Lin X-J, Bastian IN, et al. : Inflammation-induced IgA+ cells dismantle anti-liver cancer immunity. Nature 551:340–345, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Gunderson AJ, Kaneda MM, Tsujikawa T, et al. : Bruton tyrosine kinase–dependent immune cell cross-talk drives pancreas cancer. Cancer Discov 6:270–285, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Chauhan VP, Chen IX, Tong R, et al. : Reprogramming the microenvironment with tumor-selective angiotensin blockers enhances cancer immunotherapy. Proc Natl Acad Sci U S A 116:10674–10680, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Judge JL, Owens KM, Pollock SJ, et al. : Ionizing radiation induces myofibroblast differentiation via lactate dehydrogenase. Am J Physiol Lung Cell Mol Physiol 309:L879–L887, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]