Abstract
Background/Objectives
The purpose of this study was: (1) to compare body volume (BV) estimated from a 2-dimensional (2D) image analysis program (BVIMAGE), and a dual-energy x-ray absorptiometry (DXA) equation (BVDXA-Smith-Ryan) to an underwater weighing (UWW) criterion (BVUWW); (2) to compare relative adiposity (%Fat) derived from a 3-compartment (3C) model using BVIMAGE (%Fat3C-IMAGE), and a 4-compartment (4C) model using BVDXA-Smith-Ryan (%Fat4C-DXA-Smith-Ryan) to a 4C criterion model using BVUWW (%Fat4C-UWW).
Subject/Methods
Forty-eight participants were included (60% male, 22.9 ± 5.0 years, 24.2 ± 2.6 kg/m2). BVIMAGE was derived using a single digital image of each participant taken from the rear/posterior view. DXA-derived BV was calculated according to Smith-Ryan et al. Bioimpedance spectroscopy and DXA were used to measure total body water and bone mineral content, respectively, in the 3C and 4C models. A standardized mean effect size (ES) assessed the magnitude of differences between models with values of 0.2, 0.5, and 0.8 for small, moderate, and large differences, respectively. Data are presented as mean ± standard deviation.
Results
Near-perfect correlation (r = 0.998, p < 0.001) and no mean differences (p = 0.267) were observed between BVIMAGE (69.6 ± 11.5 L) and BVUWW (69.5 ± 11.4 L). No mean differences were observed between %Fat4C-DXA-Smith-Ryan and the %Fat4C-UWW criterion (p = 0.988). Small mean differences were observed between %Fat3C-IMAGE and %Fat4C-UWW (ES = 0.2, p < 0.001). %Fat3C-IMAGE exhibited smaller SEE and TE, and tighter limits of agreement than %Fat4C-DXA-Smith-Ryan.
Conclusions
The 2D image analysis program provided an accurate and non-invasive estimate of BV, and subsequently %Fat within a 3C model in generally healthy, young adults.
Introduction
Body composition analysis is a valuable predictor of clinical- and performance-related outcomes. Excessive fat mass (FM) is associated with various unfavorable health incidences, such as cardiovascular disease, diabetes, certain cancers, as well as increased morbidity and mortality [1–3]. Conversely, lower FM and higher lean mass are generally associated with the improved physical performance [4–6]. Deliberate changes in an individual’s proportions of FM and lean mass often occur via nutritional and exercise intervention programs. Thus, accurate body composition assessment is important for (1) identifying individuals at greater risk of unfavorable health outcomes associated with excess adiposity, (2) determining correlates of superior athletic performance, and (3) quantifying improvements following lifestyle interventions.
The two-compartment (2C) model is the foundation of body composition analysis, which divides the body into FM and fat-free mass (FFM) based on an individual’s body volume (BV) or total body water (TBW) and body mass [7, 8]. The primary limitation and subsequently, the greatest source of error, for 2C models is the assumption that the components of FFM remain constant across individuals [1, 9–11]. However, variations in TBW, protein mass, and bone mineral content (BMC) of FFM have been well documented [9–11]. Multicompartment models reduce the assumptions from which 2C body composition estimates are based on, by accounting for various tissues that comprise FFM [9, 11–15]. For example, a 3-compartment (3C) model derives body composition estimates from body mass, BV, and TBW; whereas a 4-compartment (4C) model includes a measure of BMC. As such, multi-compartment models are the preferred criterion reference in body composition research [9, 11–15].
The tissue components included in multi-compartment models are measured using a combination of techniques, such as underwater weighing (UWW) for BV, dual-energy x-ray absorptiometry (DXA) for BMC, and isotope dilution technique or bioimpedance spectroscopy (BIS) for TBW. Unfortunately, most of the techniques employed in laboratory-based multi-compartment models are costly, time-consuming, invasive, and are not portable. For instance, the practicality of DXA is dependent on the high cost of the equipment, the exposure of participants to low levels of radiation, and potential state-imposed radiation control regulations [16]. In addition, UWW requires substantial effort by participants as it necessitates full underwater submersion, and hence, may not be suitable for individuals who are hydrophobic or have physical limitations [16]. Thus, the use of multi-compartment modeling for body composition assessment may not be easily accessible for all practitioners.
Recent attempts to increase the practicality of multicompartment modeling have explored the potential of estimating BV from DXA [17–21]. Using DXA as a means to estimate BV decreases the amount of equipment needed, as UWW would be unnecessary. Furthermore, it would reduce a portion of the physical burden and decrease the amount of time needed for testing, as the method would allow for simultaneous assessment of both BV and BMC. Though this provides a more rapid approach for multi-compartment modeling, it still requires having access to DXA, which, as mentioned previously, may not be feasible for all practitioners.
Recently, a novel 2-dimensional (2D) image analysis program has shown to provide a valid estimate of BV estimate when compared to an UWW criterion [22]. In addition, BV derived from the 2D image analysis program was demonstrated to accurately estimate metrics of body composition when used in a 3C model [22]. This novel technique provides a simple, completely non-invasive, and portable method for estimating BV without the need for full body immersion or exposure to radiation. However, the previous validation study only focused on the relative accuracy of two 3C models and did not provide comparison to a 4C model using other BV methods, such as UWW or DXA. Currently, no research has examined the validity of BV derived from the novel 2D image analysis program when compared against a 4C criterion.
Therefore, the purpose of this study was to compare BV estimated from the novel 2D image analysis program, and a DXA equation to the criterion of UWW (BVUWW). The secondary purpose was to compare a 3C model using the 2D image analysis program and BIS, and a rapid 4C model with BV estimates from DXA to a traditional 4C model using BV derived from UWW, for relative adiposity (%Fat) determination. The hypothesis was that the 2D image and DXA would provide comparable and acceptable agreement to the criterion methods for estimating BV and subsequent %Fat via multi-compartment modeling.
Methods
Participants
This study represents data collected from a subset of participants included in a recently published study by Fedewa et al. [22]. As such, all participants met the following preestablished eligibility criteria: 18 years or older, willing to comply with the study procedures, and provide their own transportation to the testing laboratory. Forty-eight adults (29 males, 19 females) with complete body composition data (including UWW, BIS, and DXA) were included in this study. Participant characteristics are displayed in Table 1. Written informed consent was obtained from each participant prior to data collection. In addition, all participants completed a medical history form and physical activity readiness questionnaire before involvement in the current study. This study was approved by the University of Alabama’s Institutional Review Board.
Table 1.
Descriptive characteristics of study participants (n = 48).
| All (n = 48) | Female (n = 19) | Male (n = 29) | p value | |
|---|---|---|---|---|
| Age (yrs) | 22.94 ± 5.03 | 22.47 ± 4.88 | 23.24 ± 5.19 | 0.611 |
| Height (cm) | 174.37 ± 9.03 | 165.80 ± 5.35 | 179.99 ± 5.99 | <0.001 |
| Weight (kg) | 73.86 ± 12.36 | 63.06 ± 8.51 | 80.95 ± 8.95 | <0.001 |
| BMI (kg/m2) | 24.15 ± 2.56 | 22.87 ± 2.19 | 24.99 ± 2.46 | 0.002 |
| Race/ethnicity | 80.0% Caucasian | 72.7% Caucasian | 84.8% Caucasian |
Data are presented as mean ± standard deviation. p < 0.05 used to determine statistical significance.
BMI body mass index, cm centimeters, kg kilograms, yrs years.
Procedures
All testing procedures were completed during a single visit to the Exercise Physiology laboratory at the University of Alabama. Prior to arriving to the laboratory for their testing visit, participants were instructed to abstain from exercise and the ingestion of food and drink (except water) for a minimum of 12 h.
Anthropometries
For each participant, body mass was measured, to the nearest 0.1 kg, with a calibrated digital scale (Tanita BWB-800, Tanita©, Arlington Heights, IL) and standing height (without shoes) was measured to the nearest 0.1 cm with a manual stadiometer (SECA 213, Seca Ltd., Hamburg, Germany). For descriptive purposes, body mass index (BMI) was calculated as BMI = weight (kg) ÷ [height (m)]2 and reported in kg/m2.
Digital image analysis
For image procurement, participants wore snug-fitting athletic clothing that allowed for the automated 2D image analysis program to identify the necessary anatomical points of interest. Participants with long hair were instructed to pull their hair “back” and “up” to allow the digital image to show the diameter of the neck. Participants stood with their feet flat in front of a white photography background facing away from the digital camera, with weight evenly distributed on both feet. The heels were placed together with the feet pointed slightly outward at a 60° angle. Participants were required to remain motionless with arms abducted at a 45° angle away from the torso and aligned within the coronal plane, with palms facing away from the camera. Once correctly positioned, a single digital image that included the head, feet, and arms of the individual was obtained from the rear/posterior view using a 12.9 in., 64 g iPad Pro and analyzed using a commercially available application (version 0.30, made Health and Fitness, USA. www.mymadeapp.com). BV was derived from the 2D digital image (BVIMAGE), using a proprietary algorithm that automatically identifies and measures the horizontal linear diameter of various anatomical landmarks (United States Utility Patent 16/841,944), and was used in the 3C model calculation of %Fat3C-IMAGE [23]. The development of the proprietary algorithm was completed using participants not included in the current study.
Bioimpedanee speetroseopy
Hand-to-foot BIS (Imp™ SFB7, ImpediMed Limited, Queensland, Australia) was used to determine TBW for the 3C and 4C models. Multi-compartmental models of body composition assessment commonly use BIS for the estimation of TBW as it is generally accepted as a more convenient alternative to the traditional isotopic dilution criterion, when applied properly in healthy adults [24, 25]. Prior to electrode placement, sites were cleaned with alcohol pads and excess hair was removed with a razor. In accordance with the manufacturer’s specifications, electrodes were placed on the right hand and right foot with participants in a supine position with the arms ≥30° away from the body with legs separated.
Dual-energy X-ray absorptiometry
For both 4C models, BMC was estimated with a DXA scan (Lunar Prodigy, v 14.10.022, GE Healthcare, Madison, WI). Prior to each whole-body scan, DXA was calibrated according to the manufacturer’s instructions using a standard calibration block. Participants were instructed to remove shoes, all jewelry, and bulky clothing prior to the scan. Whole-body scans were performed with participants lying supine with their arms at their side and palms against their legs. Velcro straps were placed around the ankles and knees of each participant in order to prevent lower limb movement. The BMC measures from DXA were converted to total body bone mineral (Mo) [26]. In addition, BVDXA-Smith-Ryan was calculated using FM, lean mass, and BMC according to previous methods reported by Smith-Ryan et al. [17]. Specific equations for Mo and Smith-Ryan et al. are displayed in Table 2.
Table 2.
Components and calculations for all multi-compartment body composition models.
| BV (L) | TBW | BMC | FM (kg) | FFM (kg) | %Fat | |
|---|---|---|---|---|---|---|
| %Fat4C-UWW | UWW | BIS | Mo = total body BMC (kg) × 1.0436 | FM = 2.748 (BV) − 0.699 (TBW) + 1.129 (Mo) − 2.051 (BM) | FFM = BM–FM | %Fat = (FM ÷ BM) × 100 |
| %Fat4C-DXA-Smith-Ryan | BVDXA-Smith-Ryan = (FM ÷ 0.84) + (LM ÷ 1.03) + (BMC ÷ 11.63) − 3.12 | BIS | ||||
| %Fat3C-IMAGE | BVIMAGE = undisclosed proprietary algorithm | BIS | N/A | FM (kg) = 2.118 (BV) − 0.780 (TBW) − 1.354 (BM) |
BIS bioimpedance spectroscopy, BM body mass, BMC bone mineral content, BV body volume, DXA dual-energy x-ray absorptiometry, FFM fat-free mass, FM fat mass, kg kilograms, L liters, LM lean mass, Mo total body bone mineral, N/A not applicable, TBW total body water, UWW underwater weighing, %Fat relative adiposity, 3C three-compartment, 4C four-compartment.
Underwater weighing
For each participant, residual lung volume was measured on land, before UWW, using Parvo software (TrueOne 2400, Parvo Medics, Sandy, UT, UAS). All UWW occurred in a heated, custom-built tank with participants wearing form-fitted clothing or a bathing suit. Participants entered the UWW tank and were positioned into a sling seat suspended from a calibrated Chatillon® 15-kg scale (Model #1315DD-H, Largo, FL). Participants were then instructed to fully submerge underwater and maximally exhale; with all body parts submerged, the participant’s underwater weight was recorded (to the nearest 0.025 kg). Six to ten trials were completed per participant with the average of the three highest underwater weight values used to determine BVUWW for inclusion in the criterion 4C model in order to calculate %Fat4C-UWW.
Three- and four-compartment model calculations
Table 2 summarizes the components and calculations for each multi-compartment model. The BV estimates from the 2D image system (BVIMAGE) were combined with TBW and body mass for the 3C model calculation as described by Siri et al. [10]. All 4C model calculations were performed according to Wang and Shen [27]. The rapid 4C model included BV derived from DXA (BVDXA-Smith-Ryan).
Statistical analysis
Statistical analyses were performed using SPSS for Windows (SPSS 25.0, Chicago, IL). The number of participants included in the current study, exceeded the minimum number of participants (n = 34) required to detect a small difference (d = 0.2) between measures with 0.8 power, assuming a correlation between measures of 0.9 and an alpha level of 0.05. BV and %Fat values were compared using repeated measures analysis of variance, with a priori planned contrasts between the 4C criterion measure and each alternative measure (the 3C model and the DXA-based model of Smith-Ryan et al.). Bivariate correlations and univariate linear regression determined the strength of the association between BV and %Fat derived from the 4C criterion method and alternative methods. Data were screened for outliers and normal distribution with skewness or kurtosis >2 indicating non-normal distribution. Regression procedures were used to determine the Pearson’s product moment correlation coefficients (r), standard error of the estimate (SEE), and total error (TE) for the 3CIMAGE model [16, 28]. The strength of each r value was described as follows: 0.2, 0.5, and 0.8, categorized as small, moderate, and large, respectively [29]. In addition to Pearson’s product moment correlation coefficients, Lin’s concordance correlation coefficient (rc) was calculated to assess the associations of %Fat estimated from each multicompartment model. The descriptive thresholds of each rc values was defined as: <0.90 as poor, 0.90–0.95 as moderate, 0.95–0.99 as substantial, and >0.99 as almost perfect [30]. The magnitude of the differences between the 4C UWW criterion model and the alternative 3C and 4C models were assessed using a standardized mean effect size (ES), by dividing the difference between the criterion and alternative measure by the standard deviation of the criterion [31]. Threshold values for the standardized ES were 0.2, 0.5, and 0.8 for small, moderate, and large differences, respectively [29]. The Bland–Altman method was used to identify the 95% limits of agreement (LOA) for all metrics of body composition [32]. Statistical significance was determined using an alpha <0.05. All data are expressed as mean ± standard deviation (M ± SD), unless otherwise indicated.
Results
Participant age ranged from 18 to 39, with the majority of participants (79.2%) between the ages of 18 and 24. BMI ranged from 18.6 to 30.9 kg/m2, with the majority of participants (68.8%) categorized as “normal weight” (<25kg/m2). See Table 1 for additional descriptive characteristics of the study sample.
Data were normally distributed as skewness and kurtosis values were all <2. Although Mauchly’s Test of Sphericity was statistically significant, the omnibus test for potential differences between measures was statistically significant using both the Greenhouse-Geisser (p < 0.001) and Huynh-Feldt (p < 0.001) correction. Comparisons between BV and %Fat values derived from the three models are shown in Table 3. BVIMAGE (69.6 ± 11.5 L, p = 0.267) and BVDXA-Smith-Ryan (69.5 ± 11.7 L, p = 0.601) were not different than BVUWW (69.5 ± 11.4 L). In addition, BVIMAGE and BVDXA-Smith-Ryan were strongly correlated with the BVUWW criterion (r = 0.998, p < 0.001 for both) with SEE values of 0.67 and 0.73 L, respectively. The 95% LOA for BV ranged between 1.53 and −1.66%, with BVIMAGE exhibiting the lowest 95% LOA (±1.34 L) followed by BVDXA-Smith-Ryan (±1.60 L). The proportional bias indicated by the regression coefficient was statistically significant only for BVDXA-Smith-Ryan (coefficient = 0.443, p = 0.002); however, the strength of the r value was considered “moderate”.
Table 3.
Agreement between 3- and 4-compartment body composition models using body volume derived from a digital image, dual-energy x-ray absorptiometry, and underwater weighing in healthy adults.
|
|
Limits of agreement |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| M ± SD | p | ES | r | rc | SEE | TE | CE ± 1.96 SD | Upper | Lower | Trend | |
| BVUWW | 69.53 ± 11.42 | – | – | – | – | – | – | – | – | – | |
| BVDXA-Smith-Ryan | 69.59 ± 11.78 | 0.601 | 0.01 | 0.998** | 0.73 | 0.25 | 0.06 ± 1.60 | 1.53 | −1.66 | 0.443* | |
| BVIMAGE | 69.64 ± 11.57 | 0.267 | 0.01 | 0.998** | 0.67 | 0.33 | 0.11 ± 1.34 | 1.45 | −1.23 | 0.224 | |
| %Fat4C-UWW | 18.05 ± 6.40 | – | – | – | – | – | – | – | – | – | – |
| %Fat4C-DXA-Smith-Ryan | 18.04 ± 6.39 | 0.988 | 0.00 | 0.876** | 0.876 | 3.11 | 3.14 | 0.01 ± 6.24 | 6.94 | −5.54 | −0.006 |
| %Fat3C-IMAGE | 19.46 ± 6.08 | <.001 | 0.22 | 0.939** | 0.914 | 2.22 | 2.60 | 1.41 ± 4.31 | 5.72 | −2.89 | −0.152 |
BV body volume, CE constant error, DXA dual-energy x-ray absorptiometry, ES effect size, FM fat mass, FFM fat-free mass, IMAGE 2D image analysis program, M mean, r Pearson Product Moment correlation coefficient, rc Lin’s concordance correlation coefficient, SD standard deviation, SEE standard error of the estimate, TE total error, UWW underwater weighing, 3C 3-compartment, 4C 4-compartment, %Fat relative adiposity.
p < 0.05.
p < 0.001.
No statistically significant mean differences were observed for %Fat4C-DXA-Smith-Ryan when compared to the % Fat4C-UWW criterion (ES = 0.00, p = 0.988). Conversely, the mean values for %Fat3C-IMAGE were slightly higher than the criterion, however the size of the difference was small (ES = 0.22, p < 0.05). Furthermore, strong correlations were observed between methods for both body composition models, with r values of 0.88 (%Fat4C-DXA-Smith-Ryan) and 0.94 (% Fat3C-IMAGE) (p < 0.001 for both), with the strongest correlation and smallest SEE observed for the %Fat3C-IMAGE model. According to Lin’ s concordance correlation coefficient, % Fat3C-IMAGE showed substantial agreement (rc = 0.914) with the %Fat4C-UWW criterion whereas %Fat4C-DXA-Smith-Ryan exhibited poor agreement (rc = 0.876) with %Fat4C-UWW. The Bland–Altman Plots comparing the agreement of body composition metrics between the 3C and 4C models are shown in Figs. 1 and 2, with the specific values displayed in Table 3. No significant trends in regression lines of the Bland–Altman plots were observed for %Fat in either model.
Fig. 1. Bland–Altman plot of the difference between relative adiposity (%Fat) measured by the 3-compartment image-based model (%Fat3C-IMAGE) and the 4-compartment underwater weighing criterion method (%Fat4C-UWW).
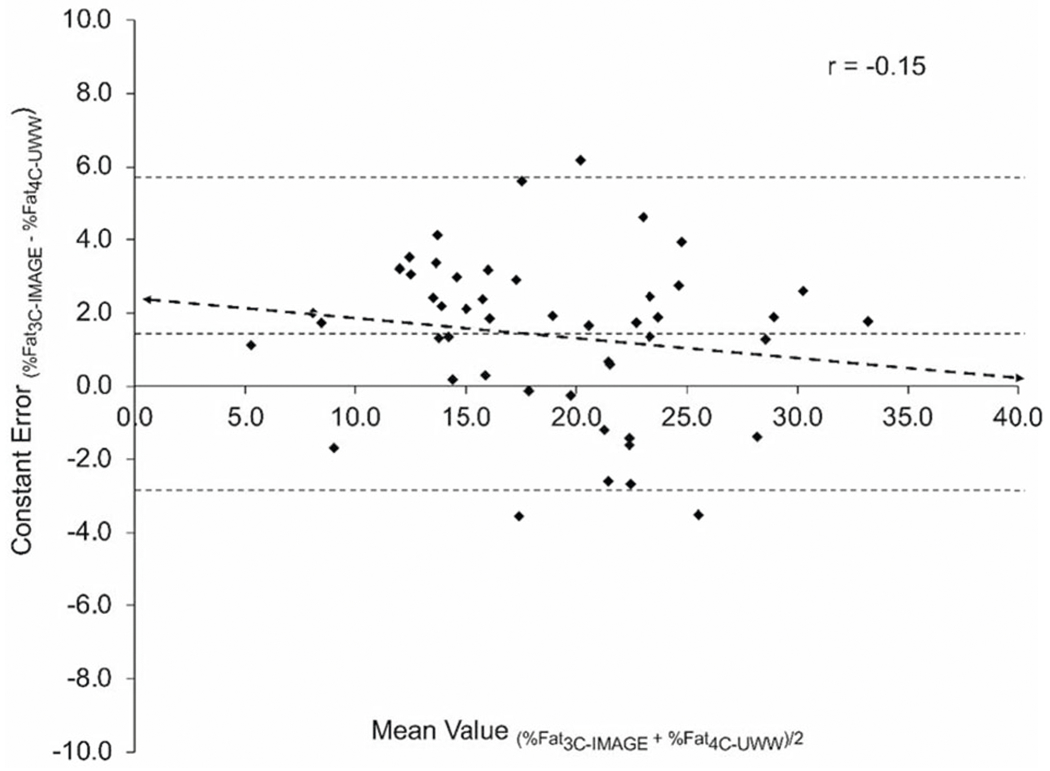
The heavy-dashed line indicates the line of best fit. The fine-dotted line in the middle indicates the mean difference, and the dotted lines at the top and bottom of the graph indicate the upper and lower 95% limits of agreement.
Fig. 2. Bland–Altman plot of the difference between relative adiposity (%Fat) measured using DXA-derived body volume (%Fat4C-Smith-Ryan) and the 4-compartment underwater weighing criterion method (%Fat4C-UWW).
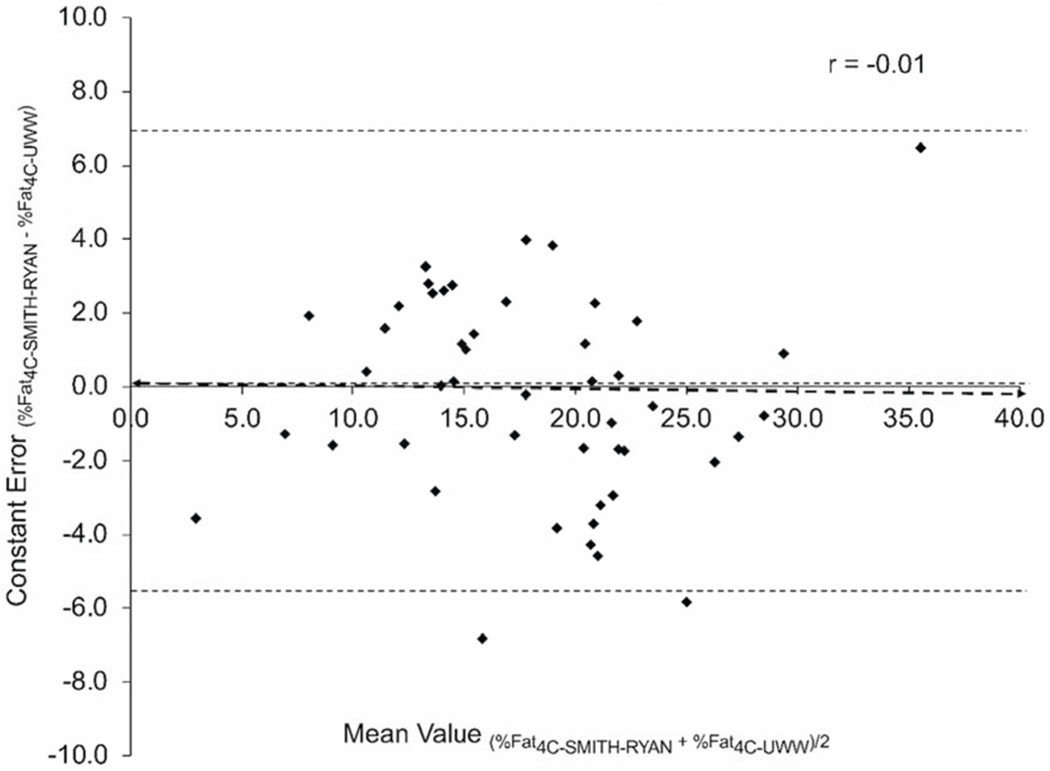
The heavy-dashed line indicates the line of best fit. The fine-dotted line in the middle indicates the mean difference, and the dotted lines at the top and bottom of the graph indicate the upper and lower 95% limits of agreement.
Discussion
The purpose of this study was to quantify the validity of BV estimates derived from a single digital image via the novel 2D image analysis program and its employment in a 3C body composition model. Furthermore, DXA-derived BV and subsequent 4C %Fat estimates were also included for comparison. Results from this study indicate that BV values derived from the novel 2D image analysis program and DXA were comparable to an UWW criterion. BV derived from the 2D image analysis program exhibited lower SEE and LOA than DXA-derived BV (BVDXA-Smith-Ryan). Despite the %Fat3C-IMAGE showing a small mean difference, it displayed the strongest correlation, lowest SEE and TE values, and tighter limits of agreement than the Smith-Ryan et al. DXA 4C %Fat method when compared to the 4C criterion. Thus, the hypotheses were accepted as these findings indicate that the novel 2D image analysis program provides a valid estimate of BV and subsequent 3C %Fat.
The current results are in line with previous research validating 2D image techniques for body composition assessment. For example, a previous investigation compared a 2D image processing system to the criterion of air displacement plethysmography for estimating BV [33]. The results showed a larger range of error compared to the current results (e.g., SEE = 1.94 L vs. 0.67 L). This discrepancy is likely attributable to differences in methodology, as the current study employed UWW as the criterion for BV assessment. Furthermore, the 2D image processing system from the previous study estimated BV from the number of pixels a person occupies within the image [33]. This process is different compared to the current 2D system that estimates BV from the dimensions of various anatomical landmarks.
In a similar investigation, Fedewa et al. demonstrated that BV could be accurately estimated with the same 2D imaging system utilized in the current study. Their results also showed excellent agreement to UWW derived BV and a laboratory 3C model for subsequent estimates of body composition when coupled with bioimpedance-derived TBW [22]. In comparison to the current study, Fedewa et al. reported lower SEE (2.07 %Fat) and TE (2.04) values, and tighter limits of agreement (±4.03) for their 2D image-derived 3C %Fat values. The slight differences in individual error analyses may be due to the different criterion methods for body composition. The previous investigation compared the agreement between a field-based and laboratory-based 3C model [22]. However, the current investigation sought to expand on previous work by comparing 3CIMAGE to the criterion of a 4C model.
Traditionally, DXA is considered the “gold standard” for BMC measures. Previous investigations have suggested DXA to be a valid method for estimating BV for use in 4C models [17–21]. However, whether 2D imaging can predict BV as well as DXA was previously unknown. Therefore, the comparison of a DXA prediction method vs. the 2D image analysis program to accepted criterion methods for BV (UWW) and body composition (laboratory 4C model) further amplify the novelty of the current study. When comparing the results of the current study, Smith-Ryan et al. reported smaller SEE and higher TE values for DXA-derived BV in their sample of 127 adults (SEE = 0.07 L, TE = 1.06) [17]. These contrasting results may be attributed to the difference in sample size as well as the criterion reference employed; Smith-Ryan et al. compared DXA-derived BV to air displacement plethysmography, whereas the current study utilized UWW as the criterion reference for BV [17]. Furthermore, the make/model of the DXA equipment used in Smith-Ryan et al.’s study (Hologic, Discovery W, Bedford, MA) was different to that of the current study (Lunar Prodigy, v 14.10.022, GE Healthcare, Madison, WI), which may have contributed towards the observed discrepancies.
Time-efficient, cost-effective, and less complex field-based measurements of BV, such as skinfold thickness (SKF), and ultrasound (US), have been well correlated with laboratory-based techniques for multi-compartment modeling. For instance, Esco et al. demonstrated that SKF and bioimpedance analysis provided acceptable agreement to UWW and BIS for the assessment of BV and TBW, respectively, when incorporated into a “field” 3C model [34]. Their results showed similar r, SEE, and TE values to the current comparisons between 3CIMAGE and the 4C criterion [34]. Additional research has further supported the use of the SKF technique for BV assessment within field-based multi-compartment modeling [9, 25]. Furthermore, Tinsley et al. suggested that a 3C model, including US for BV assessment and bioelectrical impedance analysis for TBW, was suitable for tracking changes in body composition (i.e., FM and FFM) compared to laboratory methods in resistance-trained men [35]. Most noteworthy, all of the studies in this area have shown that using a BV estimate from either SKF or US within a 3C model provided superior accuracy compared to their individual 2C prediction approaches that do not include a measure of TBW [9, 25, 34, 35].
Even though field estimates of BV may increase the utility of the 3C model among practitioners, both SKF and US methods still require a high level of technical skill and practice in order to ensure accurate results. Furthermore, because of previous research showing issues related to intrarater reliability for the SKF method [36–38], it has been suggested to be insufficient for tracking changes in body composition [38]. However, the 2D image analysis program only requires the ability to take a picture from a smartphone or tablet. Therefore, it is reasonable to consider that the potential interrater error is non-existent. Indeed, it should be noted that neither SKF nor US were included in the current study. Thus, future research is needed to compare the utility, accuracy, and reliability between multi-compartment models with BV estimated from the 2D image, SKF, and US methods.
Although thoughtfully designed, this study is not without limitations, which include the use of a small and relatively homogenous sample. The current sample included participants whose ages ranged from 18 to 29 years, predominantly classified as normal weight, and of which the vast majority were Caucasian. Therefore, results may not be sufficiently applicable to individuals outside these parameters. Thus, it is suggested that future research include a larger and more racially diverse sample. Despite its validation, it could be viewed that using BIS rather than the “gold standard” isotope dilution technique, to assess TBW, is an additional limitation of the current study. As such, it may be prudent of future research to employ isotope dilution techniques for TBW assessment. Lastly, previous research has suggested that DXA-derived BV equations may not be interchangeable across varying DXA equipment [19, 25, 39]. As such, using Smith-Ryan et al.’s DXA equation may be interpreted as a limitation despite its prevalent use in related research regardless of comparative DXA models [18, 21, 39, 40]. However, compared to the current study, Blue et al. reported smaller SEE (0.32) and TE (2.92) values for %Fat in their study which compared Smith-Ryan et al.’s DXA equation to a 4C model using a GE model DXA machine in overweight adults [21]; indicating that Smith-Ryan et al.’s DXA-based equation may be used across Hologic and GE DXA machines.
In conclusion, the 2D image analysis system appears to be an acceptable surrogate to DXA and UWW for BV measurement and multi-compartment body composition modeling. The ability to assess BV from a single digital image of an individual would simplify the assessment process immensely for clinicians and participants alike, by presenting a portable and non-invasive alternative to traditional laboratory-based techniques. The convenience that a smartphone or tablet-based method provides may allow for wider availability and increase the frequency of measurements.
Conflict of interest
MRE and MVF are co-inventors of the novel 2-dimensional image analysis system (US Utility Patent 16/841,944) which was developed as part of their ongoing research at the University of Alabama. Funding for the development of the 2-Dimensional Image Analysis Program, as well as for other laboratory equipment used in this study was provided by the University of Alabama. The University of Alabama is listed as the owner of the patent, where MRE and MVF were employed at the time of publication of this manuscript. MRE and MVF are co-owners of made Health and Fitness LLC, to which the patent is licensed for commercial use. The results of the current study do not constitute endorsement of the product by the authors. KS, BH, and CJH declare no potential conflicts of interest.
References
- 1.Borga M, West J, Bell JD, Harvey NC, Romu T, Heymsfield SB, et al. Advanced body composition assessment: from body mass index to body composition profiling. J Investig Med. 2018;66:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dhana K, Kavousi M, Ikram MA, Tiemeier HW, Hofman A, Franco OH. Body shape index in comparison with other anthropometric measures in prediction of total and cause-specific mortality. J Epidemiol Community Health. 2016;70:90–6. [DOI] [PubMed] [Google Scholar]
- 3.Jensen MD. Role of body fat distribution and the metabolic complications of obesity. J Clin Endocrinol Metab. 2008;93:S57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sternfeld B, Ngo L, Satariano WA, Tager IB. Associations of body composition with physical performance and self-reported functional limitation in elderly men and women. Am J Epidemiol. 2002;156:110–21. [DOI] [PubMed] [Google Scholar]
- 5.Hyde PN, Kendall KL, Fairman CM, Coker NA, Yarbrough ME, Rossi SJ. Use of b-mode ultrasound as a body fat estimate in collegiate football players. J Strength Conditioning Res. 2016;30: 3525–30. [DOI] [PubMed] [Google Scholar]
- 6.Suchomel TJ, Nimphius S, Stone MH. The importance of muscular strength in athletic performance. Sports Med. 2016;46:1419–49. [DOI] [PubMed] [Google Scholar]
- 7.Woodrow G Body composition analysis techniques in the aged adult: Indications and limitations. Curr Opin Clin Nutr Metab Care. 2009;12:8–14. [DOI] [PubMed] [Google Scholar]
- 8.Sheng HP, Huggins RA. A review of body composition studies with emphasis on total body water and fat. Am J Clin Nutr. 1979;32:630–47. [DOI] [PubMed] [Google Scholar]
- 9.Forslund AH, Johansson AG, Sjodin A, Bryding G, Ljunghall S, Hambraeus L. Evaluation of modified multicompartment models to calculate body composition in healthy males. Am J Clin Nutr. 1996;63:856–62. [DOI] [PubMed] [Google Scholar]
- 10.Siri WE. Body composition from fluid spaces and density: analysis of methods. Tech Measuring Body Composition. 1961;61:223–44. [PubMed] [Google Scholar]
- 11.Moon JR. Body composition in athletes and sports nutrition: An examination of the bioimpedance analysis technique. Eur J Clin Nutr. 2013;67:S54–9. [DOI] [PubMed] [Google Scholar]
- 12.Wells JC, Fewtrell MS. Measuring body composition. Arch Dis Child. 2006;91:612–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Withers RT, LaForgia J, Pillans RK, Shipp NJ, Chatterton BE, Schultz CG, et al. Comparisons of two-, three-, and four-compartment models of body composition analysis in men and women. J Appl Physiol. 1998;85:238–45. [DOI] [PubMed] [Google Scholar]
- 14.Moon JR, Smith AE, Tobkin SE, Lockwood CM, Kendall KL, Graef JL, et al. Total body water changes after an exercise intervention tracked using bioimpedance spectroscopy: a deuterium oxide comparison. Clin Nutr. 2009;28:516–25. [DOI] [PubMed] [Google Scholar]
- 15.Wilson JP, Strauss BJ, Fan B, Duewer FW, Shepherd JA. Improved 4-compartment body-composition model for a clinically accessible measure of total body protein. Am J Clin Nutr. 2013;97:497–504. [DOI] [PubMed] [Google Scholar]
- 16.Heyward V ASEP methods recommendation: body composition assessment. J Exerc Physiol Online. 2001;4:1–12. [Google Scholar]
- 17.Smith-Ryan AE, Mock MG, Ryan ED, Gerstner GR, Trexler ET, Hirsch KR. Validity and reliability of a 4-compartment body composition model using dual energy x-ray absorptiometry-derived body volume. Clin Nutr. 2017;36:825–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nickerson BS, Esco MR, Bishop PA, Kliszczewicz BM, Park KS, Williford HN. Validity of four-compartment model body fat in physically active men and women when using dxa for body volume. Int J Sport Nutr Exerc Metab. 2017;27:520–7. [DOI] [PubMed] [Google Scholar]
- 19.Nickerson BS, Fedewa MV, McLester CN, McLester JR, Esco MR. Development of a dual-energy x-ray absorptiometry-derived body volume equation in hispanic adults for administering a four-compartment model. Br J Nutr. 2020;123:1373–81. [DOI] [PubMed] [Google Scholar]
- 20.Wilson JP, Mulligan K, Fan B, Sherman JL, Murphy EJ, Tai VW, et al. Dual-energy x-ray absorptiometry-based body volume measurement for 4-compartment body composition. Am J Clin Nutr. 2012;95:25–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blue MNM, Hirsch KR, Trexler ET, Smith-Ryan AE. Validity of the 4-compartment model using dual energy x-ray absorptiometry-derived body volume in overweight individuals. Appl Physicol Nutr Metab. 2018;43:742–6. [DOI] [PubMed] [Google Scholar]
- 22.Fedewa MV, Sullivan K, Hornikel B, Holmes CJ, Metoyer CJ, Esco MR. Accuracy of a mobile 2D imaging system for body volume and subsequent composition estimates in a three-compartment model. Med Sci Sports Exerc. 2020. 10.1249/MSS.0000000000002550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fedewa MV, Esco MR. Body composition assessment using twodimensional digital image analysis. United States Provisional Patent 16/841,944 April 8, 2020.
- 24.Moon JR, Tobkin SE, Roberts MD, Dalbo VJ, Kerksick CM, Bemben MG, et al. Total body water estimations in healthy men and women using bioimpedance spectroscopy: a deuterium oxide comparison. Nutr Metab. 2008;5:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nickerson BS, Tinsley GM, Esco MR. Validity of field and laboratory three-compartment models in healthy adults. Med Sci Sports Exerc. 2019;51:1032–9. [DOI] [PubMed] [Google Scholar]
- 26.Heymsfield SB, Wang J, Heshka S, Kehayias JJ, Pierson RN. Dual-photon absorptiometry: comparison of bone mineral and soft tissue mass measurements in vivo with established methods. Am J ClinNutr. 1989;49:1283–9. [DOI] [PubMed] [Google Scholar]
- 27.Wang Z, Shen W, Withers RT, and Heymsfield SB. Multicomponent molecular-level models of body composition analysis. Champaign, IL: HumanKinetics, 2005. [Google Scholar]
- 28.Heyward VH, Stolarczyk LM. Applied body composition assessment. Champaign, IL. Human Kinetics, 1996. [Google Scholar]
- 29.Cohen J A power primer. Psychol Bull. 1992;112:155–9. [DOI] [PubMed] [Google Scholar]
- 30.McBride G A proposal for strength-of-agreement criteria for lin’s concordance correlation coefficient. NIWA Client Rep. 2005;062:62. [Google Scholar]
- 31.Lipsey MW, Wilson DB. Practical meta-analysis. Thousand Oaks, CA: SAGE publications, Inc; 2001. [Google Scholar]
- 32.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–10. [PubMed] [Google Scholar]
- 33.Moon JR, Tobkin SE, Walter AA, Smith AE, Beck TW, Cramer JT, et al. A simplified method for estimating body volume in men and women using digital image plethysmography (dip): 1667. Med Sci Sports Exerc. 2008;40:S270–S1. [Google Scholar]
- 34.Esco MR, Nickerson BS, Fedewa MV, Moon JR, Snarr RL. A novel method of utilizing skinfolds and bioimpedance for determining body fat percentage via a field-based three-compartment model. Eur J Clin Nutr. 2018;72:1431–8. [DOI] [PubMed] [Google Scholar]
- 35.Tinsley GM, Rodriguez C, White SJ, Williams AD, Stratton MT, Harty PS. A field-based three-compartment model derived from ultrasonography and bioimpedance for estimating body composition changes. Med Sci Sports Exerc. 2021;53:658–667. [DOI] [PubMed] [Google Scholar]
- 36.Wagner DR, Heyward VH. Techniques of body composition assessment: a review of laboratory and field methods. Res Q Exerc Sport. 1999;70:135–49. [DOI] [PubMed] [Google Scholar]
- 37.Lohman TG. Skinfolds and body density and their relation to body fatness: a review. Hum Biol. 1981;53:181–225. [PubMed] [Google Scholar]
- 38.Kispert CP, Merrifield HH. Interrater reliability of skinfold fat measurements. Phys Ther. 1987;67:917–20. [DOI] [PubMed] [Google Scholar]
- 39.McLester CN, Nickerson BS, Kliszczewicz BM, Hicks CS, Williamson CM, Bechke EE, et al. Validity of dxa body volume equations in a four-compartment model for adults with varying body mass index and waist circumference classifications. PLoS ONE. 2018;13:e0206866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tinsley GM. Reliability and agreement between dxa-derived body volumes and their usage in 4-compartment body composition models produced from dxa and bia values. J Sports Sci. 2018;36:1235–40. [DOI] [PubMed] [Google Scholar]


